This systematic review and meta-analysis reviews literature on the behavioral variant of Alzheimer disease and uses the outcomes to propose research criteria for this syndrome.
Key Points
Question
How is the behavioral variant of Alzheimer disease (bvAD) associated with typical AD (tAD) and behavioral variant frontotemporal dementia (bvFTD) in terms of clinical presentation and neuroimaging signatures?
Findings
This systematic review and meta-analysis found that, at time of diagnosis, bvAD showed more severe neuropsychiatric symptoms and other behavioral deficits compared with tAD. Two distinct neuroimaging phenotypes were observed across reported bvAD cases: an AD-like pattern with relative frontal sparing and a relatively more bvFTD-like pattern with both posterior and anterior involvement, with the AD-like bvAD neuroimaging phenotype being the most prevalent.
Meaning
This analysis found that bvAD is clinically most reminiscent of bvFTD, while it shares most pathophysiological features with tAD; the research criteria are aimed at improving the consistency and reliability of future research and potentially aiding in the clinical assessment of bvAD.
Abstract
Importance
The behavioral variant of Alzheimer disease (bvAD) is characterized by early and predominant behavioral deficits caused by AD pathology. This AD phenotype is insufficiently understood and lacks standardized clinical criteria, limiting reliability and reproducibility of diagnosis and scientific reporting.
Objective
To perform a systematic review and meta-analysis of the bvAD literature and use the outcomes to propose research criteria for this syndrome.
Data Sources
A systematic literature search in PubMed/MEDLINE and Web of Science databases (from inception through April 7, 2021) was performed in duplicate.
Study Selection
Studies reporting on behavioral, neuropsychological, or neuroimaging features in bvAD and, when available, providing comparisons with typical amnestic-predominant AD (tAD) or behavioral variant frontotemporal dementia (bvFTD).
Data Extraction and Synthesis
This analysis involved random-effects meta-analyses on group-level study results of clinical data and systematic review of the neuroimaging literature. The study was performed following Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.
Main Outcomes and Measures
Behavioral symptoms (neuropsychiatric symptoms and bvFTD core clinical criteria), cognitive function (global cognition, episodic memory, and executive functioning), and neuroimaging features (structural magnetic resonance imaging, [18F]fluorodeoxyglucose-positron emission tomography, perfusion single-photon emission computed tomography, amyloid positron emission tomography, and tau positron emission tomography).
Results
The search led to the assessment of 83 studies, including 13 suitable for meta-analysis. Data were collected for 591 patients with bvAD. There was moderate to substantial heterogeneity and moderate risk of bias across studies. Cases with bvAD showed more severe behavioral symptoms than tAD (standardized mean difference [SMD], 1.16 [95% CI, 0.74-1.59]; P < .001) and a trend toward less severe behavioral symptoms compared with bvFTD (SMD, −0.22 [95% CI, −0.47 to 0.04]; P = .10). Meta-analyses of cognitive data indicated worse executive performance in bvAD vs tAD (SMD, −1.03 [95% CI, −1.74 to −0.32]; P = .008) but not compared with bvFTD (SMD, −0.61 [95% CI, −1.75 to 0.53]; P = .29). Cases with bvAD showed a nonsignificant difference of worse memory performance compared with bvFTD (SMD, −1.31 [95% CI, −2.75 to 0.14]; P = .08) but did not differ from tAD (SMD, 0.43 [95% CI, −0.46 to 1.33]; P = .34). The neuroimaging literature revealed 2 distinct bvAD neuroimaging phenotypes: an AD-like pattern with relative frontal sparing and a relatively more bvFTD-like pattern characterized by additional anterior involvement, with the AD-like pattern being more prevalent.
Conclusions and Relevance
These data indicate that bvAD is clinically most similar to bvFTD, while it shares most pathophysiological features with tAD. Based on these insights, we propose research criteria for bvAD aimed at improving the consistency and reliability of future research and aiding the clinical assessment of this AD phenotype.
Introduction
Alzheimer disease (AD) is a heterogenous disease that can manifest with both amnestic and nonamnestic clinical presentations.1 Several atypical (ie, non–memory predominant) variants of AD have been described, including posterior cortical atrophy, logopenic variant primary progressive aphasia, corticobasal syndrome due to AD, and dysexecutive AD.2 The behavioral variant of Alzheimer disease (bvAD) represents another, rare variant of AD that is characterized by early and predominant behavioral deficits and personality changes caused by AD pathology. The bvAD clinical syndrome overlaps substantially with that of the behavioral variant of frontotemporal dementia (bvFTD) and approximately 10% to 40% of clinically diagnosed bvFTD cases have positive AD biomarkers and/or neuropathologically confirmed AD.3,4,5,6 This highlights a major diagnostic challenge, which is even more pertinent with the recent accelerated approval of aducanumab by the US Food and Drug Administration to reduce cerebral amyloid-β in early symptomatic AD.7 Although bvAD is acknowledged as a clinical entity in recent diagnostic and research criteria for AD dementia,8,9 currently no criteria exist that provide specific recommendations for the diagnosis of bvAD. This is in contrast with other AD variants10,11,12 and limits reliable and reproducible classification of bvAD as well as uniform scientific reporting.
The current literature on bvAD includes relatively few studies with typically small sample sizes that have reported several inconsistent findings. To better understand the bvAD phenotype, we performed a systematic review and meta-analysis of the clinical, neuroimaging, and neuropathology bvAD literature and applied the outcomes to develop research criteria for bvAD. With this work, we aim to improve the consistency and reliability of future research and potentially aid in the clinical assessment of bvAD.
Methods
Search Strategy, Selection, and Outcomes
This study was conducted following prespecified methods (PROSPERO registration number: CRD42021243497) and reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines. We performed a systematic literature search in PubMed/MEDLINE and Web of Science databases. We searched studies including clinically diagnosed (1) AD cases with so-called frontal or behavioral presentations or (2) bvFTD cases with neuropathological evidence of AD (full database queries are in eTable 1 in the Supplement). We included peer-reviewed articles, written in English, and presenting original research with human data only. Screening was first conducted at the title or abstract level in Rayyan.13 Reference lists were additionally cross-checked for eligible studies. Two independent reviewers (R.O. and E.H.S.) screened titles and abstracts. Ambiguous records were discussed with a third author (Y.A.L.P.) to reach consensus. Studies were eligible when (1) they included cases or groups of patients presenting with early and predominant behavioral changes with a clinical diagnosis, biomarker support, and/or neuropathological evidence of AD and (2) behavioral/neuropsychiatric, neuropsychological, neuroimaging, and/or neuropathological data were presented. Studies were excluded when (1) they described patients with isolated executive dysfunction in the absence of behavioral symptoms and (2) there was biomarker and/or neuropathological evidence for a non-AD pathology as the primary pathology. Studies were only eligible for the meta-analysis if a bvAD group was compared with typical AD (tAD) and/or bvFTD groups. We extracted demographic (age and sex), clinical (behavioral features per bvFTD criteria14 or neuropsychiatric symptoms per Neuropsychiatric Inventory [NPI15]), neuropsychological (Mini-Mental State Examination [MMSE] and memory and executive function tests), neuroimaging (structural magnetic resonance imaging [MRI], [18F]fluorodeoxyglucose [FDG]–positron emission tomography [PET], perfusion single-photon emission computed tomography, amyloid-PET, and tau-PET), and neuropathological (amyloid-β and tau) characteristics from all studies. After eligibility assessment for inclusion, meta-analyses were constructed using pooled clinical data (behavioral or neuropsychiatric questionnaires), neuropsychological data (MMSE and memory and executive functioning tests), and neuropathological data (amyloid-β and tau load in medial temporal lobe, occipital cortex, and frontal regions; eTable 2 in the Supplement). The lack of uniform reporting of effect sizes among neuroimaging methods across studies did not allow a meta-analysis; hence these findings were analyzed using systematic review (Table).
Table. Neuroimaging Studies in the Behavioral Variant of AD.
