Abstract
Our studies examined the effects of p27kip1 and p21cip1 on the assembly and activity of cyclin D3-cdk4 complexes and determined the composition of the cyclin D3 pool in cells containing and lacking these cyclin-dependent kinase inhibitors. We found that catalytically active cyclin D3-cdk4 complexes were present in fibroblasts derived from p27kip1-p21cip1-null mice and that immunodepletion of extracts of wild-type cells with antibody to p27kip1 and/or p21cip1 removed cyclin D3 protein but not cyclin D3-associated activity. Similar results were observed in experiments assaying cyclin D1-cdk4 activity. Data obtained using mixed cell extracts demonstrated that p27kip1 interacted with cyclin D3-cdk4 complexes in vitro and that this interaction was paralleled by a loss of cyclin D3-cdk4 activity. In p27kip1-p21cip1-deficient cells, the cyclin D3 pool consisted primarily of cyclin D3 monomers, whereas in wild-type cells, the majority of cyclin D3 molecules were complexed to cdk4 and either p27kip1 or p21cip1 or were monomeric. We conclude that neither p27kip1 nor p21cip1 is required for the formation of cyclin D3-cdk4 complexes and that cyclin D3-cdk4 complexes containing p27kip1 or p21cip1 are inactive. We suggest that only a minor portion of the total cyclin D3 pool accounts for all of the cyclin D3-cdk4 activity in the cell regardless of whether the cell contains p27kip1 and p21cip1.
Cell cycle progression is regulated by an ordered sequence of events that includes the activation of the cyclin-dependent kinases (cdk's) (37, 43). Activation of the cdk's requires both their association with cyclins, whose levels fluctuate during the cell cycle, and their phosphorylation at specific threonine residues by cdk-activating kinase, a constitutively expressed enzyme (46). cdk's also interact with a group of proteins collectively termed cdk inhibitors (CKIs); CKI levels, like cyclin levels, vary during the cell cycle and thus contribute to the timing of cdk activation (44, 45). Traverse of G0/G1 and entry into S phase is controlled by the sequential activation of complexes containing the D cyclins and cdk4 or cdk6, cyclin E and cdk2, and cyclin A and cdk2. This series of events is initiated by mitogen-induced increases in the expression of the D cyclins (D1, D2, and D3) and the formation of active cyclin D-cdk4 (or cdk6) complexes in mid-G1 (30, 50). D cyclin-containing complexes phosphorylate the antioncogene Rb, as do cyclin E-cdk2 complexes, which become active in late G1 due, at least in part, to decreases in CKI levels (16, 20, 22, 29, 49). When phosphorylated by these kinases, Rb no longer represses the activity of the E2F transcription factors, and a variety of E2F target genes, including those encoding cyclin E, cyclin A, and several DNA replication enzymes, are expressed (6, 13, 48). At this point, cells pass through the restriction point in late G1 and, in a manner dependent on cyclin E-cdk2 and cyclin A-cdk2 activity, enter and traverse S phase (36).
Two classes of CKIs have been defined: the INK proteins, which block activation of D cyclin-containing complexes (41), and the Cip/Kip proteins, which target D-, E-, and A-containing complexes (21). The INK family consists of p16INK4a, p15INK4b, p18INK4c, and p19INK4d, and the Cip/Kip family is composed of p21cip1, p27kip1, and p57kip2. The INK proteins interact with monomeric cdk4 or cdk6, whereas the Cip/Kip proteins associate with cyclin-cdk complexes. Of the Cip/Kip proteins, p27kip1 is thought to be the primary modulator of proliferative status in most cell types, where it functions to induce and maintain the quiescent state in response to suboptimal mitogenic stimuli and growth-inhibitory agents (10). In support of this role, numerous studies have shown that p27kip1 accumulates in serum-starved and density-arrested cells and that mitogen-triggered decreases in its levels are required for the resumption of G0/G1 traverse (1, 8, 14, 32, 34, 39, 40, 49). Moreover, as described by Coats et al. (9) and Rivard et al. (40), ablation of p27kip1 expression by antisense mRNA retards the entry of serum-starved cells into G0, and due to higher percentages of cycling cells and the consequent enlargement of all internal organs, mice lacking p27kip1 are larger than their control littermates (18, 24, 33).
In addition to phosphorylating Rb, the D cyclins and their cdk partners also promote proliferation by a noncatalytic process that involves sequestration of p27kip1 (44). This model proposes that cdk2 activation is dependent on both a mitogen-induced reduction in overall p27kip1 levels and the titration of residual p27kip1 molecules by cyclin D-cdk complexes. In line with the latter function, previous studies suggest that antiproliferative agents such as transforming growth factor β and lovastatin induce the formation of inactive p27kip1-bound cdk2 complexes by decreasing the size of the cyclin D-cdk reservoir (17, 39). In addition to sequestering p27kip1, cyclin D-cdk complexes also facilitate cdk2 activation by titrating the levels of p21cip1 and p57kip2 (25, 44, 45). The role of these CKIs in mitogen-regulated cell proliferation is, however, unclear. p57kip2, which is expressed in a tissue-specific manner, is thought to participate in differentiation and development (27), whereas p21cip1 has been linked primarily with radiation-induced growth arrest (15). Interestingly, levels of p21cip1 often increase after mitogenic stimulation, and regulation of cdk2 activity by p21cip1 in cycling cells (rather than restimulated quiescent cells) has been proposed elsewhere (19, 28, 34).
While the capacity of p27kip1 to inhibit cdk2 activity is well established (44), its effects on the activity of the D cyclin-associated cdk's are controversial. Cheng et al. (7, 8), for example, found that antibody to p27kip1 removed cdk4 activity from cell extracts and that ablation of p27kip1 expression reduced the association of cyclins D1 and D2 with cdk4. These data suggest that p27kip1 acts as an enabler rather than an inhibitor of cdk4 activity and provide a mechanism by which D cyclin complexes can simultaneously fulfill their sequestration and enzymatic requirements. Consistent with the data of Cheng et al. (7), Blain et al. (3) reported that in vitro-assembled complexes containing cyclin D2, cdk4, and low levels of p27kip1 were catalytically active. However, in this study, cyclin D2 efficiently interacted with cdk4 in the absence of p27kip1. Thus, according to these findings, p27kip1 neither prevents nor promotes cdk4 activity. On the other hand, LaBaer et al. (25) found that p27kip1 stabilized the interaction of cdk4 with cyclins D1, D2, and D3 and that ectopically expressed p27kip1 inhibited cyclin D1-cdk4 activity regardless of expression level. Repression of cyclin D1-cdk4 activity by p27kip1 has also been observed in recombinant systems (47), in fibroblasts inducibly expressing p27kip1 (52), and in macrophages treated with agents (e.g., cyclic AMP analogs) that increase endogenous p27kip1 levels (23). Whether the conflicting results obtained in past studies reflect differences in cell type, assay conditions, or other factors is not known. The effects of p21cip1 on the assembly and activity of D cyclin-containing complexes also remain to be resolved (3, 25, 51).
We have suggested previously that p27kip1 inhibits the activity of cyclin D3-cdk4 complexes in mouse fibroblasts (14, 52). The studies presented here further explore the effects of p27kip1, as well as p21cip1, on this process. Given the controversy regarding the role of the Cip/Kip proteins in the regulation of D cyclin-cdk activity, we used a variety of approaches, both in vivo and vitro, to address this issue and to establish a mechanism by which the D cyclins contribute to both Rb phosphorylation and cdk2 activation. Our data show that cyclin D3 associates with cdk4 in the absence of both p27kip1 and p21cip1 and that cyclin D3-cdk4 complexes containing these CKIs are catalytically inactive. We suggest that different segments of the cyclin D3 pool are responsible for Rb phosphorylation and p27kip1-p21cip1 sequestration and that enzymatically active cyclin D3-cdk4 complexes comprise only a small portion of this pool. As a result, cells contain a large reservoir of cyclin D3 molecules that facilitate cdk2 activation by forming stable ternary complexes with cdk4 and either p27kip1 or p21cip1. Additional data indicate that cyclin D1-cdk4 activity is regulated by p27kip1 and p21cip1 in a manner similar to that of cyclin D3-cdk4 activity.
MATERIALS AND METHODS
Cell culture and preparation of MEFs.
