Abstract
Swine influenza is an important disease for the swine industry. Currently used whole inactivated virus (WIV) vaccines can induce vaccine-associated enhanced respiratory disease (VAERD) in pigs when the vaccine strains mismatch with the infected viruses. Live attenuated influenza virus vaccine (LAIV) is effective to protect pigs against homologous and heterologous swine influenza virus infections without inducing VAERD but has safety concerns due to potential reassortment with circulating viruses. Herein, we used a chimeric bat influenza Bat09:mH3mN2 virus, which contains both surface HA and NA gene open reading frames of the A/swine/Texas/4199-2/1998 (H3N2) and six internal genes from the novel bat H17N10 virus, to develop modified live-attenuated viruses (MLVs) as vaccine candidates which cannot reassort with canonical influenza A viruses by co-infection. Two attenuated MLV vaccine candidates including the virus that expresses a truncated NS1 (Bat09:mH3mN2-NS1-128, MLV1) or expresses both a truncated NS1 and the swine IL-18 (Bat09:mH3mN2-NS1-128-IL-18, MLV2) were generated and evaluated in pigs against a heterologous H3N2 virus using the WIV vaccine as a control. Compared to the WIV vaccine, both MLV vaccines were able to reduce lesions and virus replication in lungs and limit nasal virus shedding without VAERD, also induced significantly higher levels of mucosal IgA response in lungs and significantly increased numbers of antigen-specific IFN-γ secreting cells against the challenge virus. However, no significant difference was observed in efficacy between the MLV1 and MLV2. These results indicate that bat influenza vectored MLV vaccines can be used as a safe live vaccine to prevent swine influenza.
Keywords: Swine influenza vaccine, bat influenza virus vectored live vaccine, cross-protection, heterologous virus challenge, safety and efficacy
Introduction
Three major subtypes of influenza A viruses (IAVs) including H1N1, H1N2 and H3N2 are endemic in swine herds worldwide. As the dynamic swine influenza viruses (SIVs) continue to evolve and generate novel IAVs, public health concerns increase with continual spillover of SIVs into human populations (1, 2). The rapidly evolving diversity of IAVs hinders effective vaccine-mediated protection due to a deficiency of antigenic matching between vaccine strains and circulating viruses (3). It is necessary and important to develop a new vaccine approach to achieve sufficient protection against circulating diverse SIVs and reducing the risk of zoonotic transmission to humans.
Whole inactivated virus (WIV) vaccines are the most widely used vaccines with oil-in-water adjuvants in pigs. WIV vaccines are bivalent or multivalent products which contain combination of antigenically distinct H1 and H3 subtypes of viruses and usually require a two-dose vaccination strategy delivered by intramuscular route. Adjuvanted WIV vaccines can provide sterilizing immunity by inducing robust neutralizing antibodies against antigenically similar HA strains (4, 5). However, only partial protection is achieved by WIV vaccines against heterologous strains (5–7). Moreover, vaccine associated enhanced respiratory disease (VAERD), characterized by the presence of high titers of non-neutralizing antibodies against the HA2 and very limited of high avidity, cross-reactive neutralizing antibodies against HA1 as well as increased lung pathology, has been observed in pigs immunized with WIV vaccine followed by infection with antigenically distinct IAVs (7–11).
In contrast to WIV vaccines, live attenuated influenza virus vaccines (LAIVs) provide improved cross-reactive immunity against antigenically distinct IAVs through inducing broader cell-mediated, humoral and mucosal immune responses without inducing VAERD (5, 12, 13). Several approaches have been developed to to produce LAIV candidates, including elastase- dependent HA cleavage, truncated NS1 gene (ΔNS1), temperature-sensitive mutations in polymerase genes and codon-pair biased-deoptimization in HA and NA genes (14–18). Among these approaches, a LAIV based on ΔNS1 was recently licensed as the first LAIV for swine in the US (19). However, major concerns regarding the use of LAIVs are the reversion to a virulent phenotype of the vaccine strain over time by natural mutations or genome reassortment between vaccine strains and circulating viruses.
Vectored vaccines are alternative approaches to produce IAV vaccines with potential application in multiple species. Replication-defective or replication-competent vectors infect cells and express the antigens of interest in infected cells, which allows the stimulation of humoral and cell mediated immunity. Furthermore, vectored vaccines can induce local immunity at the site of natural infection of IAVs through intranasal delivery (20, 21). Human adenovirus type 5- vectored and alphavirus-like replicon particle vectored vaccines have been reported to provide complete protection against homologous IAV infection and partial protection against heterologous challenge in pigs (21–24).
The genome sequences of novel H17N10 and H18N11 subtypes of IAVs were discovered from bat specimens (25, 26). Although the surface glycoproteins of these new bat influenza viruses are phylogenetically and structurally related to HA and NA of conventional IAVs, they are functionally different from them without hemagglutination and sialidase activities (26–28). Replicative chimeric bat influenza viruses have been successfully rescued, which contain HA and NA coding regions from a conventional IAV with respective gene packaging signals of bat influenza viruses (29, 30). Although the chimeric bat influenza viruses replicated efficiently in cells and mice, reassortment between chimeric bat influenza viruses and conventional IAVs was not observed in co-infection experiments (29–31). This indicates that the chimeric bat influenza virus can be a good vaccine vector to overcome the potential risk of LAIV reassortment with conventional IAVs.
In this study, we developed and evaluated novel modified live-attenuated virus (MLV) vaccine candidates using a chimeric bat influenza virus as a vaccine vector containing a truncated NS1 gene in vitro and in pigs. In addition, recombinant porcine IL-18 (rpIL-18) was also introduced into this MLV as part of the MLV vaccine because IL-18 is known to induce strong cell mediated immunity (CMI) by stimulating T helper 1 (Th1) activation and IFN-γ induction (32, 33), its effect on MLV vaccine efficacy was evaluated.
Materials and methods
Ethical statement
Animal study (IACUC no.3668) was reviewed and approved by the Institutional Animal Care and Use Committee at Kansas State University and were performed in Biosafety Level 2+ animal facilities under guidance from the Comparative Medicine Group at Kansas State University.
Viruses and vaccine preparation
The MLV with a truncated NS1 protein (Bat09:mH3mN2-NS1-128, MLV1) was generated via reverse genetics using six internal genes from the H17N10 A/little yellow-shouldered bat/Guatemala/164/2009 (Bat09) and two surface HA and NA gene coding regions from the H3N2 A/swine/Texas/4199-2/1998 (TX98) with Bat09 respective gene packaging signals (Fig. S1 A) (29). To generate the second MLV with IL-18 expression (Bat09:mH3mN2-NS1-128-IL-18, MLV2), the recombinant porcine IL-18 (rpIL-18) was incorporated between the truncated bat NS1 (NS1-128) and NEP proteins as described previously (34). Briefly, rpIL-18 was fused to the C-terminal of NS1-128 via a GSGG linker followed by a GSG linker, 2A autoproteolytic cleavage site and NEP (Fig. S1 B). The porcine IL-18 expression of Bat09:mH3mN2-NS1-128-IL-18 was confirmed by Western blotting (Fig. S1 D). Wild type TX98 H3N2 virus (cluster I) was used as WIV vaccine antigen by UV- inactivation and emulsion in 15% commercial oil-in-water adjuvant at 64 HA unites/dose (Emulsigen D, MVP Laboratories, Inc.) as described previously (9, 13). A cluster IAV H3N2 isolate, A/swine/Kansas/10-91088/2010 (KS-91088), was used as challenge virus for the pig study (35). All viruses were propagated in Madin-Darby canine kidney (MDCK) cells.
