Abstract
Objective
Selective serotonin (5-HT) reuptake inhibitors (SSRIs) are amongst the most prescribed drugs worldwide not only for psychiatric conditions, but also for medical purposes. Converging data gathered throughout the decades following their development would indicate that SSRIs have a broader side effect profile than previously assumed. Therefore, the aim of the present paper was to to review available literature highlighting less common side effects emerging with their long-term use.
Method
This systematic review, carried out according to PRISMA guidelines, was performed through searching electronic databases of PubMed, Google Scholar, Cochrane Library, Embase, MEDLINE, PsycINFO and Scopus. The keyword used was “SSRIs” combined with the following: “Side effects”, or “Emotional blunting or flattening”, or “Cognition”, or “Neuroimaging”, or “Bone”, “or “Platelet aggregation”, or “Bleeding”.
Results
The most common side effects, besides the classical ones described in the literature are represented by decreased emotional response to both adversive and pleasurable events, some cognitive impairments, bone fractures and prolonged overall bleeding time.
Conclusions
After analyzing critically the available findings, it should be noted that only the so-called “emotional blunting” is supported by converging data, while results on cognitive impairment are extremely controversial, given some evidence showing that SSRIs may improve cognition. Similarly, no agreement exists on the detrimental effects of SSRIs on bone metabolism and coagulation.
Large, prospective and long-term studies are needed to clarify the possible impact of SSRIs on emotions, cognitive functions, bone fractures and coagulation, as well to detect other possible still neglected side effects.
Keywords: antidepressants, selective serotonin reuptake inhibitors, side effects, chronic use, emotional blunting, cognition, neuroimaging, bone metabolism, coagulation
Introduction
The serendipitous observations of the euphoric properties of iproniazide, a monoamine oxidase inhibitor (MAOI), and imipramine, a tricyclic (TCA) compound, permitted the introduction into the clinical practice of the first antidepressants (ADs) (Hillhouse and Porter 2015, Lopez-Munoz and Alamo 2009). At the same time, the availability of these drugs for treating depression promoted the investigation of its biological underpinnings and related hypotheses centered on neurotransmitters, in particular norepinephrine (NE) and serotonin (5-HT) (Feighner 1999, Lopez-Munoz and Alamo 2009). Several lines of evidence gathered throughout the next decades favored a crucial role of 5-HT and of the 5-HT transporter (SERT) (Kambeitz and Howes 2015, Marazziti et al. 2013). The overall findings promoted the development and availability of the so-called selective 5-HT re-uptake inhibitors (SSRIs), fluoxetine, fluvoxamine, paroxetine, sertraline, citalopram and escitalopram that currently are amongst the most prescribed drugs worldwide (Wong et al. 1995). Indeed, SSRIs are currently prescribed not only in major depressive disorder (MDD), but also in obsessive-compulsive disorder (OCD), panic disorder, post-traumatic stress disorder (PTSD), generalized anxiety disorder (GAD), and social anxiety disorder (SAD) (Stahl 2002). Moreover, SSRIs are used to treat pre– and post-menopausal syndromes (MS), hot flushes, chronic pain and chronic fatigue syndromes, and several other conditions (Pardini et al. 2011, Patetsos and Horjales-Araujo 2016, Shams et al. 2014, Walitt et al. 2015).
The main pharmacological activity of SSRIs is the selective inhibition of the pre-synaptic SERT that provokes an acute increase of 5-HT concentration in the synaptic cleft resulting in an activation of pre-synaptic 5-HT auto-receptors: this triggers a decrease of the firing rate of the serotonergic neurons through a negative feedback mechanism (Benfield et al. 1986, Dechant and Clissold 1991, Wong et al. 1995). The therapeutic effect of SSRIs generally occurs within two weeks of treatment while consisting in desensitization of pre-synaptic 5-HT auto-receptors, leading to an increased synthesis and release of 5-HT. In addition, it is now well known that, besides the common property of blocking the SERT, each SSRI shows different secondary pharmacological characteristics, such as NE or DA reuptake blockade, 5-HT2C agonist actions, muscarinic antagonist (paroxetine) actions, interaction with the sigma receptor (sertraline), inhibition of the nitric oxide synthetase enzyme (paroxetine), and inhibition of the cytochrome P450 enzymes 2D6 (fluoxetine), 1A2, and 3A4 (fluvoxamine). The secondary binding profiles may account for the differences in efficacy and tolerability in each individual patient (Stahl 2002).
In comparison with TCAs, that bind to muscarinic, histaminergic, dopaminergic and adrenergic receptors, SSRIs have a safer tolerability profile (Dechant Clissold 1991, Kilts 1994). However, due to their high specificity for 5-HT re-uptake, they may provoke some relevant side effects, such as gastro-enteric (GE) reactions (diarrhea, nausea or vomit), central nervous system (CNS) effects (headache, insomnia, extrapyramidal symptoms (EPS) like tremors) and sexual dysfunctions (Benfield et al. 1986, Dechant and Clissold 1991, Gram 1994, Harris and Benfield 1995, Kilts 1994, Murdoch and McTavish 1992).
At least four subtypes of 5-HT receptors (5-HT2A, 5-HT2C, 5-HT3 and 5-HT4) are involved in the development of these undesirable actions that seem related to the presence of 5-HT receptors not only in the brain, but also in several parts of the body, like spinal cord, gut and heart.
In particular, acute stimulation of 5-HT2A and 5-HT2C receptors in the projections from raphe to limbic cortex may cause anxiety and induction of panic attacks that can be observed with early SSRI administration. Stimulation of 5-HT2A in the basal ganglia may lead to akathisia, psychomotor retardation, or mild extrapyramidal symptoms and dystonic movements. In the brainstem the same activity on sleep centers may cause myoclonus, disruption of slow-wave sleep and nocturnal awakening. In the spinal cord or in meso-cortical reward system may produce sexual dysfunctions. Stimulation of 5-HT3 receptors in the hypothalamus or brainstem can lead to nausea or vomit, respectively. Serotonin 3 and 4 receptors in the gastrointestinal tract may increase bowel motility and cause diarrhea; the alteration of atrio-ventricular conduction may induce bradycardia or arrhythmias, due to alterations of atrial 5-HT receptors (Drake and Gordon 1994, Kilts 1994). Moreover, despite 5-HT may vasodilate normal coronary arteries, it has been reported to provoke vasoconstriction when endothelium is damaged, worsening the course of a possible coronaropathy (McFadden et al. 1991). Furthermore, some studies showed that SSRIs are associated with the syndrome of inappropriate secretion of antidiuretic hormone (SIADH): therefore, a careful monitoring of serum electrolytes is required since SIADH may lead to hyponatremia (Adverse drug reactions advisory committee 1996, Blacksten and Birt 1993).
Nowadays, emerging data suggest that long-term use of SSRIs may could cause some side effects previously neglected or under-recognized, such as emotional blunting, cognitive impairment, bone fractures or thrombotic/hemorrhagic risks. Therefore, the aim of this paper was to present a comprehensive literature review search of these SSRIs side effects.
Methods
The systematic review was carried out according to PRISMA guidelines (Moher et al. 2009) through searching electronic databases of PubMed, Google Scholar, Cochrane Library, Embase, MEDLINE, PsycINFO and Scopus for English language papers published between January 31st, 1990 and January 31st, 2018. The keyword used was “SSRIs” combined with the following: “Side effects”, or “Emotional blunting or flattening”, or “Cognition”, or “Neuroimaging”, or “Bone”, “or “Platelet aggregation”, or “Bleeding”. All the authors agreed to include in the review conference abstracts, posters and case reports if published in indexed journals. Abstracts and/or titles were carefully evaluated, before considering the inclusion of the complete publications. The following inclusion criteria were adopted: studies carried out in clinical sample of children/adolescents and/or adults; reliable diagnosis of psychiatric disorders, according to structured interviews and standardized criteria; adoption of reliable laboratory tests or brain imaging techniques when applied. All the authors equally contributed in identifying potential information specific to this topic amongst the titles and abstracts of the publications.
