Abstract
We identified the Schizosaccharomyces pombe mex67 gene (spmex67) as a multicopy suppressor of rae1-167 nup184-1 synthetic lethality and the rae1-167 ts mutation. spMex67p, a 596-amino-acid-long protein, has considerable sequence similarity to the Saccharomyces cerevisiae Mex67p (scMex67p) and human Tap. In contrast to scMEX67, spmex67 is essential for neither growth nor nuclear export of mRNA. However, an spmex67 null mutation (Δmex67) is synthetically lethal with the rae1-167 mutation and accumulates poly(A)+ RNA in the nucleus. We identified a central region (149 to 505 amino acids) within spMex67p that associates with a complex containing Rae1p that complements growth and mRNA export defects of the rae1-167 Δmex67 synthetic lethality. This region is devoid of RNA-binding, N-terminal nuclear localization, and the C-terminal nuclear pore complex-targeting regions. The (149–505)-green fluorescent protein (GFP) fusion is found diffused throughout the cell. Overexpression of spMex67p inhibits growth and mRNA export and results in the redistribution of the diffused localization of the (149–505)-GFP fusion to the nucleus and the nuclear periphery. These results suggest that spMex67p competes for essential mRNA export factor(s). Finally, we propose that the 149–505 region of spMex67p could act as an accessory factor in Rae1p-dependent transport and that spMex67p participates at various common steps with Rae1p export complexes in promoting the export of mRNA.
Transport of macromolecules between the nucleus and the cytoplasm occurs through the nuclear pore complex (NPC). Nuclear export of mRNA is thought to be dependent upon the association of mRNA with carrier proteins in the nucleus that bear nuclear export signals (NES). This complex interacts with a receptor that mediates export of the mRNA through the NPC as an mRNP particle. In the cytoplasm, the RNP particle is disassembled, and the export receptor and RNA carrier proteins are reimported into the nucleus (25, 31, 39, 40).
Both nuclear protein import and the export of mRNA require the Ran GTPase switch system (25, 31, 39, 40). All known import and export receptors identified so far belong to the importin-β superfamily. These receptors bind Ran, and the nucleotide state of Ran affects the affinity of the receptor for its cargo. Import receptors that bind Ran-GTP in the nucleus release their cargo, whereas Ran-GTP increases the affinity of the export receptors for their cargo. Once in the cytoplasm, the hydrolysis of Ran-GTP causes dissociation of the export complex (15, 21, 25, 33). The mRNA-binding protein HIV-Rev has an NES that interacts with the export receptor, CRM1, a member of the importin β superfamily. This receptor in turn mediates the export of mRNA that binds Rev through the NPC as an mRNP particle (40). Other mRNA-binding proteins that are believed to function as carriers of mRNA from the nucleus to the cytoplasm include Npl3 in Saccharomyces cerevisiae (13, 23, 40) and hnRNP A1 in mammalian cells (30, 40). As carriers of mRNA, these proteins shuttle between the nucleus and the cytoplasm. While the import pathways of these proteins are well understood (38), little is known about their export pathways. The hnRNP A1 protein carries a domain, M9 (18), which contains both an NES and a nuclear localization signal (NLS). The NES of Npl3p is yet to be identified, and none of the export receptors for these proteins are known.
Receptors from the importin-β superfamily interact with proteins within the NPC during translocation through the pore (12). Indeed, mutations in several nucleoporins have been shown to affect mRNA export (25). How these nucleoporins participate in mRNA export is not well understood. Nucleoporin mutants altering mRNA export have also been used in genetic studies to identify other NPC-interacting proteins involved in mRNA export, including the homologues Gle2p and Rae1p, Gle1p, and scMex67p (25, 28, 29, 40).
Recently, the mRNA export factor Mex67p in S. cerevisiae (37) and its human counterpart, Tap (7, 16, 19, 20), were shown to have properties analogous to the hnRNP shuttling proteins. In S. cerevisiae, the MEX67 gene encodes a factor essential for mRNA export. Both scMex67p and Tap were shown to directly associate with poly(A)+ RNA in vivo. Tap contains NLS and NES activities for shuttling between the nucleus and cytoplasm and interacts with an NPC protein, Nup214p. Therefore, it has been suggested that Tap could mediate mRNA export by binding to mRNA and directing its export out of the nucleus (20). In addition, Tap directly associates with constitutive transport elements (CTE) encoded by the genomes of simian retroviruses. These elements direct the export of Tap from the nucleus (16). Moreover, the essential nature of scMEX67 in mRNA export and the ability of excess Tap to overcome the inhibition of cellular mRNA export in Xenopus oocytes following injection of excess CTE suggest the conservation of the Tap and scMex67p function in mRNA export (16, 34).
The scMex67p localization to the NPC requires association with the Mtr2p-Nup85p complex (36). MTR2 is an essential gene in S. cerevisiae whose protein product is located both in the nucleus and at the NPC (36). The Tap-interacting protein, p15, is a member of the Ran-GTP binding proteins and has similarity to Ntf2p. Although p15 is thought to be functionally equivalent to Mtr2p, it is unable to substitute for Mtr2p. However, the Tap-p15 complex can substitute for the scMex67-Mtr2 complex in S. cerevisiae (20).
Genetic screens in Schizosaccharomyces pombe have resulted in the identification of several NPC-associated proteins that have important roles in the nuclear export of mRNA. The genetic screens led to the identification of an essential mRNA export factor in S. pombe, Rae1p, which is evolutionarily conserved (11, 41, 42). The temperature-sensitive rae1-1 mutant rapidly accumulates poly(A)+ RNA in the nucleus at the restrictive temperature, and hRae1p can partially complement the temperature-sensitive phenotype of the rae1-1 mutation. Yeast Rae1p and its S. cerevisiae homologue, Gle2p, are predominantly located at the NPC. In human cells, Rae1p is found in both the nucleus and the cytoplasm (8, 22, 28). The notion that Rae1p and Gle2p function at the nuclear pore is supported by experiments that demonstrated the genetic and physical interactions of Rae1p and Gle2p with pore components (5, 17, 28). Gle2p and human Rae1p have been found in an NPC subcomplex that interacts with the GLFG-repeat nucleoporin proteins Nup116p and Nup98p, respectively, and this binding is necessary for the NPC localization of Rae1p and Gle2p (5, 17, 35).
The nup184 gene is a nonessential gene in S. pombe that is genetically linked with rae1. It is the S. pombe homologue of the S. cerevisiae nucleoporin gene, NUP188 (32, 41, 43). We have previously shown that the nup184-1 mutation is synthetically lethal with the rae1-167 mutation and that spNup184p is required for regulating mRNA export in response to growth in nutrient-rich media (41).
In this report, we show that spMex67p can function as a multicopy suppressor of both the temperature-sensitive phenotype of the rae1-167 mutation and the rae1-167 nup184-1 synthetic lethality. While spMex67p is not an essential gene for growth or for mRNA export in S. pombe, its function in mRNA export is required in the background of mutations that are genetically linked with the rae1 function. Overexpression of spMex67p in wild-type cells inhibits nuclear export of mRNA, suggesting that spMex67p plays an important role in mRNA export. Using crude extracts, we report the identification of a region (amino acids 149 to 505) within spMex67p that can associate with the Rae1p complex. This region, expressed from a multicopy plasmid, can complement the growth and mRNA export defect associated with the rae1-167 Δmex67 mutant, presumably by stabilizing Rae1-167p-associated RNP complexes. This region does not contain the poly(A)+ RNA binding domain, the NLS, or the NPC localization domain present in Tap. When fused to green fluorescent protein (GFP), this domain is found diffused throughout the cell, but it accumulates along with poly(A)+ RNA in the nucleus and at the nuclear periphery in cells overexpressing spMex67. These results suggest that the 149–505 domain of spMex67p can functionally interact with factors that mediate nuclear export of mRNA both within the nucleus and at the nuclear periphery.
MATERIALS AND METHODS
Strains and culture.
The strains used in the study are listed in Table 1. The basic genetic and cell culture techniques used have been described previously (1, 27). Appropriately supplemented Edinburgh minimal medium (EMM) was used to express genes from the nmt promoter. The nmt promoter was repressed by the addition of 0.5 μM thiamine in EMM (14). Strains were grown under standard growth conditions (1, 27, 42).
TABLE 1.
S. pombe strains used in this study
| Strain | Genotype(s) | Source (reference) |
|---|---|---|
| rae1-167 | h− leu1-32 ura4-D18 rae1-167 | Yoon et al. (42) |
| h+ leu1-32 ura4-D18 rae1-167 | Yoon et al. (42) | |
| Wild type | h− leu1-32 ura4-D18 | Brown et al. (11) |
| h+ leu1-32 ura4-D18 | Brown et al. (11) | |
| SL27 | h− leu1-32 ura4-D18 rae1-167 nup184-1/(pREP81X-rae1) | Yoon et al. (42) |
| nup184-1 | h− leu1-32 ura4-D18 nup184-1 | Whalen et al. (41) |
| Δnpp106 | h− leu1-32 ura4-D18 Δnpp106::ura4 | Yoon et al. (42) |
| Δmex67 | h− leu1-32 ura4-D18 Δspmex67::kanr | This study |
| Δmex67 nup184-1 | h− leu1-32 ura4-D18 Δspmex67::kanr nup184-1/(pREP81X-mex67) | This study |
| Δmex67 Δnpp106 | h− leu1-32 ura4-D18 Δspmex67::kanr/Δnup106::ura4 (pREP81X-mex67) | This study |
| Δmex67 rae1-167 | h− leu1-32 ura4-D18 rae1-167 Δspmex67::kanr/(pREP81X-mex67 or pREP81X-rae1) | This study |
| SP286 | h+/h+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-210/ade6-216 | D. Beach |
Isolation of mex67.
