Abstract
Background
Determining the best latent structure of negative symptoms in schizophrenia could benefit assessment tools, neurobiological research, and targeted interventions. However, no review systematically evaluated studies that assessed and validated latent models of negative symptoms.
Objective
To identify and evaluate existing latent structure models in the literature of negative symptoms and to determine the best model.
Method
Systematic search of MEDLINE, EMBASE, and Scopus on July 19, 2020, for confirmatory factor analysis models of negative symptoms in patients with schizophrenia. The available evidence was assessed through 2 sets of criteria: (1) study design quality—based on negative symptoms assessment and modeling strategy and (2) psychometric quality and model fit—based on fit indices and factor definition quality.
Results
In total, 22 studies (n = 17 086) from 9 countries were included. Studies differed greatly regarding symptom scales, setting, and sample size (range = 86–6889). Dimensional models included 2–6 factors (median = 4). Twelve studies evaluated competing models and adopted appropriate instruments to assess the latent structure of negative symptoms. The 5-factor and hierarchical models outperformed unitary, 2-factor, and 3-factor models on all direct comparisons, and most of the analyses derived from the Brief Negative Symptom Scale. Considering the quality criteria proposed, 5-factor and hierarchical models achieved excellent fit in just one study.
Conclusions
Our review points out that the 5-factor and hierarchical models represent the best latent structure of negative symptoms, but the immaturity of the relevant current literature may affect the robustness of this conclusion. Future studies should address current limitations regarding psychometric properties and also address biological and clinical validity to refine available models.
Keywords: schizophrenia, negative symptoms, latent structure, factor analysis, systematic review
Introduction
Negative symptoms represent core features of schizophrenia since the first descriptions of the disorder1,2 and refer to a diminution or absence of expected behaviors and inner experiences related to motivation, interest, or expression.3–7 Although a major contributor to poor real-life functioning in people with schizophrenia,3,8,9 no specific approved treatment exists for negative symptoms,10,11 which is explained both by the insufficient knowledge about its neurobiology and by the challenging assessment of these symptoms.
To address such issues, the National Institute of Mental Health (NIMH) organized, in 2005, the NIMH-MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) Consensus Statement on Negative Symptoms.12 It acknowledged 5 constructs within the negative domain: blunted affect (decrease in the observed expression of emotion, ie, facial and vocal expression, and expressive gestures),13,14 alogia (reduction in the quantity of speech and in its spontaneous elaboration)4, anhedonia (diminished capacity to experience pleasant emotions)15, asociality (reduction in social initiative due to decreased interest in forming close relationships with others)16, and avolition (reduced initiation and persistence of goal-directed activity).17
Despite improvements that allowed the development of more sophisticated tools, obtaining accurate and reliable measurements of negative symptoms remains challenging due, in part, to the overlap between the symptoms that make up this dimension. A particular negative symptom may be represented by more than one item of a scale, eg, affective flattening could influence simultaneously social withdrawal and speech spontaneity, thus being accounted for multiple times. In this scenario, factor analysis methods are useful, as they reduce measurement errors by generating latent structure models that present independent unobservable variables (factors) to explain correlations between observed variables (here, the items of a scale).18 Two possible approaches to factor analysis are exploratory factor analysis (EFA) and confirmatory factor analysis (CFA).
EFA is a data reduction technique that infers the presence of latent factors responsible for shared variance among a set of items. It can be useful to generate hypotheses about latent structures; however, EFAs do not specify an underlying structure for the observed variables but rather assume that each item could be related to each latent factor, not ensuring a true validation. EFAs using negative symptom scale items alone find support for 2 dimensions that reflect motivation and pleasure (MAP) and expression (EXP),16,19–22 but CFA models find that 1- and 2-factor solutions usually offer a poor fit for the data.23,24
CFA allows testing a priori hypothesis and objective comparison with other theoretical models. This way, CFA provides more accurate conclusions about latent models, being more appropriate to evaluate the latent structure of negative symptoms. Recently, the evidence supporting the Consensus 5-factor model has been reviewed,25 and results from CFA studies using mainly the Brief Negative Symptom Scale (BNSS) have consistently shown the robustness of the 5-factor and hierarchical models over the 2-factor or unitary models. Despite that, no study has systematically reviewed and evaluated all the literature covering CFA studies that investigated latent models for negative symptoms, even though nonsystematic reviews on the topic are available.4,19,25–28
Thus, we performed a systematic review of the literature searching for latent structure models of negative symptoms in schizophrenia and aimed to identify the best latent structure. To define the best model, we assessed the quality of each study, psychometric parameters, and direct comparisons between models obtained from the same sample.
Methods
Search Strategy
We conducted a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA)29,30 guidelines. Searching MEDLINE, EMBASE, and Scopus from database inception until July 19, 2020, we used the following search terms: (((((((“schizophrenia spectrum and other psychotic disorders”[MeSH Terms])) OR “schizophrenic disorders”) OR schizophrenia)) AND (((“negative symptoms”) OR (“negative symptom scale”)))) AND (((((((“confirmatory factor analysis”) OR “latent structure analysis”) OR “latent structure”) OR “latent model”) OR “validation study”)))))). We also reviewed manually reference lists of included articles and relevant reviews.
Inclusion and Exclusion Criteria
We included original, peer-reviewed articles without language restriction and excluded posters, letters, editorials, and reviews. We included samples with individuals aged ≥16 years old meeting diagnostic criteria for schizophrenia according to Diagnostic and Statistical Manual of Mental Disorders (DSM)-III,31 DSM-IV,32 or DSM-V,33 although the addition of non-affective psychotic disorders was not a reason for exclusion if most of the subjects (>50%) had a schizophrenia diagnosis. We selected studies that performed CFA to evaluate the latent structure of negative symptoms. We excluded studies with EFA and principal component analysis (PCA) since such approaches do not test theoretical models of latent factors.18
Data Collection
Two authors (G.K. and B.H.) independently screened all retrieved records by title and abstract and assessed selected studies in full for eligibility. Any conflicts were resolved by consensus or consulting another investigator (E.D.). Additionally, the following information was extracted independently (G.K. and B.H.) from each included study: metadata, sample characteristics, intraclass correlation coefficient (ICC), negative symptom rating scales, model origin, estimation method, competing models and discriminative criteria employed, the best models, fit indices, and shortest number of items with loadings higher than 0.5 per factor.