Results
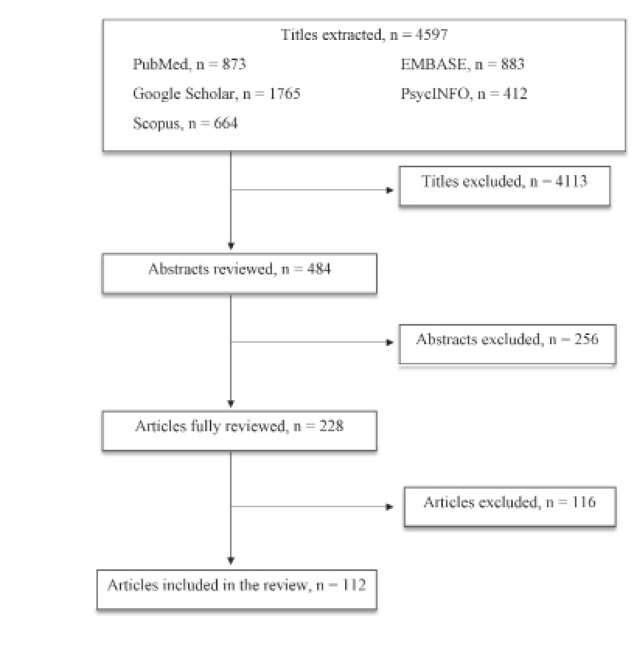
The first selection excluded 4113 titles because: a) duplicates; b) not concerning the scope of the paper; c) not informative enough. The second selection excluded 256 abstracts after being read and reviewed, as the information reported did not fulfill the scope of our paper and/or the presented information did not seem relevant to the discussed topic. Subsequently, 116 articles were excluded after being completely read and evaluated, as they did not provide enough information and/or resulted sufficiently in line with our review. Finally, 112 articles were reviewed and included in the present paper (figure 1).
Figure 1.
Article selection flow chart
Emotional Blunting
Emotional blunting, also called “reduced affect”, is a condition of diminished emotional reactivity. It is a failure to express feelings either verbally or non-verbally, especially when talking about issues that would normally be expected to engage emotions. Expressive gestures are rare, facial expression reactivity reduced and vocal inflection decreased (Opbroek et al. 2002, Reinblatt and Riddle 2006, Sansone and Sansone 2010). The “blunting of emotions” is frequently mentioned by patients taking SSRIs for long periods who report that, although they feel less emotional pain than before, they also experience a restricted range of other emotions that are a normal part of everyday life (Barnhart et al. 2004, Price et al. 2009).
Reduced affect should be distinguished from apathy, which explicitly refers to a lack of emotion, whereas reduced affect is a lack of emotional expression regardless of whether emotion is decreased or not (Marin 1990). The patients refer that they feel flattened or evened out and their emotional responses to all events seem to be reduced. They experience their emotions as thoughts rather than as feelings, as if their emotional experience had become more ‘cognitive’ or ‘intellectual’. However, they can still respond to emotional situations in an appropriate way, but without what they felt was real feeling (Liddle 2007, Price et al. 2009). The dysregulation of emotional sensitivity leads to a reduction of the quantity and quality of daily emotions with a lower capacity to experience emotions in their intensity (emotional detachment) and diminished emotionality in interpersonal relationships. Again, they feel reduced sympathy and empathy during social interactions from their friends and family, including their partner or children. Feelings were described as disconnected or detached from their own emotions and instincts with reduced or absent emotional responses.
Furthermore, some people report a reduced intensity and frequency of positive emotions, including happiness, enjoyment, excitement, anticipation, passion, love, affection and enthusiasm. Others experience lower intensity or frequency of negative emotions interpreting it as beneficial, bringing relief from distressing negative emotions and allowing normal daily life to resume. Further, some authors described reduction of sadness, emotional pain or distress, anger, irritability or aggression, anxiety, worry or fear was also reported. Although a reduction in these negative emotions was at some stage a benefit or relief, for many individuals it became an unwanted side effect, impairing their quality of life. They need to be able to feel negative emotions when appropriate, such as grief or concern (Goodwin et al. 2017, Liddle 2007, Sternat and Katzman 2016).
The investigation of the emotional response and side effects of SSRIs treatment in patients with psychiatric disorders such as depression and anxiety can be difficult because they could be residual symptoms of depression, or they could be part of the patient’s personality. In addition, moderate degrees of emotional blunting might be difficult for individuals to detect or report subjectively (retrospective distortion). Finally, they could also be the resulting of concomitant treatments with other ADs (Hoehn-Saric et al. 1990, 1991, McCabe et al. 2010, Oleshansky and Labbate 1996, Opbroek et al. 2002, Panzer and Mellow 1992).
A syndrome characterized by apathy and indifference was first diagnosed in OCD patients treated with fluoxetine or fluvoxamine. These symptoms appeared dose-related and were clearly differentiated from a sense of sedation. Additionally, the patients in that study clearly identified the symptoms of emotional blunting as being abnormal for them (Hoehn-Saric et al. 1991). Moreover, a reduction in pathological crying was also reported in patients suffering for cerebrovascular accidents out treated with SSRIs. The clinical presentation was associated with abnormal neuropsychological testing and decreased frontal lobe blood flow on SPECT, both consistent with frontal lobe impairment (Panzer and Mellow 1992).
One study carried on 192 outpatients (123 women and 69 men) with depression and treated with SSRIs and TCAs for 6 months involved in a romantic relationship, showed that only men taking SSRIs presented a reduction of love feelings. Women referred only sexual dysfunction (like anorgasmia) with TCAs (Marazziti et al. 2014). Fifteen depressed patients treated with SSRIs (fluoxetine, paroxetine and sertraline) who had developed sexual dysfunctions, showed a high percentage of emotional blunting (80%), including a decrease of creativity, sexual pleasure, interest in sex, ability to cry, expression of their own feelings. This study would indicate that sexual dysfunctions could even be a predictor of reduction of emotional reactivity in patients treated with SSRIs, and it would confirm that emotional indifference is a possible side effect of SSRIs that reduces the compliance and quality of life of patients (Opbroek et al. 2002). Another study comparing two groups of elderly depressed patients, one taking SSRIs and the other ADs, found that SSRIs patients had a greater increase of apathy symptoms (Wongpakaran et al. 2007). Additionally, it could be that some patients experience emotional blunting as part of the therapeutic effect, diminishing emotional responses to adversive life situations or stress. This may explain why in some non-depressed patients treated with SSRIs can expect improvement of symptoms like anxiety, irritability and impulse control (Elfenbein 1995).
The mechanism that may lead to the development of this syndrome is not clarified as yet. Some authors suggested that SSRIs might reduce the function of specific brain areas involved in emotional processing, such as the anterior cingulate and the amygdala (Bromfield et al. 1992, Kennedy et al. 2001, Kennedy et al. 1997, Mayberg 1994, Mayberg et al. 1991, 1992, Ring et al. 1994). Interestingly, regional brain metabolism, measured by positron emission tomography, appears to be lower in the anterior cingulate of depressed subjects when compared with non-depressed subjects, and SSRIs have been shown to further decrease activity in the anterior cingulate, rather than restore normal function (Ebert and Ebmeier 1996, Harmer et al. 2004, 2017, Kennedy et al. 1997, 2001) In another study a sample of 45 human healthy participants were randomized to two ADs, specifically citalopram and reboxetine, for one week. The functional magnetic resonance imaging (fMRI), used to measure the neural response to rewarding and aversive stimuli, showed that the serotonergic treatment causes a reduction of neural processing of both rewarding and aversive stimuli. This study supports the hypothesis that SSRIs may induce a reduction of emotional reactivity resulting in emotional indifference, not only while facing negative experiences (like ability to cry and sadness), but also with decreased positive affects (e.g., feelings of happiness and sexual pleasure) (Goodwin et al. 2017).
Taken together, these observations underline the need of large-scale clinical studies on the prevalence of SSRI-induced emotional disturbances, a problem that, given their high prescription rates, should not be neglected, but rather taken into careful consideration.
Cognitive Impairment
Cognitive symptoms represent other side effects of long-term AD treatment that may decrease compliance and quality of life (Baldwin 2006, Joss et al. 2003, Masand and Gupta 1999, Papakostas 2014, Schmitt et al. 2001), as well as they may lead to increased number of relapses and to a reduced percentage of remissions (Alexopoulos et al. 2000, Papakostas 2014).
Cognitive side effects are mainly represented by impairment of memory, concentration, attention, motivation and affective response to external stimuli (Bolling and Kohlenberg 2004, Fava et al. 2006, Hu et al. 2004), but also by loss of creativity, memory or ambition, attention deficit and problem-solving difficulties (Bolling and Kohlenberg 2004).
