Abstract
Waldenström macroglobulinemia (WM)/lymphoplasmacytic lymphoma (LPL) is often differentiated from myeloma based on the presence of lytic bone lesions (LBL). However, WM/LPL can present with LBL, and management is poorly understood. We describe a case of an 81‐year‐old woman with LPL who presented with LBL and was successfully treated with chemoimmunotherapy.
Keywords: IgM myeloma, lymphoplasmacytic lymphoma, lytic bone lesions, multiple myeloma, non‐Hodgkin's lymphoma, Waldenström macroglobulinemia
We have discussed how optimal therapeutic management of WM patients with lytic bone disease is poorly understood which necessitates the need for further investigation on the ability of novel agents and anti‐resorptive agents to reverse bone turnover in WM/LPL patients.
1. INTRODUCTION
Waldenström macroglobulinemia (WM) is a rare, indolent, lymphoproliferative disorder that represents 1%–2% of all non‐Hodgkin lymphomas (NHL). 1 It is pathologically defined as lymphoplasmacytic lymphoma (LPL) by the World Health Organization and is characterized by bone marrow infiltration with clonal lymphoplasmacytic cells and IgM monoclonal gammopathy, although non‐IgM secreting lymphoplasmacytic lymphomas have also been described. 2 , 3 Lytic bone lesions are rare in WM/LPL and are often used as a differentiating clinical feature between WM/LPL and multiple myeloma (MM), particularly IgM myeloma. Schuster et al. used strict defining criteria for IgM myeloma to make a clear distinction from WM/LPL since the approach to their treatment and prognosis vary significantly. 4 , 5 , 6 , 7 Table 1 Rothschild et al 8 documented in their clinical case study that WM/LPL has a combination of features of other hematologic malignancies such as myeloma and leukemia on both macroscopic and radiologic examination of osteolytic lesions. Papanikolaou et al 9 substantiated this rather unusual presentation in WM/LPL on imaging studies such as PET‐CT (positron emission tomography‐computed tomography) or MRI (magnetic resonance imaging), while some other studies even reported improvement of lytic lesions with treatment. 10 , 11 , 12 , 28 , 29
TABLE 1.
Comparison of key distinguishing characteristics of IgM Myeloma vs. Waldenstrom macroglobulinemia
| Characteristic | IgM myeloma | Waldenstrom’s Macroglobulinemia |
|---|---|---|
| MYD88 and CXCR4 Mutations | − | + |
| Hypercalcemia, renal failure, anemia, lytic bone lesions (CRAB) | + | −* |
| Lymphadenopathy, splenomegaly | − | + |
| CD20 Expression | − | + |
| Flow cytometry profile 45 , 46 | CD38+, CD138+ CD20−, CD19−, CD79a+, CD56, Cyclin D1+, CD117− | CD138−, CD19+, CD20+, CD22+, CD23−, CD5−, CD10− |
| Presence of t(11;14) | + | − |
| Response to anti‐CD20 monoclonal antibody therapy | − | + |
| Early autologous hematopoietic stem cell transplant (HSCT) | Yes | No, only for refractory/relapsed disease |
| Clinical course and prognosis | Aggressive | More indolent than MM |
| Overall survival 4 | Shorter (~30 months) | Longer (in years) |
| High expression of IL‐1 (Osteoclast‐activating factor) 4 | + | − |
| Association with immunological phenomenon 47 , 48 | No | Yes, with cold agglutinin disease, cryoglobulinemia, Raynaud’s syndrome, peripheral neuropathy |
WM/LPL can present with lytic bone lesions. Lytic bone lesions associated with non‐Hodgkin’s lymphomas such as CLL and WM/LPL are well reported in the literature.
There is a paucity in the literature as to whether patients with WM/LPL and lytic bone lesions should be treated with chemoimmunotherapy or novel agents and whether bone‐strengthening agents should be used. A consensus panel from the 10th International Workshop on WM has updated both first‐line and salvage treatment recommendations. The preferred primary therapy options for symptomatic patients with WM include chemoimmunotherapeutic combination regimens of rituximab with alkylating agents (ie, bendamustine, cyclophosphamide) and proteasome inhibitors (ie, bortezomib) or with Bruton's tyrosine kinase (BTK) inhibitors such as ibrutinib. Treatment options need to be customized according to the individual patient's clinical presentation and genomic features. 13 , 14 , 15 , 16 Studies for anti‐resorptive agents in WM/LPL are lacking. Herein, we describe a case of LPL with lytic bone lesions who was treated with rituximab, cyclophosphamide, and dexamethasone and had achieved a CR with complete resolution of lytic bone lesions on PET‐CT.
