Abstract
The melanopsin-expressing, intrinsically photosensitive retinal ganglion cells (ipRGCs) are a relatively recently discovered class of atypical ganglion cell photoreceptor. These ipRGCs are a morphologically and physiologically heterogeneous population that project widely throughout the brain and mediate a wide array of visual functions ranging from photoentrainment of our circadian rhythms, to driving the pupillary light reflex to improve visual function, to modulating our mood, alertness, learning, sleep/wakefulness, regulation of body temperature, and even our visual perception. The presence of melanopsin as a unique molecular signature of ipRGCs has allowed for the development of a vast array of molecular and genetic tools to study ipRGC circuits. Given the emerging complexity of this system, this review will provide an overview of the genetic tools and methods used to study ipRGCs, how these tools have been used to dissect their role in a variety of visual circuits and behaviors in mice, and identify important directions for future study.
Keywords: Intrinsically photosensitive retinal ganglion cells, Melanopsin, Non-image-forming visual pathway, Pattern vision, Circadian, Retina
Introduction
The day/night cycle is one of the most predictable changes in our daily existence, and has resulted in the evolution of robust systems to anticipate and respond to changes in environmental light. Light is required for the ability to navigate our environments, find prey, and perceive the environment around us. Beyond its role in forming image vision, light has numerous subconscious effects on our physiology and behavior ranging from photoentrainment of our circadian rhythms, to driving the pupillary light reflex (PLR) to improve visual function, to modulating our mood, alertness, learning, sleep, and thermoregulation. Despite this stunning diversity in light-evoked functions, little is understood about the specific circuits from the eye to the brain underlying them. This deficit stems in part from a lack of genetic tools to label, manipulate, and ablate single types of the retinal projections neurons: the retinal ganglion cells (RGCs).
RGCs receive synaptic inputs relayed from cones and rods and send visual information through their axons to approximately 50 brain regions, each of which processes different information about the visual environment. The melanopsin-expressing RGCs represent a specialized class of RGC that is capable of responding to light in the absence of rod and cone input (Fig. 1). These intrinsically photosensitive (ip)RGCs are a morphologically and physiologically heterogeneous population that project widely throughout the brain and mediate a wide array of visual functions from circadian photoentrainment to contrast sensitivity [1–5]. The presence of melanopsin as a unique molecular signature of ipRGCs has allowed for the development of a vast array of molecular and genetic tools to study ipRGC circuits. Given the emerging complexity of this system and the nuances of the tools that have been developed to study them, the goal of this review is to highlight the diversity of ipRGCs, their roles in behavior, the tools commonly used to manipulate, label, and ablate specific ipRGC subtypes, and identify important directions for future study.
Fig. 1.
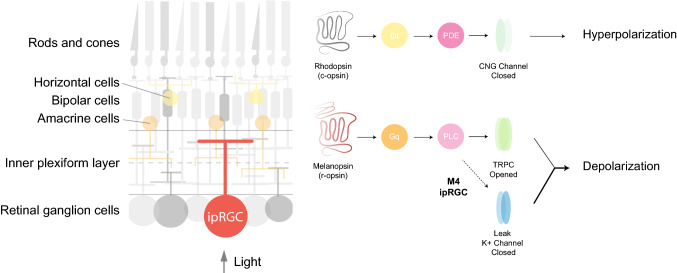
Simplified schematic representation of a cross-retinal section (left). Signaling cascade of rhodopsin and melanopsin molecules (right). When light actvates rhodopsin, Gt (transducin) triggers an activation of phosphodiesterase (PDE) that closes Na+ channels and induces hyperpolarization. In M1 ipRGCs, melanopsin activates a Gq protein that triggers the activation of phospholipase C (PLC) followed by opening of transient receptor potential channels (TRPC) which give rise to membrane depolarization [24, 92, 101, 136, 137]. In addition to TRPC cascade, in M4 ipRGCs, melanopsin increases cellular excitability via closure of potassium leak channels [55]
ipRGC diversity
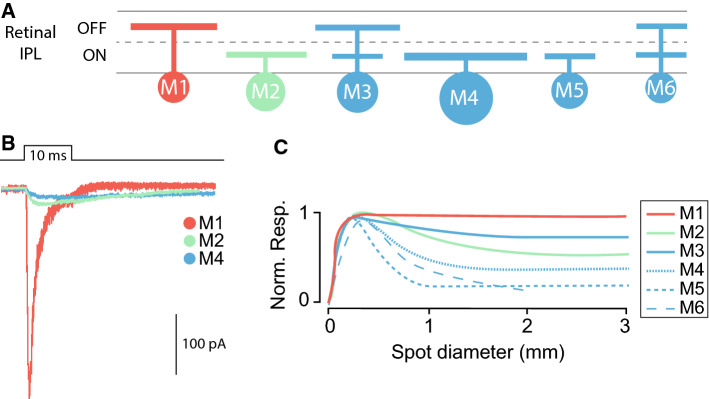
ipRGCs, like all RGCs, receive inputs from the outer retina through bipolar cells and amacrine cells (Fig. 1). The variability of the photoresponses from different ipRGC subpopulations is the result of the integration of the intrinsic melanopsin responses, and extrinsic synaptic inputs which are influenced by the location of the dendritic stratification and the degree of integration with the rod and cone circuitry. There are currently six known subtypes of ipRGC (M1–M6) (Fig. 2a). These subtypes are identified primarily by their morphological characteristics including level of dendritic stratification within the retinal inner plexiform layer (IPL) as well as their dendritic arbor size and complexity. M1 ipRGCs stratify in the OFF sublamina of the IPL, have the smallest soma size of any ipRGC subtype, and have relatively small and simple dendritic arbors (Fig. 2a). M2 ipRGCs have dendrites that stratify in the ON sublamina, and have larger somata and larger, more complex dendritic arbors than M1 cells [6]. M3 ipRGCs are similar to M2 cells in terms of soma size and dendritic arbor size and complexity, but have dendrites that bistratify in both the ON and OFF sublaminas of the IPL. M4 ipRGCs have the largest soma size and largest dendritic arbors of all ipRGCs, ramify in the ON sublamina of the IPL, and have the lowest levels of melanopsin expression [5, 7, 8]. M4 ipRGCs are synonymous with ON sustained alpha RGCs and are also the only ipRGC subtype that is labeled with an antibody to a non-phosphorylated form of the neurofilament heavy chain protein (SMI-32). ON-stratifying M5 cells have small and highly branched dendritic arbors [7], while M6 cells have the smallest soma size of any ipRGC subtype, and small, highly branched, bistratified dendritic arbors [9].
Fig. 2.
ipRGC diversity. a Schematic representation of M1–M6 ipRGC stratification in the ON and/or OFF sublaminae of the retinal inner plexiform layer (IPL). b Schematic representation of melanopsin-driven intrinsic electrophysiological responses of M1, M2, and M4 ipRGCs (drawn based on [20]). c Schematic representation of the M1–M6 ipRGCs receptive fields. M1 ipRGC receptive field lacks surround antagonism of the inhibitory input nullifying the surround of the excitatory input. M2–M6 ipRGCs have center–surround-organized receptive fields (drawn based on [9, 10])
In addition to their diverse morphological properties, each ipRGC subtype exhibits distinct responses to light. This diversity in output response between M1 and M6 ipRGCs stems from the differential ratio of melanopsin versus rod and cone contributions, and from the morphological differences between each subtype. The magnitude, latency, and duration of the light response varies across M1–M6 ipRGCs in an intensity dependent manner. The intrinsic photoresponses of M1 cells have lower threshold, higher amplitude, and faster responses than those of non-M1 ipRGCs (Fig. 2b). The receptive field of M1 cells also lacks surround antagonism of synaptic inputs [10]. M2–M6 ipRGCs, however, have center–surround-organized receptive fields which suggests a capacity to detect spatial contrast (Fig. 2c). Indeed, the response of M4 ipRGCs (i.e., ON alpha RGCs) to spatial contrast has been well studied over many decades because these cells have been identified in multiple species [5, 7, 11–15].
Beyond their diverse signaling properties, ipRGC subtypes also project to distinct downstream targets in the brain. M1 cells project primarily to non-image-forming targets such as the suprachiasmatic nucleus (SCN) (to drive circadian photoentrainment), and the olivary pretectal nucleus (OPN) (to drive the pupillary light reflex), among other targets [16, 17]. M4–M6 cells project to brain nuclei involved in image-forming vision such as dorsal geniculate nucleus (dLGN) and superior colliculus (SC). Interestingly, M2 cells project to both image forming (SC and dLGN) and non-image-forming brain areas (SCN and OPN) [7, 17–20]. While M3 ipRGCs have been reported to project to the SC, no other targets have been identified, likely because this subtype has not been as extensively studied [10]. Advances in the development of genetic tools, in combination with viral neuronal tracers, have allowed researchers to map ipRGC-brain projections and determine their role in shaping image forming and non-image-forming behaviors.
Though not the focus of this review, ipRGC subtypes have been noted in other mammalian species. M1–M5 ipRGCs have been identified in rat retinas, and seem to exhibit similar morphological variations to the parallel subtype in mouse [2, 20–29]. Similarly, ON- and OFF-stratifying ipRGCs have been identified in the macaque, marmoset, and human retina [30, 31], with some work, suggesting that there are M1, M2, M3, and M4 ipRGCs in the human retina [32]. Moreover, functional data from multielectrode recordings in human donor retinas have identified three classes of ipRGC responses that may correspond to specific ipRGC subtypes [33]. Thus, though much remains to be done regarding the properties and behavioral functions of ipRGC subtypes in species other than mouse, data thus far points to conservation of ipRGC subtypes from mice to humans, and potential parallels in behavioral roles [30, 34–37].
A diverse toolkit for studying ipRGC circuits
In ipRGCs, the photopigment melanopsin is encoded by the gene Opn4. Researchers have capitalized on this specificity to generate a host of immunohistochemical and genetic tools in mice to label, manipulate, and ablate ipRGCs. This arsenal of approaches has allowed researchers to study visual circuits with higher resolution than has been possible for many RGC types. Each of the available tools to label and/or manipulate ipRGCs has its own benefits and drawbacks, and many label or ablate specific subsets of ipRGC subtypes. In the following section, we will review many of the major tools used in ipRGC research, and provide an overview of the nuances, benefits, and drawbacks of each.
ipRGC labeling
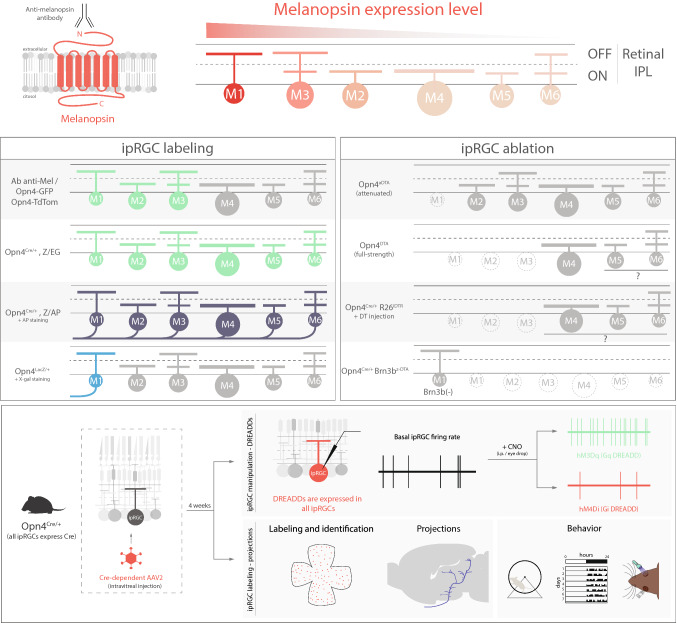
All ipRGCs express the melanopsin protein. However, M1 ipRGCs have the highest levels of melanopsin, followed by M3, then M2, and, finally, M4–M6 ipRGCs [6, 9, 38, 39] (Fig. 3). Immunohistochemistry for the melanopsin protein was one of the first methods used to identify ipRGCs [2, 40] and is still a major method used to label ipRGC distributions across the retina and stratification within the IPL. Though this method is straightforward, it is limited by the inability of the antibody to label ipRGCs that express low levels of melanopsin. Currently, the most sensitive melanopsin antibody detects the N-terminus of the melanopsin protein, and only labels M1–M3 ipRGCs. It labels very few-to-no M4–M6 ipRGCs [7, 38, 40] (Fig. 3). Given that M1 cells express the highest levels of melanopsin, they show the brightest immunolabeling and their dendrites in the OFF sublamina are readily visible. The M2 and M3 ipRGCs show detectable, but less robust, labeling of the cell body, and the dendrites of some M2 and M3 cells can be traced to the ON (or ON and OFF in the case of M3 ipRGCs) sublamina of the IPL. It is possible to detect melanopsin immunolabeling in M4–M6 ipRGCs when used in conjunction with the TSA amplification technique. Using this amplification approach, M4–M6 ipRGCs show detectable levels of melanopsin in the soma, but the labeling tapers off in the proximal dendrites [9, 20, 41].