| Source | Participants | Age, mean (SD), y | Male, % | MMSE score, mean (SD) | AD confirmation methods | Contrasts | Findings |
|---|---|---|---|---|---|---|---|
| Magnetic resonance imaging | |||||||
| Ossenkoppele et al,16 2015 | 55 With bvAD | 64.7 (8.8) | 72.7 | 22.5 (5.4) | CSF, PET, or autopsy | 58 With tAD, 59 with bvFTD, and 61 with CU | Predominant temporoparietal pattern and no differences between bvAD and tAD |
| Perry et al,17 2017a | 15 With bvAD | 62.8 (43-83)b | 66.7 | NA | Autopsy | 98 With bvFTD | Moderate atrophy in frontoinsula and anterior cingulate regions |
| Phillips et al,18 2018 | 22 With b/dAD | 64.3 (8.2) | 50.0 | 19.6 (8.4) | CSF or autopsy | 22 With tAD and with 115 CU | Staging scheme based on cross-sectional magnetic resonance imaging data indicated early frontotemporal and insular atrophy and spread toward frontotemporal and parietal cortex |
| Phillips et al,19 2019c | 12 With bvAD | 16.0 (13.5-18.0)d | 58.3 | 23.0 (17.0-26.0) | CSF or autopsy | 17 With tAD | Anterior insula, frontotemporal, angular gyrus, and middle temporal atrophy |
| Singleton et al,20 2020a | 29 With bvAD | 64.4 (9.4) | 59.0 | 22.0 (5.9) | CSF, PET, or autopsy | 28 With tAD, 28 with bvFTD, and 34 with CU | Larger amygdala gray matter volume in bvAD vs tAD |
| Therriault et al,21 2020 | 15 With b/dAD | 65.9 (8.8) | 40.0 | 19.6 (5.3) | Amyloid- and tau-PET | 25 With tAD and 131 with CU | Lateral and medial parietal and prefrontal atrophy in bvAD; no differences between bvAD vs tAD |
| Fluorodeoxyglucose-PET or hypoperfusion single-photon emission computed tomography | |||||||
| Snowden et al,22 2007 | 12 With bvAD | 49 (8) | 75.0 | NA | NA | 321 With tAD | Hypoperfusion extending into frontal regions occurred more commonly in patients with so-called frontal behavioral features |
| Woodward et al,23 2015 | 13 With bvAD | 81.6 (4.1) | 61.5 | 23.9 | NA | 40 With tAD | Greater frontotemporal and parietal hypometabolism in bvAD vs tAD |
| Wang et al,24 2019 | 13 With b/dAD | 68.0 (3.4) | 30.8 | 17.0 (5.6) | Amyloid-PET | 38 With tAD and 20 with CU | Dorsolateral hypometabolism in the medial prefrontal and dorsolateral frontal cortex |
| Bergeron et al,25 2020 | 8 With b/dAD | 61.6 | NA | 20.7 | CSF or PET | 40 With atypical AD and 12 with FTD | Most hypometabolic regions of interest in b/dAD were middle temporal gyrus, posterior cingulate, and angular gyrus |
| Singleton et al,20 2020a | 19 With bvAD | 66.1 (7.4) | 58.0 | 21.8 (5.7) | CSF, PET, or autopsy | 18 With tAD, 18 with bvFTD, and 31 with CU | No hypometabolic differences in direct contrasts but relatively greater frontoinsular involvement in bvAD vs tAD in contrast against cases with CU |
| Sala et al,26 2020 | 15 With b/dAD | 62.5 (5.7) | 66.7 | 16.5 (5.2) | CSF | 22 With tAD | Temporoparietal, dorsolateral, and orbitofrontal hypometabolism, with greatest (>90%) hypometabolism in the middle and superior frontal gyrus |
| Bergeron et al,27 2020 | 6 With b/dAD | 59.5 (7.9) | 75.0 | 22.3 (5.9) | CSF or PET | 8 With bvFTD and 10 with tAD | Two of 6 showed a predominantly frontal and temporoparietal pattern, 2 showed a mild frontal pattern, and the final 2 showed a temporoparietal-predominant pattern |
| Lehingue et al,28 2021 | 20 With bvAD | 71.5 (66-76)d | 65.0 | 25 (21-26) | CSF | 22 With tAD and 36 with bvFTD | No significant differences between bvAD vs tAD or bvFTD |
| Amyloid PET | |||||||
| Wang et al,24 2019 | 13 With b/dAD | 68.0 (3.4) | 30.8 | 17.0 (5.6) | Amyloid-PET | 38 With tAD and 20 with CU | No differences among AD groups |
| Therriault et al,21 2020 | 15 With b/dAD | 65.9 (8.8) | 40.0 | 19.6 (5.3) | Amyloid- and tau-PET | 25 With tAD and 131 with CU | No differences among AD groups |
| Tau PET | |||||||
| Therriault et al,21 2020 | 15 With b/dAD | 65.9 (8.8) | 40.0 | 19.6 (5.3) | Amyloid- and tau-PET | 25 With tAD and 131 with CU | b/dAD vs tAD: elevated uptake in the anterior cingulate, medial prefrontal, and frontal insula cortices in b/dAD vs tAD |
| Singleton et al,29 2021 | 7 With bvAD | 69.1 (8.4) | 85.7 | 21.7 (2.8) | CSF, PET, or autopsy | 205 With tAD | Three of 7 prominent lateral frontal and temporoparietal uptake, 1 with medial prefrontal uptake, 2 with lateral temporal uptake, and 1 with temporoparietal uptake |
Abbreviations: AD, Alzheimer disease; b/dAD, behavioral/dysexecutive Alzheimer disease; bvAD, behavioral variant of Alzheimer disease; bvFTD, behavioral variant of frontotemporal dementia; CU, cognitively unimpaired; CSF, cerebrospinal fluid; MMSE, Mini-Mental State Examination; NA, not applicable; PET, positron emission tomography; tAD, typical Alzheimer disease.
Cases partially overlap with Ossenkoppele et al.16
Reported as mean (range).
Cases partially overlap with Phillips et al.18
Reported as median (IQR).
Statistical Analysis
Meta-analysis was used to examine whether bvAD differed from tAD and bvFTD in terms of behavioral/neuropsychiatric and neuropsychological features and bvAD differed from tAD in the distribution of amyloid-β and tau pathology defined at autopsy. Missing data were requested from the authors of 3 studies (and all 3 responded).18,27,28 We calculated the pooled standardized mean differences and 95% CIs using Hedges g random-effects models in the “meta” package of R version 4.0.2 (R Foundation for Statistical Computing), with a significance level of P < .05. We used random effects because we assumed that the true effect size would be study dependent because of high heterogeneity in samples, methods, and outcomes among studies.
Statistical heterogeneity for the meta-analyses was assessed using the I2 statistic, with I2 greater than 75% indicating substantial heterogeneity. Heterogeneity across studies was substantial for analyses including behavioral and neuropsychiatric symptoms, memory and executive measures (I2 range, 70%-96%), and moderate for analyses including neuropathological data (I2 range, 0%-51%). Publication bias was assessed by visual inspection of funnel plots, which indicated substantial publication bias (eFigures 1-3 in the Supplement). Two authors (E.H.S. and C.G.) independently assessed risk of bias using the Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) risk of bias tool for nonrandomized studies. The overall risk of bias was serious for 2 studies and moderate for 11 studies in the meta-analysis (eTable 3 and eFigure 4 in the Supplement).
We additionally calculated the prevalence of each behavioral feature in the core clinical bvFTD criteria14 (ie, presence of disinhibition, apathy, lack of empathy, compulsiveness, and hyperorality) and the 12 items of the neuropsychiatric inventory (NPI and prevalence score ≥1) across studies and compared bvAD, bvFTD, and tAD groups using χ2 tests. For the NPI analysis only, we examined 769 amyloid-β–positive patients with tAD from the Amsterdam Dementia Cohort.30
Results
Participants
The systematic literature search yielded 1257 records, of which 116 studies were assessed at full-text level for eligibility and 83 studies5,16,17,18,19,20,21,22,23,24,26,27,28,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97 met inclusion criteria (eFigure 5 in the Supplement for flowchart). Confirmation of AD pathology was present in 91.1% of cases with bvAD based on autopsy data (36 studies5,16,17,18,19,20,22,29,31,33,34,35,39,40,42,44,49,51,52,54,55,56,57,59,62,66,69,70,76,77,79,81,82,87,90,96; n = 334), genetic data (9 studies47,50,60,63,65,67,68,80,86; n = 21), or biomarker data (31 studies3,18,19,20,21,24,25,26,27,28,29,32,38,63,70,71,72,73,74,75,77,83,84,85,88,89,91,92,94,95,97; n = 262), while no information was available in 9.9% of cases (11 studies22,23,36,37,41,43,45,46,53,64,78 including 68 cases of bvAD). Thirteen studies were eligible for meta-analysis. eTable 4 in the Supplement provides an overview of the participant characteristics for all 83 included studies. Across these studies, 591 patients with bvAD were enrolled, with a mean (SD) age at diagnosis of 62.0 (7.3) years, and 226 participants (38.2%) were women. The mean (SD) MMSE score was 20.1 (5.9), and 281 participants (47.5%) carried an APOEε4 allele.
Behavioral/Neuropsychiatric Symptoms
Meta-analysis indicated that patients with bvAD showed more severe behavioral and neuropsychiatric symptoms than patients with tAD (standardized mean difference [SMD], 1.16 [95% CI, 0.74-1.59]; P < .001) and a nonsignificant difference in severe behavioral/neuropsychiatric symptoms compared with bvFTD (SMD, −0.22 [95% CI, −0.47 to 0.04]; P = .10; Figure 1A). Results remained similar when separating bvFTD core criteria and neuropsychiatric features (eFigure 6 in the Supplement).
Figure 1. Meta-analyses of Behavioral and Neuropsychiatric Features.
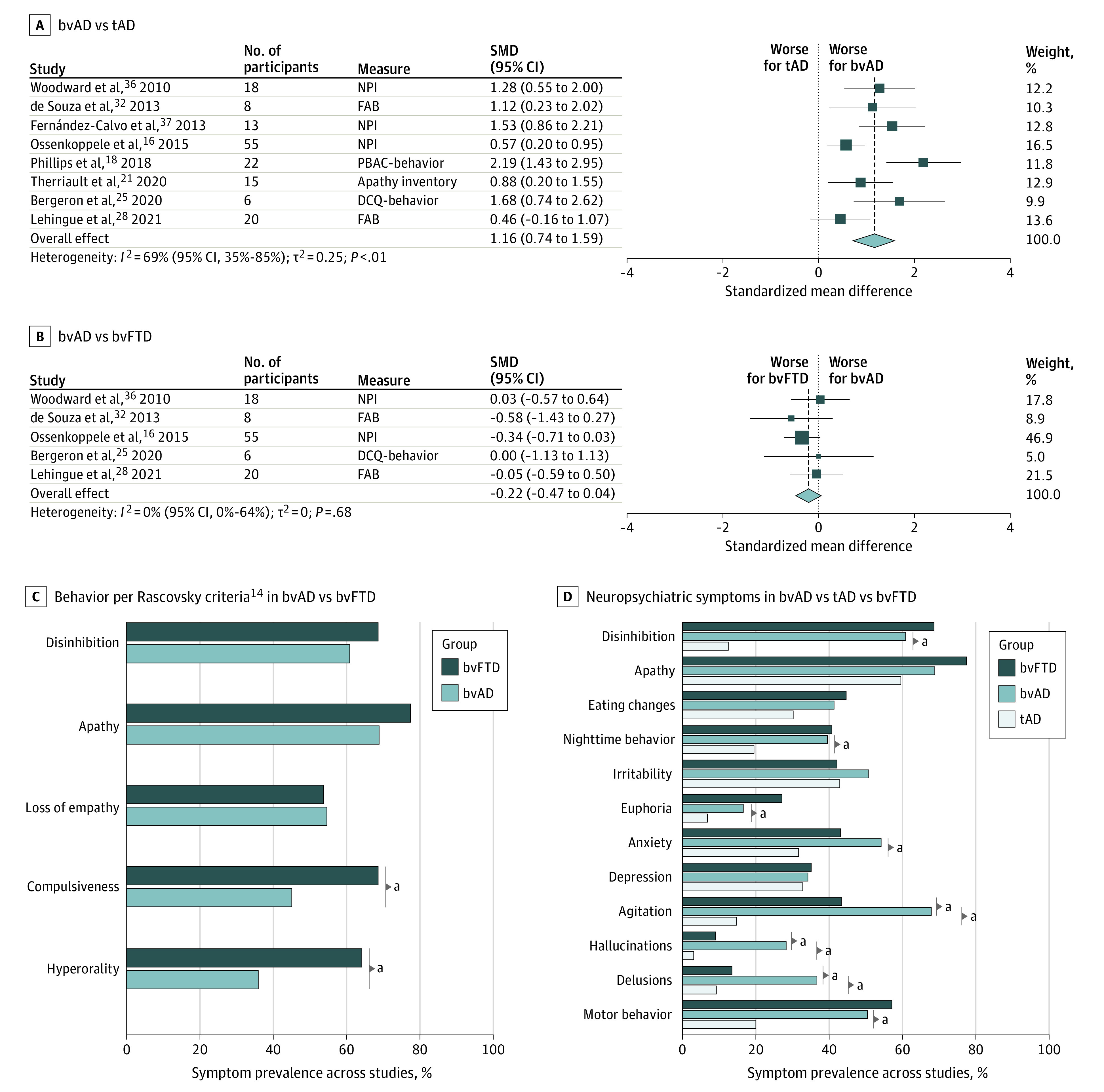
Results of meta-analyses on behavioral and neuropsychiatric total scores between the behavioral variant of Alzheimer disease (bvAD) and typical Alzheimer disease (tAD) (A) and bvAD and the behavioral variant of frontotemporal dementia (bvFTD) (B). Mean weighted percentages of participants per diagnostic group fulfilling specific bvFTD core clinical features proposed by Rascovsky et al14 (C) or presence of specific neuropsychiatric symptoms measured using the Neuropsychiatric Inventory (NPI) (D). DCQ indicates Dépistage Cognitif de Québec; FAB, Frontal Assessment Battery; PBAC, Philadelphia Brief Assessment of Cognition; SMD, standardized mean difference.
