Abstract
rRNA-based molecular phylogenetic techniques were used to identify the bacterial species present in the ear fluid from a female patient with otitis externa. We report the identification of Staphylococcus intermedius from the patient and a possible route of transmission. Analysis of 16S ribosomal DNA restriction fragment length polymorphisms indicated that the dominant species present was S. intermedius. A pet dog owned by the patient also was tested and found to harbor S. intermedius. In humans, the disease is rare and considered a zoonosis. Previously, S. intermedius has been associated with dog bite wounds, catheter-related injuries, and surgery. This study represents the first reported case of a noninvasive infection with S. intermedius.
Associated frequently with animals and rarely found in humans, Staphylococcus intermedius was first described as a new species in 1976 and differentiated from Staphylococcus aureus and Staphylococcus epidermidis based on biochemical and microbiological tests (9). S. intermedius has been isolated from the skin, hair, and gingiva of normal healthy dogs (2, 21) and has been found as a microbial inhabitant of several dog bite wounds (4, 14, 21, 22). Although this bacterium can be pathogenic in animals, it has been identified infrequently in humans (13, 15, 17, 23) and rarely causes disease. In the few instances of disease, S. intermedius was isolated only from patients who had undergone invasive procedures, for example, in a catheter-related bacteremia case (26) and following a coronary artery bypass grafting (8). Because S. intermedius is an uncommon component of the normal human microbiota (17) and can be associated with animal bite wounds, it represents a true zoonotic pathogen.
Methods for bacterial identification have been undergoing rapid change over the past decade, and molecular phylogenetic techniques are rapidly becoming the procedures of choice (10, 19, 25). Phylogenetic classification based on the 16S rRNA gene has clarified the taxonomy of many bacterial groups from genera to families and has led to the discovery of several new divisions (phyla) (12, 18). PCR amplification of the 16S rRNA gene directly from a sample of mixed microbiota alleviates the requirement for culturing (18). This is important, since most microorganisms (greater than 99% on earth) have not been cultured, and it may be very difficult to do so because many of them exist in complex natural communities, such as biofilms.
In the present study, we report the bacterial species found in the ear fluid of an otherwise healthy female patient with otitis externa, and we correlate these findings with the possible source of the infection, an indoor pet dog. S. intermedius was identified as the major bacterial component of ear fluid from this patient. It was also found that her indoor pet dog harbored S. intermedius. Following antibiotic treatment and recovery of the patient from otitis externa, the microbial population in the patient lacked S. intermedius.
Sample collection.
A sterile BBL CultureSwab (Becton Dickinson Microbiology Systems, Sparks, Md.) was used to collect ear fluid from a 38-year-old female patient with otitis externa, who was otherwise healthy. Samples from a 2-year-old dog (golden retriever) owned by the patient were collected in the same manner as above. The canine samples were from the ear, back, and chest. Both human and canine samples were stored at −80°C until processed. A control sample, S. intermedius ATCC 51874, was acquired from the American Type Culture Collection (ATCC; Manassas, Va.) and stored at −80°C.
DNA extraction and PCR.
Genomic DNA was obtained with a Soil DNA Isolation Kit according to the manufacturer's recommendation (Mo Bio Laboratories, Solana Beach, Calif.) and gave high-quality DNA suitable for PCR. The 16S and 23S ribosomal DNA (rDNA) primers used in this study are listed in Table 1. The following primer pairs were used for amplification of the genomic DNA with their specificities listed parenthetically: 515F-1492R (universal), 27F-1492R (Bacteria domain members only), StaphF-1492R (Staphylococcus and related genera), and ITS1F-ITS1R (S. intermedius and closely related species).
TABLE 1.