2. CASE
An 81‐year‐old woman with progressively deteriorating Parkinson's disease despite ongoing medical treatment for more than 5 years and osteoporosis (on denosumab every 6 months) presented to our institution's spine center for worsening back pain and frequent falls. She was initially diagnosed with a mild degenerative disc disease of her cervical, thoracic, and lumbar spine without myelopathy and severe facet arthrosis in the lumbar spine from L2‐3 to L5‐S1 as demonstrated on MRI images. Due to progressive pain and the possibility of compression fractures from her recurrent falls, a PET‐CT was performed which reported several foci of marked hypermetabolism including a dominant lesion in the right humeral head, SUV max 9.9, and additional hypermetabolic lesions were seen in the bilateral scapulae, clavicles, right hemi‐sacrum, right iliac wing, left acetabulum anterior column, left superior pubic ramus, and right femoral head (Figure 1A). The images were suggestive of MM‐associated lytic lesions and a subsequent serum protein electrophoresis and immunofixation revealed small monoclonal IgA lambda immunoglobulins on immunofixation only, no m‐spike was present. Complete blood cell count, quantitative serum‐free light chains, β2‐microglobulin, albumin, LDH, and creatinine were all within normal ranges. The patient have mild hypercalcemia with a calcium level of 10.3 mg/dl. A subsequent bone biopsy from the left anterior acetabulum was obtained, which revealed diffuse proliferation of small B‐lymphocytes with an interstitial and para‐trabecular pattern (Figure 2A). Immunohistochemistry (IHC) studies showed that the lymphocytes were positive for CD20 and PAX5 (Figure 2B, C), which proved the B‐cell lineage of the lymphoma. The neoplastic lymphocytes were negative for MUM1 and cyclin D1. Moreover, CD138 highlighted scattered plasma cells that were positive for IgA and lambda‐light chain‐restricted (Figure 2D‐F). A MYD88 L265P alteration was detected via amplification of DNA using allele‐specific polymerase chain reaction with an allele‐specific primer. A bone marrow biopsy was done and showed a plasma cell proliferative disorder with 5%–9% lambda‐light chain‐restricted plasma cells with a normocellular marrow (30%–40%) with otherwise morphologically unremarkable trilineage hematopoiesis. There were no morphologic or immunophenotypic features of a lymphoid neoplasm. Immunophenotyping by flow cytometry identified a lambda light chain‐restricted plasma cell population that expressed CD38 and CD138 and did not express CD19 or CD45. These findings were most consistent with a lymphoplasmacytic lymphoma.
FIGURE 1.
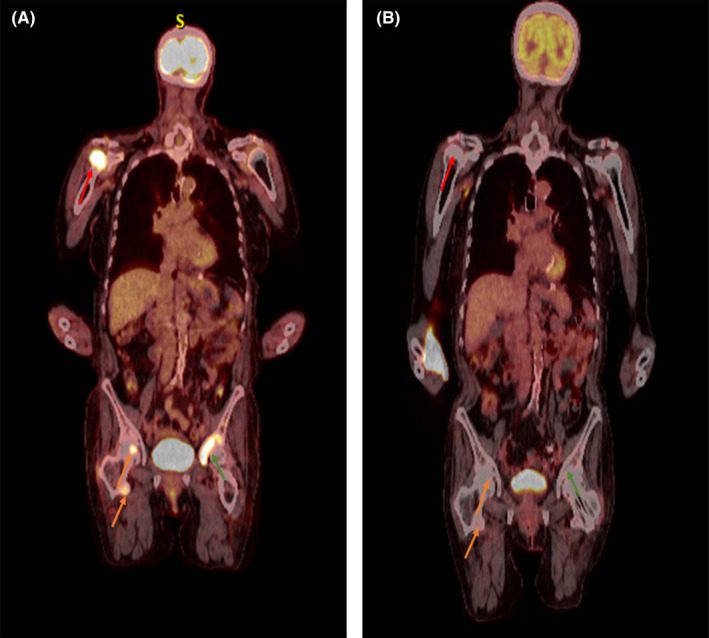
(A) Positron emission tomography‐computed tomography at diagnosis showing evidence of lytic lesions in right humeral head (red arrow), right femoral head (orange arrows) and left acetabulum (green arrow). (B) PET‐CT showing resolution of lytic lesions after six cycles of rituximab‐cyclophosphamide‐dexamethasone
FIGURE 2.
