Abstract
Background
The treatment for locally advanced breast cancer (LABC) is a severe clinical problem. The postoperative radiotherapy is a conventional treatment method for patients with LABC, whereas the effect of preoperative radiotherapy on outcome of LABC remains controversial. This study aimed to examine and compare the overall survival (OS) in patients with LABC who underwent preoperative radiotherapy or postoperative radiotherapy.
Methods
This retrospective cohort study included 41,618 patients with LABC from the National Cancer Database (NCDB) between 2010 and 2014. We collected patients’ demographic, clinicopathologic, treatment and survival information. Propensity score was used to match patients underwent pre-operative radiotherapy with those who underwent post-operative radiotherapy. Cox proportional hazard regression model was performed to access the association between variables and OS. Log-rank test was conducted to evaluate the difference in OS between groups.
Results
The estimated median follow-up of all included participants was 69.6 months (IQR: 42.84-60.22); 70.1 months (IQR: 46.85-79.97) for postoperative radiotherapy, 68.5 (IQR: 41.13-78.23) for preoperative radiotherapy, and 67.5 (IQR: 25.92-70.99) for no radiotherapy. The 5-year survival rate was 80.01% (79.56-80.47) for LABC patients who received postoperative radiotherapy, 64.08% (57.55-71.34) for preoperative radiotherapy, and 59.67% (58.60-60.77) for no radiotherapy. Compared with no radiation, patients receiving postoperative radiotherapy had a 38% lower risk of mortality (HR=0.62, 95%CI: 0.60-0.65, p<0.001), whereas those who received preoperative radiotherapy had no significant survival benefit (HR=0.88, 95%CI: 0.70-1.11, p=0.282). Propensity score matched analysis indicated that patients treated with preoperative radiotherapy had similar outcomes as those treated with postoperative radiotherapy (AHR=1.23, 95%CI: 0.88-1.72, p=0.218). Further analysis showed that in C0 (HR=1.45, 95%CI: 1.01-2.07, p=0.044) and G1-2 (AHR=1.74, 95%CI: 1.59-5.96, p=0.001) subgroup, patients receiving preoperative radiotherapy showed a worse OS than those who received postoperative radiotherapy.
Conclusions
Patients with LABC underwent postoperative radiotherapy had improved overall survival, whereas no significant survival benefit was observed in patients receiving preoperative radiotherapy. Preoperative radiotherapy did not present a better survival than postoperative radiotherapy for LABC patients.
Keywords: locally advanced breast cancer, National Cancer Database, preoperative radiotherapy, postoperative radiotherapy, overall survival
Introduction
Breast cancer has become the most common cancer worldwide. Early breast cancer accounts for an increasing proportion of new breast cancer cases, and the disease burden continues to increase over time (1). Locally advanced breast cancer (LABC) encompasses stage III of the disease and a subset of patients with stage II (2), with a maximum lesion diameter of more than 5cm or lesion involving the surrounding skin or muscle, with or without axillary lymph node fusion and intramammary node, or ipsilateral supraconavicular node involvement.
The treatment of LABC is still a major challenge in patients with breast cancer because of the large space occupied by the primary lesions and serious local adhesions (3). Due to its low rate of overall survival (OS), high rate of recurrence and distant metastasis, LABC affects the overall survival of breast cancer largely (4). Currently, common adjuvant treatments for breast cancer are postoperative chemotherapy and radiotherapy (5). Radiotherapy is an effective treatment to reduce metastasis and improve the survival rate of breast cancer (6).
Recently, with the development of radiotherapy techniques, the value of preoperative radiotherapy has been reevaluated (7–10). Preoperative radiotherapy has been proven to prolong the prognosis of many cancers, such as rectal cancer (11), cervical cancer (12), et al. Some studies stated that preoperative radiotherapy could reduce the stage of tumor, increase the rate of surgical resection, alleviate symptoms and pain in patients, and improve the life quality of patients (9, 13). At present, there are few clinical studies on preoperative radiotherapy, and its effect for LABC patients is controversial (14–16). Early studies were mainly single-center, uncontrolled retrospective studies with small sample sizes, and the results were limited (17). In terms of long-term survival, the comparison between preoperative radiotherapy and postoperative radiotherapy lacks high-grade evidence-based data, and further investigation is needed.
The Nationally recognized National Cancer Database (NCDB), co-sponsored by the American College of Surgeons and the American Cancer Society, is a clinical oncology database derived from hospital registries collected by more than 1,500 Cancer Council accredited institutions. NCDB data were used to analyze and track patients with malignant cancer, their treatment and outcomes. The data represent more than 70 percent of newly diagnosed cancer cases and more than 34 million historical records nationwide (18). Based on the NCDB, we conducted this study to determine whether preoperative radiotherapy is superior to postoperative radiotherapy for the prognosis of patients with LABC. In this study, we analyzed the radiotherapy status of LABC patients who underwent surgery, and discussed the status and role of preoperative radiotherapy and postoperative radiotherapy in the treatment of LABC, as well as their prognostic value.
Materials and Methods
Study Design and Data Sources
We performed a retrospective review of the NCDB data of LABC patients diagnosed between January 1, 2010, and December 31, 2014. All adult women with LABC were selected by the ICD-O-3 (histological code <8800), and were assigned according to the 7th AJCC TNM edition. Cases with LABC were defined as patients with stage III (T0-2N2M0, T3-4N0-2M0, T0-4N3M0) and part of stage II B (T3N0M0).
The inclusion criteria were as follows: (1) patients diagnosed with LABC in 2010-2014, microscopically confirmed, and only one malignant or in situ primary tumor in the patient’s lifetime; (2) patients who underwent breast surgery with a specific surgical procedure; (3) patients with no distant metastasis; (4) cases were females and aged ≥18.
We excluded cases for any of the following reasons: (1) lack of data on estrogen receptor, progesterone receptor, or human epidermal growth factor receptor 2 (Her-2); (2) unknown tumor grade or stage; (3) unknown status of chemotherapy, hormone therapy, or immunotherapy treatment; (3) lack of data on insurance, income, home location, vital status, or follow-up time; (4) if the patient received radiation therapy both before and after surgery or if they received intraoperative radiation with or without another therapy, in an unknow sequence except for postoperative radiotherapy, preoperative radiotherapy, and no radiation.
