Abstract
Objective:
To test the hypothesis that there are no differences in mutans streptococci (MS) adhesion between esthetic and metallic orthodontic arch wires based on their surface characteristics.
Materials and Methods:
Surface roughness (Ra) and apparent surface free energy (SFE) were measured for six wires—four esthetic, one nickel-titanium (NiTi), and one stainless-steel (SS)—using profilometry and dynamic contact angle analysis, respectively. The amount of MS (Streptococcus mutans and Streptococcus sobrinus) adhering to the wires was quantified using the colony-counting method. The surfaces, coating layers, and MS adhesion were also observed by scanning electron microscopy. Statistical significance was set at P < .05.
Results:
The Ra values of the esthetic wires were significantly different from one another depending on the coating method (P < .05). The NiTi wire showed the highest SFE, followed by the SS wire and then the four esthetic wires. The NiTi wires produced a significantly higher MS adhesion than did the SS wires (P < .05). The esthetic wires showed significantly lower MS adhesions than did the NiTi wire (P < .05). Pearson correlation analyses found moderate significant positive correlations between the SFE and the S mutans and S sobrinus adhesions (r = .636/.427, P < .001/P = .001, respectively).
Conclusions:
The hypothesis is rejected. This study indicates that some esthetic coatings on NiTi alloy might reduce MS adhesion in vitro in the short term.
Keywords: Orthodontic wire, Bacterial adhesion, Surface characteristics
INTRODUCTION
The orthodontic metal arch wire has progressed from stainless-steel (SS) and cobalt-chromium-nickel (Co-Cr-Ni) alloys to the nickel-titanium (NiTi) alloy1,2; these material changes have led to increased superelasticity, thermal shape memory, corrosion resistance, and biocompatibility.1 In addition, the growing number of adult orthodontic patients has given rise to a demand for more esthetic orthodontic appliances,3 as seen in the development of polymer-based esthetic wires, metallic arch wires coated with polymer materials,4,5 rhodium ion-implanted orthodontic wires, and gold-plated wires. However, coatings on orthodontic metal wires may influence surface characteristics, such as roughness and hardness, as well as mechanical properties, such as bending behavior.5 In practice, coated arch wires can be selected instead of uncoated counterparts in accordance with a patient's esthetic demand and clinical situation.
Oral hygiene and caries control are also important for successful orthodontic treatment. However, orthodontic wire may serve as a medium for plaque accumulation, thereby increasing the level of microorganisms in the oral cavity. Orthodontic patients with fixed appliances frequently present an abundance of Streptococcus mutans in plaque.6 Organic acids produced by mutans streptococci (MS), which include S mutans and Streptococcus sobrinus, cause enamel demineralization.7,8 Therefore, MS adhesion to orthodontic materials could be regarded as a key factor in the pathogenesis of enamel demineralization during orthodontic treatment.7
In vitro bacterial adhesion is influenced by surface characteristics of biomaterials, particularly surface roughness and surface free energy (SFE).7 It has been suggested that rougher surfaces promote bacterial adhesion to an extent that exceeds the influence of SFE by increasing the adhesion areas and preventing dislodgement of bacterial colonies.9 Some in vivo studies suggested a threshold surface roughness (Ra) for bacterial retention (Ra = 0.2 µm).10 A material with high SFE attracts more bacteria to its surface than one with low SFE, in accordance with thermodynamic rules.11 Although the physical and mechanical properties of orthodontic wires (including esthetic wires) have been extensively studied, bacterial adhesion based on their surface characteristics has not been as well investigated. Knowledge of surface characteristics of orthodontic wires will provide valuable information about bacterial adhesion and plaque-retaining capacities during orthodontic treatment.
The purpose of this in vitro study was to compare the adhesion tendency of MS to esthetic and metallic orthodontic wires in relation to their surface characteristics. The null hypothesis tested was that there would be no differences in MS adhesion or in surface characteristics between esthetic and metallic wires.
