Abstract
This study aims to evaluate the effect of non‐alcoholic fatty liver disease (NAFLD) on the susceptibility and consequences of coronavirus disease 2019 (COVID‐19). We retrospectively collected data from 218 adult COVID‐19 patients who showed no evidence of excessive alcohol consumption and underwent abdominal ultrasound examinations. Of these patients, 39.4% patients had been diagnosed with NAFLD, which indicates a much higher prevalence of NAFLD than that reported in the general population. Significantly elevated white blood cell count (p = 0.008), alanine aminotransferase (p = 0.000), aspartate aminotransferase (p = 0.006) and C reactive protein (p = 0.012) were found in the patients with NAFLD. These patients also had significantly higher proportions of hypertension (p = 0.006) and diabetes (p = 0.049) than the non‐NAFLD cases. No significant differences existed in the severity, mortality, viral shedding time and length of hospital stay between patients with or without NAFLD in the sample population. However, subgroup analyses found that in patients with normal body mass index (BMI), NAFLD sufferers were more likely to experience a severe event (30.0% vs 11.5%, p = 0.021). Kaplan‐Meier curve (log‐rank p = 0.017) and Cox regression (HR = 3.26, 95% CI: 1.17–9.04, p = 0.023) analyses confirmed that before and after adjusting for gender, age and comorbidities, NAFLD patients with normal BMI had a higher incidence of suffering severe events. People with NAFLD may have a higher proportion of COVID‐19. NAFLD may be correlated with the severity of COVID‐19 patients in the normal BMI group.
Keywords: body mass index, COVID‐19, liver injury, non‐alcoholic fatty liver disease, outcome, susceptibility
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) was first reported in Wuhan, China, in December 2019 1 , 2 , 3 , 4 , 5 and now has spread all around the world. 6 , 7 Recently, several reports have discovered that a large number of COVID‐19 patients had underlying conditions, especially in severe and lethal cases. 3 , 4 , 8 Also, pre‐existing ailments may be closely related to the susceptibility and poor outcomes of COVID‐19. 9 Liver impairment has been reported in up to 50% of patients with COVID‐19, and more than 60% of severe cases. 10 Histological examination in the liver of a COVID‐19 patient showed moderate micro‐vesicular steatosis and mild lobular and portal activity. 11 However, the causes of liver injury in COVID‐19 cases and the effects of liver‐related comorbidities on the susceptibility and outcomes of COVID‐19 remain unclear.
Non‐alcoholic fatty liver disease (NAFLD) is an acquired metabolic stress‐related liver disorder, with an overall global prevalence of about 25%. 12 The incidence of NAFLD in China is rising rapidly, from about 23.8% in the early 2000s to about 32.9% in 2018. 12 , 13 , 14 , 15 NAFLD often coexists with hyperlipidaemia and/or obesity. 16 , 17 , 18 , 19 Therefore, this study aims to evaluate the relationship between NAFLD and COVID‐19 using a retrospective cohort of Chinese individuals diagnosed with COVID‐19.
2. METHODS (SUBJECTS) AND MATERIALS
2.1. Study Design and Participants
The study protocol was subject to approval by the institutional ethics board of the Second Xiangya Hospital of Central South University (No. 2020001). We retrospectively collected data from a cohort of subjects who had laboratory‐confirmed COVID‐19 and were patients in the Public Health Treatment Center of Changsha, China, before 14 March 2020. Fatty liver was diagnosed based on the ultrasound parameters (such as parenchymal brightness, deep beam attenuation and liver‐to‐kidney contrast) by abdominal ultrasonography with a 3.5‐MHz transducer (S9, SonoScape, China). Ultrasonography was performed by the experienced radiologists for medical indications in the Public Health Treatment Center of Changsha, China. We used the following criteria to diagnose NAFLD: (1) The presence of fatty liver; (2) The absence of excessive alcohol consumption (average alcohol intake ≥30 g/day for men and ≥20 g/day for women).
2.2. Data Collection
Two authors carefully collected and reviewed the individual medical records of the patients. Detailed information on demographic data, body mass index (BMI), underlying comorbidities, medical history, symptoms, laboratory parameters, chest computed tomographic (CT) scans and outcomes were recorded. For BMI data, the medical records of 18 patients did not include height and weight.
2.3. Endpoints
The primary endpoint of this study was a severe event, which was defined using the following criteria: (1) respiratory rate ≥30 breaths/min; (2) oxygen saturation <93%; (3) PaO2/FiO2 ≤ 300 mmHg; (4) lung lesions progressed to greater than 50% within 24–48 hours; (5) mechanical ventilation was implemented; (6) shock; (7) combined with other organ failures, required intensive care. 20 Secondary endpoints included the mortality rate, virus shedding time and length of hospital stay.
