Abstract
Tripterygium hypoglaucum (Lévl.) Hutch (THH) is believed to play an important role in health care and disease treatment according to traditional Chinese medicine. Moreover, it is also the representative of medicine with both significant efficacy and potential toxicity. This characteristic causes THH hard for embracing and fearing. In order to verify its prospect for clinic, a wide variety of studies were carried out in the most recent years. However, there has not been any review about THH yet. Therefore, this review summarized its characteristic of components, pharmacological effect, pharmacokinetics and toxicity to comprehensively shed light on the potential clinical application. More than 120 secondary metabolites including terpenoids, alkaloids, glycosides, sugars, organic acids, oleanolic acid, polysaccharides and other components were found in THH based on phytochemical research. All these components might be the pharmacological bases for immunosuppression, anti-inflammatory and anti-tumour effect. In addition, recent studies found that THH and its bioactive compounds also demonstrated remarkable effect on obesity, insulin resistance, fertility and infection of virus. The main mechanism seemed to be closely related to regulation the balance of immune, inflammation, apoptosis and so on in various disease. Furthermore, the study of pharmacokinetics revealed quick elimination of the main component triptolide. The feature of celastrol was also investigated by several models. Finally, the side effect of THH was thought to be the key for its limitation in clinical application. A series of reports indicated that multiple organs or systems including liver, kidney and genital system were involved in the toxicity. Its potential serious problem in liver was paid specific attention in recent years. In summary, considering the significant effect and potential toxicity of THH as well as its components, the combined medication to inhibit the toxicity, maintain effect might be a promising method for clinical conversion. Modern advanced technology such as structure optimization might be another way to reach the efficacy and safety. Thus, THH is still a crucial plant which remains for further investigation.
Keywords: Tripterygium hypoglaucum (Lévl.) Hutch., phytochemistry, pharmacology, pharmacokinetics, toxicity
Introduction
Effect and toxicity are two important aspects of drugs. Among the compounds obtained from plants or natural sources, agents with remarkable efficacy and potential toxicity have attracted much attention in recent years. It is well-known that some traditional Chinese medicine is highly toxic such as arsenic, Aconitum carmichaelii Debx. and Tripterygium wilfordii Hook F. (TwHF). Indeed, long-term consumption of arsenic can lead to arsenic accumulation in vital organs with diabetes, atherosclerosis, hypertension, ischemic heart disease, and hepatotoxicity (Khairul et al., 2017). However, arsenic trioxide (As2O3) has recently shown significant efficacy in patients with acute promyelocytic leukemia (APL) and many other malignancies, such as adult T-cell leukemia/lymphoma (ATL) and NPM1 mutant acute myeloid leukemia (AML) (Kchour et al., 2009; Mathews et al., 2011; El Hajj et al., 2015). In addition, fatal ventricular tachycardia and asystole may occur if aconite poisoning is severe (Chan, 2012). The processed Aconitum carmichaelii Debx. plays an important role as cardiotonic, analgesic and anti-inflammatory in traditional medicine (Chan, 2011). Moreover, TwHF is frequently reported exhibiting multiple toxicity, including reproductive toxicity, renal cytotoxicity, and hepatotoxicity (Li X.-J. et al., 2014). However, due to its significant anti-inflammatory and immunosuppressive effects, TwHF preparations have been widely used for the treatment of autoimmune disorders and inflammatory diseases, such as rheumatoid arthritis (RA) (Luo et al., 2019; Xu et al., 2019). It is worth noting that multiple organ toxicity in TwHF can be resolved after dose adjustment (Zhang et al., 2021a). Therefore, the agents with both remarkable effect and potential toxicity should be paid specific focus.
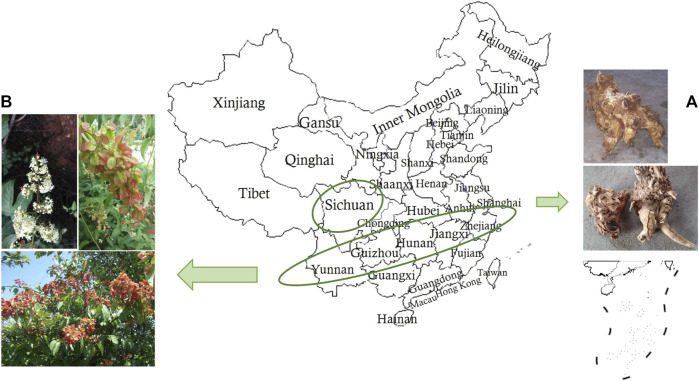
Tripterygium hypoglaucum (Lévl.) Hutch. (THH), a plant distributed in southwest regions of China and along the south bank of the Yangtze River, has similar pharmacological effects with fewer side effects and lower toxicity than TwHF in the clinic (Li, 2006; Brinker et al., 2007; Ma et al., 2008) (Figure 1). Its main chemical components include alkaloids, diterpenoids and triterpenoids. Its root bark demonstrates excellent medicinal properties and has been widely used in folk medicine in China (Li, 2006). Numerous studies have found that terpenoids isolated from THH possess various pharmacological activities including anti-tumour, immunosuppressive and anti-inflammatory effects (Liu, 2011; Lü et al., 2015). Studies have shown that celastrol, a triterpenoid discovered from THH, relieves insulin resistance, reduces body weight, and suppresses polycystic kidney disease (Booij et al., 2020). Despite significant efficacy, the clinical application of THH has a narrow therapeutic window due to severe toxicity, thereby limiting its widespread application (Dudics et al., 2018; Yuan et al., 2019a; Wang et al., 2019).
FIGURE 1.
Climatic and ecological adaptability distribution of THH in China. (A) Dried root bark of THH, (B) Aerial view of THH.
Identification of the active chemical ingredients and pharmacological effects of THH is the primary goal of most THH studies, and this information is useful for the development of efficient and low-toxicity modern traditional Chinese medicines. The current research review on THH is limited, and sufficient attention has not been given to the relationship between compounds, pharmacological activities and toxicity. The chemical compositions and pharmacological mechanisms and potential toxicity of THH were studied to provide a scientific basis for the development of a safe medication with better clinical utility. To further clarify the chemical composition, mechanism of action, pharmacological effects and toxicity characteristics of THH, we conducted the following review. We also summarized the pharmacokinetics study of THH in animals.
Methodology
Database searches using PubMed, China National Knowledge Internet (CNKI) and Wanfang database were conducted until December 2020. Tripterygium hypoglaucum (Lévl.) Hutch., Tripterygium, triptolide, celastrol and kunmingshanhaitang were searched as key words in the databases mentioned above. All of databases were retrieved twice. Experimental articles related to THH and its active components were included, and some irrelevant literature was excluded through reading abstracts or the full text. In almost all cases, the original articles were obtained and the relevant data was extracted.
Phytochemical Constituents
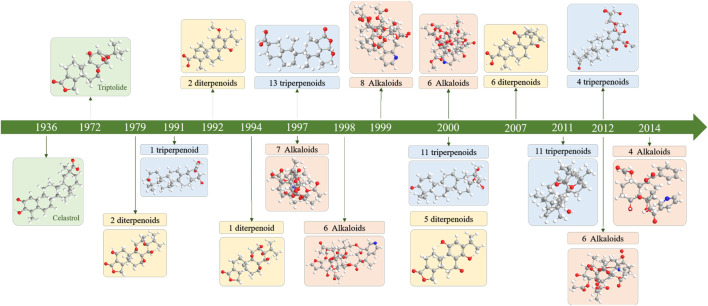
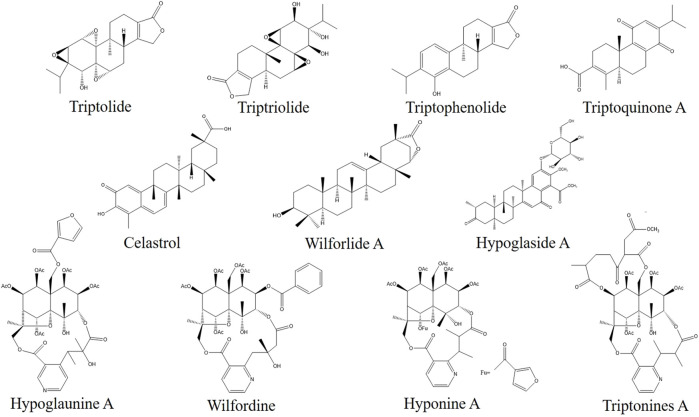
Since the 1960s, scholars have conducted in-depth research on TwHF through nearly half a century of chemical composition research on the genus Tripterygium (Figure 2). This plant genus contains more than 100 chemical components such as terpenoids (diterpenes, triterpenes, sesquiterpenes), alkaloids, glycosides, sugars, organic acids, euonymol, euonymus alkaloids, polysaccharides, β-sitosterol and other chemical components. Its characteristic active ingredients include diterpenoids such as triptolide, triptophenolide, triptriolide, triptoquinone A, and triterpenoids, such as celastrol and wilforlide A (Xie et al., 2015) (Figure 3).
FIGURE 2.
Timeline of the isolation and identification of main chemical components from the genus Tripterygium.
FIGURE 3.
Chemical structures of the existing potential quality control markers in THH.
In the earliest study (1972) of the chemical composition of THH, triptolide, triptolide diol, and triptolide ketone were isolated and identified from the ethanol-ethyl acetate extract of TwHF, a congeneric plant produced in China (Kupchan et al., 1972). In 1979, domestic studies in China first isolated trace amounts of two diterpene lactone chemical constituents, hypolide and tripterolide, from the root bark of TwHF. As a common chemical component of the three species of this genus, hypolides were isolated not only from THH but also from other species of the same genus, and it was hypothesized that hypolides might represent one of the precursors of biosynthesis in this genus (Wu and Sun, 1979).
A study reported that three rosin diterpenes, namely hypoglic acid, triptonoterpene methyl ether and triptonoterpenol could be separated from the ethyl acetate soluble portion of the root ethanol extract by silica gel column chromatography (Wang et al., 2012). An additional three ursolane triterpenes, namely hypoglaulide, triptotriterpenic acid C and regelin were obtained, and 3-acetoxy oleanolic acid was also obtained during the same separation process in 1992 (Zhang et al., 1992a). Ten crystals were obtained from the ethanol extract of the coarse powder of THH root: 3-oxofriedelan-29-oic acid methyl ester; β-sitosterol; 3-epik atonic acid methyl ester; 3β, 22α-dihydroxy-Δ12-oleanen-29-oic acid methyl ester; and 3β, 22α-dihydroxy-Δ12-ursen-30-oic acid methyl ester (Yi and Yang, 1993). In other studies, the chloroform extracts of THH roots were separated by column chromatography, and a total of 12 crystals were obtained. Of these, 8 compounds were identified in 1992, including tripteroquinone, triptolide A, triptolide B, tripterygidine, triptolide, 1-epicatechin and β-sitosterol (Zhang and Zhang, 1992). They also reported that three diterpenoid compounds, namely, triptoditerpenic acid, tritoditerpenic acid B and hypodiolide A, were obtained from the chloroform extracts of THH in the same year. In addition, other components, such as wilforgine and daucosterol, have been isolated from THH (Ding, 1991).
Sesquiterpenoids
Sesquiterpenes be isolated from THH mostly combine with pyridine to form alkaloids. At present, only two sesquiterpenoid monomeric compounds have been isolated from THH. In 2000, a sesquiterpene compound was isolated from the methanol extract of the root bark of THH. The molecular formula was C31H36O7, and the structural formula was 1β-benzoyl-8α-cinnamoyl-4α, 5α-dihydroxy dihydroagarofuran (Fujita et al., 2000). In 2014, a sesquiterpene compound was isolated from the 95% ethanol extract of the root bark of THH. The molecular formula was C31H36O8 and the structural formula was: 1α-acetoxy-6β, 9β-dibenzoyloxy-4β-hydroxy-dihydroagarofuran. These compounds exhibited weak cytotoxicity against HeLa cells with an IC50 value of 30.2 µM (Liu Z.-Z. et al., 2014). Dihydro-β-agarofuran sesquiterpenes from THH are substrates of P-gp and potential modulators of MDR. These compounds exhibit no cytotoxicity in HepG2 and HepG2/Adr cells. Moreover, these compounds restored the sensitivity of HepG2/Adr cells to Dox and increased the accumulation of intracellular Dox (Yang et al., 2019).
Diterpenoids
Diterpenoids are the most important components in THH, and triptolide exhibits the most remarkable activity. Regarding the structural types of diterpenoids, epoxy-type diterpenoids, randolactone-type diterpenoids, fulfolterpenoid diterpenoids, kaurane-type diterpenoids and abietane-type diterpenoids are primarily observed in THH.
One ranolactone-type diterpenoid, triptonolide, and two ranolterpene-type diterpenoids triptobenzene A and triptobenzene J were isolated from the methanol extract of THH (Duan et al., 1997). In addition, the following diterpenoids were isolated from the methanol extract of THH rhizomes in 2000: two ranolactone-type diterpenoids, triptobenzene K and triptophenolide; two fulfolterpenoid diterpenoids, triptobenzene D and triptobenzene L; and one abietane-type diterpenoid, quinone 21 (Fujita et al., 2000). In 2007, three fulfolterpenoid diterpenoids (triptobenzene H, triptonediol, and triptonoterpene) and three abietane-type diterpenoids (triptoquinone A, triptoquinone B, and triptoquinone H) were isolated from 95% ethanol extracts of THH (Zhang et al., 2007). In 2011, a new fulfolterpenoid diterpenoid named triptonoterpene methyl ether was isolated from a 95% ethanol extract of the root bark of THH (Liu et al., 2011). A randolactone-type diterpenoid, 11-O-β-D-glucopyranosyl neotriptophenolide was isolated from a 95% ethanol extract of THH (Li X, 2006). Four new diterpenoids, 2α, 16α-hydroxy-ent-kauran-19,20-olide, isopimara-8 (14), 15-diene-11β, 19-diol, isopimara-8 (14),15-diene-12α, 19-diol, and 3-oxo-14,15-dihydroxyabieta-8,11,13-trien-19-ol were isolated from THH. Three new terpenoid compounds, epoxyionone A, triptobenzene W and loliolide A, were isolated from the 95% EtOH extract of the twigs of THH (Zheng et al., 2020). A new diterpenoid, 19-O-β-D-glucopyranosyl-labda-8 (17), 14-dien-13-ol was isolated from the aerial parts of THH (Zhao et al., 2018) (Table 1).
TABLE 1.
Diterpenoids isolated from Tripterygium hypoglaucum.
| Serial number | Compound | Molecular formula | Plant part | References |
|---|---|---|---|---|
| 1 | Hypolide | C20H24O3 | Root | Wu and Sun (1979) |
| 2 | Triptonoterpenol | C21H30O4 | Root | Zhang et al. (1992b) |
| 3 | Hypoglic acid | C20H26O4 | Root | Zhang et al. (1992b) |
| 4 | Triptonoterpene methyl ether | C21H30O3 | Root | Zhang et al. (1992b) |
| 5 | Hypodiolide A | C24H36O3 | Root | Zhang and Zhang (1992) |
| 6 | Triptoditerpenic acid B | C21H28O3 | Root | Zhang and Zhang (1992) |
| 7 | Triptonoditerpenic acid | C21H28O4 | Root | Zhang and Zhang (1992) |
| 8 | Triptoquinone H | C20H26O3 | Root | Ding (1991) |
| 9 | Triptobenzene L | C22H34O3 | Root | Ding (1991) |
| 10 | Triptolide | C20H24O6 | Root | Duan et al. (1997) |
| 11 | Triptonolide | C20H22O4 | Root | Duan et al. (1997) |
| 12 | Triptobenzene A | C20H28O3 | Root | Duan et al. (1997) |
| 13 | Triptobenzene J | C20H30O3 | Root | Duan et al. (1997) |
| 14 | Triptobenzene K | C20H22O5 | Root | Duan et al. (1997) |
| 15 | Neotriptophenolide | C21H26O4 | Root | Duan et al. (1997) |
| 16 | 2α,16α-Hydroxy-ent-kauran-19,20-olide | C20H30O4 | Stem | Li et al. (2015b) |
| 17 | Isopimara-8 (14), 15-diene-11β,19-diol | C20H32O2 | Stem | Li et al. (2015b) |
| 18 | Isopimara-8 (14),15-diene-12α,19-diol | C20H32O2 | Stem | Li et al. (2015b) |
| 19 | 3-Oxo-14,15-dihydroxyabieta-8,11,13-trien-19-ol | C20H28O4 | Stem | Li et al. (2015b) |
| 20 | Triptobenzene W | C21H30O3 | Root | Zheng et al. (2020) |
| 21 | Epoxyionone A | C19H28O6 | Root | Zheng et al. (2020) |
| 22 | Loliolide A | C17H24O6 | Root | Zheng et al. (2020) |
| 23 | Hypoglicin A | C20H30O4 | Stem | Chen et al. (2018b) |
| 24 | Hypoglicin B | C20H24O3 | Stem | Chen et al. (2018b) |
| 25 | Hypoglicin C | C19H20O3 | Stem | Chen et al. (2018b) |
| 26 | Hypoglicin D | C20H26O5 | Stem | Chen et al. (2018b) |
| 27 | Hypoglicin E | C20H28O2 | Stem | Chen et al. (2018b) |
| 28 | Hypoglicin F | C20H28O2 | Stem | Chen et al. (2018b) |
| 29 | Hypoglicin G | C20H28O2 | Stem | Chen et al. (2018b) |
| 30 | Hypoglicin H | C20H26O3 | Stem | Chen et al. (2018b) |
| 31 | Hypoglicin I | C20H26O3 | Stem | Chen et al. (2018b) |
| 32 | Hypoglicin J | C20H26O3 | Stem | Chen et al. (2018b) |
| 33 | Hypoglicin K | C20H22O6 | Stem | Chen et al. (2018b) |
| 34 | Hypoglicin L | C20H22O6 | Stem | Chen et al. (2018b) |
| 35 | Hypoglicin M | C19H20O4 | Stem | Chen et al. (2018b) |
| 36 | 19-O-β-D-Glucopyranosyl-labda-8 (17),14-dien-13-ol | C26H44O7 | Aerial parts | Zhao et al. (2018) |
The majority of diterpenoids exhibit strong immunosuppressive and anti-inflammatory activities. The inhibitory activity towards LPS-induced NO production of these terpenoids was evaluated in microglial BV-2 cells, and all of the compounds showed inhibitory effects (Zhao et al., 2014). Triptolide is a typical representative abietane terpenoid in THH, that has been reported to have diverse pharmacological effects such as anti-inflammatory, antiproliferative, proapoptotic, immunosuppression, and tumour inhibition effects, and this compound exhibits great efficacy in the treatment of rheumatoid arthritis, asthma, and Shin disease (Shamon et al., 1997; Li J. et al., 2012; Chen S.-R. et al., 2018).
Triterpenoids
Triterpenoids are also one of the main components of THH. The triterpenoids found in THH are mainly pentacyclic triterpenoids, and three types are predominantly noted, including oleanane-type, ursane-type and friedelane-type. Such pentacyclic triterpenoids are active ingredients in the treatment of autoimmune diseases.
One new triterpenoid named 6α-hydroxy triptoline, was isolated from the 95% ethanol extract of THH roots (Song et al., 2021). One friedelane-type triterpenoid (2,3-dihydroxy-6-oxo-D:A-froedo-24 nor-1,3,5 (10), 7-oleanatetraen-29-oic acid) and other types of triterpenoids, including 23-nor-oxopristimerol and hypoglaside A, were isolated from the 95% ethanol extract of THH roots (Li X, 2006). Two oleanane-type triterpenoids, wilforlide A and 3-oxo-oleanoic acid were isolated from the chloroform emulsification layer of THH roots (Wang et al., 2011). One oleanane-type triterpenoid, triptotriterpenic acid A was isolated from 95% ethanol extract of the rhizomes of THH (Ding, 1991). The following triterpenoids were isolated from the methanol extract of THH rhizomes: four oleanane-type triterpenoids, including triptocallic acid D, triptocallic acid C, 3-epikatonic acid, oleanoic acid 3-O-acetate; two friedelane-type triterpenoids, 29-hydroxyfriedelan-3-one and polpunonic acid; one ursane-type triterpenoid, 3β-acetoxy-urs-12-ene-28-oic acid; and other types of triterpenoids, such as triptohypol D, triptohypol E, triptohypol F, and hypodiol (Fujita et al., 2000). The following triterpenoids were isolated from the methanol extract of THH roots: one oleanane-type triterpenoid, mesembryanthemoidigenic acid; ten friedelane-type triterpenoids, celastolide, triptoethylol A, wilforic acid A, triptohypol B, triptohypol C, wilforol A, wilforol B, demethylzeylasteral, wilforic acid C, and cangoronine; and other types of triterpenoids, including salaspermic acid and 23-nor-6-oxo-demethyl pristimerol (Duan et al., 1997). Three oleanane-type triterpenoids, including 3-acetoxy oleanolic acid, glut-5-en-3β, 28-diol, and 3-oxo-olean-Δ9(11), 12-diene and two friedelane-type triterpenoids, canophyllal and friedelin were isolated from 95% ethanol extracts of the root bark of THH (Liu et al., 2011) (Table 2). Celastrol, the first triterpenoid ingredient isolated from the genus Tripterygium, is classified as a friedelane-type triterpenoid. Its toxicity is much lower than that of triptolide. Celastrol has significant anticancer effects and relieves insulin resistance. Thus, celastrol has attracted considerable attention in recent years (Petronelli et al., 2009; Yang et al., 2014).