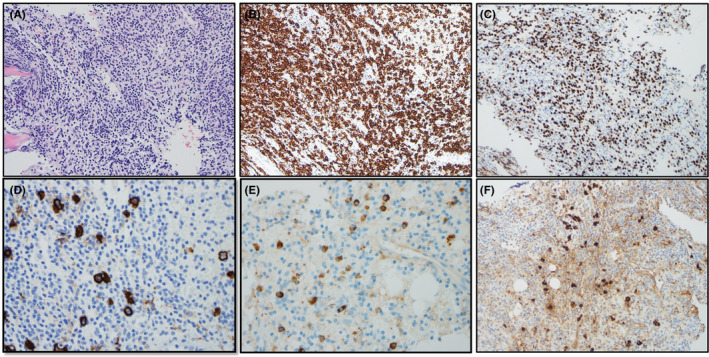
The biopsy of the lytic bone lesion at left anterior acetabulum showed diffusely proliferation of small lymphocytes (A, H&E ×20), which are positive for CD20 (B, IHC ×20) and PAX5 (C, IHC ×20). Scattered plasma cells were highlighted CD138 (D, IHC ×40), and IgA (E, IHC ×40) with lambda light chain restriction (F, IHC ×40)
Given the diagnosis of lymphoplasmacytic lymphoma in a frail patient with advanced Parkinson's disease and an ECOG (Eastern Cooperative Oncology Group) performance status of 2, it was decided to treat the patient with chemoimmunotherapy consisting of IV rituximab 375 mg/m2, IV cyclophosphamide 300 mg/m2, and IV dexamethasone 20 mg given every 21 days. The patient completed six cycles of treatment. Treatment was complicated by the development of grade 2 neutropenia and grade 1 anemia. The patient also developed a grade 2 urinary tract infection that needed treatment with oral antibiotics and grade 2 herpes labialis which required acyclovir treatment for 10 days after cycle 4 causing cycle 5 to be delayed by 1 week. Following completion of the six cycles of treatment, the patient had complete resolution of lytic lesions on PET‐CT (Figure 1B) and no detectable IgA‐kappa monoclonal protein on serum protein electrophoresis or immunofixation. The patient went on to start maintenance therapy with single‐agent rituximab every 3 months for up to 2 years. The patient still had some back pain which is likely related to her underlying degenerative joint disease and severe facet arthrosis in the lumbar spine so she will follow up with neuroradiology for possible facet joint injections and/or potential kyphoplasty.
3. DISCUSSION
We described a case of an IgA lambda, MYD88L265 mutation + lymphoplasmacytic lymphoma with lytic bone lesions that was successfully treated with rituximab, cyclophosphamide, and dexamethasone resulting in a complete response. This regimen was chosen over bendamustine and rituximab because at the age of 81, we did not think the patient would tolerate bendamustine well due to the possibility of bone marrow suppression and cytopenias which are common with bendamustine. Furthermore, rituximab cyclophosphamide, and dexamethasone have been shown to have comparable efficacy and toxicity compared to bendamustine and rituximab. 17 Ibrutinib was not chosen because the patient was having considerable bone pain and discomfort and we wanted to achieve a rapid response. Single‐agent ibrutinib is slow to act with no complete responses and a median time to best response of 7.5 months with very good partial responses occurring after a median of 15.5 months. 18 We decided to give the patient maintenance rituximab because an observation study of 248 rituximab‐naïve patients who responded to a rituximab‐containing regimen revealed that maintenance rituximab for 2 years resulted in superior progression‐free survival and overall survival. 19 It is important to note that no patients in this study received bendamustine. However, rituximab maintenance is controversial as the results of the MAINTAIN trial failed to show progression‐free or overall survival benefit for patients with Waldenstrom's macroglobulinemia who were treated with front line bendamustine and rituximab and then went on to receive either 2 years of rituximab maintenance or observation. 20 Thus, perhaps there is no benefit to rituximab maintenance when bendamustine and rituximab are given front line but there may be a benefit to rituximab maintenance if non‐bendamustine‐containing regimens are used. A multitude of novel agents are approved for the treatment of WM/LPL such as BTK inhibitors, proteasome inhibitors, and monoclonal antibodies and many more next‐generation therapies in these drug classes are under development in addition to BCL2 inhibitors such as venetoclax and phosphatidylinositol 3 kinase inhibitors such as idelalisib and umbralisib. 21 However, the efficacy of these agents in WM/LPL patients with lytic bone disease is unknown. The efficacy of agents such as proteasome inhibitors, immunomodulatory drugs, and alkylating agents on bone remodeling in MM is well established. 22 In lymphoid malignancies such as chronic lymphocytic leukemia (CLL), only BTK inhibitors such as ibrutinib have shown promising therapeutic response in patients with osteolytic lesions. 23 , 24 , 25 There is evidence that the BTK inhibitor ibrutinib can suppress bone resorption by inhibition of both osteoclast differentiation and function, predominantly by downregulation of expression of nuclear factor of activated T cells 1 (NFATc1), the key transcription factor for osteoclastogenesis, and disruption of the formation of the actin ring in mature osteoclasts. 26 In one case of an elderly woman with relapsed CLL/SLL (chronic lymphocytic leukemia/small lymphocytic lymphoma) with widespread lytic disease and pathological fractures, treatment with ibrutinib monotherapy (420 mg q.d.) with monthly denosumab (120 mg s.c.) for only 9 months resulted in remineralization of her skeletal lesions and partial disease response. The combination of a BTK Inhibitor with a bone‐resorptive agent provided significant clinical benefit with remarkable improvement in patient mobilization after about 12 months of treatment with sclerosis of skeletal lesions as noted on serial CT and MRI scans. 27
