Abstract
Annual influenza infections cause massive economic loss and pose severe threats to public health worldwide. Seasonal influenza vaccines are the most effective means of preventing influenza infections but still possess major weaknesses: requiring updating vaccine strains every year, difficulty for accurate prediction of viruses that will be circulating and low protection efficacy due to mismatching, time-consuming egg-dependent production, and ineffectiveness of protection against influenza pandemics. The ultimate quest is for a universal influenza vaccine that could be used from year to year. One approach under investigation is to design influenza vaccine immunogens based on conserved amino acid sequences or conformational epitopes, rather than strain-specific ones of influenza viruses. Such vaccines can elicit broadly reactive humoral and cellular immunity. Universal influenza vaccine development has intensively employed nanotechnology because the structural and morphological properties of nanoparticles dramatically improve vaccine immunogenicity and induced immunity duration. Layered protein nanoparticles can eliminate the possibility of off-target immune responses, integrate epitopes into different regions of the particles for differentiated antigen-recognition and processing, and facilitate comprehensive immune response induction. Herein, we reviewed the designs of effective nanoparticle universal influenza vaccines, the recent discoveries of specific nanoparticle features that contribute to immunogenicity enhancement, and recent progress in clinical trials.
1. Introduction — An Urgent Need for an Affordable Universal Influenza Vaccine.
Seasonal influenza causes 3 to 5 million cases of severe illness and up to 650,000 deaths yearly according to the World Health Organization. 1 Mismatched seasonal influenza vaccines are low protection efficacy and provide limited protection against circulating strains. For instance, by mid-October in 2017 the influenza vaccine effectiveness (VE) against H3N2 was estimated to be only 10%, which was closely associated with the predominant H3N2 activity in the southern hemisphere. 2 Not surprisingly, vaccination with this H3N2-containing vaccine had not counteracted the H3N2-predominant epidemic in the United States during the following months. 3 During the 2017–2018 influenza season, the severity of influenza B outbreaks in certain regions of the northern hemisphere was associated with the lack of a vaccine strain from the influenza B Yamagata lineage in the traditional trivalent influenza vaccine. 4–5
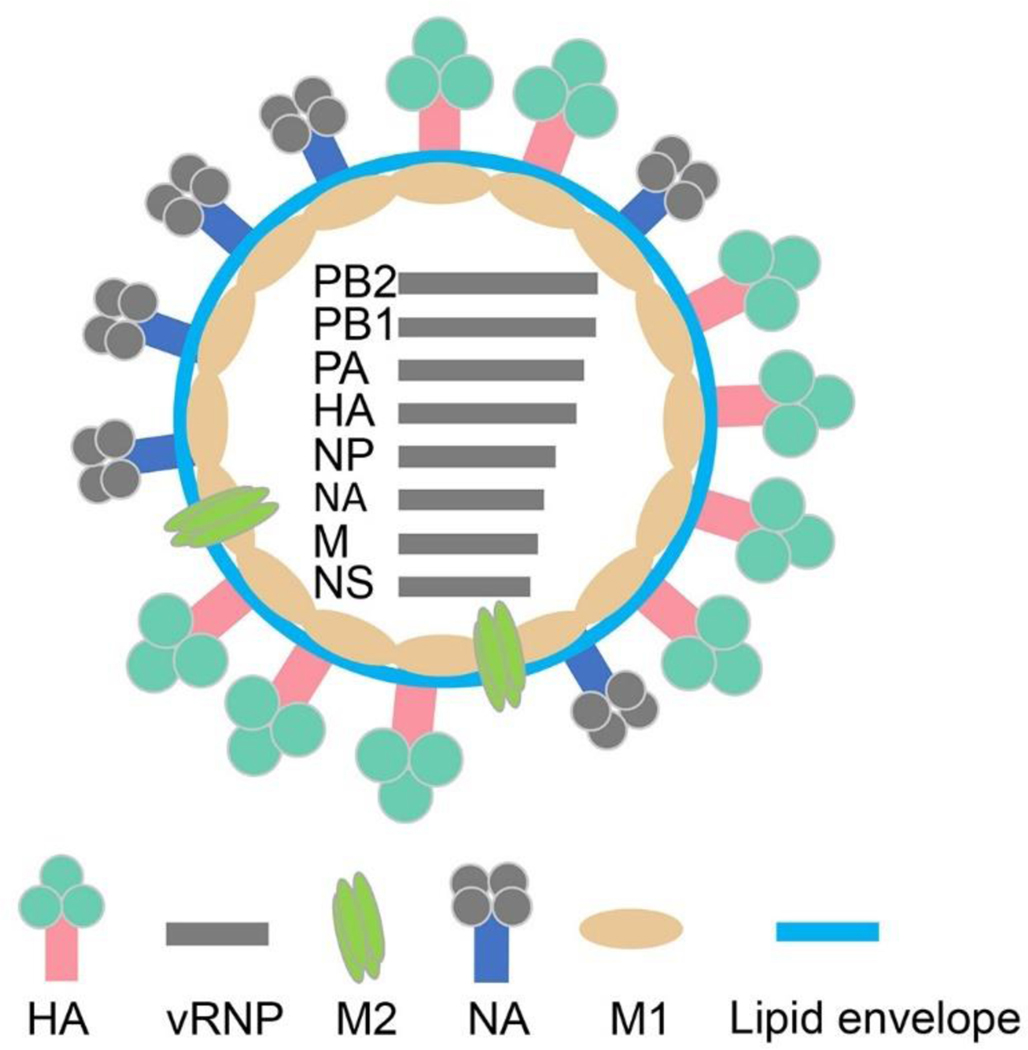
Influenza viruses contain three membrane proteins: hemagglutinin (HA or H), neuraminidase (NA or N), and matrix protein 2 (M2) (Figure 1). 6 Induction of protective immunity against influenza requires recognition of these surface proteins by the host. M2 is relatively genetically stable and possess low immunogenicity. HA and NA are much more immunogenic but also highly antigenically variable. There are 18 HA subtypes and 11 NA subtypes known for influenza A. 7 Antigenic drift — mutations in HA and/or NA — results in new influenza A virus strains over time. Antigenic shift — a major re-assortment of HA and/or NA genes — results in new subtypes of influenza A virus. As a result, influenza virus stays in a continuous state of genetic flux and displays different variants of HA and NA on its surface, enabling to evade preexisting immunity. A non-human influenza virus may also cause a pandemic in human populations by acquiring the capacity for transmission in humans. The recent infection of humans by highly pathogenic avian H5N1 and the outbreak of a novel avian H7N9 strain has reinforced this concern. 8–9 The inherent variability of HA and NA surface proteins of influenza virus creates an intractable problem for the seasonal influenza vaccine approach. For this reason, there is an urgent need for a universal influenza vaccine that will induce broad cross-protection against divergent influenza viruses.
Figure 1. Schematic diagram of influenza A virus.
The antigen name encoded by each gene segment is labeled aside. HA, hemagglutinin; NA, neuraminidase; M1, matrix protein 1; M2e, matrix protein 2 ectodomain; vRNP, viral ribonucleoprotein; PB, polymerase basic protein; PA, polymerase acid protein; NP, nucleoprotein; NS, non-structural protein.
2. Conserved Influenza Sequences as Universal Vaccine Immunogens
The suboptimal VE of conventional influenza vaccines is multifactorial. Lower VE is associated with previous vaccination with seasonal influenza vaccines 10–12 and increasing age of the recipients. 13 Vaccinologists have attempted to improve the efficacy of the seasonal vaccine through different means. Increasing the dosage of conventional influenza vaccination induced higher vaccine-specific antibody titers and interleukin - 10 levels, but has little positive impact on the development of functional T-cell memory in older adults. 14 Recent studies revealed that insect cell-produced HA restores the reduced immunogenicity caused by egg-adaptation. 15 Researchers created a novel influenza vaccine strain by introducing eight interferon-sensitive mutations — point mutations identified through quantitative high-throughput genomics analysis — and found improved in vivo immunogenicity and protectivity. 16 Nevertheless, a universal influenza vaccine is still preferable and can be used without a yearly update of vaccine components.
A universal influenza vaccine would rely on conserved amino acid sequence and/or epitope conformation from influenza to generate broadly reactive humoral and cellular immunity. M2 is a relatively conserved influenza antigen but is expressed in low copy numbers on the virion. However, infected target cells abundantly express M2 on their surfaces where it is accessible for antibodies. 17 Protection against multiple influenza A virus subtypes elicited by M2 ectodomain (M2e)-based vaccines correlates closely with M2e-specific antibody affinity to Fc gamma receptors on target cells. 18–21
The HA stalk domain is relatively more amino acid- and conformation-conserved than the HA head domain and can induce antibodies capable of binding multiple HA subtypes. 22–28 Protein structural analysis has enabled the development of some recombinant HA stalk domain vaccines with effective in vivo immunogenicity. 29–31 HA stalk-directed protective immunity prevents infection by inducing neutralizing antibodies, antibody dependent cellular cytotoxicity and antibody dependent phagocytosis. 31 T cell responses against internal influenza components and structural components correlate with cross-reactive protection and early clinical recovery from infection. 32–34
Nucleoproteins (NP) enclose the viral RNA segments, are necessary for trafficking the viral genome, and are highly conserved in comparison to the surface glycoproteins. 35–36 Blending NP with other conserved influenza components polymerase basic-2 and/or matrix protein 1 (M1) — in fusion constructs broadened the spectrum of immune protection induced by vaccine candidates. 37–41
Vaccination with multiple conserved target antigens will have advantages over using single antigens. 42 A universal vaccine providing long-term protection against heterosubtypic influenza virus strains will benefit pandemic control and routine vaccination.
