Abstract
BACKGROUND:
Data on human papillomavirus (HPV) prevalence and survival rates among HPV-infected women are scarce in Saudi Arabia.
OBJECTIVE:
Assess the prevalence of HPV genotypes in cervical biopsy specimens and its effect on survival over a 10-year timeframe.
DESIGN:
Retrospective, cross-sectional.
SETTINGS:
Saudi referral hospital.
PATIENTS AND METHODS:
Cervical biopsy specimens were collected from women aged 23-95 years old who underwent HPV detection, HPV genotyping, p16INK4a expression measurement using immunohistochemistry. Kaplan-Meier plots were constructed to analyze overall survival rates.
MAIN OUTCOME MEASURES:
Survival rate of HPV-positive cervical cancer patients.
SAMPLE SIZE:
315 cervical biopsy specimens.
RESULTS:
HPV was detected in 96 patients (30.4%): 37.3% had cervical cancer; 14.2% cervical intraepithelial neoplasia (CIN) III, 4.1% CIN II, and 17.0% CIN I. A significant association was found between HPV presence and cervical cancer (χ2=56.78; P<.001). The expression of p16INK4a was a significant predictor of survival: women who had p16INK4a overexpression had poorer survival rates (multivariate Cox regression, hazard ratio, 3.2; 95% CI, 1.1–8.8). In addition, multivariate models with HPV status and cervical cancer diagnosis showed that HPV status was a significant predictor of survival: HPV-positive women had better survival rates than HPV-negative women.
CONCLUSION:
These findings suggest that implementing cervical and HPV screening programs may decrease cervical cancer rates and improve survival rates of women in Saudi Arabia.
LIMITATION:
Single center and small sample size.
CONFLICT OF INTEREST:
None.
INTRODUCTION
Human papillomavirus (HPV), of the family Papillomaviridae, is present worldwide and is known to cause cervical cancer. More than 180 HPV genotypes have been identified, with low-risk types associated with benign hyperproliferative lesions or genital warts and high-risk types associated with pre-malignant and malignant cervical lesions.1 Of the 190 million women globally who received a diagnosis of an HPV clinical infection in 2012, approximately 528 000 also received a new diagnosis of cervical carcinoma, and 266 000 women died of this cancer.2 Approximately 85% of detected HPV cases occur in less developed countries despite current efforts to provide information about this virus and its relationship to cervical cancer to the general public as well as to health care professionals in these countries. Although it is has been estimated that 2.2% of the women in Western Asia have a cervical HPV infection, observations on the HPV burden in the more limited region of Saudi Arabia remain controversial.3 The results of some studies indicate that the rate of HPV infection in Saudi Arabia is the lowest in the world, whereas other studies show that the rate is high.4–7
Every abnormal or dysplastic lesion of the cervix should be considered potentially malignant and may develop into cervical cancer.8,9 Initial abnormal cervical epithelial cell detection is generally conducted through a Papanicolaou (Pap) test, commonly known as a Pap smear, which is considered the gold standard test, with the results reported using the Bethesda System of classification.10 A biopsy is performed, and histologic testing is used to confirm a diagnosis, with results, listed in order from the mildest to the most severe disease progression, of cervical intraepithelial neoplasia (CIN) I, CIN II, CIN III, and cervical cancer.10,11 However, the interpretation of these results is subject to intraobserver and interobserver variability.12 This subjectivity can be ameliorated by examining the expression of biomarkers in biopsied specimens to identify women with ambiguous results who should be referred for colposcopy and treatment, to distinguish transforming from productive HPV infections, and to predict disease severity.13 One such biomarker is p16INK4a, a cyclin-dependent kinase inhibitor that coordinates the cell cycle (G1 to S phase) and acts as a tumor suppressor. Overexpression of the p16INK4a protein has been used as a biomarker to distinguish transforming HPV infections and, in conjunction with histologic assessment of cervical biopsies, to confirm ambiguous results.14
Because of the high prevalence of HPV, the persistence of HPV infections, and the long latency for the development of cervical cancer, effective primary screening for detection of HPV infection and for HPV genotyping during the same clinical visit is important. Such screening would provide better outcomes for all women but especially for those in developing countries. Testing would be for the presence of HPV or HPV-associated serologic markers (HPV biomarkers) in addition to liquid-based cytology, the visual inspection of the cervix, or histologic examination.12,15
Cervical cancer is the most preventable cancer, and women with cervical cancer have a high survival rate when it is detected in the early stages.16 However, although the number of cases of cervical cancer is low in Saudi Arabia, most Saudi women do not seek medical attention until the late stages of the disease. The present study determined the prevalence of HPV and its genotypes among women attending a Saudi Arabian referral hospital between 2006 and 2016, assessed the association of HPV genotypes with cervical dysplasia and cancer, and determined whether HPV presence predicted survival.
PATIENTS AND METHODS
Cervical specimen collection
Formalin-fixed paraffin-embedded (FFPE) cervical specimens at various stages of cervical dysplasia and cancer archived from 2006 to 2016 were obtained from the Department of Pathology and Laboratory Medicine (CAP accredited) in King Faisal Specialist Hospital and Research Centre, the largest central and referral hospital in Saudi Arabia. All FFPE specimens with abnormal cervical findings were eligible for inclusion, but those with no clinical or demographic data available or those without diagnostic confirmation were excluded.
Cervical specimen processing
The FFPE specimen blocks were processed using a standard method. Briefly, four sections of tissues (4-μm thick) were obtained from each block for molecular assays. To minimize contamination, the microtome blade was disinfected after each use, and an empty paraffin-embedded block was used immediately after each disinfection step to control for any carryover contamination or inhibitors.
