Abstract
Gulf war illness (GWI) is a chronic disorder of unknown etiology characterized by multiple symptoms such as pain, fatigue, gastrointestinal disturbances and neurocognitive problems. Increasing evidence suggests that gut microbiome perturbations play a key role in the pathology of this disorder. GWI courses with gut microbiota alterations and their metabolites (e.g. short chain fatty acids -SCFA-), which can be aggravated by lifestyle risk factors such as a high fat diet (HF). To investigate the causative role of the gut microbiome, non-absorbable antibiotics (Abx) were administered to mice treated with GWI agents and concomitantly fed with a HF. In light of the wide use of Abx as pseudo-germ-free models, we evaluated the effects of Abx exposure on GWI and HF on body weight, food intake, gut microbiota changes and levels of the SCFA acetate. Results show that HF decreased food intake while increasing body weight in both controls and GWI. Exposure to Abx prevented these HF effects by offsetting the body weight gain in GWI. GWI and HF led to decreases in α-diversity, disruptions in the composition and structure of the gut bacterial community and decreases in acetate levels. This Abx-induced remodeling of the gut microbiome was characterized by an expansion of Proteobacteria, decreases in Bacteroidetes and Firmicutes, and overall increases in acetate levels, as well as by the proliferation of potential pathobionts. Therefore, the use of Abx may not represent a dependable approach to deplete the gut microbiome and its advantages as a pseudo germ-free model warrant further investigation.
Keywords: Gulf war illness, High fat diet, Antibiotics, Gut microbiome, Body weight
1. Introduction
Gulf war illness (GWI) is a chronic multisymptomatic disorder of unknown etiology characterized by a variety of symptoms such as pain, fatigue, gastrointestinal disturbances and neurocognitive problems [1]. The lack of widely accepted outcome measures complicates the study of GWI and highlights the need for identification of therapeutic targets. The influence of microbiota on host physiology has gained attention in the context of GWI and an increasing number of studies point to perturbations in the gut microbiome as key players in the pathology of this disorder [2-6]. Preclinical studies in rodents found that exposure to GWI agents caused a gut microbiome disruption, termed dysbiosis, that produced a significant decrease in intestinal tight junction proteins, leading to a leaky gut, activation of enteric glial cells, inflammation and endotoxemia [3,7,8]. In humans, a recent pilot study reported significant gut bacteria alterations in Veterans diagnosed with this disorder [9], with these gut microbiome differences extending to those individuals with GWI who also presented with gastrointestinal disturbances compared to those who did not display intestinal symptoms [9]. Dietary regimens, including those rich in fat and carbohydrates are among the most influential environmental factors with the capacity to induce gut microbiome dysbiosis [10,11]. In this sense, a cross-sectional survey conducted in a cohort of 15,000 GWI Veterans reported that nearly 50% were overweight and about 30% were obese [12], with those being obese being more prone to develop other chronic health conditions such as post-traumatic stress disorder [12]. Reports in mice have shown that although treatment with GWI agents per se does not lead to being overweight, consumption of a high fat diet (HF) post-GWI agent exposure leads to a significant increase in body weight [2] and metabolic alterations [5]. These outcomes are associated with a gut microbiome dysbiosis characterized by a reduction in bacteria which produce short chain fatty acids (SCFAs) [5]. SCFAs (e.g. acetate, propionate and butyrate) are produced via fermentation of indigestible dietary carbohydrates and fiber by the gut microbiota [13]. It has been shown that an exogenous application of SCFAs can prevent weight gain in HF-induced obese mice and overweight humans [14,15]. Given that the microbiota-related alterations in GWI can be aggravated by HF, we sought to evaluate the causative role of the gut microbiome. In an attempt to remove the intestinal microbiota as a regulatory mechanism, a cocktail of non-absorbable antibiotics (Abx) was administered to mice treated with GWI agents and concomitantly fed a HF. Abx have been widely used to generate pseudo-germ-free rodents as an alternative model for proof-of-principle studies [16], and in the present work, the effects of Abx exposure were evaluated on GWI and HF on body weight, food intake, gut microbiota remodeling and acetate levels.
2. Materials and methods
2.1. Subjects, Gulf war illness agents, diets and antibiotics
The present study employed an extensively validated GWI model and administration of a high fat diet (HF) as previously described [2]. Briefly, 2–4 male C57BL6/J mice (8 weeks of age purchased from Envigo) were housed per cage in a room with constant temperature and humidity and with alternating 12 h periods of light and darkness. All mice used in these studies were from the same cohort and assignment to treatment groups was random, with at least 2 cages per group to avoid cage effects. Half of the mice were injected with 50 μl of GWI agents in final doses of 0.7 mg/kg of pyridostigmine bromide (PB) and 200 mg/kg of permethrin (PER) solubilized in dimethyl sulfoxide (DMSO) to a final DMSO concentration of 3% just prior to intraperitoneal injection. The other half served as controls and received intraperitoneal injections of 3% DMSO in sterile physiological saline. Injections were administered once daily for 10 days. During treatment with GWI agents, mice were given ad libitum access to water and standard rodent laboratory chow ((ND); LabDiet 5001 containing 28.5% protein, 13.5% fat, and 58% carbohydrates). On the last day of treatment, the GWI and control groups were split into 4 same sized groups (N = 6–7 mice per group) and fed the following diet regimens: two groups on a ND and two groups on a HF (D12451, Research Diets with 20% protein, 45% fat, and 35% carbohydrates). Sample sizes were based on our previous study showing an effect of this HF diet on GWI-treated mice [2]. An antibiotics cocktail (Abx) or vehicle solution was administered in the drinking water simultaneously to the two diets for 21 days. Fresh solutions were prepared every other day and administered in graduated glass bottles (Braintree Scientific) containing sipper tubes with ball bearings to minimize loss of fluid to drippage. This cocktail consisted of ampicillin trihydrate (0.25 g/ml), neomycin trisulfate (0.25 g/ml), metronidazole (0.25 g/ml), and vancomycin hydrochloride (0.125 g/ml), all purchased from Sigma Aldrich, in 3% sucrose to encourage drinking. These are broad-spectrum antibiotics without systemic effects due to their poor absorption (neomycin, is not absorbed at all). Control mice received only a 3% sucrose solution, which was found to have no impact on the gut microbiome (see Supplementary Fig. 1 data). Hereafter, the groups are referred to as Con_ND −Abx, Con_ND +Abx, Con_HF −Abx, and Con_HF +Abx for controls and GWI_ND −Abx, GWI_ND +Abx, GWI_HF −Abx, and GWI_HF +Abx for PER + PB treated mice.
Food and fluids intake, as well as body weights were recorded every 2–3 days throughout the experiment. Fluid intake was determined by weighing each bottle at the start of the test period and subtracting their weights after 24 h. Consumption for each mouse was normalized to body weight and presented as g of consumed food or fluid/ g of body weight/ 24 h period. Mice were sacrificed by decapitation and the contents of the caecum were harvested and frozen at −80 °C. The Institutional Care and Use Committee of Wayne State University approved the animal care and experimental procedures (IACUC 17-08-0307). All procedures were also in compliance with the NIH Guide for the Care and Use of Laboratory Animals, with ARRIVE guidelines and under IACUC-approved protocols.
2.2. Gut microbiome analysis
16S rRNA genes in the caecum were sequenced as reported previously [2,17]. In brief, bacterial DNA was extracted and purified using the QIAamp PowerFecal DNA Kit. The V4 hypervariable region of the bacterial 16S rRNA gene was amplified using dual indexed, Illumina compatible primers and the library was loaded onto an Illumina MiSeq standard V2 flow cell for sequencing in a 2 × 250 bp paired end format. The raw 16S rRNA gene sequences from the paired fastq files were processed with the Divisive Amplicon Denoising Algorithm (DADA2) pipeline (v 1.12.1) to obtain merged, denoised, chimera-free, inferred amplicon sequence variants (ASVs) suitable to identify fine-scale variation [18]. ASVs were defined by 100% sequence similarity, and analyzed using DADA2 in R (v 3.6.2), according to the online MiSeq protocol (https://benjjneb.github.io/dada2/tutorial.html), with some modifications that included truncation lengths of 240 bp and 160 bp and a maximum number of expected errors of 2 bp for forward reads and 5 bp for reverse reads. Sequences were classified using the “silva_nr_v132_train_set” database after removal of sequences derived from Archaea, Chloroplast, or Eukaryota as previously described [19]. ASVs count were calculated for each group. Gut microbiome α-diversity was characterized using the Chao1 (i.e. community richness), Shannon and Simpson (1-D) (i.e. community heterogeneity) indices, and data were thereafter visualized and statistically analyzed with GraphPad Prism (v 9.1). Microbial β-diversity was assessed using the Jaccard (i.e. shared composition) and Bray-Curtis (i.e. shared structure) indices based on ASV relative abundance data in R. High-dimensional class comparisons were carried out with LEfSe in an on-line interface [20], using default parameters with the exception that the LDA score was set to 3.6. Taxonomic classifications of ASVs from the analyses at taxonomic level below phylum with differential abundance within groups were made using the Basic Local Alignment Search Tool (BLAST) [21]. Heat maps were generated using MetaboAnalyst 5.0 [22].
