Key Points
Question
In patients with minor ischemic stroke or transient ischemic attack, how does ticagrelor and aspirin compare with clopidogrel and aspirin in preventing recurrent ischemic strokes and death?
Findings
In this network meta-analysis including 22 098 patients from 5 randomized clinical trials, dual antiplatelet therapy combining aspirin with either ticagrelor or clopidogrel was superior to aspirin alone, but there was no statistically significant difference found between ticagrelor and clopidogrel in the prevention of recurrent strokes or death.
Meaning
Findings of this study suggest that short-term dual antiplatelet therapy with aspirin and either ticagrelor or clopidogrel is effective after minor ischemic stroke or transient ischemic attack.
This network meta-analysis compares ticagrelor and aspirin with clopidogrel and aspirin in patients with acute minor ischemic stroke or transient ischemic attack in the prevention of recurrent strokes or death.
Abstract
Importance
Dual antiplatelet therapy (DAPT) with clopidogrel and aspirin is effective in preventing recurrent strokes after minor ischemic stroke or transient ischemic attack (TIA). However, there is emerging evidence for the use of ticagrelor and aspirin, and the 2 DAPT regimens have not been compared directly.
Objective
To compare ticagrelor and aspirin with clopidogrel and aspirin in patients with acute minor ischemic stroke or TIA in the prevention of recurrent strokes or death.
Data Sources
MEDLINE, Embase, and Cochrane from database inception until February 2021.
Study Selection
Randomized clinical trials that enrolled adults with acute minor ischemic stroke or TIA and provided the mentioned interventions within 72 hours of symptom onset, with a minimum follow-up of 30 days.
Data Extraction and Synthesis
PRISMA guidelines for network meta-analyses were followed. Two reviewers independently extracted data and appraised risk of bias. Fixed-effects models were fit using a bayesian approach to network meta-analysis. Between-group comparisons were estimated using hazard ratios (HRs) with 95% credible intervals (95% CrIs). Surface under the cumulative rank curve plots were produced.
Main Outcomes and Measures
The primary outcome was a composite of recurrent stroke or death up to 90 days. Secondary outcomes include major bleeding, mortality, adverse events, and functional disability. A sensitivity analysis was performed at 30 days for the primary outcome.
Results
A total of 4014 citations were screened; 5 randomized clinical trials were included. Data from 22 098 patients were analyzed, including 5517 in the clopidogrel and aspirin arm, 5859 in the ticagrelor and aspirin arm, and 10 722 in the aspirin arm. Both clopidogrel and aspirin (HR, 0.74; 95% CrI, 0.65-0.84) and ticagrelor and aspirin (HR, 0.79; 95% CrI, 0.68-0.91) were superior to aspirin in the prevention of recurrent stroke and death. There was no statistically significant difference between clopidogrel and aspirin compared with ticagrelor and aspirin (HR, 0.94; 95% CrI, 0.78-1.13). Both DAPT regimens had higher rates of major hemorrhage than aspirin alone. Clopidogrel and aspirin was associated with a decreased risk of functional disability compared with aspirin alone (HR, 0.82; 95% CrI, 0.74-0.91) and ticagrelor and aspirin (HR, 0.85; 95% CrI, 0.75-0.97).
Conclusions and Relevance
DAPT combining aspirin with either ticagrelor or clopidogrel was superior to aspirin alone, but there was no statistically significant difference found between the 2 regimens for the primary outcome.
Introduction
Patients with minor ischemic stroke or transient ischemic attack (TIA) represent a population at high risk of recurrent stroke.1,2,3,4 In the subsequent 3 months, rates of recurrent stroke range from 10% to 20%.5 It is crucial to identify secondary prevention strategies in this high-risk population to reduce morbidity and mortality.6
Multiple clinical trials established the superior efficacy of short-term dual antiplatelet therapy (DAPT) with clopidogrel and aspirin compared with antiplatelet monotherapy for secondary stroke prevention following minor stroke or TIA.7,8 This led to revised guideline recommendations from multiple organizations, including the American Heart Association Guidelines for the Prevention of Stroke in Patients With Stroke and TIA and the Heart and Stroke Foundation of Canada Stroke Best Practice Recommendations for Stroke Prevention.9,10 Subsequently, the recent publication of the Acute Stroke or Transient Ischaemic Attack Treated with Ticagrelor and ASA [acetylsalicylic acid] for Prevention of Stroke and Death (THALES) trial11 in 2020 demonstrated similar results using an alternative DAPT regimen; the combination of ticagrelor and aspirin was also superior to aspirin monotherapy in reducing the risk of stroke or death. This prompted an expedited review from the European Stroke Organization on the early use of DAPT in patients with minor stroke or TIA, which established strong recommendations supporting the use of clopidogrel and aspirin based on multiple trials and weak recommendations supporting ticagrelor and aspirin based on a single trial alone.12
To our knowledge, there are currently no large randomized clinical trials (RCTs) directly comparing these 2 DAPT regimens. Hence, we performed a systematic review and network meta-analysis (NMA) to synthesize available evidence and compare the relative efficacy and safety of ticagrelor and aspirin vs clopidogrel and aspirin in the acute minor ischemic stroke and TIA populations.
Methods
The study protocol was registered with the Open Science Framework and published in a peer-reviewed journal.13 The completed review has been prepared in consultation with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Extension Statement for Network Meta-analysis.14
Inclusion Criteria
To be eligible, participants had to be adults presenting with a National Institutes of Health Stroke Scale (NIHSS) score of 5 or less or an ABCD2 score of 4 or higher for TIA and started DAPT within 72 hours of presentation. The intervention and comparator had to be among the following, in any dose or formulation: ticagrelor and aspirin vs aspirin, clopidogrel and aspirin vs aspirin, or ticagrelor and aspirin vs clopidogrel and aspirin. The outcomes required for inclusion were recurrent stroke or death with a minimum follow-up period of 30 days. Only RCTs were included. Studies that enrolled a pediatric population, those with moderate or severe strokes, other stroke subtypes (ie, cerebral venous sinus thrombosis), or those who were administered thrombolysis or endovascular thrombectomy were excluded.13 Observational studies and studies not written in French or English were also excluded.
