Abstract
Background
Blood culture negative infective endocarditis (BCNIE) poses both a diagnostic and therapeutic challenge. High rates of BCNIE reported in South Africa have been attributed to antibiotic use prior to blood culture sampling.
Objectives
To assess the impact of a systematic approach to organism detection and identify the causes of infective endocarditis (IE), in particular causes of BCNIE.
Design
Prospective cohort study.
Methods
The Tygerberg Endocarditis Cohort study prospectively enrolled patients with IE between November 2019 and February 2021. A set protocol for organism detection with management of patients by an endocarditis team was employed. This prospective cohort was compared with a retrospective cohort of patients with IE admitted between January 2017 and December 2018.
Results
One hundred and forty patients with IE were included, with 75 and 65 patients in the retrospective and prospective cohorts, respectively. Baseline demographic characteristics were similar with a mean age of 39.6 years and male predominance (male sex=67.1%). The rate of BCNIE was lower in the prospective group (28/65 or 43.1%) compared with the retrospective group (47/75 or 62.7%; p=0.039). The BCNIE in-hospital mortality rate in the retrospective cohort was 23.4% compared with 14.2% in the prospective cohort (p=0.35). A cause was identified (including non-culture techniques) in 86.2% of patients in the prospective cohort, with Staphylococcus aureus (26.2%), Bartonella species (20%) and the viridans streptococci (15.3%) being most common.
Conclusion
The introduction of a set protocol for organism detection, managed by an endocarditis team, has identified Staphylococcusaureus as the most common cause of IE and identified non-culturable organisms, in particular Bartonella quintana, as an important cause of BCNIE. A reduction in in-hospital mortality in patients with BCNIE was observed, but did not reach statistical significance.
Keywords: adult cardiology, valvular heart disease, diagnostic microbiology, epidemiology, microbiology
Strengths and limitations of this study.
This is the first prospective cohort study that has evaluated the impact of an endocarditis team, with a set protocol for organism detection, on patients with infective endocarditis (IE) in South Africa.
A comprehensive protocol for organism detection was employed to minimise the rate of blood culture negative infective endocarditis (BCNIE) and identify non-culturable organisms.
Causative organisms of IE, in particular BCNIE, varies geographically. This may limit the generalisability of this data.
Introduction
Infective endocarditis (IE) is an infection involving the endocardial surface of the heart. This can affect native heart valves (native valve endocarditis), prosthetic valves (prosthetic valve endocarditis), non-valvular endocardial surfaces (such as IE affecting ventricular septal defects) or any non-valvular prosthetic devices.1–4 Identification of the causative organism via blood cultures is fundamental to the diagnosis and treatment of IE.2 4 Blood cultures that fail to identify the causative organism in patients with clinical and/or imaging evidence of IE—so called blood culture negative IE (BCNIE)—pose both a diagnostic and therapeutic challenge to the treating physician. BCNIE has been associated with worse outcomes compared with patients with blood culture positive IE (BCPIE), although more recent reports have demonstrated equivalent outcomes.5–8 It is important to note that the proportion of patients with BCNIE has decreased, which is likely due to a decrease in antibiotic use prior to blood culture collection.6 7 Although BCNIE is still diagnosed in a significant proportion of patients with IE, in the majority of patients the organism or cause is identified via non-culture-dependant methods. The identification of organisms responsible for BCNIE (and thus appropriate treatment) has coincided with more equal outcomes when comparing BCNIE to BCPIE presumably due to more targeted therapy.7 9 BCNIE was previously mainly attributed to sterilised blood cultures due to antibiotic use prior to acquisition of adequate blood culture samples. Although this is still a contributor, IE caused by organisms that are either intracellular or difficult to culture with standard culture methods, has emerged as an important cause of BCNIE.7 10 11 These organisms vary according to geographic region with Coxiella burnetii more common in European cohorts in contrast to African cohorts demonstrating Bartonella species as the most common cause of BCNIE.8 10 12 Non-infectious causes, for example, non-bacterial thrombotic endocarditis are rare causes of BCNIE.9 13 14
Very high rates of BCNIE have been reported in South Africa varying from 40% to 65%.9 13 14 This was attributed to high rates of antibiotic use prior to blood culture sampling (25%–52%), although no systematic approach to organism detection was employed and thus no information is available about the other causes of BCNIE in South Africa.2 9 14 Our group recently reported the emergence and typical clinical and imaging findings of Bartonella species as a cause of BCNIE in South Africa.15
We postulated that the implementation of a set protocol for organism detection and management of patients with IE by an endocarditis team would identify causes of BCNIE and improve the short-term outcome of patients with both BCPIE and BCNIE.
