Abstract
Background & Aims:
Data have demonstrated county- and state-wide variability in mortality rates from liver disease, but data are lacking at the ‘local’ (e.g., county) level to identify factors associated with variability in liver disease-related mortality and hotspots of liver disease mortality.
Methods:
We used CDC Wonder data from 2009-2018 to calculate county-level age-adjusted liver disease-related death rates. We fit multivariable linear regression models to adjust for county-level covariates related to demographics (i.e., race and ethnicity), medical co-morbidities (e.g., obesity), access-to-care (e.g., uninsured rate), and geographic (e.g., distance to closest liver transplant center) variables. We used optimized hotspot analysis to identify clusters of liver disease mortality hotspots based on the final multivariable models.
Results:
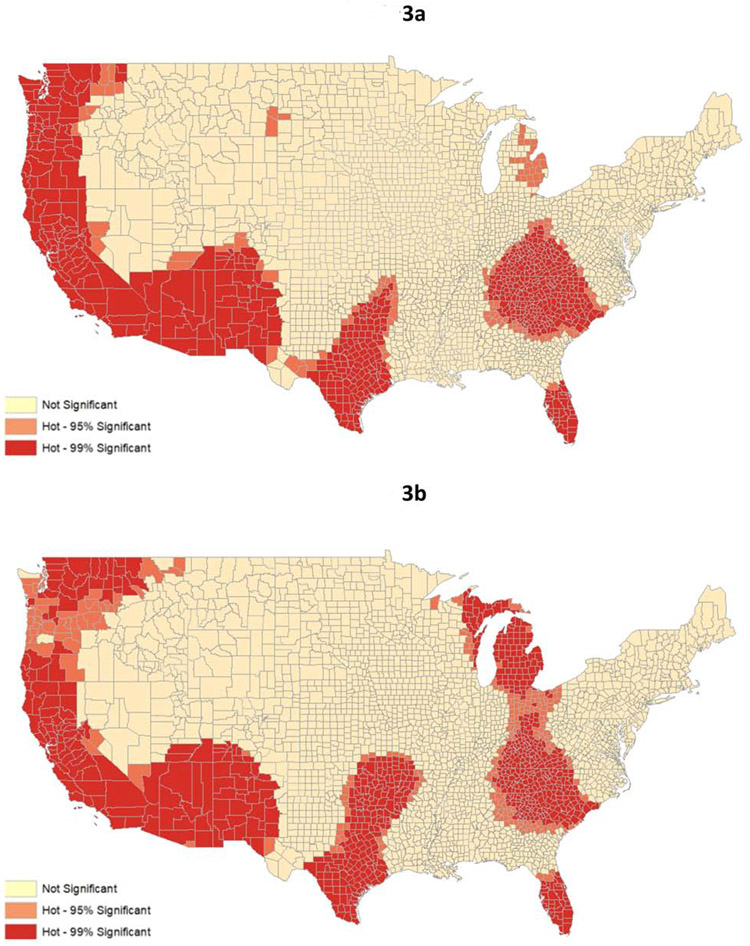
In multivariable models, 61% of the variability in among-county mortality was explained by county-level race/ethnicity, poverty, uninsured rates, distance to the closest transplant center, and local rates of obesity, diabetes, and alcohol use. Despite adjustment, there was significant within-state variability in county-level mortality rates. Of counties with the ‘top’ 5th percentile (i.e., highest mortality) of fully adjusted mortality, 60% were located in three states: Oklahoma, Texas, and New Mexico. Adjusted mortality rates were highly spatially correlated, representing five clusters: 1) South Florida; 2) Appalachia and the eastern part of the Midwest; 3) Texas and Oklahoma; 4) New Mexico, Arizona, California, and southern Oregon; and 5) parts of Washington and Montana.
Conclusions:
Our data demonstrate significant intra-state differences in liver disease-related mortality, with more than 60% of the variability being explained by patient demographics, clinical risk factors for liver disease, and access to specialty liver care.
Keywords: liver mortality, chronic liver disease, hepatocellular carcinoma, cirrhosis
Lay Summary:
Our data demonstrate significant county-level differences in liver disease-related mortality, even in the same state. More than 60% of the variability was explained by patient demographics (e.g., race), clinical risk factors for liver disease (e.g., alcohol use), and access to specialty liver care (i.e., distance needed to travel).
Introduction
Liver disease represents a worsening concern for American patients and clinicians. The number of annual deaths from cirrhosis in the US increased by 65% between 1999 and 2016, while deaths from hepatocellular carcinoma (HCC) doubled during this same period.1 Deaths rates from liver-related disease (cirrhosis and HCC) increased in 49/50 states, with disproportionate increases in five states (Alabama, Arkansas, Indiana, Kentucky, and New Mexico).1 The most notable trends in liver-related mortality were increased rates among younger people (ages 25-34), Whites, Native Americans, and Hispanics, and patients with alcohol-related liver disease.1 However, these data were only evaluated at the state level, even though variations in socioeconomic conditions and healthcare infrastructure within a given state may be associated with more local mortality differences. A 2016 study in JAMA demonstrated county-level differences in mortality from cirrhosis and chronic liver disease, however, the range in county-level mortality from liver disease was the smallest of the 10 diseases that were studied.2 In contrast to the Tapper study, this study did not include HCC deaths in the cirrhosis and chronic liver disease category, even though >90% of cases of HCC occur in the setting of cirrhosis/advanced fibrosis, and did not account for data in the era of direct acting antiviral therapy for hepatitis C virus.1-9 Additionally, neither of these studies explored the impact of socio-demographic and measures of access to care (e.g., insurance, proximity to a liver transplant center) on geographic variability in liver-related mortality.
