Abstract
Objective:
To investigate the release of metal ions from an orthodontic appliance in tests on animals (pigs).
Materials and Methods:
An animal test was conducted on 24 pigs divided equally into an experimental and a control group. In total, 12 sets of experimental orthodontic plates were surgically inserted into pig snouts in the experimental group for 6 months. Noninvasive matrices (hair [0, 3, and 6 months]) and invasive matrices (kidneys, liver, lungs, aorta, and oral mucosa) were collected for multi-elemental analysis (inductively coupled plasma optical emission spectrometry) from the experimental and control groups.
Results:
The greatest differences in the content of toxic metals were found in the aorta (Ni level was 4.8 times higher in experimental than in the control group), in the cheek (Ni 3.5 times higher), and in the hair sampled after 3 months (Cr 3.4 times higher).
Conclusions:
The obtained data indicate that the products of corrosion have passed into selected tissues of pigs; however, the doses of toxic metal ions released from the appliance did not reach toxic levels.
Keywords: Orthodontic appliances, Metal ions release, Tests on animals, Pigs, In vivo experiment
INTRODUCTION
“Warning: This product contains nickel and/or chromium. A small percentage of the population is known to be allergic to nickel and/or chromium. If an allergic reaction occurs, direct the patient to consult a physician.” This common information on orthodontic product labels makes research on the biocompatibility of orthodontic appliances a timely issue.
Since orthodontic fixed appliances have become popular, the question about the quantity and potential cytotoxicity of released metal ions is still valid. Many papers with contradictory conclusions have been published.1–5
Medical disciplines that discuss the problem of orthodontic material biocompatibility use various tools for testing. Several methods can be used to evaluate the release of metal ions from dental alloys: in vitro (eg, in the environment of artificial saliva or tissue culture)6,7 and in vivo experiments with the application of invasive (eg, blood)8 or noninvasive matrices (eg, saliva, hair, urine).9–13 None of the abovementioned methods is able to reflect the real, changeable, complex environment of the human oral cavity. In in vitro experiments, the problem lies in ensuring the proper process conditions: pH, temperature, flow rate, microflora, the composition of solution, time, and tissue contact. Also additional, sometimes difficult-to-standardize factors such as hygiene and dietary habits should be considered.14 In in vivo experiments, the problem with achieving reliable results is associated with the frequency of sampling compared with the treatment time (eg, blood, saliva, urine), the type of matrix (eg, epithelium buccal cells—contact tissue), or the sampling and preparing of the matrix (eg, hair—the lack of universal reference ranges and the difficulties in interpretation).15
Corrosion of the metal alloys from which the elements of orthodontic appliances are made mainly affect the surrounding tissues. This is confirmed by the available reports.16,17 So far, unequivocal scientific evidence has not been presented that supports the assertion that metal ions released from orthodontic appliances pose systemic toxicity.18 Such research can be undertaken by evaluation of the composition of exposed tissues, which are on the other hand invasive matrices. Blood, saliva, and urine reflect momentary exposure, and buccal epithelium cells reflect contact exposure. Invasive matrices can be used in investigation of long-term exposure. This is possible in studies on animals. The aim of the present study was to evaluate experimentally the release of metal ions from an orthodontic appliance in a living organism—pig.
MATERIALS AND METHODS
The study concept:
Preparation of plates that aimed to simulate the orthodontic appliance (appropriate weight and alloy composition);
Surgical implantation of plates in pigs' snouts for 6 months;
Sampling of noninvasive matrices (hair [0, 3, and 6 months]);
Removal of plates and sampling of invasive matrices;
The assessment of the composition of invasive and noninvasive matrices.
Previously, pilot studies on two experimental and two control pigs were conducted to investigate the construction, strength, and placement technique of the plate in pigs' snouts.19 Additionally, the contents of elements in various tissues were determined, and on this basis, the tissues were selected for the present work.
