Abstract
BACKGROUND:
SARS-CoV2/COVID-19 emerged in China and caused a global pandemic in 2020. The mortality rate has been reported to be between 0% and 14.6% in all patients. In this study, we determined the clinical and laboratory parameters of COVID-19 related morbidity and mortality in our hospital.
OBJECTIVES:
Investigate the relationship between demographic, clinical, and laboratory parameters on COVID-19-related morbidity and mortality.
DESIGN:
Retrospective observational study.
SETTINGS:
Tertiary care hospital.
PATIENTS AND METHODS:
Patients diagnosed with COVID-19 pneumonia from March until the end of December were included in the study.
MAIN OUTCOME MEASURES:
The relationship between demographic, clinical, and laboratory parameters and the morbidity and mortality rates of patients diagnosed with COVID-19.
SAMPLE SIZE:
124 patients
RESULTS:
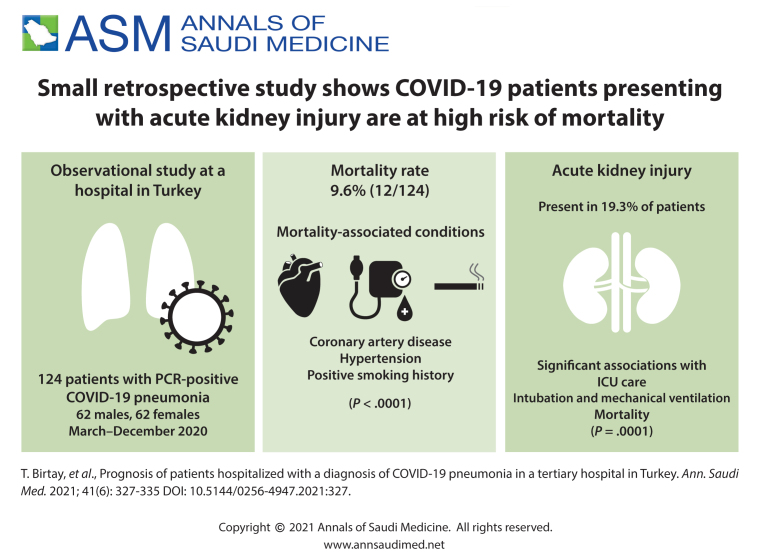
The mortality rate was 9.6% (12/124). Coronary artery disease (P<.0001) diabetes mellitus (P=.04) fever (>38.3°C) at presentation (P=.04) hypertension (P<.0001), and positive smoking history (P<.0001) were significantly associated with mortality. Patients who died were older, had a higher comorbid disease index, pneumonia severity index, fasting blood glucose, baseline serum creatinine, D-dimer, and had lower baseline haemoglobin, SaO2, percentage of lymphocyte counts and diastolic blood pressure. Patients admitted to the ICU were older, had a higher comorbidity disease index, pneumonia severity index, C-reactive protein, WBC, D-dimer, creatinine, number of antibiotics used, longer O2 support duration, lower hemoglobin, lymphocyte (%), and baseline SaO2 (%).
CONCLUSIONS:
Our results were consistent with much of the reported data. We suggest that the frequency, dosage, and duration of steroid treatment should be limited.
LIMITATIONS:
Low patient number, uncertain reason of mortality, no standard treatment regimen, limited treatment options, like ECMO.
CONFLICT OF INTEREST:
None.
INTRODUCTION
The new coronavirus disease (COVID-19) that emerged in the Hubei region of China in December 2019 and caused a pandemic that became a serious public health problem. In our country, the disease emerged after March 2020 with a variable spectrum ranging from asymptomatic cases to severe respiratory failure and death as a result of a cytokine storm. Complications that cause mortality have been defined as acute respiratory distress syndrome (ARDS), shock, acute kidney injury, acute cardiac damage, and secondary infections.1 The mortality rate has been reported between 0 and 14.6% in all patients.2,3 A retrospective study from Bulgaria revealed that most common symptoms of COVID-19 cases were fever, cough, headache and fatique.4 Severe cases had higher white blood cell counts, C-reactive protein (CRP), serum creatinine, aspartate aminotransferase (AST), lactate dehydrogenase, ferritin and fibrinogen levels compared to non-severe cases. Yang et al reported a 28-day mortality of 61.5% in 52 critical intensive care (ICU) patients.5 The most commonly suggested parameters in determining the severity of the disease were age, baseline lung computed tomography findings and co-morbid disease index.6 The most important determinant of mortality was severity of the disease, and mortality was higher in ICU patients, as expected. Patients who needed ICU were older and had a higher index of comorbid diseases. Dyspnea, abdominal pain, and anorexia are more common in these patients.3 More severe lymphopenia, higher white blood cell and neutrophil count, higher D-dimer and fibrin breakdown products were found in patients who died compared to survivors.3,5–7 Although many risk modelling systems have been developed to determine mortality, a standardized risk scoring system that can be used in clinical practice has not yet been established. Age, increased body mass index, having diabetes mellitus and diabetic complications have been shown as risk factors for increased mortality rates in cases of COVID-19 associated pneumonia.8 A meta-analysis of studies investigating the effect of smoking on the course and mortality of the disease concluded that smoking worsens the course and mortality of the disease.9 A novel systematic meta-analysis of 197 studies revealed that in diabetic patients, COVID-19 infection had a poor ARDS prognosis, more severe symptoms, and higher death rates than non-diabetic COVID-19 patients.10 In our study, we aimed to determine the possible influences of demographic features, comorbid diseases, smoking, contact history, laboratory tests, CT severity score, and penuomia severity index (PSI) on morbidity (time of stay in hospital, need and duration of oxygen therapy, need and duration of positive pressure ventilation, need for intensive care or invasive mechanical ventilation), and mortality.