Although rare, multiple studies have reported lytic bone lesions in cases of WM/LPL with little guidance on management. In the large study series of 37 patients by Schuster et al, the inclusion criteria considered were the presence of IgM monoclonal protein and ≥10% plasma cells in the bone marrow biopsy in addition to the characteristic lytic bone lesions with or without the most common cytogenetic abnormality of IgM myeloma ie, translocation t(11;14). This study did not include patients based on non‐specific clinical features of myeloma such as the presence of anemia, hypercalcemia, and renal failure or their immunophenotype. 4 , 5 , 6 , 7 The case study by Rothschild et al was used to differentiate similarly presenting cases based on the specific differences in the gross appearance of bony lesions. The lytic lesions of WM were either sharp spheroid lesions or abundant coalescing pits that were identifiable from the numerous frontally resorptive non‐spheroid leukemic lesions and the pit less although spheroid lesions of MM. 8 This is in contrast with MM, which tends to show four different forms of destructive bone changes on imaging studies—single expansile plasmacytoma, disseminated punched‐out lytic lesions, diffuse skeletal osteopenia, or osteosclerosis. 30 , 31 Based on another retrospective investigation conducted by Papanikolaou et al, focal lytic bone disease was evident in 17%–24% of WM cases on MRI or PET‐CT imaging, respectively. 29 Regardless, there is scarce literature on optimal treatment for patients with WM/LPL and lytic bone disease. The modulation of bone remodeling by anti‐myeloma agents such as immunomodulatory drugs, proteasome inhibitors, and monoclonal antibodies provide insight into their potential efficacy and mechanism of action in patients with WM/LPL. 28 Via interactions with the bone marrow microenvironment, malignant plasma cells are able to orchestrate the production of osteoclast‐activating factors (ie, RANKL) and osteoblast‐inhibitory factors which leads to asynchronous bone turnover, net bone loss, and osteolytic lesions. Proteasome inhibitors such as bortezomib, carfilzomib, and ixazomib inhibit NF‐κB (nuclear factor kappa‐B) mediated osteoclast maturation and, ultimately, bone resorption via the RANKL and OPG (osteoprotegerin) pathway. 29 Terpos et al demonstrated that bortezomib also increased bone formation markers like bone‐specific alkaline phosphatase (ALP) and osteocalcin levels with only four cycles of treatment in 34 relapsed MM patients. 32 Furthermore, bortezomib has shown inhibition of osteoclastogenesis in combination with the immunomodulatory drug lenalidomide in vitro. 33 Both of these agents have also shown the ability to reduce tumor burden in MM patients through their inhibitory effect on osteoclast‐derived growth and survival factors and blocking of RANKL secretion from bone marrow stromal cells. 33 , 34 Lenalidomide inhibits osteoclastogenesis as evidenced by decreased serum biochemical markers of bone turnover. 34 Pomalidomide, another immunomodulatory drug, has shown potent osteoclast inhibitory activity in vitro with its downregulating effect on transcription factor PU.1 and significant blunting of RANKL upregulation, thus normalizing the RANKL‐OPG ratio. 35 Daratumumab, an anti‐CD38 monoclonal antibody, inhibits bone remodeling by blocking the interaction of CD38‐expressing monocytes and osteoclast‐progenitor cells thus inhibiting bone resorption activity in bone marrow cells of MM patients. 36 , 37 In a study of 51 MM patients, high dose chemotherapy with melphalan followed by autologous stem cell transplant (ASCT) resulted in a significant reduction of sRANKL/OPG ratio, with a concomitant decrease in markers of bone resorption starting the second month post‐ASCT. 38 Further investigation is needed on whether these active anti‐MM agents have similar effects on bone turnover in patients with non‐Hodgkin lymphomas such as WM/LPL with lytic bone lesions.
