This cohort study identifies predictors for focality of the seizure-onset zone in stereoelectroencephalography based on standard presurgical epilepsy investigation to develop a score to calculate the probability of identifying a focal seizure onset in stereoelectroencephalography.
Key Points
Question
Can noninvasive presurgical investigation predict focality of the seizure-onset zone in stereoelectroencephalography?
Findings
In this cohort study of 128 patients, focal magnetic resonance imaging lesion, regional scalp ictal electroencephalographic onset, absence of bilateral independent spikes, strongly localizing semiology, and localizing neuropsychological deficit were associated with a focal seizure onset. A score using these variables was built and external validation was performed; high specificity in score development and validation showed that the 5-SENSE score predicts patients where stereoelectroencephalography is unlikely to identify a focal seizure-onset zone.
Meaning
The 5-SENSE score may be useful for clinical decision-making and can assist in identifying patients where stereoelectroencephalography is unlikely to find a focal generator.
Abstract
Importance
Stereoelectroencephalography (SEEG) has become the criterion standard in case of inconclusive noninvasive presurgical epilepsy workup. However, up to 40% of patients are subsequently not offered surgery because the seizure-onset zone is less focal than expected or cannot be identified.
Objective
To predict focality of the seizure-onset zone in SEEG, the 5-point 5-SENSE score was developed and validated.
Design, Setting, and Participants
This was a monocentric cohort study for score development followed by multicenter validation with patient selection intervals between February 2002 to October 2018 and May 2002 to December 2019. The minimum follow-up period was 1 year. Patients with drug-resistant epilepsy undergoing SEEG at the Montreal Neurological Institute were analyzed to identify a focal seizure-onset zone. Selection criteria were 2 or more seizures in electroencephalography and availability of complete neuropsychological and neuroimaging data sets. For validation, patients from 9 epilepsy centers meeting these criteria were included. Analysis took place between May and July 2021.
Main Outcomes and Measures
Based on SEEG, patients were grouped as focal and nonfocal seizure-onset zone. Demographic, clinical, electroencephalography, neuroimaging, and neuropsychology data were analyzed, and a multiple logistic regression model for developing a score to predict SEEG focality was created and validated in an independent sample.
Results
A total of 128 patients (57 women [44.5%]; median [range] age, 31 [13-58] years) were analyzed for score development and 207 patients (97 women [46.9%]; median [range] age, 32 [16-70] years) were analyzed for validation. The score comprised the following 5 predictive variables: focal lesion on structural magnetic resonance imaging, absence of bilateral independent spikes in scalp electroencephalography, localizing neuropsychological deficit, strongly localizing semiology, and regional ictal scalp electroencephalography onset. The 5-SENSE score had an optimal mean (SD) probability cutoff for identifying a focal seizure-onset zone of 37.6 (3.5). Area under the curve, specificity, and sensitivity were 0.83, 76.3% (95% CI, 66.7-85.8), and 83.3% (95% CI, 72.30-94.1), respectively. Validation showed 76.0% (95% CI, 67.5-84.0) specificity and 52.3% (95% CI, 43.0-61.5) sensitivity.
Conclusions and Relevance
High specificity in score development and validation confirms that the 5-SENSE score predicts patients where SEEG is unlikely to identify a focal seizure-onset zone. It is a simple and useful tool for assisting clinicians to reduce unnecessary invasive diagnostic burden on patients and overutilization of limited health care resources.
Introduction
Epilepsy surgery is the only option to cure patients with focal drug-resistant epilepsy.1,2 The delineation of the epileptogenic zone,3,4 the area necessary to be removed or disconnected to render the patient seizure free, is the goal of presurgical evaluation.5 Long-term video electroencephalography (EEG) monitoring, structural and functional neuroimaging, and neuropsychological assessment are the basic components of presurgical evaluation.6 The decision on a patient’s eligibility for surgery is made in a multidisciplinary case conference. In case of discordant or insufficient findings, additional invasive intracranial EEG recording is required.5,7
There is a worldwide increasing trend toward the use of stereoelectroencephalography (SEEG),8,9 which offers the advantage of a 3-dimensional exploration of the epileptogenic network with coverage of deep and bilateral brain structures without large craniotomies. Demonstration of the seizure-onset zone, defined as the area of the cortex that initiates clinical seizures4 currently offers the best approximation of the epileptogenic zone and identification of a focal resectable seizure-onset zone is the primary goal of SEEG.
However, up to 42% of patients are not eligible for subsequent epilepsy surgery9,10,11 because their epilepsy is not focal or the generator is located in eloquent cortex or cannot be identified. This is problematic because SEEG is invasive with procedure-related complications,9,10,11,12 is time consuming, and is resource intensive. Hence, identifying patients in whom this invasive investigation is unlikely to result in identifying a focal seizure-onset zone is important. We aimed to identify predictors for focality of the seizure-onset zone in SEEG based on standard presurgical epilepsy investigation and to develop a score to calculate the probability of identifying a focal seizure onset in SEEG.
Methods
Study Design and Patients
In consecutive patients who underwent SEEG at the Montreal Neurological Institute and Hospital between May 2002 and October 2018, a 5-point score was developed to predict focality of the seizure-onset zone in SEEG. We then validated this score in consecutive patients from 9 tertiary epilepsy centers (Bucharest University Hospital, Dalhousie University, Grenoble-Alpes University Hospital, University Hospital Brno, Massachusetts General Hospital, Montreal Neurological Hospital, Northwestern University, Paracelsus Medical University Salzburg, and University of Pittsburgh) with each center contributing 10 or more patients. Demographic data and clinical information were collected by medical record review. For criteria and flowcharts of patient selection of the cohorts for score development and validation, see the eFigure in the Supplement. Data on race and ethnicity were not collected. The protocol received approval from the Montreal Neurological Institute and Hospital institutional review board as lead institution and the local sites. Informed consent was waived by the Montreal Neurological Institute and Hospital Director of Professional Services because this research was retrospective.
Neuropsychological Testing and Structural Imaging
Results of neuropsychological testing at the time of presurgical evaluation were extracted from written reports and categorized as (1) no deficit, (2) localizing deficit, and (3) nonlocalizing deficit. High-resolution 3-T magnetic resonance imaging (MRI) was performed according to the in-house epilepsy protocol. MRI results were categorized as (1) no lesion, (2) focal (sublobar) lesion, (3) lobar lesion, (4) unilateral multilobar/multifocal lesion, and (5) bilateral multifocal lesion.