PATIENTS AND METHODS
Our retrospective study included patients diagnosed with COVID-19 pneumonia from March until December 2020. Inclusion criteria were hospitalization with a diagnosis of COVID-19 associated pneumonia with various symptoms, mostly through emergency services or outpatient clinics. In all patients the COVID-19 PCR (polymerase chain reaction) test was positive. Patients with typical symptoms but negative PCR test results, patients with missing clinical and laboratory data, patients who refused treatment, and patients discharged voluntarily were excluded from the study. Data was collected on age, sex, smoking history, contact history, accompanying diseases (hypertension, diabetes, chronic obstructive pulmonary disease, malignant disease, dementia, anemia, and other diseases), and all medications used; systolic and diastolic blood pressure, body temperature, and pulse; presence of arrhythmia; fingertip oxygen saturation; symptoms (fever, cough, shortness of breath, sputum, sore throat, diarrhea, abdominal pain, success, eye redness, and other symptoms). A body temperature >38.3°C was regarded as fever independently of age and underlying disease.
Baseline hemoglobin, white blood cell, thrombocyte, lymphocyte (%), monocyte (%), eosinophil (%), C-reactive protein (CRP), D-dimer, procalcitonin, creatinine, urea, alanine aminotransferase (ALT), venous bicarbonate (HCO3), pH, serum total protein, albumin values, and findings of complete urinalysis were determined from the outpatient or emergency laboratory records. All patients included in the study were contacted by phone, and verbal consent was obtained for the use of their information in the scientific study. The Başkent University Ethics Committee and the Turkish Government Ministry of Health applications were used for this study. Baskent University approval numbers: E-946603339 - 604.01.02-4007 (Date: 1/19/2021). Ministry of Health of the Republic of Turkey: 2020-12-04T19-20-27 (date: 12/20/2021)
Chest CT images were retrospectively evaluated by a board-certified radiologist in all subjects who had baseline chest CT, and the findings were recorded by calculating the CT severity score. A multi-detector CT scanner (Somatom Spirit 16; Siemens) was used for all examinations, and scanning parameters were standard, which are recommended pre-setting for a thorax routine. Diagnosis of suspected COVID-19 pneumonia was considered when ground glass opacity, crazy-paving pattern, and consolidation were observed on chest CT based on the standard lexicon for thoracic imaging reported by the Fleischner Society.11 The CT severity score, a semi-quantitative scoring method proposed by Pan et al12 was calculated based on the extension ratio of findings for each lobe as follows: 0, no involvement; 1, <5% involvement; 2, 5–25% involvement; 3, 26–50% involvement; 4, 51–75% involvement; and 5, >75% involvement. The total sum of five lobes scored between 0 and 25 and was sub-classified as 0–8 mild, 9–15 moderate and 16–25 severe involvement of the lungs. On CT images, additional findings such as lymph-adenopathy, reversed “halo sign,” pleural effusion, and subpleural lines were also reported.
Data obtained from hospital records included days of hospitalization in normal hospital wards or in intensive care units, the number of antibiotics administered during follow-up, medications for COVID-19 (favipravir, hydroxychloroquine, oseltamivir), steroid treatment (received/not received), heparin (received-\/not received), the number of days needed for oxygen treatment, the number of days ventilated with a positive pressure mask, whether there was a need for a mechanical ventilator and the number of days on mechanical ventilation, serial measurements of serum CRP, procalcitonin, ALT, creatinine, D-dimer, SaO2 (%) values during hospitalization (days 2, 3 and 4). The APACHE scores of all patients in the intensive care unit were calculated and recorded. If present, sputum, blood, and urine culture growth were recorded in all patients. Patients who healed or died at the end of the follow-up period were identified from our hospital records.