Growth kinetics
To assess the replication kinetics of MLV strains, MDCK cells were cultured in 12-well plates and infected with each virus at a multiplicity of infection (MOI.) of 0.01 in triplicate. At 12, 24, 36 and 48 h post-infection (hpi), the supernatants of infected cells were collected and virus titers were determined by calculating the 50% tissue culture infective dose (TCID50/ml) in MDCK cells using the Reed and Muench method.
Experimental design
Twenty-three 3- to 4-week-old pigs, which were confirmed to be seronegative for porcine reproductive and respiratory syndrome virus and IAVs by hemagglutination inhibition (HI) assay against currently circulating H3N2 and H1N1 viruses, were used. Pigs were randomly distributed into 4 groups (MLV1, MLV2, WIV vaccinated groups and non-vaccinated control group) and each group contained 6 pigs, while MLV2 vaccinated group had 5 pigs (Table 1). The MLV groups were intranasally vaccinated with 1.5 ml of 106 TCID50/pig once. One group of 6 pigs were intramuscularly administered with 2 ml of 64 HA units of adjuvanted WIV vaccine and boosted 21 days later with the same dose of the vaccine by the same route. Six pigs in non-vaccinated group (NV) were served as sham vaccinated controls. Clinical signs and body temperature were monitored daily for 7 days post primary vaccination. All pigs were intratracheally challenged at 28 days post-vaccination (dpv) with 105 TCID50/ml of KS-91088 virus. Rectal temperature and clinical signs were monitored daily after vaccination and challenge. Nasal swabs were collected 0, 3, 5, 7 dpv and 0, 1, 3, 5 days post-challenge (dpc) to evaluate virus nasal shedding. At 0, 14, 28 dpv and 3, 5 dpc, blood samples were collected from each pig for serological analysis. Three pigs from each group were necropsied at 3 and 5 dpc, while two pigs from the MLV2 group were necropsied at 5 dpc (Table 1). Bronchoalveolar lavage fluid (BALF) samples were collected and virus amounts of nasal swab and BALF samples were determined on MDCK cells by calculating TCID50/ml as described previously (36) . To investigate gene reassortment between MLVs and challenge virus in BALF after challenge, MDCK cells were infected with BALF samples and plaque assay was performed to select a single virus. The purified single virus plaques were amplified to identify the origin of each gene segment using gene specific reverse transcription polymerase chain reaction (RT-PCR). At necropsy, lungs were removed in toto and a single experienced veterinarian assessed the percentage of typical IAV infection gross lesions of each lobe as described previously (36). Right cardiac lung lobe and trachea samples from each pig were collected for histopathologic examination, and stained with hematoxylin and eosin as described previously (37).
Table 1:
Experimental design for vaccination and challenge
| Vaccine/group | Vaccination & boost |
Bleed |
Challenge (IT) | Necropsy |
||||
|---|---|---|---|---|---|---|---|---|
| Pig No. | Day 0 | dpva 21 | dpv 14 | dpv 28 | dpv 28 | dpcb 3 | dpc 5 | |
|
|
||||||||
| MLV1 | 6 | 106 TCID50/pig, IN | n/a | yes | yes | 105 TCID50/pig | 3 pigs | 3 pigs |
| MLV2 | 5 | 106 TCID50/pig, IN | n/a | yes | yes | 105 TCID50/pig | 3 pigs | 2 pigs |
| WIV | 6 | 2ml/pig, IM | 2ml/pig, IM | yes | yes | 105 TCID50/pig | 3 pigs | 3 pigs |
| Non-vaccine control | 6 | n/a | n/a | yes | yes | 105 TCID50/pig | 3 pigs | 3 pigs |
days post vaccination;
days post challenge;
IN: intranasal route; IM: intramuscular route; IT: intratracheal route
Antibody detection and ELISA assay
To perform HI assays, the KS-91088 virus was used as antigen to conduct the HI assay by following standard techniques. Enzyme-linked immunosorbent assays (ELISA) were performed to detect total IgG and IgA antibodies in serum and BLAF against whole virus preparation of KS-91088 as described previously (38).
IFN-γ ELISPOT assay and detection of cytokine and chemokine levels in BALF
(Whole blood samples were collected at 5 dpc to isolate peripheral blood mononuclear cells (PBMCs) that were used to perform ELISPOT assay for detecting IFN-γ secreting cells (see supplemental materials and methods). Cytokine/Chemokine levels in BALF were quantified by MILLIPLEX MAP Porcine Cytokine/Chemokine Magnetic Bead Panel using the Luminex technology according to the manufacturer’s instructions (Millipore). Each sample was analyzed in triplicate and results presented average values of pigs in each treatment group.
Statistical analysis
All statistical analysis were conducted using analysis of variance (ANOVA) with a Tukey’s multiple comparison test by GraphPad Prism version 5.0 (GraphPad Software) to compare multiple treatment groups. A P-value of 0.05 or less was considered statistically significant.
Results
Generation and characterization of MLVs
Since LAIVs with a truncated NS1 protein have been shown to provide effective protection against SIV infection with attenuated replication feature in pigs (14, 19), we generated a MLV1 based on Bat09:mH3mN2 virus expressing a truncated NS1 protein of 128 amino acids (Bat09:mH3mN2-NS1-128, MLV1) (Fig. S1 B). In parallel, MLV2 expressing both a truncated NS1 protein and rpIL-18 was generated (Bat09:mH3mN2-NS1-128-IL-18, MLV2); as IL-18 is associated with inducing a strong cell-mediated immunity (Fig. S1 B). In addition, the recombinant virus expressing full-length NS1 and rpIL-18 was also produced as a control. All recombinant viruses propagated efficiently in MDCK cells. All three recombinant viruses (Bat09:mH3mN2-NS1-IL18, Bat09:mH3mN2-NS1-128 and Bat09:mH3mN2-NS1-128-IL-18) were attenuated in vitro as a significantly lower titer was detected at 24, 36 and 48 hpi compared to the parental Bat09:mH3mN2 virus (Fig. S1 C).Interestingly, the viral yield of Bat09:mH3mN2-NS1-128-IL-18 was significantly lower than the other two recombinant viruses at 24, 36 and 48hpi (Fig. S1 C). The porcine IL-18 expression of Bat09:mH3mN2-NS1-128-IL-18 was confirmed by Western blotting (Fig. S1 D). These results indicate that all three recombinant viruses are attenuated compared to the parental Bat09:mH3mN2 virus and can be used as vaccine candidates. Both MLV1 and MLV2 were selected for further vaccine studies.