Quality Assessment
Quality assessment was based on study design, psychometrics, and model fit.
Study Design.
The study design adequacy was evaluated according to the reliability of the assessment of negative symptoms; appropriateness of the adopted instrument to assess the latent structure of negative symptoms; and modeling strategy, which encompassed model origin, estimation method employed for the CFA, and use of competing model’s approach.
Small samples produce less stable factor analysis solutions.34 However, there is no consensus regarding the minimum sample size for CFAs. This issue cannot be determined by rules of thumb35,36 and would only be adequately tested if done via sensitivity power analyses for each included study,35,36 which is far from the scope of the present study. Therefore, sample size was not included in the quality assessment.
Assessment reliability of negative symptoms was evaluated through ICC. Based on previous studies,37–39 we considered assessments with ICC ≤ 0.5 (or with ICC not informed/performed) as “hardly reliable,” 0.5 < ICC < 0.8 as “reliable,” and ICC ≥ 0.8 as “highly reliable.”
Regarding instruments appropriateness, the Positive and Negative Syndrome Scale (PANSS) and Negative Symptom Assessment (NSA) lack the items needed to cover all constructs determined by the NIMH-MATRICS Consensus and, so, cannot properly assess the latent structure of negative symptoms.23,40 On the other hand, the BNSS, the Clinical Assessment Interview for Negative Symptoms (CAINS), and the Scale for the Assessment of Negative Symptoms (SANS) possess the items to cover the Consensus’ domains, making them more suitable to answer our research question.23,40
EFA or PCA should be followed by CFA using a different sample.41–43 Therefore, studies that performed EFA/PCA to obtain the tested model and used the same sample to perform the CFA and studies that performed CFA based on theoretical models not preceded by EFA/PCA were considered “methodologically limited.”
The selection of an estimator must be based on distributional patterns of the data and assumptions, which makes appropriate to report and justify its choice, while relying on statistical software’s default settings is not advisable.18 Thus, studies that provided no information about the estimation method employed were also considered “methodologically limited.”
The last step of quality assessment of study design consisted of verifying whether the best model presented had been compared with other models and, if so, which were the competing models. The strongest test of a proposed model is to identify and test competing models that represent truly different, but highly plausible, hypothesized structural relationships.36 Using the same sample to test competing theories on the latent structure of negative symptoms provides more robust evidence than testing a single isolated model. Thus, studies that compared different models obtained from the same sample were well regarded, whereas the test of a single model reduced the quality of evidence. Studies that tested models with the same number of factors obtained by different items of the same scale were penalized, as well as studies that compared a model exclusively with the null model, since it is widely accepted that negative symptoms are a separate factor in schizophrenia.4,12,28
Psychometrics and Model Fit.
Psychometric quality and model fit were assessed considering the following parameters: descriptive fit indices—comparative fit index (CFI), non-normed fit index (NNFI), normed fit index (NFI), root mean square error of approximation (RMSEA), weighted root mean square residual (WRMR), or goodness-of-fit index (GFI) —and factor definition quality. We also evaluated information criteria—Akaike Information Criterion (AIC) and/or Bayesian Information Criterion (BIC) —when studies compared non-nested models, with the lowest values being used to determine optimal model fit.44 Chi-square values were not considered to evaluate model fit due to its high sensitivity to sample size as well as the ratio χ 2/df.45,46
The following standards for appropriate fit indices were considered:47–49 GFI > 0.90, CFI > 0.95, NFI > 0.95, NNFI > 0.95, RMSEA < 0.06, and WRMR < 1.00. Models presenting no index with adequate fit were considered to have “poor fit”; models with at least one adequate fit index were classified as “acceptable”; and models showing all indices with adequate fit were considered “excellent.”
Factor definition quality was based on the number of items with loadings > 0.536 per factor. Factors with less than 3 or 4 items per factor require larger sample sizes and are more likely to provide unstable solutions.18,50–54 On the other hand, there is support for the use of the few best indicators for the development of theoretically sophisticated models.55 Thus, we considered factors defined by a single item as “poorly defined,” factors defined by 2 indicators as “acceptable,” and factors defined by at least 3 items as “well defined.” Models obtained through an EFA in a different sample and that used the EFA’s factor loadings to define factors, instead of CFA’s, had their quality lowered.
Additional details of quality assessment are provided in the supplementary material.
Determining the Best Latent Structure
Following the quality assessment, we selected the studies that evaluated competing models derived from the SANS, BNSS, and CAINS. After that, we compiled the models tested in a single table to verify which models performed better according to each study’s criteria. Finally, we evaluated the best models according to the predefined fit quality criteria.
Results
Our search yielded 1680 records found in the database search and 9 through other sources (figure 1). After removing duplicates, 1495 records titles and abstracts were screened, out of which 69 were selected for full-text reading, resulting in 22 included studies in our qualitative analysis.
Fig. 1.
Review flow diagram.