Methods
All patients presenting to the Division of Cardiology, Department of Medicine at Tygerberg Hospital in Cape Town, South Africa, with IE by current criteria2 between November 2019 and February 2021 were prospectively included in the Tygerberg Endocarditis Cohort study as previously described15. Patients with known or newly diagnosed malignancy were excluded from this study.
The Division of Cardiology at Tygerberg Hospital is a public sector tertiary referral centre that serves a population of approximately 2.4 million people.16 All patients presenting with features of IE to hospitals within the referral network are referred to Tygerberg Hospital for definitive care.
All patients were managed by an endocarditis team2 which fulfilled all the criteria as set out by current guidelines. All patients underwent standard transthoracic echocardiography and transoesophageal echocardiography (TEE) in the absence of identifiable contra-indications to TEE. Additional imaging was performed at the discretion of the endocarditis team.
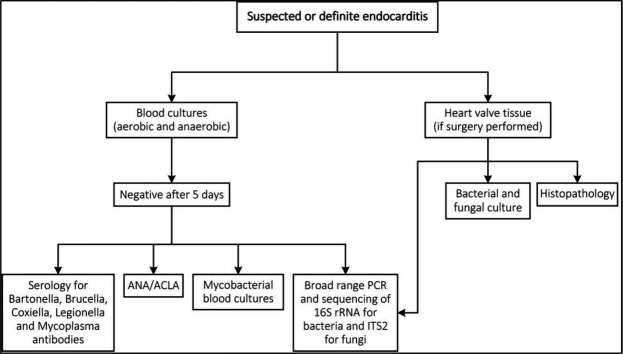
A stepwise protocol for organism detection (online supplemental file A) was used to identify the causative organisms of IE and to minimise the incidence of BCNIE (figure 1). A minimum of three sets of blood cultures (BacT/ALERT, bioMérieux, Marcy l’Etoile, France), including one aerobic and one anaerobic bottle per set, were required, with repeated cultures if clinical features of infection persisted. Further management and analysis of the samples were done according to current published guidelines.2 15 Patients without an identified organism after 5 days, using standard culture techniques, were defined as BCNIE.
Figure 1.
Protocol for organism detection. ACLA, anti-cardiolipin antibodies; ANA, antinuclear factor.
bmjopen-2021-053169supp001.pdf (76.6KB, pdf)
All patients with BCNIE underwent venous blood analysis (all tests performed in parallel) for further testing, including:
Testing for antinuclear antibodies and anti-cardiolipin antibodies.
Serology was performed using indirect immunofluorescence assays (IFA) for detection of IgM and IgG antibodies to Bartonella henselae and Bartonella quintana (FOCUS Diagnostics, Cypress, California, USA). Specific antibodies to Coxiella burnetii were also determined by IFA. Enzyme immunoassays were performed to detect IgM and IgG antibodies to Brucella species, Legionella pneumophila (EUROIMMUN, Lübeck, Germany) and Mycoplasma pneumoniae (EUROIMMUN, Lübeck, Germany).
Direct PCR was performed on blood culture bottles for detection of the universal bacterial 16S rRNA and ITS2 for fungi, followed by sequencing to identify the amplified DNA product.
BACTEC Myco/F Lytic vials (Becton Dickinson, San Jose, California, USA) were collected for the isolation of Mycobacteria, including Mycobacterium tuberculosis and non-tuberculous Mycobacteria.
A tissue sample was collected from all patients who required surgery and this was submitted for:
Bacterial and fungal culture.
Broad range PCR with 16S rRNA for bacteria and ITS2 for fungi, followed by sequencing to identify the amplified DNA product.
Histopathologic examination to detect bacteria and fungi, as well as histopathological features of IE.
All patients were managed according to current guidelines by the endocarditis team and prospectively followed.2 15 Baseline demographic and clinical features, results of special investigations including microbiological evaluation and imaging findings were documented on all patients. Treatment strategy, including specific antimicrobial therapy and surgical interventions were documented. Patients were followed until hospital discharge and all major adverse events (death, embolic events, renal failure) were recorded.
To evaluate the impact of this strategy, the prospective cohort was compared with a retrospective cohort that comprised of patients with IE admitted to Tygerberg Hospital from January 2017 to December 2018. In this latter cohort, diagnostic evaluation and treatment was not standardised and was at the discretion of the managing physician (rather than formalised in an endocarditis team), without a stepwise protocol for organism detection. Serology, blood PCR and heart valve PCR was rarely performed. All retrospective data were collected from patient folders, echocardiography, laboratory and surgical databases.
