Abstract
Background:
Necrotizing pancreatitis is a common condition with high mortality; the acute care surgeon is frequently consulted for management recommendations. Furthermore, there has been substantial change in the timing, approach, and frequency of surgical intervention for this group of patients.
Methods:
In this article we summarize key clinical and research developments regarding necrotizing pancreatitis, including current recommendations for treatment of patients requiring intensive care and those with common complications. Articles from all years were considered to provide proper historical context, and most recent management recommendations are identified.
Results:
Epidemiology, diagnosis, treatment in the acute phase, and complications (both short-term and long-term) are discussed. Images of surgical interventions are included from our institutional experience.
Conclusion:
Necrotizing pancreatitis management remains heavily based on clinical judgement, although technological advances and clinical trials have made decision making more straightforward.
Keywords: Pancreas, Pancreatitis, Necrosis
Introduction
Acute pancreatitis is amongst the most prevalent gastrointestinal conditions in patients presenting to an emergency department in the United States, and the most common reason for hospital admission.1 Although mild cases usually self-resolve with supportive care, roughly 20% of these cases are categorized as necrotizing pancreatitis. Necrotizing pancreatitis is diagnosed if a significant portion of the gland or surrounding tissue does not enhance on contrasted computed tomography scan, a finding that significantly affects treatment strategies and prognosis.2–6 This review aims to consolidate the current understanding of epidemiology, diagnosis, prognostication, treatment, and complications of necrotizing pancreatitis.
Etiology
Etiologies for necrotizing pancreatitis are similar to those for acute pancreatitis, with gallstone disease being the most common causative process in the United States, followed by alcohol abuse.1,2 The pathophysiology underlying acute pancreatitis is thought to be related to inappropriate activation of proenzymes within the gland, leading to pancreatic inflammation.7 If severe enough, this leads to a systemic inflammatory response, leading to hypotension and exacerbating pancreatic damage due to ischemia.7 Several molecular mechanisms play roles in pancreatic inflammation, including release of nuclear factor-kappa B and release of various pro- and anti-inflammatory cytokines. Nuclear factor-kappa B is thought to link local inflammation with a systemic response, which can often occur early in the disease course, manifesting with classic signs of systemic inflammatory response syndrome.8 Anti-inflammatory cytokines are released in response, but may have an undesired inhibitory effect that allows for development of infection during a period of relative immunosuppression.8
With regards to development of necrosis, specific mechanisms are elusive, as necrosis is simply thought to be achieved when pancreatic parenchyma has suffered enough insult that it cannot recover. Some studies have investigated whether genetic predisposition affects rates of necrosis development, but results have been inconclusive thus far. Alcohol abuse has been correlated with necrosis development in some studies, but this has not been definitively proven.9 Other etiologies of pancreatitis (anatomic variants, autoimmune, hypertriglyceridemia, hypercalcemia, medications, snake bites, scorpion stings, etc.) occur infrequently and have not been extensively studied with regards to development of necrosis. Risk factors are related mainly to etiologic factors (gallstones, alcohol use, etc.) and patient demographics do not appear to affect propensity for development of pancreatic necrosis. More generally, if resuscitation of patients with severe pancreatitis is suboptimal and leads to periods of hypotension and vasopressor support, this can induce or exacerbate necrosis within the gland.10,11 Necrosis may not be evident on very early imaging, but it has been postulated that it is often developing prior to manifesting radiographically.12 Thus, early recognition of systemic inflammatory response and adequate treatment is imperative in limiting pancreatic necrosis, regardless of radiographic findings.
Epidemiology
Greater than 250,000 hospital admissions occur annually, and this incidence is increasing.13 Several studies have estimated the current economic burden of pancreatitis to be around $2.5 billion.1,13 Much of this is likely spent on the roughly 20% of patients who develop necrotizing pancreatitis, as development has been correlated with increased morbidity and mortality. More extensive necrosis (as measured by contrast-enhanced computed tomography) has been correlated with increased morbidity, mortality, rate of infection, likelihood of debridement, and multi-organ dysfunction.14–16 Less extensive necrosis (less than 30% of the pancreas) is associated with significant morbidity (40%), but mortality, infection, and organ failure rates all have been reported under 20%.15,16 When greater than 50% of the pancreas is necrotic, rate of morbidity approaches 100%, mortality 40%, infection 50%, need for debridement 70%, and multi-organ dysfunction 65%.15,16 With regards to development of pancreatic necrosis in patients with multiple episodes of pancreatitis, there is no definitive evidence that more episodes lead to more severe necrosis. Indeed, certain patients may have the misfortune of experiencing severe acute pancreatitis with extensive necrosis even during their first episode of pancreatitis. Many studies have investigated laboratory values as predictors of necrosis development, but, to date, none have been robust enough to tailor therapy to specific patients.17,18
Development of acute necrotic collections and walled-off necrosis are notable potential complications of necrotizing pancreatitis. Acute necrotic collections are defined by revised Atlanta classification as associated fluid collections in the presence of necrosis less than 4 weeks after onset of pancreatitis, and walled-off necrosis developing greater than 4 weeks after onset.19 However, a recent study showed that walled-off necrosis may develop sooner than Atlanta classification definitions, with 43% of well-defined collections shown on imaging within 3 weeks of onset.12 Up to 33% of patients with necrotizing pancreatitis will develop infected necrosis.20 Infection of collections or the initial area of necrosis markedly increases morbidity and mortality rates and has traditionally mandated some form of drainage procedure.21–23 Another feared complication is hemorrhage, which occurs in approximately 5% of patients with necrotizing pancreatitis, and is thought to be due to inflammatory damage to peripancreatic vessels leading to pseudoaneurysm formation.1 Rupture of these pseudoaneurysms can lead to hemorrhagic shock and death without definitive hemorrhage control (generally via angioembolization) and blood transfusion.12 Finally, up to 30% of patients with necrotizing pancreatitis may have a main pancreatic duct disruption, though definitive links to outcomes have not been determined.24
Diagnosis
Three criteria are used to establish the diagnosis of acute pancreatitis.25 The first is abdominal pain, which is usually epigastric or left upper quadrant in location. It may radiate to the back, flank or chest although this is non-specific. The pain can be varied in intensity and may be described as dull and colicky. However, none of these findings correlate with disease severity. The first onset of pain should be obtained in history taking, as this is considered the true time of onset rather than when the patient presented to the hospital.19
The second criterion for diagnosis is serum lipase levels greater than three times the upper limit of normal. Serum amylase had been previously used for diagnosis. However, levels typically rise within a few hours of onset and return to normal after 3–5 days and may never be elevated in approximately 20% of patients as well.26,27 Thus, serum lipase has been found to be more specific and is currently recognized as part of the diagnostic criteria for acute pancreatitis.25 To be considered diagnostic, the levels must be 3 times the normal range of the laboratory performing the test. At present there is no consensus regarding a standardized upper limit of normal for serum lipase.
The final criterion is radiologic imaging consistent with pancreatitis. Contrast-enhanced computed tomography scan or magnetic resonance imaging scan is not recommended on a routine basis, but rather only for cases that remain unclear by the previous two criteria, or if a patient fails to improve after 48–72 h of treatment to evaluate for pancreatic necrosis or fluid collections and determine the extent.25,28–30 In uncomplicated acute pancreatitis, imaging will demonstrate homogenous enhancement with inflammatory changes of the peripancreatic fat. Pancreatic necrosis is defined radiographically by a failure of the pancreatic parenchyma to enhance with intravenous contrast. If greater than 30% of the parenchyma does not enhance, the diagnosis changes to necrotizing pancreatitis (Fig. 1).
Fig. 1.