2.4. Statistical Analysis
Because the continuous variables in this study were non‐normal distributed, we used the Fisher's exact test (χ2 test) and Mann‐Whitney test to compare the differences between the categorical variables and the continuous variables, separately. The Kaplan‐Meier (KM) method and the log‐rank test were applied to assess the association between NAFLD and the outcomes. Cox regression was conducted to further evaluate the effect of NAFLD after adjusting for other risk factors. All analyses were performed using IBM SPSS Statistics version 26.0 software.
3. RESULTS
In this study, we retrospectively collected the data of 230 adult patients who had contracted laboratory‐confirmed COVID‐19 before 14 March 2020, in the Public Health Treatment Center of Changsha, China. Of these patients, 218 cases had undergone abdominal ultrasound examinations and did not display the presence of excessive alcohol consumption (Figure 1). A total of 86 patients (39.4%) had been diagnosed with NAFLD, which presented a higher incidence of NAFLD than what has been reported in the general population of China. Of the NAFLD patients, 52 (60.5%) were males. Compared with the non‐NAFLD group, patients with NAFLD had a higher BMI (24.8 kg/m2 [18.7–37.2] vs 21.8 kg/m2 [11.7–29.3], p = 0.000) and higher proportions of hypertension (23.3% vs 9.1%, p = 0.004) and diabetes (10.5% vs 3.8%, p = 0.049). However, no significant differences were found in other comorbidities (including cardiovascular disease and chronic liver disease) and common COVID‐19 symptoms (including fever, cough, fatigue and expectoration). Two of the patients (1.5%) died, while the remaining 216 patients were discharged before 14 March 2020 (Table 1). The two patients who died did not suffer from NAFLD.
FIGURE 1.
Flow chart of the study population
TABLE 1.
Baseline Characteristics and laboratory findings on admission of COVID‐19 patients with and without NAFLD
| NAFLD (n = 86) | Non‐NAFLD (n= 132) | p value | |
|---|---|---|---|
| Sex (male/female) | 52/34 | 58/74 | 0.017 |
| Age, y, median (range) | 46 (19–76) | 45 (21–84) | 0.895 |
| BMI, kg/m2, median (range) | 24.8 (18.7–37.2) | 21.8 (11.7–29.3) | 0.000 |
| Hypertension (n, %) | 20 (23.3%) | 12 (9.1%) | 0.004 |
| CVD (n, %) | 3 (3.5%) | 5 (3.8%) | 1.000 |
| Diabetes (n, %) | 9 (10.5%) | 5 (3.8%) | 0.049 |
| Chronic liver disease (n, %) | 5 (5.8%) | 6 (4.5%) | 0.919 |
| Fever (n, %) | 66 (76.7%) | 97 (73.5%) | 0.588 |
| Cough (n, %) | 75 (87.2%) | 104 (78.8%) | 0.113 |
| Fatigue (n, %) | 39 (45.3%) | 61 (46.2%) | 0.901 |
| Expectoration (n, %) | 38 (44.2%) | 61 (46.2%) | 0.769 |
| WBC, x109/L, median (range) | 5.0 (1.9–13.4) | 4.3 (0.8–10.4) | 0.008 |
| Lys count, x109/L, median (range) | 1.1 (0.4–3.7) | 1.1 (0.1–3.1) | 0.344 |
| ALT, U/L, median (range) | 26.2 (11.3–93.7) | 16.8 (2.6–69.4) | 0.000 |
| AST, U/L, median (range) | 25.7 (2.0–78.8) | 23.3 (12.3–82.1) | 0.006 |
| Total bilirubin, μmol/L, medium (range) | 11.8 (4.3–38.2) | 10.3 (4.0–162.1) | 0.105 |
| Creatinine, μmol/L, medium (range) | 53.7 (28.6–105.1) | 50.5 (20.6–255.7) | 0.256 |
| Creatine kinase, U/L, medium (range) | 85.5 (11.3–986.4) | 66.5 (22.7–646.0) | 0.002 |
| Creatine kinase‐MB, U/L, medium (range) | 9.4 (0.4–82.8) | 9.6 (0.3–221.7) | 0.953 |
| Total cholesterol, mg/dl, medium (range) | 3.9 (2.3–6.9) | 3.6 (1.9–6.4) | 0.018 |
| LDL cholesterol, mg/dl, medium (range) | 2.7 (1.5–5.9) | 2.5 (1.4–4.9) | 0.006 |
| HDL cholesterol, mg/dl, medium (range) | 0.7 (0.5–1.2) | 0.8 (0.2–1.6) | 0.000 |
| Triglyceride, mg/dl, medium (range) | 1.4 (0.9–6.4) | 0.9 (0.1–2.7) | 0.000 |
| CRP, mg/L, median (range) | 21.3 (0.4–94.9) | 13.1 (0.1–101.9) | 0.012 |
| PCT, ≥0.05 ng/mL (n, %) | 31 (36.0%) | 29 (22.0%) | 0.023 |
| Chest CT positive rate (n, %) | 82 (95.3%) | 125 (94.7%) | 0.971 |
| Chest CT with ground‐glass change | 50 (58.1%) | 50 (37.9%) | 0.003 |
| Severe events (n, %) | 19 (22.1%) | 22 (16.7%) | 0.316 |
| Virus shedding time (days) | 17 (3–47) | 18 (6–53) | 0.165 |
| Length of hospital stay (days) | 15 (5–41) | 16 (5–40) | 0.407 |
| Mortality (n, %) | 0 (0%) | 2 (1.5%) | 0.251 |
Abbreviations: NAFLD, non‐alcoholic fatty liver disease; y, years; CVD, cardiovascular disease; WBC, white blood cell count; Lys, lymphocyte; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C reactive protein; PCT, procalcitonin.