Immunohistochemical assays were performed to detect levels of p16INK4a expression in the cervical specimens using an anti-p16INK4a antibody (sc-460, Santa Cruz Biotechnology, Dallas, TX, USA) by following a previously published protocol.17 Brown nuclear or cytoplasmic staining was considered positive for p16INK4a expression. The p16INK4a expression level was assigned a value from 0 to 3 as follows: 0, no staining; 1, weak focal staining; 2, strong focal or weak diffuse staining; and 3, strong diffuse staining.17,18
DNA extraction was conducted using a QIAamp DNA FFPE Tissue kit and following the manufacturer's instructions (Qiagen; Valencia, CA, USA). This kit is specialized to extract and purify DNA from FFPE tissue. The extracted genomic DNA was quantified and checked for purity using a spectrophotometer, where the ratio of the sample absorbance at wavelengths 260 nm and 280 nm is approximately 1.8 (NanoDrop Technologies; Wilmington, DE, USA). To confirm the presence of DNA in each sample, primers for the β-globin gene (i.e., a housekeeping gene) were used.
Demographic and clinical statistical analyses
Demographic and clinical data were collected, including patient age, marital status, religion, nationality, and specimen pathologic results. All data were collected and analyzed using SAS (version 9.4) software. The percentage of women in each category who were HPV-positive was calculated and is presented with corresponding 95% confidence intervals (CIs). Descriptive analyses were performed for HPV prevalence, HPV genotypes, patient age distribution, potential risk factors, and HPV status. The χ2 test of independence was used to assess the association between categorical variables. Mann-Whitney and t tests were used to assess differences between categorical groups (i.e., HPV status, p16INK4a expression level, and cervical cancer status) and numerical variables, such as age. Odds ratio estimates and their 95% CIs were generated using the Mantel-Haenszel method. The type I error rate was set at 5%. An exploratory analysis was performed to assess the association between HPV status and age, and the adjusted odds ratio (adjusted for factors that are associated with the risk of HPV infection) was calculated. Any missing data in the measured outcome were removed from the final analysis.
We also calculated the survival rate for this hospital-based cohort. The overall survival rate was defined as the time between the date of diagnosis and the date of death or of the last follow-up observation. The Kaplan-Meier method was used to generate HPV-specific survival curves, and the differences between groups (HPV-positive vs. HPV-negative) were analyzed using the log-rank test. A multivariate Cox proportional hazards analysis was used to evaluate the association of overall survival with each prognostic factor (e.g., HPV type, disease stage, and age). In this cohort analysis, 29 patients overall died within the study time frame. Thus, owing to the small sample size, these results should not be generalized to the general population.
HPV polymerase chain reaction detection
A well-established set of polymerase chain reaction (PCR) primers were used for HPV detection. The primer sets used were MY09/MY11 and GP5+/GP6+ which target sequences located within the L1 region of the HPV genome was carried out as previously described.19 The products from the nested PCR were separated using 1.5% agarose gel electrophoresis and visualized using ultraviolet light (Gel Doc EZ Gel; Bio-Rad).
HPV detection and genotyping using the GenoFlow HPV array test
The GenoFlow HPV array test (DiagCor Bioscience Inc., Hong Kong) is a reverse dot blot assay with the ability to genotype 33 types of HPV. The procedure was carried as described in Alhamlan 2019.20 The genotyping results were interpreted using the manufacturer's instructions as follows: a valid HPV-positive result was one that included visible signals at the universal, hybridization control, and amplification control probe spots; a valid HPV-negative result included signals at the hybridization control and amplification control probe spots.
This study was conducted according to the World Medical Association Declaration of Helsinki. The study protocol was approved by the Research Advisory Council (Ethics Committee) at KFSHRC (No. 2150001).
RESULTS
The 315 women included in this study were aged 23 to 95 years (mean, 49.7 years; standard deviation, 13.3 years) (Table 1). The prevalence of HPV increased with age from 23–30 years to 46–60 years, with the prevalence for women older than 60 years slightly less than that for women aged 31-45 years (Figure 1). Most of the women claimed Saudi nationality, while 11.8% were non-Saudi. In addition, 74.6% of the women were married, 11.7% were widowed, 9.8% were single, and 3.8% were divorced. The pathologic results indicated that 37.5% (n=118) collected samples were positive for cervical cancer: 14.3% were classified as CIN III (n=45), 5.1% as CIN II (n=16), and 17.1% as CIN I (n=54), and 26.0% were considered normal tissue (n=82).
Table 1.
Demographic and clinical data analyzed by HPV status and presented as percentages of each subcategory (n=315) (percentages by row).
| Category | HPV-positive (n=96) | HPV-negative (n=219) | Test statistic (P value) |
|---|---|---|---|
| Age (years) | |||
| 23–30 | 2 (12.5) | 14 (87.5) | 7.9 (.047) |
| 31-45 | 30 (26.3) | 84 (73.7) | |
| 46–60 | 37 (30.3) | 85 (69.7) | |
| >60 | 27 (42.9) | 36 (57.1) | |
| Mean (SD) age (years) | 52.51 (13.9) | 48.5 (13.2) | 2.49 (.013) |
| Religion | |||
| Muslim | 90 (31.9) | 192 (68.1) | 2.6 (.10) |
| Non-muslim | 6 (18.2) | 27 (81.8) | |
| Nationality | |||
| Saudi | 87 (32.0) | 185 (68.0) | 2.1 (.14) |
| Non-Saudi | 9 (20.9) | 34 (79.1) | |
| Marital status | |||
| Married | 66 (28.1) | 169 (71.9) | 9.2 (.03) |
| Divorced | 4 (33.3) | 8 (66.7) | |
| Widowed | 19 (51.4) | 18 (48.6) | |
| Single | 7 (22.6) | 24 (77.4) | |
| Histologic classification | |||
| Normal | 6 (7.3) | 76 (92.7) | 63.9 (<.0001) |
| CIN 1 | 6 (11.1) | 48 (88.9) | |
| CIN II | 3 (18.8) | 13 (81.2) | |
| CIN III | 17 (37.8) | 28 (62.2) | |
| Cervical cancer | 64 (54.2) | 54 (45.8) | |
| Diagnosis | |||
| Cancer cases | 64 (54.2) | 54 (45.8) | 50.3 (<.0001) |
| Non-cancer cases | 32 (16.2) | 165 (83.8) | |
Data are number (%) except age (mean, standard deviation). Categorical data analyzed by χ2 test of independence; continuous data by t test (mean ages).