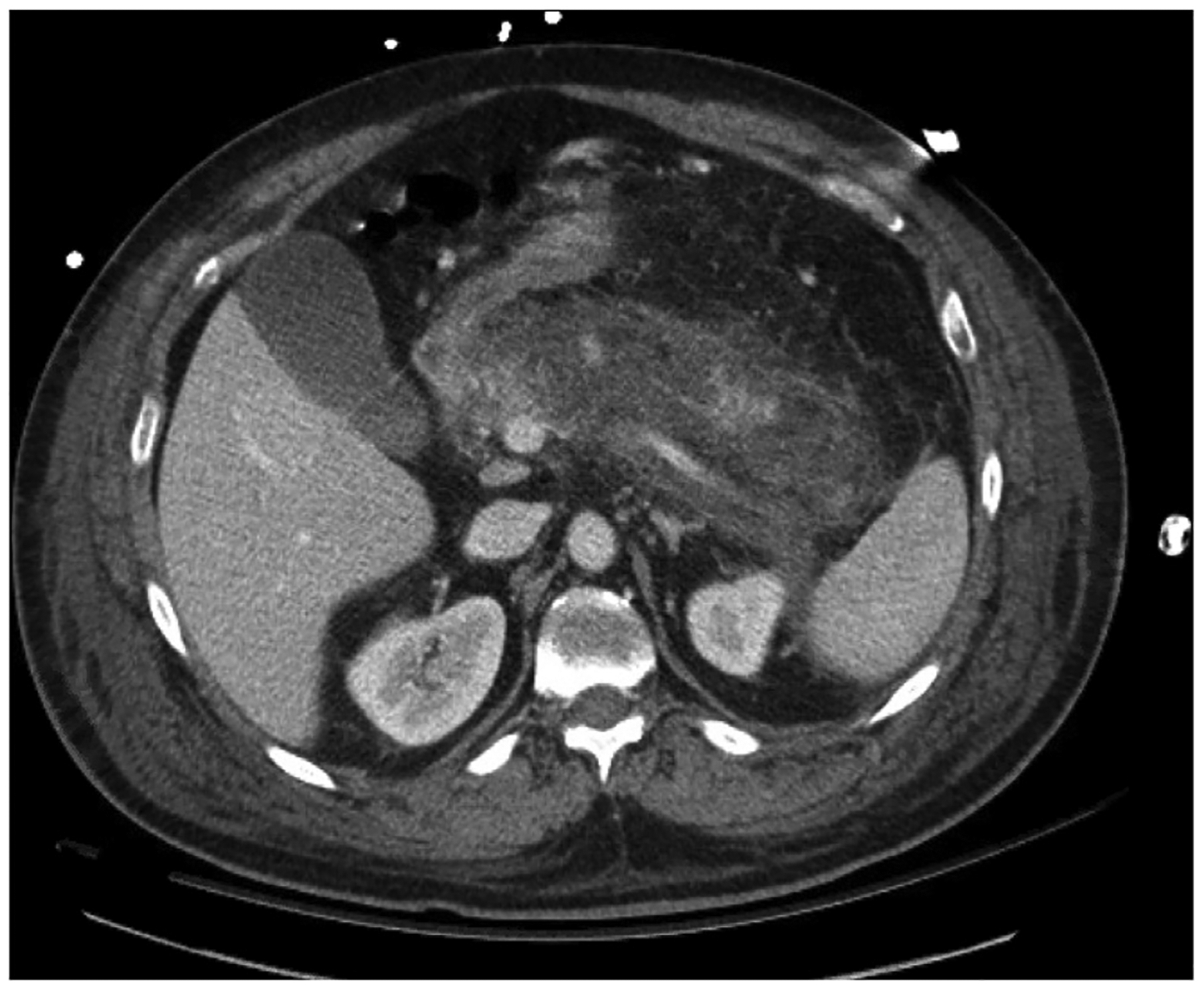
Acute necrotizing pancreatitis without associated fluid collections imaged with computed tomography scan. Note the fat stranding, implying inflammation, and lack of contrast enhancement, implying necrosis due to inadequate perfusion. Imaging is from initial presentation.
It is also essential to assess for the presence of an associated peripancreatic fluid collection. Acute peripancreatic fluid collections typically develop in the first week, are homogenous on imaging, and are confined by normal fascial planes. These fluid collections typically remain sterile and usually resolve without intervention.5,19,31 Peripancreatic fluid collections that persist beyond 4 weeks are described as pseudocysts. If imaging demonstrates a heterogeneous fluid collection associated with necrotizing pancreatitis, the fluid collection is defined to be necrotic (Fig. 2). When the collection is less than 4 weeks old, it is described as an acute necrotic collection; if present for greater than 4 weeks and contained within a wall of reactive tissue, it is described as walled-off necrosis.19 Presence of air within the fluid collection or pancreatic parenchyma is highly suspicious for infected necrosis (Fig. 3). Delayed imaging at 5–7 days after onset is usually more useful than initial imaging for distinguishing between these classes of fluid collections. Additionally, magnetic resonance imaging (including magnetic resonance cholangiopancreatography), trans-abdominal ultrasonography, and endoscopic ultrasonography may be useful for obtaining diagnosis. Magnetic resonance cholangiopancreatography has the benefit of evaluating for ductal communication with fluid collections, as management strategies may be altered by this distinction.19 In the case of suspected infected pancreatic necrosis, fine needle aspiration had been previously employed, with positive cultures used to confirm diagnosis. However, due to prohibitive false negative rates and the theoretical risk of seeding of sterile necrosis, this method has fallen out of favor. Radiographic findings in conjunction with clinical judgement is now considered adequate for diagnosis.
Fig. 2.
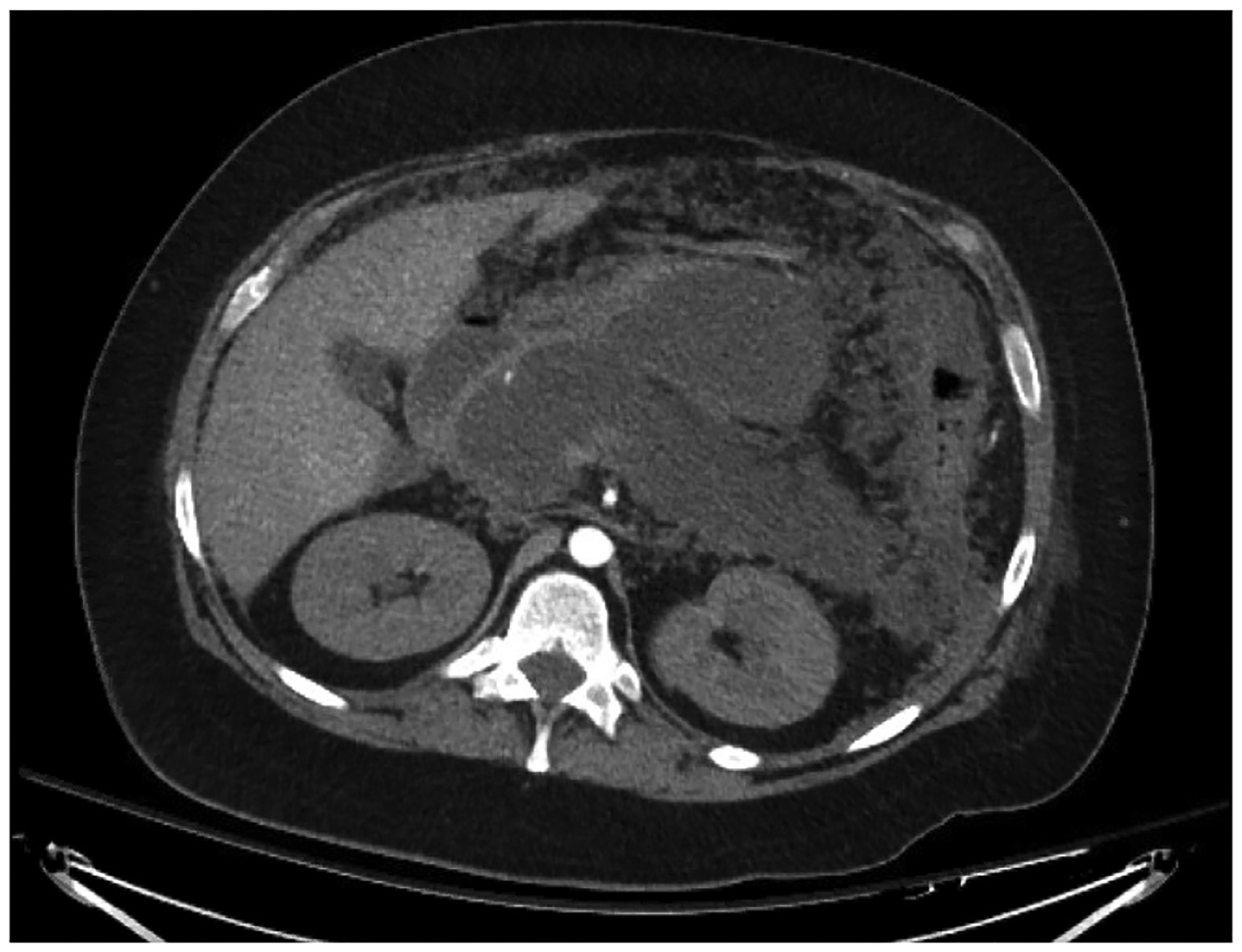
Necrotizing pancreatitis with associated walled-off necrosis imaged with computed tomography scan. Imaging is from roughly 3 weeks after initial presentation.
