Abstract
A PCR-based assay was developed to detect and identify medically important yeasts in clinical samples. Using a previously described set of primers (G. Morace et al., J. Clin. Microbiol. 35:667–672, 1997), we amplified a fragment of the ERG11 gene for cytochrome P-450 lanosterol 14α-demethylase, a crucial enzyme in the biosynthesis of ergosterol. The PCR product was analyzed in a reverse cross blot hybridization assay with species-specific probes directed to a target region of the ERG11 gene of Candida albicans (pCal), C. guilliermondii (pGui), C. (Torulopsis) glabrata (pGla), C. kefyr (pKef), C. krusei (pKru), C. parapsilosis (pPar), C. tropicalis (pTro), the newly described species C. dubliniensis (pDub), Saccharomyces cerevisiae (pSce), and Cryptococcus neoformans (pCry). The PCR-reverse cross blot hybridization assay correctly identified multiple isolates of each species tested. No cross-hybridization was detected with any other fungal, bacteria, or human DNAs tested. The method was tested against conventional identification on 140 different clinical samples, including blood and cerebrospinal fluid, from patients with suspected fungal infections. The results agreed with those of culture and phenotyping for all but six specimens (two of which grew yeasts not included in the PCR panel of probes and four in which PCR positivity-culture negativity was justified by clinical findings). Species identification time was reduced from a mean of 4 days with conventional identification to 7 h with the molecular method. The PCR-reverse cross blot hybridization assay is a rapid method for the direct detection and identification of yeasts in clinical samples.
The increasing numbers of immunocompromised patients, in particular those infected with human immunodeficiency virus and individuals receiving immunosuppressive therapy, have led to a dramatic rise in the number of cases of Candida species infections (9, 34). It is usually impossible to identify the species responsible for these infections based on clinical manifestations alone, but this information can be essential in choosing treatment. Several non-albicans Candida spp. are inherently less susceptible to commonly used antifungal drugs, and their early identification is essential for rapid initiation of empiric treatment. Other yeasts as well (e.g., Cryptococcus neoformans and Saccharomyces cerevisiae) have emerged as opportunistic pathogens (1, 35), and clinicians are increasingly in need of a method that allows rapid identification of a wide variety of fungi.
Recently, several methods involving PCR technology have been developed for this purpose (4–7, 13–16, 19, 24, 28, 30–33). The molecular approach is much more sensitive and specific than conventional procedures used to detect fungi in clinical samples. Elie et al. (5) have recently developed a very promising PCR assay that is capable of identifying DNA from a total of 18 Candida species in blood specimens, but it requires a preliminary phase of cultivation that can be time-consuming.
This paper describes a new assay based on ERG11 PCR amplification and reverse cross blot hybridization for direct detection and identification of yeasts in clinical specimens. Our new method represents an improvement over the PCR-restriction enzyme analysis (REA) assay we previously developed for the detection of candidemia (16, 17), in which amplicons were analyzed by digestion with appropriate restriction enzymes. In the present format, amplicon detection is performed by hybridization with nylon-fixed species-specific probes. The PCR-reverse cross blot hybridization assay is a simple, rapid, and sensitive method that seems to be suitable for routine use in clinical mycology laboratories.
MATERIALS AND METHODS
Microorganisms and DNA extraction.
The yeast strains used in this study included C. albicans CDC B 385 (Centers for Disease Control and Prevention, Atlanta, Ga.), C. tropicalis CBS 94 (Centraalbureau voor Schimmelcultures, Baarn, The Netherlands), C. glabrata CBS 138, and C. krusei CBS 573. At least five clinical isolates of C. albicans, C. guilliermondii, C. (Torulopsis) glabrata, C. kefyr, C. krusei, C. parapsilosis, C. tropicalis, S. cerevisiae, and C. neoformans; one isolate each of C. lusitaniae, C. rugosa, C. lambica, and Blastoschizomyces capitatus; and two isolates of C. dubliniensis (kindly furnished by Dominique Sanglard, Institut de Microbiologie, Centre Hospitalier Universitarie Vaudois, Lausanne, Switzerland) were also tested. DNA extracted from these microorganisms as previously described (16) was dissolved in TE buffer (10 mM Tris HCl, 1 mM EDTA [pH 8]), and the A260 was measured to determine the DNA concentration. The solution was then adjusted to a concentration of 20 ng/μl and further diluted in water to provide six samples with concentrations ranging from 200 pg to 2 fg of DNA per μl. DNA samples extracted from Escherichia coli, Pseudomonas aeruginosa, and human cells were also tested as negative controls (16).
Clinical samples.
