Abstract
Objective:
Oxytocin, secreted into circulation through the posterior pituitary, regulates lactation, weight, and socio-behavioral functioning. Oxytocin deficiency has been suggested in patients with hypopituitarism, however, diagnostic testing for oxytocin deficiency has not been developed. The aim of this study was to investigate known pituitary provocation tests to stimulate plasma oxytocin.
Design:
65 healthy volunteers underwent either the hypertonic saline or arginine infusion test, known to stimulate copeptin, or the oral macimorelin test, known to stimulate growth hormone. Plasma oxytocin was measured before and once plasma sodium ≥150mmol/l for the hypertonic saline, after 60 minutes for the arginine infusion and after 45 minutes for the oral macimorelin test (expected peak of copeptin and growth hormone levels, respectively). Primary outcome was change from basal to stimulated oxytocin levels using paired t-tests.
Results:
As expected, copeptin increased in response to hypertonic saline and arginine infusion (p<0.001), and growth hormone increased to oral macimorelin (p<0.001). Oxytocin increased in response to hypertonic saline infusion from 0.4pg/mL (0.2) to 0.6pg/mL (0.3) (p=0.003) but with a high variance. There was no change to arginine infusion (p=0.4), and a trend to lower stimulated levels to oral macimorelin (p=0.05).
Conclusion:
Neither the arginine infusion nor the oral macimorelin test stimulate plasma oxytocin levels, whereas there was an increase with high variance upon hypertonic saline infusion. As a predictable rise in most participants is required for a reliable pituitary provocation test, none of the investigated pituitary provocation tests can be recommended diagnostically to identify patients with an oxytocin deficiency.
Keywords: oxytocin deficiency, hypertonic saline, arginine, macimorelin, stimulation test
INTRODUCTION
Oxytocin is a neuroendocrine hormone that has primarily been described in childbirth and lactation but recent studies demonstrate important physiologic effects beyond this established role, including regulation of energy expenditure favoring weight loss [1,2], bone anabolic properties [3], and positive effects on mood, anxiety levels and social functioning [4,5]. Recent data suggest that plasma oxytocin levels are low in patients with central diabetes insipidus [6], a disorder characterized by a lack of arginine vasopressin secretion. However, identifying patients with an oxytocin deficiency is challenging. Oxytocin is secreted in a pulsatile manner and is influenced by sex hormones and socio-behavioral stimuli [7–10]. Two facts support the hypothesis of an oxytocin deficient state in patients with central diabetes insipidus: first, the proximity of oxytocin and arginine vasopressin and second, increased psychopathology. Oxytocin and arginine vasopressin are synthesized in magnocellular neurons in the supraoptic nuclei of the hypothalamus, they are stored in secretory vesicles in the posterior pituitary and released into the peripheral circulation [11]. Therefore, disruption of arginine vasopressin secretion into the peripheral circulation as seen in central diabetes insipidus could also disrupt oxytocin secretion. In fact, Aulinas and colleagues demonstrated that 1-hour pooled plasma oxytocin levels, which capture basal and pulsatile oxytocin secretion, are low in men with central diabetes insipidus compared to hypopituitary men without central diabetes insipidus and healthy controls, whereas a single fasting timepoint did not differ between groups [6]. In addition, a higher prevalence of diverse psychopathology, sense of social isolation, obesity, increased cardiovascular mortality and osteopenia has been associated with hypopituitarism [12–14] and central diabetes insipidus [6,15]. Aulinas and colleagues found that symptoms of depression, anxiety, and alexithymia were more pronounced in patients with central diabetes insipidus [6]. Together, these findings support the hypothesis of a clinically relevant oxytocin deficiency in patients with central diabetes insipidus.
For many other pituitary hormones, provocation tests are often used in the diagnosis of hormone deficiency, as pituitary hormones are often secreted in pulses with a short half-life and basal levels rarely predict hormone deficiency. So far, no reliable provocation test to detect an oxytocin deficiency is available. An oxytocin provocation test would therefore be highly desirable to identify and confirm an oxytocin deficiency in patients with hypopituitarism, as treatment with intranasal oxytocin might prove to be beneficial in some individuals [16].
The hypertonic saline infusion and arginine infusion test both stimulate arginine vasopressin release. The anatomical proximity of arginine vasopressin and oxytocin, and the fact that these tests stimulate oxytocin in animals, indicate that they might be able to provoke oxytocin release in humans as well [17–19]. Oral macimorelin, a ghrelin agonist, is established as a provocation test for growth hormone deficiency. It has been shown that ghrelin and oxytocin receptors modulate oxytocin signaling in the hippocampus and that ghrelin infusion increases plasma oxytocin levels in animals [20–23]. Therefore, macimorelin could also be useful in stimulating oxytocin release from the posterior pituitary and may have the potential as diagnostic tests for an oxytocin deficiency.
In this study, we therefore aimed at evaluating whether these three established provocation tests, i.e., the hypertonic saline infusion, the arginine infusion, and the oral macimorelin test, can be used to increase plasma oxytocin levels in healthy volunteers. Establishment of oxytocin stimulation in healthy individuals is a key step toward developing a provocative test for the diagnosis of oxytocin deficiency in clinical practice, namely in patients with central diabetes insipidus.
METHODS
Study design and participants
This study combined data from sex matched adult participants of three different prospective diagnostic studies: First, 20 healthy volunteers undergoing a test protocol of osmotic stimulation with hypertonic (3%) saline infusion [24]. Second, 20 healthy volunteers undergoing a test protocol with arginine infusion [25]. Third, 25 healthy volunteers undergoing a test protocol with oral macimorelin [26]. Full details of the studies’ rationale, design, and statistical analysis have been published elsewhere [24–26]. All three studies were preregistered on ClinicalTrials.gov (NCT02647736, NCT01879137, NCT03844217) and approved, including this secondary analysis, by the Ethical Committee Northwest and Central Switzerland, University of Basel, Basel, Switzerland and the Ethical Committee of the University of Würzburg, Würzburg, Germany. Written informed consent was obtained from each participant after full explanation of the purpose and nature of all procedures.
Exclusion criteria included a history of central and nephrogenic diabetes insipidus, primary polydipsia, hypo- or hypernatremia, any chronic or therapy-requiring diseases, chronic alcohol consumption, body mass index <18 kg/m2 or >28 kg/m2, drug intake (except for oral contraception), pregnancy, or breastfeeding.