BALB/c 3T3 mouse fibroblasts (clone A31) were cultured in Dulbecco's modified Eagle's medium supplemented with 4 mM l-glutamine, 50 U of penicillin per ml, 50 μg of streptomycin per ml, and 10% calf serum. Experiments were done on either exponentially growing cells or cells arrested at confluency for 4 to 5 days. Density-arrested cells were stimulated to reenter the cell cycle by refeeding with fresh medium containing 10% calf serum and 10 ng of platelet-derived growth factor per ml. Mice lacking the entire coding regions for p21cip1 (p21−/−) and for both p27kip1 and p21cip1 (p27/p21−/−) were obtained from Tyler Jacks (4) and James Roberts (18), respectively. Mice lacking a region within the N-terminal cyclin-cdk binding domain of p27kip1 (p27N−/−, provided by Andrew Koff [24]) were crossed with p21−/− mice to generate p21/p27N−/− double-knockout mice. Cyclin D1-null mice were obtained from Jackson Laboratories (Bar Harbor, Maine). Mouse embryo fibroblasts (MEFs) were prepared from 15- or 16-day-old embryos. Following removal of the head and internal organs, embryos were minced and plated individually in 100-mm-diameter tissue culture dishes containing medium supplemented with 10% fetal calf serum.
Preparation of cell extracts, immunoprecipitation, and immunoblotting.
Cultures were rinsed twice in ice-cold phosphate-buffered saline, harvested by scraping, and collected by centrifugation. The pellets were resuspended in lysis buffer (50 mM HEPES [pH 7.5], 100 mM NaCl, 2 mM EDTA, 0.5% NP-40, 10% glycerol, 0.1 mM sodium orthovanadate, 0.5 mM NaF, 0.1 mM phenylmethylsulfonyl fluoride, 2.5 μg of leupeptin per ml, and 1 mM dithiothreitol), vortexed, and incubated on ice for 30 min. Insoluble material was removed by centrifugation. For immunoprecipitations, cell extracts (80 to 350 μg) were incubated with the indicated antibody for 1 to 2 h at 4°C with gentle agitation. Immune complexes were recovered with protein A-agarose beads (1 to 2 h, 4°C) and washed twice with lysis buffer. For Western analysis, cell extracts (40 to 80 μg) or immune complexes were boiled in Laemmli buffer (20% glycerol, 3% sodium dodecyl sulfate [SDS], 4% β-mercaptoethanol, 0.5% bromophenol blue) and separated on 10 or 11% SDS-polyacrylamide gels. Resolved proteins were electrophoretically transferred to nitrocellulose. Membranes were blocked in PBST (phosphate-buffered saline plus 0.1% Tween 20) containing 5% instant milk and incubated with antibody in PBST for 2 h at room temperature. Proteins recognized by the antibody were detected by enhanced chemiluminescence using a horseradish peroxidase-coupled secondary antibody as specified by the manufacturer (Pierce, Rockford, Ill.). In experiments involving immunodepletion, protein removal was confirmed by Western blotting.
In vitro kinase assays.
Immune complexes were washed twice with lysis buffer and once with 2× kinase reaction buffer (100 mM HEPES [pH 7.5], 20 mM MgCl2, 10 mM MnCl2, 20 mM dithiothreitol). Washed complexes were resuspended in 1× kinase reaction buffer (50 mM HEPES [pH 7.5], 10 mM MgCl2, 5 mM MnCl2, 10 mM dithiothreitol) containing 10 μCi of [γ-32P]ATP, 10 μM ATP, and 1 μg of glutathione S-transferase (GST)–Rb and incubated for 30 min at 30°C. Reactions were stopped by boiling for 4 min in Laemmli buffer, and proteins were resolved on 11% SDS gels. Phosphoproteins were visualized by autoradiography.
Reagents and antibodies.
Platelet-derived growth factor was purchased from Pepro (Rocky Hill, N.J.). Cyclin D3, cdk6, and Stat3 polyclonal antibodies were obtained from Santa Cruz (Santa Cruz, Calif.). p21cip1 polyclonal antibody was purchased from PharMingen (San Diego, Calif.), and cdk4 and cyclin D3 monoclonal antibodies were obtained from Transduction Laboratories (Lexington, Ky.). Cyclin A monoclonal antibody was from Neomarker (Union City, Calif.). Polyclonal antibodies to cdk4, cyclin A, cyclin D3, and p27kip1 were prepared by us as described previously (1, 14). Polyclonal antibody to cyclin D1 was generated against a C-terminal peptide (EVEEEAGLACTPTDVRDVDI). Flavopiridol was obtained from the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute.
RESULTS
Cyclin D3-cdk4-p27kip1 complexes are inactive in vivo.
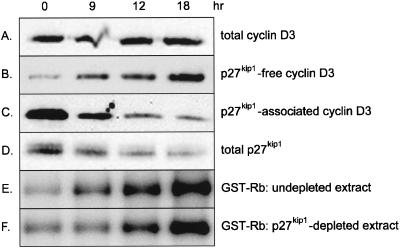
Unlike cyclin D1, which is up-regulated in response to mitogenic stimulation, cyclin D3 is expressed constitutively throughout the BALB/c 3T3 cell cycle, as is cdk4, the predominant cyclin D3 catalytic partner in these cells (14). Moreover, cyclin D3 is complexed to cdk4 in both proliferating and G0-arrested cells. Cyclin D3-cdk4 activity, however, is restricted to growing cells, and its repression in quiescent cells may reflect the presence of inhibitory proteins. Levels of p27kip1 are low in cycling cells and high in quiescent cells (1, 14, 49), and this inverse relationship between the amount of p27kip1 and the activity of cyclin D3-associated cdk4 prompted us to examine the role of p27kip1 in the regulation of this activity. Initial experiments assessed the interaction of p27kip1 with cyclin D3 in quiescent versus stimulated cells. Density-arrested BALB/c 3T3 cells received fresh medium containing 10% serum and 10 ng of platelet-derived growth factor per ml, and cell lysates were prepared at various times thereafter and immunoprecipitated with antibody to p27kip1. Immune complexes were Western blotted with antibody to cyclin D3 or p27kip1. To determine the amount of cyclin D3 not associated with p27kip1, p27kip1-depleted supernatants were immunoprecipitated and immunoblotted with antibody to cyclin D3. As shown in Fig. 1, essentially all of the cyclin D3 in unstimulated cells was complexed to p27kip1 (compare Fig. 1B and C). The amount of cyclin D3 associated with p27kip1 decreased progressively with time (Fig. 1C), as did total levels of p27kip1 (Fig. 1D), and was accompanied by an increase in the amount of p27kip1-free cyclin D3 (Fig. 1B). In addition, Western blotting of unfractionated cell lysates reaffirmed that total levels of cyclin D3 were unaltered during the time course (Fig. 1A). These findings show that BALB/c 3T3 cells contain two distinct pools of cyclin D3, one with and one without p27kip1, and that the relative proportions of these pools are governed by the amount of p27kip1 in the cell in a manner that promotes expansion of the p27kip1-free pool as cells traverse G0/G1.
FIG. 1.
Lack of effect of p27kip1 immunodepletion on cyclin D3-associated kinase activity in BALB/c 3T3 cells. Density-arrested BALB/c 3T3 cells were mitogenically stimulated with 10 ng of platelet-derived growth factor per ml and 10% serum and harvested at the indicated times. (A) Cell lysates (40 μg) were immunoblotted with antibody to cyclin D3. (B and C) Cell lysates (80 μg) were incubated with antibody to p27kip1. Immune complexes were pelleted with protein A-agarose beads and immunoblotted with antibody to cyclin D3 (C). The p27kip1-depleted supernatant was immunoprecipitated and immunoblotted with cyclin D3 antibody (B). (D) Cell lysates (40 μg) were immunoblotted with antibody to p27kip1. (E and F) Cell lysates were immunoprecipitated with preimmune serum (E) or antibody to p27kip1 (F). Immune complexes were removed by centrifugation with protein A-agarose beads, and supernatants were immunoprecipitated with cyclin D3 antibody. Immunoprecipitated material was assayed for cyclin D3-associated kinase activity using GST-Rb as substrate.
