Abstract
Background:
The Population-based HIV Impact Assessment (PHIA) surveys are among the first to estimate national adult HIV incidence, subnational prevalence of viral load suppression (VLS) and pediatric HIV prevalence. We summarize the survey methods implemented in Zimbabwe, Malawi, and Zambia, as well as response rates and quality metrics.
Methods:
Each cross-sectional, household-based survey used a two-stage cluster design. Survey preparations included sample design, questionnaire development, tablet programming for informed consent and data collection, community mobilization, establishing a network of satellite laboratories, and fieldworker training. Interviewers collected demographic, behavioral, and clinical information using tablets. Blood was collected for home-based HIV testing and counselling (HBTC) and point-of-care (PoC) CD4+ T-cell enumeration with results immediately returned. HIV-positive blood samples underwent laboratory-based confirmatory testing, HIV incidence testing, RNA PCR (viral load), DNA PCR (early infant diagnosis), and serum antiretroviral drug (ARV) detection. Data were weighted for survey design and chi square automatic interaction detection (CHAID)-based methods were used to adjust for non-response.
Results:
Each survey recruited a nationally representative, household-based sample of children and adults over a 6–10-month period in 2015 and 2016. Most (84%–90%) of the 12,000–14,000 eligible households in each country participated in the survey, with 77%–81% of eligible adults completing an interview and providing blood for HIV testing. Among eligible children, 59%–73% completed HIV testing. Across the three surveys, 97.8% of interview data were complete and had no errors.
Conclusion:
Conducting a national population-based HIV impact assessment with immediate return of HIV and other PoC test results was feasible and data quality was high.
Keywords: Population-based Surveys, HIV incidence, HIV Viral Load Suppression, Impact Assessment, Methods, Southern Africa
Introduction
The past decade has seen remarkable progress in controlling the global HIV epidemic. Between 2010 and 2017, the number of people on antiretroviral therapy (ART) worldwide nearly tripled from 7.5 million [1] to 21.7 million. [2] The progress has been substantial in Eastern and Southern Africa, where estimated coverage of ART among people living with HIV (PLHIV) increased from 24% to 66% between 2010 and 2017. [2] As of 2019, ART coverage among adults living with HIV in Zimbabwe, Malawi, and Zambia was estimated at 62%, 61%, and 63% respectively, based on programmatic data from healthcare facilities who provide ART as part of the national response. [3] However, these estimates did not directly account for the HIV-positive individuals who were not accessing HIV care and treatment at health facilities.
To address this gap through comprehensive national estimates, the Population-based HIV Impact Assessment (PHIA) surveys are evaluating the status of the HIV epidemic and the impact of HIV treatment scale-up in high-burden countries. These surveys, funded by the President’s Emergency Plan for AIDS Relief and conducted by ICAP at Columbia University in partnership with ministries of health (MOH) and the United States Centers for Disease Control and Prevention (CDC), provide direct measures of national HIV incidence among adults, as well as subnational viral load suppression (VLS). These data allow each country to identify gaps among sub-populations and benchmark their progress towards the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90–90-90 goals, namely that 90% of all PLHIV know their status, 90% of those who know their status receive ART, and 90% of those on ART achieve VLS. Modelled estimates suggest that if these goals are met by 2020 and sustained for ten years, the annual number of new HIV infections and AIDS-related deaths would each fall by 90% and 80%, respectively, by 2030. [4] Data from the PHIA surveys are critical to assess whether national and regional level programs are making progress towards epidemic control.
Previous nationally representative, population-based surveys in Zimbabwe [5], Zambia [6], and Malawi [7] measured HIV prevalence but have not estimated HIV incidence, awareness of HIV status, ART coverage, or VLS, all indicators that are needed to measure progress towards the 90-90-90 goals and epidemic control. Two nationally representative surveys, the 2011 Swaziland HIV Incidence Measurement Survey (SHIMS) [8, 9] and the 2012 Kenya AIDS Indicator Survey (KAIS) [10] included home-based HIV counseling and testing (HBTC) with return of results (RoR) and measured ART coverage, prevalence of VLS, and HIV incidence. These surveys set a new standard for detailed population-based HIV data and laid the foundation for the PHIA surveys. The PHIA surveys have built on the protocols, strategies, and procedures of SHIMS and KAIS, including the use of HBTC and RoR at the household level, in order to assess HIV impact indicators in additional countries affected by the HIV epidemic.
This paper describes the methods used in the first three PHIA surveys, implemented in Zimbabwe, Malawi, and Zambia, as well as response rates and selected quality metrics.
Methods
Objectives
The primary objectives of the PHIA surveys in Zimbabwe, Malawi, and Zambia were to estimate national HIV incidence and sub-national prevalence of VLS, defined as HIV RNA <1000 copies/ml, both among adults. Secondary objectives varied between the countries but included estimating subnational adult HIV prevalence, national pediatric HIV prevalence, and demographic and behavioral risk factors for HIV. Among HIV-positive adults, the surveys also estimated the prevalence of detectable antiretroviral (ARV) medications and transmitted drug resistance as well as the distribution of CD4+ T-cell counts. Zimbabwe and Zambia estimated the prevalence of active and past syphilis infection in adults. Zambia measured the prevalence of chronic active hepatitis B infection in adults as well (Table 1).
Table 1:
Summary of Key Objectives for Population-based HIV Impact Assessments in Zimbabwe, Malawi, and Zambia, 2015 – 2016
| Zimbabwe | Malawi | Zambia | |
|---|---|---|---|
| Adult HIV incidence | Primary | Primary | Primary |
| Adult VLS | Primary (sub-national) | Primary (sub-national) | Primary (sub-national) |
| Pediatric VL suppression | Secondary | Secondary | Secondary |
| Pediatric HIV prevalence | Secondary | Secondary | Secondary |
| Adult HIV prevalence | Secondary | Secondary | Secondary |
| CD4+ count | Secondary | Secondary | Secondary |
| Prevalence of detectable antiretrovirals (ARVs) | Secondary | Secondary | Secondary |
| Prevalence of transmitted drug resistance (DR) | Secondary | Secondary | Secondary |
| Adult prevalence of active and previous syphilis infection | Secondary | N/A | Secondary |
| Prevalence of hepatitis B surface antigen | N/A | N/A | Secondary |
| Nutritional assessment for children under 5 years using weight and height measurements | Secondary | Secondary | Secondary |
Through household and individual interviews, the surveys assessed HIV indicators related to care and treatment, behavioral risks factors, knowledge, and stigma. The questionnaires also included questions related to tuberculosis (TB) diagnosis, care and treatment, cervical cancer screening, symptoms of sexually transmitted infections, intimate partner violence, and orphans and vulnerable children (OVCs). Zimbabwe and Zambia assessed peer norms, parental support, HIV prevention interventions, HIV testing, and HIV knowledge among adolescents, ages 10–14 years.
