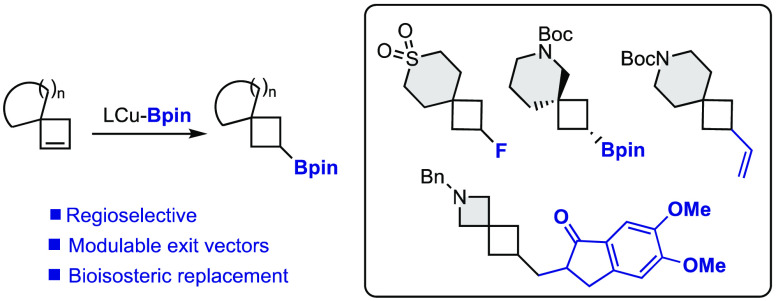
Abstract
We present a strategy for the synthesis of spirocyclic cyclobutanes with modulable exit vectors based on the regioselective monoborylation of spirocyclobutenes. Using an inexpensive copper salt and a commercially available bidentate phosphine, a broad variety of borylated spirocycles have been prepared with complete regiocontrol. The boryl moiety provides a synthetic handled for further functionalization, allowing access to a wide array of spirocyclic building blocks from a common intermediate.
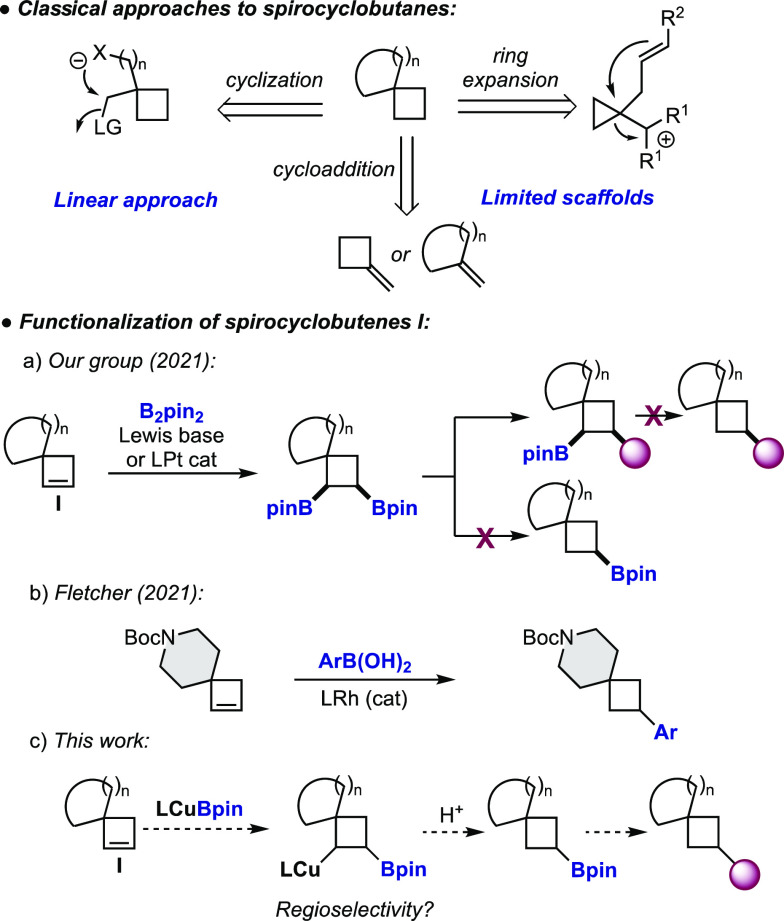
The development of tools that allow for the modulation of the physical and biological properties of lead compounds is an essential task in drug discovery programs. In this scenario, spirocyclic compounds are receiving great attention, especially those containing small rings.1 Among them, cyclobutane-containing spirocycles represent an interesting subclass, as they provide rigidity and tridimensionality with well-defined exit vectors.2 One of the most common strategies to build these spirocycles involves an intramolecular SN2 reaction or intramolecular addition to a carbonyl (Scheme 1).3 One of the drawbacks of this approach is that it is linear in design. For each spirocycle prepared, a different precursor is needed. Additionally, there is very little room to introduce substituents in the cyclobutane ring. A second strategy widely used to prepare spirocyclobutanes is the [2 + 2] or higher-order cycloaddition reaction, starting from an exocyclic alkene.4 Cyclopropanes have also been used to build the four-membered ring through ring expansion, starting from especially design precursors.5 These two approaches have the advantage of introducing structural complexity in a single step but only allow access to very specific scaffolds (Scheme 1).
Scheme 1. Synthesis of Spirocyclobutanes.