Plates
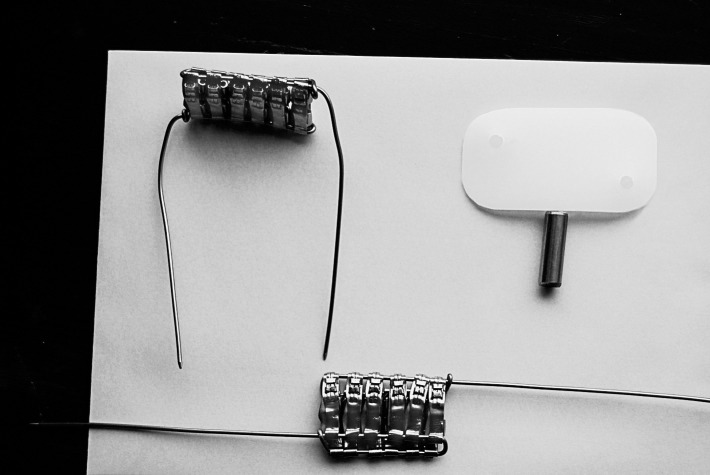
The masses of particular elements in the orthodontic appliance and experimental plate were calculated. The average orthodontic appliance used for calculation in the study consisted of: four bands (size 37+; 3M Unitek, Monrovia, Calif), 20 brackets (Victory Series 0.22; 3M Unitek), and two wires (.018 SS; 3M Unitek). The total weight of the appliance was 3.9448 g. The equivalent of a fixed orthodontic appliance (upper and lower dental arch) expressed as bands was calculated. Assuming an average weight of a patient as 55 kg and a target weight of a pig as ≈175 kg, in the snout of each pig the equivalent of 3.18 orthodontic appliances should had been placed. The plate was constructed of 12 bands (only whole bands were used), and the total mass of bands in one plate was 6.2380 g (Figure 1). The total weight of two plates was (2 × 6.2380 g) 12.4760 g. The composition of the stainless steel (SS) alloy (316L) from which the experimental plates for pigs (simulating orthodontic appliances) were formed was as follows: Fe, ≈65%; Cr, 17%; Ni, 12%; Mo, 2.5%; Mn, <2%; Si, <1%; P, <0.045%; C, <0.03%; and S, <0.03% (3M Unitek).
Figure 1.
Experimental plate.
Animal Experiments
For animal experiments, pigs (Sus scrofa domestica) as a model organism were chosen. The Polish White Landrace breed, ≈30 kg body weight, was used in the groups. The animal test was performed with the approval (120/2009/09) of the ethical committee of the Wrocław University of Environmental and Life Sciences. The animals were male pigs of 3 months of age weighing 29.0 ± 1.3 kg. The duration of tests was justified by the growth period and the target weight of the pigs. The 24 animals were randomly separated into two groups. One group (experimental) received plates on the inner buccal side, and the second group (control) did not receive plates. At the end of the experiment, the average weight of a pig was 191.3 ± 10.4 kg.
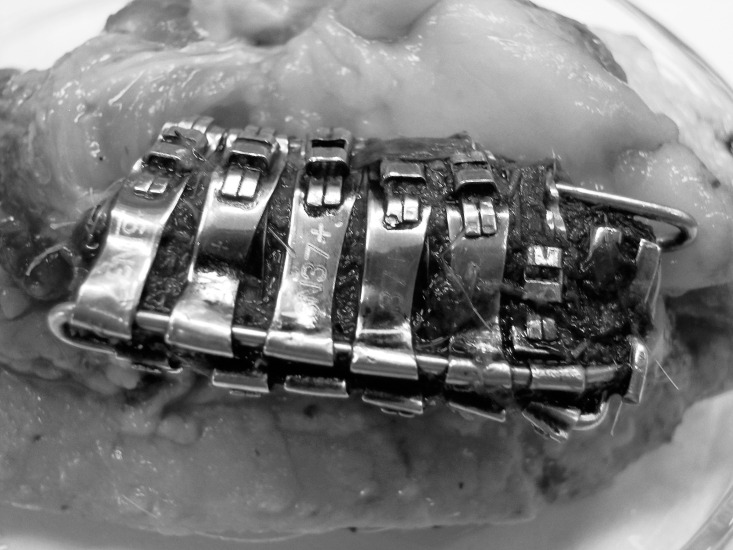
The bands were placed on a plastic plate (polyethylene) that served to alleviate skin irritation. For clamping bands, steel wire with a diameter of 0.047 inches was used. Premedication was performed using xylazine i.m. (Sedazin, Biowet Pulawy, Poland; 1 ml 20/kg body weight [BW]) and atropinum sulphuricum i.m. (0.02 mg/kg BW). Surgery was performed under general anesthesia, which was initiated with sodium thiopental i.v. (Thiopental: Biomedica Poland; 10 mg/kg BW) and maintained with ketamine i.v. (Bioketan: Vetoquinol Biowet, Ltd.; 15 mg/kg BW). Antibiotic (Betamox LA: ScanVet, Poland; amoxycillinum 1 ml/10 kg BW) and analgesic drugs (Metacam: Boehringer, Ingelheim, Germany; meloxicam 0.4 ml/12 BW) were administered s.c. for 5 days. The plates were surgically implanted on the buccal side of the pig's cheek for a period of 6 months (Figure 2).