Clinicians seeking to develop interventions to address modifiable factors leading to geographic disparities in liver-related mortality require data on a more ‘local’ level in order to identify ‘hotspots’ of liver disease. At the same time, policymakers can leverage data on ‘local’ differences in liver-related mortality, and the impact of measures of access-to-care (e.g., proximity to a transplant center) in order to develop policies to help remediate disparities in liver-related mortality across the US (e.g., opening new transplant centers, enhancing provision of telehealth coverage).10,11 In this analysis, we sought to quantify county-level differences in liver-related mortality, and to assess the determinants of variability in mortality using national liver mortality.
Methods
Study outcome
The primary outcome was county-level age-adjusted liver disease-related mortality among adults aged 25-74 years from using diagnosis codes for chronic liver disease (including HCC) from Center for Disease Control and Prevention’s Wide-ranging Online Data for Epidemiologic Research (CDC WONDER) platform. Because there are data that suggest that relying only on diagnosis codes for alcoholic liver disease (K70), chronic hepatitis (K73), and fibrosis and cirrhosis (K74) can lead to an underestimation of liver disease-related mortality,12 we sought to balance our inclusionary ICD-10 codes to capture as many liver-related deaths as possible, without including too many “garbage codes” (Supplementary Table 1).13 We feel this maximized the most accurate capture of liver-related mortality, and was more expansive than the only other study evaluating county-level differences in mortality from liver disease that did not include HCC in the grouping of liver disease-related mortality, even though >90% of deaths occur in the setting of advanced and/or chronic liver disease.1,2,14
Study period
We focused on liver disease-related deaths between 2009-2018. We included aggregated survival data from 2009-2018 because CDC WONDER does not provide age-adjusted data on counties with fewer than 20 deaths as these age-adjusted rates are considered ‘unreliable.’ Therefore, we sought to maximize the number of counties we were able to evaluate in multivariable models. Secondarily, we divided the cohort into pre-DAA (2009-2013) and post-DAA (2014-2018) eras to evaluate temporal trends in county-level liver disease-related mortality.
County-level covariates
We considered potential covariates that may be associated with liver disease-related mortality for a given individual (e.g., diabetes), or for everyone residing in a county (e.g., distance to a specialized center).15-20 However, all of these measures were assessed at the county-level because data were not available at the patient-level: 1) rural/urban status based on classification from the United States Census Bureau21; 2) poverty defined as percentage of adult population living below the federal poverty level in 2013 based on the Small Area Income and Poverty Estimates Program22; 3) race and ethnicity using population estimates from the US Census Bureau in CDC WONDER;23,24 4) uninsured rate defined as the mean annual percentage from 2009-2018 without health insurance based on the Small Area Health Insurance Estimates Program25; 5) distance from geographic centroid to closest liver transplant center; 6) number of board-certified gastroenterologists per adult population in 2015 using data from the Area Health Resources Files;26 7) local transplant waitlisting rates from 2009-2018 (calculated as the number of waitlistings per county per 100 liver disease deaths);15 8) percentage of adults with “heavy” drinking (consumption, on average, of more than one drink per day for women or two drinks per day for men in the past 30 days) from 2009-201214; 9) percentage of adults with diabetes from 2009-201227; and 10) percentage of adults classified as obese in 2011.28
Statistical analysis
Ascertainment of age-adjusted mortality: Age-adjusted liver disease mortality rates (primary outcome) were obtained from CDC Wonder, using methodology described in detail at https://wonder.cdc.gov/wonder/help/ucd.html.24 In short, age-adjusted death rates are calculated using the “direct method” using the year "2000 U.S. standard" as the default population.24 CDC Wonder provided age-adjusted rates by county aggregated over 2009-2018 for the primary analysis, and for 2009-2013 and 2014-2018 for the secondary analysis.
Mapping of mortality data: We mapped county-level mortality data using the spmap function in STATA 16.0.
Linear regression models: We fit linear regression models to identify county-level factors associated with age-adjusted liver-related mortality. We evaluated each of the covariates in univariable models, and used a backwards selection process to include covariates with a p<0.05 in the final model and/or covariates that increased the R2 of the final model.
Calculation of fully adjusted mortality rates: After fitting the final multivariable linear regression models, we calculated fully adjusted county-level liver disease-related mortality rates using the predict command in STATA 16.0
Geospatial hotspot analysis: The age-adjusted as well as the fully adjusted mortality rates were used as input for cluster analysis with optimized hotspot analysis (ArcGIS); the distance band used for analysis was identified based on incremental spatial autocorrelation.29,30 We considered clusters (not individual counties) statistically significant at a p-value of < 0.05 and a Z score of 1.96 (95% confidence level). An important note about clustering is that it is “high-high” clustering. Counties that are a part of a hotspot might not have the highest mortality but are both: 1) higher than “expected” (statistically) and 2) surrounded by other counties that are higher than expected. As a result, an individual county can be considered part of a hotspot based on its surrounding counties, even if it is not in the top 5% mortality wise.29,30
The study was considered exempt by the Institutional Review Board at the University of Miami because it only included de-identified population-level data. All authors had access to the study data and reviewed and approved the final manuscript.
Results
Overall results
From 2009-2018, there were 3,125 US counties identified by CDC WONDER.24 Among these, only a minority (n=314, 10.0%) had both population and liver-disease deaths sufficient to allow computation of annual county age-adjusted mortality rates, hence the aggregation of data from 2009-2018. There were 1,527 (48.9%) and 1,717 (54.9%) counties with available data in the pre- and post-DAA eras, respectively, but 2,124 (68.0%) in the overall aggregated cohort of 2009-2018, representing 97.6% of all liver-related deaths in the US among the population ages 25-74 during this period.