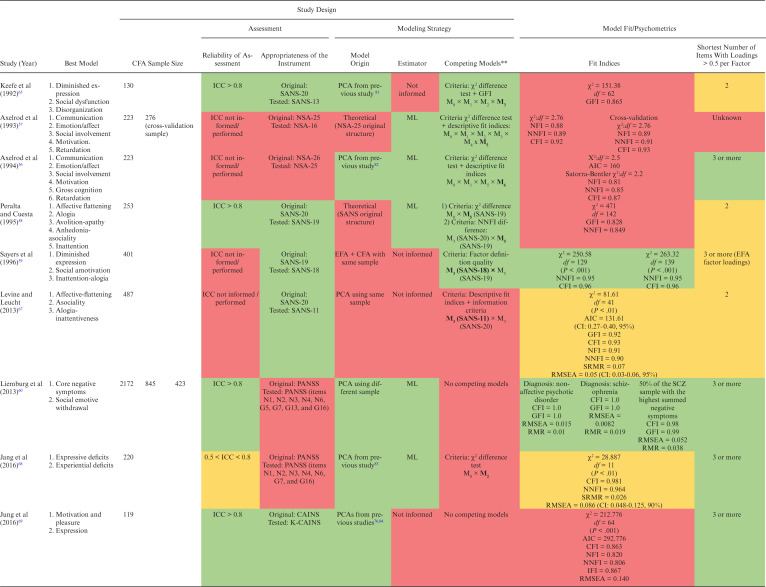
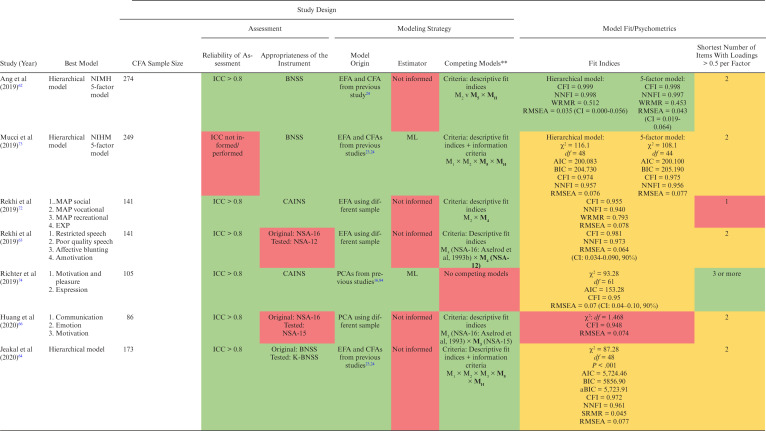
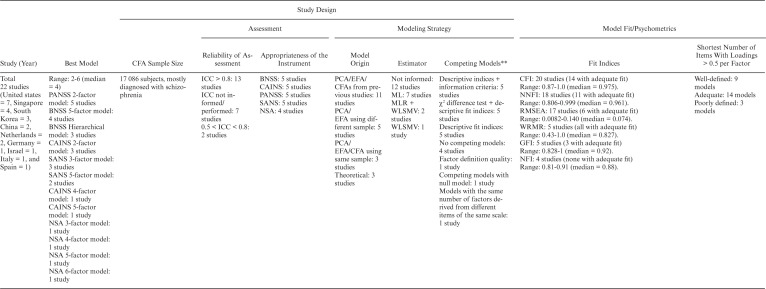
We extracted data from 26 latent models of negative symptoms. Table 1 summarizes the included studies, with 5 published between 1992 and 1996 and 17 between 2013 and 2020. Altogether, 17 086 individuals aged 16–79 years were included (range n = 86–6889), from 9 different countries (United States = 7, Singapore = 4, South Korea = 3, China = 2, Netherlands = 2, Germany = 1, Israel = 1, Italy = 1, and Spain = 1). Samples included chronic inpatients,56–59 outpatients,23,60–64 and patients in mixed settings,24,65–74 mainly diagnosed not only with schizophrenia but also with schizoaffective disorder, schizophreniform disorder, delusional disorder, and psychosis not otherwise specified.
Table 1.
Summary of the Main Findings

|
Note: AIC, Akaike information criterion; aBIC, adjusted Bayesian information criterion; BIC, Bayesian information criterion; aBIC: BNSS, Brief Negative Symptom Scale; CAINS, Clinical Assessment Interview for Negative Symptoms; CAINS-item 1, Motivation for Close Family/Spouse/Partner Relationships; CAINS-item 2, Motivation for Close Friendships/Romantic Relationships; CAINS-item 5, Motivation for Work and School Activities; CAINS-item 6, Expected Pleasurable Work and School Activities—Next Week; CFA, confirmatory factor analysis; ECVI, expected cross-validation index; CFI, comparative fit index; EXP, Expression; G5, Mannerisms and Posturing; G7, Motor Retardation; G13, Disturbance of Volition; G16, Active Social Avoidance; GFI, goodness-of-fit index; ICC, intraclass correlation coefficient; IFI, incremental fit index; K-CAINS, Korean version of the Clinical Assessment Interview for Negative Symptoms; N1, Blunted Affect; N2, Emotional Withdrawal; N3, Poor Rapport; N6, Lack of Spontaneity and Flow of Conversation; MAP, Motivation-pleasure; ML, maximum likelihood; MLR, robust maximum likelihood; NFI, normed fit index; NIMH, National Institute of Mental Health; NNFI, non-normed fit index; NOS, non otherwise specified; NSA, Negative Symptom Assessment; NSA-item 6, Affect: reduced modulation of intensity; NSA-item 16, Slowed movements; PANSS, Positive and Negative Syndrome Scale; PCA, principal component analysis; RMR, root mean square residual; RMSEA, root mean square error; SANS, Scale for the Assessment of Negative Symptoms; SANS-item 4, Poor eye contact; SANS-item 6, Inappropriate affect; SANS-item 9, Poverty of speech; SANS-item 10, Poverty of Content of Speech; SANS-item 11, Blocking; SANS-item 14, Grooming and Hygiene; SANS-item 19, Sexual activity; SANS-item 23, Social Inattentiveness; SRMR, standardized root mean square residual; WLSMV, weighted least square mean and variance; WRMR, weighted root mean square.