Scalp Video EEG Telemetry
Scalp video EEG telemetry recordings were performed according to the international 10-20 system with additional inferior temporal electrodes (F9/10, T9/10, P9/10). Interictal epileptiform discharge (IED) populations were visually identified and grouped into 3 main categories: (1) no IEDs, (2) bilateral independent, and (3) all others (unilateral regional, unilateral nonregional, bilateral synchronous, bitemporal, diffuse bilateral IEDs). For validation, interictal scalp EEG data were extracted from written reports and categorized into the same groups.
The first 3 habitual electroclinical seizures of patients in the score development cohort were analyzed by 2 epileptologists (A.A.-R. and C.A.) and reviewed by a third epileptologist (B.F.) in case of disagreement to reach consensus. If a patient had different seizure types, 3 habitual seizures of each type were analyzed. A seizure type was defined according to electroclinical criteria based on semiology and EEG pattern. For validation, data on ictal scalp EEG and semiology were extracted from written reports.
Semiology was assessed as (1) presence of an aura, (2) lateralizing and (3) localizing features of the seizure (including semiology of aura, observable semiology of recorded seizures, and peri-ictal testing), (4) time to secondary generalization, and (5) time between first ictal discharge and onset of first clinical symptoms. To assess the strength of the lateralizing and localizing value of semiological signs, a level of certainty was attributed (0 indicated not localizing; 1, weak localizing value; 2, strong localizing value). Localization of semiology was assessed from a functional and topographical perspective and divided as (1) motor, (2) somatosensory, (3) limbic, (4) autonomic, (5) visual, or (6) auditory system and (1) frontal, (2) temporal, (3) insular, (4) parietal, or (5) occipital lobe.
The ictal EEG was analyzed for (1) number and distribution of channels involved in initial ictal discharge, (2) seizure onset pattern,12 (3) interval between initial EEG change and contralateral propagation, and (4) duration of ictal EEG. Ictal EEG onset was grouped as (1) unilateral regional/lobar, (2) unilateral multilobar/hemispheric, (3) bilateral synchronous, (4) diffuse (bilateral), and (5) no ictal EEG changes. For the development of the score, these groups were merged into 3 categories: (1) unilateral regional/lobar, (2) bilateral synchronous, and (3) all others. The same groups were used for validation.
SEEG Recordings
Electrode placement was tailored based on the electroclinico-anatomical hypothesis. According to the SEEG result, patients were divided in groups of patients with a focal seizure-onset zone and nonfocal seizure-onset zone. Presence of a focal seizure-onset zone on SEEG was defined as the identification of a sublobar seizure onset, whereas nonfocal included a lobar, multilobar, multifocal, or missed seizure-onset zone.
Statistical Analysis
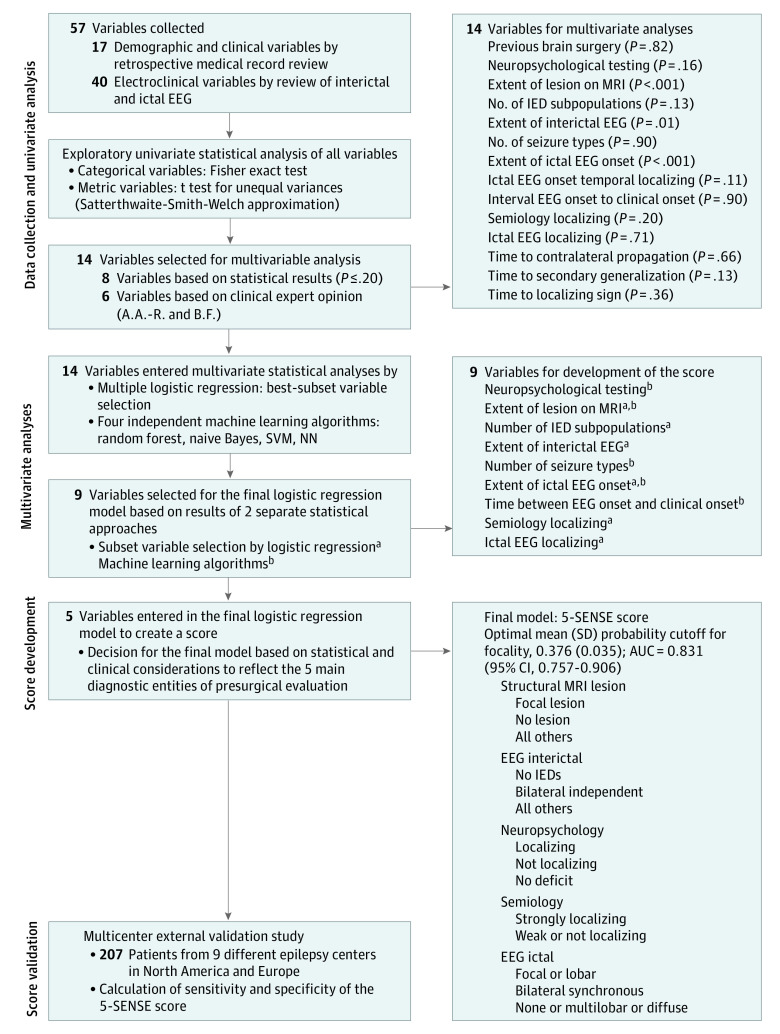
Figure 1 describes the stepwise approach used to develop the score predicting focality in SEEG. In step 1, we performed univariate statistical analysis of the potential predictors. Because statistical significance might be a misleading decision criterion with respect to inclusion of variables into multivariable models,13 subject matter expertise was also taken into account. In step 2, statistical analysis of the most influential variables was done by applying 2 different approaches: logistic regression analysis and machine learning (eAppendix in the Supplement). In step 3, the selected predictors were merged into one single list (Figure 1). The logistic regression model with 5 variables from that list, which minimized the Akaike information criterion, was considered as the final model. The decision to take 5 variables was based on a considerable drop of the event-to-variable ratio below 10 by using more variables.14 Discrimination was assessed by calculating the area under the curve and the corresponding 95% CIs. Calibration was examined by inspecting a calibration plot.15 Probability cutoff values were determined by bootstrapping: 500 samples were taken with replacement from the original data set, and for each of these samples, an optimal cutoff was obtained as the value between 0 and 1 that maximized the weighted sum 0.4 × sensitivity + 0.6 × specificity. Finally, the mean and standard deviation of the resulting cutoff value distribution was extracted, thereby proposing a range of potentially useful cutoff values for clinical practice. Analyses were conducted using the Scikit-Learn toolbox16 in Python 3.6 and R version 3.5.1 (R Foundation).17 Statistical modeling was conducted and reported following the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline.15 For validation, the 5-SENSE scores were calculated for the patients in the validation cohort. Using the cutoffs from the exploratory phase, sensitivities and specificities were estimated. Two-sided P values were statistically significant at .05. Analysis took place between May and July 2021.