MATERIALS AND METHODS
Materials
Four esthetic (Ultraesthetic [UE], Dany Coated [DC], Bioforce Sentalloy White [BW], and TruGold [TG]) and two uncoated (SE NiTi [SN] and Remanium [RE]) orthodontic arch wires with a cross-sectional dimension of 0.016 × 0.022 inches were investigated. Their codes, compositions, manufacturers, and batch numbers are summarized in Table 1.
Table 1.
Orthodontic Arch Wires Investigated
The as-received wire surfaces were examined with a field emission-scanning electron microscope (FE-SEM; JSM-6700F; Jeol, Tokyo, Japan). For the four esthetic wires, the cross-sectional images of the coating layers were also observed. Each esthetic wire was embedded in epoxy resin for cross-sectioning using a low-speed diamond saw (Isomet; Buehler, Lake Bluff, Ill) under continuous water spray. The surface was ground, polished, and observed using FE-SEM after platinum sputter coating.
Surface Roughness
The wires (coated parts for the UE and DC wires) were cut in approximately 10-mm lengths with a wire cutter and embedded longitudinally in epoxy resin, ensuring that one surface remained uncovered. The mean roughness Ra of each specimen was measured using a profilometer (Surftest SV-400; Mitutoyo Corp, Kawasaki, Japan) at a stylus speed of 0.1 mm/s, a cutoff of 0.8 mm, and a range of 600 µm. The Ra of each specimen was recorded as the average of the five readings (n = 5).
Dynamic Contact Angle Analysis
To obtain advancing and receding contact angles (CAs) for each wire, the dynamic contact angle (DCA) measurements were performed on the basis of the Wilhelmy plate technique. The force acting on a plate partially immersed in a liquid can be represented by the following equation when the balance is reset to zero after each installation of the test sample12:
where F = total force, γl = surface tension of the liquid, L = perimeter (wetted length) of the sample, Θ = CA, Fb = buoyancy force, and FW = Wilhelmy force. Because the detected force values are the sum of the buoyancy force Fb and the Wilhelmy force FW, linear regression to the zero immersion depth will eliminate Fb,12 then:
Each wire (coated parts for the UE and DC wires) was cut in approximately 20-mm lengths with a wire cutter. Each cut wire sample was fixed to the electrobalance of a tensiometer (Sigma 700; KSV instruments Ltd, Helsinki, Finland). All DCA analysis tests were made at room temperature as single-loop measurements at an immersion depth of 10 mm and an immersion velocity of 10 mm/min.12 The detected hysteresis loops were used to calculate advancing and receding CAs during immersion (wetting) and emersion (dewetting) of the wire samples in water (Figure 1).13
Figure 1.
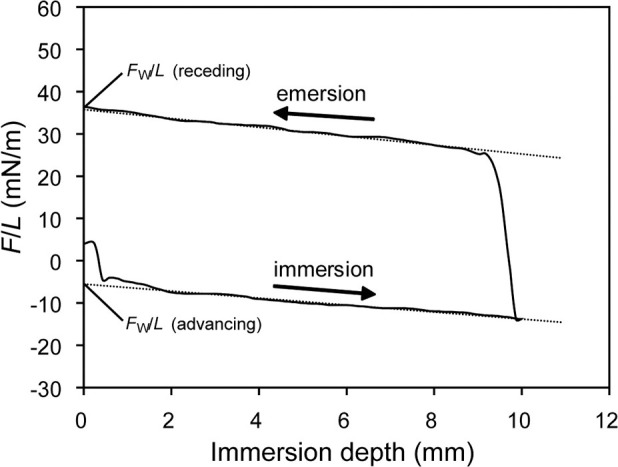
Schematic illustration of a single hysteresis loop of a dynamic contact angle experiment.
Solid total SFE (γs) of each wire was calculated from the surface tension of the probe liquid (γl; in this study, water = 72.8 mJ/m2) and the CA hysteresis of the liquid, as follows14:
where Θa and Θr are advancing and receding CAs, respectively.