Data on comorbid conditions (age, diabetes with and without complications, hypertension, coronary heart disease, heart failure, ischemic cerebrovascular diseases, malignant diseases (metastatic or localized, solid or lymphoproliferative), chronic kidney disease (CKD), CKD stage, peptic ulcer, dementia, liver disease, connective tissue diseases, chronic obstructive pulmonary disease, hemiplegia, and HIV were obtained from electronic hospital records. Smoking history was recorded as positive if the patient was an active smoker or quit. The Charlson comorbidity index (CCI) was calculated using an online calculator (https://www.mdcalc.com/charlson-comorbidity-index-cci).13
The pneumonia severity index (PSI) was implemented by the Pneumonia Patient Outcome Research Team to identify patients with community-acquired pneumonia to manage risks, outcomes, and mortality rates.14 This composite scoring index consists of 19 variables, including age, nursing home residency, coexisting illnesses, physical examination findings, and laboratory findings. The composite score classifies patients into one of five mortality risk groups and demonstrates a reduction in hospital admission rates and treatment costs and increases the security of outpatient management. The PSI scoring was performed by a chest disease specialist who initially assessed the patients. Most patients in Class I to III are suitable for outpatient management, whereas patients in Class IV and V have a higher mortality risk and should be admitted to the hospital. Additionally, all patients with hypoxemia (PaO2< 60 mmHg or SaO2 (%)< 90% on presentation) and other risk factors were admitted to the hospital.
All data were entered into the IBM SPSS version 25.0 program and subjected to statistical evaluation. Clinical characteristics and laboratory parameters were compared between patients who survived vs died with the Mann-Whitney U or the t-test where suitable. The existence of a normal distribution was investigated by the One-Sample Kolmogorov-Smirnov test, and presented as median (interquartile range, minimum-maximum) or mean (standard deviation), as appropriate. P value <.05 was the limit of statistical significance in our study.
RESULTS
After the initial case in Turkey in March, we observed 124 COVID-19 patients (62 men, 62 women) until the end of December 2020. Twelve (9.6%) patients died and 112 (90.3%) patients were discharged. Patients who died were significantly older than those who lived (P<.001) (Table 1). The PSI on admission was higher in non-survivors than in survivors. Among the 124, 39 (31.4%) had suspected contact with another COVID-19 patient, 19 (15%) had a history of smoking, 56 (45.2%) had hypertension, 22 (17.7%) had diabetes, and 33 (26.6%) had a history of coronary artery disease. Of the 124 patients, 25 (20.1%) were tourists (Scandinavian, German, and Arabic). Symptoms included 112 (90%) patients with fever, 20 (16%) patients with cough, 108 (87%) patients with shortness of breath, 34 (27.4%) patients with diarrhea, 20 (16%) patients with abdominal pain, 40 (32%) patients with throat ache, 25 (20.2%) patients with sputum, and 26 (21%) patients had loss of taste and smell sensations at presentation.
Table 1.
Clinical characteristics and laboratory parameters of COVID-19 patients by mortality.
| Variable | Died (n=12, 9.6%) | Recovered (n=112, 90.3%) | P |
|---|---|---|---|
| Age (years) | 79 (13.2, 64-91) | 58 (22.5, 14-86) | <.0001 |
| Charlson comorbidity index | 6.5 (2.1) | 2.2 (2.1) | <.0001 |
| Computerized tomography score | 6.0 (1.4) | 6.5 (3.4) | .665 |
| Pneumonia severity index | 111.9 (28.5) | 55.2 (24.7) | <.0001 |
| C-reactive protein (mg/L) | 41.4 (89.6, 3.51–170) | 25.6 (61.6, 0.1–237) | .231 |
| White blood cell count | 7513.3 (2584) | 6937.5 (2804) | .243 |
| Lymphocytes (%) | 14.6 (6.1) | 24.6 (11.9) | .006 |
| Thrombocytes (per mm3) | 197 108 (89131) | 211 734 (71981) | .426 |
| Hemoglobin (g/dL) | 10.9 (2.0) | 13.5 (1.7) | .0001 |
| Fasting glucose (mg/dL) | 134.0 (55) | 112.0 (24.9) | .04 |
| Serum creatinine (mg/dL) | 2.0 (2.2) | 0.95 (0.5) | <.001 |
| Percent change in serum creatininea (%) | 37.6 (95.5) | −6.8 (31.2) | <.0001 |
| Systolic blood pressure (mmHg) | 124.6 (16.3) | 123.3 (13.2) | .677 |
| Diastolic blood pressure (mmHg) | 72.5 (7.5) | 76.4 (6.2) | .023 |
| Procalcitonin (ng/mL) | 0.15 (0.38, 0.12–1.9) | 0.285 (0.553, 0–5.6) | .196 |
| Hospital stay (days) | 16 (6.75, 10–26) | 6 (5, 1–85) | <.0001 |
| Stay in ICU (days) | 13.5 (5.75, 3–22) | 0 (0, 0–46) | <.0001 |
| D-dimer (ng/mL) | 893 (820, 105–8600) | 1144 (568, 200–2200) | .04 |
| Alanine aminotransferase (U/L) | 20.5 (9.5, 6–114) | 24 (16, 10–154) | .28 |
| SaO2 (%) | 89.0 (9.2) | 95.0 (4.2) | .009 |
| O2 support (days) | 15.5 (3.25, 8–25) | 4.5 (5, 0–80) | <.0001 |
| APACHE scoreb | 48.5 (18.5) | 12.2 (9.7) | <.0001 |
| Number of antibiotics administered | 2.5 (0.5) | 1.4 (0.8) | <.0001 |
Data are median (interquartile range, min-mx) or mean (standard deviation).
aPercentage difference between the last serum creatinine and baseline serum creatinine.
bAPACHE score on admission to intensive care unit (ICU).