Figure 1. Flowchart of Variable Selection and Score Development.
AUC indicates area under the curve; EEG, electroencephalography; IED, interictal epileptiform discharge; MRI, magnetic resonance imaging; NN, neural network; SVM, support vector machine.
aLogistic regression.
bMachine learning algorithms.
Results
Demographic Data
A total of 128 patients (47 women [36.7%]) were analyzed for score development. Of those, 48 patients (37.5%) had a focal seizure-onset zone and 80 (62.5%) had a nonfocal seizure-onset zone. The Table and eTable in the Supplement provide demographic, imaging, and electroclinical data. Subsequent surgery was performed in 106 patients (83%), with a curative intent in 55% (58 of 106; focal vs nonfocal: 38 of 45 [88.4%] vs 21 of 61 [34.5%]; 95% CI, 3.7-31.6; P < .001). In total, 29% (31 of 106; focal vs nonfocal: 16 of 45 [36%] vs 15 of 58 [25%]; 95% CI, 0.6-4.0; P = .39) had a good surgical outcome (Engel 1).18 In patients with a focal MRI lesion (30 of 128 [23%]), SEEG was associated with a change in resection strategy or ruled out a resection in 27% of patients (n = 8).
Table. Neuroimaging and EEG Data of the Patient Cohorts Used for Score Development and Validation.
| Characteristic | Cohort for score development, No. (%) | Validation cohort, No. (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall (n = 128) | Focal (n = 48) | Nonfocal (n = 80) | P valuea | Overall (n = 207) | Focal (n = 111) | Nonfocal (n = 96) | P valuea | |
| Presence of MRI lesion | 77 (60) | 34 (71) | 43 (54) | .06 | 126 (61) | 61 (55) | 65 (68) | .03 |
| Malformation of cortical development | 41 (32) | 19 (40) | 22 (28) | 56 (27) | 23 (21) | 33 (34) | ||
| Hippocampal sclerosis | 7 (5) | 5 (10) | 2 (3) | 21 (10) | 12 (11) | 9 (9) | ||
| Encephalomalacia/posttraumatic | 7 (5) | 1 (2) | 6 (8) | 13 (6) | 5 (5) | 8 (8) | ||
| Cerebrovascular | 3 (2) | 2 (4) | 1 (1) | 11 (53) | 4 (4) | 7 (7) | ||
| Nonspecific | 17 (13) | 5 (10) | 12 (15) | 14 (7) | 9 (8) | 5 (5) | ||
| Tumor | 2 (2) | 2 (4) | 0 | 9 (43) | 9 (8) | 0 | ||
| Other | NA | NA | NA | 5 (24) | 2 (2) | 3 (3) | ||
| Extent of lesion | ||||||||
| Focal | 30 (23) | 21 (44) | 9 (11) | .001 | 53 (26) | 39 (35) | 14 (15) | <.001 |
| Lobar | 7 (5) | 1 (2) | 6 (8) | 11 (53) | 8 (7) | 3 (3) | ||
| Unilateral multilobar, multifocal | 25 (20) | 8 (17) | 17 (21) | 35 (17) | 8 (7) | 27 (28) | ||
| Multifocal bilateral | 15 (12) | 4 (8) | 11 (14) | 27 (13) | 6 (5) | 21 (22) | ||
| No lesion | 51 (40) | 14 (29) | 37 (46) | 81 (39) | 50 (45) | 31 (32) | ||
| Localization of the lesion | ||||||||
| Frontal | 23 (18) | 16 (33) | 7 (9) | .02 | 17 (8) | 8 (7) | 9 (9) | .005 |
| Temporal | 20 (16) | 8 (17) | 12 (15) | 48 (23) | 31 (28) | 17 (18) | ||
| Insular | 1 (1) | 0 | 1 (1) | 2 (1) | 2 (2) | 0 | ||
| Anterior cortex | 5 (4) | 1 (2) | 4 (5) | 9 (4) | 4 (4) | 5 (8) | ||
| Posterior cortex | 23 (18) | 7 (15) | 16 (20) | 32 (15) | 12 (11) | 20 (21) | ||
| Other | 5 (4) | 2 (4) | 3 (4) | 18 (9) | 4 (4) | 14 (15) | ||
| No lesion | 51 (40) | 14 (30) | 37 (46) | 81 (39) | 50 (45) | 31 (32) | ||
| Extent of interictal EEG, grouped | ||||||||
| No IEDs | 14 (11) | 7 (15) | 7 (9) | .03 | 18 (9) | 12 (11) | 6 (6) | .08 |
| Bilateral independent | 18 (14) | 2 (4) | 16 (20) | 28 (13) | 10 (9) | 18 (19) | ||
| All others | 96 (75) | 39 (81) | 57 (71) | 161 (78) | 89 (80) | 72 (75) | ||
| No. of IED subpopulations | ||||||||
| 0-2 | 115 (90) | 46 (96) | 69 (87) | .13 | 166 (80) | 97 (87) | 69 (72) | .003 |
| >2 | 13 (10) | 2 (4) | 11 (14) | 23 (11) | 7 (6) | 19 (20) | ||
| No. of different seizure types, median (range) | 1 (1 to 3) | 1 (1 to 2) | 1 (1 to 3) | .87 | 1 (1 to 5) | 1 (1 to 3) | 1 (1 to 5) | .11 |
| Extent of ictal EEG onset | ||||||||
| Focal/lobar | 38 (30) | 23 (48) | 15 (19) | .002 | 97 (47) | 54 (48) | 43 (45) | .68 |
| Multilobar/hemispheric | 40 (31) | 8 (17) | 32 (40) | 37 (18) | 18 (16) | 19 (20) | ||
| Bilateral synchronous | 17 (13) | 7 (15) | 10 (13) | 24 (12) | 13 (12) | 11 (11) | ||
| Diffuse | 25 (20) | 6 (13) | 19 (24) | 20 (10) | 10 (9) | 10 (10) | ||
| None | 8 (6) | 4 (8) | 4 (5) | 16 (8) | 11 (10) | 10 (10) | ||
| All others | NA | NA | NA | 13 (6) | 5 (5) | 5 (5) | ||
| Extent of ictal EEG onset grouped | ||||||||
| Focal/lobar | 38 (30) | 23 (48) | 15 (19) | <.001 | 97 (47) | 54 (48) | 43 (45) | .82 |
| Bilateral synchronous | 17 (13) | 7 (15) | 10 (13) | 24 (12) | 13 (12) | 11 (11) | ||
| All others | 73 (57) | 18 (23) | 55 (69) | 86 (41) | 44 (40) | 42 (44) | ||