Fig. 3.
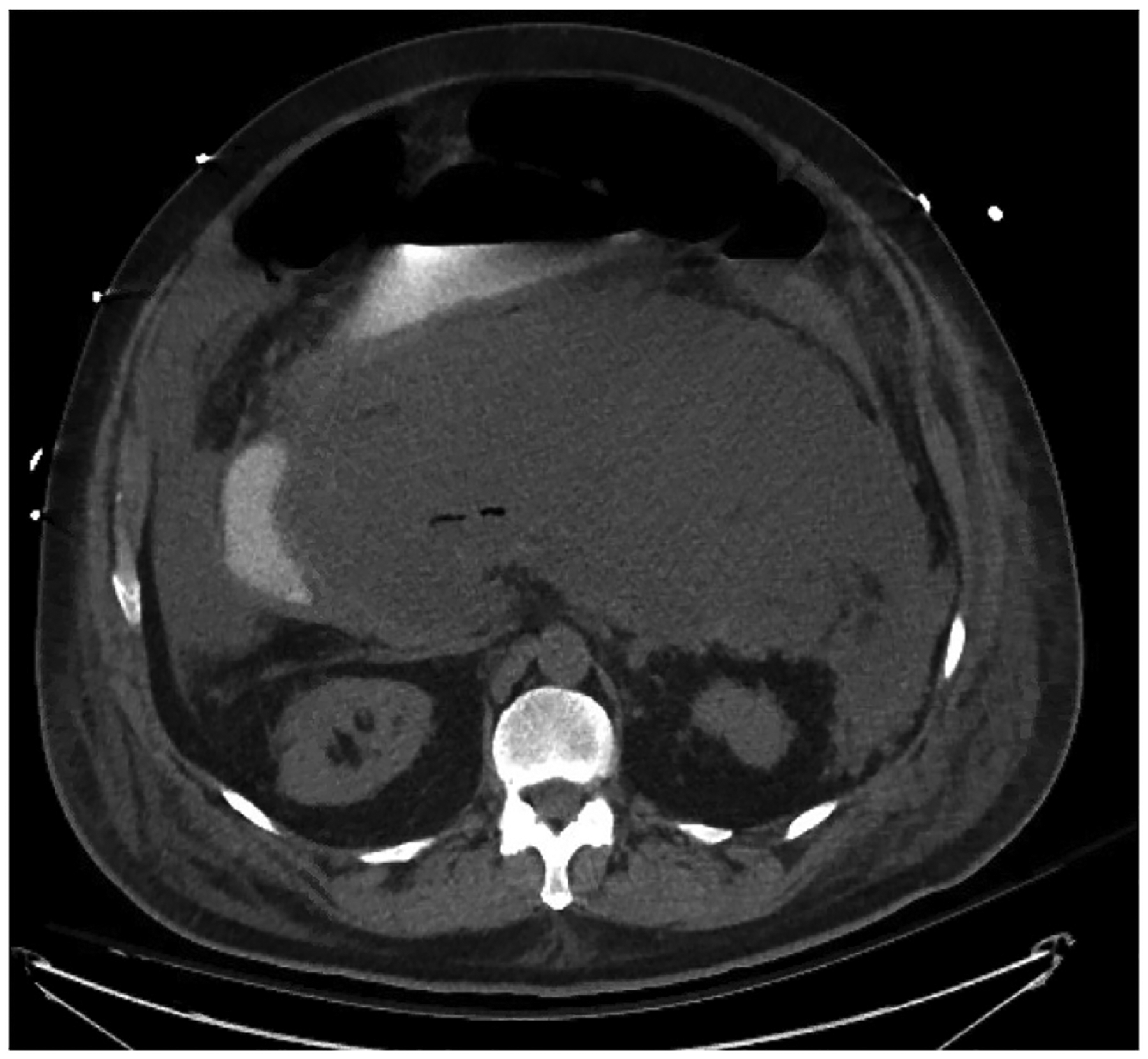
Infected pancreatic necrosis imaged with computed tomography scan. Imaging is from roughly 2 weeks after initial presentation.
Prediction of pancreatitis severity is controversial and has been attempted by multiple scoring systems, including the bedside index of severity in acute pancreatitis (BISAP), acute physiology and chronic health evaluation II (APACHE II), Ranson’s criteria, and Balthazar computed tomography severity index, among others. These scoring systems utilize various clinical, laboratory, and radiographic findings to predict the severity of pancreatitis. Each has utility, but must be considered only adjuncts to appropriate clinical judgement. As this article focuses on necrotizing pancreatitis (and thus a mostly severe subset of pancreatitis), detailed discussion of each scoring system is beyond the scope of this article.
Treatment
Non-surgical treatment of necrotizing pancreatitis initially does not differ dramatically from that for acute pancreatitis without necrosis. Early management is directed at early and aggressive hydration to address hypovolemia due to fluid sequestration in pancreatic and peripancreatic tissue along with systemic inflammation.32–35 Initial volume repletion with isotonic crystalloid should be at a rate of 250–500 mL/h, with lower rates necessary for patients with precluding comorbidities.25 Ringer’s lactated solution may be beneficial in preventing and correcting systemic inflammatory response syndrome and electrolyte imbalance.34 During repletion, repeat assessments of intravascular volume should be made frequently, particularly during the first 24 h. Non-responders in the first 6–12 h may not benefit from continued aggressive volume expansion.36 There remains no medication that specifically and effectively treats pancreatitis.32,33
In the case of biliary pancreatitis, endoscopic retrograde cholangiopancreatography (ERCP) plays an important role.25 The current indications for ERCP in the setting of acute pancreatitis include patients with a clinical picture consistent with cholangitis or evidence of ongoing biliary obstruction. In the setting of suspected choledocholithiasis without convincing evidence of cholangitis or complete biliary obstruction, magnetic resonance cholangiopancreatography or endoscopic ultrasonography may be useful in confirming diagnosis and need for ERCP. If concerned for cholangitis or biliary obstruction, ERCP should be performed within 24 h of presentation. The risk of post-ERCP pancreatitis must be considered, but should not prevent performance of the procedure if it is necessary to relieve biliary obstruction. Additionally, several adjuncts exist to decrease the incidence of post-ERCP pancreatitis, including prophylactic placement of pancreatic duct stents and post-procedural administration of rectal non-steroidal anti-inflammatory drugs.37,38
Nutrition is a critical component in the management of the patient with pancreatitis, and has been the subject of intense debate. Historically, patients were kept without oral intake until their condition clinically improved, they had resolution of inflammation on interval imaging, or until normalization of serum amylase and lipase. This was all due to fear of worsening the pancreatitis via pancreatic stimulation. These fears have since been shown to be largely unfounded, and early feeding (within 24 h) is recommended provided there is no other contraindication. In the case of mild pancreatitis, a low residue and low-fat diet may be initiated for the patient to consume as tolerated.39 Severely ill patients may require enteral feeding via nasogastric or nasojejunal tubes. Nasogastric feeding had previously been discouraged for fear of pancreatic stimulation and gastroparesis but has since been demonstrated to be non-inferior to nasojejunal feeding.40
Another area of great debate is antibiotic administration for pancreatitis. Currently, in the setting of necrotizing pancreatitis, prophylactic antibiotics do not have a role.41,42 However, if patients develop systemic signs of infection and other sources are effectively ruled out, infected pancreatic necrosis must be considered. If infected pancreatic necrosis is identified, antibiotics selected must cover pancreatic and gastrointestinal organisms, including Gram-negative enteric bacteria, Gram-positive bacteria, and, less frequently, anaerobes and fungi. First-line choices include carbapenems, with fluoroquinolones as a secondary option, and metronidazole and antifungals reserved for cases with higher suspicion for anaerobic or fungal infection. Appropriate treatment with antibiotic therapy may obviate the need for surgical intervention in a select group of patients. However, infected necrosis treated with antibiotics alone has a high mortality rate, as one meta-analysis demonstrated that 64% of patients treated conservatively for infected pancreatic necrosis had a mortality of 12%.10
When medical management proves insufficient for patients with necrotizing pancreatitis, surgical management may become pivotal to their outcome. Historically, patients with necrotizing pancreatitis were treated with early laparotomy and open necrosectomy. The morbidity and mortality associated with early necrosectomy is significant. In 1997, the concept of delayed intervention was introduced when Mier et al. randomized patients with severe necrotizing pancreatitis to early (48–72 h) versus late (>12 days) necrosectomy and found that late necrosectomy did not increase mortality.43 Other studies have since made it clear that delayed surgical intervention for necrotizing pancreatitis has a mortality benefit.44,45 Though intervention may be necessary for indications other than pancreatic necrosis, such as intestinal ischemia or abdominal compartment syndrome, delayed pancreatic intervention is the preferred approach today.