Figure 2.
Fixed experimental plate after the experiment.
Sampling
In this research, 12 control and 12 experimental female pigs were used. Two pigs died (one in each group) because of general diseases (colibacteriosis) not related to the conditions of the study. Samples from 22 pigs were collected. In total, 176 samples of hair, kidneys, liver, lungs, aorta, and oral mucosa were obtained.
Samples of hair from both groups (experimental and control) were collected from each animal at the beginning of the experiment and after 3 and 6 months. The hair samples were washed with baby shampoo and dried. The selection of the shampoo was determined by its composition. The animals were slaughtered after 6 months, and various internal tissue samples were retrieved (aorta, lung, kidney, liver, and inner part of cheek). Slaughter procedure was carried out with the required permits and according to the Minister of Agriculture and Rural Development dated 02/04/2004 by the persons entitled to carry out professional slaughter using acceptable methods of slaughter and killing of animals (Polish Journal of Laws 2004.70.643). Approved procedures involve the use of electronarcosis and exsanguination of pigs.
Analytical Methods
An internal tissue sample (1 g) was purified from organic matter with concentrated nitric acid: 69% m/m (5 ml), spectrally pure (Suprapur; Merck, Darmstadt, Germany), using Teflon bombs in the microwave oven Milestone Start D (Milestone, Sorisole, Italy). The selected parameters of the process assured the complete digestion of samples. After mineralization, samples were diluted with redemineralized water (Millipore Simplicity, Molsheim, France) to 25 g. The samples of hair (0.5 g) were solubilized with concentrated nitric acid (5 ml) and digested. After mineralization, samples were diluted with redemineralized water to 50 g. All samples were then analyzed for element content using the iCAP 6500 Duo ICP Spectrometer (Thermo Scientific, Leicetsershire, England). The concentration of the following elements was determined: Cd, Co, Cr, Cu, Fe, Mn, Mo, Ni, Si, and Zn. The limits of detection were as follows for all tissues (for liver, they were 100% higher): Cd, <0.0002; Co, <0.0002; Cr, <0.0025; Cu, <0.02; Fe, <0.0075; Mn, <0.00075; Mo, <0.005; Ni, <0.001; Si, <0.05; and Zn, <0.005. Additionally, the moisture content was determined in internal tissue samples according to PN-ISO 1442:1997.
The analyses were carried out in a quality management system according to PN-EN ISO/IEC 17025: 2005. Quality assurance of the test results was achieved by using certified reference material: Bovine Muscle BCR No. 184 from the Community Bureau of Reference and Bovine Liver SRM 1577b from the National Institute of Standard and Technology. The samples were analyzed in three repeats (the reported results of analysis were arithmetic mean, and the relative standard deviation was <5%).
Statistical Methods
Statistical analysis of the results was performed by the use of Statistica (version 10.0; StatSoft, Tulsa, Okla). Descriptive statistics (means and standard deviations) were reported. Normality of distribution of experimental results was assessed by the Shapiro-Wilk test. This was the basis for the selection of the statistical test (normal distribution with homogeneity of variance, parametric t-test; normal distribution with heterogeneity of variance, Cochran's C-test; lack of normal distribution, Mann-Whitney U-test) for assessing the significance of the differences between the experimental and control groups. The statistical significance level was assumed to be lower than 0.05 and 0.1. Correlation was considered statistically significant at P < .05.