Data Extraction and Outcomes
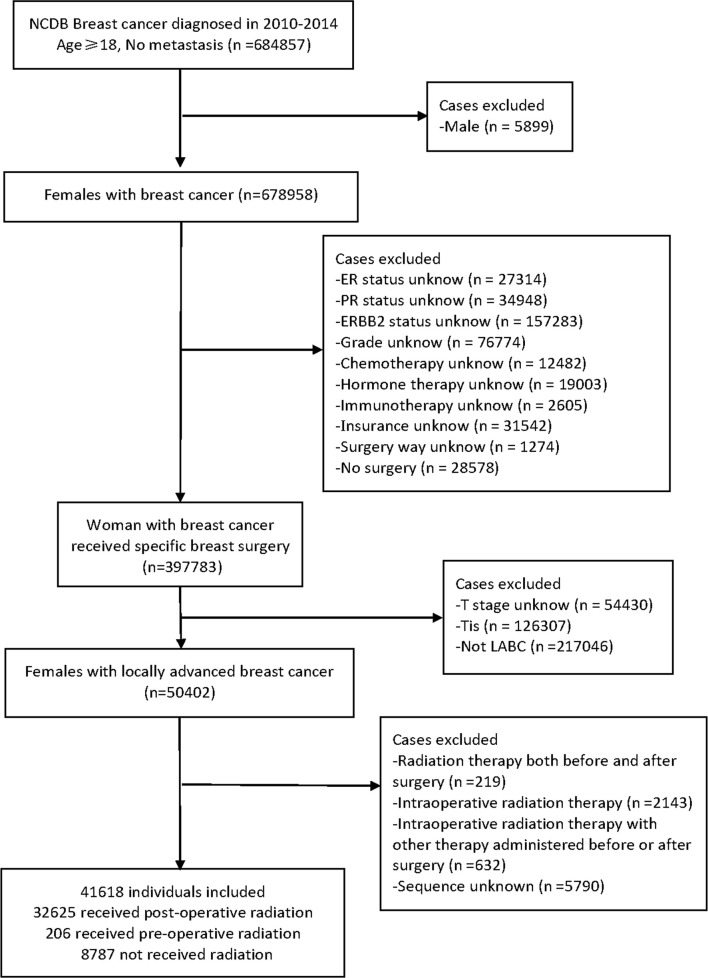
All included LABC patients were confirmed by cytology, histopathology, or microscopy and had only one lifetime history of malignancy or in situ recurrence, with no distant metastasis. We used the Charlson-Deyo Comorbidity Index (CCI) to quantify comorbid conditions. In total, eighteen factors were extracted: age at diagnosis, race, insurance provider (Medicaid, Medicare, or Private insurance/managed Care), median household income (high, high-middle, low-middle, or low), home location (rural, urban, or metro); CCI, grade (G1, well differentiated; G2, moderately differentiated; G3, poorly differentiated; G4, undifferentiated); tumor stage (T stage), nodal stage (N stage), molecular subtype (luminal, Her-2 positive, and triple-negative breast cancer); clinical stage, chemotherapy, hormone therapy, immunotherapy, surgery method; sequence of patients receiving radiotherapy and surgery, vital status, and follow-up time. The surgical procedure included total (simple) mastectomy, breast-conserving or -preserving surgery (BCS), and radical mastectomy. The race of the patients was divided into white, black, Asian/other. The pathological results of patients were classified into three categories based on ER, PR, and ERBB2 status. Luminal subtype was ER or PR positive, with or without ERBB2 positive. Her-2 positive subtype meant that both ER and PR are negative and ERBB2 is positive. Triple-negative subtype was defined as negative for estrogen receptor (ER), progesterone receptor (PR) and ERBB2 or Her-2. ER and PR were considered negative if less than 1% of cells stain positive. If the immunohistochemistry score was 0 to 1+ or fluorescence in situ hybridization and color in situ hybridization do not amplify, ERBB2 status was considered negative. The primary outcome was the rate of overall survival after breast surgery and radiotherapy. The endpoint was defined as the vital status of patients at last contact (alive or deceased). And the number of months to last contact were recorded. The diagram outlining all the selection criteria is presented in Figure 1 .
Figure 1.
Flow Chart of participants Selection in National Cancer Database.
Statistical Analysis
We used frequency (percentage) to express categorical variables data and reported quantitative variables in quartile range (IQR). χ2 test or Fisher’s exact test was used for qualitative variables, and unpaired Kruskal-Wallis test was applied in quantitative variables. The Bonferroni test was conducted to compare sociodemographic, therapeutic, and tumor characteristics between the three treatment groups. In addition, from diagnosis to the last contact or death, the OS rate was calculated on a monthly basis. Univariate and multivariate Cox proportional hazard models were used to investigate the factors affecting OS in the unmatched and matched cohort. To solve the imbalance between patients receiving postoperative and preoperative radiotherapy, we conducted propensity score matching (PSM) analysis (19). We matched the conditional probability propensity scores for adjuvant radiotherapy before and after surgery. The variables included in the PSM analysis were age, race, insurance, income, home location, CCI, grade, T stage, N stage, molecular subtype, clinical stage, chemotherapy, hormone therapy, immunotherapy, surgery method. These variables are potential factors affecting the probability of receiving radiotherapy treatment. To avoid over-fitness, items (radiation and surgery sequence) entered into the PSM were excluded from the multivariate Cox regression analysis. The Kaplan-Meier curve was fit to calculate cumulative survival in unmatched and propensity matched cohorts. A log-rank test was performed to test the differences in the cumulative proportions across different treatment groups (20). Our study was reported followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline ( eTable 1 ). All statistical tests were two-sided, the significance level of the Bonferroni test was 0.0167, and the significance level of other tests was 0.05. All statistical analyses were conducted using R software for Windows, version 4.0.5 (R Project for Statistical Computing).
Results
Patient Characteristics
A total of 41,618 cases met the inclusion criteria outlined above and were enrolled in our initial non-matched analysis ( Figure 1 ). Among these patients, 32,625 (78.39%) experienced postoperative radiotherapy, 8,787 (21.11%) received no adjuvant radiation, and 206 (0.49%) endured preoperative radiotherapy. Compared with patients experienced preoperative radiotherapy, the postoperative radiotherapy cohort was younger (mean age, 59.24 vs 59.27, p<0.001), more Asians (p<0.001), more private insurance payers (p=0.002), more luminal tumors (p<0.001); and had better differentiation levels (p<0.001), lower tumor stage (p<0.001), higher nodal stage (p=0.005), better prognosis (p<0.001); more patients received hormone therapy (p<0.001) and BCS (p<0.001). There were no significant differences of distribution between preoperative and postoperative groups in income, home location, CCI grade, clinical stage, chemotherapy, and immunotherapy ( Table 1 ).
Table 1.
Patient demographic, disease, and treatment characteristics of locally advanced breast cancer grouped by radiation status.