Additionally, minimally invasive interventions have largely replaced open necrosectomy. In a landmark paper in 2010, the “step-up approach” was demonstrated to have superior outcomes in terms of major complications and mortality.46 This study demonstrated that placement of percutaneous drains obviates the need for major abdominal intervention in approximately one-third of patients. The key to successful management when utilizing a step-up approach is a coordinated effort between the interventional radiologist placing the drains and the surgical team, who may need to utilize the drain tract for a minimally invasive necrosectomy (Fig. 4). This represents, in many ways, an evolution of the classic open flank drainage approach, which provided wide, dependent retroperitoneal drainage from either the left or right side.
Fig. 4.
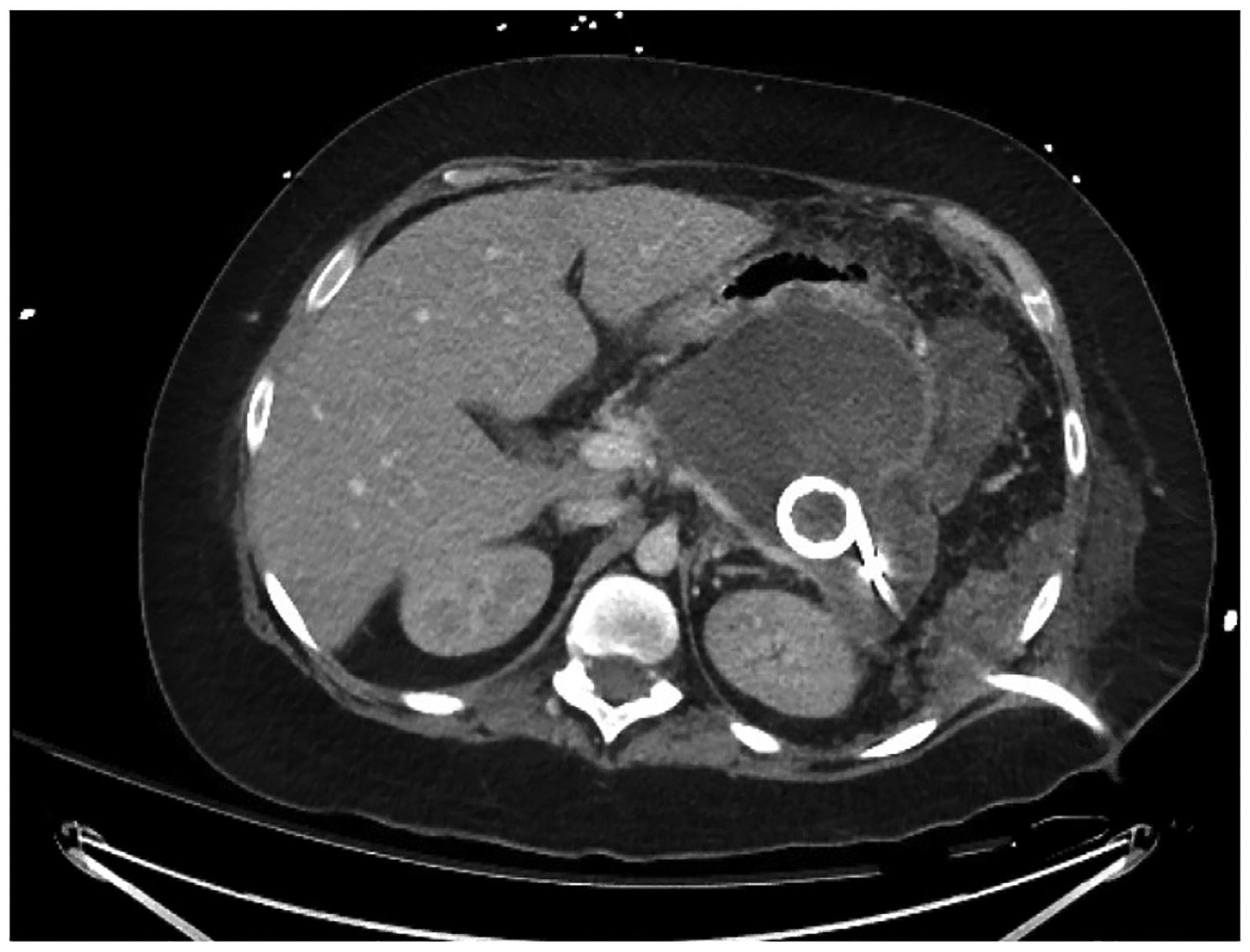
Computed tomography scan demonstrating percutaneous drain placed by interventional radiology for treatment of infected pancreatic necrosis. The trajectory was chosen to guide future minimally invasive surgical approaches.
If percutaneous drainage fails, surgical drainage must be considered. Minimally invasive options include video-assisted retroperitoneoscopic debridement (VARD) and endoscopic necrosectomy.28 In VARD, a previously placed percutaneous drain is used as a guide for incision and dissection along its tract, with eventual suctioning and removal of pancreatic necrosis (Fig. 5). Clearance of infected tissue is immediate and usually thorough (Fig. 6). In endoscopic necrosectomy entry to the retroperitoneum is gained through the posterior gastric wall, the connection stented open, and drains left between the retroperitoneum and gastric lumen (Fig. 7). This approach has similar outcomes to VARD and obviates the need for incisions, but requires more procedures to replace and remove stents and drains. VARD and endoscopic necrosectomy appear roughly equivalent in terms of mortality and overall major complications, with VARD having a higher risk of pancreatic fistula formation and longer length of stay, and endoscopic necrosectomy involving more procedures to replace and remove stents and drains.28 Choosing between endoscopic necrosectomy and VARD is ultimately dependent on patient-specific factors, including anatomic considerations (e.g., stomach-necrosis interface or previous gastrointestinal surgeries), patient preference (e.g., number of procedures or prolonged need for external drains), and experience levels of available interventionists. Finally, open necrosectomy is now less often employed, but is the traditional approach for debridement, and used most often when previous approaches have failed or the patient requires abdominal exploration for other reasons (Fig. 8).
Fig. 5.
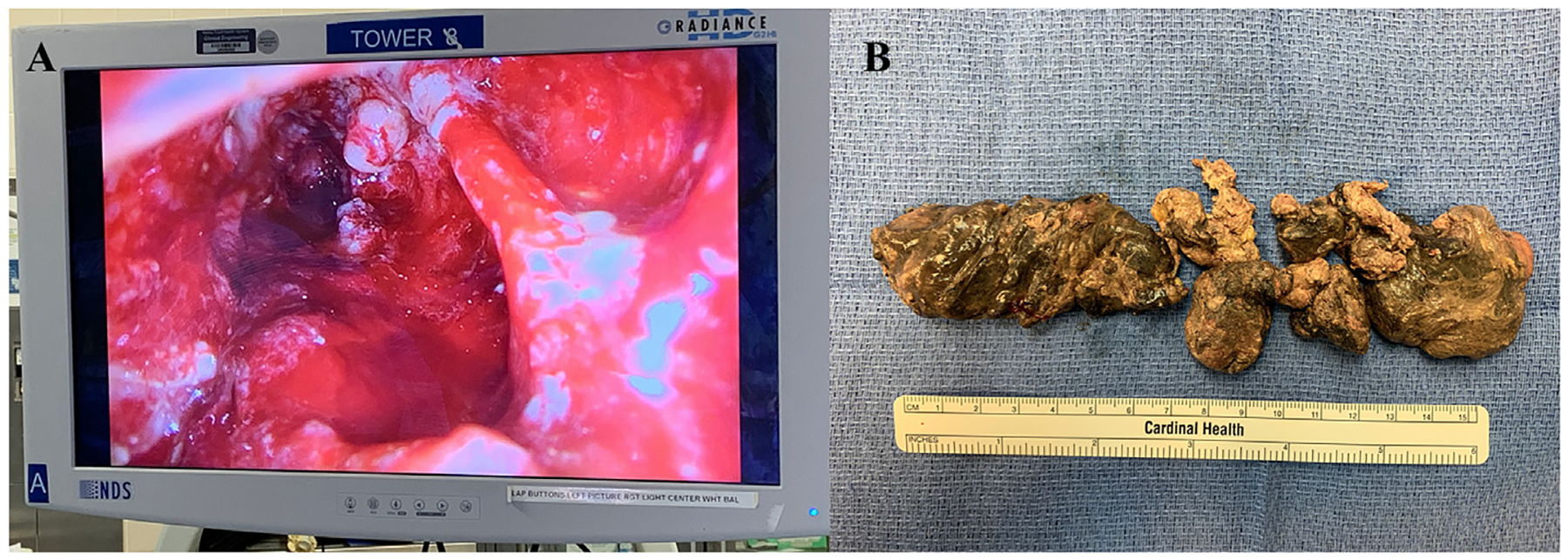
Video-assisted retroperitoneoscopic debridement for infected pancreatic necrosis. a) Completion view via laparoscope of retroperitoneum. b) Pancreatic necrosis that was removed piecemeal.
