Patient description
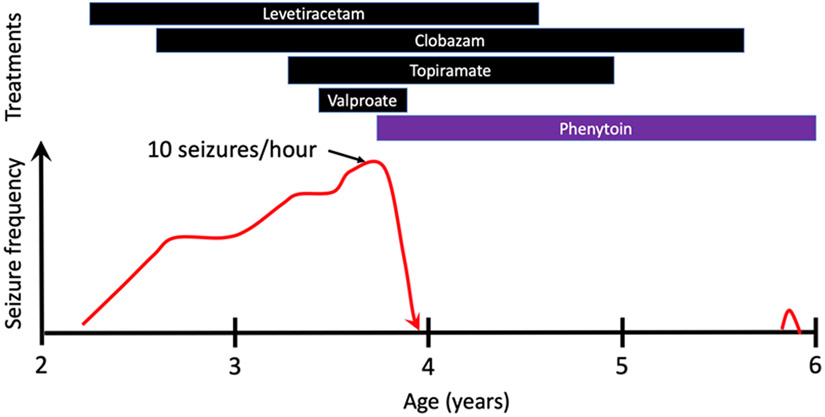
This child experienced a febrile seizure at age 26 months. At 28 months, he developed unprovoked seizures manifesting as limpness, upward eye deviation, and deep breathing, occurring every two weeks, prompting treatment with levetiracetam. At 30 months, he was hospitalized for convulsive status epilepticus. Continuous video encephalography (cvEEG) showed a background with disorganized delta and theta frequencies, continuous bitemporal slowing, paroxysmal fast activity, and generalized 1.5-Hz polyspike and wave discharges. His cvEEG at 31 months demonstrated myoclonic seizures occurring two to three times per hour, atonic seizures occurring twice a day, and bilateral tonic seizures occurring once a day. With the onset of seizures, language and memory development became impaired, and he had mild motor delay, walking at 18 to 24 months. His presentation was consistent with epilepsy with myoclonic-atonic seizures, and his seizures worsened despite the sequential addition of clobazam, topiramate, and valproate (Fig). At 42 months, cvEEG revealed atonic seizures occurring once an hour, and seizures with behavioral arrest, mild loss of tone, and myoclonic jerks occurring 10 times per hour.
Figure.
Clinical response to treatments. There was progressive worsening of seizure frequency without any sustained response to adding appropriately chosen antiseizure medications. The seizure frequency peaked at 10 times per hour, as documented on video electroencephalography. With the addition of phenytoin, seizures resolved, and other medications were weaned. There was one convulsion after weaning clobazam, in the setting of a low phenytoin level. The color version of this figure is available in the online edition.
An epilepsy gene panel (EpiSEEK, Courtagen Life Sciences) revealed a paternally inherited p.R89H monoallelic variant of uncertain significance in SCN1B. The subject’s father did not have epilepsy and completed eleventh grade education. Aside from a maternal grandmother with self-resolving childhood seizures, there was no known family history of epilepsy. There were no cardiac studies performed in the proband.
At 42 months of age, he was hospitalized because of a subacute worsening of seizure burden, and oral phenytoin was initiated. Over the ensuing month, the seizures slowed and stopped. Valproate, levetiracetam, topiramate, and then clobazam were weaned (Fig). Language development and school performance significantly improved. After two years without seizures, he had a single bilateral tonic-clonic seizure with a low phenytoin level and after weaning off clobazam.
Discussion
SCN1B was the first gene identified for genetic epilepsy with febrile seizures plus,[1] which spans a range of phenotypes including epilepsy with myoclonic-atonic seizures. SCN1B encodes the β1 and β1B subunits of voltage-gated sodium channels (VGSCs) and regulates the gating, voltage dependence, and kinetics of the pore-forming α subunit and also functions as a cell adhesion molecule.[2]
Although p.R89H was classified as a variant of uncertain significance, nearby monoallelic variants in p.R85C and p.R85H cause genetic epilepsy with febrile seizures plus[3] and biallelic p.R85C and p.R89C variants are associated with severe developmental and epileptic encephalopathy.[4,5] Several variants have been functionally evaluated using heterologous expression. Wild-type, p.R85C, and p.R85H β1 constructs were co-expressed with the VGSC α subunit Nav1.2.[6] The p.R85C and p.R85H variants resulted in loss of β1 function, including a leftward shift in the voltage dependence of sodium current activation, which can increase VGSC activity. The premise that the p.R89H variant may cause a sodium channel blocker (SCB) could help control seizures in this individual.
Because the p.R89H variant was paternally inherited and the father is not known to have epilepsy, there likely is incomplete penetrance, consistent with observations for p.C121W and p.R85C SCN1B variants.[3] Alternatively, the p.R89H variant may be unrelated to epilepsy in this individual, but his dramatic response to phenytoin suggests otherwise.
SCBs can be helpful in SCN2A- and SCN8A-related epilepsies because variants in these VGSC genes cause gain of function. In contrast, SCBs can worsen SCN1A-related Dravet syndrome, as the mechanism likely involves loss of function in inhibitory interneurons. Based on our patient’s dramatic response, we suggest that additional studies are needed to determine whether SCBs could be broadly effective in individuals with SCN1B-related epilepsy.
Comment:
This case study was deemed to be “not regulated” by the University of Michigan institutional review board (IRB), as publishing the clinical findings from a single individual does not fit the definition of human subjects research requiring IRB approval (per 45 CFR 46, 21 CFR 56 and University of Michigan policy). Written authorization from the subject’s guardian was obtained for this case report.
Louis Dang receives research funding from the NIH (NS 109289) and is a coinvestigator for a research contract from Stoke Therapeutics. Sucheta Joshi receives funding from the Health Resources and Services Administration (H98MC30374). None of these funding sources contributed to this study, nor are they relevant to this study.
Footnotes
Declarations of interest: none.
References
- [1].Wallace RH, Wang DW, Singh R, Scheffer IE, George AL, Phillips HA, et al. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B. Nat Genet 1998;19:366–70. 10.1038/1252. [DOI] [PubMed] [Google Scholar]
- [2].O’Malley HA, Isom LL. Sodium Channel β Subunits: Emerging Targets in Channelopathies. Annu Rev Physiol 2015;77:481–504. 10.1146/annurev-physiol-021014-071846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Scheffer IE, Harkin LA, Grinton BE, Dibbens LM, Turner SJ, Zielinski MA, et al. Temporal lobe epilepsy and GEFS+ phenotypes associated with SCN1B mutations. Brain 2006;130:100–9. 10.1093/brain/awl272. [DOI] [PubMed] [Google Scholar]
- [4].Aeby A, Sculier C, Bouza AA, Askar B, Lederer D, Schoonjans A-S, et al. SCN1B-linked early infantile developmental and epileptic encephalopathy. Ann Clin Transl Neurol 2019. 10.1002/acn3.50921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Darras N, Ha TK, Rego S, Martin P-M, Barroso E, Slavotinek AM, et al. Developmental and epileptic encephalopathy in two siblings with a novel, homozygous missense variant in SCN1B. Am J Med Genet 2019. 10.1002/ajmg.a.61344. [DOI] [PubMed] [Google Scholar]
- [6].Xu R, Thomas EA, Gazina EV, Richards KL, Quick M, Wallace RH, et al. Generalized epilepsy with febrile seizures plus–associated sodium channel β1 subunit mutations severely reduce beta subunit–mediated modulation of sodium channel function. Neuroscience 2007;148:164–74. 10.1016/j.neuroscience.2007.05.038. [DOI] [PubMed] [Google Scholar]