Hypertonic saline infusion test protocol
Participants underwent a standardized hypertonic saline infusion test as described in detail elsewhere [24]. In short, participants arrived at the study center in the morning after an overnight fast (no food intake for at least 8 hours and no fluid intake for at least 2 hours) and refrained from smoking and drinking alcoholic beverages within the previous 24 hours. Two indwelling catheters were placed into each antecubital vein for solute infusion and blood withdrawal, respectively. Following a 30-minute rest in supine position, clinical parameters, and basal blood for laboratory analysis of plasma sodium, copeptin, and oxytocin levels were taken. Infusion of hypertonic saline was started with a bolus of 250 ml (3% saline, 513 mOsm/L) over 15 minutes and continued thereafter at an infusion rate of 0.15 ml per kg bodyweight per minute. Plasma sodium levels were controlled every 30 minutes and hypertonic saline infusion was stopped once plasma sodium exceeded ≥150 mmol/L. This timepoint has been shown to best stimulate copeptin with still tolerable side effects due to hypernatremia. At this timepoint, clinical parameters were assessed, and blood was withdrawn for the laboratory analysis of stimulated plasma copeptin and oxytocin levels.
Arginine infusion test protocol
Participants underwent a standardized arginine infusion test as described in detail elsewhere [25]. In short, participants arrived at the study center in the morning after an overnight fast (no food intake for at least 8 hours, no fluid intake for at least 2 hours) and refrained from smoking and drinking alcoholic beverages within the previous 24 hours. An indwelling catheter was placed into an antecubital vein. Following a 30-minute rest in supine position, clinical parameters, and basal blood samples for the laboratory analysis of plasma copeptin and oxytocin levels were taken. Infusion of arginine (L-arginine-21%, Braun, B Braun Melsungen AG, Melsungen, Germany), at a dose of 0.5 g per kg bodyweight diluted in 500 ml of 0.9% sodium chloride solution, was started and infused over 30 minutes. At 60 minutes, the expected peak of arginine, clinical parameters were assessed, and blood was withdrawn for the laboratory analysis of stimulated plasma copeptin and oxytocin levels.
Macimorelin test protocol
Participants underwent a standardized oral macimorelin test as described in detail elsewhere [26]. In short, participants arrived at the study center in the morning after an overnight fast (no food or fluid intake for at least 8 hours) and refrained from smoking on the test day and from physical activity within the previous 24 hours. Clinical parameters and blood samples for the laboratory analysis of plasma growth hormone, copeptin, and oxytocin levels were taken right before participants drank in water dissolved macimorelin with a dose equivalent of 0.75 mg per kg body weight within 10 minutes. After 45 minutes, the expected peak of plasma growth hormone, clinical parameters were assessed, and blood was withdrawn for the laboratory analysis of stimulated plasma growth hormone, copeptin and oxytocin levels.
Laboratory measurements
Blood samples for plasma sodium and growth hormone were processed as routine laboratory measurements in the central laboratory of the hospital. Plasma copeptin levels were measured in a single batch analysis for the hypertonic saline and arginine infusion test and in routine for the macimorelin test using a commercially available automated immunofluorescence assay (BRAHMS CT-proAVP KRYPTOR, from BRAHMS GmbH, Hennigsdorf, Germany). Growth hormone levels were measured using an automated electrochemiluminescence immunoassay (ECLIA) (Cobas8000, Roche Diagnostics GmbH, Mannheim, Germany). Blood samples for oxytocin analysis were taken into Lithium-Heparin and EDTA plasma tubes, immediately centrifuged at 4°C and plasma was stored at −80°C until central batch analysis. For the analysis of plasma oxytocin for the hypertonic saline and arginine infusion test, a radioimmunoassay (RIA) using Lithium-Heparin plasma was performed in duplicates after acetone-ether extraction with antibody Pitt-Ab-2 [27] and HPLC purified [125I]-oxytocin. The standard curve of the assay was linear between 0.25 pg/mL and 5.0 pg/mL, the minimum limit of detection was 0.2 pg/mL, with an intra-assay coefficient of variation of 4.2% and the inter-assay coefficient of variation of 12.4%. The antiserum displayed significant cross-reactivity with arginine vasotocin but <1% cross-reactivity with arginine vasopressin, lysine vasopressin, and desmopressin [27]. For the analysis of plasma oxytocin for the macimorelin test, the Oxytocin ELISA kit (ENZO Life Sciences, Ann Arbor, MI) using EDTA plasma was performed according to the manufacturer’s protocol [28,29]. In brief, the plasma was diluted in TFA-H2O and the collected supernatant loaded onto an Oasis® PRiME HLB 96-well plate, 30 mg sorbent, (Waters Corporation, Milford, MA). The sample was eluted with 95% acetonitrile + 5% of a 0.1% TFA-H2O solution. The reconstituted samples, standards and controls were plated on the goat anti-Rabbit IgG microtiter plate and incubated with the oxytocin conjugate overnight. The plate was read at 405 nm. The oxytocin concentration was calculated with a standard curve calculated from 4 parameter logistics curve fit. The intra-assay coefficient of variation is 5.9% and the inter-assay coefficient of variation is 7.9%. The antiserum displays cross-reactivity with mesotocin of 7%, arginine vasotocin of 7.5% and <0.02% for other related molecules according to the manufacturer’s protocol.
Statistical analysis
The primary outcome was change from basal to stimulated oxytocin levels in healthy volunteers for each pituitary provocation test using paired t-tests. A mean percent change was computed based on the mean basal and stimulated hormonal levels and a patient-based percent change was computed based on the individual differences between basal and stimulated hormone levels. A percent change of 0% refers to the same basal and stimulated level, values above 0% refer to an increase in stimulated hormone levels and values below 0% refer to a decrease in stimulated hormone levels in reference to the basal level. A linear regression model was used to assess influence of age, sex, BMI, and laboratory parameters on plasma oxytocin levels.
Baseline results are shown as mean and standard deviation (SD) or median with interquartile range (IQR), and number (n) with percentage (%), accordingly. Group differences were computed using the Fisher’s Exact Test for categorical variables, and the t-test or the Wilcoxon Sign Rank Test for continuous variables, respectively. Correlations between plasma oxytocin with copeptin or growth hormone levels were computed using the Spearman’s Rank Correlation coefficient including basal and stimulated levels.
For this exploratory design and given the lack of data in humans, the sample size of 20 individuals for all three tests was estimated considering a clinically relevant difference with a Cohen’s Kappa of d=0.66, an alpha level of 0.05 and a power of 80%.
Statistical analyses were performed using the statistic program R Statistical Software [30]. Hypothesis testing was two-sided and p-values <0.05 were considered statistically significant.