Several studies reported that young depressed patients may show deficits in some components of executive functioning, such as attention, short-term and working memory and in psychomotor skills, while data on verbal memory and learning functions are controversial (Castaneda et al. 2008, Mormont and 1984, Rubinow et al. 1984, Savard et al. 1980). On the contrary, in elderly patients suffering from MDD, memory difficulties may be the chief complaint and may be mistaken for early signs of a dementia (“pseudodementia”). In addition, other disturbances of executive functions have been described in late-onset depression, in particular impairment in planning, sequencing, organizing and abstracting, sometimes associated with relapse and recurrence with residual symptoms (Herrmann et al. 2007, Pisljar et al. 2008). The cognitive impairment of older depressed patients with late-onset as opposed to early-onset illness may show important differences, in that patients with early-onset may suffer predominantly from impaired episodic memory, and those with late-onset mainly from reductions of executive functions and processing speed (Herrmann et al. 2007). According to some authors, there would be a gender-related specificity, since depressed women appear to perform significantly worse on the tests of cognitive threshold and in those of visual recall, as compared with depressed men (Sarosi et al. 2008).
The findings on the association between SSRIs and memory deficits are, however, still contradictory, as some beneficial effects on this function have been also reported (Gallassi et al. 2006, Galletta et al. 2010, Harmer et al. 2004, 2017, Herrera-Guzman et al. 2010, Levkovitz et al. 2002, Zobel et al. 2004a,b).
Others investigated the impact of SSRIs on cognitive performance in working population in everyday life using computer tasks measuring mood and cognitive functions at the start and end of a working week. SSRIs were associated with mild and episodic memory impairment. Change in cognitive processing associated with SSRIs occurred earlier than the therapeutic effect (Kent et al. 1998, Wadsworth et al. 2005). This suggested that changes in psychological functions preceded the symptom improvement (Harmer et al. 2004), even if many of these symptoms may be limited to cases of unresolved depression or anxiety. It was also suggested that cognitive changes associated with SSRIs are not due to their action on serotoninergic transmission, but rather to their acetylcholine (Ach) and DA effects (Schmitt et al. 2001).
An international study analyzed MDD patients who were responders to ADs following at least 3 months of treatment and whose illness was in partial or full remission. More than 30% of the responders reported cognitive symptoms, such as apathy, inattentiveness, forgetfulness, word-finding difficulty and mental slowing, while 40% of them reported physical symptoms of fatigue and sleepiness/sedation. However, this study did not permit to establish whether the presence of these symptoms could be attributed to an incomplete resolution of the depression itself, to the ADs treatment, or to a combination of both (Fava et al. 2006), given the phenomenological overlap between residual symptoms and side effects (Kelly et al. 2008). The regular use of citalopram, sertraline and paroxetine in MDD elderly patients and followed up for 12-months was not associated with any cognitive impairment. This might be attributed to a specific sensitivity of psychiatrists dealing with elderly patient and aware of different properties of single compounds (Han et al. 2011).
Again, neuropsychological tests’ performances were compared in amongst MDD medicated remitted patients, medication-free remitted patients and control subjects. The results showed that cognitive impairment persisted in remitted MDD patients and was related to the class of AD, as it was worse with TCAs than with SSRIs/SNRIs (Nagane et al. 2014).
More recently, cognition was explored in MDD or anxious patients successfully treated with SSRIs in monotherapy for at least six months. Between 15 % and 25 % of patients of both groups presented lack of concentration, memory impairment, such as inattentiveness, forgetfulness, mental slowing, apathy and word-finding difficulty, symptoms that were reported as moderate and severe in 10% of the cases. As cognitive symptoms were very similar and present in both depressive and anxiety disorders, it was concluded that they may be real side effects of SSRIs. In addition, cognitive impairment was even more relevant in patients with partial response and treated with drug combinations. This finding would hence suggest that cognitive symptoms should not be considered residual depressive symptoms (Popovic et al. 2015).
A recent meta-analysis reviewed the current literature on possible efficacy of ADs in different domains of cognition. The overall results showed that all ADs produce a positive and significant effect on psychomotor speed and delayed recall, while cognitive control and executive function were not changed (Rosenblat et al. 2016).
In conclusion, cognitive effects following to SSRIs remain controversial, as different studies adopted heterogeneous methods and included different patients, so that they are not easily comparable. Therefore, placebo-controlled studies are needed to disentangle the crucial question of positive or negative cognitive effects of ADs.
Bone Fractures
Serotonin seems to possess different effects on bone mass (Lavoie et al. 2017), and ADs with serotonergic effects have been related to potentially detrimental effects on bone mineral density that may increase fracture risk. There is a growing evidence of a possible link between the administration of ADs and this adverse event, despite many in vitro and in vivo studies led to controversial results, probably for the variety of confounding factors (Rizzoli et al. 2012, Schwan and Hallberg 2009). The bone cells (osteoblasts, osteocytes and osteoclasts) possess 5-HT receptors and it is known that the bone might be able to produce 5-HT in an autocrine/paracrine mode, since osteoblasts, osteocytes and osteoclasts have been found to express mRNA for tryptophan-hydroxylase 1 (TPH1), which is the limiting enzyme of 5-HT production (Lavoie et al. 2017). Serotonin appears to have opposite effects on bone mass depending on its central or peripheral origin, as shown in some studies carried out on knockout mice. Serotonin receptor of central origin seems to enhance both bone formation and bone resorption through the inhibition of sympathetic output, that is a negative regulator of bone mass accrual of the aforementioned mechanisms. Peripheral 5-HT, on the other hand, inhibits osteoblast proliferation, resulting in a direct reduction of bone formation (Bliziotes 2010, Bliziotes et al. 2002, Brand and Anderson 2011, Cui et al. 2011, Goltzman 2011, Takeda et al. 2002, Yadav et al. 2008, 2009, 2010). It would be expected that decreasing levels of 5-HT would globally reduce bone mass and increasing 5-HT levels should result in bone mass accrual. Therefore, serotonergic ADs, that increase 5-HT signaling, should induce bone mass accrual, but the results of experimental models do not fit with this prediction (Rizzoli et al. 2012, Schwan and Hallberg 2009).
Contradictory results on the possible associations between bone mineral density and SSRIs use emerged from studies on humans. No association between reduction of bone mass density and SSRIs use was found in three different studies (Cauley et al. 2005, Kinjo et al. 2005, Spangler et al. 2008). A group of women using SSRIs showed significant reduction of bone mass density at the femoral neck and trochanter (Williams et al. 2008). Similarly, a sample of elderly women treated with SSRIs led to higher rate of bone loss compared to a similar group who did not take SSRIs (Diem et al. 2007).
A sample of 1972 women (aged >42 yrs.), previously enrolled in the Study of Women’s Health Across the Nation, was recruited, in order to assess the annual bone mineral density changes among new users of selective SSRIs, new users of TCAs and non-users of ADs. No significant association was found between the use of ADs and increased bone loss. Potential confounders, such as age, race, body mass index, menopausal status and hormone therapy use were properly adjusted (Diem et al. 2013). In a recent research investigating the possible role of SSRIs and some second-generation antipsychotics on bone mass in a group of patients aged 7-17, a positive association was observed between the use of SSRIs and the reduction of bone mass density (BMD) (Calarge et al. 2015). A statistically significant increase in risk of hip fractures in serotonergic ADs users was also reported, with no relation with daily dose (Liu et al. 1998).
An analysis of the UK General Practice Research Database showed an increased risk of hip fractures in SSRI or TCA users, while highlighting a prominent increase in risk during the first six weeks of treatment, as well as a significant positive association with SSRI doses (Hubbard et al. 2003). Furthermore, it was reported a dose-dependent relationship between the risk of fracture in SSRI users in parallel with the duration of treatment (n 2.5 years) (Vestergaard et al. 2008). In a prospective randomly selected population-based community cohort, participants were asked to report current daily drug use, including SSRIs and SNRIs, at baseline, at year 5, and year 10 (Kreiger 1999). The analysis showed that higher doses of SSRIs/SNRIs at baseline were significantly related to a higher risk of fracture even after adjustment for potential confounding variables, suggesting an independent effect of SSRI/ SNRI on risk of fracture, consistent with previous studies (Rabenda et al. 2013, Richards et al. 2007, Vestergaard et al. 2013). No significant differences were found between the use of SSRIs or SNRIs, however, combined use of SSRIs and SNRIs was associated with an increased risk of fractures of about 16 (Wang et al. 2016).