RESULTS
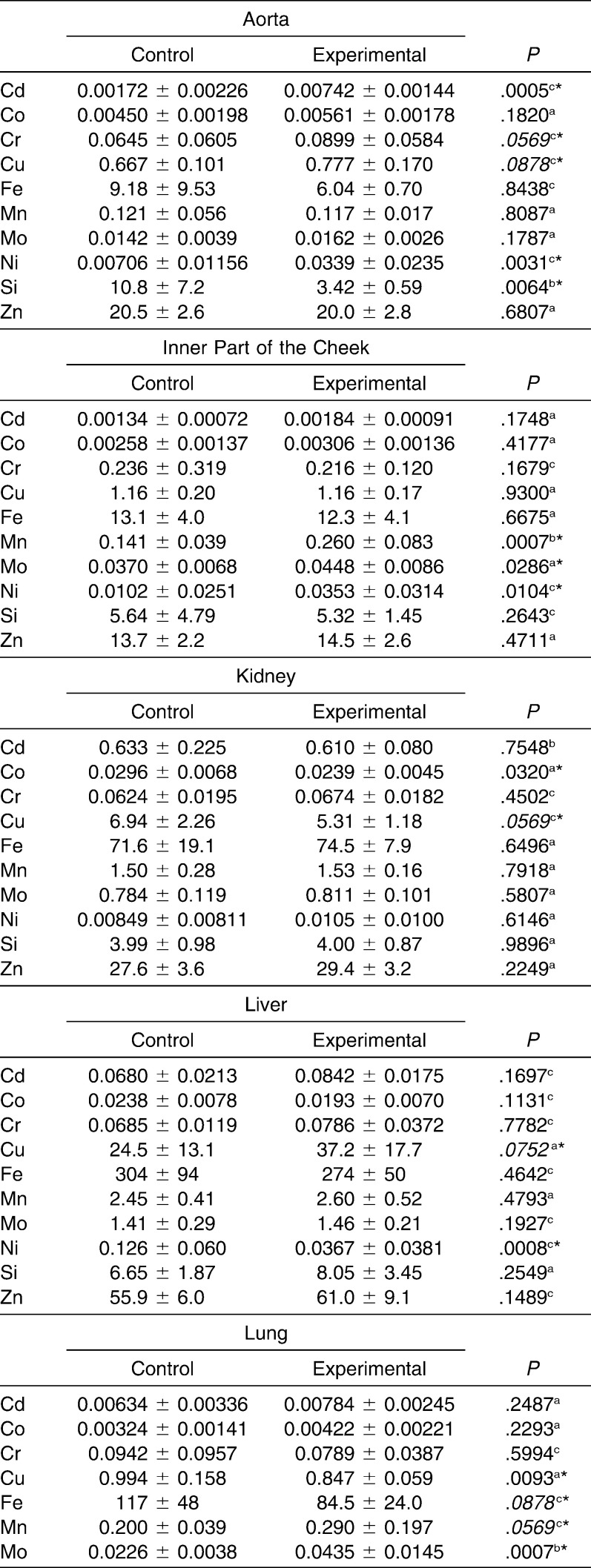
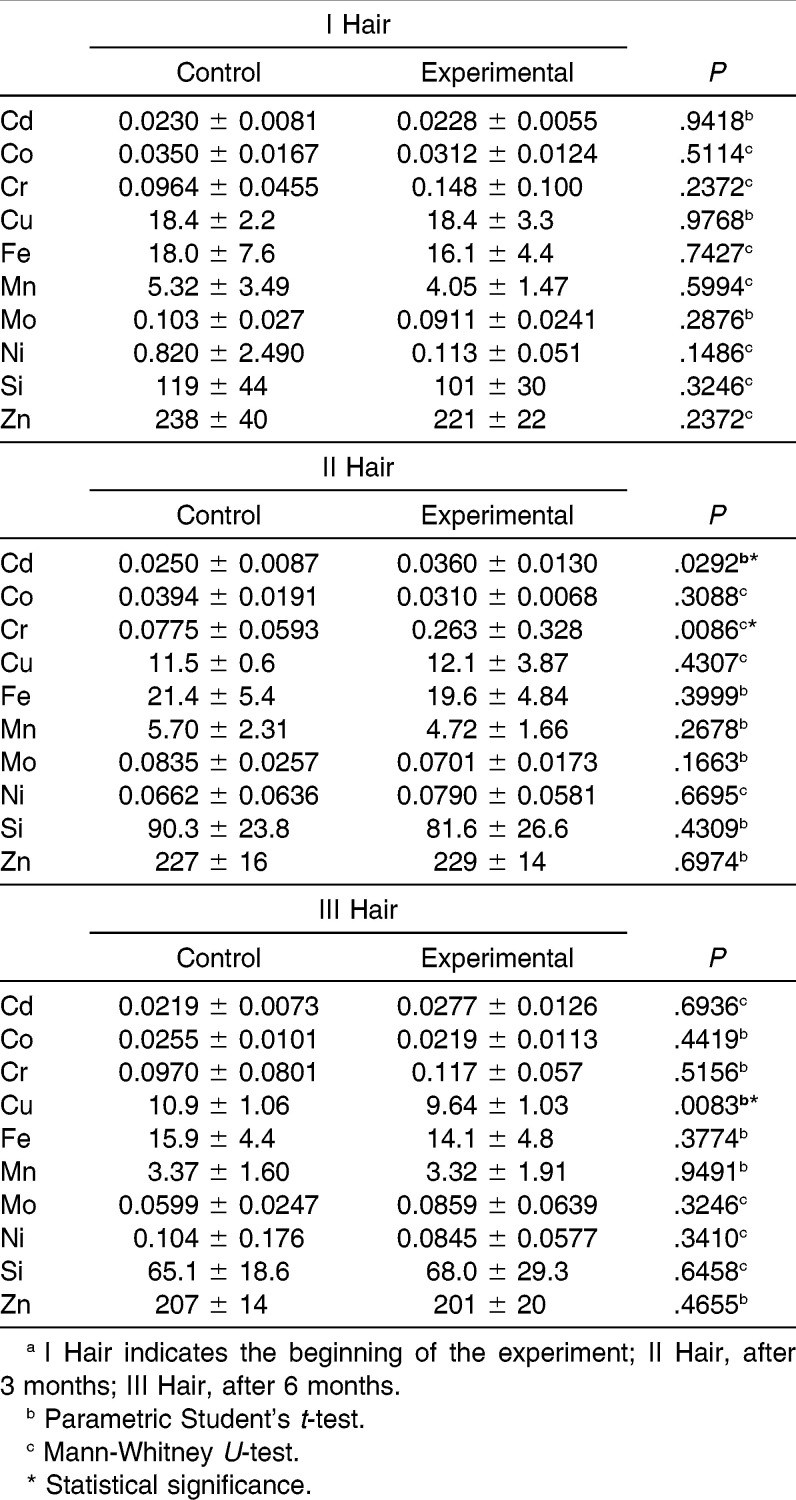
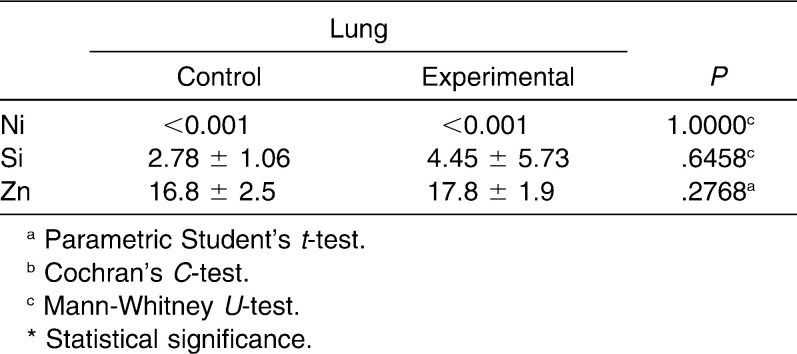
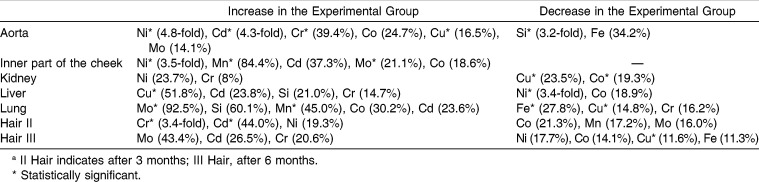
In the present paper, the release of metal ions from orthodontic appliances and their sites of accumulation in pigs were investigated. The levels of Cd, Co, Cr, Cu, Fe, Mn, Mo, Ni, Si, and Zn were determined in the aorta, lung, kidney, liver, inner part of the cheek, and hair of experimental and control pigs (Table 1 and 2). The sites of nickel and chromium accumulation were kidney, aorta, and hair sampled after 3 months of exposure. In liver and hair sampled after 6 months of exposure, Cr had accumulated. The highest differences in the content of toxic metals were found in the aorta (Ni level was 4.8 times higher in the experimental than in the control group) and in the hair sampled after 3 months (Cr, 3.4 times higher in experimental than in control). Significantly higher Cu content was found in the liver. After 3 months, the content of Cr and Ni in hair increased (3.4 times and 20%, respectively), and after 6 months as well (only Cr, 20%).