TABLE 2.
Triterpenoids isolated from Tripterygium hypoglaucum.
| Serial number | Compound | Molecular formula | Plant part | References |
|---|---|---|---|---|
| 37 | Celastrol | C29H38O5 | Root | Duan et al. (1997) |
| 38 | Wilforic acid A | C29H42O4 | Root | Duan et al. (1997) |
| 39 | Wilforol B | C29H42O4 | Root | Duan et al. (1997) |
| 40 | Wilforic acid C | C30H48O4 | Root | Duan et al. (1997) |
| 41 | Celastolide | C30H46O5 | Root | Duan et al. (1997) |
| 42 | Hypodiol | C30H50O2 | Root | Duan et al. (1997) |
| 43 | Salaspermic acid | C32H52O4 | Root | Duan et al. (1997) |
| 44 | Cangoronine | C30H44O5 | Root | Duan et al. (1997) |
| 45 | Popanonic acid | C30H48O3 | Root | Duan et al. (1997) |
| 46 | Demthylzeylasteral | C30H38O6 | Root | Duan et al. (1997) |
| 47 | Mesembryanthemoidigenic acid | C32H52O3 | Root | Duan et al. (1997) |
| 48 | 3-Hydroxy-D: A friedoolean-3-en-2-on-29-oic-acid | C30H46O4 | Root | Duan et al. (1997) |
| 49 | Triptohypol A | C30H40O6 | Root | Duan et al. (1997) |
| 50 | Triptohypol B | C30H40O5 | Root | Duan et al. (1997) |
| 51 | Triptohypol C | C29H39O4 | Root | Duan et al. (1997) |
| 52 | Triptohypol D | C32H54O2 | Root | Fujita et al. (2000) |
| 53 | Triptohypol E | C31H52O2 | Root | Fujita et al. (2000) |
| 54 | Triptohypol F | C31H52O2 | Root | Fujita et al. (2000) |
| 55 | Regelin | C31H48O4 | Root | Zhang et al. (1992a) |
| 56 | hypoglaulide | C30H44O3 | Root | Zhang et al., (1992a) |
| 57 | 3-Oxofriedelan-29-oic acid methyl ester | C32H52O3 | Root | Yi and Yang (1993) |
| 58 | 3β,22α-Dihydroxy-Δ12-ursen-30-oic acid methyl ester | C30H48O4 | Root | Yi and Yang (1993) |
| 59 | 3β,22α-Dihydroxy-Δ12-oleanen-29-oic acid methyl ester | C30H50O | Root | Yi and Yang (1993) |
| 60 | Friedelin | C30H50O | Root bark | Liu et al. (2011) |
| 61 | 3-Oxo-olean-9 (11),12-diene | C30H46O | Root bark | Liu et al. (2011) |
| 62 | Canophyllal | C30H48O2 | Root bark | Liu et al. (2011) |
| 63 | 6α-Hydroxy triptocslline | C28H42O5 | Root | Song et al. (2021) |
Alkaloids
Alkaloids are one of the strongest bioactive ingredients found in the THH extract. The alkaloids obtained from THH exhibit pyridine alkaloid structures formed by condensation of dihydroagarofuran-type sesquiterpenes with different pyridinic acids. Studies have shown that alkaloids found in THH from traditional Chinese medicine exhibit antitumour activity in animals.
In 1997, seven alkaloids were isolated from the methanol extract of THH roots: euonine, hyponine A, hyponine B, hyponine C, cangorinine E-I, regelidine, and 3-pyridinecarboxylic acid (Duan et al., 1997). Then, six alkaloids were isolated from the methanol extract of THH roots in 1998: hypoglaunine A, hypoglaunine B, hypoglaunine C, hypoglaunine D, euonymine, and wilforine (Duan and Takaishi, 1998). Two new alkaloids, wilfordine and wilformine were isolated from the ethanol extract of THH roots in 1999 (Li et al., 1999). Moreover, hyponines D, hyponines E, hyponines F, neoeunoymine and forestine were isolated from the methanol extract of THH roots (Duan and Takaishi, 1999). In 2000, six alkaloids were isolated from the methanol extract of THH roots: triptonines A, triptonines B, wilfordinines A, wilfordinines B, wilfordinines C and peritassine A (Duan et al., 2000). In 2012, two sesquiterpene pyridine alkaloids, hypoglaunine E and hypoglaunine F were isolated from 95% ethanol extracts of THH roots (Li X, 2006). Then, four alkaloids, 2-O-deacetyleuonine, wilfortrine, triptolide (wilforgine) and tripfordine C were isolated from a 95% ethanol extract of the root bark of THH (Xie et al., 2012). In 2014, four new alkaloid compounds were isolated from the methanol extract of the rhizome of THH: 9α-cinnamoyloxy-1β-furoyloxy-4-hydroxy-6α-nicotinoyloxy-β-dihydroagarofuran, 1β,9α-dibenzoyloxy-4-hydroxy-6α-nicotinoyloxy-β-dihydroagarofuran, 1β-benzoyloxy-9α-cinnamoyloxy-4-hydroxy-6α-nicotinoyloxy-β-dihydroagarofuran and 1β-acetoxy-9α-benzoyloxy-4-hydroxy-6α-nicotinoyloxy-β-dihydroagarofuran (Chen X.-L. et al., 2018) (Table 3). The alkaloid fraction in THH is characterized by high activity and low toxicity; thus, these compounds exhibit potential value in new drug development research.
TABLE 3.
Alkaloids isolated from Tripterygium hypoglaucum.
| Serial number | Compound | Molecular formula | Plant part | References |
|---|---|---|---|---|
| 64 | Hypoglaunine A | C41H47O20N | Root | Duan and Takaishi (1998) |
| 65 | Hypoglaunine B | C41H47O20N | Root | Duan and Takaishi (1998) |
| 66 | Hypoglaunine C | C43H49O19N | Root | Duan and Takaishi (1998) |
| 67 | Hypoglaunine D | C41H47O19N | Root | Duan and Takaishi (1998) |
| 68 | Wilforine | C43H49O18N | Root | Duan and Takaishi (1998) |
| 69 | Wilforgine | C41H47O19N | Root | Duan and Takaishi (1998) |
| 70 | Wilfordine | C43H49O19N | Root | Duan and Takaishi (1998) |
| 71 | Wilfortrine | C41H47O20N | Root | Duan and Takaishi (1998) |
| 72 | Euonymine | C38H47O18N | Root | Duan and Takaishi (1998) |
| 73 | Wilformine | C41H48O18N2 | Root | Duan and Takaishi (1998) |
| 74 | Wilfornine | C41H47O19N | Root | Duan and Takaishi (1998) |
| 75 | Hyponine A | C41H47O19N | Root | Duan et al. (1997) |
| 76 | Hyponine B | C41H47O19N | Root | Duan et al. (1997) |
| 77 | Hyponine C | C43H49O18N | Root | Duan et al. (1997) |
| 78 | Cangorinine E-I | C43H49O18N | Root | Duan et al. (1997) |
| 79 | Evonine | C36H43O17N | Root | Duan et al. (1997) |
| 80 | 3-Pyridinecarboxylic acid | C29H34O7N | Root | Duan et al. (1997) |
| 81 | Hyponine D | C47H50O18N2 | Root | Duan and Takaishi, (1999) |
| 82 | Hyponine E | C45H48O19N2 | Root | Duan and Takaishi (1999) |
| 83 | Hyponine F | C41H47O19N | Root | Duan and Takaishi (1999) |
| 84 | Neoeunoymine | C36H45O17N | Root | Duan and Takaishi (1999) |
| 85 | Forrestine | C43H49O18N | Root | Duan and Takaishi (1999) |
| 86 | Peritassine A | C39H49O18N | Root | Duan et al. (2000) |
| 87 | Regelidine | C35H37O8N | Root | Duan et al. (2000) |
| 88 | Triptonine A | C45H55O21N | Root | Duan et al. (2000) |
| 89 | Triptonine B | C45H55O22N | Root | Duan et al. (2000) |
| 90 | Wilfordinine A | C36H55O12N | Root | Duan et al. (2000) |
| 91 | Wilfordinine B | C38H60O13N | Root | Duan et al. (2000) |
| 92 | Wilfordinine C | C43H60O14N | Root | Duan et al. (2000) |
| 93 | 2-O-Deacetyleuonine | C36H45O17N | Root bark | Xie et al. (2012) |
| 94 | Tripfordine C | C36H45O17N | Root bark | Xie et al. (2012) |
| 95 | 1-α-Benzoyloxy-6β-nicotinoyloxy-9β-acetoxy-4β-hydroxy-dihydro-β-agarofuran | C30H35O8N | Root | Duan et al. (2000) |
Flavonoids
In 1998, two flavonoids were isolated from industrial alcohol extracts of THH stems: (+) -catechin and L-epicatechin (Zhang and Zhang, 1998). In 2012, two flavonoids were isolated from a 95% ethanol extract of the root bark of THH: 4′-O-(−)methyl-epigallocatechin and (2R, 3R)-3,5,7,3′,5′-pentahydroxyflavan (Xie et al., 2012).
Lignans
Three new lignans, 9′-O-benzoyl-lariciresinol, 9′-O-benzoyl-5′-methoxylariciresinol, and 9′-O-cinnamoyl-lariciresinol, were isolated from the 95% EtOH extract of the twigs of THH. Moreover, only 9′-O-benzoyl-lariciresinol showed weak cytotoxicity against HepG2/Adr cells, with an IC50 value of 30.1 µM in vitro (Liu et al., 2011). Recently, syringaresinol, a common bisepoxylignan, was isolated from the 95% EtOH extract of THH (Song et al., 2021).
Other Compounds
In addition to diterpenes, triterpenes and alkaloids, other compounds, such as steroids, fatty acids, tannins and glycosides, have been identified in THH. Although terpenoids are the main pharmacodynamic components of THH, other components also play synergistic roles.
In 1991, two steroidal compounds, β-sitosterol and daucosterol, and one other compound, fumaric acid, were isolated from the 95% ethanol extract of the rhizome of THH (Ding L, 1991). Two steroidal compounds, ergosta-4,6,8 (14), 22-tetraen-3-one and stigmast-4-en-3-one and three other compounds, p-hydroxyl benzoic acid, 3,4-dihydroxy-benzoic acid, and 3-meth-oxy-4-hydroxy-benzoic acid were isolated from the ether extract of THH stems (Zhang and Zhang, 1998). In 2011, three fatty acid compounds, palmitic acid, stearic acid and tricosanoic acid were isolated from the 95% ethanol extract of the root bark of THH (Liu et al., 2011). Two tannins were isolated from industrial alcohol extracts of THH stems: procyanidin B-3 and procyanidin B-4 (Zhang and Zhang, 1998). In 2011, a tannin compound, procyanidin B-2, was isolated from the chloroform emulsified layer of THH roots (Petronelli et al., 2009). Two glycosides, 3,4-dimethoxyphenyl-β-D-glucopyranoside and 3,4,5-trimethoxyphenyl-β-D-glucopyranoside were isolated from the 95% ethanol extract of the root bark of THH (Xie et al., 2012).
Pharmacological Activity
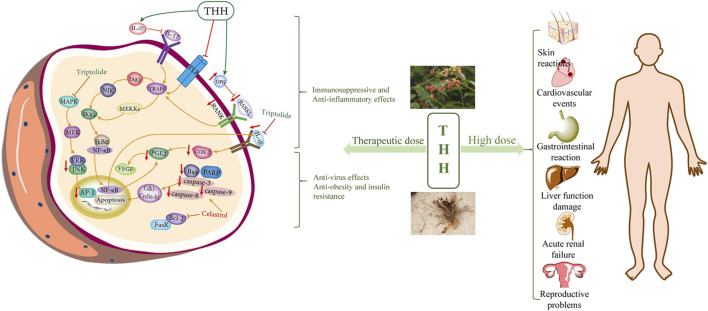
THH is a traditional medicine that exhibits various pharmacological activities, including anti-inflammation, immunosuppression, antitumour, obesity and insulin resistance, antifertility, and antiviral effects. These pharmacological effects are mainly related to the alkaloids and terpenoids of THH. These components are expected to exhibit therapeutic significance in various human immunological diseases, such as rheumatoid arthritis, systemic lupus erythematosus, acute infectious hepatitis, chronic nephritis, leukemia, neurodermatitis, and chronic urticaria (Guo et al., 2018; Tang et al., 2020). Here, we summarized several classical pharmacological activities of THH (Figure 4, Tables 4 and 5).
FIGURE 4.
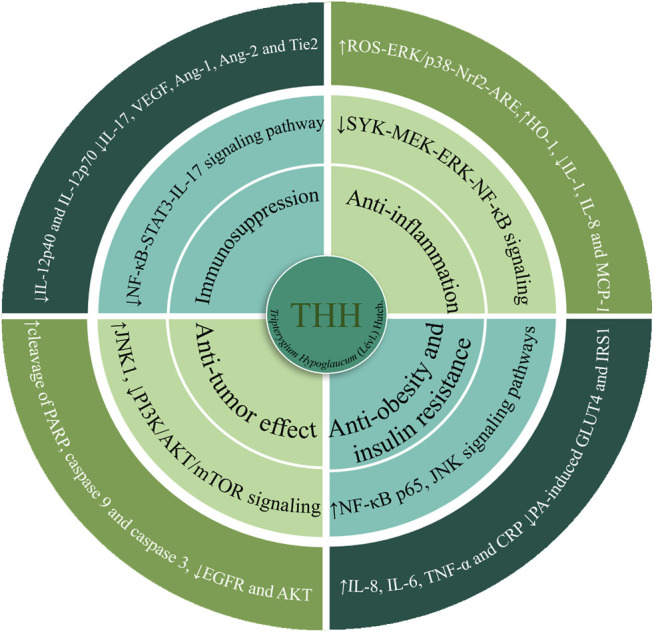
The main signalling pathways and molecular targets of the pharmacologically active components in THH.
TABLE 4.
Pharmacological effects of different compounds isolated from Tripterygium hypoglaucum in vitro studies.