Although chemoimmunotherapy combinations are current standard treatment regimens and are highly active with high response rates, they can cause immunosuppression and cytopenias which may not be well tolerated by elderly, frail patients. With a median age of diagnosis of WM/LPL being 70 years, consideration must be given to patient frailty and ability to tolerate such a treatment. However, cyclophosphamide is well tolerated in elderly patients when used as a combination regimen with rituximab and dexamethasone (DRC). This was demonstrated in a study conducted by Dimopolous et al in a large multicenter trial of 72 patients with WM, whose median age was 69 years and among which 63% patients were older than 65 years old. Based on analysis of this study, therapy with DRC was well tolerated and only about 10% of patients experienced grade 3 or 4 neutropenia, and 10% of patients developed neutropenic fever requiring hospitalization and intravenous antibiotics. No patients developed grade 3 or 4 thrombocytopenia. Therefore, DRC is a safe and well‐tolerated regimen, even in elderly frail patients. 39 The DRC regimen was also used successfully in a 64‐year‐old patient who was diagnosed with WM and had mixed lytic and sclerotic lesions on skeletal radiographs and CT scans. The patient tolerated six cycles of DRC treatment with no significant toxicity or signs of lymphoma progression after a follow‐up of 32 months. The majority of the patient's bone lesions also disappeared with treatment except for one persistent bone lesion which was treated with 8 Gy of radiation therapy. 40 Based on the available data, including our case report, DRC is an efficacious regimen for patients with WM and lytic bone lesions.
Interestingly, our patient was on denosumab every 6 months for osteoporosis, but there are no consensus guidelines on the use of anti‐resorptive agents for patients with WM/LPL and lytic bone lesions. Many preclinical and randomized control studies of bisphosphonates and RANKL (receptor activator of nuclear factor‐kappa B ligand) inhibitors in MM have demonstrated not only reduction of bone complications but also potential anti‐MM effects as well. 32 The risk of high bone turnover and premature osteoporosis in lymphoma patients due to treatment with high dose corticosteroids can be counteracted by the prophylactic use of anti‐resorptive agents. In patients with lymphoma receiving chemotherapy, treatment with the second‐generation bisphosphonate pamidronate every 3 months for 1 year reduced both bone loss and the risk of new vertebral fractures. 41 A prospective randomized phase III trial investigated the benefit of using zoledronic acid (ZA) in 74 newly diagnosed lymphoma patients undergoing chemotherapy and with a baseline bone mineral density (BMD) of ≥−2.0. A dose of 4 mg IV ZA was given at trial enrollment and at 6 months along with oral calcium (1200 mg) and vitamin D (400 or 800 IU). Fifty‐three patients were evaluable for response: 24 received ZA and had stable BMD during the observation period, whereas 29 patients in the control group had decreased BMD (p < 0.05 at lumbar spine and bilateral femoral neck). 42 Further investigation into the use of anti‐resorptive agents for patients with WM/LPL and lytic bone lesions is warranted.
4. CONCLUSION
Lytic bone lesions associated with non‐Hodgkin's lymphomas such as CLL and WM/LPL are well reported in the literature. However, the biology of these bone lesions is poorly understood as is the optimal therapeutic management of patients with lytic bone disease. Drugs and anti‐resorptive agents that are active in MM have efficacy in WM/LPL yet their role in WM/LPL patients with lytic bone lesions is unknown. Our case demonstrates the efficacy of the chemoimmunotherapy regimen DRC in causing a complete response with resolution of lytic bone lesions in a patient with LPL. Further research is warranted on the ability of novel agents to reverse bone turnover in WM/LPL patients as well as on the utility of anti‐resorptive agents in non‐Hodgkin's lymphomas with lytic bone disease.