Adhesion of MS
Each wire sample was cut into a 20-mm-long segment with a sharp wire cutter and sterilized under ultraviolet light.11 S mutans (ATCC 25175, KCTC 3065) and S sobrinus (KCTC 3288) were incubated in brain-heart infusion (BHI) medium. The optical density (OD) of the bacterial suspensions was adjusted to OD600 = 0.5.15 Each wire sample was placed into a microtube containing 1 mL of the prepared bacterial suspensions and incubated for 6 hours at 37°C.7 After washing three times with phosphate-buffered saline (PBS), each specimen was transferred to a new microtube containing 1 mL PBS. The adherent bacteria were then detached by sonication using four 30-second pulses with three 30-second intermittent coolings, following Yang et al.15 The suspensions with PBS were serially diluted up to 10−3 to 10−5, and 100 µL of each dilution was spread on a BHI agar plate.11 After incubation for 2 days, bacterial colonies on each plate were counted.15 Colony counts were expressed as a colony-forming unit (CFU) per unit area (cm2) of the wire specimen. All assays were run in triplicate and repeated three separate times (a total of 9 times for each material). The same procedures with the two strains were performed in the absence of a wire to validate the adhesion data. Whether S mutans and S sobrinus adhered to the wires was also observed using a FE-SEM after fixing, drying, and platinum sputter coating of the wire samples with the adhering MS. FE-SEM was used to check whether most of the bacteria were detached from the wires after sonication.
Statistical Analysis
For the roughness data, which did not meet the equal variance assumption (Leven's test), the Kruskal-Wallis test was employed, followed by the Mann-Whitney post hoc test, with adjustment of significance levels using the Benjamini and Hochberg method of a false discovery rate for a multiple testing correction.16 The adhesion data were log10 transformed to meet homogeneity of variance, then analyzed using one-way analysis of variance and the Fisher protected least significant difference (LSD) test for each strain. In addition, a post hoc power analysis was carried out to examine the power of the adhesion data using G*Power 3 software.17 In general, a power of about 0.80 is regarded as acceptable for most purposes.18 The degree of the correlation between the surface characteristics of the wires and the MS adhesion was determined by the Pearson correlation coefficient, r, and categorized by the following criteria: |r| > .7: strong; |r| between .3 and .7: moderate; and |r| < .3: weak.19 The statistical analyses were carried out using SPSS 17.0 for Windows (SPSS Inc, Chicago, Ill) at a significance level of .05.
RESULTS
Figure 2 presents the topographic differences of each wire using FE-SEM images of the orthodontic wires tested. BW showed a very thin coating layer as compared with the other three esthetic wires.
Figure 2.
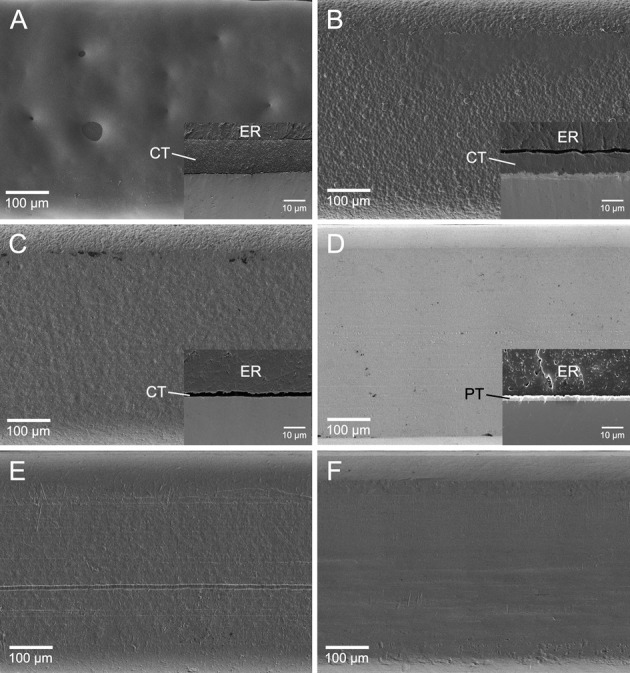
Surface SEM images of the orthodontic wires investigated (A: UE; B: DC; C: BW; D: TG; E: SN; F: RE; original magnification 150×, bar represents 100 µm). In the case of the four esthetic wires (A–D), SEM images of coating (CT) or plating (PT) are also shown in the small boxes (original magnification 2000×, bar represents 10 µm; ER indicates epoxy resin used for embedding).