| Pharmacological activity | Compound | Experimental design | Molecular targets/mode of action | References |
|---|---|---|---|---|
| Anti-inflammatory | THH alkaloids | 10 µg of total RNA isolated from THH-treated (40 µg/ml, 8 h) and untreated HL-60 cells | ↑genes related to the NF-κB signalling pathway and cell apoptosis (such as NFKBIB, PRG1 and B2M),↓c-myc binding protein and apoptosis-related cysteine proteases caspase-3 and caspase-8 | Zhuang et al. (2004) |
| — | Triptolide | Corneal fibroblasts absence or presence of IL-1 (10 ng/ml), with or without triptolide (30 nM) or dexamethasone (100 nM) | ↓IL-1, IL-8 and MCP-1 | Lu et al. (2005) |
| — | Triptolide | Raw 264.7 cells stimulated with LPS (50 ng/ml) in the presence or absence of triptolide (30 µM) for 24 h | ↓DNA binding activity of NF-κB, ↓NO production, ↓phosphorylation of c-Jun NH(2)-terminal kinase (JNK) | Kim et al. (2004) |
| — | Triptolide | Microglia were pre-treated with PBS, triptolide (10, 30, or 50 µM), with or without Bay11-7,082 for 30 min before LPS treatment (10 ng/ml, 24 h) | ↓p38-NF-kappaB-COX-2-PGE(2) and JNK-PGE(2) | Gong et al. (2008) |
| — | Triptolide | TP-mmc (15 μM) with or without TP (1.2, 12, 15, 30 µM) was added to RAW 264.7 cells (middle panel: bar, 50 μM) for 2 h | ↓TAK1-TAB1 complex kinase activity | Lu et al. (2014) |
| — | Celastrol | fibroblast-like synoviocytes (FLSs) were treated with celastrol (0.05, 0.1, 0.2, 0.4 and 0.8 mM) for 24 h | ↓MMP-9 promoter activity, ↓TLR4/MyD88/NF-κB pathway, ↓ FLS migration and invasion | Li et al. (2013a) |
| — | Celastrol | HaCaT cells were incubated with celastrol (0, 0.1, 0.5, 1 µg/ml) for 1h, and stimulated with IFN-c (100 U/ml) for 4 h (for RNA) or 12 h (for protein) | ↓IFN-γ-induced ICAM-1 mRNA and protein expression | Seo et al. (2010) |
| — | Celastrol | HaCaT cells were pretreated with DPI (10 μM), NAC (20 mM) or EUK 134 (50 μM) for 1 h, and then incubated with celastrol (1 μg/ml) for 6 h (for RNA) or 12 h (for protein) | ↑ROS-ERK/p38-Nrf2-ARE, ↑HO-1 | Seo et al. (2011) |
| — | Celastrol | RAW264.7 cells were treated with 0–1 μM of celastrol for 24 h | ↓ nitric oxide synthase and cyclooxygenase-2, ↓ MPO activity, ↓IL-6 and TNF-α | Kim et al. (2009) |
| — | Celastrol | BV-2 cells were pre-treated with various concentrations of celastrol (1, 10 and 100 nM) for 30 min prior to stimulation with LPS (10 ng/ml) for 6 h (for TNF-α) and 24 h (for IL-1β) | ↓expression of mRNAs of iNOS and cytokines; ↓NO, IL-1β and TNF-α; ↓ERK1/2 phosphorylation and NF-κB activation | Jung et al. (2007) |
| — | Celastrol | Jurkat T cells were preincubated for 30 min with celastrol (0, 0.3, 1 µg/ml) and followed by the stimulation with of TNF-a (20 ng/ml) or PMA (50 ng/ml) for 90 min | ↓IKK activity and IKKβ activity, ↓Bfl-1/A1 expression | Lee et al. (2006) |
| — | Celastrol | Neutrophils were pre-incubated with different doses of celastrol (0.5–20 μM) or vehicle only at RT for 45 min | ↓neutrophil oxidative burst and NET formation, ↓SYK-MEK-ERK-NF-κB signalling cascade | Yu et al. (2015) |
| Immuno-suppression | Triptolide | Monocytes were cultured for 5 days in the presence of various concentrations (1–20 ng/ml) of triptolide and Dex (10−8–10–6 M) | ↓CD1a, CD40, CD80, CD86 and HLA-DR expression; ↑CD14 expression | Zhu et al. (2005) |
| — | Triptolide | LPS (100 ng/ml)-stimulated U937 cells were treated with or without triptolide (12.5 nM) | ↓TREM-1 and DNAX-associated protein (DAP)12, ↓activation of JAK2 and STAT3, ↓TNF-α, IL-1β and IL-6 | Fan et al. (2016) |
| — | Celastrol | Bone marrow-derived macrophages were pre-treated with celastrol (0.1, 0.5 and 1 μM) for 1 h and treated with Alexa Fluor 594 conjugated with LPS (1.5 μg/sample) for 30 min | ↓TNF-α, IL-6, IL-12, and IL-1β, ↓LPS binding to the TLR4/MD2 complex | Lee et al. (2015) |
| — | Celastrol | Purified CD4+CD25− T cells were treated in the presence or absence (control) of Celastrol (200 nM) | ↓mTOR, HIF-1α, c-Myc and Akt expression in Th17 cells, ↑FAO of lipids by upregulating CPT1A and AMPKα expression in iTreg cells | Zhang et al. (2018) |
| — | Triptolide | Confluent synovial cells were treated with recombinant human interleukin-1α (IL-1α; 1 ng/ml), dexamethasone (Dex), and/or triptolide (2.8–140 nM) at the indicated concentrations | ↓IL-1α-induced production of proMMP- 1 and -3, ↑IL-1alpha-induced gene expression and production of TIMP-1 and -2 | Lin et al. (2001) |
| — | Triptolide | Human monocytes were cultured for 7 days with G4 medium in the presence of TPT (D2-7, 0.5–10 nM) or medium alone | ↓production of IL-12 p70 | Chen et al. (2005) |
| — | Triptolide | C57BL/6 mouse bone marrow cells were cultured with mGM-CSF and mIL-4, and triptolide (0, 1, 5, 10, 20, 50, and 100 ng/ml) added on day 3 of culture (Trip-DC) | ↑activation of p38, ↓activation of caspase 3 | Liu et al. (2004) |
| Triptolide | THP-1 cells were differentiated into macrophage-like cells upon exposure to Me2SO, and then cultured with IFN-gamma (500 kU/L) and lipopolysaccharide (LPS) (1 mg/L) with or without triptolide (2.5–0.625 µg/L) | ↓CD80 and CD86 expression on IFN-gamma- (500 kU/L) and LPS- (1 mg/L) activated THP-1 cells, ↓IL-12p40 and IL-12p70 | Liu et al. (2008) | |
| Antitumour effect | Total alkaloids | JB6 Cl41 cells were suspended containing 10% FBS, 10 μM TPA with or without THHta (1.25–10 μg/ml) | ↑activation of caspase-3 and PARP, ↓Bcl-2, Bcl-xL and XIAP | Jiang et al. (2014) |
| — | Triptonide | treatment of the pancreatic cancer cell lines Patu8988 and Panc1 with triptonide at the doses of 0–20 nM | ↓VE-cadherin and CXCL2 genes | Han et al. (2018) |
| — | Triptolide | PC-3 and DU145 cells were incubated with triptolide (0, 25, 50 nM) for 24 h | ↓RNA polymerase activity, ↓CDC25A, and MYC and Src oncogenes | Yuan et al. (2020) |
| — | Triptolide | Malignant (BxPc-3, MIA-PaCa2 and AsPC-1) and nonmalignant (pancreatic ductal cells and MSC) cells grown under normoxia or hypoxia in the presence or absence of triptolide (20 nM) | ↓epithelial-mesenchymal transition (EMT) and CSC features | Liu et al. (2014a) |
| — | Triptolide | Urothelial cancer cells were treated with CDDP at the concentration corresponding to IC25 (30 mM) with or without 1 h pretreatment of 30 nM triptolide for 12 h | ↓CDDP-induced p53 transcriptional activity | Matsui et al. (2008) |
| — | Triptolide | The JNK1 knockdown cells were treated with triptolide (14–56 nM) for 15 h | ↓phosphatidylinositol 3-kinase (PI3K) activity, ↑c-Jun NH(2)-terminal kinase 1 (JNK1) | Miyata et al. (2005) |
| — | Celastrol | SO-Rb 50 cells were treated with celastrol nanoparticles (0–54.4 µg/ml) and the same dosage of PEG-b-PCL micelles without celastrol for 48 h | ↓elevated levels of ALT, AST, ALP and AFP; ↓anti-apoptotic Bcl-2 and Bcl-xl; induced the expression of pro-apoptotic Bax, cytochrome C, PARP and caspases | Li et al. (2012b) |
| — | Celastrol | A549 cells were treated with celastrol (0–8 µM) at the indicated concentration for 24, 48, and 72 h | ↑expression of pro-apoptotic Bax, ↓anti-apoptotic Bcl-2 and Akt phosphorylation | Mou et al. (2011) |
| — | Celastrol | H1650 cells were collected for apoptosis analysis at 24 h after the treatment of celastrol (0.5, 1, 2, 4 μM) | ↓EGFR and AKT | Fan et al. (2014) |
| — | Celastrol | H1299 cells were treated with or without 4 μM celastrol in the absence or presence of 50 μM Z-VAD for 24 h | ↑cleavage of PARP, caspase 9 and caspase 3 | Chen et al. (2011) |
| — | Celastrol | Human myeloma cell line U266 cells were treated with celastrol (0.25 and 0.5 µM) for the indicated times (0–48 h) | ↑caspase-3 and NF-κB pathways | Tozawa et al. (2011) |
| Anti-obesity and insulin resistance | Celastrol | Fully differentiated 3T3-L1 adipocytes were incubated with different doses of oligomycin (5, 10, 20 and 30 µg/ml) and celastrol (5, 10, 20 and 30 μM) in DMSO for 48 h | ↓oxidative DNA damage, protein carbonylation and lipid peroxidation | Bakar et al. (2014) |
| — | Celastrol | C3A human hepatocytes were exposed to various concentration (10–50 nM) of celastrol in the serum-free media for 48 h | ↓PA-induced GLUT4 and IRS1 | Abu Bakar et al. (2017) |
| — | Celastrol | Myoblasts were treated with different concentrations (10, 20, 30, 40, 50 and 60 nM) of celastrol were prepared and mixed with 0.1% (v/v) DMSO. The vehicle consisted of an equal amount of DMSO was used as a control | ↑NF-κB p65, c-Jun NH(2)-terminal kinase (JNK) signalling pathways,↑IL-8, IL-6, TNF-α and CRP | Abu Bakar and Tan (2017) |
| Antiviral effect | Triptolide | HONE1/Akata, HK1/Akata, C666–1, and CNE1 cells were placed in 35 mm culture dishes (500 cells/dish) and cultured in standard medium with DMSO control (0.01%) or triptolide (1, 2, or 5 nM) for 2 weeks | ↓ratio of Bax/Bcl-2, ↑activated caspase-3 and Nrf2 | Zhou et al. (2018) |
| — | Triptolide | Human umbilical vascular endothelial cells (HUVEC) were trested with or without brusatol (40 nmol/L)/celastrol (50 nmol/L)/AngII(400 nmol/L) for 24 h | ↑caspase-9-dependent apoptosis | Li et al. (2017a) |
| — | Celastrol | The transfected ava5 cells were treated with celastrol (0, 0.2,0.3,0.4,0.5 μM) for 3 days | ↓iNOS, TNF-α, and NF-κB phospho-p65 expression, ↑nuclear levels of Nrf2 and HSF-1 | Tseng et al. (2017) |
| Other effects | Triptolide | The model microglial group was treated with Aβ1–40 (20 μg/ml). The low-dose triptolide microglial group was treated with Aβ1–40 (20 μg/ml) and triptolide (5 μg/ml). The high-dose triptolide microglial group was treated with Aβ1–40 (20 μg/ml) and triptolide (25 μg/ml) | ↑IL-10, ↓IL-4 | Nie et al. (2012) |
| — | Triptolide | RASF, HM, HFF, or U937 cells were incubated overnight with or without LPS (2 μg/ml) in the presence or absence of the indicated concentrations of T2 (1,2,4 μg/ml), triptolide (1,2,4 ng/ml), DEX (0.1,1,10 μM), or Indo (0.01,0.1 μg/ml) | ↓protein levels of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) | Tao et al. (1998) |
TABLE 5.
Pharmacological effects of different compounds isolated from Tripterygium hypoglaucum in vivo studies.
| Pharmacological activity | Compound | Experimental design | Molecular targets/mode of action | Duration of treatment | Route of administration | References |
|---|---|---|---|---|---|---|
| Anti-inflammation | Triptolide | Triptolide (8, 16 and 32 mg/(kg day); n = 16, respectively), dexamethasone (1 mg/(kg every 2 days); n = 16) or vehicle (n = 20) | ↓metalloproteinases-13 and -3,↓COX-2 and PGE(2),↓IL-1β, TNF-α and IL-6,↑metalloproteinases-1 and -2 | Daily for a period of 21 days | Oral administration | Lin et al. (2007) |
| — | Triptolide | Triptolide (0.1 mg/kg/d) dissolved in 5% dimethyl sulfoxide was intraperitoneally injected into the SCI rats | ↑miR-96, ↓Iba-1 and IKKβ/NF-κB-related proteins, ↓IL-1β and TNF-α | Continued for successive 10 days | Intraperitoneal injection | Huang et al. (2019) |
| — | Tripterine | tripterine (5, 10 and 20 mg kg−1 day−1), or prednisone (10 mg kg−1 day−1); 0.5% CMC solution as vehicle-treated group | ↓ IgG and delayed-type hypersensitivity (DTH), ↓IL-1β and TNF-α | 5 days | Intragastrical administration | Li et al. (2008) |
| — | Celastrol | 1 μg/g Celastrol or 2 μg/g digoxin were administrated at adjuvant-induced arthritis (AIA) rats | ↓IL-1β and TNF | After 4 days (early treatment group) and after 11 days (late treatment group) of disease induction | Intraperitoneal injection | Cascão et al. (2012) |
| — | Celastrol | Saline (20 μL) containing 4 μg of sPLA2IIA with celastrol (1, 10, 30 μM and 100 µM)/vehicle was injected into the intra-plantar surface of the right hind footpad of mice | ↓sPLA2IIA, 5-LOX and COX-2 enzymes | After 45 min, mice were anaesthetized with pentobarbitone (30 mg/kg, i.p.) and euthanized | Injected into the intra-plantar surface of the right hind footpad of mice | Joshi et al. (2016) |
| — | Celastrol | Hepa1-6 single-cell suspension cells (2 × 107/ml) were injected subcutaneously at a volume of 0.1 ml in the right flank of each mouse | ↓AKT pathway and VEGF autocrine system | 21 days of administration | Intraperitoneal injection | Zhang et al. (2019a) |
| Immuno-suppression | THH | C57BL/6 mice were used to model CIA mice received THH 420 mg/kg/day or the same amount of normal saline (NS) | ↓TNF-α, IFN-γ, and IL-17A mRNA and protein levels; ↓NF-κB-STAT3-IL-17 pathway | 20 days | Intragastrical administration | Zhou et al. (2020) |
| — | Celastrol | CIA mice were treated intraperitoneally (IP) with celastrol in phosphate buffered saline (PBS; 3 mg/kg) or PBS alone | ↓osteoclastic genes (Trap, Ctsk, Ctr, Mmp-9) and transcription factors (c-Fos, c-Jun and NFATc1), ↓NF-κB and MAPK phosphorylation | 15 days | Intraperitoneal injection | Gan et al. (2015) |
| — | Triptolide | CIA rats were treated with triptolide (11–45 µg/kg/day) starting on the day 1 after first immunization | ↓Matrigel-induced cell adhesion of HFLS-RA and HUVEC, ↓TNF-α, IL-17, VEGF, VEGFR, Ang-1, Ang-2 and Tie2, ↓IL1-β-induced ERK phosphorylation and p38 and JNK protein levels | Daily for a period of 28 days from day 1 to day 28 of first immunization | Oral administration intragastrically using syringe feeding | Kong et al. (2013) |
| — | Celastrol | Female Sprague Dawley rats were treated by celastrol (1 mg/kg/day, i.p.) | ↑IL-10, ↓TNF-α, ↓immunohistochemical expression of TLR2 and CD3+ T-lymphocytic count | 32 days | Intraperitoneal injection | Abdin and Hasby, (2014) |
| Antitumour effect | Celastrol | C57BL/6N mice were treated with 1 mg/kg of celastrol, 3 mg/kg of celastrol, or a vehicle control. Celastrol was dissolved in vehicle (10% DMSO, 70% Cremophor/ethanol (3:1), and 20% PBS) | ↑ROS-mediated caspase-dependent apoptosis; ↓PI3K/AKT/mTOR signalling | 20 days | Oral gavage every 2 days | Lee et al. (2012) |
| Anti-obesity and insulin resistance | Celastrol | C57BL/6 mice were allowed to recover for 2 weeks postsurgery before receiving intraperitoneal vehicle or celastrol (100 μg/kg) at 6 pm each day | ↓TC, TG, LDL-c and Apo B in plasma,↓NADPH oxidase activity | 10 consecutive days | Intraperitoneal injection | Kyriakou et al. (2018) |
| — | Celastrol | Sprague–Dawley rats were treated with celastrol (1.0 ml/100 g) or simvastatin (1.0 ml/100 g) | ↑protein phosphorylation of insulin signalling cascades with amplified expression of AMPK protein, ↓attenuated NF-κB and PKC θ activation | 6 weeks | Intragastrical administration | Wang et al. (2014) |
| Antiviral effect | Triptolide | Male BALB/C mice were intravenously (i.v.) treated with a single dose of TP (1.2 mg/kg) | ↓TNF-α, IL-1β, IL-6 malondialdehyde (MDA) and antioxidative superoxide dismutase (SOD), ↑glutathione (GSH) and glutathione peroxidase (GPx) | 24 h | Intravenous injection | Zhou et al. (2014) |
| Other effects | Celastrol | Celastrol (0.5 and 1.0 mg/kg, i.v.) was administered to anaesthetized rats 2 h before and 30 min after LPS challenge (10 mg/kg, i.v.) | ↑Nrf2 activation, ↓Nox2/AT1 receptor expression, ↑phosphorylation of ERK1/2 | 8 h | Intravenous injection | Wang et al. (2015) |
Anti-Inflammatory Effects
Corticosteroids are widely used as anti-inflammatory drugs, but some serious side effects often occur after long-term use. THH has been shown to be effective in a variety of inflammatory diseases. Under the condition of reasonable control of THH toxicity, THH is expected to play a huge potential as an alternative drug. The therapeutic mechanisms of THH include the inhibition of inflammation and capillary permeability as well as the reduction of infiltrates. Moreover, Hypoglicins B-G and J-M, which are isolated from the stem of THH, inhibited NO production with IC50 values ranging from 0.72 to 36.91 μM. Moreover, hypoglicin D and hypoglicin L significantly inhibit the mRNA expression of iNOS at doses of 12.5 and 3.13 μM, respectively (Chen X.-L. et al., 2018). Importantly, the compounds found in THH do not act through the pituitary-adrenal cortex system, so there will be no “rebound” phenomenon after stopping the drug, and even long-term use will not damage the immune system (Zhong et al., 2011), but the toxic accumulation of long-term use must be considered carefully.
The mechanism by which triptolide attenuates the inflammatory pathology of Crohn’s disease (CD) involves increasing IL-10 levels and Foxp3+ Tregs in the mucosa and decreasing TNF-α levels in the mucosa (Li G. et al., 2014). The pathogenesis of CD is mainly mediated by the TLR/NF-κB signalling pathway. Triptolide upregulates TLR2 and TLR4 in a CD model in IL-10-null (IL-10 −/−) mice by inhibiting TLR/NF-κB signalling pathways in vivo. TLR activation also promotes angiogenesis mediated by exogenous and endogenous ligands in different inflammatory settings (Harmey et al., 2002; Oda and Kitano, 2006; Yu et al., 2010). A key regulator of the signal transduction cascade activated by NF-κB and activator protein-1 is TAK1. However, for TAK1 to be fully activated, it must be induced by a protein activator, TAK1 binding protein (TAB1). Triptolide interferes with the formation of the TAK1TAB1 complex and thus inhibits the activity of TAK1 kinase. The interaction region between triptolide and TAB1 involves the amino acid residues between TAB1373 and 502. Triptolide inhibits MAPK pathway activation in macrophages upon binding to TAB1 (Lu et al., 2014). In addition, triptolide inhibited the activation of microglia and the secretion of inflammatory factors in BV2 cells by upregulating miR96 (Huang et al., 2019). Moreover, the γ-butyrolactone and C-14 β-hydroxyl portions of the triptolide molecules are important components responsible for the compound’s anti-inflammatory characteristics and cytotoxicity (Wong et al., 2008). Triptolide suppresses the production of IL-6, IL-8, TNF-α, and IL-1β in human bronchial epithelial cells, and inhibits staphylococcal exotoxin-stimulated T-cell proliferation. Triptolide induces the expression of monocyte chemotactic protein (MCP)-1, IFN-g, TNF, IL-6, IL-1β, macrophage inflammatory protein (MIP)-1β and MIP-1α in peripheral blood mononuclear cells (PBMCs) (Krakauer et al., 2005).
Angiogenesis is a fundamental event in inflammation. Infection leads to inflammation and growth factors released by leukocytes promote angiogenesis. Many chronic inflammatory diseases, such as retinopathy, rheumatoid arthritis, Crohn’s disease, atherosclerosis, diabetes and cancer are closely associated with angiogenesis (McDonald, 2001; Rodríguez-Martínez et al., 2006; Costa et al., 2007). Celastrol achieves its effect mainly by regulating various inflammatory mediators (Li et al., 2008; Li G. et al., 2013), inhibiting the production of proinflammatory cytokines (IL-6, IL-2, IFN-γ, TNF, IL-1β, IL-8, etc.,) and proinflammatory enzymes (COX-1, prostaglandin E2, NOS, COX-2 and iNOS, etc.,) through inhibition of NF-κB (Lee et al., 2006; Jung et al., 2007; Kim et al., 2009; Seo et al., 2010, 2011; Cascão et al., 2012; Joshi et al., 2016). In addition, celastrol interferes with the migration, proliferation, and activation of inflammatory cells (Yu et al., 2015). Celastrol suppresses the LPS-induced IκB kinase (IKK)/NF-κB pathway, significantly downregulates TLR4 expression and inhibits VEGF secretion in LP-1 cells. These results suggest that celastrol inhibits LPS-induced angiogenesis by inhibiting TLR4-triggered NF-κB activation (Zhang R. et al., 2019).
Immunosuppression
THH was used to treat various autoimmune diseases based on its action against metabolic pathways, and three metabolites, urocanate, L-glutamate and alanine played very important roles (Guo et al., 2018). The key molecular mechanism of THH against RA is related to inhibition of the inflammatory response through inactivation of the TNF and NF-κB signalling pathways (Jiang et al., 2020b). THH reduces the expression of related inflammatory cytokines in joint tissue and serum, and the mechanism of THH in the treatment of collagen II-induced arthritis (CIA) mice involves inhibition of the NF-κB-STAT3-IL-17 pathway (Zhou et al., 2020). The formation and function of osteoclasts in CIA mice are directly inhibited by celastrol. Celastrol significantly reduced joint bone damage, and inhibited arthritis, and this activity is associated with the expression of the osteoclast marker serum tartrate-resistant acid phosphatase (TRAP) 5b and osteoclastic genes (Ctr, Mmp-9, Ctsk and Trap) and transcription factors (NFATc1 c-Jun and c-Fos). Moreover, celastrol suppressed bone resorption activity and the formation of TRAP + multinucleate cells in a dose-dependent manner (Gan et al., 2015).
Triptolide and celastrol are the most important active ingredients of THH against RA, and their molecular mechanisms involve many targets, such as inhibition of inflammation, inhibition of angiogenesis, immunosuppression, protection of cartilage, and induction of apoptosis (Kong et al., 2013; Fan et al., 2016, 2018; Wang S. et al., 2018). The expression of chemokines and inflammatory cytokines in RA synovioblasts is inhibited by celastrol through modulation of the OPG/RANKL axis, thereby inhibiting RA inflammation and bone erosion (Feng et al., 2013). Celastrol also decreases the expression of NF-κB, nitrite levels, and TLR2 expression and CD3+ Tlymphocyte counts as assessed by immunohistochemistry; and the cytokine phenotype changes significantly from Th1 to Th2, with increased IL-10 and decreased TNF-α levels. (Abdin and Hasby, 2014). In addition, the binding of LPS to the TLR4/MD2 complex and TLR4 activation were also inhibited by celastrol in a thiol-dependent manner (Lee et al., 2015). Celastrol suppresses the expression of phosph-stat3, c-Myc, Glut1, mTOR, HK2, HIF-1a and Akt in Th17 cells; upregulates the expression of phosph-stat5 in iTreg cells, and upregulates the expression of CPT1A and AMPKα in iTreg cells to promote fatty acid-oxidation (Zhang et al., 2018).
Triptolide inhibits the production of proMMP-1 and -3 induced by IL-1α in human synovial fibroblasts and reduces the messenger RNA levels of IL-1α. In contrast, the IL-1alpha-induced gene expression and production of TIMP-1 and-2 were further augmented by triptolide in synovial cells. Triptolide also inhibited the IL-1alpha-induced production of PGE2 by selectively suppressing the gene expression and production of COX-2, but not COX-1 (Lin et al., 2001) (Hu et al., 2004). Triptolide inhibits the production of IL-2, IL-4, and IFN-γ and the activation of CD4+ and CD8+ T cells in peripheral blood in RA patients, thus exerting an immunosuppressive effect (Ren et al., 2010) (Nong et al., 2019). Professional antigen presenting cells (APCs), such as dendritic cells (DCs), are another target for the immunosuppressive activity of triptolide (Chen et al., 2005). DCs are induced by high concentrations (20 ng/ml) of triptolide via caspase3 activation and sequential p38 MAP kinase phosphorylation (Liu et al., 2004; Liu et al., 2006). Triptolide also inhibits T cells and DC-mediated chemoattraction of neutrophils by reducing NF-κB activation and STAT3 phosphorylation (Zhu et al., 2005). Triptolide prevents the differentiation of immature monocyte-derived DCs (MoDCs), mainly by reducing the ability of MoDCs to stimulate lymphocyte proliferation in allogeneic mixed lymphocyte reactions (MLRs) and downregulating CD86, CD40, CD1a, CD80, and HLA-DR expression. Triptolide reduces IFN-γ-induced expression of CD80 and CD86 in DCs and THP-1 cells (Liu et al., 2005). Recently, it was found that triptolide inhibited IL-12/IL-23 expression in APCs via CCAAT/enhancer-binding protein α (Liu et al., 2008).