Oligodeoxynucleotide primers used for PCR amplification of bacterial DNA (16S rDNA, ITS, or 23S rDNA) from human, canine, and control samples
| Deoxyoligonucleotide name | Deoxyoligonucleotide sequencea (5′ to 3′) | Location on 16S rRNAb | Specificity |
|---|---|---|---|
| 515F | GTGCCAGCMGCCGCGGTAA | 515–533 | Universal |
| 1492R | GGYTACCTTGTTACGACTT | 1492–1510 | Universal |
| 27F | AGAGTTTGATCMTGGCTCAG | 8–27 | Bacteria |
| StaphF | CCTAAYCAGAAAGCCACGGC | 488–507 | Staphylococcus and related genera |
| ITS1F | GATAGAGTTTTCCTCTTCGGAG | 1015–1036 | S. intermedius and closely related species |
| ITS1R | GGGTTCCCCCATTCGGAAATC | 110–130 (23S rRNA) | Staphylococcus and related genera |
M = A + C, and Y = C + T.
Escherichia coli numbering system. ITS1F anneals to the 16S rDNA, whereas ITS1R anneals to the 23S rDNA. ITS1F-ITS1R amplifies the 3′ region of the 16S rRNA gene (nucleotide numbers 1037 to 1542), the ITS, and the 5′ region of the 23S rRNA gene (nucleotide numbers 1 to 109).
PCR amplification was performed in 50-μl reaction mixtures consisting of buffer reaction mix (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 0.001% [wt/vol] gelatin), 200 μM deoxynucleotide triphosphates, 1.5 mM MgCl2, 4 μM primer, and 1 to 5 μl of template DNA. Reactions were run for 35 cycles of amplification. After an initial denaturation at 94°C for 10 min to activate the AmpliTaq Gold (Perkin-Elmer, Norwalk, Conn.), cycles of 1 min at 94°C, 1 min at 50°C, and 1.5 min at 72°C were run. There was a final extension step at 72°C to ensure complete extension for efficient cloning. Each reaction mixture contained 2.5 U of AmpliTaq Gold.
Cloning and RFLP analysis.
PCR products were purified by a QIAquick PCR purification kit (Qiagen, Chatsworth, Calif.) and cloned with the TOPO TA cloning kit pCR2.1-TOPO vector (Invitrogen Corporation, Carlsbad, Calif.), according to the manufacturer's recommendations. rDNA inserts from pCR2.1 vector clones were reamplified by PCR with vector primers to the T7 and SP6 promoter sites, approximately equidistant from the 5′ and 3′ ends of the insert. Restriction fragment length polymorphism (RFLP) analysis was used to estimate the diversity of bacteria in the patient sample. DNA was digested with MspI and HinP1I (New England Biolabs, Beverly, Mass.). The digested DNA was separated on a 3% MetaPhor gel (FMC Bioproducts, Rockland, Maine) in 1× Tris-acetate-EDTA for about 2 h at 50 V.
Sequencing and phylogenetic analysis.
PCR products were sequenced on an ABI Prism 377XL automated DNA sequencer. 16S rDNA sequences were compared to known sequences in GenBank (5) with the advanced gapped BLAST (basic local alignment search tool) algorithm (3). The sequences were compiled in Chromas version 1.3 (Conor McCarthy, Griffith University, Brisbane, Queensland, Australia), aligned with the genetic database environment alignment editor, and placed into a phylogenetic tree containing approximately 8,000 rDNA sequences. The neighbor-joining tree was generated on the ARB application (O. Strunk, O. Gross, B. Reichel, M. Max, S. Hermann, N. Struckmann, B. Nonhoff, M. Lenke, A. Vilbig, T. Ludwig, A. Bode, K. H. Schleifer, and W. Ludwig, 1996 [http://www.mikro.biologie.tu-muenchen.de/pub/ARB/documentation/arb.ps]).
Results.
PCR was used to amplify 16S rDNA from a mixture of genomic DNA isolated from the ear fluid of a 38-year-old female with otitis externa. Primer pairs were designed to detect either most bacterial species (27F-1492R) or all three domains of life (515F-1492R). The bacterium-specific PCR product was cloned, and select clones were sequenced. The majority of 16S rDNA clones sequenced (∼700 nucleotides each) were identical to the 16S rDNA sequence of S. intermedius, an organism commonly associated with dogs and other animals. We then collected samples from a canine pet belonging to the female patient and amplified the 16S rDNA from microbial contents of the ear, as well as the chest and back. Canine microbial 16S rDNA was amplified with primers StaphF-1492R to limit the 16S rDNA PCR product mixture to a population of Staphylococcus and related genera for ease of analysis. All clones tested were indeed S. intermedius. The entire swab sample from both human and canine sources was used for PCR, and so there was no sample available for culturing.
