Abstract
The natural schweinfurthins are stilbenes with significant antiproliferative activity and an uncertain mechanism of action. To obtain a fluorescent analogue with minimal deviation from the natural structure, a coumarin ring system was annulated to the D-ring, creating a new analogue of schweinfurthin F. This stilbene was prepared through a convergent synthesis, with a Horner–Wadsworth–Emmons condensation employed to form the central stilbene olefin. After preparation of a tricyclic phosphonate via a recent and more efficient modification of the classic Arbuzov reaction, condensation was attempted with an appropriately substituted bicyclic aldehyde but the coumarin system did not survive the reaction conditions. When olefin formation preceded generation of the coumarin, the stilbene formation proceeded smoothly and ultimately allowed access to the targeted coumarin-based schweinfurthin analogue. This analogue displayed the desired fluorescence properties along with significant biological activity in the National Cancer Institute’s 60-cell line bioassay, and the pattern of this biological activity mirrored that of the natural product schweinfurthin F. This approach gives facile access to new fluorescent analogues of the natural schweinfurthins and should be applicable to other natural stilbenes as well.
Introduction
The schweinfurthins (Figure 1) are a small group of natural products isolated, at least thus far, from plants of the genus Macaranga (Euphorbiaceae) directly1,2 or indirectly from propolis produced by bees visiting Macaranga plants.3 The combination of an unusual pattern of differential activity in the National Cancer Institute’s (NCI’s) 60-cell line screen4,5 and isolation efforts that have resulted in limited and sometimes poorly reproducible quantities6 has encouraged us to pursue efforts to synthesize these compounds and a variety of analogues. To date, we have reported the total synthesis of several natural schweinfurthins that include the hexahydroxanthene system [A (1),7 B (2),8 E (3),8 F (5),9 G (6),10 and vedelianin (4)],11 including one as both enantiomers to allow determination of the absolute stereochemistry of the natural products (Figure 1). We also have prepared approximately 90 analogues that have been evaluated in the NCI’s 60-cell line screen for structure activity studies.7−9,12,13 Results of these studies indicate that the A/B/C ring system and a stilbene in the trans orientation are essential to the selective antiproliferative activity of these compounds, while modifications of the D-ring are generally better tolerated. Of special significance, studies of structure–activity relationships and chemical stability have revealed that the D-ring resorcinol may limit the schweinfurthins’ stability. Thus, optimum placement of a coumarin system might preserve the biological activity and simultaneously improve the chemical stability. Methylation of one of the symmetric D-ring phenolic groups has been shown to significantly increase chemical stability and testing in the NCI-60 assay revealed that there is little or no loss in activity relative to the corresponding non-methylated compounds. Furthermore, the indole analogues 7 and 8 also have shown significant activity, suggesting a tolerance for substitution at a single phenolic position.14
Figure 1.
Relevant natural schweinfurthins and two indole analogues.
The NCI created the COMPARE algorithm to associate bioactivity data from each candidate in the NCI-60 bioassay with those from other compounds that have also been through the screening process and function by a similar mechanism of action rather than by structural similarity.15 In the COMPARE analysis, the schweinfurthin family did not pose biological resemblance to that of any chemotherapeutic agent currently in use, but rather, the activity of the schweinfurthin family most closely resembles that of the cephalostatins (e.g. 9), the ritterazines (e.g. 10), the stellettins (e.g. 11), and OSW-1 (12, Figure 2).13 Deeper investigation into the mechanism of action of the schweinfurthins by several groups has not yet provided a complete and clear mode of action. Studies have suggested interactions between several targets including oxysterol binding proteins,16−18 trans-Golgi-network trafficking,19 and the production and export of cholesterol20 and other products of isoprenoid biosynthesis.21
Figure 2.
Natural products that display biological activity similar to that of schweinfurthins, based on COMPARE analyses.
To increase understanding of the mechanism of action for the schweinfurthins, it might be useful to prepare a fluorescent analogue, as long as that analogue displays significant biological activity. To maximize the possibility of activity, it appeared prudent to anneal a coumarin ring to the D-ring, as suggested in structure 13 (Figure 3). Compound 13 would preserve the complete hexahydroxanthene system of a mono-methylated schweinfurthin F (14), the trans-stilbene, and one free phenol in the D-ring. Thus, preparation of the schweinfurthin analogues in the form of structure 13 became our goal.
Figure 3.
Comparison of a coumarin-containing schweinfurthin (13) with a mono-methylated schweinfurthin F (14).
Results and Discussion
Although several disconnections have been explored for schweinfurthin assembly,1,22,23 we have favored use of late-stage Horner–Wadsworth–Emmons (HWE) condensation to form the central stilbene olefin because this approach allows a highly convergent synthesis. From this perspective, the coumarin-containing schweinfurthin F analogue 13 could be seen arising from an HWE olefination between phosphonate 15 and aldehyde 16 (Scheme 1). Phosphonate 15 may be formed from the known tricyclic alcohol 17,10 which can be prepared in high enantiomeric excess from commercial vanillin (18) through an enantioselective Shi epoxidation.24 If the HWE condensation were postponed to the end of the synthetic sequence, the complementary aldehyde 16 would be required. Coumarin 16 could be prepared via a Knoevenagel condensation between the aldehyde 19 and a β-ketoester of the general structure 20, with ethyl acetoacetate providing the methyl ketone and extended acetoacetates giving larger analogues. Aldehyde 19 could be seen to arise from bromide 21 by halogen metal exchange followed by reaction with dimethylformamide (DMF). Finally, the benzyl methyl ether 21 could be obtained from commercial 3,5-dihydroxybenzoic acid (22).
Scheme 1. One Retrosynthesis to the Schweinfurthin Analogue 13.

Initial synthetic efforts were focused on the coumarin component because previous syntheses of other schweinfurthins provided confidence that an appropriate tricyclic component could be prepared. When compounds 13 and 16 include a methyl ketone, this group can be viewed as a mimic of the prenyl group in schweinfurthin F and a homoprenyl ketone could be imagined to mimic the geranyl substituent of larger schweinfurthins. Our efforts began with the prenyl mimic where R is a methyl group because this allowed use of commercially available ethyl acetoacetate as the ketoester 20.
Although the brominated resorcinol 23 is commercially available, it can be easily prepared in virtually quantitative yield by treatment of 3,5-dihydroxybenzoic acid (22) with bromine (Scheme 2). The benzoic acid 23 has been converted to the benzylic alcohol 25 through a three-step sequence via the methyl ester,25,26 but it also was possible to accomplish this overall transformation in just two steps by formation of the acyloxyester 24 while concurrently protecting the phenols as MOM ethers, followed by reduction of this intermediate to the desired alcohol 25. Protection of the benzylic alcohol 25 as the methyl ether 21 proceeded smoothly,27 and halogen metal exchange followed by treatment with DMF afforded the desired aldehyde 19.
Scheme 2. Synthesis of the Aldehyde Intermediate 19.
With the aldehyde 19 in hand, attention was turned to formation of the desired coumarin ring system through a Knoevenagel condensation. All efforts to form a coumarin directly from the bis-MOM-protected compound 19 went unrewarded. Fortunately, treatment of compound 19 with sodium bisulfate on silica resulted in cleavage of a single MOM protecting group in reasonably good yield (70%, Scheme 3).28,29 Grinding the resulting ortho-hydroxy benzaldehyde 26 with ethyl acetoacetate (27) and piperidine resulted in condensation and cyclization to afford the coumarin 28. Because ketones can undergo reaction with DDQ via their tautomeric enol forms, the ketone 28 was protected as its acetal 29. Subsequent reaction with DDQ gave the desired coumarin aldehyde 30 in modest yield. Preparation of the tricyclic phosphonate 15 then was investigated because aldehyde 30 appeared to be an appropriate substrate for an HWE condensation.
Scheme 3. Formation of the Coumarin Aldehyde 30.