Fig. 3.
Diverse toolkit for studying ipRGCs circuits. Top, differential melanopsin expression in M1–M6 ipRGCs. Anti-melanopsin antibody binds to the N-terminus extreme, labeling M1–M3 ipRGC subtypes. ipRGC labeling box: Opn4-GFP line labels M1–M3 ipRGCs in the adult mouse retina and also M4 cells prior to P14 [43]. The Opn4-tdTomato line labels similar subtypes to Opn4-GFP. Opn4Cre/+; Z/EG labels M1–M6 ipRGCs [7]. Opn4Cre/+; Z/AP line labels somas and axons of M1–M6 cells after AP staining [7]. Opn4LacZ/+ line labels M1 ipRGCs somas and axons after X-gal staining [2]. ipRGC ablation box: in the Opn4aDTA line M1 ipRGCs are ablated [19]; in Opn4DTA, M1–M3 cells are likely ablated and Opn4Cre/+; R26iDTR mice potentially all ipRGCs are ablated [49, 53]. In Opn4Cre/+; Brn3bZ-DTA, all ipRGCs are ablated except Brn3b-negative M1ipRGCs [99]. Bottom, use of adeno-associated viruses (AAV) to manipulate ipRGC activity through DREADDs (top) or to label somata and axons of ipRGCs (bottom) [50–52, 55–60]
There are now multiple genetic tools to label ipRGCs. Depending on the sensitivity of each tool, different ipRGC subtypes are labeled. Two knock-in lines, Opn4LacZ and Opn4Cre, and three BAC transgenic lines, two Opn4-GFP lines and one Opn4-tdTomato line, have been developed (Fig. 3). In the Opn4LacZ line, the melanopsin gene has been replaced with the gene for tau-lacZ. Tau-lacZ codes for a protein containing the beta-galactosidase enzyme fused to a signal sequence from tau that preferentially targets the protein to the axon (Fig. 3). This allows for visualization of ipRGC axons at their downstream brain targets using immunolabeling for B-gal or X-gal (5-bromo-4-chloro-3-indolyl-b-d-galactopyranoside) labeling. In this mouse line, the amount of protein expression is under control of the melanopsin promoter, and only M1 ipRGCs (and potentially M3 ipRGCs, [42]) are visible using either antibodies (immunolabeling) or X-gal labeling [2].
There are two different Opn4-GFP BAC transgenic lines currently available. The first of these was developed by the Kofuji lab. In this line, a BAC transgene contains 29 kb of upstream and 155 kb of downstream regulatory regions of the melanopsin locus, but the melanopsin gene itself has been replaced by GFP [43]. In this mouse line, M1–M3 ipRGCs are labeled, but M4 ipRGCs (and likely M5–M6 ipRGCs) are not labeled in the adult retina [44]. However, early in development, M4 ipRGCs also show detectable GFP expression, likely because melanopsin levels in M4 ipRGCs are higher during development [44, 45]. A major benefit to the BAC transgenic approach is that it can be used to label ipRGCs, but leaves both copies of the melanopsin locus itself intact for further genetic manipulation (i.e., knockout of melanopsin or use with a Cre or LacZ allele). A second Opn4-GFP BAC transgenic line was generated as part of the GENSAT project [46]. This mouse line was also generated using a BAC containing the melanopsin gene, but GFP was inserted upstream of the melanopsin gene on the BAC, leaving a copy of the melanopsin gene intact on the transgene. Thus, in the GENSAT Opn4-GFP line, it is impossible to generate a melanopsin null animal that also contains this transgene. However, the GFP signal in this mouse line is such that some ipRGC axons are detectable in the brain with immunolabeling, allowing for mapping some projections of M1–M3 ipRGCs. Thus, it is critical that the Opn4-GFP line is chosen with care depending on experimental needs. The Opn4-tdTomato line was developed in the Yau lab [47]. This line primarily labels M1 and M2 (and presumably M3) ipRGCs, but has been reported to label some M4 ipRGCs, as well [47, 48]. While Opn4-GFP lines have the disadvantage that GFP excitation and melanopsin excitation share a preferred wavelength and thus strongly activate the melanopsin phototransduction cascade if epifluorescence is used, the Opn4-tdTomato line has the advantage of allowing for the use of longer wavelength light to excite the tdTomato fluorophore.
The Opn4Cre line is by far the most sensitive mouse line for ipRGC identification and manipulation that has been developed and has allowed for the discovery of new ipRGC subtypes [7, 49]. In this mouse line, Cre recombinase is knocked into the melanopsin locus. The best characterized of the two developed Cre lines was generated by the Hattar Lab [7] and is described in detail here. When crossed with either the Z/EG or Z/AP reporters, this mouse line labels all currently known ipRGC subtypes (Fig. 3). There is evidence of wider Cre expression in the retina when this mouse line is crossed to a stronger, Rosa-based reporter such as tdTomato (Ai9), AP (R26iAP), and synaptophysin–tdTomato [50, 51]. The identity of these additional retinal neurons has not been reported and it is unclear if these are additional M1–M6 ipRGCs, new ipRGC subtypes, or ‘ectopic’ labeling of other retinal neurons (amacrine and/or cone cells). The Opn4Cre line can also be combined with intravitreal injection of Cre-dependent AAV reporters and used to label somata and projections. This strategy is very effective for driving reporter expression in ipRGCs [52] (Fig. 3). Although the high sensitivity of this Cre-loxP-based genetic approach of Opn4Cre line has allowed the identification of different subtypes of ipRGCs, using Z/EG or Z/AP reporters, it also labels some cone photoreceptors [7]. Furthermore, the Opn4Cre; Z/EG lines show extraretinal expression in areas that are not thought to express melanopsin like brainstem, diencephalon, and cortex [7]. This evidence suggests either leaky expression of Cre recombinase or that these non-retinal cells transiently express melanopsin during some stage of development [7]. These issues can be partially circumvented by intravitreal injection of AAV into adult animals to restrict expression to retinal neurons that express melanopsin in adulthood. Intravitreal AAV also avoids transgene expression in cones. Regardless of these caveats, the Opn4Cre line has greatly expanded our understanding of how ipRGCs function within their specific circuits.
ipRGC ablation
Given the ability to successfully introduce transgenes into the endogenous melanopsin locus, researchers have been able to develop multiple lines for ipRGC ablation. These models have been used to great effect in understanding the functions of ipRGCs in light-evoked behavior. These advances will be discussed in detail in a subsequent section, but, here, we will review the tools for ipRGC ablation that have been developed thus far. In 2008, Güler et al. developed the first mouse line designed to ablate ipRGCs, the Opn4aDTA line [19]. To generate this mouse line, researchers replaced the melanopsin locus with an attenuated version of the diphtheria toxin (aDTA). Using this strategy in combination with the Opn4LacZ line, researchers determined that Opn4aDTA/+ eliminated mostly the M1 ipRGCs at adult ages (Fig. 3). ipRGC loss in Opn4aDTA/aDTA mice cannot be further tested without crossing to a BAC transgenic line, because the two copies of aDTA mean a loss of melanopsin protein expression, making antibody immunolabeling impossible. Opn4aDTA/aDTA animals also preclude crossing with other knock-in lines to assess the extent of ipRGC loss. However, behavioral analyses (described below) suggest that the loss of ipRGCs in aDTA animals is mostly due to loss of M1 ipRGCs. The Hattar lab has since generated an Opn4DTA line that expresses the full-strength diphtheria toxin in the melanopsin locus [53]. The Opn4DTA mouse line shows greater loss of ipRGCs and more severe behavioral phenotypes, though as with the Opn4aDTA line, only Opn4DTA/DTA homozygous mice show free running circadian rhythms [53]. Moreover, SMI-32 labeling is normal in Opn4DTA/DTA mice, suggesting that at least M4 ipRGCs survive [53]. Based on comparably low melanopsin expression levels between M4 and M5/6 ipRGCs, it is possible, even likely, that M5 and M6 ipRGCs also escape ablation in the Opn4DTA/DTA line, though this has not been explicitly tested. Importantly, the Opn4aDTA animals show ipRGC loss only after 3–6 months of age, while Opn4DTA animals show ipRGC loss by the first postnatal week [19, 53] (Fig. 3).
Researchers have also taken the strategy of crossing the Opn4Cre line with other ablation lines. For example, the Opn4Cre line has been crossed with a Brn3bZ−DTA line. This knock-in line expresses a Cre-dependent, full-strength DTA under the control of the Brn3b promoter. Therefore, when crossed with the Opn4Cre line to generate Opn4Cre/+; Brn3bZ−DTA/+ mice, this mouse line ablates all RGCs that express both Brn3b and melanopsin. This mouse line results in ablation of all M2–M6 ipRGCs (all of which express Brn3b) and a subset of M1 ipRGCs that are Brn3b-positive (Fig. 3). Researchers also crossed the Opn4Cre line with a mouse strain in which the inducible diphtheria toxin receptor (iDTR) is knocked-in to the ROSA26 locus (R26iDTR) [49]. This knock-in mouse line expresses a Cre-dependent diphtheria toxin receptor under the Rosa26 promoter. Therefore, when crossed to the Opn4Cre line, this mouse line results in ablation of M1, M2, and M3 ipRGC subtypes when mice are injected with diphtheria toxin (Fig. 3). Göz et al. also took the strategy of using saporin-conjugated melanopsin antibodies to ablate ipRGCs and study their role in behavior [54].
ipRGC manipulation
The development of the Opn4Cre line has made all ipRGC subtypes amenable to cell-type specific manipulations to either activate or silence ipRGCs themselves or to manipulate ipRGC gene expression. One widely used tool to manipulate the activity of neurons are the Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) activated in the presence of artificially designed, specific ligands [e.g., clozapine N-oxide (CNO)]. Particularly, for ipRGC manipulation, Cre-dependent DREADDs sequences are packaged in adenovirus vectors (AAV2) and injected intravitreally into the eyes of Opn4Cre mice (Fig. 3). Gq-DREADDs (hM3Dq) have been used to mimic melanopsin phototransduction in M4 ipRGCs to assess the role of melanopsin in ipRGC physiology [55] or to activate all ipRGCs and assess behavioral outcomes [56–59]. On the other hand, it can also be leveraged to chemogenetically silence ipRGCs with inhibitory Gi-DREADDs (hM4Di) [60]. AAV can also be used to selectively express channelrhodopsins in ipRGCs to map their postsynaptic targets and assess their neurotransmitter content in specific brain regions [61–63] or to study neurite outgrowth of postnatal retinal explants [64]. Sulfonamide compounds been also used as antagonists of melanopsin [65]. These opsinamides are able to inhibit melanopsin-mediated photoresponses and behaviors such as PLR and phototaxis in neonatal mice [65].