Amplification of microbial 16S rDNA by PCR is very sensitive to contamination by microbial DNA in laboratory reagents and solutions (24, 27). Therefore, we analyzed negative controls by two methods. First, a control was prepared without the addition of sample (i.e., ear fluid) and worked up in the same manner as the true sample. Second, a control was performed during PCR that lacked input genomic DNA. No amplification was observed in these negative controls.
We performed RFLP analysis to provide more informative data on the population of bacteria in the otitis externa sample. An analysis of 20 samples indicated a population dominated by Staphylococcus species, particularly S. intermedius (RFLP type A, 50%). The other staphylococcal species present was identified as Staphylococcus capitis (RFLP type B, 15%)—a species related to S. epidermidis and a common inhabitant of the human integument (17). Additional species identified included Pseudomonas sp., Corynebacterium tuberculostearicum, and Dolosigranulum pigrum (RFLP types C, D, and E, respectively). All the sequenced 16S rDNA clones were 99% or greater in identity to known species except for the unknown Pseudomonas sp. (98% identity to Pseudomonas fluorescens).
To examine the similarities between S. intermedius from the human ear and the canine ear, we compared their 16S rDNA sequences with an external sample of S. intermedius isolated from a canine furuncle (ATCC 51874). Comparison of the 16S rDNA (1,211 nucleotides) of S. intermedius isolated in this study from both the human and the canine indicated that the sequences were identical at every position to the sequence of the ATCC sample. These sequences were nearly identical to the GenBank entry D83369, except at position 1260. Sequence D83369 has an A at position 1260, whereas the sequences from this study have a G.
In an attempt to identify greater heterogeneity, we amplified and cloned the 16S-23S intergenic spacer (ITS) of S. intermedius from the three samples. Primers ITS1F-ITS1R amplified the ITS (390 nucleotides long) and portions of the 16S and 23S rDNA genes (Table 1). However, the sequences were nearly identical in all three samples. One region at position 242 of the ITS differed in several clones examined. At this position, we observed a G-to-A change when comparing the samples from this study to the sample from the ATCC. A variable nucleotide at position 50 in the 23S rDNA changed from a U in the human and canine ear samples to a C in the ATCC sample.
The female patient was treated with the topical antibiotics neomycin and polymyxin B and quickly recovered from the otitis externa (approximately 4 days). We again tested an ear sample by PCR to examine the organisms present in the healthy ear. Examination of the 16S rDNA sequences indicated that the ear still harbored S. capitis and Pseudomonas sp., but S. intermedius was absent.
Discussion.