No clinical signs and limited virus shedding in vaccinated pigs with MLVs
Pigs were intranasally inoculated with either MLV1 or MLV2 did not show obvious clinical signs post vaccination and prior to challenge. No fever was detected in all experimental pigs for 7 days post primary vaccination. Virus shedding with a low titer was detected in a few pigs only at 3 dpv, not at later time points. Virus was found in 2 out of 6 pigs infected with the MLV1 with a titer of 101.7 or 102.3 TCID50/ml, while only 1 out of 5 animals infected with the MLV2 candidate shed virus with a titer of 101.7 TCID50/ml. These low amounts of virus detected in a few pigs could be the residual of the inoculated virus.
Serological response in pigs vaccinated with MLVs or WIV
At 14 dpv, both MLVs induced a low HI antibody titer against the heterologous KS-91088 H3N2 virus following a single dose of vaccination, while substantial HI antibody titers were detected from the WIV vaccinated group after booster vaccination at 28 dpv (Fig. 1A). However, the HI titers in pigs of all vaccinated groups were lower than 1:40 at all tested time points prior to challenge. Although HI titers from all immunized groups increased to higher than 1:100 following challenge, no significant difference in titers was observed between vaccinated groups (Fig. 1A). The non-vaccinated control group had no HI titers before challenge, and lower HI antibody titers were detected following challenge compared to the vaccinated groups. We next investigated IgG levels against KS-91088 H3N2 virus in sera at 28 dpv using a whole-virus ELISA assay. WIV vaccinated pigs produced significantly higher IgG serum levels to KS-91088 than those of MLV vaccinated and non-vaccinated pigs (Fig. 1B). There was no significant difference in serum IgG levels between the two MLV vaccinated groups and the non-vaccinated control group. Together, these data show that the two MLV vaccine candidates applied as a single dose and the WIV vaccine applied as two doses induced minimal levels of heterologous HI antibody titers (≤ 40) following vaccination, while the WIV vaccine elicited significantly higher levels of IgG in sera compared to the other groups at 28 dpv.
Fig 1. Serum antibody responses in pigs after vaccination.
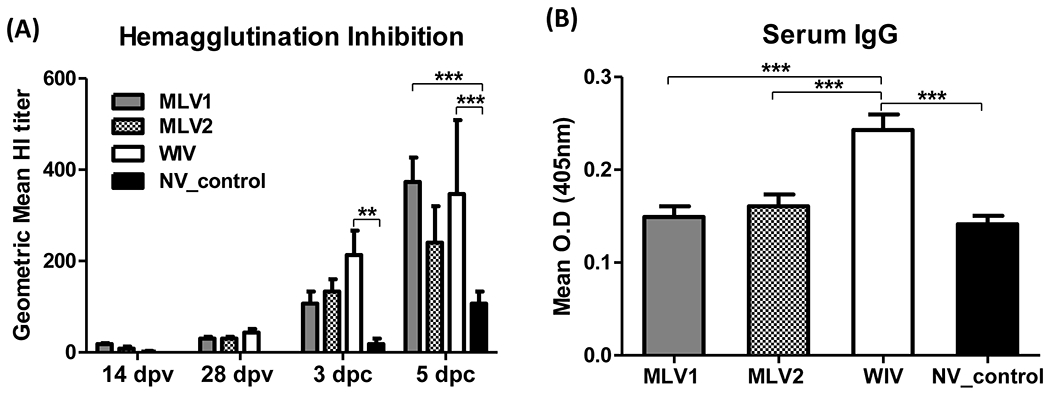
(A) Geometric mean reciprocal titers of hemagglutination inhibition (HI) antibodies to heterologous challenge virus (KS-91088) were determined in sera from all pigs following vaccination and challenge. The reported HI titers are the average titers for each group. (B) At 28 dpv, serum samples were collected from all pigs to evaluate the serum IgG levels against KS-91088 virus using a whole-virus ELISA assay. Antibody levels were analyzed by average of each triplicate sample and reported as the mean of OD values of each group. The error bars indicate standard errors of the mean (SEM). The asterisks (*) represent a statistically significant difference between groups (**: p<0.01 and ***: p<0.001).
Clinical signs, virus replication and shedding, and lung pathology in pigs after challenge
After challenge with heterologous KS-91088 H3N2 virus, all vaccinated pigs and non-vaccinated controls did not show obvious respiratory clinical signs. However, 3 out of 6 pigs immunized with the WIV vaccine showed fever starting at 1 dpc, and all pigs in this group displayed fever at 2 dpc which lasted for 2 days, while no pigs in other groups had fever at 1 dpc. Four or 5 pigs in either the non-vaccinated control group or in the MVL1 group, respectively, showed fever starting at 2 dpc, while all pigs in both groups had fever at 3 dpc. Interestingly, all five pigs in the MLV2 group had fever only at 3 dpc, not at any other days. Pigs immunized with either MLV1 or MLV2 exhibited minimal macroscopic lung lesions with an average of less than 3% (Fig. 2A and B). The WIV vaccine group had enhanced macroscopic lung lesions compared to the two MLV groups and the non-vaccination control group at 3 dpc, and the average percentage of lung lesions (over 50%) was significantly higher than those observed with the other groups (Fig. 2A and B). At 5 dpc, no significant differences in lung lesion profiles were observed among the different groups although a higher lung lesion percentage was found in WIV-immunized pigs and minimal lung lesions were found in both MLV vaccinated animals (Fig. 2B).
Fig 2. Macroscopic lung lesions in pigs after challenge.
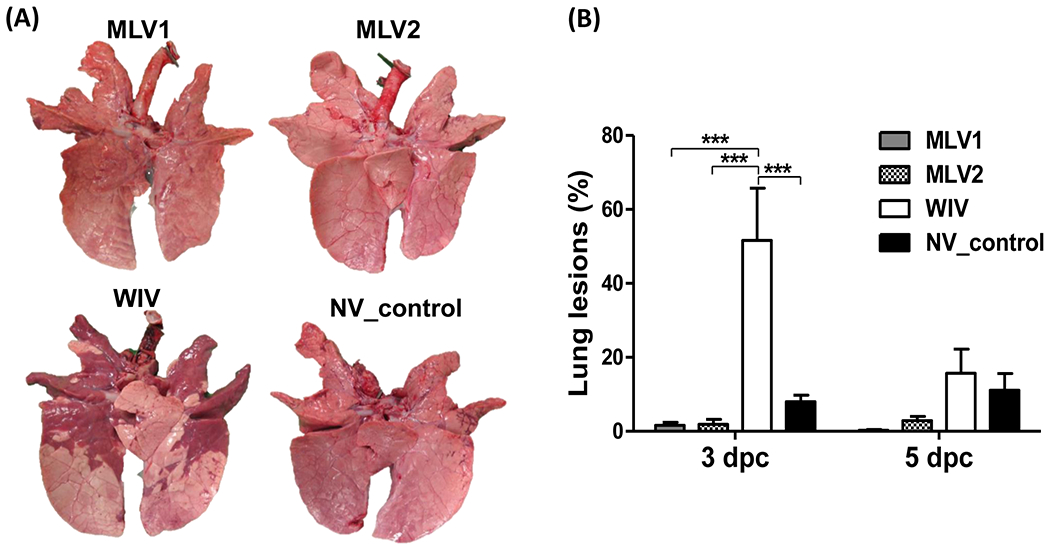
(A) Ventral surfaces of lungs from representative pigs in each group at 3 dpc are shown. (B) Macroscopic lung lesions of challenged pigs are presented as the average percentage ± SEM of gross lesions of three pigs in each group at 3 and 5 dpc. The asterisks (*) represent a statistically significant difference between groups (***: p<0.001).