The tricyclic phosphonate 15 was employed in our original synthesis of schweinfurthin F,10 where it was prepared from the corresponding alcohol 17 in an overall yield of 62% via a classical approach involving formation of the mesylate, displacement by sodium iodide, and an Arbuzov reaction with triethyl phosphite. Instead, a shortened Arbuzov approach was followed. After the aldehyde 31 was prepared via DDQ oxidation of the corresponding benzyl methyl ether, the C-2 alcohol was protected as the MOM ether (32) and the aldehyde was reduced to the benzylic alcohol 17 (Scheme 4). Then the alcohol 17 simply was treated with zinc iodide and triethyl phosphite, modernized Arbuzov conditions30 for benzylic alcohols, which gave phosphonate 15 in a single step and 69% isolated yield.
Scheme 4. Direct Conversion of Benzylic Alcohol 17 to Phosphonate 15.
From all perspectives, the HWE condensation of phosphonate 15 and aldehyde 30 was expected to be straightforward, and parallel reactions have been employed in multiple schweinfurthin syntheses. To our disappointment, this specific HWE condensation failed despite repeated attempts (Scheme 5) perhaps because the coumarin subunit was not stable under the reaction conditions. Whatever the root cause, the failure of this reaction necessitated a redesign of the synthetic sequence.
Scheme 5. Initially Attempted HWE Condensation.
To take full advantage of the intermediates in hand and the experience gained with the successful reactions described above, introduction of the coumarin ring system was postponed until after the formation of the central stilbene. Therefore, the alcohol 25 was protected as its TBS ether 34 to avoid potential side-reactions during DDQ oxidation (Scheme 6). Compound 34 undergoes lithium halogen exchange under standard conditions, and a subsequent reaction with DMF gave the aldehyde 35. After protection of the carbonyl group as its acetal 36, treatment with TBAF generated the primary alcohol 37. Final MnO2 oxidation provided the new HWE coupling partner, aldehyde 38.
Scheme 6. Synthesis of the Aldehyde 38.
The HWE condensation of phosphonate 15 and aldehyde 38 proceeded smoothly upon treatment with sodium hexamethyldisilazide, affording the schweinfurthin analogue 39 in quantitative yield based on recovered phosphonate 15 (Scheme 7). The three MOM acetals and the ethylene glycol-protected aldehyde underwent hydrolysis under acidic conditions to afford the aldehyde 40. After aldehyde 40 was combined with ethyl acetoacetate (27) and piperidine and the reaction was allowed to stir in anhydrous MeOH, the desired fluorescent coumarin schweinfurthin analogue 41 was obtained in high enantiomeric excess.10 The spectral data for coumarin 41 indicates that the compound has an absorption maximum at ∼420 nm and an emission maximum at ∼590 nm, showing the expected fluorescence.
Scheme 7. Assembly of the First Coumarin-Based Schweinfurthin (41).
In principal, synthesis of other coumarin-based schweinfurthin analogues could be based on C-alkylation of the methyl ketone in compound 41. However this would certainly require protection of the free phenol and probably the C-2 hydroxyl group as well. A more attractive approach might involve extension of ethyl acetoacetate (27) prior to the Knoevenagel condensation. To test this possibility, the β-ketoester 42 was prepared via alkylation of the ethyl acetoacetate dianion (Scheme 8).31 When compound 42 was ground in a mortar with a pestle in the presence of piperidine to induce condensation and cyclization by mechanochemical means, the extended coumarin 43 was obtained in an attractive yield. Although synthesis of compound 43 demonstrates the accessibility of more extended coumarins, pursuit of additional schweinfurthin analogues was postponed pending the results of bioassays on the new analogues in hand.
Scheme 8. Synthesis of Coumarin 43 with an Extended Isoprenoid Chain.
Both schweinfurthin analogues 40 and 41 were tested in the NCI-60 cell line bioassay. Both compounds were first tested in a single-dose assay and demonstrated sufficient activity to warrant testing in the full five-dose assay. The aldehyde 40 shows modest activity against SF-295 with a GI50 of 3.0 μM (Table 1 and Supporting Information). This activity is 270 times less potent than that of natural schweinfurthin A (1). Although aldehyde 40 is not as active as most schweinfurthins and schweinfurthin analogues sent to the NCI, it does show a pattern of activity similar to that of other schweinfurthins, with a Pearson correlation coefficient of 0.66 to schweinfurthin A.32 The GI50 against each cell line in the NCI-60 assay for compound 40 shows a pattern similar to that of other schweinfurthins. Of the cell lines tested, analogue 40 also has the greatest activity against the CNS cancer cell line SF-539 with a GI50 of 1.3 μM, which aligns with our expectation that the schweinfurthins have selective activity against CNS malignancies.
Table 1. Comparison of the Activity (GI50) of Compounds 40 and 41 to Representative Schweinfurthins against Selected CNS Cell Lines.
| compound # (NSC number) | SF-295 (μM) | SF-539 (μM) | SNB-75 (μM) | Pearson correlation to 1 |
|---|---|---|---|---|
| 1 (696119) | 0.011 | 0.010 | 0.015 | 1.00 |
| 44 (730430)a | 1.5 | – | 15.8 | 0.39 |
| 14 (740545) | 0.066 | 0.28 | 0.18 | 0.78 |
| 40 (819974) | 3.0 | 1.3 | 1.8 | 0.66 |
| 41 (823234) | 0.51 | 0.98 | 1.0 | 0.66 |
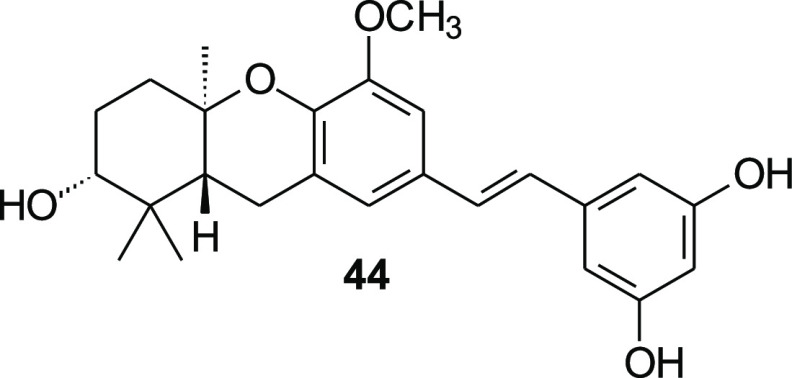
The five-dose assay of coumarin 41 also shows selective activity toward some cancer cell lines over others (c.f. Supporting Information) in a pattern of activity strikingly similar to that of other schweinfurthins that carry a substituent para to the stilbene linkage (i.e. more substituted variations on the parent compound 44, Figure 4). Among the most sensitive cell lines were the SF-295 and SF-539 human-derived glioblastoma lines, with GI50 values of 0.51 and 0.98 μM respectively, but cells in the leukemia (RPMI-8226, 0.32 μM) and renal (RXF 393, 0.43 μM) panels also were sensitive as is often the case with other schweinfurthins. Conversely, the ovarian cancer panel was uniformly resistant, which is also typical of the schweinfurthins. The three-fold increase in potency of the analogue 41 relative to its precursor, aldehyde 40, also is striking and encouraging.
Figure 4.
Parent compound 44.