Antitumour Effects
The alkaloid fraction of THH induced apoptosis at lower concentrations and significantly inhibited tumour growth in vitro and in vivo. In addition, the apoptotic effect of total alkaloids in tumour cells is potentially mediated through both the extrinsic death receptor pathway and the intrinsic mitochondrial pathway. The total alkaloids fraction of THH induced apoptosis by inhibiting Bcl-xL, Bcl-2, and XIAP and activating PARP and caspase-3, thereby inhibiting the growth of colon cancer cells in a dose-dependent manner in vitro (Jiang et al., 2014). Triptonide potently inhibited vasculogenic mimicry mediated by pancreatic cancer cells (Han et al., 2018). In addition, triptonide inhibited the epithelial-mesenchymal transition of gastric cancer-associated fibroblasts (GCAFs) by correcting abnormalities in microRNA expression and subsequently eliminating the epithelial-mesenchymal transition in gastric cancer cells (Wang et al., 2017). Moreover, a cytotoxicity of triptolide and 2-epilactone was observed, particularly in A549, DU145, KB, KBvin, and MDA-MB-231 human tumour cell lines, with IC50 values of 0.0012–0.1306 μM in vitro (Li X.-L. et al., 2015). The mRNA and protein levels of CD147 and MMPs were reduced, and triptonide also inhibited the expression of caveolin-1, thereby inhibiting the invasion and migration of prostate cancer cells (Yuan et al., 2020).
Pancreatic cancer (PC) cell lines SNU-213, Capan-1 and Capan-2 exhibit different sensitivities to triptolide with IC50 values of 0.01, 0.02, and 0.0096 μM, respectively. Phosphorylation of Chk2 was more significant in SNU-213 sensitive to triptolide but, not in SNU-410 insensitive to triptolide, suggesting that the effect of triptolide on different PC cell lines may be related to gene expression (Liu L. et al., 2014; Kim et al., 2018). In addition, triptolide inhibited CRC growth through induction of cleavage and perinuclear translocation of 14-3-3e protein (Liu et al., 2012). Furthermore, triptolide dose-dependently inhibited the proliferation of human scale-cell carcinoma SAS, human cervical carcinoma SKG-II, and human fibrosarcoma HT-1080 cells, an important target molecule in the antitumour effect of triptolide is phosphatidylinositol 3-kinase (PI3K). The reduction of PI3K activity leads to inhibition of tumour cell proliferation, which subsequently leads to enhanced JNK1 phosphorylation through Akt and/or PKC-independent pathways (Miyata et al., 2005).
As an important compound of THH, celastrol exhibits tumour-inhibiting effects in a variety of tumour models, and its antitumour mechanisms have been demonstrated in various cancers in vitro (Li Z. et al., 2012; Lee et al., 2012). There are two main pathways by which celastrol induces apoptosis in cancer cell lines, namely, through the activation of intrinsic pathways (mitochondrial pathways) and extrinsic apoptotic pathways (death receptor pathways) (Mou et al., 2011). Apoptosis, paraptosis, and necrosis are modes of cell death induced by celastrol (Chen et al., 2011); the expression of ERα target genes was suppressed, celastrol also decreased the expression of ERα at the protein and mRNA levels in MCF7 and T47D human breast cancer cells, leading to cell cycle arrest and inhibition of breast cancer cell growth (Jang et al., 2011). Moreover, celastrol induced apoptosis by cleaving the PARP proteins, caspase-3, caspase-9, and caspase-8; increasing FasL and Fas expression; decreasing mitochondrial membrane potential; upregulating proapoptotic Bax expression; and downregulating antiapoptotic Bcl-2 (Mou et al., 2011). In addition, the activation of caspase-3 also involved in the induces myeloma cell apoptosis, and celastrol inhibited NF-κB migration into the nucleus, and treatment of the human myeloma cell line U266 with celastrol induces cell cycle at the G1 phase followed by apoptosis (Tozawa et al., 2011). Moreover, celastrol suppressed the migration of AGS and YCC-2 cells in gastric tumour induced G1 arrest in cell-cycle populations, and increased phosphorylated AMPK levels after reducing the levels of AKT, mTOR, and S6K phosphorylation (Lee et al., 2014). In addition, celastrol can significantly reduce tumour nodules in the liver by decreasing DEN-induced MDM2 levels and activating the p53 pathway, thereby improving DEN-induced liver pathology (Chang et al., 2016). It also induces apoptosis in Bel-7402 cells by the mitochondrial apoptosis pathway (Li P.-P. et al., 2015). Moreover, it upregulates both the mRNA and protein levels of DR4 and DR5, which induce TRAIL/Apo-2 L-mediated apoptosis in cancer cells (Zhu et al., 2010; Lin et al., 2017).
In the last 10 years, given the increased accessibility of oncometabolites, the idea that cancer is a metabolic disorder has gradually emerged (Collins et al., 2017). In reports on colon cancer, many oncometabolites such as histidine, isoleucine, phenylalanine, tyrosine, threonine, glutamate, and pyruvate have been found to be closely related to cancer-associated metabolic pathways (Mishra and Ambs, 2015; Wishart et al., 2016). Treatment with triptolide may help to restore normal metabolite levels in these pathways. Triptolide increases isoleucine, plasma proline, methionine, 2-hydroxyisovalerate (2-HIV), low-density lipoprotein/very low-density lipoprotein (LDL/VLDL) and 2-hydroxyisobutyrate (2-HIB) levels, and reduces plasma pyruvate, 3-hydroxybutyric acid, and glutamine levels. Because 2-HIV, LDL/VLDL and 2-HIB are related to branched-chain amino acid metabolism, lactate and pyruvate metabolism, and cholesterol metabolism, respectively, the antitumour mechanism of triptolide may rely on correcting alterations in serine/glycine/methionine biosynthesis, branched-chain amino acid metabolism, and ketone body metabolism (Li et al., 2019).
Anti-Obesity and Insulin Resistance Effects
Celastrol targets mitochondria, inhibits the inflammatory response and prevents obesity induced by specific diets in mice. Celastrol-administered mice at 100 μg/kg decrease food consumption and body weight via a leptin-dependent mechanism (Kyriakou et al., 2018). In 2015, the Umut Ozcan team at Harvard Medical School screened more than 1,000 compounds that reduce ER stress (insulin resistance is a major risk factor for obesity, and ER stress is an important cause of resistance) and found that celastrol can be used as a weight-loss drug. Obese mice treated with celastrol lost 45% of their body weight, and improved insulin sensitivity was observed. Thus, celastrol may exhibit potential therapeutic effects in various diseases, such as type 2 diabetes and fatty liver (Liu et al., 2015). The weight-loss mechanism of celastrol is mediated through interleukin 1 receptor 1 (IL1R1) (Feng X. et al., 2019), it has no effects on weight loss, diabetes or fatty liver in mice if IL1R1 is absent.
Mitochondrial dysfunction and inflammation tend to occur at high levels of fatty acid saturation in insulin-sensitive tissues (Hernández-Aguilera et al., 2013). Celastrol reduces the body weight of obese mice induced by a high leptin diet by up to 45%, increases insulin sensitivity and energy expenditure, and inhibits food intake. Celastrol is a leptin sensitizer, but it has shown effects in leptin-deficient (ob/ob) and leptin receptor-deficient (db/db) mouse models (Liu et al., 2015). Palmitate-mediated insulin resistance could be blocked by celastrol by improving metabolic activity associated with mitochondrial function (Bakar et al., 2014). In addition, celastrol significantly reduced mitochondrial superoxide production; enhanced the cellular fatty acid oxidation rate, intracellular ATP content and mitochondrial membrane potential citrate synthase activity; increased levels of tricarboxylic acid cycle intermediates; improved mitochondrial function; increased mitochondrial DNA content, and inhibited oxidative DNA damage, and glucose uptake activity and enhances the expression of AMPK and GLUT4 proteins were also improved by decreasing the activation of NF-κB and PKCθ (Abu Bakar et al., 2015; Abu Bakar and Tan, 2017).
Importantly, Celastrol showed an anti-IR activity, it was achieved by reducing miR-223, and celastrol re-upregulated the downregulation of miR-150 and miR-223 in HepG2 cells (human hepatocellular carcinoma cell lines) through the GLUT4 pathway, reversing palmitate-induced insulin resistance (Zhang X. et al., 2019). Celastrol treatment significantly enhanced tyrosine-612 phosphorylation of IRS-1 protein and decreased serine-307 in C3A hepatocytes, protecting cells from mitochondrial dysfunction and insulin resistance (Abu Bakar et al., 2017). Meanwhile, it also reduces serum malondialdehyde (MDA) and reactive oxygen species levels, increases antioxidant enzyme activities, reduces oxidative stress and improves lipid metabolism in a dose-dependent manner by alleviating oxidative damage, and inhibiting NADPH oxidase activity by improving ABCA1 expression. These actions inhibit increases in body weight and alleviate cardiovascular damage (Abu Bakar and Tan, 2017). The inhibition of negative regulators protein tyrosine phosphatase (PTP) 1B (PTP1B) and T cell PTP (TCPTP) in the hypothalamic arcuate nucleus (ARC) were also invovled in body weight reduction. The reversible non-competitive binding of celastrol to these proteins is mediated by an allosteric pocket close to the active site (Kyriakou et al., 2018).
Antifertility Effect
In 1986, reversible anti-fertility effects of TwHF extract in male rats were discovered, sparking global interest. The researchers performed bioassay-guided subfractionation of material extracted from plants and isolated a series of six male antifertility diterpene epoxides: triptolide, tripdiolide, triptolidenol, tripchlorolide, 16 hydroxytriptolide and T7/19 (Zhen et al., 1995). At the ED95 dosage levels, metamorphic spermatids as well as testicular and epididymal sperm excoriation and late spermatid basic nuclear protein turnover were inhibited. In addition, treatment with these compounds delayed spermatogenesis and sperm head-to-tail separation as well as microtubule, microfilament, and membrane damage (Zhen et al., 1995). Triptolide inhibited GATA4-mediated glycolysis by suppressing Sp1-dependent PFKP expression in SCs and induced testicular toxicity (Zhang et al., 2021b).
Oral THH reversibly induces infertility in men without significant side effects and does not affect libido or potency (Qian et al., 1988). Triptolide induced complete infertility in adult rats with minimal adverse effect on the testis, and it mainly targets epididymal sperm. No effects on testicular and accessory organ weights, tubular lumen volume and total number of Leydig cells, spermatogonia, tubule diameter, and number of Sertoli cells, pachytene (P) spermatocytes, and preleptotene (PL) were observed. Triptolide significantly reduced tubule volume and the number of round spermatids, making it an attractive lead agent as a post-testicular male contraceptive (Lue et al., 1998). We look forward to more reliable data to verify this possibility.
Antiviral Effects
Many natural products have been found to effectively inhibit unique enzymes and proteins that are essential for the viral life cycle (Vlietinck and Vanden Berghe, 1991; Baker et al., 1995). Hypoglaunine A, hypoglaunine B, triptonine A, and hyponine E have represented compounds extracted and purified from THH roots with anti-HIV viral effects (Cheng et al., 2002). The total alkaloid extracts of THH showed potent anti-HSV-1 activity and low cytotoxicity in Vero cells (Tao et al., 2007). Triptobenzene J and quinone 21 have anti-A/PR/8/34 (H1N1) influenza virus (oseltamivir-resistance) activity with EC50 values of 38.6 ± 10.7 μM, and 22.9 ± 6.4 μM, respectively. Quinone 21 exhibited anti-A/Hong Kong/8/68 (H3N2) influenza virus (sensitivity) activity with an EC50 value of 21.6 ± 0.6 Μm (Wang et al., 2020).
Moreover, triptolide treatment inhibited macrophage infiltration; inhibited the overproduction of malondialdehyde (MDA), IL-6, TNF-α and IL-1β in reperfusion myocardial tissue; and upregulated the activities of glutathione peroxidase (GPx), antioxidant superoxide dismutase (SOD), and glutathione (GSH), and the nuclear accumulation of Nrf2 and the activity of its downstream target haem oxygenase-1 (HO-1) were enhanced in ischaemic myocardial tissues (Yu et al., 2016). Additionally, triptolide significantly inhibited Epstein-Barr virus (EBV)-positive cell-induced tumour proliferation in vivo, and low-dose triptolide significantly decreased the half-life of EBV nuclear antigen 1, obviously reduced the expression of EBV nuclear antigen 1, and enhanced triptolide-induced apoptosis by activating the proteasome-ubiquitin pathway (Zhou et al., 2018). However, mitochondrial dysfunction, apoptotic damage, oxidative stress, and histopathological changes in the heart were noted in normal BALB/C mice treated with 1.2 mg/kg triptolide (Zhou et al., 2014). Therefore, the determination of safe dose is the key to the clinical application of triptolide.
Celastrol also showed effective activities in antivirals, it upregulated ERK1/2 phosphorylation, inhibited reactive oxygen species (ROS) generation, and improved endothelial cell activity and Ang II-mediated HUVEC injury by activating Nrf2 (Li M. et al., 2017); and the nuclear levels of Nrf2, nuclear levels of HSF-1 and cardiac GSH levels are also increased (Wang et al., 2015). Moreover, celastrol also inhibited hepatitis C virus (HCV) replication in both the HCV subgenomic and HCVcc infection systems with EC50 values of 0.37 ± 0.022 mM and 0.43 ± 0.019 mM, respectively, the NS3/4A protease activity is inhibited, and further enhancing anti-HCV activity, thereby blocking HCV replication. Interestingly, celastrol showed synergistic effects in combination with the NS5B inhibitors sofosbuvir and interferon-α and the NS5A inhibitor daclatasvir targeting the JNK/Nrf2/HO-1 axis with celastrol presents a promising strategy against HCV infection that could serve as a potential supplement to block HCV replication (Tseng et al., 2017). This result suggests that synergistic use could be a topic in future research.
Other Effects
THH promoted the expression of Foxp3, increased the level of CD4+/CD25 + T cells, decreased the occurrence of aGVHD, and prolonged survival time in mice by regulating cytokine secretion (Li Z. Y. et al., 2013). Syringaresinol has significant protective activity against excitatory damage induced by sodium glutamate in SH-SY5Y neurons, and the mechanism potentially involves antioxidative stress and repair of mitochondrial function and DNA damage to significantly reduce sodium glutamate-induced neuronal apoptosis (Yan et al., 2019).
In recent years, researchers have gradually started to pay attention to the effect of triptolide in AD. Regarding the neuroinflammatory pathology of the AD brain, triptolides potentially exert neuroprotective effects on synapses by partially inhibiting the mechanism of the Aβ-induced immunoinflammatory response, which may effectively slow the neurodegenerative process of AD (Nie et al., 2012). Triptolide inhibits Aβ-induced elevation of IL-1β and TNF-α levels in cultured rat microglia (Jiao et al., 2008). Triptolide promotes synaptophysin expression in hippocampal neurons in an AD cell model (Nie et al., 2012). Tripchlorolide, a novel analogue of triptolide, protected neuronal cells by blocking microglial inflammatory responses to oligomeric Abeta (1–42), inhibited nuclear translocation of nuclear NF-κB without affecting I-κBα phosphorylation and inhibited Aβ-induced JNK phosphorylation, but not ERK or p38 MAPK (Pan et al., 2009). Triptolide also inhibits the upregulated expression of prostaglandin E2 and IL-1β in lymphocytes and AD cell models (Tao et al., 1998; Li et al., 2011).
Pharmacokinetics
Over the years, scholars have conducted numerous studies on the pharmacodynamics of THH, but studies on the pharmacokinetics of THH compounds in animals, especially those administered intravenously, are limited. Antitumour pharmacodynamics and toxicological studies of triptolide are closely related to the pharmacokinetics and tissue distribution of triptolide, which are important components of new drug evaluation studies. Oral triptolide is rapidly and highly absorbed and distributed in the liver, heart, spleen, lung and kidney. Biotransformation of triptolide in rats includes hydroxylation, sulfate, glucuronide, N-acetylcysteine (NAC) and glutathione (GSH) conjugation and combinations of these pathways. Less than 4% of triptolide was recovered from the faces, bile and urine within 24 h. After repeating the dosage, triptolide was eliminated quickly without accumulation in vivo. As a substrate for P-glycoprotein (P-gp) and CYP3A4, triptolide could have clinically significant pharmacokinetic interactions with protein substrates/inhibitors (Song et al., 2019). After injection of three doses (100 µg/kg, 200 µg/kg, 300 µg/kg) of triptolide into the veins of rats, the corresponding t1/2α of each subsequent dose group was: 0.033, 0.021, and 0.026 h, respectively, and the t1/2β values were 0.753, 0.630, and 0.574 h. The AUC was related to the dose, and the compartmental model was a two-compartment model. After intravenous injection of an effective dose (200 µg/kg) of triptolide in rats, rapid and extensive tissue distribution was observed. After 5 min of administration, the highest triptolide levels were noted in lung tissue followed by liver, kidney, heart, brain, spleen, small intestine, gonads, skeletal muscle, and stomach. Fifteen min after administration, the drug concentrations in all tissues decreased with higher concentrations in the lung, kidney, heart and liver. The distribution order is similar to that noted 5 min after administration. One hour after administration, the drug concentration in each tissue decreased significantly, and high drug concentration was still maintained in the liver and small intestine (Shao et al., 2007).
Celastrol exhibited linearity in the concentration range of 0.05–5 µg/ml with R2 of 0.999. Rats were intravenously (IV) administered 1 mg/kg of pure CL in PEG 300 solution, which resulted in a maximum concentration (Cmax) value of 0.17 µg/ml at 5 min following administration (Onyeabor et al., 2019). After oral administration of Tripterygium wilfordii tablets (1 tablet/kg) to beagle dogs, the changes in plasma celastrol levels followed a one-compartment model (w = 1/cc), and the main pharmacokinetic parameters were Cmax (35.64 ± 9.540) μg·L−1, Tmax (2.62 ± 0.69) h, T1/2 (2.93 ± 0.29) h, CL (0.308 ± 0.056) Lkg −1·h−1, AUC0–12 (131.16 ± 31.94) μg·L·h−1, and AUC0–∞ (142.83 ± 37.57) μg·L·h−1 (Zhang et al., 2016). Glycyrrhizin significantly decreased the plasma concentration (from 64.36 ng/ml to 38.42 ng/ml) and AUC0-t (from 705.39 to 403.43 μg h/L) of celastrol in rats, and increased the efflux ratio of celastrol (4.02 vs. 6.51). Moreover, glycyrrhizin significantly increased the intrinsic clearance rate of celastrol from 20.3 ± 3.37 to 38.8 ± 4.18 μL/min/mg proteins (Yan et al., 2017).
Toxicity
The targets of toxicity were closely associated with the p53 signalling pathway, PI3K-Akt signalling pathway and other pathways. Although THH has multiple phytochemical activities and pharmacological effects, it is also limited in clinical application given its toxicities associated with multiple targets (Xu et al., 2004). Although triptolide has attracted remarkable efficacy against diseases, the safety margin of the triptolide medication dose is very narrow, and the accumulation of triptolide in the body can lead to severe hepatotoxicity, renal toxicity, and reproductive toxicity (Sun et al., 2013; Kong et al., 2015; Ma et al., 2015). Therefore, dose control is particularly important in clinical treatment (Figure 5).
FIGURE 5.
Illustration of the main signalling pathways involved in THH-based therapy and organ toxicities observed at high doses.
Triptolide mainly causes abnormal purine and pyrimidine metabolites in the body through the metabolism of hypoxanthine, allantoic acid, ADA and other proteins. The mechanism of triptolide-induced liver injury exhibits an important relationship with oxidative stress, and excessive ROS and anionic peroxide production, depletion, autophagy, hepatocyte apoptosis, and suppression of antioxidant enzyme activities are the main causes of oxidative stress (Fu et al., 2011; Xi et al., 2017; Wei et al., 2019). Thus, hepatocyte injury is mainly caused by high levels of ROS and reactive nitrogen species (RNS) inhibiting sodium, ATPase, and calcium pump activities in the cell membrane, resulting in decreased mitochondrial membrane potential (MMP) and release of the apoptosis factor caspase-3 (Li J. et al., 2014; Wang Y. et al., 2018; Tabeshpour et al., 2018; Feng Z. et al., 2019; Yuan et al., 2019b; Hasnat et al., 2019). Moreover, triptolide significantly increased serum ALP, AST, and ALT levels; increased hepatic MDA, IL-6, TNF-α, IFN-γ, and IL-1β levels, induced nuclear translocation of NF-κB; and decreased hepatic GSH-Px, CAT, and SOD activities (Yang et al., 2017). It also regulates the metabolism of sphingolipids and glycerophospholipids by affecting metabolites and key proteins such as sphingosine, PC, PKC, PAH, LPC, and PLC, which cause disturbances in Ca2+ channels and stimulate oxidative stress levels, leading to kidney damage (Xie et al., 2020). Therefore, reducing liver toxicity and nephrotoxicity through chemical modification is an important topic for triptolide to be applied.