2.3. Acetate measurements
Quantification of the SCFA acetate was assessed in caecum samples by using a colorimetric assay kit (Sigma, MAK086), according to the manufacturer's specifications. In this test, acetate concentration is determined by a coupled enzyme assay, which results in a colorimetric (450 nm) product proportional to the acetate present.
2.4. Data analysis
Food intake was analyzed with two-way ANOVA followed by Tukey's post hoc tests using GraphPad Prism (v9) for Windows (GraphPad Software, La Jolla, CA, USA, www.graphpad.com). Due to munching behavior unrelated to the treatment (it was detected in a random fashion), values associated with such behavior had to be excluded and repeated-measures analyses were not performed. Fluid intake and body weight data were not affected by this behavior and were analyzed with repeated-measures two-way ANOVA with Tukey's post hoc comparisons. The indices for microbiota α-diversity were obtained using PAST software (v3.20; free software for scientific data analysis). The results for α-diversity and ASV counts were analyzed statistically with a two-way ANOVA and subsequent Tukey's post hoc comparisons, using Prism. The indices for β-diversity were calculated and plotted in 3D using R, and statistical analyses were carried out using PAST. The results were analyzed using a two-way NPMANOVA, and post hoc comparisons were made using one-way NPMANOVAs. Taxonomic distributions at phylum and lower taxonomic levels, as well as data from acetate levels in caecum were analyzed in Prism with a two-way ANOVA and subsequent Tukey's multiple comparison tests.
3. Results
3.1. Effect of Abx on food/fluids intake and body weight in a GWI model fed with HF
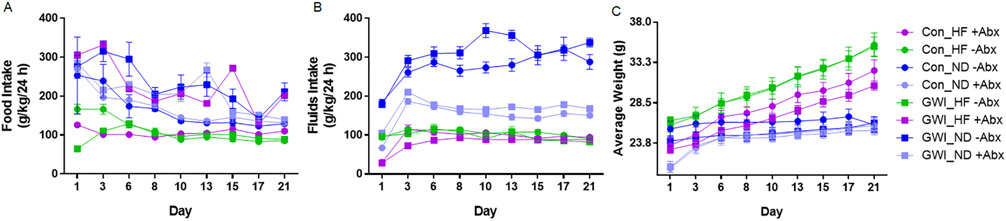
There were significant main effects of treatment (F7,402 = 67.17, p < 0.0001) and time (F8,402 = 16.64, p < 0.0001), and a significant treatment X time interaction (F56,402 = 2.38, p < 0.0001) on food intake (Fig. 1A). In the absence of Abx, HF produced a decrease in food intake compared to ND in both controls and GWI-treated mice (p < 0.0001 Tukey's test for both pairwise comparisons). While the administration of Abx still resulted in a significant decrease in food intake by HF in the controls (p < 0.001), these effects were not present in the GWI mice. For fluids intake, there were significant main effects of treatment (F7,42 = 150, p < 0.0001), time (F9,378 = 123.9, p < 0.0001), subjects matching (F42,378 = 14.61, p < 0.0001), and a significant treatment X time interaction (F63,378 = 13.55, p < 0.0001) (Fig. 1B). Feeding mice a HF reduced the amount of fluids taken by control and GWI-treated mice compared to mice on ND, regardless whether they received Abx or not (p < 0.0001 for both groups comparisons with and without Abx). In addition, both control and GWI groups receiving ND +Abx had a significantly lower intake compared to their corresponding group on ND −Abx (p < 0.0001 for both controls and GWI). Similarly, controls and GWI on ND +Abx had a significantly higher fluids intake than any of their analogs on HF with or without Abx (p < 0.01 for all comparisons). Lastly, there were significant main effects of treatment (F7,42 = 10.09, p < 0.0001), time (F8,336 = 425.3, p < 0.0001), subjects matching (F42,336 = 84.66, p < 0.0001), and a significant treatment X time interaction (F56,336 = 22.22, p < 0.0001), for body weight (Fig. 1C). Control and GWI subjects receiving HF in the absence of Abx exhibited an increase in body weight compared to their corresponding groups receiving ND (p < 0.01 for Con_ND −Abx vs Con_HF −Abx and p < 0.001 for GWI_ND −Abx vs GWI_HF −Abx). Abx administration maintained this body weight increase in controls fed with HF versus ND (p < 0.001) but this diet effect disappeared for the GWI group.
Fig. 1.
Effect of diet on food intake (A), fluids intake (B), and average body weight (C). Mice were treated with GWI agents or Con (control) and then fed a normal diet (ND) or a high fat diet (HF), concomitantly with antibiotics (+Abx) or without antibiotics (Abx) for 21 days. Food and fluids intake measures were calculated based on food or fluids consumption (g), mouse body weight (kg) for a 24 h period and reported as g/kg/24 h. Results are mean ± SEM, N = 6–7.
3.2. Effects of Abx on the gut microbiome alterations induced by GWI and HF
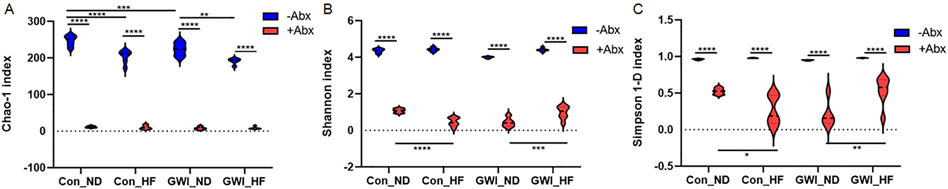
The number of sequences exceeded an average of 9562 per group. The ASV counts ± standard error for each group were the following: 261 ± 29.6 for Con_ND −Abx, 11.7 ± 2.3 for Con_ND +Abx, 205.8 ± 16.6 for Con_HF −Abx, 10.3 ± 5.3 for Con_HF +Abx, 206.4 ± 23.1 for GWI_ND −Abx, 7.8 ± 3.5 for GWI_ND +Abx, 196.3 ± 12.5 for GWI_HF −Abx, and 8.7 ± 3.1 for GWI_HF + Abx. As expected, Abx administration significantly reduced the number of sequences for the Con_ND, Con_HF, GWI_ND and GWI_HF when compared to their analog group without Abx (p < 0.0001 for each pairwise comparison, Tukey's tests). In light of this, and to ensure that any observed differences in microbial diversity among treatment groups were not due to differential sequence depth, we subsampled each sample to 6831 sequences, which was the lowest number of sequences obtained from any of the samples included in α-diversity analyses. Two-way ANOVA analyses of microbial α-diversity revealed significant main effects of GWI + diet (F1,36 = 42 42, p < 0.0001), Abx (F3,36 = 17.73, p < 0.0001) and the interaction between GWI + diet and Abx (F3,36 = 15.01, p < 0.0001) for bacterial richness (Chao-1 index, Fig. 2A). Abx administration drastically reduced the richness for Con_ND, Con_HF, GWI_ND and GWI_HF when compared to their analog group without Abx (p < 0.0001 for each pairwise comparison, Tukey's tests). In the absence of Abx, HF significantly reduced the richness in the bacterial communities of Con (p < 0.0001) and GWI (p < 0.01) when compared to their corresponding ND group, and treatment with GWI agents produced a richness decrease in the ND group versus the control (p < 0.001).
Fig. 2.
Violin plots of the microbial α-diversity indexes Chao-1 (A), Shannon (B), and inverse Simpson (C) in mice treated with GWI agents or Con (control) and then fed a NORMAL diet (ND) or a high fat diet (HF), concomitantly with antibiotics (+Abx) or without antibiotics (−Abx) for 21 days. Values are mean ± SEM. Symbols represent significance levels for the indicated post hoc comparisons as p<: *0.5, **0.01, ***0.001, and ****0.0001.