Figure 1.
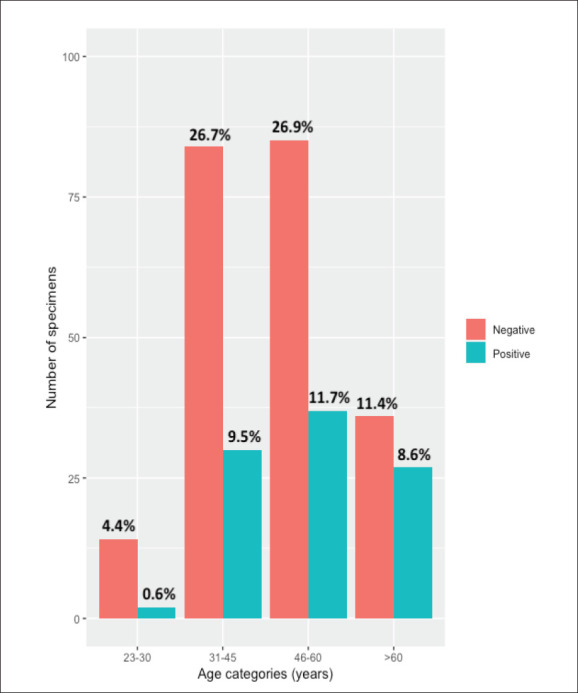
Distribution of HPV status by age category (n=315).
HPV status was analyzed with the other demographic and clinical data (age group, religion, nationality, marital status, histology grades, and cervical cancer diagnosis) using the χ2 test. Marital status, age groups, histologic grades, and cancer diagnosis were significantly associated with HPV status (P<.05 for each) (Table 1). Of the 315 tested cervical specimens, HPV was detected in 96 specimens (30.4%). The most common HPV high-risk types detected were HPV-16 (56.3%), and HPV-18 (7.3%). Nineteen percent of the cervical specimens had multiple infections (Table 2). The low-risk HPV types, including HPV-6, 11, 57, and 71, were detected mostly as co-infections with the high-risk types.
Table 2.
Distribution of high- and low-risk HPV subtypes as detected by GenoFlow HPV array assay in 96 formalin fixed paraffin-embedded specimens.
| High-risk genotypes detected | |
| 16 | 54 (56.3) |
| 18 | 7 (7.3) |
| 31 | 4 (4.2) |
| 33 | 2 (2.1) |
| 35 | 1 (1.0) |
| 45 | 1 (1.0) |
| 56 | 2 (2.1) |
| 58 | 3 (3.1) |
| 82 | 1 (1.0) |
| Multiple HPV Infections detected | |
| 11, 33 | 2 (2.1) |
| 16, 11, 33 | 1 (1.0) |
| 16, 18 | 3 (3.1) |
| 16, 31 | 1 (1.0) |
| 16, 51 | 1 (1.0) |
| 16, 57, 71 | 2 (2.1) |
| 16, 58 | 1 (1.0) |
| 16, 66, 68 | 1 (1.0) |
| 11, 31 | 1 (1.0) |
| 31, 33, 18 | 1 (1.0) |
| 53, 56 | 1 (1.0) |
| 6, 16 | 1 (1.0) |
| 6, 58 | 1 (1.0) |
| Unknown | 1 (1.0) |
| Negative | 3 (3.1) |
Data are number (%) or m.
Figure 2 shows the distribution of HPV status (positive or negative) by classification of cervical dysplasia or the presence of cervical cancer. Of the total specimens tested for HPV, 54.2% of cervical cancer cases tested positive: 37.8% of those classified as CIN III tested positive, 18.8% of those classified as CIN II tested positive, and 11.1% of those classified as CIN I tested positive. In addition, 92.7% of the specimens were both normal cervical tissue and negative for HPV.
Figure 2.
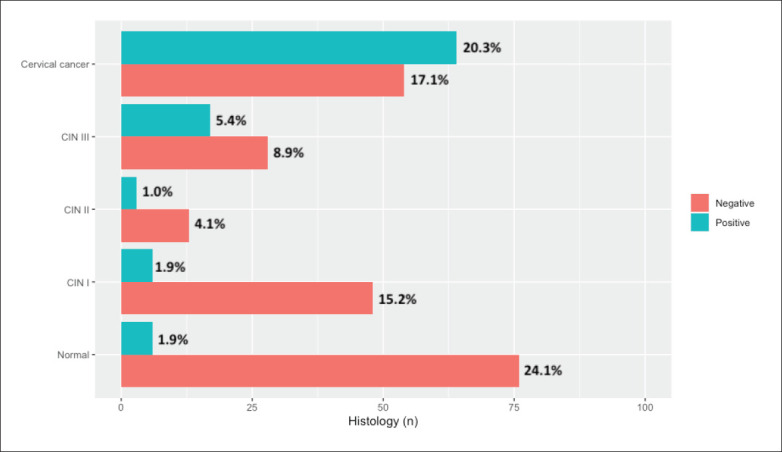
Distribution of the histologic classification by HPV status in 315 cervical specimens. Percentages calculated using the 315 specimens processed are shown on the bars. CIN: cervical intraepithelial neoplasia.
The p16INK4a immunohistochemistry staining was strongly positive in 212 samples (Table 3). The p16INK4a marker was significantly associated with both histologic abnormalities and HPV status (P<.0001). For the histologic abnormalities, approximately 86.9% of cervical cancer cases were positive for the p16INK4a marker, and the majority of both CIN II and CIN III specimens were positive for this marker. For the HPV status, 23.2% were both HPV- and p16INK4a-positive, and approximately 44.1% were negative for both HPV and p16INK4a (Table 3).