Strain SL27 (rae1-167 nup184-1) was transformed with a partial Sau3A genomic library cloned into the SalI site of pUR18, and Ura+ colonies that could grow in the presence of thiamine at 28°C were isolated (41). Plasmids were rescued into Escherichia coli, and those that were able to complement SL27 were analyzed by restriction analysis. Analysis of the maps showed that eight different genes had been isolated. Among these, the gene encoding p27N2 was able to complement SL27 in the presence of B1 at 28°C and suppress the temperature-sensitive phenotype of the rae1-167 mutation under the lower restrictive temperature of 32°C. This genomic clone was used as a probe to isolate cDNA clones. Sequence analysis (SAIC, Frederick, Md.) of genomic and cDNA clones revealed an intronless gene with a 596-amino-acid open reading frame with sequence similarity to scMEX67.
The spmex67::ura4 null mutation was constructed by first cloning the spmex67 gene into a pBluescript vector (Stratagene). A HindIII site downstream of the termination codon was created. The sequences between the HindIII site at amino acid 55 and the newly created HindIII site near the termination codon were removed and replaced with a ura4 gene. The SalI-Δmex67::ura4-EcoRI fragment was transformed into the h+/h+ diploid, SP826. Stable Ura+ transformants were screened by PCR and Southern blotting for the replacement of one of the mex67 genes. To determine if the spmex67 gene was essential, the ura4+ strain was sporulated and 24 tetrads were dissected. All spores formed colonies, and the ura4 marker segregated 2:2, indicating that the spmex67 gene was not essential. The Δmex67::kan mutation was generated by PCR using the kanMX6 module as a template (4). The resulting PCR products were transformed into the haploid JBP16, and the G418-resistant transformants were screened. PCR and Southern blotting confirmed the replacement of the mex67 gene.
Plasmid constructions.
For construction of pmex67, a 3-kb genomic DNA fragment containing the spmex67 gene was inserted into the plasmid pDW232 (11) at the KpnI and SphI site. In-frame deletions (pmex1 to pmex8) were introduced within the spmex67 coding region by PCR. The 5′ PCR products were generated from the KpnI site in the multicloning site of plasmid pmex67, and a PstI site was introduced at the C terminus of the PCR product. The 3′ PCR products contained a PstI site at the N terminus and a SphI site in the multicloning site of pmex67. The respective KpnI-PstI upstream fragments and the PstI-SphI downstream fragments were purified and ligated into KpnI and SphI within pDW232. The resulting deletions place a PstI site (Leu-Gln) at the junction site, which contains the deletion (see Fig. 4). For the construction of pmex9, PstI sites were created immediately downstream of the initiation codon and immediately upstream of the termination codon of spmex67 on the plasmid pmex67. The PCR product that carries amino acid residues 149 to 505 flanked by PstI sites was then ligated into the above-described plasmid at the PstI site. The deletions within spmex67 clones were sequenced to confirm their presence and the fidelity of the PCR products. For overexpression of spMex67p and its deletions, the coding sequences of the full-length mex67 open reading frame and its deletions were ligated into the XhoI and BglII sites of the pREP3X vector and to the pREP3X-HA-tagged vector containing a strong wild-type thiamine-repressible nmt1 promoter (26). spMex67p and its deletions (pRM, pRMΔ1, pRMΔ2, pRMΔ3, pRMΔ4, pRMΔ5, pRMΔ6, pRMΔ7, and pRMΔ8) can be overexpressed in the absence of thiamine.
FIG. 4.
Complementation of rae1-167 Δmex67/pREP81X-rae1 synthetic lethality by spMex67 deletions. (A) A map of the regions surrounding the spMex67p deletions is shown (see Materials and Methods for details). These deletions were expressed from the genomic spmex67 promoter in the pDW232 vector. Under synthetic lethal conditions, expression of Rae1p is repressed by addition of thiamine (+B1). Growth was monitored for 4 days. ++, normal growth; −, no growth; ±, intermediate growth. LRR, leucine-rich repeats. (B) Crude extracts were prepared from the rae1-167 Δmex67 strain under synthetic lethal conditions expressing spMex67p and its truncations, shown in panel A. The amount of spMex67p staining in these experiments was determined by Western blot analysis using antibodies generated against an spMex67p peptide. As a control for the amount of protein used, the amount of Rae1p was determined by Western blotting using polyclonal antibodies against Rae1p.
GFP was fused to spMex67p at the C terminus by first creating a KpnI site and a SacI site immediately upstream of the termination codon on the p27N2 plasmid. A KpnI-GFP-SacI fragment was inserted between the KpnI and SacI sites. The resulting pGmex67 plasmid was capable of complementing the SL27 synthetic lethality and the temperature-sensitive phenotype of rae1-167 cells. For integration of GFP at the C terminus of mex67, the BamHI-mex67-GFP-EcoRI fragment was inserted into a derivative of pDW232 lacking the ARS sequence (pDW234). The resulting plasmid was integrated into the spmex67 locus following linearization with AvaI and transformation into JBP16. Ura+ transformants were screened for proper insertion of this fusion by Southern blotting. For the construction of other GFP-tagged deletions, the GFP was tagged at the C terminus of mex67 deletion constructs between the SphI and KpnI sites. For the construction of spMex67p tagged with GFP at the N terminus, XhoI and BglII sites were created immediately upstream of the initiation codon of spmex67 and downstream of the termination codon. The XhoI-spmex67-BglII fragment was inserted into the SalI and BamHI sites of vector pZA69U, which has GFP(S65T) expressed from the wild-type nmt1 promoter. The GFP(S65T) is fused in frame at the C terminus to a Gly-Ala linker followed by the multicloning site. Plasmid constructs used in the heterologous nuclear export assay were derived from the pXRGG vector (24). spMex67p deletions were cloned into this vector by replacing HIV-Rev with the PCR products of spMex67p truncations.
Transient transfections using pMex67-Gr-GFP and nuclear translocation.
HeLa cells (ATCC, CCL2) were transiently transfected using Superfect (Qiagen) according to the manufacturer's instructions. Briefly, 2 μg of DNA was added to Optimem (Life Technologies) to a final volume of 100 μl. Ten microliters of Superfect was added to the DNA and incubated for 10 min at room temperature. Following incubation, 600 μl of Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (Life Technologies) was added to the DNA-Superfect suspension, which was then added to HeLa cells grown to a 50% confluent monolayer on a six-well dish. After a 3-h incubation, the DNA-Superfect suspension was removed and replaced with Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. Eighteen hours following transfection, cells were used in transport assays as described previously (24).
Immunoprecipitations.
Δspmex67 cells were transformed with plasmids expressing an AU1-epitope-tagged (11) Rae1p from the pREP3X vector along with hemagglutinin (HA)-epitope-tagged full-length spMex67p or different spMex67p truncations from the pREP4X vector. Cultures were grown to 8 × 106 cells/ml in the absence of thiamine for 16 h, spun down, and washed in water. Cell walls were partially digested with lyticase (5,000 U; Sigma) for 15 min in a buffer containing 1.2 M sorbitol–20 mM potassium phosphate (pH 7.4). Cells were washed and resuspended in a lysis buffer containing 150 mM NaCl, 20 mM Tris-HCL (pH 7.5), 5 mM MgCl2, 0.2% Triton X-100, 10% glycerol, and a protease inhibitor cocktail (Sigma). Lysates were prepared by breaking the cells with glass beads. Cell debris and unlysed cells were removed by centrifugation. Protein content in the supernatants was determined by the Bradford assay (Bio-Rad Laboratories). The supernatants were incubated with monoclonal antibodies against the HA epitope at 4°C for 3 h and then with Gammabind G-Sepharose (Pharmacia) for 1 h. The beads were washed 6 times with lysis buffer. The samples were then resuspended in loading buffer and electrophoresed on a 4 to 20% polyacrylamide sodium dodecyl sulfate (SDS) gel (Novex, San Diego, Calif.). Rae1p was probed by Western blotting using polyclonal antibodies against Rae1p and spMex67, and its truncations were probed by using polyclonal antibodies against the HA epitope (Babco, Berkeley, Calif.). Rae1p and spMex67p were detected on X-ray film by using ECL (Amersham), a light-emitting nonradioactive method.
In vitro binding assay.
rae1 cDNA was cloned into the pET14b vector and transformed into the BL21/pLys strain (Stratagene). Rae1p was induced by addition of 0.5 mM isopropyl-β-d-thiogalactopyranoside. Washed cells from 1 liter of culture were lysed in universal buffer (20 mM HEPES-KOH [pH 7.0], 100 mM KoAc, 2 mM Mg(oAc)2, 0.1% Tween 20, 10% glycerol, 5 mM β-mercaptoethanol) (20) containing 1 mM phenylmethylsulfonyl fluoride and protease cocktail set I (Calbiochem) by passing twice through a French press (12,000 lb/in2). The extract was clarified first by spinning in an Eppendorf Microfuge at 14,000 rpm for 15 min at 4°C followed by a spin at 20,000 rpm in a Beckman ultracentrifuge for 30 min at 4°C. The extract was stored at −70°C. Glutathione S-transferase (GST) and GST-spMex67p were expressed from the pGex5X-3 plasmid in E. coli strain BL21 (Pharmacia). The proteins were purified according to the protocol provided by the supplier (Amersham Pharmacia), except that universal buffer instead of phosphate-buffered saline (PBS) was used. In vitro binding assays were performed in universal buffer (20). Proteins were identified by staining the SDS-polyacrylamide gel electrophoresis (PAGE) gels with Coomassie blue or by Western blot analysis using polyclonal anti-Rae1p and monoclonal anti-GST antibodies followed by the ECL detection kit (NEN).