3. Different Types of Nanoparticles Accommodating Influenza Conserved Epitopes Benefit for Enhanced Immunogenicity
Conserved influenza antigens typically induce weak immune responses. There is a need to develop novel delivery systems and adjuvants that increase the immunogenicity of conserved components for next-generation vaccines. The application of nanotechnology in vaccinology has been increasing dramatically over the past decades. 43 The common benefits of nanoparticles are prolonged circulation and directed targeting by modification of nanoparticle surfaces with dendritic cell or T cell targeting ligands. 44 Nanoparticles are an efficient delivery system which facilitate the enhancement of immune cell uptake and the sustaining antigen release. 45–46
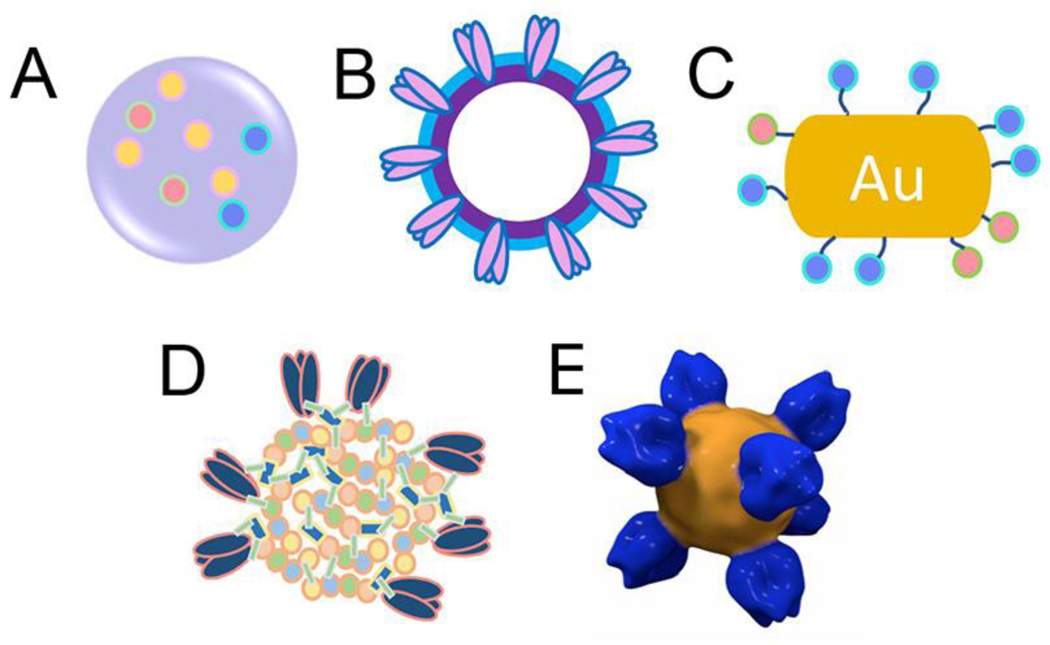
Our recent study indicated that gradual intracellular disassembly of protein nanoparticles led to sustained specific immune responses. 47 Physiologically controlled, stimuli-sensitive release is a major benefit of this nano system. An optimized eudragit-S/Trehalose polymer nanoparticle influenza vaccine released influenza antigens during in vitro simulation of the pH conditions of the mouse gastrointestinal tract. Mice experiments indicated that oral immunization with this nanoparticle vaccine induced protective immune responses. 48 Thermo-sensitive, redox condition-sensitive, and near infrared light activated controlled release has recently been developed in the field of anti-cancer research. 44, 49 Comparatively, specific features of different nanoparticle types can benefit prophylactic universal influenza vaccine designs (Figure 2). One or multiple features can be synergistically integrated into one nanoparticle system for an optimal immune response. Divergent nanoparticle universal influenza vaccines under investigation are summarized in Table 1.
Figure 2. Nanoparticle universal influenza vaccine types.
(A) Polymer nanoparticle; (B) Virus like particle; (C) Gold nanoparticle; (D) Chemically assembled double-layered protein nanoparticle; (E) Naturally assembled protein nanoparticle (EMDB EMD-6332).
Table 1.
Overview of nanoparticle universal influenza A vaccines.
| Overview of nanoparticle universal influenza A vaccines | ||||
|---|---|---|---|---|
| Vaccine types | Vectors | Antigen types | Immunogenicity readout in animal models | References |
| Synthetic polymers | PLGA | Norovirus P particle containing M2e, Peptides of HA, NP and PA; outermembrane vesicles displaying M2e | Pigs, mice, | 57, 58, 59 |
| poly-γ-glutamic acid/chitosan | M2, HA fusion peptide; H1N1 split vaccine | Mice | 63, 64 | |
| VLPs | Virosome | Headless HA; M2e; M2e/FliC; | Mice | 83, 84, 85, 86, 87 |
| Hepatitis B core | M2e | Mice, pigs, ferrets, human | 18, 80 | |
| MaMV | M2e | Canine | 88 | |
| Tobacco mosaic virus coat protein | M2e | Mice | 89 | |
| Papaya mosaic virus | M2e | Mice | 92 | |
| F88 bacteriophage | M2e | Mice | 81 | |
| Woodchuck hepatitis VLP vectored in Salmonella Typhimurium | M2e | Mice | 94 | |
| T7 bacteriophage | M2e | Mice | 82 | |
| Q-β | M2e | Mice | 88 | |
| Self-assembling nanoparticles | Ferritin | Headless HA; M2e | Mice, ferrets | 30, 97, 99 |
| Self-Antigen | M2e, headless HA; M2e-NA tetramer; NP | Mice | 31, 47, 87, 100, 101 | |
| Metal nanoparticles | Gold nanoparticle | M2e/CpG | Mice | 108 |
1). Polymer nanoparticles.
Synthetic polymers are widely used to fabricate nanoparticle vaccines due to their favorable properties such as biocompatibility and biodegradability. US Food and Drug Administration approved poly(D,L-lactic-co-glycolic acid) (PLGA) has been extensively characterized in animal models and is widely used in vaccine development as an encapsulation matrix 50 to co-deliver 51–52 and sustain controlled release of antigens. 46, 53 Other synthetic polymers with similar features include poly(D, L-lactide-co-glycolide), 54 poly(D,L-lactic-co-hydroxymethyl glycolic acid) 55 and polystyrene. 56
In universal influenza vaccine development, PLGA nanoparticles encapsulating conserved influenza A virus components including norovirus P particle containing M2e and synthetic peptides of HA, NP and polymerase acidic protein, elicited strong prophylactic protection and eliminated influenza symptoms in pigs against swine H1N1 challenge, 57 which significantly improves the previously reported immunization strategy with M2e and NP antigens in pigs. 58 PLGA can be formulated into larger sizes and facilitate the release of nanometer-sized constructs. Recombinant outer membrane vesicles displaying M2e released from PLGA microparticles over 30 days, induced sustained protective M2e specific immunity in mice. 59
Natural polymers based on polysaccharides have also been used to prepare nanoparticles, such as pullulan, alginate, inulin and chitosan. 43 Non-toxic chitosan nanoparticles have been widely studied as vaccine vectors owing to its advantages, biocompatibility, biodegradability and amenability to ease modification into desired shape and size. 60–62 Poly-γ-glutamic acid/chitosan nanoparticles containing truncated M2 and a fusion peptide of influenza HA and mucosal adjuvant cholera toxin subunit A1 acted as an effective mucosal vaccine against diverse influenza A viruses. 63 Nanogel particles encapsulating pandemic H1N1 split vaccine antigen increased cross-protection against homologous and heterosubtypic influenza A viral infection. 64 The diverse biocompatible polymers for nanoparticles expand our ability to accommodate different immunogens and adjuvants. However, because a major portion of the vaccine-loaded nanoparticles is the polymer materials, the low immunogen load of the polymer nanoparticles could be a limitation for some immunogens needing high dosage for a robust immune response.
2). Virus-like particles.
Virus-like particle (VLP) formation is driven by the same mechanisms used during virus assembly — viral self-assembling proteins or domains. The morphological features of VLPs simulate the features of viruses that the host immune system has evolved to combat. This makes VLPs highly immunogenic. 65–68
The first VLP vaccine for hepatitis B virus, developed by Glaxo Smith Kline, was commercialized in 1986 69 followed by commercialization of other VLP vaccines including Cervarix (human papillomavirus, Glaxo Smith Kline), Recombivax HB (hepatitis B virus, Merck & Co. Inc) and Gardasil (human papillomavirus, Merck & Co. Inc). Many others are currently in the clinical trial or research stage.