Analyses of bacterial profile heterogeneity and evenness showed a significant main effect of Abx (F3,39 = 14.21, p < 0.0001 for the Shannon index, Fig. 2B, and F3,40 = 6.1, p < 0.01 for the Simpson 1-D index, Fig. 2C), of GWI + diet (F1,39 = 3601, p < 0.0001 for the Shannon index, and F1,40 = 266.9, p < 0.0001 for the Simpson 1-D index) and the interaction between GWI + diet and Abx (F3,39 = 6.92, p < 0.001 for the Shannon index and F3,40 = 5.45, p < 0.01 for the Simpson 1-D index). As was seen for the Chao-1 index, α-diversity measured with the Shannon and Simpson indexes was also reduced by Abx administration for all groups compared to their analog without Abx (p < 0.0001 for all, Tukey's test). Interestingly, while HF in the presence of Abx caused a reduction of the microbial heterogeneity and evenness in controls (p < 0001 for Shannon and p < 0.05 for Simpson 1-D, Tukey's tests), this diet in combination with Abx was associated with diversity increases in the GWI group (p < 001 for Shannon and p < 0.01 for Simpson 1-D, Tukey's tests).
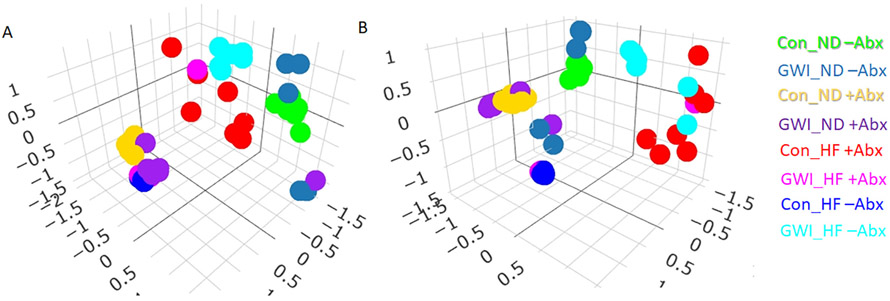
Two-way NPMANOVA analyses of β-diversity revealed main effects of GWI + diet (F1,48 = 25.5, p < 0.0001), Abx (F1,48 = 45.92, p < 0.0001) and their interaction (F1,48 = 21.85, p < 0.0001). The Jaccard index (Fig. 3A), which reflects bacterial community composition showed that the ASV profiles of groups clustered by GWI treatment, diet regimen and Abx (all post hoc comparisons among groups were statistically significant at p < 0.05). The Bray-Curtis index (Fig. 3B), which indicates the structure of the microbial community showed a similar ASV clustering by the same factor with all group comparisons reaching statistical significance (p < 0.01) but one: Con_ND +Abx vs GWI_ND +Abx. This indicates that Abx administration made the differences between Con and GWI-treated mice on ND disappear.
Fig. 3.
3D Non-metric multidimensional scaling analyses of the microbial β-diversity indices Jaccard (A) and Bray-Curtis (B) in mice treated with GWI agents or Con (control) and then fed a normal diet (ND) or a high fat diet (HF), concomitantly with antibiotics (+Abx) or without antibiotics (−Abx) for 21 days. N = 5–7.
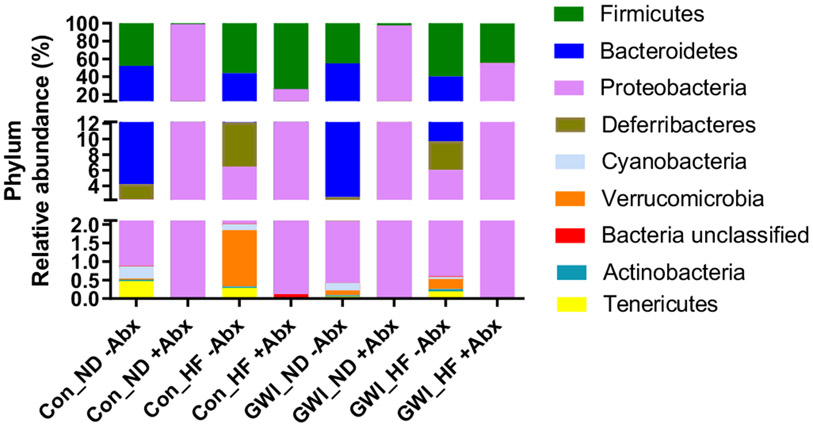
Analyses at the level of bacterial phyla showed that the main effects of phylum (F8,369 = 266.4, p < 0.0001) and the interaction between treatment X phylum (F56,369 = 42.45, p < 0.0001) were significant, whereas the effects of treatment alone were not (Fig. 4A). Abx were effective in decreasing the relative abundance of almost the entire set of the most prominent phyla in the gut with the exception of Proteobacteria. However, only Firmicutes, Bacteroidetes and Proteobacteria reached statistical significance. Without Abx, no differences were found by diet regimen or GWI treatment for Proteobacteria. However, in the presence of Abx, the abundance of Proteobacteria was lower in HF groups compared to their ND analog for both controls and GWI-treated mice (p < 0.0001 for both pairwise comparisons).
Fig. 4.
Percent relative abundance of the 8 most prominent bacterial phyla in mice treated with GWI agents or Con (control) and then fed a normal diet (ND) or a high fat diet (HF), concomitantly with antibiotics (+Abx) and without antibiotics (−Abx) for 21 days. N = 6–7.
For ND groups on Abx, the differences between controls and GWI were not significant, whereas for HF with Abx, controls had a lower relative abundance of Proteobacteria compared to GWI-treated mice (p < 0.0001). In controls and GWI group without Abx, the relative abundance of Bacteroidetes was higher when receiving ND compared to HF (p < 0.01 for controls, and p < 0.0001 for GWI). No differences were found between controls and GWI-treated mice when receiving the same diet regimen, whether that was ND or HF. These patterns on Bacteroidetes relative abundance changed in the presence of Abx, when the diet regimen had no effect on either controls or GWI-treated animals. In addition, Abx administration resulted in no differences between controls and GWI within the same diet regimen. In the absence of Abx, diet regimen had no effects on the relative abundance of the Firmicutes phylum in controls, whereas in GWI subjects, HF produced a decrease in this phylum compared to its ND analog (p < 0.05). Comparisons between control and GWI groups on the same diet without Abx did not show any significant differences. This indicates that in the absence of Abx, GWI treatment did not cause any effects on the relative abundance of Firmicutes. However, in the presence of Abx HF increased the abundance of Firmicutes in both controls and GWI (p < 0.0001 for both pairwise tests) compared to their respective ND groups. GWI treatment did not have any effect on Firmicutes abundance when mice received ND, but it decreased when receiving HF (p < 0.0001, Tukey's test).
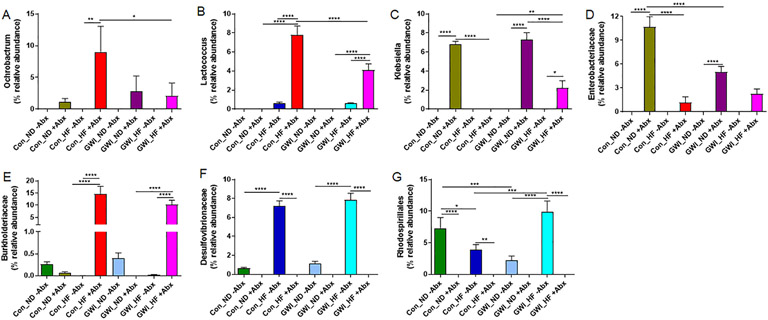
Analyses of gut bacteria at taxonomic levels below phylum (Fig. 5), showed significant effects for 6 members of Proteobacteria (Ochrobactrum, Klebsiella, Enterobacteriaceae, Burkholderiaceae, Desulfovibrionaceae, and Rhodospirillales), and a member of Firmicutes (Lactococcus). The main effects of GWI treatment (F1,35 = 10.72, p = 0.002 for Ochrobactrum; F3,41 = 33.91, p < 0.0001 for Klebsiella; F3,34 = 23.06, p < 0.0001 for Enterobacteriaceae; F3,32 = 30.59, p < 0.0001 for Burkholderiaceae; F3,41 = 71.23, p < 0.0001 for Desulfovibrionaceae; F3,35 = 10.64, p < 0.0001 for Rhodospirillales; and F3,40 = 41.7, p < 0.0001 for Lactococcus), Abx (F1,34 = 12.69, p = 0.01 for Ochrobactrum; F1,41 = 178.1, p < 0.0001 for Klebsiella; F1,34 = 127, p < 0.0001 for Enterobacteriaceae; F1,32 = 89.62, p < 0.0001 for Burkholderiaceae; F1,41 = 344.2, p < 0.0001 for Desulfovibrionaceae; F1,35 = 129.3, p < 0.0001 for Rhodospirillales; and F1,40 = 68.85, p < 0.0001 for Lactococcus) and GWI treatment X Abx interaction (F3,34 = 3.1, p < 0.05 for Ochrobactrum; F3,41 = 33.9, p < 0.0001 for Klebsiella; F3,34 = 26.14, p < 0.0001 for Enterobacteriaceae; F3,32 = 33.93, p < 0.0001 for Burkholderiaceae; F3,41 = 71.2, p < 0.0001 for Desulfovibrionaceae; F3,35 = 10.6, p < 0.0001 for Rhodospirillales; and F3,40 = 29.18, p < 0.0001 for Lactococcus) were all significant. Although and increase in the abundance of the genus Ochrobactrum (Fig. 5A) by HF did not reach statistical significance in controls treated with Abx, this increase was significant in Con_HF +Abx for Burkholderiaceae (p < 0.0001, Fig. 5E), and Lactococcus (p < 0.0001, Fig. 5B) compared to Con_ND +Abx. However, the opposite effect of HF was observed in controls exposed to Abx, where the abundance of the genus Klebsiella (p < 0.0001, Fig. 5C) and the Enterobacteriaceae family (p < 0.0001, Fig. 5D) were significantly decreased. Similar patterns of increased abundance of Burkholderiaceae (p < 0.0001) and Lactococcus (p < 0.0001) were observed after HF in GWI-treated mice exposed to Abx compared to their corresponding group fed with ND. The administration of Abx to controls and GWI mice did not cause any changes in Desulfovibrionaceae (Fig. 5F) or Rhodospirillales (Fig. 5G) regardless of the diet. Increases in Klebsiella and Enterobacteriaceae were observed in both controls (p < 0.001 for both taxa) and GWI (p < 0.0001 for both taxa) mice fed with ND in the presence of Abx. While the abundance of Ochrobactrum and Lactococcus was significantly decreased by HF in GWI compared to controls in the presence of Abx (p < 0.05 for both taxa), the relative abundance of Klebsiella was significantly increased (p < 0.01).