A total of 140 clinical samples from 140 patients with suspected yeast infections were sent for culture to the Microbiology Laboratory of the Catholic University Medical Center in Rome. The samples, which included blood (n = 30), cerebrospinal fluid (n = 10), urine (n = 40), vaginal swabs (n = 30), and pharyngeal exudate (n = 30), were cultured using standard procedures (18). Prior to cultivation, a 1-ml aliquot of each liquid specimen, with the exception of blood, was collected and stored at −80°C until it was used for DNA isolation. Specimens collected with sterile swabs were vortexed in 1 ml of Sabouraud broth, and the latter was frozen. For each specimen of blood (13 ml), 3 ml (collected in tubes containing EDTA) was immediately processed to separate leukocytes and yeasts as previously described (17); the remainder was inoculated into a Mycosis IC/F* bottle (Becton Dickinson, Cockeysville, Md.) and incubated in a BACTEC 9240 automated system (Becton Dickinson). Yeasts detected in cultures were identified by the germination tube test in fetal bovine serum using the yeast biochemical cards of the Vitek Automicrobic System (BioMerieux, Marcy l'Etoile, France), and results were confirmed by analysis of micromorphology on rice extract agar (18).
DNA extraction from clinical samples.
All of the samples were subjected to DNA extraction as previously described (16). The purified DNA preparation (20 μl) was kept at −20°C until PCR. Universal precautions (12), including physical separation of laboratory areas used to handle samples, prepare PCRs, and analyze PCR products, were used to prevent sample contamination.
Oligonucleotides.
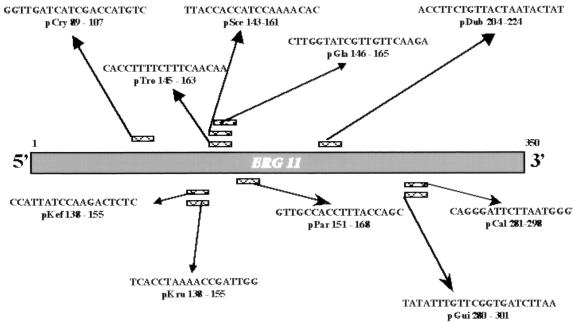
The oligonucleotides used were the same primers used in our earlier PCR-based assay (16), but in the new system the forward primer P4501 was biotin labeled at the 5′ end (P4501bio). Using Oligo Software (v. 4.0), we designed probes based on the ERG11 gene region chosen for PCR amplification. In developing these probes, many length combinations for each probe were evaluated to obtain probes with comparable annealing temperatures. The specificities of these sequences were evaluated by comparing them with the ERG11 sequences previously published (16), using DNASIS for Windows software (Hitachi Software Engineering, San Bruno, Calif.). For ERG11 sequences of C. neoformans and C. dubliniensis, see below. The probes used in the hybridization assay were pCal (5′-CAG GGA TTC TTA ATG GGT-3′), pDub (5′-ACC TTC TGT TAC TAA TAC TAT-3′), pGla (5′-CTT GGT ATC GTT GTT CAA GA-3′), pGui (5′-TAT ATT TGT TCG GTG ATC TTA A-3′), pKef (5′-CCA TTA TCC AAG ACT CTC-3′), pKru (5′-TCA CCT AAA ACC GAT TGG-3′), pPar (5′-GTT GCC ACC TTT ACC AGA-3′), pTro (5′-CAC CCT TTT CTT TCA ACA A-3′), pCry (5′-GGT TGA TCA TCG ACC ATG TC-3′), and pSce (5′-TTA CCA CCA TCC AAA ACA C-3′) (Fig. 1).
FIG. 1.
Sequences of oligonucleotide probes and their positions in the amplified ERG11 gene fragment.
PCR.
PCR was performed in 50 μl of a reaction mixture containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate (dATP, dCTP, dGTP, and dTTP), 50 ng (each) of primers P4501bio and P4502, and 1 U of Taq DNA polymerase (Boehringer Mannheim, Mannheim, Germany). A 10-μl aliquot of extracted DNA (from clinical samples or yeast isolates) was added to the mixture. PCR was performed in a thermocycler (GeneAmp PCR system 2400; Perkin-Elmer, Foster City, Calif.) for 40 cycles under conditions that have been previously described (17). A 10-μl aliquot of the amplified product was immediately analyzed on a 2% agarose gel stained with ethidium bromide, and another 15 μl was used for reverse cross blot hybridization (see below). To detect the presence of inhibitors, one part (10 μl) of each sample was spiked with 5 μl of a positive DNA control (C. albicans DNA) and subjected to PCR. Several negative controls, consisting of water, were included in each PCR run to exclude contamination (17).