In Zimbabwe, 4,000 randomly selected participants were invited to complete a Computer Assisted Self Interview module repeating 26 sensitive questions from the main interview in order to compare responses to these questions using face-to-face versus anonymous question delivery methods.
Study Design and Sample Size
The PHIA surveys employed a cross-sectional, two-stage, cluster sampling design to obtain a nationally representative sample of individuals living in households in each country. For the first-stage sampling, 500 census enumeration areas (EAs) each in Malawi and Zimbabwe, and 515 EAs in Zambia were selected using probability proportional to estimated size sampling based on the most recent census. The sample was stratified by area of residence (urban/rural) and subnational area. A household was defined as a group of individuals who reside in a physical structure such as a house, apartment, compound, or homestead, and share housekeeping arrangements. All households within the boundaries of the selected EA were listed by trained staff in January 2015 for Zimbabwe, April 2015 for Malawi, and August-September 2015 for Zambia. In Zimbabwe, household listing data were compiled on a paper form and EA maps were sketched by hand. In Malawi and Zambia, household listing data were collected using a tablet application that recorded the Global Positioning System (GPS) coordinates of each household and EA centroid. In the second stage of sampling, households were selected from each EA using an equal probability approach that allowed the number of selected households to vary based on the total number of households in the EA. On average, 30 households were selected per EA, with a minimum of 15 households in all three countries and a maximum of 60 in Zimbabwe and Malawi and 50 in Zambia.
Survey sample sizes were powered to provide annualized national HIV incidence estimates among adults, aged 15–49 years, with a relative standard error (RSE) of 30% or less, and subnational prevalence of VLS among HIV-positive adults, aged 15–49 years, with 95% confidence intervals (95% CIs) of ±10% (no more than 10% greater and less than the point estimate) in each subnational area. In Malawi, high-prevalence zones in the south of the country were oversampled to obtain more precise estimates with 95% CIs of ±5–8% (no more than 5–8% greater and less than the point estimate). The number of selected households needed to achieve target precision levels for these two measures were estimated based on subnational population counts, HIV prevalence, and response rates from previous national, population-based surveys of the three countries. All eligible adults in selected households were asked to participate in the survey. To provide a national estimate of pediatric HIV prevalence with an RSE of 20% or less, half of the sampled households in each country were randomly selected for the inclusion of children aged 0–14 years in the biomarker component of the survey. In Zimbabwe and Zambia, all adolescents aged 10–14 were invited to participate in the interview component.
Eligibility, Consent and Recruitment
At selected households, field staff introduced the survey to the household head and obtained his/her consent for the household interview. Eligibility criteria for individual participants included having slept in the selected household the night before the survey, being literate or providing a literate witness in one of the survey languages, and being willing and able to provide consent. Adults self-reporting their age as 15 years and older in Zimbabwe, 15–64 years in Malawi and 15–59 years in Zambia were eligible to participate. Emancipated minors, as defined by local law, were also eligible to participate as adults even if their recorded age was less than 15 years. In households sampled for children, children aged 0–14 years were eligible to participate if a parent or guardian provided consent or permission as described below. PHIA field teams returned to selected households, varying days and times of re-visits, up to three times to recruit participants who may have been unavailable on prior visits.
Written informed consent was documented via electronic signature on the data collection tablet (described below) at each of three stages: the head-of-household interview, individual interview, and blood draw for biomarker testing. Older minors (see Table 2 for ages) were asked to provide written assent for the interview and blood draw components after permission was granted by their parent or guardian. Parents were asked to provide written permission for minors below the age of assent. Refusal at any stage ended survey procedures for that household member.
Table 2:
Interview1 instruments and ages of consent, assent and disclosure for Population-based HIV Impact Assessments in Zimbabwe, Malawi, and Zambia, 2015 – 2016
| Zimbabwe | Malawi | Zambia | |
|---|---|---|---|
| Household Interview | X | X | X |
| Adult Interview | X | X | X |
| Adolescent Interview (Ages 10–14) | X | Not done | X |
| Computer Assisted Self Interview (CASI) Module | X | Not done | Not done |
| Age of consent2 | 16 years | 18 years | 18 years |
| Age of assent2 | 7–15 years | 10–17 years | 10–17 years |
| Age of disclosure3 | 15 years | 18 years | 18 years |
All interviews were administered face to face with the exception of CASI which was supported by survey staff.
Consent/assent: informed consent or assent to participate with full knowledge of risk and benefits was obtained from all participants. Assent (approval or agreement) was obtained from children between the ages of 10–17 after parental permission was obtained.
Disclosure indicates the age at which participants could be informed of their HIV status without a parent/guardian present.
All participants were informed during the consent process that they would receive the rapid HIV test result as part of the survey. HIV testing without RoR was not offered. Participants worked with survey staff to identify a health facility to which results of additional clinically actionable testing, including viral load, early infant diagnosis (EID) and drug resistance, could be returned. Mobile phone numbers were collected from consenting participants in order to notify them when results were available. Finally, participants were asked for written consent to contact them for future studies and to store their blood samples in a repository for future testing.
Training
PHIA field staff included laboratory coordinators, laboratory technicians, regional field work coordinators, team leaders, interviewers, HIV testers and counsellors, community mobilization coordinators (CMCs), and drivers. In all countries, field teams included two interviewers and four staff qualified to conduct biomarker procedures and RoR in addition to a team leader and driver. Each team was assigned the task of collecting all the survey data in a set of sampled EAs.
A two-month training program, supplemented by refresher trainings, was critical in ensuring procedural fidelity and data quality. The survey training process began with a training of trainers for local facilitators on survey procedures and tablet use. This was followed by two weeks of training interviewers on survey procedures, interviewing skills and electronic data capture. CMCs received three days of training on community mobilization procedures and in turn trained Community Mobilizers (CMs) in each survey area. Supervisors and team leaders attended two to three days of survey management training in addition to participating in the two-week interviewer training sequence. At the end of training, each field staff member participated in at least two days of field practice to pilot all survey procedures in one or two EAs that had not been selected for the survey. Laboratory staff training is described in detail by Patel et al. 2019. [11]
The total number of staff trained included 245 field staff, 16 Satellite Lab Technicians, 4 Regional Coordinators, 16 CMCs, and roughly 1,000 CMs in Zimbabwe; 150 field staff, 16 Satellite Lab Technicians, 6 Regional Coordinators, 18 CMCs, and roughly 1000 CMs in Malawi; and 330 field staff, 48 Satellite Lab Technicians, 32 Regional Coordinators, 16 CMCs, and roughly 1,000 CMs in Zambia.