Recently, we wondered if spirocyclobutenes I could serve as a template for the preparation of functionalized spirocycles through selective functionalization of the double bond. Although a few spirocyclobutenes had been prepared in the literature, we were surprised to find that their use in catalytic transformations remained at the time virtually unexplored. With this idea, we recently developed the diboration of spirocyclobutenes I promoted by a Lewis base or a platinum catalyst (Scheme 1, a).6
Selective functionalization of the two boryl moieties in the products allowed us to prepare a wide array of spirocyclic building blocks, 2,3-disubstituted, with control in the nature and the directionality of the substituents in the cyclobutane ring. One of the limitations that we found was the inability to monofunctionalize the double bond using this approach. Despite significant experimentation, we could not prepare 3-monosubstituted derivatives through protodeboration of diborylated or monoborylated products (Scheme 1, a). During the preparation of this manuscript, in the context of a wider study, Fletcher and co-workers reported an elegant regioselective rhodium-catalyzed hydroarylation of spirocyclic piperidine and pyrrolidine derivatives (Scheme 1, b).7 We thought that the monoborylation of spirocyclobutenes could provide access to a variety of novel drug-like building blocks that nicely complement those prepared through diboration and hydroarylation.
Inspired by our previous work on the enantioselective desymmetrization of meso cyclobutenes,8 we envisioned that in situ generated copper–boryl complexes could react with the strained alkene in I through a migratory insertion/protonation sequence to provide monoborylated spirocycles (Scheme 1, c).9 The main challenge here, that was not present in our previous study, was the control of the regioselectivity in the insertion step.10
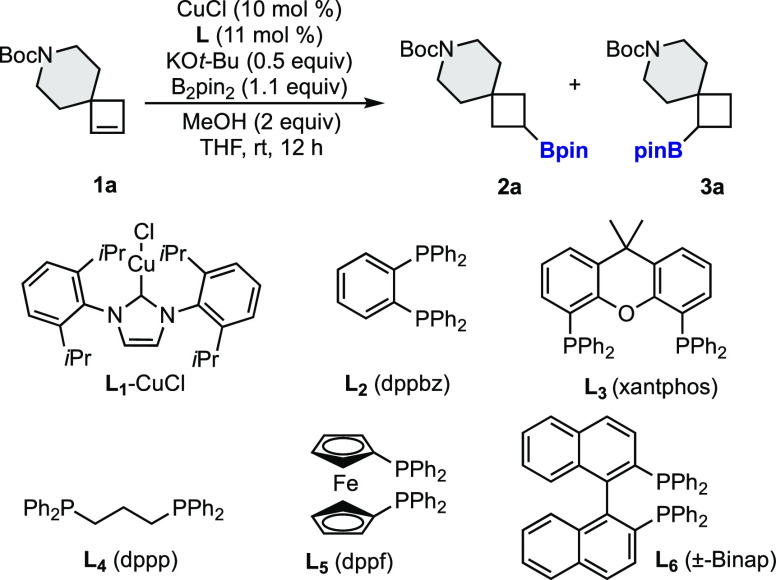
At the outset of our study, it was not obvious that the presence of the spirocyclic quaternary carbon would discriminate between the ligand–copper unit and the boryl moiety of the copper–boryl complex. Indeed, we found that the ligand had a profound effect on the regioselectivity outcome (Table 1). Mixing spirocyclobutene 1a with the NHC–CuCl complex L1–CuCl (10 mmol %), B2pin2 (1.1 equiv), and MeOH (2 equiv) in THF provided monoborylated compounds 2a and 3a in good yield but with low regioselectivity (65:35 mixture, Table 1, entry 1). Using dppbz (L2), a bidentate phosphine with a small bite angle (βn = 83°), a 50:50 mixture of regioisomers was obtained (Table 1, entry 2). Remarkably, L3 xantphos (βn = 108°) afforded borylated spirocycle 2a in 86% yield as a single regioisomer (Table 1, entry 3). Dppp (L4, βn = 91°), dppf (L5, βn = 99°), and BINAP (L6, βn = 93°), bidentate phosphines with bite angles between L2 and L3, provided moderate regioselectivities (Table 1, entries 4–6). The catalyst loading could be reduced to 5 mol %, providing 2a as a single regioisomer although in lower yield. We also tested the possibility of preparing boronic ester 2a through borylation of the corresponding cyclobutyl bromide.11 Under the conditions optimized for the cyclobutene, compound 2a was obtained in 39% yield.12 The structural assignment of regioisomer 2a was confirmed by oxidation of the C–B bond and comparison of the 1H NMR data of the product with those of the same alcohol prepared through reduction of the corresponding cyclobutanone.12
Table 1. Effect of the Ligand in the Copper-Catalyzed Borylation.
| entrya | L | 2a:3ab | yield (%)c |
|---|---|---|---|
| 1 | L1 | 65:35 | 71 |
| 2 | L2 | 50:50 | 84 |
| 3 | L3 | ≥98:2 | 86 |
| 4 | L4 | 83:17 | 58 |
| 5 | L5 | 64:36 | 79 |
| 6 | L6 | 73:27 | 69 |
| 7d | L3 | ≥98:2 | 69 |
| 8 | — | 60:40 | 13 |
Reaction conditions: 1a (0.1 mmol), B2pin2 (0.11 mmol), KOt-Bu (0.5 equiv), CuCl (10 mol %), L (11 mol %), MeOH (0.2 mmol), THF (0.2 M).
Determined by 1H NMR.
Isolated yields.