Literature Search
We used the following databases: MEDLINE, Embase, and Cochrane Registry of Clinical Trials and included articles from database inception until February 2021. Additionally, we searched the abstracts database from both the World Stroke Congress and International Stroke Conference for potentially relevant abstracts over the last 20 years. The search strategy was developed with the assistance of a health science librarian with expertise in systematic reviews and is detailed in eTable 1 in the Supplement.
Article and Data Extraction
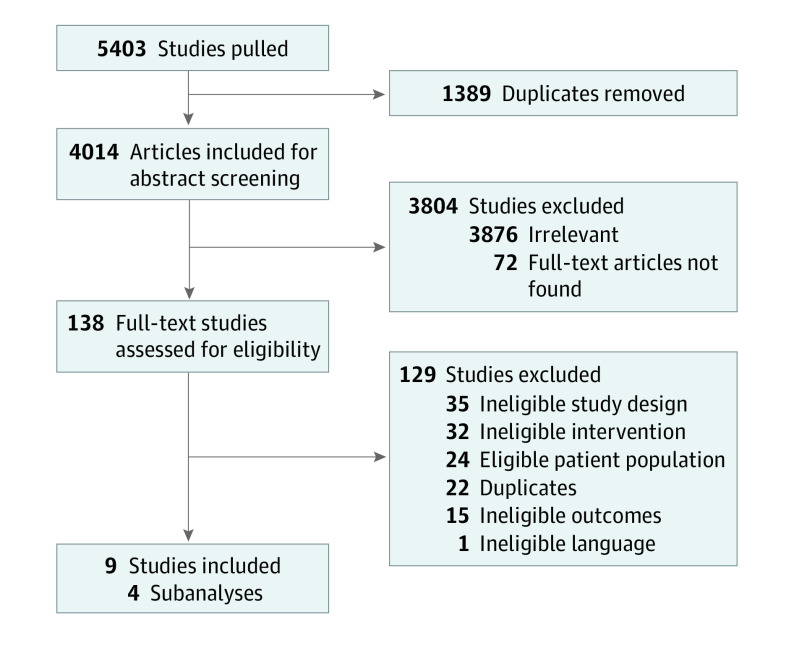
Two reviewers (S.D. and D.C.R.) independently completed level 1 (title and abstract) and level 2 (full-text) screening for articles using Covidence Systematic Review Software. A pilot exercise was conducted initially for both levels of screening to ensure consistency between reviewers. Discrepancies were settled by discussion with a third reviewer (R.L.). Findings from the screening process were summarized using a flow diagram (Figure 1).15
Figure 1. PRISMA Flow Diagram.
A data extraction form was created a priori and piloted independently by all reviewers. The data extracted included journal article characteristics (journal name, publication year, authors, and country of origin), study methodology (article type), participant characteristics (age, presence of risk factors, and intervention) and comparator details (medication name, dosage [loading dose and maintenance dose], and duration), outcome data (death, number of strokes [ischemic and hemorrhagic], functional dependence, bleeding, and adverse events), and study results (odds ratios and hazard ratios [HRs], if available).
Risk of Bias Assessment
Risk of bias of individual studies was assessed using the Cochrane Risk of Bias tool for randomized trials version 2 (RoB2).16 Raters independently implemented the tool, and any disagreement in the rating was resolved by discussion. The effect of interest that was used for the RoB2 assessment was the effect of assignment to the intervention at baseline. The main outcome assessed for risk of bias was the results of the primary outcome: recurrent stroke or death. The domains of the RoB2 assessment tool were random sequence generation, effect of assignment, missing outcome data, measurement of the outcome, and selective outcome reporting. These domains were judged using high, some concerns, or low risk of bias judgments. Findings from these assessments have been summarized pictorially.
Statistical Analysis
Prior to performance of the NMAs, we appraised the clinical and methodologic characteristics of the included studies to judge the appropriateness of the transitivity assumption. We also conducted fixed-effects (FE) pairwise meta-analyses of the available data to inspect for statistical heterogeneity of treatment effects using I2 values (eTable 2 in the Supplement).
We performed all NMAs using BUGSnet version 1.0.4 and JAGS packages in RStudio version 1.4.1106 (RStudio), evaluated under a bayesian framework with Markov Chain Monte Carlo simulation.17,18,19 Because of the limited number of studies in all connections of the treatment network and given model fit was adequate, FE models were used for all primary analyses; analyses using random-effects (RE) models have also been provided (eTable 3 in the Supplement). All NMAs were performed using a model for binary outcomes using a cloglog link function to account for differences in follow-up time. We ran the estimation with a burn-in of 25 000 iterations and sampling of 50 000 iterations from 3 chains of initial values. Model fit was assessed by comparing the posterior total residual deviance with the number of unconstrained data points.20 The selection between models was based on the deviance information criteria (DIC), with smaller values indicative of a greater fit and a difference greater than 5 points suggesting an important difference.21,22 We evaluated consistency of direct and indirect evidence by fitting an unrelated means model and comparing DIC and residuals with those from the corresponding consistency model (eTable 4 in the Supplement).21 Convergence was visually inspected using trace plots and Gelman-Rubin diagnostics.20,23 HRs with 95% credible intervals (95% CrIs) were estimated using arm-based analyses based on aggregate data from intention-to-treat analyses presented in eligible articles. We also calculated secondary measures of treatment effect for each intervention in the form of surface under the cumulative rank curve (SUCRA) probabilities and treatment rankings.19 P values were calculated using the χ2 test of heterogeneity. Significance was set at P < .05, and all P values were 2-tailed.