Table 3.
p16INK4a Immunohistochemistry results for 212 samples by histologic classification and HPV status with percentages calculated using the total number in each subcategory (row).
| Histologic classification | p16INK4a-positive | p16INK4a-negative | Chi-square test statistic (P value) |
|---|---|---|---|
| Normal | 2 (3.6) | 53 (96.4) | 113.1 (<.0001) |
| CIN 1 | 9 (24.3) | 28 (75.7) | |
| CIN II | 7 (70.0) | 3 (30.0) | |
| CIN III | 20 (80.0) | 5 (20.0) | |
| Cervical cancer | 73 (86.9) | 11 (13.1) | |
| HPV status | |||
| Positive | 49 (87.5) | 7 (12.5) | 37.2 (<.0001) |
| Negative | 62 (40.0) | 93 (60.0) | |
Data are number (%).
The survival analysis was conducted using data from 315 women, of which 29 died and 2 were excluded from the analysis. The initial summary of the survival analysis using Kaplan-Meier analysis (Supplementary material, Table S1), indicated that 2 patients had significantly longer survival time, exceeding 5 years, than the remaining patients, who had survival times of 3 years or less; therefore, these two patients were excluded from the analysis. Table 4 summarizes the Kaplan-Meier survival analyses of 313 patients. Figure 2 shows the Kaplan-Meier plot for cervical cancer clinical groups, Cervical cancer patients had a higher risk and lower survival rates than other patients (other examined groups are shown in the Supplementary Figures S1-S3).
Table 4.
Kaplan-Meier and Cox regression analysis summaries.
| Category | Mean overall survival (days) | Hazard ratio (95% Cl) | Log rank test P value | Maximum likelihood test P value |
|---|---|---|---|---|
| HPV status | ||||
| Positive (deceased=20) | 1278.8 | 0.9 (0.4–2.0) | .70 | .70 |
| Negative (deceased=7) | 1179.4 | 1 | ||
| Age group (years) | ||||
| 23–30 (deceased =3) | 552.9 | 1 | <.0001 | .0003 |
| 31–45 (deceased=4) | 1142.2 | 0.3 (0.1–1.1) | ||
| 46–60 (deceased =10) | 1229.2 | 0.7 (0.2–2.5) | ||
| >60 (deceased=10) | 659.5 | 2.8 (0.8–10.3) | ||
| P16ink4a expressiona | ||||
| Positive (deceased =14) | 825.2 | 3.2 (1.1–8.8) | .021 | .0003 |
| Negative (deceased=5) | 959.2 | 1 | ||
| Cervical cancer | ||||
| Positive (deceased=25) | 1343.6 | 17.9 (4.1–77.5) | <.0001 | <.0001 |
| Negative (deceased=2) | 2524.4 | 1 |
aFrom the 212 samples strongly determined by the p16 marker.
The distributions of survival in several groups were tested using the Kaplan-Meier method and validated with the log-rank test (univariate modeling) and using hazard ratios (HRs) validated with the maximum likelihood test. The Kaplan-Meier plots showed that a significant difference in survival distribution was detected by the presence of cervical cancer, age, p16INK4a expression level, and histologic grade (log-rank test, P<.05). For the HPV status, a lower HR was detected in positive HPV samples (HR, 0.9; 95% CI, 0.4–2.0). For the age groups, the HR was highest in the oldest group of women (HR, 2.8; 95% CI, 0.8–10.3). For cervical cancer, a higher HR was found in women with cervical cancer (HR, 17.9; 95% CI, 4.1–77.5), and the HR was statistically significant (χ2, 33.5; P<.0001). For the histologic grades, there was insufficient data to conduct an analysis because we did not include patients in the CIN II and CIN III groups who died during the time frame of the study. A higher HR was found in p16INK4a expression positive cervical specimens versus negative samples (HR, 3.2; 95% CI, 1.1–8.8). Kaplan-Meier plots generated for p16INK4a status by survival rate showed that the survival rate was higher among women whose specimens were negative for p16INK4a than among those positive for p16INK4a (log-rank test, P<.001). This finding indicated that p16INK4a expression directly reflected cervical specimens infected with high-risk HPV and thus added substantial diagnostic accuracy in the evaluation of CIN (Table 4).
A multivariate Cox regression analysis was conducted to determine the effect of all variables. Using a stepwise method, the model that best predicted survival was one that consisted of HPV status (HR, 0.24; 95% CI, 0.10–0.59) and cervical cancer diagnosis (HR, 39.51; 95% CI, 9.1–171.50). Other models are described in Table 5 (Figure 3).
Table 5.
Multivariate Cox regression model analyses.
| Multivariate Cox model using cervical cancer and HPV status | ||
|---|---|---|
| Factor | Hazard ratio (95% Cl) | P Value |
| HPV status | <.0001 | |
| Positive | 0.2 (0.1–0.6) | |
| Negative | 1 | |
| Cervical cancer | ||
| Positive | 39.5 (9.1–171.5) | |
| Negative | 1 | |
| Multivariate Cox model using cervical cancer and p16INK4a expression | ||
| p16INK4a expression | <.0001 | |
| Positive | 0.5 (0.2–1.6) | |
| Negative | 1 | |
| Carvical cancer | ||
| Positive | 27.9 (5.3–147.1) | |
| Negative | 1 | |
| Multivariate Cox model using HPV status, age group, and p16INK4a expression | ||
| p16INK4a expression | .015 | |
| Positive | 4.3 (1.5–12.4) | |
| Negative | 1 | |
| Age group, years | ||
| <30 | 1 | |
| 31–45 | 1.2 (0.1–11.3) .015 | |
| 46–60 | 2.3 (0.3–19.5) | |
| >60 | 12.3 (1.4–106.5) | |
| HPV status | ||
| Positive | 0.2 (0.05–0.7) | |
| Negative | 1 | |
Figure 3.