Community Mobilization
Materials for publicizing the survey and encouraging participation, such as brochures and posters, were disseminated at national, sub-national, and community levels. Each country held a national launch accompanied by press releases, media interviews, and the airing of songs [12], jingles, TV spots, and radio announcements explaining the purpose and procedures of the survey. Activities within communities were organized by CMCs over the duration of the survey. Approximately one week before field teams entered a selected EA, CMCs conducted a brief training with, on average, two community health workers in each EA to work as local CMs. Together, CMCs and CMs met with community leaders to explain the survey and secure support, went door-to-door and organized community meetings to share information and answer questions. CMs disseminated written materials and, in some cases, organized music and drama performances about the survey. Additional efforts were made to sensitize young people and men through informational activities in schools with youth groups and in targeted locations where men were likely to congregate, such as marketplaces and sporting events.
Data Collection
The PHIA survey staff conducted interviews in a private location in or around selected homes using Google Nexus 9 tablets (HTC, Taipei, Taiwan). The tablets were password-protected, and a data collection application programmed with Open Data Kit 1.4.5 (ODK)-hosted eligibility screening forms, consent forms with space to record electronic signatures, household and individual questionnaires and PoC biomarker results and refusal forms. A separate application, Maps.me, stored interactive maps with information on the location of selected households.
The wording and flow of questionnaires were informed by established surveys (Demographic and Health Surveys [5–7], and KAIS 2012 [13]), cognitive interviews, and pre-testing conducted in Zimbabwe and Malawi. Questionnaires, consent forms, and other tools used with participants were translated from English into local languages (2 in Malawi, 2 in Zimbabwe, and 8 in Zambia). Where possible, participants and interviewers were matched by gender and age.
The household questionnaire collected information on household residents, assets, economic support, and recent deaths. The individual adult interview included modules on antenatal care, HIV care and treatment indicators, male circumcision, and HIV knowledge and stigma. Parents and guardians answered questions about their children’s health and participation in HIV testing and care services. Behavioral risk factors associated with HIV prevalence and incidence, as well as questions related to TB infection, cervical cancer, and information about OVCs were also included. In Zimbabwe and Zambia, adolescent participants, aged 10–14 years, were interviewed with age-appropriate questions on peer social norms, parental support, HIV prevention interventions, alcohol and drug use, HIV testing, and HIV knowledge.
A module designed to assess experiences of violence was included in the individual adult and adolescent questionnaires. One randomly selected adult woman per household (in households with at least one female participant) was asked about her experiences of violence, and all children, aged 13–14 years (12–14 years in Zambia), were asked about their experiences of violence using questions adapted from the Violence Against Children Survey (VACS)[14]. All participants disclosing experience with violence were referred to the national social service system.
The PHIA questionnaires were designed to ensure alignment with key concepts in prior demographic and health surveys and included questions needed for the measurement of standard national indicators.
Collection of Blood Samples and Anthropometric Assessments
All blood samples were collected by MOH-credentialed and trained nurses or laboratory technicians who were part of each PHIA field team. Whole blood was collected from adults and children (aged 2–14 years) via venipuncture. For infants and young children, capillary blood was collected via heel stick (0–6 months) or finger prick (7–24 months). Among participants for whom venous blood draw was not possible, capillary blood was collected using a finger prick. Bar-coded labels with a unique participant ID (PTID) were affixed to each blood tube as well as to a sample tracking form that accompanied the blood specimens from the field to the laboratory for processing.
Children under the age of five years who tested HIV positive or who were HIV exposed were assessed for undernutrition using weight and height measurements. Weight was measured with a flat, electronic SECA 874 Mother and Baby scale. Using a ShorrBoard®, recumbent height was measured for children under 24 months while standing height was measured for children two years and older. Five percent of HIV-negative children were also assessed for nutritional status to avoid revealing the HIV status of those selected and to establish a baseline for comparison. Children who met World Health Organization criteria for undernutrition were referred to the nearest health facility.
Biomarker Testing
Detailed information on training, procedures, specimen transport, and quality assurance for PHIA biomarker testing can be found in Patel et al. 2019 [11]. In brief, biomarker testing was conducted at four locations: households, satellite laboratories, central laboratory, and international laboratories. At the household level, participants over the age of 18 months received HBTC using the national HIV rapid testing algorithm. Blood samples from HIV-positive participants underwent PoC CD4+ T-cell enumeration using the Alere PIMA Analyzer (Abbott Molecular Inc., Des Plaines, IL, United States). PoC CD4+ T-cell enumeration was also performed on 5% of randomly-selected HIV-negative participants to reduce the potential stigma of CD4+ cell count testing. Blood samples from infants under 18 months were screened with Determine™ HIV-1/2 (Abbott Molecular Inc., Des Plaines, IL, United States). Infants who tested seropositive for HIV were immediately referred to care while their blood samples underwent confirmatory HIV DNA PCR testing at a central laboratory. All blood samples from adult participants in Zimbabwe and Zambia underwent PoC syphilis testing (DPP® Syphilis Screen & Confirm Assay, Chembio Diagnostics, Medford, NY, United States) which distinguished between active and previous infections. In Zambia, blood samples from participants ages 0–59 years were tested for evidence of chronic active hepatitis B virus infection using the PoC Determine™ Hepatitis B Surface Antigen (HBsAg) rapid test (Abbott Molecular Inc., Des Plaines, IL, United States). Syphilis and HBsAg PoC results were returned during the household visit and participants with positive test results were referred for care and treatment.
In satellite laboratories, established within MOH district laboratories, health facilities, and, in Zambia, mobile laboratories, all HIV-positive samples underwent confirmatory testing using Geenius HIV 1/2 Supplemental Assay (Bio-Rad, Hercules, CA, United States) and quality assurance testing.
In one central laboratory in each country, all confirmed HIV-positive samples underwent HIV incidence testing using the HIV-1 Limiting Antigen Avidity enzyme immunoassay (Sedia Biosciences Corporation, Portland, OR, United States). Samples from HIV-positive adults and HIV-exposed infants underwent HIV RNA and HIV DNA testing respectively using the Abbott m2000 System (Abbott Molecular Inc., Chicago, IL, United States). HIV RNA and HIV DNA results were returned to the participant’s preferred healthcare facility.
Testing for resistance to ARVs and qualitative screening for detectable concentrations of selected ARVs (based on country guidelines, used as an indicator of participant use of a given drug at the time of blood collection) were conducted at the International Laboratory Branch of the CDC and the laboratory in the Department of Clinical Pharmacology of the Department of Medicine at the University of Cape Town in South Africa, respectively, using standard methods and references. ARV results were used in the HIV recency testing algorithm (along with other assays) to distinguish recent from long-term HIV infections and to adjust self-reported population-based measures (eg. ART uptake, awareness of status, etc) Drug resistance results were returned to MOH-led Technical Working Groups who advised clinicians on how to interpret and respond in line with national guidelines.
Referrals and Return of Results
All participants and parents or guardians of participating children received their HIV test results in writing to take to a health facility for follow-up care. The form included the participant’s PTID so that the participant could be linked to additional results, such as viral load and EID, at the health facility. Viral load and EID results were sent to the health facility within approximately 6–10 weeks linked to the PTID to protect confidentiality.