Because VLPs lack genomic components or contain premature termination codon, 70 they are safe compared with other replicating vaccine vectors. Due to viral features like repetitive structures, naturally retained antigen conformation, and virion sizes, VLPs induce innate and adaptive immune responses. 71–72 Influenza lacks a capsid. Instead, it has a core bridged with viral envelop by the matrix protein M1. VLPs are assembled in the physiological conditions on host membranes and released into the environment via budding. Our influenza VLPs are formed in SF9 insect cells by the same manner that influenza envelopes are formed: a budding process organized by the M1 protein. Enveloped VLPs can be produced by co-expression of structure proteins in mammalian cells, insect cells, or plants. 73–79 Furthermore, the self-assembling biocompatible viral capsid proteins can be adapted to various cell systems, like mammalian cells, insect cells, plants, yeasts and Escherichia coli (E. coli) for VLP production. 80–82
A vaccine incorporating PR8 (A/Puerto Rico/8/1934) headless HA into human immunodeficiency virus Gag-based VLPs expressed in 293T cells protected mice against influenza viral challenges. 83 M2 VLPs have been produced in insect cells coinfected with recombinant baculoviruses expressing M1 and wild type M2 protein 84 or multiple M2e. 85 Supplemental M2 VLP immunization with inactivated H1N1 vaccine enhanced cross-protection against influenza viral challenges. 84–85 Proper presentation of ligands or agonists in nanoparticles targeting immune cells potentially make antigens more immunogenic in vivo. 86–87 Appropriate antigen presentation in a nanoparticle form and coadministration of adjuvant molecules, often targeting specific receptors of immune cells, elicits strong specific immune response. We designed a membrane-anchored fusion protein by replacing the hyperimmunogenic region of Salmonella enterica serovar Typhimurium flagellin with four repeats of M2e (4M2e-tFliC) and fusing it to the membrane anchoring domain of influenza HA. The fusion protein was incorporated into influenza M1-driven VLPs. Immunization with these VLP greatly enhanced the M2e specific immune responses. 86
Non-enveloped VLPs have been widely developed with chimeric capsid proteins displaying influenza conserved epitopes which provide effective bystander T-helper responses and induce protective immune responses specific to such epitopes. 80–82, 88–94 Immunization with E. coli produced hepatitis B core VLPs displaying M2e cross-protected mice and ferrets against diverse influenza virus challenge and efficiently induced protective M2e-specific immunity in volunteers. 18 VLP-display are frequently investigated as a promising vaccine platform for presenting conserved surface proteins in a highly immunogenic form.
3). Self-assembling protein nanoparticles.
Naturally occurring self-assembling protein nanoparticles have been identified from a wide variety of sources. 95 Self-assembling motifs can enable fusion proteins to assemble into protein nanoparticles. 96–98 Ferritin can be self-organized into a nano scale structure (nanocage) with intracellular iron storage functionality. It serves as an ideal epitope presentation platform with repetitive symmetrical structure and an ordered matrix. It was previously reported that headless HA trimers and tandem copies of M2e displayed on ferritin nanoparticles retained native conformation and induced protective homosubtypic and heterosubtypic immunity in vivo. 30, 99 Because most of large self-assembling domains are adopted from non-human species, off-target immune responses are a major weakness of such self-assembling protein nanoparticles. 30, 96–97, 99
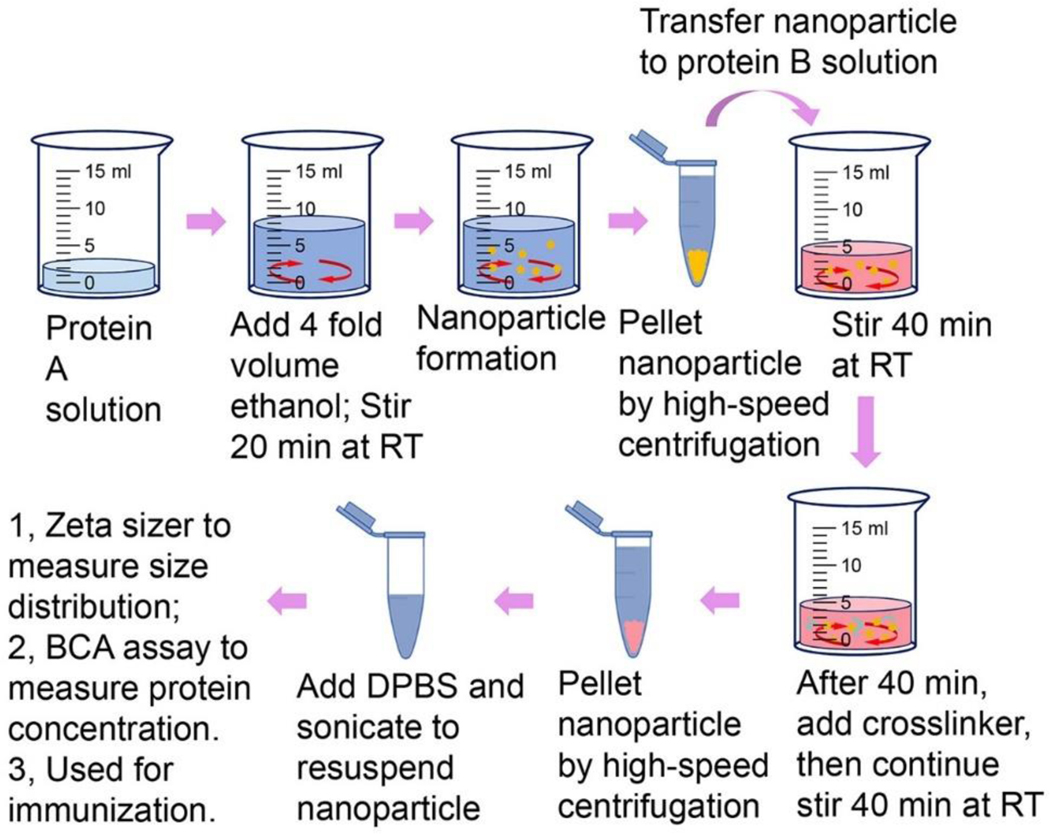
Changes in the physicochemical condition of protein solutions can drive the formation protein nanoparticles. Because changes in physicochemical condition are dynamic processes, nanoparticles assembled this way are generally not homogenous in size but do show a normally distributed range of sizes. We first used ethanol desolvation to assemble influenza M2e or influenza HA into protein nanoparticles. 31, 87, 100–101 Because these particles have solid structures without non-antigen components, they have the highest possible antigen-load for protein nanoparticles. Another advantage of these nanoparticles is that they can go through multiple cycles of particle assembly processes with different antigenic proteins to assemble proteins into different layers around the particles. Layered protein nanoparticles are particularly suitable for protein antigens with different stability in solution. We have generated double-layered protein nanoparticles by ethanol desolvation of M2e or NP peptides into nanoparticles as the cores and chemically crosslinking structure-stabilized influenza HA stalk antigens or M2e onto the outer layers. 31, 47 The fabrication process is summarized in figure 3. These physicochemical layered protein nanoparticles can maximize the different immunological role of the different antigens. 100
Figure 3. Schematic diagram of double-layered nanoparticle fabrication process.
RT, room temperature; DPBS, Dulbecco’s phosphate-buffered saline.
The desolvated nanoparticle size is 50 to 300 nm, depending on the desolvent composition 102 and desolvated protein materials. 31 Because this technique is compatible with most proteins, other proteins such as innate signaling initiators (immune stimulators) or immune cell-targeting molecules can be crosslinked onto the outer layers to endow additional beneficial immunological features. 103
The combination of dissolving microneedle patch technology with such protein nanoparticles enables convenient skin vaccination, which is syringe-free, painless, and can be self-administrated. Mouse skin immunization experiments demonstrated that this protein nanoparticle vaccine conferred at least four months of universal immunity against diverse influenza virus challenges. Furthermore, this approach allows for cold-chain independent storage and triggered long-lasting protection. 31
4). Metal nanoparticles.
Metal nanoparticles are rigid in structure and nearly non-biodegradable. The shape and growth of metal nanoparticles are controlled by fine-tuning the rates of surface diffusion and deposition. 104 The inorganic nanoparticle is frequently investigated as a vaccine development platform in order to improve antigen immunogenicity and avoid antibody production against the platform materials. Gold nanoparticles have attracted attention in the nanomedical field due to their unique pros, their biocompatibility and easy fabrication in terms of size and shape. Gold nanoparticles less than 100 nm in diameter can be synthesized, which is preferentially recognized and engulfed by dendritic cells. Gold nanoparticles can also be formed into different shapes like star, spherical, cube and rod to control the induced, shape-dependent modulation of immune responses. 105 However, the cons of gold nanoparticle vaccines include expensive manufacture cost and less drug loading capacity than polymer nanoparticles, VLPs and desolvated nanoparticles.