The primary outcome was recurrent stroke or death at 90 days. Secondary outcomes included individual components of the composite outcome (ischemic stroke, hemorrhagic stroke, and mortality), functional disability, and safety outcomes (such as major bleeding and reports of adverse events). We defined functional disability as modified Rankin Scale scores of 2 to 6. A sensitivity analysis was done of the primary outcome at 30 days to account for varying lengths of treatment duration and follow-up time.
Results
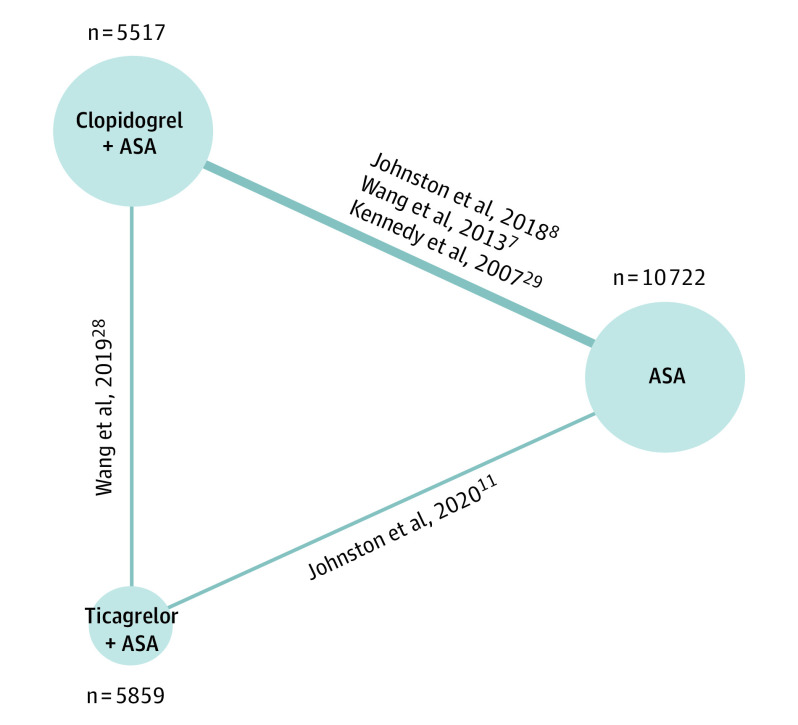
The search identified 5403 studies, and after removing duplicates, we screened 4014 unique titles and abstracts. Subsequently, we assessed 138 articles in full-text review; reasons for exclusion are provided in Figure 1. Nine articles met eligibility for extraction, representing 5 RCTs and 4 subgroup analyses (Figure 1).7,8,11,24,25,26,27,28,29 We found 3 RCTs comparing clopidogrel and aspirin with aspirin,7,8,29 1 comparing ticagrelor and aspirin with aspirin,11 and 1 comparing the 2 DAPT regimens against each other.28 In total, we analyzed data from 22 098 individuals: 10 722 received aspirin, 5517 received clopidogrel and aspirin, and 5859 received ticagrelor and aspirin; the corresponding network diagram is presented in Figure 2.
Figure 2. Network Diagram of the Total Number of Patients Analyzed in Each Treatment Arm for All 90-Day Outcomes, Excluding Functional Disability.
ASA indicates acetylsalicylic acid (aspirin).
Overview of Study and Patient Characteristics
The mean and median ages were similar across all studies (Table 1). Inclusion criteria for stroke and TIA severity were comparable across studies with ranges of 0 to 5 and 4 to 7 for NIHSS and ABCD2 scores, respectively; the Fast Assessment of Stroke and Transient Ischaemic Attack to Prevent Early Recurrence (FASTER) trial was an exception, as the ABCD2 score had not been developed at the time of the trial (Table 1).29,30 The timing from symptom onset to medication initiation was similar across studies—either within 12 or 24 hours for all trials (Table 1). The trials used similar loading and maintenance doses of clopidogrel and ticagrelor, while loading and maintenance doses for aspirin varied slightly between studies, although still within the known effective range (Table 1).31 Overall treatment periods ranged from 21 days to 90 days. The definitions of stroke, bleeding events, and adverse events for each study are outlined in eTable 5 in the Supplement and were deemed homogeneous across studies. In terms of methodological homogeneity, all studies had a low risk of bias across all RoB2 domains (eFigure 1 in the Supplement).
Table 1. Study and Patient Characteristics of Included Studies for Analysis.