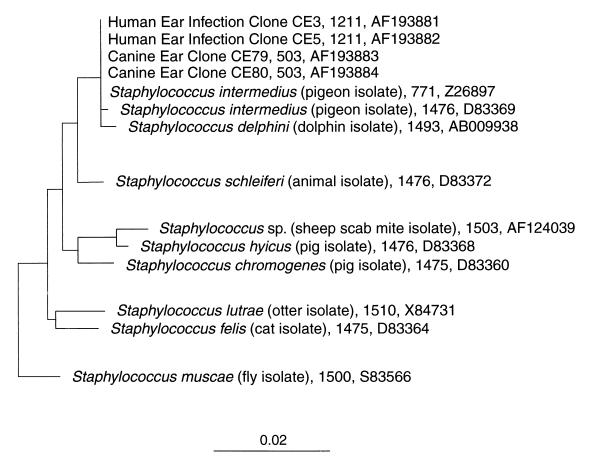
Molecular phylogenetic approaches based on PCR and sequence analysis of the 16S rRNA gene were applied to identify the bacteria present in an ear fluid sample from a patient with otitis externa. We analyzed 25 cloned 16S rDNA sequences from the 27F-1492R-amplified PCR product and noticed an undiversified population of bacteria dominated by one type. This bacterium was identified as S. intermedius, an organism normally associated with healthy animals but capable of causing disease in both animals and humans (2, 4, 6, 8, 26). S. intermedius is related to staphylococcal species isolated from various animals and falls into a cluster referred to as the Staphylococcus hyicus-intermedius cluster group (Fig. 1) (20). Four additional species were identified as S. capitis, Pseudomonas sp., C. tuberculostearicum, and D. pigrum. S. capitis and C. tuberculostearicum are inhabitants of the human integument and likely represent the normal microbiota. D. pigrum, isolated originally from the spinal cord of a multiple sclerosis patient and the eye swab from a neurotopic cornea (1), appears to be rare, as few descriptions exist. However, out of 25 clones, the D. pigrum 16S rDNA sequence was identified only once, and thus it is probably a minor component of the bacterial population. Interestingly, D. pigrum is closely related to the genus Alloiococcus, which includes the single species Alloiococcus otitis, a bacterium identified as a commensal frequently found in ear samples from healthy adults (data not shown).
FIG. 1.
Selected sequences of the genus Staphylococcus showing the S. hyicus-intermedius cluster group. The number of nucleotides analyzed and the corresponding GenBank number are to the right of the sequence. A neighbor-joining algorithm from the ARB software determined phylogenetic distances and topology. A local tree consisting of the approximately 45 Staphylococcus species was generated with Bacillus subtilis as an outgroup (data not shown). The bar indicates nucleotide changes per site.
Since we speculated that S. intermedius from the ear fluid of the human patient was acquired from the canine pet, we compared the sequences of the 16S rRNA genes from the human and canine samples to an outside source. We chose an ATCC S. intermedius strain originating from the furuncle (boil) of a dog. All three 16S rDNA sequences were identical in the regions analyzed. However, it is known that some species of bacteria have highly variable ITS sequences in the rRNA operon that allow for strain differentiation (16). Therefore, we amplified the ITS region between the 16S and 23S genes in the desire to uncover greater sequence heterogeneity. Although highly similar in all three samples, the ITS had one position of variation at nucleotide 242. This region of the ITS was identical in the two ear samples but changed in the ATCC sample. The ITS has been known to be highly conserved between different isolates in some species, such as Tropheryma whippelii (11) and Mycobacterium leprae (7).
Fortuitously, additional evidence for the similarity of S. intermedius from the human and canine ear sample clones was noticed at the extreme 5′ end of the 23S rDNA. A variable position in the 23S rDNA (position 50) changed from a U to a C in the human and canine clones but not the ATCC clones. Due to its greater length and larger number of variable positions, further sequence analysis of the 23S rDNA may be helpful in species and strain identification.
Talan et al. identified S. intermedius as a potential pathogen in dog bite wounds (21) and reported the first confirmed cases of human infection presenting S. intermedius (22) a decade ago. Since then, there have been additional reports of bite wounds harboring S. intermedius (4, 14). In humans, however, S. intermedius is rarely found as part of the normal microbiota, even in people who have contact with animals. In a study of the nasopharyngeal flora of 144 veterinary staff members, only one person was identified as harboring S. intermedius (23). Another study reported just two cases of S. intermedius out of 3,397 isolates from samples of hospitalized patients (15). A recent report on the distribution of Staphylococcus species within human clinical samples indicated the absence of S. intermedius from 1,230 strains tested (13). Interestingly, there has been a report of a dog with otitis media harboring S. intermedius (6), providing evidence that S. intermedius may be involved in canine ear infections.