| Time, median (range), s | ||||||||
| To contralateral spread | 8.7 (0 to 78.3) | .6 (0.2-78.3) | 8.5 (0 to 52.3) | .10 | NA | NA | NA | NA |
| Between EEG onset and clinical onset | 3 (−10 to 37.7) | 3 (−6.5 to 37.7) | 3.3 (−10 to 24.3) | .81 | NA | NA | NA | NA |
| To localizing sign | 6.7 (0 to 84) | 6 (0 to 84) | 6.8 (0 to 44.7) | .48 | NA | NA | NA | NA |
| To secondary generalization | 26 (1 to 417) | 49 (16 to 196) | 20.3 (1 to 417) | .28 | NA | NA | NA | NA |
| Semiology localizing | ||||||||
| Strong | 73 (57) | 31 (65) | 42 (53) | .10 | 85 (41) | 56 (50) | 29 (30) | .01 |
| Weak | 49 (38) | 17 (35) | 32 (40) | 96 (46) | 45 (41) | 51 (53) | ||
| Not localizing | 6 (5) | 0 | 6 (7) | 26 (13) | 10 (9) | 16 (17) | ||
| Semiology lateralizing | ||||||||
| Strong | 64 (50) | 24 (50) | 40 (50) | .50 | 91 (44) | 51 (46) | 40 (42) | .54 |
| Weak | 38 (30) | 12 (25) | 26 (32) | 71 (34) | 43 (39) | 28 (29) | ||
| Not lateralizing | 26 (20) | 12 (25) | 14 (18) | 45 (22) | 17 (15) | 18 (19) | ||
Abbreviations: EEG, electroencephalography; IED, interictal epileptiform discharge; NA, not applicable.
For metric/ordinal variables, the t test for unequal variances (ie, the Satterthwaite-Smith-Welch approximation) was used. For nominal variables, Fisher exact test was applied.
Data of 207 patients (97 women [46.9%]) were analyzed in the validation cohort. A total of 111 patients (54%) had a focal and 96 (46%) had a nonfocal seizure-onset zone (Table and eTable in the Supplement provide electroclinical information). Surgery was performed following SEEG exploration in 80% of all patients (164 of 207) (focal vs nonfocal: 102 of 111 [92%] vs 61 of 96 [64%]; 95% CI, 2.8-16.3; P < .001) with a curative intent in 72% (80 of 111) in the focal and 35% (34 of 96) in the nonfocal group (95% CI, 2.5-8.9; P < .001). Good outcome (Engel 1) was achieved in 49% (81 of 164; focal vs nonfocal: 54 of 102 [53%] vs 27 of 61 [39%]; 95% CI, 0.7-2.8; P = .33) of patients undergoing surgery (median [range] follow-up, 24 [12-79] months).
Score Development
Step 1: Univariate Analysis
Fourteen variables were selected for multivariate analyses (Figure 1). A focal MRI lesion was associated with a focal (21 of 48 [44%]) vs nonfocal seizure-onset zone (9 of 80 [11%]) (95% CI, 2.3-17.0; P < .001). A lobar ictal EEG onset was more frequently found in the focal (23 of 48 [48%]) vs the nonfocal (15 of 80 [19%]) group (95% CI, 1.7-9.6; P < .001). In contrast, bilateral independent interictal EEG changes were associated with a nonfocal seizure-onset zone (nonfocal vs focal: 16 of 80 [20%] vs 2 of 48 [4%]; 95% CI, 1.2-53.4; P = .03). Trends were found for a nonfocal seizure-onset zone and more than 2 IED subpopulations (focal vs nonfocal: 2 of 48 [4%] vs 11 of 80 [14%]; 95% CI, 0-1.3; P = .13) and for a focal seizure-onset zone and strongly localizing semiology (focal vs nonfocal: 31 of 48 [65%] vs 42 of 80 [53%]; 95% CI, 0.7-3.7; P = .20), a temporal ictal EEG onset (focal vs nonfocal: 14 of 48 [29%] vs 41 of 80 [51%]; 95% CI, 0.2-1.9; P = .11), longer duration between seizure onset and generalization (focal: median [range], 49 [16-196] seconds vs nonfocal: 20.3 [1-417] seconds; P = .13) and a localizing neuropsychological deficit (focal vs nonfocal: 21 of 48 [44%] vs 24 of 80 [30%]; 95% CI, 0.8-4.1; P = .16).
Step 2: Multivariate Logistic Regression and Machine Learning
A focal MRI lesion and a focal ictal EEG onset were associated with a focal seizure-onset zone in both approaches; hence, considered essential components of the final model. Moreover, a longer duration between EEG onset and clinical onset was identified as an important predictor of focality by 4 machine learning algorithms, whereas a strong localizing value of the ictal EEG was significantly associated with focality based on logistic regression. The localizing value of semiology as well as the interictal EEG extent were almost equally important. To a lesser extent, the analyses revealed the importance of more than 2 independent IED subpopulations, a lower number of different seizure types and a localizing neuropsychological deficit (eAppendix in the Supplement). Subsequently, a reduced list of 9 variables ranked by their clinical and statistical importance served as the basis for the final score (Figure 1). Owing to correlation of the variables localizing ictal EEG and extent of ictal EEG onset, the variable localizing ictal EEG was excluded.