At 3 dpc, virus was detected in BALF samples collected from pigs of each group except for one pig from the MLV1 group. In contrast to WIV-vaccinated pigs, a lower virus titer was detected in BALF samples of other three groups of pigs. However, a significant difference in virus titers was only observed between the MLV2-vaccinated and the WIV-vaccinated groups (Fig. 3A). At 5 dpc, virus was not detected in BALF from pigs receiving either the MLV1 or MLV2 candidate vaccines, whereas virus was detected in one out of three pigs in the WIV vaccine group and from all 3 pigs in the non-vaccinated control group (Fig. 3A). No virus was detected in nasal samples collected from all pigs at 1 dpc (Fig. 3B). At 3 dpc, virus was detected in nasal swabs collected from 5 out of 6 pigs in the MLV1 group and from all pigs in other 3 groups. A significantly lower titer was found in the MLV1 group compared to those of the other 3 groups at this time point post challenge. No virus was detected in nasal swabs of 2 pigs immunized with the MLV2 vaccine candidate at 5 dpc, whereas virus was found in all pigs in either the WIV or non-vaccinated control groups (Fig. 3B). In contrast, significant less virus was detected in 2 out of 3 nasal swabs collected in the MVL1 immunized pigs at 5 dpc. In summary, both MLV vaccine candidates reduced virus replication in lungs and nasal shedding after challenge with a heterologous H3N2 virus in contrast to the WIV vaccinated group (Fig. 3).
Fig 3. Virus replication in BALF and nasal shedding in nasal swabs after challenge.
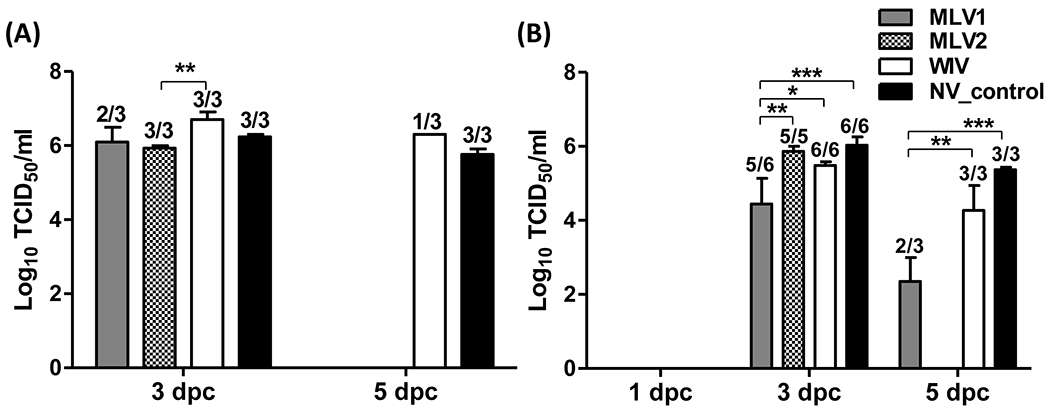
Mean of virus titers in BALF (A) and in nasal swabs (B) from challenged pigs were evaluated on the days indicated. Virus titers were determined by calculating the 50% tissue culture infective dose (TCID50)/ml in MDCK cells. The number of pigs with positive virus isolation out of the total number of tested pigs is presented above of each bar. The asterisks (*) represent a statistically significant difference between groups (*: p<0.05, **: p<0.01 and ***: p<0.001).
Histopathological lesions were examined in lungs and trachea collected from pigs at 5 dpc. Microscopic lung lesion scores were not significantly different between the groups, however, a lower score trend was observed in pigs immunized with either the MLV1 or MLV2 vaccine (Fig. S2 A). The MLV2 group and the non-vaccinated control group demonstrated the statistically less microscopic damages to the trachea when compared to the WIV vaccine group (Fig. S2 B). However, there was no significant difference in microscopic trachea lesions between the two MLV groups and the non-vaccinated control groups. Similar to the above described enhanced macroscopic lung lesions, the WIV vaccine group exhibited more severe histopathological lung and trachea damages as characterized by marked peribrochiolar lymphocytic cuffing, degenerated airway epithelium, extensive infiltration of inflammatory cells within the lumen of airways, attenuated mucosa of trachea and infiltration of lymphocytes and plasma cells in the lamina propria of the trachea with transepethelial migration of inflammatory cells (Fig. S2 C). These data suggest that both MLV vaccine candidates can prevent virus replication in lungs and limit virus transmission more efficiently than the WIV vaccine when challenged with heterologous virus in the absence of enhanced lung pathology and disease.
Assessment of reassortment between MLVs and challenge virus in BALF
Previous studies have shown that chimeric bat influenza viruses fail to reassort with conventional IAVs by co-infection experiments (29–31). Herein, we investigated reassortment events between MLVs and challenge virus in BALF samples post challenge. Sixty and ninety single virus plaques isolated from BALF of the MLV1 group and MLV2 group, respectively, were purified and the origin of the gene segments of each isolated virus was determined using gene specific RT-PCR assays. The results showed that all segments of the 150 tested isolates belong to the KS-91088 challenge virus and no reassortant viruses with the MLV candidate vaccines were detected.
Antigen-specific T-cell response after challenge
An ELISPOT assay was performed to detect antigen-specific IFN-γ secreting cells (IFN-γ SCs) in response to heterologous KS-91088 antigens. All immunized pigs showed significantly higher numbers of antigen-specific IFN-γ SCs compared to the non-vaccinated control pigs. However, both MLV candidates induced significantly greater antigen-specific IFN-γ recall responses to the heterologous KS-91088 antigens when compared to the WIV vaccinated pigs (Fig. 4A).
Fig 4. Antigen-specific T-cell response in PBMCs and antibody response to heterologous challenge virus in BLAF at 5 dpc.