Patients who presented within the Tygerberg Hospital referral network but demised due to IE before referral to Tygerberg Hospital were included to minimise selection bias.
Statistical analysis
Statistical analysis was done using SPSS V.27 for iOS and JASP (V.0.14.1) for iOS.
Descriptive statistics were calculated, nominal data were compared via cross tabulation and χ2 tests, parametric data were compared using independent-sample t-tests (Cohen’s d) and non-parametric data were compared using independent-samples t-test (Mann-Whitney U or Kruskal-Wallis one-way analysis of variance).
Patient and public involvement
It was not possible to involve patients or the public in the design, conduct, reporting or dissemination plans of our research.
Results
A total of 140 patients with IE were included, with 75 and 65 patients in the retrospective and prospective cohorts, respectively. The baseline characteristics of patients in both cohorts are summarised in table 1. The mean age was 39.6 years with a male predominance (male sex=67.1%). Fourteen of the 75 patients (21.5%) in the retrospective cohort were HIV-positive compared with 18 of 65 (29%) in the prospective cohort (p=0.21). There was no difference in absolute CD4 count (442 cells/µL vs 402 cells/µL; p=0.96) or use of antiretroviral therapy (10/14 vs 13/18; p=0.98). The rate of BCNIE (table 2) was significantly lower in the prospective group (28/65 or 43.1%) as compared with the retrospective group (47/75 or 62.7%; p=0.04). The number of patients with BCNIE with no organism or cause detected was significantly lower in the prospective cohort compared with the retrospective cohort (13.8% vs 57.4%; p<0.01). The in-hospital mortality rate was 23.4% in the retrospective group with BCNIE compared with 14.2% in the prospective cohort (p=0.35).
Table 1.
Demographic profile
| All patients, n=140 | Retrospective cohort, n=75 | Prospective cohort, n=65 | P value | |
| Age in years, mean (SD) | 39.6 (12.8) | 39.6 (12.4) | 39.5 (13.1) | 0.80 |
| Male sex | 94 (67.1%) | 51 (68%) | 43 (67%) | 0.81 |
| Diabetes | 7 (5%) | 3 (4%) | 4 (6.3%) | 0.55 |
| Hypertension | 25 (17.9%) | 15 (20%) | 10 (15.6%) | 0.51 |
| Current smokers | 51 (34.4%) | 27 (36%) | 24 (37.5%) | 0.88 |
| PLHIV | 32 (22.9%) | 14 (21.5%) | 18 (29%) | 0.21 |
| CD4 count in cells/µL, median (Q1;Q3) | 423 | 442 (137;568) | 409 (204;568) | 0.96 |
| c-ART | 23 (71.8%) | 10/14 (71.4%) | 13/18 (72%) | 0.98 |
| History of intravenous drug use | 10 (7.1%) | 5 (6.7%) | 5 (7.7%) | 0.93 |
| History of valvular heart disease | 40 (28.6%) | 24 (32%) | 16 (25%) | 0.37 |
| Previous cardiac surgery | 20 (14.3%) | 10 (13.3%) | 10 (15.6%) | 0.70 |
| Definite IE by the modified Duke/ ESC 2015 clinical criteria2 | 83 (59.3%) | 35 (46.7%) | 48 (73.8%) | <0.01 |
c-ART, combination anti-retroviral therapy; IE, infective endocarditis; PLHIV, people living with HIV.
Table 2.
Results of blood cultures and short-term mortality
| All patients, n=140 | Retrospective cohort, n=75 | Prospective cohort, n=65 | P value | |
| BCNIE | 75 (53.6%) | 47 (62.7%) | 28 (43.1%) | 0.02 |
| BCNIE with no organism or cause detected | 53 (37.9%) | 44 (57.4%) | 9 (13.8%) | <0.01 |
| In-hospital mortality BCPIE | 10/65 (15.4%) | 5/28 (17.9%) | 5/37 (13.5%) | 0.64 |
| In-hospital mortality BCNIE | 15/75 (20%) | 11/47 (23.4%) | 4/28 (14.2%) | 0.35 |
BCNIE, blood culture negative infective endocarditis; BCPIE, blood culture positive infective endocarditis.
The baseline comparison of patients with BCPIE and BCNIE in the prospective cohort is summarised in table 3. The baseline characteristics of these groups were similar, except for the number of intravenous drug abusers that was significantly higher in the BCPIE group (5 vs 0) and the number of current smokers that was significantly higher in the BCNIE group (27% vs 60.3%; p<0.01). The rate of antibiotic use prior to blood culture sampling was not significantly different in the BCPIE group when compared with the BCNIE group (19.4 vs 35.7%; p=0.15).