In conclusion, two new schweinfurthin analogues, coumarin 41 and its immediate precursor aldehyde 40, have been synthesized and tested for biological activity in the NCI-60 cell bioassay. Although the initial approach to the central stilbene demonstrated only that this coumarin system did not survive the standard HWE reaction conditions, it proved possible to incorporate the coumarin ring system after formation of the central stilbene. Both traditional Knoevenagel condensation and mechanical grinding of an ortho hydroxy aldehyde with a β-ketoester allowed formation of the coumarin system. Furthermore, the target compound 41 displays both significant antiproliferative activity in the NCI 60-cell line screen and fluorescent properties that may help illuminate the mechanism of action for the schweinfurthins. Finally, the reactions and strategies reported here might be applicable to preparation of fluorescent analogues of other natural stilbenes, including compounds such as combretastatin,33 resveratrol34,35 and its myriad derivatives,36 the chiricanines,37 the arachidins and arahypins,38 and the pawhuskins.39
Experimental Section
General Section
Diethyl ether (Et2O) and tetrahydrofuran (THF) were distilled from sodium and benzophenone, and dichloromethane (CH2Cl2) was distilled from calcium hydride prior to use. Solutions of n-BuLi were purchased from commercial sources and titrated with diphenylacetic acid to determine molar concentrations prior to use. All other reagents and solvents were purchased from commercial sources and used without further purification. The nuclear magnetic resonance (NMR) spectra were obtained on 300, 400, or 600 MHz Bruker spectrometers with Si(CH3)4 (1H, δ 0.00), CDCl3 (1H, δ 7.26; 13C, δ 77.2), CD3CN (1H, δ 1.94; 13C, δ 118.3, 1.32), or (CD3)2CO (1H, δ 2.05; 13C, δ 206.3, 29.8) as internal standards. To assign signals as C, CH, CH2, or CH3, DEPT-135 NMR spectra were obtained. High-resolution mass spectra were obtained at the University of Iowa Mass Spectrometry Facility. Silica gel (60 Å, 0.040–0.063 mm) was used for flash column chromatography. The UV–vis spectra were obtained on a Cary UV–vis NIR spectrophotometer, and fluorescence data were collected on HORIBA Scientific FluroMax-4. A quartz (200–2500 nm) 1400 μL Hellma Analytics cuvette (semi-micro cell type 114F-QS) with a 10 mm × 4 mm path length fitted with a PTFE stopper was used for UV–vis and fluorometry.
4-Bromo-3,5-dihydroxybenzoic Acid (23)
To an oven-dried and argon-purged round-bottom flask containing 3,5-dihydroxybenzoic acid (22, 10.0 g, 64.5 mmol) was added aqueous 20% HCl (110 mL) followed by a dropwise addition of bromine (3.31 mL, 64.5 mmol). The reaction was heated in an oil bath under reflux for 3 h and then was quenched by addition of ice. The solution was washed with Et2O (3 × 50 mL), and the combined organic layers were dried (Na2SO4) and then filtered through a bed of Celite, and the filtrate was concentrated on a rotary evaporator to afford aryl bromide 23 as an off-white solid (14.9 g, 99%). Both the 1H NMR and 13C NMR spectra were in agreement with the reported data.40
Methoxymethyl 4-Bromo-3,5-bis(methoxymethoxy)benzoate (24)
To an oven-dried and argon-purged round-bottom flask containing the carboxylic acid 23 (400 mg, 1.7 mmol) in CH2Cl2 (20 mL) at 0 °C was added dropwise DIPEA (1.0 mL, 6.0 mmol) followed by a dropwise addition of MOMCl (520 μL, 6.9 mmol). The reaction was allowed to stir at 0 °C for 2.5 h, and then the reaction was quenched by addition of saturated NH4Cl (10 mL) and the organic compounds were extracted into CH2Cl2 (3 × 20 mL). The combined organic layers were washed with 3 N NaOH (15 mL) and dried (Na2SO4), and the solids were removed by filtration. The filtrate was concentrated on a rotary evaporator to afford compound 24 as a white solid (0.58 g, 93%): 1H NMR (400 MHz, CDCl3): δ 7.51 (s, 2H), 5.46 (s, 2H), 5.30 (s, 4H), 3.53 (s, 3H), 3.52 (s, 6H); 13C{1H} NMR (101 MHz, CDCl3): δ 165.3, 154.9 (2C), 130.0, 110.2 (2C), 109.9, 95.1 (2C), 91.2, 57.9, 56.6 (2C). HRMS (ESI) m/z: [M + Na]+ calcd for C13H17O7BrNa, 387.0055; found, 387.0060.
[4-Bromo-3,5-bis(methoxymethoxy)phenyl]methanol (25)
To an oven-dried and argon-purged round-bottom flask containing compound 24 (4.56 g, 12.5 mmol) in THF (60 mL) was slowly added solid NaBH4 (4.02 g, 106 mmol), and the reaction was allowed to stir at 65 °C for 15 min. After MeOH (60 mL) was added dropwise, the reaction was heated under reflux in an oil bath for an additional 2 h. After cooling to rt, the reaction was quenched by dropwise addition of saturated NH4Cl (50 mL). The organic compounds were extracted into EtOAc, the combined organic layers were dried over Na2SO4, and the solids were removed by filtration. The filtrate was concentrated on a rotary evaporator to afford the benzylic alcohol 25 as a white solid (2.76 g, 72%): 1H NMR (400 MHz, CDCl3): δ 6.85 (s, 2H), 5.26 (s, 4H), 4.64 (s, 2H), 3.52 (s, 6H); 13C{1H} NMR (100 MHz, (CD3)2CO): δ 154.8 (2C), 143.6, 107.3 (2C), 101.15, 95.4 (2C), 63.3, 55.6 (2C).25
2-Bromo-1,3-bis(methoxymethoxy)-5-(methoxymethyl)benzene (21)
To a solution of the alcohol 25 (8.66 g, 28.2 mmol) in THF (200 mL) was slowly added 60% NaH in oil (1.27 g, 53.0 mmol), and the reaction then was allowed to stir at 0 °C for 5 min. To the solution was added iodomethane (2.19 mL, 35.3 mmol), and the solution was allowed to stir for 2 h. After the reaction was quenched by addition of H2O, it was extracted with EtOAc. The combined organic layers were washed with 1 N NaOH, dried over Na2SO4, and filtered, and the filtrate was concentrated on a rotary evaporator to afford the methyl ether 21 as colorless crystals (8.71 g, 96%): 1H NMR (400 MHz, (CD3)2CO): δ 6.88 (s, 2H), 5.30 (s, 4H), 4.40 (s, 2H), 3.49 (s, 6H), 3.36 (s, 3H); 13C{1H} NMR (100 MHz, (CD3)2CO): δ 154.9 (2 C), 152.0 (C), 139.8 (C), 108.0 (2 CH), 94.9 (2 CH2), 73.5 (CH2), 57.4 (CH3), 55.6 (2 CH3).27
2,6-Bis(methoxymethoxy)-4-(methoxymethyl)benzaldehyde (19)
To a flame-dried and argon-purged round-bottom flask containing n-BuLi (2.48 M, 5.5 mL, 13 mmol) at −78 °C was added a −78 °C solution of the aryl bromide 21 (3.55 g, 11.1 mmol) in Et2O (200 mL) using a cannula. The solution was allowed to stir for 15 min and then DMF (0.94 mL, 12 mmol) was added dropwise. Once the reaction reached rt, it was quenched by addition of NH4Cl and extracted with Et2O. The combined organic layers were dried over Na2SO4 and filtered, and the filtrate was concentrated on a rotary evaporator to afford a yellow oil. Final purification was achieved using an ISCO auto-column (0%–100% EtOAc in hexanes) to afford aldehyde 19 as a yellow oil (1.52 g, 50%): 1H NMR (400 MHz, CDCl3): δ 10.51 (s, 1H), 6.82 (s, 2H), 5.27 (s, 4H), 4.42 (s, 2H), 3.50 (s, 6H), 3.41 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3): δ 188.5 (CH), 159.4 (2 CH), 147.1 (C), 114.9 (C), 106.6 (2 C), 94.6 (2 CH2), 73.8 (CH2), 58.3 (CH3), 56.3 (2 CH3). HRMS (ESI) m/z: [M + Na]+ calcd for C13H18O6Na, 293.0996; found, 293.0995.
2-Hydroxy-6-(methoxymethoxy)-4-(methoxymethyl)benzaldehyde (26)
A mixture of compound 19 (1.38 g, 5.09 mmol) and NaHSO4 on SiO2 (3.06 g)28,29 was allowed to stir in CH2Cl2 (75 mL) at rt for 10 min. The mixture was filtered through Celite, washed with CH2Cl2, and the filtrate was concentrated on a rotary evaporator to afford the phenol 26 (806 mg, 70%): 1H NMR (300 MHz, CDCl3): δ 11.85 (s, 1H), 10.23 (s, 1H), 6.51 (s, 1H), 6.44 (s, 1H), 5.23 (s, 2H), 4.31 (s, 2H), 3.43 (s, 3H), 3.32 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 193.7 (CH), 163.3 (C), 160.0 (C), 150.3 (C), 110.3 (C), 108.4 (CH), 102.2 (CH), 94.5 (CH2), 73.7 (CH2), 58.3 (CH3), 56.4 (CH3). HRMS (ESI) m/z: [M + H]+ calcd for C11H15O5, 227.0920; found, 227.0913.