Triptolide induced cytotoxicity in L02 cells, caused mitochondrial dysfunction and altered mitochondrial dynamics, mainly characterized by an imbalance in mitochondrial fusion and fission protein mitochondrial fragmentation. Mitochondrial fragmentation can lead to increasing autophagic flux by triptolide. Changes in mitochondrial dynamics were associated with increasing expression of the Drp1 fission protein. Despite no significant effect on L02 cells, triptolide doses of 12.5, 25 and 50 nM were significantly toxic. Mdivi-1 is a potent mitochondrial fission inhibitor that reverses mitophagy by inhibiting mitochondrial fission and increasing mitophagy in vitro and in vivo (Hasnat et al., 2019). Treatment of mice with the PPARα agonist fenofibrate alleviated triptolide-induced liver injury, whereas the PPARα antagonist GW6471 increased hepatotoxicity and increased long-chain acylcarnitine through activation of the NOTCH-NRF2 pathway to protect against triptolide-induced liver damage (Hu et al., 2019). The hepatotoxicity of triptolide is closely related to its dose, indicating that the liver is more sensitive to other drugs (CYP450 inhibitors) and their accumulation. Thus, celastrol, which is also a hepatotoxic component of THH and an inhibitor of CYP450, could aggravate triptolide-induced hepatotoxicity, which may explain why the combination of celastrol and triptolide causes more severe liver injury. In summary, celastrol and triptolide damaged primary rat hepatocytes by decreasing cell viability, enhancing cellular stress, decreasing MMP, increasing LDH and ROS levels, and inevitably damaging the cell membrane (Jin C. et al., 2015; Jin et al., 2019).
However, Arctiin exerts antioxidant effects mediated by the Nrf2/ARE pathway and alleviates triptolide-induced hepatotoxicity, and its molecular mechanism may be related to its antioxidative stress ability (Jin J. et al., 2015; Wei et al., 2019). A novel mechanism of triptolide-induced hepatotoxicity is mitochondrial fission associated mitophagy, and triptolide-induced hepatotoxicity can be targeted by mitochondrial fission and mitochondrial autophagy signalling pathways (Hasnat et al., 2019). Specifically, 18β glycyrrhetinic acid (GA) is the main bioactive component of liquorice (Glycyrrhiza glabra L.), and low-dose GA (50 mg/kg) effectively reduces triptolide-induced hepatotoxicity in rats through its inflammatory, antioxidative, and antiapoptotic effects (Yang et al., 2017). GA reduced the accumulation of triptolide in HK-2 cells, which were related to P-gp (Li Z. et al., 2017). Pretreatment with GL significantly accelerates the metabolic elimination of triptolide from the body mainly through induction of hepatic CYP3A activity (Tai et al., 2014). Thus, the combination of triptolide with other natural compounds may be an effective measure to control its toxicity and ensure its safety, there still need more further research to investigate its mechanism of combination.
Discussion and Conclusion
Traditional Chinese medicine (TCM) has unique therapeutic advantages in the treatment of a variety of complex diseases and major diseases, such as cholestasis, nonalcoholic fatty liver disease, liver fibrosis, and ischaemic stroke (Jiang et al., 2020a; Hu et al., 2020). The genus Tripterygium contains only 3 species, i.e. Tripterygium wilfordii Hook. f., Tripterygium hypoglaucum (H. Lév.) Hutch. and Tripterygium regelii Sprague and Takeda. In the last 10 years, many studies have discussed the plants related to the genus Tripterygium. Among them, Tripterygium wilfordii Hook. f., as one of the widely reported plants, has occupied almost all the focus of the review of the genus Tripterygium. Individual scholars have included the chemical constituents and pharmacological activities of THH in their studies on the genus Tripterygium (Lv et al., 2019). In contrast, our study separately investigated the related active components and pharmacological effects of THH, providing more selectivity for the extraction of components from the genus Tripterygium. In addition, we updated the recently published experimental studies and supplemented the pharmacokinetic studies of compounds in THH, so as to provide some ideas for the development of new drugs.
In this review, we summarized the various bioactive components of THH, such as sesquiterpenes, diterpenes, triterpenoids, flavonoids, and lignans. In this review, we introduced the structural grouping of 120 secondary metabolites of THH and described the activity data obtained from THH research in the past 2 decades. The compounds isolated from TwHF, including triptolide and tripdiolide, induce male infertility (Wang et al., 2014). Although the prospects of male antifertility drugs have been explored, further safety evaluation results of this drug are needed. Therefore, THH is likely to be a genotoxic agent. Chinese investigators are cautious and suggest that patients should avoid reproduction for 6 months after THH therapy (Liang et al., 2006). In addition, we emphasized the pharmacological effects of triptolide, celastrol and other important bioactive components, and great progress has been made in the identification of the mechanisms associated with these components, such as anti-inflammatory, immunosuppressive, and anti-tumour effects. At 50 nM, triptolide could completely block the LPS-induced PGE2 release. Further, triptolide at 50 or 100 nM in primary microglial cells was not toxic (Jiao et al., 2008). In addition, the animals pretreated with triptolide (100 mg/kg) show significant attenuation of LPS-induced proinflammatory cytokine release in serum, including TNF-a, IL-6, and IL-12, but not IL-1β (Huang et al., 2019). Triptolide could suppress CD80 and CD86 expressions on IFN-γ (500 kU/L) and LPS (1 mg/L)-activated THP-1 cells at 2.5–0.625 µg/L (Liu et al., 2005). Celastrol caused a dose- and time-dependent growth inhibition of A549 cells with an IC50 of 2.12 μM at 48 h treatment (Mou et al., 2011). Celastrol at the optimum concentration of 30 nM was able to protect the cells from mitochondrial dysfunction and insulin resistance (Abu Bakar et al., 2017).
At present, the studies in vitro and in vivo are relatively separate from each other. There is a lack of relevance between experiments in vitro and in vivo especially in the aspect of dosage. In future studies, more attention should be paid to research to understand whether the concentration used in vitro can achieve the same efficacy in vivo. In conclusion, some data suggest that triptolide may possess anti-angiogenic effect in RA both in vivo and in vitro assay systems by downregulating the angiogenic activators and inhibiting the activation of mitogen-activated protein kinase downstream signal pathway (Kong et al., 2013). Triptonide effectively inhibited pancreatic cancer cell-formed capillary-like structures in vitro and blood vessels in vivo through suppressing pancreatic cancer cell migration, invasion, and VM via inhibiting expression of tumour VM master gene VE-cadherin and pro-migratory gene chemokine C-X-C motif ligand 2 (CXCL2), mainly via reduction of gene promoter activity (Han et al., 2018). This is particularly crucial for the exploration of anti-tumour effect. However, this promising effect seems less to be displayed in vivo. It is to be due to the complex system of the body. Therefore, from the perspective of animals or even humans, we should construct a systematic experiment synchronously carried out the experiment in vitro and in vivo. Furthermore, the dosage for these studies should confirm the stability of the effect. In addition, researchers have discovered new pharmacological effects of these compounds, including alleviation of AD and insulin resistance as well as anti-obesity, and cardioprotective effects. However, these herbs also have potential multiorgan toxicity that has not been addressed for many years (Wang L. et al., 2018). Pharmacodynamic and toxicological studies of THH are closely related to pharmacokinetics and tissue distribution. Due to the lack of studies on human pharmacokinetics, it is unclear whether the pharmacological effects obtained at the concentrations of compounds used in vitro can achieve the same efficacy in humans. Therefore, various toxic reactions might occur in clinical application. Many studies have reported the metabolic processing of toxic active compounds in vitro and in vivo, aiming to reduce drug toxicity and improve the safety of these agents from a metabolic point of view or using different methods.
Alternatively, many low toxicity active analogues have been developed by drug synthesis researchers from natural active products of the genus Tripterygium (Shan et al., 2017; Ning et al., 2018). The Chinese pharmacopoeia indicates that THH patent medicine (Kunming Shanhaitang tablet) is made from extracts of THH dried roots, and this is a legally licenced drug in China. Kunming Shanhaitang tablet is commonly used to treat RA. Inhibition of IL-2 production by human peripheral blood lymphocytes through nuclear inhibition of transcriptional activation of NF-κB, prevention of T cell proliferation, induction of apoptotic death of T lymphocytes, reduction of PGE2 production in human monocytes and rheumatoid arthritis synovial fibroblasts, suppression of complement C3 and the expression of CD40 and B7 in activated human proximal tubular epithelial cells by triptolide indicates a wide range therapeutic target cells of this Chinese herb. Dex is a well-known classical glucocorticoid with potent anti-inflammatory and immunosuppressive effects when gave in pharmacological doses. Though the effect of triptolide is not so strong as Dex in the clinical managements, it shares the properties of Dex such as anti-inflammation and immunosuppression. Here, we demonstrated that DC is a target of triptolide. Due to DC has the unique property to activate native T cells and is required for the induction of a primary response, the suppression of DC function may very efficiently control the specific immune response. One of the mechanisms by which triptolide can suppress the immune response in humans is by inhibiting differentiation, terminal maturation and function of DC. In addition, triptolide abrogates the capacity of mature DCs to secrete IL-12 and also promotes DC apoptosis. These effects result in inhibition of alloreactive T cell activation. Suppression of DC may contribute to the actions of triptolide in the treatment of immune-related diseases and for the prevention of allograft rejection. Adjuvant treatment of facial corticosteroid addiction dermatitis with Kunming Shanhaitang tablet effectively improved symptoms and manifestations within 2 months and was more beneficial than topical drugs alone in significantly improving symptoms in the first 2 weeks. In addition, the effect was comparable to that of antihistamine combined with topical drugs or topical drugs alone (Xue and Wu, 2008). The injection of THH demonstrates a significant inhibitory effect on the production of haemolysin antibodies in mice, and the effect is more obvious with a long period of dosing (Li X, 2006). Tripterygium agents (TAs) extracted from TwHF are a complementary therapy used to treat eczema based on previous experience. However, TA has significant side effects in the treatment of specific eczema, but seems to be effective only in combination with some therapies. Thus, researchers believe that TA cannot be used clinically for eczema in general (Liu et al., 2019). Less toxic preparation of THH could potentially be used as an alternative. In previous separation and extraction experiments, most of the chemical active ingredients of THH were extracted with 95% ethanol, but it was found that column chromatography was replaced by sodium carbonate extraction for removing the acidic compounds and enriching epoxyditerpenoids and alkaloids in the extract. The therapeutic index (IC50/EC50) on murine macrophage Raw 264.7 cells and rat mesangial HBZY-1 cells of the extract prepared by sodium carbonate extraction was significantly higher than that of Tripterygium glycosides (0.8 and 5.2 vs. 0.3 and 2.6), while its cytotoxicity on human liver HL7702 cells was significantly lower (14.5 ± 1.4 vs. 6.8 ± 0.9,). Therefore, Tripterygium extract prepared by sodium carbonate extraction may represent a potentially optimal source of medicine with good therapeutic index (Fang et al., 2012). Therefore, many studies have shown that THH is a potential source of various drugs. Here, we consulted the literature and summarized the useful information and evidence. From the current research, it can be seen that a variety of active components of THH show significant efficacy and great potential in many major diseases. Toxicity is the main factor limiting THH in drug development. To date, the research on the mechanism of toxicity caused by THH is not sufficient. A full understanding of the mechanism of toxicity induced by THH is the premise to effectively reduce the toxicity of THH, and it is also the key to the development of THH efficacy. So do other plants of the genus Tripterygium. Through continuous in-depth studies of THH and TwHF, our understanding of their structure, pharmacology, toxicology and other aspects will be further advanced, which will provide a solid theoretical basis for their clinical application. In the future, if we focus on preserving the active ingredients and minimizing the toxic components of the preparations, these agents will certainly have broader prospects in various fields.
In conclusion, THH has abundant chemically active components, and some representative components (triptolide, celastrol, etc.,) have been demonstrated to have significant anti-inflammatory, immunosuppressive and anti-tumour effects. Meanwhile, the toxicity of THH cannot be underestimated. How to effectively control the toxicity of THH and obtain the maximum therapeutic effect is still a problem to be solved. The combination of traditional theory and modern advanced technology will be an important goal for the efficacy and safety of traditional Chinese medicine.
Acknowledgments
The authors would like to thank the reviewers and also the authors of all references. The reviewer’s advice really makes the great improvement of this paper.
Author Contributions
JZ is the major contributor to this manuscript. JZ conducted the analytical part, wrote the first version of the manuscript, FZ, XX, and ZW finalized the manuscript. XX, YJ, and WZ, downloaded the reference and processed the graph and the table in the manuscript. SW, QH, collected the data. XM and XZ (corresponding author) conceived and coordinated the study, and critically evaluated the data. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (81874365, 81703725), Sichuan Science and Technology Program (2019YJ0492), Beijing Medical and Health Foundation (YWJKJJHKYJJ-B20645FN), Chengdu University of TCM Found Grant (QNXZ2018025), National Major New Drug Creation Major Science and Technology Projects (2017ZX09101002-002-004), Natural Science Foundation of Chongqing (cstc2017jcyjAX0001, cstc2018jcyjAX0142).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- 17β-HSD
17β-hydroxysteroid dehydrogenase
- 2-HIB
2-hydroxyisobutyrate
- 2-HIV
2-hydroxyisovalerate
- 3β-HSD
3β-hydroxysteroid dehydrogenase
- ABCA1
ATP-binding cassette transporter A1
- AD
Alzheimer’s disease
- aGVHD
acute graft versus host disease
- ALP
alkaline phosphatase
- ALT
alanine aminotransfease
- AML
acute myeloid leukaemia
- AMPKα
Adenosine 5′-monophosphate (AMP)-activated protein kinase
- APCs
antigen presenting cells
- APL
acute promyelocytic leukemia
- ARC
hypothalamic arcuate nucleus
- As 2 O 3
arsenic trioxide
- AST
aspartate aminotransferase
- ATL
adult T-cell leukaemia/lymphoma
- AUC
Area Under Curve
- Bcl-xL
B-cell lymphoma-2
- CD
Crohn’s disease
- c-Fos
Cellular oncogene fos
- CIA
collagen II-induced arthritis
- c-Jun
Cellular Jun
- c-Myc
cancer-Myc
- COX
cyclo-oxygen-ase
- CPT1A
Carnitine Palmitoyl transferase 1A
- CRC
colorectal carcinoma
- Ctr
calcitonin receptor
- Ctsk
cathepsin K
- CXCL2
C-X-C motif ligand 2
- DCs
dendritic cells
- Drp1
dynamin-related protein 1
- EBV
Epstein-Barr virus
- ERK
extracellular regulated protein kinases
- Foxp3
Forkhead box P3
- GA
glycyrrhetinic acid
- GCAFs
gastric cancer-associated fibroblasts
- GLUT1
glucose transporter 1
- GLUT4
glucose transporter 4
- GPx
glutathione peroxidase;
- GSH
glutathione
- HCV
hepatitis C virus
- HIF-1α
hypoxia-inducible factor 1-α
- HK2
hexokinase 2
- HLA-DR
human leukocyte antigen DR
- HO-1
haem oxygenase-1
- HSF1
heatshocktranscription factor 1
- HSP70
Heat shock 70 kDa protein
- HUVEC
human umbilical vein endothelial cells
- IFN-g
interferon gamma
- IKK
IκB kinase
- IL1R1
interleukin 1 receptor 1
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- iNOS
inducible Nitric Oxide Synthase (enzyme)
- JNK1
c-Jun N-terminal kinase 1
- LDH
Lactic Dehydrogenase
- LDL/VLDL
low-density lipoprotein/very low-density lipoprotein
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MCP
monocyte chemotactic protein
- MDA
malondialdehyde
- MDM2
murine double minute2
- MDR
multidrug resistance
- MIP
macrophage inflammatory protein
- miR-96
microRNA-96
- MLRs
mixed lymphocyte reactions
- Mmp-9
matrix metalloprotein 9
- MMPs
matrix metalloproteinases
- MoDCs
monocyte-derived DCs
- mTOR
mammalian target of Rapamycin
- NAC
N-acetylcysteine
- NADPH
nicotinamide adenine dinucleotide phosphate
- NFATc
nuclear factor activated T-cells
- NF-κB
nuclear factor kappa-B
- NO
nitric oxide
- OPG
osteoprotegerin
- P450c17
cytochrome P450 17-hydroxylase
- P450scc
P450 side-chain cleavage enzyme
- PARP
poly ADP-ribose polymerase
- PBMCs
peripheral blood mononuclear cells
- PC
Pancreatic cancer
- PFKP
Phosphofructokinase Platelet
- PGE2
prostaglandin E2
- P-gp
P-glycoprotein
- PI3K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- PKCθ
Protein Kinase C θ
- PLC
phospholipase C
- proMMP-1
pro-matrix metalloproteinase-1
- PTP
protein tyrosine phosphatase
- RA
rheumatoid arthritis
- RANKL
receptor activator of NF-κB ligand
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- StAR
steroidogenic acute regulatory protein
- STAT
signal transducers and activators of transcription
- TAB1
TAK1 binding protein
- TAK1
transforming growth factor β-activated kinase 1
- TAs
Tripterygium agents
- TCM
Traditional Chinese medicine
- TCPTP
T cell PTP
- THH
Tripterygium hypoglaucum (Lévl.) Hutch.
- TLR
Toll-like receptors
- TNF-α
tumour necrosis factor-α
- TRAIL
TNF-related apoptosis-inducing ligand
- TRAP
tartrate-resistant acid phosphatase
- Tregs
T regulatory cells
- TwHF
Tripterygium wilfordii Hook F.