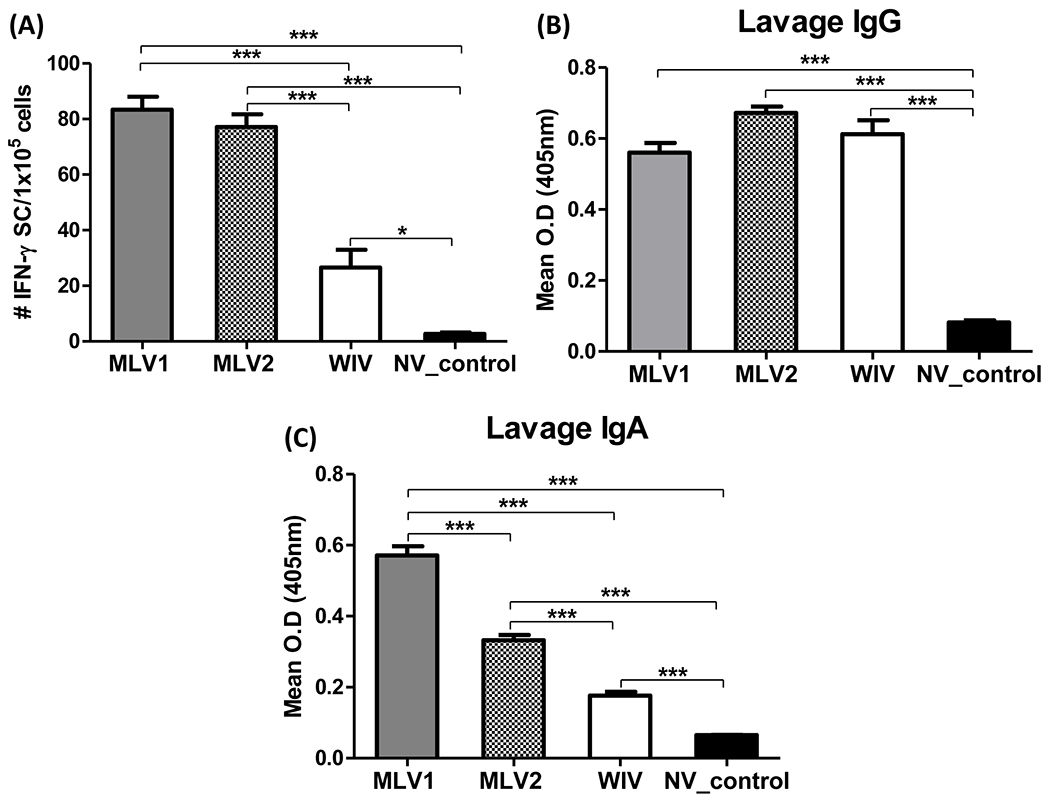
(A) PBMCs were collected from pigs at 5 dpc and ELISPOT assay was performed to detect antigen-specific IFN-γ secreting cells (IFN-γ SCs) which respond to heterologous KS-91088 antigen. The average numbers of counted spots for triplicates well of individual sample were used to present the data of the mean numbers of each group. Levels of KS-91088 specific IgG (B) and IgA (C) antibodies in BALF at 5 dpc were evaluated by ELISA. Antibody levels were analyzed by average of each triplicate sample and expressed as the mean of OD values of each group. The error bars indicate standard errors of the mean (SEM). The asterisks (*) represent a statistically significant difference between groups (*: p<0.05, **: p<0.01 and ***: p<0.001).
Antibody response to challenge virus in BALF samples
Levels of IgG and IgA antibodies reactive to the KS-91088 challenge virus in BALF at 5 dpc were measured using an isotype-specific ELISA. Results showed that there were significantly higher levels of IgG in BALF from all vaccinated pigs compared to non-vaccinated control pigs (Fig. 4B). However, both MLVs and the WIV vaccine elicited similar levels of cross-reactive IgG antibodies in BALF samples. Like the IgG levels in BALF, the KS-91088 reactive IgA levels of all vaccinated pigs were significantly higher than those of non-vaccinated pigs. In contrast, significantly higher levels of IgA antibodies were detected in BALF from pigs immunized with either MLV1 or MLV2 compared to the WIV vaccine group (Fig. 4C).
Cytokine and chemokine levels in BALF following challenge
Pulmonary levels of cytokines and chemokines in BALF collected from pigs at 3 and 5 dpc were assessed using the Luminex technology. All measured cytokine and chemokine levels in BALF from the WIV vaccine group were significantly higher than those detected from both the MLV1 and MLV2 groups as well as the non-vaccinated control group at either 3 or 5 dpc. Significant differences in all tested cytokine and chemokine levels between the MLV groups and the non-vaccinated control group were not observed except for IL-6 and IL-12 at 3 dpc (Fig. S3).
Discussion
In North America, the complexity and diverse ecology of SIVs lead to repeated failure of WIV vaccines including farm-specific autogenous WIV vaccines used (3). Furthermore, WIV ineffective protection together with induction of VAERD are observed when WIV vaccinated pigs are infected with antigenically mismatched strains (4–7). LAIVs using a variety of genetic modifications have been shown to provide superior protection against homologous and heterologous SIV challenges compared to WIV vaccines, without causing VAERD (13, 21, 38, 39). However, there are safety concerns of using LAIVs that potentially reassort with circulating SIV strains. In this study, we developed new MLV vaccine candidates for swine influenza using an attenuated chimeric bat influenza virus and showed that they conferred better protection than the WIV vaccine against heterologous virus challenge as evidenced by reduced virus replication in lungs and limiting nasal virus shedding. Interestingly, significantly reduced virus replication and shedding was observed in the MLV vaccinated pigs at 5 dpc, not at 3 dpc (Fig. 3), the lack of early protection in the MLV groups may be due to the high, intra-tracheal challenge dose used and/or the antigenic mismatch between challenge and vaccine strains. Importantly, VAERD was only observed in WIV vaccinated pigs but not in pigs immunized with the MLV candidatea upon heterologous challenge. Moreover, no reassortment was detected between MLVs and the challenge virus in BALF after challenge. These data suggest that the bat influenza vectored MLV vaccine candidates can provide protection from heterologous SIV infection without safety concerns compared to traditional WIV and LAIV vaccines by overcoming the risks of VAERD and virus reassortment.