Individual county-level
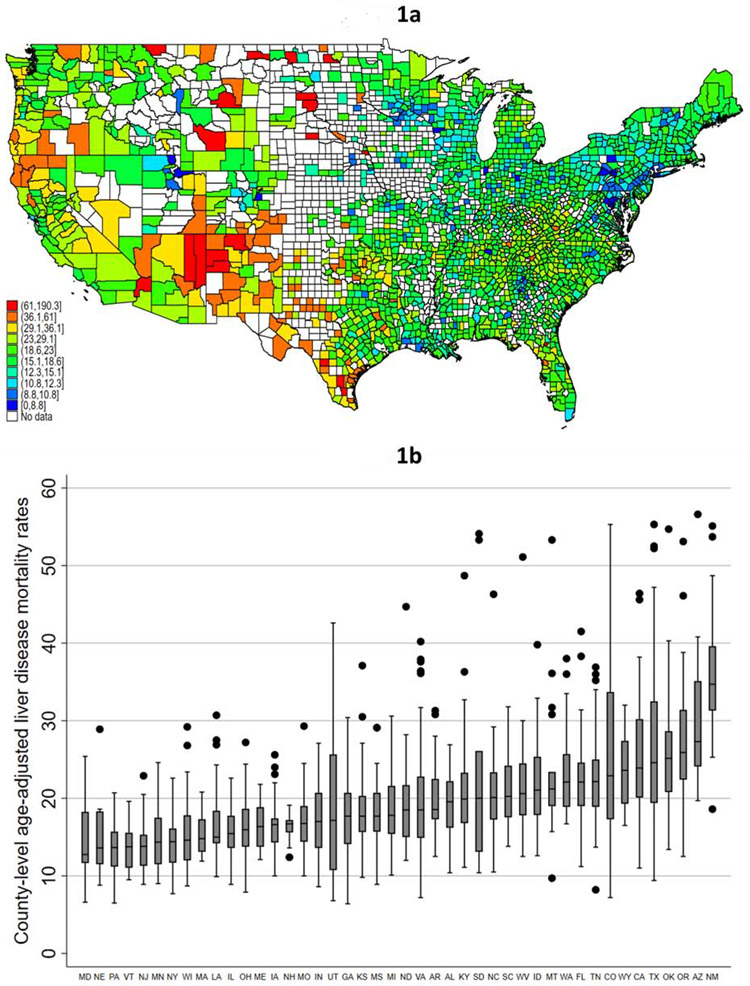
At the individual county level, the median age-adjusted liver disease death rate from 2009-2018 was 18.6 deaths per 100,000 population (interquartile range [IQR]: 15.1-23.0).The counties with the top 5% of mortality rates (n=103 counties) were found in 22 different states, however more than 40% of the counties with the highest mortality rates were located in either Texas (n=33, 32.1%) or New Mexico (n=12, 11.7%; Figure 1a). The counties with the lowest death rates (bottom 5th percentile) were spread across 28 states, and were more evenly distributed, with 13 (12.3% of total) in Pennsylvania,, with no other state contributing more than 6% of the total. Only 1 (0.9%) of these counties were in Texas, and none were in New Mexico.
Figure 1 (two panels): County-level variability in liver disease-related mortality rates.
- Figure 1a: County-level age-adjusted liver disease-related mortality rates; 2009-2018*†
- Figure 1b: Within-state variability in age-adjusted county-level liver disease-related mortality rates; 2009-2018*‡
- * CDC WONDER age-adjusted mortality rates excludes counties with fewer than 20 liver disease-related deaths due to “unreliable” age-adjusted rates
- † Legend categories based on 1st, 5th, 10th, 25th, 50th, 75th, 90th, 95th, and 99th percentile of mortality
- ‡ Figure y-axis excludes top 1% of counties, and only states (n=45) with 10 or more counties with available data were included
Within-state variability in county-level mortality
There were marked differences in the within-state among-county liver-related mortality (limited to states with at least 10 counties with available data; Figure 1b). Three states (MA, ME, NH) had a less than two-fold difference in mortality among counties with the highest and lowest mortality, while three states (NE, ND, SD) had a more than ten-fold difference (Figure 1b). There were 8 states (CO, KS, NE, SD, TN, TX, UT) that had at least one county in the top and bottom 5th percentile of age-adjusted liver-related mortality from 2009-2018.
Multivariable models
In univariable linear regression models, all of the covariates were associated with the outcome of county-level age-adjusted liver disease mortality rate (p<0.1; Supplementary Table 2). However, in multivariable linear regression models, county rural/urban classification, number of board-certified gastroenterologists, and percentage of the county population classified as obese were no longer significant (p>0.1). Several county-level variables were significantly associated with county-specific age-adjusted liver related mortality rates (Table 1). Those with a positive beta coefficient were associated with increased adjusted county-level liver disease-related mortality (e.g., counties with an increased percentage of American Indians or counties located farther from a liver transplant center had higher liver disease-related mortality rates), while those with a negative beta coefficient had lower rates of liver disease-related mortality (e.g., counties with an increased percentage of Black non-Hispanics had lower mortality). Together, these variables accounted for 62% of the observed proportion of variance in county-level mortality rates (Table 1, R2=0.617). County-level liver transplant waitlisting rates were significantly associated with age-adjusted mortality rates in multivariable models and increased the R2.
Table 1:
Multivariable linear regression model evaluating factors associated with county-level age-adjusted liver disease-related mortality rates
| County-level variable | Beta coefficient | P-value | Unadjusted R2 |
|---|---|---|---|
| Racial/ethnic composition | |||
| % White Hispanic | 0.20 (0.16, 0.23) | <0.001 | 0.08 |
| % Black non-Hispanic | −0.10 (−0.14, −0.07) | <0.001 | 0.008 |
| % Black Hispanic | −0.93 (−1.65, −0.21) | 0.01 | 0.002 |
| % Asian | −0.14 (−0.27, −0.02) | 0.03 | 0.01 |
| % American Indian | 0.97 (0.91, 1.03) | <0.001 | 0.40 |
| % adults living below poverty level | 0.20 (0.11, 0.30) | <0.001 | 0.11 |
| % uninsured | 0.10 (0.02, 0.18) | 0.01 | 0.17 |
| Miles to closest liver transplant center† | 0.12 (0.07, 0.17) | <0.001 | 0.08 |
| % with diabetes | 1.01 (0.70, 1.33) | <0.001 | 0.09 |
| % with heavy alcohol use | 0.33 (0.16, 0.51) | <0.001 | 0.002 |
| Listings per 100 deaths | −0.05 (−0.06, −0.04) | <0.001 | 0.09 |
Model did not include county rural/urban classification, the number of board-certified gastroenterologists, and percentage of county residents classified as obese as they were not associated with county-level age-adjusted liver disease related mortality (p>0.1 for all variables), and did not change the overall R2 of the multivariable model. Beta coefficients with a positive value associated with increased county-level liver disease-related mortality. Overall R2 of the multivariable model=0.62.