CONFLICT OF INTEREST
M.B, R.D.P, U.B, L.J, V.A, V.R, T.S, and A. C‐K have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
M.B. and R.D.P wrote the manuscript; L.J obtained the pathology images for the case; M.B, R.D.P, U.B, L.J, V.A, V.R, T.S, A.C‐K, and S.A edited and finalized the manuscript.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGEMENT
None.
Baksh M, Jiang L, Bhatia U, et al. Management of lytic bone disease in lymphoplasmacytic lymphoma: A case report and review of the literature. Clin Case Rep. 2021;9:e05181. doi: 10.1002/ccr3.5181
Funding information
S.A receives honoraria from Celgene and Takeda, as well as research funding from Amgen, Janssen, Pharmacyclics, Cellectar, Bristol Myers Squibb, Medimmune and Phosplatin
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Sekhar J, Sanfilippo K, Zhang Q, Trinkaus K, Vij R, Morgensztern D. Waldenström macroglobulinemia: a surveillance, epidemiology, and end results database review from 1988 to 2005. Leuk Lymphoma. 2012;53(8):1625‐1626. [DOI] [PubMed] [Google Scholar]
- 2. Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019‐5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Owen RG, Treon SP, Al‐Katib A, et al. Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the second international workshop on Waldenstrom's macroglobulinemia. Semin Oncol. 2003;30(2):110‐115. [DOI] [PubMed] [Google Scholar]
- 4. Schuster SR, Rajkumar SV, Dispenzieri A, et al. IgM multiple myeloma: disease definition, prognosis, and differentiation from Waldenstrom's macroglobulinemia. Am J Hematol. 2010;85(11):853‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feyler S, O'Connor SJ, Rawstron AC, et al. IgM myeloma: a rare entity characterized by a CD20‐CD56‐CD117‐ immunophenotype and the t(11;14). Br J Haematol. 2008;140(5):547‐551. [DOI] [PubMed] [Google Scholar]
- 6. Avet‐Loiseau H, Garand R, Lodé L, Robillard N, Bataille R. 14q32 translocations discriminate IgM multiple myeloma from Waldenstrom's macroglobulinemia. Semin Oncol. 2003;30(2):153‐155. [DOI] [PubMed] [Google Scholar]
- 7. Bonilla‐Valentín FJ, Cerra J, Cáceres‐Perkins W, Alsina M. Case report of IgM multiple myeloma: diagnosing a rare hematologic entity. Cancer Control. 2018;25(1):1073274817744448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rothschild BM, Ruhli F, Rothschild C. Skeletal clues apparently distinguishing Waldenstrom's macroglobulinemia from multiple myeloma and leukemia. Am J Hum Biol. 2002;14(4):532‐537. [DOI] [PubMed] [Google Scholar]
- 9. Papanikolaou X, Waheed S, Barlogie B, et al. Waldenstrom's macroglobulinemia associated bone disease the UAMS experience. Blood. 2014;124(21):2999. [Google Scholar]
- 10. Marks MA, Tow DE, Jay M. Bone scanning in Waldenstrom's macroglobulinemia. J Nucl Med. 1985;26(12):1412‐1414. [PubMed] [Google Scholar]
- 11. Banwait R, Aljawai Y, Cappuccio J, et al. Extramedullary Waldenström macroglobulinemia. Am J Hematol. 2015;90(2):100‐104. [DOI] [PubMed] [Google Scholar]
- 12. Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med. 2015;372(15):1430‐1440. [DOI] [PubMed] [Google Scholar]
- 13. Mehmood K. Waldenstroms macroglobulinemia patient presenting with rare ‘lytic’ lesions and hypercalcemia: a diagnostic dilemma. J Clin Diagn Res. 2014;8(11):Fd10‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pujani M, Kushwaha S, Sethi N, Beniwal A, Shukla S. Waldenstrom's macroglobulinemia presenting with lytic bone lesions: a rare presentation. Blood Res. 2013;48:230‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Castillo JJ, Advani RH, Branagan AR, et al. Consensus treatment recommendations from the tenth international workshop for Waldenström macroglobulinaemia. Lancet Haematol. 2020;7(11):e827‐e837. [DOI] [PubMed] [Google Scholar]