3-Acetyl-5-(methoxymethoxy)-7-(methoxymethyl)chromen-2-one (28)
The aldehyde 26 (515 mg, 2.28 mmol) was combined with ethyl acetoacetate (27, 580 μL, 4.55 mmol) and piperidine (nine drops) in a mortar, and the solution was ground with a pestle for 30 min. The residue was transferred to a round-bottom flask with EtOAc, and the solvent was removed using a rotary evaporator. Final purification by crystallization from hot EtOH and H2O gave the coumarin 28 (329 mg, 50%): 1H NMR (300 MHz, CDCl3): δ 8.87 (s, 1H), 6.96 (s, 1H), 6.93 (s, 1H), 5.33 (s, 2H), 4.50 (s, 2H), 3.51 (s, 3H), 3.44 (s, 3H), 2.70 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 195.3 (C), 159.6 (C), 157.2 (C), 156.5 (C), 149.1 (C), 142.2 (CH), 123.6 (C), 109.9 (C), 108.1 (CH), 108.0 (CH), 95.1 (CH2), 74.3 (CH2), 59.0 (CH3), 57.0 (CH3), 30.4 (CH3). HRMS (ESI) m/z: [M + H]+ calcd for C15H17O6, 293.1020; found, 293.1019.
5-(Methoxymethoxy)-7-(methoxymethyl)-3-(2-methyl-1,3-dioxolan-2-yl)chromen-2-one (29)
To a round-bottom flask containing the ketone 28 (250 mg, 850 μmol) in benzene (20 mL) were added ethylene glycol (0.34 mL, 6.0 mmol) and pyridinium p-toluenesulfonate (43 mg, 0.17 mmol). The solution was heated in an oil bath under reflux with a Dean–Stark trap in place for 18 h. After it had cooled to rt, the reaction was quenched by addition of saturated NaHCO3 (5 mL) and extracted with EtOAc, and the combined organic layers were dried (Na2SO4) and filtered. The filtrate was concentrated by rotary evaporation. Final purification was achieved through flash column chromatography (20% EtOAc in hexanes) to afford the acetal 29 as colorless crystals (110 mg, 39%) and recovered ketone 28 (44 mg, 18%). For the acetal: 1H NMR (400 MHz, CDCl3): δ 8.27 (s, 1H), 6.96 (s, 1H), 6.93 (s, 1H), 5.33 (s, 2H), 4.40 (s, 2H), 4.10 (m, 2H), 3.92 (m, 2H), 3.55 (s, 3H), 3.44 (s, 3H), 1.83 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3): δ 159.4 (C), 154.9 (C), 154.2 (C), 143.8 (C), 133.6 (CH), 126.7 (C), 109.0 (C), 108.1 (CH), 107.2 (CH), 107.1 (C), 94.9 (CH2), 74.0 (CH2), 65.0 (2 CH2), 58.5 (CH3), 56.6 (CH3), 24.4 (CH3). HRMS (ESI) m/z: [M + H]+ calcd for C17H21O7, 337.1282; found, 337.1278.
5-(Methoxymethoxy)-7-methyl-3-(2-methyl-1,3-dioxolan-2-yl)chromen-2-one (30)
To a solution of the methyl ether 29 (110 mg, 330 μmol), CH2Cl2 (5 mL), and H2O (0.5 mL) was added DDQ (210 mg, 900 μmol), and the mixture was allowed to stir at rt for 24 h. The mixture was quenched by addition of saturated NaHCO3, extracted with CH2Cl2, and the combined organic layers were washed with brine, dried (Na2SO4), and filtered. The filtrate was concentrated on a rotary evaporator. Final purification was achieved through flash column chromatography (20% EtOAc in hexanes) to afford the aldehyde 30 as a yellow oil (45 mg, 43%): 1H NMR (400 MHz, CDCl3): δ 10.00 (s, 1H), 8.29 (s, 1H), 7.48 (s, 1H), 7.43 (s, 1H), 5.38 (s, 2H), 4.12 (m, 2H), 3.95 (m, 2H), 3.53 (s, 3H), 1.82 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3): δ 190.7 (CH), 158.5 (C), 154.8 (C), 154.7 (C), 138.8 (C), 132.7 (CH), 129.8 (C), 118.1 (C), 111.9 (CH), 107.4 (CH), 107.0 (C), 95.1 (CH2), 65.1 (2 CH2), 56.8 (CH3), 24.5 (CH3). HRMS (ESI) m/z: [M + H]+ calcd for C16H16O7Na, 343.0788; found, 343.0796.
(7R,8aR,10aR)-4-Methoxy-7-(methoxymethoxy)-8,8,10a-trimethyl-6,7,8a,9-tetrahydro-5H-xanthene-2-carbaldehyde (32)
To a vial containing aldehyde 31(8) (177 mg, 580 μmol) in CH2Cl2 (5 mL) at 0 °C was added dropwise DIPEA (110 μL, 0.64 mmol). The solution was allowed to stir for 10 min, and then MOMCl (50 μL, 0.64 mmol) was added dropwise. After the solution was allowed to stir for 72 h, it was quenched by addition of NH4Cl and was extracted with CH2Cl2. The combined organic layers were washed with 1 N NaOH, dried (Na2SO4), and filtered, and the filtrate was concentrated in vacuo to afford aldehyde 32 (140 mg, 70%): 1H NMR (400 MHz, CDCl3): δ 9.80 (s, 1H), 7.26–7.23 (m, 2H), 4.79 (d, J = 6.9 Hz, 1H), 4.66 (d, J = 7.0 Hz, 1H), 3.90 (s, 3H), 3.41 (s, 3H), 3.31–3.24 (m, 1H), 2.80–2.78 (m, 2H), 2.18 (td, J = 12.9, 3.6 Hz, 1H), 2.02 (dq, J = 14.4, 4.0 Hz, 1H), 1.82 (ddd, J = 13.8, 13.8, 4.1 Hz, 1H), 1.72 (dd, J = 11.3, 7.5 Hz, 1H), 1.65–1.58 (m, 1H), 1.28 (s, 3H), 1.10 (s, 3H), 0.92 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 191.2, 149.6, 148.9, 129.0, 127.4, 122.6, 107.4, 96.3, 83.8, 78.5, 56.1, 55.7, 46.7, 38.4, 37.5, 27.5, 25.4, 23.1, 20.1, 15.2. HRMS (ESI) m/z: [M + H]+ calcd for C20H29O5, 349.2015; found, 349.2006.