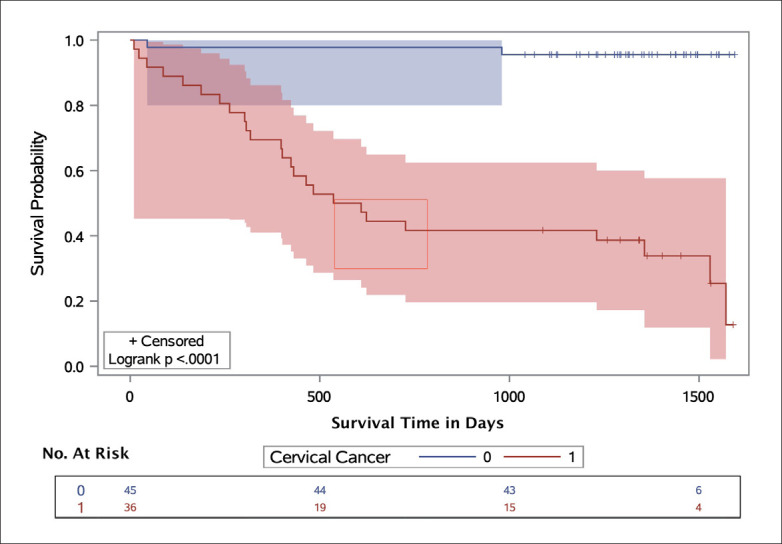
Kaplan-Meier (survival) plot by cervical cancer diagnosis in the KFSHRC cohort, with Hall-Wellner 95% confidence bands and survival rate test. Cervical cancer cases are represented by the red band, and patients negative for cervical cancer are represented by the blue band. The number of cases in the high-risk groups are shown below the figure.
DISCUSSION
HPV infection is the most common sexually transmitted infection and is particularly common among sexually active young women. The results of the present study showed slight differences in age-specific HPV prevalence, with HPV infection more common in middle-aged women (31–45 years) than in younger women (20–30 years). This discrepancy with the worldwide trend could be attributable to low sample size or differences in sexual practice, lifestyle, or genetic predisposition in Saudi Arabia.16 However, further studies and evaluation will be required because of the current changes in socioeconomic status, lifestyles, and sexual behaviors in regions that have been traditionally considered to be conservative societies.
HPV was detected in 96 of the 315 cervical specimens examined in the present study. The three most common HPV types detected were HPV-16 (56.3%), HPV-18 (7.3%), and HPV-31 (4.2%), with 19% of the cervical specimens having multiple infections. These data are consistent with previous studies reporting that HPV-16 is the most common type followed by HPV-18 where there was a significant association between the presence of HPV and cervical cancer.20
The present study also evaluated the association of HPV with the prognosis of women with cervical dysplasia. A multivariate Cox regression analysis showed the model with HPV status and cervical cancer diagnosis to be a significant predictor of survival. In this model, women with HPV-negative cervical specimens had a poorer survival rate than those with HPV-positive specimens. The results of many previous studies agree with our results, and those studies that are not in agreement do not have larger sample sizes than that of the present study. Rodrigues-Carunchio et al found that patients with cervical cancer and confirmed HPV negativity had significantly worse disease-free survival than women with HPV-positive tumors.21 In their multivariate analysis, HPV-negativity and International Federation of Gynecology and Obstetrics staging were associated with increased risk of progression and mortality.22 Li et al published a comprehensive meta-analysis showing the prognosis significance of HPV DNA status in cervical cancer.23 They evaluated survival data from 2838 women with cervical cancer and found that women who tested positive for high-risk HPV had a better cervical cancer prognosis (pooled HR, 0.4; 95% CI, 0.3–0.5; P<.001) and better overall survival (pooled HR, 0.6; 95% CI, 0.5–0.8; P=.001) than those who tested negative for high-risk HPV. Previous studies have discussed the better survival outcomes for women who are HPV-positive and have cervical cancer.24 Infection with a high-risk HPV is strongly associated with the development of cervical carcinoma. The gene sequences encoding the two major viral oncoproteins, E6 and E7, are consistently retained and expressed in cervical cancer cases. The E6 and E7 genes are thought to play causative roles because E6 promotes the degradation of p53, and E7 binds to the retinoblastoma protein and disrupts its complex formation with E2F transcription factors.25 However, there are independent factors that cause p53 mutations. The association of p53 mutation with metastases may explain the poor prognosis reported for HPV-negative primary cancers, many of which already contain mutant p53. A high proportion of p53 mutations detected in both primary and meta-static cancers are GC to TA transversions, strongly suggesting a role for external carcinogens in the development of these cancers. The evidence supports the idea that HPV-negative cancers, which frequently carry somatic p53 mutations, show a worse prognosis than HPV-positive, wild-type p53 tumors.26
Our data showed unexpectedly high numbers of CIN-III and cervical cancer cases that were negative for the presence of HPV. For specimens that had cervical cancer, these results may be explained by cervical cancer tumors that were not induced by the presence of a persistent HPV infection, including the misclassification of endometrial cancers or the metastasis of other cancers to the cervix, the loss of HPV expression, or the existence of cervical cancers that are not induced by HPV.27 However, many reports have indicated that HPV is a necessary but not sufficient cause of invasive cervical cancer; a variable proportion of tumors have been reported to be negative for high-risk HPV.28 In addition, a negative HPV test result from a cervical cancer specimen does not necessarily mean that HPV was never involved in the etiology of that cancer; an HPV viral infection may have been involved in the early development of the cancer but thereafter resolved. Nevertheless, we acknowledge that there are limitations in the current testing for detection of HPV, especially between the DNA and mRNA testing approaches. Currently, a DNA-based assay is considered best for HPV detection and typing in early infection stages; however, once the disease progresses, RNA-based assays are considered more accurate.29 Moreover, to obtain a higher number of samples, we used FFPE specimens, rather than fresh samples, and this may have affected our results.