VAERD is a major adverse effect of WIV vaccines when immunized pigs are infected with heterologous virus, especially HA mismatched virus. The WIV vaccine used in this study is cluster I of H3 virus, whereas the challenge KS-91088 (H3N2) virus belongs to the cluster IV of H3 virus that has no or limited cross-reactivity with the vaccine strain. Previous studies demonstrated that VAERD correlated with the presence of high level of non-neutralizing antibodies against HA2 and the absence of neutralizing antibodies against HA1 of antigenically mismatched virus (8, 10, 38, 40). In particular, cross-reactive HA2 antibodies which target the HA2 domain but not the HA1 globular head domain contributed to enhanced IAV infection in cells through promoting the fusion process between virus and cell membrane, and this infection enhancement is associated with VAERD (38, 40). In this study, all vaccine groups displayed similarly low levels of heterologous HI antibody titers (≤ 40) before challenge. However, high cross-reactive IgG levels against the KS-91088 virus were detected in sera from WIV vaccinated pigs while the MLV vaccinated groups produced minimal levels of serum IgG antibodies similar to those of the non-vaccinated group at 28 dpv. These results are consistent with the findings in previous LAIV studies (13, 38, 41). Moreover, a study found that sera from WIV vaccinated pigs contained high titers of cross-reactive antibodies against the HA2 domain of a heterologous challenge virus, which was consistent with high cross-reactive serum IgG levels and low titers of cross-reactive HA1 antibodies which corresponded with limited HI antibody response and low serum neutralizing antibodies to a heterologous challenge virus (38). VAERD observed in WIV vaccinated pigs in this study could be due to the same reason, i.e., the high anti-HA2 antibodies in WIV vaccinated pigs enhance virus replication in epithelial cells, resulting in longer duration and higher titers of lung virus replication, nasal virus shedding as well as severe lung damage in the WIV vaccine group compared to the MLV groups. In contrast, the MLV groups likely do not develop elevated fusion-enhancing HA2 antibodies and therefore, did not develop VAERD when challenged with a heterologous virus.
Intranasally administered LAIVs have been shown to elicit cross-reactive T-cell responses and robust mucosal antibodies which play an important role in LAIV efficacy for heterologous challenge protection (5, 19, 21, 39, 42, 43). In this study, MLV vaccine candidates induced significantly more antigen-specific IFN-γ SCs in the blood in response to heterologous antigen and significantly higher levels of cross-reactive IgA antibodies compared to WIV vaccinated pigs after challenge (Fig. 1A and Fig. 4). This indicates that cross-reactive T cell-mediated and mucosal immune responses induced by the MLV vaccines might contribute to providing better cross-protection for heterologous challenge compared to a WIV vaccine. Since the majority of T-cell responses are directed towards epitopes which are highly conserved between different influenza virus strains, T-cell immunity has the potential to elicit heterologous cross-protective immune responses (44, 45) and has been shown to confer cross-protection to heterologous challenge with reduced disease severity (46). This suggests that bat influenza internal proteins likely have conserved viral epitopes similar as those in internal proteins of classical IAVs. However, the degree of conservation of viral epitopes between bat influenza viruses and conventional IAVs needs to be determined in future studies. Our study demonstrated that the novel MLV candidates were able to elicit robust cross-reactive IgA antibodies in BALF even with a single intranasal vaccination (Fig. 4C). Since IgG antibody levels in BALF were similar between all vaccine groups, mucosal cross-reactive IgA antibodies were most likely crucial for the local immunity in lungs to limit virus replication and reduce lung pathology under heterologous challenge conditions. This is an agreement with findings using intranasal administration of LAIVs against SIV infections (5, 21, 38, 39). A study showed that a two dose LAIV vaccination was able to induce increased antigen-specific serum HI, IgG and mucosal IgA antibodies compared to a one dose of intranasal LAIV vaccination (39). Whether a boosting regimen of bat influenza vectored MLV candidates can induce improved immune responses and a better protection from subsequent SIV infection in pigs needs to be investigated.
Previous studies have demonstrated that influenza virus infection can induce IL-18 expression in human alveolar macrophages and IL-18 expression was necessary for optimal cytokine production and adequate protection against influenza infections (33, 47). Additionally, one study reported that mucosal associated invariant T cells were activated and produced antiviral effectors including IFN-γ via a IL-18 dependent mechanism during influenza infection and contributed to antiviral influenza immunity (48). However, IL-18 expression in young piglets up to one month old is significantly reduced at their respiratory mucosal epithelium, which is the major infection site of IAVs (49). Therefore, we tested whether exogenous IL-18 expression in conjunction with vaccine administration enables to provide enhanced protective immunity against SIV infection. However, no significant difference was observed in vaccine efficacy between two MLV vaccine candidates, regardless of rpIL-18 expression. Most likely the MLV2 virus (Bat09:mH3mN2-NS1-128-IL-18) did not replicate as efficiently as needed to express enough IL-18 to show an obvious functional effect of IL-18 (Fig. S1 C and 1D). Further studies are required to investigate the effect of IL-18 on vaccine efficacy using less attenuated MLVs, a higher dose of vaccine or a booster regimen.
We have demonstrated protective immunity of two new MLV candidates against SIV infection with several advantages. One advantage of developed MLV vaccines is the mode of intranasal immunization which mimics natural infection. It induces not only protective local immunity including IgA production at the site of natural IAV infection but also a broad cross protective CMI without causing VAERD. Another advantage is no reassortment with conventional IAVs to resolve safety issues of normal LAIVs faced. Taken together, bat influenza vectored MLV candidate vaccines is able to provide protective immunity against heterologous challenge and can be used as safe and efficacious live virus vaccines to prevent SIV infections in pigs and might be also used in other species.
Conclusions:
Our results demonstrate that bat influenza vectored MLV vaccines are effective and safe to protect swine from SIV infection without risks of VAERD and reassortment.
Supplementary Material
Acknowledgments
The authors thank staffs from the Comparative Medicine Group at Kansas State University for supporting the animal studies, and Jennifer Phinney from the Kansas State Veterinary Diagnostic Lab for technical assistance with H&E and IHC staining. This work was supported by grants from NIH NIAID 1R01AI134768-01A1 and the NIAID-funded Center of Excellence for Influenza Research and Surveillance (CEIRS, #HHSN272201400006C). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Declaration of Competing Interest
Wenjun Ma and Jingjiao Ma have a patent entitled: Modified bat influenza viruses and their uses at Kansas State University.