aP < .05.
Next, we compared proportions of bvFTD features and NPI items as reported in previous studies (Figure 1B; eTable 5 in the Supplement16,17,19,31,32,33,34). We used amyloid-β–positive patients with tAD from the Amsterdam Dementia Cohort (mean [SD] age, 65.9 [7.7] years; 403 women [52.4%]; mean [SD] MMSE score, 20.3 [5.1]). Compared with bvFTD, patients with bvAD less frequently showed compulsive behaviors (45.0% vs 68.5%; χ2 = 22.5; P < .001) and hyperorality (35.9% vs 64.1%; χ2 = 32.8; P < .001) but showed no differences on disinhibition (60.8% vs 68.6%; χ2 = 2.8; P = .10), apathy (68.8% vs 77.4%; χ2 = 3.7; P = .05), and lack of empathy (54.6% vs 53.6%; χ2 = 0.1; P = .83). On the NPI, patients with bvAD more frequently showed agitation (67.9% vs 43.4%; χ2 = 8.8), hallucinations (28.2% vs 9.0%; χ2 = 12.8), and delusions (36.6% vs 13.4%; χ2 = 13.4) compared with bvFTD (P < .001). Furthermore, those with bvAD more frequently showed nighttime behaviors (39.6% vs 19.5%; χ2 = 12.9), euphoria (16.6% vs 6.8%; χ2 = 7.9), anxiety (54.2% vs 31.7%; χ2 = 10.8), agitation (67.9% vs 14.8%; χ2 = 90.3), hallucinations (28.2% vs 3.1%; χ2 = 71.2), delusions (36.6% vs 9.2%; χ2 = 37.2), and motor behaviors (50.4% vs 19.8%; χ2 = 26.2) compared with patients with tAD (P < .01).
Cognition
Meta-analyses of cognitive data indicated that at initial assessment bvAD, patients showed no differences on MMSE compared with tAD (SMD, −0.18 [95% CI, −0.56 to 0.20]; P = .35) and bvFTD (SMD, −0.22 [95% CI, −0.78 to 0.35]; P = .46; Figure 2). Patients with bvAD showed worse executive performance compared with tAD (SMD, −1.03 [95% CI, −1.74 to −0.32]; P = .008) but not compared with bvFTD (SMD, −0.61 [95% CI, −1.75 to 0.53]; P = .29). Finally, bvAD showed a trend toward worse memory performance compared with bvFTD (SMD, −1.31 [95% CI, −2.75 to 0.14]; P = .08) but did not differ from tAD (SMD, 0.43 [95% CI, −0.46 to 1.33]; P = .34).
Figure 2. Meta-analyses of Cognitive Performance.
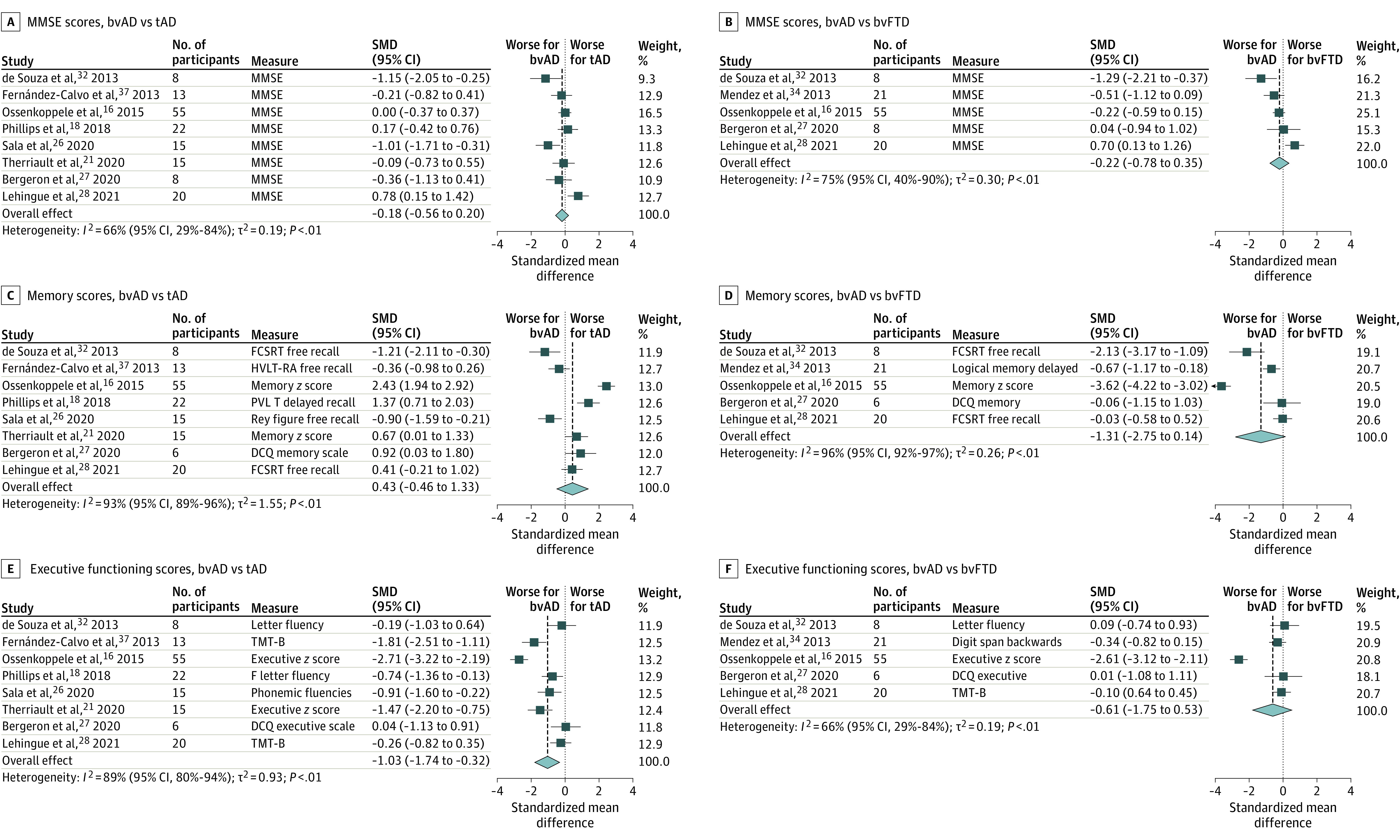
Results of meta-analyses on Mini-Mental State Examination (MMSE; A and B), episodic memory (C and D), and executive function (E and F) for the contrast of behavioral variant of Alzheimer disease (bvAD) vs typical Alzheimer disease (tAD) and the behavioral variation of frontotemporal dementia (bvFTD). SMD indicates standardized mean difference.
Neuroimaging
The Table provides an overview of neuroimaging studies in bvAD. Structural MRI studies (16 studies; 92 participants) showed temporoparietal-predominant,16 frontotemporal-predominant and insular-predominant,17,18,19 or frontoparietal-predominant21 atrophy patterns across patients with bvAD. Cases of bvAD did not differ from tAD in 3 studies16,20,21 and showed moderately more involvement of frontal regions in bvAD compared with tAD in 3 other studies.17,18,19 Studies assessing glucose metabolism with [18F]FDG-PET or perfusion with single-photon emission computed tomography (7 studies; 88 participants) also showed heterogeneous results, ranging from a predominantly temporoparietal hypometabolic pattern20,25 to a mixed frontal and temporoparietal23,26,27,28 or predominantly frontal pattern.24 Amyloid-PET studies (2 studies21,24; 28 participants) showed no differences in amyloid-β burden or distribution between patients with bvAD vs tAD. For tau-PET (2 studies; 22 participants), 1 study21 showed a temporoparietal pattern with higher uptake in anterior regions in bvAD compared with tAD, whereas another29 study showed heterogeneous patterns across patients with bvAD. Findings on functional connectivity (3 studies19,20,24; 54 participants) and white matter hyperintensities (1 study20; 29 participants) in bvAD are presented in eTable 6 in the Supplement.