**Models in bold outperformed other models.
Study Design
Negative symptom assessment was hardly reliable in 7 studies,56,57,59,61,67,70,73 reliable in 2 studies,68,71 and highly reliable in 13 studies.23,24,58,60,62–66,69,72,74,75
Negative symptom scales varied greatly. Four models were based on the NSA,56,57,63,66 7 on the BNSS,23,24,62,64,73 and the others were equally divided between the SANS,23,58,59,65,67 the PANSS,60,61,68,70,71 and the CAINS.23,69,72,74,75
Regarding models origins, most of the studies based the CFA on a PCA, EFA, and/or CFA from previous studies; 56,61,62,64,65,68–70,73–75 5 studies obtained the CFA model from a PCA or EFA performed in a different sample,24,60,63,66,72 while 3 studies used the same sample of the CFA; 59,67,71 and 3 studies performed the CFA based on a theoretical model not preceded by a PCA or EFA.23,57,58
The most employed estimation method was the maximum likelihood,56–58,60,68,73,74 followed by a combination of robust maximum likelihood and robust weighted least squares,23,24 and the robust weighted least squares alone.61 Twelve studies did not provide information about the estimator.59,62–67,69–72,75
The competing model’s approach was adopted by 18 studies. The most used discriminative criteria were based on the analysis of descriptive fit indices and information criteria,23,24,64,67,73,75 followed by the chi-square difference test combined with the analysis of descriptive fit indices,56–58,65,71 the analysis only of descriptive fit indices,62,63,66,70,72 and factor definition quality.59
Psychometrics and Model Fit
Dimensional models ranged from 260,61,68–71,74,75 to 656 dimensions (median = 4). Two and 5-factor23,24,57,58,62,73 models were the most depicted among studies, followed by hierarchical,62,64,73 3-factor,59,65–67 and 4-factor63,72 structures. The hierarchical model consists of 2 second-order factors reflecting EXP and MAP and 5 first-order factors reflecting the domains of the NIMH consensus development conference.12
We observed high heterogeneity in terms of models and factors composition. For example, none of the studies that evaluated the 3-factor model using the SANS presented a common factor composed of the same indicators. In addition, none of the models derived from the NSA presented the same number of factors. On the other hand, models obtained from the PANSS, BNSS, and CAINS showed greater uniformity.
Fit indices used and their values varied. To assess model fit, older studies used the chi-square test and few descriptive fit indices, whereas more recent articles used modern fit measures and information criteria. The summary of descriptive fit indices is presented in table 2. Regarding factor definition quality, 9 models were considered well-defined,56,60,61,68–71,74,75 14 were adequate,23,24,58,59,62–67,73 and 3 were poorly defined.23,57,72
Table 2.
Summary of Fit Indices
| Fit Index | Range | Median | Reference | Used (nº of Studies) | Adequate (nº of Studies) | Adequacy Rate (%) |
|---|---|---|---|---|---|---|
| CFI | 0.870-1.0 | 0.975 | >0.95 | 20 | 1423,24,59–64,68,70,72–75 | 70.0 |
| NNFI | 0.806-0.999 | 0.961 | >0.95 | 19 | 1123,24,59,61–64,68,70,73,75 | 61.1 |
| RMSEA | 0.0082-0.140 | 0.074 | <0.06 | 17 | 660–62,67,71,75 | 35.3 |
| WRMR | 0.430–1.0 | 0.827 | <1.0 | 5 | 523,61,62,70,72 | 100.0 |
| GFI | 0.828-1.0 | 0.920 | >0.9 | 5 | 361,67,71 | 60.0 |
| NFI | 0.810-0.910 | 0.880 | >0.95 | 4 | 0 | 0 |
Note: CFI, confirmatory factor analysis; GFI, goodness-of-fit index; NFI, normed fit index; NNFI, non-normed fit index; RMSEA, root mean square error of approximation; WRMR, weighted root mean square.
Six models presented poor fit,56–58,65,66,69 14 presented acceptable fit,23,24,63,64,67,68,70–74 and 6 models met requirements for excellent fit.59–62,75
The Best Latent Structure of Negative Symptoms
Table 3 summarizes the studies that used the SANS, BNSS, or CAINS to assess competing models of negative symptoms. The 5-factor and hierarchical models based on the NIMH-MATRICS Consensus outperformed other models in all 5 comparisons using the BNSS and in a single comparison both with the CAINS and the SANS.
Table 3.
Competing models of the SANS, CAINS, and BNSS
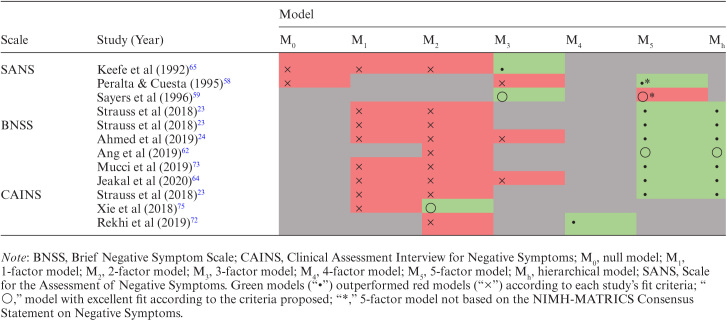
|
Among these studies that directly compared the Consensus 5-factor and hierarchical models with other models, only Ang et al62 obtained models with excellent fit according to the fit criteria proposed, whereas the others presented models with acceptable fit. Ang et al62 used the BNSS and, based on models generated by Strauss et al23 and Ahmed et al24, concluded that the hierarchical model and the 5-factor model outperformed the 2-factor model, with the hierarchical model having an advantage over the 5-factor model. Regarding the studies with acceptable fit, according to the predefined criteria, the superiority of the hierarchical model in relation to the 5-factor model was not unanimous.