References
- 1.Lindstrom S, Garten R, Balish A, Shu B, Emery S, Berman L, Barnes N, Sleeman K, Gubareva L, Villanueva J, Klimov A. 2012. Human infections with novel reassortant influenza A(H3N2)v viruses, United States, 2011. Emerg Infect Dis 18:834–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JS, Guan Y, Rambaut A. 2009. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459:1122–5. [DOI] [PubMed] [Google Scholar]
- 3.Van Reeth K, Ma W. 2013. Swine influenza virus vaccines: to change or not to change-that’s the question. Curr Top Microbiol Immunol 370:173–200. [DOI] [PubMed] [Google Scholar]
- 4.Vincent AL, Ciacci-Zanella JR, Lorusso A, Gauger PC, Zanella EL, Kehrli ME Jr., Janke BH, Lager KM. 2010. Efficacy of inactivated swine influenza virus vaccines against the 2009 A/H1N1 influenza virus in pigs. Vaccine 28:2782–7. [DOI] [PubMed] [Google Scholar]
- 5.Loving CL, Lager KM, Vincent AL, Brockmeier SL, Gauger PC, Anderson TK, Kitikoon P, Perez DR, Kehrli ME Jr. 2013. Efficacy in pigs of inactivated and live attenuated influenza virus vaccines against infection and transmission of an emerging H3N2 similar to the 2011-2012 H3N2v. J Virol 87:9895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent AL, Lager KM, Janke BH, Gramer MR, Richt JA. 2008. Failure of protection and enhanced pneumonia with a US H1N2 swine influenza virus in pigs vaccinated with an inactivated classical swine H1N1 vaccine. Vet Microbiol 126:310–23. [DOI] [PubMed] [Google Scholar]
- 7.Reeth KV, Brown I, Essen S, Pensaert M. 2004. Genetic relationships, serological cross-reaction and cross-protection between H1N2 and other influenza A virus subtypes endemic in European pigs. Virus Res 103:115–24. [DOI] [PubMed] [Google Scholar]
- 8.Gauger PC, Vincent AL, Loving CL, Lager KM, Janke BH, Kehrli ME Jr., Roth JA. 2011. Enhanced pneumonia and disease in pigs vaccinated with an inactivated human-like (delta-cluster) H1N2 vaccine and challenged with pandemic 2009 H1N1 influenza virus. Vaccine 29:2712–9. [DOI] [PubMed] [Google Scholar]
- 9.Gauger PC, Vincent AL, Loving CL, Henningson JN, Lager KM, Janke BH, Kehrli ME Jr., Roth JA. 2012. Kinetics of lung lesion development and pro-inflammatory cytokine response in pigs with vaccine-associated enhanced respiratory disease induced by challenge with pandemic (2009) A/H1N1 influenza virus. Vet Pathol 49:900–12. [DOI] [PubMed] [Google Scholar]
- 10.Rajao DS, Chen H, Perez DR, Sandbulte MR, Gauger PC, Loving CL, Shanks GD, Vincent A. 2016. Vaccine-associated enhanced respiratory disease is influenced by haemagglutinin and neuraminidase in whole inactivated influenza virus vaccines. J Gen Virol 97:1489–99. [DOI] [PubMed] [Google Scholar]
- 11.Souza CK, Rajao DS, Loving CL, Gauger PC, Perez DR, Vincent AL. 2016. Age at Vaccination and Timing of Infection Do Not Alter Vaccine-Associated Enhanced Respiratory Disease in Influenza A Virus-Infected Pigs. Clin Vaccine Immunol 23:470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loving CL, Vincent AL, Pena L, Perez DR. 2012. Heightened adaptive immune responses following vaccination with a temperature-sensitive, live-attenuated influenza virus compared to adjuvanted, whole-inactivated virus in pigs. Vaccine 30:5830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent AL, Ma W, Lager KM, Richt JA, Janke BH, Sandbulte MR, Gauger PC, Loving CL, Webby RJ, Garcia-Sastre A. 2012. Live attenuated influenza vaccine provides superior protection from heterologous infection in pigs with maternal antibodies without inducing vaccine-associated enhanced respiratory disease. J Virol 86:10597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solorzano A, Webby RJ, Lager KM, Janke BH, Garcia-Sastre A, Richt JA. 2005. Mutations in the NS1 protein of swine influenza virus impair anti-interferon activity and confer attenuation in pigs. J Virol 79:7535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masic A, Babiuk LA, Zhou Y. 2009. Reverse genetics-generated elastase-dependent swine influenza viruses are attenuated in pigs. J Gen Virol 90:375–85. [DOI] [PubMed] [Google Scholar]
- 16.Pena L, Vincent AL, Ye J, Ciacci-Zanella JR, Angel M, Lorusso A, Gauger PC, Janke BH, Loving CL, Perez DR. 2011. Modifications in the polymerase genes of a swine-like triple-reassortant influenza virus to generate live attenuated vaccines against 2009 pandemic H1N1 viruses. J Virol 85:456–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman JR, Papamichail D, Skiena S, Futcher B, Wimmer E, Mueller S. 2008. Virus attenuation by genome-scale changes in codon pair bias. Science 320:1784–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan BS, Souza CK, Gauger PC, Stauft CB, Robert Coleman J, Mueller S, Vincent AL. 2018. Vaccination of pigs with a codon-pair bias de-optimized live attenuated influenza vaccine protects from homologous challenge. Vaccine 36:1101–1107. [DOI] [PubMed] [Google Scholar]
- 19.Vincent AL, Ma W, Lager KM, Janke BH, Webby RJ, Garcia-Sastre A, Richt JA. 2007. Efficacy of intranasal administration of a truncated NS1 modified live influenza virus vaccine in swine. Vaccine 25:7999–8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tatsis N, Fitzgerald JC, Reyes-Sandoval A, Harris-McCoy KC, Hensley SE, Zhou D, Lin SW, Bian A, Xiang ZQ, Iparraguirre A, Lopez-Camacho C, Wherry EJ, Ertl HC. 2007. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood 110:1916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braucher DR, Henningson JN, Loving CL, Vincent AL, Kim E, Steitz J, Gambotto AA, Kehrli ME Jr. 2012. Intranasal vaccination with replication-defective adenovirus type 5 encoding influenza virus hemagglutinin elicits protective immunity to homologous challenge and partial protection to heterologous challenge in pigs. Clin Vaccine Immunol 19:1722–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wesley RD, Tang M, Lager KM. 2004. Protection of weaned pigs by vaccination with human adenovirus 5 recombinant viruses expressing the hemagglutinin and the nucleoprotein of H3N2 swine influenza virus. Vaccine 22:3427–34. [DOI] [PubMed] [Google Scholar]
- 23.Vander Veen RL, Loynachan AT, Mogler MA, Russell BJ, Harris DL, Kamrud KI. 2012. Safety, immunogenicity, and efficacy of an alphavirus replicon-based swine influenza virus hemagglutinin vaccine. Vaccine 30:1944–50. [DOI] [PubMed] [Google Scholar]
- 24.Vander Veen RL, Mogler MA, Russell BJ, Loynachan AT, Harris DL, Kamrud KI. 2013. Haemagglutinin and nucleoprotein replicon particle vaccination of swine protects against the pandemic H1N1 2009 virus. Vet Rec 173:344. [DOI] [PubMed] [Google Scholar]