To determine if p27kip1-associated cyclin D3 complexes were catalytically active, p27kip1-depleted and undepleted cell extracts were immunoprecipitated with antibody to cyclin D3, and in vitro kinase assays were performed on immune complexes using GST-Rb as substrate. As shown in Fig. 1E, cyclin D3-cdk4 activity was low in undepleted extracts of quiescent cells and increased significantly upon mitogenic stimulation in parallel with the decrease in p27kip1 levels. Immunodepletion of p27kip1 prior to assay had no effect on either the extent or the timing of cyclin D3-cdk4 activation (compare Fig. 1E and F), thus indicating that cyclin D3 complexes that contain p27kip1 do not contribute substantially to this process. These data suggest that cyclin D3-cdk4 activity is restricted to p27kip1-free complexes, which are present in stimulated but not quiescent BALB/c 3T3 cells.
Experiments similar to those described above were also performed on MEFs prepared from p21cip1-null mice. Although p27kip1 antibody coprecipitated cyclin D3 protein (∼50% of total) from extracts of mitogenically stimulated p21−/− MEFs, it did not remove GST-Rb-phosphorylating activity as determined in cyclin D3 immunoprecipitates (Fig. 2). As shown in Fig. 2A, levels of cyclin D3-cdk4 activity were similar in both mock-depleted and p27kip1-depleted extracts of p21−/− MEFs. This result indicates that cyclin D3-cdk4 complexes lacking both p27kip1 and p21cip1 are enzymatically active and account for most if not all of the cyclin D3-cdk4 activity in the cell.
FIG. 2.
Lack of effect of p27kip1 immunodepletion on cyclin D3-associated kinase activity in p21−/− MEFs. Confluent p21−/− MEFs were incubated in medium containing 0.1% serum for 30 h and subsequently stimulated with 10 ng of PDGF per ml and 10% serum for 18 h. Cell lysates were immunoprecipitated with preimmune serum (mock depletion, left panel) or antibody to p27kip1 (right panel). Immune complexes were removed by centrifugation with protein A-agarose beads and immunoblotted with monoclonal antibody to cyclin D3 (C). Supernatants were immunoprecipitated with polyclonal antibody to cyclin D3, and immunoprecipitated material was assayed for cyclin D3-associated kinase activity using GST-Rb as substrate (A) or immunoblotted with monoclonal antibody to cyclin D3 (B).
Cyclin D3-cdk4 activity in cells lacking both p27kip1 and p21cip1.
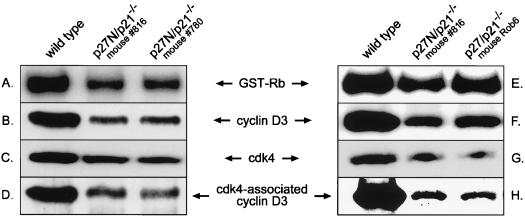
Previous studies (7) did not detect Rb kinase activity in either cyclin D1 or cdk4 immune complexes derived from MEFs lacking both p27kip1 and p21cip1. On the other hand, we observed cyclin D3-cdk4 activity in BALB/c 3T3 extracts immunodepleted of both p27kip1 and p21cip1 and thus predict that this activity should be readily apparent in p27kip1-p21cip1 double-null cells. Two mouse models were used to test this premise: one in which the entire coding regions of p27kip1 and p21cip1 were eliminated (termed p27/p21−/−) and the other in which the entire coding region of p21cip1 and the N-terminal cyclin-cdk binding domain of p27kip1 (termed p27N/p21−/−) were deleted (4, 18, 24). Cell lysates were prepared from asynchronously growing wild-type, p27/p21−/−, and p27N/p21−/− MEFs, and cyclin D3-associated kinase activity was determined. Although less than that of wild-type MEFs, considerable cyclin D3-cdk4 activity was seen in cells derived from both types of knockout mice (Fig. 3A and E). In accord with this observation, antibody to cdk4 coprecipitated cyclin D3 from extracts of both p27/p21−/− and p27N/p21−/− cells, albeit to a reduced extent compared to that for wild-type cells (Fig. 3D and H). These findings demonstrate that cyclin D3-cdk4 complexes are formed and become active in the absence of both p27kip1 and p21cip1. The lower levels of cyclin D3-cdk4 association and activity in the double-null cells may reflect the lower levels of cyclin D3 (Fig. 3B and F) and cdk4 (Fig. 3C and G) in these cells.
FIG. 3.
Cyclin D3-cdk4 association and activity in MEFs lacking both p27kip1 and p21cip1. Asynchronously cycling wild-type MEFs, p27N/p21−/− MEFs (derived from mice 816 and 780), and p27/p21−/− MEFs (derived from mouse Rob6) were harvested and assayed as follows. (A and E) Cell lysates were immunoprecipitated with antibody to cyclin D3, and immune complexes were assayed for kinase activity using GST-Rb as substrate. (B and F) Cell lysates (80 μg) were immunoblotted with antibody to cyclin D3. (C and G) Cell lysates (80 μg) were immunoblotted with antibody to cdk4. (D and H) Cell lysates (200 μg) were immunoprecipitated with antibody to cdk4 and immunoblotted with antibody to cyclin D3. As different amounts of lysate were used for the assays in panels B and F versus those in panels D and H, the results obtained cannot be compared on a quantitative basis.
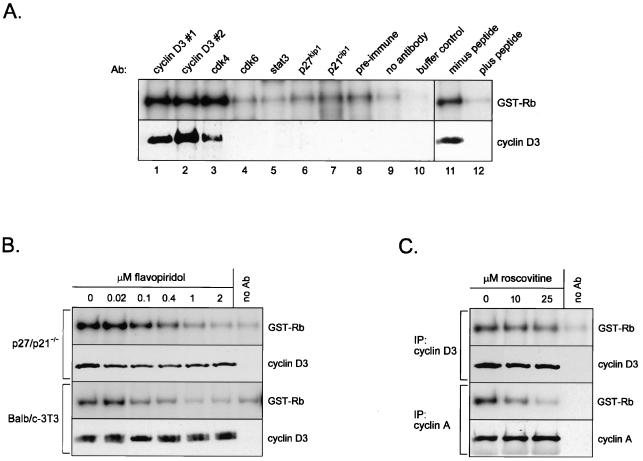
Additional experiments were performed to conclusively demonstrate that the GST-Rb-phosphorylating activity seen in our experiments on p27kip1-p21cip1-deficient MEFs was indeed representative of cyclin D3-cdk4 activity. In the first set of experiments, extracts of asynchronously cycling p27/p21−/− cells were immunoprecipitated with a panel of different antibodies, and the capacity of the precipitated material to phosphorylate GST-Rb was assessed. A very low level of GST-Rb phosphorylation was observed in mock precipitates (“no antibody”) and in precipitates of lysates preincubated with preimmune serum or with antibody to Stat3, a transcription factor that does not possess kinase activity or associate with cyclin-cdk complexes (Fig. 4A, top panel) (11). Immune complexes prepared using antibodies to p27kip1, p21cip1, or cdk6 also exhibited basal levels of GST-Rb phosphorylation. On the other hand, kinase activity resulting in greater than basal amounts of GST-Rb phosphorylation was evident in immunoprecipitates obtained with two different antibodies to cyclin D3, as well as with an antibody to cdk4. Increased activity correlated with the appearance of cyclin D3 in the immune complex (Fig. 4A, bottom panel), and neither increased activity nor cyclin D3 protein was seen in extracts precipitated with cyclin D3 antibody prebound to the immunizing peptide (Fig. 4A, compares lanes 11 and 12). These results demonstrate that a background level of Rb-phosphorylating activity is present in immune complexes regardless of the antibody used for precipitation. Despite this background, an increase in activity that specifically reflects the presence of cyclin D3 in the immune complex is observed.
FIG. 4.