Table 3.
Baseline characteristics of BCPIE versus BCNIE in the prospective cohort
| BCPIE (n=37) | BCNIE (n=28) | P value | |
| Age (mean) | 39.75 | 38.2 | 0.64 |
| Male sex | 23 (63.9%) | 20 (71.4%) | 0.44 |
| Diabetes | 4 (11.1%) | 0 | |
| Hypertension | 5 (13.9%) | 5 (17.9%) | 0.68 |
| Current smokers | 7 (27%) | 17 (60.7%) | <0.01 |
| PLHIV | 9 (25.7%) | 9 (33.3%) | 0.49 |
| CD4 count cells/µL (mean) | 347 | 470 | 0.67 |
| c-ART | 6 (66.7%) | 7 (77.8%) | 0.65 |
| History of intravenous drug use | 5 (13.5%) | 0 | |
| History of valvular heart disease | 9 (25%) | 7 (25%) | 1 |
| Previous cardiac surgery | 7 (19.4%) | 3 (10.7%) | 0.35 |
| Antibiotic therapy prior to blood culture sampling | 7 (19.4%) | 10 (35.7%) | 0.15 |
| Surgery performed | 19 | 20 | 0.11 |
| In-hospital mortality | 5 (13.5%) | 4 (14.2%) | 0.95 |
BCNIE, blood culture negative infective endocarditis; BCPIE, blood culture positive infective endocarditis; c-ART, combination anti-retroviral therapy; PLHIV, people living with HIV.
Serology for Bartonella and Mycoplasma species (15/28; 53.5%) and heart valve PCR (9/20; 45%) had the highest yield for identifying the causative organism in patients with BCNIE (table 4).
Table 4.
Results of set protocol for organism detection in patients with BCNIE in the prospective cohort
| Test performed | n=28 |
| Mycobacterium specific blood cultures positive | 0 |
| Anti-nuclear antibodies positive (ANA) | 1 |
| Serology indicating acute infection | 15 (53.5%) |
| Bartonella species | 13 |
| Mycoplasma species | 2 |
| Blood PCR positive | 1/22 (4.5%) |
| Mycoplasma hominis | 1 |
| Heart valve PCR positive | 9/20 (45%) |
| Bartonella quintana | 6 |
| Bartonella henselae | 1 |
| Mycoplasma hominis | 1 |
| Alternaria species | 1 |
| Histopathological confirmed IE | 20/20 (100%) |
| Cause identified (Mycobacterium tuberculosis) | 1/20 |
| Heart valve culture positive | 0/20 |
BCNIE, blood culture negative infective endocarditis; IE, infective endocarditis.
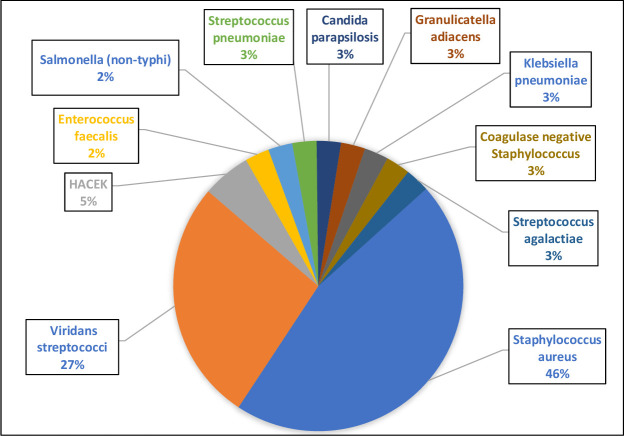
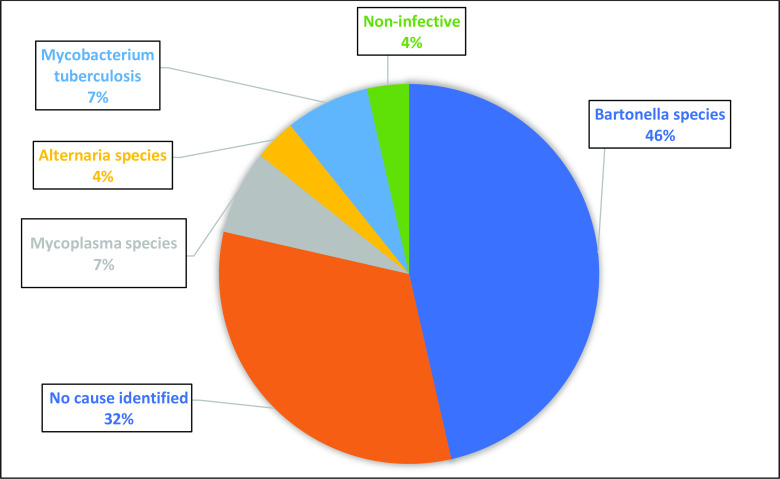
The most common causes of BCPIE (figure 2) in the prospective cohort were Staphylococcus aureus (45.9%) and viridans streptococci (27%). The causes of BCPIE were similar in the retrospective cohort with Staphylococcus aureus (43%) and viridans streptococci (32%) the most common. The most common cause of BCNIE (figure 3) in the prospective cohort was Bartonella species (46%).