Cloning of PCR products.
The PCR products of C. neoformans and C. dubliniensis were cloned using the T/A Cloning System (Invitrogen, San Diego, Calif.) in accordance with the manufacturer's instructions and sequenced in a Sequigen apparatus (Bio-Rad Laboratories, Hercules, Calif.) with the Sequenase sequencing kit, version 2.0 (U.S. Biochemicals) as previously described (16).
Tailing of oligonucleotide probes with dTTP.
Tailing of the oligonucleotide probes was necessary to ensure efficient capture of the PCR products in the reverse cross blot hybridization assay. The tailing reactions were performed with 200 pmol of the oligonucleotide probe in a solution containing 25 mM Tris-HCl (pH 6.6), 200 mM potassium cacodylate, 0.025% (wt/vol) bovine serum albumin, 5 mM CoCl2, 0.5 mM dTTP, and 25 U of terminal transferase (Boehringer Mannheim) per 40-μl reaction volume. The reaction mixtures were incubated at 37°C for 2 h, after which 4 μl of a 0.2 mM EDTA solution was added to stop the reaction.
Reverse cross blot hybridization assay.
The reverse cross blot hybridization assay was performed with a cross blotter apparatus (Accutran-Cross ACC 100/0; Schleicher & Schuell, Dassel, Germany) as described by Kox et al. (11). Briefly, 25 pmol of a dTTP-tailed oligonucleotide probe was blotted onto a positively charged nylon membrane (Boehringer Mannheim) in each slot of the cross blotter apparatus. The probes were fixed to the membrane by baking at 80°C for 2 h. The PCR products (15 μl) were then denatured by heating (100°C for 10 min) and applied to the membrane in the hybridization solution (5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 1% blocking reagent [Boehringer Mannheim], 0.1% N-lauroylsarcosine, 0.02% sodium dodecyl sulfate), at 50°C for 1 h. The hybridized PCR products were detected by incubation with streptavidin-alkaline phosphatase and a color substrate in accordance with the manufacturer's (Boehringer Mannheim) instructions.
REA.
Aliquots (10 μl) of the PCR products were subjected to REA and electrophoresed as previously described (16). The patterns were visualized under UV light after staining with ethidium bromide (0.5 μg/ml).
Nucleotide sequence accession numbers.
The EMBL GenBank accession numbers for the C. dubliniensis and C. neoformans sequences described here are AJ012573 and AJ249605, respectively.
RESULTS
PCR amplification of the P-450 lanosterol 14α-demethylase (ERG11) gene and detection of the PCR product by reverse cross blot hybridization.
Our primers (16) were able to amplify a wide variety of yeast species, including, in the present study, C. dubliniensis. In developing probes to be used in the reverse cross blot hybridization assay, we limited our efforts to those yeast species most commonly involved in human infections, such as C. albicans, C. tropicalis, C. krusei, C. (Torulopsis) glabrata, C. guilliermondii, C. parapsilosis, C. kefyr, C. dubliniensis, C. neoformans, and S. cerevisiae. These 10 oligonucleotide probes were designed based on species-specific sequence variations observed in the ERG11 gene target region (20). Figure 1 shows the locations of the probes in the ERG11 gene region chosen for PCR amplification. In the reverse cross blot hybridization assay, membranes were spotted with the dTTP-tailed probes pCal, pDub, pGla, pGui, pKef, pKru, pPar, pTro, pCry, and pSce.
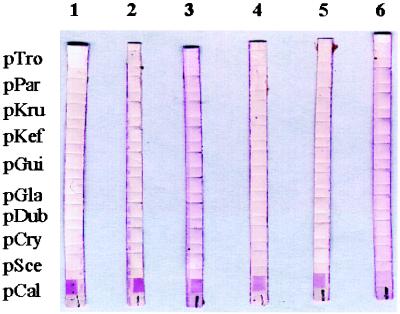
The sensitivity of the reverse cross blot hybridization assay was tested in duplicate with 200 pg, 20 pg, 2 pg, 200 fg, 20 fg, and 2 fg of DNA from C. albicans, C. tropicalis, C. krusei, C. glabrata, C. guilliermondii, C. parapsilosis, C. kefyr, C. dubliniensis, C. neoformans, and S. cerevisiae. Figure 2 shows that 20 fg of C. albicans DNA, the equivalent of two cells, could be detected with the pCal probe. For the other nine yeast species tested, the detection limits of the hybridization assays with the corresponding species-specific probe were similar (data not shown). Figure 3 and Table 1 show the results of hybridization of the probes with PCR products from all of the species tested. All of the probes demonstrated absolute species specificity. None of the PCR products from the yeast species for which no probes had been designed and none of the human or bacterial DNAs used as negative controls hybridized with any of the species-specific probes (Table 1).