Per unit increase of 10 miles
Fully adjusted county-level mortality rates
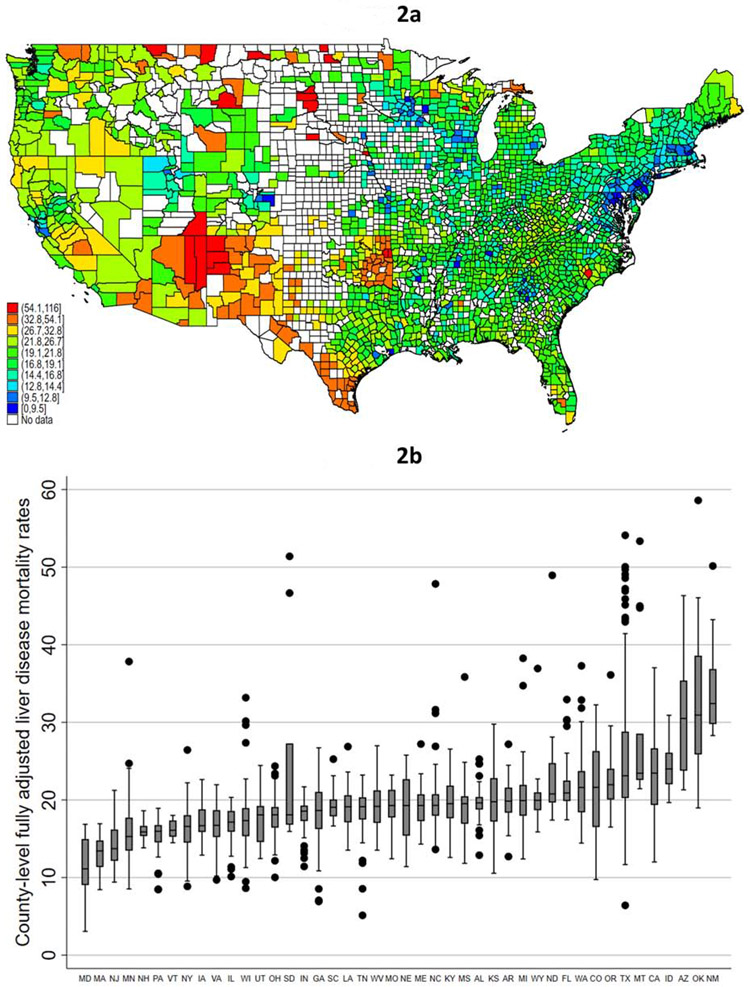
Despite adjustment for the county-level variables associated with age-adjusted liver mortality rates, there was continued variability in county-level mortality rates, although this was somewhat attenuated (Figure 2a). The counties with fully adjusted mortality rates in the ‘bottom’ 5th percentile (i.e., lowest mortality) were distributed across 26 states, led by Maryland (n=13, 12.9% of total), New York (n=10, 9.9%), and Georgia (7.9%). In contrast, the counties with the ‘top’ 5th percentile (i.e., highest mortality) of fully adjusted mortality encompassed 27 states, with more than 50% of them located in Texas (n=29, 23.6%), Oklahoma (n=25, 20.3%), and New Mexico (n=13, 10.6%). Notably, 13 (50.0%) of counties in New Mexico were in the top 5th percentile for highest fully adjusted liver disease mortality. Although there continued to be within-state among-county differences in liver-related mortality after adjusting for all of the covariates in the multivariable model, the within-state variability was attenuated. Twelve states had a less than two-fold difference in mortality among the counties with the highest and lowest mortality in the respective state, while only two (SD, WI) had a more than ten-fold difference, and five others with a more than five-fold difference (AR, NE, ND, TX, and UT; Figure 2b).
Figure 2 (two panels): County-level variability in liver disease-related mortality rates.
- Figure 2a: County-level fully adjusted liver disease-related mortality rates; 2009-2018*†**
- Figure 2b: Within-state variability in fully adjusted county-level liver disease-related mortality rates; 2009-2018*‡**
- * CDC WONDER age-adjusted mortality rates excludes counties with fewer than 20 liver disease-related deaths due to “unreliable” age-adjusted rates
- † Legend categories based on 1st, 5th, 10th, 25th, 50th, 75th, 90th, 95th, and 99th percentile of mortality
- ‡ Figure y-axis excludes top 1% of counties, and only states (n=46) with 10 or more counties with available data were included
- ** Fully adjusted mortality rates based on post-estimation predictions of multivariable linear regression models. Legend categories based on 1st, 5th, 10th, 25th, 50th, 75th, 90th, 95th, and 99th percentile of mortality
Geospatial hotspot analysis
Adjusted mortality rates were highly spatially correlated, representing five distinct clusters across the United States (Figures 3a and 3b). The age-adjusted hotspot analysis identified statistically significant clusters of counties with high age-adjusted mortality rates from liver disease (figure 3a), while the fully adjusted hotspot analysis identified statistically significant clusters of counties with high mortality rates from liver disease that are not explained by variation in the county population characteristics accounted for in our multivariable models (Figure 3b). Statistically significant hotspots were identified that encompassed: 1) South Florida; 2) Appalachia and the eastern part of the Midwest (fully adjusted hotspot analysis extended through all of Michigan and far eastern Wisconsin); 3) Texas and Oklahoma; 4) New Mexico, Arizona, California, and southern Oregon; and 5) parts of Washington and Montana. Notably, these areas had significantly higher than expected mortality from liver disease even after adjusting for factors that might traditionally explain this relationship, such as population composition and socioeconomic status, in the multivariable linear regression model, even if individual counties in the cluster did not have the highest mortality (e.g., Broward County in South Florida was at the 54th percentile for mortality).