With 5 mol % of CuCl and 6 mol % of L3.
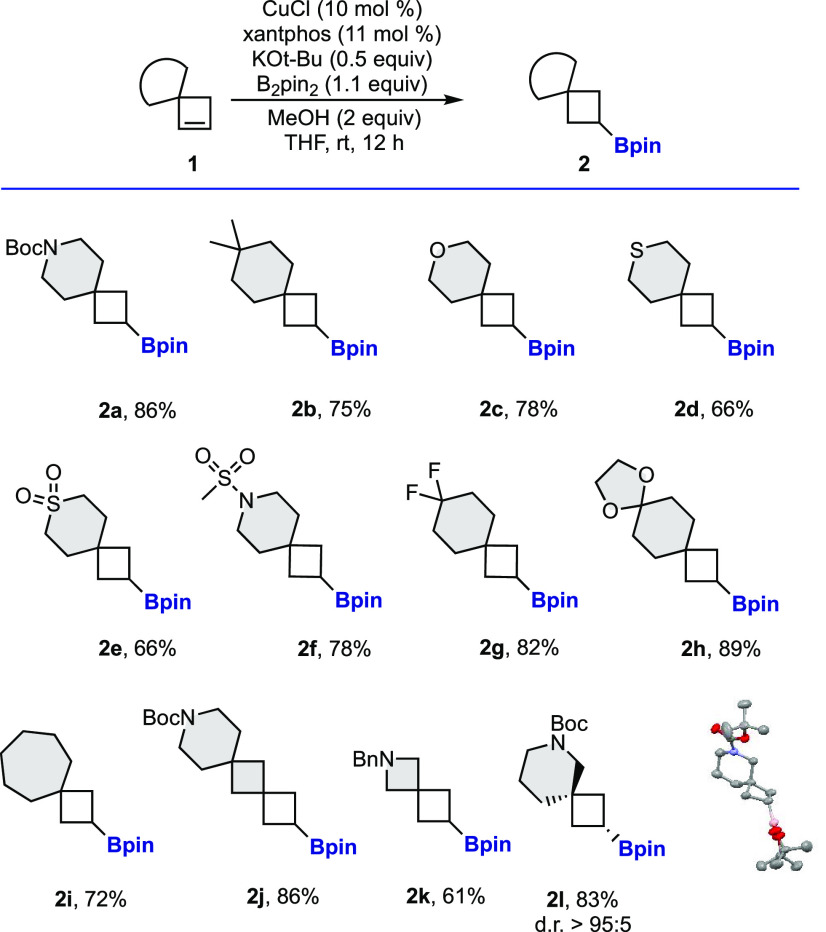
With these conditions in hand, we prepared in a straightforward manner a wide variety of novel borylated building blocks (Scheme 2). Monoborylated spiro[3.5]nonanes (2a–2h) with different functional groups were synthesized. Ether, thioether, sulfone, sulfonamide, difluoromethane, and acetal are different connectors that can be embedded in the spirocyclic framework. Additionally, the size of the ring attached to the cyclobutene could be modified. Spiro[3.3]heptane (2j, 2k) and spiro[3.6]decane (2i) ring systems were prepared as single regioisomers in good yields. Moreover, a nonsymmetric spirocyclobutene afforded diborylated spirocycle 2l as a single diastereomer.13
Scheme 2. Regioselective Borylation of Spirocyclobutenes,
Reaction conditions: 1 (0.2 mmol), B2pin2 (0.22 mmol), KOt-Bu (0.5 equiv), CuCl (10 mol %), xantphos (11 mol %), MeOH (0.4 mmol), and THF (0.2 M).
Isolated yields.
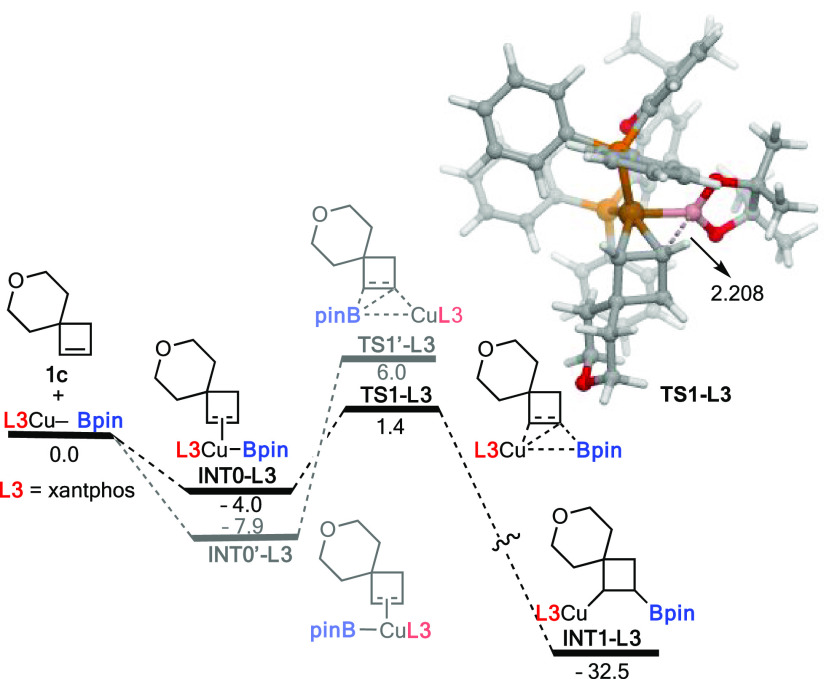
Density functional theory (DFT) calculations were carried out at the dispersion-corrected PCM (tetrahydrofuran)/B3LYP-D3/def2-SVP level (see computational details in the SI) to understand the complete regioselectivity observed in the transformation when xantphos was used as a ligand. According to the computed reaction profile involving 1c and L3Cu-Bpin (Figure 1), the regioselectivity takes place in the initial migratory insertion step, where the associated transition state leading to the observed regioisomer (TS1-L3) lies 4.6 kcal/mol below that leading to the opposite regioisomer (TS1′-L3). This is very likely due to unfavorable steric interactions between the BPin and tetrahydropyran fragments in the latter saddle point which are not present in the favored TS1-L3.14
Figure 1.