RESULTS
Baseline Characteristics
Of the 65 included participants in this study, median age was 24 years (IQR 22, 28), 32 (49%) were male and median BMI was 21.7 kg/m2 (IQR 20.4, 24.1). Baseline characteristics of the respective tests are presented in Table 1.
Table 1:
Baseline characteristics of the three different provocation tests.
| Hypertonic saline infusion | Arginine infusion | Oral macimorelin | p-value | |
|---|---|---|---|---|
|
| ||||
| Number of patients | 20 | 20 | 25 | |
| Age (years) | 26.5 [23.0, 31.2] | 27.0 [23.8, 29.0] | 22.0 [22.0, 24.0] | <0.001 |
| Female sex (%) | 11 (55) | 10 (50) | 12 (48) | 0.894 |
| BMI (kg/m2) | 22.4 (2.1) | 21.4 (2.3) | 23.3 (2.7) | 0.041 |
| Systolic blood pressure (mmHg) | 118 (14) | 122 (17) | 114 (10) | 0.170 |
| Diastolic blood pressure (mmHg) | 73 (9) | 74 (8) | 65 (7) | 0.001 |
| Heart rate (bpm) | 67 (10) | 73 (13) | 62 (9) | 0.006 |
Continuous variables are presented as mean (SD) or median (IQR); categorical variables are presented as frequency (percentage). Three group difference was computed using the Kruskal-Wallis Rank Sum Test for continuous variables and the Fisher’s Exact Test for categorical variables.
Abbreviations: BMI = body mass index; bpm = beats per minute
Copeptin and oxytocin upon hypertonic saline infusion test
Twenty healthy volunteers underwent the hypertonic saline infusion. Median time until serum sodium ≥150 mmol/l was 150 minutes (IQR 148, 150). Upon hypertonic saline infusion test, mean (SD) plasma copeptin levels increased from 4.8 pmol/L (2.4) to 33.5 pmol/L (14.8) (p-value <0.001), corresponding to a mean percent change of 598% (516.7) and a patient-based percent change of 819.2% (762.4). Mean (SD) plasma oxytocin levels increased from 0.4 pg/mL (0.2) to 0.6 pg/mL (0.3) (p-value = 0.003), corresponding to a mean percent change of 50% (50) and a patient-based percent change of 89.7% (136.6) (Table 2, Figure 1 a and b). The results were attenuated after exclusion of high outliers and measurements below the lower detection limit of 0.2 pg/ml but remained statistically significant. There was no difference in baseline characteristics between participants who increased (n = 15, 75%) compared to those who did not increase (n = 5, 25%) upon hypertonic saline (data not shown). There was a moderate correlation for copeptin and oxytocin levels (R = 0.4, p-value 0.01).
Table 2:
Laboratory levels upon provocation tests.
| Basal | Stimulated | Mean difference (95% CI) | Percent change | p-value | ||
|---|---|---|---|---|---|---|
| Hypertonic saline infusion | ||||||
| Plasma copeptin (pmol/l) | 4.8 (2.4) | 33.5 (14.8) | 28.7 (21.7, 35.6) | 819.2 (762.4) | <0.001 | |
| Plasma oxytocin (pg/ml) | 0.4 (0.2) | 0.6 (0.3) | 0.2 (0.08–0.34) | 89.7 (136.6) | 0.003 | |
| Arginine infusion | ||||||
| Plasma copeptin (pmol/l) | 5.4 (3.8) | 9.4 (4.9) | 4.0 (3.1, 4.8) | 79.1 (54.3) | <0.001 | |
| Plasma oxytocin (pg/ml) | 0.5 (0.3) | 0.4 (0.2) | −0.04 (−0.15, 0.06) | 2.1 (55.6) | 0.4 | |
| Oral macimorelin test | ||||||
| Plasma growth hormone (ng/ml) | 12.5 (16.6) | 97.1 (40.0) | 85.3 (67.9, 102.7) | 13514.0 (19061.8) | <0.001 | |
| Plasma copeptin (pmol/l) | 5.0 (2.3) | 4.6 (2.3) | −0.38 (−0.6, −0.2) | −7.8 (11.1) | 0.002 | |
| Plasma oxytocin (pg/ml) | 49.7 (23.3) | 40.2 (16.1) | −10.6 (−21.4, 0.2) | −9.5 (36.7) | 0.05 | |
Basal and stimulated laboratory levels upon the hypertonic saline infusion, the arginine infusion and the oral macimorelin test with mean difference and percent change between basal and stimulated levels.
Continuous variables are presented as mean (standard deviation). Percent change represents the percent increase or decrease from basal and stimulated levels. Two group comparison was computed using the paired t-test.
Figure 1:

Percent change in plasma copeptin (a) and oxytocin levels (b) upon hypertonic saline infusion test. The middle line represents the median, the outer lines of the box the interquartile range (IQR). The lines between basal and stimulated hormonal levels indicate each individual participant. A percent change of 0% refers to the same basal and stimulated level, values >0% refer to an increase and values >0% refer to a decrease in stimulated hormone levels in reference to the basal level.
Copeptin and oxytocin upon arginine infusion test
Upon arginine infusion test, mean (SD) copeptin levels increased from 5.4 pmol/L (3.8) to 9.4 pmol/L (4.9) (p-value <0.001), corresponding to a mean percent change of 74% (28.9) and a patient-based percent change of 79.1% (54.3), while plasma oxytocin levels remained unchanged (basal plasma oxytocin levels 0.5 pg/mL (0.3), stimulated plasma oxytocin levels 0.4 pg/mL (0.2), p-value = 0.4, median percent change −20% (33), patient-based percent change 2.1 % (55.6)) (Table 2, Figure 2 a and b). There was no difference in oxytocin response when correcting for age, sex, BMI or copeptin levels (data not shown). There was a weak correlation for copeptin and oxytocin levels (R = 0.32, p-value = 0.05).
Figure 2:

Percent change of plasma copeptin (A) and oxytocin (B) levels upon arginine infusion test. The middle line represents the median, the outer lines of the box the interquartile range (IQR). The lines between basal and stimulated hormonal levels indicate each individual participant. A percent change of 0% refers to the same basal and stimulated level, values >0% refer to an increase and values >0% refer to a decrease in stimulated hormone levels in reference to the basal level.