UV cross-linking of Mex67p to poly(A)+ RNA.
To determine the ability of spMex67p and its deletions to UV cross-link with poly(A)+ RNA, the full-length spMex67p and its deletions were introduced into the pSLF273 vector containing an HA epitope (2). The coding sequences of mex67 and its deletions were amplified by PCR using pmex67 and its pmex deletions as templates; the resulting PCR fragments contained an XhoI site at the N terminus and a BglII site at the C terminus and were inserted into the SalI-BglII sites within the pSLF273 vector (ATCC). They all contained an in-frame HA epitope at the N terminus, whose expression is under the control of a weaker version of the thiamine-repressible nmt1 promoter (14). The resulting plasmids were transformed into Δmex67 cells. UV-cross-linked poly(A)+ RNA-RNP complexes were isolated and analyzed by SDS-PAGE and Western blotting as described previously (2). One liter of transformed Δmex67 cells grown to 5 × 106 cells/ml was harvested, washed in PBS, and UV irradiated two times (3 min each) on ice in the Stratalinker 2400 (Stratagene). Cells were resuspended in lysis buffer (20 mM Tris [pH 7.4], 1 mM EDTA, 50 mM LiCl, 1% SDS, 1% β-mercaptoethanol, 10 mM Vanadyl complex, 1 mM phenylmethylsulfonyl fluoride, 1× protease inhibitor complex [Sigma]) and passed twice through a French press (18,000 lb/in2). Total cell extracts from cross-linked or non-cross-linked cells were purified twice on oligo(dT) cellulose columns. After the second elution, poly(A)+ RNA-containing fractions were pooled, ethanol precipitated, and digested with RNase A (100 μg/ml; Sigma). The final protein samples were separated by SDS-PAGE, probed with anti-HA monoclonal antibody (Babco), and detected with an ECL Western blotting kit (Amersham).
Fluorescence microscopy.
Fluorescent in situ hybridization for Poly(A)+ RNA was performed as previously described (11), using an oligo-dT(50)-labeled probe followed by a fluorescein isothiocyanate-labeled anti-digoxigenin Fab antibody. Indirect immunofluorescence was performed as described previously (11). GFP fusion proteins were visualized either in live cells or following fixation of cells for 5 min in 2% formaldehyde and 0.05% glutaraldehyde in PBS at room temperature. Cells were visualized using a Zeiss Axiophot microscope with 63× and 100× objectives and photographed with Kodak Ektachrome 200 slide film. The slides were digitized with a Polaroid Sprint Scan scanner, and the images were processed with the Image Pro Plus software.
RESULTS
Suppression of rae1-167 nup184 synthetic lethality and the temperature-sensitive phenotype of the rae1-167 mutation by spMex67p.
Rae1p and Nup184p have been shown to play important roles in the nuclear export of mRNA in S. pombe (8, 11, 41). The S. pombe mRNA export factor Nup184p is homologous to S. cerevisiae Nup188p (32, 43). The S. pombe rae1-167 mutation is synthetically lethal with the nup184-1 mutation. For growth, the rae1-167 nup184-1 synthetic lethal mutant, SL27, carries a plasmid expressing rae1+ from a weak thiamine-repressible nmt1 promoter on the pREP81X vector (6, 41). Repression of Rae1p expression in SL27 cells by addition of thiamine results in a growth defect that is accompanied by poly(A)+ RNA accumulation in the nucleus.
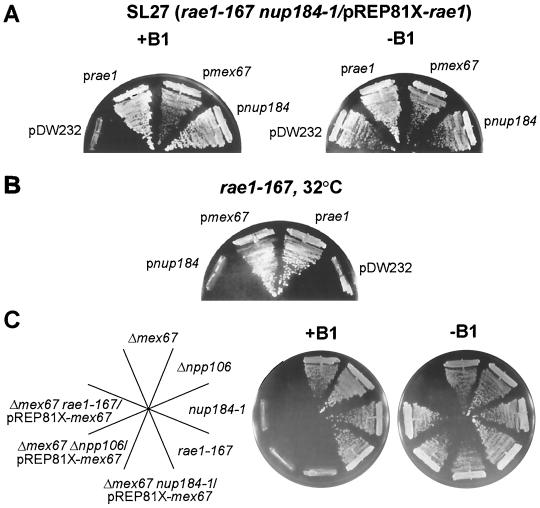
We isolated an extragenic suppressor of the rae1-167 nup184-1 synthetic lethality from a partial Sau3A S. pombe genomic library (Fig. 1A). This suppressor was also able to suppress the temperature-sensitive phenotype of the rae1-167 mutation at the lower restrictive temperature of 32°C (Fig. 1B). Isolation and sequencing of the genomic and cDNA clones revealed an intronless 596-amino-acid open reading frame encoding a 67-kDa protein. Upon conducting sequence searches in the database, we discovered that the newly isolated gene shares 35% identity and 54% similarity with Mex67p of S. cerevisiae (scMex67p). Therefore, the gene was named S. pombe mex67 (spmex67). Sequence searches in the S. pombe database did not reveal any other genes that share sequence similarities with spMex67p. Sequence alignment of spMex67p, scMex67p, and Tap (human and Caenorhabditis elegans homologues) (37) revealed a conservation of structure (Fig. 2).
FIG. 1.
(A) Suppression of the growth defect in synthetic lethal mutant SL27 by spMex67p. SL27 cells carrying pDW232, prae1, pmex67, and pnup184 were streaked onto EMM agar in the absence (−B1) and presence (+B1) of thiamine (0.5 μM). rae1 (prae1), spmex67 (pmex67), and nup184 (pnup184) genes are expressed from their genomic promoters carried in the pDW232 vector. (B) Suppression of the ts phenotype of the rae1-167 mutation by spMex67p expressed from a multicopy plasmid. The mutant rae1-167 cells expressing different genes are indicated. Cells were grown at 32°C for 4 days. (C) Δmex67 is synthetically lethal with rae1-167, nup184-1, or Δnpp106. Growth of different mutants, Δmex67, Δnpp106, nup184-1, and rae1-167, carrying pREP81X-mex67, was compared to that of synthetic lethal mutants of Δmex67 as indicated. The synthetic lethal mutants expressed spMex67p from a vector pREP81X-mex67. spMex67p is expressed in these cells in the absence of thiamine (−B1) and repressed in the presence of thiamine (+B1).
FIG. 2.
spMex67p shows conservation of amino acid sequences with Tap and scMex67. Multiple alignment of spMex67p homologues using the CLUSTAL-W2.0 program is shown. Identical (dark shade) and similar (light shade) amino acids are indicated. The accession number of S. pombe spMex67p is A055036. The human, S. cerevisiae, and C. elegans protein sequences were derived from reference 37.
An spmex67 gene product is not essential for growth.
In order to determine the phenotype of the spmex67 knockout, a null mutant in a diploid strain was constructed by replacing the spmex67-coding region with a ura4 gene. Tetrad analysis of the diploids showed a 2:2 segregation of the ura4 marker. All Ura+ colonies were viable and grew normally in a range of temperatures (18 to 37°C; data not shown). The S. pombe database searches did not show that there was any other gene with similarities with spMex67p. In contrast to MEX67 in S. cerevisiae, which is essential (37), the spmex67 gene in S. pombe is not essential for growth (Fig. 1C).
spmex67 is genetically linked with genes encoding the mRNA export factors rae1, nup184, and npp106.
The viability of the Δspmex67 mutant suggested that spMex67p may have an accessory role in mRNA export in S. pombe. To test this possibility, we determined if there was a genetic interaction between spmex67 and other mRNA export factors in S. pombe. We crossed the Δspmex67 strain with the rae1-167, nup184-1, and Δnpp106 mutant strains. The S. pombe mRNA export factor Npp106p is homologous to the S. cerevisiae nucleoporin, Nic96p (42). To allow growth in a synthetically lethal background, the double mutant cells also carry a plasmid expressing spmex67 from the weak thiamine-repressible, nmt1 promoter on the pREP81X vector. The single mutants grew normally. However, the combination of the Δspmex67 mutation with either rae1-167, nup184-1, or Δnpp106 resulted in synthetic lethality when the expression of spmex67 in the double mutant was repressed by addition of thiamine (Fig. 1C). These results suggest that spmex67 genetically interacts with nup184, npp106, and rae1 for growth in S. pombe. We have previously demonstrated that a rae1-167 mutation is synthetically lethal with either the npp106 or nup184 mutants and that the double mutants accumulate poly(A)+ RNA in the nucleus under synthetic lethal conditions (41, 42). Taken together, these results suggest a genetic linkage among the mRNA export factors rae1, npp106, nup184, and spmex67 in S. pombe.
spmex67 is required for poly(A)+ RNA export in mutant backgrounds that are genetically linked with rae1.