Patients with loss of smell and taste dysfunction had lower CRP and WBC counts and higher baseline SaO2 (%) values (P>.05). Age, sex, smoking status, prevalence of DM and hypertension, comorbidity index, and AKI at presentation were similar between patients with and without smell and taste dysfunction. Other symptoms and history of contact with COVID-19 positive subjects were not correlated with mortality. Stomach ache, throat ache and sputum, baseline systolic blood pressure, CRP, WBC count, procalcitonin, computed tomography score, and ALT were not different between patients who died and healed (Table 1), but differences in the Charlson comorbidity index, PSI, and other laboratory values were statistically significant. The percentage of lymphocytes at admission had an insignificant P value of negative correlation with mortality (OR: 0.77; P=.051).
The median (IQR) time from hospitalization to death was 16 (6.75) days in non-survivors. Twelve patients (9.6%) required intubation and mechanical ventilation. Acute kidney injury (AKI) (serum creatinine >1.2 mg/dL) was detected in 24 (19.3%) patients on admission. Patients with AKI had longer hospital stays, higher D-dimer, CRP, comorbidities, pneumonia severity index, number of antibiotics administered, need for O2 support (days), APACHE score on admission to ICU, procalcitonin and lower HCO3, thrombocyte count, SaO2 (%), and lymphocyte (%) on admission (P<.05). AKI was positively associated with male sex (P=.036), mortality (P=.0001), need for intubation and mechanical ventilation (P=.0001), hypertension (P=.004), smoking status (P=.029), and need for ICU care (P=.0001). There was no relationship between AKI and any symptoms (fever, diarrhea, headache, taste, and smell dysfunction), steroid, or heparin treatment (P>.05).
One patient did not receive antiviral treatment. Of the remaining patients, 85 (68.5%) were administered favipiravir only, four were administered hydroxychloroquine only, and 33 (26.6%) were given a combination of favipiravir, hydroxychloroquine, and oseltamivir. Forty (11%) patients were given no antibiotics, 52 (42%) patients were given one antibiotic, 44 (35.4%) patients were given two antibiotics, and 14 (11%) patients were given three antibiotics during follow-up. Steroid treatment was administered to 70 (56%) patients, and systemic low molecular weight heparin was administered to 105 (84.7%) patients. The mean steroid dosage was 34.4 (16.5) mg prednisolone (28 patients), 5.2 (3.4) mg dexamethasone (42 patients), and the mean time of steroid treatment was 10.2 (4.4) days.
Parameters related to mortality
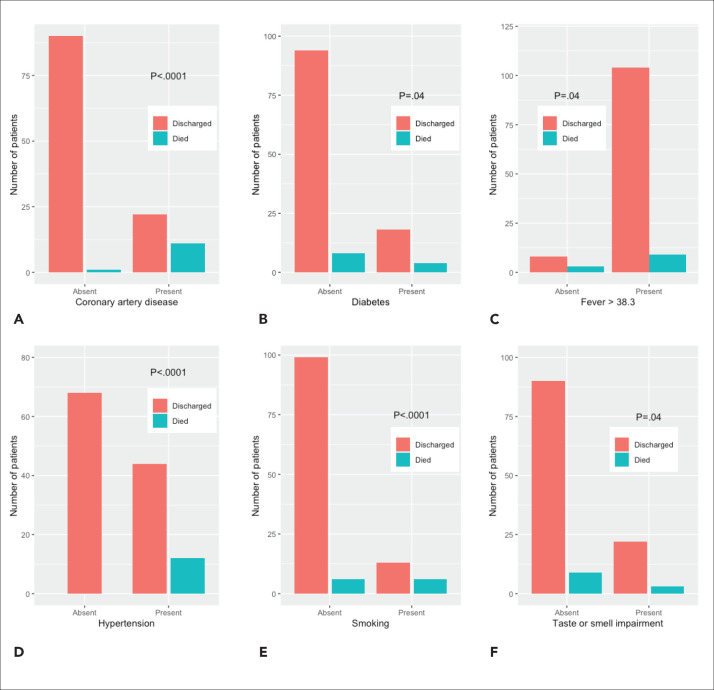
When ICU patients were separately analyzed, steroid treatment was still positively associated with mortality (P=.007). Coronary artery disease (P=.0001), diabetes mellitus (P=.04), fever (>38.3°C) at presentation, hypertension (P=.0001) and a positive smoking history (P<.001) were significantly associated with mortality (Figure 1). Loss of taste or smell as an initial symptom was negatively associated with mortality (P=.04). Patients with loss of smell and taste dysfunction had lower CRP and WBC counts and higher baseline SaO2 (%) values (P>.05). Age, sex, smoking status, prevalence of DM and hypertension, comorbidity index, and AKI at presentation were similar between patients with and without these symptoms. Other symptoms and history of contact with COVID-19 positive subjects were not correlated with mortality. Baseline systolic blood pressure, CRP, WBC count, procalcitonin, computed tomography score, and ALT were not different between patients who died and healed (Table 1), but differences in the Charlson comorbidity index, PSI, and other laboratory values were statistically significant. The percentage of lymphocytes at admission had an insignificant P value of negative correlation with mortality (OR: 0.77; P=.051).