Growth hormone and oxytocin upon oral macimorelin test
Upon oral macimorelin test, mean (SD) plasma growth hormone levels increased significantly from 12.5 ng/mL (16.6) to 97.1 ng/mL (40.0) (p-value <0.001), corresponding to a mean percent change of 6,768% (141) and a patient-based percent change of 13,514 % (19,062). Mean (SD) plasma copeptin levels decreased from 5.0 pmol/L (2.3) to 4.6 pmol/L (2.3) (p-value = 0.4), corresponding to a mean percent change of −8% (0) and a patient-based percent change of −7.8 % (11.1). Plasma oxytocin tended to decrease in response to oral macimorelin test (basal plasma oxytocin levels 49.7 pg/mL (23.3) and stimulated plasma oxytocin levels 40.2 pg/mL (16.1), p-value = 0.05, mean based percent change of −19.1% (29.6) and a patient-based percent change of −9.5 % (36.7) (Table 2, Figure 3 a, b and c). There was no difference in oxytocin response correcting for age, sex, BMI, growth hormone or copeptin levels (data not shown). There was a poor correlation for growth hormone and oxytocin levels (R = −0.16, p-value = 0.3) as well as for copeptin and oxytocin levels (R = −0.13, p-value = 0.4).
Figure 3:
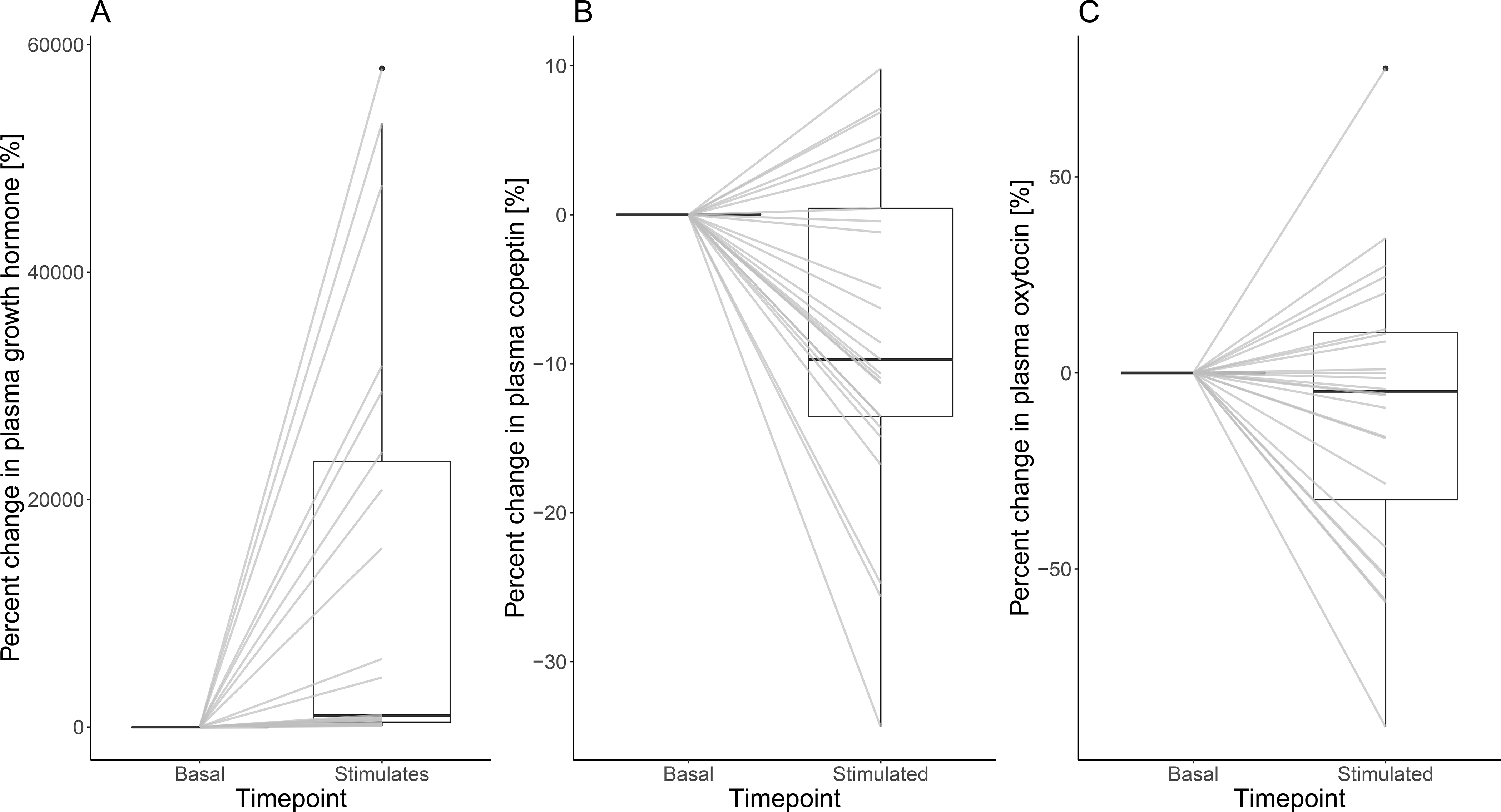
Percent change of growth hormone (A), copeptin (B) and oxytocin (C) levels upon oral macimorelin test. The middle line represents the median, the outer lines of the box the interquartile range (IQR). The lines between basal and stimulated hormonal levels indicate each individual participant. A percent change of 0% refers to the same basal and stimulated level, values >0% refer to an increase and values >0% refer to a decrease in stimulated hormone levels in reference to the basal level.
DISCUSSION
Rising interest in oxytocin, namely a potential oxytocin deficiency in patients with hypopituitarism, supports the need for a reliable oxytocin provocation test. Our results indicate that neither the arginine infusion nor the oral macimorelin test stimulate plasma oxytocin levels in healthy volunteers. The increase upon hypertonic saline was statistically significant, but small and with a high inter-individual variance. A clear and predictable rise for most participants is required for a reliable pituitary provocation test. Based on our results, the hypertonic saline infusion test is also not a useful provocation test to detect an oxytocin deficiency.
Provocation tests are established tools in clinical routine to diagnose pituitary hormone deficiencies, but no clinically useful provocation test for oxytocin is yet available. The insulin-induced hypoglycemia test is one of the strongest stimuli for the pituitary and leads to an increase in various pituitary hormones such as growth hormone, cortisol, and prolactin [31]. Chiodera et al. has previously shown that the insulin-induced hypoglycemia test significantly increases plasma oxytocin levels in healthy volunteers from approximately 3 pmol/L to 6 pmol/L following 30 minutes of profound hypoglycemia [32]. However, this test is associated with severe side effects due to profound hypoglycemia and is contra-indicated in patients with cardiac disorders or epilepsy [31,33]. This is why, even though increasing plasma oxytocin levels, this test is not ideal as provocation test for oxytocin in clinical routine. The same authors showed a significant, but much smaller response to administration of angiotensin II, but in association with significant increases in mean blood pressure [32]. Nevertheless, these results indicate that a pituitary provocation test could be useful to stimulate pituitary oxytocin secretion. Investigating established provocation tests of the pituitary for oxytocin is, therefore, a reasonable approach to identify a test for oxytocin deficiency.