Figure 2.
Causes of BCPIE (%). BCPIE, blood culture positive infective endocarditis; HACEK, haemophilus, aggregatibacter, cardiobacterium, eikenella, kingella.
Figure 3.
Causes of BCNIE (%) detected by non-culture dependent techniques. BCNIE, blood culture negative infective endocarditis.
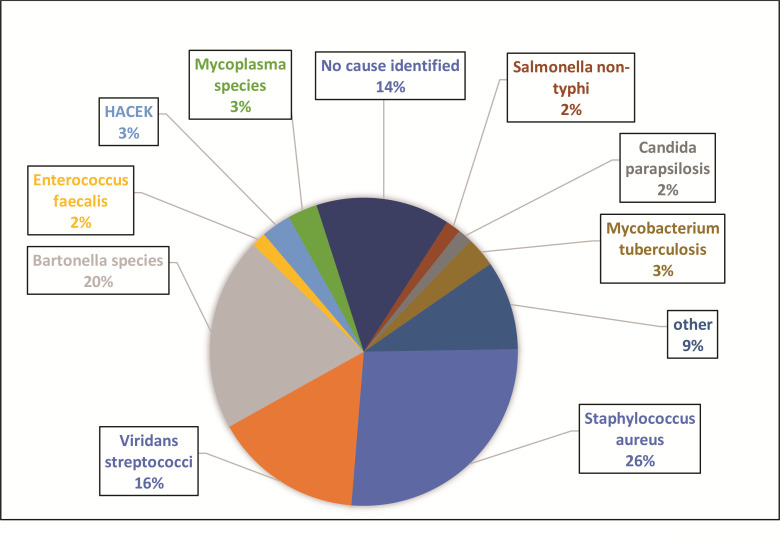
Considering the comprehensive microbiological evaluation, including serology and PCR data, a causative organism was identified in 86.2% of patients (figure 4) in the prospective cohort, with Staphylococcus aureus (26.2%), Bartonella species (20%) and the viridans streptococci (15.3%) being the most common.
Figure 4.
Causes of IE after set protocol for organism detection. HACEK, haemophilus, aggregatibacter, cardiobacterium, eikenella, kingella; IE, infective endocarditis.
Discussion
The establishment of a set protocol for organism detection significantly decreased the number of patients with IE where no causative organism or disease was detected. This was achieved by a significant reduction in the incidence of BCNIE and an improvement in non-culture identification of Bartonella species (Bartonella quintana in particular), Mycoplasma species and Mycobacterium tuberculosis as causes of BCNIE in the Western Cape region of South Africa. The finding of Bartonella species as the most common cause of BCNIE contrasts with European data where Coxiella burnetii has been demonstrated to be the most common cause of BCNIE.2 6 10 17 No previous study has systematically evaluated the causes of BCNIE in South Africa. However, evaluation of patients with BCNIE in Algeria and Ethiopia, developing nations similar to South Africa, also found Bartonella species to be the the most common cause of BCNIE.2 10 12 This finding has important implications for future diagnostic algorithms and empirical therapy in South Africa.