SSRIs were also reported to be significantly associated with higher risk of fractures in women who recently received SSRIs for the treatment of vasomotor menopausal syndrome (VMS), especially after several months of treatment (Sheu et al. 2015). Possibly, a decrease in bone density could be directly related to depression and its symptoms. Indeed, hypothalamus-pituitary-adrenal (HPA) axis activation, sympathetic nervous system hyperactivity, and subclinical inflammation are some of the pathophysiological alterations arising within an MD that may promote bone resorption (Calarge et al. 2015, Catena-Dell’Osso et al. 2013, Marazziti et al. 2014, Rosenblat et al. 2016). A significant lower total body less head (TBLH) during MDD has been confirmed in a study in which was measured bone mass using dual-energy x-ray absorptiometry (DXA) in a sample of young people aged 15-20, most of them naïve for any psychotropic-drug treatment. The lower bone mass appeared to be due to smaller cortical thickness, perhaps secondary to a larger endosteal circumference (Calarge et al. 2015).
Evidence supporting the effect of sedentary lifestyle on bone metabolism has been also reported (Ho and Kung 2005, Korpelainen et al. 2006). Physical inactivity is common in depressed patients, although studies focusing on depressive patients and BMD are rare (Harvey et al. 2018, Malik et al. 2013, Petronijevic et al. 2008). Although the majority of the literature seems in line to conclude that long-term treatment with SRIs could be related to decreased BMD, it is still difficult to define if the effect of SSRIs is actually detrimental for BMD or a confounder factor within the context of a complex syndrome, involving different neuro-endocrinal systems. Depression is associated with hypothalamic dysfunction and hypercortisolism, diminished secretion of growth hormone, hypothalamic hypogonadism and anorexia that are already risk factors for bone loss (Gold et al. 1988a,b, 2015). Depression is related also to a dysregulation of pro– and anti-inflammatory cytokines, such as increased levels of IL-1β, interleukin-2 (IL-2), IL-6, tumor necrosis factor-α (TNF-α), the soluble IL-2 and IL-6 receptors (Dantzer et al. 1999, Frommberger et al. 1997, Kiecolt-Glaser and Glaser 2002, Lanquillon et al. 2000, Maes 1999, Maes et al. 1997, Marazziti et al. 2014, van West and Maes 1999). IL-6 and TNF-α seem to play a role in activating osteoclasts and, therefore, in promoting bone resorption (Cizza et al. 2001, Dentino et al. 1999, Hofbauer et al. 2000). Moreover, an increased IL-6 secretion may be triggered by sympathetic activity, which is often increased in depression (Wong et al. 2000).
To summarize, the limited and controversial information on possible induction of bone fractures by SSRIs do not permit to draw definitive conclusions on this question that remains open and requiring specific and controlled investigations to be answered.
Coagulation/Bleeding
A series of studies showed that treatment with some ADs, particularly SRIs, may alter platelet aggregation and bleeding time by modifying the intracellular levels of 5-HT in platelets. The increased risk of bleeding under treatment with SRIs is possible due to the decreased intraplatelet 5-HT concentration (Hergovich et al. 2000, McCloskey et al. 2008, Ross et al. 1980). Serotonin is involved in platelet activation and vasoconstriction, which could be attributable to receptor-independent mechanisms (Walther et al. 2003). The platelets possess the 5-HT2A and 5-HT3 (Hoyer et al. 2002, Stratz et al. 2008) receptors and the SERT (Carneiro and Blakely 2006) in their membranes. Once activated they release 5-HT from the dense granules increasing the activation of the platelets themselves and potentiating their overall pro-coagulant activity. Despite these observations, the implications of the serotonergic mechanisms are not considered relevant for hemostasis, as no hemorrhagic disorder has been associated with alterations of 5-HT or its receptors: some studies, based on standard aggregometry, show that 5-HT is a weak platelet agonist, with the ability to enhance the aggregating response by interacting with other procoagulant drugs (Gomez-Gil et al. 2002, Jin and Kunapuli 1998).
Several investigations (aggregation studies, flow cytometry studies, thrombin generation assay, thromboelastometry studies, perfusion studies, generation of prothrombin fragment F 1+2) showed that 5-HT is not a weak agonist, instead it increases platelet activation, enhances procoagulant responses and increases thrombogenesis on damaged vascular surfaces (Galan et al. 2009).
Different studies have been carried out to explore the possibility of SRIs to prolong overall bleeding time, to alter platelet adhesion or to modify the general coagulation profile. One of them conducted on eight patients (seven of them treated with fluoxetine and one treated with paroxetine), for a period of four weeks, showed that there were no significant changes in the platelet aggregation profile, international normalized ratio (INR), activated partial thromboplastin time (aPT) or platelet count (Alderman et al. 1996). In 1998, a study conducted on blood of five healthy subjects taking fluoxetine did not show any prothrombin time (PT) alterations (Bondurant et al. 1998). Another study in a slightly larger sample of 19 patients taking paroxetine showed a decrease of platelet 5-HT and ß-thromboglobulin (ß-TG) without any modification of the overall bleeding time (Abdelmalik et al. 2008).
Controversial results are present in the international literature. In 2011, 20 patients treated with fluoxetine and 20 treated with escitalopram for three months were investigated while highlighting an increase of bleeding time in those taking fluoxetine (Siddiqui et al. 2011). Further, an in-vitro study explored the effect of citalopram, sertraline, reboxetine and venlafaxine on coagulation and showed how the first three decreased platelet adhesion capacity (Hallback et al. 2012). A case report underlined how paroxetine for two weeks reduce platelet plug formation by decreasing intraplatelet 5-HT (Hergovich et al. 2000).
More agreement exists on the interaction of SRIs with drugs acting on coagulation, in particular warfarin and acenocoumarol, as SRIs would enhance the anticoagulant action of the drugs. In particular, it has been shown that citalopram and fluvoxamine (Borras-Blasco et al. 2002, Hallback et al. 2012, Teichert et al. 2011) increased significantly INR, while raising the risk of bleeding. Likewise, patients taking SRIs show an increased risk of any bleeding event, by increasing the anticoagulant effect of warfarin (Cochran et al. 2011, Limke et al. 2002, Woolfrey et al. 1993). A particular case emphasizes the action of fluvoxamine on the increase of the INR that may last for several days after the discontinuation of the drug itself (Limke et al. 2002). On the contrary, a study in 2007, through a study conducted on 2441 patients with previous intracerebral hemorrhage (ICH) and on 894 patients with previous subarachnoid hemorrhage (SAH), in treatment with aspirin, lasting two weeks, shows how the SRIs did not lead to an increased risk of hemorrhagic stroke (Kharofa et al. 2007).
Conclusions
Selective serotonin reuptake inhibitors (SSRIs) have been developed as new-generation drugs for the treatment of depression, as compounds characterized by a better tolerability compared to more traditional drugs (TCAs and MAOIs). For this reason, in the last years they have become amongst the most prescribed drugs in the general population. Nowadays, after more than three decades of their intensive and long-term prescription, there is a growing evidence that, besides the “classical” and well described side effects, such as gastrointestinal distress, sexual problems, headache, weight gain, just to mention the main ones, SSRIs can provoke other that can be labeled “emergent”. These include emotional blunting, cognitive impairment, bone fractures and interference with coagulation process. The literature in this field is accumulating and, although some data can be considered anecdotal, as based on case study reports or small size samples, in the case of emotional blunting, supporting evidence suggests that it is a quite common phenomenon that may contribute to worsen patients’ clinical picture, and impair their quality of life and compliance to treatments. On the contrary the findings related to cognitive impairment are still controversial, as are those related to bone fractures and coagulation.
In any case, it seems that only large, prospective, and long-term studies might permit to answer the questions whether or not they are really due to SSRIs or just innocent casualties.