| Parameter | CHANCE7 | FASTER29 | POINT8 | PRINCE28 | THALES11 |
|---|---|---|---|---|---|
| Study design | Parallel, double-blind, placebo-controlled RCT | Factorial, double-blind RCT | Parallel, double-blind, placebo-controlled RCT | Parallel, open-label RCT | Parallel, double-blind placebo-controlled RCT |
| Sample size of active/control arms | Clopidogrel and ASA: 2584; ASA: 2586 | Clopidogrel and ASA: 198; ASA: 194 | Clopidogrel and ASA: 2432; ASA: 2449 | Ticagrelor and ASA: 336; Clopidogrel and ASA: 339 | Ticagrelor and ASA: 5523; ASA: 5493 |
| Treatment window from symptom onset, h | 24 | 24 | 12 | 24 | 24 |
| NIHSS score, range | 0-3 | 0-3 | 0-3 | 0-3 | 0-5 |
| ABCD2 score, range | 4-7 | NA | 4-7 | 4-7 | 6-7 |
| DAPT treatment duration, d | 21 | 90 | 90 | 21 | 30 |
| Country of publication | China | Canada | US | China | US |
| Countries that attended the trial | China | Canada, US | Australia, Canada, Finland, France, Germany, Mexico, New Zealand, Spain, United Kingdom, US | China | Argentina, Australia, Belgium, Brazil, Bulgaria, Canada, China, Czechia, France, Germany, Hong Kong, Hungary, India, Italy, Mexico, Peru, Poland, Romania, Russia, Saudi Arabia, Slovakia, South Korea, Spain, Sweden, Taiwan, Thailand, Ukraine, Vietnam |
| Clopidogrel | |||||
| Loading dose, mg | 300 | 300 | 600 | 300 | NA |
| Maintenance dose, mg | 75 | 75 | 75 | 75 | NA |
| Maintenance duration, d | 2-90 | 2-90 | 2-90 | 2-90 | NA |
| Ticagrelor | |||||
| Loading dose, mg | NA | NA | NA | 180 | 180 |
| Maintenance dose, mg | NA | NA | NA | 90 | 90 |
| Maintenance duration, d | NA | NA | NA | 2-90 | 2-30 |
| ASA | |||||
| Loading dose, mg | 75-300 | 162 | 50-325 | 100-300 | 300-325 |
| Maintenance dose, mg | 75 | 81 | 50-325 | 100 | 75-100 |
| Maintenance duration, d | Clopidogrel and ASA: 2-21; ASA: 2-90 | Clopidogrel and ASA: 2-90; ASA: 2-90 | Clopidogrel and ASA: 2-90; ASA: 2-90 | Ticagrelor and ASA: 2-21; Clopidogrel and ASA: 2-21 | Ticagrelor and ASA: 2-30; ASA: 2-30 |
| Follow-up period, d | 90 | 90 | 90 (76-104) | 7-90 | 30-60 |
| Median age, y | Clopidogrel and ASA: 63; ASA: 62 | Clopidogrel and ASA mean age: with simvastatin, 67.1; without simvastatin, 68.9; ASA mean age: with simvastatin, 66.6; without simvastatin, 69.8 | Clopidogrel and ASA: 65 ASA: 65 | Ticagrelor and ASA: 62; clopidogrel and ASA: 61 | Ticagrelor and ASA mean age: 65.2; ASA mean age: 65.1 |
| Male, % | 66.2 | 52.8 | 55.1 | 73.2 | 61.7 |
| White, % | NA | 91.8 | 75.1 | NA | 53.8 |
Abbreviations: ASA, acetylsalicylic acid (aspirin); DAPT, dual antiplatelet therapy; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale; RCT, randomized clinical trial.
Figure 2 shows the networks of eligible treatment comparisons for the primary outcome—a composite of stroke or death up to 90 days. All other outcomes, except for functional disability and mortality, were reported in all 22 098 patients from a total of 5 trials evaluating 3 classes of interventions (Figure 2).7,8,11,28,29 Functional disability and 30-day composite outcomes were both reported in 21 031 of 22 098 patients (95.2%) from a total of 3 trials (eFigure 2 in the Supplement),7,8,11 and mortality up to 90 days was reported in 21 706 of 22 098 patients (98.2%) from a total of 4 trials (eFigure 3 in the Supplement).7,8,11,28
For all outcomes, comparisons of total posterior residual deviance with the number of unconstrained data points for all FE and RE NMAs suggested fit was adequate. Comparisons of DIC suggested no important differences in fit between FE and RE consistency models, and therefore results from FE models are presented for all analyses (eTable 4 in the Supplement). Comparisons of DIC and inspection of leverage plots provided no suggestions of violation of the consistency assumption. Details of model fit statistics and checks for inconsistency are provided in eTable 4 and eFigure 4 in the Supplement.
Primary Outcome: 90-Day Composite of Recurrent Stroke or Mortality
Clopidogrel and aspirin and ticagrelor and aspirin were both more efficacious in preventing stroke or death compared with aspirin alone (clopidogrel: HR, 0.74; 95% CrI, 0.65-0.84; ticagrelor: HR, 0.79; 95% CrI, 0.68-0.91; Table 2). There was no significant difference when comparing clopidogrel and aspirin with ticagrelor and aspirin (HR, 0.94; 95% CrI, 0.78-1.13). Clopidogrel and aspirin had the highest SUCRA value for our primary outcome at 0.76, followed by ticagrelor and aspirin (SUCRA, 0.24), and aspirin alone (SUCRA, 0; Table 2).
Table 2. Fixed-Effects Model Measures for Efficacy and Safety Up to 90 Days and 30 Days for Studies With Available Dataa.
| Outcome measure | Estimates from NMA | |||||
|---|---|---|---|---|---|---|
| HR (95% CrI) | SUCRAb | |||||
| Clopidogrel and ASA vs ASA | Ticagrelor and ASA vs ASA | Clopidogrel and ASA vs ticagrelor and ASA | Clopidogrel and ASA | Ticagrelor and ASA | ASA | |
| Outcomes up to 90 d | ||||||
| Primary composite outcome of stroke and death | 0.74 (0.65-0.84)c | 0.79 (0.68-0.91)c | 0.94 (0.78-1.13) | 0.76 | 0.24 | 0 |
| Ischemic stroke only | 0.71 (0.62-0.82)c | 0.74 (0.64-0.86)c | 0.96 (0.79-1.17) | 0.66 | 0.34 | 0 |
| Hemorrhagic stroke only | 0.99 (0.54-1.83) | 1.48 (0.63-3.61) | 0.67 (0.26-1.70) | 0.46 | 0.12 | 0.42 |
| Mortality | 0.64 (0.45-0.91)c | 0.53 (0.36-0.77)c | 1.20 (0.76-1.90) | 0.22 | 0.78 | 0 |
| Major hemorrhage | 1.78 (1.09-2.92)c | 2.63 (1.51-4.82)c | 0.68 (0.33-1.33) | 0.01 | 0 | 0.99 |
| Adverse events | 1.03 (0.89-1.18) | 0.86 (0.75-1.00) | 1.19 (0.98-1.44) | 0.04 | 0.94 | 0.02 |
| Functional disability | 0.82 (0.74-0.91)c | 0.96 (0.89-1.04) | 0.85 (0.75-0.97)c | 0.99 | 0.01 | 0 |
| Outcomes up to 30 d | ||||||
| Composite outcome of stroke and death | 0.68 (0.59-0.79)c | 0.82 (0.71-0.95)c | 0.83 (0.68-1.02) | 0.96 | 0.04 | 0 |
Abbreviations: ASA, acetylsalicylic acid (aspirin); CrI, credible interval; NMA, network meta-analysis; HR, hazard ratio; SUCRA, surface under the cumulative rank curve.