The Opn4Cre line has also been combined to knock out specific genes in ipRGCs, most of them related to neurotransmitters glutamate, pituitary adenylate cyclase-activating polypeptide (PACAP), and gamma-aminobutyric acid (GABA) to study their role in specific behaviors such as PLR and photoentrainment [63, 66]. These conditional knockout tools have also been used to study the importance of ipRGC apoptosis during development [67] and as well as to rescue melanopsin expression in ipRGCs to study the role of human melanopsin polymorphisms associated with abnormal behaviors [68]. Researchers have used AAVs to rescue melanopsin expression in melanopsin knockout mice (Opn4Cre/Cre) with a full or modified version of the melanopsin sequence (lacking combinations of C-terminal phosphorylation sites) using AAV2 vectors to study the role of signal cascade on the intrinsic light response, the PLR, and photoentrainment [69, 70]. The possibilities for using these strategies are vast and allow for molecular level insights into ipRGC function. As the ability to specifically manipulate subsets of ipRGC subtypes is expanded, these approaches will provide important insight into the contribution of single RGC subtype to visual behavior.
Knock-in lines that target the melanopsin locus also provide the opportunity to knockout the melanopsin gene using homozygous mice such as Opn4LacZ/LacZ and Opn4Cre/Cre. These lines allow for a determination of the role of melanopsin in various behaviors and cellular functions [3, 7, 69–71]. One concern with these manipulations is the global knockout of melanopsin from early developmental stages. In studies that have addressed this question, behavioral or cellular deficits that are attributed to melanopsin can be rescued by acute activation of the melanopsin phototransduction cascade in ipRGCs [55] or by expressing melanopsin in adult ipRGCs in melanopsin null animals [70]. The ability to acutely rescue behavioral deficits argues that at least in the behaviors tested thus far, deficits are not likely to arise from circuit level rewiring due to melanopsin knockout.
ipRGC projections and behavior
Non-image-forming visual system
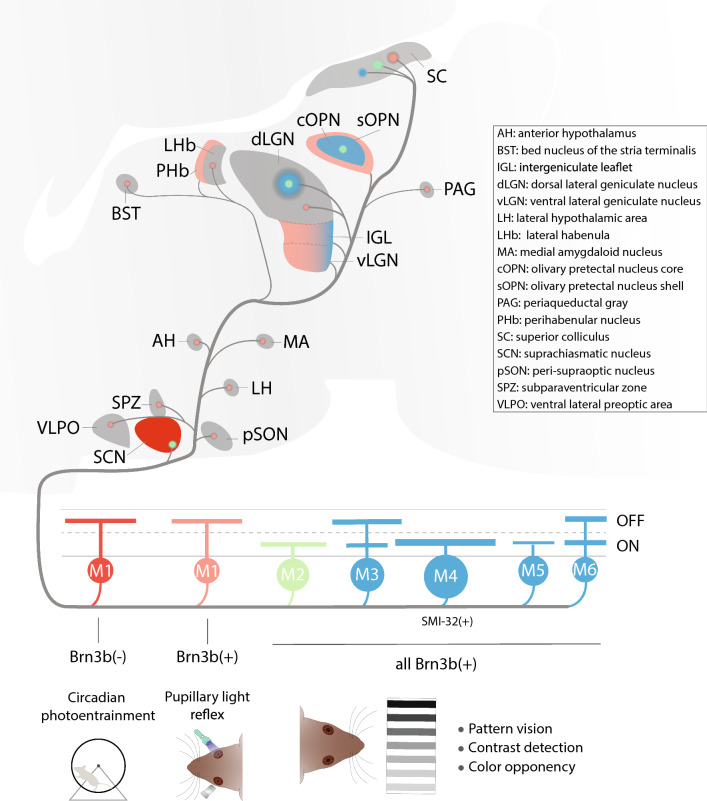
The non-image-forming system is a visual circuit that operates in parallel to conscious visual perception. This non-image-forming visual system regulates numerous subconscious effects on our physiology and behaviors such as photoentrainment of our circadian rhythms, the regulation of light-dependent pupillary constriction, sleep—wake cycles, body temperature, and improvement of learning and visual function. Almost a century ago, Clyde Keeler observed that blind mice were still able to constrict their pupils in response to light [72]. In 1999, the Foster lab demonstrated in two different papers that mice lacking rods and cones were able to adjust their circadian rhythms to light and maintain a robust PLR [73, 74]. In clinics, it was observed that some blind patients maintained circadian entrainment and suppressed melatonin secretion despite an apparently complete loss of visual function [75]. These observations suggested that there must exist a third class of non-rod, non-cone photoreceptor. Taking a cue from the continued photoentrainment of blind individuals, the Berson lab tested whether the retinal ganglion cells that project to the central circadian pacemaker, the SCN, were in fact directly light sensitive. To do this, they recorded from SCN-projecting RGCs and found that these responded to light in the absence of rod/cone input [1]. Concurrently, the Yau lab used Opn4LacZ animals to show that melanopsin-positive RGCs project axons to a variety of non-image brain targets including the SCN. Researchers initially concluded that ipRGCs comprised a uniform population of RGC that primarily influenced non-image-forming behaviors. As mentioned earlier in this review, however, we now know that there are multiple ipRGC subtypes that mediate a variety of behavioral functions. Subsequent research showed that the ipRGC input to the non-image-forming visual system primarily arises from M1 ipRGCs which project to brain areas involved in circadian photoentrainment (SCN, intergeniculate leaflet (IGL)), ventral lateral geniculate nucleus (vLGN), the pupillary light reflex (OPN), mood (perihabenular nucleus (pHb)), sleep (ventrolateral preoptic area (VLPO) and lateral hypothalamic area (LH)), and multiple other hypothalamic regions such as peri-supraoptic nucleus (pSON), IGL, anterior hypothalamus (AH), subparaventricular zone (SPZ) among others [16, 17] (Fig. 4). Below, we outline what is known about ipRGC contributions to non-image-forming behaviors.
Fig. 4.
Schematic representation of the main brain targets of ipRGCs. Brn3b-negative M1 ipRGCs are sufficient to drive circadian photoentrainment and project mainly to the SCN, while Brn3b-positive M1 ipRGCs project to sOPN and are necessary for the PLR [99]. Brn3b-positive M1 ipRGCs constitute the majority of sparse M1 ipRGC innervation of the thalamus, hypothalamus, and midbrain (VLPO, SPZ, pSON, AH, MA, vLGN, IGL, dLGN, PAG, BST, PHb, and LHb) [16, 17]. M2 cells project to both image forming (SC and dLGN) and non-image forming (SCN and OPN) visual areas and M4–M6 cells project to brain nuclei involved in image-forming vision such as dorsal geniculate nucleus (dLGN) and superior colliculus (SC) [7, 17–20]. While M3 ipRGCs have been reported to project to the SC [10], no other targets have yet been identified
Circadian photoentrainment, sleep, and alertness
Originally, it was assumed that ipRGCs played a major role in circadian photoentrainment, because they were found to project to the SCN. However, when the melanopsin gene was removed, abolishing ipRGC intrinsic sensitivity to light, circadian photoentrainment remained largely intact with mild impacts on phase shifting [76, 77] and period lengthening in constant light [76, 77]. This indicated that rod/cone signaling was sufficient for circadian photoentrainment [78]. Rods, cones, and melanopsin are the only photoreceptors that contribute to circadian photoentrainment, because a triple knockout of phototransduction in rods, cones, and ipRGCs results in a complete loss of this behavior.
The sufficiency of both rod/cone signaling and melanopsin phototransduction for photoentrainment led to a critical question: is all light information routed through ipRGCs or are melanopsin versus rod/cone signals routed through separate types of RGCs? This question was tackled directly by ablation of ipRGCs. Using the Opn4aDTA line, researchers demonstrated that ablation of ipRGCs resulted in a complete loss of circadian photoentrainment, demonstrating conclusively that ipRGCs are the principal conduit for light to drive this behavior. These conclusions were supported by studies in the same year using Opn4Cre; RosaiDTR mice, or saporin-conjugated melanopsin antibodies to ablate ipRGCs, and later by removing synaptic transmission from ipRGCs or using the full-strength Opn4DTA line [49, 53, 54, 79]. Subsequent dissection of the specific rod versus cone contributions to circadian photoentrainment revealed that rod photoreceptors play a critical role in photoentrainment. Rods are required for circadian photoentrainment from low-light intensities of at least 0.1 lux to surprisingly bright light intensities (10 lux) where rod signaling was not thought to influence behavior [78, 80]. When rod phototransduction is abolished, animals show deficits in photoentrainment below 10 lux. At high light intensities, melanopsin signaling is required for proper circadian photoentrainment. Mice lacking cone phototransduction show no deficits in photoentrainment, while mice where cones are the only functional photoreceptor fail to photoentrain [78]. Moreover, spectral analysis of circadian photoentrainment also indicates a major role for rod, but not cone, photoreceptors [80].
ipRGCs also play a major role in sleep. Mice are nocturnal rodents; they are active during subjective night and sleep during the subjective day. A light pulse presented during the early part of the subjective night (i.e., ZT 14–17) results in increased sleep. The circadian photoentrainment of sleep and the induction of sleep by light are both absent when ipRGCs are ablated using Opn4aDTA mice [3]. Furthermore, the induction of sleep is attenuated in melanopsin null (Opn4LacZ/LacZ or Opn4Cre/Cre) animals or animals lacking rods, though photoentrainment of sleep rhythms remained intact, indicating that rod/cone inputs can compensate for the loss of melanopsin to photoentrain sleep rhythms [3, 71]. Moreover, melanopsin null animals showed a reduction in c-Fos expression in neurons from sleep promoting areas such as VLPO and SC [71]. Sleep and circadian brain regions are heavily innervated by M1 ipRGCs, which are likely to be the primary ipRGC subtype lost in Opn4aDTA line. Because photoentrainment of sleep rhythms and circadian rhythms along with sleep induction by light is lost in the Opn4aDTA line, the available evidence suggests that M1 ipRGCs are necessary for these behaviors.