Hypertonic saline infusion has been shown to be the best provocation test for copeptin, a surrogate marker for arginine vasopressin, with a high diagnostic accuracy to identify patients with central diabetes insipidus, i.e., arginine vasopressin deficiency [34,35]. The similarity between oxytocin and arginine vasopressin is supported by the significant, although moderate, correlation of plasma oxytocin and copeptin levels upon hypertonic saline infusion as seen in our study. In contrast, animal data have shown a strong increase in central and peripheral oxytocin levels following hypertonic saline infusion, and oxytocin infusion seems to have natriuretic effects at physiological oxytocin levels in rats [17–19]. In humans, the effect of oxytocin on the sodium-water homeostasis is less clear, with most studies indicating no change or small decreases in plasma oxytocin levels following increased osmolality [36,37], and no altered water excretion in the kidneys following oxytocin infusions [38,39]. One study investigating plasma sodium, oxytocin, arginine vasopressin, and urinary sodium levels in response to exercise of differing intensities found no correlation between plasma oxytocin and arginine vasopressin levels, hypothesizing that, in humans, arginine vasopressin regulates water and sodium homeostasis independently of oxytocin [40]. Our results indicate a small but statistically significant increase in plasma oxytocin levels upon hypertonic saline infusion in healthy volunteers. Excluding outliers and measurements below the detection limit attenuated the results, however, the results remained statistically significant. Additionally, all oxytocin levels were below the expected range (1.25–160 pg/ml). The observed difference might therefore be distorted by the proximity to the lower detection limit. Additionally, the spread of oxytocin levels is highly variable, and we found no difference in baseline characteristics between participants who showed increased oxytocin levels compared to those who did not. A clear and predictable rise for nearly all participants is required for a reliable pituitary provocation test, as seen with copeptin and growth hormone changes in validated pituitary provocation tests. In conclusion, even though we found a small but significant increase in plasma oxytocin levels in response to hypertonic saline in our study, this increase is questionably clinically relevant and in view of the literature insufficient to consider hypertonic saline infusion as a diagnostic test for oxytocin deficiency.
An alternative substance to stimulate arginine vasopressin and copeptin is arginine [25]. Arginine is an endogenous precursor of nitric oxide, an important signaling molecule in endocrine regulatory pathways [41,42]. Arginine infusion is primarily known to stimulate the anterior pituitary and provokes secretion of prolactin and growth hormone and is thus used in clinical practice as a standard provocation test in the diagnosis of growth hormone deficiency [33,43,44]. Increasing also arginine vasopressin and copeptin, it has been suggested as an alternative test for the diagnosis of central diabetes insipidus [25]. Additionally, in rats, centrally administered arginine increases peripheral oxytocin levels [45]. However, our data indicate that, in humans, plasma oxytocin is not stimulated by arginine infusion. We speculate that the arginine stimulus leading to arginine vasopressin release activates pathways that do not stimulate oxytocin in humans, in contrast to animals [45].
Other growth hormone secretagogues (e.g., ghrelin, hexarelin) have been shown to stimulate the release of hypothalamic and pituitary arginine vasopressin [22,23]. Animal data indicate that ghrelin infusion increases plasma oxytocin levels [20,21], and that a heterocomplex formation of the ghrelin and oxytocin receptor in the hypothalamus and hippocampus modulates oxytocin signaling [46]. In our study, macimorelin, a ghrelin agonist, led to a significant increase in growth hormone levels, as expected, but did not increase oxytocin levels. One explanation could be that even though macimorelin is a ghrelin agonist and potent in stimulating growth hormone, it cannot bind to the oxytocin receptor. In contrast to our previous study on copeptin in response to macimorelin, we see decreased copeptin levels in this analysis, which is likely explained by investigating one timepoint and a subset of the original study population [26]. Taken together, in our study, none of the growth hormone secretagogues investigated provoked an increase in plasma oxytocin levels.
Other stimuli for oxytocin secretion have been described in humans. Specifically, psychological arousal or stress may increase oxytocin levels both in plasma and saliva. Peripheral oxytocin increase has most commonly been described using the Trier Social Stress Test, a standardized psychological stress instrument [47]. Physical exercise, especially when psychologically challenging, also increase peripheral oxytocin levels both in plasma and saliva [40,47]. Lastly, sexual-stimulation and orgasm has long been known to increase peripheral oxytocin levels in plasma and saliva in both males and females [9,47,48]. However, the described tests and situations are impractical as provocation tests for oxytocin in clinical practice, since standardization would be difficult.
Our findings have several important physiological implications about oxytocin secretion and its assessment in humans. First, these results add to previous studies showing that some stimuli that cause robust oxytocin secretion in experimental animal studies result in no or minor peripheral oxytocin secretion in humans. Second, stimulated arginine vasopressin secretion, as indicated by copeptin levels, by many provocative tests suggests evolutionary preservation of arginine vasopressin secretion over oxytocin secretion. One example is cholecystokinin, which selectively stimulates oxytocin secretion in rats, and clearly arginine vasopressin secretion but inconsistently oxytocin in monkey and humans [49–51].
Some limitations should be considered for our study. First, this is a secondary analysis of three prospective diagnostic studies investigating peripheral oxytocin levels in human plasma. Despite careful blood sampling, immediate centrifugation, and quick storage at −80°C, a certain decay of oxytocin is not excluded, presenting a certain bias as the oxytocin assay requires standardized procedures in blood sampling and storage. However, as pre and poststimulation samples have been handled the same way, we expect the percent change to be reliable. Second, we measured oxytocin with two different assays, a RIA for the hypertonic saline and arginine infusion test and an ELISA for the oral macimorelin test. Reliable measurement of oxytocin is not straightforward and different oxytocin ranges and a poor correlation have been described between RIA and ELISA [52,53]. However, the relative change between basal and stimulated oxytocin levels should still indicate oxytocin activity and we therefore consider our results reliable. Third, we measured only a single timepoint pre- and post-oxytocin stimulation and might have missed an oxytocin peak. This could furthermore explain why some individuals responded with increased oxytocin levels to hypertonic saline infusion, while others did not. Fourth, we did not systematically assess socio-behavioral factors, oral contraceptives, menopausal status, and smoking which are all factors that could influence plasma oxytocin levels. However, these factors are of less concern when considering relative oxytocin change on a within-individual difference.