A preliminary study reported that M2e-immobilized gold nanoparticles formulated with CpG induced cross-protection against diverse influenza viral challenges. 106–107 Our studies have demonstrated that gold nanoparticles can conjugate influenza HA and adjuvant protein flagellin and trigger strong immune responses conferring heterologous protection in mice. 108–109
5). Important Physical Features Benefit to Enhanced Immunogenecity
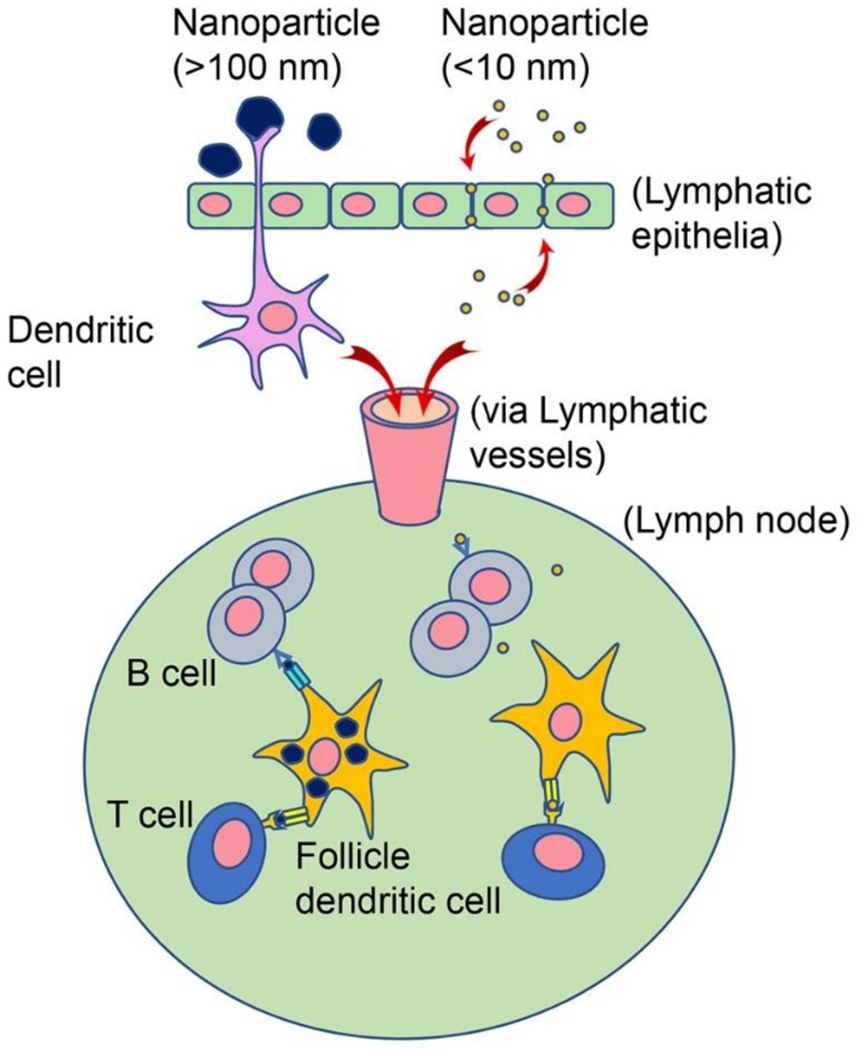
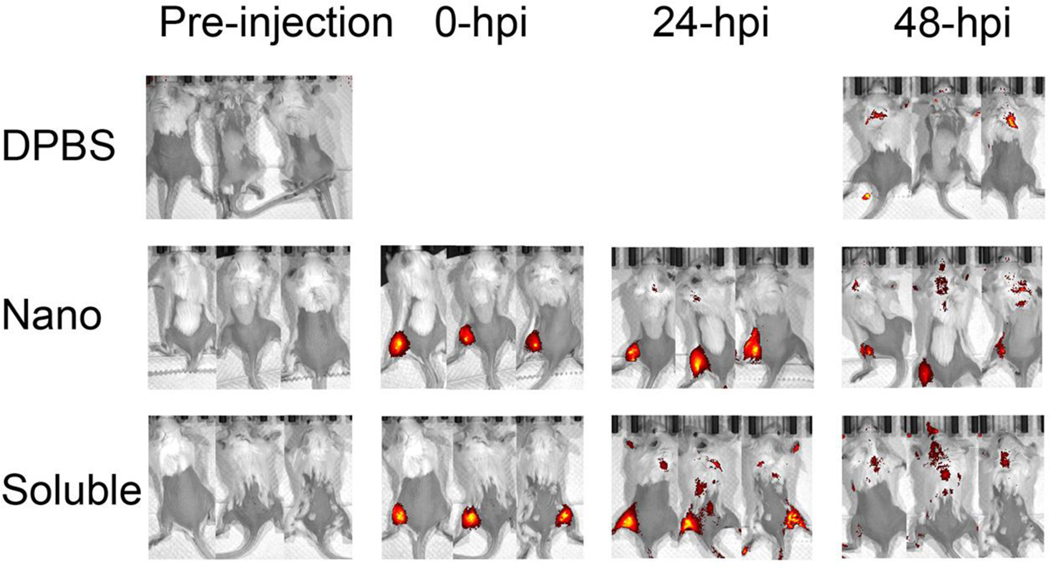
The properties of nanoparticles, such as size and steric conformation, play an active role in mediating the biological effects and the adaptive immunity induction. 110–113 Ultra-small nanoparticles less than 10 nm in diameter or soluble antigens can rapidly diffuse into and out of lymph organs, which decreases the opportunity for uptake by antigen presenting cells (APCs). 114 Nanoparticles larger than 100 nm can be trapped in the injection site, require active transport, and are eventually scavenged by tissue-resident APCs (Figure 4). 115 We found that intramuscularly injected fluorescent NP nanoparticles remained at the injection site much longer than fluorescent soluble NP protein (Figure 5). Smaller nanoparticles, optimally size around 50 – 200 nm in diameter, were shown to be engulfed more efficiently by dendritic cells while the larger, micrometer-scale nanoparticles were preferentially internalized by macrophages. 105, 116–117
Figure 4. Nanoparticle transport route changes with nanoparticle sizes.
Nanoparticles with larger sizes over 100 nm are captured by dendritic cells from outside of lymphatic epithelia, in comparison, soluble protein antigen and smaller nanoparticles less than 10 nm are able to penetrate through lymphatic epithelia. Once antigens transported into lymph nodes via lymphatic vessels, they are presented to B cells and T cells by follicle dendritic cells. The larger nanoparticles are much more efficiently taken up by dendritic cells than smaller nanoparticles and potentially induce stronger and long-term specific immunity.
Figure 5. In vivo visualization in mice.
The fabrications of fluorescent NP protein and fluorescent NP nanoparticle were described previously. (46) Mice were fed alfalfa-free diets for two weeks to reduce autofluorescence background before intramuscular injection with five micrograms of fluorescent antigen each mouse. Biodistributions of intramuscularly injected Alexa Fluor 700 succinimidyl ester dye-conjugated fluorescent NP nanoparticle and fluorescent soluble NP protein were analyzed in vivo using Perkin-Elmer IVIS Spectrum In Vivo Imaging System at the following time points: pre-injection, 0-hour post injection (hpi), 24-hpi and 48-hpi. (46) Mice injected with DPBS were used as negative control.
The strength of induced immunity is also affected by the efficiency of dendritic cells migration towards the draining lymph nodes (LNs) and the length of antigen retention period in LNs. 118 We recently found that ~200 nm protein nanoparticles had an increased chance to be presented by APCs due to their efficient drainage into inguinal LNs and spleens and relatively long residence at those sites compared with soluble antigens. The soluble molecules diffused rapidly from the injection sites and disappeared earlier from the LNs. 47
We also found that nanoparticle vaccines delivered by dissolvable microneedle patches conferred mice long-lasting anti-influenza A virus immunity. We speculated that the entrapment in immune tissues and the intracellularly-activated disassembly of nanoparticles within APCs allowed for the sustained processing and releasing of peptides for a longer period versus soluble antigens, shaping memory T cells and long-term immunity. Sustained antigen release is a critical characteristic for long-lasting memory immune responses to nanoparticle vaccines. 114
Particle shape also plays important roles in the mediation of immunity induction, especially in the phagocytosis of particles. 119–120 The local shape at the interface between particles and phagocytes determines the efficiency of particle uptake. 121–123 For example, spherical gold nanoparticles (40 nm) more efficiently induced antibody responses against delivered antigens than did cube- and rod-shaped nanoparticles with similar sizes. 124
A phase I clinical trial of the universal influenza A nanoparticle vaccine, M2eHBc (ACAM-FLU-A™) sponsored by Acambis (later acquired by Sanofi Pasteur), generated interesting results. The intramuscularly injected ACAM-FLU-A™, adjuvanted with QS21, was well tolerated and able to stimulate anti-M2e seroconversion in up to 90% of healthy volunteers. However, the induced M2e-specific antibody titers dropped rapidly over 10 months. 18 Resolving the short-term persistence issue of the M2e-specific immune responses is the last piece of the puzzle in M2e-based vaccine designs. We found that desolvated M2e nanoparticles have an order of magnitude higher levels of M2e antigen loading than the M2eHBc construct, potentially inducing stronger and longer sustained protective M2e-specific T cell immune responses in humans.
Concluding remarks
Even if proof-of-concept has been demonstrated in pre-clinical data, a vaccine product candidate still be potentially incompetitive in the market if costly or inscalable manufacturing process. The baculovirus expression vector/insect cell (BEVS/IC) system constitutes an optimal expression system for VLP manufactures, through which Glaxo Smith Kline’s Cervarix human papillomavirus VLP cancer vaccine is produced. Insect cell large-scale suspension cultures established in either stirred or rocked bioreactors have been shown to be one of the best VLP production system. Furthermore, improved insect cell lines has been ‘humanized’, which perform mammalian-like post-translation glycosylation modifications. 125 There are no other marketed types of nanoparticle vaccines.
Because of a series of appreciated features, nanoparticles are potent for the development of an affordable universal vaccines. They are safe, biocompatible, flexible to be fabricated in variable sizes, relatively high surface area for immune ligand-receptor ligation-recognition, capable of accommodating high load of different conserved antigens in the same particle, and sustaining longer period of antigen-supplement, processing and presentation. All these features will be interpreted into a broadly protective universal influenza vaccine.
Acknowledgment
This work is supported by the Institute of Biomedical Science, Georgia State University and by grants R01AI101047 and R01AI116835 (to BZW) from US National Institutes of Health.
Footnotes
Competing interest statement
There are no conflicting interests among all co-authors.
References:
- 1.WHO, World Health Organization. http://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (Access on June/20/2018). 2018.