Examination of the poly(A)+ RNA distribution in the Δspmex67 mutant revealed no detectable accumulation of poly(A)+ RNA in the nucleus in a range of temperatures from 25 to 36°C (Fig. 3a). Therefore, spmex67 apparently has no essential role in mRNA export in S. pombe. However, since spMex67p genetically interacts with Rae1p and Nup184p (Fig. 1), we sought to evaluate the function of spMex67p in mRNA export by monitoring poly(A)+ RNA export in Δspmex67 rae1-167 and Δspmex67 nup184-1 strains under synthetically lethal conditions. The double-mutant cells incubated under these conditions (16 h in the presence of thiamine) accumulated significant amounts of poly(A)+ RNA in the nucleus (Fig. 3, compare b and c with e and f). This observation suggests that the spMex67p function is required for the nuclear export of poly(A)+ RNA in either a rae1-167 or nup184-1 mutant background. Furthermore, spMex67p expressed from a multicopy plasmid was able to significantly suppress the poly(A)+ RNA export defects associated with the rae1-167 nup184-1 synthetic lethality (SL27) (Fig. 3, m to o) and the temperature-sensitive phenotype of the rae1-167 mutation (Fig. 3, p to r). This suggests a correlation between suppression of the growth defect and suppression of their poly(A)+ RNA export defects by spMex67p in these mutants (compare Fig. 1 and 3). Even though spMex67p is not an essential protein in S. pombe, our results point to spMex67p's importance in the nuclear export of poly(A)+ RNA, particularly in the background of mutant genes that affect rae1 function.
FIG. 3.
Poly(A)+ RNA localization in SL27, rae1-167, Δmex67, and Δmex67 synthetic lethal mutants. The Δmex67 (a), Δmex67 rae1-167 synthetic lethal mutant cells expressing rae1 from pREP81X-rae1 were grown for 16 h each in the absence (−B1) and presence (+B1) of thiamine (b and c). The synthetic lethal mutant expressing amino acids 145 to 505 of spMex67p from a genomic promoter on a multicopy plasmid is indicated (c). Δmex67 nup184-1 synthetic lethal mutant cells expressing spmex67 from a weak promoter in the pREP81X-mex67 plasmid were grown to mid-log phase in the absence (−B1) (e) and presence (+B1) of thiamine (f) for 16 h. Corresponding 4′,6′-diamidino-2-phenylindole (DAPI) stainings are shown in panels g to l. The rae1-167 nup184-1 synthetic lethal mutant (SL27) cells expressing rae1 from a weak promoter in plasmid pREP81X-rae1, also carrying an empty vector, were grown to mid-log phase in EMM medium in the absence (−B1) (m) and presence (+B1) of thiamine for 16 h (n). SL27 cells carrying plasmid pREP81X-rae1 and pmex67 were grown in the presence of thiamine (+B1) for 16 h (o). rae1-167 carrying an empty vector was grown at 27°C (p) and shifted to 32°C for 3 h (q). The rae1-167 strain carrying the pmex67 plasmid was grown at 32°C in appropriately supplemented EMM medium (r). Cells were fixed, and the localization of poly(A)+ RNA was visualized by fluorescent in situ hybridization. Coincident DAPI staining is shown in the bottom panels, s to x.
Identification of a minimal region within spMex67p that can complement the rae1-167 Δmex67 synthetic lethality.
To gain a better understanding of the genetic interactions between rae1 and spmex67, we decided to determine whether the full-length spMex67p was necessary for complementing the rae1-167 Δmex67 synthetic lethality. The rae1-167 Δmex67 synthetic lethal mutant carries a pREP81X-rae1 plasmid, expressing Rae1p from a weak, thiamine-repressible promoter. A set of spMex67p deletions was constructed, and their ability to complement the rae1-167 Δmex67 synthetic lethality was tested by expressing the deleted genes from a multicopy plasmid (Fig. 4). Among the deletions of spMex67p tested, shown in Fig. 4A, we found that a region between amino acids 149 and 505 (pmex9) was sufficient to complement the rae1-167 Δmex67 synthetic lethality (Fig. 4) as well as the poly(A)+ RNA export defects associated with the synthetic lethality (Fig. 3, b to d). In contrast, expression of other deletions, e.g., pmex2, pmex4, pmex5, and pmex6, was unable to complement the rae1-167 Δmex67 synthetic lethality of the double mutant. We compared the steady-state levels of spMex67p and its deletions under permissive conditions to determine whether the inability of the deletions to complement the rae1-167 Δmex67 synthetic lethality was due to their reduced steady-state levels. The spMex67p protein was detected by using a polyclonal peptide antibody against spMex67p. Rae1p expression was used as a control for the amount of protein loaded onto gels (Fig. 4B). The expression of spMex67p truncations was comparable to the expression of full-length spMex67p (Fig. 4B). Therefore, the inability to complement the rae1-167 Δmex67 synthetic lethality was not due to their reduced steady-state levels. These results, therefore, define a core region in spMex67p from amino acid 149 to 505 that is necessary and sufficient for growth in a rae1-167 mutant background when expressed from a multicopy plasmid.
Since spMex67p expressed from a multicopy plasmid can complement the temperature-sensitive phenotype of the rae1-167 mutation and the rae1-167 nup184-1 synthetic lethality, we wanted to know whether this region (149 to 505) expressed from a multicopy plasmid can also suppress their phenotypes. We found that the 149–505 region (pmex9) was unable to suppress either the temperature-sensitive phenotype of the rae1-167 mutation or the synthetic lethality of the rae1-167 nup184-1 mutant (data not shown). These results suggest that full-length spMex67p has domains, in addition to that present within the 149–505 region, that are required to suppress the temperature-sensitive phenotype of rae1-167 and the lethality caused by a combination of rae1-167 and nup184-1 mutations.
Overexpression of spMex67p inhibits growth and nuclear export of mRNA.
Even though spMex67p is not essential, if it is involved in the nuclear export of mRNA, its overexpression could inhibit nuclear export of poly(A)+ RNA by interacting and titrating out essential mRNA export factors. We decided to overexpress spMex67p from a strong thiamine-repressible nmt1 promoter in the pREP3X vector (14) and test if nuclear export of poly(A)+ RNA is inhibited in the wild-type cells. Interestingly, we found that overexpression of spMex67p (pRM) inhibited growth of wild-type cells (Fig. 5A and B). This inhibition was accompanied by accumulation of poly(A)+ RNA in the nucleus and at the nuclear periphery (Fig. 5D). In the nucleus, the poly(A)+ RNA was also found concentrated in discrete foci (Fig. 5D, inset panel). These results suggest that regions within spMex67p likely interact with and compete for proteins that are essential for growth and nuclear export of mRNA.
FIG. 5.
Overexpression of spMex67p inhibits growth and mRNA export. (A) The schematic diagram of spMex67p and its deletions (the extent of each deletion is shown in parentheses) expressed from a strong thiamine-repressible promoter, nmt1 in the pREP3X-vector. The abilities of these deletions to inhibit growth following overexpression in wild-type cells are indicated. −, complete inhibition; +, no inhibition; ±, intermediate inhibition. (B) Wild-type S. pombe cells carrying the plasmids as indicated were grown on EMM agar plates at 28°C for 4 days. Growth of cells overexpressing spMex67p and their deletions in the absence of thiamine (−B1) was compared to growth in the presence of thiamine (+B1) when their expression was repressed. A plasmid (pRGST) expressing GST was used as a control. (C) Determination of the steady-state levels of overexpressed spMex67p and its truncations in crude extracts. The upper panel shows the amount of spMex67p detected by a peptide antibody against spMex67p. The lower panel shows the amount of Rae1p as a control for protein loading. (D) Localization of poly(A)+ RNA in wild-type cells overexpressing spMex67 or its deletions, as indicated. The poly(A)+ RNA in the inset within the pRM panel was visualized using a 100× objective. Coincident 4′,6′-diamidino-2-phenylindole (DAPI) staining is shown in the lower panels.
To identify regions which could be important for interaction with these presumptive factors related to mRNA export and growth, we overexpressed a series of deletions of spMex67p in wild-type cells. From these experiments, we identified deletions that no longer inhibited growth and that also restored mRNA export. Interestingly, cells overexpressing spMex67p carrying a deletion of positions 439 to 470 (pRMΔ5) or 509 to 555 (pRMΔ7) had no defect in growth or in the nuclear export of poly(A)+ RNA (Fig. 5A and B). Moreover, overexpression of the 149–505 domain also did not inhibit growth and mRNA export (data not shown). In contrast, growth was inhibited in cells overexpressing any one of Mex67p deletions of 145 to 202 (pRMΔ2), 226 to 314 (pRMΔ3), 323 to 434 (pRMΔ4) or 469 to 505 (pRMΔ6) (Fig. 4B and 5A). Partial inhibition of growth occurred in cells overexpressing spMex67p carrying a deletion of 3 to 148 (pRMΔ1) or 561 to 596 (pRMΔ8) (Fig. 5B). We ensured that the deletion constructs expressed comparable levels of protein, and thus, the observed difference in growth inhibition by pRMΔ5 and pRMΔ7 was not due to unequal amounts of functional proteins (Fig. 5C). We conclude from these results that regions between 439 and 470 and between 509 and 555, at least in the context of full-length spMex67p, likely interact with essential mRNA export factors.
spMex67p can be UV cross-linked with poly(A)+ RNA.