References
- Abdelmalik N, Ruhe HG, Barwari K, van den Dool EJ, Meijers JC, Middeldorp S, Buller HR, Schene AH, Kamphu-isen PW (2008). Effect of the selective serotonin reuptake inhibitor paroxetine on platelet function is modified by a slc6a4 serotonin transporter polymorphism. Journal of Thrombosis and Haemostasis 6, 12, 2168-2174. [DOI] [PubMed] [Google Scholar]
- Adverse drug reactions advisory committee. Selective serotonin reuptake inhibitors and siadh (1996). The Medical Journal of Australia 164, 9, 562. [PubMed] [Google Scholar]
- Alderman CP, Seshadri P, Ben-Tovim DI (1996). Effects of serotonin reuptake inhibitors on hemostasis. Annals of Pharmacotherapy 30, 11, 1232-1234. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Kalayam B, Kakuma T, Gabrielle M, Sirey JA, Hull J (2000). Executive dysfunction and long-term outcomes of geriatric depression. Archives Of General Psychiatry 57, 3, 285-290. [DOI] [PubMed] [Google Scholar]
- Baldwin DS (2006). The importance of long-term tolerability in achieving recovery. International Journal of Psychiatry in Clinical Practice 10 Suppl 1, 31-37. [DOI] [PubMed] [Google Scholar]
- Barnhart WJ, Makela EH, Latocha MJ (2004). Ssri-induced apathy syndrome: A clinical review. Journal of Psychiatric Practice 10, 3, 196-199. [DOI] [PubMed] [Google Scholar]
- Benfield P, Heel RC, Lewis SP (1986). Fluoxetine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depressive illness. Drugs 32, 6, 481-508. [DOI] [PubMed] [Google Scholar]
- Blacksten JV, Birt JA (1993). Syndrome of inappropriate secretion of antidiuretic hormone secondary to fluoxetine. Annals of Pharmacotherapy 27, 6, 723-724. [DOI] [PubMed] [Google Scholar]
- Bliziotes M (2010). Update in serotonin and bone. The Journal of Clinical Endocrinology & Metabolism 95, 9, 4124-4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliziotes M, Gunness M, Eshleman A, Wiren K (2002). The role of dopamine and serotonin in regulating bone mass and strength: Studies on dopamine and serotonin transporter null mice. Journal of musculoskeletal & neuronal interactions 2, 3, 291-295. [PubMed] [Google Scholar]
- Bolling MY, Kohlenberg RJ (2004). Reasons for quitting serotonin reuptake inhibitor therapy: Paradoxical psychological side effects and patient satisfaction. Psychotherapy and Psychosomatics 73, 6, 380-385. [DOI] [PubMed] [Google Scholar]
- Bondurant T, Darrell MJ, el Asyouty S, Hartman WR, Jones B, Steiner PM, Vincent KM, Li XP, Huff MO, el-Mallakh RS (1998). Effect of fluoxetine on prothrombin time. Psychosomatics 39, 3, 296-298. [DOI] [PubMed] [Google Scholar]
- Borras-Blasco J, Marco-Garbayo JL, Bosca-Sanleon B, Navarro-Ruiz A (2002). Probable interaction between citalopram and acenocoumarol. Annals of Pharmacotherapy 36, 2, 345. [DOI] [PubMed] [Google Scholar]
- Brand T, Anderson GM (2011). The measurement of platelet-poor plasma serotonin: A systematic review of prior reports and recommendations for improved analysis. Clinical Chemistry 57, 10, 1376-1386. [DOI] [PubMed] [Google Scholar]
- Bromfield EB, Altshuler L, Leiderman DB, Balish M, Ketter TA, Devinsky O, Post RM, Theodore WH (1992). Cerebral metabolism and depression in patients with complex partial seizures. Archives of neurology 49, 6, 617-623. [DOI] [PubMed] [Google Scholar]
- Calarge CA, Burns TL, Schlechte JA, Zemel BS (2015). Longitudinal examination of the skeletal effects of selective serotonin reuptake inhibitors and risperidone in boys. Journal of Clinical Psychiatry 76, 5, 607-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro AM, Blakely RD (2006). Serotonin-, protein kinase C-, and Hic-5-associated redistribution of the platelet serotonin transporter. The Journal of Biological Chemistry 281, 34, 24769-24780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lonnqvist J (2008). A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. Journal of Affective Disorders 106, 1-2, 1-27. [DOI] [PubMed] [Google Scholar]
- Catena-Dell’Osso M, Rotella F, Dell’Osso A, Fagiolini A, Marazziti D (2013). Inflammation, serotonin and major depression. Current Drug Targets 14, 5, 571-577. [DOI] [PubMed] [Google Scholar]
- Cauley JA, Fullman RL, Stone KL, Zmuda JM, Bauer DC, Barrett-Connor E, Ensrud K, Lau EM, Orwoll ES, Mr OSRG (2005). Factors associated with the lumbar spine and proximal femur bone mineral density in older men. Osteoporosis International 16, 12, 1525-1537. [DOI] [PubMed] [Google Scholar]
- Cizza G, Ravn P, Chrousos GP, Gold PW (2001). Depression: A major, unrecognized risk factor for osteoporosis? Trends in Endocrinology and Metabolism 12, 5, 198-203. [DOI] [PubMed] [Google Scholar]
- Cochran KA, Cavallari LH, Shapiro NL, Bishop JR (2011). Bleeding incidence with concomitant use of antidepressants and warfarin. Therapeutic Drug Monitoring 33, 4, 433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Niziolek PJ, MacDonald BT, Zylstra CR, Alenina N, Robinson DR, Zhong Z, Matthes S, Jacobsen CM, Conlon RA, Brommage R, Liu Q, Mseeh F, Powell DR, Yang QM, Zambrowicz B, Gerrits H, Gossen JA, He X, Bader M, Williams BO, Warman ML, Robling AG (2011). Lrp5 functions in bone to regulate bone mass. Nature Medicine 17, 6, 684-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Wollman E, Vitkovic L, Yirmiya R (1999). Cytokines and depression: Fortuitous or causative association? Molecular Psychiatry 4, 4, 328-332. [DOI] [PubMed] [Google Scholar]
- Dechant KL, Clissold SP (1991). Paroxetine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in depressive illness. Drugs 41, 2, 225-253. [DOI] [PubMed] [Google Scholar]
- Dentino AN, Pieper CF, Rao MK, Currie MS, Harris T, Blazer DG, Cohen HJ (1999). Association of interleukin-6 and other biologic variables with depression in older people living in the community. Journal of the American Geriatrics Society 47, 1, 6-11. [DOI] [PubMed] [Google Scholar]
- Diem SJ, Blackwell TL, Stone KL, Yaffe K, Haney EM, Bliziotes MM, Ensrud KE (2007). Use of antidepressants and rates of hip bone loss in older women: The study of osteoporotic fractures. Archives of Internal Medicine 167, 12, 1240-1245. [DOI] [PubMed] [Google Scholar]
- Diem SJ, Ruppert K, Cauley JA, Lian Y, Bromberger JT, Finkelstein JS, Greendale GA, Solomon DH (2013). Rates of bone loss among women initiating antidepressant medication use in midlife. The Journal of Clinical Endocrinology & Metabolism 98, 11, 4355-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake WM, Gordon GD (1994). Heart block in a patient on propranolol and fluoxetine. Lancet 343, 8894, 425-426. [DOI] [PubMed] [Google Scholar]
- Ebert D, Ebmeier KP (1996). The role of the cingulate gyrus in depression: From functional anatomy to neurochemistry. Biological Psychiatry 39, 12, 1044-1050. [DOI] [PubMed] [Google Scholar]
- Elfenbein D. (1995). Living with prozac and other serotonin reuptake inhibitors (ssris): Personal accounts of life on antidepressants. In: San Francisco: Harper 1995. [Google Scholar]
- Fava M, Graves LM, Benazzi F, Scalia MJ, Iosifescu DV, Alpert JE, Papakostas GI (2006). A cross-sectional study of the prevalence of cognitive and physical symptoms during long-term antidepressant treatment. Journal of Clinical Psychiatry 67, 11, 1754-1759. [DOI] [PubMed] [Google Scholar]
- Feighner JP (1999). Mechanism of action of antidepressant medications. Journal of Clinical Psychiatry 60 Suppl 4, 4-11; discussion 12-13. [PubMed] [Google Scholar]
- Frommberger UH, Bauer J, Haselbauer P, Fraulin A, Riemann D, Berger M (1997). Interleukin-6-(il-6) plasma levels in depression and schizophrenia: Comparison between the acute state and after remission. European Archives of Psychiatry and Clinical Neuroscience 247, 4, 228-233. [DOI] [PubMed] [Google Scholar]
- Galan AM, Lopez-Vilchez I, Diaz-Ricart M, Navalon F, Gomez E, Gasto C, Escolar G (2009). Serotonergic mechanisms enhance platelet-mediated thrombogenicity. Thrombosis and Haemostasis 102, 3, 511-519. [DOI] [PubMed] [Google Scholar]
- Gallassi R, Di Sarro R, Morreale A, Amore M (2006). Memory impairment in patients with late-onset major depression: The effect of antidepressant therapy. Journal of Affective Disorders 91, 2-3, 243-250. [DOI] [PubMed] [Google Scholar]
- Galletta M, Baldini L, Sansone F, Ugozzoli F, Ungaro R, Casnati A, Mariani M (2010). Calix[6]arene-picolinamide extractants for radioactive waste: Effect of modification of the basicity of the pyridine n atom on the extraction efficiency and an/ln separation. Dalton Transactions 39, 10, 2546-2553. [DOI] [PubMed] [Google Scholar]