Current guideline empirical therapy for IE has limited efficacy against Bartonella and Mycoplasma species.2 This would suggest that a significant number of patients with BCNIE may previously have been inadequately treated.2 In this relatively small cohort of patients with BCNIE, we demonstrated a 39.3% reduction in in-hospital mortality (23.4% vs 14.2%; p=0.35). This reduction may be due to the introduction of an endocarditis team2 and the increased detection and subsequent effective treatment of the causative organism. A variety of factors may have contributed to the fact that this did not reach statistical significance. Our protocol dictated that we only perform additional investigations if initial blood cultures remained negative. This meant that additional investigations were only done 5 days after presentation and the addition of appropriate antibiotic therapy in patients with Bartonella species and other fastidious organisms were necessarily delayed beyond 5 days. During the COVID-19 pandemic, strain on healthcare resources also caused some delay in surgical intervention and the performing of blood and heart valve PCR. We would propose that serology for both Bartonella and Mycoplasma species be performed as part of the initial work up of patients with suspected IE. The addition of doxycycline to current guideline empirical therapy for BCNIE in countries with known or likely high rates of these organisms should be considered.2 18 19 Doxycycline has proven effective in the treatment of both Bartonella-and-Mycoplasma associated IE, although current guidelines propose levofloxacin as first-line therapy for Mycoplasma associated IE.2 19 The availability, low cost and favourable side effect profile of doxycycline makes it an ideal add on therapy in South Africa.20
This is the first report of the effect that a set protocol for organism detection, managed by an endocarditis team, has on the incidence of BCNIE in South Africa and it mimics the reduction reported from other groups.9 Although the rate of antibiotic administration prior to blood culture sampling was still high (25.6%), the introduction of an endocarditis team managed to reduce the rate of antibiotic use prior to blood culture sampling compared with a previous prospective study at our institution (25.6% vs 52.2%).9 More specific data regarding antibiotic use prior to blood culture sampling were unfortunately not available for the retrospective cohort. This effect of the endocarditis team may be due to increased awareness and upskilling of the initial treating physicians as well as improving pathways for referral and further management.21 The reduction in antibiotic use prior to blood culture sampling was an important contributor to the decrease in patients with BCNIE in the prospective cohort compared with the retrospective cohort (61.3%; p=0.039) and previous prospective cohort study (55.3%) performed at our institution.9 13 Additional factors that may have contributed to the lowering of the BCNIE rate was the mandatory collection of a minimum of three sets of blood cultures, repeated sampling if clinical features of infection persist and the routine use of both aerobic and anaerobic blood culture bottles.
The spectrum of BCPIE has changed in South Africa, with a profile similar to developed countries. In both our retrospective and prospective cohorts, Staphylococcus aureus was the most common causative organism, which contrasts with a previous series from our centre.9 The demographic profile of patients in both our cohorts was similar to previous series,9 except for the significant increase in intravenous drug users. All patients who volunteered an intravenous drug use history were culture positive for Staphylococcus aureus (10/10; 100%). However, even if intravenous drug users were excluded, Staphylococcus aureus remained the most common causative organism in both cohorts. Some empirical protocols for the treatment of IE in South Africa still exclude specific Staphylococcus aureus targeted antimicrobials (no addition of cloxacillin) because of previous data demonstrating the viridans streptococci to be the most common cause of IE with low rates of Staphylococcus aureus associated IE.1 9 Our data strongly support the empirical use of antimicrobial drugs that specifically target Staphylococcus aureus, as this is now established as the most common cause of IE in South Africa.
The different additional investigations to identify causes of BCNIE yielded contrasting results. Serology (53.5%) and heart valve PCR (45%) had the highest yield for identifying causes of BCNIE. Of the 13 patients with serological evidence of active Bartonella infection22 in the setting of BCNIE, eight patients underwent surgery. Heart valve PCR was positive in seven of the eight patients (88%); confirming Bartonella quintana (six patients) and Bartonella henselae (one patient) as the causative organism. This would suggest that serology for Bartonella species should be added to the initial venous blood analysis of all patients with suspected IE in South Africa. In addition, all patients with BCNIE undergoing surgery should have heart valve PCR performed in addition to culture, as the yield for Bartonella species is much higher with heart valve PCR.
Histological evaluation, especially microscopy, is an important additional investigation in patients undergoing valve surgery. Histopathology confirmed endocarditis in all of the patients in whom surgery was performed and confirmed Mycobacterium tuberculosis associated IE in one patient with clinical and imaging features typical of Mycobacterium tuberculosis associated IE.1 The diagnosis of tuberculous IE is usually suspected on typical clinical and imaging findings1 and confirmed with histopathology. Microbiological confirmation remains difficult.2 A second patient was diagnosed with Mycobacterium tuberculosis associated IE on the basis of typical clinical and imaging findings combined with a positive urinary lipoarabinomannan test. Histopathology revealed typical features of IE, but no typical features of Mycobacterium tuberculosis associated IE. This patient had been treated with anti-tuberculous antimicrobials for 31 days before surgery and this might have contributed to the inability of histopathology to identify Mycobacterium tuberculosis as the causative organism.
Both patients with Mycobacterium tuberculosis associated IE returned negative Mycobacterium specific (BACTEC Myco/F Lytic) blood cultures; none of the Mycobacterium-specific blood cultures was positive in any of the patients in the BCNIE group.