- 2.Sullivan SG; Chilver MB; Carville KS; Deng YM; Grant KA; Higgins G; Komadina N; Leung VK; Minney-Smith CA; Teng D; Tran T; Stocks N; Fielding JE, Low interim influenza vaccine effectiveness, Australia, 1 May to 24 September 2017. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin 2017, 22 (43). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garten R; Blanton L; Elal AIA; Alabi N; Barnes J; Biggerstaff M; Brammer L; Budd AP; Burns E; Cummings CN; Davis T; Garg S; Gubareva L; Jang Y; Kniss K; Kramer N; Lindstrom S; Mustaquim D; O’Halloran A; Sessions W; Taylor C; Xu X; Dugan VG; Fry AM; Wentworth DE; Katz J; Jernigan D, Update: Influenza Activity in the United States During the 2017–18 Season and Composition of the 2018–19 Influenza Vaccine. MMWR. Morbidity and mortality weekly report 2018, 67 (22), 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machado A; Kislaya I; Nunes B; Rodrigues AP; Guiomar R; Euro E. V. A. p., Moderate influenza vaccine effectiveness in a B mismatch season: Preliminary results from the 2017/2018 season in Portugal. Pulmonology 2018. [DOI] [PubMed] [Google Scholar]
- 5.CNIC, Chinese National Influenza Centre, http://www.chinaivdc.cn/cnic/en/ (Access on January/30/2018). 2018.
- 6.Ebrahimi SM; Tebianian M, Influenza A viruses: why focusing on M2e-based universal vaccines. Virus Genes. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H; Wang L; Compans RW; Wang BZ, Universal influenza vaccines, a dream to be realized soon. Viruses 2014, 6 (5), 1974–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mei L; Song PP; Tang Q; Shan K; Tobe RG; Selotlegeng L; Ali AH; Cheng YY; Xu LZ, Changes in and shortcomings of control strategies, drug stockpiles, and vaccine development during outbreaks of avian influenza A H5N1, H1N1, and H7N9 among humans. Biosci Trends 2013, 7 (2), 64–76. [PubMed] [Google Scholar]
- 9.Gao R; Cao B; Hu Y; Feng Z; Wang D; Hu W; Chen J; Jie Z; Qiu H; Xu K; Xu X; Lu H; Zhu W; Gao Z; Xiang N; Shen Y; He Z; Gu Y; Zhang Z; Yang Y; Zhao X; Zhou L; Li X; Zou S; Zhang Y; Li X; Yang L; Guo J; Dong J; Li Q; Dong L; Zhu Y; Bai T; Wang S; Hao P; Yang W; Zhang Y; Han J; Yu H; Li D; Gao GF; Wu G; Wang Y; Yuan Z; Shu Y, Human Infection with a Novel Avian-Origin Influenza A (H7N9) Virus. N Engl J Med 2013. [DOI] [PubMed] [Google Scholar]
- 10.Ohmit SE; Petrie JG; Malosh RE; Fry AM; Thompson MG; Monto AS, Influenza vaccine effectiveness in households with children during the 2012–2013 season: assessments of prior vaccination and serologic susceptibility. The Journal of infectious diseases 2015, 211 (10), 1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLean HQ; Thompson MG; Sundaram ME; Meece JK; McClure DL; Friedrich TC; Belongia EA, Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2014, 59 (10), 1375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferdinands JM; Fry AM; Reynolds S; Petrie J; Flannery B; Jackson ML; Belongia EA, Intraseason waning of influenza vaccine protection: Evidence from the US Influenza Vaccine Effectiveness Network, 2011–12 through 2014–15. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2017, 64 (5), 544–550. [DOI] [PubMed] [Google Scholar]
- 13.Olafsdottir TA; Alexandersson KF; Sveinbjornsson G; Lapini G; Palladino L; Montomoli E; Del Giudice G; Gudbjartsson DF; Jonsdottir I, Age and Influenza-Specific Pre-Vaccination Antibodies Strongly Affect Influenza Vaccine Responses in the Icelandic Population whereas Disease and Medication Have Small Effects. Frontiers in immunology 2017, 8, 1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merani S; Kuchel GA; Kleppinger A; McElhaney JE, Influenza vaccine-mediated protection in older adults: Impact of influenza infection, cytomegalovirus serostatus and vaccine dosage. Experimental gerontology 2018, 107, 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zost SJ; Parkhouse K; Gumina ME; Kim K; Diaz Perez S; Wilson PC; Treanor JJ; Sant AJ; Cobey S; Hensley SE, Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proceedings of the National Academy of Sciences of the United States of America 2017, 114 (47), 12578–12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du Y; Xin L; Shi Y; Zhang TH; Wu NC; Dai L; Gong D; Brar G; Shu S; Luo J; Reiley W; Tseng YW; Bai H; Wu TT; Wang J; Shu Y; Sun R, Genome-wide identification of interferon-sensitive mutations enables influenza vaccine design. Science 2018, 359 (6373), 290–296. [DOI] [PubMed] [Google Scholar]
- 17.Lamb RA; Zebedee SL; Richardson CD, Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell 1985, 40 (3), 627–33. [DOI] [PubMed] [Google Scholar]
- 18.Deng L; Cho KJ; Fiers W; Saelens X, M2e-Based Universal Influenza A Vaccines. Vaccines (Basel) 2015, 3 (1), 105–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutchinson EC; Charles PD; Hester SS; Thomas B; Trudgian D; Martinez-Alonso M; Fodor E, Conserved and host-specific features of influenza virion architecture. Nature communications 2014, 5, 4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Bakkouri K; Descamps F; De Filette M; Smet A; Festjens E; Birkett A; Van Rooijen N; Verbeek S; Fiers W; Saelens X, Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. J Immunol 2011, 186 (2), 1022–31. [DOI] [PubMed] [Google Scholar]
- 21.Schepens B; De Vlieger D; Saelens X, Vaccine options for influenza: thinking small. Current opinion in immunology 2018, 53, 22–29. [DOI] [PubMed] [Google Scholar]
- 22.Throsby M; van den Brink E; Jongeneelen M; Poon LL; Alard P; Cornelissen L; Bakker A; Cox F; van Deventer E; Guan Y; Cinatl J; ter Meulen J; Lasters I; Carsetti R; Peiris M; de Kruif J; Goudsmit J, Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PloS one 2008, 3 (12), e3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dreyfus C; Laursen NS; Kwaks T; Zuijdgeest D; Khayat R; Ekiert DC; Lee JH; Metlagel Z; Bujny MV; Jongeneelen M; van der Vlugt R; Lamrani M; Korse HJ; Geelen E; Sahin O; Sieuwerts M; Brakenhoff JP; Vogels R; Li OT; Poon LL; Peiris M; Koudstaal W; Ward AB; Wilson IA; Goudsmit J; Friesen RH, Highly conserved protective epitopes on influenza B viruses. Science 2012, 337 (6100), 1343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corti D; Voss J; Gamblin SJ; Codoni G; Macagno A; Jarrossay D; Vachieri SG; Pinna D; Minola A; Vanzetta F; Silacci C; Fernandez-Rodriguez BM; Agatic G; Bianchi S; Giacchetto-Sasselli I; Calder L; Sallusto F; Collins P; Haire LF; Temperton N; Langedijk JP; Skehel JJ; Lanzavecchia A, A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 2011, 333 (6044), 850–6. [DOI] [PubMed] [Google Scholar]
- 25.Sui J; Hwang WC; Perez S; Wei G; Aird D; Chen LM; Santelli E; Stec B; Cadwell G; Ali M; Wan H; Murakami A; Yammanuru A; Han T; Cox NJ; Bankston LA; Donis RO; Liddington RC; Marasco WA, Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol 2009, 16 (3), 265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan GS; Lee PS; Hoffman RM; Mazel-Sanchez B; Krammer F; Leon PE; Ward AB; Wilson IA; Palese P, Characterization of a broadly neutralizing monoclonal antibody that targets the fusion domain of group 2 influenza A virus hemagglutinin. Journal of virology 2014, 88 (23), 13580–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friesen RH; Lee PS; Stoop EJ; Hoffman RM; Ekiert DC; Bhabha G; Yu W; Juraszek J; Koudstaal W; Jongeneelen M; Korse HJ; Ophorst C; Brinkman-van der Linden EC; Throsby M; Kwakkenbos MJ; Bakker AQ; Beaumont T; Spits H; Kwaks T; Vogels R; Ward AB; Goudsmit J; Wilson IA, A common solution to group 2 influenza virus neutralization. Proceedings of the National Academy of Sciences of the United States of America 2014, 111 (1), 445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tharakaraman K; Subramanian V; Viswanathan K; Sloan S; Yen HL; Barnard DL; Leung YH; Szretter KJ; Koch TJ; Delaney JC; Babcock GJ; Wogan GN; Sasisekharan R; Shriver Z, A broadly neutralizing human monoclonal antibody is effective against H7N9. Proceedings of the National Academy of Sciences of the United States of America 2015, 112 (35), 10890–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Impagliazzo A; Milder F; Kuipers H; Wagner MV; Zhu X; Hoffman RM; van Meersbergen R; Huizingh J; Wanningen P; Verspuij J; de Man M; Ding Z; Apetri A; Kukrer B; Sneekes-Vriese E; Tomkiewicz D; Laursen NS; Lee PS; Zakrzewska A; Dekking L; Tolboom J; Tettero L; van Meerten S; Yu W; Koudstaal W; Goudsmit J; Ward AB; Meijberg W; Wilson IA; Radosevic K, A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 2015, 349 (6254), 1301–6. [DOI] [PubMed] [Google Scholar]