- 16. Dimopoulos MA, Panayiotidis P, Moulopoulos LA, Sfikakis P, Dalakas M. Waldenström's macroglobulinemia: clinical features, complications, and management. J Clin Oncol. 2000;18(1):214‐226. [DOI] [PubMed] [Google Scholar]
- 17. Dimopoulos MA, Gertz MA, Kastritis E, et al. Update on treatment recommendations from the fourth international workshop on Waldenstrom's macroglobulinemia. J Clin Oncol. 2009;27(1):120‐126. [DOI] [PubMed] [Google Scholar]
- 18. Treon SP, Hatjiharissi E, Leleu X, et al. Novel agents in the treatment of Waldenström's macroglobulinemia. Clin Lymphoma Myeloma. 2007;7(Suppl 5):S199‐S206. [DOI] [PubMed] [Google Scholar]
- 19. Paludo J, Abeykoon JP, Shreders A, et al. Bendamustine and rituximab (BR) versus dexamethasone, rituximab, and cyclophosphamide (DRC) in patients with Waldenström macroglobulinemia. Ann Hematol. 2018;97(8):1417‐1425. [DOI] [PubMed] [Google Scholar]
- 20. Treon SP, Meid K, Gustine J, et al. Long‐term follow‐up of ibrutinib monotherapy in symptomatic, previously treated patients with Waldenström macroglobulinemia. J Clin Oncol. 2021;39(6):565‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Treon SP, Hanzis C, Manning RJ, et al. Maintenance rituximab is associated with improved clinical outcome in rituximab naïve patients with Waldenstrom macroglobulinaemia who respond to a rituximab‐containing regimen. Br J Haematol. 2011;154(3):357‐362. [DOI] [PubMed] [Google Scholar]
- 22. Rummel MJ, Lerchenmüller C, Hensel M, et al. Two years rituximab maintenance vs. observation after first line treatment with bendamustine plus rituximab (B‐R) in patients with Waldenström's macroglobulinemia (MW): results of a prospective, randomized, multicenter phase 3 study (the StiL NHL7‐2008 MAINTAIN trial). Blood. 2019;134:343. [Google Scholar]
- 23. Castillo JJ, Treon SP. What is new in the treatment of Waldenstrom macroglobulinemia? Leukemia. 2019;33(11):2555‐2562. [DOI] [PubMed] [Google Scholar]
- 24. Raje N, Roodman GD. Advances in the biology and treatment of bone disease in multiple myeloma. Clin Cancer Res. 2011;17(6):1278‐1286. [DOI] [PubMed] [Google Scholar]
- 25. Dimopoulos MA, Trotman J, Tedeschi A, et al. Ibrutinib for patients with rituximab‐refractory Waldenström's macroglobulinaemia (iNNOVATE): an open‐label substudy of an international, multicentre, phase 3 trial. Lancet Oncol. 2017;18(2):241‐250. [DOI] [PubMed] [Google Scholar]
- 26. Shumilov E, Wulf G, Ströbel P, et al. Osteolytic lesions occur rarely in patients with B‐CLL and may respond well to ibrutinib. Leuk Lymphoma. 2016;57(10):2476‐2480. [DOI] [PubMed] [Google Scholar]
- 27. O'Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open‐label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15(1):48‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shinohara M, Chang BY, Buggy JJ, et al. The orally available Btk inhibitor ibrutinib (PCI‐32765) protects against osteoclast-mediated bone loss. Bone. 2014;60:8‐15. [DOI] [PubMed] [Google Scholar]
- 29. Tucker DL, Mihailescu L, Riordan R, Rule S. Remineralization of lytic bone disease in a patient with small lymphocytic lymphoma using ibrutinib. Br J Haematol. 2017;178(1):153‐153. [DOI] [PubMed] [Google Scholar]
- 30. Angtuaco EJC, Fassas ABT, Walker R, Sethi R, Barlogie B. Multiple myeloma: clinical review and diagnostic imaging. Radiology. 2004;231(1):11‐23. [DOI] [PubMed] [Google Scholar]
- 31. Hanrahan CJ, Christensen CR, Crim JR. Current concepts in the evaluation of multiple myeloma with MR imaging and FDG PET/CT. Radiographics. 2010;30(1):127‐142. [DOI] [PubMed] [Google Scholar]
- 32. Silbermann R, Roodman GD. Current controversies in the management of myeloma bone disease. J Cell Physiol. 2016;231(11):2374‐2379. [DOI] [PubMed] [Google Scholar]
