Abstract
Synthetic chemicals with endocrine disrupting properties are pervasive in the environment and are present in the bodies of humans and in wildlife. As thyroid hormones (THs) control normal brain development, and maternal hypothyroxinemia is associated with neurological impairments in children, chemicals that interfere with TH signaling are of considerable concern for children’s health. However, identifying thyroid disrupting chemicals (TDCs) in vivo is largely based on measuring serum tetraiodothyronine in rats, which may be inadequate to assess TDCs with disparate mechanisms of action and insufficient to evaluate the potential neurotoxicity of TDCs. In this review 2 neurodevelopmental processes that are dependent on TH action are highlighted, neuronal migration and maturation of gamma amino butyric acid-ergic interneurons. We discuss how interruption of these processes by TCDs may contribute to abnormal brain circuitry following developmental TH insufficiency. Finally, we identify issues in evaluating the developmental neurotoxicity of TDCs and the strengths and limitations of current approaches designed to regulate them. It is clear that an enhanced understanding of how THs affect brain development will lead to refined toxicity testing, reducing uncertainty and improving our ability to protect children’s health.
Introduction
The World Health Organization defines an endocrine disrupting chemical (EDC) as “an exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub) populations” (1). Although key characteristics of EDCs have been defined, the complexities of the endocrine system and the diverse biological processes it controls challenge our ability to identify the risks EDCs may pose to human health (2–4). The effects of EDCs may go undetected in traditional in vivo regulatory testing schemes, the adverse health effects may only manifest after a significant delay, and human exposure to complex EDC mixtures can often mask the identity of the causative agent(s) (5). These challenges are even more acute for chemicals that disrupt the thyroid system because of the significant role thyroid hormones play in the development of the most complex organ in the body, the brain.
Thyroid hormones (THs) are essential to maintain pregnancy and for optimal fetal development (6). The necessity of TH action throughout neurodevelopment is well established in humans (7–9). Countries around the world have implemented screening initiatives to detect TH dysfunction in pregnant women and newborns; these serum/plasma thyroid function tests have been highly successful in circumventing severe intellectual disabilities resulting from overt developmental hypothyroidism (7–11). However, clinical studies have also shown that modest perturbations in circulating THs during pregnancy can also lead to altered brain structure and function in the child (12–14). Psychomotor deficits, lower intelligent quotient scores, reduced cognitive function, and abnormal hippocampal activity have all been reported in children born to women with serum thyroid function tests falling in the lower range of the normal population distribution (13,15–19). These changes in brain function are also accompanied by morphological abnormalities, specifically in the volume of the cortex and hippocampus (14, 15, 20). These findings in humans have raised concern that environmental exposure to TDCs may represent a significant threat to children’s health.
A wide variety of manufactured chemicals have the potential to disrupt the thyroid system. Currently, identified TDCs include various pesticides, plasticizers, flame retardants, alkylphenols, metals, dioxins, and per- and polyfluoroalkyl substances. All of these substances affect serum TH concentrations and/or TH signaling in vitro and/or in vivo (21–24). Human biomonitoring data have revealed the presence of these chemicals in urine, breastmilk, amniotic fluid, and umbilical cord and newborn blood, indicating ubiquitous exposure to these substances in the mother, fetus, newborn, and child (3, 25–28). Human studies have also correlated TDC exposure(s) to adverse neurodevelopmental consequences, as reviewed by Mughal et al. (23). Epidemiological studies must rely on associations between exposures and observed effects, making animal models essential to bolster these observations by establishing causal linkages and elucidating underlying mechanisms. However, disconnects in the attributions of TH action to impaired neurodevelopment in clinical studies, epidemiology, and outcomes of thyroid dysregulation in animal studies are not uncommon. This is not surprising given that rodent models and in vitro systems cannot fully recapitulate the complexities of the human brain; these translational limitations leading to increased uncertainty in the regulation of TDCs.
In this review we discuss how developmental TH insufficiency induced by TDCs may lead to abnormal brain circuitry in rodents and humans. In laboratory animals, functional disruptions in neural circuitry can be reflected in tests of synaptic transmission (29–35). We propose that neural circuit formation is impaired by abnormal TH action in developing brain and contributes to cognitive impairments observed in children born to women with mild TH insufficiency. Specifically, we explore how two developmental processes, neuronal migration and maturation of gamma amino butyric acid (GABA)-ergic interneurons, are controlled by TH action, and when perturbed can lead to the formation of abnormal brain circuitry. Disruption of these processes is believed to underlie an array of complex neurodevelopmental disorders in humans, including autism, schizophrenia, and epilepsy (36). Importantly, distinct from numerous other mechanistic reports of TH-mediated effects in brain, quantifiable effects in cell migration and interneuron maturation are evident following moderate TH deficits in rodent models, suggesting that dysregulation of these processes represent sensitive and clinically relevant readouts of TH action. The study of TH-dependent developmental processes in more clinically relevant animal models of TH disruption are essential to identify TDCs and protect human health.