- 30.Yassine HM; Boyington JC; McTamney PM; Wei CJ; Kanekiyo M; Kong WP; Gallagher JR; Wang L; Zhang Y; Joyce MG; Lingwood D; Moin SM; Andersen H; Okuno Y; Rao SS; Harris AK; Kwong PD; Mascola JR; Nabel GJ; Graham BS, Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat Med 2015, 21 (9), 1065–70. [DOI] [PubMed] [Google Scholar]
- 31.Deng L; Mohan T; Chang TZ; Gonzalez GX; Wang Y; Kwon YM; Kang SM; Compans RW; Champion JA; Wang BZ, Double-layered protein nanoparticles induce broad protection against divergent influenza A viruses. Nat Commun 2018, 9 (1), 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng M; Luo J; Chen Z, Development of universal influenza vaccines based on influenza virus M and NP genes. Infection 2014, 42 (2), 251–62. [DOI] [PubMed] [Google Scholar]
- 33.Sridhar S; Begom S; Bermingham A; Hoschler K; Adamson W; Carman W; Bean T; Barclay W; Deeks JJ; Lalvani A, Cellular immune correlates of protection against symptomatic pandemic influenza. Nature medicine 2013, 19 (10), 1305–12. [DOI] [PubMed] [Google Scholar]
- 34.Eliasson DG; Omokanye A; Schon K; Wenzel UA; Bernasconi V; Bemark M; Kolpe A; El Bakkouri K; Ysenbaert T; Deng L; Fiers W; Saelens X; Lycke N, M2e-tetramer-specific memory CD4 T cells are broadly protective against influenza infection. Mucosal immunology 2017. [DOI] [PubMed] [Google Scholar]
- 35.Morens DM; Taubenberger JK; Fauci AS, Pandemic influenza viruses--hoping for the road not taken. The New England journal of medicine 2013, 368 (25), 2345–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chenavas S; Estrozi LF; Slama-Schwok A; Delmas B; Di Primo C; Baudin F; Li X; Crepin T; Ruigrok RW, Monomeric nucleoprotein of influenza A virus. PLoS pathogens 2013, 9 (3), e1003275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antrobus RD; Berthoud TK; Mullarkey CE; Hoschler K; Coughlan L; Zambon M; Hill AV; Gilbert SC, Coadministration of seasonal influenza vaccine and MVA-NP+M1 simultaneously achieves potent humoral and cell-mediated responses. Molecular therapy : the journal of the American Society of Gene Therapy 2014, 22 (1), 233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang W; Li R; Deng Y; Lu N; Chen H; Meng X; Wang W; Wang X; Yan K; Qi X; Zhang X; Xin W; Lu Z; Li X; Bian T; Gao Y; Tan W; Ruan L, Protective Efficacy of the Conserved NP, PB1, and M1 Proteins as Immunogens in DNA- and Vaccinia Virus-Based Universal Influenza A Virus Vaccines in Mice. Clinical and vaccine immunology : CVI 2015, 22 (6), 618–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lillie PJ; Berthoud TK; Powell TJ; Lambe T; Mullarkey C; Spencer AJ; Hamill M; Peng Y; Blais ME; Duncan CJ; Sheehy SH; Havelock T; Faust SN; Williams RL; Gilbert A; Oxford J; Dong T; Hill AV; Gilbert SC, Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2012, 55 (1), 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coughlan L; Sridhar S; Payne R; Edmans M; Milicic A; Venkatraman N; Lugonja B; Clifton L; Qi C; Folegatti PM; Lawrie AM; Roberts R; de Graaf H; Sukhtankar P; Faust SN; Lewis DJM; Lambe T; Hill A; Gilbert SC, Heterologous Two-Dose Vaccination with Simian Adenovirus and Poxvirus Vectors Elicits Long-Lasting Cellular Immunity to Influenza Virus A in Healthy Adults. EBioMedicine 2018, 29, 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Mario G; Sciaraffia E; Facchini M; Gubinelli F; Soprana E; Panigada M; Bernasconi V; Garulli B; Siccardi A; Donatelli I; Castrucci MR, Protective immunity against influenza in HLA-A2 transgenic mice by modified vaccinia virus Ankara vectored vaccines containing internal influenza proteins. Pathogens and global health 2017, 111 (2), 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price GE; Lo CY; Misplon JA; Epstein SL, Reduction of influenza virus transmission from mice immunized against conserved viral antigens is influenced by route of immunization and choice of vaccine antigen. Vaccine 2018, 36 (32 Pt B), 4910–4918. [DOI] [PubMed] [Google Scholar]
- 43.Zhao L; Seth A; Wibowo N; Zhao CX; Mitter N; Yu C; Middelberg AP, Nanoparticle vaccines. Vaccine 2014, 32 (3), 327–37. [DOI] [PubMed] [Google Scholar]
- 44.Torchilin VP, Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nature reviews. Drug discovery 2014, 13 (11), 813–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh SM; Alkie TN; Abdelaziz KT; Hodgins DC; Novy A; Nagy E; Sharif S, Characterization of Immune Responses to an Inactivated Avian Influenza Virus Vaccine Adjuvanted with Nanoparticles Containing CpG ODN. Viral immunology 2016, 29 (5), 269–75. [DOI] [PubMed] [Google Scholar]
- 46.Wang Q; Tan MT; Keegan BP; Barry MA; Heffernan MJ, Time course study of the antigen-specific immune response to a PLGA microparticle vaccine formulation. Biomaterials 2014, 35 (29), 8385–93. [DOI] [PubMed] [Google Scholar]
- 47.Deng L; Chang TZ; Wang Y; Li S; Wang S; Matsuyama S; Yu G; Compans RW; Li JD; Prausnitz MR; Champion JA; Wang BZ, Heterosubtypic influenza protection elicited by double-layered polypeptide nanoparticles in mice. Proceedings of the National Academy of Sciences of the United States of America 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shastri PN; Kim MC; Quan FS; D’Souza MJ; Kang SM, Immunogenicity and protection of oral influenza vaccines formulated into microparticles. Journal of pharmaceutical sciences 2012, 101 (10), 3623–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang C; Zhang J; Shi G; Song H; Shi S; Zhang X; Huang P; Wang Z; Wang W; Wang C; Kong D; Li C, A Light Responsive Nanoparticle-Based Delivery System Using Pheophorbide A Graft Polyethylenimine for Dendritic Cell-Based Cancer Immunotherapy. Molecular pharmaceutics 2017, 14 (5), 1760–1770. [DOI] [PubMed] [Google Scholar]
- 50.Dhakal S; Hiremath J; Bondra K; Lakshmanappa YS; Shyu DL; Ouyang K; Kang KI; Binjawadagi B; Goodman J; Tabynov K; Krakowka S; Narasimhan B; Lee CW; Renukaradhya GJ, Biodegradable nanoparticle delivery of inactivated swine influenza virus vaccine provides heterologous cell-mediated immune response in pigs. Journal of controlled release : official journal of the Controlled Release Society 2017, 247, 194–205. [DOI] [PubMed] [Google Scholar]
- 51.Rosalia RA; Cruz LJ; van Duikeren S; Tromp AT; Silva AL; Jiskoot W; de Gruijl T; Lowik C; Oostendorp J; van der Burg SH; Ossendorp F, CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials 2015, 40, 88–97. [DOI] [PubMed] [Google Scholar]
- 52.Monkare J; Pontier M; van Kampen EEM; Du G; Leone M; Romeijn S; Nejadnik MR; O’Mahony C; Slutter B; Jiskoot W; Bouwstra JA, Development of PLGA nanoparticle loaded dissolving microneedles and comparison with hollow microneedles in intradermal vaccine delivery. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V 2018, 129, 111–121. [DOI] [PubMed] [Google Scholar]
- 53.Danhier F; Ansorena E; Silva JM; Coco R; Le Breton A; Preat V, PLGA-based nanoparticles: an overview of biomedical applications. Journal of controlled release : official journal of the Controlled Release Society 2012, 161 (2), 505–22. [DOI] [PubMed] [Google Scholar]
- 54.Thomas C; Rawat A; Hope-Weeks L; Ahsan F, Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Molecular pharmaceutics 2011, 8 (2), 405–15. [DOI] [PubMed] [Google Scholar]
- 55.Rahimian S; Fransen MF; Kleinovink JW; Christensen JR; Amidi M; Hennink WE; Ossendorp F, Polymeric nanoparticles for co-delivery of synthetic long peptide antigen and poly IC as therapeutic cancer vaccine formulation. Journal of controlled release : official journal of the Controlled Release Society 2015, 203, 16–22. [DOI] [PubMed] [Google Scholar]
- 56.Minigo G; Scholzen A; Tang CK; Hanley JC; Kalkanidis M; Pietersz GA; Apostolopoulos V; Plebanski M, Poly-L-lysine-coated nanoparticles: a potent delivery system to enhance DNA vaccine efficacy. Vaccine 2007, 25 (7), 1316–27. [DOI] [PubMed] [Google Scholar]
- 57.Hiremath J; Kang KI; Xia M; Elaish M; Binjawadagi B; Ouyang K; Dhakal S; Arcos J; Torrelles JB; Jiang X; Lee CW; Renukaradhya GJ, Entrapment of H1N1 Influenza Virus Derived Conserved Peptides in PLGA Nanoparticles Enhances T Cell Response and Vaccine Efficacy in Pigs. PloS one 2016, 11 (4), e0151922. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Heinen PP; Rijsewijk FA; de Boer-Luijtze EA; Bianchi AT, Vaccination of pigs with a DNA construct expressing an influenza virus M2-nucleoprotein fusion protein exacerbates disease after challenge with influenza A virus. The Journal of general virology 2002, 83 (Pt 8), 1851–9. [DOI] [PubMed] [Google Scholar]