The present study also assessed p16INK4a expression in cervical biopsy specimens and found that expression of p16INK4a increased with the severity of histologic abnormality. This finding is consistent with previous studies that reported evidence that p16INK4a immunostaining correlates with the severity of histological abnormalities.30 Previous studies have shown that p16INK4a is a highly sensitive and specific marker of high-grade squamous and glandular neoplasia of the cervix owing to its overexpression in cancerous and precancerous cervical lesions.31 However, the reproducibility of this finding may be limited by the lack of a standardized method to quantify and interpret p16INK4a immunostaining. Interestingly, the results of the immunohistochemistry biomarker p16INK4a were more strongly correlated with abnormal histologic classification than with HPV status.
To our knowledge, this is the first study examining the association between HPV infection and cervical cancer survival among women in Saudi Arabia. This study used a sensitive method capable of detecting a wide range (33 types) of HPV types, enabling the detection of most HPV types that have been associated with genital and sexual contacts. In addition, the 10-year retrospective analysis is a substantial strength of the study. However, there are several limitations of this study that should be considered when interpreting our results. Because of the low number of cervical cancer cases and late diagnoses, the number of study specimens was relatively small. This small number also informed our decision to use FFPE specimens, rather than fresh samples, to obtain a higher number of samples for the analyses.
The present study determined the prevalence of HPV among women attending a main referral hospital in Saudi Arabia over a 10-year timeframe. The results suggest that women with HPV-negative cervical specimens may have a poorer mean survival rate than those with HPV-positive specimens. Our study also highlighted the importance of accurate molecular diagnostic techniques for HPV detection and identification, which is crucial for diagnosing patients at risk. These findings contribute to the scientific and medical database and suggest that implementing cervical cancer and HPV screening programs in developing countries may help control cervical cancer and improve survival rates.
ACKNOWLEDGMENTS
We thank the Infectious Diseases Program, National Center for Biotechnology in King Abdulaziz City for Science and Technology for their financial and intellectual support. We also thank the members of the pathology department, chaired by Dr. Fouad Aldayel, for their help and efforts to retrieve the archived cervical specimens. We thank Dr. Angela Cox from Sheffield Medical School for her advice on statistical analysis. The financial support of the Research Centre administration at KFSHRC is highly appreciated.
Table S1.
Survival analysis summaries of Kaplan-Meier and Cox regression analyses including the two excluded patients.
| Mean overall survival (days) | Hazard ratio (95% Cl) | Log-Rank test P value | Maximum-likelihood test P value | |
|---|---|---|---|---|
| HPV status | ||||
| Positive | 1471.8 | 0.68 (0.30–1.6) | .40 | .395 |
| Negative | 12 349.7 | 1 | ||
| Age group (years) | ||||
| <30 | 644.5 | 1 | .0310 | .0248 |
| 31–45 | 1205 | 0.18 (0.04–0.80) | ||
| 46–60 | 2382 | 0.95 (0.20–1.90) | ||
| >60 | 1363.3 | 0.81 (0.22–2.93) | ||
| p16INK4a expression | ||||
| Positive | 2291.5 | 2.74 (0.99–7.52) | .0423 | .0371 |
| Negative | 951.88 | 1 | ||
| Cervical cancer | ||||
| Positive | 1343.6 | 11.79 (4.10–33.9) | <.0001 | <.0001 |
| Negative | 2524.4 | 1 | ||
| Histology | ||||
| CIN 1 | NA* | NA* | <.0001 | <.0001 |
| CIN II | 45 | 1 | ||
| CIN III | 2546.0 | 0.33 (0.02–5.3) | ||
| Cervical cancer | 1343.6 | 3.56 (0.48–26.3) |
*No observations recorded.
Figure S1.
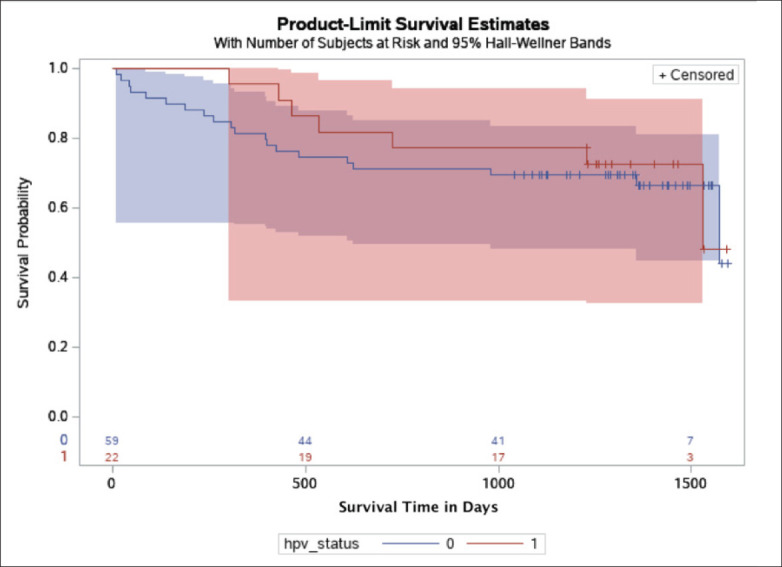
Kaplan-Meier (survival) plot by HPV status in the KFSHRC cohort, with Hall-Wellner 95% confidence bands. Patients positive for HPV (HPV status, 1) are represented as the red band, and patients negative for HPV are represented as the blue band (HPV status, 0). The numbers on the x axis are the number of patients at high risk by the HPV status groups.
Figure S2.