Fig. 6.
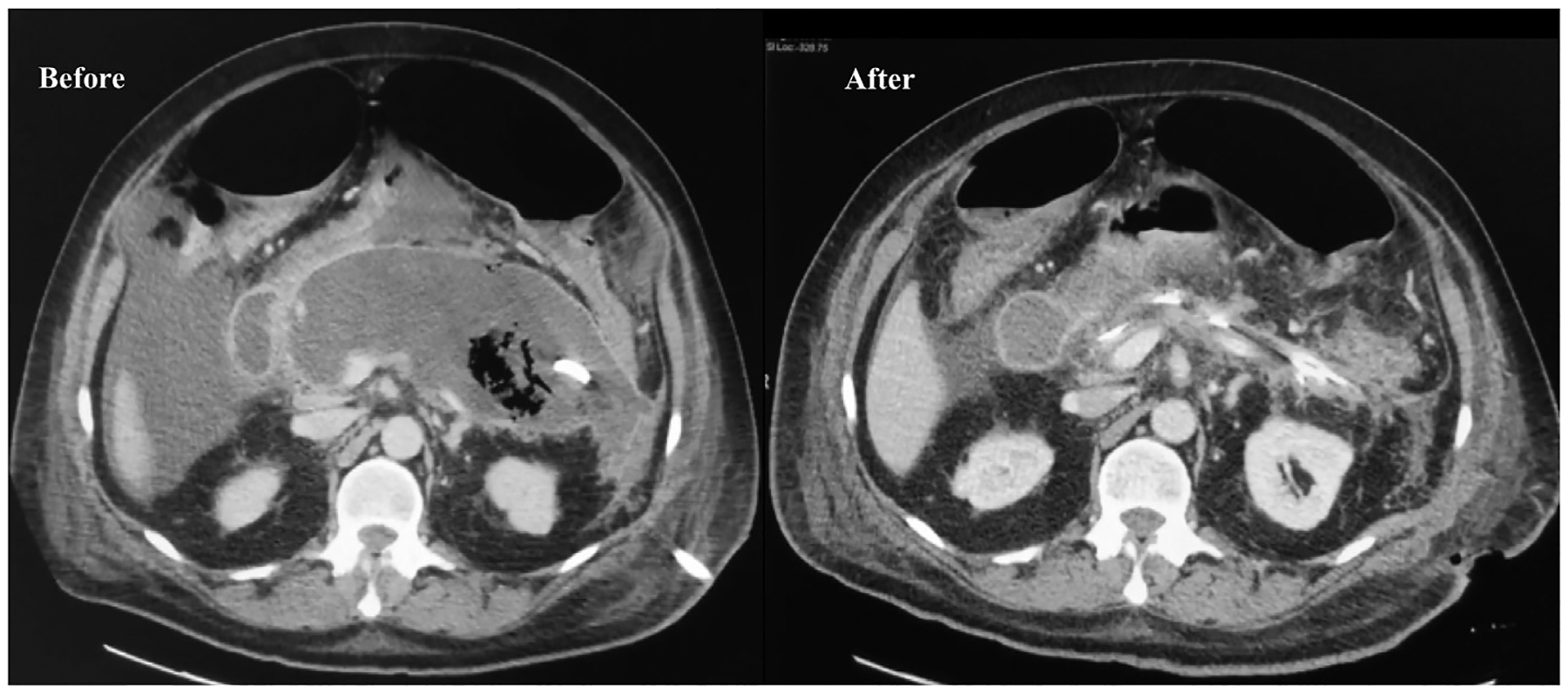
Computed tomography scans before and after video-assisted retroperitoneoscopic debridement (VARD) of infected pancreatic necrosis.
Fig. 7.
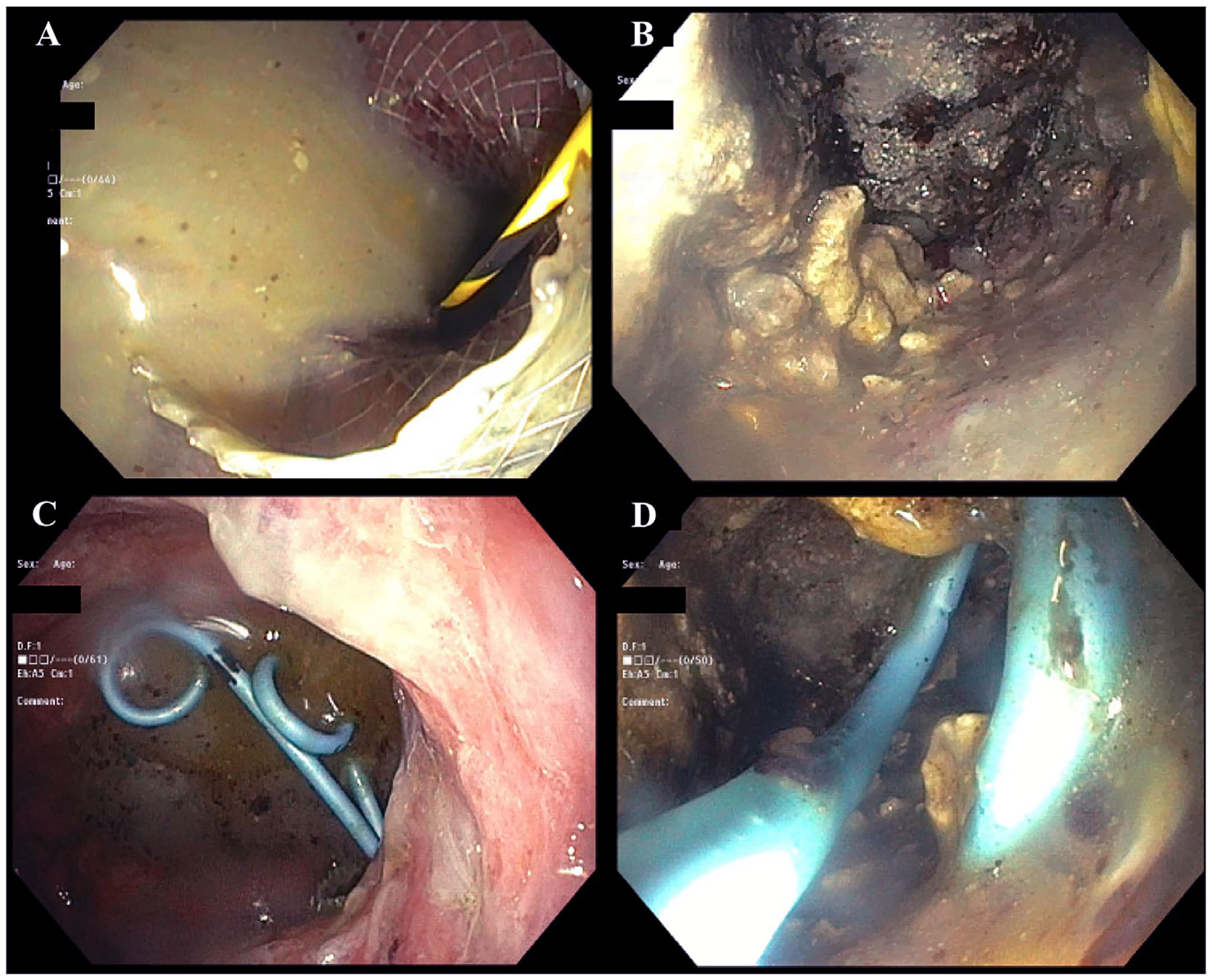
Images of endoscopic necrosectomy for infected pancreatic necrosis. a) Purulence visualized in stomach after deployment of metal trans-gastric stent. b) Pancreatic necrosis within the retroperitoneum. c) Trans-gastric drains within the stomach. d) Trans-gastric drains visualized within the retroperitoneum.