Pupillary light reflex
The PLR is a non-image-forming behavior that is critical to regulate the amount of light entering the eye for proper vision. The OPN is a midbrain nucleus, the shell of which plays a central role in PLR pathway. Neurons in the OPN shell project to the Edinger–Westphal nucleus which, in turn, sends projections to the ciliary ganglion. The ciliary ganglion neurons innervate the iris muscle to induce contraction [35–37, 81–84]. For many decades, it was believed that PLR was driven only by rod and cone signaling. However, in 2001, Lucas et al. showed that animals lacking rods and cones continue to show a PLR, albeit with greatly reduced sensitivity [85]. Loss of rod, cone, and melanopsin phototransduction results in a complete loss of the PLR [86], indicating that the PLR in rodless/coneless animals arises from ipRGCs. In agreement with the reduced sensitivity of the PLR in rodless/coneless animals, animals that lack only melanopsin signaling show a normal PLR except at the brightest light levels, but cannot maintain pupil diameter for long periods in constant light [66, 80, 85, 86].
Multiple papers revealed that the OPN receives innervation from multiple ipRGCs subtypes. Studies using Opn4LacZ mice showed that M1 ipRGCs heavily innervate the OPN shell but avoid the core [2, 17, 18]. However, ipRGC labeling using a Z/AP reporter line combined with the Opn4Cre line (Opn4Cre/+; Z/AP mice) (Fig. 3) revealed that the OPN core region is heavily innervated by non-M1 ipRGCs [7, 18], and a retrograde tracing study has indicated that M2 ipRGCs may contribute to the OPN core innervation [18]. The role of ipRGC innervation of the OPN core in behavior is currently unknown. In addition to the conventional PLR pathway, multiple studies in fish, amphibians, birds, and some mammals suggest that a PLR can be generated by intrinsic phototransduction within the iris muscle even when the eye is physically separated from the brain [87–91]. This intrinsic (i) PLR is very insensitive to light and is dependent upon both melanopsin phototransduction and cholinergic neurotransmission [92, 93]. ipRGCs from the ciliary marginal zone, mainly from the nasal retina, project directly to the cilliary body to induce pupillary constriction [93].
Mood and learning
M1 ipRGCs directly project to limbic regions such as the lateral habenula (LHb), the medial amygdala (MA) [2, 17], and different brain region involved in mood and behavioral state such as bed nucleus of the stria terminalis (BST), paraventricular nucleus (PVN), and periaqueductal gray (PAG) [52] (Fig. 4). Moreover, circadian rhythms and sleep can be disrupted by manipulation of the light environment and lead to mood and learning defects in mice and humans [94–96]. These two lines of evidence prompted the hypothesis that ipRGCs may drive direct effects of aberrant light on mood and learning. To model aberrant light, researchers used long-term exposure to an ultradian cycle consisting of 3.5-h light and 3.5-h dark (T7). This paradigm provides light input at multiple times in the circadian cycle where light would not normally be encountered but does so while leaving the endogenous circadian and sleep rhythms intact [97]. Exposing mice to this T7 cycle induces depression-like behaviors and hippocampal-dependent learning deficits. Opn4aDTA/aDTA mice where M1 ipRGCs are ablated, fail to show these deficits when exposed to the T7 light/dark cycle, indicating that ipRGCs relay this aberrant light information. These direct effects on mood are driven by a direct projection of (likely M1) ipRGCs to the PHb and LHb [61, 97] (Fig. 4). There is also evidence that a disynaptic connection between M4 ipRGCs and GABAergic neurons in the vLGN and IGL, which in turn project to the LHb, may decrease depression-like behavior in mice and provide a potential mechanistic explanation for the treatment of depression by light [98].
Direct effects of ipRGCs on behaviors related to anxiety and alertness have also been studied using molecular genetic strategies (Fig. 3). Milosavljevic et al. selectively activated ipRGCs by intravitreally injecting AAVs containing a Cre-dependent hM3Dq (Gq-DREADD) into the eyes of Opn4Cre animals [56] (Fig. 3). Induction of ipRGC activity through injection of the specific Gq-DREADD agonist CNO resulted in alertness and anxiety-like behaviors in mice. Moreover, ipRGC activation induced downstream neuronal c-Fos induction in multiple brain regions in the hypothalamus, thalamus, and limbic system that are commonly associated with aspects of autonomic and neuroendocrine activity. While the specific pathways underlying these behaviors are yet unknown, this evidence indicates that ipRGCs could drive a light-dependent switch in behavioral motivation toward a more alert, risk-averse state [56].
Molecular subtypes of ipRGC: differential expression of Brn3b
Until 2002, M1 ipRGCs were considered a homogenous population with uniform molecular, morphological, and functional properties. However, this homogeneity was questioned in 2011 when Chen et al. demonstrated that M1 ipRGCs differentially express the transcription factor Brn3b (encoded by Pou4f2 gene), while all non-M1 ipRGCs express Brn3b. Taking advantage of this molecular distinction, researchers generated mice to either selectively label (Opn4Cre; Brn3bcKOAP/+) or selectively ablate (Opn4Cre; Brn3bZ-DTA/+) Brn3b-positive ipRGCs. Researchers found that Brn3b-positive M1 ipRGCs innervate the shell of the OPN, while Brn3b-negative M1 ipRGCs innervate the SCN. Ablation of Brn3b-positive ipRGCs resulted in a loss of the PLR but retention of nearly normal circadian light responses [99]. Thus, Brn3b-positive ipRGCs are necessary for the PLR, while Brn3b-negative ipRGCs are sufficient to drive circadian photoentrainment.
Li and Schmidt studied the projection patterns of M1 ipRGC subtypes beyond regions associated with circadian photoentrainment and the PLR [16]. They found that Brn3b-positive M1 ipRGCs constitute the majority of sparse M1 ipRGC innervation of the thalamus, hypothalamus, and midbrain (Fig. 4). This work demonstrated that Brn3b-positive and Brn3b‐negative M1 ipRGCs have very little overlap in their projection patterns. In agreement with non-SCN projections arising largely from Brn3b-positive M1 ipRGCs, Fernandez et al. demonstrated that a Brn3b-positive M1 ipRGC projection to the PHb is primarily responsible for the effects of aberrant light exposure on mood [61]. Moreover, Rupp et al. examined the role of Brn3b-positive and Brn3b-negative ipRGCs in driving the circadian and acute effects of light on body temperature and sleep [59]. They found that Brn3b-positive ipRGCs are necessary for the acute light induction of sleep and acute light induction of body temperature decrease. However, Brn3b-negative M1 ipRGCs are sufficient for the photoentrainment of both sleep/wake cycles and body temperature rhythms. Collectively, these data point to distinct and diverse functions for these molecularly sub-divisible Brn3b-positive and Brn3b-negative ipRGCs.
Image forming visual system
While M1 ipRGCs contribute primarily to non-image-forming vision, non-M1 ipRGCs project to brain regions involved in conscious visual perception such as the dLGN and SC, and these regions receive only very sparse M1 ipRGC inputs [7] (Fig. 4). Ecker et al. were the first to demonstrate that non-M1 ipRGCs projected to image-forming brain regions. As a first test of whether melanopsin signaling was sufficient to drive rudimentary pattern vision, they demonstrated that mice lacking classical rod-cone photoreception and, therefore, entirely dependent on melanopsin for light detection were able to discriminate coarse grating stimuli from equiluminant gray [7]. Therefore, animals lacking rod/cone phototransduction had measurable, albeit very low, visual acuity, indicating that ipRGCs could, in principle, play a role in visual perception in the intact animal. Soon after this study, the M4 ipRGCs arose as a candidate to influence visual perception. Indeed, melanopsin null (Opn4LacZ/LacZ and Opn4Cre/Cre) mice showed a reduction in two measures of behavioral contrast sensitivity but normal visual acuity. These results indicated that melanopsin phototransduction in non-M1 ipRGCs was necessary for normal contrast sensitivity, even in the presence of functional rod/cone signaling. Ablation of the non-M1 ipRGCs using Opn4Cre/+; Brn3bZ-DTA/+ animals showed a further reduction in contrast sensitivity, consistent with non-M1 ipRGCs relaying rod, cone, and melanopsin signals to influence contrast sensitivity [5].
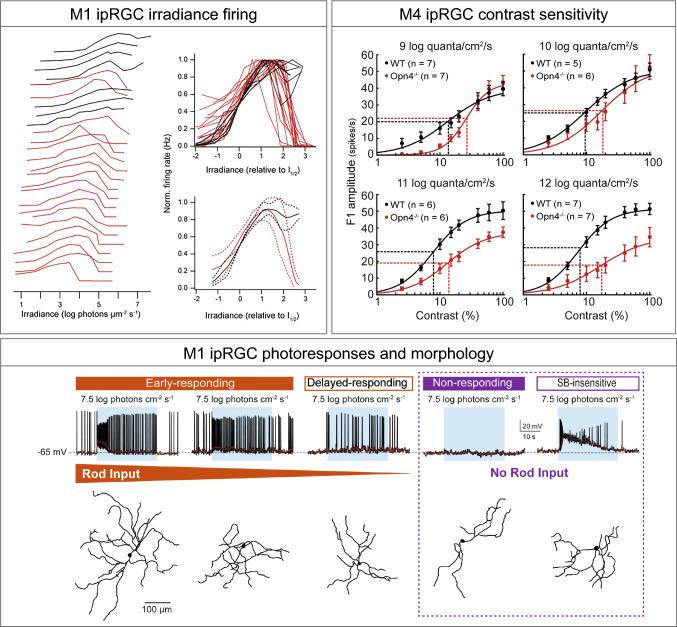
Our recent work demonstrated that melanopsin phototransduction is also necessary for normal contrast sensitivity within M4 cells themselves [55]. Melanopsin phototransduction in ipRGCs had been well characterized as slow in onset and offset, decaying on the order of tens of seconds or even minutes, as well as very sustained [6, 7, 10, 20, 100]. Moreover, in light-adapted tissue, melanopsin phototransduction had been demonstrated to be active only at very bright, photopic, light levels. Each of these three features (slow kinetics, sustained signaling, and insensitivity to light) seemed, at face value, to be inconsistent with a potential role for melanopsin in enhancing visual perception, which requires spatial and temporal precision that melanopsin phototransduction lacks. Sonoda et al. found that melanopsin phototransduction acts across a range of physiological light intensities from photopic to scotopic to enhance the contrast sensitivity of M4 ipRGCs [55] (Fig. 5). Melanopsin phototransduction does this through the use of a novel phototransduction cascade in M4 ipRGCs that serves to both depolarize the resting membrane potential of these cells as well as increase their excitability through the closure of potassium channels. Combined, these influences serve to boost the response of M4 ipRGCs to the incoming temporally and spatially precise rod/cone signals, in effect enhancing the contrast sensitivity of M4 ipRGCs through modulation of its cellular properties [55]. This mechanism is distinct from that reported in M1 ipRGCs [48, 92, 101] (Fig. 1), and indicates that different ipRGC subtypes utilize distinct melanopsin phototransduction cascades that properly tune each cell for its unique behavioral functions. Moreover, this influence of melanopsin was detectable even at dim, scotopic light intensities where only rods were thought to influence RGC responses. These data demonstrate that melanopsin phototransduction is active and influencing cellular function at much lower light intensities than previously appreciated (Fig. 5). The role of melanopsin, rod, and cone inputs to shaping the M4 ipRGC response is still an active area of research, with much yet to be resolved [5, 20, 48, 102].
Fig. 5.