Verification of cyclin D3-associated cdk4 activity as the GST-Rb-phosphorylating activity in p27kip1-p21cip1-deficient MEFs. (A) Lysates of exponentially proliferating p27/p21−/− MEFs were immunoprecipitated with the indicated antibodies (lanes 1 to 7) or preimmune serum (lane 8) or were mock precipitated (lane 9). Immune complexes were assayed for kinase activity using GST-Rb as substrate (top panel) or were immunoblotted with antibody to cyclin D3 (bottom panel). The buffer control (lane 10) contained kinase buffer in place of resuspended immune complex. Cyclin D3 antibodies 1 and 2 were purchased from Santa Cruz and prepared by us, respectively. Cell extracts were also immunoprecipitated with antibody to cyclin D3 (Santa Cruz) that had been preincubated with either buffer alone (lane 11) or the peptide against which the antibody was generated (lane 12). (B) Cell extracts prepared from logarithmically growing p27/p21−/− MEFs (top panels) and BALB/c 3T3 cells (bottom panels) were immunoprecipitated with antibody to cyclin D3. Immunoprecipitated material was resuspended in kinase buffer containing the indicated concentrations of flavopiridol and incubated for 30 min at 30°C. Immune complexes were assayed for cyclin D3-associated activity using GST-Rb as substrate or were immunoblotted with antibody to cyclin D3. (C) Cell extracts prepared from asynchronously cycling p27/p21−/− cells were immunoprecipitated with antibody to cyclin D3 (top panels) or cyclin A (bottom panels). Immune complexes were resuspended in kinase buffer containing the indicated concentrations of roscovitine and incubated for 30 min at 30°C. Immunoprecipitated material was assayed for kinase activity using GST-Rb as substrate or was immunoblotted with antibody to cyclin D3 (cyclin D3 immunoprecipitates) or cyclin A (cyclin A immunoprecipitates). Ab, antibody; IP, immunoprecipitation.
In a second set of experiments, extracts of exponentially proliferating p27/p21−/− cells (or, as a positive control, BALB/c 3T3 cells) were immunoprecipitated with antibody to cyclin D3, and immune complexes were incubated with the cdk4 inhibitor flavopiridol (5) prior to determination of kinase activity. For both cell lines, addition of flavopiridol to immune complexes resulted in a dose-dependent decrease in activity, with essentially complete inhibition occurring at 2 μM (Fig. 4B). Flavopiridol also inhibits the activity of cdk2 (12); however, as assessed in immune complexes derived from p27/p21−/− MEFs, the cdk2 inhibitor roscovitine (2, 31) had no effect on cyclin D3-associated activity (Fig. 4C). In contrast, cyclin A-associated activity (presumably cdk2) was markedly repressed by roscovitine. As confirmed by Western blotting, equal levels of cyclin D3 and cyclin A were present in each sample. Although additional unsuspected actions of flavopiridol cannot be excluded, the susceptibility of cyclin D3-associated activity to flavopiridol (but not roscovitine) strongly suggests that cdk4 is the catalytic entity responsible for this activity in p27/p21−/− cells. This supposition is supported by the presence of both cyclin D3 protein and GST-Rb-phosphorylating activity in cdk4 immune complexes prepared from double-null cells (Fig. 4A, lane 3). Although cdk6 is a potential target of flavopiridol, we did not detect cdk6 activity in p27/p21−/− MEFs (Fig. 4A, lane 4). A similar finding was reported by Cheng et al. (7).
Cyclin D1-cdk4 activity in p27/p21−/− MEFs.
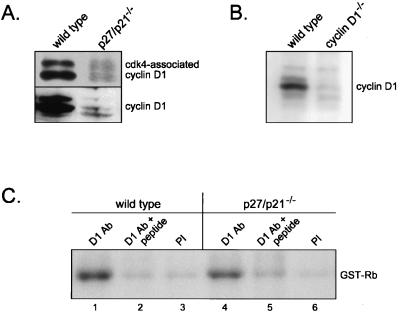
Although cdk4 interacted with cyclin D3 in cells lacking p27kip1 and p21cip1, it is possible that it does not associate with other D cyclins in the absence of these CKIs. To test this, we examined the association of cdk4 with cyclin D1 in wild-type and p27/p21−/− MEFs by immunoprecipitation-immunoblot analysis. As shown in Fig. 5A, cyclin D1-cdk4 complexes were present in p27/p21−/− cells, albeit at lower levels than in wild-type cells. Total amounts of cyclin D1 were substantially reduced in the double-null cells and thus may limit the extent of cyclin D1-cdk4 complex formation in these cells. Using various amounts of cell extract, we determined that cyclin D1 levels were ∼15-fold lower in p27/p21−/− MEFs (data not shown). The cyclin D1 antibody used in these experiments was prepared in our laboratory, and Western analysis of cell extracts derived from cyclin D1+/+ and cyclin D1−/− MEFs confirmed that this antibody specifically recognizes cyclin D1 (Fig. 5B). To determine if cyclin D1-cdk4 complexes in p27/p21−/− MEFs were enzymatically active, kinase assays were performed on cell extracts immunoprecipitated with cyclin D1 antibody or, as negative controls, preimmune serum or cyclin D1 antibody plus blocking peptide. A low level of kinase activity was observed in the negative controls for both wild-type and p27/p21−/− MEFs (Fig. 5C). Kinase activity greater than background levels was, however, clearly evident in cyclin D1 immune complexes prepared from wild-type cells and, to a somewhat lesser extent, p27/p21−/− cells. Additional experiments showed that depletion of p27kip1 and p21cip1 from extracts of wild-type cells did not remove cyclin D1-cdk4 activity, as assessed in both cyclin D1 (data not shown) and cdk4 (see Fig. 8C) immunoprecipitates. Collectively, these results suggest that the activity of cyclin D1 complexes is regulated similarly to that of cyclin D3 complexes. Both complexes are present and active in cells lacking p27kip1 and p21cip1 and are not active when bound to p27kip1 or p21cip1.
FIG. 5.
Cyclin D1-cdk4 activity in p27/p21−/− MEFs. (A) Cell lysates of growing wild-type and p27/p21−/− MEFs were immunoprecipitated with antibody to cdk4 and immunoblotted with antibody to cyclin D1 (top panel) or were only immunoblotted with antibody to cyclin D1 (bottom panel). (B) Extracts of wild-type and cyclin D1-deficient MEFs were immunoblotted with antibody to cyclin D1. (C) Extracts of wild-type and p27/p21−/− MEFs were immunoprecipitated with antibody to cyclin D1 (lanes 1 and 4), preimmune serum (PI) (lanes 3 and 6), or cyclin D1 antibody that had been preincubated with the peptide against which the antibody was generated (lanes 2 and 5). Immune complexes were assayed for kinase activity using GST-Rb as substrate. Ab, antibody.
FIG. 8.
Low levels of binary cyclin D3-cdk4 complexes in wild-type MEFs. (A) Extracts of growing wild-type MEFs were immunodepleted of p27kip1, p21cip1, or cdk4 or were mock depleted. Depleted extracts were immunoprecipitated with antibody to cdk4 (lanes 1 to 4) or cyclin D3 (lanes 5 to 9) and immunoblotted with antibody to cyclin D3. Depleted extracts were also immunoblotted with antibody to actin (lanes 10 to 13). A longer exposure of lanes 1 to 4 is also shown (lanes denoted by asterisks). (B) Extracts were immunodepleted of p27kip1, p21cip1, or both or were mock depleted. Depleted extracts were immunoprecipitated with antibody to cyclin D3 and assayed for kinase activity using GST-Rb as substrate. (C) Extracts of wild-type MEFs were immunodepleted using preimmune serum (−) or antibodies to p27kip1 and p21cip1 (+). Depleted extracts were immunoprecipitated with antibody to cdk4 or preimmune serum (PI), and immune complexes were assayed for kinase activity (top panel) or immunoblotted with cdk4 antibody (bottom panel). (D) Extracts of growing p21−/− MEFs were depleted of p27kip1 or mock depleted. Depleted extracts were immunoprecipitated with antibody to cyclin D3 for determination of kinase activity (top panel) or were immunoprecipitated with antibody to cdk4 and immunoblotted with antibody to cyclin D3 (bottom panel). Ab, antibody. IP, immunoprecipitation.
Inhibition of cyclin D3-cdk4 activity by p27kip1 in vitro.