- 33. Delgado‐Calle J, Bellido T, Roodman GD. Role of osteocytes in multiple myeloma bone disease. Curr Opin Support Palliat Care. 2014;8(4):407‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Terpos E, Zamagni E, Lentzsch S, et al. Treatment of multiple myeloma‐related bone disease: recommendations from the bone working group of the international myeloma working group. Lancet Oncol. 2021;22(3):e119‐e130. [DOI] [PubMed] [Google Scholar]
- 35. Terpos E, Mihou D, Szydlo R, et al. The combination of intermediate doses of thalidomide with dexamethasone is an effective treatment for patients with refractory/relapsed multiple myeloma and normalizes abnormal bone remodeling, through the reduction of sRANKL/osteoprotegerin ratio. Leukemia. 2005;19(11):1969‐1976. [DOI] [PubMed] [Google Scholar]
- 36. Breitkreutz I, Raab MS, Vallet S, et al. Lenalidomide inhibits osteoclastogenesis, survival factors and bone‐remodeling markers in multiple myeloma. Leukemia. 2008;22(10):1925‐1932. [DOI] [PubMed] [Google Scholar]
- 37. Anderson G, Gries M, Kurihara N, et al. Thalidomide derivative CC‐4047 inhibits osteoclast formation by down‐regulation of PU.1. Blood. 2006;107(8):3098‐3105. [DOI] [PubMed] [Google Scholar]
- 38. Costa F, Toscani D, Chillemi A, et al. Expression of CD38 in myeloma bone niche: a rational basis for the use of anti‐CD38 immunotherapy to inhibit osteoclast formation. Oncotarget. 2017;8(34):56598‐56611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moreau P, Touzeau C. Multiple myeloma: from front‐line to relapsed therapies. Am Soc Clin Oncol Educ Book. 2015;35:e504‐e511. [DOI] [PubMed] [Google Scholar]
- 40. Terpos E, Politou M, Szydlo R, et al. Autologous stem cell transplantation normalizes abnormal bone remodeling and sRANKL/osteoprotegerin ratio in patients with multiple myeloma. Leukemia. 2004;18(8):1420‐1426. [DOI] [PubMed] [Google Scholar]
- 41. Dimopoulos MA, Anagnostopoulos A, Kyrtsonis M‐C, et al. Primary treatment of Waldenström macroglobulinemia with dexamethasone, rituximab, and cyclophosphamide. J Clin Oncol. 2007;25(22):3344‐3349. [DOI] [PubMed] [Google Scholar]
- 42. Koehler M, Moita F, Cabeçadas J, Gomes da Silva M. Mixed lytic and blastic bone lesions as a presenting feature of Waldenström macroglobulinemia: case report and review of the literature. Clin Lymphoma Myeloma Leuk. 2020;20(2):e87‐e91. [DOI] [PubMed] [Google Scholar]
- 43. Kim SH, Lim SK, Hahn JS. Effect of pamidronate on new vertebral fractures and bone mineral density in patients with malignant lymphoma receiving chemotherapy. Am J Med. 2004;116(8):524‐528. [DOI] [PubMed] [Google Scholar]
- 44. Westin JR, Thompson MA, Cataldo VD, et al. Zoledronic acid for prevention of bone loss in patients receiving primary therapy for lymphomas: a prospective, randomized controlled phase III trial. Clin Lymphoma Myeloma Leuk. 2013;13(2):99‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Elba S, Castellino A, Soriasio R, et al. Immunoglobulin M (IgM) multiple myeloma versus Waldenström macroglobulinemia: diagnostic challenges and therapeutic options: two case reports. J Med Case Rep. 2020;14(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lu H, Durkin L, Zhao X, Nakashima MO. IgM plasma cell myeloma: clinicopathologic features including evaluation for MYD88 and CXCR4 mutations. Am J Clin Pathol. 2021. doi: 10.1093/ajcp/aqab095 PMID: 34508562 [DOI] [Google Scholar]
- 47. Swiecicki PL, Hegerova LT, Gertz MA. Cold agglutinin disease. Blood. 2013;122(7):1114‐1121. [DOI] [PubMed] [Google Scholar]
- 48. Berentsen S. Cold agglutinin‐mediated autoimmune hemolytic anemia in Waldenström's macroglobulinemia. Clin Lymphoma Myeloma. 2009;9(1):110‐112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