In terms of laboratory findings, considerably higher levels of white blood cells (5.0 x 109/L [1.9–13.4] vs 4.3 x 109/L [0.8–10.4]; p = 0.008), alanine aminotransferase (26.2 U/L [11.3–93.7] vs 16.8 U/L [2.6–69.4]; p = 0.000), aspartate aminotransferase (25.7 U/L [2.0–78.8] vs 23.3 U/L [12.3–82.1]; p = 0.006), creatine kinase (85.5 U/L [11.3–986.4] vs 66.5 U/L [22.7–646.0], p = 0.002) and C reactive protein (21.3 mg/L [0.4–94.9] vs 13.1 mg/L [0.1–101.9]; p = 0.012) were detected in the patients with NAFLD than in those without NAFLD. Meanwhile, the proportions of procalcitonin positive subjects (36.0% vs 22.0%, p = 0.023) and the incidence of ground‐glass opacity obtained from chest CTs (58.1% vs 37.9%, p = 0.003) were significantly higher in the NAFLD group than in the non‐NAFLD group (Table 1). This may suggest that patients with NAFLD had a higher inflammatory response to SARS‐CoV‐2 infection and a higher incidence of liver injury. Moreover, significantly differences were observed in the serum levels of lipid metabolism, including total cholesterol (3.9 mg/dL [2.3–6.9] vs 3.6 mg/dL [1.9–6.4], p = 0.018), low‐density lipoprotein (LDL) cholesterol (2.7 mg/dL [1.5–5.9] vs 2.5 mg/dL [1.4–4.9], p = 0.006), high‐density lipoprotein (HDL) cholesterol (0.7 mg/dL [0.5–1.2] vs 0.8 mg/dL [0.2–1.6], p = 0.000) and triglyceride (1.4 mg/dL [0.9–6.4] vs 0.9 mg/dL [0.1–2.7], p = 0.000; Table 1).
With regard to the outcomes, there was no significant difference between NAFLD and non‐NAFLD patients, including the severe events (22.1% vs 16.7%, p = 0.316), virus shedding time (17 days [3–47] vs 18 days [6–53], p = 0.165), length of hospital stay (15 days [5–41] vs 16 days [5–40], p = 0.407) and mortality (0% vs 1.5%, p = 0.251).
Next, to investigate whether the effect of NAFLD on the outcomes of COVID‐19 patients was dependent on gender, age, BMI or comorbidities, we divided our patients into subgroups according to these factors. We found that in patients with normal BMI (18.5 ≤ BMI <24 kg/m2), NAFLD patients were more likely to develop severe events than those without NAFLD (30.0% vs 11.5%, p = 0.021). However, no significant differences were found in patients with low (< 18.5 kg/m2) or high (≥ 24 kg/m2) BMI values (Table 2). Regarding laboratory parameters, NAFLD patients in the normal BMI group had significantly higher levels of alanine aminotransferase (26.2 U/L [12.1–93.7] vs 15.8 U/L [4.9–58.4], p = 0.000), aspartate aminotransferase (25.7 U/L [11.5–58.5] vs 22.6 U/L [12.6–69.0], p = 0.009), total bilirubin (12.6 μmol/L [6.9–30.1] vs 9.7 μmol/L [4.0–26.1], p = 0.010), C reactive protein (20.8 mg/L [1.0–94.9] vs 11.1 mg/L [0.1–101.9], p = 0.012) and triglyceride (1.4 mg/dL [0.9–6.1] vs 0.9 mg/dL [0.1–2.7], p = 0.000). Conversely, they had slightly lower HDL cholesterol levels (0.8 mg/dL [0.6–1.2] vs 0.9 mg/dL [0.5–1.6], p = 0.008) than those without NAFLD (Table 3). Thus, a KM analysis was performed and confirmed that NAFLD patients were more likely to experience a severe event (log‐rank p = 0.017; Figure 2). After adjusting for gender, age, hypertension, cardiovascular disease, diabetes and chronic liver disease, NAFLD is significantly correlated with the severity of COVID‐19 (HR =3.26, 95% CI: 1.17–9.04, p = 0.023; Table 4) in the normal BMI group.