Step 3: Development of a 5-Point Score to Predict Focality
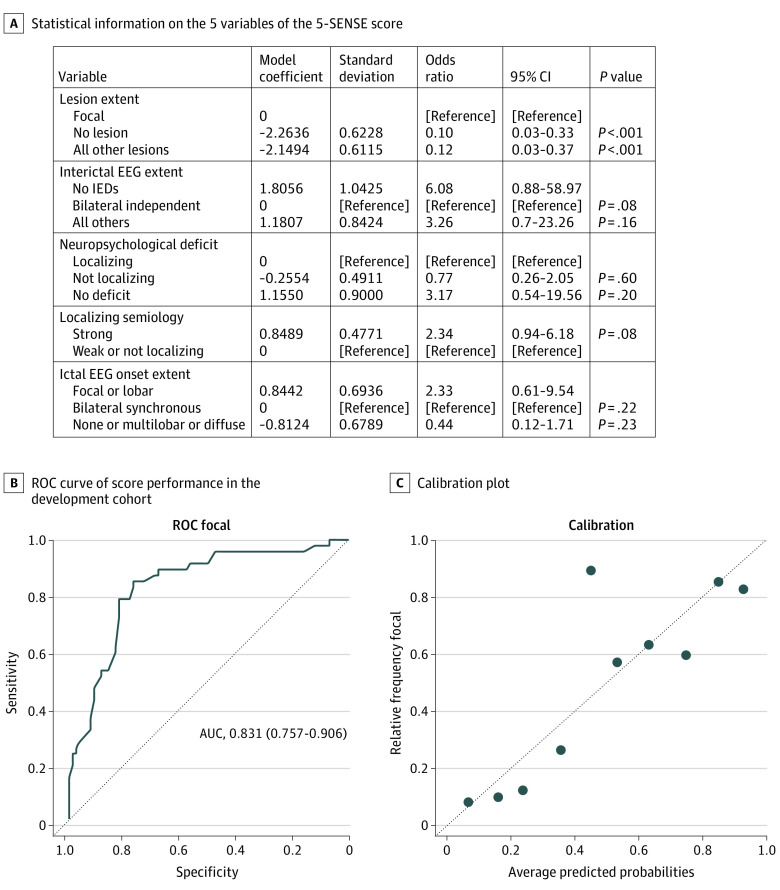
Figure 2 provides information on variables of the 5-SENSE score. No lesion (odds ratio [OR], 0.1 [95% CI, 0.03-0.33]; P < .001; model coefficient = −2.26) or a nonfocal lesion on MRI (OR, 0.12 [95% CI, 0.03-0.37]; P < .001; model coefficient = −2.15) strongly point to a nonfocal seizure-onset zone on SEEG, whereas a focal lesion on MRI was strongly associated with a focal seizure-onset zone. A focal or lobar ictal scalp EEG onset was associated with focality (OR, 2.33 [95% CI, 0.61-9.54]; P = .22; model coefficient = 0.844), compared with a bilateral synchronous ictal EEG onset, no ictal EEG changes, and a multilobar or diffuse ictal EEG onset (OR, 0.44 [95% CI, 0.12-1.7]; P = .23; model coefficient = −0.81). Bilateral independent interictal EEG changes increase the probability of a nonfocal seizure-onset zone compared with no IEDs (OR, 6.08 [95% CI, 0.88-58.97]; P = .08; model coefficient = 1.806) or all others (OR, 3.26 [95% CI, 0.7-23.26]; P = .16; model coefficient = 1.18). A strongly localizing semiology points to a focal seizure-onset zone (OR, 2.34 [95% CI, 0.94-6.18]; P = .08; model coefficient = 0.85). Compared with a localizing neuropsychological deficit, a nonlocalizing neuropsychological deficit was associated with a nonfocal seizure-onset zone (OR, 0.77 [95% CI, 0.26-2.05]; P = .60; model coefficient = −0.26), whereas no deficit points to focality (OR, 3.17 [95% CI, 0.54-19.56]; P = .20; model coefficient = 1.15).
Figure 2. The 5-SENSE Score for Prediction of SEEG Focality and Its Performance.
The 5-SENSE score includes extent of the lesion on magnetic resonance imaging, extent of the ictal discharge, extent of interictal epileptiform discharges (IEDs), strength of localizing semiology, and localizing neuropsychological deficit. The area under the curve (AUC) demonstrates good discrimination (0.831 [95% CI, 0.757-0.906]) and good calibration. Sensitivity and specificity were 83.3% (95% CI, 72.3-94.1) and 76.3% (95% CI, 66.7-85.8), respectively. For classifying predicted probabilities into the focal and nonfocal categories, the optimal mean (SD) probability cutoff was 37.6 (3.5). EEG indicates electroencephalography; ROC, receiver operating characteristic curve.
The 5-SENSE score demonstrated high discrimination based on the area under the curve value of 0.83 (95% CI, 0.757-0.906; Figure 2), with sensitivity of 83.3% (95% CI, 72.3%-94.1%) and specificity of 76.3% (95% CI, 66.7%-85.8%). We calculated the probability of focality in 2 individual patients by inserting their characteristics into the model (Figure 3). Hereby, each variable was weighted based on the model coefficients derived from multivariate analysis (Figure 3). The final score was calculated by applying the standard logistic regression model formula (eAppendix in the Supplement). For classifying the score into focal and no focal categories, the optimal mean (SD) cutoff value was 37.6 (3.5), meaning that values above 37.6 point to focality, whereas all values below 37.6 are in favor of finding no focal seizure-onset zone on SEEG. The 5-SENSE score calculator is publicly available.19
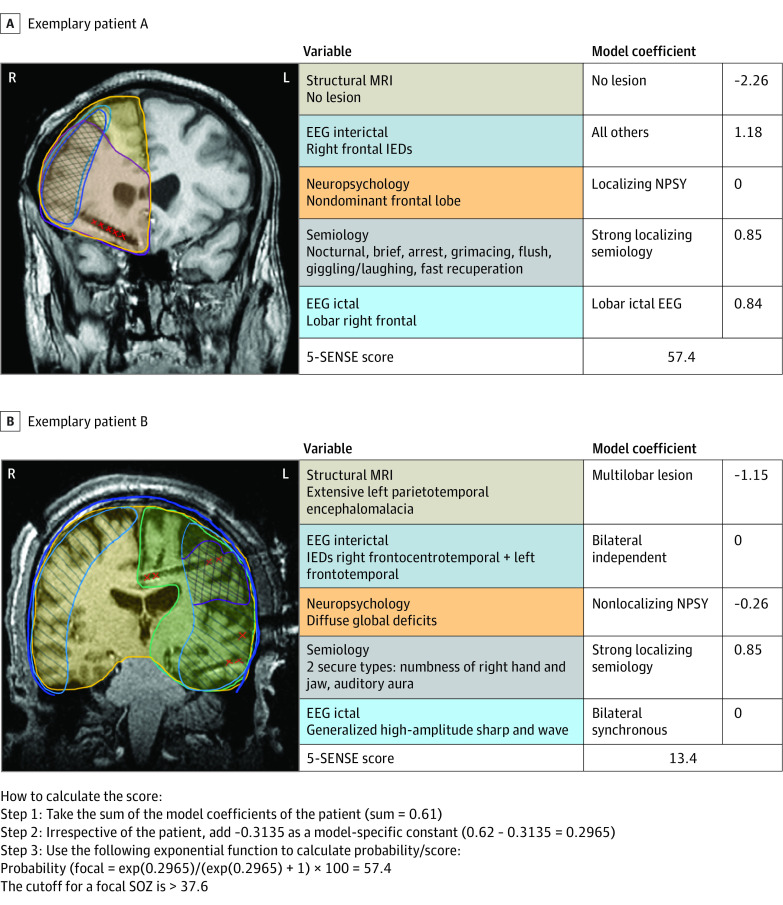
Figure 3. Illustration of the Use of the 5-SENSE Score in 2 Exemplary Patients.