Fig. 5.
Relative abundance of taxa below the level of phylum in treatment and diet groups. Results are presented as % relative abundance for each taxon. Con = control; GWI = PER + PB; ND = normal diet; HF = high fat diet; −Abx = without antibiotics; +Abx = with antibiotics. Symbols represent significance levels for the indicated post hoc comparisons as p<: *0.05, **0.01, ***0.001; ****0.0001.
In the absence of Abx, HF caused an increase in Desulfovibrionaceae in both controls and GWI-treated mice compared to their analog groups fed with ND (p < 0.0001 for both). Comparisons between Con_HF −Abx and Con_HF +Abx in controls and GWI-treated subjects show significant decreases for Desulfovibrionaceae and Rhrodospirillales (p < 0.01). Interestingly, the effect of HF in controls without Abx was associated with decreases in Rhodospirillales (p < 0.05), whereas in the GWI group, this taxon was increased after HF (p < 0.0001). Furthermore, treatment with GWI agents produced a decrease compared to controls when both groups received ND in the absence of Abx (p < 0.0001).
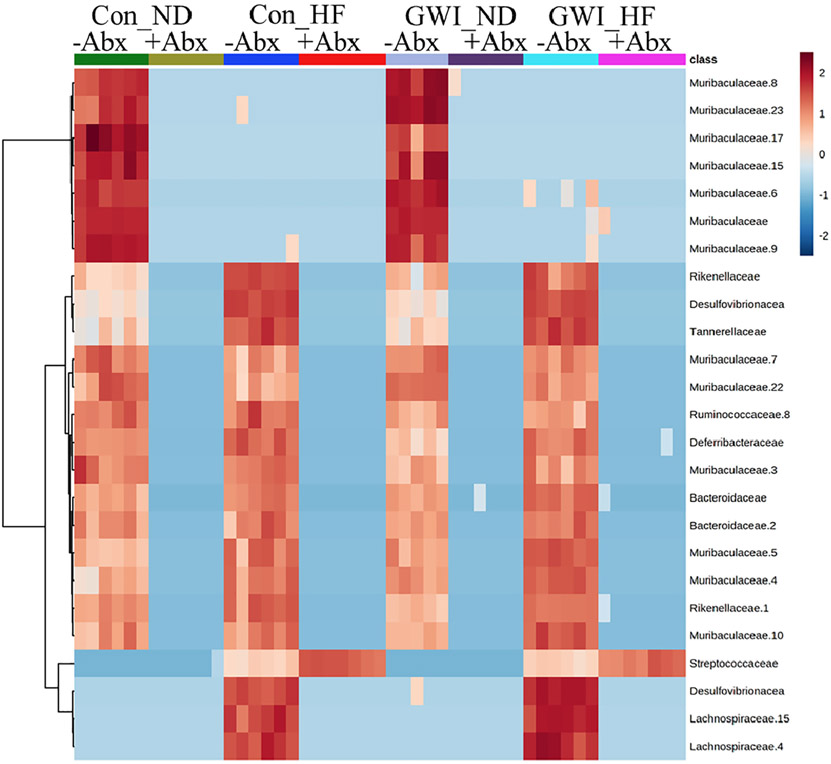
These treatment-driven differences in taxa composition can be visualized in a heat map (Fig. 6), where the presence of Abx is associated with an overall lower abundance of bacteria (dominated by blue intensities) compared to the groups without Abx (dominated by orange-red intensities). The bottom section of this heat map shows that members of Streptococcaceae are the only ones which are increased in HF +Abx groups.
Fig. 6.
Heat map illustrating patterns in relative abundance of ASVs among the treatment groups. All subjects in each group are arrayed in columns and bacterial taxonomies are indicated in rows. Con = control; GWI = PER + PB; ND = normal diet; HF=HF diet; −Abx = without antibiotics; +Abx = with antibiotics. Clustering along the y-axis was done using the Ward algorithm. N = 6–7.
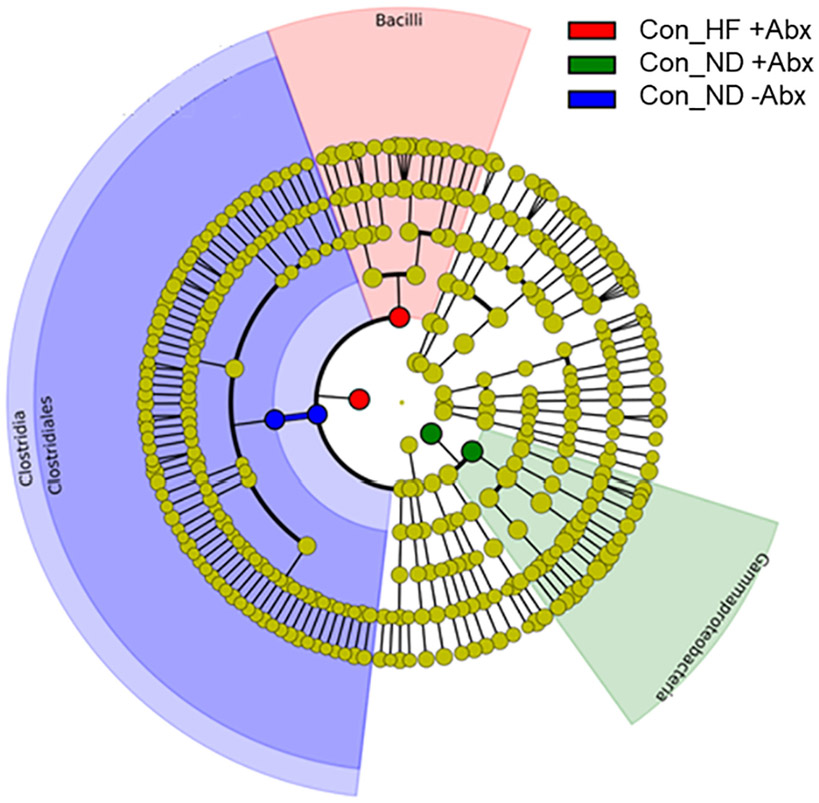
LEfSe analyses identified specific taxa associated with 3 control groups with categories down to bacterial order level (Fig. 7). Members of the Clostridia class and the Clostridiales order were more abundant in Con_ND −Abx, whereas Gammaproteobacteria were representative of Con_ND +Abx, and Bacilli members were representative of the Con_HF +Abx group.
Fig. 7.
Bacterial taxa that were differentially abundant according to LEfSe analysis. Results are displayed as a cladogram where taxa values in each treatment group are highlighted by small circles and by shading. All groups shown are statistically different compared to each other (LDA > 3.6). Control (Con), High fat diet (HF), with (+Abx) and without (−Abx) antibiotics.