Re-evaluating the roles of ipRGCs. M1 ipRGCs irradiance firing box: every individual M1 cell operates over a narrow range and the population covers irradiances from moonlight to full daylight (left); relations overlaid and aligned by I1/2 for comparison of shape (right) [109]. Melanopsin signaling in both M1 and M4 is active into the scotopic range. M4 ipRGC contrast sensitivity box: contrast response functions of M4 cells in WT (black) and Opn4−/− (red) retinas recorded at background light levels from 9 to 12 log quanta/cm2/s. Vertical dotted lines indicate C50 and horizontal dotted lines indicate half-maximal response [55]. M1 ipRGC photoresponses and morphology box: top, representative recording examples, each from various types of M1 response arranged based on degree of rod input (orange gradient). Blue rectangle indicates start and end of light stimulus. Bottom, dendrite tracing image for each recorded cell [111]
Recently, new evidence suggests that M5 ipRGCs, like M4 ipRGCs cells, contribute to ‘image-forming’ or spatial vision. In 2018, Stabio et al. reported M5 ipRGCs to exhibit opponent responses to different wavelengths of light (color opponency) [39]. They showed that M5 ipRGCs had sustained ON responses, receptive-field centers driven by balanced input from UV and mid-wavelength cone (M-cone) opsins, and a strong suppressive surround dominated by input from M-cones. M5 ipRGCs project axons to the image-forming visual thalamic nuclei, and it could provide chromatic signal to the primary visual cortex of mice and could contribute to color vision [103–105]. These cells can be distinguished from the temporal M4 ipRGCs (which are also bushy and stratify in the ON sublamina) by their lack of SMI-32 labeling and a higher number of branch points and smaller somata [39, 106]. These morphological and molecular distinctions are important as it has been reported that strong light adaptation of the ex-vivo retina can lead to color-opponent responses even in M4 ipRGCs [106]. Future work will be needed to elucidate the mechanisms behind ipRGC influences at a cellular and circuit level and, ultimately, on image-forming visual behaviors.
Re-evaluating the roles of ipRGCs
As our understanding of ipRGCs has evolved, the field has made exciting discoveries about ipRGC diversity and their functions. In this section, we will touch on some of the recent advances that are reshaping our understanding of ipRGCs.
A spectrum of M1 ipRGC properties
As mentioned before, there are important interactions between melanopsin phototransduction or rod and cone inputs to ipRGCs that are required to modulate the visual responses that will ultimately be relayed to the brain. Many studies have shown that the ipRGCs (M1 ipRGCs in particular) can individually track light intensity over a wide range [1, 30, 107, 108]. However, instead of all M1 ipRGCs each encoding a wide range of irradiance, the recent evidence from the Do lab suggests that every individual M1 cell operates over a relatively narrow intensity range (Fig. 5). However, the M1 ipRGC population covers irradiances from moonlight to full daylight [109] (Fig. 5). In parallel, the same group also demonstrated that M1 ipRGCs vary widely across other morphological and biophysical properties [110]. In addition to the intrinsic photoresponse of M1 ipRGCs, rod pathway input is necessary for normal pupil constriction and normal circadian photoentrainment across a relatively broad range of light intensities [66, 78]. Recent work from our lab indicates that M1 ipRGCs also exhibit variability in their processing of signals arising from the rod pathway, and that the degree of rod pathway input correlates with certain morphological features of M1 ipRGCs [111] (Fig. 5). As with M4 cells, we also find that M1 ipRGCs show melanopsin-evoked responses well into the scotopic regime, again indicating that melanopsin phototransduction at the cellular level is much more sensitive than previously appreciated (Fig. 5), and may only be detectable when tissue is dark adapted and cells are identified with 2-photon excitation of GFP [55, 111]. Collectively, these recent data and the molecular evidence for variability in Brn3b expression in M1 ipRGCs suggest that these are not a uniform population of cell, but perhaps exist on a wide continuum of properties.
A subset of ipRGCs release GABA
All RGCs, including ipRGCs, were thought to influence behavior through the release of the excitatory neurotransmitter glutamate at downstream targets in the brain. M1 ipRGCs, and perhaps other ipRGC subtypes, also release the neuropeptide PACAP, which is critical for sustained pupil constriction in constant light [66]. However, our recent work has demonstrated that a subset of ipRGCs (likely of the M1 subtype) release the inhibitory neurotransmitter GABA [63]. We found that ipRGC GABA release serves to dampen the sensitivity of both circadian photoentrainment and the pupillary light reflex in dim light. These new findings explain, at least in part, why ipRGCs in the retina are, in fact, quite sensitive to light, but their associated non-image-forming behaviors are so insensitive to light.
ipRGCs play critical roles in visual system development
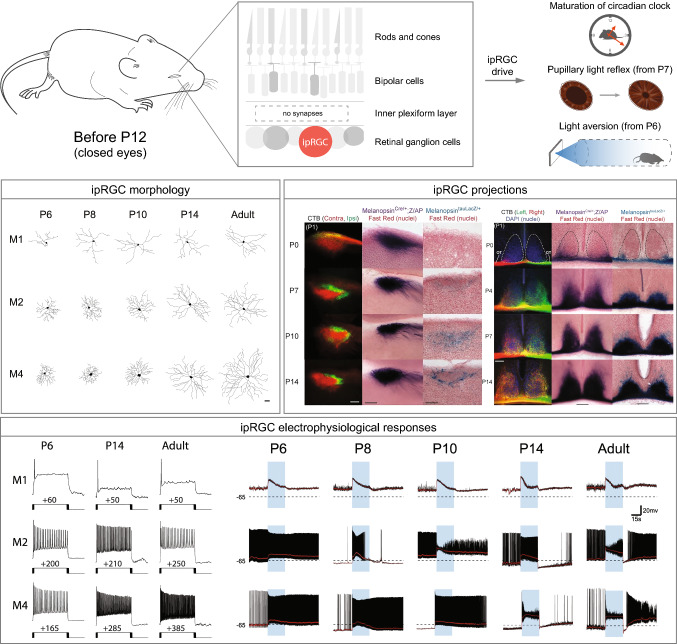
In addition to their role in visual circuits in the adult retina, increasing amounts of evidence link ipRGCs to early retinal and visual circuit development. ipRGCs are photosensitive from embryonic stages, and are the only functional photoreceptors before eye opening, at which point the rod/cone circuitry is functionally connected to the RGCs [43, 112–117] (Fig. 6). Moreover, ipRGCs connect with the SCN and dLGN as early as P0, positioning ipRGCs to relay light information to the brain from late early postnatal stages [114, 118] (Fig. 6). Light signaling by ipRGCs has been linked to multiple developmental effects on visual system development. For example, ipRGCs modulate the development and branching of the retinal vasculature, influence spontaneous activity during retinal development, send retinofugal axons to the outer retina, couple widely to amacrine and RGCs, and even regulate refinement of retinofugal projections [53, 119–125]. Researchers have begun to dissect the properties of ipRGC development and how they signal within the retina and to the brain. Recently, the Hattar group has proposed a new role for ipRGCs during development controlling non-photic entrainment [126]. Innervation of ipRGCs to IGL in early postnatal stages tunes the SCN to allow entrainment to time-restricted feeding [126]. Currently, the mechanisms behind these influences during retinal and visual system development, and the ipRGC subtypes involved are unknown. ipRGC subtypes exhibit many of their distinguishing features relatively early in development, making a study of ipRGC signaling at a subtype specific level feasible [44]. Moreover, because melanopsin expression occurs early in retinal development, ipRGCs are amenable to genetic manipulation throughout most of retinal development, making an understanding of their role in visual system development an achievable future goal.
Fig. 6.
ipRGC morphology, projections, and behavior during development. Top, ipRGCs are the only photosensitive cells up to ~ P12, prior to the opening of the eyes and before the connection between bipolar cells and RGCs [115]. ipRGCs set the length of the circadian period [53], and drive rudimentary PLR at P7 [114] and light aversion in mice as young as P6 [138]. ipRGC morphology box: representative cell tracings of M1, M2, and M4 subtypes for P6, P8, P10, P14, and adult time points [44]. ipRGC projections box: left, representative images of olivary pretectal nucleus (OPN) innervation through contralateral (red) and ipsilateral (green) eye injection with fluorescent cholera toxin B subunit (CTB) at P0; Opn4Cre; Z/AP-labeled axons show a similar innervation pattern from P0. In contrast, Opn4LacZ/+-labeled axons are not detected until P7 and show adult-like innervation of the OPN shell (sOPN) by P14 [114]. Right: representative images of suprachiasmatic nucleus (SCN) innervation using CTB. Retinal fibers fill the SCN by P7 and innervation becomes bilateral by P14. Similar innervation pattern was observed in both Opn4Cre; Z/AP and Opn4LacZ/+ lines [114]. ipRGCs electrophysiological responses box: left, representative traces from depolarizing current injection that elicited the maximum spike output for M1, M2, and M4 subtypes at P6, P14, and adult time points [44]. Right, representative light response traces from M1, M2, and M4 cells at P6, P8, P10, P14, and adult time points. Blue rectangle indicates start and end of light stimulus. Black dotted line indicates − 65 mV [44]
Concluding remarks
In this review, we synthesized what is known about the contribution of ipRGCs to diverse visual circuits. We highlighted the complex diversity of ipRGC subtypes and discussed the different molecular, histological, genetic, circuitry, and behavioral approaches that researchers have used to classify them. Functionally, the ipRGCs can be classified into those that influence non-image-forming behaviors (e.g., photoentrainment and the PLR; largely driven by M1 ipRGCs), and into those that influence image-forming vision (largely driven by non-M1 ipRGCs). Interestingly, the M2 ipRGCs project to nuclei involved in both forming and non-forming image visual pathways, indicating that they may influence both classes of visual behavior. While this simplified functional classification can be useful in a practical sense, each subtype has unique morphological features (dendritic field and soma size, as well as level of dendritic stratification), melanopsin expression level, electrophysiological characteristics, and axonal projection patterns, which suggest possible specific functional roles for each subtype within these broad categories.
Much has yet to be uncovered regarding how ipRGCs function within their associated visual circuits. For example, how does melanopsin function within each ipRGC subtype and how does that integrate with rod/cone input at the cellular level? How do each of these individual subtypes modulate their associated light-evoked effects on physiology and behavior? What are the downstream cellular synaptic targets of each ipRGC within the ~ 20 brain regions innervated by ipRGCs? How does this feed into more complex downstream circuits to influence physiology?
A final remaining major question is how this system functions within humans. ipRGCs have been identified in non-human primate and human retina. ON and OFF subtypes of ipRGC have been identified, and they seem to target both image and non-image-forming brain regions in the primate and influence the PLR, circadian system, and even conscious visual perception in humans [33, 34, 127–132]. Work has also indicated that ipRGCs play a role in photophobia in migraines, sleep disorders, anxiety, and depression in humans [94, 133, 134]. A recent molecular analysis of RGCs from both primate retina and mouse retina indicated that the ipRGCs are one of the few cell types that appear well conserved across these species [135], bolstering the rationale for continued in-depth study of these cells in the rodent, making use of the suite of genetic tools that have been (and likely will be) developed.
Acknowledgements
This work was funded by Sloan Research Fellowship to T.M.S. and NIH grant 1DP2EY022584 to T.M.S. We would like to thank Schmidt Lab members Kayla Miguel, Ely Contreras, and Jarildy Javier for helpful comments on the manuscript.