Nurses or counselors on each team shared a list of local health facilities and social service providers with all participants. Participants testing positive for any biomarkers were encouraged to commit to a plan to seek care. Participants reporting an experience with violence or people under the age of 18 years who reported abuse including experience with commercial sex were offered to have their name shared with a local social service organization that could provide additional support.
Data Architecture & Data Management
To minimize errors in field work, the ODK tablet application used for consent forms, interview data, and PoC biomarker data collection was pre-programmed with skip patterns, range checks, and logical constraints to ensure the internal consistency of the data. Unique PTIDs pre-printed on barcode labels were scanned directly to both the tablet and the CD4 machine to reduce transcription errors. Figure 1 details the data architecture and flow of data from the field and the respective laboratories and to a cloud server.
Figure 1: Data Architecture, for Population-based HIV Impact Assessments in Zimbabwe, Malawi, and Zambia, 2015 – 2016.
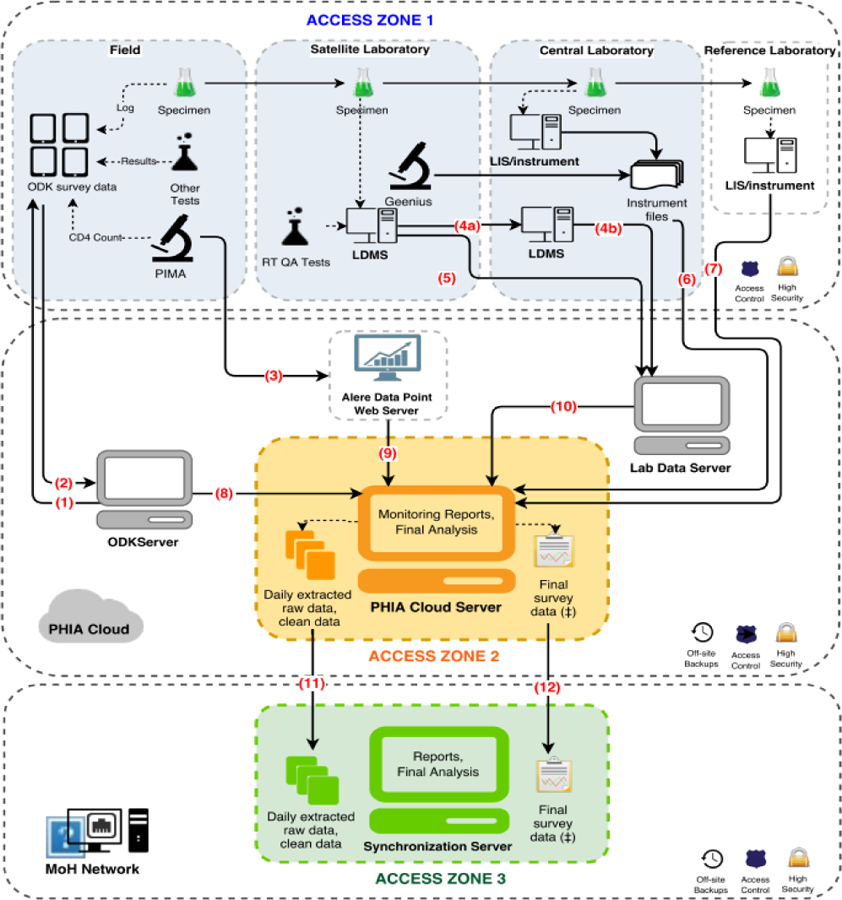
At completion of all study activities in the household, supervisors uploaded data from the tablets (1) to a secure cloud server via a Wi-Fi or 3G network connection using a pocket wireless router (2). CD4+ cell counts and their associated participant identification number (PTIDs) were uploaded from the CD4 PIMA analyzer via Wi-Fi or 3G network to a secure cloud server (3) and subsequently merged with the survey database. Both satellite and central laboratories used a Laboratory Data Management System (LDMS) (Frontier Science, Boston, Massachusetts) database to track specimen receipt, processing, freezing times, quantity, quality assurance testing data, storage location. and shipment details which were transmitted to Frontier Science using encrypted flash drives (4a, 4b) or directly (5). Central laboratory-based test results, including viral load, early infant diagnosis, and HIV recency were either pulled directly from the local laboratory information management system (LIMS) or sent in files extracted from the test instruments and uploaded to a secure FTP server and appended to the database (6, 7). Questionnaire data from the ODK server, CD4 data and LDMS quality assurance/quality control (QA/QC) data that went through intermediary servers were all linked with the overall database (8, 9, 10). The overall survey database was sent daily to an in-country server for local stakeholders to access and monitor (11). After completion of data cleaning, finalized data was also transferred to an in-country server (12).
Throughout the study, investigators in all locations had access to analytical files produced from the study database allowing review of data while the survey was in progress. The centralized survey database also populated an electronic dashboard reporting process indicators daily. These included coverage and completion of EAs and sampled households, household response, eligible household members providing consent to the interview and biomarker components of the survey, number of blood draws, and response rates both survey-wide and by team. The dashboard also summarized biomarker data, including the quantity and quality of specimens as well as preliminary results of tests. The aggregation of data from tablets and laboratory information systems into a central server and display of summarized process indicators on a dashboard allowed near real-time monitoring and prompt troubleshooting of survey activities.
Weighting and Statistical Analysis
Following data cleaning to eliminate duplicates and correctly link all household, individual interview and biomarker responses and test results, PHIA data were weighted to account for selection probabilities, nonresponse and noncoverage. Probabilities of selection were calculated hierarchically for EAs, households, individual interviews, and blood draws. Weighting adjustments for individual interview nonresponse and blood draw nonresponse were based on a combination of variables available for both participants and nonparticipants that were potential predictors of survey response and HIV status. The nonresponse adjustment cells were constructed using a chi-square automatic interaction detection (CHAID) algorithm with the SI-CHAID software® (Statistical Innovations, Belmont, MA, United States). Post-stratification adjustments were also implemented to compensate for noncoverage in the sampling process. This final adjustment calibrated the weighted survey population to national population totals by sex and five-year age groups based on population projections provided by each national statistical bureau.
Jackknife replicate weights were generated for variance estimation and used for all PHIA estimates unless otherwise specified. Taylor series weights were also generated as an alternative for variance estimation.
All steps of data cleaning and weighting were performed and verified by two independent teams.
Ethical Approval
All PHIA survey protocols, consent forms, screening forms, refusal forms, referral forms, recruitment materials, and questionnaires were reviewed and approved by in-country ethics and regulatory bodies and the institutional review boards (IRBs) of Columbia University Medical Center, Westat, and the CDC. External monitoring was conducted twice per survey. Survey management teams conducted continuous surveillance for protocol deviations and adverse events which were promptly reported to IRBs.