The data presented in Fig. 1 and 2 demonstrate that cyclin D3-cdk4-p27kip1 complexes are inactive in vivo. As a corollary to these studies, we also examined the effect of exogenously supplied p27kip1 on cyclin D3-cdk4 activity in vitro. As our source of p27kip1, we used extracts of G0-arrested BALB/c 3T3 cells. As we reported previously (49), these extracts contain a large pool of p27kip1 molecules that are not bound to cyclin-cdk complexes. In addition, we boiled G0 extracts to release cyclin-cdk-sequestered p27kip1, and it is noted that boiling also results in a cyclin D3-containing precipitate, which is removed by centrifugation. Different amounts of boiled and clarified G0 extracts were mixed with extracts of exponentially growing p27/p21−/− cells; as shown above (Fig. 3), these cells contain active cyclin D3-cdk4 complexes. After a 30-min incubation at 30°C, cyclin D3 (or for comparative purposes, cyclin A) was immunoprecipitated from mixed cell extracts, and the amounts of coprecipitated p27kip1 and kinase activity were determined.
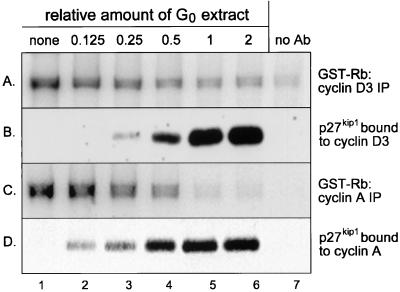
As shown in Fig. 6, addition of boiled and clarified G0 extracts to extracts of growing p27/p21−/− cells resulted in the association of p27kip1 with cyclin D3-containing complexes (Fig. 6B) and the repression of cyclin D3-cdk4 activity (Fig. 6A). The extent of inhibition of cyclin D3-cdk4 activity was directly proportional to the amount of p27kip1 bound to cyclin D3-cdk4 complexes, with maximal interaction and inactivation occurring at a 1:1 ratio of G0 extract to growing cell extract (lane 5). In terms of dose dependency, and although less striking, inhibition of cyclin D3-cdk4 activity by G0 extract was comparable to that of cyclin A-cdk2 activity (Fig. 6C and D). We have shown previously that the inhibitory activity in G0 extracts is removed by antibody to p27kip1 (49). Thus, similar to results obtained in vivo, p27kip1 inhibits the activity of cyclin D3-cdk4 complexes in vitro. We also found that recombinant p27kip1 repressed cyclin D3-cdk4 activity when added to extracts of proliferating p27/p21−/− cells (T. K. Bagui and W. J. Pledger, unpublished data).
FIG. 6.
Inhibition of cyclin D3-cdk4 activity by p27kip1 in mixed cell extracts. Extracts of density-arrested BALB/c 3T3 cells were boiled for 5 min and clarified by centrifugation (designated “G0 extracts”). G0 extracts were mixed at the indicated ratios with extracts (200 μg) of proliferating p27/p21−/− cells and incubated at 30°C for 30 min. Mixed cell extracts were immunoprecipitated with antibody to cyclin D3 (A and B) or cyclin A (C and D). Immune complexes were assayed for cyclin D3-associated activity (A) or cyclin A-associated activity (C) or were immunoblotted with antibody to p27kip1 (B and D). Based on comparisons with known amounts of recombinant p27kip1, 100 μg of G0 extract contains approximately 7 ng of p27kip1. Ab, antibody. IP, immunoprecipitation.
Limited amounts of cyclin D3-cdk4 complexes in p27kip1-p21cip1-deficient MEFs.
Additional experiments assessed the stoichiometry of cyclin D3 binding to p27kip1 in mixed cell extracts. In these experiments, as in those above, extracts of exponentially proliferating p27/p21−/− MEFs were combined with boiled and clarified extracts of G0-arrested BALB/c 3T3 cells. As shown in Fig. 7A (top panel), similar amounts of cyclin D3 were present in mixed cell extracts regardless of the amount of G0 extract added. This observation verifies the removal of cyclin D3 from boiled G0 extracts and thus indicates that extracts of growing p27/p21−/− MEFs are the sole source of cyclin D3 in mixed cell extracts. As shown in Fig. 7A (middle panel) and similar to data presented in Fig. 6, cyclin D3 and p27kip1 interacted in vitro. However, even in conditions in which cyclin D3-cdk4 activity was maximally inhibited (Fig. 6, lanes 5 and 6), only a low percentage of the total cyclin D3 pool was associated with p27kip1 (Fig. 7A, compare top and middle panels). Consistent with this observation, immunodepletion of p27kip1 from mixed cell extracts had no discernible effect on cyclin D3 levels (bottom panel), again indicating that the amount of cyclin D3 bound to p27kip1 was limited. These data demonstrate that p27/p21−/− cells contain a large pool of cyclin D3 molecules that do not bind p27kip1.
FIG. 7.
High levels of uncomplexed cyclin D3 in p27/p21−/− MEFs. (A) Boiled and clarified extracts of G0-arrested BALB/c 3T3 cells were mixed with extracts of exponentially growing p27/p21−/− cells at the indicated ratios and incubated for 30 min at 30°C. (Top panel) Mixed cell extracts were immunoprecipitated and immunoblotted with antibody to cyclin D3. (Middle panel) Mixed cell extracts were immunoprecipitated with antibody to p27kip1 and immunoblotted with antibody to cyclin D3. (Bottom panel) Mixed cell extracts were immunodepleted of p27kip1, and depleted extracts were immunoprecipitated and immunoblotted with antibody to cyclin D3. (B) Mixed cell extracts were prepared and incubated as for panel A and were immunodepleted of p27kip1 (+) or mock depleted (−). Depleted extracts were immunoprecipitated (IP) with preimmune serum (PI) or antibody to cdk4 and immunoblotted with antibody to cyclin D3.
Although p27kip1 binds with high affinity to cyclin-cdk complexes, it does not interact efficiently with individual cyclin or cdk subunits (38, 39, 42). Thus, it is possible that the cyclin D3 pool in p27/p21−/− MEFs that does not bind p27kip1 consists of cyclin D3 monomers (defined as cyclin D3 not bound to cdk4). To test this, extracts of proliferating p27/p21−/− cells and boiled, clarified extracts of quiescent BALB/c 3T3 cells were combined, and the amounts of cdk4-associated cyclin D3 in mock-depleted and p27kip1-depleted mixed cell extracts were determined. Although comprising only a small fraction of the total cyclin D3 pool, cyclin D3-cdk4 complexes were evident in mock-depleted extracts (Fig. 7B, compare lanes 1 and 2). In contrast, cyclin D3-cdk4 complexes were not detectable in mixed cell extracts depleted of p27kip1 (lane 4). Removal of p27kip1, however, had little effect on total levels of cyclin D3 (compare lanes 1 and 3) or cdk4 (data not shown). These results indicate that most of the cyclin D3 molecules in p27/p21−/− cells are not bound to cdk4 and thus are unavailable for interaction with p27kip1.
Active cyclin D3-cdk4 complexes comprise only a minor portion of the cyclin D3 pool in wild-type MEFs.
The observation that only a minor portion of total cyclin D3 molecules are complexed to cdk4 in p27/p21−/− cells implies that only a minor portion of the total cyclin D3 pool is (or can be) active. Thus, one would predict that cyclin D3-cdk4 activity in p27/p21−/− cells would be, at best, barely detectable. However, as shown in Fig. 3, considerable cyclin D3-cdk4 activity was observed in two different types of p27kip1-p21cip1-deficient MEFs. As an explanation of this paradox, we considered the possibility that the cyclin D3 pool in wild-type cells consists primarily of cyclin D3 monomers and inactive complexes (e.g., cyclin D3-cdk4-p27kip1). Thus, in both p27/p21−/− and p27/p21+/+ cells, only a seemingly inconsequential fraction of total cyclin D3 molecules would account for all of the cyclin D3-associated activity in the cells. To test this possibility, we determined the relative amounts of cyclin D3 monomers and of binary (cyclin D3-cdk4) and ternary (cyclin D3-cdk4-CKI) cyclin D3 complexes in asynchronously growing wild-type MEFs. In these experiments, cell extracts were immunodepleted with antibody to p27kip1, p21cip1, or both, and depleted extracts were immunoprecipitated with antibody to cyclin D3 (to assess cyclin D3) or cdk4 (to assess cdk4-associated cyclin D3); immunoprecipitated material was immunoblotted with antibody to cyclin D3.