Thirty-day outcomes and functional disability only include data from the THALES, POINT, and CHANCE trials; mortality only included data from the THALES, POINT, CHANCE, and PRINCE trials.
Values nearest 1 denote preferred treatment.
P < .05.
Ischemic Stroke
Ischemic strokes contributed most to the primary composite outcome. Compared with aspirin, clopidogrel and aspirin (HR, 0.71; 95% CrI, 0.62-0.82) and ticagrelor and aspirin (odds ratio, 0.74; 95% CrI, 0.64-0.86) resulted in significant reductions in recurrent ischemic stroke (Table 2). SUCRA values suggest that clopidogrel and aspirin may be associated with the lowest risk of recurrent ischemic stroke at 90 days (SUCRA, 0.66), while aspirin appeared to have the highest risk (SUCRA, 0; Table 2).
Hemorrhagic Stroke
Neither DAPT regimen resulted in a significant increase of hemorrhagic stroke compared with aspirin (Table 2). There was also no significant difference in the number of hemorrhagic strokes between the 2 DAPT regimens (HR, 0.67; 95% CrI, 0.26-1.70; Table 2). Based on SUCRA values, ticagrelor and aspirin was associated with the highest risk of hemorrhagic stroke (SUCRA, 0.12; Table 2).
Major Hemorrhage
Clopidogrel and aspirin and ticagrelor and aspirin both resulted in a significant increase in major hemorrhage compared with aspirin alone (clopidogrel: HR, 1.78; 95% CrI, 1.09-2.92; ticagrelor: HR, 2.63; 95% CrI, 1.51-4.82; Table 2). SUCRA values further suggest that aspirin is associated with the lowest risk of major hemorrhage (SUCRA, 0.99), followed by clopidogrel and aspirin (SUCRA, 0.01) and ticagrelor and aspirin (SUCRA, 0; Table 2).
Functional Disability
Clopidogrel and aspirin was associated with a lower risk of 90-day functional disability compared with aspirin alone and compared with ticagrelor and aspirin (clopidogrel: HR, 0.82; 95% CrI, 0.74-0.91; ticagrelor: HR, 0.85; 95% CrI, 0.75-0.97). SUCRA values confirmed clopidogrel and aspirin to be the best treatment, with a SUCRA value of 0.99, compared with 0.01 for ticagrelor and aspirin and 0 for aspirin monotherapy (Table 2).
Mortality and Serious Adverse Events
There was no difference in the risk of 90-day mortality or adverse events between any of the treatment regimens (Table 2). However, SUCRA values can provide some indication of possible ordering of treatments based on probabilities of being the best treatment: ticagrelor and aspirin was associated with the highest SUCRA value (0.78) for mortality, while aspirin alone was associated with the lowest SUCRA value (0.09). The complete definitions for individual outcomes in each included trial are included in eTable 5 in the Supplement; while the FASTER and Clopidogrel in High-Risk Patients With Acute Non-Disabling Cerebrovascular Events (CHANCE) trials included study outcomes (ie, hemorrhagic strokes) in their definitions for adverse events,7,29 the Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) and THALES trials excluded components of the primary efficacy outcome measures, including strokes.8,11
Sensitivity Analysis for 30-Day Recurrent Stroke and Mortality
Compared with aspirin, both clopidogrel and aspirin as well as ticagrelor and aspirin resulted in a significant reduction of recurrent stroke or mortality at 30 days (clopidogrel: HR, 0.68; 95% CrI, 0.59-0.79; ticagrelor: HR, 0.82; 95% CrI, 0.71-0.95; Table 2). There was no significant difference when comparing clopidogrel and aspirin with ticagrelor and aspirin (HR, 0.83; 95% CrI, 0.68-1.02; Table 2). Based on SUCRA values, clopidogrel and aspirin was associated with the lowest risk of recurrent stroke or death up to 30 days (SUCRA, 0.96), followed by ticagrelor and clopidogrel (SUCRA, 0.04) and aspirin (SUCRA, 0).
Discussion
In this systematic review and NMA, we found no statistically significant difference in efficacy between clopidogrel and aspirin as well as ticagrelor and aspirin in the prevention of recurrent strokes or death up to 90 days. Moreover, we have confirmed that either regimen of DAPT was superior to aspirin alone. Ischemic strokes made up most of the events in the composite primary outcome, and there was no difference noted between the 2 DAPT regimens in preventing their recurrence. While both DAPT regimens were associated with higher rates of major bleeding compared with aspirin alone, no difference was noted between ticagrelor and aspirin and clopidogrel and aspirin. Lastly, clopidogrel and aspirin was associated with a lower risk of functional disability compared with aspirin alone and ticagrelor and aspirin.
Calculated probabilities using SUCRA plots revealed that ranking of the regimens assessed in the prevention of the primary outcome is as follows: clopidogrel and aspirin had the highest SUCRA value (ie, suggesting it is likely the best treatment), followed by ticagrelor and aspirin and aspirin monotherapy. Rankings based on SUCRA values should be interpreted with caution, particularly since comparisons of HRs across treatments were not significant for most secondary outcomes.