- 59.Watkins HC; Pagan CL; Childs HR; Posada S; Chau A; Rios J; Guarino C; DeLisa MP; Whittaker GR; Putnam D, A single dose and long lasting vaccine against pandemic influenza through the controlled release of a heterospecies tandem M2 sequence embedded within detoxified bacterial outer membrane vesicles. Vaccine 2017, 35 (40), 5373–5380. [DOI] [PubMed] [Google Scholar]
- 60.Arca HC; Gunbeyaz M; Senel S, Chitosan-based systems for the delivery of vaccine antigens. Expert review of vaccines 2009, 8 (7), 937–53. [DOI] [PubMed] [Google Scholar]
- 61.Dhakal S; Renu S; Ghimire S; Shaan Lakshmanappa Y; Hogshead BT; Feliciano-Ruiz N; Lu F; HogenEsch H; Krakowka S; Lee CW; Renukaradhya GJ, Mucosal Immunity and Protective Efficacy of Intranasal Inactivated Influenza Vaccine Is Improved by Chitosan Nanoparticle Delivery in Pigs. Frontiers in immunology 2018, 9, 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi GN; Zhang CN; Xu R; Niu JF; Song HJ; Zhang XY; Wang WW; Wang YM; Li C; Wei XQ; Kong DL, Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials 2017, 113, 191–202. [DOI] [PubMed] [Google Scholar]
- 63.Chowdhury MYE; Kim TH; Uddin MB; Kim JH; Hewawaduge CY; Ferdowshi Z; Sung MH; Kim CJ; Lee JS, Mucosal vaccination of conserved sM2, HA2 and cholera toxin subunit A1 (CTA1) fusion protein with poly gamma-glutamate/chitosan nanoparticles (PC NPs) induces protection against divergent influenza subtypes. Veterinary microbiology 2017, 201, 240–251. [DOI] [PubMed] [Google Scholar]
- 64.Yang J; Shim SM; Nguyen TQ; Kim EH; Kim K; Lim YT; Sung MH; Webby R; Poo H, Poly-gamma-glutamic acid/chitosan nanogel greatly enhances the efficacy and heterosubtypic cross-reactivity of H1N1 pandemic influenza vaccine. Scientific reports 2017, 7, 44839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang BZ; Gill HS; He C; Ou C; Wang L; Wang YC; Feng H; Zhang H; Prausnitz MR; Compans RW, Microneedle delivery of an M2e-TLR5 ligand fusion protein to skin confers broadly cross-protective influenza immunity. J Control Release 2014, 178, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang BZ; Quan FS; Kang SM; Bozja J; Skountzou I; Compans RW, Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses. J Virol 2008, 82 (23), 11813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paust S; Gill HS; Wang BZ; Flynn MP; Moseman EA; Senman B; Szczepanik M; Telenti A; Askenase PW; Compans RW; von Andrian UH, Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol 2010, 11 (12), 1127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L; Wang YC; Feng H; Ahmed T; Compans RW; Wang BZ, Virus-like particles containing the tetrameric ectodomain of influenza matrix protein 2 and flagellin induce heterosubtypic protection in mice. BioMed research international 2013, 2013, 686549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andre FE, Overview of a 5-year clinical experience with a yeast-derived hepatitis B vaccine. Vaccine 1990, 8 Suppl, S74–8; discussion S79–80. [DOI] [PubMed] [Google Scholar]
- 70.Si L; Xu H; Zhou X; Zhang Z; Tian Z; Wang Y; Wu Y; Zhang B; Niu Z; Zhang C; Fu G; Xiao S; Xia Q; Zhang L; Zhou D, Generation of influenza A viruses as live but replication-incompetent virus vaccines. Science 2016, 354 (6316), 1170–1173. [DOI] [PubMed] [Google Scholar]
- 71.Zeltins A, Construction and characterization of virus-like particles: a review. Molecular biotechnology 2013, 53 (1), 92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roldao A; Mellado MC; Castilho LR; Carrondo MJ; Alves PM, Virus-like particles in vaccine development. Expert review of vaccines 2010, 9 (10), 1149–76. [DOI] [PubMed] [Google Scholar]
- 73.D’Aoust MA; Couture MM; Charland N; Trepanier S; Landry N; Ors F; Vezina LP, The production of hemagglutinin-based virus-like particles in plants: a rapid, efficient and safe response to pandemic influenza. Plant biotechnology journal 2010, 8 (5), 607–19. [DOI] [PubMed] [Google Scholar]
- 74.Pillet S; Aubin E; Trepanier S; Bussiere D; Dargis M; Poulin JF; Yassine-Diab B; Ward BJ; Landry N, A plant-derived quadrivalent virus like particle influenza vaccine induces cross-reactive antibody and T cell response in healthy adults. Clinical immunology 2016, 168, 72–87. [DOI] [PubMed] [Google Scholar]
- 75.Klausberger M; Wilde M; Palmberger D; Hai R; Albrecht RA; Margine I; Hirsh A; Garcia-Sastre A; Grabherr R; Krammer F, One-shot vaccination with an insect cell-derived low-dose influenza A H7 virus-like particle preparation protects mice against H7N9 challenge. Vaccine 2014, 32 (3), 355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith GE; Flyer DC; Raghunandan R; Liu Y; Wei Z; Wu Y; Kpamegan E; Courbron D; Fries LF 3rd; Glenn GM, Development of influenza H7N9 virus like particle (VLP) vaccine: homologous A/Anhui/1/2013 (H7N9) protection and heterologous A/chicken/Jalisco/CPA1/2012 (H7N3) cross-protection in vaccinated mice challenged with H7N9 virus. Vaccine 2013, 31 (40), 4305–13. [DOI] [PubMed] [Google Scholar]
- 77.Milian E; Kamen AA, Current and emerging cell culture manufacturing technologies for influenza vaccines. BioMed research international 2015, 2015, 504831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beljanski V; Chiang C; Kirchenbaum GA; Olagnier D; Bloom CE; Wong T; Haddad EK; Trautmann L; Ross TM; Hiscott J, Enhanced Influenza Virus-Like Particle Vaccination with a Structurally Optimized RIG-I Agonist as Adjuvant. Journal of virology 2015, 89 (20), 10612–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chia MY; Hu AY; Tseng YF; Weng TC; Lai CC; Lin JY; Chen PL; Wang YF; Chao SR; Chang JY; Hwang YS; Yeh CT; Yu CP; Chen YC; Su IJ; Lee MS, Evaluation of MDCK cell-derived influenza H7N9 vaccine candidates in ferrets. PloS one 2015, 10 (3), e0120793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Neirynck S; Deroo T; Saelens X; Vanlandschoot P; Jou WM; Fiers W, A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med 1999, 5 (10), 1157–63. [DOI] [PubMed] [Google Scholar]
- 81.Deng L; Ibanez LI; Van den Bossche V; Roose K; Youssef SA; de Bruin A; Fiers W; Saelens X, Protection against Influenza A Virus Challenge with M2e-Displaying Filamentous Escherichia coli Phages. PloS one 2015, 10 (5), e0126650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hashemi H; Pouyanfard S; Bandehpour M; Noroozbabaei Z; Kazemi B; Saelens X; Mokhtari-Azad T, Immunization with M2e-displaying T7 bacteriophage nanoparticles protects against influenza A virus challenge. PloS one 2012, 7 (9), e45765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Steel J; Lowen AC; Wang TT; Yondola M; Gao Q; Haye K; Garcia-Sastre A; Palese P, Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio 2010, 1 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Song JM; Van Rooijen N; Bozja J; Compans RW; Kang SM, Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proceedings of the National Academy of Sciences of the United States of America 2011, 108 (2), 757–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim MC; Song JM; O E; Kwon YM; Lee YJ; Compans RW; Kang SM, Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol Ther 2013, 21 (2), 485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang BZ; Gill HS; Kang SM; Wang L; Wang YC; Vassilieva EV; Compans RW, Enhanced influenza virus-like particle vaccines containing the extracellular domain of matrix protein 2 and a Toll-like receptor ligand. Clin Vaccine Immunol 2012, 19 (8), 1119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang L; Hess A; Chang TZ; Wang YC; Champion JA; Compans RW; Wang BZ, Nanoclusters self-assembled from conformation-stabilized influenza M2e as broadly cross-protective influenza vaccines. Nanomedicine 2014, 10 (2), 473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bessa J; Schmitz N; Hinton HJ; Schwarz K; Jegerlehner A; Bachmann MF, Efficient induction of mucosal and systemic immune responses by virus-like particles administered intranasally: implications for vaccine design. European journal of immunology 2008, 38 (1), 114–26. [DOI] [PubMed] [Google Scholar]
- 89.Petukhova NV; Gasanova TV; Stepanova LA; Rusova OA; Potapchuk MV; Korotkov AV; Skurat EV; Tsybalova LM; Kiselev OI; Ivanov PA; Atabekov JG, Immunogenicity and protective efficacy of candidate universal influenza A nanovaccines produced in plants by Tobacco mosaic virus-based vectors. Current pharmaceutical design 2013, 19 (31), 5587–600. [DOI] [PubMed] [Google Scholar]
- 90.Leclerc D; Rivest M; Babin C; Lopez-Macias C; Savard P, A novel M2e based flu vaccine formulation for dogs. PloS one 2013, 8 (10), e77084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tyulkina LG; Skurat EV; Frolova OY; Komarova TV; Karger EM; Atabekov IG, New viral vector for superproduction of epitopes of vaccine proteins in plants. Acta naturae 2011, 3 (4), 73–82. [PMC free article] [PubMed] [Google Scholar]