A, A male patient in his late 30s with seizure onset during his late teen years. On high-resolution magnetic resonance imaging (MRI), there was no visible lesion. He experienced brief, nocturnal seizures with arrest and grimacing, followed by a flush, shaking of his head, and giggling, with immediate recuperation afterward. Ictal scalp electroencephalography (EEG) revealed a lobar onset over the right frontal head region. Interictal EEG showed spikes in the same area. His neuropsychological profile showed a mild nondominant frontal lobe disturbance. On stereo EEG (SEEG), a focal right orbitofrontal seizure-onset zone (SOZ) was discovered with subsequent resection. Histology revealed a focal cortical dysplasia (FCD) type 2b; the patient was completely seizure free following surgery. His 5-SENSE score for predicting a focal EEG results was 57.4. B, A male patient in his early 30s with seizure onset in his early 20s, following bacterial meningoencephalitis with empyema and residual extensive left parietotemporal encephalomalacia on MRI. Ictal scalp EEG showed a generalized discharge at seizure onset; interictally, there were independent epileptiform discharges over the right frontocentrotemporal as well as left frontotemporal regions, with right-sided predominance. He experienced 2 different seizure types with strongly localizing aura, consisting of numbness of his right hand and jaw or an auditory aura. Neuropsychology showed diffuse global deficits. SEEG revealed a widespread SOZ involving the left insula as well as the frontotemporoparietal cortex and immediate involvement of the left supplementary motor area (SMA); hence, no surgery was proposed. The patient’s 5-SENSE score precited a nonfocal SEEG result (13.4; cutoff for focality >37.6). IED indicates interictal epileptiform discharge; NPSY, neuropsychology.
Score Validation
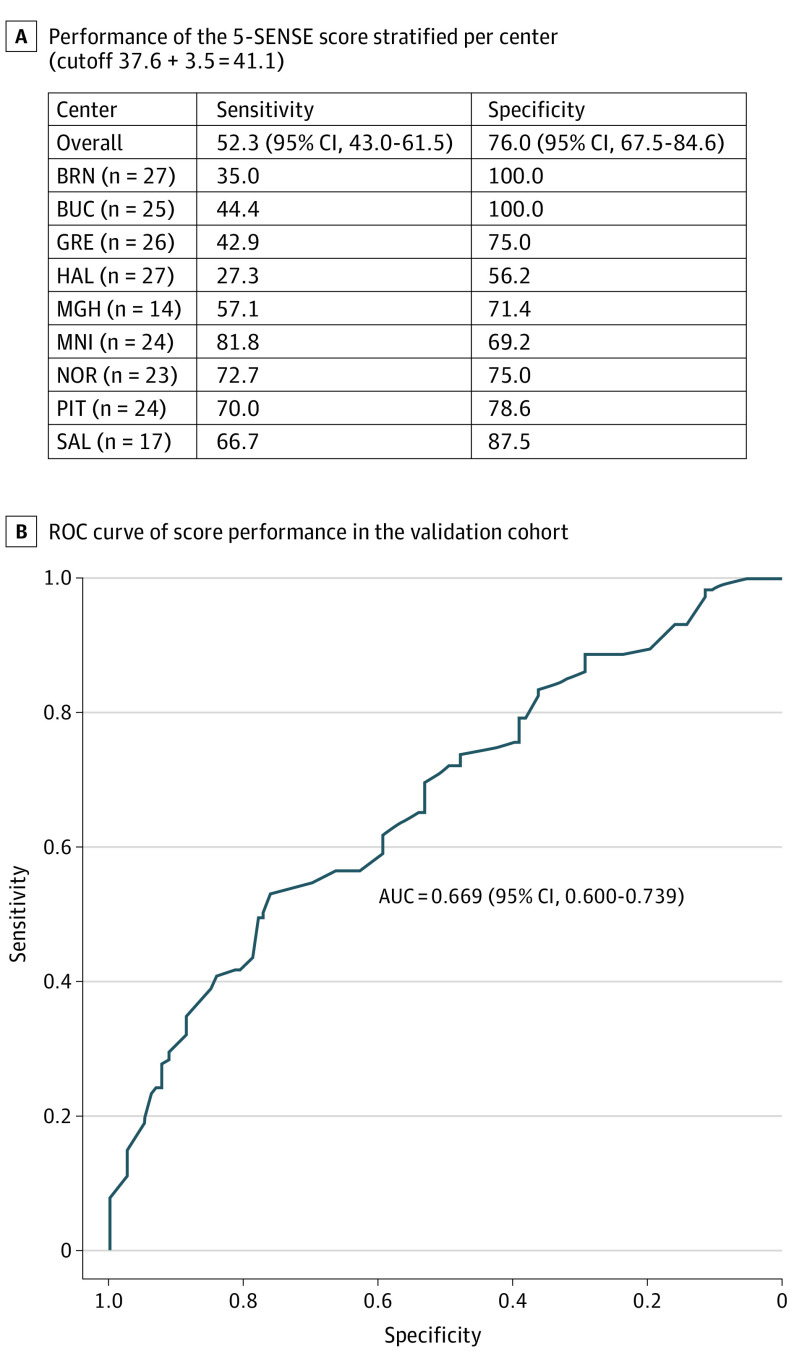
Predictions were calculated for the 207 patients in the validation cohort, using the model obtained in the exploratory model building steps. Subsequently, sensitivity and specificity were calculated. Using a cutoff of 37.6, sensitivity was 54% (95% CI, 44.8%-63.3%) and specificity 69% (95% CI, 59.5%-78.0%). Applying the 3.5 SD, which raised the cutoff to 41.1, specificity increased to 76% (95% CI, 67.5%-84.6%), with minor decrease of sensitivity to 52% (95% CI, 43.0%-61.5%) (Figure 4).
Figure 4. Performance of the 5-SENSE Score in the Validation Cohort.