We distilled 2 distinct bvAD neuroimaging phenotypes from the literature, characterized by either relative frontal sparing (more AD-like) or by both posterior and anterior involvement (more bvFTD-like) (Figure 3A). We propose that these phenotypes occur on a continuum (Figure 3B), with the more AD-like phenotype being most prevalent (Figure 3C).
Figure 3. Neuroimaging Features in the Behavioral Variant of Alzheimer Disease (bvAD).
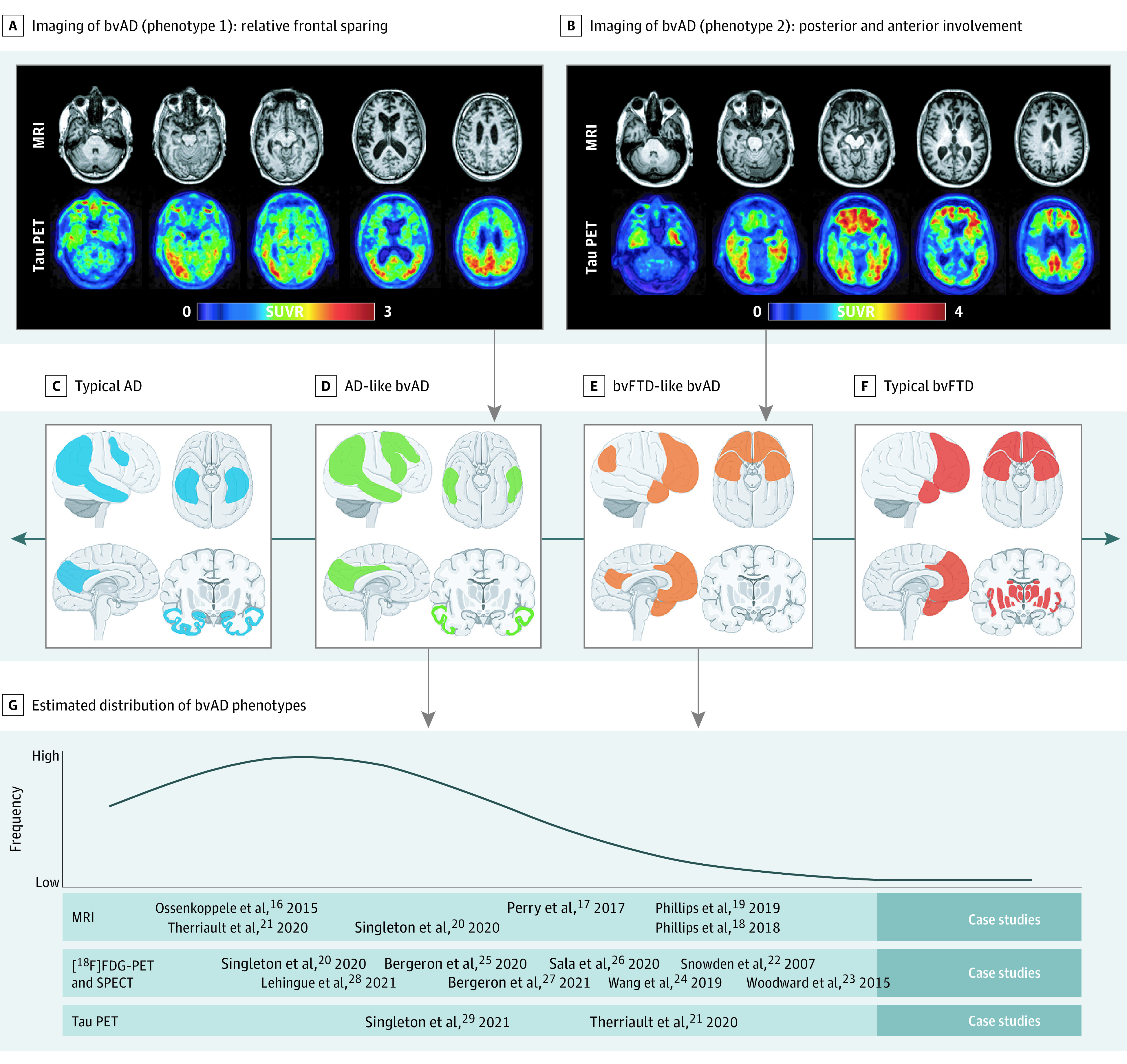
A and B, Two cases that serve as examples of 2 distinct bvAD neuroimaging phenotypes: an Alzheimer disease–like atrophy and tau load pattern with relative frontal sparing and a more behavioral variant of frontotemporal dementia (bvFTD)–like atrophy and tau load pattern with both posterior and anterior involvement. The tau positron emission tomography (PET) scans were performed using [18F]flortaucipir, and magnetic resonance imaging (MRI) was conducted on a 3-T scanner. C-F, Proposed neuroimaging phenotypes as part of a spectrum that ranges from a typical Alzheimer disease regional distribution to a classical bvFTD regional distribution. The brain template images were obtained from https://smart.servier.com/. G, Literature-informed estimated distribution of the regional distribution in bvAD, indicating that typical AD and bvAD-AD–like patterns are more common than bvAD-bvFTD–like and typical bvFTD. FDG indicates fluorodeoxyglucose; SPECT, single-photon emission computed tomography; SUVR, standardized uptake value ratio.
Neuropathology
In line with amyloid and tau PET findings, the meta-analyses on neuropathological data18,29,31,35 showed that bvAD and tAD did not differ in the neuropathological burden of amyloid-β (3 studies18,31,35; 20 participants) across frontal regions (SMD, 0.23 [95% CI, −0.36 to 0.81]; P = .45), medial temporal lobe (SMD, −0.06 [95% CI, −0.65 to 0.53]; P = .84), or occipital cortex (SMD, −0.16 [95% CI, −1.05 to 0.73]; P = .73; eFigure 7 in the Supplement). Furthermore, there was no difference in tau burden (4 studies18,29,31,35; 28 participants) across frontal regions (SMD, −0.05 [95% CI, −0.56 to 0.46]; P = .84), medial temporal lobe (SMD, 0.32 [95% CI, −0.19 to 0.83]; P = .22), or occipital cortex (SMD, −0.36 [95% CI, −0.95 to 0.23]; P = .24; eFigure 7 in the Supplement).
Discussion
In this systematic review and meta-analysis, we found that bvAD is clinically most reminiscent of bvFTD while it shares most pathophysiological features with tAD. Based on these insights, we provide research criteria for bvAD aimed at improving the consistency and reliability of future research and aiding in future clinical assessments.
Systematic Review and Meta-analyses
bvAD phenotype typically presents at a young age (mean [SD] age, 62.0 [7.3] years at diagnosis), is more frequently found in men than women (61.7% vs 38.2%, in line with bvFTD but in contrast with tAD98), and has a lower frequency of APOEε4 carriership compared with tAD (47.5% vs 66.1%99). Clinically, bvAD shows a milder behavioral profile compared with bvFTD, with less compulsivity and hyperorality but greater prevalence of neuropsychiatric symptoms, such as agitation, delusions, and hallucinations. By definition, bvAD shows greater impairment on a range of behavioral and neuropsychiatric measures compared with tAD. The directionality of findings in the meta-analyses of cognitive data suggest that bvAD might show greater memory and executive function deficits compared with bvFTD and relatively better memory function and worse executive functioning compared with tAD, but further research in larger cohorts is needed to confirm the significance of these findings. The neuroimaging methods were too heterogenous across studies to conduct a formal meta-analysis, but a systematic review revealed 2 distinct phenotypes of brain atrophy, hypometabolism, and tau pathology in bvAD, with many cases likely occurring on a continuum. The most prevalent bvAD neuroimaging phenotype is an AD-like pattern involving bilateral temporoparietal regions with limited involvement of the frontal cortex. This observation is congruent with our meta-analysis on neuropathological data showing that patients with bvAD were indistinguishable from patients with tAD in both amyloid-β and tau load and spatial distribution. The other bvAD phenotype is characterized by a more bvFTD-like neuroimaging pattern, including posterior and anterior regions (eg, anterior cingulate cortex, frontal insula, temporal poles) located in brain networks (eg, the salience network) that are engaged during socioemotional processing of information.100 Altogether, our systematic review and meta-analyses further refine the bvAD phenotype but also highlight the need for larger studies with more uniform methods and inclusion and exclusion criteria.
Research Criteria for bvAD
Our main objective was to propose research criteria for bvAD guided by the results of the systematic review and meta-analyses. The criteria are based on consensus between all authors, including neurologists, neuropsychologists, neuropathologists, and neuroscientists. To facilitate widespread use but also take into account the complexity of this phenotype, we offer 4 levels of evidence (Box). The first level (clinical bvAD) can be established solely based on clinical information, while the second and third levels (possible bvAD and probable bvAD) add biomarker confirmation of amyloid-β and tau pathology. The fourth level (definite bvAD) is assigned through histopathological or genetic confirmation of AD (ie, by the presence of pathogenic APP, PSEN1, or PSEN2 genetic variations) in conjunction with a bvAD clinical syndrome.
Box. Research Criteria for the Behavioral Variant of Alzheimer Disease (bvAD).
Clinical bvAD
-
The clinical syndrome is characterized by:
-
Early, persistent, predominant, and progressive change or exacerbation of at least 2 of 5 core behavioral features of the diagnostic criteria for behavioral variant frontotemporal dementia (Rascovsky et al14):
-
Behavioral disinhibition (1 of the following symptoms must be present):
Socially inappropriate behavior
Loss of manners or decorum
Impulsive, rash, or careless actions
-
Apathy or inertia (1 of the following symptoms must be present):
Apathy
Inertia
-
Loss of empathy or sympathy (1 of the following symptoms must be present):
Diminished response to other people’s needs and feelings
Diminished social interest, interrelatedness, or personal warmth
-
Perseverative, stereotyped, or compulsive or ritualistic behavior (1 of the following symptoms must be present):
Simple, repetitive movements
Complex, compulsive, or ritualistic behaviors
Stereotypy of speech
-
Hyperorality and dietary changes (1 of the following symptoms must be present):
Altered food preferences
Binge eating or increased consumption of alcohol or cigarettes
Oral exploration or consumption of inedible objects
-
In addition, documented impairment in executive functions and/or episodic memory with relatively preserved language and visuospatial abilities.