In conclusion, our data indicate that none of the known pituitary provocation tests, i.e., neither the hypertonic saline infusion, the arginine infusion, nor the oral macimorelin test, can be used to reliably stimulate oxytocin in healthy volunteers and are not useful to detect a possible oxytocin deficiency in clinical practice. However, before final conclusions can be made, reproducibility of our findings are of utmost importance, both in healthy individuals and patients with a suspected oxytocin deficiency, i.e., central diabetes insipidus. In combination with earlier findings, our results confirm the difficulty of assessing stimulated pituitary oxytocin secretion in humans, in contrast to experimental animal studies.
ACKNOWLEDGEMENTS
We thank all participants for their valuable contribution to our study. We thank the clinical staff, especially Cemile Bathelt, Nina Hutter, Joyce Santos de Jesus and Anna Schrader for their valuable help during the study conduction. We thank all participants for their participation in the study.
FINANCIAL SUPPORT
The study was investigator-initiated and was supported by a grant from the Swiss National Science Foundation to M. Christ-Crain (SNF-162608) and the University Hospital Basel, Switzerland. W. Fenske was supported by the Federal Ministry of Education and Research (BMBF) Germany (FKZ: 01EO1501) and Deutsche Forschungsgemeinschaft (DFG) Germany (AOBJ: 624808). C. O. Sailer was supported by a Young Talents in Clinical Research project grant of the Swiss Academy of Medical Sciences and of the G.& J. Bangerter-Rhyner Foundation, Switzerland. E.A. Lawson received a K24 mentoring grant (MH120568) from the National Institutes of Health, USA.
Footnotes
DISCLOSURE SUMMARY
Dr. Lawson has a financial interest in OXT Therapeutics, a company developing oxytocin-based therapeutics for obesity and metabolic disease. Dr. Lawson’s interests were reviewed and are managed by MGH and Mass General Brigham (f/k/a/ Partners Healthcare) in accordance with their conflict-of-interest policies. 6Martin Fassnacht is on the editorial board of EJE. 6Martin Fassnacht was not involved in the review or editorial process for this paper, on which he/she is listed as an author.
References
- 1.Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides [Internet]. 1989. [cited 2020 Sep 8];10(1):89–93. Available from: https://pubmed.ncbi.nlm.nih.gov/2748428/ [DOI] [PubMed] [Google Scholar]
- 2.Herisson FM, Waas JR, Fredriksson R, Schiöth HB, Levine AS, Olszewski PK. Oxytocin Acting in the Nucleus Accumbens Core Decreases Food Intake. J Neuroendocrinol [Internet]. 2016. April 1 [cited 2021 Jan 26];28(4). Available from: https://pubmed.ncbi.nlm.nih.gov/27114001/ [DOI] [PubMed] [Google Scholar]
- 3.Tamma R, Colaianni G, Zhu L, DiBenedetto A, Greco G, Montemurro G, Patano N, Strippoli M, Vergari R, Mancini L, et al. Oxytocin is an anabolic bone hormone. Natl Acad Sci [Internet]. [cited 2019 Aug 9]; Available from: https://www.pnas.org/content/106/17/7149/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Maréchal P, Pequeux C, Ansseau M, Legros JJ. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology [Internet]. 2007. May [cited 2019 Aug 9];32(4):407–10. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0306453007000297 [DOI] [PubMed] [Google Scholar]
- 5.Hoge EA, Lawson EA, Metcalf CA, Keshaviah A, Zak PJ, Pollack MH, Simon NM. Plasma oxytocin immunoreactive products and response to trust in patients with social anxiety disorder. Depress Anxiety [Internet]. 2012. November [cited 2019 Aug 9];29(11):924–30. Available from: 10.1002/da.21973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aulinas A, Plessow F, Asanza E, Silva L, Marengi DA, Fan W, Abedi P, Verbalis J, Tritos NA, Nachtigall L, et al. Low Plasma Oxytocin Levels and Increased Psychopathology in Hypopituitary Men With Diabetes Insipidus. J Clin Endocrinol Metab [Internet]. 2019. August 1 [cited 2019 Jun 24];104(8):3181–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30882859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belin V, Moos F. Paired recordings from supraoptic and paraventricular oxytocin cells in suckled rats: recruitment and synchronization. J Physiol. 1986. August 1;377(1):369–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNeilly AS, Robinson IC, Houston MJ, Howie PW. Release of oxytocin and prolactin in response to suckling. BMJ [Internet]. 1983. January 22 [cited 2020 Jun 10];286(6361):257–9. Available from: 10.1136/bmj.286.6361.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab. 1987;64(1):27–31. [DOI] [PubMed] [Google Scholar]
- 10.Baskaran C, Plessow F, Silva L, Asanza E, Marengi D, Eddy KT, Sluss PM, Johnson ML, Misra M, Lawson EA. Oxytocin secretion is pulsatile in men and is related to social-emotional functioning. Psychoneuroendocrinology. 2017. November 1;85:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci [Internet]. 2011. September 1 [cited 2018 Nov 28];12(9):524–38. Available from: http://www.nature.com/articles/nrn3044 [DOI] [PubMed] [Google Scholar]
- 12.Daughters K, Manstead ASR, Rees DA. Hypopituitarism is associated with lower oxytocin concentrations and reduced empathic ability. Endocrine [Internet]. 2017. July 1 [cited 2020 Dec 31];57(1):166–74. Available from: https://pubmed.ncbi.nlm.nih.gov/28597171/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebert D, Auer MK, Stieg MR, Freitag MT, Lahne M, Fuss J, Schilbach K, Schopohl J, Stalla GK, Kopczak A. De-masking oxytocin-deficiency in craniopharyngioma and assessing its link with affective function. Psychoneuroendocrinology [Internet]. 2018. February 1 [cited 2020 Dec 31];88:61–9. Available from: https://pubmed.ncbi.nlm.nih.gov/29175721/ [DOI] [PubMed] [Google Scholar]