As shown in Fig. 8A, the amount of cyclin D3 precipitated by cyclin D3 antibody (lane 5) was only slightly higher than that coprecipitated by cdk4 antibody (lane 1). This finding indicates that, unlike p27/p21−/− cells, most (although not all) of the cyclin D3 molecules in p27/p21+/+ cells are complexed to cdk4. The cdk4-associated cyclin D3 pool was reduced by ∼50% following depletion of either p27kip1 or p21cip1 (lanes 2 and 3) and was essentially undetectable following removal of both CKIs (lane 4). However, longer exposures of the Western blot demonstrated the presence of cyclin D3 in this lane (lane 4*). Thus, nearly all of the cyclin D3-cdk4 complexes in wild-type MEFs contain either p27kip1 or p21cip1. Analysis of the total cyclin D3 pool confirmed that the majority of cyclin D3 molecules were associated with p27kip1 or p21cip1 (lanes 6 and 7) and directly demonstrated the presence of a small amount of cyclin D3 monomers (lane 9). Together, these data demonstrate that the cyclin D3 pool in exponentially growing wild-type MEFs consists primarily of cyclin D3-cdk4-CKI complexes, contains a lesser subfraction of cyclin D3 monomers, and also includes a minute amount of cyclin D3-cdk4 complexes that are not associated with either p27kip1 or p21cip1. Consistent with the low percentage of binary complexes, the amount of cyclin D3 observed after depletion of p27kip1 and p21cip1 (representative of cyclin D3 plus cyclin D3-cdk4 [lane 8]) was only marginally higher than the amount of cyclin D3 observed after depletion of p27kip1, p21cip1, and cdk4 (representative of cyclin D3 alone [lane 9]). To ensure that our immunodepletion protocol did not nonspecifically remove proteins that do not associate with p27kip1 or p21cip1, depleted extracts were immunoblotted with antibody to actin. As shown in Fig. 8A, actin levels were comparable in control extracts (lane 10) and in extracts depleted of p27kip1, p21cip1, or both (lanes 11 to 14).
Although nearly undetectable, binary cyclin D3-cdk4 complexes accounted for essentially all of the cyclin D3-associated activity in extracts of wild-type MEFs. As shown in Fig. 8B, cyclin D3 immunoprecipitates containing only binary complexes (i.e., not containing p27kip1 or p21cip1 [lane 4]) exhibited levels of activity similar to those of immunoprecipitates derived from mock-depleted extracts (lane 1). In addition, the inability of p21cip1 antibody to remove activity (lane 3) indicates that cyclin D3-cdk4 complexes containing p21cip1, like those containing p27kip1, are inactive. The lack of effect of p27kip1-p21cip1 depletion on D cyclin-associated activity was also apparent in experiments in which kinase activity was determined in cdk4 (rather than cyclin D3) immune complexes (Fig. 8C). Despite the removal of cdk4 protein by p27kip1-p21cip1 depletion, levels of cdk4 activity were similar in control and depleted extracts. Because cdk4 interacts with cyclins D1, D2, and D3, all of which are present in MEFs (this study and reference 7), the data in Fig. 8C indicate that cdk4 activity is inhibited by p27kip1 and p21cip1 regardless of which D cyclin is present in the complex.
Similar experiments were done on logarithmically growing p21−/− MEFs. As shown in Fig. 8D (bottom panel), levels of cdk4-associated cyclin D3 were reduced substantially by p27kip1 depletion, thus indicating that most of the cyclin D3-cdk4 complexes in these cells are bound to p27kip1. As seen with wild-type MEFs, removal of p27kip1-associated cyclin D3-cdk4 complexes from extracts of p21−/− MEFs was not accompanied by a corresponding loss of cyclin D3-cdk4 activity (top panel). These observations, coupled with those presented above, demonstrate that irrespective of whether MEFs contain p27kip1 and/or p21cip1, the majority of cyclin D3 molecules in the cell are not present in enzymatically active complexes.
DISCUSSION
Our studies establish a model of cyclin D3-cdk4 activation in which p27kip1 and p21cip1 play inhibitory roles. Our data show that cyclin D3-cdk4 complexes containing these CKIs are catalytically inactive both in vivo and in vitro. Moreover, these CKIs are not required for the formation of cyclin D3-cdk4 complexes, as has been proposed previously (7). Both p27kip1 and p21cip1 may, however, enhance the stability of these complexes (see below). We also found that enzymatically active cyclin D3-cdk4 complexes comprised only a small fraction of the total cyclin D3 pool regardless of whether cells contained p27kip1 and p21cip1. We conclude that cdk2 activation in wild-type cells is implemented by the presence of a large pool of cyclin D3 molecules that (with cdk4) sequester p27kip1 and p21cip1. On the other hand, in p27kip1-p21cip1-deficient cells, the inherent instability of cyclin D3-cdk4 complexes limits the extent of cyclin D3-cdk4 activity and thus prevents unregulated cell proliferation.
Using two different mouse models, we found that MEFs lacking both p27kip1 and p21cip1 contained cyclin D3-cdk4 complexes and exhibited cyclin D3-cdk4 activity. Levels of both parameters were lower in double-null cells than in wild-type cells, as were levels of cyclin D3 and cdk4. We also observed cyclin D3-cdk4 activity in p27kip1-immunodepleted extracts of p21−/− MEFs and in p27kip1-p21cip1-depleted extracts of wild-type MEFs. These data clearly demonstrate that neither p27kip1 nor p21cip1 is required for the assembly or activation of cyclin D3-cdk4 complexes. These CKIs were also dispensable for cyclin D1-cdk4 activation; as shown above, substantial amounts of cyclin D1-associated activity were seen in p27/p21−/− cells. Based on these results, and although not examined in this study, it is likely that cyclin D2-cdk4 complexes are also present and active in p27/p21−/− MEFs.
In a previous investigation, Cheng et al. (7) found that cdk4 did not appreciably associate with cyclin D1 or cyclin D2 (cyclin D3 was not examined) in p27/p21−/− MEFs. Moreover, these investigators did not detect cdk4 activity in p27/p21−/− cells by in vitro kinase assay (i.e., by the method used in our study). However, residual levels of cdk4 activity were apparent in other assays (e.g., Western blotting of cell lysates with an antibody that recognizes D cyclin-specific Rb phosphorylation). Thus, both our study and that of Cheng et al. (7) demonstrate that D cyclin complexes are active, at least to some extent, in MEFs lacking p27kip1 and p21cip1. The differences between these studies appear to be more quantitative than qualitative, with Cheng et al. (7) showing a more severe reduction in cdk4 activity in the double-null cells than was seen by us. Because p27kip1-p21cip1-deficient MEFs proliferate and are susceptible to p16INK4a-mediated growth inhibition (7), it is evident that these cells retain a level of D cyclin-associated activity that, while less than that of wild-type MEFs, is sufficient for cell cycle traverse.
Based on the apparent absence of cyclin D-cdk4 complexes from p27/p21−/− MEFs, Cheng et al. (7) concluded that these CKIs were required for the efficient assembly of these complexes and, consequently, functioned as activators of cyclin D-dependent kinases. Consistent with this role, Cheng et al. (7) found that p27kip1 and p21cip1 did not inhibit cdk4 activity when bound to cdk4 complexes, a finding that has been both corroborated (3, 25, 35, 50) and challenged (25). Our data show that cyclin D-cdk4 complexes containing p27kip1 or p21cip1 are not active, and thus it is unlikely that the formation of these complexes would be dependent on their association with proteins that would ultimately prevent their activation.
In our studies, two approaches were used to determine the effect of p27kip1 on the activity of cyclin D3-cdk4 complexes. In the in vitro approach, we examined the effect of exogenously added p27kip1 on cyclin D3-cdk4 activity in mixed cell extracts. Our results show that p27kip1 interacts with cyclin D3-cdk4 complexes in vitro and that this interaction is accompanied by a decrease in cyclin D3-cdk4 activity. p27kip1 also repressed the activity of cyclin A-cdk2 complexes present in mixed cell extracts, and the loss of both activities occurred at similar concentrations of p27kip1. In the in vivo approach, we found that mitogenic stimulation of quiescent BALB/c 3T3 cells resulted in a decrease in cyclin D3-p27kip1 association and a corresponding increase in cyclin D3-cdk4 activity. More importantly, immunodepletion of extracts of stimulated or cycling cells with p27kip1 antibody did not remove cyclin D3-cdk4 activity, thus indicating that active cyclin D3-cdk4 complexes do not contain p27kip1. Depletion of p21cip1, either alone or with p27kip1, also had no effect on cyclin D3-cdk4 activity. Moreover, additional experiments examining kinase activity in cyclin D1 and cdk4 immune complexes indicate that p27kip1 and p21cip1 inhibit the activity of all D cyclin-cdk4 complexes.