Results
Survey Response Rate
Data collection took place from October 18, 2015 to August 5, 2016 in Zimbabwe, from November 27, 2015 to August 28, 2016 in Malawi, and from March 1 to August 31, 2016 in Zambia.
The weighted household response rate, defined as the percentage of selected, occupied dwellings for which the head of household completed a household interview, was 83.9% among eligible households in Zimbabwe, 90.2% in Malawi, and 89.4% in Zambia. The weighted percentage of eligible adults completing an interview was 88.8% in Zimbabwe, 88.5% in Malawi, and 86.0% in Zambia. The weighted percentage of eligible adults completing an interview and providing a blood sample was 80.9% in Zimbabwe, 77.1% in Malawi, and 77.0% in Zambia. The weighted percentage of eligible adolescents, ages 10–14 years, completing a questionnaire was 78.8% in Zimbabwe and 76.8% in Zambia. The weighted percentage of children ages 0–14 providing a blood sample was 72.8% in Zimbabwe, 58.9% in Malawi, and 67.8% in Zambia (Table 3).
Table 3:
Interview and Blood Response by country for Population-based HIV Impact Assessments in Zimbabwe, Malawi, and Zambia, 2015 – 2016
| Total Eligible for participation, N | % Completing an Interview among total eligible (weighted) | % Completing an Interview AND Providing Blood Sample among total eligible (weighted) | |
|---|---|---|---|
| ZIMBABWE | |||
| Households | |||
| Urban | 4419 | 77.9 | |
| Rural | 9409 | 86.9 | |
| TOTAL households 1 | 13828 | 83.9 | |
| Individuals | |||
| Children (0–14 years) | 9599 | 78.82 | 72.8 |
| Adults (15–64 years) | 25131 | 88.8 | 80.9 |
| -Gender | |||
| Male | 11098 | 82.3 | 74.3 |
| Female | 14033 | 93.9 | 86.3 |
| -Residence | |||
| Urban | 7932 | 83.5 | 75.1 |
| Rural | 17199 | 91.7 | 84.2 |
| MALAWI | |||
| Households | |||
| Urban | 4689 | 85.1 | |
| Rural | 8042 | 91.2 | |
| TOTAL households 1 | 12731 | 90.2 | |
| Individuals | |||
| Children (0–14 years) | 9993 | 58.9 | |
| Adults (15–64 years) | 22405 | 88.5 | 77.1 |
| -Gender | |||
| Male | 10170 | 82.7 | 72.2 |
| Female | 12235 | 93.2 | 81.0 |
| -Residence | |||
| Urban | 8811 | 84.3 | 73.8 |
| Rural | 13594 | 89.6 | 77.9 |
| ZAMBIA | |||
| Households | |||
| Urban | 4989 | 89.5 | |
| Rural | 7204 | 89.2 | |
| TOTAL households 1 | 12193 | 89.4 | |
| Individuals | |||
| Children (0–14 years) | 11646 | 76.82 | 67.8 |
| Adults (15–59 years) | 24,679 | 86.0 | 77.0 |
| -Gender | |||
| Male | 11,354 | 80.4 | 71.2 |
| Female | 13,325 | 90.8 | 82.0 |
| -Residence | |||
| Urban | 11,224 | 82.2 | 73.9 |
| Rural | 13,455 | 89.2 | 79.7 |
AAPOR RR4 method of response rate calculation was used. https://www.aapor.org/AAPOR_Main/media/publications/Standard-Definitions2015_8theditionwithchanges_April2015_logo.pdf
Adolescent Interviews among eligible 10–14 year-olds only: N= 3000 in Zimbabwe; N= 3593 in Zambia
Among adults, the interview and blood response rate was higher among women and among people in rural areas. In Zimbabwe, Malawi and Zambia, respectively, 86.3%, 81.0%, and 82.0% of eligible women compared to 74.3%, 72.2%, and 71.2% of eligible men in consenting households gave blood. Likewise, among all those eligible to participate in the PHIA survey, a higher proportion of adults in rural areas, in Malawi and Zimbabwe particularly, completed an interview and gave blood as compared to those in urban areas (Table 3). The biomarker response rate among children aged 0–14 years was lower than adults in all countries (Table 3).
Figure 2 displays blood draw response rates by self-reported HIV status. In all three countries, over 95% of individuals who self-reported HIV positive during the interview consented to survey biomarker procedures including blood draw and HIV testing. A slightly lower percentage of those who self-reported HIV negative during the interview consented to biomarker procedures: 91% in Zimbabwe, 87% in Malawi, and 90% in Zambia.
Figure 2: Among adults interviewed, blood draw response rates by self-reported HIV status.
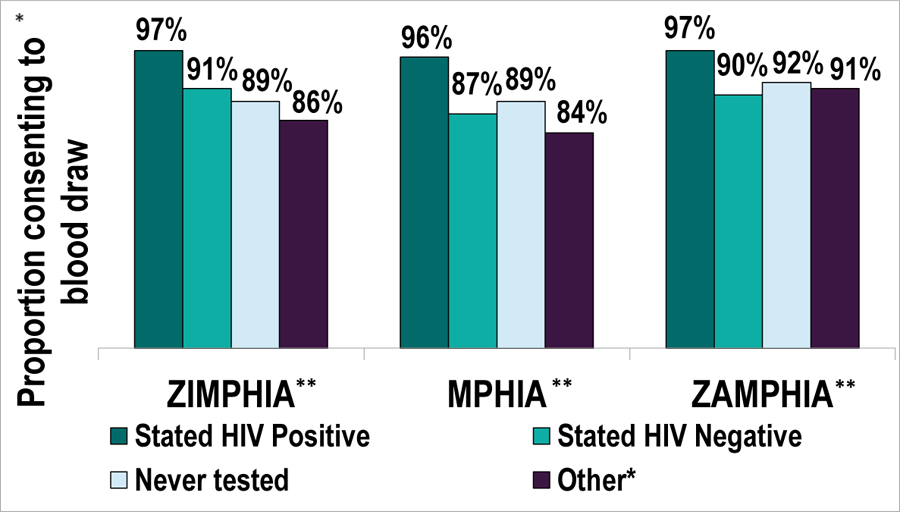
*Other includes the response categories refused, don’t know, and missing
** Zimbabwe Population-based HIV Impact Assessment (ZIMPHIA); Malawi Population-based HIV Impact Assessment (MPHIA); Zambia Population-based HIV Impact Assessment (ZAMPHIA)
The target sample sizes were 16,650 adults ages 15–64 and 7,309 children ages0–14 in Zimbabwe; 18,700 adults and 8,949 children ages 0–14 in Malawi, and 19,168 adults and 8,974 children ages 0–14 in Zambia. As seen in the Figure 3, Zimbabwe surpassed recruitment targets for adults by nearly 4,000 participants but fell short of the child target by 277 participants. Zambia recruited almost exactly the target number of adults (19,115) but fell short of the child target by 959 participants. Malawi fell 1,513 participants short of its adult target and 2,833 participants short of its child target.