Computed reaction profile for the copper-catalyzed borylation of 1c. Relative free energies (ΔG, at 298 K) are given in kcal/mol. All data have been computed at the PCM (tetrahydrofuran)/B3LYP-D3/def2-SVP level.
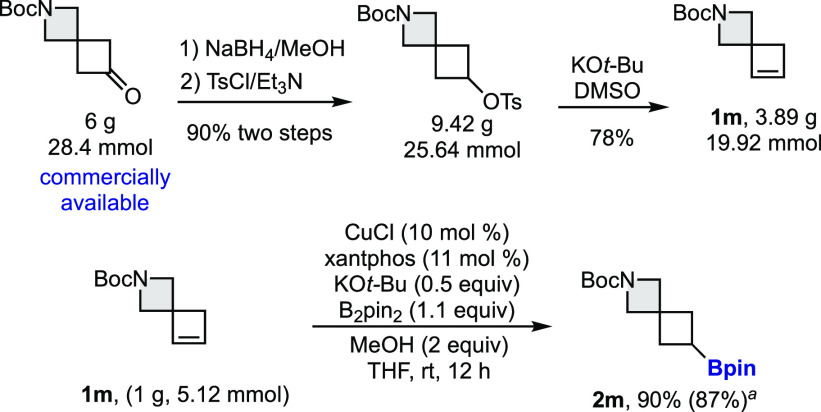
The preparation of the spirocyclobutenes and the copper-catalyzed borylation was effectively scaled up to prepare compounds 1m and 2m (Scheme 3). The borylation of 1g of cyclobutene 1m afforded borylated spirocycle 2m in 87% yield (Scheme 3).
Scheme 3. Gram-Scale Reactions.
Yield starting from 0.2 mmol of 1m.
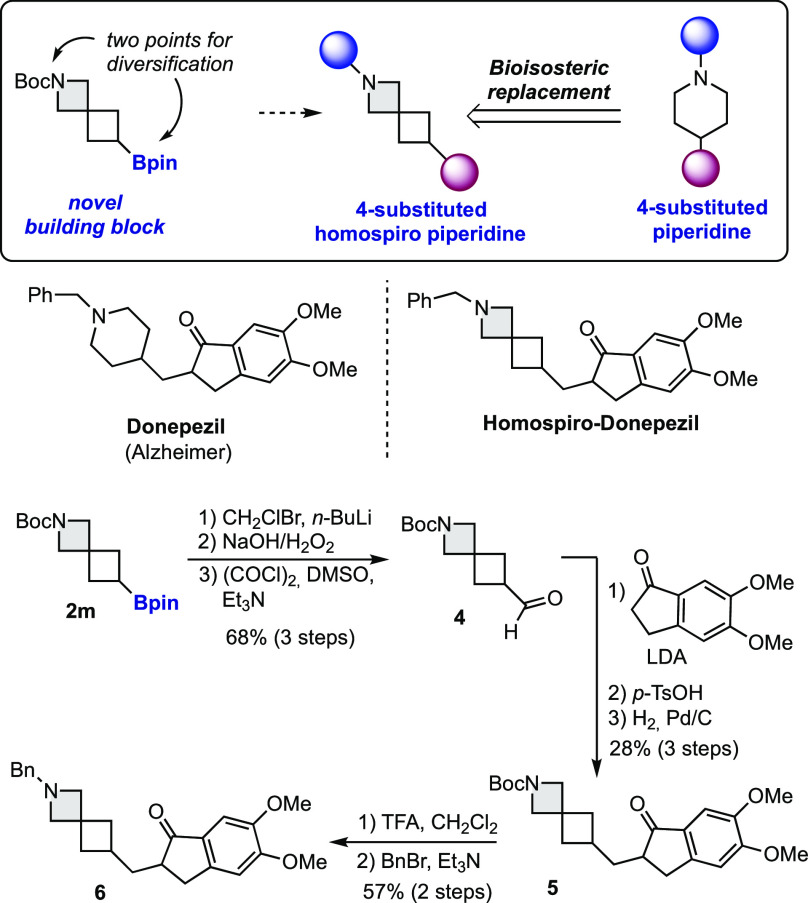
One of the interesting features of small ring spirocycles is their potential use for bioisosteric replacement of commonly used heterocycles.2 In particular, the 2-azaspiro[3.3]heptane ring present in compound 2m has been proposed as a bioisostere of the piperidine ring with improved water solubility.1b 4-Substituted piperidines are widely present in commercialized drugs and lead compounds.15 Spirocycle 2m, with two handles for diversification, represents an ideal novel building block to substitute the piperidine ring for the homospiro moiety in libraries of compounds (Scheme 4). To highlight the synthetic potential of this approach, we have prepared a conformationally restricted analogue of the FDA-approved drug donepezil in which the 4-substituted piperidine ring has been replaced by the homospiro piperidine framework. Starting from monoborylated spirocycle 2m, Matteson homologation followed by double oxidation afforded aldehyde 4. Then, aldol reaction, dehydration, and hydrogenation of the double bond provided intermediate 5. Finally, deprotection of the azaspirocycle and benzylation afforded donepezil derivative 6.