Our results are in line with the latest rapid review from the European Stroke Organization stating stronger evidence for the acute, short-term use of clopidogrel and aspirin compared with ticagrelor and aspirin in the minor ischemic stroke and TIA population.12 This is largely because of a greater number of trials evaluating the use of clopidogrel and aspirin in our study cohort.7,8,29 Conversely, the THALES trial was the only RCT evaluating the use of ticagrelor and aspirin compared with aspirin,11 resulting in a lower level of evidence recommendation. We provide further evidence to support this recommendation, as our rankings suggest clopidogrel and aspirin was the best treatment in preventing recurrent strokes and death, both at 30 days and 90 days. Furthermore, there was a trend toward favoring clopidogrel and aspirin compared with ticagrelor and aspirin for prevention of functional disability and bleeding-related complications. This is similar to previous meta-studies in the cardiology literature, reporting higher events of bleeding with ticagrelor DAPT compared with clopidogrel DAPT following myocardial infarction and percutaneous coronary intervention.32,33
Despite strong existing evidence to support the use of clopidogrel and aspirin in acute minor ischemic stroke and TIA,1,9,10 clopidogrel may not be a universal therapy, as genetic variations may render the drug ineffective. Clopidogrel is a prodrug that requires enzyme activation by CYP2C19 to be converted to its active metabolite form.34 Individuals who carry nonfunctional copies of CYP2C19 are considered nonmetabolizers and may clinically present with recurrent ischemic events despite medication compliance.35,36 The prevalence of clopidogrel resistance in the general population is highly variable in the literature, largely depending on the race and ethnicity of the population under study and definitions used.37 It is estimated that approximately 30% of the US population carries a loss-of-function allele for the CYP2C19 gene, and in Chinese populations, it may be as high as 60%.36,38 Despite this, clopidogrel and aspirin seems to be an effective therapy for stroke prevention even when tested in a predominantly Chinese population (eg, CHANCE trial7), and therefore, the clinical significance of clopidogrel resistance remains unknown. Recently, the introduction of genetic testing in medicine has opened the door to new possibilities for personalized medicine: the genetic substudy of the CHANCE trial showed that clopidogrel and aspirin reduced the risk of stroke recurrence only in noncarriers of the CYP2C19 loss-of-function allele and not in carriers.39 However, routine genetic testing for clopidogrel resistance is not currently recommended for any indication. If a patient clinically presents with clopidogrel failure, an effective and safe alternative medication may be needed.38,39 Our results support the use of ticagrelor and aspirin as an effective alternative therapy for these patients, given its comparable efficacy in prevention of recurrent strokes and death. The ongoing CHANCE-2 clinical trial in China will provide further insight after completion,40 as it randomizes patients with minor stroke or TIA with CYP2C19 loss-of-function allele to either ticagrelor and aspirin or clopidogrel and aspirin.41 In the future, the choice of DAPT may be more precise and individualized based on genotype testing of CYP2C19. Until then, our results provide a direct comparison between the efficacy and safety of clopidogrel and aspirin vs ticagrelor and aspirin in the minor stroke and TIA populations.
Strengths and Limitations
The strengths of our study include the publication of an a priori protocol and comprehensive search strategy encompassing 3 databases.13 Furthermore, the NMA is unique in its ability to produce rankings of treatments based on calculated probabilities, which is novel for this topic.12,42 Nonetheless, there are a number of limitations that must be considered. First, by focusing on RCTs, we may have overlooked potential data from nonrandomized and gray literature. However, we believe this is also a strength, as it focuses only on article types with the highest hierarchy of evidence. Second, treatment durations differed between studies: patients enrolled in the CHANCE and Platelet Reactivity in Acute Stroke or Transient Ischaemic Attack (PRINCE) trials received DAPT for 21 days,7,28 those in the THALES trial received DAPT for 30 days,11 and patients in the POINT and FASTER trials were on DAPT for 90 days.8,29 Furthermore, the THALES trial only reported outcomes up to 30 days. While our statistical analysis strategy using HRs accounts for varying follow-up times, this does not replace RCT-level data that actually follows patients for the entire 90-day period. To account for differences in treatment durations, we performed a post hoc analysis looking at the primary composite outcome at 30 days, which revealed similar findings. Owing to incomplete reporting of specific outcomes across trials, we were unable to report our prespecified primary outcome (ie, ischemic stroke alone at 30 days). However, we believe that the composite outcome of recurrent strokes or death is more clinically impactful than recurrent ischemic strokes and is in line with the primary outcomes reported across individual trials. Next, assessment of publication bias is difficult in an NMA owing to limited numbers of studies for each pairwise comparison.14 Additionally, given the slight differences in inclusion and exclusion criteria between studies, there may be residual clinical and statistical heterogeneity between trials, although this is felt to be minimal.
Conclusions
In this NMA, DAPT was superior to aspirin in the prevention of recurrent strokes or death, but no statistically significant difference was found between clopidogrel and aspirin and ticagrelor and aspirin. The use of clopidogrel and aspirin was associated with a decreased risk for functional disability compared with ticagrelor and aspirin. Our study suggests aspirin and ticagrelor is a reasonable alternative to aspirin and clopidogrel where there is clopidogrel failure or intolerance.
eTable 1. Search strategy used for all databases (Embase, Cochrane, and Medline).
eTable 2. Fixed-effects pairwise meta-analysis and homogeneity for outcomes.
eTable 3. Random-effects (RE) model measures for efficacy and safety for studies with available data.
eTable 4. Summary of fixed-effects and random-effects model fit statistics from network meta-analysis by outcome.
eTable 5. Definitions of stroke and bleeding events across all included trials.
eFigure 1. Risk of bias assessment for all included randomized clinical trials using the Cochrane Risk of Bias tool for randomized trials.
eFigure 2. Network diagram of total number of patients analyzed in each treatment arm for 30-day sensitivity analysis and functional disability up to 90 days.
eFigure 3. Network diagram of total number of patients analyzed in each treatment arm for mortality.
eFigure 4. Leverage plots of fixed-effects consistency and inconsistency models for network meta-analysis by outcome.