Figure 3: Households and participants by country for Population-based HIV Impact Assessments in Zimbabwe, Malawi, and Zambia, 2015 – 2016.
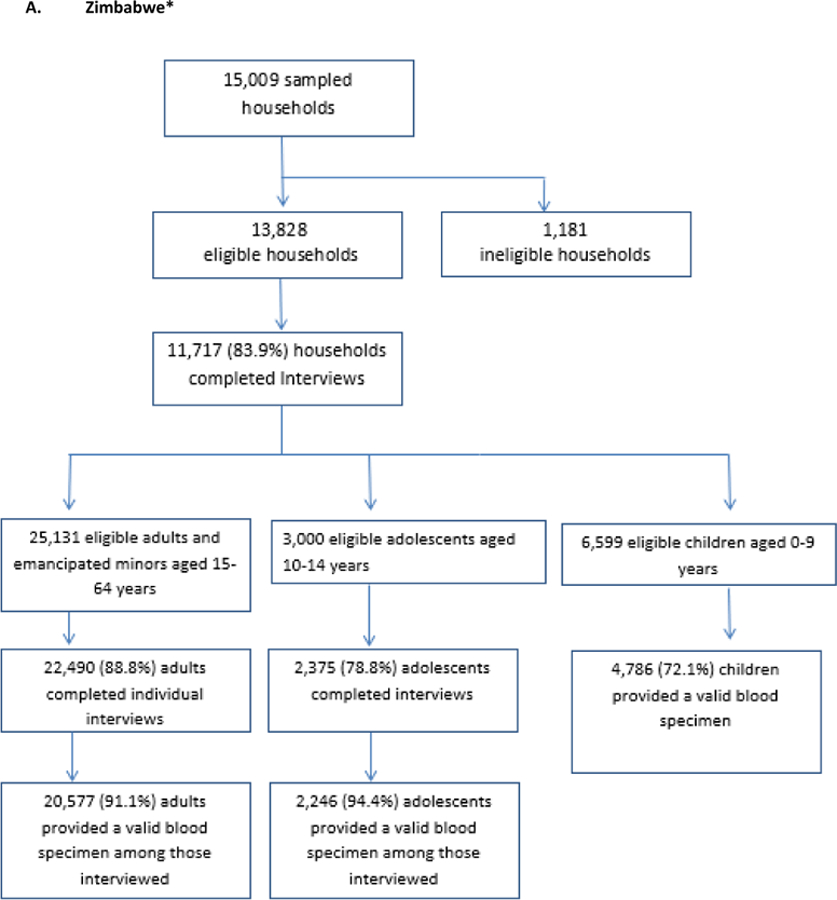
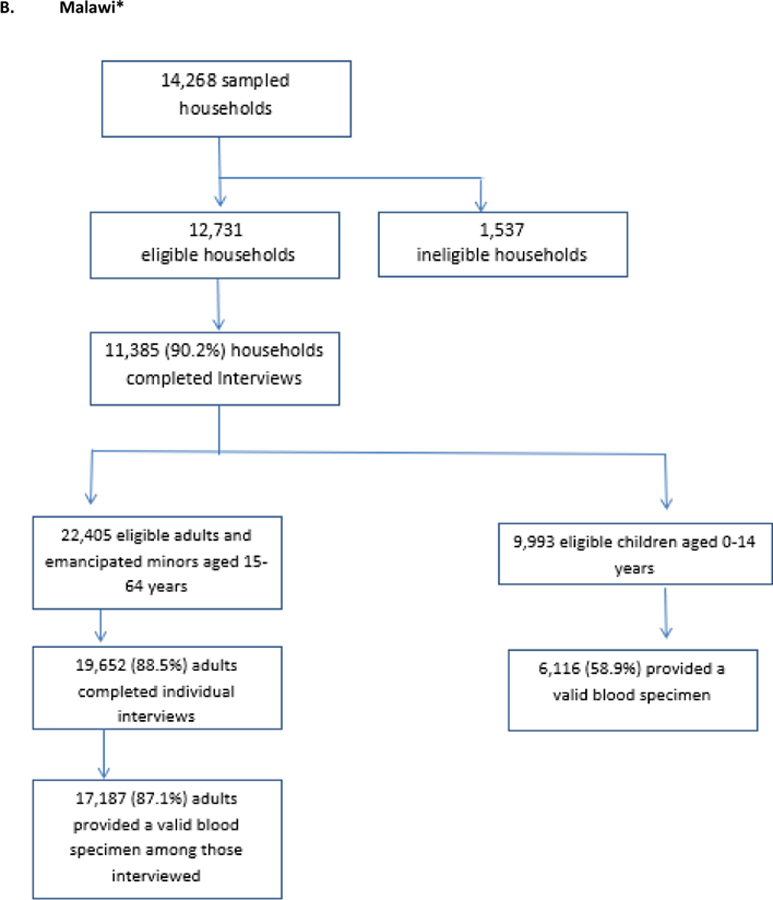
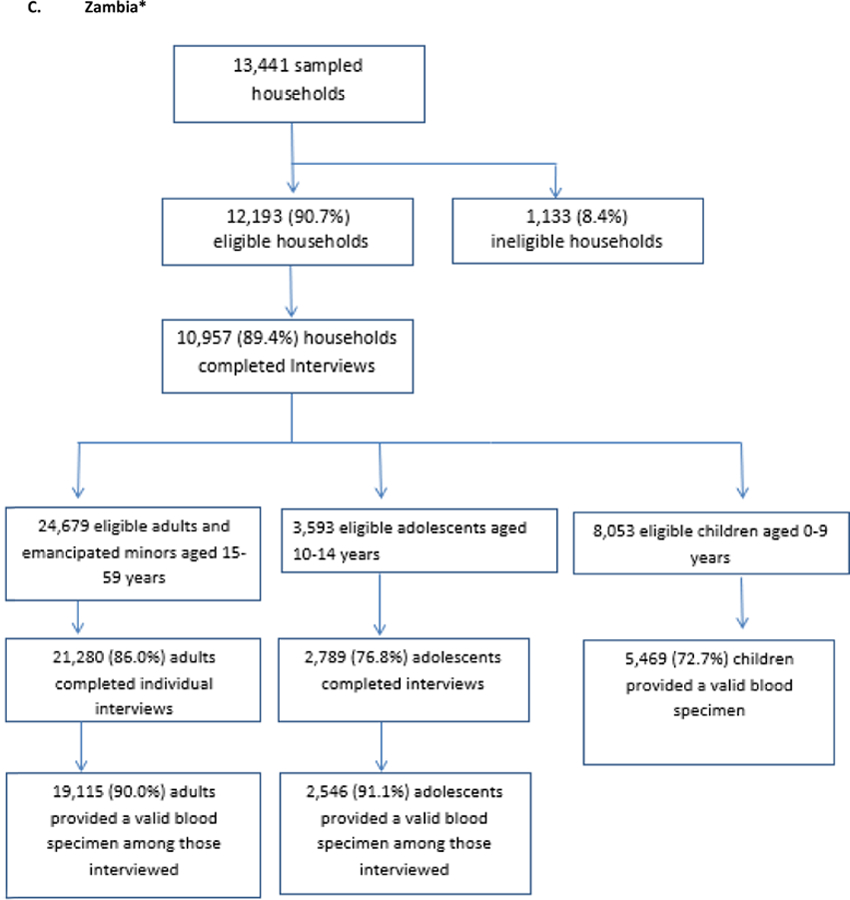
*all percentages are weighted percentages
Questionnaire Data Quality and Return of Results
Data quality and completeness was high in the first three PHIA surveys: 97.8% of individual questionnaires were completed without errors and 98.1% of household questionnaires were completed without errors in all three countries. This was facilitated by the tablet application which automated skip patterns, required responses to advance, and included range checks for many questions.