| Variable | Total population (No.) | No radiation | Postoperative radiotherapy | Preoperative radiotherapy | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (No.) | % | (No.) | % | (No.) | % | No radiation vs Postoperative radiotherapy | No radiation vs Preoperative radiotherapy | Postoperative radiotherapy vs Preoperative radiotherapy | ||
| Age, mean (SD), years | 59.82(12.91) | 60.98 (14.64) | 59.24 (11.89) | 56.27 (13.00) | <0.001 a | <0.001 a | <0.001 a | |||
| Age distribution (years) | ||||||||||
| <35 | 1415 | 187 | 13.22 | 1212 | 85.65 | 16 | 1.13 | <0.001 a | <0.001 a | 0.015 a |
| 35-50 | 10860 | 1575 | 14.50 | 9236 | 85.05 | 49 | 0.45 | |||
| 50-70 | 20345 | 3434 | 16.88 | 16804 | 82.60 | 107 | 0.53 | |||
| ≥70 | 8998 | 3591 | 39.91 | 5373 | 59.71 | 34 | 0.38 | |||
| Race | ||||||||||
| White | 33144 | 6927 | 20.90 | 26073 | 78.67 | 144 | 0.43 | <0.001 a | 0.001a | <0.001 a |
| Asia/other | 2140 | 401 | 18.74 | 1731 | 80.89 | 8 | 0.37 | |||
| Black | 6334 | 1459 | 23.03 | 4821 | 76.11 | 54 | 0.85 | |||
| Insurance | ||||||||||
| Not insured | 1436 | 267 | 18.59 | 1153 | 80.29 | 16 | 1.11 | <0.001 a | <0.001 a | 0.002 a |
| Medicaid | 4800 | 941 | 19.60 | 3830 | 79.79 | 29 | 0.60 | |||
| Medicare | 12995 | 4258 | 32.77 | 8676 | 66.76 | 61 | 0.47 | |||
| Private Insurance/Managed Care | 22387 | 3321 | 14.83 | 18966 | 84.72 | 100 | 0.45 | |||
| Income | ||||||||||
| Low | 7839 | 1937 | 24.71 | 5856 | 74.70 | 46 | 0.59 | <0.001 a | <0.001 a | 0.381 |
| High | 15333 | 2878 | 18.77 | 12384 | 80.77 | 71 | 0.46 | |||
| High-middle | 9612 | 1980 | 20.60 | 7583 | 78.89 | 49 | 0.51 | |||
| Low-middle | 8834 | 1992 | 22.55 | 6802 | 77.00 | 40 | 0.45 | |||
| Home location | ||||||||||
| Rural/urban | 5720 | 1207 | 21.10 | 4491 | 78.51 | 22 | 0.38 | 0.957 | 0.246 | 0.238 |
| Metro | 35898 | 7580 | 21.12 | 28134 | 78.37 | 184 | 0.51 | |||
| Charlson Comorbidity Index | ||||||||||
| C0 | 34199 | 6684 | 19.54 | 27344 | 79.96 | 171 | 0.50 | <0.001 a | 0.053 | 0.11 |
| C1 | 5921 | 1574 | 26.58 | 4323 | 73.01 | 24 | 0.41 | |||
| C2-3 | 1498 | 529 | 35.31 | 958 | 63.95 | 11 | 0.73 | |||
| Grade | ||||||||||
| G1-2 | 22435 | 4344 | 19.36 | 18008 | 80.27 | 83 | 0.37 | <0.001 a | 0.0116 a | <0.001 a |
| G3-4 | 19183 | 4443 | 23.16 | 14617 | 76.20 | 123 | 0.64 | |||
| Tumor stage | ||||||||||
| T0-1 | 7016 | 767 | 10.93 | 6224 | 88.71 | 25 | 0.36 | <0.001 a | <0.001 a | <0.001 a |
| T2 | 13610 | 2007 | 14.75 | 11551 | 84.87 | 52 | 0.38 | |||
| T3 | 17616 | 4828 | 27.41 | 12718 | 72.20 | 70 | 0.40 | |||
| T4 | 3376 | 1185 | 35.10 | 2132 | 63.15 | 59 | 1.75 | |||
| Nodal stage | ||||||||||
| N0 | 8255 | 3153 | 38.20 | 5053 | 61.21 | 49 | 0.59 | <0.001 a | <0.001 a | 0.005 a |
| N1 | 5787 | 1558 | 26.92 | 4198 | 72.54 | 31 | 0.54 | |||
| N2 | 19298 | 2771 | 14.36 | 16437 | 85.17 | 90 | 0.47 | |||
| N3 | 8278 | 1305 | 15.76 | 6937 | 83.80 | 36 | 0.43 | |||
| Stage | ||||||||||
| S0-2 | 8050 | 2883 | 35.81 | 5134 | 63.78 | 33 | 0.41 | <0.001 a | <0.001 a | 0.988 |
| S3-4 | 33568 | 5904 | 17.59 | 27491 | 81.90 | 173 | 0.52 | |||
| Chemotherapy | ||||||||||
| No | 8054 | 4207 | 52.23 | 3827 | 47.52 | 20 | 0.25 | <0.001 a | <0.001 a | 0.429 |
| Yes | 33564 | 4580 | 13.65 | 28798 | 85.80 | 186 | 0.55 | |||
| Hormone therapy | ||||||||||
| No | 12563 | 4408 | 35.09 | 8055 | 64.12 | 100 | 0.80 | <0.001 a | 0.697 | <0.001 a |
| Yes | 29055 | 4379 | 15.07 | 24570 | 84.56 | 106 | 0.36 | |||
| Immunotherapy | ||||||||||
| No | 39252 | 8457 | 21.55 | 30597 | 77.95 | 198 | 0.50 | <0.001 a | 0.99 | 0.215 |
| Yes | 2366 | 330 | 13.95 | 2028 | 85.71 | 8 | 0.34 | |||
| Subtype | ||||||||||
| Luminal | 31257 | 5895 | 18.86 | 25247 | 80.77 | 115 | 0.37 | <0.001 a | <0.001 a | <0.001 a |
| Triple negative | 7677 | 2209 | 28.77 | 5391 | 70.22 | 77 | 1.00 | |||
| Her-2 | 2684 | 683 | 25.45 | 1987 | 74.03 | 14 | 0.52 | |||
| Surgery | ||||||||||
| Simple mastectomy | 13582 | 3586 | 26.40 | 9938 | 73.17 | 58 | 0.43 | <0.001 a | <0.001 a | <0.001 a |
| BCS/other | 9374 | 1200 | 12.80 | 8150 | 86.94 | 24 | 0.26 | |||
| Radical mastectomy | 18662 | 4001 | 21.44 | 14537 | 77.90 | 124 | 0.66 | |||
| Vital status | ||||||||||
| Alive | 30352 | 5108 | 16.83 | 25116 | 82.75 | 128 | 0.42 | <0.001 a | 0.28 | <0.001 a |
| Deceased | 11266 | 3679 | 32.66 | 7509 | 66.65 | 78 | 0.69 | |||
BCS, breast-conserving surgery; SD, standard deviation.
Significance was evaluated using Bonferroni test. The statistical tests were two-sided, the significance level was 0.0167.
Univariate and Multivariate Analysis
The estimated median follow-up time was 70.1 months (IQR: 46.85-79.97, range: 2.92-112.95, 95%CI: 69.7-70.5) for postoperative radiotherapy, 68.5 (IQR: 41.13-78.23, range: 4.99-111.57, 95%CI: 65.2- 74.8) for preoperative radiotherapy. The 5-year survival rate was 80.01% (79.56-80.47) for LABC patients receiving postoperative radiotherapy, 64.08% (57.55-71.34) for preoperative radiotherapy. In the survival analysis of the unmatched cohort, postoperative radiotherapy was related associated with improved OS compared to no radiation (p<0.0001) ( Figure 2A ). Similarly, in the multivariable Cox analysis adjusted for confounders, patients who received postoperative radiotherapy had a 38% lower risk of mortality [Adjusted HR (AHR) =0.62, 95%CI: 0.60-0.65, p<0.001]. However, there was no significant difference in prognosis between patients who received preoperative radiotherapy and those who did not (HR=0.85, 95%CI: 0.68-1.06, p=0.148; AHR=0.88, 95%CI: 0.70-1.11, p=0.282, Table 2 ).
Figure 2.
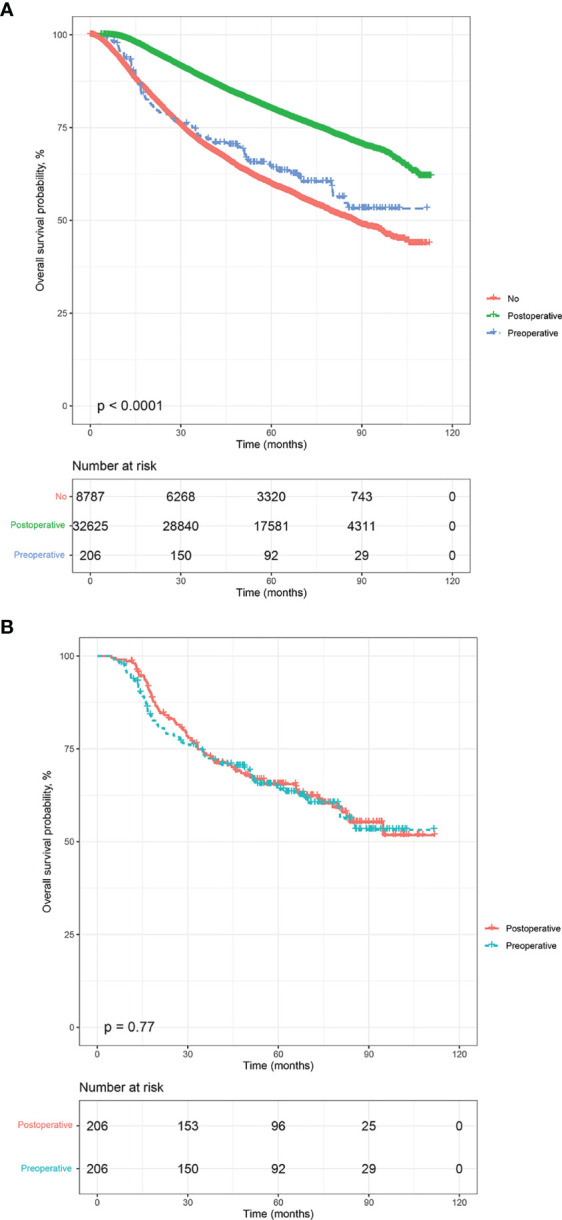
Kaplan-Meier overall survival analysis, before and after propensity score matching. (A) all participants, (B) matched population.