Figure 3 (two panels): Geospatial hotspot analysis of liver disease-related mortality from 2009-2018.
- Figure 3a: Geospatial hotspot analysis based on age-adjusted liver disease-related mortality rates*
- Figure 3b: Geospatial hotspot analysis based on fully adjusted liver disease-related mortality rates*†
- *Hotspot analysis identifies clusters with significantly increased mortality based on county-level Z-scores and p-values (p<0.05 and p<0.01)
- † Mortality rates based on fully adjusted liver disease-related mortality from multivariable linear regression model
Temporal trends
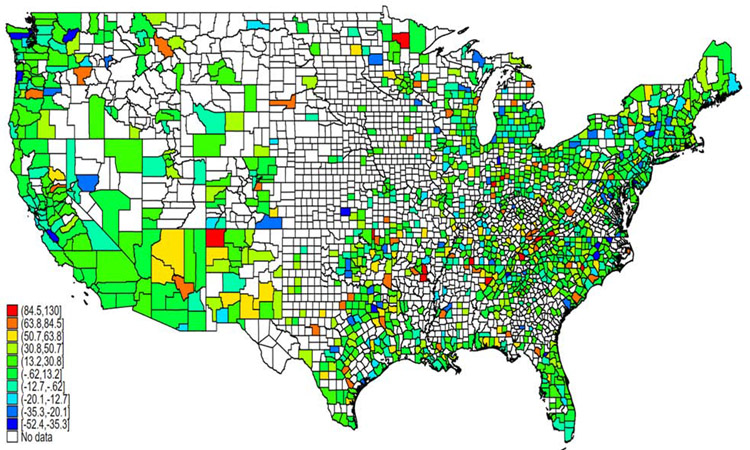
There were 1,337 counties (42.8% of all counties and 62.9% of the counties in the 2011-2018 analysis) with sufficient data in both the 2009-2013 and 2014-2018 periods. Among those 1,337 counties, 984 (73.6%) had a higher age-adjusted mortality rate in 2014-2018 compared to 2009-2013, of which 773 counties had a relative increase in the age-adjusted mortality rate of ≥10%, and 426 with a relative increase in the age-adjusted mortality rate of ≥25% (Figure 4). Conversely, there were 158 counties with a relative decrease in the age-adjusted mortality rate of ≥10%, and 42 counties with a relative decrease of ≥25% (Figure 4). Of the 42 counties (across 22 states) with the greatest relative decrease in age-adjusted liver disease-related mortality, only 20 were in a state that expanded Medicaid under the Affordable Care Act in 2014, while 22 were in states that did not expand during the study period (TX: 6; GA: 3; NC: 3; VA: 3; FL, ID, KS, MS, OK, SC, and WI: 1 each).
Figure 4: Relative percent change in county-level age-adjusted liver disease-related mortality from 2009-2013 to 2014-2018.
- Map includes data on 1,472 counties with ≥20 liver disease-related deaths in both time periods
Discussion
Using ten years of county-level mortality data, we demonstrate that there is substantial local variability in liver disease-related mortality rates, with five geographic clusters of mortality in the US. Although prior studies have shown state-level differences in mortality from cirrhosis and HCC and county-level differences from cirrhosis and chronic liver disease-related mortality (excluding HCC), these studies did not evaluate differences in mortality from all liver disease (cirrhosis + HCC) at the local level, did not examine the population-level sociodemographic and geographic variables that are contributing to the local variability in liver-related mortality, and did not perform formal hotspot analyses.1,2 Our data highlight that county-level racial composition and socioeconomic conditions (e.g., poverty, insurance) and remoteness from specialty care together account for nearly 60% of the variance in county-level mortality. These data have important public health and policy implications that help to identify hotspots of liver disease-related mortality that is not explained by the socio-demographic characteristics of the population and require further study and interventions to help mitigate these disparities.
Our findings are consistent with state-level data published in 2018.1 The age-adjusted liver disease mortality rates differed across the 50 states, and the temporal changes in liver disease mortality were not uniform across the US. Most notably, mortality rates increased the most in the South and West of the US.1 Furthermore, this previous work identified differences in liver disease mortality across specific populations (e.g., American Indians). The range in mortality across counties seen in our study is similar to that from an analysis of cirrhosis and chronic liver disease-related mortality published in 2016. That study demonstrated a difference of 14.0 deaths per 100,000 population between the 90th and 10th percentile counties (25.5 vs 11.5), while we found a difference of 16.8 deaths per 100,000 population (29.1 vs 12.3). 2 The difference in results is attributable to our study including HCC in the grouping of liver disease-related mortality, given that >90% of deaths occur in the setting of advanced and/or chronic liver disease.1,2,14 That study found that the difference in age-standardized cirrhosis and chronic liver disease-related mortality in the 90th vs 10th percentile counties in 2014 was the smallest among the 10 disease they studied.2 In contrast to the Tapper study, this study did not include HCC deaths in the cirrhosis and chronic liver disease category, even though >90% of cases of HCC occur in the setting of cirrhosis/advanced fibrosis, and did not account for data in the era of direct acting antiviral therapy for hepatitis C virus. 1-6 Neither of these studies however explored the determinants of the variability in liver disease mortality rates. By focusing on county-level data, we demonstrated significant differences in mortality among counties within the same state, and specific hotspots of liver disease mortality even after accounting for important county-level socio-demographic and access-to-care variables.