Discussion
The PHIA surveys built on the work of SHIMS and KAIS, bringing the population-based measurement of national HIV incidence among adults, prevalence of VLS and pediatric HIV prevalence to scale across Africa. The PHIA surveys have provided high-quality, nationally representative results that are guiding national and global HIV program planning. The response rates demonstrate the feasibility of estimating impact-level indicators of the HIV epidemic through population-based surveys with HBTC and universal RoR.
The PHIA surveys were novel in several respects. They were the first to estimate national HIV incidence as a primary objective and provincial prevalence of VLS alongside socio-demographic factors, history of clinical care, and behavioral risk factors in these three countries. They were also among the first to measure HIV prevalence for the entire pediatric age range including infants, as well as risk factors and knowledge and attitudes among adolescents ages 10–14 years old. Having high quality data on young people ages 10–24 years is particularly important, given the projected doubling of the 15–24-year-old population in sub-Saharan Africa by 2030 as compared to the start of the HIV epidemic. [15] The data from the large samples of adolescents and young adults in each surveyed country will assist in understanding epidemiological trends and risk factors among these age groups and guide prevention, testing, and treatment strategies.
Operationally, PHIA surveys were the first series of national household-based surveys to draw whole blood from participants with consistently high quality and to return all clinically-actionable test results. The PHIA surveys also included several PoC tests for the first time in national HIV impact assessment surveys including chronic and active syphilis, HBsAg and CD4 testing. Additional biomarkers including presence of ARVs and transmitted drug resistance were also novel measures in national surveys in these three countries.
The PHIA data architecture and electronic dashboard were innovations which facilitated high-quality fieldwork and data by enabling real time monitoring and quality control. The data architecture allowed data from multiple instruments and locations, including the field-based tablets, PoC analyzers, and laboratory testing platforms, to be merged into a single, secure, cloud-based database. Automated analyses of these aggregated survey data were presented through the PHIA dashboard, which study teams used to rapidly identify challenges and shortfalls in response and accrual rates, and, in turn, guide field teams with increased efficiency to meet targets.
The response rates observed in the first three PHIAs speak to the feasibility of the universal HBTC and RoR model, as well as the use of comprehensive personal interviews to understand key programmatic gaps and HIV risk factors. In all three countries, nearly 90% of adults who completed an interview opted to provide a blood sample for the biomarker component of the survey and receive HBTC. Further, as displayed in Figure 2, the high proportion of self-reported PLHIV providing blood samples demonstrates that neither the blood draw nor the RoR were important deterrents to HIV-positive individuals who were willing to disclose their status. On the contrary, given that participants self-reporting HIV positive were more likely to consent to blood draw than those self-reporting HIV-negative, it is possible that the opportunity to receive PoC CD4 results and have viral load results sent to their clinic may have motivated participation.
This manuscript reports response rates in two different ways in order to highlight different findings. Table 3 presents household response rates, individual interview response rates, and biomarker response rates among all who were eligible for the survey, including those who refused or were unavailable at the interview stage. This calculation reveals that approximately 8 out of 10 adults who were eligible members of a consenting household gave blood and were therefore included in the estimation of primary outcomes. Conversely, Figure 3 presents response rates only among those participants completing the previous stage: household consent among eligible households, interview consents among those eligible for interviews, and biomarker consents among those interviewed. This approach highlights the attrition at each specific stage where consent was possible. While the attrition was fairly evenly spread across the three stages, it is notable that attrition was highest at the household stage in Zimbabwe, at the interview stage in Zambia and at the blood draw stage in Malawi. This approach also allows comparison with other population-based surveys, including KAIS and Demographic and Health Surveys, which present response rates at each stage. [5–7, 10] Table 3 and Figure 3 show two of many ways of presenting response rates and highlights the importance of considering the denominator when interpreting response to surveys involving multi-stage consent and when comparing results across surveys.
The total numbers of participants recruited compared to sample size targets from the first three PHIA surveys speak to both strengths and challenges. Zimbabwe and Zambia met or very nearly met recruitment targets for adults despite somewhat lower response rates among men and in urban areas. Malawi did not meet recruitment targets for adults, with shortfalls noted among men and people in urban areas. As Malawi oversampled in the densely populated south of the country, including Blantyre city, the shortfall among men in urban areas had a particularly pronounced impact on overall recruitment.
None of the three countries met recruitment targets for children, ranging from a shortfall of less than 0.5% (approximately 300 participants) in Zimbabwe to a shortfall of over 20% in Malawi. Field teams consistently reported parental concerns that blood draws would weaken children who were already suffering from food insecurity, especially in Malawi, due to drought at the time of data collection. In Malawi, this concern was addressed by offering biscuits and juice to households where children were invited to participate. Other obstacles to recruiting children included an inability of field teams to meet children who left the household who travelled long distances to school, leaving the household early and returning late at night, and reluctance of parents who already knew their HIV-negative status to consent to having their child tested. In addition to lower response rates, relatively low HIV prevalence (<3.0%) has been observed among children ages 0–14 in all 10 PHIA surveys reporting pediatric results to date. [16] As a result, extensive efforts are required to recruit even small numbers of HIV-positive children. Accordingly, recent UNAIDS guidance on measuring HIV in pediatric populations notes that even well-resourced general-population surveys in high prevalence countries yield estimates of pediatric prevalence with limited precision, citing CIs as large as the prevalence estimates in some PHIA surveys. [17] For future general-population surveys, it may be worth targeting higher risk pediatric sub-populations [18], particular age-groups of interest or measuring pediatric outcomes through different study designs altogether.