- 25.Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, Recuenco S, Ellison JA, Davis CT, York IA, Turmelle AS, Moran D, Rogers S, Shi M, Tao Y, Weil MR, Tang K, Rowe LA, Sammons S, Xu X, Frace M, Lindblade KA, Cox NJ, Anderson LJ, Rupprecht CE, Donis RO. 2012. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci U S A 109:4269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, Chen LM, Johnson A, Tao Y, Dreyfus C, Yu W, McBride R, Carney PJ, Gilbert AT, Chang J, Guo Z, Davis CT, Paulson JC, Stevens J, Rupprecht CE, Holmes EC, Wilson IA, Donis RO. 2013. New world bats harbor diverse influenza A viruses. PLoS Pathog 9:e1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Sun X, Li Z, Liu Y, Vavricka CJ, Qi J, Gao GF. 2012. Structural and functional characterization of neuraminidase-like molecule N10 derived from bat influenza A virus. Proc Natl Acad Sci U S A 109:18897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun X, Shi Y, Lu X, He J, Gao F, Yan J, Qi J, Gao GF. 2013. Bat-derived influenza hemagglutinin H17 does not bind canonical avian or human receptors and most likely uses a unique entry mechanism. Cell Rep 3:769–78. [DOI] [PubMed] [Google Scholar]
- 29.Zhou B, Ma J, Liu Q, Bawa B, Wang W, Shabman RS, Duff M, Lee J, Lang Y, Cao N, Nagy A, Lin X, Stockwell TB, Richt JA, Wentworth DE, Ma W. 2014. Characterization of uncultivable bat influenza virus using a replicative synthetic virus. PLoS Pathog 10:e1004420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juozapaitis M, Aguiar Moreira E, Mena I, Giese S, Riegger D, Pohlmann A, Hoper D, Zimmer G, Beer M, Garcia-Sastre A, Schwemmle M. 2014. An infectious bat-derived chimeric influenza virus harbouring the entry machinery of an influenza A virus. Nat Commun 5:4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Lee J, Ma J, Lang Y, Nietfeld J, Li Y, Duff M, Yang Y, Liu H, Zhou B, Wentworth DE, Richt JA, Li Z, Ma W. 2017. Pathogenicity of modified bat influenza virus with different M genes and its reassortment potential with swine influenza A virus. J Gen Virol 98:577–584. [DOI] [PubMed] [Google Scholar]
- 32.Dinarello CA. 1999. IL-18: A TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol 103:11–24. [DOI] [PubMed] [Google Scholar]
- 33.Denton AE, Doherty PC, Turner SJ, La Gruta NL. 2007. IL-18, but not IL-12, is required for optimal cytokine production by influenza virus-specific CD8+ T cells. Eur J Immunol 37:368–75. [DOI] [PubMed] [Google Scholar]
- 34.Manicassamy B, Manicassamy S, Belicha-Villanueva A, Pisanelli G, Pulendran B, Garcia-Sastre A. 2010. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc Natl Acad Sci U S A 107:11531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma J, Shen H, Liu Q, Bawa B, Qi W, Duff M, Lang Y, Lee J, Yu H, Bai J, Tong G, Hesse RA, Richt JA, Ma W. 2015. Pathogenicity and transmissibility of novel reassortant H3N2 influenza viruses with 2009 pandemic H1N1 genes in pigs. J Virol 89:2831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma W, Vincent AL, Gramer MR, Brockwell CB, Lager KM, Janke BH, Gauger PC, Patnayak DP, Webby RJ, Richt JA. 2007. Identification of H2N3 influenza A viruses from swine in the United States. Proc Natl Acad Sci U S A 104:20949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, Henningson J, Ma J, Duff M, Lang Y, Li Y, Nagy A, Sunwoo S, Bawa B, Yang J, Bai D, Richt JA, Ma W. 2017. Effects of PB1-F2 on the pathogenicity of H1N1 swine influenza virus in mice and pigs. J Gen Virol 98:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gauger PC, Loving CL, Khurana S, Lorusso A, Perez DR, Kehrli ME Jr., Roth JA, Golding H, Vincent AL. 2014. Live attenuated influenza A virus vaccine protects against A(H1N1)pdm09 heterologous challenge without vaccine associated enhanced respiratory disease. Virology 471–473:93–104. [DOI] [PubMed] [Google Scholar]
- 39.Masic A, Lu X, Li J, Mutwiri GK, Babiuk LA, Brown EG, Zhou Y. 2010. Immunogenicity and protective efficacy of an elastase-dependent live attenuated swine influenza virus vaccine administered intranasally in pigs. Vaccine 28:7098–108. [DOI] [PubMed] [Google Scholar]
- 40.Khurana S, Loving CL, Manischewitz J, King LR, Gauger PC, Henningson J, Vincent AL, Golding H. 2013. Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci Transl Med 5:200ra114. [DOI] [PubMed] [Google Scholar]
- 41.Sandbulte MR, Platt R, Roth JA, Henningson JN, Gibson KA, Rajao DS, Loving CL, Vincent AL. 2014. Divergent immune responses and disease outcomes in piglets immunized with inactivated and attenuated H3N2 swine influenza vaccines in the presence of maternally-derived antibodies. Virology 464–465:45–54. [DOI] [PubMed] [Google Scholar]
- 42.Cheng X, Zengel JR, Suguitan AL Jr., Xu Q, Wang W, Lin J, Jin H. 2013. Evaluation of the humoral and cellular immune responses elicited by the live attenuated and inactivated influenza vaccines and their roles in heterologous protection in ferrets. J Infect Dis 208:594–602. [DOI] [PubMed] [Google Scholar]
- 43.Sun K, Ye J, Perez DR, Metzger DW. 2011. Seasonal FluMist vaccination induces cross-reactive T cell immunity against H1N1 (2009) influenza and secondary bacterial infections. J Immunol 186:987–93. [DOI] [PubMed] [Google Scholar]
- 44.Soema PC, van Riet E, Kersten G, Amorij JP. 2015. Development of cross-protective influenza a vaccines based on cellular responses. Front Immunol 6:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sridhar S 2016. Heterosubtypic T-Cell Immunity to Influenza in Humans: Challenges for Universal T-Cell Influenza Vaccines. Front Immunol 7:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kappes MA, Sandbulte MR, Platt R, Wang C, Lager KM, Henningson JN, Lorusso A, Vincent AL, Loving CL, Roth JA, Kehrli ME Jr. 2012. Vaccination with NS1-truncated H3N2 swine influenza virus primes T cells and confers cross-protection against an H1N1 heterosubtypic challenge in pigs. Vaccine 30:280–8. [DOI] [PubMed] [Google Scholar]
- 47.Pirhonen J, Sareneva T, Kurimoto M, Julkunen I, Matikainen S. 1999. Virus infection activates IL-1 beta and IL-18 production in human macrophages by a caspase-1-dependent pathway. J Immunol 162:7322–9. [PubMed] [Google Scholar]
- 48.Loh L, Wang Z, Sant S, Koutsakos M, Jegaskanda S, Corbett AJ, Liu L, Fairlie DP, Crowe J, Rossjohn J, Xu J, Doherty PC, McCluskey J, Kedzierska K. 2016. Human mucosal-associated invariant T cells contribute to antiviral influenza immunity via IL-18-dependent activation. Proc Natl Acad Sci U S A 113:10133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muneta Y, Goji N, Tsuji NM, Mikami O, Shimoji Y, Nakajima Y, Yokomizo Y, Mori Y. 2002. Expression of interleukin-18 by porcine airway and intestinal epithelium. J Interferon Cytokine Res 22:883–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