PCR and sequence analysis have allowed us to rapidly identify the dominant species present in the ear samples from a human with otitis externa and the ear samples from the patient's pet dog. To our knowledge, this is the first reported case of a noninvasive infection with S. intermedius (i.e., not the result of bite wounds or surgical procedures). Since S. intermedius is not detected unless specific biochemical tests are done (9), it may be misclassified as S. aureus. We believe that S. intermedius may be an important zoonotic pathogen in humans who are in close contact with indoor pets. PCR of the 16S rDNA gene could be an effective alternative in detecting S. intermedius in human infections where zoonosis is suspected. It is not clear how widespread S. intermedius involvement in otitis externa may be, but patients who are in close contact with house pets may benefit from a more detailed analysis. This type of analysis does not require any culturing, and once DNA is prepared, there are no biohazard dangers. Regular bathing and cleansing of house pets and immediate treatment of pet ear infections with antibiotics may help to prevent zoonotic transmission to their owners.
Nucleotide sequence accession numbers.
The rDNA sequences of select clones from this study have the GenBank accession no. AF193881 to AF193888.
Acknowledgments
We thank Edward Bylina, William Coleman, and Mary Yang for comments on the manuscript.
This work was supported by grant R43GM60209-01 from the National Institutes of Health and by Office of Basic Energy Research grant 99ER20211 from the U.S. Department of Energy.
REFERENCES
- 1.Aguirre M, Morrison D, Cookson B D, Gay F W, Collins M D. Phenotypic and phylogenetic characterization of some Gemella-like organisms from human infections: description of Dolosigranulum pigrum gen. nov., sp. nov. J Appl Bacteriol. 1993;75:608–612. doi: 10.1111/j.1365-2672.1993.tb01602.x. [DOI] [PubMed] [Google Scholar]
- 2.Allaker R P, Lloyd D H, Simpson A I. Occurrence of Staphylococcus intermedius on the hair and skin of normal dogs. Res Vet Sci. 1992;52:174–176. doi: 10.1016/0034-5288(92)90006-n. [DOI] [PubMed] [Google Scholar]
- 3.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnham M, Holmes B. Isolation of CDC group M-5 and Staphylococcus intermedius from infected dog bites. J Infect. 1992;25:332–334. doi: 10.1016/0163-4453(92)91759-5. [DOI] [PubMed] [Google Scholar]
- 5.Benson D A, Karsch-Mizrachi I, Lipman D J, Ostell J, Rapp B A, Wheeler D L. GenBank. Nucleic Acids Res. 2000;28:15–18. doi: 10.1093/nar/28.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole L K, Kwochka K W, Kowalski J J, Hillier A. Microbial flora and antimicrobial susceptibility patterns of isolated pathogens from the horizontal ear canal and middle ear in dogs with otitis media. J Am Vet Med Assoc. 1998;212:534–538. [PubMed] [Google Scholar]
- 7.de Wit M Y L, Klatser P R. Mycobacterium leprae isolates from different sources have identical sequences of the spacer region between the 16S and 23S ribosomal RNA genes. Microbiology. 1994;140:1983–1987. doi: 10.1099/13500872-140-8-1983. [DOI] [PubMed] [Google Scholar]
- 8.Gerstadt K, Daly J S, Mitchell M, Wessolossky M, Cheeseman S H. Methicillin-resistant Staphylococcus intermedius pneumonia following coronary artery bypass grafting. Clin Infect Dis. 1999;29:218–219. doi: 10.1086/520168. [DOI] [PubMed] [Google Scholar]
- 9.Hájek V. Staphylococcus intermedius, a new species isolated from animals. Int J Syst Bacteriol. 1976;26:401–408. [Google Scholar]
- 10.Head I M, Saunders J R, Pickup R W. Microbial evolution, diversity, and ecology: a decade of ribosomal RNA analysis of uncultivated microorganisms. Microb Ecol. 1998;35:1–21. doi: 10.1007/s002489900056. [DOI] [PubMed] [Google Scholar]