While the comparison of target versus recruited sample sizes highlights disparities in response rates across demographic groups, the PHIA surveys compensated for differential response by leveraging the full complement of available variables to construct nonresponse adjustment cells using the CHAID method. This nuanced and context-specific approach to non-response weighting was particularly suitable in view of the sensitive nature of the survey questions and methods and the risk that HIV outcomes and non-response may be correlated. Indeed, a comparison of weighting methods suggests that, for the Zimbabwe, Malawi, and Zambia PHIA surveys, the CHAID weighting approach produced a greater variation in weight adjustments and better accounted for differential nonresponse than using a limited number of standard predictors of nonresponse such as region, urban/rural, and sex, as in the Demographic and Health Surveys. [19]
While response rates suggest that measuring national HIV incidence and subnational VLS with RoR and adequate precision is feasible, obtaining these direct measures required concerted efforts. First and foremost, achieving adequate precision on primary objectives depended on sample sizes of 20,000 to 30,000 participants per country. Collecting high quality data on this scale requires rigorously training and supervising hundreds of field staff to conduct informed consent, administer questionnaires, conduct HBTC, and upload data with high fidelity. Additionally, laboratory staff had to be trained and deployed to conduct satellite and central laboratory testing and laboratory data management procedures. Throughout field work, a robust, continuously operational data management system was required to capture, compile, transmit, and store data from tablets and laboratory instruments. Optimizing response rates required close collaboration with national and local stakeholders to conduct sensitization activities in each of 500 EAs per country in advance of the arrival of survey teams. Finally, all clinically-actionable test results, including testing performed in distant laboratories, were returned to the participant and/or their care provider within a reasonable timeframe. This required extensive coordination between laboratory, data management, and field teams [20]. Moreover, because HBTC was a new approach in some countries, national policies had to be developed before the surveys could start. While conducting nationally representative, general-population surveys at this scale may be viewed as complex and resource intensive, the information gained makes it possible to benchmark progress, steer HIV programming, and inform the targeting of resources. [21] With repeat surveys planned for each of these countries in 2020, there is additional value from the systematic, ongoing data collection, analysis, and interpretation of results which has the potential to reveal patterns of progress, gaps, and trends over time.
Limitations:
PHIA surveys, like all cross-sectional population surveys have several important limitations. As with all household-based surveys, some selected participants will be unavailable or will choose not to participate. Survey weighting helps to account for this however non-participation remains a source of potential bias. As general population surveys it is also important to note that these surveys do not focus on institutionalized populations or key populations at risk for HIV and should not be used to draw conclusions about these groups. As cross sectional surveys, the PHIAs are not designed to identify trends or changes over time. Lastly, while interviewers were trained in techniques to put participants at ease and to support accurate reporting of dates and events, self-reported data remains susceptible to recall bias as well as social desirability bias in relation to sensitive topics..
Conclusion
Measuring impact-level indicators of the HIV epidemic in a national survey is feasible and achievable. The response rates attained in the initial three PHIA surveys demonstrate that HBTC and RoR at the household level are not deterrents to survey participation. Despite concerns that HBTC and RoR would prove financially and operationally overwhelming as well as unacceptable to a large number of participants, the PHIA surveys have demonstrated the feasibility of this approach. Data management systems are critical to ensuring quality and completeness of data. By providing directly-measured estimates of key impact indicators and critical insights about national and sub-national progress towards epidemic control, the PHIA surveys are empowering countries to address gaps in their national response to the HIV epidemic and chart a path towards achieving an AIDS-free generation.
Funding:
This research has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Centers for Diseases Control and Prevention under the terms of Cooperative Agreements 1U2GGH000994 and 5NU2GGH001226. The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the funding agencies.
References
- 1.UNAIDS. Global AIDS Update 2016. 2016.
- 2.UNAIDS. Miles to Go: Closing Gaps Breaking Barriers Righting Injustices. 2018.
- 3.UNAIDS. AIDSinfo. Available from: http://aidsinfo.unaids.org/. Accessed 11 June, 2019.
- 4.UNAIDS. 90–90-90: An ambitious treatment target to help end the AIDS epidemic. 2014.
- 5.Zimbabwe National Statistics Agency and ICF International, Zimbabwe Demographic and Health Survey 2015. Rockville, Maryland, USA. 2016. [Google Scholar]
- 6.Central Statistical Office and Ministry of Health and ICF International, Zambia Demographic and Health Survey 2013–14. Rockville, Maryland, USA. 2015. [Google Scholar]
- 7.National Statistical Office and ICF International. Malawi Demographic and Health Survey 2015–16. Zomba, Malawi. 2017. [Google Scholar]
- 8.Bicego GT, et al. , Recent Patterns in Population-Based HIV Prevalence in Swaziland. PLoS ONE, 2013. 8: p. e77101. Accessed June 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Justman J, et al. , Swaziland HIV Incidence Measurement Survey (SHIMS): a prospective national cohort study. The lancet. HIV, 2017. 4(2): p. e83–e92. Accessed June 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waruiru W, et al. , The Kenya AIDS Indicator Survey 2012: rationale, methods, description of participants, and response rates. J Acquir Immune Defic Syndr, 2014. 66 Suppl 1: p. S3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel H, et al. , PHIA Laboratory Methods. JAIDS, 2020. [Google Scholar]
- 12.Nyathi A, et al. , ZIMPHIA Song: Knock, Knock, Knock. 2015: Harare, Zimbabwe. [Google Scholar]
- 13.National AIDS and STI Control Programme (NASCOP) Kenya, Kenya AIDS Indicator Survey 2012: Final Report. 2014.
- 14.U.S. Centers for Disease Control and Prevention. Violence against Children Surveys: Our Methods. Violence against Children Surveys; [cited 2019 August 2]; Available from: https://www.cdc.gov/violenceprevention/childabuseandneglect/vacs/methods.html. [Google Scholar]
- 15.The U.S. Department of State Office of the Global AIDS Coordinator, PEPFAR 3.0: Controlling the Epidemic, Delivering on the Promise of an AIDS-free Generation. 2014: Washington DC. [Google Scholar]
- 16.The PHIA Project: Summary Sheets. [cited 2019 July 19]; Available from: https://phia.icap.columbia.edu/resources/.
- 17.UNAIDS, Improving UNAIDS’ paediatric and adolescent estimates. 2018.
- 18.Reid G, Voetsch A, Kalton G, Saito S, Improving the Efficiency of Sampling for Pediatric HIV Prevalence: Evidence from 9 Sub-Saharan African Countries. JAIDS, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin T-H, et al. , Developing Nonresponse Weighting Adjustments for Population-Based HIV Impact Assessments Surveys in Three African Countries, in JSM Proceedings. 2017. [Google Scholar]
- 20.Saito S, et al. , Returning HIV-1 viral load results to participant-selected health facilities in national Population-based HIV Impact Assessment (PHIA) household surveys in three sub-Saharan African Countries, 2015 to 2016. Journal of the International AIDS Society, 2017. 20: p. e25004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Justman JE, Mugurungi O, and El-Sadr WM, HIV Population Surveys - Bringing Precision to the Global Response. N Engl J Med, 2018. 378(20): p. 1859–1861. [DOI] [PubMed] [Google Scholar]