FIG. 2.
Sensitivity of the PCR-reverse cross blot hybridization assay. Lanes 1 to 6 contained PCR products from 200 pg, 20 pg, 2 pg, 200 fg, 20 fg, and 2 fg of C. albicans genomic DNA, respectively. The probes used are identified on the left.
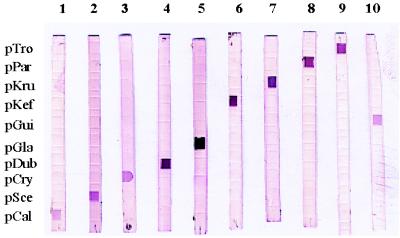
FIG. 3.
Analysis by reverse cross blot hybridization of PCR products from 10 yeast species. Lanes: 1, C. albicans CDC B 385; 2, S. cerevisiae clinical isolate; 3, C. neoformans clinical isolate; 4, C. dubliniensis clinical isolate; 5, C. glabrata CBS 138; 6, C. kefyr clinical isolate; 7, C. krusei CBS 573; 8, C. parapsilosis clinical isolate; 9, C. tropicalis CBS 94; 10, C. guilliermondii clinical isolate. The probes used are identified on the left.
TABLE 1.
Species specificities of the oligonucleotide probes used in the reverse cross blot hybridization assay
| Genomic DNA source (no. of samples tested) | Result of hybridization with probe:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pCal | pDub | pGla | pGui | pKef | pKru | pPar | pTro | pCry | pSce | |
| C. albicans (20) | + | − | − | − | − | − | − | − | − | − |
| C. dubliniensis (2) | − | + | − | − | − | − | − | − | − | − |
| C. glabrata (9) | − | − | + | − | − | − | − | − | − | − |
| C. guilliermondii (5) | − | − | − | + | − | − | − | − | − | − |
| C. kefyr (5) | − | − | − | − | + | − | − | − | − | − |
| C. krusei (8) | − | − | − | − | − | + | − | − | − | − |
| C. parapsilosis (10) | − | − | − | − | − | − | + | − | − | − |
| C. tropicalis (9) | − | − | − | − | − | − | − | + | − | − |
| C. neoformans (5) | − | − | − | − | − | − | − | − | + | − |
| S. cerevisiae (10) | − | − | − | − | − | − | − | − | − | + |
| C. lusitaniae (1) | − | − | − | − | − | − | − | − | − | − |
| C. rugosa (1) | − | − | − | − | − | − | − | − | − | − |
| C. lambica (1) | − | − | − | − | − | − | − | − | − | − |
| B. capitatus (1) | − | − | − | − | − | − | − | − | − | − |
| Staphylococcus aureus (2) | − | − | − | − | − | − | − | − | − | − |
| E. coli (2) | − | − | − | − | − | − | − | − | − | − |
| P. aeruginosa (2) | − | − | − | − | − | − | − | − | − | − |
| Human skin biopsy | − | − | − | − | − | − | − | − | − | − |
Results of PCR-reverse cross blot hybridization versus routine phenotypic methods.
The PCR-reverse cross blot hybridization assay was performed on 140 clinical samples from 140 patients. As shown in Table 2, 48 samples produced positive results in the ERG11-based PCR assay (27 reacted with probe pCal, 9 reacted with probe pGla, 3 reacted with probe pTro, 1 reacted with probe pKef, 2 reacted with probe pCry, 4 reacted with probe pSce, and 2 reacted with both pCal and pGla). For the 47 specimens that were positive on routine culture, phenotypic identification procedures yielded the following results: 25 isolates were identified as C. albicans, 9 were identified as C. glabrata, 3 were identified as C. tropicalis, 1 was identified as C. kefyr, 2 were identified as C. neoformans, 1 was identified as C. lusitaniae, 2 were identified as Yarrowia lipolytica, and 4 were identified as S. cerevisiae (Table 2).
TABLE 2.