The area under the curve (AUC) demonstrates good discrimination (0.655 [95% CI, 0.581-0.730]) and good calibration. Sensitivity and specificity were 52.3% (95% CI, 43.0-61.5) and 76.0% (95% CI, 67.5-84.6), respectively. For classifying predicted probabilities into the focal and nonfocal categories, the optimal mean (SD) probability cutoff was 41.1 (3.5). BRN indicates Masaryk University, Brno, Czech Republic; BUC, Carol Davila University of Medicine and Pharmacy Bucharest, Romania; GRE, Grenoble Institute of Neurosciences Centre Hospitalier Universitaire Grenoble Alpes, Grenoble, France; HAL, Dalhousie University and Hospital, Halifax, Canada; MGH, Massachusetts General Hospital, Boston; MNI, Montreal Neurological Institute and Hospital, Montreal, Canada; NOR, Northwestern University, Chicago, Illinois; PIT, University of Pittsburgh, Pittsburgh, Pennsylvania; ROC, receiver operating characteristic curve; SAL, Christian Doppler Clinic, Paracelsus Medical University Hospital, Salzburg, Austria.
Discussion
The decision for implantation of electrodes for SEEG is challenging and despite a reasonable preimplantation hypothesis, the rate of patients in whom no focal seizure-onset zone can be identified is high. The identification of a focal seizure-onset zone is the primary goal of SEEG, as it is the main prerequisite for subsequent surgery. To avoid unnecessary invasive intracranial investigations, we developed a score based on standard presurgical epilepsy evaluation to support whether SEEG will allow identifying a focal seizure-onset zone. We included only the basic diagnostic modalities in the score because we wanted to provide a tool that is easily applicable and independent from auxiliary methods that are not available at all epilepsy centers.
Variables Predictive of a Focal Seizure-Onset Zone
The 2 most significant predictors of a focal seizure-onset zone are a focal extent of the ictal scalp EEG onset and a focal MRI lesion. This is in keeping with the literature,11,20,21 showing better postsurgical outcome when a focal MRI lesion is present.11 However, one has to keep in mind that there is only partial or no overlap of the MRI visible lesion and the seizure-onset zone detected by SEEG in up to 56% of patients.22 In our series, SEEG was associated with a change in resection strategy or ruled out resection in nearly one-third of patients with a focal lesion, underlining that SEEG is not redundant in case of a visible MRI lesion. Combining these data with a strong localizing semiology and a localizing neuropsychological deficit further increases the probability of identifying a focal seizure-onset zone. In contrast, bilateral independent IEDs, with the exception of bilateral temporal IEDs frequently observed in unilateral temporal lobe epilepsy being of different significance,23,24 decrease the probability of a focal generator.25 Also, diffuse, multilobar or no ictal scalp EEG changes at seizure onset are inversely associated with SEEG focality.
Focal or lobar interictal EEG changes did not show a high predictive value for focality on SEEG. One possible explanation is that patients with lobar interictal and ictal scalp EEG changes, especially in case of concordant findings on MRI, are direct surgical candidates. Second, extratemporal lobe epilepsies tend to show more widespread interictal changes.26,27,28 Because we aimed to investigate whether SEEG will allow to identify a focal seizure-onset zone, patients with a primary multifocal hypothesis after phase 1 workup due to highly discordant findings in different diagnostic modalities were not included.
Primary Outcome
Identification of a focal seizure-onset zone strongly associates with the decision to proceed with curative surgery. Therefore, we decided to define seizure-onset zone focality as our primary outcome parameter. We opted not to choose seizure-free outcome as the primary outcome parameter because the decision for surgery following SEEG is not only determined by the identification of a focal seizure-onset zone but depends also on numerous other factors, such as non-EEG–based presurgical investigations, the localization of the seizure-onset zone in relation to eloquent cortex, anatomical conditions, patient factors as well as the complete resectability. Good postsurgical outcome (Engel 1) in our score development cohort was 29%, which is lower compared with other studies11 as well as the validation cohort. This likely reflects the complexity of patients referred to the Montreal Neurological Institute and Hospital, as a high proportion of 38% of patients was offered a palliative approach owing to widespread or multifocal epileptogenicity.
5-SENSE Score
The identification of variables for the 5-point score to estimate probability of a focal seizure-onset zone, derived from a multistage approach, incorporates conventional and modern statistical methods. In addition, because relying only on significance-based variable selection should be avoided,13 we added clinical expert opinion to guide variable selection. This innovative multistage approach allows creation of a robust statistical basis for a score that incorporates the most important diagnostic methods of presurgical evaluation. Convergence of evidence is needed to increase the chances to identify a focal seizure-onset zone.6 This is reflected in the 5-SENSE score putting different weights on the various test results allowing less experienced epileptologists to objectify their decision to implant. The simplified way of calculating the 5-point score should facilitate its use in clinical practice.
External Validation
The 5-SENSE score was developed to predict patients in whom SEEG is unlikely to identify a focal seizure-onset zone. High specificity in score development and validation confirms that it correctly predicts these patients. This is promising and could aid clinicians to estimate the probability that SEEG will not allow to identify a focal seizure-onset zone. Future work will show if integration of auxiliary diagnostic methods will further improve the model.
Limitations
To minimize the risk of overfitting, we used cross-validation for machine learning–based variable selection. Moreover, for logistic regression, multiple models with similar Akaike information criterion values were considered, and in the final model building step, we used a bootstrap approach to select the optimal cutoff value. By validating the score independently, we demonstrated generalizability with reasonable performance on a heterogeneous patient sample.