- 11.Hinrikson H P, Dutly F, Altwegg M. Homogeneity of 16S-23S ribosomal intergenic spacer regions of Tropheryma whippelii in Swiss patients with Whipple's disease. J Clin Microbiol. 1999;37:152–156. doi: 10.1128/jcm.37.1.152-156.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hugenholtz P, Goebel B M, Pace N R. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J Bacteriol. 1998;180:4765–4774. doi: 10.1128/jb.180.18.4765-4774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawamura Y, Hou X-G, Sultana F, Hirose K, Miyake M, Shu S-E, Ezaki T. Distribution of Staphylococcus species among human clinical specimens and emended description of Staphylococcus caprae. J Clin Microbiol. 1998;36:2038–2042. doi: 10.1128/jcm.36.7.2038-2042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J. Staphylococcus intermedius isolated from dog-bite wounds. J Infect. 1994;29:105. doi: 10.1016/s0163-4453(94)95276-0. [DOI] [PubMed] [Google Scholar]
- 15.Mahoudeau I, Delabranche X, Prevost G, Monteil H, Piemont Y. Frequency of isolation of Staphylococcus intermedius from humans. J Clin Microbiol. 1997;35:2153–2154. doi: 10.1128/jcm.35.8.2153-2154.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naïmi A, Beck G, Branlant C. Primary and secondary structures of rRNA spacer regions in enterococci. Microbiology. 1997;143:823–834. doi: 10.1099/00221287-143-3-823. [DOI] [PubMed] [Google Scholar]
- 17.Noble W C. The human skin microflora and disease. In: Tannock G W, editor. Medical importance of the normal microflora. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1999. pp. 24–46. [Google Scholar]
- 18.Pace N R. A molecular view of microbial diversity and the biosphere. Science. 1997;276:734–740. doi: 10.1126/science.276.5313.734. [DOI] [PubMed] [Google Scholar]
- 19.Relman D A. Detection and identification of previously unrecognized microbial pathogens. Emerg Infect Dis. 1998;4:382–389. doi: 10.3201/eid0403.980310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi T, Satoh I, Kikuchi N. Phylogenetic relationships of 38 taxa of the genus Staphylococcus based on 16S rRNA gene sequence analysis. Int J Syst Bacteriol. 1999;49:725–728. doi: 10.1099/00207713-49-2-725. [DOI] [PubMed] [Google Scholar]
- 21.Talan D A, Staatz D, Staatz A, Goldstein E J C, Singer K, Overturf G D. Staphylococcus intermedius in canine gingiva and canine-inflicted human wound infections: laboratory characterization of a newly recognized zoonotic pathogen. J Clin Microbiol. 1989;27:78–81. doi: 10.1128/jcm.27.1.78-81.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talan D A, Staatz D, Staatz A, Overturf G D. Staphylococcus intermedius: clinical presentation of a new human dog bite pathogen. Ann Emerg Med. 1989;18:410–413. doi: 10.1016/s0196-0644(89)80582-7. [DOI] [PubMed] [Google Scholar]
- 23.Talan D A, Staatz D, Staatz A, Overturf G D. Frequency of Staphylococcus intermedius as human nasopharyngeal flora. J Clin Microbiol. 1989;27:2393. doi: 10.1128/jcm.27.10.2393-.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanner M A, Goebel B M, Dojka M A, Pace N R. Specific ribosomal DNA sequences from diverse environmental settings correlate with experimental contaminants. Appl Environ Microbiol. 1998;64:3110–3113. doi: 10.1128/aem.64.8.3110-3113.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanner M A, Shoskes D, Shahed A, Pace N R. Prevalence of corynebacterial 16S rRNA sequences in patients with bacterial and “nonbacterial” prostatitis. J Clin Microbiol. 1999;37:1863–1870. doi: 10.1128/jcm.37.6.1863-1870.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vandenesch F, Célard M, Arpin D, Bes M, Greenland T, Etienne J. Catheter-related bacteremia associated with coagulase-positive Staphylococcus intermedius. J Clin Microbiol. 1995;33:2508–2510. doi: 10.1128/jcm.33.9.2508-2510.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wintzingerode F, Göbel U B, Stackebrandt E. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev. 1997;21:213–229. doi: 10.1111/j.1574-6976.1997.tb00351.x. [DOI] [PubMed] [Google Scholar]