References
- 1.Hao Q, Tampi M, O’Donnell M, Foroutan F, Siemieniuk RA, Guyatt G. Clopidogrel plus aspirin versus aspirin alone for acute minor ischaemic stroke or high risk transient ischaemic attack: systematic review and meta-analysis. BMJ. 2018;363:k5108. doi: 10.1136/bmj.k5108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khare S. Risk factors of transient ischemic attack: an overview. J Midlife Health. 2016;7(1):2-7. doi: 10.4103/0976-7800.179166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amarenco P, Lavallée PC, Monteiro Tavares L, et al. ; TIAregistry.org Investigators . Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378(23):2182-2190. doi: 10.1056/NEJMoa1802712 [DOI] [PubMed] [Google Scholar]
- 4.Zhang C, Zhao X, Wang C, et al. ; Chinese IntraCranial AtheroSclerosis (CICAS) Study Group . Prediction factors of recurrent ischemic events in one year after minor stroke. PLoS One. 2015;10(3):e0120105. doi: 10.1371/journal.pone.0120105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coutts SB, Hill MD, Campos CR, et al. ; VISION study group . Recurrent events in transient ischemic attack and minor stroke: what events are happening and to which patients? Stroke. 2008;39(9):2461-2466. doi: 10.1161/STROKEAHA.107.513234 [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Johnston SC, Bath PM, et al. Acute dual antiplatelet therapy for minor ischaemic stroke or transient ischaemic attack. BMJ. 2019;364:l895. doi: 10.1136/bmj.l895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Wang Y, Zhao X, et al. ; CHANCE Investigators . Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369(1):11-19. doi: 10.1056/NEJMoa1215340 [DOI] [PubMed] [Google Scholar]
- 8.Johnston SC, Easton JD, Farrant M, et al. ; Clinical Research Collaboration, Neurological Emergencies Treatment Trials Network, and the POINT Investigators . Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379(3):215-225. doi: 10.1056/NEJMoa1800410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 10.Wein T, Lindsay MP, Côté R, et al. ; Heart and Stroke Foundation Canadian Stroke Best Practice Committees . Canadian stroke best practice recommendations: secondary prevention of stroke, sixth edition practice guidelines, update 2017. Int J Stroke. 2018;13(4):420-443. doi: 10.1177/1747493017743062 [DOI] [PubMed] [Google Scholar]
- 11.Johnston SC, Amarenco P, Denison H, et al. ; THALES Investigators . Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. N Engl J Med. 2020;383(3):207-217. doi: 10.1056/NEJMoa1916870 [DOI] [PubMed] [Google Scholar]
- 12.Dawson J, Merwick Á, Webb A, Dennis M, Ferrari J, Fonseca AC; European Stroke Organisation . European Stroke Organisation expedited recommendation for the use of short-term dual antiplatelet therapy early after minor stroke and high-risk TIA. Eur Stroke J. 2021;6(2):CLXXXVII-CXCI. doi: 10.1177/23969873211000877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zitikyte G, Roy DC, Dhaliwal S, et al. Ticagrelor vs clopidogrel in addition to aspirin in minor ischemic stroke/transient ischemic attack—protocol for a systematic review and network meta-analysis. PLoS One. 2021;16(4):e0250553. doi: 10.1371/journal.pone.0250553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(n71):n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 17.Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing meta-analysis in R. Accessed April 29, 2021. https://bookdown.org/MathiasHarrer/Doing_Meta_Analysis_in_R/
- 18.Tonin FS, Rotta I, Mendes AM, Pontarolo R. Network meta-analysis: a technique to gather evidence from direct and indirect comparisons. Pharm Pract (Granada). 2017;15(1):943. doi: 10.18549/PharmPract.2017.01.943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163-171. doi: 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 20.Béliveau A, Boyne DJ, Slater J, Brenner D, Arora P. BUGSnet: an R package to facilitate the conduct and reporting of Bayesian network Meta-analyses. BMC Med Res Methodol. 2019;19(1):196. doi: 10.1186/s12874-019-0829-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU technical support document 4: inconsistency in networks of evidence based on randomised controlled trials. Accessed March 10, 2021. https://research-information.bris.ac.uk/en/publications/nice-dsu-technical-support-document-4-inconsistency-in-networks-o [PubMed]
- 22.Toft N, Innocent GT, Gettinby G, Reid SWJ. Assessing the convergence of Markov Chain Monte Carlo methods: an example from evaluation of diagnostic tests in absence of a gold standard. Prev Vet Med. 2007;79(2-4):244-256. doi: 10.1016/j.prevetmed.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 23.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7(4):434-455. doi: 10.1080/10618600.1998.10474787 [DOI] [Google Scholar]
- 24.Johnston SC, Elm JJ, Easton JD, et al. ; POINT and Neurological Emergencies Treatment Trials Network Investigators . Time course for benefit and risk of clopidogrel and aspirin after acute transient ischemic attack and minor ischemic stroke. Circulation. 2019;140(8):658-664. doi: 10.1161/CIRCULATIONAHA.119.040713 [DOI] [PubMed] [Google Scholar]