- Gold PW, Goodwin FK, Chrousos GP (1988a). Clinical and biochemical manifestations of depression. Relation to the neurobiology of stress (1). The New England Journal of Medicine 319, 6, 348-353. [DOI] [PubMed] [Google Scholar]
- Gold PW, Goodwin FK, Chrousos GP (1988b). Clinical and biochemical manifestations of depression. Relation to the neurobiology of stress (2). The New England Journal of Medicine 319, 7, 413-420. [DOI] [PubMed] [Google Scholar]
- Gold PW, Machado-Vieira R, Pavlatou MG (2015). Clinical and biochemical manifestations of depression: Relation to the neurobiology of stress. Neural Plasticity 2015, 581976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goltzman D (2011). Lrp5, serotonin, and bone: Complexity, contradictions, and conundrums. Journal of Bone and Mineral Research 26, 9, 1997-2001. [DOI] [PubMed] [Google Scholar]
- Gomez-Gil E, Gasto C, Diaz-Ricart M, Carretero M, Salamero M, Catalan R, Escolar G (2002). Platelet 5-ht2a-receptor-mediated induction of aggregation is not altered in major depression. Human Psychopharmacology: Clinical and Experimental 17, 8, 419-424. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Price J, De Bodinat C, Laredo J (2017). Emotional blunting with antidepressant treatments: A survey among depressed patients. Journal of Affective Disorders 221, 31-35. [DOI] [PubMed] [Google Scholar]
- Gram L (1994). Fluoxetine. The New England Journal of Medicine 331, 20, 1354-1361. [DOI] [PubMed] [Google Scholar]
- Hallback I, Hagg S, Eriksson AC, Whiss PA (2012). In vitro effects of serotonin and noradrenaline reuptake inhibitors on human platelet adhesion and coagulation. Pharmacological Reports 64, 4, 979-983. [DOI] [PubMed] [Google Scholar]
- Han L, McCusker J, Cole M, Capek R, Abrahamowicz M (2011). Antidepressant use and cognitive functioning in older medical patients with major or minor depression: A prospective cohort study with database linkage. Journal of Clinical Psychopharmacology 31, 4, 429-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Duman RS, Cowen PJ (2017). How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry 4, 5, 409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM (2004). Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. American Journal of Psychiatry 161, 7, 1256-1263. [DOI] [PubMed] [Google Scholar]
- Harris MG, Benfield P (1995). Fluoxetine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in older patients with depressive illness. Drugs & Aging 6, 1, 64-84. [DOI] [PubMed] [Google Scholar]
- Harvey SB, Overland S, Hatch SL, Wessely S, Mykletun A, Hotopf M (2018). Exercise and the prevention of depression: Results of the hunt cohort study. American Journal of Psychiatry 175, 1, 28-36. [DOI] [PubMed] [Google Scholar]
- Hergovich N, Aigner M, Eichler HG, Entlicher J, Drucker C, Jilma B (2000). Paroxetine decreases platelet serotonin storage and platelet function in human beings. Clinical Pharmacology & Therapeutics 68, 4, 435-442. [DOI] [PubMed] [Google Scholar]
- Herrera-Guzman I, Herrera-Abarca JE, Gudayol-Ferre E, Herrera-Guzman D, Gomez-Carbajal L, Pena-Olvira M, Villuendas-Gonzalez E, Joan GO (2010). Effects of selective serotonin reuptake and dual serotonergic-noradrenergic reuptake treatments on attention and executive functions in patients with major depressive disorder. Psychiatry Research 177, 3, 323-329. [DOI] [PubMed] [Google Scholar]
- Herrmann LL, Goodwin GM, Ebmeier KP (2007). The cognitive neuropsychology of depression in the elderly. Psychological Medicine 37, 12, 1693-1702. [DOI] [PubMed] [Google Scholar]
- Hillhouse TM, Porter JH (2015). A brief history of the development of antidepressant drugs: From monoamines to glutamate. Experimental and Clinical Psychopharmacology 23, 1, 1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AY, Kung AW (2005). Determinants of peak bone mineral density and bone area in young women. Journal of Bone and Mineral Metabolism 23, 6, 470-475. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, Harris GJ, Pearlson GD, Cox CS, Machlin SR, Camargo EE (1991). A fluoxetine-induced frontal lobe syndrome in an obsessive compulsive patient. Journal of Clinical Psychiatry 52, 3, 131-133. [PubMed] [Google Scholar]
- Hoehn-Saric R, Lipsey JR, McLeod DR (1990). Apathy and indifference in patients on fluvoxamine and fluoxetine. Journal of Clinical Psychopharmacology 10, 5, 343-345. [PubMed] [Google Scholar]
- Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL (2000). The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. Journal of Bone and Mineral Research 15, 1, 2-12. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR (2002). Molecular, pharmacological and functional diversity of 5-ht receptors. Pharmacology Biochemistry and Behavior 71, 4, 533-554. [DOI] [PubMed] [Google Scholar]
- Hu XH, Bull SA, Hunkeler EM, Ming E, Lee JY, Fireman B, Markson LE (2004). Incidence and duration of side effects and those rated as bothersome with selective serotonin reuptake inhibitor treatment for depression: Patient report versus physician estimate. Journal of Clinical Psychiatry 65, 7, 959-965. [DOI] [PubMed] [Google Scholar]
- Hubbard R, Farrington P, Smith C, Smeeth L, Tattersfield A (2003). Exposure to tricyclic and selective serotonin reuptake inhibitor antidepressants and the risk of hip fracture. American Journal of Epidemiology 158, 1, 77-84. [DOI] [PubMed] [Google Scholar]
- Jin J, Kunapuli SP (1998). Coactivation of two different gprotein-coupled receptors is essential for adp-induced platelet aggregation. Proceedings of the National Academy of Sciences of the United States of America 95, 14, 8070-8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joss JD, Burton RM, Keller CA (2003). Memory loss in a patient treated with fluoxetine. Annals of Pharmacotherapy 37, 12, 1800-1803. [DOI] [PubMed] [Google Scholar]
- Kambeitz JP, Howes OD (2015). The serotonin transporter in depression: Meta-analysis of in vivo and post mortem findings and implications for understanding and treating depression. Journal of Affective Disorders 186, 358-366. [DOI] [PubMed] [Google Scholar]
- Kelly K, Posternak M, Alpert JE (2008). Toward achieving optimal response: Understanding and managing antidepressant side effects. Dialogues in Clinical Neuroscience 10, 4, 409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S, Arifuzzman AI, Houle S, Vaccarino FJ (2001). Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. American Journal of Psychiatry 158, 6, 899-905. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Javanmard M, Vaccarino FJ (1997). A review of functional neuroimaging in mood disorders: Positron emission tomography and depression. The Canadian Journal of Psychiatry 42, 5, 467-475. [DOI] [PubMed] [Google Scholar]
- Kent JM, Coplan JD, Gorman JM (1998). Clinical utility of the selective serotonin reuptake inhibitors in the spectrum of anxiety. Biological Psychiatry 44, 9, 812-824. [DOI] [PubMed] [Google Scholar]
- Kharofa J, Sekar P, Haverbusch M, Moomaw C, Flaherty M, Kissela B, Broderick J, Woo D (2007). Selective serotonin reuptake inhibitors and risk of hemorrhagic stroke. Stroke 38, 11, 3049-3051. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R (2002). Depression and immune function: Central pathways to morbidity and mortality. Journal of Psychosomatic Research 53, 4, 873-876. [DOI] [PubMed] [Google Scholar]
- Kilts CD (1994). Recent pharmacologic advances in antidepressant therapy. The American Journal of Medicine 97, 6A, 3S-12S. [DOI] [PubMed] [Google Scholar]
- Kinjo M, Setoguchi S, Schneeweiss S, Solomon DH (2005). Bone mineral density in subjects using central nervous system-active medications. The American Journal of Medicine 118, 12, 1414. [DOI] [PubMed] [Google Scholar]
- Korpelainen R, Korpelainen J, Heikkinen J, Vaananen K, Keinanen-Kiukaanniemi S (2006). Lifelong risk factors for osteoporosis and fractures in elderly women with low body mass index--a population-based study. Bone 39, 2, 385-391. [DOI] [PubMed] [Google Scholar]
- Kreiger N TA, Joseph L et al. (1999). The canadian multicentre osteoporosis study (camos): Background, rationale, methods. Canadian Journal of Aging, 18(13): 376–387. [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H (2000). Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 22, 4, 370-379. [DOI] [PubMed] [Google Scholar]