Table 2.
Univariable and multivariable Cox analysis of overall survival for patients with locally advanced breast cancer.
| Variable | Total population | Alive | Deceased | Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|---|---|---|
| No. | No. | % | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age distribution (years) | ||||||||
| 35-50 | 10860 | 8731 | 2129 | 19.60 | 1 (Ref.) | 1 (Ref.) | ||
| <35 | 1415 | 1055 | 360 | 25.44 | 1.36 (1.22-1.53) | <0.001 | 1.23 (1.10-1.37) | <0.001 a |
| 50-70 | 20345 | 15582 | 4763 | 23.41 | 1.22 (1.16-1.28) | <0.001 | 1.11 (1.05-1.17) | <0.001 a |
| ≥70 | 8998 | 4984 | 4014 | 44.61 | 2.80 (2.65-2.95) | <0.001 | 1.63 (1.52-1.76) | <0.001 a |
| Race | ||||||||
| White | 33144 | 24421 | 8723 | 26.32 | 1 (Ref.) | |||
| Asia/other | 2140 | 1758 | 382 | 17.85 | 0.68 (0.61-0.75) | <0.001 | 0.77 (0.69-0.85) | <0.001 a |
| Black | 6334 | 4173 | 2161 | 34.12 | 1.42 (1.36-1.49) | <0.001 | 1.16 (1.10-1.22) | <0.001 a |
| Insurance | ||||||||
| Not insured | 1436 | 1049 | 387 | 26.95 | 1 (Ref.) | 1 (Ref.) | ||
| Medicaid | 4800 | 3416 | 1384 | 28.83 | 1.07 (0.95-1.19) | 0.264 | 0.99 (0.89-1.11) | 0.898 |
| Medicare | 12995 | 7975 | 5020 | 38.63 | 1.50 (1.35-1.66) | <0.001 | 0.97 (0.87-1.09) | 0.646 |
| Private Insurance/Managed Care | 22387 | 17912 | 4475 | 19.99 | 0.66 (0.60-0.74) | <0.001 | 0.78 (0.70-0.86) | <0.001 a |
| Income | ||||||||
| Low | 7839 | 5336 | 2503 | 31.93 | 1 (Ref.) | 1 (Ref.) | ||
| High | 15333 | 11767 | 3566 | 23.26 | 0.67 (0.63-0.70) | <0.001 | 0.94 (0.89-0.99) | 0.041 a |
| High-middle | 9612 | 7020 | 2592 | 26.97 | 0.81 (0.76-0.85) | <0.001 | 0.97 (0.92-1.03) | 0.344 |
| Low-middle | 8834 | 6229 | 2605 | 29.49 | 0.90 (0.85-0.95) | <0.001 | 1.03 (0.98-1.092) | 0.27 |
| Home location | ||||||||
| Rural/urban | 5720 | 4041 | 1679 | 29.35 | 1 (Ref.) | 1 (Ref.) | ||
| Metro | 35898 | 26311 | 9587 | 26.71 | 0.88 (0.84-0.93) | <0.001 | 0.93 (0.88-0.99) | 0.014 a |
| Charlson Comorbidity Index | ||||||||
| C0 | 34199 | 25771 | 8428 | 24.64 | 1 (Ref.) | 1 (Ref.) | ||
| C1 | 5921 | 3843 | 2078 | 35.10 | 1.54 (1.46-1.61) | <0.001 | 1.27 (1.21-1.33) | <0.001 a |
| C2-3 | 1498 | 738 | 760 | 50.73 | 2.59 (2.41-2.79) | <0.001 | 1.67 (1.55-1.80) | <0.001 a |
| Grade | ||||||||
| G1-2 | 22435 | 18100 | 4335 | 19.32 | 1 (Ref.) | 1 (Ref.) | ||
| G3-4 | 19183 | 12252 | 6931 | 36.13 | 2.18 (2.09-2.26) | <0.001 | 1.53 (1.46-1.60) | <0.001 a |
| Tumor stage | ||||||||
| T0-1 | 7016 | 5454 | 1562 | 22.26 | 1 (Ref.) | 1 (Ref.) | ||
| T2 | 13610 | 9939 | 3671 | 26.97 | 1.25 (1.18-1.33) | <0.001 | 1.12 (1.05-1.19) | <0.001 a |
| T3 | 17616 | 13198 | 4418 | 25.08 | 1.19 (1.12-1.26) | <0.001 | 1.57 (1.47-1.68) | <0.001 a |
| T4 | 3376 | 1761 | 1615 | 47.84 | 2.75 (2.56-2.95) | <0.001 | 2.18 (2.02-2.36) | <0.001 a |
| Nodal stage | ||||||||
| N0 | 8255 | 6478 | 1777 | 21.53 | 1 (Ref.) | 1 (Ref.) | ||
| N1 | 5787 | 4329 | 1458 | 25.19 | 1.18 (1.10-1.26) | <0.001 | 1.39 (1.24-1.54) | <0.001 a |
| N2 | 19298 | 14438 | 4860 | 25.18 | 1.14 (1.08-1.21) | <0.001 | 2.35 (2.12-2.62) | <0.001 a |
| N3 | 8278 | 5107 | 3171 | 38.31 | 1.90 (1.79-2.01) | <0.001 | 3.49 (3.14-3.89) | <0.001 a |
| Stage | ||||||||
| S0-2 | 8050 | 6400 | 1650 | 20.50 | 1 (Ref.) | 1 (Ref.) | ||
| S3-4 | 33568 | 23952 | 9616 | 28.65 | 1.44 (1.37-1.52) | <0.001 | 1.14 (1.03-1.26) | 0.012 a |
| Chemotherapy | ||||||||
| No | 8054 | 4794 | 3260 | 40.48 | 1 (Ref.) | <0.001 | 1 (Ref.) | |
| Yes | 33564 | 25558 | 8006 | 23.85 | 0.49 (0.47-0.51) | <0.001 | 0.57 (0.54-0.60) | <0.001 a |
| Hormone therapy | ||||||||
| No | 12563 | 7024 | 5539 | 44.09 | 1 (Ref.) | 1 (Ref.) | ||
| Yes | 29055 | 23328 | 5727 | 19.71 | 0.34 (0.33-0.35) | <0.001 | 0.62 (0.58-0.66) | <0.001 a |
| Immunotherapy | ||||||||
| No | 39252 | 28387 | 10865 | 27.68 | 1 (Ref.) | |||
| Yes | 2366 | 1965 | 401 | 16.95 | 0.69 (0.63-0.77) | <0.001 | 0.85 (0.77-0.95) | 0.003 a |
| Subtype | ||||||||
| Luminal | 31257 | 24683 | 6574 | 21.03 | 1 (Ref.) | 1 (Ref.) | ||
| Triple negative | 7677 | 3822 | 3855 | 50.21 | 3.34 (3.21-3.48) | <0.001 | 1.94 (1.81-2.09) | <0.001 a |
| Her-2 | 2684 | 1847 | 837 | 31.18 | 1.60 (1.49-1.72) | <0.001 | 0.93 (0.84-1.02) | 0.125 |
| Surgery | ||||||||
| Simple mastectomy | 13582 | 10482 | 3100 | 22.82 | 1 (Ref.) | 1 (Ref.) | ||
| BCS/other | 9374 | 7279 | 2095 | 22.35 | 0.96 (0.91-1.01) | 0.137 | 0.95 (0.90-1.01) | 0.085 |
| Radical mastectomy | 18662 | 12591 | 6071 | 32.53 | 1.48 (1.42-1.55) | <0.001 | 1.12 (1.07-1.17) | <0.001 a |
| Radiotherapy | ||||||||
| No | 8787 | 5108 | 3679 | 41.87 | 1 (Ref.) | 1 (Ref.) | ||
| Postoperative radiotherapy | 32625 | 25116 | 7509 | 23.02 | 0.43 (0.42-0.45) | <0.001 | 0.62 (0.60-0.65) | <0.001 a |
| Preoperative radiotherapy | 206 | 128 | 78 | 37.86 | 0.85 (0.68-1.06) | 0.148 | 0.88 (0.70-1.11) | 0.282 |
BCS, breast-conserving surgery; HR, hazard ratio.