After contrasting the best 2 models, Strauss et al23 concluded that the 5-factor model obtained a better fit both with the BNSS and the CAINS, based on the information criteria, but found that the hierarchical model performed slightly better with the SANS. Ahmed et al24 considered both models adequate, based on descriptive fit indices and information criteria, but extended their analyzes of cultural invariance of the BNSS exclusively to the 5-factor model. Mucci et al73 and Jeakal et al64 also used the BNSS and considered both models equally adequate, but Jeakal et al64 only provided the data about the hierarchical model.
Regarding the studies that did not directly compared the Consensus 5-factor and hierarchical models with other models, only Sayers et al59 and Xie et al75 presented models with excellent fit. Sayers et al59 obtained excellent fit both with a 3-factor and a 5-factor SANS model but considered the latter less adequate because it had a factor composed of 2 items. Xie et al75 used the CAINS and concluded that the 2-factor model by Kring et al16 outperformed the unitary model and the 2-factor model by Chan et al76.
Discussion
We analyzed 22 publications addressing the dimensionality of negative schizophrenia symptoms, 12 of which used the SANS, the BNSS, or the CAINS to assess competing models of negative symptoms. The Consensus 5-factor and hierarchical models outperformed unitary, 2-factor, and 3-factor models on direct comparisons, and our results suggest that they currently represent the best latent structure of negative symptoms. However, the reduced number of available studies may affect the robustness of this conclusion.
Twelve studies directly compared models using appropriate instruments to assess the latent structure of negative symptoms, of which only 5 compared the Consensus 5-factor and hierarchical structure to other models, resulting in 5 comparative analysis derived from the BNSS, 1 from the CAINS, and 1 from the SANS. Of note, Strauss et al23 assessed competing models from the BNSS, CAINS, and SANS using a different sample for each instrument. Peralta and Cuesta58 and Sayers et al59 also tested SANS 5-factor models, but both studies presented latent structures with an inattention factor (no longer included in the negative dimension) and grouped anhedonia and asociality into a single factor. Moreover, among the 5-factor and hierarchical Consensus-based models, only those obtained by Ang et al62 showed excellent fit according to the proposed criteria. In sum, direct comparisons favored the Consensus 5-factor and hierarchical model, but psychometric properties are limited.
Indeed, few studies fulfilled all the standard fit requirements proposed, and the RMSEA was the fit index with the lowest adequacy. Since it assesses how well a model fits in the population,18 the RMSEA determines how a proposed model is apart from a perfect model.77 It represents one of the most informative fit indices78 for being sensitive to the number of estimated parameters in the model and relatively little influenced by sample size.18 RMSEA seems to improve—indicating better fit—as more variables are added to the model, which means that the lower the number of items per factor, the worse the RMSEA.79 As most of the instruments have few items to assess each factor, the psychometric criteria did not fully favor the 5-factor and hierarchical model, which should not be considered a criterion for rejecting these models but rather an example of the contrast between requirements for good fit and available instruments. In fact, the only fit index that separated the models obtained by Ahmed et al,24 Strauss et al,23 Mucci et al,73 and Jeakal et al64 from an excellent fit, according to the proposed quality criteria, was the RMSEA, whose cutoff value of 0.08 is also accepted by some authors.18,80
Despite the superiority of the 5-factor and hierarchical models over the 2-factor model in studies that performed comparative analyzes, the neurobiological and clinical evidence for specific constructs of the 5-factor model is still limited, although promising. Previous investigations regarding the underlying neurobiology of negative symptoms may have failed to find neural correlates for the 5 domains due to assessments based on scores of constructs with fewer factors.25 Similarly, past unsuccessful attempts to promote targeted treatments for negative symptoms may, in fact, have been effective for 1 or more of the 5 domains, with positive results being masked by assessments not specific enough for each domain.25 Further studies could fill these gaps assessing negative symptoms with greater granularity, which could, in turn, provide domain-specific therapeutic and neurobiological advances.
Although the present study strengthens the thesis that negative symptoms should be evaluated with greater granularity matching clinical experience, conclusions derived from only 5 studies. Therefore, the field may benefit from additional studies investigating unanswered questions: the 5-factor/hierarchical models are invariant across different disease stages or over time? Other 5-factor models could fit similarly or best? Can a multilevel structure affect the results?
Psychometrics, clinical experience, and neurobiology have convergences and tensions. No single source of evidence can be sufficient. The balance between seeking psychometric and clinical relevance has been especially controversial. We acknowledge that the rejection of models solely based on one single fit index can be excessive. However, merely disregarding fit indices as inadequate can overlook opportunities for new research questions. In the end, we believe that data and clinical utility provide the best guides to the current discussion. In this sense, our results identify some caveats when framing available evidence on the dimensional nature of negative symptoms.
Future investigations of latent structures for negative symptoms should adopt strict procedures regarding the study design, such as calculating the minimum sample size, using modern instruments (BNSS and CAINS), ensuring the highest possible reliability for the assessment of negative symptoms by performing and reporting the ICC, not using the CFA sample in a previous EFA or PCA, reporting the estimation method and justifying its use, and adopting the competing models’ approach. Additionally, we encourage future studies to provide comparative analysis between the CAINS 2-factor, 5-factor, and hierarchical models and, ideally, also between the BNSS and the CAINS using samples jointly assessed by these instruments.
The limitations of this review include the high heterogeneity among studies’ samples and designs (eg, assessment tools and modeling strategies), the small number of studies that analyzed competing latent models of negative symptoms using appropriate instruments, and the lack of a validated method to measure the quality of factor analysis studies. Nonetheless, we operationalized a unified quality assessment according to criteria previously recommended by methodological papers, which enabled a systematic comparison among the included studies. We adopted standard and widely used cutoff points but that may be considered overly rigorous and not completely unanimous in literature. Psychometrics represents an important perspective to validate a construct but does not provide the final answer alone. Thus, we added additional perspectives, ie, the study design and instruments used, to bring a comprehensive view of the field.