Scheme 4. Synthesis of Homospiro-donepezil.
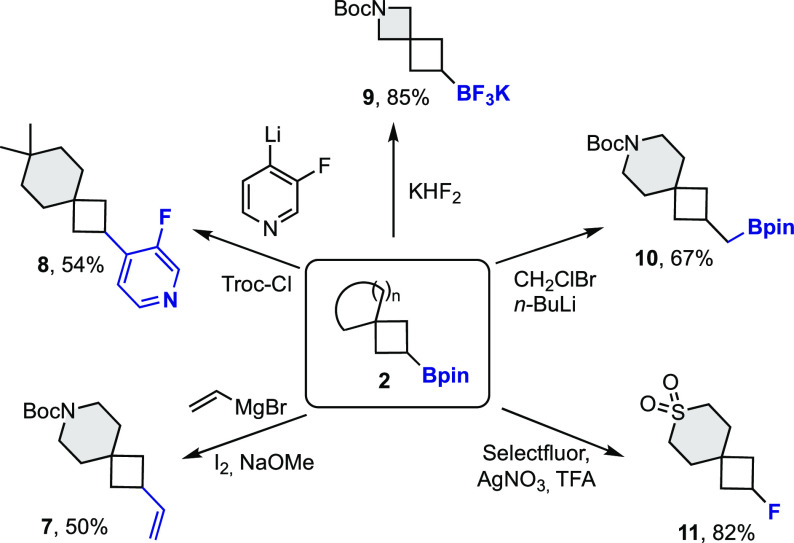
Finally, we have explored the possibility to introduce different functional groups in the spirocycle through C–B bond functionalization. Zweifel olefination (compound 7),16 Aggarwal’s cross coupling (compound 8),17 trifluoroborate formation (compound 9), Matteson homologation (compound 10), and fluorination (compound 11)18 have been efficiently carried out with spirocyles 2 (Scheme 5). These transformations highlight the potential of the method to prepare a broad set of novel spirocyclic compounds from a common intermediate.
Scheme 5. Carbon–Boron Bond Functionalization.
In summary, we have shown that spirocyclobutenes are suitable substrates for the regioselective preparation of monoborylated spirocycles under copper-catalyzed conditions. The regioselectivity of the transformation is highly dependent on the ligand used, and it can be controlled completely with xantphos, a commercially available bidentate phosphine. This strategy allows easy access to a broad variety of spirocyclic building blocks, most of them not accessible by known methods.
Acknowledgments
We thank the European Research Council (ERC-337776) and MINECO and MICIIN (CTQ2016-78779-R and PID2019-107380GB-I00 to M.T.; PID2019-106184GB-I00 and RED2018-102387-T to I.F.) for financial support. L.N. thanks Comunidad de Madrid for a predoctoral fellowship. We acknowledge Dr. Josefina Perles (UAM) for X-ray structure analysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.1c02645.