- Lavoie B, Lian JB, Mawe GM (2017). Regulation of bone metabolism by serotonin. Advances in Experimental Medicine and Biology 1033, 35-46. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Caftori R, Avital A, Richter-Levin G (2002). The ssris drug fluoxetine, but not the noradrenergic tricyclic drug desipramine, improves memory performance during acute major depression. Brain Research Bulletin 58, 4, 345-350. [DOI] [PubMed] [Google Scholar]
- Liddle PF. (2007). Schizophrenia: The clinical picture. Paper presented at the Seminars in General Adult Psychiatry London: Royal College of Psychiatrists. [Google Scholar]
- Limke KK, Shelton AR, Elliott ES (2002). Fluvoxamine interaction with warfarin. Annals of Pharmacotherapy 36, 12, 1890-1892. [DOI] [PubMed] [Google Scholar]
- Liu B, Anderson G, Mittmann N, To T, Axcell T, Shear N (1998). Use of selective serotonin-reuptake inhibitors or tricyclic antidepressants and risk of hip fractures in elderly people. Lancet 351, 9112, 1303-1307. [DOI] [PubMed] [Google Scholar]
- Lopez-Munoz F, Alamo C (2009). Monoaminergic neurotransmission: The history of the discovery of antidepressants from 1950s until today. Current Pharmaceutical Design 15, 14, 1563-1586. [DOI] [PubMed] [Google Scholar]
- Maes M (1999). Major depression and activation of the inflammatory response system. Advances in Experimental Medicine and Biology 461, 25-46. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H (1997). Increased serum il-6 and il-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 9, 11, 853-858. [DOI] [PubMed] [Google Scholar]
- Malik P, Gasser RW, Moncayo RC, Kandler C, Koudouovoh-Tripp P, Giesinger J, Sperner-Unterweger B (2013). Bone mineral density and bone metabolism in patients with major depressive disorder without somatic comorbidities. Progress in Neuro-Psychopharmacology & Biological Psychiatry 44, 58-63. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Akiskal HS, Udo M, Picchetti M, Baroni S, Massimetti G, Albanese F, Dell’Osso L (2014). Dimorphic changes of some features of loving relationships during long-term use of antidepressants in depressed outpatients. Journal of Affective Disorders 166, 151-155. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Landi P, Baroni S, Vanelli F, Bartolommei N, Picchetti M, Dell’Osso L (2013). The role of platelet/lymphocyte serotonin transporter in depression and beyond. Current Drug Targets 14, 5, 522-530. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Rutigliano G, Baroni S, Landi P, Dell’Osso L (2014). Metabolic syndrome and major depression. CNS Spectrums 19, 4, 293-304. [DOI] [PubMed] [Google Scholar]
- Marin RS (1990). Differential diagnosis and classification of apathy. American Journal of Psychiatry 147, 1, 22-30. [DOI] [PubMed] [Google Scholar]
- Masand PS, Gupta S (1999). Selective serotonin-reuptake inhibitors: An update. Harvard Review of Psychiatry 7, 2, 69-84. [PubMed] [Google Scholar]
- Mayberg HS (1994). Functional imaging studies in secondary depression. Psychiatric Annals, 24: 643–647. [Google Scholar]
- Mayberg HS, Parikh RM, Morris PL, Robinson RG (1991). Spontaneous remission of post-stroke depression and temporal changes in cortical s2-serotonin receptors. The Journal of Neuropsychiatry and Clinical Neurosciences 3, 1, 80-83. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Starkstein SE, Peyser CE, Brandt J, Dannals RF, Folstein SE (1992). Paralimbic frontal lobe hypometabolism in depression associated with huntington’s disease. Neurology 42, 9, 1791-1797. [DOI] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Cowen PJ, Harmer CJ (2010). Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biological Psychiatry 67, 5, 439-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DJ, Postolache TT, Vittone BJ, Nghiem KL, Mon-sale JL, Wesley RA, Rick ME (2008). Selective serotonin reuptake inhibitors: Measurement of effect on platelet function. Translational Research 151, 3, 168-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden EP, Clarke JG, Davies GJ, Kaski JC, Haider AW, Maseri A (1991). Effect of intracoronary serotonin on coronary vessels in patients with stable angina and patients with variant angina. The New England Journal of Medicine 324, 10, 648-654. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009). Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. Journal of Clinical Epidemiology 62, 10, 1006-1012. [DOI] [PubMed] [Google Scholar]
- Mormont C (1984). The influence of age and depression on intellectual and memory performances. Acta Psychiatrica Belgica 84, 2, 127-134. [PubMed] [Google Scholar]
- Murdoch D, McTavish D (1992). Sertraline. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in depression and obsessive-compulsive disorder. Drugs 44, 4, 604-624. [DOI] [PubMed] [Google Scholar]
- Nagane A, Baba H, Nakano Y, Maeshima H, Hukatsu M, Ozawa K, Suzuki T, Arai H (2014). Comparative study of cognitive impairment between medicated and medication-free patients with remitted major depression: Class-specific influence by tricyclic antidepressants and newer antidepressants. Psychiatry Research 218, 1-2, 101-105. [DOI] [PubMed] [Google Scholar]
- Oleshansky MA, Labbate LA (1996). Inability to cry during sri treatment. Journal of Clinical Psychiatry 57, 12, 593. [DOI] [PubMed] [Google Scholar]
- Opbroek A, Delgado PL, Laukes C, McGahuey C, Katsanis J, Moreno FA, Manber R (2002). Emotional blunting associated with ssri-induced sexual dysfunction. Do ssris inhibit emotional responses? International Journal of Neuropsychopharmacology 5, 2, 147-151. [DOI] [PubMed] [Google Scholar]
- Panzer MJ, Mellow AM (1992). Antidepressant treatment of pathologic laughing or crying in elderly stroke patients. Journal of Geriatric Psychiatry and Neurology 5, 4, 195-199. [DOI] [PubMed] [Google Scholar]
- Papakostas GI (2014). Cognitive symptoms in patients with major depressive disorder and their implications for clinical practice. Journal of Clinical Psychiatry 75, 1, 8-14. [DOI] [PubMed] [Google Scholar]
- Pardini M, Guida S, Primavera A, Krueger F, Cocito L, Gialloreti LE (2011). Amisulpride vs. Fluoxetine treatment of chronic fatigue syndrome: A pilot study. European Neuropsychopharmacology 21, 3, 282-286. [DOI] [PubMed] [Google Scholar]
- Patetsos E, Horjales-Araujo E (2016). Treating chronic pain with ssris: What do we know? Pain Research and Management 2016, 2020915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronijevic M, Petronijevic N, Ivkovic M, Stefanovic D, Radonjic N, Glisic B, Ristic G, Damjanovic A, Paunovic V (2008). Low bone mineral density and high bone metabolism turnover in premenopausal women with unipolar depression. Bone 42, 3, 582-590. [DOI] [PubMed] [Google Scholar]
- Pisljar M, Pirtosek Z, Repovs G, Grgic M (2008). Executive dysfunction in late-onset depression. Psychiatria Danubina 20, 2, 231-235. [PubMed] [Google Scholar]
- Popovic D, Vieta E, Fornaro M, Perugi G (2015). Cognitive tolerability following successful long term treatment of major depression and anxiety disorders with ssri antidepressants. Journal of Affective Disorders 173, 211-215. [DOI] [PubMed] [Google Scholar]
- Price J, Cole V, Goodwin GM (2009). Emotional side-effects of selective serotonin reuptake inhibitors: Qualitative study. The British Journal of Psychiatry 195, 3, 211-217. [DOI] [PubMed] [Google Scholar]
- Rabenda V, Nicolet D, Beaudart C, Bruyere O, Reginster JY (2013). Relationship between use of antidepressants and risk of fractures: A meta-analysis. Osteoporosis International 24, 1, 121-137. [DOI] [PubMed] [Google Scholar]
- Reinblatt SP, Riddle MA (2006). Selective serotonin reuptake inhibitor-induced apathy: A pediatric case series. Journal of Child and Adolescent Psychopharmacology 16, 1-2, 227-233. [DOI] [PubMed] [Google Scholar]
- Richards JB, Papaioannou A, Adachi JD, Joseph L, Whitson HE, Prior JC, Goltzman D, Canadian Multicentre Osteoporosis Study Research G (2007). Effect of selective serotonin reuptake inhibitors on the risk of fracture. Archives of Internal Medicine 167, 2, 188-194. [DOI] [PubMed] [Google Scholar]
- Ring HA, Bench CJ, Trimble MR, Brooks DJ, Frackowiak RS, Dolan RJ (1994). Depression in Parkinson’s disease. A positron emission study. The British Journal of Psychiatry 165, 3, 333-339. [DOI] [PubMed] [Google Scholar]