Blood PCR for patients with BCNIE had a very low yield (4.5%), with only a single positive result. The organism (Mycoplasma hominis) was also detected with heart valve PCR putting into question whether blood PCR is a cost-effective test for patients with BCNIE. Although low positive serology for Coxiella burnetii, Legionella pneumonia and Brucella species were common, not a single patient fulfilled criteria for regarding these as the causative organism.2 One patient with systemic lupus erythematosus returned positive serology for Brucella and Bartonella species; on final analysis these were considered false positive due to cross reactivity.
Conclusion
The introduction of a set protocol for organism detection with diagnosis and management by an endocarditis team not only lowered the rate of BCNIE but detected causative organisms that are difficult or impossible to culture. Bartonella species, and Bartonella quintana in particular, is the most common cause of BCNIE in the Western Cape, South Africa and empirical therapy directed at Bartonella species should be considered in patients with BCNIE. Future trials should evaluate if early therapy directed at Bartonella species as part of empirical therapy for IE improve short-term and long-term outcomes. Staphylococcus aureus has now been established as the most common cause of BCPIE in South Africa and all empirical regiments should include specific anti-staphylococcal therapy.
Limitations
The causative organisms associated with IE, in particular BCNIE, varies according to geographic region.1 7 This may limit the generalisability of our results.
During a large part of the study, the COVID-19 pandemic had a significant influence on healthcare services in South Africa.23–25 The exact influence of this pandemic on the treatment of IE in our institution is difficult to quantify, but it is safe to assume that delays from diagnosis to surgery were contributed to by the pandemic. The fact that we could still demonstrate a reduction in in-hospital mortality, although not statistically significant, strengthens the argument that the introduction of an endocarditis team with a set protocol for organism detection should improve patient outcomes. The inability of this study to demonstrate a statistically significant in-hospital mortality benefit is likely due to the small sample size and thus type II statistical error.
Supplementary Material
Acknowledgments
Dr M McCaul and the Division of Epidemiology and Biostatistics, Stellenbosch University for support with statistical analysis. Dr D Zaharie and the Division of Anatomical Pathology, Stellenbosch University. Dr E Ngarande, Research coordinator, Division of Cardiology, Department of Medicine, Stellenbosch University.
Footnotes
Twitter: @alfonsopecorar1
Contributors: All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing or revision of the manuscript. AJKP as the primary investigator was responsible for the conception and design of the study, acquisition of data, analysis and interpretation as well as drafting of the manuscript. CP, PGH and AFD contributed to the conception and design of the study, acquisition of data, analysis and interpretation as well as revising the manuscript critically for important intellectual content. SP, LJ, HP, JT, JJ and MN-F contributed to the acquisition, analysis and interpretation of data as well as revising the manuscript critically for important intellectual content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the Health Research Ethics Committee (HREC) of Stellenbosch University (Project numbers S19/08/162 and S19/10/234) and performed in accordance with the Declaration of Helsinki (2013 version). All patients in the prospective cohort signed written informed consent; a waiver of consent was obtained from HREC to include patients in the retrospective cohort.
References
- 1.Pecoraro AJ, Doubell AF. Infective endocarditis in South Africa. Cardiovasc Diagn Ther 2020;10:252–61. 10.21037/cdt.2019.06.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 2015;36:3075–128. 10.1093/eurheartj/ehv319 [DOI] [PubMed] [Google Scholar]
- 3.Cahill TJ, Prendergast BD. Infective endocarditis. Lancet 2016;387:882–93. 10.1016/S0140-6736(15)00067-7 [DOI] [PubMed] [Google Scholar]
- 4.Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017;69:325–44. 10.1016/j.jacc.2016.10.066 [DOI] [PubMed] [Google Scholar]
- 5.Zamorano J, Sanz J, Moreno R, et al. Comparison of outcome in patients with culture-negative versus culture-positive active infective endocarditis. Am J Cardiol 2001;87:1423–5. 10.1016/S0002-9149(01)01570-3 [DOI] [PubMed] [Google Scholar]