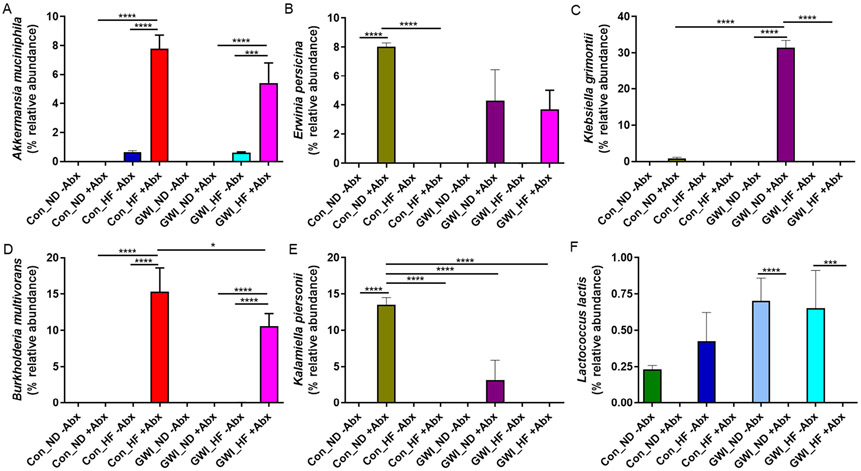
The sequences of ASVs with differential abundance within groups from the analyses at taxonomic level below phylum were searched in BLAST and 6 classifications were made at the species level (100% genetic identity match; Fig. 8). In the presence of Abx, Akkermansia muciniphila was more abundant in HF groups regardless of being a control or GWI-treated (p < 0.0001 vs their respective ND −Abx analog group; Fig. 8A), but administration of Abx caused a larger expansion of A. muciniphila in both controls and GWI (p < 0.001). While the abundance of Erwinia persicina was increased in both Con_ND +Abx and GWI_ND +Abx compared to their analog groups without Abx, only controls reached statistical significance (p < 0.0001; Fig. 8B). In the presence of Abx, HF was associated with a decrease in E. persicina in controls (p < 0.0001; Fig. 8C) but had no effect in GWI-treated mice. Although the species Klebsiella grimontii was only present in Con_ND +Abx and GWI_ND +Abx, when compared to their corresponding analog without Abx or with HF, only the GWI group was significantly increased (p < 0.001 vs GWI_ND −Abx and vs GWI_HF +Abx). In addition, the abundance of K. grimontii was higher in GWI_ND compared to Con_ND in the presence of Abx (p < 0.0001). The combination of HF with Abx whether in Con or GWI lead to increases in Burkholderia multivorans (p < 0.0001 for all; Fig. 8D), as this bacterium was not present with either HF or Abx alone in either group. Moreover, there was a significant increase in Con_HF +Abx when compared to GWI_HF +Abx (p < 0.05). Increases in Kalamiella piersonii were exclusively present in Con_ND +Abx and GWI_ND +Abx (Fig. 8E) but they were only significant for the control group when compared to its control analogs without Abx (p < 0.0001) or with HF (p < 0.0001), and to GWI_ND +Abx and GWI_HF +Abx (p < 0.0001 for both). Lastly, the presence of Lactococcus lactis was observed in all treatment groups without Abx (Fig. 8F), but when comparing to their corresponding group in either controls or GWI without Abx, the differences were significant only for the GWI groups (p <0.0001 vs both ND and HF).
Fig. 8.
Relative abundance of bacterial species in treatment and diet groups. Results are presented as % relative abundance for each taxon. Con = control; GWI = PER + PB; ND = normal diet; HF = high fat diet; −Abx = without antibiotics; +Abx = with antibiotics. Symbols represent significance levels for the indicated post hoc comparisons as p<: *0.05, ***0.001; ****0.0001.
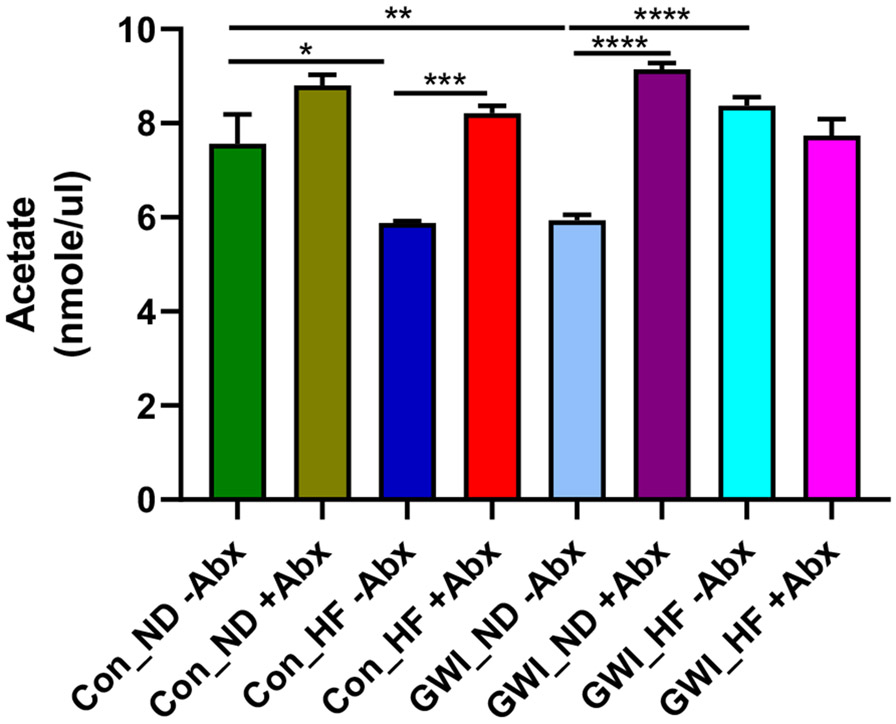
3.3. Effects of Abx on acetate levels in the caecum of animals treated with GWI agents and fed with HF
Two-way ANOVA analyses revealed significant effects of GWI + diet (F3,23 = 6.74, p < 0.01), Abx (F1,23 = 62.35, p < 0.0001) and their interaction (F3,23 = 19.31, p < 0.0001) on the levels of the SCFA acetate (Fig. 9). Post hoc tests showed that in the absence of Abx, HF decreased the amount of acetate compared to controls (p < 0.05 for Con_ND −Abx vs Con_HF −Abx), and treatment with GWI agents in mice receiving a ND also caused an acetate reduction compared to its corresponding Con_ND group (p < 0.01). In controls fed with HF, the acetate levels were lower in the group without Abx compared to the one receiving Abx (p < 0.001). Treatment with GWI agents is associated with significant increases in acetate levels when combined with either HF (p < 0.0001 for GWI_ND −Abx vs GWI_HF −Abx) or Abx (p < 0.001 for GWI_ND −Abx vs GWI_ND +Abx).
Fig. 9.
Levels of acetate in caecum of mice treated with GWI agents or Con (control) and then fed a normal diet (ND) or high fat diet (HF), concomitantly with antibiotics (+Abx) or without antibiotics (−Abx) for 21 days. Results are mean ± SEM, N = 4–5. Symbols represent significance levels for the indicated post hoc comparisons as p <: *0.05, **0.01, ***0.001, ****0.0001.
4. Discussion
Results show that while treatment with GWI agents does not change the HF-driven increase in body weight, administration of Abx was effective in counteracting this increase only in GWI subjects as controls showed a tendency towards a body weight decrease with Abx but this was not statistically significant (see Fig. 1). This offsetting effect of Abx on the body weight increase by HF in GWI subjects was unexpected in light of the significant increase in food intake observed in the GWI group receiving HF +Abx. While this finding is counterintuitive and requires future investigation, the fact that it only took place in the presence of Abx pinpoints the gut microbiota as a mediator in the body weight effects of HF in GWI. A similar capacity of Abx to decrease the body weight gain induced by HF was documented in C57Bl/6 mice [23] but no reports on the effects of Abx on body weight of GWI Veterans was found.
While the use of Abx was effective in reducing the ASV counts to less than 5% of the groups without Abx (see ASV counts above), this drop favored the proliferation of certain bacterial taxa. In this sense, a previous study in mice using Abx and a sequencing approach to assess gut bacteria alterations yielded a significant reduction of about 50% of bacterial species [24]. When quantifying colony-forming units (CFU) from fecal cultures, the bacterial decreases after Abx exposure are more pronounced, ranging from 1 million-fold to non-detectable [25,26]. These discrepancies are explained by the CFU method being restricted to the measurement of culturable gut microbes. Strikingly, a large number of published reports employing the broad-spectrum Abx as an approach to deplete the gut microbiota, do not document any data on the extent of such depletion [27-30].