- 25.Tillman H, Johnston SC, Farrant M, et al. Risk for major hemorrhages in patients receiving clopidogrel and aspirin compared with aspirin alone after transient ischemic attack or minor ischemic stroke: a secondary analysis of the POINT randomized clinical trial. JAMA Neurol. 2019;76(7):774-782. doi: 10.1001/jamaneurol.2019.0932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Zhao X, Johnston SC, et al. ; CHANCE investigators . Effect of clopidogrel with aspirin on functional outcome in TIA or minor stroke: CHANCE substudy. Neurology. 2015;85(7):573-579. doi: 10.1212/WNL.0000000000001844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cucchiara B, Elm J, Easton JD, et al. Disability after minor stroke and transient ischemic attack in the POINT trial. Stroke. 2020;51(3):792-799. doi: 10.1161/STROKEAHA.119.027465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Chen W, Lin Y, et al. ; PRINCE Protocol Steering Group . Ticagrelor plus aspirin versus clopidogrel plus aspirin for platelet reactivity in patients with minor stroke or transient ischaemic attack: open label, blinded endpoint, randomised controlled phase II trial. BMJ. 2019;365:l2211. doi: 10.1136/bmj.l2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuk AM, Buchan AM; FASTER Investigators . Fast Assessment of Stroke and Transient Ischaemic Attack to Prevent Early Recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6(11):961-969. doi: 10.1016/S1474-4422(07)70250-8 [DOI] [PubMed] [Google Scholar]
- 30.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283-292. doi: 10.1016/S0140-6736(07)60150-0 [DOI] [PubMed] [Google Scholar]
- 31.Johnson ES, Lanes SF, Wentworth CE III, Satterfield MH, Abebe BL, Dicker LW. A metaregression analysis of the dose-response effect of aspirin on stroke. Arch Intern Med. 1999;159(11):1248-1253. doi: 10.1001/archinte.159.11.1248 [DOI] [PubMed] [Google Scholar]
- 32.Alfredsson J, Omar K, Csog J, Venetsanos D, Janzon M, Ekstedt M. Bleeding complications with clopidogrel or ticagrelor in ST-elevation myocardial infarction patients—a real life cohort study of two treatment strategies. Int J Cardiol Heart Vasc. 2020;27:100495. doi: 10.1016/j.ijcha.2020.100495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan W, Lu H, Yang K. Choosing between ticagrelor and clopidogrel following percutaneous coronary intervention: a systematic review and meta-analysis (2007-2017). Medicine (Baltimore). 2018;97(43):e12978. doi: 10.1097/MD.0000000000012978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasan MS, Basri HB, Hin LP, Stanslas J. Genetic polymorphisms and drug interactions leading to clopidogrel resistance: why the Asian population requires special attention. Int J Neurosci. 2013;123(3):143-154. doi: 10.3109/00207454.2012.744308 [DOI] [PubMed] [Google Scholar]
- 35.Bonello L, Tantry US, Marcucci R, et al. ; Working Group on High On-Treatment Platelet Reactivity . Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56(12):919-933. doi: 10.1016/j.jacc.2010.04.047 [DOI] [PubMed] [Google Scholar]
- 36.Pan Y, Elm JJ, Li H, et al. Outcomes associated with clopidogrel-aspirin use in minor stroke or transient ischemic attack: a pooled analysis of Clopidogrel in High-Risk Patients With Acute Non-Disabling Cerebrovascular Events (CHANCE) and Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) trials. JAMA Neurol. 2019;76(12):1466-1473. doi: 10.1001/jamaneurol.2019.2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Topçuoglu MA, Arsava EM, Ay H. Antiplatelet resistance in stroke. Expert Rev Neurother. 2011;11(2):251-263. doi: 10.1586/ern.10.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein MD, Williams AK, Lee CR, Stouffer GA. Clinical utility of CYP2C19 genotyping to guide antiplatelet therapy in patients with an acute coronary syndrome or undergoing percutaneous coronary intervention. Arterioscler Thromb Vasc Biol. 2019;39(4):647-652. doi: 10.1161/ATVBAHA.118.311963 [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Zhao X, Lin J, et al. ; CHANCE investigators . Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA. 2016;316(1):70-78. doi: 10.1001/jama.2016.8662 [DOI] [PubMed] [Google Scholar]
- 40.Clopidogrel with aspirin in high-risk patients with acute non-disabling cerebrovascular events II (CHANCE-2). ClinicalTrials.gov identifier: NCT04078737. Updated August 3, 2021. Accessed August 3, 2021. https://clinicaltrials.gov/ct2/show/NCT04078737
- 41.Wang Y, Johnston C, Bath PM, et al. ; CHANCE-2 Investigators . Clopidogrel With Aspirin in High-Risk Patients With Acute Non-Disabling Cerebrovascular Events II (CHANCE-2): rationale and design of a multicentre randomised trial. Stroke Vasc Neurol. 2021;6(2):280-285. doi: 10.1136/svn-2020-000791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhatia K, Jain V, Aggarwal D, et al. Dual antiplatelet therapy versus aspirin in patients with stroke or transient ischemic attack: meta-analysis of randomized controlled trials. Stroke. 2021;52(6):e217-e223. doi: 10.1161/STROKEAHA.120.033033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Search strategy used for all databases (Embase, Cochrane, and Medline).
eTable 2. Fixed-effects pairwise meta-analysis and homogeneity for outcomes.
eTable 3. Random-effects (RE) model measures for efficacy and safety for studies with available data.
eTable 4. Summary of fixed-effects and random-effects model fit statistics from network meta-analysis by outcome.
eTable 5. Definitions of stroke and bleeding events across all included trials.
eFigure 1. Risk of bias assessment for all included randomized clinical trials using the Cochrane Risk of Bias tool for randomized trials.
eFigure 2. Network diagram of total number of patients analyzed in each treatment arm for 30-day sensitivity analysis and functional disability up to 90 days.
eFigure 3. Network diagram of total number of patients analyzed in each treatment arm for mortality.
eFigure 4. Leverage plots of fixed-effects consistency and inconsistency models for network meta-analysis by outcome.