-
Criteria for clinical bvAD are not met if the behavioral deficits are (better) accounted for by another concurrent (active) neurological (eg, Lewy body dementia) or nonneurological medical (eg, psychiatric) comorbidity, a known genetic variant associated with familial behavioral variant of frontotemporal dementia, or the use of medication.
-
Supportive features (not mandatory; categories A and B must be met):
Presence of hallucinations and/or delusions.
Alzheimer disease–specific (ie, temporoparietal pattern) and/or behavioral variant of frontotemporal dementia–specific neuroimaging features (ie, frontotemporal pattern) on magnetic resonance imaging, computed tomography, perfusion single-photo emission computed tomography, and/or fluorodeoxyglucose–positron emission tomography.
Possible bvAD
Meets criteria for clinical bvAD and
There is in vivo biomarker evidence for the presence of (1) β-amyloid pathology on amyloid positron emission tomography and/or in cerebrospinal fluid and/or (2) tau pathology in cerebrospinal fluid and/or plasma.
Probable bvAD
Meets criteria for clinical bvAD or possible bvAD, with additional in vivo tau positron emission tomography evidence for the presence of neocortical tau aggregates.
Definite bvAD
Meets criteria for clinical bvAD, possible bvAD, or probable bvAD, and
-
Presence of AD is established by
Histopathological indication of AD as the primary pathology on biopsy or at autopsy, or
Presence of a known genetic variant associated with familial AD.
Several issues warrant further explanation. First, both the literature and our clinical experience align with the notion that bvAD is a combined cognitive and behavioral clinical syndrome. We previously showed that cognitive impairment was among the first symptoms reported by patients and caregivers in approximately 75% of bvAD cases.16 In addition, our meta-analysis suggests that episodic memory performance in bvAD is intermediate between tAD and bvFTD, while bvAD shows greater executive dysfunction compared with bvFTD (Figure 2). To enhance the discriminative accuracy between bvAD and bvFTD, objectively confirmed impairment in either memory or executive domains is therefore mandatory. To achieve this, we recommend a full neuropsychological evaluation rather than use of relatively crude dementia screening tests. In addition, 2 of 5 behavioral features of the diagnostic criteria for bvFTD14 (ie, disinhibition, apathy, lack of empathy, compulsiveness, and hyperorality) must be present. Note that the sixth bvFTD criterion (ie, a dysexecutive neuropsychological profile) was removed because documented memory and/or executive function deficits are required for a bvAD diagnosis. The 2-of-5 criterion was selected to sufficiently distinguish bvAD from tAD but also acknowledge the generally milder behavioral profile in bvAD compared with bvFTD (in which 3 of 6 bvFTD criteria must be present). Second, despite clinically significant differences between bvAD and both bvFTD and tAD (Figure 1), we deemed it premature to include hallucinations and delusions in the core research criteria because these observations were derived from only 2 studies.33,34 Instead, they were added as supportive features, and future prospective studies are needed to assess whether they should be incorporated in the core criteria for bvAD. Third, most AD variants have a clear neurodegenerative signature on MRI and/or [18F]FDG-PET that corresponds with their clinical phenotype, such as left-hemispheric predominance in logopenic variant primary progressive aphasia or occipitotemporal or occipitoparietal damage in posterior cortical atrophy.10,11 However, the neuroimaging literature in bvAD is highly inconsistent. Some studies (mainly case studies or case series) showed anterior neurodegenerative patterns that resemble bvFTD, but most group studies showed either a mix of anterior and posterior involvement or a posterior-predominant pattern.16,17,18,19,20,21,22,25,36,37 Contrary to posterior cortical atrophy and logopenic variant primary progressive aphasia, we therefore did not incorporate MRI, computed tomography, single-photon emission computed tomography, or [18F]FDG-PET readouts into the core bvAD research criteria but only added them as supportive features. Fourth, evidence of amyloid-β pathology provided by PET, cerebrospinal fluid, or plasma biomarkers can upgrade the diagnosis from clinical bvAD to possible bvAD. Positive amyloid-β biomarkers substantially increase the likelihood that AD is the primary causative mechanism, but given their limited specificity, the possibility of amyloid-β as comorbid pathology cannot be ruled out, especially in older individuals and those who carry APOEε4.38,101 The addition of biomarker evidence for tau pathology further increases the certainty for a bvAD diagnosis (ie, probable bvAD). Here, we make the distinction between biofluid and neuroimaging markers of tau pathology. For cerebrospinal fluid and plasma biomarkers of tau pathology, the differential diagnostic value for distinguishing AD from bvFTD is less well established, and as with amyloid-β markers, they become abnormal relatively early in the disease course, which lowers their specificity.102,103 Hence, a full AD-like fluid biomarker profile with abnormalities in both amyloid-β and phosphorylated tau supports a level II diagnosis of possible bvAD. Instead, the currently most widely used tau PET ligands (ie, [18F]flortaucipir, [18F]MK6240, and [18F]RO948) have consistently shown to bind selectively and with high affinity to the tau aggregates formed in AD (ie, combinations of 3R/4R tau in paired helical filaments), while neocortical tau PET uptake in sporadic bvFTD is negligible, resulting in excellent discriminative accuracy between AD and bvFTD.104,105 Furthermore, since tau PET uptake in the neocortex almost exclusively occurs in individuals positive for amyloid-β104,106 we consider a level I diagnosis (clinical bvAD) plus tau PET–positive results in an AD-like pattern107 supportive of a level III diagnosis of probable bvAD. Given the rapid developments in the blood-based biomarker field, the current distinction between neuroimaging and biofluid markers should be reevaluated in the future. Fifth, although the question of whether bvAD and dysexecutive AD exist on a single continuum or represent distinct clinical entities is yet unresolved, we deliberately developed criteria specific to bvAD. This was motivated by our previous study showing that only approximately 25% of bvAD cases additionally met dysexecutive AD criteria16; hence, bvAD occurs in isolation in most cases, as well as a recent article12 proposing specific dysexecutive AD criteria that explicitly exclude behavioral features. Therefore, while dysexecutive AD is considered if dysexecutive functioning and positive AD biomarkers are present in the absence of behavioral deficits, a diagnosis of bvAD is established when early behavioral alterations are observed in conjunction with either memory or executive functioning deficits and positive AD biomarkers (eFigure 8 in the Supplement).
Limitations
There are several limitations. First, bvAD is a rare AD phenotype that, for the most part, has been described in single case studies and case series. The bvAD literature therefore consists of relatively few cohort studies generally characterized by modest sample sizes, which resulted in reduced statistical power to detect differences between bvAD vs bvFTD and tAD. This was further complicated by substantial heterogeneity in patient samples and outcome measures and subsequent substantial risk of bias across studies. Second, the variability across neuroimaging studies did not allow a meta-analytical approach; hence, we interpreted this literature using a systematic review. Third, in the behavioral, cognitive, and neuropathological meta-analyses, we combined comparable yet distinct study outcome measures, such as different neuropsychological tests for memory and executive functions, questionnaires for neuropsychiatric/behavioral features, or staining methods and selection of brain regions for histopathological assessment of amyloid-β and tau. Fourth, we did not account for possible copathologies (eg, Lewy bodies) that may contribute to the clinical phenotype. Fifth, the classification of possible bvAD and probable bvAD may be influenced by inherent differences in diagnostic accuracy of various amyloid and tau PET tracers, as well as assays for cerebrospinal fluid and plasma analysis, and centers likely vary in the reliability of their biomarker result interpretation. Sixth, there were only limited data on behavioral presentations of AD in diverse populations.
Future Directions
Akin to the development of diagnostic criteria for posterior cortical atrophy, we consider the currently proposed research criteria as a stepping stone toward internationally established consensus criteria for bvAD. For posterior cortical atrophy, research criteria were first proposed by 2 research groups and were subsequently applied by other groups to establish a posterior cortical atrophy diagnosis for several years,108,109 followed by widely supported formal diagnostic criteria based on consensus by an international working group.10 Similarly, our bvAD criteria should improve the consistency and reliability of future research and possibly aid in the clinical assessment of bvAD, which in turn would enhance the diagnostic accuracy of future bvAD criteria to be established by a working group of worldwide experts. There are several promising novel biomarkers and behavioral features that could be included in future bvAD criteria, such as more objective measurements of behavior, such as social cognition in conjunction with biometric information (eg, eye tracking, face reading, galvanic skin response)110 or blood-based biomarkers of AD pathology (eg, phosphorylated tau, amyloid-β) and neurodegeneration (eg, neurofilament light chain).111 Furthermore, the diagnostic utility of potential bvAD-specific features (eg, relatively preserved disease insight, presence of hallucinations, and delusions) or measures of disease severity (eg, the frontotemporal lobar degeneration-modified Clinical Dementia Rating scale112) should be further investigated.