- Rizzoli R, Cooper C, Reginster JY, Abrahamsen B, Adachi JD, Brandi ML, Bruyere O, Compston J, Ducy P, Ferrari S, Harvey NC, Kanis JA, Karsenty G, Laslop A, Rabenda V, Vestergaard P (2012). Antidepressant medications and osteoporosis. Bone 51, 3, 606-613. [DOI] [PubMed] [Google Scholar]
- Rosenblat JD, Gregory JM, Carvalho AF, McIntyre RS (2016). Depression and disturbed bone metabolism: A narrative review of the epidemiological findings and postulated mechanisms. Current Molecular Medicine 16, 2, 165-178. [DOI] [PubMed] [Google Scholar]
- Ross SB, Aperia B, Beck-Friis J, Jansa S, Wetterberg L, Aberg A (1980). Inhibition of 5-hydroxytryptamine uptake in human platelets by antidepressant agents in vivo. Psycho-pharmacology (Berlin) 67, 1, 1-7. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Post RM, Savard R, Gold PW (1984). Cortisol hypersecretion and cognitive impairment in depression. Archives Of General Psychiatry 41, 3, 279-283. [DOI] [PubMed] [Google Scholar]
- Sansone RA, Sansone LA (2010). Ssri-induced indifference. Psychiatry (Edgmont) 7, 10, 14-18. [PMC free article] [PubMed] [Google Scholar]
- Sarosi A, Gonda X, Balogh G, Szekely A, Sasvari M, Faludi G (2008). Gender differences in the neurocognitive components of depression. Neuropsychopharmacologia hungarica 10, 4, 191-199. [in Hungarian] [PubMed] [Google Scholar]
- Savard RJ, Rey AC, Post RM (1980). Halstead-Reitan Category test in bipolar and unipolar affective disorders. Relationship to age and phase of illness. J Nerv Ment Dis 168, 5, 297-304. [DOI] [PubMed] [Google Scholar]
- Schmitt JA, Kruizinga MJ, Riedel WJ (2001). Non-serotonergic pharmacological profiles and associated cognitive effects of serotonin reuptake inhibitors. Journal of Psycho-pharmacology 15, 3, 173-179. [DOI] [PubMed] [Google Scholar]
- Schwan S, Hallberg P (2009). Ssris, bone mineral density, and risk of fractures--a review. European Neuropsychopharmacology 19, 10, 683-692. [DOI] [PubMed] [Google Scholar]
- Shams T, Firwana B, Habib F, Alshahrani A, Alnouh B, Murad MH, Ferwana M (2014). Ssris for hot flashes: A systematic review and meta-analysis of randomized trials. Journal of General Internal Medicine 29, 1, 204-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu YH, Lanteigne A, Sturmer T, Pate V, Azrael D, Miller M (2015). Ssri use and risk of fractures among perimenopausal women without mental disorders. Injury Prevention 21, 6, 397-403. [DOI] [PubMed] [Google Scholar]
- Siddiqui R, Gawande S, Shende T, Tadke R, Bhave S, Kirpekar V (2011). Ssri-induced coagulopathy: Is it reality? Therapeutic Advances in Psychopharmacology 1, 6, 169-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler L, Scholes D, Brunner RL, Robbins J, Reed SD, Newton KM, Melville JL, Lacroix AZ (2008). Depressive symptoms, bone loss, and fractures in postmenopausal women. Journal of General Internal Medicine 23, 5, 567-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM (2014). Stahls essential psychopharmacology. Cambridge University Press, Cambridge. [Google Scholar]
- Sternat T, Katzman MA (2016). Neurobiology of hedonic tone: The relationship between treatment-resistant depression, attention-deficit hyperactivity disorder, and substance abuse. Neuropsychiatric Disease and Treatment 12, 2149-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratz C, Trenk D, Bhatia HS, Valina C, Neumann FJ, Fiebich BL (2008). Identification of 5-ht3 receptors on human platelets: Increased surface immunoreactivity after activation with adenosine diphosphate (adp) and thrombin receptor-activating peptide (trap). Thrombosis and Haemostasis 99, 4, 784-786. [DOI] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G (2002). Leptin regulates bone formation via the sympathetic nervous system. Cell 111, 3, 305-317. [DOI] [PubMed] [Google Scholar]
- Teichert M, Visser LE, Uitterlinden AG, Hofman A, Buhre PJ, Straus S, De Smet PA, Stricker BH (2011). Selective serotonin re-uptake inhibiting antidepressants and the risk of overanticoagulation during acenocoumarol maintenance treatment. British Journal of Clinical Pharmacology 72, 5, 798-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van West D, Maes M (1999). Activation of the inflammatory response system: A new look at the etiopathogenesis of major depression. Neuro endocrinology letters 20, 1-2, 11-17. [PubMed] [Google Scholar]
- Vestergaard P, Prieto-Alhambra D, Javaid MK, Cooper C (2013). Fractures in users of antidepressants and anxiolytics and sedatives: Effects of age and dose. Osteoporosis International 24, 2, 671-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard P, Rejnmark L, Mosekilde L (2008). Selective serotonin reuptake inhibitors and other antidepressants and risk of fracture. Calcified Tissue International 82, 2, 92-101. [DOI] [PubMed] [Google Scholar]
- Wadsworth EJ, Moss SC, Simpson SA, Smith AP (2005). Ss-RIs and cognitive performance in a working sample. Human Psychopharmacology: Clinical and Experimental 20, 8, 561-572. [DOI] [PubMed] [Google Scholar]
- Walitt B, Urrutia G, Nishishinya MB, Cantrell SE, Hauser W (2015). Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database of Systematic Reviews 6, CD011735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Winter S, Holtje M, Paulmann N, Grohmann M, Vowinckel J, Alamo-Bethencourt V, Wilhelm CS, Ahnert-Hilger G, Bader M (2003). Serotonylation of small gtpases is a signal transduction pathway that triggers platelet alpha-granule release. Cell 115, 7, 851-862. [DOI] [PubMed] [Google Scholar]
- Wang CY, Fu SH, Wang CL, Chen PJ, Wu FL, Hsiao FY (2016). Serotonergic antidepressant use and the risk of fracture: A population-based nested case-control study. Osteoporosis International 27, 1, 57-63. [DOI] [PubMed] [Google Scholar]
- Williams LJ, Henry MJ, Berk M, Dodd S, Jacka FN, Kotowicz MA, Nicholson GC, Pasco JA (2008). Selective serotonin reuptake inhibitor use and bone mineral density in women with a history of depression. International Clinical Psychopharmacology 23, 2, 84-87. [DOI] [PubMed] [Google Scholar]
- Wong DT, Bymaster FP, Engleman EA (1995). Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: Twenty years since its first publication. Life Sciences 57, 5, 411-441. [DOI] [PubMed] [Google Scholar]
- Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti, Jr. TD, DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW (2000). Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: Relation to hypercortisolism and corticotropin-releasing hormone. Proceedings of the National Academy of Sciences of the United States of America 97, 1, 325-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongpakaran N, van Reekum R, Wongpakaran T, Clarke D (2007). Selective serotonin reuptake inhibitor use associates with apathy among depressed elderly: A case-control study. Annals of General Psychiatry 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfrey S, Gammack NS, Dewar MS, Brown PJ (1993). Fluoxetine-warfarin interaction. British Medical Journal 307, 6898, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Balaji S, Suresh PS, Liu XS, Lu X, Li Z, Guo XE, Mann JJ, Balapure AK, Gershon MD, Medhamurthy R, Vidal M, Karsenty G, Ducy P (2010). Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nature Medicine 16, 3, 308-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G (2009). A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138, 5, 976-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G (2008). Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135, 5, 825-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel A, Barkow K, Schulze-Rauschenbach S, Von Widdern O, Metten M, Pfeiffer U, Schnell S, Wagner M, Maier W (2004a). High neuroticism and depressive temperament are associated with dysfunctional regulation of the hypothalamic-pituitary-adrenocortical system in healthy volunteers. Acta Psychiatrica Scandinavica 109, 5, 392-399. [DOI] [PubMed] [Google Scholar]
- Zobel AW, Schulze-Rauschenbach S, von Widdern OC, Metten M, Freymann N, Grasmader K, Pfeiffer U, Schnell S, Wagner M, Maier W (2004b). Improvement of working but not declarative memory is correlated with hpa normalization during antidepressant treatment. Journal of Psychiatric Research 38, 4, 377-383. [DOI] [PubMed] [Google Scholar]