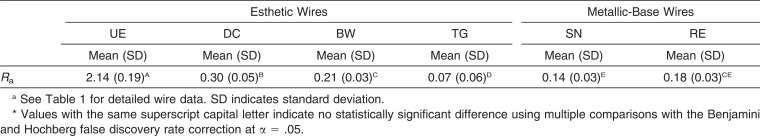
The surface roughness of the wires is summarized in Table 2. UE and TG showed the highest and lowest Ra values, respectively, among all the wires tested, the difference being statistically significant (both P = .033).
Table 2.
Surface Roughness (Ra, µm) of the Orthodontic Wires Tested in This Study (n = 5)
Figure 3 shows the resulting CA and apparent SFE calculated from the results of the DCA experiments. The calculations using Equation (3) revealed the highest and lowest apparent SFE in SN and DC, respectively, among the wires investigated.
Figure 3.
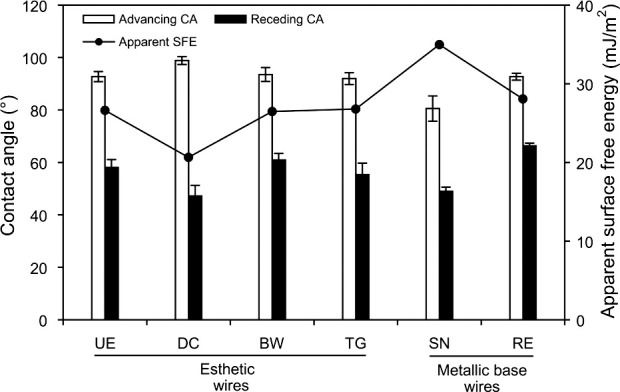
CA and apparent SFE calculated from the results of the DCA experiments (n = 3).
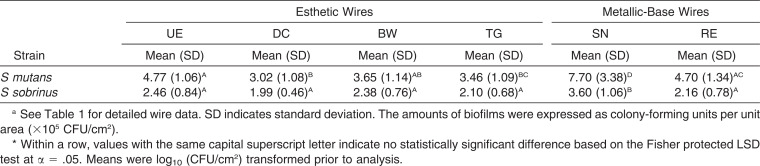
The results of MS adhesion to the wires are summarized in Table 3. It was verified that the CFU/cm2 values between the two strains (in the absence of a wire) were not significantly different, and few bacteria remained on the wire surfaces after sonication. According to the post hoc power analysis, the power values for both species were higher than 0.80 (0.99 and 0.86 for S mutans and S sobrinus, respectively). In general, adhesion of S mutans to wires was greater than that of S sobrinus. SN produced a significantly higher MS adhesion than RE (P < .05). The esthetic wires showed significantly lower MS adhesions than did SN (P < .05). Representative FE-SEM images (Figure 4) also support such bacterial-adhesion patterns.
Table 3.
Adhesion of Streptococcus mutans and Streptococcus sobrinus to Six Orthodontic Wires (n = 9)a
Figure 4.