Figure 1.
Association of mortality with a) coronary artery disease, b) diabetes, c) fever, d) hypertension, e) smoking, and f) taste and smell impairments.
Parameters related to ICU admission
Twenty-four (19%) patients required ICU follow-up during hospitalization (Table 2). Of these 24 patients, 2 (8.3%) died. Patients who needed ICU were older, had higher comorbidity disease index, pneumonia severity index, CRP, WBC count, D-dimer, creatinine, number of antibiotics used, and number of days for O2 support, had lower hemoglobin, lymphocyte (%), and lower baseline SaO2 (%).
Table 2.
Demographic, clinic and laboratory parameters that showed statistically significant differences between patients who were admitted or not to the intensive care unit.
| ICU (n=24, 19%) | No ICU (n=95, 80%) | P | |
|---|---|---|---|
| Age | 70 (17.8, 34–90) | 58 (23.5, 14–91) | <.0001 |
| Charlson comorbidity index | 5.2 (2.8) | 1.8 (1.8) | <.0001 |
| Pneumonia severity index | 95.1 (27.9) | 52.3 (22.7) | <.0001 |
| C-reactive protein (mg/L) | 65.5 (87.8, 0.51–200) | 20 (56.6, 0.1–237) | <.0001 |
| White blood cell count | 8006 (2722) | 6810 (3106) | .034 |
| Lymphocytes (%) | 14.2 (7.3) | 26.0 (11.5) | <.0001 |
| Hemoglobin (g/dL) | 12.3 (2.2) | 14.2 (7.3) | .018 |
| D-dimer (ng/mL) | 1001 (638, 159–3600) | 890 (842, 105–8600) | .017 |
| Serum creatinine (mg/dL) | 1.6 (1.9) | 0.9 (0.3) | .038 |
| Days of O2 support | 7 (5, 1–80) | 4 (6, 0–29) | <.0001 |
| SaO2 (%) | 91.1 (6.9) | 95.4 (3.4) | .003 |
| Number of antibiotics | 2.1 (0.7) | 1.3 (0.8) | <.0001 |
| Mortality | 2 (8.3) | 10 (10.5) | .750 |
Data are median (interquartile range, min-mx) or mean (standard deviation). Missing: 5 observations.
DISCUSSION
The present study demonstrated 9.6% mortality in our consecutively hospitalized population of PCR-confirmed COVID-19 patients of different ethnicities, different symptoms, and different grades of lung involvement from Alanya, a touristic region of Turkey. Because of the retrospective nature of our study and the lack of post-mortem biopsy or autopsy examinations, the causes of mortality were not clear. A wide range of mortality in COVID-19 patients between 0% and 14.6% has been reported in the literature.1 Therefore, our study population had a moderate rate of mortality rate compared to the literature. Yang et al. revealed 61.5% (32 of 52) mortality in critically ill ICU patients.5 Mortality of our patients who were admitted to ICU was consistent with Yang et al. In other studies, ICU patients with COVID-19 were older, had more comorbid conditions, and had higher plasma cytokine levels like IL 2, IL 7, IL 10, TNF-α, MCP 1, IP 10, G-CSF, and MIP 1A.2–4 Likewise, patients admitted in the present study were older and had a higher comorbid disease index, WBC count, D-dimer, serum creatinine, and CRP levels at baseline. Our patients had lower lymphocyte (%) and lower SaO2 (%) and hemoglobin levels on admission compared to non-ICU patients. Non-survivors had more severe lymphopenia, higher neutrophil counts, D-dimer, and fibrin degradation products than survivors.3,5–7 In our study, non-survivors were older and had higher D-dimer, blood glucose, PSI, comorbid disease index, serum creatinine level, longer stays in the hospital and ICU, higher APACHE score on admission to ICU, higher number of antibiotics, lower lymphocyte (%), SaO2 (%), diastolic blood pressure, and hemoglobin levels on admission. Survivors showed a stable recovery in kidney function (6.8% decrease in serum creatinine) despite non-survivors having worsening kidney function (37.6% increase in serum creatinine) during the hospitalization period. Baseline CRP, WBC count, procalcitonin, ALT, and pneumonic infiltration score on chest CT parameters were similar between survivors and non-survivors. All of these parameters could be seen as signals of worse general clinical condition or increased probability of worsening over time. High blood glucose levels were shown to be related to mortality in the CORONADA study and in another study from Wuhan.8–34 This might be considered a marker of the severity of infection.