Conclusions
Many epilepsy centers face the challenging decision of whether a patient should undergo implantation for identifying a focal seizure-onset zone. The 5-SENSE score provides an easily applicable tool to guide clinicians in predicting if SEEG will unlikely identify a focal seizure-onset zone. Thereby, patients with small likelihood to benefit from this invasive and resource-intensive investigation can be identified earlier avoiding unnecessary procedure-related burden on patients and overutilization of health care resources.
eFigure. Flow Chart of Patient Selection of cohort for score development and validation cohort
eAppendix. Statistical Approaches
eTable. Demographic data of the patient cohorts used for score development and validation
eReferences
References
- 1.Wiebe S, Blume WT, Girvin JP, Eliasziw M; Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group . A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311-318. doi: 10.1056/NEJM200108023450501 [DOI] [PubMed] [Google Scholar]
- 2.Dwivedi R, Ramanujam B, Chandra PS, et al. Surgery for drug-resistant epilepsy in children. N Engl J Med. 2017;377(17):1639-1647. doi: 10.1056/NEJMoa1615335 [DOI] [PubMed] [Google Scholar]
- 3.Lüders HO, Najm I, Nair D, Widdess-Walsh P, Bingman W. The epileptogenic zone: general principles. Epileptic Disord. 2006;8(suppl 2):S1-S9. [PubMed] [Google Scholar]
- 4.Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain. 2001;124(Pt 9):1683-1700. doi: 10.1093/brain/124.9.1683 [DOI] [PubMed] [Google Scholar]
- 5.Frauscher B. Localizing the epileptogenic zone. Curr Opin Neurol. 2020;33(2):198-206. doi: 10.1097/WCO.0000000000000790 [DOI] [PubMed] [Google Scholar]
- 6.Vakharia VN, Duncan JS, Witt J-A, Elger CE, Staba R, Engel J Jr. Getting the best outcomes from epilepsy surgery. Ann Neurol. 2018;83(4):676-690. doi: 10.1002/ana.25205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jayakar P, Gotman J, Harvey AS, et al. Diagnostic utility of invasive EEG for epilepsy surgery: Indications, modalities, and techniques. Epilepsia. 2016;57(11):1735-1747. doi: 10.1111/epi.13515 [DOI] [PubMed] [Google Scholar]
- 8.Abou-Al-Shaar H, Brock AA, Kundu B, Englot DJ, Rolston JD. Increased nationwide use of stereoencephalography for intracranial epilepsy electroencephalography recordings. J Clin Neurosci. 2018;53:132-134. doi: 10.1016/j.jocn.2018.04.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Martinez J, Bulacio J, Alexopoulos A, Jehi L, Bingaman W, Najm I. Stereoelectroencephalography in the “difficult to localize” refractory focal epilepsy: early experience from a North American epilepsy center. Epilepsia. 2013;54(2):323-330. doi: 10.1111/j.1528-1167.2012.03672.x [DOI] [PubMed] [Google Scholar]
- 10.Hall JA, Khoo HM. Robotic-assisted and image-guided MRI-compatible stereoelectroencephalography. Can J Neurol Sci. 2018;45(1):35-43. doi: 10.1017/cjn.2017.240 [DOI] [PubMed] [Google Scholar]
- 11.Cardinale F, Rizzi M, Vignati E, et al. Stereoelectroencephalography: retrospective analysis of 742 procedures in a single centre. Brain. 2019;142(9):2688-2704. doi: 10.1093/brain/awz196 [DOI] [PubMed] [Google Scholar]
- 12.Cossu M, Cardinale F, Castana L, et al. Stereoelectroencephalography in the presurgical evaluation of focal epilepsy: a retrospective analysis of 215 procedures. Neurosurgery. 2005;57(4):706-718. doi: 10.1227/01.NEU.0000176656.33523.1e [DOI] [PubMed] [Google Scholar]
- 13.Heinze G, Wallisch C, Dunkler D. Variable selection: a review and recommendations for the practicing statistician. Biom J. 2018;60(3):431-449. doi: 10.1002/bimj.201700067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373-1379. doi: 10.1016/S0895-4356(96)00236-3 [DOI] [PubMed] [Google Scholar]
- 15.Moons KGM, Altman DG, Reitsma JB, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1-73. doi: 10.7326/M14-0698 [DOI] [PubMed] [Google Scholar]
- 16.scikit-learn: Machine learning in Python. Accessed October 22, 2021. https://scikit-learn.org/stable/index.html.
- 17.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engel J. Surgical Treatment of the Epilepsies: Lippincott Williams & Wilkins; 1993. [Google Scholar]
- 19.SENSE. The 5-SENSE calculator. Accessed October 22, 2021. https://lab-frauscher.github.io/Sense_calc/
- 20.Jobst BC, Cascino GD. Resective epilepsy surgery for drug-resistant focal epilepsy: a review. JAMA. 2015;313(3):285-293. doi: 10.1001/jama.2014.17426 [DOI] [PubMed] [Google Scholar]
- 21.Tanaka H, Gotman J, Khoo HM, Olivier A, Hall J, Dubeau F. Neurophysiological seizure-onset predictors of epilepsy surgery outcome: a multivariable analysis. J Neurosurg. 2019;1-10. doi: 10.3171/2019.9.JNS19527 [DOI] [PubMed] [Google Scholar]
- 22.Arévalo-Astrada M, McLachlan RS, Suller-Marti A, et al. All that glitters: contribution of stereo-EEG in patients with lesional epilepsy. Epilepsy Res. 2021;170:106546. doi: 10.1016/j.eplepsyres.2020.106546 [DOI] [PubMed] [Google Scholar]
- 23.Krendl R, Lurger S, Baumgartner C. Absolute spike frequency predicts surgical outcome in TLE with unilateral hippocampal atrophy. Neurology. 2008;71(6):413-418. doi: 10.1212/01.wnl.0000310775.87331.90 [DOI] [PubMed] [Google Scholar]
- 24.Aghakhani Y, Liu X, Jette N, Wiebe S. Epilepsy surgery in patients with bilateral temporal lobe seizures: a systematic review. Epilepsia. 2014;55(12):1892-1901. doi: 10.1111/epi.12856 [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald Z, Morita-Sherman M, Hogue O, et al. Improving the prediction of epilepsy surgery outcomes using basic scalp EEG findings. Epilepsia. 2021;62(10):2439-2450. doi: 10.1111/epi.17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bautista RE, Spencer DD, Spencer SS. EEG findings in frontal lobe epilepsies. Neurology. 1998;50(6):1765-1771. doi: 10.1212/WNL.50.6.1765 [DOI] [PubMed] [Google Scholar]
- 27.Salanova V, Andermann F, Olivier A, Rasmussen T, Quesney LF. Occipital lobe epilepsy: electroclinical manifestations, electrocorticography, cortical stimulation and outcome in 42 patients treated between 1930 and 1991. Surgery of occipital lobe epilepsy. Brain. 1992;115(Pt 6):1655-1680. doi: 10.1093/brain/115.6.1655 [DOI] [PubMed] [Google Scholar]
- 28.Blume WT, Wiebe S, Tapsell LM. Occipital epilepsy: lateral versus mesial. Brain. 2005;128(Pt 5):1209-1225. doi: 10.1093/brain/awh458 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Flow Chart of Patient Selection of cohort for score development and validation cohort
eAppendix. Statistical Approaches
eTable. Demographic data of the patient cohorts used for score development and validation
eReferences