Our previously reported findings that treatment with GWI agents as well as with HF caused a significant reduction in microbial richness [2] were corroborated, and the present study found that exposure to Abx accentuated even more these decreases in α-diversity. Such reduction in microbial α-diversity measures has been consistently reported as a result of HF [2,31,32] and Abx exposure [24,25,33].
Besides altering α-diversity, treatment with GWI and HF changed the composition and structure of the microbial gut community in a way that was not prevented by administration of Abx (see Fig. 3). More specifically, it was found that GWI and HF each had an effect on the relative abundance of the most prevalent bacterial phyla (Fig. 4A). HF reduced the abundance of Bacteroidetes while increasing that of Firmicutes, and this effect was potentiated with the administration of Abx, which caused a complete depletion of Bacteroidetes (Fig. 4B). Beyond their capacity to alter microbial β-diversity [33,34], Abx favored the expansion of Proteobacteria members. These overall findings have been documented [24,34] but the extent of the shifts in each phylum differ, depending on the type of samples used (i.e. adolescent vs adult age, or rat vs mouse samples). Increases in the phylum Proteobacteria have been reported in the aged gut microbiome [35], and in conditions coursing with inflammation such as inflammatory bowel disease and Alzheimer's disease [36,37]. Similar shifts in bacterial phyla favoring a Proteobacteria outgrowth have been reported in studies with broad-spectrum Abx [24,34]. In terms of gut microbiome alterations attributed to GWI, preliminary data from a pilot study of Gulf War (GW) Veterans with and without gastrointestinal symptoms reported increases in Proteobacteria in a subset of individuals with both GWI and gastrointestinal symptoms [9]. Furthermore, this proliferation of Proteobacteria was associated with greater levels of inflammatory cytokines in plasma [9]. A doubleblind, placebo-controlled trial in GW Veterans with irritable bowel syndrome (IBS), a common condition in GWI, reported that the use of Abx was not associated with significant improvement in IBS-related symptoms [38]. However, a close analysis of these results called for a more cautionary interpretation as albeit having multiple strengths, this study also had weaknesses associated with an underpowered sample size [39]. The Abx-induced restructuring of the microbial community demonstrates that Abx administration is one of the most pervasive ways to disrupt the gut microbiome, as observed by the capacity of these compounds to induce dysbiosis.
Deeper analyses of bacterial taxa below the phylum level evidenced increases in the genera Ochrobactrum and Lactococcus produced by the combination of HF with Abx, which were of greater magnitude in controls than in GWI subjects (Fig. 5A and B). This is consistent with reports of Ochrobactrum increases in the intestinal mucosa of mice fed with HF [40]. Interestingly, the presence of Lactococcus has been consistently documented as an outcome of HF consumption [41], but it was later found that this was the result of dietary contaminants in most commercially available HF (i.e. casein), and that the high levels of bacteria found in casein-containing HF were intact but likely dead cells as they failed to proliferate in culture [41]. While casein was a component of the HF formulation employed in the present study, and this factor could explain the increases in Lactococcus observed in the HF groups, the fact that such increases were further heightened by Abx in both control and GWI groups points to a synergic effect of Abx with HF, and suggests that either Lactococcus in the HF groups were not dead and their outgrowth was promoted by Abx in the absence of other competitors, or that baseline levels of Lactococcus were not only resistant to Abx but also capable of proliferating in a non-adversarial environment. It is noteworthy that Lactococcus is significantly more abundant in controls than in GWI subjects exposed to HF and Abx. Given that Lactococcus is considered a beneficial bacterial genus with members that constitute probiotic strains [32], the decreases in this genus in GWI relative to controls could be associated with worse outcomes.
Two taxa of gram-negative bacteria, including the genus Klebsiella and the family Enterobacteriacea were overrepresented not only in the groups treated with Abx in a fashion that seemed diet-independent (Fig. 5C and D). While the abundance of these two bacteria was greater in the groups receiving a ND with Abx, the combination of HF with Abx also led to increases. Exposure to Abx in GWI-treated mice was associated with lower abundance of Enterobacteriacea than in controls. These two taxa belong to the betaproteobacteria and gammaproteo-bacterial classes respectively, and contain members that cause human disease and are resistant to Abx [42,43]. The multiplication of bacteria capable of causing disease in the control groups receiving Abx points to the adverse effects of this seemingly innocuous intervention. Although the families Burkholderiaceae and Desulfovibrionaceae were significantly altered by HF, neither diet nor treatment with GWI seemed to be determinant factors (Fig. 5E and F). However, the order Rhodospirillales, seemed sensitive to treatment with GWI, diet and Abx exposure. This taxon was only detected in the absence of Abx, and was found less abundant in the GWI group fed with ND compared to HF, while in controls Rhodospirillales was more abundant with ND. This order of bacteria comprises members that produce acetic acid [44] but also some strains capable of utilizing acetate as a growth source [45]. In light of the increased acetate levels observed in the groups exposed to Abx (see Fig. 9), it is likely that the members of Rhodospirillales thriving in the absence of Abx are the strains that produce acetic acid. The lower abundance of this order in GWI-treated mice on ND and the subsequent increase in its analog fed with a HF supports this hypothesis.
The increased abundance of the Clostridia class, and the Clostridiales order particularly in controls fed with ND without Abx (considered the true control in this study), in comparison to the rest of the groups with HF or GWI treatment, indicates that these two interventions alter host's health (see LEfSE cladogram, Fig. 7). Clostridia, a class of 20–30 beneficial bacteria has been identified as a crucial factor for maintenance of gut homeostasis [46] and can prevent mice from becoming obese [47]. Similarly, decreases in Clostridiales has been associated with type 2 diabetes in mouse models [48]. HF in combination with Abx in controls was associated with increases in gram-positive Bacilli, which contain several well-known pathogens. As revealed by LEfSe, Abx also disrupted gut homeostasis and led to a proliferation of Gammaproteobacteria not only in controls, but also in subjects treated with GWI as evidenced by increases in K. grimontii and K piersonii (see Fig. 8C and E). E. persicina was also increased in the control group by Abx independent of the diet (Fig. 8B). Preliminary data from an infection model with E. Persicina suggest that this bacterium causes diarrhea and increases liver inflammation [49]. In addition, L. lactis was overrepresented in the GWI group with and without HF, which is consistent with data from GWI mouse models showing increases in L. lactis in GWI fed with a Western-style diet (WD) [5], that is rich is fat and carbohydrates. This study also found increases in A. muciniphila in the group treated with GWI + WD, which we observed increased with HF, regardless of treatment (Fig. 8A). While A. muciniphila, a mucin-degrading bacterium is suggested to play a protective role in the gut and in reversing the metabolic alterations induced by HF, mucin degradation could compromise the integrity of the entire mucus barrier, which is vital for health maintenance [50]. Thus, a HF-driven increase in A. muciniphila could also be interpreted as a possible mechanism for this detrimental diet to disrupt the gastrointestinal tract. Moreover, B. multivorans was characteristic of the groups fed with HF and exposed to Abx (see Fig. 8D). The genus Burkholderia comprises metabolically diverse bacteria that are known to thrive in adversarial environments. This is the case of B. multivorans, which is an opportunistic pathogen displaying significant Abx resistance [51]. The increased abundance of this microbe in the Abx group could be a result of its capacity to survive in the presence of these compounds.
Treatment with GWI produced a clear reduction in the acetate levels compared to controls but the effects of HF on acetate depended on the treatment (see Fig. 9). In controls without Abx, HF significantly reduced the levels of acetate whereas in the GWI group without Abx HF caused an increase. The reports indicating that HF is associated with decreases in SCFAs, including acetate [52] are consistent with the effects observed in controls. Furthermore, the acetate concentration was reduced in feces of overweight and obese humans compared to healthy controls [15]. In the case of GWI_HF −Abx group, the dysbiosis favoring an increase in acetate-producing bacteria such as Ochrobactrum [53], could explain the increases in this SCFA. Exposure to Abx tended to increase the levels acetate in a diet-independent manner. The only exception was the GWI_HF +Abx group, which was not significantly different from its analog without Abx. These results stand in contrast to a study showing that the production of the SCFAs acetate, propionate and butyrate by the colonic microbiota are significantly reduced by Abx [33]. These overall increases in acetate in the groups exposed to Abx could also be enhanced by an expansion of potential acetate consumers in groups without Abx, such as Rhodospirillales.