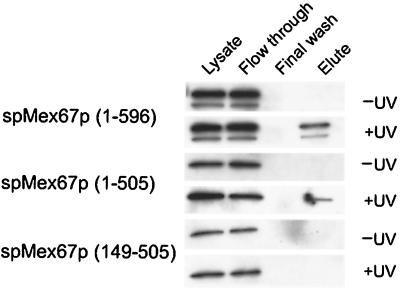
The N-terminal region of Tap and scMex67p directly associates with RNA (10, 19, 20, 37). We next wanted to determine whether spMex67p or the spMex67p(149–505) domain could be UV cross-linked to poly(A)+ RNA. Δspmex67 cells expressing a functional HA-tagged epitope at the amino terminus of either full-length spMex67p, spMex67p(149–505), or spMex67p(Δ509–596) were treated with UV to test their ability to cross-link with poly(A)+ RNA. UV cross-linking of poly(A)+ RNA was detected with spMex67p and spMex67p carrying a deletion of 509 to 596 but not with the 149–505-tagged protein (Fig. 6). Therefore, it appears likely that the suppression of rae1-167 Δmex67 lethality by the 149–505 domain of spMex67p doesn't require a direct association with poly(A)+ RNA.
FIG. 6.
UV cross-linking of spMex67p and its deletions with poly(A)+ RNA. Purified poly(A)+ RNA was loaded from untreated and UV-treated cells (2) expressing full-length spMex67, amino acids 1 to 505, and amino acids 149–569 of spMex67p carrying the HA-tagged epitope at the N terminus. The proteins cross-linking with poly(A)+ RNA were visualized by Western blot analysis using antibodies raised against the HA epitope.
spMex67p fusion protein predominantly localizes in the nucleus.
To gain further insights into the function of spMex67p, we determined the subcellular localization of spMex67p tagged at the N terminus or the C terminus with GFP. Both fusions were functional and complemented the synthetic lethality of rae1-167 nup184-1 and the temperature-sensitive phenotype of rae1-167 cells (data not shown). An integrated version of the pmex67-GFP fusion was then constructed at the spmex67 locus, and the localization of the fusion protein was determined. In contrast to the nuclear peripheral localization of scMex67p-GFP, the spMex67p-GFP cellular localization was most similar to that of Tap, where both proteins are present predominantly in the nucleus with a trace amount at the nuclear periphery (Fig. 7). The staining at the nuclear periphery is difficult to visualize, however, because of intense fluorescence staining in the nucleus (due to the strong nuclear localization signal [NLS] present at the N terminus of spMex67p; see below).
FIG. 7.
Cellular localization of spMex67p and its deletions. (A) A schematic map of the different deletions of spMex67p fused to GFP and their cellular localization. N, NPC, C, and D stand for nucleus, nuclear periphery, cytoplasm, and diffused throughout the cell, respectively. (B) GFP was fused to the C terminus of proteins with deletions, and these GFP-fusion proteins were expressed from a thiamine-repressible promoter in the pREP81-vector. Cells were grown in appropriately supplemented EMM in the absence of thiamine, and their localization was visualized in fixed cells. Coincident DAPI staining is shown in the lower panel.
To identify regions within spMex67p that could be important for its cellular localization, we made deletions within spMex67p that were fused to GFP or to LacZ-GFP and examined their cellular localization in Δspmex67 cells (Fig. 7A). A strong NLS was identified near the N terminus of spMex67p between amino acids 1 and 111 (pGmex10). Further deletion analysis localized the NLS near the N terminus (residues 1 to 20) (pGmex11) (Fig. 7B, lower panel). When the NLS was deleted in spMex67p(Δ3–148)-GFP (pGmex1), the fusion protein was found predominantly at the nuclear periphery with some localization in the cytoplasm and in the nucleus (Fig. 7B, upper panel), indicating that regions downstream of positions 1 to 148 in spMex67p contain sequences for its localization to the nuclear periphery and also for nuclear import. When the sequences near the C terminus were deleted in pGmex7 (Δ509–555) or pGmex8 (Δ556–596), the fusion protein was diffused throughout the cell, suggesting that the C-terminal region (509 to 596) is important for localization to the nuclear periphery. In addition, the GFP fusion of the 149–505 region (pGmex9), lacking the NPC localization sequences, was also found throughout the cell (Fig. 7B, lower panel). These results show that similar to Tap, spMex67p contains an NLS near the N terminus, and the C terminus contains sequences for localization to the nuclear periphery.
The 149–505 region of spMex67p can be located within the nucleus and to the nuclear periphery in cells expressing saturating amounts of spMex67p.
Since the 149–505 region of spMex67p can suppress growth and the mRNA export defect of the rae1-167 Δmex67 lethality, we wanted to know if it could shuttle between the nucleus and the cytoplasm. Since overexpression of full-length spMex67p inhibits mRNA export, it might also affect the steady-state localization of the (149–505)-GFP fusion in the cytoplasm and the nucleus.
Wild-type cells were transformed with the plasmid expressing (149–505)-GFP from the genomic spMex67p promoter, and full-length spMex67p was expressed from the nmt1-promoter in the pREP3X vector. spMex67p(149–505)-GFP localized to both the nucleus and the cytoplasm in cells in which the expression of spMex67p was repressed (Fig. 8). In contrast, in cells overexpressing spMex67p, the fusion protein accumulated in the nucleus and at the nuclear periphery with minor localization in the cytoplasm (Fig. 8b, top inset). In the nucleus of these cells, the fusion protein was also found concentrated in one or two foci (Fig. 8 inset). Interestingly, the pattern of nuclear accumulation of both poly(A)+ RNA (Fig. 5D, inset) and spMex67p(149–505)-GFP (Fig. 8 inset) in cells overexpressing spMex67p was very similar. These results are consistent with the suggestion that the 149–505 region of spMex67p likely shuttles between the nucleus and the cytoplasm. The inhibition of poly(A)+ RNA and the localization of the (149–505)-GFP fusion at the nuclear periphery and within the nucleus is likely a consequence of depletion of essential export factors following overexpression of spMex67p.
FIG. 8.
Analysis of the nucleocytoplasmic localization of spMex67p(149–505) in cells overexpressing spMex67p. The localization of the spMex67p(149–505)-GFP fusion was determined in cells overexpressing spMex67p (pRM) or spMex67p(Δ509–505) (pRM7) from a thiamine-repressible strong nmt1-promoter in the pREP3X vector. Cells were visualized for GFP staining following growth in the presence (+B1) and absence (−B1) of thiamine for 16 h. Coincident DAPI staining is shown for each panel.
As mentioned above, overexpression of spMex67p carrying deletions of either 439 to 470 or 509 to 555 did not inhibit nuclear export of poly(A)+ RNA (Fig. 5, pRMΔ5 and pRMΔ7). We next wanted to test whether these regions would affect the dynamics of the 149–505 fusion by competing for export factors. Interestingly, overexpression of spMex67p(Δ509–555) led to the accumulation of the spMex67p(149–505)-GFP fusion in the nucleus but not at the nuclear periphery (Fig. 8). But in cells overexpressing spMex67p(Δ439–470), only a small amount of fusion protein was detected at the nuclear periphery and in the nucleus (data not shown). Taken together, these results suggest that spMex67p can likely interact with factors whose function affects the dynamics of spMex67p.
spMex67p amino acids 439 to 505 can direct nuclear export in HeLa cells.
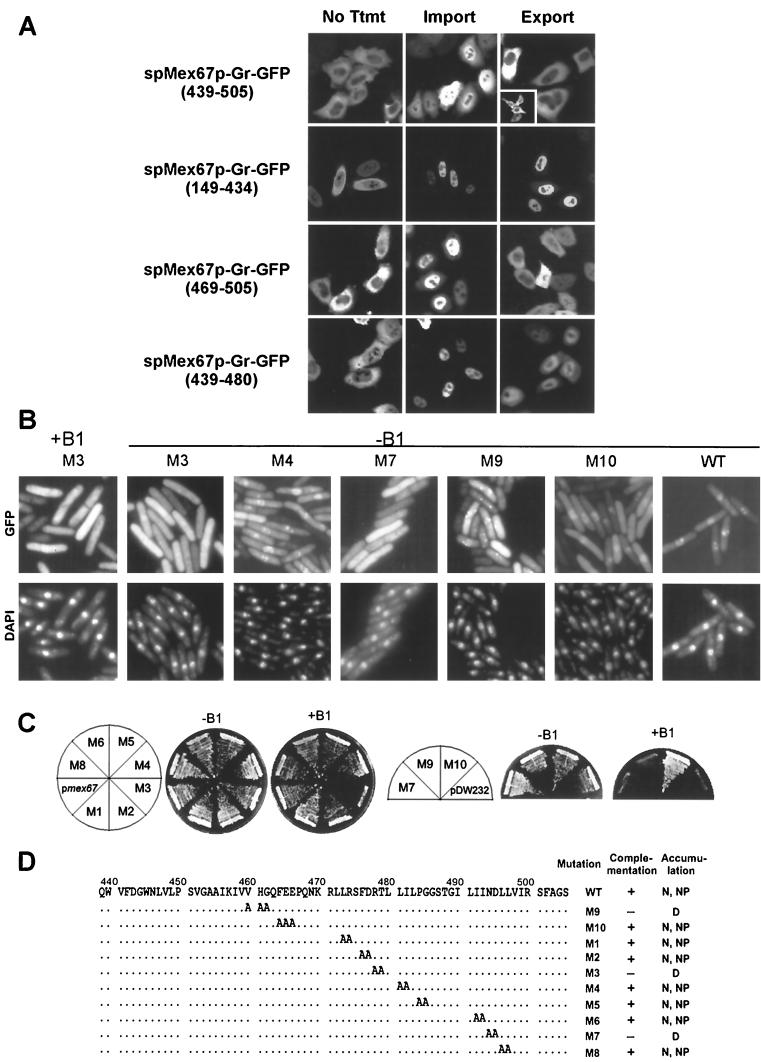
The above-described results indicate that the 149–505 region of spMex67p can be imported into the nucleus and that its nuclear export is likely inhibited in cells overexpressing spMex67p or spMex67p carrying a deletion of 509 to 555. However, we found no obvious sequences that could direct nuclear import or export within the 149–505 region in the context of S. pombe (data not shown). This observation could be explained if the import and export signals overlap. We therefore analyzed deletions within the 149–505 region for nuclear export activity using an established heterologous assay in HeLa cells (24). Deletions within the 149–505 region of spMex67p were fused to the N terminus of a hormone-responsive-GFP chimeric protein. The responsive element is the steroid-binding region of the rat glucocorticoid receptor (Gr) (24). Inclusion of the domain allows for control of import by the addition of the steroid. Removal of the steroid induces export, assuming the protein of interest contains an export sequence. Gr-GFP is not exported unless specific export sequences are added (24). Deletions within the 149–505 region of spMex67p were fused to Gr-GFP and expressed in HeLa cells. The fusion proteins were found predominantly in the cytoplasm and upon treatment with the steroid translocated to the nucleus. Thirty minutes following removal of the hormone and treatment with cycloheximide to prevent new protein synthesis, cells were assayed for nuclear export. A region between 439 and 505 of spMex67p was able to direct nuclear export of Gr-GFP, whereas the 149–434 region was unable to do so (Fig. 9A). Furthermore, addition of leptomycin B did not inhibit the export activity associated with amino acids 439 to 505, suggesting that the Crm1 export receptor is not involved in export of spMex67p (see the insert in Fig. 9). To further define the nuclear export sequence, sequential deletions within the 439–505 region were examined. We found a reduced nuclear export activity when the 469–505 or 439–480 region was fused to Gr-GFP. Thus, the 439–505 region of spMex67p could contain more than one region that may contribute either to its nuclear export activity or to its stable association with export complexes.