Results of analysis of 140 samples by ERG11-based PCR assay and conventional methods
| Sample type (no.), culture and phenotypic identification | No. of positive samples | ERG11-based PCR assay probe(s) | No. of positive samples |
|---|---|---|---|
| Blood (30) | |||
| C. albicans | 4 | pCal | 5 |
| C. glabrata | 2 | pGla | 2 |
| C. albicans, C. glabrata | 0 | pCal, pGla | 1 |
| Cerebrospinal fluid (10) | |||
| C. neoformans | 2 | pCry | 2 |
| Urine (40) | |||
| C. albicans | 8 | pCal | 8 |
| C. glabrata | 3 | pGla | 3 |
| Y. lipolytica | 2 | 0 | |
| C. albicans, C. glabrata | 0 | pCal, pGla | 1 |
| Vaginal secretion (30) | |||
| C. albicans | 8 | pCal | 8 |
| S. cerevisiae | 4 | pSce | 4 |
| C. glabrata | 3 | pGla | 3 |
| Pharyngeal exudate (30) | |||
| C. albicans | 5 | pCal | 6 |
| C. tropicalis | 3 | pTro | 3 |
| C. kefyr | 1 | pKef | 1 |
| C. lusitaniae | 1 | 0 | |
| C. glabrata | 1 | pGla | 1 |
| Total (140) | 47 | 48 |
Comparison of the results of the ERG11-based PCR assay and those obtained with conventional methods showed concordant identification for 44 of the 47 culture-positive samples. The remaining three samples (Tables 2 and 3), which were found to contain Y. lipolytica (n = 2) and C. lusitaniae (n = 1) by phenotypic methods, were negative by the PCR assay because the P4501 and P4502 primers do not amplify DNA from Y. lipolytica, and a probe specific for C. lusitaniae was not included in this study. Four other samples that were negative by culture were positive by the molecular assay. Samples U18 (urine specimen) and B17 (blood specimen) (Table 3), which were obtained from two different patients, reacted positively with both probes pCal and pGla, suggesting the simultaneous presence of C. albicans and C. glabrata. The validity of this presumptive diagnosis is supported by the fact that both species had, in fact, been previously cultured from specimens of the same type in both cases. The absence of yeast growth in the specimens used in our study (U18 and B17) is probably due to the fact that they were collected after both patients had been started on azole antifungal agents. Sample F20, a pharyngeal exudate (Table 3), was also negative in culture, but the PCR product hybridized with probe pCal. Again, the culture negativity is probably due to inhibition by azole therapy. The fourth specimen, a blood sample that was PCR positive for C. albicans (Table 3), produced only gram-positive cocci in cultures, but a sample drawn some days later from the same patient was culture positive for C. albicans.
TABLE 3.
Discordance between ERG11-based PCR assay and conventional phenotyping results
| Patient no. | Sample code | Sample type | Identification by:
|
|
|---|---|---|---|---|
| ERG11-based PCR assay | Phenotypic method | |||
| 8 | B8 | Blood | C. albicans | Negative |
| 111 | B17 | Blood | C. albicans, C. glabrata | Negative |
| 18 | U4 | Urine | Negative | Y. lipolytica |
| 29 | U15 | Urine | Negative | Y. lipolytica |
| 41 | U18 | Urine | C. albicans, C. glabrata | Negative |
| 131 | P11 | Pharyngeal exudate | Negative | C. lusitaniae |
| 138 | P20 | Pharyngeal exudate | C. albicans | Negative |
The mean time from the first positive culture to species identification by routine phenotypic methods (subculture, germ tube and chlamydospore formation tests, and Vitek system identification) was 4 days. For PCR-reverse cross blot hybridization, 1 h was required to isolate DNA from the samples (blood processing required an additional 2 h), 3 h was required for PCR amplification of isolated DNA, and 2 h was required for reverse cross blot hybridization assay of the PCR product. In short, the yeast species could be identified in as little as 7 h by the molecular method, including the time required to prepare PCR and hybridization assay reagent mixtures.
Results of PCR-reverse cross blot hybridization versus PCR-REA results.
All of the samples which produced positive results in the ERG11-based PCR assay were also evaluated by PCR-REA (16). REA patterns were consistent with the species identified by the ERG11-based PCR assay in all cases, with the exception of those samples that simultaneously hybridized with probes for C. albicans and C. glabrata: in these two cases, the results of the REA were unintelligible (data not shown).
DISCUSSION
PCR amplification of a small number of Candida organisms in blood or other clinical specimens is a promising approach to overcome the limits of currently available culture methods (25). It is most important that a PCR-based system be able to distinguish several non-albicans Candida spp. as well because these species are often innately resistant to azole antifungal drugs (10, 26). Different target genes have been used to develop PCR-based assays (2–8, 15, 21), and efforts have been made to improve the means of detecting the PCR amplicons (22). For maximum sensitivity and specificity, dot blot and microtitration plate capture assays are attractive alternatives to ethidium bromide staining (25). In this study, we used the reverse dot blot method described by Saiki et al. (27), which has been successfully applied for rapid detection and identification of mycobacteria by Kox et al. (11). In a previous report (16), we demonstrated that Candida spp. can be detected in clinical specimens with primers P4501 and P4502. In the present study, we attempted to increase the sensitivity of this method and simplify it to render it more suitable for routine diagnostic use.