Fig. 8.
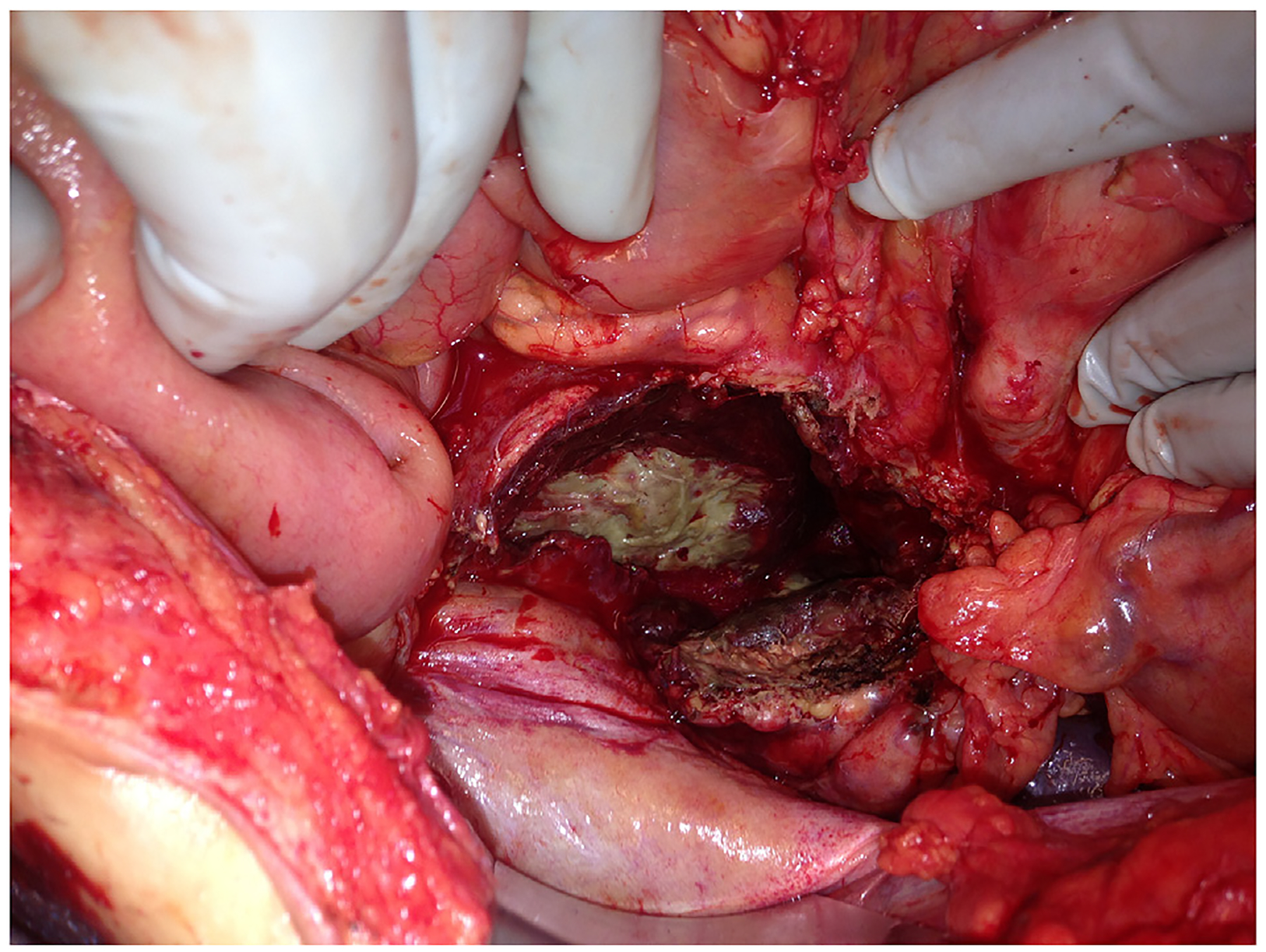
Open necrosectomy for infected and hemorrhagic necrotizing pancreatitis. The lesser sac is open and exposed with abdominal viscera retracted.
Long-term sequelae
In addition to the potentially severe acute illness, necrotizing pancreatitis is accompanied by several potential long-term complications, many of which are related to necrosectomy.1,4,47 Though percutaneous drains are sometimes adequate, necrosectomy is often necessary for complete debridement. Without source control of infected pancreatic necrosis, mortality is extremely high; necrosectomy reduces this mortality rate dramatically and is thus an essential procedure in many cases.48 The necessity of this procedure makes characterization of long-term complications similarly essential. Sequelae are often related to the degree of necrosectomy and amount of remaining functional pancreatic tissue. In a retrospective study of acute necrotizing pancreatitis patients who underwent necrosectomy, Connor et al. showed that 62% of patients developed multiple late complications, including diabetes mellitus (33%), exocrine pancreatic insufficiency (25%), pancreatic fistula (13%), pseudocyst (8%), delayed fluid collection (5%), biliary stricture (6%), gastrointestinal stricture (2%), and incisional hernia (2%).49 Hemorrhage is an additional late complication in up to 6% of patients with necrotizing pancreatitis and can occur within the gastrointestinal tract, peritoneal cavity, or pancreatic parenchyma.4
Pancreatic endocrine dysfunction manifests as new-onset diabetes. In a systemic review and meta-analysis consisting of 31 studies and 13,894 patients, Zhi et al. showed that new onset diabetes is frequently seen in patients with severe acute pancreatitis, acute necrotizing pancreatitis, and alcoholic pancreatitis. Factors associated with an increased incidence of new-onset diabetes included severe vs mild acute pancreatitis (39% vs 14%), presence vs absence of pancreatic necrosis (37% vs 11%), and alcoholic vs biliary etiology (28% vs 12%). The use of insulin therapy was required in 21% and 18% of severe acute pancreatitis and alcoholic acute pancreatitis, respectively.50 Tu et al. assessed endocrine and exocrine pancreatic function of discharged patients with acute pancreatitis. On multivariate logistic regression, pancreatic necrosis <30%, presence of walled-off necrosis, and insulin resistance were protective of endocrine pancreatic insufficiency.51 Pancreatic endocrine function may be assessed through oral glucose tolerance test, homeostatic model assessment-beta and C-peptide levels. Homeostatic model assessment is used to quantify beta-cell function and insulin resistance. Treatment usually involves insulin supplementation as the disease process mimics type 1 rather than type 2 diabetes mellitus.
Acute severe pancreatitis, necrotizing pancreatitis and alcoholic pancreatitis are associated with a higher incidence of developing pancreatic exocrine insufficiency.52 Furthermore, the development of exocrine dysfunction correlates with the extent of pancreatic necrosis and the severity of pancreatic endocrine dysfunction.53 Pancreatic exocrine insufficiency was more frequent in alcoholic pancreatitis compared to biliary pancreatitis and other etiologies.54,55 Pancreatic exocrine function is typically assessed by measuring the fecal pancreatic elastase-1 level. Garip et al. showed lower fecal pancreatic elastase-1 levels in patients with severe acute and necrotizing pancreatitis, pancreatic head necrosis, near-total necrosis, and in post-necrosectomy patients.52 Treatment is with pancreatic enzymatic supplementation. Some have reported, however, that pancreatic exocrine and endocrine dysfunction following an episode of acute necrotizing pancreatitis may be transient, with complete recovery achieved in some cases within a few years.56
The long-term vascular complications of pancreatitis include hemorrhage secondary to arterial erosion and pseudoaneurysm, ischemic complications, and venous splanchnic thrombosis.57 These vascular incidents are associated with high morbidity.58 More than half of the patients with acute necrotizing pancreatitis will develop vascular abnormalities.57 It is believed that the inflammatory reaction associated with pancreatitis compresses adjacent vessels and activates the coagulation cascade. Given its close proximity to the pancreas, splenic artery erosion and pseudoaneurysm formation is relatively common.58 Acute mesenteric venous thrombosis is a rare but extremely morbid complication of pancreatitis, with mortality rates approaching 90% in the presence of bowel ischemia.59 Early diagnosis and treatment are crucial. Diagnoses of vascular abnormalities are best achieved with contrast-enhanced multiphasic computed-tomography scan, with the portal venous phase diagnosing mesenteric thrombosis, and arterial phase diagnosing incidental pseudoaneurysms that may complicate the decision to anticoagulated. Ruptured pseudoaneurysms are most commonly treated with endovascular embolization of feeding vessels. In cases of extremis, open control of hemorrhage may be necessary. Treatment of mesenteric thrombosis is with anticoagulation, with surgery reserved for bowel compromise. In the case of the patient with concurrent hemorrhagic pancreatitis or pseudoaneurysms and acute mesenteric venous thrombosis there is no accepted algorithm for treatment; clinicians must weigh risks and benefits to decide which issue poses a more immediate threat to life.