Older age, having more frequent diabetes, hyper-tension, coronary artery disease, or smoking history were features of patients who had worse outcomes in our study. These are also components of the CCI. Age, comorbid diseases, and some symptoms (dyspnea, abdominal pain, etc.) and some laboratory findings such as lymphopenia and elevated D-dimer have been reported as risk factors for mortality in COVID-19.3,5–37 Several studies have shown a higher risk of complications and death from COVID-19 in patients with diabetes mellitus, hypertension, and severe obesity.10–19 Similar to our results, smoking history has been shown to increase disease severity and mortality, with a hazard ratio of 1.4–2.4 times.9–20 Tobacco smoking is suggested to cause dose-dependent upregulation of angiotensin-converting-enzyme up regulation (ACE-2), the virus cellular entry receptor, which may explain the increased risk of severe COVID-19 in smokers. Similarly, an association between ACE-2 expression and hypertension was confirmed in a study.34 A study from Wuhan reported that hypertension was the only comorbidity associated with the severity of COVID-19.34
Some studies in the literature have shown favorable outcomes for females compared to males with COVID-19.16–21 However, others could not find a relationship between sex, mortality, and other unfavorable outcomes in COVID-19.17,18 In the present study, mortality was similar between male and female patients. Although the prevalence of AKI and smoking was lower in women, the need for ICU, intubation, and mechanical ventilation was similar between male and female patients.
In the present study, we calculated the CCI in all patients from the electronic patient records. The CCI value was similar between male and female patients, higher in non-survivors than in survivors, and higher in patients who need ICU care. Similarly, several studies have revealed that CCI is an independent predictor of mortality in patients with COVID-19 infection.16–21
Our results showed that non-survivors had higher baseline creatinine levels than the survivors. COVID-19 patients on the first admission had a 19.3% prevalence of AKI. AKI is positively associated with age, mortality, male sex, need for mechanical ventilation, hypertension, smoking status, and need or ICU care. Patients with AKI also had worse clinical and laboratory indicators for mortality, such as higher CCI, PSI, procalcitonin, D-dimer, CRP, and lower baseline SaO2 (%), and lymphocyte (%). Although steroid treatment was associated with increased mortality in the present study, the frequency of steroid and heparin administration was similar between patients with and without AKI. In the literature, AKI prevalence in COVID-19 patients was between 9.4% and 40.6%, and similar to our present results, AKI has been shown to be associated with mortality in COVID-19.22,23
The present study showed that PSI on admission was an important predictor of mortality. Respiratory failure was shown to be the leading cause of mortality in patients with COVID-19.24 Satici et al revealed that PSI and CRP levels on admission were significant predictors of mortality, similar to our results.25 Some other studies proved that the CT-based semi-quantitative score of pulmonary involvement was a predictor of mortality in patients with COVID-19.26,27 However, our study revealed no significant relationship between chest CT scores and mortality. The CT score was only weakly correlated with the need for O2 support (days).
Our results demonstrated that fever (>38.3°C) on admission was a positive predictor of in-hospital mortality. Fever was the most common (90%) symptom in our patient population, similar to other studies (frequency reported between 77.4–98.6%.1–18 There are limited data showing an association between fever (body temperature) and mortality. A retrospective analysis of 9417 patients showed that low (<36°C) body temperature (BT) on admission was significantly associated with mortality.28 However, maximum BT during COVID-19 infection, especially over 40°C, also had a significant correlation with mortality. Another study showed no significant difference in BT on admission between alive and deceased patients.25 One study reported that not the degree but the duration of fever during COVID-19 course was related to severity of infection.34 We observed a 21% prevalence of self-reported smell and taste dysfunction in our study population. Anosmia is a characteristic symptom of COVID-19 infection with a reported frequency between 5.1–98.3% depending on the study, study population, and study method.29–38 Taste dysfunction (gustatory loss) was also reported between 59–88% of patients with severe COVID-19.39,40 Some studies revealed that patients with anosmia had a milder course of COVID-19 with a lower probability of hospitalization.30 Talavera et al reported a lower mortality rate and less severe disease course in COVID-19 patients presenting with anosmia.29 In our patient group, the comorbidity disease index, age, and sex were similar between patients with or without symptoms, the main cause of this association is still unknown. Milder immune or inflammatory responses or different cytokine-releasing genotypes may be the underlying mechanisms of these different disease patterns.