Table 1.
Contents of Elements in Invasive Biomarkers of Experimental and Control Groups, mg/kg (Fresh Weight), n = 11
Table 2.
Contents of Elements in a Noninvasive Biomarker of Experimental and Control Groups, mg/kg, n = 11a
Table 1.
Continued
The mineral levels (mean and standard deviation) of selected tissues for the experimental and control groups were presented in Tables 1 and 2. The moisture content in internal tissue samples was as follows: inner part of the cheek, 82.6%; lung, 79.8%; kidney, 76.4%; liver, 73.8%; and aorta, 67.3%. The elements were bioaccumulated in various tissues to different extents. The studied elements in invasive and noninvasive matrices were accumulated.
In hair samples that were collected at the beginning of the experiment, the levels of the elements were similar in both groups, and the differences were not statistically significant. In other hair samples and in the invasive matrices, statistically significant differences between both groups were observed. The differences in the mean values were markedly high for Ni in the aorta and the inner part of the cheek (4.8 times and 3.5 times more in the experimental group with respect to control; P = .0031 and .0104, respectively; Table 3). Other statistically significant differences between both groups concerned the following elements: Cd (hair samples collected after 3 months and aorta), Cr (hair samples collected after 3 months and aorta), Mo (lung and inner part of the cheek), Mn (inner part of the cheek and lung), Cu (liver, kidney, aorta, lung, and hair samples collected at the end of the experiment), Fe (lung), and Co (kidney).
Table 3.
Differences of Element Contents in Studied Matrices
DISCUSSION
Many factors facilitate the release of metal ions from an orthodontic appliance in the oral cavity.3 There are also several possible consequences: hypersensitivity, cytotoxicity, or genotoxicity.17 Consequently, to investigate long-term biocompatibility, it is essential to carry out in vivo studies.20
Animal studies allow simultaneous collection in invasive and noninvasive matrices via exposure to essential, toxic, and other elements, thereby enabling determination of the degree of bioaccumulation in the internal tissues.21 Pigs correspond to a high extent to humans when considering the development of the dentition, teeth mineralization and function, skin physical and anatomical properties, and bone ossification. Consequently, the data obtained in studies on pigs can be transferred to humans.22 Such studies are used, for example, in radiological diagnostics in dentistry.23
Sahmali et al.24 investigated the effects of dental alloys containing Ni on the level of this element in the serum, liver, kidney, and oral mucosa of guinea pigs. The test was conducted for 15 days. It was found that guinea pigs sensitized to Ni had higher levels of Ni in the serum, oral mucosa, liver, and, slightly, in kidney as compared with the control group. Statistically significant differences were found between liver and oral mucosa Ni content in the experimental and control groups.
Faccioni et al.16 reported that Ni and Co levels in buccal mucosa cells of patients with fixed appliances had increased 3.4-fold and 2.8-fold, respectively, after 2–4 years. In the present study, oral mucosa cells were identified as the sites of accumulation of Ni, with a 3.5-fold increase.
Amini et al.4 found that the level of Ni increased in oral mucosa cells in orthodontic patients (from 12.23 ng/ml in the control to 21.74 ng/ml in the experimental group; sampling, 16 months). Cr and Co levels were not statistically significantly higher. Similarly, in the present work, the level of Co slightly increased (20%) and was not statistically significant. The content of Cr in invasive biomarkers of exposure did not increase. In another study, elevated levels of some metal ions in oral mucosa cells were confirmed (Cr, Fe, Mn, and Ti).14 The genotoxic effect on oral mucosa cells at the debonding phase of the orthodontic fixed appliance was found, although increased levels of Ni and Cr 30 days after debonding were not confirmed. Directly after debonding, mean Cr and Ni contents were slightly higher in the test group, but the results were not statistically significant.17 In our study, the increase in the content of Ni was statistically significant, and Cr did not increase.