FIG. 9.
(A) Identification of NES activity in spMex67p using a heterologous system. spMex67p (439–505), spMex67p (149–434), spMex67p (469–505), and spMex67p (439–480) each were expressed as a hormone-inducible Gr-GFP chimeric protein in HeLa cells (spMex67p-Gr-GFP). In the absence of corticosteroid (No Ttmt), the chimera was mainly cytoplasmic. Import, treatment with 2 μM corticosteroid for 30 min at 37°C. Export, cells were washed three times with PBS to remove the steroid and incubated for an additional hour at 37°C. Cells expressing spMex67p (439–505) were also incubated with 2 nM leptomycin B to inhibit Crm1-dependent export (inset, top row right). Cycloheximide (25 μg/ml) was added to all cells to prevent new protein synthesis. Following incubations, cells were fixed in 4% formaldehyde for 30 min at room temperature and visualized by fluorescence microscopy (24). (B) Localization of spMex67p(149–505)-GFP and different mutations as indicated in cells overexpressing spMex67p. Coincident DAPI staining is shown in the lower panel. (C) Complementation of rae1-167 Δmex67 synthetic lethality by different plasmids expressing full-length spMex67p (pmex67) and mutations (M1 through M10) introduced into full-length spMex67p from the genomic promoter. rae1-167 Δmex67 synthetic lethal strains expressing wild-type spMex67 and different mutations as indicated were streaked onto EMM agar plates in the absence (−B1) and presence (+B1) of thiamine and incubated at 28°C for 4 days. (D) Summary of the mutational analyses of a sequence in the 439–505 region of spMex67p. Site-directed mutations were introduced into spMex67p(149–505)-GFP and into full-length spMex67 as indicated, and both were expressed from a spmex67 genomic promoter. + and − indicate the ability or inability, respectively, of full-length mutant versions of spMex67p to complement the rae1-167 Δmex67 synthetic lethality. N, NP, and D stand for nucleus, nuclear periphery, and diffused, respectively.
The 439–505 region of spMex67 can mediate interactions with the nuclear periphery.
We next tested whether the 439–505 region contains sites for interaction with putative export factors in the context of S. pombe. We introduced mutations into conserved amino acids within the 439–505 region (Fig. 2) of spMex67p(149–505)-GFP. The localization of the mutants was examined in the context of either overexpression or repression of full-length spMex67p. The same mutations were introduced into full-length spMex67p, and complementation of the rae1-167 Δmex67 synthetic lethality was assessed (Fig. 9B, C, and D). All the spMex67(149–505)-GFP fusions that contained mutations within the 439–505 region were found diffused throughout the cell. However, in contrast to (149–505)-GFP, fusion proteins carrying any one of the mutations VHG460–462 AAA (M9), RT478,479 AA (M3), and ND494–495 AA (M7) were unable to accumulate in the nucleus and at the nuclear periphery in cells overexpressing spMex67p (Fig. 9B and D). Moreover, these mutations introduced into the full-length spMex67p were also unable to complement the rae1-167 Δmex67 synthetic lethality (Fig. 9B and C). These amino acids may contribute to important mRNA export functions of spMex67p. Fusion proteins carrying any one of the mutations FEE464–466 AAA (M10), LR473,474 AA (M1), LI481,482 AA (M2), PG484,485 AA (M4), II492,493 AA (M5), and LL495,496 AA (M8) accumulated to varying degrees within the nucleus and at the nuclear periphery in cells overexpressing spMex67p. In addition, these mutations introduced in the context of full-length spMex67p were also able to complement the rae1-167 Δmex67 lethality (summarized in Fig. 9B and C). Taken together, these results suggest that the 439–505 region of spMex67p contains important determinants for mediating interactions with export factors both within the nucleus and at the nuclear periphery.
spMex67p and Rae1p physically interact.
Coimmunoprecipitation was used to determine whether the genetic and functional interactions between the 149–505 region of spMex67p and Rae1p in growth and mRNA export also involve a physical interaction. Various HA-epitope-tagged spMex67p and deletion constructs were expressed in S. pombe cells, immunoprecipitated, and analyzed by Western blot analysis using polyclonal antibodies against Rae1p. Rae1p coimmunoprecipitated in cells expressing spMex67p or different deletions within spMex67p, with the exception of a deletion in the 469–505 region. No Rae1p was detected in cells carrying an empty vector (Fig. 10A, upper panel). The amount of spMex67p or its truncations immunoprecipitated in these experiments was determined by Western blot analysis of duplicate gels with polyclonal antibodies against the HA tag (Fig. 10A, lower panel). The relative amounts of Rae1p and of spMex67p and its truncations present in the extracts before immunoprecipitation were similar (data not shown). These results suggest that the 149–505 region within spMex67p can interact with the Rae1p complex. The inability to detect an association between spMex67p carrying a deletion for the 469–505 region and Rae1p could be explained by the fact that region 469–505 is important for association with a putative Rae1p complex or that the interactions are unstable or transient.
FIG. 10.
Coimmunoprecipitation of Rae1p with spMex67p. Whole-cell extracts were prepared from the transformants of the Δmex67 strain expressing Rae1p from the pREP4X plasmid and N-terminal HA-epitope-tagged spMex67p and different deletions within full-length spMex67p, as indicated. Extract prepared from cells expressing the HA tag from an empty vector was used as a control for immunoprecipitations. (A) Crude extracts were immunoprecipitated with monoclonal antibodies against the HA epitope, and the coimmunoprecipitation of Rae1p was detected by using polyclonal antibodies against Rae1p (upper panel). spMex67p and its deletions were detected by using polyclonal antibodies against the HA epitope (lower panel). (B) Coomassie staining of the GST-Mex67p pull-down assay. GST and GST-spMex67p expressed in E. coli and purified on GSH beads were incubated with clarified extracts of E. coli containing Rae1p. Lanes 1 and 5 show Rae1p-containing extracts that did not bind to GST-spMex67p and GST immobilized to GSH beads, respectively. Lanes 2 and 6 show input GST-spMex67 and GST, lanes 3 and 7 are GST-spMex67p and GST incubated with Rae1p-containing extracts, and lane 4 shows Rae1p input used in the binding experiments. Western blot analysis was performed on duplicate gels using anti-Rae1p (b) and anti-GST (c) antibodies as indicated. Asterisks indicate in vivo degradation products of GST-spMex67p. (C) Wild-type cells expressing spMex67p from a thiamine-repressible pREP4X promoter and Rae1p from a pREP3-promoter. Growth as indicated was compared in the presence (+B1) and absence (−B1) of thiamine.
We next tested whether Rae1p directly interacts with spMex67p under in vitro binding conditions. GST and spMex67p fused to GST were expressed in E. coli and purified on glutathione (GSH) beads. Clarified extracts prepared from bacterial cells expressing Rae1p were incubated separately with GST or GST-spMex67p. Unbound and bound fractions were assayed by SDS-PAGE analysis by staining with Coomassie blue (Fig. 10B, top, panel a). No Rae1p was visible in the bound fraction with spMex67 (lane 3), and the amount of Rae1 in the input was essentially the same as in the unbound fraction (lanes 1 and 4). However, there were several endogenous breakdown products of GST-spMex67p (Fig. 10B, top panel), including one that migrated slightly faster than Rae1p. To ascertain whether they are Rae1p or spMex67p related, we performed Western blot analysis on duplicate gels using anti-GST and anti-Rae1p antibodies and confirmed that the suspected breakdown products were spMex67p related (Fig. 10B, middle and bottom panels). The Western data clearly showed the absence of Rae1p bound to Mex67p (middle panel, lane 3). These results showed no detectable association between Rae1p and spMex67p under in vitro binding conditions. While these results do not completely rule out direct interaction between the two proteins, based on our coimmunoprecipitation experiments, we favor the notion that the bulk of the Rae1p-spMex67p interactions occur through common complexes.