Conclusions
Although the existence of bvAD is acknowledged in the most recent diagnostic and research criteria for AD dementia,8,9 there currently does not exist a set of criteria that provide specific recommendations for the diagnosis of bvAD. Our systematic review and meta-analyses of the current bvAD literature indicate that bvAD is clinically most similar to bvFTD, while it shares most pathophysiological features with tAD. Based on these insights, we provide the first research criteria for bvAD aimed at improving the consistency and reliability of future research and potentially facilitating clinical assessment of bvAD.
eTable 1. Full database queries
eTable 2. Selection of frontal regions in autopsy studies
eTable 3. Risk of bias assessment
eTable 4. Characteristics of included studies
eTable 5. Percentage of bvFTD features and NPI items in bvAD, bvFTD and tAD
eTable 6. Functional connectivity and white matter hyperintensities in bvAD
eFigure 1. Funnel plots for behavioral/neuropsychiatric data in meta-analysis
eFigure 2. Funnel plots for cognitive data in meta-analysis
eFigure 3. Funnel plots for neuropathological data in meta-analysis
eFigure 4. Risk of bias assessment summary
eFigure 5. Flow chart of study inclusion
eFigure 6. Results of meta-analysis for behavioral and neuropsychiatric separately
eFigure 7. Meta-analyses for neuropathological data in bvAD vs typical AD
eFigure 8. Differences and overlap between bvAD and dysexecutive AD
eReferences. Reference list Supplement
References
- 1.Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet. 2021;397(10284):1577-1590. doi: 10.1016/S0140-6736(20)32205-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graff-Radford J, Yong KXX, Apostolova LG, et al. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 2021;20(3):222-234. doi: 10.1016/S1474-4422(20)30440-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabinovici GD, Rosen HJ, Alkalay A, et al. Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology. 2011;77(23):2034-2042. doi: 10.1212/WNL.0b013e31823b9c5e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ossenkoppele R, Prins ND, Pijnenburg YA, et al. Impact of molecular imaging on the diagnostic process in a memory clinic. Alzheimers Dement. 2013;9(4):414-421. doi: 10.1016/j.jalz.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 5.Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006;59(6):952-962. doi: 10.1002/ana.20873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71(4):266-273. doi: 10.1097/NEN.0b013e31824b211b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings J, Aisen P, Apostolova LG, Atri A, Salloway S, Weiner M. Aducanumab: appropriate use recommendations. J Prev Alzheimers Dis. 2021;8(4):398-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubois B, Villain N, Frisoni GB, et al. Clinical diagnosis of Alzheimer’s disease: recommendations of the International Working Group. Lancet Neurol. 2021;20(6):484-496. doi: 10.1016/S1474-4422(21)00066-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jack CR Jr, Bennett DA, Blennow K, et al. ; Contributors . NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535-562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crutch SJ, Schott JM, Rabinovici GD, et al. ; Alzheimer’s Association ISTAART Atypical Alzheimer’s Disease and Associated Syndromes Professional Interest Area . Consensus classification of posterior cortical atrophy. Alzheimers Dement. 2017;13(8):870-884. doi: 10.1016/j.jalz.2017.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006-1014. doi: 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Townley RA, Graff-Radford J, Mantyh WG, et al. Progressive dysexecutive syndrome due to Alzheimer’s disease: a description of 55 cases and comparison to other phenotypes. Brain Commun. 2020;2(1):a068. doi: 10.1093/braincomms/fcaa068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rayyan. Accessed October 29, 2021. https://www.rayyan.ai/
- 14.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456-2477. doi: 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummings J. The Neuropsychiatric Inventory: development and applications. J Geriatr Psychiatry Neurol. 2020;33(2):73-84. doi: 10.1177/0891988719882102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ossenkoppele R, Pijnenburg YA, Perry DC, et al. The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain. 2015;138(pt 9):2732-2749. doi: 10.1093/brain/awv191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perry DC, Brown JA, Possin KL, et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain. 2017;140(12):3329-3345. doi: 10.1093/brain/awx254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips JS, Da Re F, Dratch L, et al. Neocortical origin and progression of gray matter atrophy in nonamnestic Alzheimer's disease. Neurobiol Aging. 2018;63:75-87. doi: 10.1016/j.neurobiolaging.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips JS, Da Re F, Irwin DJ, et al. Longitudinal progression of grey matter atrophy in non-amnestic Alzheimer’s disease. Brain. 2019;142(6):1701-1722. doi: 10.1093/brain/awz091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singleton EH, Pijnenburg YAL, Sudre CH, et al. Investigating the clinico-anatomical dissociation in the behavioral variant of Alzheimer disease. Alzheimers Res Ther. 2020;12(1):148. doi: 10.1186/s13195-020-00717-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Therriault J, Pascoal TA, Savard M, et al. Topographic distribution of amyloid-β, tau, and atrophy in patients with behavioral/dysexecutive Alzheimer disease. Neurology. 2020;96(1):e81-e92. doi: 10.1212/WNL.0000000000011081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snowden JS, Stopford CL, Julien CL, et al. Cognitive phenotypes in Alzheimer’s disease and genetic risk. Cortex. 2007;43(7):835-845. doi: 10.1016/S0010-9452(08)70683-X [DOI] [PubMed] [Google Scholar]
- 23.Woodward MC, Rowe CC, Jones G, Villemagne VL, Varos TA. Differentiating the frontal presentation of Alzheimer’s disease with FDG-PET. J Alzheimers Dis. 2015;44(1):233-242. doi: 10.3233/JAD-141110 [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Shi Z, Zhang N, et al. Spatial patterns of hypometabolism and amyloid deposition in variants of Alzheimer’s disease corresponding to brain networks: a prospective cohort study. Mol Imaging Biol. 2019;21(1):140-148. doi: 10.1007/s11307-018-1219-6 [DOI] [PubMed] [Google Scholar]
- 25.Bergeron D, Beauregard JM, Soucy JP, et al. Posterior cingulate cortex hypometabolism in non-amnestic variants of Alzheimer’s disease. J Alzheimers Dis. 2020;77(4):1569-1577. doi: 10.3233/JAD-200567 [DOI] [PubMed] [Google Scholar]
- 26.Sala A, Caprioglio C, Santangelo R, et al. Brain metabolic signatures across the Alzheimer’s disease spectrum. Eur J Nucl Med Mol Imaging. 2020;47(2):256-269. doi: 10.1007/s00259-019-04559-2 [DOI] [PubMed] [Google Scholar]
- 27.Bergeron D, Sellami L, Poulin S, Verret L, Bouchard RW, Laforce R Jr. The behavioral/dysexecutive variant of Alzheimer’s disease: a case series with clinical, neuropsychological, and FDG-PET characterization. Dement Geriatr Cogn Disord. 2020;49(5):518-525. [DOI] [PubMed] [Google Scholar]
- 28.Lehingue E, Gueniat J, Jourdaa S, et al. Improving the diagnosis of the frontal variant of Alzheimer’s disease with the DAPHNE scale. J Alzheimers Dis. 2021;79(4):1735-1745. doi: 10.3233/JAD-201088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singleton E, Hansson O, Pijnenburg YAL, et al. Heterogeneous distribution of tau pathology in the behavioural variant of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2021;92(8):872-880. doi: 10.1136/jnnp-2020-325497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eikelboom WS, van den Berg E, Singleton EH, et al. Neuropsychiatric and cognitive symptoms across the Alzheimer disease clinical spectrum: cross-sectional and longitudinal associations. Neurology. 2021;97(13):e1276-e1287. doi: 10.1212/WNL.0000000000012598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blennerhassett R, Lillo P, Halliday GM, Hodges JR, Kril JJ. Distribution of pathology in frontal variant Alzheimer’s disease. J Alzheimers Dis. 2014;39(1):63-70. doi: 10.3233/JAD-131241 [DOI] [PubMed] [Google Scholar]
- 32.de Souza LC, Bertoux M, Funkiewiez A, et al. Frontal presentation of Alzheimer’s disease: a series of patients with biological evidence by CSF biomarkers. Dement Neuropsychol. 2013;7(1):66-74. doi: 10.1590/S1980-57642013DN70100011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Léger GC, Banks SJ. Neuropsychiatric symptom profile differs based on pathology in patients with clinically diagnosed behavioral variant frontotemporal dementia. Dement Geriatr Cogn Disord. 2014;37(1-2):104-112. doi: 10.1159/000354368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendez MF, Joshi A, Tassniyom K, Teng E, Shapira JS. Clinicopathologic differences among patients with behavioral variant frontotemporal dementia. Neurology. 2013;80(6):561-568. doi: 10.1212/WNL.0b013e3182815547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balasa M, Gelpi E, Antonell A, et al. ; Neurological Tissue Bank/University of Barcelona/Hospital Clínic NTB/UB/HC Collaborative Group . Clinical features and APOE genotype of pathologically proven early-onset Alzheimer disease. Neurology. 2011;76(20):1720-1725. doi: 10.1212/WNL.0b013e31821a44dd [DOI] [PubMed] [Google Scholar]
- 36.Woodward M, Jacova C, Black SE, Kertesz A, Mackenzie IR, Feldman H; ACCORD investigator group . Differentiating the frontal variant of Alzheimer’s disease. Int J Geriatr Psychiatry. 2010;25(7):732-738. doi: 10.1002/gps.2415 [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Calvo B, Ramos F, de Lucena VM. Frontal variant of Alzheimer's disease and typical Alzheimer's disease: a comparative study. Anales De Psicologia. 2013;29(1):293-300. [Google Scholar]