Although the P4501 and P4502 primers amplified a highly conserved region of the ERG11 gene, certain nucleotide variations in this region allowed us to design species-specific probes that could be used in hybridization analysis for the identification of the most important pathogenic and opportunistic Candida species, as well as that of C. neoformans and S. cerevisiae. One of the prerequisites of this analytical method is that all of the bound oligonucleotides must be sequence specific under the same hybridization conditions. This requirement can be met by adjusting the length, position, and strand specificity of the probes (27). In this study, several combinations of length and strand position were evaluated and 10 probes with similar annealing temperatures were selected. The specificities of the probes were tested with multiple isolates of yeast and non-yeast species, and the results confirmed that each probe reacted exclusively with the species for which it was specific with no sign of cross-hybridization. Moreover, none of the 10 probes hybridized with the PCR product from a specimen that grew C. lusitaniae, although the specimen was positive by agarose gel electrophoresis. In addition, the combined use of the P4501 and P4502 primers and hybridization analysis increased the sensitivity of the system by approximately 10-fold over that of our previous assay (16).
To our knowledge, this is the first reported use of reverse cross blot hybridization for detection of PCR-amplified yeast DNA in clinical specimens. Analysis of PCR products by reverse hybridization has several advantages. It is simple to perform, several PCR products derived from different samples can be analyzed simultaneously, and the results are easy to interpret; i.e., positive samples can be distinguished from truly negative ones without the so-called “gray zone” observed with enzyme-linked immunosorbent assay-based detection formats (13). Presumptive identification can save valuable time in the initiation of treatment, and mixed yeast infections can be easily detected without any interference among the species present in the same sample.
In our assay, the menu of identifiable species included C. dubliniensis, a species first described by Sullivan et al. (29) on the basis of detailed molecular studies that demonstrated distinct differences between the genomic structure of this species and those of both C. albicans and C. stellatoidea. This phylogenetic distance from C. albicans was verified when we analyzed the ERG11 gene of clinical isolates of C. dubliniensis from Switzerland (see Materials and Methods). Thanks to the variability of the target region of this gene, we were able to develop a probe specific for C. dubliniensis (pDub) which did not react with DNA from C. albicans or any of the other Candida species.
A specific probe was also developed for S. cerevisiae. Morrison's group recently developed a panel of probes for identification of 18 Candida species (5), and cross-reactions with S. cerevisiae DNA were observed for the probe specific for C. glabrata. These investigators maintain that the inability to distinguish between these two yeasts should have little impact on either diagnosis or treatment in clinical settings because the incidence of S. cerevisiae infections is quite low and both species are innately resistant to fluconazole. However, S. cerevisiae appears to be increasingly associated with severe disease in immunocompromised patients (35) and our experience indicates that its isolation from immunocompetent patients is also becoming more common (23).
Although this study was not designed to be a clinical evaluation of the method, one of its greatest advantages is that it can be used directly on clinical samples without resorting to time-consuming cultures. The latter are not only the foundation of traditional typing methods, but they are also a preliminary step in the PCR method recently developed by Elie et al. (5). The savings, in terms of time, is accompanied by excellent reliability. The results of the ERG11-based PCR assay were fully concordant with those of conventional culture and biochemical typing for 44 specimens of various types. As for the PCR positivity of four other samples that were culture negative, we cannot exclude the possibility that the assay detected naked DNA from damaged or inhibited organisms. However, it is entirely possible that three of these specimens were indeed true positives that were missed with conventional methods because the patient was on antifungal therapy at the time the specimen was collected. In the fourth case, cultures of blood drawn 2 days after the study specimen was collected were also positive for C. albicans, the species that had been identified by the molecular assay. There were only three false negatives, which were caused by the presence of yeasts that were not included in the PCR-hybridization menu of identifiable organisms. We are now performing a prospective study on clinical samples from patients with malignancies.
In an era in which chip array technology is becoming an attractive means for identifying a large number of different organisms simply and rapidly, a system that can be used directly on clinical specimens with the potential to identify a sufficiently broad panel of medically important Candida and/or yeast species can provide a powerful tool in clinical mycology.
ACKNOWLEDGMENT
This work was supported by grant 50B.17 (AIDS National Research Program) from the Istituto Superiore di Sanità-Ministero della Sanità.