We wanted to know if the defects associated with overexpression of spMex67p were due to the titration of a factor(s) that also interacts with Rae1p. In this scenario, if Rae1p was overexpressed in cells that also overexpressed spMex67p, Rae1p could compete for a common factor(s). However, overexpression of Rae1p did not rescue the growth and mRNA export defects associated with the overexpression of spMex67p (Fig. 10C). This observation suggests that a factor(s) that interacts with spMex67p specifically is the likely cause of the phenotypes associated with overexpression of spMex67p. In addition, cells overexpressing spMex67p carrying a deletion of the 469–505 region had growth and mRNA export inhibition (Fig. 5B and C). This region within spMex67p is required for coimmunoprecipitation with Rae1p (Fig. 10A). Together these results suggest that overexpression of spMex67p does not titrate Rae1p or a Rae1p interacting factor(s).
DISCUSSION
Conservation of spmex67p function.
The essential role of the scmex67 gene in mRNA export in S. cerevisiae (37) and the ability of Tap to overcome the inhibition of mRNA export following overexpression of CTE RNA in Xenopus oocytes (16) strongly suggest that Mex67p and Tap play important roles in mRNA export. Tap has been shown to shuttle between the nucleus and the cytoplasm; it also associates with mRNA and the NPC (7, 19, 20). Some of the signals that contribute to its shuttling have recently been identified (7, 19). Overlapping nuclear import and export activities have been reported to be present near the N terminus around amino acids 61 to 102 (NLS) and 83 to 110 (NES) and near the C terminus between amino acids 540 and 614 (NLS and NES) of Tap (7, 19). It has been suggested that Tap and scMex67p could function in mRNA export by interacting with poly(A)+ RNA and/or the RNP complex and direct their export from the nucleus (7, 19, 20, 37). The nucleocytoplasmic shuttling of Tap or Mex67p could be critical for its mRNA export function. Similar to Tap (7, 19, 20), the N terminus of spMex67p also contains an import signal that contributes to its steady-state localization in the nucleus (Fig. 7 and 11A). Removal of this import signal (amino acids 1 to 145) from spMex67p results in an increase in its NPC localization with a concomitant reduction in its nuclear accumulation (Fig. 7). Other than this NLS present at the N terminus of spMex67p, no short sequences having either import or export signals were apparent in spMex67p (Fig. 7). In addition, the 540–619 region of Tap has been suggested to contain import and export activities. The C terminus (509 to 596) of spMex67p also contains sequences that contribute to the localization to the nuclear periphery (Fig. 11A) (7, 20).
FIG. 11.
(A) Schematic diagram comparing the functional domains of spMex67p and Tap. Relative locations of the mRNA binding domain, NLS, and NES identified in Tap and spMex67p are indicated. The spMex67p (149–505) domain that can complement the rae1-167 mutation in the Δspmex67 background is shown. I, II, and III represent functional domains defined in this work (see the text for details). (B) A model of spMex67p function in mRNA export. spMex67p may have multiple roles in the assembly of mRNP within the nucleus (step 1), translocation of the mRNP through the NPC (step 2), and release of RNP in the cytoplasm (step 3) (see Discussion).
In S. pombe, domains I and III (Fig. 11A) play important roles, presumably by interacting with a factor(s) whose functions are required for cell growth, mRNA export, and the dynamics of spMex67p. Interestingly, mutations within scMex67p having effects on growth, mRNA export, and localization of spMex67p map onto corresponding regions. A temperature-sensitive mutation, scmex67-5 (H400Y) of scMex67p (corresponding to domain I of spMex67p), at the restrictive temperature, accumulates mRNA in the nucleus and the mutant fusion in the cytoplasm (7, 20). Mutations within the putative NES region in scMex67p (549–557 residues) that correspond to the domain III of spMex67p also have similar phenotypes: poly(A)+ RNA accumulates in the nucleus, and the mutant proteins locate predominantly in the cytoplasm and the nucleus instead of the NPC (20). Thus our results indicate both structural and functional conservation among Mex67p from S. cerevisiae, Mex67p from S. pombe, and Tap.
Shuttling of the core region of spMex67p.
Since Tap has been shown to shuttle, we wanted to know if the core-complementing region of spMex67p (149–505 domain) could also shuttle. Our experimental support for import came from the observation that while the 149–505 region is found diffused throughout the cell, it accumulates in the nucleus and at the nuclear periphery in cells expressing saturating amounts of spMex67p (Fig. 8). While the export of this domain out of the nucleus was not demonstrable in the context of S. pombe, we show that this region can exit out of the nucleus in a heterologous assay (Fig. 9). However, the 149–505 region does not contain a short sequence found in classical NES and NLS (data not shown). Therefore, a likely mechanism by which this domain could move in and out of the nucleus is by piggybacking on other factors.
Interactions between spmex67 and rae1.
Overexpression of spMex67p carrying a deletion of domain II inhibited growth and mRNA export (Fig. 5 and 11A) and localized the reporter 149–505 fusion in the nucleus and at the nuclear periphery (data not shown). Furthermore, mutations M3 and M7 (Fig. 9C and D) within the (149–505)-GFP fusion no longer accumulate within the nucleus and at the nuclear periphery following overexpression of spMex67p. Domain II also plays an important role in mediating interaction with Rae1p. Indeed, spMex67p with a deletion of domain II is unable to coimmunoprecipitate Rae1p (Fig. 10A). However, domain II may not be the only region capable of associating with a Rae1p complex; spMex67p could still associate with Rae1p through Nup98-Rae1p. For example, the C-terminal region of full-length spMex67p (509–596 region) also associates with the nuclear periphery, presumably through its interactions with Nup98p. The 149–505 domain of spMex67p associates with Rae1p and it does not UV cross-link with poly(A)+ RNA (Fig. 6 and 10). Moreover, it is devoid of the N-terminal NLS and the C-terminal NPC localization signal. These results raise the possibility that the complementing functions and localization to the nuclear periphery of the 149–505 domain are dependent on its association with Rae1p.
How does spMex67p function in mRNA export?
Overexpression of spMex67p resulted in the localization defects of both the poly(A)+ RNA and the (149–505)-GFP fusion within the nucleus and at the nuclear periphery. These results could be interpreted as resulting from a simple depletion of mRNA export factors or factors involved specifically in the trafficking of the 149–505 fusion. However, the trafficking of the 149–505 fusion does not directly correlate with mRNA export. (i) In cells overexpressing spMex67p carrying a deletion of either domain I or III, mRNA export is unaffected while the fusion is retained in the nucleus (Fig. 5, 8, and 11A). (ii) Under conditions when mRNA export is inhibited, e.g., inactivation of Rae1p or under synthetic lethal conditions, the diffuse localization of the 149–505 fusion is not altered (11, 41). These results are consistent with a model in which spMex67p plays an accessory role in mRNA export in S. pombe and may be associated with the RNP only transiently. We propose that spMex67p mediates multiple associations at various steps in RNP assembly.
A model depicting some of the possible functions of spMex67p is shown in Fig. 11B. Based on results presented in this work and others (36, 37), we propose that the 149–505 region within spMex67p could function by bridging different RNP export factors for promoting their assembly and translocation both within the nucleus and at the nuclear pore (shown as steps 1, 2, and 3). Suppression of the mRNA export defects associated with rae1-167 Δmex67 lethality could be achieved simply by stabilizing interactions among different factors of the RNP export complex in the Rae1-167p mutant background. Depletion of an export factor(s) in cells expressing saturating amounts of spMex67p could lock the RNP-spMex67p complex into a permanently bound form. This may result in the accumulation of both the poly(A)+ RNA and the 149–505 fusion protein within the nucleus and at the nuclear periphery in cells overexpressing spMex67p (Fig. 5, 8, and 11B). The spMex67p (149–505)-GFP protein with mutations in domain I or II may be unable to form stable interactions with functional mRNA export complexes or the nuclear periphery and therefore does not accumulate in the nucleus or at the nuclear periphery in cells overexpressing spMex67p (Fig. 9).
Our studies, aimed at dissecting the functions of spMex67p, complement those previously performed with Tap and spMex67p. This family of proteins likely performs an array of functions critical to mRNA assembly and transport. Although not essential in S. pombe, our results demonstrate an accessory role for spMex67p in Rae1-dependent mRNA export. The minimal 149–505 region of spMex67p may act as an accessory, stabilizing factor in Rae1p-dependent export of mRNA.
ACKNOWLEDGMENTS
We thank Craig Whiteford, Henry Levin, and Janet Duval for critical reading of the manuscript.