TABLE 2.
Influence of NAFLD on the severe event in subgroups of COVID‐19 patients according to gender, age, BMI and comorbidities
| Severe event (n, %) | p value | Virus shedding time (days) | p value | Length of hospital stay (days) | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
|
NAFLD (n=86) |
Non‐NAFLD (n = 132) |
NAFLD (n=86) |
Non‐NAFLD (n = 130) |
NAFLD (n=86) |
Non‐NAFLD (n = 130) |
||||
| Gender | |||||||||
| Male | 12 (23.1%) | 13 (22.4%) | 0.934 | 17 (6–47) | 17.5 (6–42) | 0.696 | 14 (5–41) | 17 (5–37) | 0.689 |
| Female | 7 (20.6%) | 9 (12.2%) | 0.252 | 17.5 (1–38) | 19 (5–53) | 0.131 | 17 (5–37) | 16 (5–40) | 0.569 |
| Age | |||||||||
| Elderly (≥ 60 years) | 5 (25%) | 13 (35.1%) | 0.432 | 16 (4–38) | 23 (11–43) | 0.011 | 17 (6–37) | 21 (6–40) | 0.155 |
| Non‐elderly (< 60years) | 14 (21.2%) | 9 (10.5%) | 0.036 | 17.5 (3–47) | 17 (6–53) | 0.975 | 15 (5–41) | 15 (5–40) | 0.986 |
| BMI | |||||||||
| BMI ≥24 kg/m2 | 7 (14.6%) | 7 (23.3%) | 0.327 | 18.5 (8–47) | 21 (6–45) | 0.595 | 15 (5–41) | 17 (6–40) | 0.643 |
| 18.5 ≤ BMI <24 kg/m2 | 9 (30%) | 9 (11.5%) | 0.021 | 17 (3–38) | 17 (8–53) | 0.939 | 16.5 (5–37) | 16 (5–37) | 0.697 |
| BMI <18.5 kg/m2 | NA | 3 (21.4%) | NA | NA | 18.5 (8–40) | NA | NA | 14.5 (8–40) | NA |
| Hypertension | |||||||||
| Yes | 9 (45%) | 4 (33.3%) | 0.780 | 16.5 (11–45) | 18 (12–43) | 0.366 | 16.5 (5–41) | 21 (8–35) | 0.387 |
| No | 10 (15.2%) | 18 (15.0%) | 0.978 | 17.5 (3–47) | 18.5 (6–53) | 0.138 | 16.5 (11–45) | 18 (12–43) | 0.348 |
| Cardiovascular disease | |||||||||
| Yes | 2 (66.7%) | 2 (40.0%) | 1.000 | 21 (19–29) | 35 (22–29) | 0.114 | 22 (19–27) | 24.5 (14–35) | 0.857 |
| No | 17 (20.5%) | 20 (15.7%) | 0.379 | 17 (3–47) | 18 (6–53) | 0.205 | 15 (5–41) | 16 (5–40) | 0.411 |
| Diabetes | |||||||||
| Yes | 2 (22.2%) | 2 (40.0%) | 0.580 | 15 (9–29) | 27 (15–43) | 0.112 | 15 (6–30) | 28 (17–32) | 0.060 |
| No | 17 (22.1%) | 20 (15.7) | 0.255 | 17 (3–47) | 18 (6–53) | 0.259 | 15 (5–41) | 16 (5–40) | 0.560 |
| Chronic liver disease | |||||||||
| Yes | 2 (40.0%) | 1 (16.7%) | 0.545 | 16 (14–29) | 24 (11–34) | 0.537 | 13 (9–27) | 22 (8–36) | 0.662 |
| No | 17 (21.0%) | 21 (16.7%) | 0.433 | 17 (3–47) | 18 (6–53) | 0.188 | 15 (5–41) | 15 (5–40) | 0.496 |
Abbreviations: NAFLD, Non‐alcohol fatty liver disease; BMI, Body mass index; NA, Not available.