REFERENCES
- 1.Anaissie E. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin Infect Dis. 1992;14(Suppl. 1):S43–S53. doi: 10.1093/clinids/14.supplement_1.s43. [DOI] [PubMed] [Google Scholar]
- 2.Buchman T G, Rossier M, Merz W G, Charache P. Detection of surgical pathogens by in vitro DNA amplification. Part 1. Rapid identification of Candida albicans by in vitro amplification of a fungus-specific gene. Surgery. 1990;108:338–347. [PubMed] [Google Scholar]
- 3.Crampin A C, Matthews R C. Application of the polymerase chain reaction to the diagnosis of candidosis by amplification of an HSP-90 gene fragment. J Med Microbiol. 1993;39:233–238. doi: 10.1099/00222615-39-3-233. [DOI] [PubMed] [Google Scholar]
- 4.Einsele H, Hebart H, Roller G, Loffler J, Rothenhofer I, Muller C A, Bowden R A, van Burik J, Engelhard D, Kanz L, Schumacher U. Detection and identification of fungal pathogens in blood by using molecular probes. J Clin Microbiol. 1997;35:1353–1360. doi: 10.1128/jcm.35.6.1353-1360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elie C M, Lott T J, Reiss E, Morrison C J. Rapid identification of Candida species with species-specific DNA probes. J Clin Microbiol. 1998;36:3260–3265. doi: 10.1128/jcm.36.11.3260-3265.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flahaut M, Sanglard D, Monod M, Bille J, Rossier M. Rapid detection of Candida albicans in clinical samples by DNA amplification of common regions from C. albicans-secreted aspartic proteinase genes. J Clin Microbiol. 1998;36:395–401. doi: 10.1128/jcm.36.2.395-401.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita S-H, Lasker B A, Lott T J, Reiss E, Morrison C J. Microtitration plate enzyme immunoassay to detect PCR-amplified DNA from Candida species in blood. J Clin Microbiol. 1995;33:962–967. doi: 10.1128/jcm.33.4.962-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopfer R L, Walden P, Setterquist S, Highsmith W E. Detection and differentiation of fungi in clinical specimens using polymerase chain reaction (PCR) amplification and restriction enzyme analysis. J Med Vet Mycol. 1993;31:65–75. doi: 10.1080/02681219380000071. [DOI] [PubMed] [Google Scholar]
- 9.Jarvis W R. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin Infect Dis. 1995;20:1526–1530. doi: 10.1093/clinids/20.6.1526. [DOI] [PubMed] [Google Scholar]
- 10.Johnson E M, Warnock D W, Luker J, Porter S R, Scully C. Emergence of azole drug resistance in Candida species from HIV-infected patients receiving prolonged fluconazole therapy for oral candidosis. J Antimicrob Chemother. 1995;35:103–114. doi: 10.1093/jac/35.1.103. [DOI] [PubMed] [Google Scholar]
- 11.Kox L F F, van Leeuwen J, Knijper S, Jansen H M, Kolk A H J. PCR assay based on DNA coding for 16S rRNA for detection and identification of mycobacteria in clinical samples. J Clin Microbiol. 1995;33:3225–3233. doi: 10.1128/jcm.33.12.3225-3233.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwok S, Higuchi R. Avoiding false positives with PCR. Nature (London) 1989;339:237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- 13.Löffler J, Hebart H, Sepe S, Schumacher U, Klingebiel T, Einsele H. Detection of PCR-amplified fungal DNA by using a PCR-ELISA system. Med Mycol. 1998;36:275–279. doi: 10.1080/02681219880000441. [DOI] [PubMed] [Google Scholar]
- 14.Mannarelli B M, Kurtzman C P. Rapid identification of Candida albicans and other human pathogenic yeasts by using short oligonucleotides in a PCR. J Clin Microbiol. 1998;36:1634–1641. doi: 10.1128/jcm.36.6.1634-1641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyakawa Y, Mabuchi T, Fukazawa Y. New method for detection of Candida albicans in human blood by polymerase chain reaction. J Clin Microbiol. 1993;31:3344–3347. doi: 10.1128/jcm.31.12.3344-3347.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morace G, Sanguinetti M, Posteraro B, Lo Cascio G, Fadda G. Identification of various medically important Candida species in clinical specimens by PCR-restriction enzyme analysis. J Clin Microbiol. 1997;35:667–672. doi: 10.1128/jcm.35.3.667-672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morace G, Pagano L, Sanguinetti M, Posteraro B, Mele L, Equitani F, D'Amore G, Leone G, Fadda G. PCR-restriction enzyme analysis for detection of Candida DNA in blood from febrile patients with hematological malignancies. J Clin Microbiol. 1999;37:1871–1875. doi: 10.1128/jcm.37.6.1871-1875.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 5th ed. Washington, D.C.: American Society for Microbiology; 1995. [Google Scholar]