The within-state variability in mortality that we demonstrate differed across the US, with some states having little variability, and others having a more than ten-fold difference in age-adjusted liver disease-related mortality. In addition, beyond demographics, we identified important measures of access-to-care that help to explain nearly 60% of the variance in county-level mortality, although other factors (e.g., rates of HCV, access to primary care) may explain the residual variance. It is also important to note that the covariates in our model were examined at the county-, rather than patient-level, and therefore must be interpreted differently than they would be in a typical model using patient-level data. The data on race must also be interpreted in a similar fashion, although prior studies have shown lower rates of mortality from cirrhosis in Blacks.1 However, other variables that apply to all residents of a county, notably distance (or rurality), can be interpreted similarly to a model with patient-level data as the covariate applies the same to everyone in that geographic area.
Although prior studies have demonstrated that mortality rates for many chronic diseases are higher for those living in rural areas, including heart disease, cancer, stroke, chronic respiratory disease, and even HCC, our study found that rurality was not significant, but rather distance to a transplant center was significantly associated with county-level mortality rates.1,5,20,31-41 However, those prior studies focused only on rural/urban status, rather than proximity to specialty care, which is important in patients with cirrhosis and HCC, who have significantly lower mortality when they are treated by hepatology specialists, who almost exclusively practice at a liver transplant center.16-18,31,42-45 The association between distance to a transplant center and liver disease-related survival on a population level validates published data from our group that shows that increased distance from a transplant center, rather than rurality, is associated with increased mortality among Veterans with end-stage liver disease,16 and commercially insured patients with decompensated cirrhosis and/or HCC.17 However, even after accounting for distance, and other county-level variables, we identified five geographic hotspots of liver disease-related mortality that cannot be explained solely by the variables in our model. Mortality may be higher in these areas due to factors that we could not account for in our model (e.g., prevalence of HCV, access to primary care, or other socioeconomic, cultural, and/or biological factors in the population that we could not fully adjust for).46 Nevertheless, there are several policy and care initiatives that could be considered based on these findings. First, efforts to enhance telehealth coverage and outreach is needed for geographically isolated patients, which has been shown to improve care and survival for patients with HCV47-56 and to improve access to transplantation for patients with end-stage liver disease.57 Second, data on county-level mortality and distance to a liver transplant center could better inform decisions when opening new liver transplant center(s), a strategy that has been proposed in other countries, or at the minimum satellite clinics to provide liver care to those with the highest mortality rates.10 Third, these county-level data can be used to apply area-need variables to promote equitable distribution of transplant grafts based on the perspective of mortality of the broader population with end-stage liver disease.58
Our analyses demonstrated that counties with a higher percentage of uninsured patients had higher liver disease-related mortality rates. Differential access to insurance under the Affordable Care Act, both in terms of lower-cost health insurance through healthcare exchanges and Medicaid for states that expanded Medicaid may have impacted both county- and state-level mortality rates. It is possible expanded access to health insurance could help to remediate disparities in counties with low uninsured rates. However, to fully address this requires a difference-in-difference analysis that evaluates changes in mortality across states as a function of Medicaid expansion, which is beyond the scope of this manuscript.
Our study did have limitations. First, because of the small number of liver disease-related deaths in many counties in any individual year, we combined ten years of data for the primary analyses. Although there have been temporal changes in mortality, the results of our multivariable model are unlikely to have been biased by aggregation of 10 years of data. Secondly, we evaluated mortality and potential explanatory variables in aggregate at the county-level due to a lack of patient-level data for mortality and exposures. Although this does not allow us to conclude if individual factors are associated with mortality at the patient level (e.g., obesity), several of the variables are uniform across the county (e.g., distance), and the goal was to evaluate geographic differences in mortality at a county-level. Third, we were unable to evaluate local rates of specific diseases (e.g., HCV). Additionally, death certificate data have substantial missingness related to the underlying cause of liver disease (e.g., in 2018 <10% of the deaths had a diagnosis of HCV or NASH in the CDC dataset), and therefore we couldn’t evaluate for temporal changes in the cause of liver disease-related deaths. Although these factors may have explained the differences in mortality that we found (e.g., certain counties have higher prevalence of HCV and therefore higher rates of liver disease-related mortality), they would have been mediators in the causal pathway rather than confounders to be adjusted for in models. Fourth, certain variables were not available for the entire study period (e.g., obesity, drinking), although this is unlikely to have biased our findings substantially. Lastly, we are unable to evaluate local factors that may explain the striking mortality differences in the same state (e.g., Oklahoma), and this identifies a research future direction to obtain more granular data to evaluate factors influencing differences in mortality in the same geographic area. Ultimately, the limitations of the data do not allow us to conclude whether the higher mortality rates in certain “hotspots” is due to a higher prevalence of liver disease, more severe/aggressive cases, poor access-to-care, substandard care, or a combination of these factors. These analyses, which would be stratified by key variables, requires a different data source that is not constrained by CDC limitations of reporting data for areas with a small number of deaths (<20) for a given group of interest. Additionally, county-level data may obscure local, neighborhood-level disparities that cannot be address using these data, nor can we address the potential beneficial (or detrimental) effect of movement from one area to another on an individual’s risk of liver-related mortality. Therefore, further work is needed to explore these issues.
In conclusion, our data clearly demonstrates significant intra-state differences in liver disease-related mortality, and several socio-demographic and access-to-care variables that help to explain this variability. These data help to identify hotspots of liver disease while identifying potentially modifiable factors (e.g., proximity to a liver transplant center) that could help to remediate observed disparities, including improving access to specialized liver care. Further studies are needed to examine interplay of these factors, and to identify other variables that contribute to the substantial county-level differences in liver disease-related mortality.
Supplementary Material
What You Need to Know.
Background & Context: Data are lacking at the ‘local’ (e.g., county) level to identify factors associated with variability in liver disease-related mortality and hotspots of liver disease mortality.
New Findings: In multivariable models, 61% of the variability in among-county mortality was explained by county-level race/ethnicity, poverty, uninsured rates, distance to the closest transplant center, and local rates of obesity, diabetes, and alcohol use. Despite adjustment, there was significant within-state variability in county-level mortality rates.
Limitations: The analysis relied on county-, rather than individual patient-level data.