TABLE 3.
Baseline Characteristics and laboratory findings on admission of COVID‐19 patients of normal BMI with and without NAFLD
| NAFLD (n = 30) | Non‐NAFLD (n=78) | p value | |
|---|---|---|---|
| Sex (male/female) | 15/15 | 45/33 | 0.471 |
| Age, y, median (range) | 47 (27–72) | 43 (21–84) | 0.929 |
| Hypertension (n, %) | 8 (26.7%) | 9 (11.5%) | 0.101 |
| CVD (n, %) | 2 (6.7%) | 3 (3.8%) | 0.616 |
| Diabetes (n, %) | 3 (10.0%) | 4 (5.1%) | 0.628 |
| Chronic liver disease (n, %) | 3 (10.0%) | 4 (5.1%) | 0.628 |
| Fever (n, %) | 25 (83.3%) | 56 (71.8%) | 0.173 |
| Cough (n, %) | 26 (86.7%) | 55 (70.5%) | 0.082 |
| Fatigue (n, %) | 18 (60.0%) | 34 (43.6%) | 0.126 |
| Expectoration (n, %) | 16 (53.3%) | 27 (34.6%) | 0.075 |
| WBC, x109/L, median (range) | 4.6 (2.3–9.35) | 4.5 (0.8–10.4) | 0.384 |
| Lys count, x109/L, median (range) | 1.0 (0.4–2.7) | 1.2 (0.1–3.1) | 0.131 |
| ALT, U/L, median (range) | 26.2 (12.1–93.7) | 15.8 (4.9–58.4) | 0.000 |
| AST, U/L, median (range) | 25.7 (11.5–58.5) | 22.6 (12.6–69.0) | 0.009 |
| Total bilirubin, μmol/L, medium (range) | 12.6 (6.9–30.1) | 9.7 (4.0–26.1) | 0.010 |
| Creatinine, μmol/L, medium (range) | 52.0 (30.0–83.3) | 50.9 (20.6–213.9) | 0.970 |
| Creatine kinase, U/L, medium (range) | 104.7 (17.4–513.3) | 68.4 (22.7–449.5) | 0.054 |
| Creatine kinase‐MB, U/L, medium (range) | 11.2 (1.1–34.1) | 9.7 (0.3–35.2) | 0.259 |
| CRP, mg/L, median (range) | 20.8 (1.0–94.9) | 11.1 (0.1–101.9) | 0.012 |
| Total cholesterol, mg/dL, medium (range) | 3.9 (2.5–6.9) | 3.8 (2.5–5.9) | 0.380 |
| LDL cholesterol, mg/dL, medium (range) | 2.7 (1.6–5.9) | 2.6 (1.4–4.4) | 0.344 |
| HDL cholesterol, mg/dL, medium (range) | 0.8 (0.6–1.2) | 0.9 (0.5–1.6) | 0.008 |
| Triglyceride, mg/dL, medium (range) | 1.4 (0.9–6.1) | 0.9 (0.1–2.7) | 0.000 |
| PCT, ≥0.05 ng/mL (n, %) | 9 (30.0%) | 16 (20.5%) | 0.295 |
| Chest CT positive rate (n, %) | 29 (96.7%) | 74 (94.9%) | 1.000 |
| Chest CT with ground‐glass change | 18 (60%) | 31 (39.7%) | 0.058 |
| Virus shedding time (days) | 17 (3–38) | 17 (6–53) | 0.989 |
| Length of hospital stay (days) | 16.5 (5–37) | 16 (5–37) | 0.695 |
Abbreviations: NAFLD, non‐alcoholic fatty liver disease; y, years; CVD, cardiovascular disease; WBC, white blood cell count; Lys, lymphocyte; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C reactive protein; PCT, procalcitonin.
FIGURE 2.
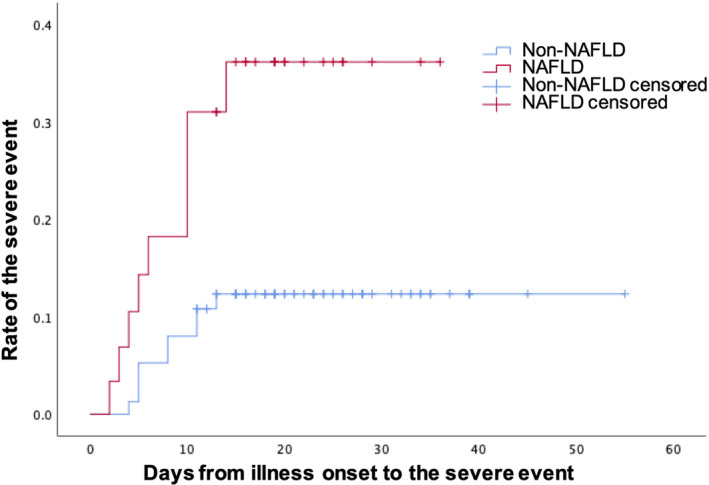
Time‐dependent risk of suffering a severe event between COVID‐19 patients of normal BMI with non‐alcoholic fatty liver disease (NAFLD) and without NAFLD
TABLE 4.