Hafez et al.3 collected buccal mucosa cells 3 and 6 months after placement of the appliance. The content of Ni increased from 0.52 to 0.78 ng/ml, and that of Cr from 0.31 to 0.78 ng/ml, after 6 months. Only the content of Cr after 3 months (0.41 ng/ml) as related to the beginning of the experiment was statistically significant. From the present work, similar conclusions can be derived: after 3 months, higher levels of Cr and Ni in hair were observed. After 3 months, only a slight increase in Cr was found.
Mikulewicz et al.11 conducted a study on orthodontic patients with hair sampling. The level of Ni was statistically significantly higher than that in the control group. The level of Cr was elevated but not statistically significant.
Among the studies on invasive matrices, the results of serum analysis sampled from orthodontic patients were reported.8 The concentration of Cr in the second year of treatment was statistically significantly higher.
In the available literature, the body burden of Ni averages ≈7.3 µg/kg body weight25 and that of Cr ≈8–72 µg/kg tissue weight.26 Assuming that, it can be stated out that in our study the orthodontic alloys were not a significant source of exposure.
CONCLUSION
Metal ions were released from the appliances in low doses, in particular at the beginning of the experiment.
The doses of toxic metal ions did not reach toxic levels.
Hair was found to be a noninvasive biomarker of exposure to metal ions released from orthodontic appliances.
ACKNOWLEDGMENTS
This research was financially supported by The National Centre for Research and Development in Poland (N R130006 10).
REFERENCES
- 1.Karnam SK, Reddy AN, Manjith CM. Comparison of metal ion release from different bracket archwire combinations: an in vitro study. J Contemp Dent Pract. 2012;13:376–381. doi: 10.5005/jp-journals-10024-1154. [DOI] [PubMed] [Google Scholar]
- 2.Milheiro A, Kleverlaan C, Muris J, Feilzer A, Pallav P. Nickel release from orthodontic retention wires—the action of mechanical loading and pH. Dent Mater. 2012;28:548–553. doi: 10.1016/j.dental.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Hafez HS, Selim EMN, Eid FHK, Tawfik WA, Al-Ashkar EA, Mostafa YA. Cytotoxicity, genotoxicity, and metal release, in patients with fixed orthodontic appliances: a longitudinal in-vivo study. Am J Orthod Dentofacial Orthop. 2011;140:298–308. doi: 10.1016/j.ajodo.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Amini F, Borzabadi Farahani A, Jafari A, Rabbani M. In vivo study of metal content of oral mucosa cells in patients with and without fixed orthodontic appliances. Orthod Craniofac Res. 2008;11:51–56. doi: 10.1111/j.1601-6343.2008.00414.x. [DOI] [PubMed] [Google Scholar]
- 5.Geurtsen W. Biocompatibility of dental casting alloys. Crit Rev Oral Biol Med. 2002;13:71–84. doi: 10.1177/154411130201300108. [DOI] [PubMed] [Google Scholar]
- 6.Okazaki Y, Gotoh E. Comparison of metal release from various metallic biomaterials in vitro. Biomaterials. 2005;26:11–21. doi: 10.1016/j.biomaterials.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Huang HH, Chiu YH, Lee TH, et al. Ion release from NiTi orthodontic wires in artificial saliva with various acidities. Biomaterials. 2003;24:3585–3592. doi: 10.1016/s0142-9612(03)00188-1. [DOI] [PubMed] [Google Scholar]
- 8.Ağaoğlu G, Arun T, Izgi B, Yarat A. Nickel and chromium levels in the saliva and serum of patients with fixed orthodontic appliances. Angle Orthod. 2001;71:375–379. doi: 10.1043/0003-3219(2001)071<0375:NACLIT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Ousehal L, Lazrak L. Change in nickel levels in the saliva of patients with fixed orthodontic appliances. Int Orthod. 2012;10:190–197. doi: 10.1016/j.ortho.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Amini F, Jafari A, Amini P, Sepasi S. Metal ion release from fixed orthodontic appliances—an in vivo study. Eur J Orthod. 2012;34:126–130. doi: 10.1093/ejo/cjq181. [DOI] [PubMed] [Google Scholar]