Abbreviations
- AAV
Adeno-associated virus
- aDTA
Attenuated version of the diphtheria toxin
- AH
Anterior hypothalamus
- AP
Alkaline phosphatase
- BST
Bed nucleus of the stria terminalis
- CNO
Clozapine N-oxide
- cOPN
Olivary pretectal nucleus core
- CTB
Cholera toxin B subunit
- dLGN
Dorsal lateral geniculate nucleus
- DREADDs
Designer receptors exclusively activated by designer drugs
- DTA
Diphtheria toxin
- GABA
Gamma-aminobutyric acid
- GFP
Green fluorescent protein
- iDTR
Inducible diphtheria toxin receptor
- IGL
Intergeniculate leaflet
- IPL
Inner plexiform layer
- iPLR
Intrinsic pupillary light reflex
- ipRGC
Intrinsically photosensitive retinal ganglion cell
- LH
Lateral hypothalamic area
- LHb
Lateral habenula
- MA
Medial amygdaloid nucleus
- PACAP
Pituitary adenylate cyclase-activating polypeptide
- PAG
Periaqueductal gray
- PDE
Phosphodiesterase
- PHb
Perihabenular nucleus
- PLC
Phospholipase C
- PLR
Pupillary light reflex
- pSON
Peri-supraoptic nucleus
- R26
Rosa26
- RGC
Retinal ganglion cell
- SC
Superior colliculus
- SCN
Suprachiasmatic nucleus
- sOPN
Olivary pretectal nucleus shell
- SPZ
Subparaventricular zone
- TRPC
Transient receptor potential channels
- vLGN
Ventral lateral geniculate nucleus
- VLPO
Ventral lateral preoptic area
- X-gal
5-Bromo- 4-chloro-3-indolyl-b-D-galactopyranoside
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 2.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295(5557):1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altimus CM, Guler AD, Villa KL, McNeill DS, Legates TA, Hattar S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci USA. 2008;105(50):19998–20003. doi: 10.1073/pnas.0808312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15(7):443–454. doi: 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt TM, Alam NM, Chen S, Kofuji P, Li W, Prusky GT, Hattar S. A role for melanopsin in alpha retinal ganglion cells and contrast detection. Neuron. 2014;82(4):781–788. doi: 10.1016/j.neuron.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci. 2009;29(2):476–482. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67(1):49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viney TJ, Balint K, Hillier D, Siegert S, Boldogkoi Z, Enquist LW, Meister M, Cepko CL, Roska B. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr Biol. 2007;17(11):981–988. doi: 10.1016/j.cub.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 9.Quattrochi LE, Stabio ME, Kim I, Ilardi MC, Michelle Fogerson P, Leyrer ML, Berson DM. The M6 cell: a small-field bistratified photosensitive retinal ganglion cell. J Comp Neurol. 2019;527(1):297–311. doi: 10.1002/cne.24556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao X, Stafford BK, Godin AL, King WM, Wong KY. Photoresponse diversity among the five types of intrinsically photosensitive retinal ganglion cells. J Physiol. 2014;592(7):1619–1636. doi: 10.1113/jphysiol.2013.262782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boycott BB, Wassle H. The morphological types of ganglion cells of the domestic cat's retina. J Physiol. 1974;240(2):397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peichl L, Buhl EH, Boycott BB. Alpha ganglion cells in the rabbit retina. J Comp Neurol. 1987;263(1):25–41. doi: 10.1002/cne.902630103. [DOI] [PubMed] [Google Scholar]
- 13.Huxlin KR, Goodchild AK. Retinal ganglion cells in the albino rat: revised morphological classification. J Comp Neurol. 1997;385(2):309–323. doi: 10.1002/(SICI)1096-9861(19970825)385:2<309::AID-CNE9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Berson DM, Isayama T, Pu M. The Eta ganglion cell type of cat retina. J Comp Neurol. 1999;408(2):204–219. doi: 10.1002/(SICI)1096-9861(19990531)408:2<204::AID-CNE5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 15.Sun W, Deng Q, Levick WR, He S. ON direction-selective ganglion cells in the mouse retina. J Physiol. 2006;576(Pt 1):197–202. doi: 10.1113/jphysiol.2006.115857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li JY, Schmidt TM. Divergent projection patterns of M1 ipRGC subtypes. J Comp Neurol. 2018;526(13):2010–2018. doi: 10.1002/cne.24469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497(3):326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27(7):1763–1770. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- 19.Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453(7191):102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estevez ME, Fogerson PM, Ilardi MC, Borghuis BG, Chan E, Weng S, Auferkorte ON, Demb JB, Berson DM. Form and function of the M4 cell, an intrinsically photosensitive retinal ganglion cell type contributing to geniculocortical vision. J Neurosci. 2012;32(39):13608–13620. doi: 10.1523/JNEUROSCI.1422-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boudard DL, Mendoza J, Hicks D. Loss of photic entrainment at low illuminances in rats with acute photoreceptor degeneration. Eur J Neurosci. 2009;30(8):1527–1536. doi: 10.1111/j.1460-9568.2009.06935.x. [DOI] [PubMed] [Google Scholar]
- 22.Li RS, Chen BY, Tay DK, Chan HH, Pu ML, So KF. Melanopsin-expressing retinal ganglion cells are more injury-resistant in a chronic ocular hypertension model. Invest Ophthalmol Vis Sci. 2006;47(7):2951–2958. doi: 10.1167/iovs.05-1295. [DOI] [PubMed] [Google Scholar]
- 23.Ostergaard J, Hannibal J, Fahrenkrug J. Synaptic contact between melanopsin-containing retinal ganglion cells and rod bipolar cells. Invest Ophthalmol Vis Sci. 2007;48(8):3812–3820. doi: 10.1167/iovs.06-1322. [DOI] [PubMed] [Google Scholar]
- 24.Warren EJ, Allen CN, Brown RL, Robinson DW. The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells. Eur J Neurosci. 2006;23(9):2477–2487. doi: 10.1111/j.1460-9568.2006.04777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reifler AN, Chervenak AP, Dolikian ME, Benenati BA, Meyers BS, Demertzis ZD, Lynch AM, Li BY, Wachter RD, Abufarha FS, Dulka EA, Pack W, Zhao X, Wong KY. The rat retina has five types of ganglion-cell photoreceptors. Exp Eye Res. 2015;130:17–28. doi: 10.1016/j.exer.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engelund A, Fahrenkrug J, Harrison A, Hannibal J. Vesicular glutamate transporter 2 (VGLUT2) is co-stored with PACAP in projections from the rat melanopsin-containing retinal ganglion cells. Cell Tissue Res. 2010;340(2):243–255. doi: 10.1007/s00441-010-0950-3. [DOI] [PubMed] [Google Scholar]
- 27.Hannibal J, Georg B, Fahrenkrug J. Differential expression of melanopsin mRNA and protein in Brown Norwegian rats. Exp Eye Res. 2013;106:55–63. doi: 10.1016/j.exer.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Ingham ES, Gunhan E, Fuller PM, Fuller CA. Immunotoxin-induced ablation of melanopsin retinal ganglion cells in a non-murine mammalian model. J Comp Neurol. 2009;516(2):125–140. doi: 10.1002/cne.22103. [DOI] [PubMed] [Google Scholar]
- 29.Esquiva G, Lax P, Cuenca N. Impairment of intrinsically photosensitive retinal ganglion cells associated with late stages of retinal degeneration. Invest Ophthalmol Vis Sci. 2013;54(7):4605–4618. doi: 10.1167/iovs.13-12120. [DOI] [PubMed] [Google Scholar]
- 30.Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433(7027):749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 31.Jusuf PR, Lee SC, Hannibal J, Grunert U. Characterization and synaptic connectivity of melanopsin-containing ganglion cells in the primate retina. Eur J Neurosci. 2007;26(10):2906–2921. doi: 10.1111/j.1460-9568.2007.05924.x. [DOI] [PubMed] [Google Scholar]
- 32.Hannibal J, Christiansen AT, Heegaard S, Fahrenkrug J, Kiilgaard JF. Melanopsin expressing human retinal ganglion cells: subtypes, distribution, and intraretinal connectivity. J Comp Neurol. 2017;525(8):1934–1961. doi: 10.1002/cne.24181. [DOI] [PubMed] [Google Scholar]
- 33.Mure LS, Vinberg F, Hanneken A, Panda S. Functional diversity of human intrinsically photosensitive retinal ganglion cells. Science. 2019;366(6470):1251–1255. doi: 10.1126/science.aaz0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47(7):946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gamlin PD. The pretectum: connections and oculomotor-related roles. Prog Brain Res. 2006;151:379–405. doi: 10.1016/S0079-6123(05)51012-4. [DOI] [PubMed] [Google Scholar]
- 36.Gamlin PD, Zhang H, Clarke RJ. Luminance neurons in the pretectal olivary nucleus mediate the pupillary light reflex in the rhesus monkey. Exp Brain Res. 1995;106(1):169–176. doi: 10.1007/bf00241367. [DOI] [PubMed] [Google Scholar]
- 37.Gamlin PD, Clarke RJ. The pupillary light reflex pathway of the primate. J Am Optom Assoc. 1995;66(7):415–418. [PubMed] [Google Scholar]
- 38.Berson DM, Castrucci AM, Provencio I. Morphology and mosaics of melanopsin-expressing retinal ganglion cell types in mice. J Comp Neurol. 2010;518(13):2405–2422. doi: 10.1002/cne.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stabio ME, Sabbah S, Quattrochi LE, Ilardi MC, Fogerson PM, Leyrer ML, Kim MT, Kim I, Schiel M, Renna JM, Briggman KL, Berson DM. The M5 cell: a color-opponent intrinsically photosensitive retinal ganglion cell. Neuron. 2018;97(1):251. doi: 10.1016/j.neuron.2017.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415(6871):493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- 41.Renna JM, Chellappa DK, Ross CL, Stabio ME, Berson DM. Melanopsin ganglion cells extend dendrites into the outer retina during early postnatal development. Dev Neurobiol. 2015;75(9):935–946. doi: 10.1002/dneu.22260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pires SS, Hughes S, Turton M, Melyan Z, Peirson SN, Zheng L, Kosmaoglou M, Bellingham J, Cheetham ME, Lucas RJ, Foster RG, Hankins MW, Halford S. Differential expression of two distinct functional isoforms of melanopsin (Opn4) in the mammalian retina. J Neurosci. 2009;29(39):12332–12342. doi: 10.1523/JNEUROSCI.2036-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt TM, Taniguchi K, Kofuji P. Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development. J Neurophysiol. 2008;100(1):371–384. doi: 10.1152/jn.00062.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lucas JA, Schmidt TM. Cellular properties of intrinsically photosensitive retinal ganglion cells during postnatal development. Neural Dev. 2019;14(1):8. doi: 10.1186/s13064-019-0132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sexton TJ, Bleckert A, Turner MH, Van Gelder RN. Type I intrinsically photosensitive retinal ganglion cells of early post-natal development correspond to the M4 subtype. Neural Dev. 2015;10:17. doi: 10.1186/s13064-015-0042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425(6961):917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 47.Do MT, Kang SH, Xue T, Zhong H, Liao HW, Bergles DE, Yau KW. Photon capture and signalling by melanopsin retinal ganglion cells. Nature. 2009;457(7227):281–287. doi: 10.1038/nature07682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang Z, Yue WWS, Chen L, Sheng Y, Yau KW (2018) Cyclic-nucleotide- and HCN-channel-mediated phototransduction in intrinsically photosensitive retinal ganglion cells. Cell 175(3):652–664 e612. 10.1016/j.cell.2018.08.055 [DOI] [PMC free article] [PubMed]