The statistical tests were two-sided, the significance level was 0.05.
Multivariable Cox analysis revealed that some factors were independently associated with improved or worse OS in LABC patients. Among these, the highest HR was for high nodal stage of LABC (N1/N2/N3 vs. N0), with N3 patients having an AHR of 3.49 (95%CI: 3.14-3.89, p<0.001, Table 2 ) and tumor stage ≥T2, and those with T4 having an AHR of 2.18 (95%CI: 2.02-2.36, p<0.001). Compared with well or moderately differentiated LABC, patients with poorly differentiated or undifferentiated histology had a 53% higher mortality risk (AHR= 1.53, 95%CI: 1.46-1.60, p<0.001). Compared with patients aged 35-50 years, patients aged <35 years, 50-70 years, and ≥70 years had a 23% (p<0.001), 11% (p<0.001), and 63% (p<0.001) higher mortality risk, respectively. Black patients had a 16% higher mortality risk (AHR= 1.16, 95%CI: 1.10-1.22, p<0.001) than white patients. Patients classified as C1 and C2-3 on the CCI had higher mortality risk values compared to C0 patients (C1: AHR=1.27, 95%CI: 1.21-1.33, p<0.001; C2-3: AHR=1.67, 95%CI: 1.55-1.80, p<0.001). Other factors associated with poor survival included clinical stage (stage 0-2 vs 3-4: AHR=1.14, 95%CI: 1.03-1.26, p=0.012), triple-negative subtype (triple-negative vs. luminal: AHR=1.94, 95%CI: 1.81-2.09, p<0.001), and the receipt of radical mastectomy (radical vs. simple: AHR=1.12, 95%CI: 1.07-1.17, p<0.001). In addition, some factors were associated with improved survival of patients with LABC. Asian and other races had a 23% lower mortality risk than white patients (AHR= 0.77, 95%CI: 0.69-0.85, p<0.001). Compared with patients who were not insured, private insurance payers had a 22% lower mortality risk (AHR= 0.78, 95%CI: 0.70-0.86, p<0.001). In addition, compared with low-income patients, those who carried a high median household income had a 6% lower mortality risk (AHR= 0.94, 95%CI: 0.89-0.99, p=0.041). Patients who lived in metro had a 7% lower mortality risk (AHR= 0.93, 95%CI: 0.88-0.99, p=0.014) than those who lived in rural or urban areas. As presented in eTable 2 , patients who received preoperative radiotherapy combined with chemotherapy (HR= 0.34, 95%CI: 0.19-0.62, p<0.001) or hormone therapy (HR= 0.56, 95%CI: 0.36-0.88, p=0.012) showed better outcomes compared with their counterparts without corresponding treatments. The univariate Cox analysis results of patients who received postoperative radiotherapy were shown in eTable 3 .
Propensity Score–Matched Analysis and Outcomes
The estimated median follow-up time was 71.4 months (IQR: 34.37-75.22, range: 4.50-107.04, 95%CI: 67.40-75.20) for patients who received postoperative radiotherapy and 68.5 months (IQR: 65.20-74.80, range: 4.99-111.57, 95%CI: 65.2- 74.8) for those who experienced preoperative radiotherapy. The 5-year survival rate was 66.29% (59.82-73.47) for those who received postoperative radiotherapy and 64.08% (57.55-71.34) for those who endured preoperative radiotherapy. In the multivariable analysis of the matched cohort ( Table 3 ), patients aged ≥70 years had a three times higher risk of mortality (AHR= 3.83, 95%CI: 1.81-8.11, p<0.001) compared to those aged 35-50 years. Black patients had a 59% worse OS (AHR= 1.59, 95%CI: 1.07-2.37, p<0.001) than white patients. In addition, factors associated with poor OS in the matched cohort included tumor stages T3 (T3 vs T0-1: AHR= 2.09, 95%CI: 1.09-4.02, p=0.027) and T4 (T4 vs T0-1:AHR= 3.45, 95%CI: 1.82-6.54, p<0.001), nodal stages N1 (N1 vs N0: AHR= 3.37, 95%CI: 1.48-7.68, p=0.004), N2 (N2 vs N0: AHR= 10.01, 95%CI: 4.59-21.83, p<0.001), and N3 (N3 vs N0: AHR= 10.26, 95%CI: 4.62-22.78, p<0.001), triple-negative subtype (Triple negative vs Luminal: AHR= 9.02, 95%CI: 3.90-20.86, p<0.001), Her-2 positive subtype (Her-2 positive vs Luminal: AHR= 4.17, 95%CI: 1.48-11.72, p=0.007), and patients underwent radical mastectomy (AHR= 1.71, 95%CI: 1.10-2.66, p=0.017). Finally, patients who endured preoperative radiotherapy had a statistically similar prognosis to those who received postoperative radiotherapy (AHR=1.23, 95%CI: 0.88-1.72, p=0.218). Survival analysis indicated no difference existed in the OS of LABC patients between preoperative radiotherapy and postoperative radiotherapy (p=0.77, Figure 2B ). In addition, patients in C0 (HR=1.45, 95%CI: 1.01-2.07, p=0.044) and G1-2 subgroup (AHR=1.74, 95%CI: 1.59-5.96, p=0.001) experienced preoperative radiotherapy showed a worse OS than those who received postoperative radiotherapy ( Figure 3 ).
Table 3.
Propensity-adjusted multivariable Cox regression analysis of overall survival for locally advanced breast cancer.
| Variable | HR (95% CI) | P value |
|---|---|---|
| Age distribution (years) | ||
| 35-50 | 1 (Ref.) | |
| <35 | 1.09 (0.54-2.21) | 0.814 |
| 50-70 | 1.33 (0.83-2.14) | 0.233 |
| ≥70 | 3.83 (1.81- 8.11) | <0.001 a |
| Race | ||
| White | 1 (Ref.) | |
| Asia/other | 1.31 (0.45-3.81) | 0.625 |
| Black | 1.59 (1.072-2.37) | 0.021 a |
| Insurance | ||
| Not insured | 1 (Ref.) | |
| Medicaid | 1.27 (0.48-3.35) | 0.63 |
| Medicare | 0.67 (0.26-1.74) | 0.406 |
| Private Insurance/Managed Care | 1.09 (0.44-2.67) | 0.858 |
| Income | 1 (Ref.) | |
| Low | 1.35 (0.80-2.27) | 0.259 |
| High | 1.09 (0.64-1.88) | 0.741 |
| High-middle | 1.70 (0.99-2.90) | 0.054 |
| Low-middle | ||
| Home location | ||
| Rural/urban | 1 (Ref.) | |
| Metro | 1.13 (0.59-2.19) | 0.71 |
| Charlson Comorbidity Index | ||
| C0 | 1 (Ref.) | |
| C1 | 1.12 (0.62-2.02) | 0.706 |
| C2-3 | 1.48 (0.74-2.97) | 0.266 |
| Grade | ||
| G1-2 | 1 (Ref.) | |
| G3-4 | 1.07 (0.72-1.60) | 0.736 |
| Tumor stage | ||
| T0-1 | 1 (Ref.) | |
| T2 | 1.76 (0.96-3.25) | 0.07 |
| T3 | 2.09 (1.09-4.02) | 0.027 a |
| T4 | 3.45 (1.82-6.54) | <0.001 a |
| Nodal stage | ||
| N0 | 1 (Ref.) | |
| N1 | 3.37 (1.48-7.68) | 0.004 a |
| N2 | 10.01 (4.59-21.83) | <0.001 a |
| N3 | 10.26 (4.62-22.78) | <0.001 a |
| Stage | ||
| S0-2 | 1 (Ref.) | |
| S3-4 | 0.71 (0.30-1.68) | 0.436 |
| Chemotherapy | ||
| No | 1 (Ref.) | |
| Yes | 1.06 (0.58-1.94) | 0.855 |
| Hormone therapy | ||
| No | 1 (Ref.) | |
| Yes | 1.60 (0.71-3.58) | 0.254 |
| Immunotherapy | ||
| No | 1 (Ref.) | |
| Yes | 1.46 (0.52-4.13) | 0.472 |
| Subtype | ||
| Luminal | 1 (Ref.) | |
| Triple negative | 9.02 (3.90-20.86) | <0.001 a |
| Her-2 | 4.17 (1.48-11.72) | 0.007 a |
| Surgery | ||
| Simple mastectomy | 1 (Ref.) | |
| BCS/other | 1.80 (0.89-3.62) | 0.1 |
| Radical mastectomy | 1.71 (1.10-2.66) | 0.017 a |
| Radiotherapy | ||
| Postoperative radiotherapy | 1 (Ref.) | |
| Preoperative radiotherapy | 1.23 (0.88-1.72) | 0.218 |
BCS, breast-conserving surgery; HR, hazard ratio.