An important result of our retrospective observational study was the association between steroid use and mortality. Dexamethasone (mean dose 5.4 mg) or prednisolone (mean dose of 35 mg) was used for a mean time of 10 days. Our steroid dosages and time of steroid treatment were similar to those in previous studies.31–33 The RECOVERY trial revealed that 6 mg daily dexamethasone (equivalent to 150 mg hydrocortisone) decreased the 28 days mortality in COVID-19 patients under respiratory support with or without invasive mechanical ventilation.31 On the contrary, similar to our present results, a meta-analysis with a total of 15 754 patients showed that systemic steroid treatment in COVID-19 increased mortality with an OR of 1.9.32 This meta-analysis also revealed that systemic steroid treatment increased hospital stay in hospital and prolonged viral shedding. Matthey et al proved that the timing, dosage of steroids, and severity of COVID-19 pneumonia would determine the immune response and viral outcome and suggested the use of moderate dose corticosteroids in moderate to severe COVID-19 patients late in the disease course.33 Another study from Wuhan reported that high-dose prednisone (1 mg/kg) during hospitalization was a significant risk factor associated with death in patients with severe COVID-19.34 the frequency of steroid administration was higher in our patients (56%) than in their study (11.9%). Although in the present study, our mean steroid dosage was lower than prednisone 1 mg/kg or equivalent, steroid treatment was still associated with mortality. One the 12 patients who died was not using a steroid, but the reminder where using a steroid (P=.007).
The limitations of our study were the small sample size, lack of knowledge about the reason for mortality in deceased cases, and the lack of a standard treatment protocol for all patients due to the retrospective nature of the study. However, all of our patients were admitted and followed up in the hospital by two chest disease specialists. Therefore, treatment choices were similar. Because of the limited treatment options, we could not apply plasmapheresis or immune plasma treatment, tocalisumab, cytokine adsorption treatment, or extracorporeal membrane oxygenation.
In conclusion, our present data show that older age, higher comorbidity disease index, PSI, smoking, AKI at presentation, higher number of antibiotics and higher frequency of steroid usage during hospitalization, presence of fever (>38.3°C), lower SaO2 (%), hemoglobin, and diastolic blood pressure on admission were associated with increased mortality. Smell and taste dysfunction can be seen as signs of a milder disease course. We suggest that the frequency, dosage, and duration of steroid treatment should be limited. Inflammatory parameters, especially CRP and procalcitonin levels, should be frequently monitored to prevent opportunistic infections, especially in older patients with comorbid diseases. Further prospective studies should be conducted to determine whether the minimum effective dosage can be used safely. Furthermore, determination of viral load and inflammatory and anti-inflammatory cytokine profiling might be a safer way to use steroids. Anti-inflammatory medications such as colchicine and IL-6 receptor blocker (tocilizumab) have been shown to be effective in lowering mortality in COVID-1935,36 In future treatment plans, these alternatives may be combined with low-dose steroid treatment to prevent the undesirable effects of steroids on viral replication.
Funding Statement
None
REFERENCES
- 1.Ge H, Wang X, Yuan X, Xiao G, Wang C, Deng T, et al. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis 2020; 39(6):1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C,Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 15;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel corona virus-infected pneumonia in Wuhan, China. JAMA. 2020; 17; 323(11):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Popov GT, Baymakova M, Vaseva V, Kundurzhiev T, Mutafchiyski V. Clinical Characteristics of Hospitalized Patients with COVID-19 in Sofia, Bulgaria. Vector Borne Zoonotic Dis. 2020; 20(12):910–915. [DOI] [PubMed] [Google Scholar]
- 5.Yang X, Yu Y, Xu J,Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8(5):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, et al. Prediction models for diagnosis and of COVID-19 infection: systematic review and critical appraisal. BMJ. 2020; 7; 369: 1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020; 8(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheen AJ, Marre M, Thivolet C. Prognostic factors in patients with diabetes hospitalized for COVID-19: Findings from the CORONADO study and other recent reports. Diabetes Metab. 2020; 46(4):265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vardavas CI, Nikitara K. COVID-19 and smoking: A systematic review of the evidence. Tob Induc Dis. 2020; 20; 18:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdi A, Jalilian M, Sarbarzeh PA, Vlaisavljevic Z. Diabetes and COVID-19. A systematic review on the current evidences. Diabetes Res Clin Pract. 2020; 166:108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. J. Fleischner society: glossary of terms for thoracic imaging. Radiology 2008; Epub 2008 Jan 14: 246:697–722. [DOI] [PubMed] [Google Scholar]
- 12.Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time course of lung changes on chest CT during recovery from 2019 novel corona virus (COVID19) pneumonia. Radiology 2020; 295(3):715–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic co morbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 14.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia N Engl J Med. 1997; 23; 336(4):243–50. [DOI] [PubMed] [Google Scholar]