Overall, we conclude that the 5-factor and hierarchical models are currently the best conceptualization of negative symptoms. We believe that these results may guide future psychometric studies and facilitate the search for biological and clinical validity of the negative dimension in schizophrenia.
Supplementary Material
Acknowledgments
The authors would like to thank the team of the Interdisciplinary Laboratory of Integrative Neurosciences (Linc) for their support and participation in this research. The research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Dr Correll has been a consultant and/or advisor to or has received honoraria from Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He has provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Rovi, Supernus, and Teva. He received grant support from the Berlin Institute of Health (BIH), Janssen, the National Institute of Mental Health (NIMH), Patient Centered Outcomes Research Institute (PCORI), Takeda, and the Thrasher Foundation. He received royalties from UpToDate and is also a stock option holder of LB Pharma. Dr Lacerda reports personal fees from Daiichi Sankyo Brasil, Pfizer, Janssen Pharmaceutical, Cristalia Produtos Químicos e Farmacêuticos, Mantecorp Indústria Química e Farmacêutica, Sanofi-Aventis, Libbs Farmacêutica, and Eli Lilly and grants from Janssen Pharmaceutical, H Lundbeck A/S, Cristalia Produtos Químicos e Farmacêuticos, Eli Lilly, H Lundbeck A/S, Servier Laboratories, FQM Farma, Hoffman-La Roche, and Forum Pharmaceuticals, outside the submitted work. Dr Gadelha has been a speaker and/or advisor to or has received honoraria from Janssen, Daiichi-Sankyo, Aché, Cristália, and Torrent.
References
- 1.Bleuler E.Dementia Praecox or the Group of Schizophrenias, translated by Zinkin J. New York, NY: International Universities Press; 1950. [Google Scholar]
- 2.Kraepelin E.Dementia praecox and paraphrenia (1919), translated by Barclay RM. Huntingdon. New York, NY: Robert E Publishing Co.; 1971. [Google Scholar]
- 3.Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. 2020;16:519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marder SR, Galderisi S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry. 2017;16(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brian Kirkpatrick BK. Developing concepts in negative symptoms: primary vs secondary and apathy vs expression. J Clin Psychiatry. 2014;75:3–7. [DOI] [PubMed] [Google Scholar]
- 6.Fervaha G, Foussias G, Agid O, Remington G. Amotivation and functional outcomes in early schizophrenia. Psychiatry Res. 2013;210(2):665–668. [DOI] [PubMed] [Google Scholar]
- 7.Konstantakopoulos G, Ploumpidis D, Oulis P, et al. Apathy, cognitive deficits and functional impairment in schizophrenia. Schizophr Res. 2011;133(1–3):193–198. [DOI] [PubMed] [Google Scholar]
- 8.Harvey PD, Strassnig M. Predicting the severity of everyday functional disability in people with schizophrenia: cognitive deficits, functional capacity, symptoms, and health status. World Psychiatry. 2012;11(2):73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galderisi S, Rossi A, Rocca P, et al. ; Italian Network For Research on Psychoses. The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry. 2014;13(3):275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correll CU, Rubio JM, Inczedy-Farkas G, Birnbaum ML, Kane JM, Leucht S. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry. 2017;74(7):675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krogmann A, Peters L, von Hardenberg L, Bödeker K, Nöhles VB, Correll CU. Keeping up with the therapeutic advances in schizophrenia: a review of novel and emerging pharmacological entities. CNS Spectr. 2019;24(S1):38–69. [DOI] [PubMed] [Google Scholar]
- 12.Kirkpatrick B, Fenton WS, Carpenter WT Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32(2):214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry JD, Green MJ, de Lucia A, Restuccia C, McDonald S, O′Donnell M. Emotion dysregulation in schizophrenia: reduced amplification of emotional expression is associated with emotional blunting. Schizophr Res. 2007;95(1–3):197–204. [DOI] [PubMed] [Google Scholar]
- 14.Kirkpatrick B. Progress in the study of negative symptoms. Schizophr Bull. 2014;40(Suppl 2):S101–S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meehl PE. Hedonic capacity: some conjectures. Bull Menninger Clin. 1975;39(4):295–307. [PubMed] [Google Scholar]
- 16.Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. Am J Psychiatry. 2013;170(2):165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam’s razor. Schizophr Bull. 2010;36(2):359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown TA.Confirmatory Factor Analysis for Applied Research. 2nd ed. New York, NY: The Guilford Press; 2015. [Google Scholar]
- 19.Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32(2):238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirkpatrick B, Strauss GP, Nguyen L, et al. The Brief Negative Symptom Scale: psychometric properties. Schizophr Bull. 2011;37(2):300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mucci A, Galderisi S, Merlotti E, et al. ; Italian Network for Research on Psychoses. The Brief Negative Symptom Scale (BNSS): independent validation in a large sample of Italian patients with schizophrenia. Eur Psychiatry. 2015;30(5):641–647. [DOI] [PubMed] [Google Scholar]