Synthetic procedures and full characterization for all new compounds and NMR spectra (PDF)
Accession Codes
CCDC 2041652 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Author Contributions
§ L.N. and L.T. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- a Lovering F.; Bikker J.; Humblet C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]; b Burkhard J. A.; Wagner B.; Fischer H.; Schuler F.; Müller K.; Carreira E. M. Synthesis of Azaspirocycles and their Evaluation in Drug Discovery. Angew. Chem., Int. Ed. 2010, 49, 3524–3527. 10.1002/anie.200907108. [DOI] [PubMed] [Google Scholar]; c Zheng Y.; Tice C. M.; Singh S. B. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. 10.1016/j.bmcl.2014.06.081. [DOI] [PubMed] [Google Scholar]; d Zheng Y.-J.; Tice C. M. The utilization of spirocyclic scaffolds in novel drug discovery. Expert Opin. Drug Discovery 2016, 11, 831–834. 10.1080/17460441.2016.1195367. [DOI] [PubMed] [Google Scholar]; e Voss F.; Schunk S.; Steinhagen H. Spirocycles as Privileged Structural Motifs in Medicinal Chemistry. RSC Drug Discovery Series 2015, 439–458. 10.1039/9781782622246-00439. [DOI] [Google Scholar]; f Kirichok A. A.; Shton I.; Kliachyna M.; Pishel I.; Mykhailiuk P. K. 1-Substituted 2-Azaspiro[3.3]heptanes: Overlooked Motifs for Drug Discovery. Angew. Chem., Int. Ed. 2017, 56, 8865–8869. 10.1002/anie.201703801. [DOI] [PubMed] [Google Scholar]; g Muller G.; Berkenbosch T.; Benningshof J. C. J.; Stumpfe D.; Bajorath J. Charting Biologically Relevant Spirocyclic Compound Space. Chem. - Eur. J. 2017, 23, 703–710. 10.1002/chem.201604714. [DOI] [PubMed] [Google Scholar]; h Kotha S.; Panguluri N. R.; Ali R. Design and Synthesis of Spirocycles. Eur. J. Org. Chem. 2017, 2017, 5316–5342. 10.1002/ejoc.201700439. [DOI] [Google Scholar]
- Carreira E. M.; Fessard T. C. Four-Membered Ring-Containing Spirocycles: Synthetic Strategies and Opportunities. Chem. Rev. 2014, 114, 8257–8322. 10.1021/cr500127b. [DOI] [PubMed] [Google Scholar]
- Selected recent examples:; a Burkhard J. A.; Guérot C.; Knust H.; Rogers-Evans M.; Carreira E. M. Synthesis and Structural Analysis of a New Class of Azaspiro[3.3]heptanes as Building Blocks for Medicinal Chemistry. Org. Lett. 2010, 12, 1944–1947. 10.1021/ol1003302. [DOI] [PubMed] [Google Scholar]; b Burkhard J. A.; Guérot C.; Knust H.; Carreira E. M. Expanding the Azaspiro[3.3]heptane Family: Synthesis of Novel Highly Functionalized Building Blocks. Org. Lett. 2012, 14, 66–69. 10.1021/ol2028459. [DOI] [PubMed] [Google Scholar]; c Kubota K.; Yamamoto E.; Ito H. Copper(I)-Catalyzed Borylative exo-Cyclization of Alkenyl Halides Containing Unactivated Double Bond. J. Am. Chem. Soc. 2013, 135, 2635–2640. 10.1021/ja3104582. [DOI] [PubMed] [Google Scholar]; d Royes J.; Ni S.; Farré A.; La Cascia E.; Carbó J. J.; Cuenca A. B.; Maseras F.; Fernández E. Copper-Catalyzed Borylative Ring Closing C–C Coupling toward Spiro- and Dispiroheterocycles. ACS Catal. 2018, 8, 2833–2838. 10.1021/acscatal.8b00257. [DOI] [Google Scholar]; e Reddy L. R.; Waman Y.; Kallure P.; Nalivela K. S.; Begum Z.; Divya T.; Kotturi S. Asymmetric synthesis of 1-substituted 2-azaspiro[3.3]heptanes: important motifs for modern drug discovery. Chem. Commun. 2019, 55, 5068–5070. 10.1039/C9CC00863B. [DOI] [PubMed] [Google Scholar]; f Whyte A.; Mirabi B.; Torelli A.; Prieto L.; Bajohr J.; Lautens M. Asymmetric Synthesis of Boryl-Functionalized Cyclobutanols. ACS Catal. 2019, 9, 9253–9258. 10.1021/acscatal.9b03216. [DOI] [Google Scholar]
- Selected recent examples:; a Qi L.-W.; Yang Y.; Gui Y.-Y.; Zhang Y.; Chen F.; Tian F.; Peng L.; Wang L.-X. Asymmetric Synthesis of 3,3′-Spirooxindoles Fused with Cyclobutanes through Organocatalytic Formal [2 + 2] Cycloadditions under H-Bond-Directing Dienamine Activation. Org. Lett. 2014, 16, 6436–6439. 10.1021/ol503266q. [DOI] [PubMed] [Google Scholar]; b Halskov K. S.; Kniep F.; Lauridsen V. H.; Iversen E. H.; Donslund B. S.; Jørgensen K. A. Organocatalytic Enamine-Activation of Cyclopropanes for Highly Stereoselective Formation of Cyclobutanes. J. Am. Chem. Soc. 2015, 137, 1685–1691. 10.1021/ja512573q. [DOI] [PubMed] [Google Scholar]; c Chalyk B. A.; Butko M. V.; Yanshyna O. O.; Gavrilenko K. S.; Druzhenko T. V.; Mykhailiuk P. K. Chem. - Eur. J. 2017, 23, 16782–16786. 10.1002/chem.201702362. [DOI] [PubMed] [Google Scholar]; d Poplata S.; Bach T. Enantioselective Intermolecular [2 + 2] Photocycloaddition Reaction of Cyclic Enones and Its Application in a Synthesis of (−)-Grandisol. J. Am. Chem. Soc. 2018, 140, 3228–3231. 10.1021/jacs.8b01011. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Demchuk O. P.; Hryshchuk O. V.; Vashchenko B. V.; Kozytskiy A. V.; Tymtsunik A. V.; Komarov I. V.; Grygorenko O. O. Photochemical [2 + 2] Cycloaddition of Alkenyl Boronic Derivatives: An Entry into 3-Azabicyclo[3.2.0]heptane Scaffold. J. Org. Chem. 2020, 85, 5927–5940. 10.1021/acs.joc.0c00265. [DOI] [PubMed] [Google Scholar]