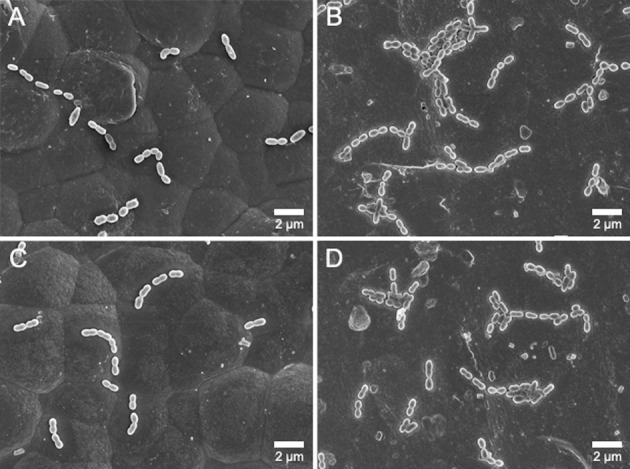
FE-SEM images of bacterial adhesion to the orthodontic wires (original magnification 5000×, bar represents 2 µm). Low S mutans and S sobrinus adhesion to the DC wire (A and C, respectively). High S mutans and S sobrinus adhesion to the SN wire (B and D, respectively).
Figure 5 shows the results of the Pearson correlation analyses between the apparent SFE and the bacterial adhesion. Moderate, significant, positive correlations were found between the SFE and the S mutans and S sobrinus adhesion (r = .636, P < .001; r = .427, P = .001; respectively).
Figure 5.
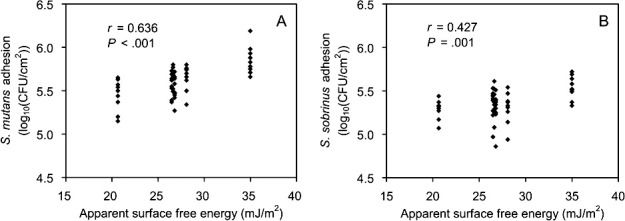
Pearson correlations between the apparent SFE and the S mutans adhesion (A) and S sobrinus adhesion (B) to the six orthodontic wires tested. r indicates the Pearson correlation coefficient.
DISCUSSION
Our findings suggest that esthetic coating of metallic wire decreases MS adhesion. Hence, the null hypothesis tested—that there would be no difference in MS adhesion between esthetic and metallic orthodontic arch wires based on their surface characteristics—was rejected.
Although the esthetic wire UE showed a significantly higher Ra value than did the other five materials, it did not produce significantly higher MS adhesion (Tables 2 and 3). This suggests that surface roughness is not the single factor determining MS adhesion. It is known that changes in roughness influence the CA, thereby changing SFE values.11 However, Busscher et al.20 suggested that, even in such cases, changes in solid-surface Ra below 0.1 µm have no effect on CA. As shown in Figure 2, the wire surfaces or coating/plating surfaces were far from ideal, and the differences in Ra value were larger than 0.1 µm (Table 2). In this study, thus, apparent SFEs derived from the DCA analysis were employed to characterize the wire surface or coating/plating layer surfaces. As the flat surface of the wires was too shallow to allow goniometric methods for measuring CAs, DCA measurements were performed on the basis of the Wilhelmy plate technique (Figure 1). Although the thicknesses of the coating or plating layers in the esthetic wires are different from one another and very thin in BW, DCA measurements used in this study still seem valid because the analysis depth of CA measurements is typically 0.3–2 nm.21
The apparent SFE results show trends that are different from those of the surface roughness results (Figure 3; Table 2). The NiTi-alloy wire, SN, showed the highest apparent SFE values among the wires tested. Oxide films present on the surface of SN and RE are responsible for their corrosion resistance.2 The oxide passive layer for NiTi alloys (such as SN) is mainly TiO2, whereas Cr-rich oxide passive film is present on SS alloy (such as RE) surfaces.2 SN and RE had significantly similar Ra values. It can, thus, be assumed that the substantially higher SFE for SN as compared to RE reflects a difference in the inherent chemical reactivity of the surfaces between the two metallic wires. MS adhesion results (Table 3) also showed a significantly higher adhesion in SN than in RE. Thus, SS orthodontic wires may be preferable to NiTi wires in terms of reducing MS adhesion. Demling et al.22 suggested that coating of the SS bracket with polytetrafluoroethylene (PTFE) reduces long-term in vivo biofilm formation. MS adhesion to PTFE-coated arch wires, not evaluated in the present study, needs further investigation.