- 38.Rabinovici GD, Furst AJ, O’Neil JP, et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007;68(15):1205-1212. doi: 10.1212/01.wnl.0000259035.98480.ed [DOI] [PubMed] [Google Scholar]
- 39.Brun A, Gustafson L. Distribution of cerebral degeneration in Alzheimer’s disease: a clinico-pathological study. Arch Psychiatr Nervenkr (1970). 1976;223(1):15-33. doi: 10.1007/BF00367450 [DOI] [PubMed] [Google Scholar]
- 40.Shibayama H, Kitoh J. Electron microscopic structure of the Alzheimer’s neurofibrillary changes in case of atypical senile dementia. Acta Neuropathol. 1978;41(3):229-234. doi: 10.1007/BF00690441 [DOI] [PubMed] [Google Scholar]
- 41.Shuttleworth EC. Atypical presentations of dementia of the Alzheimer type. J Am Geriatr Soc. 1984;32(7):485-490. doi: 10.1111/j.1532-5415.1984.tb02232.x [DOI] [PubMed] [Google Scholar]
- 42.Brun A. Frontal lobe degeneration of non-Alzheimer type: I: neuropathology. Arch Gerontol Geriatr. 1987;6(3):193-208. doi: 10.1016/0167-4943(87)90021-5 [DOI] [PubMed] [Google Scholar]
- 43.Perani D, Di Piero V, Vallar G, et al. Technetium-99m HM-PAO-SPECT study of regional cerebral perfusion in early Alzheimer’s disease. J Nucl Med. 1988;29(9):1507-1514. [PubMed] [Google Scholar]
- 44.Bird TD, Sumi SM, Nemens EJ, et al. Phenotypic heterogeneity in familial Alzheimer’s disease: a study of 24 kindreds. Ann Neurol. 1989;25(1):12-25. doi: 10.1002/ana.410250104 [DOI] [PubMed] [Google Scholar]
- 45.Grady CL, Haxby JV, Schapiro MB, et al. Subgroups in dementia of the Alzheimer type identified using positron emission tomography. J Neuropsychiatry Clin Neurosci. 1990;2(4):373-384. doi: 10.1176/jnp.2.4.373 [DOI] [PubMed] [Google Scholar]
- 46.Molchan SE, Martinez RA, Lawlor BA, Grafman JH, Sunderland T. Reflections of the self: atypical misidentification and delusional syndromes in two patients with Alzheimer’s disease. Br J Psychiatry. 1990;157:605-608. doi: 10.1192/bjp.157.4.605 [DOI] [PubMed] [Google Scholar]
- 47.Raux G, Gantier R, Thomas-Anterion C, et al. Dementia with prominent frontotemporal features associated with L113P presenilin 1 mutation. Neurology. 2000;55(10):1577-1578. doi: 10.1212/WNL.55.10.1577 [DOI] [PubMed] [Google Scholar]
- 48.Rippon GA, Crook R, Baker M, et al. Presenilin 1 mutation in an african american family presenting with atypical Alzheimer dementia. Arch Neurol. 2003;60(6):884-888. doi: 10.1001/archneur.60.6.884 [DOI] [PubMed] [Google Scholar]
- 49.Yokota O, Terada S, Ishizu H, et al. Variability and heterogeneity in Alzheimer’s disease with cotton wool plaques: a clinicopathological study of four autopsy cases. Acta Neuropathol. 2003;106(4):348-356. doi: 10.1007/s00401-003-0737-7 [DOI] [PubMed] [Google Scholar]
- 50.Doran M, Larner AJ. Prominent behavioural and psychiatric symptoms in early-onset Alzheimer’s disease in a sib pair with the presenilin-1 gene R269G mutation. Eur Arch Psychiatry Clin Neurosci. 2004;254(3):187-189. doi: 10.1007/s00406-004-0467-4 [DOI] [PubMed] [Google Scholar]
- 51.Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005;128(Pt 9):1996-2005. doi: 10.1093/brain/awh598 [DOI] [PubMed] [Google Scholar]
- 52.Shi J, Shaw CL, Du Plessis D, et al. Histopathological changes underlying frontotemporal lobar degeneration with clinicopathological correlation. Acta Neuropathol. 2005;110(5):501-512. doi: 10.1007/s00401-005-1079-4 [DOI] [PubMed] [Google Scholar]
- 53.Larner AJ. “Frontal variant Alzheimer’s disease”: a reappraisal. Clin Neurol Neurosurg. 2006;108(7):705-708. doi: 10.1016/j.clineuro.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 54.Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer’s disease. Brain. 2007;130(Pt 10):2636-2645. doi: 10.1093/brain/awm213 [DOI] [PubMed] [Google Scholar]
- 55.Taylor KI, Probst A, Miserez AR, Monsch AU, Tolnay M. Clinical course of neuropathologically confirmed frontal-variant Alzheimer’s disease. Nat Clin Pract Neurol. 2008;4(4):226-232. doi: 10.1038/ncpneuro0746 [DOI] [PubMed] [Google Scholar]
- 56.Kile SJ, Ellis WG, Olichney JM, Farias S, DeCarli C. Alzheimer abnormalities of the amygdala with Klüver-Bucy syndrome symptoms: an amygdaloid variant of Alzheimer disease. Arch Neurol. 2009;66(1):125-129. doi: 10.1001/archneurol.2008.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bigio EH, Mishra M, Hatanpaa KJ, et al. TDP-43 pathology in primary progressive aphasia and frontotemporal dementia with pathologic Alzheimer disease. Acta Neuropathol. 2010;120(1):43-54. doi: 10.1007/s00401-010-0681-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Habek M, Hajnsek S, Zarković K, Chudy D, Mubrin Z. Frontal variant of Alzheimer’s disease: clinico-CSF-pathological correlation. Can J Neurol Sci. 2010;37(1):118-120. doi: 10.1017/S0317167100009768 [DOI] [PubMed] [Google Scholar]
- 59.Lehmann M, Rohrer JD, Clarkson MJ, et al. Reduced cortical thickness in the posterior cingulate gyrus is characteristic of both typical and atypical Alzheimer’s disease. J Alzheimers Dis. 2010;20(2):587-598. doi: 10.3233/JAD-2010-1401 [DOI] [PubMed] [Google Scholar]
- 60.Piscopo P, Talarico G, Crestini A, et al. A novel mutation in the predicted TMIII domain of the PSEN2 gene in an Italian pedigree with atypical Alzheimer’s disease. J Alzheimers Dis. 2010;20(1):43-47. doi: 10.3233/JAD-2010-1369 [DOI] [PubMed] [Google Scholar]
- 61.Snowden JS, Thompson JC, Stopford CL, et al. The clinical diagnosis of early-onset dementias: diagnostic accuracy and clinicopathological relationships. Brain. 2011;134(pt 9):2478-2492. doi: 10.1093/brain/awr189 [DOI] [PubMed] [Google Scholar]
- 62.Whitwell JL, Jack CR Jr, Przybelski SA, et al. Temporoparietal atrophy: a marker of AD pathology independent of clinical diagnosis. Neurobiol Aging. 2011;32(9):1531-1541. doi: 10.1016/j.neurobiolaging.2009.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borroni B, Pilotto A, Bonvicini C, et al. Atypical presentation of a novel Presenilin 1 R377W mutation: sporadic, late-onset Alzheimer disease with epilepsy and frontotemporal atrophy. Neurol Sci. 2012;33(2):375-378. doi: 10.1007/s10072-011-0714-1 [DOI] [PubMed] [Google Scholar]
- 64.Duker AP, Espay AJ, Wszolek ZK, Rademakers R, Dickson DW, Kelley BJ. Atypical motor and behavioral presentations of Alzheimer disease: a case-based approach. Neurologist. 2012;18(5):266-272. doi: 10.1097/NRL.0b013e3182675376 [DOI] [PubMed] [Google Scholar]
- 65.Wallon D, Rousseau S, Rovelet-Lecrux A, et al. ; collaborators of GMAJ project . The French series of autosomal dominant early onset Alzheimer’s disease cases: mutation spectrum and cerebrospinal fluid biomarkers. J Alzheimers Dis. 2012;30(4):847-856. doi: 10.3233/JAD-2012-120172 [DOI] [PubMed] [Google Scholar]
- 66.Herrero-San Martín A, Villarejo-Galende A, Rábano-Gutiérrez A, Guerrero-Márquez C, Porta-Etessam J, Bermejo-Pareja F. [Frontal variant of Alzheimer’s disease. Two pathologically confirmed cases and a literature review]. Rev Neurol. 2013;57(12):542-548. [PubMed] [Google Scholar]
- 67.Marini S, Lucidi G, Tedde A, Bessi V, Nacmias B. A case of atypical early-onset Alzheimer's disease carrying the missense mutation Thr354Ile in exon 10 of the PSEN1 gene. Neurol Sci. 2013;34(9):1691-1692. doi: 10.1007/s10072-012-1260-1 [DOI] [PubMed] [Google Scholar]
- 68.Nygaard HB, Lippa CF, Mehdi D, Baehring JM. A novel presenilin 1 mutation in early-onset Alzheimer’s disease with prominent frontal features. Am J Alzheimers Dis Other Demen. 2014;29(5):433-435. doi: 10.1177/1533317513518653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balasa M, Gelpi E, Martín I, et al. Diagnostic accuracy of behavioral variant frontotemporal dementia consortium criteria (FTDC) in a clinicopathological cohort. Neuropathol Appl Neurobiol. 2015;41(7):882-892. doi: 10.1111/nan.12194 [DOI] [PubMed] [Google Scholar]
- 70.Paterson RW, Toombs J, Slattery CF, et al. Dissecting IWG-2 typical and atypical Alzheimer’s disease: insights from cerebrospinal fluid analysis. J Neurol. 2015;262(12):2722-2730. doi: 10.1007/s00415-015-7904-3 [DOI] [PubMed] [Google Scholar]
- 71.Li P, Zhou YY, Lu D, Wang Y, Zhang HH. Correlated patterns of neuropsychological and behavioral symptoms in frontal variant of Alzheimer disease and behavioral variant frontotemporal dementia: a comparative case study. Neurol Sci. 2016;37(5):797-803. doi: 10.1007/s10072-015-2405-9 [DOI] [PubMed] [Google Scholar]
- 72.Ossenkoppele R, Schonhaut DR, Scholl M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139(pt 5):1551-1567. doi: 10.1093/brain/aww027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scialò C, Ferrara M, Accardo J, et al. Frontal variant Alzheimer disease or frontotemporal lobe degeneration with incidental amyloidosis? Alzheimer Dis Assoc Disord. 2016;30(2):183-185. doi: 10.1097/WAD.0000000000000123 [DOI] [PubMed] [Google Scholar]
- 74.Dickerson BC, McGinnis SM, Xia C, et al. Approach to atypical Alzheimer’s disease and case studies of the major subtypes. CNS Spectr. 2017;22(6):439-449. doi: 10.1017/S109285291600047X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duclos H, de La Sayette V, Bonnet AL, et al. Social cognition in the frontal variant of Alzheimer’s disease: a case study. J Alzheimers Dis. 2017;55(2):459-463. doi: 10.3233/JAD-160690 [DOI] [PubMed] [Google Scholar]
- 76.Kawakatsu S, Kobayashi R, Hayashi H. Typical and atypical appearance of early-onset Alzheimer's disease: a clinical, neuroimaging and neuropathological study. Neuropathology. 2017;37(2):150-173. doi: 10.1111/neup.12364 [DOI] [PubMed] [Google Scholar]