The statistical tests were two sided, the significance level was 0.05.
Figure 3.
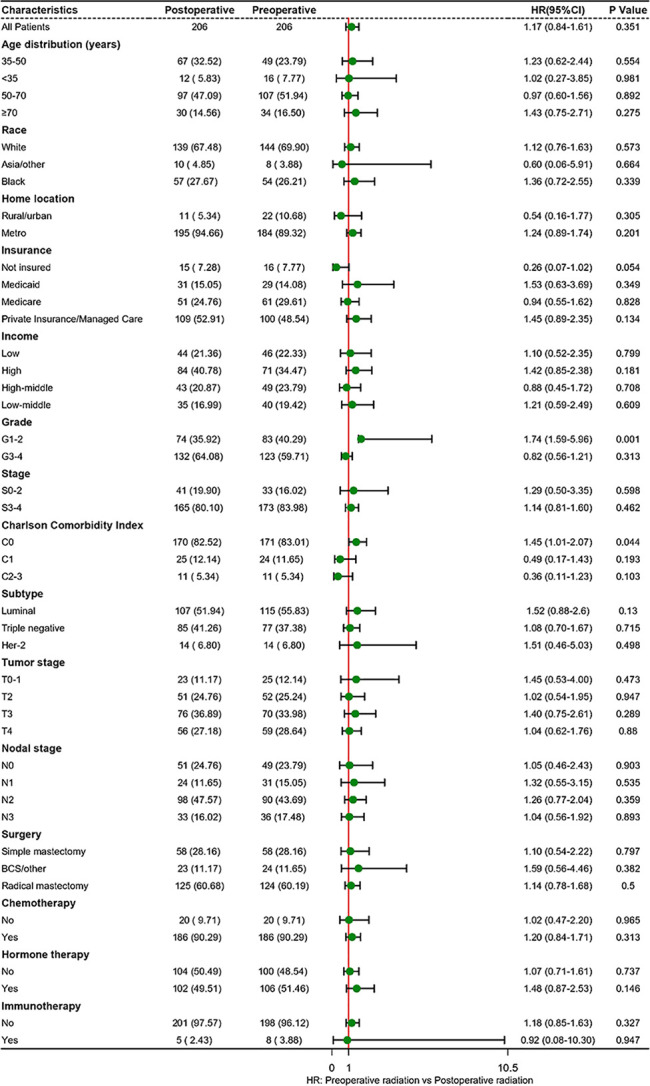
Subgroup analyses of radiotherapy treatment based on matched population. BCS, breast-conserving surgery; HR, hazard ratio.
Discussion
In this hospital-based registry analysis, postoperative radiotherapy presented a significant benefit for improved OS of LABC patients compared to no radiation, which appears to be consistent with a previous study (21). The benefit was also observed in patients who endured preoperative radiotherapy. However, the benefit was not statistically significant. PSM matched analysis indicated that, compared with postoperative radiotherapy, no survival improvement was observed in LABC patients who experienced preoperative radiotherapy. The effect of postoperative radiotherapy for LABC patients had been confirmed by several large clinical trials, which could significantly increase the local control rates and improve their OS rates (22).
In recent years, the value of preoperative radiotherapy in the treatment of LABC patients has been reassessed. Studies showed that new adjuvant chemotherapy improved the pathological complete response of tumors (23). A Previous study reported on the benefits of preoperative radiotherapy or chemotherapy on tumor treatment (24) and the impact of breast reconstruction surgery, as well as its value in tumor biology and translational medicine research. Our analysis illustrated that patients receiving preoperative radiotherapy combined with chemotherapy or hormone therapy showed prognosis benefit, which is consistent with published studies (25, 26). Through the combined use of preoperative radiotherapy and drugs, clinicians can obtain a clinical effect evaluation in a relatively short period of time and guide follow-up treatment by observing lesion changes (27, 28). However, approximately 1/3 of LABC patients are resistant to neoadjuvant chemotherapy, and there is still no manual resection opportunity for the tumor after chemotherapy. In this case, preoperative radiotherapy (21) or preoperative concurrent chemoradiotherapy is an important salvage treatment measure which could reduce the tumor load in some patients and provide the opportunity for surgical resection (2). Preoperative radiotherapy could increase the sensitivity of radiotherapy (29), cause tumor tissue fibrosis, reduce the risk of intraoperative implantation and metastasis, change the tumor microenvironment, transform the tumor immune escape state into a tumor immune attack state, and activate the immune system to produce long-distance effects (9, 30). However, the high incidence of acute toxic reactions is attributed to the lack of therapeutic experience and/or technical limitations due to factors such as concurrent chemotherapy, a high total dose of radiotherapy, and the limit of radiation techniques. Severe toxic reactions are the most important reason for the limited clinical application of preoperative radiotherapy or preoperative neoadjuvant concurrent chemoradiotherapy (31). Several studies have demonstrated the favorable effect of preoperative radiotherapy on tumor treatment and breast reconstruction surgery, as well as its value in tumor biology and translational medicine research.
Radiotherapy is important for the treatment of breast cancer, improving the local control rate and OS of patients at a high risk of recurrence. For advanced breast cancer (16), preoperative radiotherapy can reduce tumor stage, increase the resection rate, and alleviate the symptoms of patients.
Clinically, the selection of neoadjuvant radiotherapy for patients is limited to a certain extent, and there is currently no unified standard. Most clinical decisions depend on the clinical experience of doctors, so there may be the possibility of overtreatment. In our analysis, black patients with LABC were more inclined to endure preoperative radiotherapy, especially for patients with T4 stage tumors, aged 50-70 years, uninsured, triple negative subtype, poorly or undifferentiated. As for surgery method, the proportion of patients undergoing radical breast cancer resection undergoing preoperative radiotherapy was higher than that of patients undergoing other surgical procedures. Besides, neoadjuvant radiotherapy or chemoradiotherapy may lead to vascular injury and microcirculation disturbance, resulting in tissue cell degeneration and necrosis, breast fibrosis and skin injury. However, the fibrotic and damaged skin of the breast increases the difficulty of operation and prolongs the operation time, making radiotherapy as a neoadjuvant therapy method not widely employed for breast cancer (7). Suitable and safe treatment plans timelines, and treatment modalities with long survival rates, short and convenient reconstruction processes, and good appearance should be determined for LABC patients. In addition, biomarkers that are sensitive to radiation and chemotherapy should be ascertained.
A study based on 129,692 patients supported that breast-conserving surgery with radiation therapy improved the survival of breast cancer patients (26). Patients with stage IIB-IIIA breast cancer are generally considered having “operable breast cancer”. In contrast, those receiving postoperative radiotherapy or with stage IIIB and IIIC cancer are likely to be classified as inoperable cases; this is due to the presence of inflammation and/or extensive skin involvement, immobilization, or very large axillary lymph node disease, and/or the involvement of supraclavicular or internal breast lymph nodes (32). However, preoperative radiotherapy provides LABC patients with no chance of surgery with the opportunity of surgical treatment, as well as the opportunity of breast-conserving surgery for patients who cannot initially undergo breast-conserving surgery (24), thus improving their quality of life (33). By comparing the tumor tissues before and after radiotherapy and analyzing the various differences at the molecular level, biological information related to the radio sensitivity of tumor cells can be obtained, which helps to understand the changes in the immune microenvironment (34).