Impact: Our study identifies potential modifiable risk factors to mitigate county-level mortality from liver disease (e.g., increase access to specialty care) and hotspots of liver disease mortality in the US.
Acknowledgments
Dr. Goldberg receives funding from NIH R01 DK120561.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None of the authors have any conflicts of interests as it relates to this manuscript
References
- 1.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ. 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. US County-Level Trends in Mortality Rates for Major Causes of Death, 1980-2014. JAMA. 2016;316(22):2385–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiang X, You XM, Zhong JH, Li LQ. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. Journal of hepatology. 2017;67(4):885–886. [DOI] [PubMed] [Google Scholar]
- 4.Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2016;14(1):124–131.e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127. [DOI] [PubMed] [Google Scholar]
- 6.Shah SA, Smith JK, Li Y, Ng SC, Carroll JE, Tseng JF. Underutilization of therapy for hepatocellular carcinoma in the medicare population. Cancer. 2011;117(5):1019–1026. [DOI] [PubMed] [Google Scholar]
- 7.Backus LI, Belperio PS, Shahoumian TA, Mole LA. Direct-acting antiviral sustained virologic response: Impact on mortality in patients without advanced liver disease. Hepatology. 2018;68(3):827–838. [DOI] [PubMed] [Google Scholar]
- 8.Backus LI, Belperio PS, Shahoumian TA, Mole LA. Impact of Sustained Virologic Response with Direct-Acting Antiviral Treatment on Mortality in Patients with Advanced Liver Disease. Hepatology. 2019;69(2):487–497. [DOI] [PubMed] [Google Scholar]
- 9.Belperio PS, Shahoumian TA, Loomis TP, Mole LA, Backus LI. Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3. Journal of hepatology. 2019;70(1):15–23. [DOI] [PubMed] [Google Scholar]
- 10.Webb GJ, Hodson J, Chauhan A, et al. Proximity to transplant center and outcome among liver transplant patients. American Journal of Transplantation. 2019;19(1):208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arora S, Thornton K, Murata G, et al. Outcomes of Treatment for Hepatitis C Virus Infection by Primary Care Providers. New England Journal of Medicine. 2011;364(23):2199–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asrani SK, Larson JJ, Yawn B, Therneau TM, Kim WR. Underestimation of liver-related mortality in the United States. Gastroenterology. 2013;145(2):375–382.e371–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naghavi M, Makela S, Foreman K, O'Brien J, Pourmalek F, Lozano R. Algorithms for enhancing public health utility of national causes-of-death data. Popul Health Metr. 2010;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dwyer-Lindgren L, Flaxman AD, Ng M, Hansen GM, Murray CJ, Mokdad AH. Drinking Patterns in US Counties From 2002 to 2012. Am J Public Health. 2015;105(6):1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch RJ, Ye F, Sheng Q, Zhao Z, Karp SJ. State-Based Liver Distribution: Broad Sharing With Less Harm to Vulnerable and Underserved Communities Compared With Concentric Circles. Liver transplantation. 2019;25(4):588–597. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg DS, French B, Forde KA, et al. Association of distance from a transplant center with access to waitlist placement, receipt of liver transplantation, and survival among US veterans. JAMA. 2014;311(12):1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg DS, Newcomb C, Gilroy R, et al. Increased Distance to a Liver Transplant Center Is Associated With Higher Mortality for Patients With Chronic Liver Failure. Clinical gastroenterology and hepatology. 2017;15(6):958–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross K, Patzer RE, Goldberg DS, Lynch RJ. Sociodemographic Determinants of Waitlist and Posttransplant Survival Among End-Stage Liver Disease Patients. American Journal of Transplantation. 2017;17(11):2879–2889. [DOI] [PubMed] [Google Scholar]
- 19.Ross KH, Patzer RE, Goldberg D, Osborne NH, Lynch RJ. Rural-Urban Differences in In-Hospital Mortality Among Admissions for End-Stage Liver Disease in the United States. Liver transplantation. 2019;25(9):1321–1332. [DOI] [PubMed] [Google Scholar]
- 20.Cross SH, Mehra MR, Bhatt DL, et al. Rural-Urban Differences in Cardiovascular Mortality in the US, 1999-2017. JAMA. 2020;323(18):1852–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.United States Census Bureau Urban and Rural. https://www.census.gov/programssurveys/geography/guidance/geo-areas/urban-rural.html. Accessed July 28, 2020.
- 22.United States Census Bureau Small Area Income and Poverty Estimates (SAIPE) Program. https://www.census.gov/programs-surveys/saipe.html. Accessed July 9, 2020.
- 23.US Department of Health and Human Services. Centers for Disease Control and Prevention. National Center for Health Statistics. The Validity of Race and Hispanic-origin Reporting on Death Certificates in the United States: An Update. https://www.cdc.gov/nchs/data/series/sr_02/sr02_172.pdf. Accessed July 28, 2020.
- 24.Centers for Disease Control and Prevention Underlying Cause of Death 1999-2018. https://wonder.cdc.gov/wonder/help/ucd.html#. Accessed July 28, 2020.
- 25.United States Census Bureau Small Area Health Insurance Estimates (SAHIE). https://www.census.gov/content/census/en/programs-surveys/sahie.html/. Accessed July 7, 2020.
- 26.HRSA Area Health Resources Files. https://data.hrsa.gov/topics/health-workforce/ahrf. Accessed July 26, 2020.