[(7R,8aR,10aR)-4-Methoxy-7-(methoxymethoxy)-8,8,10a-trimethyl-6,7,8a,9-tetrahydro-5H-xanthen-2-yl]methanol (17)
To an oven-dried and argon-purged round-bottom flask containing the aldehyde 32 (350 mg, 1.00 mmol) in THF (10 mL) and MeOH (2 mL) at 0 °C was added solid NaBH4 (234 mg, 1.60 mmol). The solution was allowed to stir for 40 min, was quenched by addition of H2O, and then was extracted with EtOAc. The combined organic layers were washed with saturated NaHCO3 and brine and dried (MgSO4). After filtration, the filtrate was concentrated on a rotary evaporator to afford the benzylic alcohol 17 (352 mg, 100%) as a colorless oil. Both the 1H and 13C NMR spectra were in agreement with data reported in the literature.10
[(2R,4aR,9aR)-7-(Diethoxyphosphonylmethyl)-5-methoxy-1,1,4a-trimethyl-3,4,9,9a-tetrahydro-2H-xanthen-2-yl]oxymethanol (15)
To an oven-dried and argon-purged round-bottom flask containing ZnI2 (1.1 g, 3.5 mmol) and triethyl phosphite (400 μL, 2.3 mmol) in THF (15 mL) was added benzylic alcohol 17 (410 mg, 1.2 mmol). The reaction was heated in an oil bath under reflux for 17 h. The solution was concentrated in vacuo to 1 mL, and then the residue was dissolved in Et2O, which caused formation of a solid that was removed by filtration. After the filtrate was washed with 1 N NaOH (0.5 mL), the organic layer was dried (Na2SO4) and filtered, and the filtrate was concentrated on a rotary evaporator. Excess triethyl phosphite was removed using high vacuum to afford phosphonate 15 (370 mg, 69%) as a colorless oil. Both the 1H and 31P NMR spectra were in agreement with data reported for compound 15 prepared by a traditional Arbuzov sequence.10
Attempted Preparation of 7-[(E)-2-[(7R,8aR,10aR)-4-Methoxy-7-(methoxymethoxy)-8,8,10a-trimethyl-6,7,8a,9-tetrahydro-5H-xanthen-2-yl]vinyl]-3-(2-methyl-1,3-dioxolan-2-yl)chromen-2-one (33)
To a flame-dried round-bottom flask containing the phosphonate 15 (78 mg, 0.17 mmol) in THF (1 mL) at 0 °C was added NaH (60% dispersion in oil, 10 mg, 0.25 mmol). To the stirring solution was added the aldehyde 30 (8.6 mg, 26 μmol) in THF (2 mL). The reaction was allowed to warm to rt naturally and then was quenched by addition of H2O. The organic compounds were extracted into EtOAc, dried (Na2SO4), and filtered, and the filtrate was concentrated in vacuo. The desired stilbene 33 could not be detected in the resulting material.
[4-Bromo-3,5-bis(methoxymethoxy)phenyl]methoxy tert-Butyldimethylsilane (34)
To an oven-dried and argon-purged round-bottom flask containing alcohol 25 (1.3 g, 3.4 mmol) in anhydrous CH2Cl2 (200 mL) was added imidazole (470 mg, 6.9 mmol) followed by TBSCl (560 mg, 3.8 mmol), and the reaction was allowed to stir at rt for 14 h. After the reaction was quenched by addition of water, the aqueous layer was extracted with CH2Cl2. The combined organic layers were washed with brine, dried (Na2SO4), and filtered through a pad of Celite, and the filtrate was concentrated under reduced pressure to afford the silyl ether 34 as a yellow oil (1.4 g, 97%): 1H NMR (400 MHz, CDCl3): δ 6.84 (s, 2H), 5.24 (s, 4H), 4.68 (s, 2H), 3.52 (s, 6H), 0.94 (s, 9H), 0.10 (s, 6H). 13C{1H} NMR (75 MHz, CDCl3): δ 158.5 (2 C), 144.4 (C), 107.3 (2 CH), 103.5 (C), 94.7 (2 CH2), 64.9 (CH2), 56.1 (2 CH3), 26.1 (3 CH3), 18.6 (C), −5.1 (2 CH3).41
4-(((tert-Butyldimethylsilyl)oxy)methyl)-2,6-bis(methoxymethoxy)benzaldehyde (35)
An oven-dried round-bottom flask containing aryl bromide 34 (5.6 g, 13 mmol) in Et2O (150 mL) was cooled to −78 °C for 20 min. To the solution was added n-BuLi (7.4 mL, 18 mmol, 2.4 M). Immediately after the addition was complete, anhydrous DMF (1.4 mL, 18 mmol) was added dropwise and the reaction was allowed to stir and warm to rt overnight. After the reaction was quenched by addition of saturated NH4Cl (50 mL), the organic compounds were extracted into Et2O (3 × 50 mL). The combined organic layers were dried (Na2SO4), the solids were removed by filtration, and the filtrate was concentrated on a rotary evaporator. Final purification was achieved by ISCO normal-phase auto-chromatography (0–5% EtOAc in hexanes), which gave aldehyde 35 as a yellow oil (2.61 g, 53%). Both the 1H and 13C NMR spectra match the literature data for material prepared by a different route.421H NMR (300 MHz, CDCl3): δ 10.34 (s, 1H), 6.70 (s, 2H), 5.09 (s, 4H), 4.56 (s, 2H), 3.32 (s, 6H), 0.80 (s, 9H), 0.05 (s, 6H).
tert-Butyl-[[4-(1,3-dioxolan-2-yl)-3,5-bis(methoxymethoxy)phenyl]methoxy]dimethylsilane (36)
A round-bottom flask containing aldehyde 35 (680 mg, 1.8 mmol), ethylene glycol (660 μL, 11 mmol), and PPTS (110 mg, 370 μmol) in benzene (50 mL) was fitted with a Dean–Stark trap and heated in an oil bath under reflux. The reaction progress was monitored periodically by thin-layer chromatography. After 1 h, the reaction was allowed to cool to rt, quenched by addition of saturated NaHCO3 (5 mL), and extracted with EtOAc (3 × 20 mL). The combined organic layers were dried (Na2SO4), the solid was removed by gravity filtration, and the resulting filtrate was concentrated on a rotary evaporator to afford acetal 36 (720 mg, 96%): 1H NMR (400 MHz, CDCl3) 6.74 (2H, s), 6.40 (1H, s), 5.09 (4H, s), 4.63 (2H, s), 4.11 (2H, m), 3.89 (2H, m), 3.39 (6H, s), 0.89 (9H, s), 0.03 (6H, s); 13C NMR (101 MHz, CDCl3) 157.4 (C), 145.0 (C), 114.1 (2 CH), 106.5 (CH), 98.9 (2 C), 94.7 (2 CH2), 65.6 (2 CH2), 64.4 (CH2), 56.1 (2 CH3), 26.2 (3 CH3), 18.4 (C), −5.4 (2 CH3). HRMS (ESI) m/z: [M + H]+ calcd for C20H35O7Si, 417.2147; found, 415.2145.
[4-(1,3-Dioxolan-2-yl)-3,5-bis(methoxymethoxy)phenyl]methanol (37)
To a flask containing the silyl ether 36 (2.5 g, 6.0 mmol) in THF (150 mL) was added TBAF (1.0 M in THF, 6.0 mL, 6.0 mmol), and the solution was allowed to stir at 0 °C and naturally warm to rt over 1 h. The reaction was quenched by addition of water, and the organic compounds were extracted into EtOAc (3 × 100 mL), washed with brine, and dried (Na2SO4). The solids were removed by gravity filtration, and the filtrate was concentrated on a rotary evaporator to afford alcohol 37 as a pale yellow solid (1.7 g, 94%): 1H NMR (400 MHz, CDCl3) 6.81 (2H, s), 6.48 (1H, s), 5.20 (4H, s), 4.57 (2H, s), 4.24–4.19 (2H, m), 4.04–4.01 (2H, m), 3.50 (6H, s); 13C NMR (101 MHz, CDCl3): δ 157.3 (2 C), 144.2 (C), 114.8 (C), 106.9 (2 CH), 98.5 (CH), 94.7 (2 CH2), 66.1 (2 CH2), 65.4 (CH2), 56.3 (2 CH3). HRMS (ESI) m/z: [M + H]+ calcd for C14H21O7, 301.1282; found, 301.1280.
4-(1,3-Dioxolan-2-yl)-3,5-bis(methoxymethoxy)benzaldehyde (38)
To a flame-dried and argon-purged round-bottom flask containing alcohol 37 (210 mg, 690 μmol) in anhydrous CH2Cl2 (20 mL) was slowly added manganese dioxide (1.5 g, 14 mmol). The resulting mixture was allowed to stir at rt for 26 h. The mixture then was filtered through a bed of Celite, and the filtrate was concentrated to afford aldehyde 38 (180 mg, 86%): 1H NMR (400 MHz, CDCl3): δ 9.89 (s, 1H), 7.29 (s, 2H), 6.50 (s, 1H), 5.24 (s, 4H), 4.23–4.20 (m, 2H), 4.04–4.00 (m, 2H), 3.49 (s, 6H); 13C NMR (101 MHz, CDCl3): δ 191.7 (CH), 158.0 (2 C), 138.1 (C), 121.5 (C), 109.7 (2 CH), 98.1 (CH), 94.5 (2 CH2), 66.5 (2 CH2), 56.6 (2 CH3). HRMS (ESI) m/z: [M + H]+ calcd for C14H19O7, 299.1125; found, 299.1127.