There are some limitations inevitable in this study. A small percentage of patients with LABC received preoperative radiation. Due to the limited data, we could not perform further subgroup analysis on the radiotherapy duration and dose of patients. The study population included patients who were diagnosed with LABC and underwent breast surgery. It should be emphasized that the application of these results cannot be expanded to general breast cancer. Although we used a retrospective paired study to select the control group, there is still an unavoidable selection bias, and there are some unknown influencing factors that will affect the final study conclusion. Besides, due to the limited data of preoperative radiotherapy, we had not been able to do a preoperative and postoperative analysis of other treatments (e.g., chemotherapy, endocrine therapy, immunotherapy) in LABC patients. However, we had enrolled those potential factors into the PSM analysis, and the effect of the variables has been largely balanced. In addition, we analyzed the relationship between the two types of radiotherapy combined with other treatments independently. We recommend that patients with LABC be treated in combination with chemotherapy or hormone therapy, regardless of preoperative or postoperative radiotherapy. Nevertheless, the role and value of preoperative radiotherapy or concurrent radio-chemotherapy for the treatment of LABC under the application of novel radiotherapy technologies and medicines requires confirmation and investigation by prospective, multi-center, randomized controlled clinical studies with large sample sizes.
In this study, patients with LABC who received postoperative radiotherapy were associated with improved OS, while those who received preoperative radiotherapy had no significant benefit. In the matched analysis, there was no significant difference in survival between patients receiving postoperative radiotherapy and those who receiving preoperative radiotherapy. The conclusions still need to be confirmed in large prospective clinical trials.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by The First Affiliated Hospital of Zhejiang University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
All authors read, critically reviewed and approved the final manuscript. ZD and HD designed the research. YD, HL, JL, and ZZ collected the data. MW and SL verified the accuracy of the data. YD, SY, YL, and BW performed the statistical analysis. PX, YW, JH, and XD conducted the visualization. YD wrote the manuscript. YD and HL contributed equally to this work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer QT has declared a shared parent affiliation with the authors MW, SL, PX, and YW at the time of review.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all members of our study team for their wonderful cooperation and the National Cancer Database for their works.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.779185/full#supplementary-material
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Reporting Guideline Checklist.
Univariate cox analysis of overall survival in patients with locally advanced breast cancer received preoperative radiation.
Univariate cox analysis of overall survival in patients with locally advanced breast cancer received postoperative radiation.
Abbreviations
LABC, locally advanced breast cancer; OS, overall survival; NCDB, National Cancer Database; Her-2, human epidermal growth factor receptor 2; BCS, breast-conserving or -preserving surgery; IQR inter-quartile range; PSM, propensity score matching; AHR, adjusted hazard ratio.
References
- 1. Li N, Deng Y, Zhou L, Tian T, Yang S, Wu Y, et al. Global Burden of Breast Cancer and Attributable Risk Factors in 195 Countries and Territories, From 1990 to 2017: Results From the Global Burden of Disease Study 2017. J Hematol Oncol (2019) 12(1):140. doi: 10.1186/s13045-019-0828-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tryfonidis K, Senkus E, Cardoso MJ, Cardoso F. Management of Locally Advanced Breast Cancer-Perspectives and Future Directions. Nat Rev Clin Oncol (2015) 12(3):147–62. doi: 10.1038/nrclinonc.2015.13 [DOI] [PubMed] [Google Scholar]
- 3. Klein J, Tran W, Watkins E, Vesprini D, Wright FC, Look Hong NJ, et al. Locally Advanced Breast Cancer Treated With Neoadjuvant Chemotherapy and Adjuvant Radiotherapy: A Retrospective Cohort Analysis. BMC Cancer (2019) 19(1):306. doi: 10.1186/s12885-019-5499-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sikov WM. Locally Advanced Breast Cancer. Curr Treat Options Oncol (2000) 1(3):228–38. doi: 10.1007/s11864-000-0034-9 [DOI] [PubMed] [Google Scholar]
- 5. Veronesi U, Boyle P, Goldhirsch A, Orecchia R, Viale G. Breast Cancer. Lancet (2005) 365(9472):1727–41. doi: 10.1016/s0140-6736(05)66546-4 [DOI] [PubMed] [Google Scholar]
- 6. Rutqvist LE, Pettersson D, Johansson H. Adjuvant Radiation Therapy Versus Surgery Alone in Operable Breast Cancer: Long-Term Follow-Up of a Randomized Clinical Trial. Radiother Oncol (1993) 26(2):104–10. doi: 10.1016/0167-8140(93)90090-u [DOI] [PubMed] [Google Scholar]
- 7. Riet FG, Fayard F, Arriagada R, Santos MA, Bourgier C, Ferchiou M, et al. Preoperative Radiotherapy in Breast Cancer Patients: 32 Years of Follow-Up. Eur J Cancer (2017) 76:45–51. doi: 10.1016/j.ejca.2017.01.022 [DOI] [PubMed] [Google Scholar]
- 8. Bollet MA, Belin L, Reyal F, Campana F, Dendale R, Kirova YM, et al. Preoperative Radio-Chemotherapy in Early Breast Cancer Patients: Long-Term Results of a Phase II Trial. Radiother Oncol (2012) 102(1):82–8. doi: 10.1016/j.radonc.2011.08.017 [DOI] [PubMed] [Google Scholar]
- 9. Adams S, Chakravarthy AB, Donach M, Spicer D, Lymberis S, Singh B, et al. Preoperative Concurrent Paclitaxel-Radiation in Locally Advanced Breast Cancer: Pathologic Response Correlates With Five-Year Overall Survival. Breast Cancer Res Treat (2010) 124(3):723–32. doi: 10.1007/s10549-010-1181-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corradini S, Krug D, Meattini I, Matuschek C, Bölke E, Francolini G, et al. Preoperative Radiotherapy: A Paradigm Shift in the Treatment of Breast Cancer? A Review of Literature. Crit Rev Oncol Hematol (2019) 141:102–111. doi: 10.1016/j.critrevonc.2019.06.003 [DOI] [PubMed] [Google Scholar]
- 11. Marijnen CA, Kapiteijn E, van de Velde CJ, Martijn H, Steup WH, Wiggers T, et al. Acute Side Effects and Complications After Short-Term Preoperative Radiotherapy Combined With Total Mesorectal Excision in Primary Rectal Cancer: Report of a Multicenter Randomized Trial. J Clin Oncol (2002) 20(3):817–25. doi: 10.1200/jco.2002.20.3.817 [DOI] [PubMed] [Google Scholar]
- 12. Beskow C, Agren-Cronqvist AK, Granath F, Frankendal B, Lewensohn R. Pathologic Complete Remission After Preoperative Intracavitary Radiotherapy of Cervical Cancer Stage Ib and IIa Is a Strong Prognostic Factor for Long-Term Survival: Analysis of the Radiumhemmet Data 1989-1991. Int J Gynecol Cancer (2002) 12(2):158–70. doi: 10.1046/j.1525-1438.2002.01089.x [DOI] [PubMed] [Google Scholar]