- 92.Denis J; Acosta-Ramirez E; Zhao Y; Hamelin ME; Koukavica I; Baz M; Abed Y; Savard C; Pare C; Lopez Macias C; Boivin G; Leclerc D, Development of a universal influenza A vaccine based on the M2e peptide fused to the papaya mosaic virus (PapMV) vaccine platform. Vaccine 2008, 26 (27–28), 3395–403. [DOI] [PubMed] [Google Scholar]
- 93.Matic S; Rinaldi R; Masenga V; Noris E, Efficient production of chimeric human papillomavirus 16 L1 protein bearing the M2e influenza epitope in Nicotiana benthamiana plants. BMC biotechnology 2011, 11, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ameiss K; Ashraf S; Kong W; Pekosz A; Wu WH; Milich D; Billaud JN; Curtiss R 3rd, Delivery of woodchuck hepatitis virus-like particle presented influenza M2e by recombinant attenuated Salmonella displaying a delayed lysis phenotype. Vaccine 2010, 28 (41), 6704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee LA; Wang Q, Adaptations of nanoscale viruses and other protein cages for medical applications. Nanomedicine : nanotechnology, biology, and medicine 2006, 2 (3), 137–49. [DOI] [PubMed] [Google Scholar]
- 96.Jardine J; Julien JP; Menis S; Ota T; Kalyuzhniy O; McGuire A; Sok D; Huang PS; MacPherson S; Jones M; Nieusma T; Mathison J; Baker D; Ward AB; Burton DR; Stamatatos L; Nemazee D; Wilson IA; Schief WR, Rational HIV immunogen design to target specific germline B cell receptors. Science 2013, 340 (6133), 711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kanekiyo M; Wei CJ; Yassine HM; McTamney PM; Boyington JC; Whittle JR; Rao SS; Kong WP; Wang L; Nabel GJ, Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 2013, 499 (7456), 102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Georgiev IS; Joyce MG; Chen RE; Leung K; McKee K; Druz A; Van Galen JG; Kanekiyo M; Tsybovsky Y; Yang ES; Yang Y; Acharya P; Pancera M; Thomas PV; Wanninger T; Yassine HM; Baxa U; Doria-Rose NA; Cheng C; Graham BS; Mascola JR; Kwong PD, Two-Component Ferritin Nanoparticles for Multimerization of Diverse Trimeric Antigens. ACS infectious diseases 2018, 4 (5), 788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qi M; Zhang XE; Sun X; Zhang X; Yao Y; Liu S; Chen Z; Li W; Zhang Z; Chen J; Cui Z, Intranasal Nanovaccine Confers Homo- and Hetero-Subtypic Influenza Protection. Small 2018. [DOI] [PubMed] [Google Scholar]
- 100.Wang L; Chang TZ; He Y; Kim JR; Wang S; Mohan T; Berman Z; Tompkins SM; Tripp RA; Compans RW; Champion JA; Wang BZ, Coated protein nanoclusters from influenza H7N9 HA are highly immunogenic and induce robust protective immunity. Nanomedicine 2017, 13 (1), 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Deng L; Kim JR; Chang TZ; Zhang H; Mohan T; Champion JA; Wang BZ, Protein nanoparticle vaccine based on flagellin carrier fused to influenza conserved epitopes confers full protection against influenza A virus challenge. Virology 2017, 509, 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Storp B; Engel A; Boeker A; Ploeger M; Langer K, Albumin nanoparticles with predictable size by desolvation procedure. Journal of microencapsulation 2012, 29 (2), 138–46. [DOI] [PubMed] [Google Scholar]
- 103.Chang TZ; Diambou I; Kim JR; Wang B; Champion JA, Host- and pathogen-derived adjuvant coatings on protein nanoparticle vaccines. Bioeng Transl Med 2017, 2 (1), 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Konuk M; Durukanoglu S, Shape-controlled growth of metal nanoparticles: an atomistic view. Physical chemistry chemical physics : PCCP 2016, 18 (3), 1876–85. [DOI] [PubMed] [Google Scholar]
- 105.Kumar S; Anselmo AC; Banerjee A; Zakrewsky M; Mitragotri S, Shape and size-dependent immune response to antigen-carrying nanoparticles. Journal of controlled release : official journal of the Controlled Release Society 2015, 220 (Pt A), 141–148. [DOI] [PubMed] [Google Scholar]
- 106.Tao W; Ziemer KS; Gill HS, Gold nanoparticle-M2e conjugate coformulated with CpG induces protective immunity against influenza A virus. Nanomedicine (Lond) 2014, 9 (2), 237–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tao W; Gill HS, M2e-immobilized gold nanoparticles as influenza A vaccine: Role of soluble M2e and longevity of protection. Vaccine 2015, 33 (20), 2307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chao W; Wandi Z; W. B, Dual-linker gold nanoparticles as adjuvanting carriers for multivalent display of recombinant influenza hemagglutinin trimer and flagellin improve the immunological responses in vivo and in vitro. International journal of nanomedicine 2017, 12, 4747–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang C; Zhu W; Luo Y; Wang BZ, Gold nanoparticles conjugating recombinant influenza hemagglutinin trimer and flagellin enhanced mucosal cellular immunity. Nanomedicine : nanotechnology, biology, and medicine 2018, 14 (4), 1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dobrovolskaia MA; Aggarwal P; Hall JB; McNeil SE, Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Molecular pharmaceutics 2008, 5 (4), 487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Toy R; Roy K, Engineering nanoparticles to overcome barriers to immunotherapy. Bioengineering & translational medicine 2016, 1 (1), 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Benne N; van Duijn J; Kuiper J; Jiskoot W; Slutter B, Orchestrating immune responses: How size, shape and rigidity affect the immunogenicity of particulate vaccines. Journal of controlled release : official journal of the Controlled Release Society 2016, 234, 124–34. [DOI] [PubMed] [Google Scholar]
- 113.Hammond PT, Nano Tools Pave the Way to New Solutions in Infectious Disease. ACS infectious diseases 2017, 3 (8), 554–558. [DOI] [PubMed] [Google Scholar]
- 114.Demento SL; Cui W; Criscione JM; Stern E; Tulipan J; Kaech SM; Fahmy TM, Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials 2012, 33 (19), 4957–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Manolova V; Flace A; Bauer M; Schwarz K; Saudan P; Bachmann MF, Nanoparticles target distinct dendritic cell populations according to their size. European journal of immunology 2008, 38 (5), 1404–13. [DOI] [PubMed] [Google Scholar]
- 116.Xiang SD; Scholzen A; Minigo G; David C; Apostolopoulos V; Mottram PL; Plebanski M, Pathogen recognition and development of particulate vaccines: does size matter? Methods 2006, 40 (1), 1–9. [DOI] [PubMed] [Google Scholar]
- 117.Joshi VB; Geary SM; Salem AK, Biodegradable particles as vaccine antigen delivery systems for stimulating cellular immune responses. Human vaccines & immunotherapeutics 2013, 9 (12), 2584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.MartIn-Fontecha A; Sebastiani S; Hopken UE; Uguccioni M; Lipp M; Lanzavecchia A; Sallusto F, Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. The Journal of experimental medicine 2003, 198 (4), 615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xie X; Liao J; Shao X; Li Q; Lin Y, The Effect of shape on Cellular Uptake of Gold Nanoparticles in the forms of Stars, Rods, and Triangles. Scientific reports 2017, 7 (1), 3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gong T; Xie J; Liao J; Zhang T; Lin S; Lin Y, Nanomaterials and bone regeneration. Bone research 2015, 3, 15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Anselmo AC; Zhang M; Kumar S; Vogus DR; Menegatti S; Helgeson ME; Mitragotri S, Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS nano 2015, 9 (3), 3169–77. [DOI] [PubMed] [Google Scholar]
- 122.Akei H; Whitsett JA; Buroker M; Ninomiya T; Tatsumi H; Weaver TE; Ikegami M, Surface tension influences cell shape and phagocytosis in alveolar macrophages. American journal of physiology. Lung cellular and molecular physiology 2006, 291 (4), L572–9. [DOI] [PubMed] [Google Scholar]
- 123.Patel B; Gupta N; Ahsan F, Particle engineering to enhance or lessen particle uptake by alveolar macrophages and to influence the therapeutic outcome. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V 2015, 89, 163–74. [DOI] [PubMed] [Google Scholar]
- 124.Niikura K; Matsunaga T; Suzuki T; Kobayashi S; Yamaguchi H; Orba Y; Kawaguchi A; Hasegawa H; Kajino K; Ninomiya T; Ijiro K; Sawa H, Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS nano 2013, 7 (5), 3926–38. [DOI] [PubMed] [Google Scholar]
- 125.hang GD; Chen CJ; Lin CY; Chen HC; Chen H, Improvement of glycosylation in insect cells with mammalian glycosyltransferases. J Biotechnol 2003, 102 (1), 61–71. [DOI] [PubMed] [Google Scholar]