- 19.Nagai H, Yamakami Y, Hashimoto A, Tokimatsu I, Nasu M. PCR detection of DNA specific for Trichosporon species in serum of patients with disseminated trichosporonosis. J Clin Microbiol. 1999;37:694–699. doi: 10.1128/jcm.37.3.694-699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson D R, Koymans L, Kamataki T, Stegeman J J, Feyereisen R, Waxman D J, Waterman M R, Gotoh O, Coon M J, Estabrook R W, Gunsalus L C, Nebert D W. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996;6:1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Niesters H G M, Goessens W H F, Meis J F M G, Quint W G V. Rapid, polymerase chain reaction-based identification assays for Candida species. J Clin Microbiol. 1993;31:904–910. doi: 10.1128/jcm.31.4.904-910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Persing D H. Polymerase chain reaction: trenches to benches. J Clin Microbiol. 1991;29:1281–1285. doi: 10.1128/jcm.29.7.1281-1285.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Posteraro B, Sanguinetti M, D'Amore G, Masucci L, Morace G, Fadda G. Molecular and epidemiological characterization of vaginal Saccharomyces cerevisiae isolates. J Clin Microbiol. 1999;37:2230–2235. doi: 10.1128/jcm.37.7.2230-2235.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reichard U, Margraf S, Hube B, Ruchel R. A method for recovery of Candida albicans DNA from larger blood samples and its detection by polymerase chain reaction on proteinase genes. Mycoses. 1997;40:249–253. doi: 10.1111/j.1439-0507.1997.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 25.Reiss E, Morrison C J. Nonculture methods for diagnosis of disseminated candidiasis. Clin Microbiol Rev. 1993;6:311–323. doi: 10.1128/cmr.6.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saiki R K, Walsh P S, Levenson C H, Erlich H A. Genetic analysis of amplified DNA with immobilized sequence-specific oligonucleotide probes. Proc Natl Acad Sci USA. 1989;86:6230–6234. doi: 10.1073/pnas.86.16.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin J H, Nolte F S, Morrison C J. Rapid identification of Candida species in blood cultures by a clinically useful PCR method. J Clin Microbiol. 1997;35:1454–1459. doi: 10.1128/jcm.35.6.1454-1459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan D J, Westerneng T J, Haynes K A, Bennett D E, Coleman D C. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology. 1995;141:1507–1521. doi: 10.1099/13500872-141-7-1507. [DOI] [PubMed] [Google Scholar]
- 30.Talluri G, Mangone C, Freyle J, Shirazian D, Lehman H, Wise G J. Polymerase chain reaction used to detect candidemia in patients with candiduria. Urology. 1998;51:501–505. doi: 10.1016/s0090-4295(97)00641-9. [DOI] [PubMed] [Google Scholar]
- 31.Urata T, Kobayashi M, Imamura J, Tanaka Y, Muneishi H, Iwahara Y, Uemura Y, Taguchi H, Miyoshi I. Polymerase chain reaction amplification of Asp f I and alkaline protease genes from fungus balls: clinical application in pulmonary aspergillosis. Intern Med. 1997;36:19–27. doi: 10.2169/internalmedicine.36.19. [DOI] [PubMed] [Google Scholar]
- 32.Van Burik J A, Myerson D, Schreckhise R W, Bowden R A. Panfungal PCR assay for detection of fungal infection in human blood specimens. J Clin Microbiol. 1998;36:1169–1175. doi: 10.1128/jcm.36.5.1169-1175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verweij P E, Brinkman K, Kremer H P H, Kullberg B J, Meis J F. Aspergillus meningitis: diagnosis by non-culture-based microbiological methods and management. J Clin Microbiol. 1999;37:1186–1189. doi: 10.1128/jcm.37.4.1186-1189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wingard J R. Importance of Candida species other than C. albicans as pathogens in oncology patients. Clin Infect Dis. 1995;20:115–125. doi: 10.1093/clinids/20.1.115. [DOI] [PubMed] [Google Scholar]
- 35.Zerva L, Hollis R J, Pfaller M A. In vitro susceptibility testing and DNA typing of Saccharomyces cerevisiae clinical isolates. J Clin Microbiol. 1996;34:3031–3034. doi: 10.1128/jcm.34.12.3031-3034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]