- 22.Bischof M, Obermann C, Hartmann MN, et al. The Brief Negative Symptom Scale: validation of the German translation and convergent validity with self-rated anhedonia and observer-rated apathy. BMC Psychiatry. 2016;16(1):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strauss GP, Nuñez A, Ahmed AO, et al. The latent structure of negative symptoms in schizophrenia. JAMA Psychiatry. 2018;75(12):1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed AO, Kirkpatrick B, Galderisi S, et al. Cross-cultural validation of the 5-factor structure of negative symptoms in schizophrenia. Schizophr Bull. 2019;45(2):305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strauss GP, Ahmed AO, Young JW, Kirkpatrick B. Reconsidering the latent structure of negative symptoms in schizophrenia: a review of evidence supporting the 5 consensus domains. Schizophr Bull. 2019;45(4):725–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peralta V, Cuesta MJ. How many and which are the psychopathological dimensions in schizophrenia? Issues influencing their ascertainment. Schizophr Res. 2001;49(3):269–285. [DOI] [PubMed] [Google Scholar]
- 27.Galderisi S, Mucci A, Buchanan RW, Arango C. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry. 2018;5(8):664–677. [DOI] [PubMed] [Google Scholar]
- 28.Blanchard JJ, Kring AM, Horan WP, Gur R. Toward the next generation of negative symptom assessments: the collaboration to advance negative symptom assessment in schizophrenia. Schizophr Bull. 2011;37(2):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269, W64. [DOI] [PubMed] [Google Scholar]
- 30.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Publishing; 1980. [Google Scholar]
- 32.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Publishing; 1994. [Google Scholar]
- 33.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 34.MacCallum RC, Widaman KF, Zhang S, Hong S. Sample size in factor analysis. Psychol Methods. 1999;4:84–99. [Google Scholar]
- 35.Kyriazos TA. Applied psychometrics: sample size and sample power considerations in factor analysis (EFA, CFA) and SEM in general. Psychology. 2018;9:2207–2230. [Google Scholar]
- 36.Hair JF, Black WC, Babin BJ, Anderson RE.. Multivariate Data Analysis, Andover. Hampshire, United Kingdom: Cengage Learning; 2019. [Google Scholar]
- 37.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. [DOI] [PubMed] [Google Scholar]
- 38.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1:30–46. [Google Scholar]
- 39.Portney LG, Watkins MP.. Foundations of Clinical Research: Applications to Practice. Upper Saddle River, NJ: Pearson; 2000. [Google Scholar]
- 40.Marder SR, Kirkpatrick B. Defining and measuring negative symptoms of schizophrenia in clinical trials. Eur Neuropsychopharmacol. 2014;24(5):737–743. [DOI] [PubMed] [Google Scholar]
- 41.Costello AB, Osborne JW. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pract Ass Res Eval. 2005;10(1):7. [Google Scholar]
- 42.Henson RK, Roberts JK. Use of exploratory factor analysis in published research: common errors and some comment on improved practice. Educ Psychol Meas. 2006;66:393–416. [Google Scholar]
- 43.Worthington RL, Whittaker TA. Scale development research: a content analysis and recommendations for best practices. Couns. Psychol. 2006;34:806–838. [Google Scholar]
- 44.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- 45.Schermelleh-Engel K, Moosbrugger H, Müller H. Evaluating the fit of structural equation models: tests of significance and descriptive goodness-of-fit measures. Methods Psychol Res Online. 2003;8:23–74. [Google Scholar]
- 46.Bollen KA. Structural Equations with Latent Variables. New York, NY: Wiley; 1989. [Google Scholar]
- 47.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107(2):238–246. [DOI] [PubMed] [Google Scholar]
- 48.Hu LT, Bentler PM. Evaluating model fit. In: Hoyle RH, ed. Structural Equation Modeling: Concepts, Issues, and Applications. Thousand Oaks, CA: Sage Publications. Inc; 1995:76–99. [Google Scholar]
- 49.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model A Multidiscip J. 1999;6:1–55. [Google Scholar]
- 50.Marsh HW, Hau KT, Balla JR, Grayson D. Is more ever too much? The number of indicators per factor in confirmatory factor analysis. Multivariate Behav Res. 1998;33(2):181–220. [DOI] [PubMed] [Google Scholar]
- 51.Boomsma A, Hoogland JJ. The robustness of LISREL modeling revisited. Struct Equ Model Present Futur. 2001:139–168. [Google Scholar]
- 52.Thompson B.Exploratory and Confirmatory Factor Analysis: Understanding Concepts and Applications. Washington, DC: American Psychological Association; 2004. [Google Scholar]
- 53.Dimitrov DM. Statistical Methods for Validation of Assessment Scale Data in Counseling and Related Fields. Alexandria, VA: American Counseling Association; 2012. [Google Scholar]
- 54.Tabachnick BG, Fidell LS.. Using Multivariate Statistics. Boston, MA: Pearson Education, Inc.; 2013. [Google Scholar]
- 55.Hayduk LA, Littvay L. Should researchers use single indicators, best indicators, or multiple indicators in structural equation models? BMC Med Res Methodol. 2012;12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Axelrod BN, Goldman RS, Woodard JL, Alphs LD. Factor structure of the negative symptom assessment. Psychiatry Res. 1994;52(2):173–179. [DOI] [PubMed] [Google Scholar]
- 57.Axelrod BN, Goldman RS, Alphs LD.. Validation of the 16-item negative symptom assessment. J Psychiatr Res. 1993;27(3):253–258. [DOI] [PubMed] [Google Scholar]
- 58.Peralta V, Cuesta MJ. Negative symptoms in schizophrenia: a confirmatory factor analysis of competing models. Am J Psychiatry. 1995;152(10):1450–1457. [DOI] [PubMed] [Google Scholar]
- 59.Sayers SL, Curran PJ, Mueser KT. Factor structure and construct validity of the Scale for the Assessment of Negative Symptoms. Psychol. Assess. 1996;8:269–280. [Google Scholar]