Multivariate analysis of factors related to severe events of COVID‐19 patients with normal body mass index using the COX regression model
| HR (hazard ratio) | 95% confidence interval | p value | |
|---|---|---|---|
| NAFLD | 3.26 | 1.17, 9.04 | 0.023 |
| Gender | 1.91 | 0.71, 5.20 | 0.203 |
| Age | 1.04 | 1.00, 1.08 | 0.049 |
| Hypertension | 1.12 | 0.29, 4.32 | 0.873 |
| Cardiovascular disease | 0.36 | 0.07, 1.85 | 0.220 |
| Diabetes | 0.72 | 0.15, 3.44 | 0.678 |
| Chronic liver disease | 0.90 | 0.14, 5.69 | 0.913 |
Abbreviation: NAFLD, non‐alcoholic fatty liver disease.
Moreover, the results showed that in non‐elderly patients (< 60 years), those with NAFLD had a higher incidence of severe events (21.2% vs 10.5%, p = 0.036; Table 2). Subsequently, a KM analysis confirmed that non‐elderly NAFLD patients had a significantly elevated risk of suffering a severe event compared with those without NAFLD (log‐rank p = 0.035; Figure S1). However, after adjusting for gender, hypertension, cardiovascular disease, diabetes and chronic liver disease, NAFLD had no significant influence on the likelihood of the severe events in non‐elderly patients (HR =1.79, 95% CI: 0.72–4.44, p = 0.208; Table S2).
No significant effects of NAFLD were found on the virus shedding time or length of hospital stay in the patient cohort. Thus, we performed subgroup analyses according to gender, age, BMI and the common comorbidities. Results showed that in elderly patients, the virus shedding time was significantly lower in NAFLD patients than in non‐NAFLD individuals (16 days [4–38] vs 23 days [11–43], p = 0.011; Table 2). In terms of laboratory findings among elderly patients, alanine aminotransferase was conspicuously higher in patients with NAFLD, compared to those without (25.0 U/L [12.8–58.1] vs 15.6 U/L [4.9–69.4], p = 0.002; Table S1).
4. DISCUSSION
This study investigated the susceptibility of patients with NAFLD to SARS‐CoV‐2 and the association between NAFLD and outcomes of COVID‐19. We found that individuals with NAFLD may be more susceptible to SARS‐CoV‐2 than those without NAFLD. In patients with normal BMI, those with NAFLD who were infected by COVID‐19 may have a higher proportion of developing severe diseases than other patients.
In our study, 39.4% of the COVID‐19 patients we investigated had NAFLD, which is significantly higher than the rate reported in the general population. 12 , 13 , 14 , 15 This finding is consistent with another study that was done in China. 21 It suggests that NAFLD sufferers generally seem to be more susceptible to SARS‐CoV‐2 infection than people without NAFLD. Although the reason for this is not yet known, immune system disorders in NALFD may be an important cause, such as a decrease in CD4 + T cells and abnormal macrophage function. 22 , 23
NAFLD often coexists with hyperlipidaemia and obesity. 17 , 18 People with NAFLD usually have a high incidence of hypertension, diabetes and cardiovascular disease. All of these conditions have been demonstrated to be independent risk factors of COVID‐19. 24 In this study, compared with the non‐NAFLD patients, the proportions of hypertension and diabetes were also significantly higher in the NAFLD group, which is similar to the conclusions of previous studies.
Despite no evidence of increased liver uptake of SARS‐CoV‐2 in NAFLD patients, 25 in our study, the COVID‐19 cases with NAFLD had higher levels of alanine aminotransferase and aspartate aminotransferase than the other patients. Moreover, patients with abnormal liver tests are at a higher risk of developing to the severe events. 26 It has been reported that patients with NAFLD have a higher risk of disease progression, 21 while NAFLD patients with an increased fibrosis‐4 index and NAFLD fibrosis score are at a higher risk of suffering a severe event due to COVID‐19. 27 However, the influence of NAFLD on the COVID‐19 in subgroups of age, gender, BMI and comorbidities remains unclear.