In addition to vascular erosion, necrotizing pancreatitis and its associated inflammation can lead to fistula formation to surrounding gastrointestinal structures. It has not yet been clarified whether infection causes further inflammation that results in fistula formation, or if microscopic fistula is the etiology for many cases of infected necrosis.60 Regardless, gastrointestinal fistula formation may be a more common occurrence than has been previously reported. In one observational study, 928 patients were admitted for necrotizing pancreatitis and 119 (12.8%) patients developed a gastrointestinal fistula; 160 total fistulae were identified.61 Pancreaticocolonic fistula was most common (72, 45.0%), followed by pancreaticoduodenal fistula (53, 33.1%). Several patients developed pancreatic fistulae to multiple organs (36, 22.5%).61 Treatment is usually non-surgical, with a combination of percutaneous drainage and optimization of nutritional status to aid in spontaneous closure. If this fails, surgical options may be considered, but must be weighed against the risk of new fistula creation. In the series mentioned, upper gastrointestinal fistulae were all managed non-surgically, and 65.3% of pancreaticocolonic fistulae were treated with diverting ostomy formation.61 If fistulae have matured and there is no ongoing extraluminal contamination, fistulae may be asymptomatic and not require any intervention.
Pancreatic necrosis is an independent risk factor for developing recurrent pancreatitis and chronic pancreatitis.62 Repetitive inflammation and necrosis, as seen in recurrent pancreatitis, leads to glandular scarring, fibrosis and resultant ductal obstruction observed in chronic pancreatitis. This etiologic linkage is further supported by the necrosis-fibrosis theory.63 Furthermore, the Sentinel Acute Pancreatitis Event theory hypothesizes that sentinel pancreatitis sensitizes the pancreas to permanent fibrosis, and recurrent episodes result in progressive inflammation and fibrosis that affects glandular structure and function.64 In a large multi-center cross-sectional cohort study of 669 patients with first episodes of acute pancreatitis, Ahmed Ali et al. found that first episodes of acute pancreatitis led to recurrent pancreatitis in 17% of patients; 8% subsequently developed chronic pancreatitis within 5 years.62 In another study that followed patients after sentinel acute pancreatitis for a 20-year period, the rate of recurrent pancreatitis was 16.5% and progression to chronic pancreatitis was seen in 13% and 16% of patients at 10 and 20 years, respectively.65 Independent risk factors associated with the development of chronic pancreatitis included male sex, alcohol as the etiology, smoking, severity of first episode, necrotizing pancreatitis, organ failure, modified Glasgow score, surgical intervention, and recurrent pancreatitis. Necrotizing pancreatitis, alcoholic etiology, and recurrent pancreatitis were found to be independent predictors of developing chronic pancreatitis.62
Conclusion
Necrotizing pancreatitis is an often-severe disease process that may cause severe systemic illness requiring intensive management. Historical dogma regarding several therapeutic strategies has been debunked in the past several decades, but mortality for patients with the disease remains high. Appropriate recognition and treatment are necessary to optimize short- and long-term outcomes.
Acknowledgements
We would like to thank Stephanie Stebens, MLIS, Sladen Library, for assistance with editing and formatting.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Declaration of competing interest
None declared.
References
- 1.Sabo A, Goussous N, Sardana N, et al. Necrotizing pancreatitis: a review of multidisciplinary management. JOP. 2015;16:125–135. [DOI] [PubMed] [Google Scholar]
- 2.Forsmark Ch E, Vege SS, Wilcox CM. Acute pancreatitis. N Engl J Med. 2017;376: 598–599. [DOI] [PubMed] [Google Scholar]
- 3.Hackert T, Buchler MW. Decision making in necrotizing pancreatitis. Dig Dis. 2016;34:517–524. [DOI] [PubMed] [Google Scholar]
- 4.Bugiantella W, Rondelli F, Boni M, et al. Necrotizing pancreatitis: a review of the interventions. Int J Surg. 2016;28:S163–S171. [DOI] [PubMed] [Google Scholar]
- 5.Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331–336. [DOI] [PubMed] [Google Scholar]
- 6.Shyu JY, Sainani NI, Sahni VA, et al. Necrotizing pancreatitis: diagnosis, imaging, and intervention. Radiographics. 2014;34:1218–1239. [DOI] [PubMed] [Google Scholar]
- 7.Lowenfels AB, Maisonneuve P, Sullivan T. The changing character of acute pancreatitis: epidemiology, etiology, and prognosis. Curr Gastroenterol Rep. 2009;11:97–103. [DOI] [PubMed] [Google Scholar]
- 8.Boumitri C, Brown E, Kahaleh M. Necrotizing pancreatitis: current management and therapies. Clin Endosc. 2017;50:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papachristou GI, Papachristou DJ, Morinville VD, et al. Chronic alcohol consumption is a major risk factor for pancreatic necrosis in acute pancreatitis. Am J Gastroenterol. 2006;101:2605–2610. [DOI] [PubMed] [Google Scholar]
- 10.Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta-analysis. Gastroenterology. 2013;144:333–340.e2. [DOI] [PubMed] [Google Scholar]
- 11.Kokosis G, Perez A, Pappas TN. Surgical management of necrotizing pancreatitis: an overview. World J Gastroenterol. 2014;20:16106–16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trikudanathan G, Wolbrink DRJ, van Santvoort HC, et al. Current concepts in severe acute and necrotizing pancreatitis: an evidence-based approach. Gastroenterology. 2019;156:1994–2007.e3. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg A, Steensma EA, Napolitano LM. Necrotizing pancreatitis: new definitions and a new era in surgical management. Surg Infect. 2015;16:1–13. [DOI] [PubMed] [Google Scholar]
- 14.Bendersky VA, Mallipeddi MK, Perez A, Pappas TN. Necrotizing pancreatitis: challenges and solutions. Clin Exp Gastroenterol. 2016;9:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg PK, Madan K, Pande GK, et al. Association of extent and infection of pancreatic necrosis with organ failure and death in acute necrotizing pancreatitis. Clin Gastroenterol Hepatol. 2005;3:159–166. [DOI] [PubMed] [Google Scholar]
- 16.Babu RY, Gupta R, Kang M, et al. Predictors of surgery in patients with severe acute pancreatitis managed by the step-up approach. Ann Surg. 2013;257: 737–750. [DOI] [PubMed] [Google Scholar]
- 17.Yalcin MS, Tas A, Kara B, et al. New predictor of acute necrotizing pancreatitis: red cell distribution width. Adv Clin Exp Med. 2018;27:225–228. [DOI] [PubMed] [Google Scholar]
- 18.Unal Y, Barlas AM. Role of increased immature granulocyte percentage in the early prediction of acute necrotizing pancreatitis. Ulus Travma Acil Cerrahi Derg. 2019;25:177–182. [DOI] [PubMed] [Google Scholar]
- 19.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. [DOI] [PubMed] [Google Scholar]
- 20.van Grinsven J, van Brunschot S, van Baal MC, et al. Natural history of gas configurations and encapsulation in necrotic collections during necrotizing pancreatitis. J Gastrointest Surg. 2018;22:1557–1564. [DOI] [PubMed] [Google Scholar]
- 21.Sion MK, Davis KA. Step-up approach for the management of pancreatic necrosis: a review of the literature. Trauma Surg Acute Care Open. 2019;4, e000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beger HG, Bittner R, Block S, Buchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986;91:433–438. [DOI] [PubMed] [Google Scholar]
- 23.Baron TH, Morgan DE. Acute necrotizing pancreatitis. N Engl J Med. 1999;340: 1412–1417. [DOI] [PubMed] [Google Scholar]
- 24.Jang JW, Kim MH, Oh D, et al. Factors and outcomes associated with pancreatic duct disruption in patients with acute necrotizing pancreatitis. Pancreatology. 2016;16:958–965. [DOI] [PubMed] [Google Scholar]