- VEGF
vascular endothelial growth factor
- XIAP
X-linked inhibitor of apoptosis protein
References
- Abdin A. A., Hasby E. A. (2014). Modulatory Effect of Celastrol on Th1/Th2 Cytokines Profile, TLR2 and CD3+ T-Lymphocyte Expression in a Relapsing-Remitting Model of Multiple Sclerosis in Rats. Eur. J. Pharmacol. 742, 102–112. 10.1016/j.ejphar.2014.09.001 [DOI] [PubMed] [Google Scholar]
- Abu Bakar M. H., Cheng K.-K., Sarmidi M. R., Yaakob H., Huri H. Z. (2015). Celastrol Protects against Antimycin A-Induced Insulin Resistance in Human Skeletal Muscle Cells. Molecules 20, 8242–8269. 10.3390/molecules20058242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu Bakar M. H., Sarmidi M. R., Tan J. S., Mohamad Rosdi M. N. (2017). Celastrol Attenuates Mitochondrial Dysfunction and Inflammation in Palmitate-Mediated Insulin Resistance in C3A Hepatocytes. Eur. J. Pharmacol. 799, 73–83. 10.1016/j.ejphar.2017.01.043 [DOI] [PubMed] [Google Scholar]
- Abu Bakar M. H., Tan J. S. (2017). Improvement of Mitochondrial Function by Celastrol in Palmitate-Treated C2C12 Myotubes via Activation of PI3K-Akt Signalling Pathway. Biomed. Pharmacother. 93, 903–912. 10.1016/j.biopha.2017.07.021 [DOI] [PubMed] [Google Scholar]
- Bakar M. H. A., Sarmidi M. R., Kai C. K., Huri H. Z., Yaakob H. (2014). Amelioration of Mitochondrial Dysfunction-Induced Insulin Resistance in Differentiated 3T3-L1 Adipocytes via Inhibition of NF-Κb Pathways. Int. J. Mol. Sci. 15, 22227–22257. 10.3390/ijms151222227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. T., Borris R. P., Carté B., Cordell G. A., Soejarto D. D., Cragg G. M., et al. (1995). Natural Product Drug Discovery and Development: New Perspectives on International Collaboration. J. Nat. Prod. 58, 1325–1357. 10.1021/np50123a003 [DOI] [PubMed] [Google Scholar]
- Booij T. H., Leonhard W. N., Bange H., Yan K., Fokkelman M., Plugge A. J., et al. (2020). In Vitro 3D Phenotypic Drug Screen Identifies Celastrol as an Effective In Vivo Inhibitor of Polycystic Kidney Disease. J. Mol. Cel Biol. 12, 644–653. 10.1093/jmcb/mjz029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinker A. M., Ma J., Lipsky P. E., Raskin I. (2007). Medicinal Chemistry and Pharmacology of Genus Tripterygium (Celastraceae). Phytochemistry 68, 732–766. 10.1016/j.phytochem.2006.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascão R., Vidal B., Raquel H., Neves-Costa A., Figueiredo N., Gupta V., et al. (2012). Effective Treatment of Rat Adjuvant-Induced Arthritis by Celastrol. Autoimmun. Rev. 11, 856–862. 10.1016/j.autrev.2012.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T. Y. K. (2012). Aconitum Alkaloid Content and the High Toxicity of Aconite Tincture. Forensic Sci. Int. 222, 1–3. 10.1016/j.forsciint.2012.02.026 [DOI] [PubMed] [Google Scholar]
- Chan T. Y. K. (2011). Causes and Prevention of Herb-Induced Aconite Poisonings in Asia. Hum. Exp. Toxicol. 30, 2023–2026. 10.1177/0960327111407224 [DOI] [PubMed] [Google Scholar]
- Chang W., He W., Li P.-P., Song S.-S., Yuan P.-F., Lu J.-T., et al. (2016). Protective Effects of Celastrol on Diethylnitrosamine-Induced Hepatocellular Carcinoma in Rats and its Mechanisms. Eur. J. Pharmacol. 784, 173–180. 10.1016/j.ejphar.2016.04.045 [DOI] [PubMed] [Google Scholar]
- Chen G., Zhang X., Zhao M., Wang Y., Cheng X., Wang D., et al. (2011). Celastrol Targets Mitochondrial Respiratory Chain Complex I to Induce Reactive Oxygen Species-dependent Cytotoxicity in Tumour Cells. BMC Cancer 11, 170. 10.1186/1471-2407-11-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.-R., Dai Y., Zhao J., Lin L., Wang Y., Wang Y. (2018a). A Mechanistic Overview of Triptolide and Celastrol, Natural Products from Tripterygium Wilfordii Hook F. Front. Pharmacol. 9, 104. 10.3389/fphar.2018.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.-L., Liu F., Xiao X.-R., Yang X.-W., Li F. (2018b). Anti-inflammatory Abietanes Diterpenoids Isolated from Tripterygium Hypoglaucum. Phytochemistry 156, 167–175. 10.1016/j.phytochem.2018.10.001 [DOI] [PubMed] [Google Scholar]
- Chen X., Murakami T., Oppenheim J. J., Howard O. M. Z. (2005). Triptolide, a Constituent of Immunosuppressive Chinese Herbal Medicine, Is a Potent Suppressor of Dendritic-Cell Maturation and Trafficking. Blood 106, 2409–2416. 10.1182/blood-2005-03-0854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H.-Y., Lin C.-C., Lin T.-C. (2002). Antiherpes Simplex Virus Type 2 Activity of Casuarinin from the Bark of Terminalia Arjuna Linn. Antivir. Res 55, 447–455. 10.1016/s0166-3542(02)00077-3 [DOI] [PubMed] [Google Scholar]
- Collins R. R. J., Patel K., Putnam W. C., Kapur P., Rakheja D. (2017). Oncometabolites: A New Paradigm for Oncology, Metabolism, and the Clinical Laboratory. Clin. Chem. 63, 1812–1820. 10.1373/clinchem.2016.267666 [DOI] [PubMed] [Google Scholar]
- Costa C., Incio J., Soares R. (2007). Angiogenesis and Chronic Inflammation: Cause or Consequence? Angiogenesis 10, 149–166. 10.1007/s10456-007-9074-0 [DOI] [PubMed] [Google Scholar]
- Ding L., Zhang Z. (1991). Studies on Chemical Constituents of Tripterygium Hypoglaucum Stems II. Nanjing J. China Pharm. Univ., 25–26. [PubMed] [Google Scholar]
- Duan H., Kawazoe K., Bando M., Kido M., Takaishi Y. (1997). Di- and Triterpenoids from Tripterygium Hypoglaucum. Phytochemistry 46, 103–110. 10.1016/s0031-9422(97)00288-4 9276982 [DOI] [Google Scholar]
- Duan H., Takaishi Y., Imakura Y., Jia Y., Li D., Cosentino L. M., et al. (2000). Sesquiterpene Alkaloids from Tripterygium Hypoglaucum and Tripterygium Wilfordii: a New Class of Potent Anti-HIV Agents. J. Nat. Prod. 63, 357–361. 10.1021/np990281s [DOI] [PubMed] [Google Scholar]
- Duan H., Takaishi Y. (1999). Sesquiterpene Evoninate Alkaloids from Tripterygium Hypoglaucum. Phytochemistry 52, 1735–1738. 10.1016/s0031-9422(99)00179-x [DOI] [Google Scholar]
- Duan H., Takaishi Y. (1998). Structures of Sesquiterpene Polyol Alkaloids from Tripterygium Hypoglaucum. Phytochemistry 49, 357. 10.1016/s0031-9422(98)00439-7 [DOI] [Google Scholar]
- Dudics S., Langan D., Meka R. R., Venkatesha S. H., Berman B. M., Che C.-T., et al. (2018). Natural Products for the Treatment of Autoimmune Arthritis: Their Mechanisms of Action, Targeted Delivery, and Interplay with the Host Microbiome. Int. J. Mol. Sci. 19, 2508. 10.3390/ijms19092508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hajj H., Dassouki Z., Berthier C., Raffoux E., Ades L., Legrand O., et al. (2015). Retinoic Acid and Arsenic Trioxide Trigger Degradation of Mutated NPM1, Resulting in Apoptosis of AML Cells. Blood 125, 3447–3454. 10.1182/blood-2014-11-612416 [DOI] [PubMed] [Google Scholar]
- Fan D., Guo Q., Shen J., Zheng K., Lu C., Zhang G., et al. (2018). The Effect of Triptolide in Rheumatoid Arthritis: From Basic Research towards Clinical Translation. Int. J. Mol. Sci. 19, 376. 10.3390/ijms19020376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D., He X., Bian Y., Guo Q., Zheng K., Zhao Y., et al. (2016). Triptolide Modulates TREM-1 Signal Pathway to Inhibit the Inflammatory Response in Rheumatoid Arthritis. Int. J. Mol. Sci. 17, 498. 10.3390/ijms17040498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X.-X., Li N., Wu J.-L., Zhou Y.-L., He J.-X., Liu L., et al. (2014). Celastrol Induces Apoptosis in Gefitinib-Resistant Non-small Cell Lung Cancer Cells via Caspases-dependent Pathways and Hsp90 Client Protein Degradation. Molecules 19, 3508–3522. 10.3390/molecules19033508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W., Peng F., Yi T., Zhang C., Wan C., Xu H., et al. (2012). Biological Activity and Safety of Tripterygium Extract Prepared by Sodium Carbonate Extraction. Molecules 17, 11113–11123. 10.3390/molecules170911113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., Guan D., Auen T., Choi J. W., Salazar Hernández M. A., Lee J., et al. (2019a). IL1R1 Is Required for Celastrol’s Leptin-Sensitization and Antiobesity Effects. Nat. Med. 25, 575–582. 10.1038/s41591-019-0358-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., Lv C., Wang F., Gan K., Zhang M., Tan W. (2013). Modulatory Effect of 1,25-dihydroxyvitamin D 3 on IL1 β -induced RANKL, OPG, TNF α , and IL-6 Expression in Human Rheumatoid Synoviocyte MH7A. Clin. Dev. Immunol. 2013, 160123. 10.1155/2013/160123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z., Zhou C., Dong S., Liu Z., Liu T., Zhou L., et al. (2019b). Catalpol and Panax Notoginseng Saponins Synergistically Alleviate Triptolide-Induced Hepatotoxicity through Nrf2/ARE Pathway. Toxicol. Vitro 56, 141–149. 10.1016/j.tiv.2019.01.016 [DOI] [PubMed] [Google Scholar]
- Fu Q., Huang X., Shu B., Xue M., Zhang P., Wang T., et al. (2011). Inhibition of Mitochondrial Respiratory Chain Is Involved in Triptolide-Induced Liver Injury. Fitoterapia 82, 1241–1248. 10.1016/j.fitote.2011.08.019 [DOI] [PubMed] [Google Scholar]
- Fujita R., Duan H., Takaishi Y. (2000). Terpenoids from Tripterigyum Hypoglaucum. Phytochemistry 53, 715–722. 10.1016/s0031-9422(99)00557-9 [DOI] [PubMed] [Google Scholar]
- Gan K., Xu L., Feng X., Zhang Q., Wang F., Zhang M., et al. (2015). Celastrol Attenuates Bone Erosion in Collagen-Induced Arthritis Mice and Inhibits Osteoclast Differentiation and Function in RANKL-Induced RAW264.7. Int. Immunopharmacol. 24, 239–246. 10.1016/j.intimp.2014.12.012 [DOI] [PubMed] [Google Scholar]
- Gong Y., Xue B., Jiao J., Jing L., Wang X. (2008). Triptolide Inhibits COX-2 Expression and PGE2 Release by Suppressing the Activity of NF-kappaB and JNK in LPS-Treated Microglia. J. Neurochem. 107, 779–788. 10.1111/j.1471-4159.2008.05653.x [DOI] [PubMed] [Google Scholar]
- Guo Y., Wang Y., Shi X., Qin W., Zhang X., Xu J., et al. (2018). A Metabolomics Study on the Immunosuppressive Effect of Tripterygium Hypoglaucum (Levl.) Hutch in Mice: The Discovery of Pathway Differences in Serum Metabolites. Clin. Chim. Acta 483, 94–103. 10.1016/j.cca.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Han H., Du L., Cao Z., Zhang B., Zhou Q. (2018). Triptonide Potently Suppresses Pancreatic Cancer Cell-Mediated Vasculogenic Mimicry by Inhibiting Expression of VE-Cadherin and Chemokine Ligand 2 Genes. Eur. J. Pharmacol. 818, 593–603. 10.1016/j.ejphar.2017.11.019 [DOI] [PubMed] [Google Scholar]
- Harmey J. H., Bucana C. D., Lu W., Byrne A. M., McDonnell S., Lynch C., et al. (2002). Lipopolysaccharide-induced Metastatic Growth Is Associated with Increased Angiogenesis, Vascular Permeability and Tumour Cell Invasion. Int. J. Cancer 101, 415–422. 10.1002/ijc.10632 [DOI] [PubMed] [Google Scholar]
- Hasnat M., Yuan Z., Naveed M., Khan A., Raza F., Xu D., et al. (2019). Drp1-associated Mitochondrial Dysfunction and Mitochondrial Autophagy: a Novel Mechanism in Triptolide-Induced Hepatotoxicity. Cell Biol. Toxicol. 35, 267–280. 10.1007/s10565-018-9447-8 [DOI] [PubMed] [Google Scholar]
- Hernández-Aguilera A., Rull A., Rodríguez-Gallego E., Riera-Borrull M., Luciano-Mateo F., Camps J., et al. (2013). Mitochondrial Dysfunction: a Basic Mechanism in Inflammation-Related Non-communicable Diseases and Therapeutic Opportunities. Mediators Inflamm. 2013, 135698. 10.1155/2013/135698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D.-D., Zhao Q., Cheng Y., Xiao X.-R., Huang J.-F., Qu Y., et al. (2019). The Protective Roles of PPARα Activation in Triptolide-Induced Liver Injury. Toxicol. Sci. 171, 1–12. 10.1093/toxsci/kfz146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q., Wei S., Wen J., Zhang W., Jiang Y., Qu C., et al. (2020). Network Pharmacology Reveals the Multiple Mechanisms of Xiaochaihu Decoction in the Treatment of Non-alcoholic Fatty Liver Disease. BioData Min 13, 11. 10.1186/s13040-020-00224-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Zhu N., Chen T., Chen W., Kong J., Zheng W., et al. (2019). Triptolide Suppressed the Microglia Activation to Improve Spinal Cord Injury through miR-96/ikkβ/nf-Κb Pathway. Spine (Phila. Pa. 1976) 44, E707–E714. 10.1097/BRS.0000000000002989 [DOI] [PubMed] [Google Scholar]
- Jang S. Y., Jang S.-W., Ko J. (2011). Celastrol Inhibits the Growth of Estrogen Positive Human Breast Cancer Cells through Modulation of Estrogen Receptor α. Cancer Lett. 300, 57–65. 10.1016/j.canlet.2010.09.006 [DOI] [PubMed] [Google Scholar]
- Jiang X., Huang X., Ao L., Liu W., Han F., Cao J., et al. (2014). Total Alkaloids of Tripterygium Hypoglaucum (levl.) Hutch Inhibits Tumour Growth Both In Vitro and In Vivo . J. Ethnopharmacol. 151, 292–298. 10.1016/j.jep.2013.10.045 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Wen J., Zhang W., Ma Z., Zhang C., Wang J., et al. (2020a). Metabolomics Coupled with Integrative Pharmacology Reveals the Therapeutic Effect of L-Borneolum against Cerebral Ischaemia in Rats. J. Pharm. Pharmacol. 72, 1256–1268. 10.1111/jphp.13294 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Zhong M., Long F., Yang R. (2020b). Deciphering the Active Ingredients and Molecular Mechanisms of Tripterygium Hypoglaucum (Levl.) Hutch against Rheumatoid Arthritis Based on Network Pharmacology. Evid. Based. Complement. Alternat. Med. 2020, 2361865. 10.1155/2020/2361865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J., Xue B., Zhang L., Gong Y., Li K., Wang H., et al. (2008). Triptolide Inhibits Amyloid-Beta1-42-Induced TNF-Alpha and IL-1beta Production in Cultured Rat Microglia. J. Neuroimmunol. 205, 32–36. 10.1016/j.jneuroim.2008.08.006 [DOI] [PubMed] [Google Scholar]
- Jin C., He X., Zhang F., He L., Chen J., Wang L., et al. (2015a). Inhibitory Mechanisms of Celastrol on Human Liver Cytochrome P450 1A2, 2C19, 2D6, 2E1 and 3A4. Xenobiotica 45, 571–577. 10.3109/00498254.2014.1003113 [DOI] [PubMed] [Google Scholar]
- Jin C., Wu Z., Wang L., Kanai Y., He X. (2019). CYP450s-Activity Relations of Celastrol to Interact with Triptolide Reveal the Reasons of Hepatotoxicity of Tripterygium Wilfordii. Molecules 24, 2162. 10.3390/molecules24112162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Sun X., Zhao Z., Wang W., Qiu Y., Fu X., et al. (2015b). Activation of the Farnesoid X Receptor Attenuates Triptolide-Induced Liver Toxicity. Phytomedicine 22, 894–901. 10.1016/j.phymed.2015.06.007 [DOI] [PubMed] [Google Scholar]
- Joshi V., Venkatesha S. H., Ramakrishnan C., Nanjaraj Urs A. N., Hiremath V., Moudgil K. D., et al. (2016). Celastrol Modulates Inflammation through Inhibition of the Catalytic Activity of Mediators of Arachidonic Acid Pathway: Secretory Phospholipase A(2) Group IIA, 5-lipoxygenase and Cyclooxygenase-2. Pharmacol. Res. 113, 265–275. 10.1016/j.phrs.2016.08.035 [DOI] [PubMed] [Google Scholar]
- Jung H. W., Chung Y. S., Kim Y. S., Park Y.-K. (2007). Celastrol Inhibits Production of Nitric Oxide and Proinflammatory Cytokines through MAPK Signal Transduction and NF-kappaB in LPS-Stimulated BV-2 Microglial Cells. Exp. Mol. Med. 39, 715–721. 10.1038/emm.2007.78 [DOI] [PubMed] [Google Scholar]
- Kchour G., Tarhini M., Kooshyar M.-M., El Hajj H., Wattel E., Mahmoudi M., et al. (2009). Phase 2 Study of the Efficacy and Safety of the Combination of Arsenic Trioxide, Interferon Alpha, and Zidovudine in Newly Diagnosed Chronic Adult T-Cell Leukemia/lymphoma (ATL). Blood 113, 6528–6532. 10.1182/blood-2009-03-211821 [DOI] [PubMed] [Google Scholar]
- Khairul I., Wang Q. Q., Jiang Y. H., Wang C., Naranmandura H. (2017). Metabolism, Toxicity and Anticancer Activities of Arsenic Compounds. Oncotarget 8, 23905–23926. 10.18632/oncotarget.14733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. H., Shin E. K., Kim Y. H., Lee B. W., Jun J.-G., Park J. H. Y., et al. (2009). Suppression of Inflammatory Responses by Celastrol, a Quinone Methide Triterpenoid Isolated from Celastrus Regelii. Eur. J. Clin. Invest. 39, 819–827. 10.1111/j.1365-2362.2009.02186.x [DOI] [PubMed] [Google Scholar]
- Kim S. T., Kim S. Y., Lee J., Kim K., Park S. H., Park Y. S., et al. (2018). Triptolide as a Novel Agent in Pancreatic Cancer: the Validation Using Patient Derived Pancreatic Tumour Cell Line. BMC Cancer 18, 1103. 10.1186/s12885-018-4995-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-H., Lee S.-H., Lee J.-Y., Choi S.-W., Park J.-W., Kwon T. K. (2004). Triptolide Inhibits Murine-Inducible Nitric Oxide Synthase Expression by Down-Regulating Lipopolysaccharide-Induced Activity of Nuclear Factor-Kappa B and C-Jun NH2-terminal Kinase. Eur. J. Pharmacol. 494, 1–9. 10.1016/j.ejphar.2004.04.040 [DOI] [PubMed] [Google Scholar]
- Kong L.-L., Zhuang X.-M., Yang H.-Y., Yuan M., Xu L., Li H. (2015). Inhibition of P-Glycoprotein Gene Expression and Function Enhances Triptolide-Induced Hepatotoxicity in Mice. Sci. Rep. 5, 11747. 10.1038/srep11747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X., Zhang Y., Liu C., Guo W., Li X., Su X., et al. (2013). Anti-angiogenic Effect of Triptolide in Rheumatoid Arthritis by Targeting Angiogenic cascade. PLoS One 8, e77513. 10.1371/journal.pone.0077513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer T., Chen X., Howard O. M. Z., Young H. A. (2005). Triptolide Attenuates Endotoxin- and Staphylococcal Exotoxin-Induced T-Cell Proliferation and Production of Cytokines and Chemokines. Immunopharmacol. Immunotoxicol. 27, 53–66. 10.1081/iph-51294 [DOI] [PubMed] [Google Scholar]