The prevalence and severity of NAFLD vary between different age groups. 15 , 28 A large sample study in China found that, compared to non‐elderly adults (18–59 years), the elderly (≥ 60 years) showed a lower overall prevalence of NAFLD, but a higher proportion of NAFLD accompanied with diabetes, hypertension and hyperlipidaemia. 29 Elderly NAFLD patients are more likely to develop non‐alcoholic steatohepatitis and fibrosis than non‐elderly patients. 30 Moreover, NAFLD is considered to be associated with increased mortality in elderly people. 31 Interestingly, in the non‐elderly group of our study, NAFLD is significantly associated with the severity of COVID‐19. After adjusting for gender, hypertension and BMI, the difference in frequency of the severe events becomes statistically insignificant, which may be a result of both the high incidence of comorbidities and the small sample size.
The susceptibility to COVID‐19 of NAFLD sufferers increases linearly with BMI. It increases 5‐ to 10‐fold in obese patients and by a factor of 10‐ to 14‐fold in the morbidly obese. 32 However, a significant proportion of NAFLD patients have relatively normal BMI, and it has been reported that obese NAFLD has different clinical characteristics from non‐obese NAFLD. 33 Therefore, in this study, we performed a subgroup analysis on BMI and found that in patients with a normal BMI (18.5–24 kg/m2), NAFLD was associated with an increased risk of severe disease. However, we did not find similar results in patients with higher and lower BMI, which may be related to their different metabolic states. This is an interesting result, but well‐designed studies with larger sample sizes are required to investigate this assumption.
This study contains several limitations. Firstly, we analysed the effect of NAFLD on the susceptibility and outcome of COVID‐19 in one of the nearest provincial capitals to Wuhan, but we did not have the data on the prevalence of NAFLD in Changsha, China. Also, in most of the subgroups that were created according to the common comorbidities, no significant differences were found. However, the results did show certain trends. Univariate analysis indicated that non‐elderly NAFLD patients had a higher incidence of severe events. Nevertheless, after adjusting for gender, hypertension and BMI, the difference became statistically insignificant. This may be because of the small sample size and the high incidence of comorbidities. Another limitation is that in the subgroup analysis on BMI, we only divided our patients into three groups. Overweight and obese patients were pooled together in one group (BMI ≥24 kg/m2), due to the small number of obese patients (BMI ≥28 kg/m2). Finally, as this study was retrospective, data regarding abdominal adiposity was not available. Therefore, well‐designed studies with larger sample sizes are still needed to more clearly demonstrate the association between NAFLD and the outcomes of COVID‐19.
In summary, people with NAFLD may have a higher proportion of SARS‐CoV‐2 infection, with a higher rate of liver injury, as well as a higher incidence of severe COVID‐19 in normal‐weight patients. People who have NAFLD may need stronger protection and more aggressive treatment to decrease the risk of infection, reduce the chance of liver injury and improve the outcome. However, due to the small sample size, especially for elderly patients, further studies with a larger sample size are required to confirm these findings.
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interest.
AUTHOR CONTRIBUTION
Guyi Wang: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Project administration (equal); Resources (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Shangjie Wu: Funding acquisition (equal); Investigation (equal); Supervision (equal); Validation (equal); Writing‐review & editing (equal). Chenfang Wu: Data curation (equal); Investigation (equal); Supervision (equal); Writing‐review & editing (equal). Quan Zhang: Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Resources (equal). Fang Wu: Formal analysis (equal); Investigation (equal); Supervision (equal); Writing‐review & editing (equal). Bo Yu: Conceptualization (equal); Data curation (equal); Methodology (equal). Siye Zhang: Data curation (equal); Formal analysis (equal); Methodology (equal). Chao Wu: Data curation (equal); Formal analysis (equal); Writing‐original draft (equal). Guobao Wu: Supervision (equal); Writing‐review & editing (equal). Yanjun Zhong: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (equal); Resources (equal); Supervision (equal); Validation (equal); Visualization (equal); Writing‐original draft (equal); Writing‐review & editing (equal).
Supporting information
Fig S1
Table S1
Table S2
ACKNOWLEDGEMENT
Not available.