- 25.Tenner S, Baillie J, DeWitt J, et al. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108: 1400–1415. [DOI] [PubMed] [Google Scholar]
- 26.Winslet M, Hall C, London NJ, Neoptolemos JP. Relation of diagnostic serum amylase levels to aetiology and severity of acute pancreatitis. Gut. 1992;33: 982–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clavien PA, Robert J, Meyer P, et al. Acute pancreatitis and normoamylasemia. Not an uncommon combination. Ann Surg. 1989;210:614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Brunschot S, van Grinsven J, van Santvoort HC, et al. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet. 2018;391:51–58. [DOI] [PubMed] [Google Scholar]
- 29.Perez A, Whang EE, Brooks DC, et al. Is severity of necrotizing pancreatitis increased in extended necrosis and infected necrosis? Pancreas. 2002;25: 229–233. [DOI] [PubMed] [Google Scholar]
- 30.Banks PA, Gerzof SG, Langevin RE, et al. CT-guided aspiration of suspected pancreatic infection: bacteriology and clinical outcome. Int J Pancreatol. 1995;18:265–270. [DOI] [PubMed] [Google Scholar]
- 31.Lenhart DK, Balthazar EJ. MDCT of acute mild (nonnecrotizing) pancreatitis: abdominal complications and fate of fluid collections. AJR Am J Roentgenol. 2008;190:643–649. [DOI] [PubMed] [Google Scholar]
- 32.Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med. 1994;330:1198–1210. [DOI] [PubMed] [Google Scholar]
- 33.Banks PA, Freeman ML. Practice parameters committee of the American college of gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–2400. [DOI] [PubMed] [Google Scholar]
- 34.Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:710–717.e1. [DOI] [PubMed] [Google Scholar]
- 35.Tenner S Initial management of acute pancreatitis: critical issues during the first 72 hours. Am J Gastroenterol. 2004;99:2489–2494. [DOI] [PubMed] [Google Scholar]
- 36.Eckerwall G, Olin H, Andersson B, Andersson R. Fluid resuscitation and nutritional support during severe acute pancreatitis in the past: what have we learned and how can we do better? Clin Nutr. 2006;25:497–504. [DOI] [PubMed] [Google Scholar]
- 37.Andriulli A, Forlano R, Napolitano G, et al. Pancreatic duct stents in the prophylaxis of pancreatic damage after endoscopic retrograde cholangiopancreatography: a systematic analysis of benefits and associated risks. Digestion. 2007;75:156–163. [DOI] [PubMed] [Google Scholar]
- 38.Elmunzer BJ, Waljee AK, Elta GH, et al. A meta-analysis of rectal NSAIDs in the prevention of post-ERCP pancreatitis. Gut. 2008;57:1262–1267. [DOI] [PubMed] [Google Scholar]
- 39.Petrov MS, Kukosh MV, Emelyanov NV. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg. 2006;23:336–345. [DOI] [PubMed] [Google Scholar]
- 40.Oláh A, Romics L Jr. Enteral nutrition in acute pancreatitis: a review of the current evidence. World J Gastroenterol. 2014;20:16123–16131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villatoro E, Mulla M, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. 2010;2010:CD002941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jafri NS, Mahid SS, Idstein SR, et al. Antibiotic prophylaxis is not protective in severe acute pancreatitis: a systematic review and meta-analysis. Am J Surg. 2009;197:806–813. [DOI] [PubMed] [Google Scholar]
- 43.Mier J, Leon EL, Castillo A, et al. Early versus late necrosectomy in severe necrotizing pancreatitis. Am J Surg. 1997;173:71–75. [DOI] [PubMed] [Google Scholar]
- 44.Besselink MG, Verwer TJ, Schoenmaeckers EJ, et al. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg. 2007;142:1194–1201. [DOI] [PubMed] [Google Scholar]
- 45.Mowery NT, Bruns BR, MacNew HG, et al. Surgical management of pancreatic necrosis: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;83:316–327. [DOI] [PubMed] [Google Scholar]
- 46.van Santvoort HC, Besselink MG, Bakker OJ, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491–1502. [DOI] [PubMed] [Google Scholar]
- 47.Aparna D, Kumar S, Kamalkumar S. Mortality and morbidity in necrotizing pancreatitis managed on principles of step-up approach: 7 years experience from a single surgical unit. World J Gastrointest Surg. 2017;9:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gotzinger P, Sautner T, Kriwanek S, et al. Surgical treatment for severe acute pancreatitis: extent and surgical control of necrosis determine outcome. World J Surg. 2002;26:474–478. [DOI] [PubMed] [Google Scholar]
- 49.Connor S, Alexakis N, Raraty MG, et al. Early and late complications after pancreatic necrosectomy. Surgery. 2005;137:499–505. [DOI] [PubMed] [Google Scholar]
- 50.Zhi M, Zhu X, Lugea A, et al. Incidence of new onset diabetes mellitus secondary to acute pancreatitis: a systematic review and meta-analysis. Front Physiol. 2019;10:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tu J, Zhang J, Ke L, et al. Endocrine and exocrine pancreatic insufficiency after acute pancreatitis: long-term follow-up study. BMC Gastroenterol. 2017;17: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garip G, Sarandol E, Kaya E. Effects of disease severity and necrosis on pancreatic dysfunction after acute pancreatitis. World J Gastroenterol. 2013;19: 8065–8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boreham B, Ammori BJ. A prospective evaluation of pancreatic exocrine function in patients with acute pancreatitis: correlation with extent of necrosis and pancreatic endocrine insufficiency. Pancreatology. 2003;3:303–308. [DOI] [PubMed] [Google Scholar]
- 54.Migliori M, Pezzilli R, Tomassetti P, Gullo L. Exocrine pancreatic function after alcoholic or biliary acute pancreatitis. Pancreas. 2004;28:359–363. [DOI] [PubMed] [Google Scholar]
- 55.Hollemans RA, Hallensleben NDL, Mager DJ, et al. Pancreatic exocrine insufficiency following acute pancreatitis: systematic review and study level meta-analysis. Pancreatology. 2018;18:253–262. [DOI] [PubMed] [Google Scholar]
- 56.Uomo G, Gallucci F, Madrid E, et al. Pancreatic functional impairment following acute necrotizing pancreatitis: long-term outcome of a non-surgically treated series. Dig Liver Dis. 2010;42:149–152. [DOI] [PubMed] [Google Scholar]
- 57.Mendelson RM, Anderson J, Marshall M, Ramsay D. Vascular complications of pancreatitis. ANZ J Surg. 2005;75:1073–1079. [DOI] [PubMed] [Google Scholar]
- 58.Chait J, Duffy E, Marks N, et al. Superior mesenteric artery thrombosis after necrotizing pancreatitis. Ann Vasc Surg. 2019;59:307.e17–.e20. [DOI] [PubMed] [Google Scholar]
- 59.Stone JR, Wilkins LR. Acute mesenteric ischemia. Tech Vasc Intervent Radiol. 2015;18:24–30. [DOI] [PubMed] [Google Scholar]
- 60.Kochhar R, Jain K, Gupta V, et al. Fistulization in the GI tract in acute pancreatitis. Gastrointest Endosc. 2012;75:436–440. [DOI] [PubMed] [Google Scholar]
- 61.Jiang W, Tong Z, Yang D, et al. Gastrointestinal fistulas in acute pancreatitis with infected pancreatic or peripancreatic necrosis. Medicine. 2016;95:e3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahmed Ali U, Issa Y, Hagenaars JC, et al. Risk of recurrent pancreatitis and progression to chronic pancreatitis after a first episode of acute pancreatitis. Clin Gastroenterol Hepatol. 2016;14:738–746. [DOI] [PubMed] [Google Scholar]
- 63.Stevens T, Conwell DL, Zuccaro G. Pathogenesis of chronic pancreatitis: an evidence-based review of past theories and recent developments. Am J Gastroenterol. 2004;99:2256–2270. [DOI] [PubMed] [Google Scholar]
- 64.Schneider A, Whitcomb DC. Hereditary pancreatitis: a model for inflammatory diseases of the pancreas. Best Pract Res Clin Gastroenterol. 2002;16:347–363. [DOI] [PubMed] [Google Scholar]
- 65.Lankisch PG, Breuer N, Bruns A, et al. Natural history of acute pancreatitis: a long-term population-based study. Am J Gastroenterol. 2009;104:2797–2805. quiz 2806. [DOI] [PubMed] [Google Scholar]