- 11.Mikulewicz M, Chojnacka K, Zielińska A, Michalak I. Exposure to metals from orthodontic appliances by hair mineral analysis. Environ Toxicol Pharmacol. 2011;32:10–16. doi: 10.1016/j.etap.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Matos de Souza R, Macedo de Menezes L. Nickel, chromium and iron levels in the saliva of patients with simulated fixed orthodontic appliances. Angle Orthod. 2008;78:345–350. doi: 10.2319/111806-466.1. [DOI] [PubMed] [Google Scholar]
- 13.Menezes LM, Quintão CA, Bolognese AM. Urinary excretion levels of nickel in orthodontic patients. Am J Orthod Dentofacial Orthop. 2007;131:635–638. doi: 10.1016/j.ajodo.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Minano E, Vicente COA, Calvo JL, Ortiz AJ. Metallic ion content and damage to the DNA in oral mucosa cells of children with fixed orthodontic appliances. Biometals. 2011;24:935–941. doi: 10.1007/s10534-011-9448-z. [DOI] [PubMed] [Google Scholar]
- 15.Mikulewicz M, Chojnacka K. Trace metal release from orthodontic appliances by in vivo studies: a systematic literature review. Biol Trace Elem Res. 2010;137:127–138. doi: 10.1007/s12011-009-8576-6. [DOI] [PubMed] [Google Scholar]
- 16.Faccioni F, Franceschetti P, Cerpelloni M, Fracasso ME. In vivo study on metal release from fixed orthodontic appliances and DNA damage in oral mucosa cells. Am J Orthod Dentofacial Orthop. 2003;124:687–694. doi: 10.1016/j.ajodo.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Natarajan M, Padmanabhan S, Chitharanjan A, Narasimhan M. Evaluation of the genotoxic effects of fixed appliances on oral mucosal cells and the relationship to nickel and chromium concentrations: an in-vivo study. Am J Orthod Dentofacial Orthop. 2011;140:383–388. doi: 10.1016/j.ajodo.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Wataha JC. Biocompatibility of dental casting alloys: a review. J Prosthet Dent. 2000;83:223–234. doi: 10.1016/s0022-3913(00)80016-5. [DOI] [PubMed] [Google Scholar]
- 19.Mikulewicz M, Chojnacka K, Janeczek M, Wołowiec P, Ziętek M, Górecki H. Bioaccumulation of toxic elements in pigs tissues. Pilot studies. Przem Chem. 2013;92:724–726. [Google Scholar]
- 20.Baričević M, Ratkaj I, Mladinić M, et al. In vivo assessment of DNA damage induced in oral mucosa cells by fixed and removable metal prosthodontic appliances. Clin Oral Investig. 2012;16:325–331. doi: 10.1007/s00784-010-0489-4. [DOI] [PubMed] [Google Scholar]
- 21.Spears JW, Jones EE, Samsell LJ, Armstrong D. Effect of dietary nickel on growth, urease activity, blood parameters and tissue mineral concentrations in the neonatal pig. J Nutr. 1984;114:845–853. doi: 10.1093/jn/114.5.845. [DOI] [PubMed] [Google Scholar]
- 22.Douglas WR. Of pigs and men and research: a review of applications and analogies of the pig, Sus scrofa, in human medical research. Orig Life Evol Biosph. 1972;3:226–234. doi: 10.1007/BF00928167. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann E, Medelnik J, Fink M, Lell M, Hirschfelder U. Three-dimensional volume tomographic study of the imaging accuracy of impacted teeth: MSCT and CBCT comparison—an in vitro study. Eur J Orthod. 2013;35:286–294. doi: 10.1093/ejo/cjr030. [DOI] [PubMed] [Google Scholar]
- 24.Sahmali SM, Kural O, Kilic Z. Systemic effects of nickel-containing dental alloys: analysis of nickel levels in serum, liver, and oral mucosa of guinea pigs. Quintessence Int. 1991;22:961–966. [PubMed] [Google Scholar]
- 25.Sunderman FW., Jr Biological monitoring of nickel in humans. Scand J Work Environ Health. 1993;19:34–38. [PubMed] [Google Scholar]
- 26.Iyengar V, Woittiez J. Trace elements in human clinical specimens: evaluation of literature data to identify reference values. Clin Chem. 1988;34:474–481. [PubMed] [Google Scholar]