REFERENCES
- 1.Alfa C, Fantes P, Hams J, McLeod M, Warbrick E. Experiments in fission yeast. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1993. [Google Scholar]
- 2.Anderson J T, Wilson S M, Datar K V, Swanson M S. NAB2: a yeast nuclear polyadenylated RNA-binding protein essential for cell viability. Mol Cell Biol. 1993;13:2730–2741. doi: 10.1128/mcb.13.5.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachi A, Braun I C, Rodrigues J P, Pante N, Ribbeck K, von Kobbe C, Kutay U, Wilm M, Gorlich D, Carmo-Fonseca M, Izaurralde E. The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. RNA. 2000;6:136–158. doi: 10.1017/s1355838200991994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahler J, Wu J Q, Longtine M S, Shah N G, McKenzie A, Steever A B, Wach A, Philippsen P, Pringle J R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 5.Bailer S M, Siniossoglou S, Podtelejnikov A, Hellwig A, Mann M, Hurt E. Nup116p and Nup100p are interchangeable through a conserved motif which constitutes a docking site for the mRNA transport factor Gle2p. EMBO J. 1998;17:1107–1119. doi: 10.1093/emboj/17.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basi G, Schmid E, Maundrell K. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene. 1993;123:131–136. doi: 10.1016/0378-1119(93)90552-e. [DOI] [PubMed] [Google Scholar]
- 7.Bear J, Tan W, Zolotukhin A S, Tabernero C, Hudson E A, Felber B K. Identification of novel import and export signals of human TAP, the protein that binds to the constitutive transport element of the type D retrovirus mRNAs. Mol Cell Biol. 1999;19:6306–6317. doi: 10.1128/mcb.19.9.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bharathi A, Ghosh A, Whalen W A, Yoon J H, Pu R, Dasso M, Dhar R. The human RAE1 gene is a functional homologue of Schizosaccharomyces pombe rae1 gene involved in nuclear export of poly(A)+ RNA. Gene. 1997;198:251–258. doi: 10.1016/s0378-1119(97)00322-3. [DOI] [PubMed] [Google Scholar]
- 9.Black B E, Vesque L, Holaska J M, Wood T C, Paschal B M. Identification of an NTF2-related factor that binds Ran-GTP and regulates nuclear protein export. Mol Cell Biol. 1999;19:8616–8624. doi: 10.1128/mcb.19.12.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun I C, Rohrbach E, Schmitt C, Izaurralde E. TAP binds to the constitutive transport element (CTE) through a novel RNA-binding motif that is sufficient to promote CTE-dependent RNA export from the nucleus. EMBO J. 1999;18:1953–1965. doi: 10.1093/emboj/18.7.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown J A, Bharathi A, Ghosh A, Whalen W, Fitzgerald E, Dhar R. A mutation in the Schizosaccharomyces pombe rae1 gene causes defects in poly(A)+ RNA export and in the cytoskeleton. J Biol Chem. 1995;270:7411–7419. doi: 10.1074/jbc.270.13.7411. [DOI] [PubMed] [Google Scholar]
- 12.Fabre E, Hurt E. Yeast genetics to dissect the nuclear pore complex and nucleocytoplasmic trafficking. Annu Rev Genet. 1997;31:277–313. doi: 10.1146/annurev.genet.31.1.277. [DOI] [PubMed] [Google Scholar]
- 13.Flach J, Bossie M, Vogel J, Corbett A, Jinks T, Willins D A, Silver P A. A yeast RNA-binding protein shuttles between the nucleus and the cytoplasm. Mol Cell Biol. 1994;14:8399–8407. doi: 10.1128/mcb.14.12.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsburg S L. Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 1993;21:2955–2956. doi: 10.1093/nar/21.12.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorlich D, Mattaj I W. Nucleocytoplasmic transport. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- 16.Gruter P, Tabernero C, von Kobbe C, Schmitt C, Saavedra C, Bachi A, Wilm M, Felber B K, Izaurralde E. TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol Cell. 1998;1:649–659. doi: 10.1016/s1097-2765(00)80065-9. [DOI] [PubMed] [Google Scholar]
- 17.Ho A K, Raczniak G A, Ives E B, Wente S R. The integral membrane protein snl1p is genetically linked to yeast nuclear pore complex function. Mol Biol Cell. 1998;9:355–373. doi: 10.1091/mbc.9.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izaurralde E, Jarmolowski A, Beisel C, Mattaj I W, Dreyfuss G, Fischer U. A role for the M9 transport signal of hnRNP A1 in mRNA nuclear export. J Cell Biol. 1997;137:27–35. doi: 10.1083/jcb.137.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang Y, Cullen B R. The human Tap protein is a nuclear mRNA export factor that contains novel RNA-binding and nucleocytoplasmic transport sequences. Genes Dev. 1999;13:1126–1139. doi: 10.1101/gad.13.9.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katahira J, Strasser K, Podtelejnikov A, Mann M, Jung J U, Hurt E. The Mex67p-mediated nuclear mRNA export pathway is conserved from yeast to human. EMBO J. 1999;18:2593–2609. doi: 10.1093/emboj/18.9.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koepp D M, Silver P A. A GTPase controlling nuclear trafficking: running the right way or walking RANdomly? Cell. 1996;87:1–4. doi: 10.1016/s0092-8674(00)81315-x. [DOI] [PubMed] [Google Scholar]
- 22.Kraemer D, Blobel G. mRNA binding protein mrnp 41 localizes to both nucleus and cytoplasm. Proc Natl Acad Sci USA. 1997;94:9119–9124. doi: 10.1073/pnas.94.17.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee M S, Henry M, Silver P A. A protein that shuttles between the nucleus and the cytoplasm is an important mediator of RNA export. Genes Dev. 1996;10:1233–1246. doi: 10.1101/gad.10.10.1233. [DOI] [PubMed] [Google Scholar]
- 24.Love D C, Sweitzer T D, Hanover J A. Reconstitution of HIV-1 rev nuclear export: independent requirements for nuclear import and export. Proc Natl Acad Sci USA. 1998;95:10608–10613. doi: 10.1073/pnas.95.18.10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattaj I W, Englmeier L. Nucleocytoplasmic transport: the soluble phase. Annu Rev Biochem. 1998;67:265–306. doi: 10.1146/annurev.biochem.67.1.265. [DOI] [PubMed] [Google Scholar]
- 26.Maundrell K. nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J Biol Chem. 1990;265:10857–10864. [PubMed] [Google Scholar]
- 27.Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 28.Murphy R, Watkins J L, Wente S R. GLE2, a Saccharomyces cerevisiae homologue of the Schizosaccharomyces pombe export factor RAE1, is required for nuclear pore complex structure and function. Mol Biol Cell. 1996;7:1921–1937. doi: 10.1091/mbc.7.12.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy R, Wente S R. An RNA-export mediator with an essential nuclear export signal. Nature. 1996;383:357–360. doi: 10.1038/383357a0. [DOI] [PubMed] [Google Scholar]
- 30.Nakielny S, Dreyfuss G. Nuclear export of proteins and RNA. Curr Opin Cell Biol. 1997;9:420–429. doi: 10.1016/s0955-0674(97)80016-6. [DOI] [PubMed] [Google Scholar]
- 31.Nakielny S, Dreyfuss G. Transport of proteins and RNAs in and out of the nucleus. Cell. 1999;99:677–690. doi: 10.1016/s0092-8674(00)81666-9. [DOI] [PubMed] [Google Scholar]
- 32.Nehrbass U, Rout M P, Maguire S, Blobel G, Wozniak R W. The yeast nucleoporin Nup188p interacts genetically and physically with the core structures of the nuclear pore complex. J Cell Biol. 1996;133:1153–1162. doi: 10.1083/jcb.133.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nigg E A. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- 34.Pasquinelli A E, Ernst R K, Lund E, Grimm C, Zapp M L, Rekosh D, Hammarskjold M L, Dahlberg J E. The constitutive transport element (CTE) of Mason-Pfizer monkey virus (MPMV) accesses a cellular mRNA export pathway. EMBO J. 1997;16:7500–7510. doi: 10.1093/emboj/16.24.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pritchard C E, Fornerod M, Kasper L H, van Deursen J M. RAE1 is a shuttling mRNA export factor that binds to a GLEBS-like NUP98 motif at the nuclear pore complex through multiple domains. J Cell Biol. 1999;145:237–254. doi: 10.1083/jcb.145.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santos-Rosa H, Moreno H, Simos G, Segref A, Fahrenkrog B, Pante N, Hurt E. Nuclear mRNA export requires complex formation between Mex67p and Mtr2p at the nuclear pores. Mol Cell Biol. 1998;18:6826–6838. doi: 10.1128/mcb.18.11.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segref A, Sharma K, Doye V, Hellwig A, Huber J, Luhrmann R, Hurt E. Mex67p, a novel factor for nuclear mRNA export, binds to both poly(A)+ RNA and nuclear pores. EMBO J. 1997;16:3256–3271. doi: 10.1093/emboj/16.11.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Senger B, Simos G, Bischoff F R, Podtelejnikov A, Mann M, Hurt E. Mtr10p functions as a nuclear import receptor for the mRNA-binding protein Np13p. EMBO J. 1998;17:2196–2207. doi: 10.1093/emboj/17.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strasser K, Hurt E. Nuclear RNA export in yeast. FEBS Lett. 1999;452:77–81. doi: 10.1016/s0014-5793(99)00537-2. [DOI] [PubMed] [Google Scholar]
- 40.Stutz F, Rosbash M. Nuclear RNA export. Genes Dev. 1998;12:3303–3319. doi: 10.1101/gad.12.21.3303. [DOI] [PubMed] [Google Scholar]
- 41.Whalen W A, Yoon J H, Shen R, Dhar R. Regulation of mRNA export by nutritional status in fission yeast. Genetics. 1999;152:827–838. doi: 10.1093/genetics/152.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoon J H, Whalen W A, Bharathi A, Shen R, Dhar R. Npp106p, a Schizosaccharomyces pombe nucleoporin similar to Saccharomyces cerevisiae Nic96p, functionally interacts with Rae1p in mRNA export. Mol Cell Biol. 1997;17:7047–7060. doi: 10.1128/mcb.17.12.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zabel U, Doye V, Tekotte H, Wepf R, Grandi P, Hurt E C. Nic96p is required for nuclear pore formation and functionally interacts with a novel nucleoporin, Nup188p. J Cell Biol. 1996;133:1141–1152. doi: 10.1083/jcb.133.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]