- 60.Liemburg E, Castelein S, Stewart R, van der Gaag M, Aleman A, Knegtering H; Genetic Risk and Outcome of Psychosis (GROUP) Investigators . Two subdomains of negative symptoms in psychotic disorders: established and confirmed in two large cohorts. J Psychiatr Res. 2013;47(6):718–725. [DOI] [PubMed] [Google Scholar]
- 61.Stiekema APM, et al. Confirmatory factor analysis and differential relationships of the two subdomains of negative symptoms in chronically ill psychotic patients. PLoS One. 2016;11:e0149785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ang MS, Rekhi G, Lee J. Validation of the Brief Negative Symptom Scale and its association with functioning. Schizophr Res. 2019;208:97–104. [DOI] [PubMed] [Google Scholar]
- 63.Rekhi G, Alphs L, Ang MS, Lee J. Clinical utility of the Negative Symptom Assessment-16 in individuals with schizophrenia. Eur Neuropsychopharmacol. 2019;29(12):1433–1441. [DOI] [PubMed] [Google Scholar]
- 64.Jeakal E, Park K, Lee E, Strauss GP, Choi KH. Validation of the Brief Negative Symptom Scale in Korean patients with schizophrenia. Asia Pac Psychiatry. 2020;12(3):e12382. [DOI] [PubMed] [Google Scholar]
- 65.Keefe RS, Harvey PD, Lenzenweger MF, et al. Empirical assessment of the factorial structure of clinical symptoms in schizophrenia: negative symptoms. Psychiatry Res. 1992;44(2):153–165. [DOI] [PubMed] [Google Scholar]
- 66.Huang BJ, Wang Y, Miao Q, Yu X, Pu CC, Shi C. Validation of the Chinese version of the 16-item negative symptom assessment. Neuropsychiatr Dis Treat. 2020;16:1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levine SZ, Leucht S. Psychometric analysis in support of shortening the Scale for the Assessment of Negative Symptoms. Eur Neuropsychopharmacol. 2013;23(9):1051–1056. [DOI] [PubMed] [Google Scholar]
- 68.Jang SK, Choi HI, Park S, et al. A two-factor model better explains heterogeneity in negative symptoms: evidence from the Positive and Negative Syndrome Scale. Front Psychol. 2016;7:707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jung SI, Woo J, Kim YT, Kwak SG. Validation of the Korean-version of the Clinical Assessment Interview for Negative Symptoms of Schizophrenia (CAINS). J Korean Med Sci. 2016;31(7):1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lim J, Lee SA, Lam M, et al. The relationship between negative symptom subdomains and cognition. Psychol Med. 2016;46(10):2169–2177. [DOI] [PubMed] [Google Scholar]
- 71.Khan A, Liharska L, Harvey PD, Atkins A, Ulshen D, Keefe RSE. Negative symptom dimensions of the Positive and Negative Syndrome Scale across geographical regions: implications for social, linguistic, and cultural consistency. Innov Clin Neurosci. 2017;14(11–12):30–40. [PMC free article] [PubMed] [Google Scholar]
- 72.Rekhi G, Ang MS, Yuen CKY, Ng WY, Lee J. Assessing negative symptoms in schizophrenia: validity of the clinical assessment interview for negative symptoms in Singapore. Schizophr Res. 2019;206:177–182. [DOI] [PubMed] [Google Scholar]
- 73.Mucci A, Vignapiano A, Bitter I, et al. A large European, multicenter, multinational validation study of the Brief Negative Symptom Scale. Eur Neuropsychopharmacol. 2019;29(8):947–959. [DOI] [PubMed] [Google Scholar]
- 74.Richter J, Hesse K, Schreiber L, et al. Evidence for two distinct domains of negative symptoms: confirming the factorial structure of the CAINS. Psychiatry Res. 2019;271:693–701. [DOI] [PubMed] [Google Scholar]
- 75.Xie DJ, Shi HS, Lui SSY, et al. Cross cultural validation and extension of the Clinical Assessment Interview for Negative Symptoms (CAINS) in the Chinese context: evidence from a spectrum perspective. Schizophr Bull. 2018;44(suppl_2):S547–S555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chan RCK, et al. Validation of the Chinese version of the Clinical Assessment Interview for Negative Symptoms (CAINS): a preliminary report. Front. Psychol. 2015:6:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xia Y, Yang Y. RMSEA, CFI, and TLI in structural equation modeling with ordered categorical data: the story they tell depends on the estimation methods. Behav Res Methods. 2019;51(1):409–428. [DOI] [PubMed] [Google Scholar]
- 78.Diamantopoulos A, Siguaw JA.. Introducing LISREL: A Guide for the Uninitiated. Thousand Oaks, CA: SAGE Publications; 2000. [Google Scholar]
- 79.Kenny DA, McCoach DB. Effect of the number of variables on measures of fit in structural equation modeling. Struct Equ Model A Multidiscip. J. 2003;10:333–351. [Google Scholar]
- 80.Browne MW, Cudeck R. Alternative ways of assessing model fit. Sociol Methods Res. 1992;21:230–258. [Google Scholar]
- 81.Liddle PF. The symptoms of chronic schizophrenia: a re-examination of the positive-negative dichotomy. Brit J Psychiat. 1987;151(2):145–151. [DOI] [PubMed] [Google Scholar]
- 82.Alphs LD, Summerfelt A, Lann H, Muller RJ. The negative symptom assessment: a new instrument to assess negative symptoms of schizophrenia. Psychopharmacol Bull. 1989;25:159–163. [PubMed] [Google Scholar]
- 83.Fervaha G, Foussias G, Agid O, Remington G. Motivational and neurocognitive deficits are central to the prediction of longitudinal functional outcome in schizophrenia. Acta Psychiatr Scand. 2014;130:290–299. [DOI] [PubMed] [Google Scholar]
- 84.Engel M, Fritzsche A, Lincoln TM. Validation of the German version of the clinical assessment interview for negative symptoms (CAINS). Psychiatry Res. 2014;220: 659–663. [DOI] [PubMed] [Google Scholar]
- 85.Jiang J, Sim K, Lee J. Validated five-factor model of positive and negative syndrome scale for schizophrenia in Chinese population. Schizophr Res. 2013;143(1):38–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