- 6.Trichine A, Foudad H, Bouaguel I, et al. 0175: reassessment of blood culture-negative endocarditis: its profile is similar to that of blood culture-positive endocarditis. Archives of Cardiovascular Diseases Supplements 2015;7:46–7. 10.1016/S1878-6480(15)71619-8 [DOI] [Google Scholar]
- 7.Tattevin P, Watt G, Revest M, et al. Update on blood culture-negative endocarditis. Med Mal Infect 2015;45:1–8. 10.1016/j.medmal.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 8.Suardi LR, de Alarcón A, García MV, et al. Blood culture-negative infective endocarditis: a worse outcome? results from a large multicentre retrospective Spanish cohort study. Infect Dis 2021;53:755–63. 10.1080/23744235.2021.1925342 [DOI] [PubMed] [Google Scholar]
- 9.Koegelenberg CFN, Doubell AF, Orth H, et al. Infective endocarditis in the Western Cape Province of South Africa: a three-year prospective study. QJM 2003;96:217–25. 10.1093/qjmed/hcg028 [DOI] [PubMed] [Google Scholar]
- 10.Fournier P-E, Gouriet F, Casalta J-P, et al. Blood culture-negative endocarditis: improving the diagnostic yield using new diagnostic tools. Medicine 2017;96:e8392. 10.1097/MD.0000000000008392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subedi S, Jennings Z, Chen SC-A. Laboratory approach to the diagnosis of culture-negative infective endocarditis. Heart, Lung and Circulation 2017;26:763–71. 10.1016/j.hlc.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 12.Tasher D, Raucher-Sternfeld A, Tamir A, et al. Bartonella quintana, an unrecognized cause of infective endocarditis in children in Ethiopia. Emerg Infect Dis 2017;23:1246–52. 10.3201/eid2308.161037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koshy J, Engel M, Carrara H, et al. Long term outcome and EuroSCORE II validation in native valve surgery for active infective endocarditis in a South African cohort. SA Heart 2018;15:116–26. 10.24170/15-2-3045 [DOI] [Google Scholar]
- 14.De Villiers MC, Viljoen CA, Manning K, et al. The changing landscape of infective endocarditis in South Africa. S Afr Med J 2019;109:592–6. 10.7196/SAMJ.2019.v109i8.13888 [DOI] [PubMed] [Google Scholar]
- 15.Pecoraro AA, Herbst PP, Pienaar CC, et al. Bartonella species as a cause of culture-negative endocarditis in South Africa. Eur J Clin Microbiol Infect Dis 2021;40:1873–9. 10.1007/s10096-021-04239-w [DOI] [PubMed] [Google Scholar]
- 16.Western Cape Government . City of Cape town 2017, 2017. Available: https://www.westerncape.gov.za/assets/departments/treasury/Documents/Socio-economic-profiles/2017/city_of_cape_town_2017_socio-economic_profile_sep-lg_-_26_january_2018.pdf
- 17.et alEdouard S, Nabet C, Lepidi H. Bartonella, a common cause of endocarditis: a report on 106 cases and review, 2015. Available: http://dx.doi.org/10.1128 [Accessed 04 Jun 2020]. [DOI] [PMC free article] [PubMed]
- 18.Moodley VM, Zeeman TTS, Van Greune CHJ, et al. Culture-Negative endocarditis due to Bartonella quintana. S Afr Med J 2016;106:470–1. 10.7196/SAMJ.2016.v106i5.9062 [DOI] [PubMed] [Google Scholar]
- 19.Fenollar F, Gauduchon R, Casalta J-P. Mycoplasma Endocarditis: Two Case Reports and a Review [Internet]. Available: https://academic.oup.com/cid/article/38/3/e21/292747 [Accessed 21 Feb 2021].
- 20.Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol 2013;112:1171–6. 10.1016/j.amjcard.2013.05.060 [DOI] [PubMed] [Google Scholar]
- 21.van Deventer JD, Herbst PG, Doubell AF, et al. Evaluation of the SUNHEART cardiology outreach programme: cardiology outreach programme. SA Heart 2015;12:82–6. [Google Scholar]
- 22.Bartonella Serology interpretation [Internet]. Available: https://www.childrensmn.org/references/Lab/serology/bartonella-antibody.pdf [Accessed 10 Nov 2020].
- 23.Society of Cardiology E . ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic [Internet], 2020. Available: https://www.escardio.org/static_file/Escardio/Education-General/Topicpages/Covid-19/ESCGuidanceDocument/ESC-Guidance-COVID-19-Pandemic.pdf [Accessed 31 May 2020].
- 24.Abdool Karim SS. The South African response to the pandemic. N Engl J Med 2020;382:e95. 10.1056/NEJMc2014960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pecoraro AJK, Herbst PG, Joubert LH. Dwindling myocardial infarctions: lessons from a pandemic. Eur Heart J 2020;41:3497–9. 10.1093/eurheartj/ehaa619 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-053169supp001.pdf (76.6KB, pdf)
Data Availability Statement
Data are available upon reasonable request.