- Kupchan S. M., Court W. A., Dailey R. G. J., Gilmore C. J., Bryan R. F. (1972). Triptolide and Tripdiolide, Novel Antileukemic Diterpenoid Triepoxides from Tripterygium Wilfordii. J. Am. Chem. Soc. 94, 7194–7195. 10.1021/ja00775a078 [DOI] [PubMed] [Google Scholar]
- Kyriakou E., Schmidt S., Dodd G. T., Pfuhlmann K., Simonds S. E., Lenhart D., et al. (2018). Celastrol Promotes Weight Loss in Diet-Induced Obesity by Inhibiting the Protein Tyrosine Phosphatases PTP1B and TCPTP in the Hypothalamus. J. Med. Chem. 61, 11144–11157. 10.1021/acs.jmedchem.8b01224 [DOI] [PubMed] [Google Scholar]
- Lee H.-W., Jang K. S. Bin., Choi H. J., Jo A., Cheong J.-H., Chun K.-H. (2014). Celastrol Inhibits Gastric Cancer Growth by Induction of Apoptosis and Autophagy. BMB Rep. 47, 697–702. 10.5483/bmbrep.2014.47.12.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-H., Koo T. H., Yoon H., Jung H. S., Jin H. Z., Lee K., et al. (2006). Inhibition of NF-Kappa B Activation through Targeting I Kappa B Kinase by Celastrol, a Quinone Methide Triterpenoid. Biochem. Pharmacol. 72, 1311–1321. 10.1016/j.bcp.2006.08.014 [DOI] [PubMed] [Google Scholar]
- Lee J.-H., Won Y.-S., Park K.-H., Lee M.-K., Tachibana H., Yamada K., et al. (2012). Celastrol Inhibits Growth and Induces Apoptotic Cell Death in Melanoma Cells via the Activation ROS-dependent Mitochondrial Pathway and the Suppression of PI3K/AKT Signalling. Apoptosis 17, 1275–1286. 10.1007/s10495-012-0767-5 [DOI] [PubMed] [Google Scholar]
- Lee J. Y., Lee B. H., Kim N. D., Lee J. Y. (2015). Celastrol Blocks Binding of Lipopolysaccharides to a Toll-like Receptor4/myeloid Differentiation Factor2 Complex in a Thiol-dependent Manner. J. Ethnopharmacol. 172, 254–260. 10.1016/j.jep.2015.06.028 [DOI] [PubMed] [Google Scholar]
- Li C., Li Z., Zhang T., Wei P., Li N., Zhang W., et al. (2019). (1)H NMR-Based Metabolomics Reveals the Antitumour Mechanisms of Triptolide in BALB/c Mice Bearing CT26 Tumours. Front. Pharmacol. 10, 1175. 10.3389/fphar.2019.01175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Liu D., Zhang Y., Qian Y., Zhang H., Guo S., et al. (2013a). Celastrol Inhibits Lipopolysaccharide-Stimulated Rheumatoid Fibroblast-like Synoviocyte Invasion through Suppression of TLR4/NF-Κb-Mediated Matrix Metalloproteinase-9 Expression. PLoS One 8, e68905. 10.1371/journal.pone.0068905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Ren J., Wang G., Gu G., Hu D., Ren H., et al. (2014a). T2 Enhances In Situ Level of Foxp3+ Regulatory Cells and Modulates Inflammatory Cytokines in Crohn’s Disease. Int. Immunopharmacol. 18, 244–248. 10.1016/j.intimp.2013.12.014 [DOI] [PubMed] [Google Scholar]
- Li H., Zhang Y.-Y., Tan H.-W., Jia Y.-F., Li D. (2008). Therapeutic Effect of Tripterine on Adjuvant Arthritis in Rats. J. Ethnopharmacol. 118, 479–484. 10.1016/j.jep.2008.05.028 [DOI] [PubMed] [Google Scholar]
- Li J., Jin J., Li M., Guan C., Wang W., Zhu S., et al. (2012a). Role of Nrf2 in protection against Triptolide-Induced Toxicity in Rat Kidney Cells. Toxicol. Lett. 213, 194–202. 10.1016/j.toxlet.2012.07.008 [DOI] [PubMed] [Google Scholar]
- Li J., Shen F., Guan C., Wang W., Sun X., Fu X., et al. (2014b). Activation of Nrf2 Protects against Triptolide-Induced Hepatotoxicity. PLoS One 9, e100685. 10.1371/journal.pone.0100685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Liu X., He Y., Zheng Q., Wang M., Wu Y., et al. (2017a). Celastrol Attenuates Angiotensin II Mediated Human Umbilical Vein Endothelial Cells Damage through Activation of Nrf2/ERK1/2/Nox2 Signal Pathway. Eur. J. Pharmacol. 797, 124–133. 10.1016/j.ejphar.2017.01.027 [DOI] [PubMed] [Google Scholar]
- Li P.-P., He W., Yuan P.-F., Song S.-S., Lu J.-T., Wei W. (2015a). Celastrol Induces Mitochondria-Mediated Apoptosis in Hepatocellular Carcinoma Bel-7402 Cells. Am. J. Chin. Med. 43, 137–148. 10.1142/S0192415X15500093 [DOI] [PubMed] [Google Scholar]
- Li X.-J., Jiang Z.-Z., Zhang L. (2014c). Triptolide: Progress on Research in Pharmacodynamics and Toxicology. J. Ethnopharmacol. 155, 67–79. 10.1016/j.jep.2014.06.006 [DOI] [PubMed] [Google Scholar]
- Li X.-L., Gao L.-H., Li H.-M., Wang L.-T., Lee K.-H., Li R.-T. (2015b). Diterpenoids from the Stems of Tripterygium Hypoglaucum (Celastraceae) and Cytotoxic Evaluation. Phytochem. Lett. 12. 10.1016/j.phytol.2015.02.018 [DOI] [Google Scholar]
- Li X. G., He L. (2006). Pharmacological Control Study between Tripterygium Hypoglaucum Hutch and Tripterygium Wilfordii Hook F. J. Kunming Med. Coll. 12, 527723. 10.3969/j.issn.1005-9202.2011.18.040 [DOI] [Google Scholar]
- Li Y. B., Nie J., Lü C., Hu X. L., Xue Gy L. C. (2011). Influence of Triptolide on the Expression of Interleukin-1β and Prostaglandin E2 in Rat Microglia Induced by Beta-Amyloid Protein. Chin. J. Gerontol. 31, 3521–3523. [Google Scholar]
- Li Z., Wu X., Li J., Yao L., Sun L., Shi Y., et al. (2012b). Antitumour Activity of Celastrol Nanoparticles in a Xenograft Retinoblastoma Tumour Model. Int. J. Nanomedicine 7, 2389–2398. 10.2147/IJN.S29945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Yan M., Cao L., Fang P., Guo Z., Hou Z., et al. (2017b). Glycyrrhetinic Acid Accelerates the Clearance of Triptolide through P-Gp In Vitro . Phytother. Res. 31, 1090–1096. 10.1002/ptr.5831 [DOI] [PubMed] [Google Scholar]
- Li Z. Y., Wu Q., Yan Z., Li D., Pan X., Qiu T., et al. (2013b). Prevention of Acute GVHD in Mice by Treatment with Tripterygium Hypoglaucum Hutch Combined with Cyclosporin A. Hematology 18, 352–359. 10.1179/1607845413Y.0000000076 [DOI] [PubMed] [Google Scholar]
- Liang Z.-Q., Cao N., Song Z.-K., Wang X. (2006). In Vitro porcine Brain Tubulin Assembly Inhibition by Water Extract from a Chinese Medicinal Herb, Tripterygium Hypoglaucum Hutch. World J. Gastroenterol. 12, 1133–1135. 10.3748/wjg.v12.i7.1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.-F., Hsieh M.-J., Hsi Y.-T., Lo Y.-S., Chuang Y.-C., Chen M.-K., et al. (2017). Celastrol-induced Apoptosis in Human Nasopharyngeal Carcinoma Is Associated with the Activation of the Death Receptor and the Mitochondrial Pathway. Oncol. Lett. 14, 1683–1690. 10.3892/ol.2017.6346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N., Liu C., Xiao C., Jia H., Imada K., Wu H., et al. (2007). Triptolide, a Diterpenoid Triepoxide, Suppresses Inflammation and Cartilage Destruction in Collagen-Induced Arthritis Mice. Biochem. Pharmacol. 73, 136–146. 10.1016/j.bcp.2006.08.027 [DOI] [PubMed] [Google Scholar]
- Lin N., Sato T., Ito A. (2001). Triptolide, a Novel Diterpenoid Triepoxide from Tripterygium Wilfordii Hook. f., Suppresses the Production and Gene Expression of Pro-matrix Metalloproteinases 1 and 3 and Augments Those of Tissue Inhibitors of Metalloproteinases 1 and 2 in Human Synovia. Arthritis Rheum. 44, 2193–2200. [DOI] [PubMed] [Google Scholar]
- Liu H.-K., Perrier S., Lipina C., Finlay D., McLauchlan H., Hastie C. J., et al. (2006). Functional Characterisation of the Regulation of CAAT Enhancer Binding Protein Alpha by GSK-3 Phosphorylation of Threonines 222/226. BMC Mol. Biol. 7, 14. 10.1186/1471-2199-7-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Lee J., Salazar Hernandez M. A., Mazitschek R., Ozcan U. (2015). Treatment of Obesity with Celastrol. Cell 161, 999–1011. 10.1016/j.cell.2015.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Wu Q.-L., Feng Y.-H., Wang Y.-F., Li X.-Y., Zuo J.-P. (2005). Triptolide Suppresses CD80 and CD86 Expressions and IL-12 Production in THP-1 Cells. Acta Pharmacol. Sin. 26, 223–227. 10.1111/j.1745-7254.2005.00035.x [DOI] [PubMed] [Google Scholar]
- Liu L., Luo Y., Zhou M., Lu Y., Xing M., Ru Y., et al. (2019). Tripterygium Agents for the Treatment of Atopic Eczema: A Bayesian Analysis of Randomized Controlled Trials. Phytomedicine 59, 152914. 10.1016/j.phymed.2019.152914 [DOI] [PubMed] [Google Scholar]
- Liu L., Salnikov A. V., Bauer N., Aleksandrowicz E., Labsch S., Nwaeburu C., et al. (2014a). Triptolide Reverses Hypoxia-Induced Epithelial-Mesenchymal Transition and Stem-like Features in Pancreatic Cancer by NF-Κb Downregulation. Int. J. Cancer 134, 2489–2503. 10.1002/ijc.28583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Chen T., Chen H., Zhang M., Li N., Lu Z., et al. (2004). Triptolide (PG-490) Induces Apoptosis of Dendritic Cells through Sequential P38 MAP Kinase Phosphorylation and Caspase 3 Activation. Biochem. Biophys. Res. Commun. 319, 980–986. 10.1016/j.bbrc.2004.04.201 [DOI] [PubMed] [Google Scholar]
- Liu Q. (2011). Triptolide and its Expanding Multiple Pharmacological Functions. Int. Immunopharmacol. 11, 377–383. 10.1016/j.intimp.2011.01.012 [DOI] [PubMed] [Google Scholar]
- Liu Y., Chen Y., Liu F. Q., Lamb J. R., Tam P. K. H. (2008). Combined Treatment with Triptolide and Rapamycin Prolongs Graft Survival in a Mouse Model of Cardiac Transplantation. Transpl. Int. Off. J. Eur. Soc. Organ. Transpl. 21, 483–494. 10.1111/j.1432-2277.2007.00630.x [DOI] [PubMed] [Google Scholar]
- Liu Y., Song F., Wu W. K. K., He M., Zhao L., Sun X., et al. (2012). Triptolide Inhibits colon Cancer Cell Proliferation and Induces Cleavage and Translocation of 14-3-3 Epsilon. Cell Biochem. Funct. 30, 271–278. 10.1002/cbf.2793 [DOI] [PubMed] [Google Scholar]
- Liu Z.-Z., Zhao R.-H., Liu Y.-T., Zhang H.-W., Ding G., Ma Z., et al. (2014b). A New Dihydroagarofuranoid Sesquiterpene from the Roots of Tripterygium Hypoglaucum. J. Asian Nat. Prod. Res. 16, 327–331. 10.1080/10286020.2013.873410 [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhao R., Zou Z. (2011). Chemical Constituents from Root Bark of Tripterygium Hypoglaucum. Zhongguo Zhong Yao Za Zhi 36, 2503–2506. [PubMed] [Google Scholar]
- Lü S., Wang Q., Li G., Sun S., Guo Y., Kuang H. (2015). The Treatment of Rheumatoid Arthritis Using Chinese Medicinal Plants: From Pharmacology to Potential Molecular Mechanisms. J. Ethnopharmacol. 176, 177–206. 10.1016/j.jep.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Lu Y., Fukuda K., Nakamura Y., Kimura K., Kumagai N., Nishida T. (2005). Inhibitory Effect of Triptolide on Chemokine Expression Induced by Proinflammatory Cytokines in Human Corneal Fibroblasts. Invest. Ophthalmol. Vis. Sci. 46, 2346–2352. 10.1167/iovs.05-0010 [DOI] [PubMed] [Google Scholar]
- Lu Y., Zhang Y., Li L., Feng X., Ding S., Zheng W., et al. (2014). TAB1: a Target of Triptolide in Macrophages. Chem. Biol. 21, 246–256. 10.1016/j.chembiol.2013.12.009 [DOI] [PubMed] [Google Scholar]
- Lue Y., Sinha Hikim A. P., Wang C., Leung A., Baravarian S., Reutrakul V., et al. (1998). Triptolide: a Potential Male Contraceptive. J. Androl. 19, 479–486. [PubMed] [Google Scholar]
- Luo D., Zuo Z., Zhao H., Tan Y., Xiao C. (2019). Immunoregulatory Effects of Tripterygium Wilfordii Hook F and its Extracts in Clinical Practice. Front. Med. 13, 556–563. 10.1007/s11684-018-0649-5 [DOI] [PubMed] [Google Scholar]
- Lv H., Jiang L., Zhu M., Li Y., Luo M., Jiang P., et al. (2019). The Genus Tripterygium: A Phytochemistry and Pharmacological Review. Fitoterapia 137, 104190. 10.1016/j.fitote.2019.104190 [DOI] [PubMed] [Google Scholar]
- Ma B., Qi H., Li J., Xu H., Chi B., Zhu J., et al. (2015). Triptolide Disrupts Fatty Acids and Peroxisome Proliferator-Activated Receptor (PPAR) Levels in Male Mice Testes Followed by Testicular Injury: A GC-MS Based Metabolomics Study. Toxicology 336, 84–95. 10.1016/j.tox.2015.07.008 [DOI] [PubMed] [Google Scholar]
- Ma J., Schmidt B. M., Poulev A., Raskin I. (2008). Determination of Tripdiolide in Root Extracts of Tripterygium Wilfordii by Solid-phase Extraction and Reversed-phase High-Performance Liquid Chromatography. Phytochem. Anal. 19, 348–352. 10.1002/pca.1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews V., Chendamarai E., George B., Viswabandya A., Srivastava A. (2011). Treatment of Acute Promyelocytic Leukemia with Single-Agent Arsenic Trioxide. Mediterr. J. Hematol. Infect. Dis. 3, e2011056. 10.4084/MJHID.2011.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y., Watanabe J., Ikegawa M., Kamoto T., Ogawa O., Nishiyama H. (2008). Cancer-specific Enhancement of Cisplatin-Induced Cytotoxicity with Triptolide through an Interaction of Inactivated Glycogen Synthase Kinase-3beta with P53. Oncogene 27, 4603–4614. 10.1038/onc.2008.89 [DOI] [PubMed] [Google Scholar]
- McDonald D. M. (2001). Angiogenesis and Remodeling of Airway Vasculature in Chronic Inflammation. Am. J. Respir. Crit. Care Med. 164, S39–S45. 10.1164/ajrccm.164.supplement_2.2106065 [DOI] [PubMed] [Google Scholar]
- Mishra P., Ambs S. (2015). Metabolic Signatures of Human Breast Cancer. Mol. Cel. Oncol. 2, e992217. 10.4161/23723556.2014.992217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata Y., Sato T., Ito A. (2005). Triptolide, a Diterpenoid Triepoxide, Induces Antitumour Proliferation via Activation of C-Jun NH2-terminal Kinase 1 by Decreasing Phosphatidylinositol 3-kinase Activity in Human Tumour Cells. Biochem. Biophys. Res. Commun. 336, 1081–1086. 10.1016/j.bbrc.2005.08.247 [DOI] [PubMed] [Google Scholar]
- Mou H., Zheng Y., Zhao P., Bao H., Fang W., Xu N. (2011). Celastrol Induces Apoptosis in Non-small-cell Lung Cancer A549 Cells through Activation of Mitochondria- and Fas/FasL-Mediated Pathways. Toxicol. Vitro 25, 1027–1032. 10.1016/j.tiv.2011.03.023 [DOI] [PubMed] [Google Scholar]
- Nie J., Zhou M., Lü C., Hu X., Wan B., Yang B., et al. (2012). Effects of Triptolide on the Synaptophysin Expression of Hippocampal Neurons in the AD Cellular Model. Int. Immunopharmacol. 13, 175–180. 10.1016/j.intimp.2012.03.021 [DOI] [PubMed] [Google Scholar]
- Ning C., Mo L., Chen X., Tu W., Wu J., Hou S., et al. (2018). Triptolide Derivatives as Potential Multifunctional Anti-alzheimer Agents: Synthesis and Structure-Activity Relationship Studies. Bioorg. Med. Chem. Lett. 28, 689–693. 10.1016/j.bmcl.2018.01.019 [DOI] [PubMed] [Google Scholar]
- Nong C., Wang X.-Z., Jiang Z.-Z., Zhang L.-Y. (2019). [Progress of Effect and Mechanisms of Tripterygium Wilfordii on Immune System]. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 44, 3374–3383. 10.19540/j.cnki.cjcmm.20190419.401 [DOI] [PubMed] [Google Scholar]
- Oda K., Kitano H. (2006). A Comprehensive Map of the Toll-like Receptor Signalling Network. Mol. Syst. Biol. 2, 0015. 10.1038/msb4100057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyeabor F., Paik A., Kovvasu S., Ding B., Lin J., Wahid M. A., et al. (2019). Optimization of Preparation and Preclinical Pharmacokinetics of Celastrol-Encapsulated Silk Fibroin Nanoparticles in the Rat. Molecules 24, 3271. 10.3390/molecules24183271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X.-D., Chen X.-C., Zhu Y.-G., Chen L.-M., Zhang J., Huang T.-W., et al. (2009). Tripchlorolide Protects Neuronal Cells from Microglia-Mediated Beta-Amyloid Neurotoxicity through Inhibiting NF-kappaB and JNK Signalling. Glia 57, 1227–1238. 10.1002/glia.20844 [DOI] [PubMed] [Google Scholar]
- Petronelli A., Pannitteri G., Testa U. (2009). Triterpenoids as New Promising Anticancer Drugs. Anticancer. Drugs 20, 880–892. 10.1097/CAD.0b013e328330fd90 [DOI] [PubMed] [Google Scholar]
- Qian S. Z., Hu Y. Z., Wang S. M., Luo Y., Tang A. S., Shu S. Y., et al. (1988). Effects of Tripterygium Hypoglaucum (Lévl.) Hutch on Male Fertility. Adv. Contracept. Off. J. Soc. Adv. Contracept. 4, 307–310. 10.1007/BF01849272 [DOI] [PubMed] [Google Scholar]
- Ren Z., Zhang C., Wang L., Cui Y., Qi R., Yang C., et al. (2010). In Vitro anti-viral Activity of the Total Alkaloids from Tripterygium Hypoglaucum against Herpes Simplex Virus Type 1. Virol. Sin. 25, 107–114. 10.1007/s12250-010-3092-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Martínez S., Cancino-Diaz M. E., Miguel P.-S., Cancino-Diaz J. C. (2006). Lipopolysaccharide from Escherichia coli Induces the Expression of Vascular Endothelial Growth Factor via Toll-like Receptor 4 in Human Limbal Fibroblasts. Exp. Eye Res. 83, 1373–1377. 10.1016/j.exer.2006.07.015 [DOI] [PubMed] [Google Scholar]
- Seo W. Y., Goh A. R., Ju S. M., Song H. Y., Kwon D.-J., Jun J.-G., et al. (2011). Celastrol Induces Expression of Heme Oxygenase-1 through ROS/Nrf2/ARE Signalling in the HaCaT Cells. Biochem. Biophys. Res. Commun. 407, 535–540. 10.1016/j.bbrc.2011.03.053 [DOI] [PubMed] [Google Scholar]
- Seo W. Y., Ju S. M., Song H. Y., Goh A. R., Jun J.-G., Kang Y.-H., et al. (2010). Celastrol Suppresses IFN-Gamma-Induced ICAM-1 Expression and Subsequent Monocyte Adhesiveness via the Induction of Heme Oxygenase-1 in the HaCaT Cells. Biochem. Biophys. Res. Commun. 398, 140–145. 10.1016/j.bbrc.2010.06.053 [DOI] [PubMed] [Google Scholar]
- Shamon L. A., Pezzuto J. M., Graves J. M., Mehta R. R., Wangcharoentrakul S., Sangsuwan R., et al. (1997). Evaluation of the Mutagenic, Cytotoxic, and Antitumour Potential of Triptolide, a Highly Oxygenated Diterpene Isolated from Tripterygium Wilfordii. Cancer Lett. 112, 113–117. 10.1016/S0304-3835(96)04554-5 [DOI] [PubMed] [Google Scholar]
- Shan W.-G., Wang H.-G., Chen Y., Wu R., Wen Y.-T., Zhang L.-W., et al. (2017). Synthesis of 3- and 29-substituted Celastrol Derivatives and Structure-Activity Relationship Studies of Their Cytotoxic Activities. Bioorg. Med. Chem. Lett. 27, 3450–3453. 10.1016/j.bmcl.2017.05.083 [DOI] [PubMed] [Google Scholar]
- Shao F., Wang G., Xie H., Zhu X., Sun J., Jiye A. (2007). Pharmacokinetic Study of Triptolide, a Constituent of Immunosuppressive Chinese Herb Medicine, in Rats. Biol. Pharm. Bull. 30, 702–707. 10.1248/bpb.30.702 [DOI] [PubMed] [Google Scholar]