- 14.Daubenbüchel AMM, Hoffmann A, Eveslage M, Özyurt J, Lohle K, Reichel J, Thiel CM, Martens H, Geenen V, Müller HL. Oxytocin in survivors of childhood-onset craniopharyngioma. Endocrine [Internet]. 2016. November 1 [cited 2020 Dec 31];54(2):524–31. Available from: https://pubmed.ncbi.nlm.nih.gov/27585663/ [DOI] [PubMed] [Google Scholar]
- 15.Aulinas A, Guarda FJ, Yu EW, Haines MS, Asanza E, Silva L, Tritos NA, Verbalis J, Miller KK, Lawson EA. Lower oxytocin levels are associated with lower bone mineral density and less favorable hip geometry in hypopituitary men. Neuroendocrinology [Internet]. 2020. February 20 [cited 2020 Feb 26]; Available from: https://www.karger.com/Article/FullText/506638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker KJ, Oztan O, Libove RA, Sumiyoshi RD, Jackson LP, Karhson DS, Summers JE, Hinman KE, Motonaga KS, Phillips JM, et al. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc Natl Acad Sci U S A. 2017. July 25;114(30):8119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balment RJ, Brimble MJ, Forsling ML. Release of oxytocin induced by salt loading and its influence on renal excretion in the male rat. J Physiol. 1980. November 1;308(1):439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brimble MJ, Dyball REJ, Forsling ML. Oxytocin release following osmotic activation of oxytocin neurones in the paraventricular and supraoptic nuclei. J Physiol. 1978. May 1;278(1):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackburn RE, Samson WK, Fulton RJ, Stricker EM, Verbalis JG. Central oxytocin inhibition of salt appetite in rats: Evidence for differential sensing of plasma sodium and osmolality. Proc Natl Acad Sci U S A. 1993. November 1;90(21):10380–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pekary AE, Sattin A. Rapid modulation of TRH and TRH-like peptide release in rat brain and peripheral tissues by ghrelin and 3-TRP-ghrelin. Peptides. 2012. August;36(2):157–67. [DOI] [PubMed] [Google Scholar]
- 21.Szabó R, Ménesi R, Molnár AH, Szalai Z, Daruka L, Tóth G, Gardi J, Gálfi M, Börzsei D, Kupai K, et al. New metabolic influencer on oxytocin release: The ghrelin. Molecules. 2019. February 18;24(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korbonits M, Little JA, Forsling ML, Tringali G, Costa A, Navarra P, Trainer PJ, Grossman AB. The effect of growth hormone secretagogues and neuropeptide Y on hypothalamic hormone release from acute rat hypothalamic explants. J Neuroendocrinol [Internet]. 1999. July [cited 2020 Feb 10];11(7):521–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10444309 [DOI] [PubMed] [Google Scholar]
- 23.Korbonits M, Kaltsas G, Perry LA, Putignano P, Grossman AB, Besser GM, Trainer PJ. The growth hormone secretagogue hexarelin stimulates the hypothalamo-pituitary-adrenal axis via arginine vasopressin. J Clin Endocrinol Metab [Internet]. 1999. July [cited 2020 Feb 10];84(7):2489–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10404825 [DOI] [PubMed] [Google Scholar]
- 24.Fenske WK, Schnyder I, Koch G, Walti C, Pfister M, Kopp P, Fassnacht M, Strauss K, Christ-Crain M. Release and Decay Kinetics of Copeptin vs AVP in Response to Osmotic Alterations in Healthy Volunteers. J Clin Endocrinol Metab [Internet]. 2018. February 1 [cited 2018 Dec 3];103(2):505–13. Available from: https://academic.oup.com/jcem/article/103/2/505/4743224 [DOI] [PubMed] [Google Scholar]
- 25.Winzeler B, Cesana-Nigro N, Refardt J, Vogt DR, Imber C, Morin B, Popovic M, Steinmetz M, Sailer CO, Szinnai G, et al. Arginine-stimulated copeptin measurements in the differential diagnosis of diabetes insipidus: a prospective diagnostic study. Lancet [Internet]. 2019. August 17 [cited 2019 Aug 29];394(10198):587–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31303316 [DOI] [PubMed] [Google Scholar]
- 26.Urwyler SA, Lustenberger S, Drummond JR, Soares BS, Vogt DR, Ammer N, Yuen KCJ, Ribeiro-Oliveira A, Christ-Crain M. Effects of oral macimorelin on copeptin and anterior pituitary hormones in healthy volunteers. Pituitary [Internet]. 2021. [cited 2021 May 27]; Available from: https://pubmed.ncbi.nlm.nih.gov/33615399/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stricker EM, Verbalis JG. Interaction of osmotic and volume stimuli in regulation of neurohypophyseal secretion in rats. Am J Physiol - Regul Integr Comp Physiol. 1986;250(2). [DOI] [PubMed] [Google Scholar]
- 28.Holze F, Vizeli P, Müller F, Ley L, Duerig R, Varghese N, Eckert A, Borgwardt S, Liechti ME. Distinct acute effects of LSD, MDMA, and d-amphetamine in healthy subjects. Neuropsychopharmacology [Internet]. 2020. February 1 [cited 2020 Oct 5];45(3):462–71. Available from: https://pubmed.ncbi.nlm.nih.gov/31733631/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holt-Lunstad J, Birmingham WA, Light KC. Influence of a “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosom Med [Internet]. 2008. November [cited 2020 Oct 5];70(9):976–85. Available from: https://pubmed.ncbi.nlm.nih.gov/18842740/ [DOI] [PubMed] [Google Scholar]
- 30.R Core T. R: A Language and Environment for Statistical Computing. 2018. [cited 2019 Jan 14]; Available from: https://www.r-project.org/
- 31.Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML. Evaluation and treatment of adult growth hormone deficiency: An endocrine society clinical practice guideline. Vol. 96, Journal of Clinical Endocrinology and Metabolism. Oxford Academic; 2011. p. 1587–609. [DOI] [PubMed] [Google Scholar]
- 32.Chiodera P, Volpi R, Capretti L, Caiazza A, Marchesi M, Caffarri G, Rossi G, Coiro V. Oxytocin response to challenging stimuli in elderly men. Regul Pept. 1994. May 5;51(2):169–76. [DOI] [PubMed] [Google Scholar]
- 33.Society GR. Consensus Guidelines for the Diagnosis and Treatment of Growth Hormone (GH) Deficiency in Childhood and Adolescence: Summary Statement of the GH Research Society 1. J Clin Endocrinol Metab. 2000. November;85(11):3990–3. [DOI] [PubMed] [Google Scholar]