- 49.Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Buch T, Waisman A, Schmedt C, Jegla T, Panda S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE. 2008;3(6):e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandez DC, Chang YT, Hattar S, Chen SK. Architecture of retinal projections to the central circadian pacemaker. Proc Natl Acad Sci USA. 2016;113(21):6047–6052. doi: 10.1073/pnas.1523629113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Q, Yue WWS, Jiang Z, Xue T, Kang SH, Bergles DE, Mikoshiba K, Offermanns S, Yau KW (2017) Synergistic signaling by light and acetylcholine in mouse iris sphincter muscle. Curr Biol 27(12):1791–1800 e1795. 10.1016/j.cub.2017.05.022 [DOI] [PMC free article] [PubMed]
- 52.Delwig A, Larsen DD, Yasumura D, Yang CF, Shah NM, Copenhagen DR. Retinofugal projections from melanopsin-expressing retinal ganglion cells revealed by intraocular injections of Cre-dependent virus. PLoS ONE. 2016;11(2):e0149501. doi: 10.1371/journal.pone.0149501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chew KS, Renna JM, McNeill DS, Fernandez DC, Keenan WT, Thomsen MB, Ecker JL, Loevinsohn GS, VanDunk C, Vicarel DC, Tufford A, Weng S, Gray PA, Cayouette M, Herzog ED, Zhao H, Berson DM, Hattar S. A subset of ipRGCs regulates both maturation of the circadian clock and segregation of retinogeniculate projections in mice. Elife. 2017 doi: 10.7554/eLife.22861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goz D, Studholme K, Lappi DA, Rollag MD, Provencio I, Morin LP. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS ONE. 2008;3(9):e3153. doi: 10.1371/journal.pone.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sonoda T, Lee SK, Birnbaumer L, Schmidt TM (2018) Melanopsin phototransduction is repurposed by ipRGC subtypes to shape the function of distinct visual circuits. Neuron 99(4):754–767 e754. 10.1016/j.neuron.2018.06.032 [DOI] [PMC free article] [PubMed]
- 56.Milosavljevic N, Cehajic-Kapetanovic J, Procyk CA, Lucas RJ. Chemogenetic activation of melanopsin retinal ganglion cells induces signatures of arousal and/or anxiety in Mice. Curr Biol. 2016;26(17):2358–2363. doi: 10.1016/j.cub.2016.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Milosavljevic N, Storchi R, Eleftheriou CG, Colins A, Petersen RS, Lucas RJ. Photoreceptive retinal ganglion cells control the information rate of the optic nerve. Proc Natl Acad Sci USA. 2018;115(50):E11817–E11826. doi: 10.1073/pnas.1810701115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keenan WT, Fernandez DC, Shumway LJ, Zhao H, Hattar S. Eye-drops for activation of DREADDs. Front Neural Circuits. 2017;11:93. doi: 10.3389/fncir.2017.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rupp AC, Ren M, Altimus CM, Fernandez DC, Richardson M, Turek F, Hattar S, Schmidt TM. Distinct ipRGC subpopulations mediate light's acute and circadian effects on body temperature and sleep. Elife. 2019 doi: 10.7554/eLife.44358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Storchi R, Milosavljevic N, Eleftheriou CG, Martial FP, Orlowska-Feuer P, Bedford RA, Brown TM, Montemurro MA, Petersen RS, Lucas RJ. Melanopsin-driven increases in maintained activity enhance thalamic visual response reliability across a simulated dawn. Proc Natl Acad Sci USA. 2015;112(42):E5734–5743. doi: 10.1073/pnas.1505274112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernandez DC, Fogerson PM, Lazzerini Ospri L, Thomsen MB, Layne RM, Severin D, Zhan J, Singer JH, Kirkwood A, Zhao H, Berson DM, Hattar S (2018) light affects mood and learning through distinct retina-brain pathways. Cell 175(1):71–84 e18. 10.1016/j.cell.2018.08.004 [DOI] [PMC free article] [PubMed]
- 62.Bhandari A, Smith JC, Zhang Y, Jensen AA, Reid L, Goeser T, Fan S, Ghate D, Van Hook MJ. Early-stage ocular hypertension alters retinal ganglion cell synaptic transmission in the visual thalamus. Front Cell Neurosci. 2019;13:426. doi: 10.3389/fncel.2019.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sonoda T, Li JY, Hayes NW, Chan JC, Okabe Y, Belin S, Nawabi H, Schmidt TM. A noncanonical inhibitory circuit dampens behavioral sensitivity to light. Science. 2020;368(6490):527–531. doi: 10.1126/science.aay3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin CI, Chiao CC. Blue light promotes neurite outgrowth of retinal explants in postnatal ChR2 Mice. eNeuro. 2019 doi: 10.1523/ENEURO.0391-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones KA, Hatori M, Mure LS, Bramley JR, Artymyshyn R, Hong SP, Marzabadi M, Zhong H, Sprouse J, Zhu Q, Hartwick AT, Sollars PJ, Pickard GE, Panda S. Small-molecule antagonists of melanopsin-mediated phototransduction. Nat Chem Biol. 2013;9(10):630–635. doi: 10.1038/nchembio.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keenan WT, Rupp AC, Ross RA, Somasundaram P, Hiriyanna S, Wu Z, Badea TC, Robinson PR, Lowell BB, Hattar SS. A visual circuit uses complementary mechanisms to support transient and sustained pupil constriction. Elife. 2016 doi: 10.7554/eLife.15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen SK, Chew KS, McNeill DS, Keeley PW, Ecker JL, Mao BQ, Pahlberg J, Kim B, Lee SC, Fox MA, Guido W, Wong KY, Sampath AP, Reese BE, Kuruvilla R, Hattar S. Apoptosis regulates ipRGC spacing necessary for rods and cones to drive circadian photoentrainment. Neuron. 2013;77(3):503–515. doi: 10.1016/j.neuron.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodgers J, Peirson SN, Hughes S, Hankins MW. Functional characterisation of naturally occurring mutations in human melanopsin. Cell Mol Life Sci. 2018;75(19):3609–3624. doi: 10.1007/s00018-018-2813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mure LS, Hatori M, Zhu Q, Demas J, Kim IM, Nayak SK, Panda S. Melanopsin-encoded response properties of intrinsically photosensitive retinal ganglion cells. Neuron. 2016;90(5):1016–1027. doi: 10.1016/j.neuron.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Somasundaram P, Wyrick GR, Fernandez DC, Ghahari A, Pinhal CM, Simmonds Richardson M, Rupp AC, Cui L, Wu Z, Brown RL, Badea TC, Hattar S, Robinson PR. C-terminal phosphorylation regulates the kinetics of a subset of melanopsin-mediated behaviors in mice. Proc Natl Acad Sci USA. 2017;114(10):2741–2746. doi: 10.1073/pnas.1611893114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lupi D, Oster H, Thompson S, Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci. 2008;11(9):1068–1073. doi: 10.1038/nn.2179. [DOI] [PubMed] [Google Scholar]
- 72.Keeler CE. Iris movements in blind Mice. Am J Physiol. 1927;81(1):107–112. doi: 10.1152/ajplegacy.1927.81.1.107. [DOI] [Google Scholar]
- 73.Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284(5413):502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 74.Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284(5413):505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- 75.Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, Klein T, Rizzo JF., 3rd Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med. 1995;332(1):6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 76.Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298(5601):2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 77.Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O'Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298(5601):2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- 78.Altimus CM, Guler AD, Alam NM, Arman AC, Prusky GT, Sampath AP, Hattar S. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat Neurosci. 2010;13(9):1107–1112. doi: 10.1038/nn.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kofuji P, Mure LS, Massman LJ, Purrier N, Panda S, Engeland WC. Intrinsically photosensitive retinal ganglion cells (ipRGCs) are necessary for light entrainment of peripheral clocks. PLoS ONE. 2016;11(12):e0168651. doi: 10.1371/journal.pone.0168651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lall GS, Revell VL, Momiji H, Al Enezi J, Altimus CM, Guler AD, Aguilar C, Cameron MA, Allender S, Hankins MW, Lucas RJ. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66(3):417–428. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trejo LJ, Cicerone CM. Cells in the pretectal olivary nucleus are in the pathway for the direct light reflex of the pupil in the rat. Brain Res. 1984;300(1):49–62. doi: 10.1016/0006-8993(84)91340-4. [DOI] [PubMed] [Google Scholar]
- 82.Young MJ, Lund RD. The anatomical substrates subserving the pupillary light reflex in rats: origin of the consensual pupillary response. Neuroscience. 1994;62(2):481–496. doi: 10.1016/0306-4522(94)90381-6. [DOI] [PubMed] [Google Scholar]
- 83.Distler C, Hoffmann KP. The pupillary light reflex in normal and innate microstrabismic cats, II: Retinal and cortical input to the nucleus praetectalis olivaris. Vis Neurosci. 1989;3(2):139–153. doi: 10.1017/s0952523800004454. [DOI] [PubMed] [Google Scholar]
- 84.Distler C, Hoffmann KP. The pupillary light reflex in normal and innate microstrabismic cats, I: Behavior and receptive-field analysis in the nucleus praetectalis olivaris. Vis Neurosci. 1989;3(2):127–138. doi: 10.1017/s0952523800004442. [DOI] [PubMed] [Google Scholar]
- 85.Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4(6):621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- 86.Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299(5604):245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 87.Barr L, Alpern M. Photosensitivity of the Frog Iris. J Gen Physiol. 1963;46:1249–1265. doi: 10.1085/jgp.46.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Seliger HH. Direct action of light in naturally pigmented muscle fibers. I. Action spectrum for contraction in eel iris sphincter. J Gen Physiol. 1962;46:333–342. doi: 10.1085/jgp.46.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tu DC, Batten ML, Palczewski K, Van Gelder RN. Nonvisual photoreception in the chick iris. Science. 2004;306(5693):129–131. doi: 10.1126/science.1101484. [DOI] [PubMed] [Google Scholar]
- 90.Bito LZ, Turansky DG. Photoactivation of pupillary constriction in the isolated in vitro iris of a mammal (Mesocricetus auratus) Comp Biochem Physiol A Comp Physiol. 1975;50(2):407–413. doi: 10.1016/0300-9629(75)90034-1. [DOI] [PubMed] [Google Scholar]
- 91.Lau KC, So KF, Campbell G, Lieberman AR. Pupillary constriction in response to light in rodents, which does not depend on central neural pathways. J Neurol Sci. 1992;113(1):70–79. doi: 10.1016/0022-510x(92)90267-o. [DOI] [PubMed] [Google Scholar]
- 92.Xue T, Do MT, Riccio A, Jiang Z, Hsieh J, Wang HC, Merbs SL, Welsbie DS, Yoshioka T, Weissgerber P, Stolz S, Flockerzi V, Freichel M, Simon MI, Clapham DE, Yau KW. Melanopsin signalling in mammalian iris and retina. Nature. 2011;479(7371):67–73. doi: 10.1038/nature10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Semo M, Gias C, Ahmado A, Vugler A. A role for the ciliary marginal zone in the melanopsin-dependent intrinsic pupillary light reflex. Exp Eye Res. 2014;119:8–18. doi: 10.1016/j.exer.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 94.Foster RG, Wulff K. The rhythm of rest and excess. Nat Rev Neurosci. 2005;6(5):407–414. doi: 10.1038/nrn1670. [DOI] [PubMed] [Google Scholar]