- Zhao C.-G.; Feng Z.-T.; Xu G.-Q.; Gao A.; Chen J.-W.; Wang Z.-Y.; Xu P.-F. Highly Enantioselective Construction of Strained Spiro[2,3]hexanes through a Michael Addition/Ring Expansion/Cyclization Cascade Angew. Angew. Chem., Int. Ed. 2020, 59, 3058–3062. 10.1002/anie.201912834. [DOI] [PubMed] [Google Scholar]
- Nóvoa L.; Trulli L.; Parra A.; Tortosa M. Stereoselective Diboration of Spirocyclobutenes: A Platform for the Synthesis of Spirocycles with Orthogonal Exit Vectors. Angew. Chem., Int. Ed. 2021, 60, 11763–11768. 10.1002/anie.202101445. [DOI] [PubMed] [Google Scholar]
- Goetzke F. W.; Hell A. M. L.; van Dijk L.; Fletcher S. P. A Catalytic Asymmetric Cross-coupling Approach to the Synthesis of Cyclobutanes. Nat. Chem. 2021, 10.1038/s41557-021-00725-y. [DOI] [PubMed] [Google Scholar]
- Guisán-Ceinos M.; Parra A.; Martín-Heras V.; Tortosa M. Enantioselective Synthesis of Cyclobutylboronates via a Copper-Catalyzed Desymmetrization Approach. Angew. Chem., Int. Ed. 2016, 55, 6969–6972. 10.1002/anie.201601976. [DOI] [PubMed] [Google Scholar]
- For a copper-catalyzed hydroboration of benzylidenecyclobutenes, see:Kang T.; Erbay T. G.; Xu K. L.; Gallego G. M.; Burtea A.; Nair S. K.; Patman R. L.; Zhou R.; Sutton S. C.; McAlpine I. J.; Liu P.; Engle K. M. Multifaceted Substrate–Ligand Interactions Promote the Copper-Catalyzed Hydroboration of Benzylidenecyclobutanes and Related Compounds. ACS Catal. 2020, 10, 13075–13083. 10.1021/acscatal.0c03622. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this case, the regioselectivity is controlled by the presence of an aryl group in the exocyclic alkene, which places the copper atom in the benzylic position after the migratory insertion.
- For seminal contributions:; a Ito H.; Yamanaka H.; Tateiwa J.; Hosomi A. Boration of an α,β-Enone Using a Diboron Promoted by a Copper(I)–Phosphine Mixture Catalyst. Tetrahedron Lett. 2000, 41, 6821–6825. 10.1016/S0040-4039(00)01161-8. [DOI] [Google Scholar]; b Takahashi K.; Ishiyama T.; Miyaura N. Addition and Coupling Reactions of Bis(Pinacolato)Diboron Mediated by CuCl in the Presence of Potassium Acetate. Chem. Lett. 2000, 29, 982–983. 10.1246/cl.2000.982. [DOI] [Google Scholar]; For recent reviews on copper-catalyzed borylations, see:; c Neeve E. C.; Geier S. J.; Mkhalid I. A. I.; Westcott S. A.; Marder T. B. Diboron(4) Compounds: From Structural Curiosity to Synthetic Workhorse. Chem. Rev. 2016, 116, 9091–9161. 10.1021/acs.chemrev.6b00193. [DOI] [PubMed] [Google Scholar]; d Yoshida H. Borylation of Alkynes under Base/Coinage Metal Catalysis: Some Recent Developments. ACS Catal. 2016, 6, 1799–1811. 10.1021/acscatal.5b02973. [DOI] [Google Scholar]; e Hemming D.; Fritzemeier R.; Westcott S. A.; Santos W. L.; Steel P. G. Copper-Boryl Mediated Organic Synthesis. Chem. Soc. Rev. 2018, 47, 7477–7494. 10.1039/C7CS00816C. [DOI] [PubMed] [Google Scholar]; f Hoveyda A. H.; Koh M. J.; Lee K.; Lee J.. Catalytic, Enantioselective, Copper-Boryl Additions to Alkenes. Organic Reactions; Wiley, 2019; pp 959–1056. [Google Scholar]; g Parra A.; Trulli L.; Tortosa M.. Copper-Catalyzed Addition of Diboron Species. PATAI’S Chemistry of Functional Groups; Wiley, 2020; pp 1–82. [Google Scholar]; h Whyte A.; Torelli A.; Mirabi B.; Zhang A.; Lautens M. Copper-Catalyzed Borylative Difunctionalization of π-Systems. ACS Catal. 2020, 10, 11578–11622. 10.1021/acscatal.0c02758. [DOI] [Google Scholar]; i Xue W.; Oestreich M. Beyond Carbon: Enantioselective and Enantiospecific Reactions with Catalytically Generated Boryl- and Silylcopper Intermediates. ACS Cent. Sci. 2020, 6, 1070–1081. 10.1021/acscentsci.0c00738. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Corpas J.; Mauleón P.; Gómez-Arrayás R.; Carretero J. C. Transition-Metal-Catalyzed Functionalization of Alkynes with Organoboron Reagents: New Trends, Mechanistic Insights, and Applications. ACS Catal. 2021, 11, 7513–7551. 10.1021/acscatal.1c01421. [DOI] [Google Scholar]
- Ito H.; Kubota K. Copper(I)-Catalyzed Boryl Substitution of Unactivated Alkyl Halides. Org. Lett. 2012, 14, 890–893. 10.1021/ol203413w. [DOI] [PubMed] [Google Scholar]
- See Supporting Information for details.