- 34.Fenske W, Refardt J, Chifu I, Schnyder I, Winzeler B, Drummond J, Ribeiro-Oliveira A, Drescher T, Bilz S, Vogt DR, et al. A Copeptin-Based Approach in the Diagnosis of Diabetes Insipidus. N Engl J Med [Internet]. 2018. August 2 [cited 2018 Dec 3];379(5):428–39. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30067922 [DOI] [PubMed] [Google Scholar]
- 35.Timper K, Fenske W, Kühn F, Frech N, Arici B, Rutishauser J, Kopp P, Allolio B, Stettler C, Müller B, et al. Diagnostic Accuracy of Copeptin in the Differential Diagnosis of the Polyuria-polydipsia Syndrome: A Prospective Multicenter Study. J Clin Endocrinol Metab [Internet]. 2015. June [cited 2018 Dec 3];100(6):2268–74. Available from: 10.1210/jc.2014-4507 [DOI] [PubMed] [Google Scholar]
- 36.Seckl JR, Johnson MR, Lightman SL. Vasopressin and oxytocin responses to hypertonic saline infusion: effect of the opioid antagonist naloxone. Clin Endocrinol (Oxf) [Internet]. 1989. May [cited 2020 Jan 27];30(5):513–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2605788 [DOI] [PubMed] [Google Scholar]
- 37.Williams TDM, Abel DC, King CMP, Jelley RY, Lightman SL. Vasopressin and oxytocin responses to acute and chronic osmotic stimuli in man. J Endocrinol. 1986. January 1;108(1):163–8. [DOI] [PubMed] [Google Scholar]
- 38.Rasmussen MS, Simonsen JA, Sandgaard NCF, Høilund-Carlsen PF, Bie P. Effects of oxytocin in normal man during low and high sodium diets. Acta Physiol Scand. 2004. June;181(2):247–57. [DOI] [PubMed] [Google Scholar]
- 39.Thomson WB. The effect of oxytocin and vasopressin and of phenylalanyl3‐oxytocin on the urinary excretion of water and electrolytes in man. J Physiol. 1960. February 1;150(2):284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hew-Butler T, Noakes TD, Soldin SJ, Verbalis JG. Acute changes in endocrine and fluid balance markers during high-intensity, steady-state, and prolonged endurance running: Unexpected increases in oxytocin and brain natriuretic peptide during exercise. Eur J Endocrinol [Internet]. 2008. December [cited 2020 Aug 25];159(6):729–37. Available from: https://pubmed.ncbi.nlm.nih.gov/18794210/ [DOI] [PubMed] [Google Scholar]
- 41.Reid IA. Role of nitric oxide in the regulation of renin and vasopressin secretion. Front Neuroendocrinol. 1994;15(4):351–83. [DOI] [PubMed] [Google Scholar]
- 42.Vargas F, Moreno JM, Wangensteen R, Rodríquez-Gómez I, Garcia-Estan J. The endocrine system in chronic nitric oxide deficiency. Vol. 156, European Journal of Endocrinology. Eur J Endocrinol; 2007. p. 1–12. [DOI] [PubMed] [Google Scholar]
- 43.Garcia JM, Swerdloff R, Wang C, Kyle M, Kipnes M, Biller BMK, Cook D, Yuen KCJ, Bonert V, Dobs A, et al. Macimorelin (AEZS-130)-stimulated growth hormone (GH) test: Validation of a novel oral stimulation test for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab. 2013. June;98(6):2422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia JM, Biller BMK, Korbonits M, Popovic V, Luger A, Strasburger CJ, Chanson P, Medic-Stojanoska M, Schopohl J, Zakrzewska A, et al. Macimorelin as a diagnostic test for adult GH deficiency. J Clin Endocrinol Metab. 2018;103(8):3083–93. [DOI] [PubMed] [Google Scholar]
- 45.Summy-Long JY, Bui V, Gestl S, Koehler-Stec E, Liu H, Terrell ML, Kadekaro M. Effects of central injection of kyotorphin and L-arginine on oxytocin and vasopressin release and blood pressure in conscious rats. Brain Res Bull [Internet]. 1998. [cited 2019 Jun 24];45(4):395–403. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9527014 [DOI] [PubMed] [Google Scholar]
- 46.Wallace Fitzsimons SE, Chruścicka B, Druelle C, Stamou P, Nally K, Dinan TG, Cryan JF, Schellekens H. A ghrelin receptor and oxytocin receptor heterocomplex impairs oxytocin mediated signalling. Neuropharmacology. 2019. July 1;152:90–101. [DOI] [PubMed] [Google Scholar]
- 47.de Jong TR, Menon R, Bludau A, Grund T, Biermeier V, Klampfl SM, Jurek B, Bosch OJ, Hellhammer J, Neumann ID. Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: The Regensburg Oxytocin Challenge (ROC) study. Psychoneuroendocrinology [Internet]. 2015. December 1 [cited 2020 Aug 25];62:381–8. Available from: https://pubmed.ncbi.nlm.nih.gov/26385109/ [DOI] [PubMed] [Google Scholar]
- 48.MURPHY MR, SECKL JR, BURTON S, CHECKLEY SA, LIGHTMAN SL. Changes In Oxytocin and Vasopressin Secretion During Sexual Activity in Men. J Clin Endocrinol Metab [Internet]. 1987. October 1 [cited 2020 Sep 17];65(4):738–41. Available from: 10.1210/jcem-65-4-738 [DOI] [PubMed] [Google Scholar]
- 49.Verbalis JG, McCann MJ, McHale CM, Stricker EM. Oxytocin secretion in response to cholecystokinin and food: Differentiation of nausea from satiety. Science (80- ). 1986;232(4756). [DOI] [PubMed] [Google Scholar]
- 50.Miaskiewicz SL, Stricker EM, Verbalis JG. Neurohypophyseal secretion in response to cholecystokinin but not meal-induced gastric distention in humans. J Clin Endocrinol Metab. 1989;68(4). [DOI] [PubMed] [Google Scholar]
- 51.Ohlsson B, Forsling ML, Rehfeld JF, Sjölund K. Cholecystokinin stimulation leads to increased oxytocin secretion in women. Eur J Surg [Internet]. 2002. [cited 2021 Feb 11];168(2):114–8. Available from: https://pubmed.ncbi.nlm.nih.gov/12113268/ [DOI] [PubMed] [Google Scholar]
- 52.Leng G, Sabatier N. Measuring Oxytocin and Vasopressin: Bioassays, Immunoassays and Random Numbers. J Neuroendocrinol [Internet]. 2016. October 1 [cited 2020 Jun 10];28(10). Available from: 10.1111/jne.12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacLean EL, Wilson SR, Martin WL, Davis JM, Nazarloo HP, Carter CS. Challenges for measuring oxytocin: The blind men and the elephant? [Internet]. Vol. 107, Psychoneuroendocrinology. Elsevier Ltd; 2019. [cited 2021 May 23]. p. 225–31. Available from: https://pubmed.ncbi.nlm.nih.gov/31163380/ [DOI] [PMC free article] [PubMed] [Google Scholar]