- 95.Fonken LK, Finy MS, Walton JC, Weil ZM, Workman JL, Ross J, Nelson RJ. Influence of light at night on murine anxiety- and depressive-like responses. Behav Brain Res. 2009;205(2):349–354. doi: 10.1016/j.bbr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 96.Ma WP, Cao J, Tian M, Cui MH, Han HL, Yang YX, Xu L. Exposure to chronic constant light impairs spatial memory and influences long-term depression in rats. Neurosci Res. 2007;59(2):224–230. doi: 10.1016/j.neures.2007.06.1474. [DOI] [PubMed] [Google Scholar]
- 97.LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Kirkwood A, Weber ET, Hattar S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491(7425):594–598. doi: 10.1038/nature11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang L, Xi Y, Peng Y, Yang Y, Huang X, Fu Y, Tao Q, Xiao J, Yuan T, An K, Zhao H, Pu M, Xu F, Xue T, Luo M, So KF, Ren C (2019) A visual circuit related to habenula underlies the antidepressive effects of light therapy. Neuron 102(1):128–142 e128. 10.1016/j.neuron.2019.01.037 [DOI] [PubMed]
- 99.Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476(7358):92–95. doi: 10.1038/nature10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wong KY. A retinal ganglion cell that can signal irradiance continuously for 10 hours. J Neurosci. 2012;32(33):11478–11485. doi: 10.1523/JNEUROSCI.1423-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Perez-Leighton CE, Schmidt TM, Abramowitz J, Birnbaumer L, Kofuji P. Intrinsic phototransduction persists in melanopsin-expressing ganglion cells lacking diacylglycerol-sensitive TRPC subunits. Eur J Neurosci. 2011;33(5):856–867. doi: 10.1111/j.1460-9568.2010.07583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schroeder MM, Harrison KR, Jaeckel ER, Berger HN, Zhao X, Flannery MP, St Pierre EC, Pateqi N, Jachimska A, Chervenak AP, Wong KY. The roles of rods, cones, and melanopsin in photoresponses of M4 intrinsically photosensitive retinal ganglion cells (ipRGCs) and optokinetic visual behavior. Front Cell Neurosci. 2018;12:203. doi: 10.3389/fncel.2018.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Denman DJ, Siegle JH, Koch C, Reid RC, Blanche TJ. Spatial organization of chromatic pathways in the mouse dorsal lateral geniculate nucleus. J Neurosci. 2017;37(5):1102–1116. doi: 10.1523/JNEUROSCI.1742-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jacobs GH, Williams GA, Fenwick JA. Influence of cone pigment coexpression on spectral sensitivity and color vision in the mouse. Vision Res. 2004;44(14):1615–1622. doi: 10.1016/j.visres.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 105.Rhim I, Coello-Reyes G, Ko HK, Nauhaus I. Maps of cone opsin input to mouse V1 and higher visual areas. J Neurophysiol. 2017;117(4):1674–1682. doi: 10.1152/jn.00849.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sonoda T, Okabe Y, Schmidt TM. Overlapping morphological and functional properties between M4 and M5 intrinsically photosensitive retinal ganglion cells. J Comp Neurol. 2020;528(6):1028–1040. doi: 10.1002/cne.24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Do MT, Yau KW. Adaptation to steady light by intrinsically photosensitive retinal ganglion cells. Proc Natl Acad Sci USA. 2013;110(18):7470–7475. doi: 10.1073/pnas.1304039110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Do MTH. Melanopsin and the intrinsically photosensitive retinal ganglion cells: biophysics to behavior. Neuron. 2019;104(2):205–226. doi: 10.1016/j.neuron.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Milner ES, Do MTH (2017) A population representation of absolute light intensity in the mammalian retina. Cell 171(4):865–876 e816. 10.1016/j.cell.2017.09.005 [DOI] [PMC free article] [PubMed]
- 110.Emanuel AJ, Kapur K, Do MTH. Biophysical variation within the M1 type of ganglion cell photoreceptor. Cell Rep. 2017;21(4):1048–1062. doi: 10.1016/j.celrep.2017.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee SK, Sonoda T, Schmidt TM (2019) M1 intrinsically photosensitive retinal ganglion cells integrate rod and melanopsin inputs to signal in low light. Cell Rep 29(11):3349–3355 e3342. 10.1016/j.celrep.2019.11.024 [DOI] [PMC free article] [PubMed]
- 112.Sekaran S, Lupi D, Jones SL, Sheely CJ, Hattar S, Yau KW, Lucas RJ, Foster RG, Hankins MW. Melanopsin-dependent photoreception provides earliest light detection in the mammalian retina. Curr Biol. 2005;15(12):1099–1107. doi: 10.1016/j.cub.2005.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang XM, Chen BY, Ng AH, Tanner JA, Tay D, So KF, Rachel RA, Copeland NG, Jenkins NA, Huang JD. Transgenic mice expressing Cre-recombinase specifically in retinal rod bipolar neurons. Invest Ophthalmol Vis Sci. 2005;46(10):3515–3520. doi: 10.1167/iovs.04-1201. [DOI] [PubMed] [Google Scholar]
- 114.McNeill DS, Sheely CJ, Ecker JL, Badea TC, Morhardt D, Guido W, Hattar S. Development of melanopsin-based irradiance detecting circuitry. Neural Dev. 2011;6:8. doi: 10.1186/1749-8104-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hoon M, Okawa H, Della Santina L, Wong RO. Functional architecture of the retina: development and disease. Prog Retin Eye Res. 2014;42:44–84. doi: 10.1016/j.preteyeres.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003;39(1):85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- 117.Sernagor E, Eglen SJ, Wong RO. Development of retinal ganglion cell structure and function. Prog Retin Eye Res. 2001;20(2):139–174. doi: 10.1016/s1350-9462(00)00024-0. [DOI] [PubMed] [Google Scholar]
- 118.Osterhout JA, El-Danaf RN, Nguyen PL, Huberman AD. Birthdate and outgrowth timing predict cellular mechanisms of axon target matching in the developing visual pathway. Cell Rep. 2014;8(4):1006–1017. doi: 10.1016/j.celrep.2014.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rao S, Chun C, Fan J, Kofron JM, Yang MB, Hegde RS, Ferrara N, Copenhagen DR, Lang RA. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature. 2013;494(7436):243–246. doi: 10.1038/nature11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Renna JM, Weng S, Berson DM. Light acts through melanopsin to alter retinal waves and segregation of retinogeniculate afferents. Nat Neurosci. 2011;14(7):827–829. doi: 10.1038/nn.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tufford AR, Onyak JR, Sondereker KB, Lucas JA, Earley AM, Mattar P, Hattar S, Schmidt TM, Renna JM, Cayouette M. Melanopsin retinal ganglion cells regulate cone photoreceptor lamination in the mouse retina. Cell Rep. 2018;23(8):2416–2428. doi: 10.1016/j.celrep.2018.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Caval-Holme F, Zhang Y, Feller MB (2019) Gap junction coupling shapes the encoding of light in the developing retina. Curr Biol 29(23):4024–4035 e4025. 10.1016/j.cub.2019.10.025 [DOI] [PMC free article] [PubMed]
- 123.Muller LP, Do MT, Yau KW, He S, Baldridge WH. Tracer coupling of intrinsically photosensitive retinal ganglion cells to amacrine cells in the mouse retina. J Comp Neurol. 2010;518(23):4813–4824. doi: 10.1002/cne.22490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reifler AN, Chervenak AP, Dolikian ME, Benenati BA, Li BY, Wachter RD, Lynch AM, Demertzis ZD, Meyers BS, Abufarha FS, Jaeckel ER, Flannery MP, Wong KY. All spiking, sustained ON displaced amacrine cells receive gap-junction input from melanopsin ganglion cells. Curr Biol. 2015;25(21):2763–2773. doi: 10.1016/j.cub.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Arroyo DA, Feller MB. Spatiotemporal features of retinal waves instruct the wiring of the visual circuitry. Front Neural Circuits. 2016;10:54. doi: 10.3389/fncir.2016.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fernandez DC, Komal R, Langel J, Ma J, Duy PQ, Penzo MA, Zhao H, Hattar S. Retinal innervation tunes circuits that drive nonphotic entrainment to food. Nature. 2020 doi: 10.1038/s41586-020-2204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zaidi FH, Hull JT, Peirson SN, Wulff K, Aeschbach D, Gooley JJ, Brainard GC, Gregory-Evans K, Rizzo JF, 3rd, Czeisler CA, Foster RG, Moseley MJ, Lockley SW. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol. 2007;17(24):2122–2128. doi: 10.1016/j.cub.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mure LS, Cornut PL, Rieux C, Drouyer E, Denis P, Gronfier C, Cooper HM. Melanopsin bistability: a fly's eye technology in the human retina. PLoS ONE. 2009;4(6):e5991. doi: 10.1371/journal.pone.0005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hankins MW, Lucas RJ. The primary visual pathway in humans is regulated according to long-term light exposure through the action of a nonclassical photopigment. Curr Biol. 2002;12(3):191–198. doi: 10.1016/s0960-9822(02)00659-0. [DOI] [PubMed] [Google Scholar]
- 130.Brown TM, Tsujimura S, Allen AE, Wynne J, Bedford R, Vickery G, Vugler A, Lucas RJ. Melanopsin-based brightness discrimination in mice and humans. Curr Biol. 2012;22(12):1134–1141. doi: 10.1016/j.cub.2012.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vandewalle G, van Ackeren MJ, Daneault V, Hull JT, Albouy G, Lepore F, Doyon J, Czeisler CA, Dumont M, Carrier J, Lockley SW, Collignon O. Light modulates oscillatory alpha activity in the occipital cortex of totally visually blind individuals with intact non-image-forming photoreception. Sci Rep. 2018;8(1):16968. doi: 10.1038/s41598-018-35400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Allen AE, Martial FP, Lucas RJ. Form vision from melanopsin in humans. Nat Commun. 2019;10(1):2274. doi: 10.1038/s41467-019-10113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, Burstein R. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13(2):239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vandewalle G, Maquet P, Dijk DJ. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13(10):429–438. doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 135.Dhande OS, Stafford BK, Franke K, El-Danaf R, Percival KA, Phan AH, Li P, Hansen BJ, Nguyen PL, Berens P, Taylor WR, Callaway E, Euler T, Huberman AD. Molecular fingerprinting of On-Off direction-selective retinal ganglion cells across species and relevance to primate visual circuits. J Neurosci. 2019;39(1):78–95. doi: 10.1523/JNEUROSCI.1784-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Graham DM, Wong KY, Shapiro P, Frederick C, Pattabiraman K, Berson DM. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J Neurophysiol. 2008;99(5):2522–2532. doi: 10.1152/jn.01066.2007. [DOI] [PubMed] [Google Scholar]
- 137.Hartwick AT, Bramley JR, Yu J, Stevens KT, Allen CN, Baldridge WH, Sollars PJ, Pickard GE. Light-evoked calcium responses of isolated melanopsin-expressing retinal ganglion cells. J Neurosci. 2007;27(49):13468–13480. doi: 10.1523/JNEUROSCI.3626-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Johnson J, Wu V, Donovan M, Majumdar S, Renteria RC, Porco T, Van Gelder RN, Copenhagen DR. Melanopsin-dependent light avoidance in neonatal mice. Proc Natl Acad Sci USA. 2010;107(40):17374–17378. doi: 10.1073/pnas.1008533107. [DOI] [PMC free article] [PubMed] [Google Scholar]