- The relative stereochemistry of compound 2l was assigned by single crystal X-ray crystallography. See Supporting Information for details.
- With dppbz as ligand (L2, Table 1), the ΔΔG≠ between the corresponding transition states (TS1-L2 and TS1′-L2) decreased to 2 kcal/mol, which is in qualitative agreement with the lower regioselectivity observed for this ligand. See Supporting Information for details.
- For a recent review, see:; a Goel P.; Alam O.; Naim M. J.; Nawaz F.; Iqbal M.; Alam M. I. Recent advancement of piperidine moiety in treatment of cancer- A review. Eur. J. Med. Chem. 2018, 157, 480–502. 10.1016/j.ejmech.2018.08.017. [DOI] [PubMed] [Google Scholar]; For selected recent references, see:; b Velcicky J.; Miltz W.; Oberhauser B.; Orain D.; Vaupel A.; Weigand K.; King J. D.; Littlewood-Evans A.; Nash M.; Feifel R.; Loetscher P. Development of Selective, Orally Active GPR4 Antagonists with Modulatory Effects on Nociception, Inflammation, and Angiogenesis. J. Med. Chem. 2017, 60, 3672–3683. 10.1021/acs.jmedchem.6b01703. [DOI] [PubMed] [Google Scholar]; c Menhaji-Klotz E.; Hesp K. D.; Londregan A. T.; Kalgutkar A. S.; Piotrowski D. W.; Boehm M.; Song K.; Ryder T.; Beaumont K.; Jones R. M.; Atkinson K.; Brown J. A.; Litchfield J.; Xiao J.; Canterbury D. P.; Burford K.; Thuma B. A.; Limberakis C.; Jiao W.; Bagley S. W.; Agarwal S.; Crowell D.; Pazdziorko S.; Ward J.; Price D. A.; Clerin V. Discovery of a Novel Small-Molecule Modulator of C–X–C Chemokine Receptor Type 7 as a Treatment for Cardiac Fibrosis. J. Med. Chem. 2018, 61, 3685–3696. 10.1021/acs.jmedchem.8b00190. [DOI] [PubMed] [Google Scholar]; d Shi J.; Gu Z.; Jurica E. A.; Wu X.; Haque L. E.; Williams K. N.; Hernandez A. S.; Hong Z.; Gao Q.; Dabros M.; Davulcu A. H.; Mathur A.; Rampulla R. A.; Gupta A. K.; Jayaram R.; Apedo A.; Moore D. B.; Liu H.; Kunselman L. K.; Brady E. J.; Wilkes J. J.; Zinker B. A.; Cai H.; Shu Y.-Z.; Sun Q.; Dierks E. A.; Foster K. A.; Xu C.; Wang T.; Panemangalore R.; Cvijic M. E.; Xie C.; Cao G. G.; Zhou M.; Krupinski J.; Whaley J. M.; Robl J. A.; Ewing W. R.; Ellsworth B. A. Discovery of Potent and Orally Bioavailable Dihydropyrazole GPR40 Agonists. J. Med. Chem. 2018, 61, 681–694. 10.1021/acs.jmedchem.7b00982. [DOI] [PubMed] [Google Scholar]
- Armstrong R. J.; Aggarwal V. K. 50 Years of Zweifel Olefination: A Transition-Metal-Free Coupling. Synthesis 2017, 49, 3323–3336. 10.1055/s-0036-1589046. [DOI] [Google Scholar]
- Llaveria J.; Leonori D.; Aggarwal V. K. Stereospecific Coupling of Boronic Esters with N-Heteroaromatic Compounds. J. Am. Chem. Soc. 2015, 137, 10958–10961. 10.1021/jacs.5b07842. [DOI] [PubMed] [Google Scholar]
- Li Z.; Wang Z.; Zhu L.; Tan X.; Li C. Silver-Catalyzed Radical Fluorination of Alkylboronates in Aqueous Solution. J. Am. Chem. Soc. 2014, 136, 16439–16443. 10.1021/ja509548z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.