- 27.Institute for Health Metrics and Evaluation (IHME). Diagnosed and Undiagnosed Diabetes Prevalence by County in the U.S., 1999-2012. Seattle, United States: Institute for Health Metrics and Evaluation (IHME), 2016. [Google Scholar]
- 28.Institute for Health Metrics and Evaluation (IHME). Life Expectancy, Physical Activity, and Obesity by County in the U.S., 2001-2011. Seattle, United States: Institute for Health Metrics and Evaluation (IHME), 2016. [Google Scholar]
- 29.Patel RS, Walker T, Weber ZT, Kelley SD, Hansen R. A pilot study using geospatial analysis to identify hot-spot of populations utilizing services at university based counseling centers. J Am Coll Health. 2020:1–6. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez A, Branscum AJ, Li J, MacKinnon NJ, Hincapie AL, Cuadros DF. Epidemiological and geospatial profile of the prescription opioid crisis in Ohio, United States. Sci Rep. 2020;10(1):4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Potentially Excess Deaths from the Five Leading Causes of Death in Metropolitan and Nonmetropolitan Counties – United States, 2010–2017. https://www.cdc.gov/mmwr/volumes/68/ss/ss6810a1.htm?s_cid=ss6810a1_w. Accessed August 3, 2020. [DOI] [PubMed]
- 32.Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the Case for Investment in Rural Cancer Control: An Analysis of Rural Cancer Incidence, Mortality, and Funding Trends. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2017;26(7):992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoehn RS, Hanseman DJ, Jernigan PL, et al. Disparities in care for patients with curable hepatocellular carcinoma. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2015;17(9):747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology (Baltimore, Md). 2011;53(3):1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. [DOI] [PubMed] [Google Scholar]
- 36.Artinyan A, Mailey B, Sanchez-Luege N, et al. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer. 2010;116(5):1367–1377. [DOI] [PubMed] [Google Scholar]
- 37.Mathur AK, Osborne NH, Lynch RJ, Ghaferi AA, Dimick JB, Sonnenday CJ. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg. 2010;145(12):1158–1163. [DOI] [PubMed] [Google Scholar]
- 38.Stewart SL, Kwong SL, Bowlus CL, et al. Racial/ethnic disparities in hepatocellular carcinoma treatment and survival in California, 1988-2012. World J Gastroenterol. 2016;22(38):8584–8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109(4):542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu L, Kim Y, Spolverato G, Gani F, Pawlik TM. Racial disparities in treatment and survival of patients with hepatocellular carcinoma in the United States. Hepatobiliary Surgery and Nutrition. 2016;5(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rich NE, Hester C, Odewole M, et al. Racial and Ethnic Differences in Presentation and Outcomes of Hepatocellular Carcinoma. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cicalese L, Shirafkan A, Jennings K, Zorzi D, Rastellini C. Increased Risk of Death for Patients on the Waitlist for Liver Transplant Residing at Greater Distance From Specialized Liver Transplant Centers in the United States. Transplantation. 2016;100(10):2146–2152. [DOI] [PubMed] [Google Scholar]
- 43.Patwardhan V, Paul S, Corey KE, et al. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and involvement of gastrointestinal sub-specialists. Digestive diseases and sciences. 2011;56(11):3316–3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34(6):1089–1095. [DOI] [PubMed] [Google Scholar]
- 45.Kanwal F, Kramer JR, Buchanan P, et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012;143(1):70–77. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen GC, Thuluvath PJ. Racial disparity in liver disease: Biological, cultural, or socioeconomic factors. Hepatology. 2008;47(3):1058–1066. [DOI] [PubMed] [Google Scholar]
- 47.Page K, Qeadan F, Qualls C, Thornton K, Arora S. Project ECHO Revisited: Propensity Score Analysis And HCV Treatment Outcomes. Hepat Med. 2019;11:149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohsen W, Chan P, Whelan M, et al. Hepatitis C treatment for difficult to access populations: can telementoring (as distinct from telemedicine) help? Intern Med J. 2019;49(3):351–357. [DOI] [PubMed] [Google Scholar]
- 49.Mendizabal M, Ridruejo E, Ceballos S, et al. The ECHO model proved to be a useful tool to increase clinicians' self-effectiveness for care of patients with Hepatitis C in Argentina. Journal of viral hepatitis. 2019;26(11):1284–1292. [DOI] [PubMed] [Google Scholar]
- 50.Marciano S, Haddad L, Plazzotta F, et al. Implementation of the ECHO(®) telementoring model for the treatment of patients with hepatitis C. J Med Virol. 2017;89(4):660–664. [DOI] [PubMed] [Google Scholar]
- 51.Jayasekera CR, Arora S, Ahmed A. Hepatitis C Treatment Delivery Mandates Optimizing Available Health Care Human Resources: A Case for Task Shifting. JAMA. 2016;315(18):1947–1948. [DOI] [PubMed] [Google Scholar]
- 52.Dhiman RK, Grover GS, Premkumar M, et al. Decentralized care with generic direct-acting antivirals in the management of chronic hepatitis C in a public health care setting. Journal of hepatology. 2019;71(6):1076–1085. [DOI] [PubMed] [Google Scholar]
- 53.Arora S, Thornton K, Jenkusky SM, Parish B, Scaletti JV. Project ECHO: linking university specialists with rural and prison-based clinicians to improve care for people with chronic hepatitis C in New Mexico. Public Health Rep. 2007;122 Suppl 2(Suppl 2):74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arora S, Thornton K. Novel Models of Hepatitis C Virus Care Delivery: Telemedicine, Project ECHO, and Integrative Care. Clin Liver Dis (Hoboken). 2020;16(1):5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arora S, Kalishman S, Thornton K, et al. Expanding access to hepatitis C virus treatment--Extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology. 2010;52(3):1124–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arora S, Kalishman S, Dion D, et al. Partnering urban academic medical centers and rural primary care clinicians to provide complex chronic disease care. Health Aff (Millwood). 2011;30(6):1176–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.John BV, Love E, Dahman B, et al. Use of Telehealth Expedites Evaluation and Listing of Patients Referred for Liver Transplantation. Clinical gastroenterology and hepatology. 2020;18(8):1822–1830.e1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldberg DS, French B, Sahota G, Wallace AE, Lewis JD, Halpern SD. Use of Population-based Data to Demonstrate How Waitlist-based Metrics Overestimate Geographic Disparities in Access to Liver Transplant Care. American Journal of Transplantation. 2016;16(10):2903–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.