5. Conclusion
In light of these results, it can be concluded that the perturbations caused by GWI and HF were only partially prevented by Abx. Feeding a HF decreased both food intake and body weight gain in the GWI groups exposed to Abx. However, these offsetting effects of Abx were not translated into restitution of microbial diversity or normalization of beneficial bacterial byproducts such as acetate. Furthermore, Abx themselves caused a remodeling of the gut microbiome that was associated with decreases in α-diversity, changes in the composition and structure of the microbial community characterized by a large expansion of Proteobacteria members, and acetate levels. While Abx were very effective in reducing the two dominant gut microbiome phyla (i.e. Firmicutes and Bacteroidetes), they favored the proliferation of potential pathobionts. Although the detrimental effects of treatment with GWI on the gut microbiome and their aggravation by HF were tangible, the use of Abx may not represent a dependable approach to deplete the gut microbiome and its advantages as a pseudo germ-free model warrant further investigation.
Supplementary Material
Funding
This work was supported by the Department of Veterans Affairs: 1 I01 R01 DA044564; the US Department of Defense CDMRP: GW170034.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influenced the work reported in this paper.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.lfs.2021.119675.
CRediT authorship contribution statement
Mariana Angoa-Pérez: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration. Branislava Zagorac: Investigation, Data curation. Dina M. Francescutti: Investigation, Formal analysis. Kevin R. Theis: Formal analysis, Data curation, Writing – review & editing. Donald M. Kuhn: Conceptualization, Writing – review & editing, Supervision, Project administration, Funding acquisition.
References
- [1].Keating JA, Shaughnessy C, Baubie K, Kates AE, Putman-Buehler N, Watson L, Dominguez N, Watson K, Cook DB, Rabago D, Suen G, Gangnon R, Safdar N, Characterising the gut microbiome in veterans with Gulf War Illness: a protocol for a longitudinal, prospective cohort study, BMJ Open 9 (2019), e031114, 10.1136/bmjopen-2019-031114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Angoa-Pérez M, Zagorac B, Francescutti DM, Winters AD, Greenberg JM, Ahmad MM, Manning SD, Gulbransen BD, Theis KR, Kuhn DM, Effects of a high fat diet on gut microbiome dysbiosis in a mouse model of Gulf War Illness, Sci. Rep 10 (2020) 9529, 10.1038/s41598-020-66833-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alhasson F, Das S, Seth R, Dattaroy D, Chandrashekaran V, Ryan CN, Chan LS, Testerman T, Burch J, Hofseth LJ, Horner R, Nagarkatti M, Nagarkatti P, Lasley SM, Chatterjee S, Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation, PLoS One 12 (2017), e0172914, 10.1371/journal.pone.0172914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bose D, Mondal A, Saha P, Kimono D, Sarkar S, Seth RK, Janulewicz P, Sullivan K, Horner R, Klimas N, Nagarkatti M, Nagarkatti P, Chatterjee S, TLR antagonism by Sparstolonin B alters microbial signature and modulates gastrointestinal and neuronal inflammation in gulf war illness preclinical model, Brain Sci 10 (2020), 10.3390/brainsci10080532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bose D, Saha P, Mondal A, Fanelli B, Seth RK, Janulewicz P, Sullivan K, Lasley S, Horner R, Colwell RR, Shetty AK, Klimas N, Chatterjee S, Obesity worsens gulf war illness symptom persistence pathology by linking altered gut microbiome species to long-term gastrointestinal, hepatic, and neuronal inflammation in a mouse model, Nutrients 12 (2020), 10.3390/nul2092764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mote RS, Carpenter JM, Dockman RL, Steinberger AJ, Suen G, Norberg T, Harn DA, Wagner JJ, Filipov NM, Assessing the beneficial effects of the immunomodulatory glycan LNFPIII on gut microbiota and health in a mouse model of gulf war illness, Int. J. Environ. Res. Public Health 17 (2020), 10.3390/ijerph17197081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Seth RK, Kimono D, Alhasson F, Sarkar S, Albadrani M, Lasley SK, Horner R, Janulewicz P, Nagarkatti M, Nagarkatti P, Sullivan K, Chatterjee S, Increased butyrate priming in the gut stalls microbiome associated-gastrointestinal inflammation and hepatic metabolic reprogramming in a mouse model of Gulf War Illness, Toxicol. Appl. Pharmacol 350 (2018) 64–77, 10.1016/j.taap.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kimono D, Bose D, Seth RK, Mondal A, Saha P, Janulewicz P, Sullivan K, Lasley S, Horner R, Klimas N, Chatterjee S, Host Akkermansia muciniphila abundance correlates with gulf war illness symptom persistence via NLRP3-mediated neuroinflammation and decreased brain-derived neurotrophic factor, Neurosci Insights 15 (2020), 2633105520942480, 10.1177/2633105520942480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Janulewicz PA, Seth RK, Carlson JM, Ajama J, Quinn E, Heeren T, Klimas N, Lasley SM, Horner RD, Sullivan K, Chatterjee S, The gut-microbiome in gulf war veterans: a preliminary report, Int. J. Environ. Res. Public Health 16 (2019), 10.3390/ijerph16193751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Al-Daihan S, Ben Bacha A, Al-Dbass AM, Alonazi MA, Bhat RS, High-fat diet stimulates the gut pathogenic microbiota and maintains hepatic injury in antibiotic-treated rats, Cell Mol Biol (Noisy-le-grand) 64 (2018) 103–106, 10.14715/cmb/2018.64.1.18. [DOI] [PubMed] [Google Scholar]
- [11].Miller AW, Orr T, Dearing D, Monga M, Loss of function dysbiosis associated with antibiotics and high fat, high sugar diet, Isme j 13 (2019) 1379–1390, 10.1038/s41396-019-0357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Coughlin SS, Kang HK, Mahan CM, Selected health conditions among overweight, obese, and non-obese veterans of the 1991 gulf war: results from a survey conducted in 2003-2005, Open Epidemiol J. 4 (2011) 140–146, 10.2174/1874297101104010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lu Y, Fan C, Li P, Lu Y, Chang X, Qi K, Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota, Sci. Rep 6 (2016) 37589, 10.1038/srep37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lin HV, Frassetto A, Kowalik EJ, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, Marsh DJ, Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms, PLoS One 7 (2012), e35240, 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD, Microbiota and SCFA in lean and overweight healthy subjects, Obesity (Silver Spring) 18 (2010) 190–195, 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- [16].Liu D, Wen B, Zhu K, Luo Y, Li J, Li Y, Lin H, Huang J, Liu Z, Antibiotics-induced perturbations in gut microbial diversity influence metabolic phenotypes in a murine model of high-fat diet-induced obesity, Appl. Microbiol. Biotechnol 103 (2019) 5269–5283, 10.1007/s00253-019-09764-5. [DOI] [PubMed] [Google Scholar]
- [17].Angoa-Pérez M, Zagorac B, Francescutti DM, Theis KR, Kuhn DM, Responses to chronic corticosterone on brain glucocorticoid receptors, adrenal gland, and gut microbiota in mice lacking neuronal serotonin, Brain Res. 1751 (2021), 147190, 10.1016/j.brainres.2020.147190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP, DADA2: high-resolution sample inference from Illumina amplicon data, Nat. Methods 13 (2016) 581–583, 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Angoa-Pérez M, Zagorac B, Anneken JH, Briggs DI, Winters AD, Greenberg JM, Ahmad M, Theis KR, Kuhn DM, Repetitive, mild traumatic brain injury results in a progressive white matter pathology, cognitive deterioration, and a transient gut microbiota dysbiosis, Sci. Rep 10 (2020) 8949, 10.1038/S41598-020-65972-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Afgan E, Baker D, Batut B, van den Beek M, Bouvier D, Cech M, Chilton J, Clements D, Coraor N, Gruning BA, Guerler A, Hillman-Jackson J, Hiltemann S, Jalili V, Rasche H, Soranzo N, Goecks J, Taylor J, Nekratenko A, Blankenberg D, The galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update, Nucleic Acids Res. 46 (2018) W537–w544, 10.1093/nar/gky379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ, Basic local alignment search tool, J. Mol. Biol 215 (1990) 403–410, 10.1016/s0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- [22].Pang Z, Zhou G, Chong J, Xia J, Comprehensive meta-analysis of COVID-19 global metabolomics datasets, Metabolites 11 (2021), 10.3390/metabo11010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R, Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice, Diabetes 57 (2008) 1470–1481, 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- [24].Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD, Cotter PD, Dinan TG, Cryan JF, Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour, Brain Behav. Immun 48 (2015) 165–173, 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- [25].Wang S, Huang M, You X, Zhao J, Chen L, Wang L, Luo Y, Chen Y, Gut microbiota mediates the anti-obesity effect of calorie restriction in mice, Sci. Rep 8 (2018) 13037, 10.1038/s41598-018-31353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rios-Arce ND, Schepper JD, Dagenais A, Schaefer L, Daly-Seiler CS, Gardinier JD, Britton RA, McCabe LR, Parameswaran N, Post-antibiotic gut dysbiosis-induced trabecular bone loss is dependent on lymphocytes, Bone 134 (2020), 115269, 10.1016/j.bone.2020.115269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJ, de Boer JD, Hoogendijk AJ, de Beer R, de Vos A, Belzer C, de Vos WM, van der Poll T, Wiersinga WJ, The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia, Gut 65 (2016) 575–583, 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Emal D, Rampanelli E, Stroo I, Butter LM, Teske GJ, Claessen N, Stokman G, Florquin S, Leemans JC, Dessing MC, Depletion of gut microbiota protects against renal ischemia-reperfusion injury, J. Am. Soc. Nephrol 28 (2017) 1450–1461, 10.1681/asn.2016030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li G, Xie C, Lu S, Nichols RG, Tian Y, Li L, Patel D, Ma Y, Brocker CN, Yan T, Krausz KW, Xiang R, Gavrilova O, Patterson AD, Gonzalez FJ, Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota, Cell Metab. 26 (2017) 672–685 (e674), 10.1016/j.cmet.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Suárez-Zamorano N, Fabbiano S, Chevalier C, Stojanović O, Colin DJ, Stevanović A, Veyrat-Durebex C, Tarallo V, Rigo D, Germain S, Ilievska M, Montet X, Seimbille Y, Hapfelmeier S, Trajkovski M, Microbiota depletion promotes browning of white adipose tissue and reduces obesity, Nat. Med 21 (2015) 1497–1501, 10.1038/nm.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cândido FG, Valente FX, Grześkowiak Ł M, Moreira APB, Rocha D, Alfenas RCG, Impact of dietary fat on gut microbiota and low-grade systemic inflammation: mechanisms and clinical implications on obesity, Int. J. Food Sci. Nutr 69 (2018) 125–143, 10.1080/09637486.2017.1343286. [DOI] [PubMed] [Google Scholar]
- [32].Kong C, Gao R, Yan X, Huang L, Qin H, Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet, Nutrition 60 (2019) 175–184, 10.1016/j.nut.2018.10.002. [DOI] [PubMed] [Google Scholar]
- [33].Fröhlich EE, Farzi A, Mayerhofer R, Reichmann F, Jačan A, Wagner B, Zinser E, Bordag N, Magnes C, Fröhlich E, Kashofer K, Gorkiewicz G, Holzer P, Cognitive impairment by antibiotic-induced gut dysbiosis: analysis of gut microbiota-brain communication, Brain Behav. Immun 56 (2016) 140–155, 10.1016/j.bbi.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hoban AE, Moloney RD, Golubeva AV, McVey Neufeld KA, O'Sullivan O, Patterson E, Stanton C, Dinan TG, Clarke G, Cryan JF, Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat, Neuroscience 339 (2016) 463–477, 10.1016/j.neuroscience.2016.10.003. [DOI] [PubMed] [Google Scholar]
- [35].Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkïla J, Monti D, Satokari R, Franceschi C, Brigidi P, De Vos W, Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians, PLoS One 5 (2010), e10667, 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hughes ER, Winter MG, Duerkop BA, Spiga L, Furtado de Carvalho T, Zhu W, Gillis CC, Büttner L, Smoot MP, Behrendt CL, Cherry S, Santos RL, Hooper LV, Winter SE, Microbial respiration and formate oxidation as metabolic signatures of inflammation-associated dysbiosis, Cell Host Microbe 21 (2017) 208–219, 10.1016/j.chom.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bäuerl C, Collado MC, Diaz Cuevas A, Viña J, Pérez Martínez G, Shifts in gut microbiota composition in an APP/PSS1 transgenic mouse model of Alzheimer’s disease during lifespan, Lett. Appl. Microbiol 66 (2018) 464–471, 10.1111/lam.12882. [DOI] [PubMed] [Google Scholar]
- [38].Tuteja AK, Talley NJ, Stoddard GJ, Verne GN, Double-blind placebo-controlled study of Rifaximin and lactulose hydrogen breath test in gulf war veterans with irritable bowel syndrome, Dig. Dis. Sci 64 (2019) 838–845, 10.1007/s10620-018-5344-5. [DOI] [PubMed] [Google Scholar]
- [39].Harris LA, Rifaximin for irritable bowel syndrome (IBS) in gulf war veterans: losing the battle but winning the war? Dig. Dis. Sci 64 (2019) 609–610, 10.1007/s10620-019-05505-w. [DOI] [PubMed] [Google Scholar]
- [40].Li X, Peng X, Guo K, Tan Z, Bacterial diversity in intestinal mucosa of mice fed with Dendrobium officinale and high-fat diet, 3 Biotech 11 (2021) 22, 10.1007/s13205-020-02558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bisanz JE, Upadhyay V, Turnbaugh JA, Ly K, Turnbaugh PJ, Meta-analysis reveals reproducible gut microbiome alterations in response to a high-fat diet, Cell Host Microbe 26 (2019) 265–272 (e264), 10.1016/j.chom.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schaefers MM, Regulation of virulence by two-component systems in pathogenic Burkholderia, Infect. Immun 88 (2020), 10.1128/iai.00927-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mizrahi A, Delerue T, Morel H, Le Monnier A, Carbonnelle E, Pilmis B, Zahar JR, Infections caused by naturally AmpC-producing Enterobacteriaceae: can we use third-generation cephalosporins? A narrative review, Int. J. Antimicrob. Agents 55 (2020), 105834, 10.1016/j.ijantimicag.2019.10.015. [DOI] [PubMed] [Google Scholar]
- [44].Garrity GB, Don J, Krieg Noel R., Staley James T. (Eds.), Bergey’s Manual of Systematic Bacteriology, the Proteobacteria, Part C: The Alpha-, Beta-, Delta-, and Epsilonproteobacteria vol. 2, Springer US, 2005, p. 1388 (LVI). [Google Scholar]
- [45].Blasco R, Cardenas J, Castillo F, Acetate metabolism in purple non-sulfur bacteria, FEMS Microbiol. Lett 58 (1989) 129–132 (https://). [Google Scholar]
- [46].Lopetuso LR, Scaldaferri F, Petito V, Gasbarrini A, Commensal clostridia: leading players in the maintenance of gut homeostasis, Gut Pathog 5 (2013) 23, 10.1186/1757-4749-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Petersen C, Bell R, Klag KA, Lee SH, Soto R, Ghazaryan A, Buhrke K, Ekiz HA, Ost KS, Boudina S, O'Connell RM, Cox JE, Villanueva CJ, Stephens WZ, Round JL, T cell-mediated regulation of the microbiota protects against obesity, Science 365 (2019), 10.1126/science.aat9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mora-Ortiz M, Oregioni A, Claus S, Functional Characterisation of Gut Microbiota and Metabolism in Type 2 Diabetes Indicates thatClostridialesandEnterococcuscould Play a Key Role in the Disease, 2019. (bioRxiv). [Google Scholar]
- [49].Mohamaden WI, Zhen-Fen Z, Hegab IM, Shang-Li S, Experimental infection in mice with Erwinia persicina, Microb. Pathog 130 (2019) 38–43, 10.1016/j.micpath.2019.01.050. [DOI] [PubMed] [Google Scholar]
- [50].Paone P, Cani PD, Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut 69 (2020) 2232–2243, 10.1136/gutjnl-2020-322260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rhodes KA, Schweizer HP, Antibiotic resistance in Burkholderia species, Drug Resist. Updat 28 (2016) 82–90, 10.1016/j.drup.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yin J, Li Y, Han H, Chen S, Gao J, Liu G, Wu X, Deng J, Yu Q, Huang X, Fang R, Li T, Reiter RJ, Zhang D, Zhu C, Zhu G, Ren W, Yin Y, Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice, J. Pineal Res 65 (2018), e12524, 10.1111/jpi.12524. [DOI] [PubMed] [Google Scholar]
- [53].Hou X, Huang L, Zhou P, Tian F, Tao Y, Li Puma G, Electrosynthesis of acetate from inorganic carbon (HCO(3)(−)) with simultaneous hydrogen production and Cd(II) removal in multifunctional microbial electrosynthesis systems (MES), J. Hazard. Mater 371 (2019) 463–473, 10.1016/j.jhazmat.2019.03.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.