(2R,4aR,9aR)-7-[(E)-2-[4-(1,3-Dioxolan-2-yl)-3,5-bis(methoxymethoxy)phenyl]vinyl]-5-methoxy-2-(methoxymethoxy)-1,1,4a-trimethyl-3,4,9,9a-tetrahydro-2H-xanthene (39)
To a flame-dried and argon-purged round-bottom flask containing the phosphonate 15 (116 mg, 250 μmol) and the aldehyde 38 (61 mg, 0.21 mmol) in THF (12 mL) at 0 °C was added dropwise NaHMDS (1.0 M in THF, 680 μL, 680 μmol), and the mixture was allowed to stir at 0 °C for 32 h. The reaction was quenched by a dropwise addition of saturated NH4Cl to reach a pH of 8, and the organic compounds were extracted into EtOAc (3 × 20 mL). The combined organic layers were washed with brine and dried (MgSO4), the solids were removed by filtration, and the filtrate was concentrated on a rotary evaporator. Final purification was achieved by column chromatography (15–100% EtOAc in hexanes) to afford the recovered phosphonate 15 (98 mg, 83%) and the desired stilbene 39 as a fluorescent yellow oil (25 mg, 16%): 1H NMR (400 MHz, CDCl3): δ 6.98 (d, J = 14.8 Hz, 1H), 6.92 (s, 2H), 6.90–6.84 (m, 3H), 6.47 (s, 1H), 5.22 (s, 4H), 4.77 (d, J = 7.1 Hz, 1H), 4.65 (d, J = 6.4 Hz, 1H), 4.24–4.19 (m, 2H), 4.02–3.99 (m, 2H), 3.89 (s, 3H), 3.51 (s, 6H), 3.41 (s, 3H), 3.28 (dd, J = 11.3, 3.5 Hz, 1H), 2.71 (d, J = 8.6 Hz, 1H), 2.15 (d, J = 12.9 Hz, 1H), 2.02–1.92 (m, 1H), 1.84–1.75 (m, 1H), 1.75–1.69 (m, 1H), 1.65–1.54 (m, 2H), 1.25 (s, 3H), 1.10 (s, 3H), 0.91 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 157.5 (2C), 149.3, 142.9, 140.6, 130.1, 126.1, 122.7, 121.1, 114.8, 109.7, 107.0, 106.8 (2C), 98.8, 96.5, 94.9 (2C), 84.1, 66.0 (2C), 56.3, 56.0, 55.6, 47.0, 38.3, 37.6, 29.8, 27.5, 25.4, 23.1, 19.9, 15.2, 14.2. HRMS (ESI) m/z: [M + H]+ calcd for C34H47O17, 615.3164; found, 615.3171.
4-[(E)-2-[(7R,8aR,10aR)-7-Hydroxy-4-methoxy-8,8,10a-trimethyl-6,7,8a,9-tetrahydro-5H-xanthen-2-yl]vinyl]-2,6-dihydroxybenzaldehyde (40)
To the acetal 39 (35 mg, 57 μmol) was added 5 M HCl (5 mL) and anhydrous MeOH (15 mL); the solution was allowed to stir at rt for 69 h, and then was quenched by addition of saturated NaHCO3 to a pH of 7. The organic compounds were extracted into EtOAc (2 × 25 mL), the combined organic layers were dried (MgSO4), and the solids were removed by filtration. The filtrate was concentrated to afford the aldehyde 40 as an orange solid (26 mg, 100%): 1H NMR (400 MHz, CDCl3): δ 10.27 (s, 1H), 7.05 (d, J = 15.9 Hz, 1H), 6.85 (m, 2H), 6.76 (d, J = 15.4 Hz, 1H), 6.47 (s, 2H), 3.87 (s, 3H), 3.44 (d, J = 6.5 Hz, 1H), 2.71 (d, J = 8.6 Hz, 2H), 2.18–2.07 (m, 2H), 1.92–1.80 (m, 1H), 1.73–1.49 (m, 2H), 1.24 (s, 3H), 1.09 (s, 3H), 0.87 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 193.1 (CH), 149.1 (2 C), 148.1, 143.7, 133.2, 128.0, 125.1 (2 C), 123.0, 121.7, 109.5, 107.4, 105.2, 78.4, 77.5, 56.1, 46.8, 38.5, 37.6, 29.7, 28.2, 27.3, 23.1, 19.9, 14.3. HRMS (ESI) m/z: [M – H]– calcd for C26H29O6, 437.1964; found, 437.1965.
7-[(E)-2-[(7R,8aR,10aR)-7-Hydroxy-4-methoxy-8,8,10a-trimethyl-6,7,8a,9-tetrahydro-5H-xanthen-2-yl]vinyl]-3-acetyl-5-hydroxychromen-2-one (41)
To a flame-dried round-bottom flask containing the aldehyde 40 (19 mg, 41 μmol) in anhydrous MeOH (2 mL) were added ethyl acetoacetate (27, 5.2 μL, 41 μmol) and piperidine (2.0 μL, 20 μmol) and the sides of the flask were washed with 1 mL of anhydrous MeOH. The solution was allowed to stir in a foil-covered flask at rt for 75 h, and then the reaction was quenched by addition of H2O (10 mL). The organic compounds were extracted into CH2Cl2 (3 × 20 mL), the combined organic layers were dried (Na2SO4), and the solids were removed by filtration. The filtrate was concentrated on a rotary evaporator, and the resulting material was purified by column chromatography (50–100% EtOAc in hexanes). Final purification was achieved by washing the solid with pentane (3 × 2 mL) to afford coumarin 41 as a fluorescent orange solid (22 mg, 100%): 1H NMR (400 MHz, CD3CN): δ 8.66 (s, 1H), 7.27 (d, J = 15.9 Hz, 1H), 7.07–7.01 (m, 3H), 6.94 (s, 2H), 3.86 (s, 3H), 3.71–3.67 (m, 1H), 2.76 (d, J = 8.8 Hz, 2H), 2.61 (s, 3H), 1.80–1.71 (m, 3H), 1.71–1.65 (m, 2H), 1.22 (s, 3H), 1.09 (s, 3H), 0.87 (s, 3H); 13C NMR (151 MHz, CD3CN): δ 195.3, 169.0, 166.5, 160.8, 159.1, 156.2, 149.4, 148.9, 141.7, 133.2, 124.3, 122.8, 121.9, 121.1, 108.1, 108.0, 107.7, 104.4, 77.1, 76.9, 55.2, 46.5, 38.2, 37.5, 29.2, 26.7, 25.9, 23.4, 19.3, 13.9. HRMS (ESI) m/z: [M + Na]+ calcd for C30H32O7Na, 527.2046; found, 527.2051.