- 15.Muniyappa R, Gubbi S. COVID-19 pandemic, corona viruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020; 1; 318(5):E736–E741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho KH, Kim SW, Park JW, Do JY, Kang SH, et al. Effect of Sex on Clinical Outcomes in Patients with Corona virus Disease: A Population-Based Study. J Clin Med. 2020:24;10(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J Infect.2020; 80(6):639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allameh SF, Nemati S, Ghalehtaki R, Mohammadnejad E, Aghili SM, Khajavirad N, et al. Clinical Characteristics and Outcomes of 905 COVID-19 Patients Admitted to Imam Khomeini Hospital Complex in the Capital City of Tehran, Iran. Arch Iran Med. 2020; 1; 23(11):766–775. [DOI] [PubMed] [Google Scholar]
- 19.Guo L, Xiong W, Liu D, Feng Y, Wang P, Dong X, et al. The mNCP-SPI Score Predicting Risk of Severe COVID-19 among Mild-Pneumonia Patients on Admission. Infect Drug Resist. 2020; 14; 13:3593–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cattaruzza MS, Zagà V, Gallus S, D'Argenio P, Gorini G. Tobacco smoking and COVID-19 pandemic: old and new issues. A summary of the evidence from the scientific literature. Acta Biomed. 2020; 11; 91(2):106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holler JG, Eriksson R, Jensen TØ, van Wijhe M, Fischer TK, Søgaard OS, et al. First wave of COVID-19 hospital admissions in Denmark: a nationwide population-based cohort study. BMC Infect Dis. 2021; 9; 21(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Li J, Su J, Yang J, Jiang X, Jiang N, et al. Clinical characteristics and risk factors of acute kidney injury in corona virus disease 2019. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020; 32(4):407–411. [DOI] [PubMed] [Google Scholar]
- 23.Hansrivijit P, Gadhiya KP. Gangireddy M, Goldman JD. Risk Factors, Clinical Characteristics, and of Acute Kidney Injury in Hospitalized COVID-19 Patients: A Retrospective Cohort Study. Medicines (Basel). 2021; 7; 8(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang F, Shi S, Zhu J, Shi J, Dai K, Chen X: Analysis of 92 deceased patients with COVID-19. J Med Virol. 2020; 92(11):2511–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satici S, Demirkol MA, Altunok SE, Gursoy B, Alkan M, Kamat S, et al. Performance of pneumonia severity index and CURB-65 in predicting 30-day mortality in patients with COVID-19. Int J Infect Dis. 2020; 98: 84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francone M, Iafrate F, Masci G, Coco S, Cilia F, Manganaro L, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term. Eur Radiol. 2020; 30(12):6808–6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanza E, Muglia R, Bolengo I, Giuseppe Santonocito O, C, Lisi O, Angelotti G, et al. Quantitative chest CT analysis in COVID-19 to predict the need for oxygenation support and intubation. Eur Radiol. 2020; 30(12):6770–6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tharakan S, Nomoto K, Miyashita S, Ishikawa K. Body temperature correlates with mortality in COVID-19 patients. Crit Care. 2020; 24(1):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talavera B, García-Azorín D, Martínez-Pías E, Trigo J, Hernández-Pérez I, Valle-Peñacoba G, et al. Anosmia is associated with lower in-hospital mortality in COVID-19. J Neurol Sci. 2020; 419: 117–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan CH, Faraji F, Prajapati D, Ostrander BD, DeConde AS, et al. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int Forum Allergy Rhinol. 2020; 10(7):821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19. New England Journal of Medicine. 2021. Feb 25;384(8):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarkar S, Khanna P, Soni KD. Are the steroids a blanket solution for COVID-19 A systematic review and meta-analysis. J Med Virol. 2020: Sep 3, doi: 10.1002/jmv.26483. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Matthay MA, Wick KD. Corticosteroids, COVID-19 pneumonia, and acute respiratory distress syndrome. J Clin Invest. 2020; 130(12):6218–6221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020; 146(1):110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vrachatis DA, Giannopoulos GV, Giotaki SG, Raisakis K, Kossyvakis C, Iliodromitis KE, et al. Impact of colchicine on mortality in patients with COVID-19. A meta-analysis. Hellenic J Cardiol 2021: Epub ahead of print. doi: 10.1016/j.hjc.2020.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mikulska M, Nicolini LA, Signori A, Di Biagio A, Sepulcri C, Russo C, et al. Tocilizumab and steroid treatment in patients with COVID-19 pneumonia. PLoS One. 2020; 15(8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel corona virus (2019-nCoV) in Wuhan, China. J Med Virol 2020:92: 441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngology–Head and Neck Surgery. 2020. Jul;163(1):3–11. [DOI] [PubMed] [Google Scholar]
- 39.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the corona virus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020; 277(8):2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menni C, Valdes A, Freydin MB, Ganesh S, El-Sayed Moustafa J, Visconti A, et al. Loss of smell and taste in combinations with other symptoms is a strong predictor of COVID-19 infection. Nature Medicine, Online publication, doi: 10.1038/s41591-020-0916-2. [DOI] [Google Scholar]