Various esthetic coatings on the NiTi alloy wire (UE, DC, and BW vs SN) significantly decreased S mutans adhesion, probably by decreasing the SFE (Figure 3; Table 3). On the other hand, gold plating on SS (TG vs RE) did not significantly influence S mutans adhesion, probably because the gold-plated surfaces and SS surfaces had a similar apparent SFE. A similar tendency was also found in S sobrinus adhesion. Pearson correlation analyses (Figure 5) indicate that S mutans is more sensitive than S sobrinus to the surface characteristics of the materials with respect to initial adhesion.
Our short-term in vitro study suggests that the MS adhesion can be accounted for by the SFE of the materials rather than by their surface roughness. The influence of saliva coating, which might potentially affect the adhesion process,7,23 was not included in the present study, although it obviously requires further investigation. Moreover, coating layers on esthetic wires may peel off during orthodontic treatment,4 adversely promoting bacterial adhesion in such cases. Thus, long-term clinical observation of orthodontic wires will provide the most accurate information on the surface characteristics of the materials and their resultant susceptibility to bacterial adhesion.
Esthetic coating of NiTi wires may have an additional benefit for patients allergic to nickel.24 Coating treatments should primarily decrease surface roughness of the materials to improve the sliding of the wire.25 The advantages and drawbacks of such esthetic coating should be carefully and comprehensively evaluated by investigating various biomechanical properties of coated esthetic wires.
CONCLUSIONS
Some esthetic coatings on orthodontic arch wires (in particular, NiTi wires) are favorable for decreasing MS adhesion.
Future studies should investigate the influence of surface coating on in vivo biofilm adhesion.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2012-0009330). Authors are also grateful to the Korea Institute of Ceramic Engineering & Technology for the use of the tensiometry and to Dany BMT Co Ltd, for donating their materials. However, we have no conflict of interest that we should disclose.
REFERENCES
- 1.Kao CT, Ding SJ, He H, Chou MY, Huang TH. Cytotoxicity of orthodontic wire corroded in fluoride solution in vitro. Angle Orthod. 2007;77:349–354. doi: 10.2319/0003-3219(2007)077[0349:COOWCI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Mirjalili M, Momeni M, Ebrahimi N, Moayed MH. Comparative study on corrosion behaviour of Nitinol and stainless steel orthodontic wires in simulated saliva solution in presence of fluoride ions. Mater Sci Eng C Mater Biol Appl. 2013;33:2084–2093. doi: 10.1016/j.msec.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Imai T, Watari F, Yamagata S, Kobayashi M, Nagayama K, Nakamura S. Effects of water immersion on mechanical properties of new esthetic orthodontic wire. Am J Orthod Dentofacial Orthop. 1999;116:533–538. doi: 10.1016/s0889-5406(99)70185-x. [DOI] [PubMed] [Google Scholar]
- 4.Elayyan F, Silikas N, Bearn D. Ex vivo surface and mechanical properties of coated orthodontic archwires. Eur J Orthod. 2008;30:661–667. doi: 10.1093/ejo/cjn057. [DOI] [PubMed] [Google Scholar]
- 5.Iijima M, Muguruma T, Brantley W, et al. Effect of coating on properties of esthetic orthodontic nickel-titanium wires. Angle Orthod. 2012;82:319–325. doi: 10.2319/021511-112.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee HJ, Park HS, Kim KH, Kwon TY, Hong SH. Effect of garlic on bacterial biofilm formation on orthodontic wire. Angle Orthod. 2011;81:895–900. doi: 10.2319/121010-713.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SP, Lee SJ, Lim BS, Ahn SJ. Surface characteristics of orthodontic materials and their effects on adhesion of mutans streptococci. Angle Orthod. 2009;79:353–360. doi: 10.2319/021308-88.1. [DOI] [PubMed] [Google Scholar]