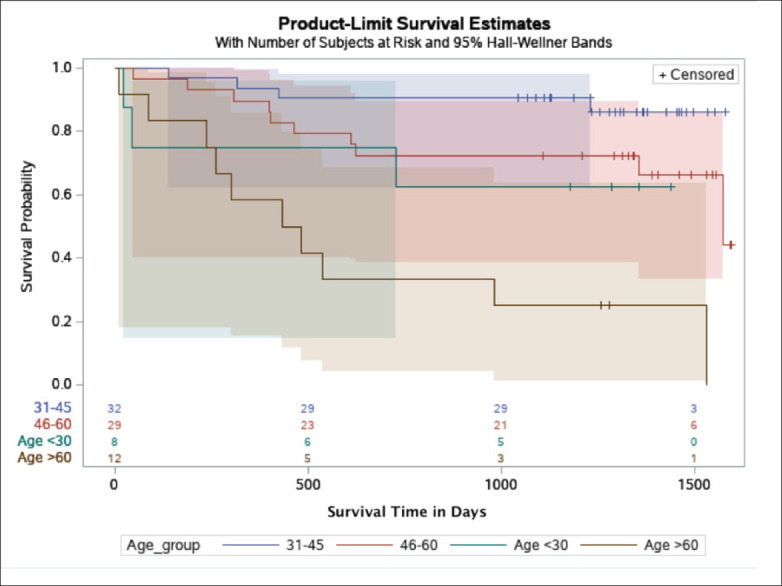
Kaplan-Meier (survival) plot by age group in the KFSHRC cohort, with Hall-Wellner 95% confidence bands. Green band represents patients who are younger than 30 years old; blue band, patients aged 31-45 years; red band, patients aged 46-60 years; and brown band, patients aged 60 years or older. The numbers on the x axis are the number of patients at high risk by the age groups.
Figure S3.
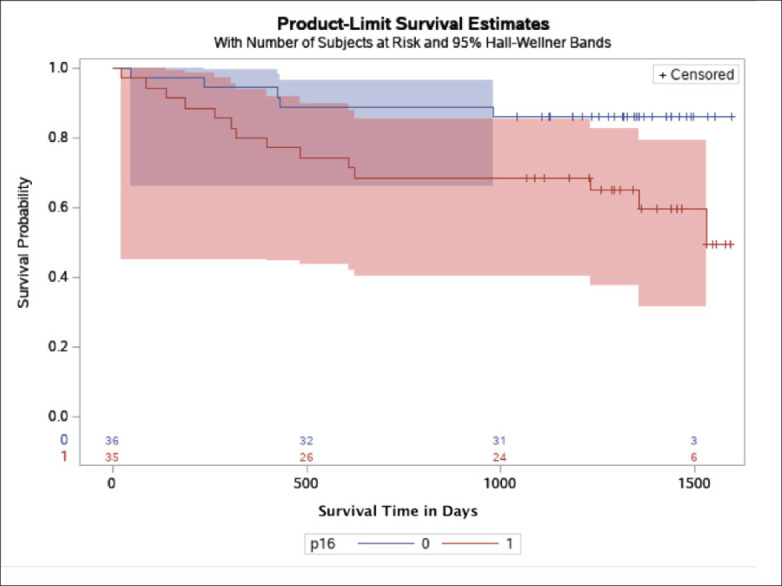
Kaplan-Meier (survival) plot by p16INK4a expression in the KFSHRC cohort, with Hall-Wellner 95% confidence bands. Cases with positive p16INK4a expression are represented by the red band; cases negative for p16INK4a expression are represented by the blue band. The numbers on the x axis are the number of patients at high risk by the p16INK4a expression groups.
Funding Statement
King Abdulaziz City for Science and Technology 2150001
REFERENCES
- 1.Fernandes J, Galvão de Araújo J, Allyrio Araújo de Medeiros Fernandes T.. Biology and natural history of human papillomavirus infection. Open Access Journal of Clinical Trials. 2013;:1
- 2.International Agency for Research on Cancer and World Health Organization. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. Available from: http://globocan.iarc.fr/Default.aspx
- 3.Canadian Cancer Survivor Network. WHO/ICO information centre on human papillomavirus (HPV) and cervical cancer. Available from: http://survivornet.ca/learn/health-concerns-for-cancer-patients/hpvand-cancer/whoico-information-centre-on-human-papilloma-virus-hpv-and-cervical-cancer/
- 4.Alsbeih G, Al-Harbi N, El-Sebaie M, Al-Badawi I.. HPV prevalence and genetic predisposition to cervical cancer in Saudi Arabia. Infect Agent Cancer [Internet]. 2013;8(1):15. Available from: 10.1186/1750-9378-8-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bondagji NS, Gazzaz FS, Sait K, Abdullah L.. Prevalence of high-risk human papillomavirus infections in healthy Saudi women attending gynecologic clinics in the western region of Saudi Arabia. Ann Saudi Med. 2013;33. [DOI] [PMC free article] [PubMed]
- 6.Al-Ahdal M, Al-Arnous W, Bohol M, Abuzaid S, Shoukri M, Elrady K, et al.. Human papillomaviruses in cervical specimens of women residing in Riyadh, Saudi Arabia: a hospital-based study. J Infect Dev Ctries [Internet]. 2014. Mar 13;8(03 SE-Original Articles). Available from: https://jidc.org/index.php/journal/article/view/24619263 [DOI] [PubMed]
- 7.Alhamlan FS, Khayat HH, Ramisetty-Mikler S, Al-Muammar TA, Tulbah AM, Al-Badawi IA, et al.. Sociodemographic characteristics and sexual behavior as risk factors for human papillomavirus infection in Saudi Arabia. Int J Infect Dis [Internet]. 2016. May 1;46:94–9. Available from: 10.1016/j.ijid.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 8.Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al.. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. The Journal of Pathology. 1999;189(1):12–9. [DOI] [PubMed] [Google Scholar]
- 9.Steenbergen RD, Wilde JD, Wilting SM, Brink AA, Snijders PJ, Meijer CJ.. HPV-mediated transformation of the anogenital tract. Journal of Clinical Virology. 2005;32:25–33. [DOI] [PubMed] [Google Scholar]
- 10.S. Apgar, L. Zoschnick, Wright T.C. Jr.. The 2001 Bethesda System terminology Am. Fam. Physician. 2003;68:992–1998. [PubMed] [Google Scholar]
- 11.C.P. Crum, E.E. Meserve, Peters W.A. III. Cervical squamous neoplasia. In: Diagnostic Gynecologic and Obstetric Pathology, 3rd Edition Elsevier. 2018:298–374 [Google Scholar]
- 12.Molijn A, Kleter B, Quint W, van Doorn LJ.. Molecular diagnosis of human papilloma-virus (HPV) infections J. Clin. Virol. 2005;32 Supp1:43–51. [DOI] [PubMed] [Google Scholar]
- 13.Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, et al.. Overexpression of p16INK4A as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. International Journal of Cancer. 2001;92(2):276–84. [DOI] [PubMed] [Google Scholar]