Our in vivo data are in agreement with a previous study (25) showing undiminished levels of cyclin D1-cdk4 activity in p27kip1-depleted extracts of U2OS cells. On the other hand, Cheng et al. (7) reported that p27kip1 depletion of wild-type MEF extracts significantly reduced cdk4 activity. The reason for these differences is not known at present. In our studies, we tested a variety of different cyclin D1 antibodies and found that the capacity of these antibodies to recognize low amounts of cyclin D1 protein and activity varied widely. Thus, it is possible that the disparate results obtained here and in past studies reflect, at least in part, the antibodies used. As shown above and in a previous publication (14), p27kip1 is associated with cyclin D3 (and thus with cdk4) in quiescent BALB/c 3T3 cells. If cyclin D3-cdk4-p27kip1 complexes are active as suggested by Cheng et al. (7), one would expect to see cyclin D3-cdk4 activity in growth-arrested BALB/c 3T3 cells, which is not the case. On the other hand, the restriction of cyclin D3-cdk4 activity to p27kip1-free complexes, as proposed here, provides a means by which the activity of constitutively expressed D cyclin complexes can be repressed in noncycling cells. The lack of cyclin D1-cdk4 activity in serum-starved NIH 3T3 cells ectopically expressing cyclin D1 was also thought to result from p27kip1-mediated inhibition (26).
Additional studies compared the composition of the cyclin D3 pools in logarithmically growing wild-type and p27/p21−/− MEFs. We found that the majority of cyclin D3 molecules in wild-type cells were complexed to cdk4 and either p27kip1 or p21cip1. Wild-type MEFs also contained a small subpopulation of cyclin D3 monomers (i.e., cyclin D3 molecules not bound to p27kip1, p21cip1, or cdk4) and a nearly imperceptible amount of binary cyclin D3-cdk4 complexes. Although comprising only a minor fraction of the total cyclin D3-cdk4 pool, binary cyclin D3-cdk4 complexes appeared to be the sole source of cyclin D3-cdk4 activity in wild-type MEFs. As shown above, cyclin D3 complexes containing p27kip1 or p21cip1 were not active (i.e., removal of these complexes did not deplete activity), and cyclin D3 monomers have no intrinsic kinase activity. We suggest that cyclin D3 fulfills its sequestration requirement in wild-type cells by devoting the major portion of its cdk-associated pool to p27kip1-p21cip1 interaction. We also note that proliferating wild-type MEFs contained approximately equal amounts of cyclin D3-cdk4-p27kip1 and cyclin D3-cdk4-p21cip1. Whereas G0 entrance and exit is controlled primarily by p27kip1 (10), this finding, in agreement with previous data (34), suggests that G1 traverse in cycling cells is regulated by both p27kip1 and p21cip1.
In contrast to p27/p21+/+ MEFs, the majority of cyclin D3 molecules in p27/p21−/− MEFs were not coupled to cdk4. This finding implies that in the absence of p27kip1 and p21cip1, cyclin D3-cdk4 complexes are not readily formed or are not stable. The preferential association of p27kip1 and p21cip1 with cyclin-cdk complexes as opposed to individual subunits (37, 38, 42) argues against a role of these CKIs in complex assembly. Moreover, using purified proteins, LaBaer et al. (25) found that p27kip1 and p21cip1 significantly increased the stability of cyclin D1-cdk4 complexes but had little effect on complex formation. Regardless of the mechanism involved, the limited association of cyclin D3 with cdk4 in p27/p21−/− cells indicates that in these cells, as in wild-type cells, only a small fraction of the total cyclin D3 pool supplies the enzymatic activity required for Rb phosphorylation and G1 traverse. These findings suggest that loss of p27kip1 and/or p21cip1 would not necessarily affect the absolute amount of cyclin D-cdk4 activity in the cell; i.e., loss of these CKIs would simply convert catalytically inactive ternary complexes to catalytically inactive monomers. In our studies, cyclin D3-cdk4 activity was lower in p27/p21−/− cells than in wild-type cells; this may simply reflect the reduced levels of cyclin D3 and cdk4 in the double-knockout cells. Decreased amounts of cyclin D1 and cyclin D2 were also observed in p27/p21−/− cells, the result perhaps of accelerated degradation due to glycogen synthase kinase-3β-mediated phosphorylation (7). Removal of p27kip1 and p21cip1 from cells obviates the need for high levels of cyclin D-cdk4 complexes, and in response to elimination of these CKIs, cells may readjust their levels of D cyclins and their cdk partners.
In our experiments on mixed cell extracts, enhancement of cyclin D3-cdk4 complex formation by exogenously added p27kip1 was not observed. In contrast, in studies by others (25), both p27kip1 and p21cip1 promoted cyclin D-cdk4 assembly in in vitro systems. In the cell, an equilibrium most likely exists between the monomeric and binary forms of the D cyclins and their cdk partners. As noted above, binary complexes have higher dissociation rates than do ternary complexes containing p27kip1 or p21cip1 (25). Generation of stable ternary complexes, however, presumably requires the initial association of cyclin and cdk and the subsequent interaction of p27kip1 or p21cip1 with the preassembled cyclin D-cdk complex. It is possible, therefore, that the capacity of the Cip/Kip proteins to promote assembly in vitro reflects the capacity of the D cyclins and cdk4 or cdk6 to transiently dimerize, a phenomenon that may be governed by assay conditions; such differences would account for the differences between our results and those of others (25).
Lastly, it is emphasized that, although cyclin D-cdk4 complexes may be more stable when bound to p27kip1 or p21cip1, cyclin D-associated kinases are active only in the absence of these CKIs. We suggest that the D cyclins fulfill their sequestration requirement in wild-type cells by devoting the major portion of their cdk-associated pool to p27kip1-p21cip1 interaction. The remainder of this pool is sufficient to supply all the necessary activity required for Rb phosphorylation. In addition, in wild-type cells, cyclin D-cdk4 activation indicates that levels of free p27kip1 and p21cip1 are reduced sufficiently to allow activation of cdk2-containing complexes; thus, cyclin D-cdk4 activation serves as a permissive signal for cdk2 activation. In cells lacking p27kip1 and p21cip1, levels of cyclin D-cdk4 activity are kept in check by the inherent instability of binary cyclin D-cdk4 complexes. Thus, irrespective of whether p27kip1 and p21cip1 are present in cells, mechanisms capable of limiting the extent and duration of cyclin D-cdk4 activity are operative.
ACKNOWLEDGMENTS
This work was supported by the Cortner-Couch Endowed Chair for Cancer Research and NIH grants CA67360 and CA72694 to W.J.P. R.J.J. was supported by ACS grant PF-99-320-01-CNE.
We thank James Roberts, Andrew Koff, and Tyler Jacks for generously providing knockout mice and Nancy Olashaw for manuscript preparation. We also acknowledge the helpful service of the Molecular Imaging Core Laboratory at the Moffitt Cancer Center.