- 8.Kwon TY, Hong SH, Kim YK, Kim KH. Antibacterial effects of 4-META/MMA-TBB resin containing chlorhexidine. J Biomed Mater Res B Appl Biomater. 2010;92:561–567. doi: 10.1002/jbm.b.31553. [DOI] [PubMed] [Google Scholar]
- 9.Mei L, Busscher HJ, van der Mei HC, Ren Y. Influence of surface roughness on streptococcal adhesion forces to composite resins. Dent Mater. 2011;27:770–778. doi: 10.1016/j.dental.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Bollen CM, Papaioanno W, Van Eldere J, Schepers E, Quirynen M, van Steenberghe D. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin Oral Implants Res. 1996;7:201–211. doi: 10.1034/j.1600-0501.1996.070302.x. [DOI] [PubMed] [Google Scholar]
- 11.Kang SH, Lee HJ, Hong SH, Kim KH, Kwon TY. Influence of surface characteristics on the adhesion of Candida albicans to various denture lining materials. Acta Odontol Scand. 2013;71:241–248. doi: 10.3109/00016357.2012.671360. [DOI] [PubMed] [Google Scholar]
- 12.Rupp F, Axmann D, Ziegler C, Geis-Gerstorfer J. Adsorption/desorption phenomena on pure and Teflon AF-coated titania surfaces studied by dynamic contact angle analysis. J Biomed Mater Res. 2002;62:567–578. doi: 10.1002/jbm.10198. [DOI] [PubMed] [Google Scholar]
- 13.Rupp F, Scheideler L, Eichler M, Geis-Gerstorfer J. Wetting behavior of dental implants. Int J Oral Maxillofac Implants. 2011;26:1256–1266. [PubMed] [Google Scholar]
- 14.Kim YK, Son JS, Kim KH, Kwon TY. Influence of surface energy parameters of dental self-adhesive resin cements on bond strength to dentin. J Adhes Sci Technol. 2013;27:1778–1789. [Google Scholar]
- 15.Yang IH, Lim BS, Park JR, Hyun JY, Ahn SJ. Effect of orthodontic bonding steps on the initial adhesion of mutans streptococci in the presence of saliva. Angle Orthod. 2011;81:326–333. doi: 10.2319/062210-343.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryou DB, Park HS, Kim KH, Kwon TY. Use of flowable composites for orthodontic bracket bonding. Angle Orthod. 2008;78:1105–1109. doi: 10.2319/013008-51.1. [DOI] [PubMed] [Google Scholar]
- 17.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 18.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 19.Zhou ZJ, Fan SW. Letter to the editor: new equations for predicting postoperative risk in patients with hip fracture. Clin Orthop Relat Res. 2010;468:1181–1182. doi: 10.1007/s11999-010-1248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Busscher HJ, van Pelt AWJ, de Boer P, de Jong HP, Arends J. The effect of surface roughening of polymers on measured contact angles of liquids. Colloids Surf. 1984;9:319–331. [Google Scholar]
- 21.Ratner BD. Characterization of biomaterial surfaces. Cardiovasc Pathol. 1993;2:87–100. [Google Scholar]
- 22.Demling A, Elter C, Heidenblut T, et al. Reduction of biofilm on orthodontic brackets with the use of a polytetrafluoroethylene coating. Eur J Orthod. 2010;32:414–418. doi: 10.1093/ejo/cjp142. [DOI] [PubMed] [Google Scholar]
- 23.Buergers R, Schneider-Brachert W, Hahnel S, Rosentritt M, Handel G. Streptococcal adhesion to novel low-shrink silorane-based restorative. Dent Mater. 2009;25:269–275. doi: 10.1016/j.dental.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Rahilly G, Price N. Nickel allergy and orthodontics. J Orthod. 2003;30:171–174. doi: 10.1093/ortho/30.2.171. [DOI] [PubMed] [Google Scholar]
- 25.D'Antò V, Rongo R, Ametrano G, et al. Evaluation of surface roughness of orthodontic wires by means of atomic force microscopy. Angle Orthod. 2012;82:922–928. doi: 10.2319/100211-620.1. [DOI] [PMC free article] [PubMed] [Google Scholar]