Wang G, Wu S, Wu C, et al. Association between non‐alcoholic fatty liver disease with the susceptibility and outcome of COVID‐19: A retrospective study. J Cell Mol Med. 2021;25:11212–11220. 10.1111/jcmm.17042
Funding information
Emergency project for COVID‐19 prevention and control of Central South University, China (No 160260005)
REFERENCES
- 1. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92(4):401‐402. 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hui DS, I Azhar E, Madani TA, et al. The continuing 2019‐nCoV epidemic threat of novel coronaviruses to global health — The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264‐266. 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061– 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoon SH, Lee KH, Kim JY, et al. Chest Radiographic and CT Findings of the 2019 Novel Coronavirus Disease (COVID‐19): Analysis of Nine Patients Treated in Korea. Korean J Radiol. 2020;21(4):494‐ 10.3348/kjr.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Albarello F, Pianura E, Di Stefano F, et al. 2019‐novel Coronavirus severe adult respiratory distress syndrome in two cases in Italy: An uncommon radiological presentation. Int J Infect Dis. 2020;93:192‐197. 10.1016/j.ijid.2020.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1,590 patients with COVID‐19 in China: A Nationwide Analysis. medRxiv. 2020.10.1101/2020.02.25.20027664v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang C, Shi L, Wang FS. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428‐430. 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li ZZ, Xue J, Chen P, et al. Prevalence of nonalcoholic fatty liver disease in mainland of China: A meta‐analysis of published studies. J Gastroenterol Hepatol. 2014;29(1):42‐51. 10.1111/jgh.12428. [DOI] [PubMed] [Google Scholar]
- 13. Fan JG, Farrell GC. Epidemiology of non‐alcoholic fatty liver disease in China. J Hepatol. 2009;50(1):204‐210. 10.1016/j.jhep.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 14. Fan JG. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol. 2013;28(Suppl 1):11‐17. 10.1111/jgh.12036. [DOI] [PubMed] [Google Scholar]
- 15. Zhou J, Zhou F, Wang W, et al. Epidemiological feature of NAFLD from 1999 to 2018 in China. Hepatology. 2020. [DOI] [PubMed] [Google Scholar]
- 16. Sarwar R, Pierce N, Koppe S. Obesity and nonalcoholic fatty liver disease: current perspectives. Diabetes Metab Syndr Obes. 2018;11:533‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 2019;92:82‐97. 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 18. Alves‐Bezerra M, Cohen DE. Triglyceride Metabolism in the Liver. Compr Physiol. 2017;8:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu EL, Golshan S, Harlow KE, et al. Prevalence of Nonalcoholic Fatty Liver Disease in Children with Obesity. J Pediatr. 2019;207:64‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National health commission, National administration of traditional Chinese medicine. Diagnosis and treatment of new coronavirus pneumonia (trial sixth edition). Chinese Journal of Viral Diseases. 2020; 10: 1‐5.
- 21. Ji D, Qin E, Xu J, et al. Non‐alcoholic fatty liver diseases in patients with COVID‐19: A retrospective study. J Hepatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma C, Kesarwala AH, Eggert T, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:253‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oates JR, McKell MC, Moreno‐Fernandez ME, et al. Macrophage Function in the Pathogenesis of Non‐alcoholic Fatty Liver Disease: The Mac Attack. Front Immunol. 2019;10:2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guan WJ, Liang WH, Zhao Y, et al. China Medical Treatment Expert Group for C. Comorbidity and its impact on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J. 2020;55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Biquard L, Valla D, Rautou PE. No evidence for an increased liver uptake of SARS‐CoV‐2 in metabolic associated fatty liver disease. J Hepatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cai Q, Huang D, Yu H, et al. COVID‐19: Abnormal liver function tests. J Hepatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Targher G, Mantovani A, Byrne CD, et al. Risk of severe illness from COVID‐19 in patients with metabolic dysfunction‐associated fatty liver disease and increased fibrosis scores. Gut. 2020. [DOI] [PubMed] [Google Scholar]
- 28. Gan L, Chitturi S, Farrell GC. Mechanisms and implications of age‐related changes in the liver: nonalcoholic Fatty liver disease in the elderly. Curr Gerontol Geriatr Res. 2011;2011: 831536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cai MJ, Kong XN, Zhao XY. Influences of Gender and Age on the Prevalence and Complications of Nonalcoholic Fatty Liver Disease. Zhongguo Yi Xue Ke Xue Yuan Xue Bao Acta Academiae Medicinae Sinicae. 2017;39:499‐505. [DOI] [PubMed] [Google Scholar]
- 30. Noureddin M, Yates KP, Vaughn IA, et al. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology. 2013;58:1644‐1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Golabi P, Paik J, Reddy R, Bugianesi E, Trimble G, Younossi ZM. Prevalence and long‐term outcomes of non‐alcoholic fatty liver disease among elderly individuals from the United States. BMC Gastroenterol. 2019;19:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loomis AK, Kabadi S, Preiss D, et al. Body Mass Index and Risk of Nonalcoholic Fatty Liver Disease: Two Electronic Health Record Prospective Studies. J Clin Endocrinol Metab. 2016;101:945‐952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862‐873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1
Table S2