- 13. Paillocher N, Florczak AS, Richard M, Classe JM, Oger AS, Raro P, et al. Evaluation of Mastectomy With Immediate Autologous Latissimus Dorsi Breast Reconstruction Following Neoadjuvant Chemotherapy and Radiation Therapy: A Single Institution Study of 111 Cases of Invasive Breast Carcinoma. Eur J Surg Oncol (2016) 42(7):949–55. doi: 10.1016/j.ejso.2016.03.024 [DOI] [PubMed] [Google Scholar]
- 14. Mladenovic J, Susnjar S, Tanic M, Jankovic R, Karadzic K, Gavrilovic D, et al. Tumor Response and Patient Outcome After Preoperative Radiotherapy in Locally Advanced Non-Inflammatory Breast Cancer Patients. J buon (2017) 22(2):325–33. [PubMed] [Google Scholar]
- 15. Lerouge D, Touboul E, Lefranc JP, Genestie C, Moureau-Zabotto L, Blondon J. [Locally Advanced non Inflammatory Breast Cancer Treated by Combined Chemotherapy and Preoperative Irradiation: Updated Results in a Series of 120 Patients]. Cancer Radiother (2004) 8(3):155–67. doi: 10.1016/j.canrad.2004.01.001 Cancer du sein localement évolué non inflammatoire traité par association de chimiothérapie et de radiothérapie à dose préopératoire: réactualisation des résultats d’une série de 120 patientes. fre. [DOI] [PubMed] [Google Scholar]
- 16. Lerouge D, Touboul E, Lefranc JP, Genestie C, Moureau-Zabotto L, Blondon J. Combined Chemotherapy and Preoperative Irradiation for Locally Advanced Noninflammatory Breast Cancer: Updated Results in a Series of 120 Patients. Int J Radiat Oncol Biol Phys (2004) 59(4):1062–73. doi: 10.1016/j.ijrobp.2003.12.034 [DOI] [PubMed] [Google Scholar]
- 17. Gerlach B, Audretsch W, Gogolin F, Königshausen T, Rohn R, Schmitt G, et al. Remission Rates in Breast Cancer Treated With Preoperative Chemotherapy and Radiotherapy. Strahlenther Onkol (2003) 179(5):306–11. doi: 10.1007/s00066-003-1019-y [DOI] [PubMed] [Google Scholar]
- 18. Boffa DJ, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol (2017) 1 3(12):1722–8. doi: 10.1001/jamaoncol.2016.6905 [DOI] [PubMed] [Google Scholar]
- 19. Duhamel A, Labreuche J, Gronnier C, Mariette C. Statistical Tools for Propensity Score Matching. Ann Surg (2017) 265(6):E79–e80. doi: 10.1097/sla.0000000000001312 [DOI] [PubMed] [Google Scholar]
- 20. Zhai Z, Zheng Y, Yao J, Liu Y, Ruan J, Deng Y, et al. Evaluation of Adjuvant Treatments for T1 N0 M0 Triple-Negative Breast Cancer. JAMA Netw Open (2020) 3(11):e2021881. doi: 10.1001/jamanetworkopen.2020.21881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang J, Shi M, Ling R, Xia Y, Luo S, Fu X, et al. Adjuvant Chemotherapy and Radiotherapy in Triple-Negative Breast Carcinoma: A Prospective Randomized Controlled Multi-Center Trial. Radiother Oncol (2011) 100(2):200–4. doi: 10.1016/j.radonc.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 22. Pondé NF, Zardavas D, Piccart M. Progress in Adjuvant Systemic Therapy for Breast Cancer. Nat Rev Clin Oncol (2019) 16(1):27–44. doi: 10.1038/s41571-018-0089-9 [DOI] [PubMed] [Google Scholar]
- 23. Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, et al. Clinical Course of Breast Cancer Patients With Complete Pathologic Primary Tumor and Axillary Lymph Node Response to Doxorubicin-Based Neoadjuvant Chemotherapy. J Clin Oncol (1999) 17(2):460–9. doi: 10.1200/jco.1999.17.2.460 [DOI] [PubMed] [Google Scholar]
- 24. Matuschek C, Nestle-Kraemling C, Haussmann J, Bölke E, Wollandt S, Speer V, et al. Long-Term Cosmetic Outcome After Preoperative Radio-/Chemotherapy in Locally Advanced Breast Cancer Patients. Strahlenther Onkol (2019) 195(7):615–28. doi: 10.1007/s00066-019-01473-2 Langfristiges kosmetisches Ergebnis nach neoadjuvanter Radio−/Chemotherapie bei lokal fortgeschrittenen Brustkrebspatientinnen. [DOI] [PubMed] [Google Scholar]
- 25. Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM. Breast-Conserving Surgery With or Without Irradiation in Women Aged 65 Years or Older With Early Breast Cancer (PRIME II): A Randomised Controlled Trial. Lancet Oncol (2015) 16(3):266–73. doi: 10.1016/s1470-2045(14)71221-5 [DOI] [PubMed] [Google Scholar]
- 26. Lagendijk M, van Maaren MC, Saadatmand S, Strobbe LJA, Poortmans PMP, Koppert LB, et al. Breast Conserving Therapy and Mastectomy Revisited: Breast Cancer-Specific Survival and the Influence of Prognostic Factors in 129,692 Patients. Int J Cancer (2018) 142(1):165–75. doi: 10.1002/ijc.31034 [DOI] [PubMed] [Google Scholar]
- 27. Sharma RA, Plummer R, Stock JK, Greenhalgh TA, Ataman O, Kelly S, et al. Clinical Development of New Drug-Radiotherapy Combinations. Nat Rev Clin Oncol (2016) 13(10):627–42. doi: 10.1038/nrclinonc.2016.79 [DOI] [PubMed] [Google Scholar]
- 28. Ataman OU, Sambrook SJ, Wilks C, Lloyd A, Taylor AE, Wedge SR. The Clinical Development of Molecularly Targeted Agents in Combination With Radiation Therapy: A Pharmaceutical Perspective. Int J Radiat Oncol Biol Phys (2012) 84(4):e447–54. doi: 10.1016/j.ijrobp.2012.05.019 [DOI] [PubMed] [Google Scholar]
- 29. Zaidi M, Fu F, Cojocari D, McKee TD, Wouters BG. Quantitative Visualization of Hypoxia and Proliferation Gradients Within Histological Tissue Sections. Front Bioeng Biotechnol (2019) 7:397. doi: 10.3389/fbioe.2019.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bauer JA, Chakravarthy AB, Rosenbluth JM, Mi D, Seeley EH, De Matos Granja-Ingram N, et al. Identification of Markers of Taxane Sensitivity Using Proteomic and Genomic Analyses of Breast Tumors From Patients Receiving Neoadjuvant Paclitaxel and Radiation. Clin Cancer Res (2010) 16(2):681–90. doi: 10.1158/1078-0432.Ccr-09-1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brunt AM, Wheatley D, Yarnold J, Somaiah N, Kelly S, Harnett A, et al. Acute Skin Toxicity Associated With a 1-Week Schedule of Whole Breast Radiotherapy Compared With a Standard 3-Week Regimen Delivered in the UK FAST-Forward Trial. Radiother Oncol (2016) 120(1):114–8. doi: 10.1016/j.radonc.2016.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matsen CB, Neumayer LA. Breast Cancer: A Review for the General Surgeon. JAMA Surg (2013) 148(10):971–9. doi: 10.1001/jamasurg.2013.3393 [DOI] [PubMed] [Google Scholar]
- 33. Engel J, Kerr J, Schlesinger-Raab A, Sauer H, Hölzel D. Quality of Life Following Breast-Conserving Therapy or Mastectomy: Results of a 5-Year Prospective Study. Breast J (2004) 10(3):223–31. doi: 10.1111/j.1075-122X.2004.21323.x [DOI] [PubMed] [Google Scholar]
- 34. Chakravarthy AB, Kelley MC, McLaren B, Truica CI, Billheimer D, Mayer IA, et al. Neoadjuvant Concurrent Paclitaxel and Radiation in Stage II/III Breast Cancer. Clin Cancer Res (2006) 12(5):1570–6. doi: 10.1158/1078-0432.Ccr-05-2304 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Reporting Guideline Checklist.
Univariate cox analysis of overall survival in patients with locally advanced breast cancer received preoperative radiation.
Univariate cox analysis of overall survival in patients with locally advanced breast cancer received postoperative radiation.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.