- Song Q., Xiang Y., Gu J., Chen J., Li X. (2021). Chemical Constituents from the Rhizomes of Tripterygium Hypoglaucum. J. Chinese Med. Mater., 355–358. 10.13863/j.issn1001-4454.2021.02.018 [DOI] [Google Scholar]
- Song W., Liu M., Wu J., Zhai H., Chen Y., Peng Z. (2019). Preclinical Pharmacokinetics of Triptolide: A Potential Antitumour. Drug. Curr. Drug Metab. 20, 147–154. 10.2174/1389200219666180816141506 [DOI] [PubMed] [Google Scholar]
- Sun L., Li H., Huang X., Wang T., Zhang S., Yang J., et al. (2013). Triptolide Alters Barrier Function in Renal Proximal Tubular Cells in Rats. Toxicol. Lett. 223, 96–102. 10.1016/j.toxlet.2013.08.014 [DOI] [PubMed] [Google Scholar]
- Tabeshpour J., Mehri S., Shaebani Behbahani F., Hosseinzadeh H. (2018). Protective Effects of Vitis vinifera (Grapes) and One of its Biologically Active Constituents, Resveratrol, against Natural and Chemical Toxicities: A Comprehensive Review. Phytother. Res. 32, 2164–2190. 10.1002/ptr.6168 [DOI] [PubMed] [Google Scholar]
- Tai T., Huang X., Su Y., Ji J., Su Y., Jiang Z., et al. (2014). Glycyrrhizin Accelerates the Metabolism of Triptolide through Induction of CYP3A in Rats. J. Ethnopharmacol. 152, 358–363. 10.1016/j.jep.2014.01.026 [DOI] [PubMed] [Google Scholar]
- Tang Y., Zhang Y., Li L., Xie Z., Wen C., Huang L. (2020). Kunxian Capsule for Rheumatoid Arthritis: Inhibition of Inflammatory Network and Reducing Adverse Reactions through Drug Matching. Front. Pharmacol. 11, 485. 10.3389/fphar.2020.00485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Hu Q., Yang J., Li R., Li X., Lu C., et al. (2007). In Vitro anti-HIV and -HSV Activity and Safety of Sodium Rutin Sulfate as a Microbicide Candidate. Antivir. Res 75, 227–233. 10.1016/j.antiviral.2007.03.008 [DOI] [PubMed] [Google Scholar]
- Tao X., Schulze-Koops H., Ma L., Cai J., Mao Y., Lipsky P. E. (1998). Effects of Tripterygium Wilfordii Hook F Extracts on Induction of Cyclooxygenase 2 Activity and Prostaglandin E2 Production. Arthritis Rheum. 41, 130–138. [DOI] [PubMed] [Google Scholar]
- Tozawa K., Sagawa M., Kizaki M. (2011). Quinone Methide Tripterine, Celastrol, Induces Apoptosis in Human Myeloma Cells via NF-Κb Pathway. Int. J. Oncol. 39, 1117–1122. 10.3892/ijo.2011.1161 [DOI] [PubMed] [Google Scholar]
- Tseng C.-K., Hsu S.-P., Lin C.-K., Wu Y.-H., Lee J.-C., Young K.-C. (2017). Celastrol Inhibits Hepatitis C Virus Replication by Upregulating Heme Oxygenase-1 via the JNK MAPK/Nrf2 Pathway in Human Hepatoma Cells. Antivir. Res 146, 191–200. 10.1016/j.antiviral.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y. H., Zeng K. Q., Zhang M. M., Tu S. H., Lai X. Y., Zhang W. C., et al. (2004). Effects of Triptolide on the Expression and Activity of Nuclear Factor κB in Synovium of Collagen-Induced Arthritis Rats. Chin. J. Rheumatol. 25 (5), 543–545. 10.1007/BF02896012 [DOI] [PubMed] [Google Scholar]
- Vlietinck A. J., Vanden Berghe D. A. (1991). Can Ethnopharmacology Contribute to the Development of Antiviral Drugs? J. Ethnopharmacol. 32, 141–153. 10.1016/0378-8741(91)90112-q [DOI] [PubMed] [Google Scholar]
- Wang C., Shi C., Yang X., Yang M., Sun H., Wang C. (2014). Celastrol Suppresses Obesity Process via Increasing Antioxidant Capacity and Improving Lipid Metabolism. Eur. J. Pharmacol. 744, 52–58. 10.1016/j.ejphar.2014.09.043 [DOI] [PubMed] [Google Scholar]
- Wang F., Zhang Y., Zhao Y. Q., (2011). Study on Chemical Constituents of Tripterygium Hypoglaucum. Chin. Herb. Med. 42, 46–49. [Google Scholar]
- Wang J.-M., Li J.-Y., Cai H., Chen R.-X., Zhang Y.-Y., Zhang L.-L., et al. (2019). Nrf2 Participates in Mechanisms for Reducing the Toxicity and Enhancing the Antitumour Effect of Radix Tripterygium Wilfordii to S180-Bearing Mice by Herbal-Processing Technology. Pharm. Biol. 57, 437–448. 10.1080/13880209.2019.1634106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Xu D., Li L., Xing X., Liu L., Ismail Abdelmotalab M., et al. (2018a). Possible Role of Hepatic Macrophage Recruitment and Activation in Triptolide-Induced Hepatotoxicity. Toxicol. Lett. 299, 32–39. 10.1016/j.toxlet.2018.08.017 [DOI] [PubMed] [Google Scholar]
- Wang S., Wang L., Chen X., (2020). Diterpenoids of the Stem and Leaf of Tripterygium Hypoglaucum and Their Biological Activities. J. Kunming Univ. Sci. Technol. Sci. Ed. 45, 108–114. 10.16112/j.cnki.53-1223/n.2020.02.014 [DOI] [Google Scholar]
- Wang S., Zuo S., Liu Z., Ji X., Yao Z., Wang X. (2018b). Study on the Efficacy and Mechanism of Triptolide on Treating TNF Transgenic Mice with Rheumatoid Arthritis. Biomed. Pharmacother. 106, 813–820. 10.1016/j.biopha.2018.07.021 [DOI] [PubMed] [Google Scholar]
- Wang W.-B., Feng L.-X., Yue Q.-X., Wu W.-Y., Guan S.-H., Jiang B.-H., et al. (2012). Paraptosis Accompanied by Autophagy and Apoptosis Was Induced by Celastrol, a Natural Compound with Influence on Proteasome, ER Stress and Hsp90. J. Cel. Physiol. 227, 2196–2206. 10.1002/jcp.22956 [DOI] [PubMed] [Google Scholar]
- Wang Y.-L., Lam K.-K., Cheng P.-Y., Lee Y.-M. (2015). Celastrol Prevents Circulatory Failure via Induction of Heme Oxygenase-1 and Heat Shock Protein 70 in Endotoxemic Rats. J. Ethnopharmacol. 162, 168–175. 10.1016/j.jep.2014.12.062 [DOI] [PubMed] [Google Scholar]
- Wang Y., Guo S.-H., Shang X.-J., Yu L.-S., Zhu J.-W., Zhao A., et al. (2018c). Triptolide Induces Sertoli Cell Apoptosis in Mice via ROS/JNK-dependent Activation of the Mitochondrial Pathway and Inhibition of Nrf2-Mediated Antioxidant Response. Acta Pharmacol. Sin. 39, 311–327. 10.1038/aps.2017.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Ma D., Wang C., Zhu Z., Yang Y., Zeng F., et al. (2017). Triptonide Inhibits the Pathological Functions of Gastric Cancer-Associated Fibroblasts. Biomed. Pharmacother. 96, 757–767. 10.1016/j.biopha.2017.10.046 [DOI] [PubMed] [Google Scholar]
- Wei Y. M., Luan Z. H., Liu B. W., Wang Y. H., Chang Y. X., Xue H. Q., et al. (2019). Autophagy in Triptolide-Mediated Cytotoxicity in Hepatic Cells. Int. J. Toxicol. 38, 436–444. 10.1177/1091581819864518 [DOI] [PubMed] [Google Scholar]
- Li W. W., Li B. G., Chen Y. Z. (1999). Sesquiterpene Alkaloids from Tripterygium Hypoglaucum. Phytochemistry 40. 10.1016/S0031-9422(98)00618-9 [DOI] [Google Scholar]
- Wishart D. S., Mandal R., Stanislaus A., Ramirez-Gaona M. (2016). Cancer Metabolomics and the Human Metabolome Database. Metabolites 6, 10. 10.3390/metabo6010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K.-F., Chan J. K., Chan K.-L., Tam P., Yang D., Fan S.-T., et al. (2008). Immunochemical Characterization of the Functional Constituents of Tripterygium Wilfordii Contributing to its Anti-inflammatory Property. Clin. Exp. Pharmacol. Physiol. 35, 55–59. 10.1111/j.1440-1681.2007.04740.x [DOI] [PubMed] [Google Scholar]
- Wu D. G., Sun X. C., Li F. (1979). New Diterpene Lactones of Tripterygium Wilfordii - Hypoglaucine and Triptolide C Kunming Acta Bot. Yunnanica. 29–36. [Google Scholar]
- Xi C., Peng S., Wu Z., Zhou Q., Zhou J. (2017). Toxicity of Triptolide and the Molecular Mechanisms Involved. Biomed. Pharmacother. 90, 531–541. 10.1016/j.etap.2017.09.013 [DOI] [PubMed] [Google Scholar]
- Xie C. Q., Zhou P., Li X. (2015). Research Progress on Chemical Constituents, Pharmacological Effects, and Clinical Application of Tripterygium Hypoglaucum. Chin. Tradit. Herbal Drugs. 46. 1996-2010. 10.7501/j.issn.0253-2670.2015.13.024 [DOI] [Google Scholar]
- Xie F. G., Li C. J., Yang J. Z., Luo Y. M., Zhang D. M. (2012). Study on Chemical Constituents from the Root Bark of Tripterygium Hypoglaucum. J. Chin. Med. Mater. 35, 1083–1087. 10.13863/j.issn1001-4454.2012.07.023 [DOI] [PubMed] [Google Scholar]
- Xie L., Zhao Y., Duan J., Fan S., Shu L., Liu H., et al. (2020). Integrated Proteomics and Metabolomics Reveal the Mechanism of Nephrotoxicity Induced by Triptolide. Chem. Res. Toxicol. 33, 1897–1906. 10.1021/acs.chemrestox.0c00091 [DOI] [PubMed] [Google Scholar]
- Xu H.-Y., Zhang Y.-Q., Liu Z.-M., Chen T., Lv C.-Y., Tang S.-H., et al. (2019). ETCM: an Encyclopaedia of Traditional Chinese Medicine. Nucleic Acids Res. 47, D976–D982. 10.1093/nar/gky987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Ziqing L., Yinrun D., Xiaoyan W., Jinglun X. (2004). Tripterygium Hypoglaucum (Level) Hutch Induces Aneuploidy of Chromosome 8 in Mouse Bone Marrow Cells and Sperm. Mutagenesis 19, 379–382. 10.1093/mutage/geh044 [DOI] [PubMed] [Google Scholar]
- Xue S., Wu T. (2008). Chinese Herbal Medicine Tripterygium Hypoglaucum Hutch Tablet for Facial Corticosteroid Addiction Dermatitis. J. Altern. Complement. Med. 14, 619. 10.1089/acm.2008.0142 [DOI] [PubMed] [Google Scholar]
- Yan G., Zhang H., Wang W., Li Y., Mao C., Fang M., et al. (2017). Investigation of the Influence of Glycyrrhizin on the Pharmacokinetics of Celastrol in Rats Using LC-MS and its Potential Mechanism. Xenobiotica 47, 607–613. 10.1080/00498254.2016.1211773 [DOI] [PubMed] [Google Scholar]
- Yan Q., Li Y., Fan Y., Zhang M., Yang L., Yao Y., et al. (2019). Protective Effect of Syringaresinol on Excitatory Damage Induced by Sodium Glutamate in SH-Sy5y Cells. Chin. J. Exp. Tradit. Med. Formulae 25, 76–82. 10.13422/j.cnki.syfjx.20191839 [DOI] [Google Scholar]
- Yang G., Wang L., Yu X., Huang Y., Qu C., Zhang Z., et al. (2017). Protective Effect of 18β-Glycyrrhetinic Acid against Triptolide-Induced Hepatotoxicity in Rats. Evid. Based. Complement. Alternat. Med. 2017, 3470320. 10.1155/2017/3470320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Li Y., Ren J., Zhu C., Fu J., Lin D., et al. (2014). Celastrol Attenuates Inflammatory and Neuropathic Pain Mediated by Cannabinoid Receptor Type 2. Int. J. Mol. Sci. 15, 13637–13648. 10.3390/ijms150813637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Wang S., Zheng H., Wang L., Liu D., Chen X., et al. (2019). Understanding Dihydro-β-Agarofuran Sesquiterpenes from Tripterygium Hypoglaucum as the Modulators of Multi-Drug Resistance in HepG2/Adr Cells. Biochem. Biophys. Res. Commun. 508, 742–748. 10.1016/j.bbrc.2018.11.188 [DOI] [PubMed] [Google Scholar]
- Yi J. H., Yang H., Zhang Q. L., Pei T. R., Chen Z. Z., Hu Y. B., (1993). Studies on Chemical Constituents of Tripterygium Hypoglaucum. Chin. Tradit. Herb. Drugs 24, 398–400+446. [Google Scholar]
- Yu H., Shi L., Zhao S., Sun Y., Gao Y., Sun Y., et al. (2016). Triptolide Attenuates Myocardial Ischemia/Reperfusion Injuries in Rats by Inducing the Activation of Nrf2/HO-1 Defense Pathway. Cardiovasc. Toxicol. 16, 325–335. 10.1007/s12012-015-9342-y [DOI] [PubMed] [Google Scholar]
- Yu L., Wang L., Chen S. (2010). Endogenous Toll-like Receptor Ligands and Their Biological Significance. J. Cel. Mol. Med. 14, 2592–2603. 10.1111/j.1582-4934.2010.01127.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Koehn C. D., Yue Y., Li S., Thiele G. M., Hearth-Holmes M. P., et al. (2015). Celastrol Inhibits Inflammatory Stimuli-Induced Neutrophil Extracellular Trap Formation. Curr. Mol. Med. 15, 401–410. 10.2174/1566524015666150505160743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S., Wang L., Chen X., Fan B., Yuan Q., Zhang H., et al. (20202016). Corrigendum to “Triptolide Inhibits the Migration and Invasion of Human Prostate Cancer Cells via Caveolin-1/CD147/MMPs Pathway”. Biomed. Pharmacother. 84, 1776–1782. 10.1016/j.biopha.2019.109782 [DOI] [PubMed] [Google Scholar]
- Yuan Z., Hasnat M., Liang P., Yuan Z., Zhang H., Sun L., et al. (2019a). The Role of Inflammasome Activation in Triptolide-Induced Acute Liver Toxicity. Int. Immunopharmacol. 75, 105754. 10.1016/j.intimp.2019.105754 [DOI] [PubMed] [Google Scholar]
- Yuan Z., Zhang H., Hasnat M., Ding J., Chen X., Liang P., et al. (2019b). A New Perspective of Triptolide-Associated Hepatotoxicity: Liver Hypersensitivity upon LPS Stimulation. Toxicology 414, 45–56. 10.1016/j.tox.2019.01.005 [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu S.-J., Hu J.-H., Xu M.-J., Liu Z.-X., Zhou L., et al. (2016). [LC-MS/MS Method for Determination of Tripterine in Plasma Pharmacokinetic Study in Beagles]. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 41, 2727–2731. 10.4268/cjcmm20161426 [DOI] [PubMed] [Google Scholar]
- Zhang J., Shan J., Chen X., Li S., Long D., Li Y. (2018). Celastrol Mediates Th17 and Treg Cell Generation via Metabolic Signalling. Biochem. Biophys. Res. Commun. 497, 883–889. 10.1016/j.bbrc.2018.02.163 [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang Z. X., (1992). Studies on Chemical Constituents of Tripterygium Hypoglaucum. Chinese Tradit. Herb. Drugs. 23, 339–340+360+390. [Google Scholar]
- Zhang L., Zhang Z. X., An D. K., (1998). Study on Ether Soluble Chemical Constituents of Tripterygium Hypoglaucum. Chinese Tradit. Herb. Drugs 35, 441–442. 10.3321/j.issn:0253-2670.2007.04.004 [DOI] [Google Scholar]
- Zhang R., Chen Z., Wu S.-S., Xu J., Kong L.-C., Wei P. (2019a). Celastrol Enhances the Anti-liver Cancer Activity of Sorafenib. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 25, 4068–4075. 10.12659/MSM.914060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. M., Wang C. F., Wu D. G. (1992a). Aconitine Triterpenes from the Roots of Tripterygium Hypoglaucum. Kunming Acta Bot. Yunnanica., 211–214. [Google Scholar]
- Zhang X. M., Wang C. F., Wu D. G. (1992b). Rosin Alkane Diterpenoids from the Roots of Tripterygium Hypoglaucum. Kunming Acta Bot. Yunnanica. 319–322. [Google Scholar]
- Zhang X., Xue X.-C., Wang Y., Cao F.-F., You J., Uzan G., et al. (2019b). Celastrol Reverses Palmitic Acid-Induced Insulin Resistance in HepG2 Cells via Restoring the miR-223 and GLUT4 Pathway. Can. J. Diabetes 43, 165–172. 10.1016/j.jcjd.2018.07.002 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Mao X., Li W., Chen W., Wang X., Ma Z., et al. (2021a). Tripterygium Wilfordii: An Inspiring Resource for Rheumatoid Arthritis Treatment. Med. Res. Rev. 41, 1337–1374. 10.1002/med.21762 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Tang Y., Luo Y., Luo L., Shen F., Huang Z. (2021b). Triptolide Impairs Glycolysis by Suppressing GATA4/Sp1/PFKP Signalling axis in Mouse Sertoli Cells. Toxicol. Appl. Pharmacol. 425, 115606. 10.1016/j.taap.2021.115606 [DOI] [PubMed] [Google Scholar]
- Zhang Y. W., Fan Y. S., Wang X. D., Gao W. Y., Duan H. Q. (2007). Diterpenoids with Immunosuppressive Activity from Tripterygium Hypoglaucum. Chinese Tradit. Herb. Drugs 38, 493–496. 10.3321/j.issn:0253-2670.2007.04.004 [DOI] [Google Scholar]
- Zhao P., Wang H., Jin D.-Q., Ohizumi Y., Xu J., Guo Y. (2014). Terpenoids from Tripterygium Hypoglaucum and Their Inhibition of LPS-Induced NO Production. Biosci. Biotechnol. Biochem. 78, 370–373. 10.1080/09168451.2014.890035 [DOI] [PubMed] [Google Scholar]
- Zhao Q., Li H.-M., Chen X.-Q., Li R.-T., Liu D. (2018). Terpenoids from Tripterygium Hypoglaucum and Their Anti-inflammatory Activity. Chem. Nat. Compd. 54. 10.1007/s10600-018-2381-4 [DOI] [Google Scholar]
- Zhen Q. S., Ye X., Wei Z. J. (1995). Recent Progress in Research on Tripterygium: a Male Antifertility Plant. Contraception 51, 121–129. 10.1016/0010-7824(94)00018-r [DOI] [PubMed] [Google Scholar]
- Zheng H., Wang L., Yang T., Liu D., Li H.-M., Chen X.-Q., et al. (2020). New Terpenoids and Lignans from the Twigs of Tripterygium Hypoglaucum. Nat. Prod. Res. 34, 1853–1861. 10.1080/14786419.2018.1564297 [DOI] [PubMed] [Google Scholar]
- Zhong J., Xian D., Xu Y., Liu J. (2011). Efficacy of Tripterygium Hypoglaucum Hutch in Adults with Chronic Urticaria. J. Altern. Complement. Med. 17, 459–464. 10.1089/acm.2009.0648 [DOI] [PubMed] [Google Scholar]
- Zhou H., Liu Y., Wang C., Liu L., Wang H., Zhang Y., et al. (2018). Triptolide Inhibits Epstein-Barr Nuclear Antigen 1 Expression by Increasing Sensitivity of Mitochondria Apoptosis of Nasopharyngeal Carcinoma Cells. J. Exp. Clin. Cancer Res. 37, 192. 10.1186/s13046-018-0865-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Xi C., Wang W., Fu X., Jinqiang L., Qiu Y., et al. (2014). Triptolide-induced Oxidative Stress Involved with Nrf2 Contribute to Cardiomyocyte Apoptosis through Mitochondrial Dependent Pathways. Toxicol. Lett. 230, 454–466. 10.1016/j.toxlet.2014.08.017 [DOI] [PubMed] [Google Scholar]
- Zhou X., Liu Q., Zhou X., Zhang J., Liu W., Zhao X., et al. (2020). THH Relieves CIA Inflammation by Reducing Inflammatory-Related Cytokines. Cell Biochem. Biophys. 78, 367–374. 10.1007/s12013-020-00911-8 [DOI] [PubMed] [Google Scholar]
- Zhu H., Liu X.-W., Ding W.-J., Xu D.-Q., Zhao Y.-C., Lu W., et al. (2010). Up-regulation of Death Receptor 4 and 5 by Celastrol Enhances the Anti-cancer Activity of TRAIL/Apo-2L. Cancer Lett. 297, 155–164. 10.1016/j.canlet.2010.04.030 [DOI] [PubMed] [Google Scholar]
- Zhu K.-J., Shen Q.-Y., Cheng H., Mao X.-H., Lao L.-M., Hao G.-L. (2005). Triptolide Affects the Differentiation, Maturation and Function of Human Dendritic Cells. Int. Immunopharmacol. 5, 1415–1426. 10.1016/j.intimp.2005.03.020 [DOI] [PubMed] [Google Scholar]
- Zhuang W. J., Fong C. C., Cao J., Ao L., Leung C. H., Cheung H. Y., et al. (2004). Involvement of NF-kappaB and C-Myc Signalling Pathways in the Apoptosis of HL-60 Cells Induced by Alkaloids of Tripterygium Hypoglaucum (levl.) Hutch. Phytomedicine 11, 295–302. 10.1078/0944711041495128 [DOI] [PubMed] [Google Scholar]