REFERENCES
- 1.Agrawal D, Hauser P, McPherson F, Dong F, Garcia A, Pledger W J. Repression of p27kip1 synthesis by platelet-derived growth factor in BALB/c 3T3 cells. Mol Cell Biol. 1996;16:4327–4336. doi: 10.1128/mcb.16.8.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi F, Quarta S, Savio M, Riva F, Rossi L, Stivala L A, Scovassi A I, Meijer L, Prosperi E. The cyclin-dependent kinase inhibitors olomoucine and roscovitine arrest human fibroblasts in G1 phase by specific inhibition of CDK2 kinase activity. Exp Cell Res. 1998;245:8–18. doi: 10.1006/excr.1998.4216. [DOI] [PubMed] [Google Scholar]
- 3.Blain S W, Montalvo E, Massague J. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J Biol Chem. 1997;272:25863–25872. doi: 10.1074/jbc.272.41.25863. [DOI] [PubMed] [Google Scholar]
- 4.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 5.Carlson B A, Dubay M M, Sausville E A, Brizuela L, Worland P J. Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res. 1996;56:2973–2978. [PubMed] [Google Scholar]
- 6.Chellappan S P, Hiebert S, Mudryj M, Horowitz J M, Nevins J R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- 7.Cheng M, Olivier P, Diehl J A, Fero M, Roussel M F, Roberts J M, Sherr C J. The p21Cip1 and p27Kip1 CDK “inhibitors” are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng M, Sexl V, Sherr C J, Roussel M F. Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1) Proc Natl Acad Sci USA. 1998;95:1091–1096. doi: 10.1073/pnas.95.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coats S, Flanagan W M, Nourse J, Roberts J M. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- 10.Coats S, Whyte P, Fero M L, Lacy S, Chung G, Randel E, Firpo E, Roberts J M. A new pathway for mitogen-dependent cdk2 regulation uncovered in p27Kip1-deficient cells. Curr Biol. 1999;9:163–173. doi: 10.1016/s0960-9822(99)80086-4. [DOI] [PubMed] [Google Scholar]
- 11.Darnell J E., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 12.De Azevedo W F, Jr, Mueller-Dieckmann H J, Schulze-Gahmen U, Worland P J, Sausville E, Kim S H. Structural basis for specificity and potency of a flavonoid inhibitor of human CDK2, a cell cycle kinase. Proc Natl Acad Sci USA. 1996;93:2735–2740. doi: 10.1073/pnas.93.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeGregori J, Kowalik T, Nevins J R. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol. 1995;15:4215–4224. doi: 10.1128/mcb.15.8.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong F, Agrawal D, Bagui T, Pledger W J. Cyclin D3-associated kinase activity is regulated by p27kip1 in BALB/c-3T3 cells. Mol Biol Cell. 1998;9:2081–2092. doi: 10.1091/mbc.9.8.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dulic V, Kaufmann W K, Wilson S J, Tlsty T D, Lees E, Harper J W, Elledge S J, Reed S I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 16.Ewen M E, Sluss H K, Sherr C J, Matsushime H, Kato J, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 17.Ewen M E, Sluss H K, Whitehouse L L, Livingston D M. TGFβ inhibition of Cdk4 synthesis is linked to cell cycle arrest. Cell. 1993;74:1009–1020. doi: 10.1016/0092-8674(93)90723-4. [DOI] [PubMed] [Google Scholar]
- 18.Fero M L, Rivkin M, Tasch M, Porter P, Carow C E, Firpo E, Polyak K, Tsai L H, Broudy V, Perlmutter R M, Kaushansky K, Roberts J M. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 19.Firpo E J, Koff A, Solomon M J, Roberts J M. Inactivation of a Cdk2 inhibitor during interleukin 2-induced proliferation of human T lymphocytes. Mol Cell Biol. 1994;14:4889–4901. doi: 10.1128/mcb.14.7.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatakeyama M, Brill J A, Fink G R, Weinberg R A. Collaboration of G1 cyclins in the functional inactivation of the retinoblastoma protein. Genes Dev. 1994;8:1759–1771. doi: 10.1101/gad.8.15.1759. [DOI] [PubMed] [Google Scholar]
- 21.Hengst L, Reed S I. Inhibitors of the Cip/Kip family. Curr Top Microbiol Immunol. 1998;227:25–41. doi: 10.1007/978-3-642-71941-7_2. [DOI] [PubMed] [Google Scholar]
- 22.Kato J, Matsushime H, Hiebert S W, Ewen M E, Sherr C J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 23.Kato J Y, Matsuoka M, Polyak K, Massague J, Sherr C J. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell. 1994;79:487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 24.Kiyokawa H, Kineman R D, Manova-Todorova K O, Soares V C, Hoffman E S, Ono M, Khanam D, Hayday A C, Frohman L A, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 25.LaBaer J, Garrett M D, Stevenson L F, Slingerland J M, Sandhu C, Chou H S, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 26.Ladha M H, Lee K Y, Upton T M, Reed M F, Ewen M E. Regulation of exit from quiescence by p27 and cyclin D1-CDK4. Mol Cell Biol. 1998;18:6605–6615. doi: 10.1128/mcb.18.11.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M H, Reynisdottir I, Massague J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995;9:639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Jenkins C W, Nichols M A, Xiong Y. Cell cycle expression and p53 regulation of the cyclin-dependent kinase inhibitor p21. Oncogene. 1994;9:2261–2268. [PubMed] [Google Scholar]
- 29.Lundberg A S, Weinberg R A. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsushime H, Roussel M F, Ashmun R A, Sherr C J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65:701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- 31.Meijer L, Borgne A, Mulner O, Chong J P, Blow J J, Inagaki N, Inagaki M, Delcros J G, Moulinoux J P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 32.Millard S S, Yan J S, Nguyen H, Pagano M, Kiyokawa H, Koff A. Enhanced ribosomal association of p27Kip1 mRNA is a mechanism contributing to accumulation during growth arrest. J Biol Chem. 1997;272:7093–7098. doi: 10.1074/jbc.272.11.7093. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh D Y, Nakayama K. Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 34.Nourse J, Firpo E, Flanagan W M, Coats S, Polyak K, Lee M H, Massague J, Crabtree G R, Roberts J M. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 35.Parry D, Mahony D, Wills K, Lees E. Cyclin D-cdk subunit arrangement is dependent on the availability of competing INK and p21 class inhibitors. Mol Cell Biol. 1999;19:1775–1783. doi: 10.1128/mcb.19.3.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Planas-Silva M D, Weinberg R A. The restriction point and control of cell proliferation. Curr Opin Cell Biol. 1997;9:768–772. doi: 10.1016/s0955-0674(97)80076-2. [DOI] [PubMed] [Google Scholar]
- 37.Pledger W J, Stiles C D, Antoniades H N, Scher C D. An ordered sequence of events is required before BALB/c-3T3 cells become committed to DNA synthesis. Proc Natl Acad Sci USA. 1978;75:2839–2843. doi: 10.1073/pnas.75.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polyak K, Kato J Y, Solomon M J, Sherr C J, Massague J, Roberts J M, Koff A. p27kip1, a cyclin-Cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 39.Poon R Y, Toyoshima H, Hunter T. Redistribution of the CDK inhibitor p27 between different cyclin-CDK complexes in the mouse fibroblast cell cycle and in cells arrested with lovastatin or ultraviolet irradiation. Mol Biol Cell. 1995;6:1197–1213. doi: 10.1091/mbc.6.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rivard N, L'Allemain G, Bartek J, Pouyssegur J. Abrogation of p27Kip1 by cDNA antisense suppresses quiescence (G0 state) in fibroblasts. J Biol Chem. 1996;271:18337–18341. doi: 10.1074/jbc.271.31.18337. [DOI] [PubMed] [Google Scholar]
- 41.Ruas M, Peters G. The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim Biophys Acta. 1998;1378:F115–F177. doi: 10.1016/s0304-419x(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 42.Russo A A, Jeffrey P D, Patten A K, Massague J, Pavletich N P. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature. 1996;382:325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- 43.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 44.Sherr C J, Roberts J M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 45.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 46.Solomon M J, Harper J W, Shuttleworth J. CAK, the p34cdc2 activating kinase, contains a protein identical or closely related to p40MO15. EMBO J. 1993;12:3133–3142. doi: 10.1002/j.1460-2075.1993.tb05982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 48.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 49.Winston J, Dong F, Pledger W J. Differential modulation of G1 cyclins and the Cdk inhibitor p27kip1 by platelet-derived growth factor and plasma factors in density-arrested fibroblasts. J Biol Chem. 1996;271:11253–11260. doi: 10.1074/jbc.271.19.11253. [DOI] [PubMed] [Google Scholar]
- 50.Winston J T, Pledger W J. Growth factor regulation of cyclin D1 mRNA expression through protein synthesis-dependent and -independent mechanisms. Mol Biol Cell. 1993;4:1133–1144. doi: 10.1091/mbc.4.11.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Hannon G J, Beach D. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 1994;8:1750–1758. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X, Wharton W, Donovan M, Coppola D, Croxton R, Cress W D, Pledger W J. Density-dependent growth inhibition of fibroblasts ectopically expressing p27kip1. Mol Biol Cell. 2000;11:2117–2130. doi: 10.1091/mbc.11.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]