Ethyl 7-Methyl-3-oxo-6-octenoate (42)
To an oven-dried and argon-purged round-bottom flask containing NaH (2.08 g, 51.9 mmol) in THF (400 mL) at 0 °C was added ethyl acetoacetate (27, 6.02 mL, 47.2 mmol), and the solution was stirred for 10 min. To the reaction flask was added dropwise n-BuLi (21.6 mL, 51.9 mmol) followed by a dropwise addition of prenyl bromide (6.00 mL, 51.9 mmol). The reaction was stirred at rt for 20 min and then quenched by addition of saturated NH4Cl and extracted with Et2O. The combined organic layers were dried (Na2SO4) and filtered, and the filtrate was concentrated on a rotary evaporator. Final purification was achieved by column chromatography (10% EtOAc in hexanes) to afford the β-ketoester 42 as a pale yellow oil (4.69 g, 50%). Both the 1H and 13C NMR spectra matched those in the literature.43
5-(Methoxymethoxy)-7-(methoxymethyl)-3-(5-methylhex-4-enoyl)chromen-2-one (43)
To a mortar were added aldehyde 26 (806 mg, 3.56 mmol), the β-ketoester 42 (1.41 g, 7.12 mmol), and piperidine (three drops). The solution was ground with a pestle intermittently over 12 h. Purification was achieved by column chromatography (20–40% EtOAc in hexanes). The desired fractions were combined and heated (oil bath) under reflux in benzene with a Dean–Stark trap overnight. After cooling to rt, the resulting solution was concentrated in vacuo to afford compound 43 (1.12 g, 87%): 1H NMR (400 MHz, CDCl3): δ 8.83 (s, 1H), 6.93 (s, 1H), 6.90 (s, 1H), 5.30 (s, 2H), 5.13 (tt, J = 7.2, 1.3 Hz, 1H) 4.47 (s, 2H), 3.49 (s, 3H), 3.41 (s, 3H), 3.12 (t, J = 7.2 Hz, 2H), 2.34 (q, J = 7.2 Hz, 2H), 1.64 (s, 3H), 1.60 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3): δ 197.7 (C), 159.2 (C), 156.1 (C), 155.6 (C), 147.5 (CH), 142.8 (C), 132.5 (C), 123.0 (CH), 122.3 (C), 109.0 (C), 107.6 (CH), 107.0 (CH), 94.7 (CH2), 73.8 (CH2), 58.7 (CH3), 56.7 (CH3), 42.6 (CH2), 25.7 (CH3), 22.6 (CH2), 17.7 (CH3). HRMS (ESI) m/z: [M + H]+ calcd for C20H25O6, 361.1646; found, 361.1624.
Acknowledgments
We thank Vic Parcell for his help in securing the HRMS data and Dr. Santhana Velupillai (at the UI) and Dr. Dayani Senadeera (at NCI-F) for their help with the NMR spectra. We thank the University of Iowa Graduate College for fellowship support for C.M.S. This research was supported in part by the Intramural Research Program of NIH, National Cancer Institute, Center for Cancer Research. We thank the Developmental Therapeutics Program, NCI, for the 60-cell testing. Financial support from the Roy J. Carver Charitable Trust as a Research Program of Excellence (to D.F.W.) is gratefully acknowledged. The Q-Exactive mass spectrometer used in this research was acquired through the National Science Foundation Major Research Instrumentation and the Chemical Instrumentation Programs (CHE-1919422).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.1c02046.
1H and 13C NMR spectra for all new compounds and full tables of the bioassay data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Harmalkar D. S.; Mali J. R.; Sivaraman A.; Choi Y.; Lee K. Schweinfurthins A-Q: Isolation, Synthesis, and Biochemical Properties. RSC Adv. 2018, 8, 21191–21209. 10.1039/c8ra02872a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoison O.; Hnawia E.; Guiéritte-Voegelein F.; Sévenet T. Vedelianin, a hexahydroxanthene derivative isolated from Macaranga vedeliana. Phytochemistry 1992, 31, 1439–1442. 10.1016/0031-9422(92)80315-6. [DOI] [Google Scholar]
- Petrova A.; Popova M.; Kuzmanova C.; Tsvetkova I.; Naydenski H.; Muli E.; Bankova V. New Biologically Active Compounds from Kenyan Propolis. Fitoterapia 2010, 81, 509–514. 10.1016/j.fitote.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Beutler J. A.; Shoemaker R. H.; Johnson T.; Boyd M. R. Cytotoxic Geranyl Stilbenes from Macaranga schweinfurthii. J. Nat. Prod. 1998, 61, 1509–1512. 10.1021/np980208m. [DOI] [PubMed] [Google Scholar]
- Yoder B. J.; Cao S.; Norris A.; Miller J. S.; Ratovoson F.; Razafitsalama J.; Andriantsiferana R.; Rasamison V. E.; Kingston D. G. I. Antiproliferative Prenylated Stilbenes and Flavonoids from Macaranga alnifolia from the Madagascar Rainforest,1. J. Nat. Prod. 2007, 70, 342–346. 10.1021/np060484y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler J. A.; Jato J.; Cragg G. M.; Boyd M. R. Schweinfurthin D, A Cytotoxic Stilbene from Macaranga schweinfurthii. Nat. Prod. Lett. 2000, 14, 399–404. 10.1080/10575630008043774. [DOI] [Google Scholar]
- Topczewski J. J.; Kodet J. G.; Wiemer D. F. Exploration of Cascade Cyclizations Terminated by Tandem Aromatic Substitution: Total Synthesis of (+)-Schweinfurthin A. J. Org. Chem. 2011, 76, 909–919. 10.1021/jo1022102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topczewski J. J.; Neighbors J. D.; Wiemer D. F. Total Synthesis of (+)-Schweinfurthins B and E. J. Org. Chem. 2009, 74, 6965–6972. 10.1021/jo901161m. [DOI] [PubMed] [Google Scholar]
- Mente N. R.; Wiemer A. J.; Neighbors J. D.; Beutler J. A.; Hohl R. J.; Wiemer D. F. Total synthesis of (R,R,R)- and (S,S,S)-schweinfurthin F: Differences of bioactivity in the enantiomeric series. Bioorg. Med. Chem. Lett. 2007, 17, 911–915. 10.1016/j.bmcl.2006.11.096. [DOI] [PubMed] [Google Scholar]
- Mente N. R.; Neighbors J. D.; Wiemer D. F. BF3·Et2O-Mediated Cascade Cyclizations: Synthesis of Schweinfurthins F and G. J. Org. Chem. 2008, 73, 7963–7970. 10.1021/jo800951q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topczewski J. J.; Wiemer D. F. First Total Synthesis of (+)-Vedelianin, a Potent Antiproliferative Agent. Tetrahedron Lett. 2011, 52, 1628–1630. 10.1016/j.tetlet.2011.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neighbors J. D.; Beutler J. A.; Wiemer D. F. Synthesis of Nonracemic 3-Deoxyschweinfurthin B. J. Org. Chem. 2005, 70, 925–931. 10.1021/jo048444r. [DOI] [PubMed] [Google Scholar]
- Neighbors J. D.; Salnikova M. S.; Beutler J. A.; Wiemer D. F. Synthesis and Structure-Activity Studies of Schweinfurthin B Analogs: Evidence for the Importance of a D-Ring Hydrogen Bond Donor in Expression of Differential Cytotoxicity. Bioorg. Med. Chem. 2006, 14, 1771–1784. 10.1016/j.bmc.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Kodet J. G.; Wiemer D. F. Synthesis of Indole Analogues of the Natural Schweinfurthins. J. Org. Chem. 2013, 78, 9291–9302. 10.1021/jo4014244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharevitz D. W.; Holbeck S. L.; Bowerman C.; Svetlik P. A. COMPARE: A Web Accessible Tool for Investigating Mechanisms of Cell Growth Inhibition. J. Mol. Graph. Model. 2002, 20, 297–303. 10.1016/s1093-3263(01)00126-7. [DOI] [PubMed] [Google Scholar]