- 14.Tsoumpou I, Arbyn M, Kyrgiou M, Wentzensen N, Koliopoulos G, Martin-Hirsch P, et al.. p16INK4a immunostaining in cytological and histological specimens from the uterine cervix: A systematic review and meta-analysis. Cancer Treatment Reviews. 2009;35(3):210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Darwish AA, Al-Naim AF, Al-Mulhim KS, Al-Otaibi NK, Morsi MS, Aleem AM.. Knowledge about Cervical Cancer Early Warning Signs and Symptoms, Risk Factors and Vaccination among Students at a Medical School in Al-Ahsa, Kingdom of Saudi Arabia. Asian Pacific Journal of Cancer Prevention. 2014;15(6):2529–32. [DOI] [PubMed] [Google Scholar]
- 16.Alhamlan FS, Alahdal MNA, Al-Zahrani AS, Almatrrouk SA.. Human papillomaviruses: The cervical cancer saga in developing countries. The Journal of Infection in Developing Countries. 2017;11(11):819–25. [DOI] [PubMed] [Google Scholar]
- 17.Tan G, Norlatiffah S, Sharifah N, Razmin G, Shiran M, Hatta A, et al.. Immunohistochemical study of p16INK4A and survivin expressions in cervical squamous neoplasm. Indian Journal of Pathology and Microbiology. 2010;53(1):1. [DOI] [PubMed] [Google Scholar]
- 18.M.K. Chaloob, A.G. Hussein, B.J.. Qasim Correlation of p16 (INK4A) and CK17 to HPV (16E6+18E6) in premalignant and malignant lesions of uterine cervix: a clinicopathologic study Iran J. Pathol., 2016;11:377–390 [PMC free article] [PubMed] [Google Scholar]
- 19.Husman A-MDR, Walboomers JMM, Van Den Brule AAJC, Meijer CJLM, Snijders PJF.. The use of general primers GP5 and GP6 elongated at their 3’ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. Journal of General Virology. 1995;76(4):1057–62. [DOI] [PubMed] [Google Scholar]
- 20.Alhamlan FS, Khayat HH, Obeid DA, Tulba AM, Baduwais TS, Alfageeh MB, et al.. Clinical comparison of two human papil-lomavirus detection assays: GenoFlow and reverse line blot. J Infect Dev Ctries [Internet]. 2020. Jan 31;14(01 SE-Original Articles). Available from: https://jidc.org/index.php/journal/article/view/32088690 [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez-Carunchio L, Soveral I, Steen-bergen R, Torné A, Martinez S, Fusté P, et al.. HPV-negative carcinoma of the uterine cervix: a distinct type of cervical cancer with poor prognosis. BJOG: An International Journal of Obstetrics & Gynaecology. 2014;122(1):119–27. [DOI] [PubMed] [Google Scholar]
- 23.Lei J, Ploner A, Lagheden C, Eklund C, Kleppe SN, Andrae B, et al.. High-risk human papillomavirus status and prognosis in invasive cervical cancer: A nationwide cohort study. PLOS Medicine. 2018;15(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.M. Narisawa-Saito, T, Kiyono. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins Cancer Sci. 2007;98:1505–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.T. Crook, K.H. Vousden. Properties of p53 mutations detected in primary and secondary cervical cancers suggest mechanisms of metastasis and involvement of environmental carcinogens EMBO J. 1992;11:3935–3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.W. Tjalma HPV negative cervical cancers and primary HPV screening Facts Views Vis. Obgyn., 2018;10:107–113. [PMC free article] [PubMed] [Google Scholar]
- 27.Schiffman M, Kinney WK, Cheung LC, Gage JC, Fetterman B, Poitras NE, et al.. Relative Performance of HPV and Cytology Components of Cotesting in Cervical Screening. JNCI: Journal of the National Cancer Institute. 2018;110(5):501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.K. Cuschieri, N.. Wentzensen Human papillomavirus mRNA and p16 detection as bio-markers for the improved diagnosis of cervical neoplasia Cancer Epidemiol. Biomarkers Prev. 2008;17:2536–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xi LF, Hughes JP, Castle PE, Edelstein ZR, Wang C, Galloway DA, et al.. Viral Load in the Natural History of Human Papillomavirus Type 16 Infection: A Nested Case–Control Study. The Journal of Infectious Diseases. 2011;203(10):1425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarwath H, Bansal D, Husain NE, Mohamed M, Sultan AA, Bedri S.. Introduction of p16INK4a as a surrogate biomarker for HPV in women with invasive cervical cancer in Sudan. Infectious Agents and Cancer. 2017;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahasrabuddhe VV, Luhn P, Wentzensen N.. Human papillomavirus and cervical cancer: biomarkers for improved prevention efforts. Future Microbiology. 2011;6(9):1083–98. [DOI] [PMC free article] [PubMed] [Google Scholar]