- 77.Oboudiyat C, Gefen T, Varelas E, et al. Cerebrospinal fluid markers detect Alzheimer’s disease in nonamnestic dementia. Alzheimers Dement. 2017;13(5):598-601. doi: 10.1016/j.jalz.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rawtaer I, Krishnamoorthy A. Co-occurring frontal variant Alzheimer's dementia and carrier of Huntington's disease allele with reduced penetrance. Psychogeriatrics. 2017;17(6):488-490. doi: 10.1111/psyg.12246 [DOI] [PubMed] [Google Scholar]
- 79.Sawyer RP, Rodriguez-Porcel F, Hagen M, Shatz R, Espay AJ. Diagnosing the frontal variant of Alzheimer’s disease: a clinician’s yellow brick road. J Clin Mov Disord. 2017;4:2. doi: 10.1186/s40734-017-0052-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bagyinszky E, Lee HM, Van Giau V, et al. PSEN1 p.Thr116Ile variant in two Korean families with young onset Alzheimer’s dsease. Int J Mol Sci. 2018;19(9). doi: 10.3390/ijms19092604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boon BDC, Hoozemans JJM, Lopuhaä B, et al. Neuroinflammation is increased in the parietal cortex of atypical Alzheimer’s disease. J Neuroinflammation. 2018;15(1):170. doi: 10.1186/s12974-018-1180-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seo SW, Thibodeau M-P, Perry DC, et al. Early vs late age at onset frontotemporal dementia and frontotemporal lobar degeneration. Neurology. 2018;90(12):e1047-e1056. doi: 10.1212/WNL.0000000000005163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Whitwell JL, Graff-Radford J, Tosakulwong N, et al. Imaging correlations of tau, amyloid, metabolism, and atrophy in typical and atypical Alzheimer’s disease. Alzheimers Dement. 2018;14(8):1005-1014. doi: 10.1016/j.jalz.2018.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Souza LC, Mariano LI, de Moraes RF, Caramelli P. Behavioral variant of frontotemporal dementia or frontal variant of Alzheimer’s disease? a case study. Dement Neuropsychol. 2019;13(3):356-360. doi: 10.1590/1980-57642018dn13-030015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Foiani MS, Cicognola C, Ermann N, et al. Searching for novel cerebrospinal fluid biomarkers of tau pathology in frontotemporal dementia: an elusive quest. J Neurol Neurosurg Psychiatry. 2019;90(7):740-746. doi: 10.1136/jnnp-2018-319266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Monacelli F, Martella L, Parodi MN, Odetti P, Fanelli F, Tabaton M. Frontal variant of Alzheimer’s disease: a report of a novel PSEN1 mutation. J Alzheimers Dis. 2019;70(1):11-15. doi: 10.3233/JAD-190363 [DOI] [PubMed] [Google Scholar]
- 87.Nolan A, Resende EDF, Petersen C, et al. Astrocytic tau deposition is frequent in typical and atypical Alzheimer disease presentations. J Neuropathol Exp Neurol. 2019;78(12):1112-1123. doi: 10.1093/jnen/nlz094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pawlowski M, Joksch V, Wiendl H, Meuth SG, Duning T, Johnen A. Apraxia screening predicts Alzheimer pathology in frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2019;90(5):562-569. doi: 10.1136/jnnp-2018-318470 [DOI] [PubMed] [Google Scholar]
- 89.Pillai JA, Bonner-Jackson A, Bekris LM, Safar J, Bena J, Leverenz JB. Highly elevated cerebrospinal fluid total tau level reflects higher likelihood of non-amnestic subtype of Alzheimer’s disease. J Alzheimers Dis. 2019;70(4):1051-1058. doi: 10.3233/JAD-190519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tan RH, Yang Y, McCann H, Shepherd C, Halliday GM. Von economo neurons in behavioral variant frontotemporal dementia with underlying Alzheimer’s disease. J Alzheimers Dis. 2019;69(4):963-967. doi: 10.3233/JAD-180900 [DOI] [PubMed] [Google Scholar]
- 91.Wong S, Strudwick J, Devenney E, Hodges JR, Piguet O, Kumfor F. Frontal variant of Alzheimer’s disease masquerading as behavioural-variant frontotemporal dementia: a case study comparison. Neurocase. 2019;25(1-2):48-58. doi: 10.1080/13554794.2019.1609523 [DOI] [PubMed] [Google Scholar]
- 92.Cai H, Ning S, Li W, Li X, Xiao S, Sun L. Patient with frontal-variant syndrome in early-onset Alzheimer’s disease. Gen Psychiatr. 2020;33(2):e100173. doi: 10.1136/gpsych-2019-100173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cousins KAQ, Irwin DJ, Wolk DA, et al. ATN status in amnestic and non-amnestic Alzheimer’s disease and frontotemporal lobar degeneration. Brain. 2020;143(7):2295-2311. doi: 10.1093/brain/awaa165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li CH, Fan SP, Chen TF, Chiu MJ, Yen RF, Lin CH. Frontal variant of Alzheimer’s disease with asymmetric presentation mimicking frontotemporal dementia: case report and literature review. Brain Behav. 2020;10(3):e01548. doi: 10.1002/brb3.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paquin V, Therriault J, Pascoal TA, Rosa-Neto P, Gauthier S. Frontal variant of Alzheimer disease differentiated from frontotemporal dementia using in vivo amyloid and tau imaging. Cogn Behav Neurol. 2020;33(4):288-293. doi: 10.1097/WNN.0000000000000251 [DOI] [PubMed] [Google Scholar]
- 96.Scarioni M, Gami-Patel P, Timar Y, et al. Frontotemporal dementia: correlations between psychiatric symptoms and pathology. Ann Neurol. 2020;87(6):950-961. doi: 10.1002/ana.25739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu L, Sun LM, Sun L, Xiao SF. Case of early-onset Alzheimer’s disease with atypical manifestation. Gen Psychiatr. 2021;34(1):e100283. doi: 10.1136/gpsych-2020-100283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Johnson JK, Diehl J, Mendez MF, et al. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol. 2005;62(6):925-930. doi: 10.1001/archneur.62.6.925 [DOI] [PubMed] [Google Scholar]
- 99.Mattsson N, Groot C, Jansen WJ, et al. Prevalence of the apolipoprotein E ε4 allele in amyloid β positive subjects across the spectrum of Alzheimer’s disease. Alzheimers Dement. 2018;14(7):913-924. doi: 10.1016/j.jalz.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 100.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349-2356. doi: 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. ; Amyloid PET Study Group . Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313(19):1939-1949. doi: 10.1001/jama.2015.4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ashton NJ, Janelidze S, Al Khleifat A, et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun. 2021;12(1):3400. doi: 10.1038/s41467-021-23620-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leuzy A, Ashton NJ, Mattsson-Carlgren N, et al. update on the clinical validity of cerebrospinal fluid amyloid, tau, and phospho-tau as biomarkers for Alzheimer’s disease in the context of a structured 5-phase development framework. Eur J Nucl Med Mol Imaging. 2020;2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ossenkoppele R, Rabinovici GD, Smith R, et al. Discriminative accuracy of [18F]flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2018;320(11):1151-1162. doi: 10.1001/jama.2018.12917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bischof GN, Dodich A, Boccardi M, et al. Clinical validity of second-generation tau PET tracers as biomarkers for Alzheimer’s disease in the context of a structured 5-phase development framework. Eur J Nucl Med Mol Imaging. 2021;48(7):2110-2120. doi: 10.1007/s00259-020-05156-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jack CR, Wiste HJ, Botha H, et al. The bivariate distribution of amyloid-β and tau: relationship with established neurocognitive clinical syndromes. Brain. 2019;142(10):3230-3242. doi: 10.1093/brain/awz268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fleisher AS, Pontecorvo MJ, Devous MD Sr, et al. ; A16 Study Investigators . Positron emission tomography imaging with [18F]flortaucipir and postmortem assessment of Alzheimer disease neuropathologic changes. JAMA Neurol. 2020;77(7):829-839. doi: 10.1001/jamaneurol.2020.0528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer’s disease. Dement Geriatr Cogn Disord. 2002;14(1):33-40. doi: 10.1159/000058331 [DOI] [PubMed] [Google Scholar]
- 109.Tang-Wai DF, Graff-Radford NR, Boeve BF, et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63(7):1168-1174. doi: 10.1212/01.WNL.0000140289.18472.15 [DOI] [PubMed] [Google Scholar]
- 110.Adolphs R. Conceptual challenges and directions for social neuroscience. Neuron. 2010;65(6):752-767. doi: 10.1016/j.neuron.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hansson O. Biomarkers for neurodegenerative diseases. Nat Med. 2021;27(6):954-963. doi: 10.1038/s41591-021-01382-x [DOI] [PubMed] [Google Scholar]
- 112.Borroni B, Agosti C, Premi E, et al. The FTLD-modified Clinical Dementia Rating scale is a reliable tool for defining disease severity in frontotemporal lobar degeneration: evidence from a brain SPECT study. Eur J Neurol. 2010;17(5):703-707. doi: 10.1111/j.1468-1331.2009.02911.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Full database queries
eTable 2. Selection of frontal regions in autopsy studies
eTable 3. Risk of bias assessment
eTable 4. Characteristics of included studies
eTable 5. Percentage of bvFTD features and NPI items in bvAD, bvFTD and tAD
eTable 6. Functional connectivity and white matter hyperintensities in bvAD
eFigure 1. Funnel plots for behavioral/neuropsychiatric data in meta-analysis
eFigure 2. Funnel plots for cognitive data in meta-analysis
eFigure 3. Funnel plots for neuropathological data in meta-analysis
eFigure 4. Risk of bias assessment summary
eFigure 5. Flow chart of study inclusion
eFigure 6. Results of meta-analysis for behavioral and neuropsychiatric separately
eFigure 7. Meta-analyses for neuropathological data in bvAD vs typical AD
eFigure 8. Differences and overlap between bvAD and dysexecutive AD
eReferences. Reference list Supplement