- Péresse T.; Kovacs D.; Subra M.; Bigay J.; Tsai M.-C.; Polidori J.; Gautier R.; Desrat S.; Fleuriot L.; Debayle D.; Litaudon M.; Pham V.-C.; Bignon J.; Antonny B.; Roussi F.; Mesmin B. Molecular and Cellular Dissection of the Oxysterol-Binding Protein Cycle through a Fluorescent Inhibitor. J. Biol. Chem. 2020, 295, 4277–4288. 10.1074/jbc.ra119.012012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgett A. W. G.; Poulsen T. B.; Wangkanont K.; Anderson D. R.; Kikuchi C.; Shimada K.; Okubo S.; Fortner K. C.; Mimaki Y.; Kuroda M.; Murphy J. P.; Schwalb D. J.; Petrella E. C.; Cornella-Taracido I.; Schirle M.; Tallarico J. A.; Shair M. D. Natural Products Reveal Cancer Cell Dependence on Oxysterol-Binding Proteins. Nat. Chem. Biol. 2011, 7, 639–647. 10.1038/nchembio.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosavi B.; Gao M.; Zhu X.-L.; Yang G.-F. The Anti-Cancer Compound Schweinfurthin a Targets Osh2 and Disrupts Lipid Metabolism in the Yeast Model. Bioorg. Chem. 2020, 94, 103471. 10.1016/j.bioorg.2019.103471. [DOI] [PubMed] [Google Scholar]
- Bao X.; Zheng W.; Sugi N. H.; Agarwala K. L.; Xu Q.; Wang Z.; Tendyke K.; Lee W.; Parent L.; Li W.; Cheng H.; Shen Y.; Taylor N.; Dezso Z.; Du H.; Kotake Y.; Zhao N.; Wang J.; Postema M.; Woodall-Jappe M.; Takase Y.; Uenaka T.; Kingston D. G. I.; Nomoto K. Small Molecule Schweinfurthins Selectively Inhibit Cancer Cell Proliferation and Mtor/Akt Signaling by Interfering with Trans-Golgi-Network Trafficking. Cancer Biol. Ther. 2015, 16, 589–601. 10.1080/15384047.2015.1019184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy R. M.; Kuder C. H.; Bachman Z.; Hohl R. J. Calcium and P-Glycoprotein Independent Synergism between Schweinfurthins and Verapamil. Cancer Biol. Ther. 2015, 16, 1259–1268. 10.1080/15384047.2015.1056420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein S. A.; Kuder C. H.; Tong H.; Hohl R. J. Pleiotropic Effects of a Schweinfurthin on Isoprenoid Homeostasis. Lipids 2011, 46, 907–921. 10.1007/s11745-011-3572-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M.; Argade N. P. Palladium-Catalyzed Routes to Geranylated or Farnesylated Phenolic Stilbenes: Synthesis of Pawhuskin C and Schweinfurthin J. Synthesis 2012, 44, 2895–2902. 10.1055/s-0032-1316765. [DOI] [Google Scholar]
- Roos D.; Maier M. E. Approach to the Core Structure of Schweinfurthin B. Chemistryselect 2020, 5, 14747–14752. 10.1002/slct.202003749. [DOI] [Google Scholar]
- Shi Y. Organocatalytic Asymmetric Epoxidation of Olefins by Chiral Ketones. Acc. Chem. Res. 2004, 37, 488–496. 10.1021/ar030063x. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Huang Q.; Chen B.; Lu J.; Wang H.; She X.; Pan X. Total Synthesis of (+)-Machaeriol D with a Key Regio- and Stereoselective SN2′ Reaction. Angew. Chem. Int. Ed. 2006, 45, 3651–3653. 10.1002/anie.200600006. [DOI] [PubMed] [Google Scholar]
- Lélias-Vanderperre A.; Aubert E.; Chambron J.-C.; Espinosa E. Effect of Substituents on the Molecular Shapes of π-Basic Macrotricyclic Receptors. Eur. J. Org. Chem. 2010, 2010, 2701–2708. 10.1002/ejoc.200901398. [DOI] [Google Scholar]
- Sakakura A.; Sakuma M.; Ishihara K. Chiral Lewis Base-Assisted Brønsted Acid (LBBA)-Catalyzed Enantioselective Cyclization of 2-Geranylphenols. Org. Lett. 2011, 13, 3130–3133. 10.1021/ol201032t. [DOI] [PubMed] [Google Scholar]
- Ramesh C.; Ravindranath N.; Das B. Simple, Efficient, and Selective Deprotection of Phenolic Methoxymethyl Ethers Using Silica-Supported Sodium Hydrogen Sulfate as a Heterogeneous Catalyst1. J. Org. Chem. 2003, 68, 7101–7103. 10.1021/jo030088+. [DOI] [PubMed] [Google Scholar]
- Niu Y.; Wang N.; Cao X. P.; Ye X. S. Efficient Formation and Cleavage of Benzylidene Acetals by Sodium Hydrogen Sulfate Supported on Silica Gel. Synlett 2007, 13, 2116–2120. 10.1055/s-2007-984903. [DOI] [Google Scholar]
- Barney R. J.; Richardson R. M.; Wiemer D. F. Direct Conversion of Benzylic and Allylic Alcohols to Phosphonates. J. Org. Chem. 2011, 76, 2875–2879. 10.1021/jo200137k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sum F. W.; Weiler L. Stereoselective Synthesis of Latia Luciferin. Tetrahedron Lett. 1979, 20, 707–708. 10.1016/s0040-4039(01)93551-8. [DOI] [Google Scholar]
- Ulrich N. C.Cascade cyclizations in total synthesis: applications to synthesis of cytotoxic natural products. Ph.D. Thesis, University of Iowa, 2010. [Google Scholar]
- Pettit G. R.; Singh S. B.; Niven M. L.; Hamel E.; Schmidt J. M. Isolation, Structure, and Synthesis of Combretastatins A-1 and B-1, Potent New Inhibitors of Microtubule Assembly, Derived from Combretum caffrum. J. Nat. Prod. 1987, 50, 119–131. 10.1021/np50049a016. [DOI] [PubMed] [Google Scholar]
- Aggarwal B. B.; Bhardwaj A.; Aggarwal R. S.; Seeram N. P.; Shishodia S.; Takada Y. Role of Resveratrol in Prevention and Therapy of Cancer: Preclinical and Clinical Studies. Anticancer Res. 2004, 24, 2783–840. [PubMed] [Google Scholar]
- Poltronieri P.; Xu B.; Giovinazzo G. Resveratrol and Other Stilbenes: Effects on Dysregulated Gene Expression in Cancers and Novel Delivery Systems. Anti-Cancer Agents Med. Chem. 2021, 21, 567–574. 10.2174/1871520620666200705220722. [DOI] [PubMed] [Google Scholar]
- Keylor M. H.; Matsuura B. S.; Stephenson C. R. J. Chemistry and Biology of Resveratrol-Derived Natural Products. Chem. Rev. 2015, 115, 8976–9027. 10.1021/cr500689b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioset J.-R.; Marston A.; Gupta M. P.; Hostettmann K. Five New Prenylated Stilbenes from the Root Bark of Lonchocarpus chiricanus. J. Nat. Prod. 2001, 64, 710–715. 10.1021/np000597w. [DOI] [PubMed] [Google Scholar]
- Sobolev V. S. Production of Phytoalexins in Peanut (Arachis hypogaea) Seed Elicited by Selected Microorganisms. J. Agric. Food Chem. 2013, 61, 1850–1858. 10.1021/jf3054752. [DOI] [PubMed] [Google Scholar]
- Belofsky G.; French A. N.; Wallace D. R.; Dodson S. L. New Geranyl Stilbenes from Daleapurpurea with in Vitro Opioid Receptor Affinity. J. Nat. Prod. 2004, 67, 26–30. 10.1021/np030258d. [DOI] [PubMed] [Google Scholar]
- Hume P. A.; Furkert D. P.; Brimble M. A. Total Synthesis of Virgatolide B. Org. Lett. 2013, 15, 4588–4591. 10.1021/ol402191t. [DOI] [PubMed] [Google Scholar]
- Chakrapani L.; Jung E. M.; Lee Y. R. First Total Synthesis of Mappain with a Prenylated and Geranylated Stilbene. Helv. Chim. Acta 2010, 93, 829–836. 10.1002/hlca.200900318. [DOI] [Google Scholar]
- Little A.; Porco J. A. Total Syntheses of Graphisin A and Sydowinin B. Org. Lett. 2012, 14, 2862–2865. 10.1021/ol301107m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada A.; Wang F.; Suhara Y.; Yamano Y.; Okitsu T.; Nakagawa K.; Okano T. Efficient Synthesis and Biological Evaluation of Demethyl Geranylgeranoic Acid Derivatives. Bioorg. Med. Chem. 2010, 18, 5795–5806. 10.1016/j.bmc.2010.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.