Overview of The Thyroid System.
Many detailed reviews are available on the physiology of the thyroid system (10, 37–42), so only a brief summary will be provided here. THs are initially synthesized and stored in the thyroid gland as iodinated tyrosol residues of thyroglobulin. These residues form the 2 main iodothyronines found in blood, possessing 4 (tetraiodothyronine, T4) or 3 (triiodothyronine, T3) iodine atoms; T4 is present at much higher concentrations than T3 in the blood. Upon release from the gland and into the blood stream, the majority of these hormones remain tightly bound to TH distributing proteins, which facilitate their delivery to target cells. THs bound to these distributing proteins are in dynamic equilibrium with unbound or “free” hormones. The free hormone is the fraction available for uptake into cells via specialized membrane-bound transporters; in the brain, the most notably being monocarboxylate transporter 8 (MCT8/SLC16A2) and organic anion transporter 1c1 (OATP1C1/SLCO1C1) (43, 44). Circulating levels of THs are maintained under tight regulatory control through a negative feedback loop between the hypothalamus, pituitary, and thyroid gland, typically referred to as the HPT axis. When blood T4 and T3 concentrations are low, pituitary release of thyrotropin (TSH) is triggered, signaling the thyroid gland to increase the synthesis and release of additional THs to maintain hormone levels within a narrow range in the blood. THs are metabolized in both the liver and the kidney by glucuronide and sulfatase enzymes, which deactivate THs and facilitate clearance from the body. At the cellular level, TH concentrations are tightly controlled by deiodinating enzymes that regulate ligand concentrations in the cell. T4 is converted to T3 by deiodinase 1 or 2 (DIO1, DIO2), whereas T3 is deactivated to diiodothyronine by DIO1 and deiodinase 3 (DIO3).
Clinically, thyroid function is assessed by serum or plasma concentrations of TSH/T4/T3, which are compared with a population reference range. Hypothyroxinemia is a condition where serum T4 is reduced but not accompanied by an increase in TSH; overt hypothyroidism is reduced T4 and elevated TSH. Serum/plasma T3 may also be measured, but it is less common and is not necessarily required for disease diagnosis. In developmental toxicology guideline/guidance studies total T4 is the most common suggested measurement, and, thus, reductions in serum total T4 are often how many TDCs are identified (45, 46).
The primary role of THs is to control gene transcription. Canonically T3 acts as a ligand to activate thyroid hormone receptors alpha and beta (TRα/THRA1 and TRβ/THRB2) but may also activate other nuclear receptors. Following ligand activation, the receptor homo-or heterodimerizes and translocates from the cytosol to the nucleus where it acts as a transcription factor to control gene expression (37, 47). A large number of T3-responsive genes have been identified in the rodent brain, some of which are directly regulated by genomic signaling (48–50). As the functions of THs are often evolutionarily conserved (51), homologous genes controlled by THs may drive similar neurodevelopmental processes in rodents and in humans (39, 52–54).
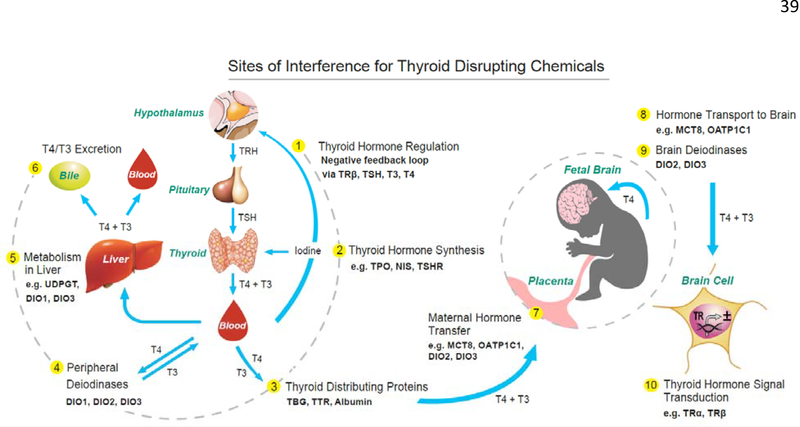
The presence of thyroid receptors, transporters, and deiodinases are spatiotemporally regulated, varying in presence and concentrations across tissues, life stages, and species (44, 52, 55–59). The precise regulation of thyroid signaling machinery is particularly critical during the protracted period of mammalian brain development- certain subregions of the brain requiring differing degrees of TH across time (40, 52). In early pregnancy, maternal T4 represents the sole source of hormones for the developing fetus, and is strictly controlled by transporters and deiodinases in the placenta (60, 61). Additionally, only T4 can cross to the fetal brain, and, thus, brain T3 concentrations are completely dependent on deiodinase activity within the brain during this period (40, 43, 62, 63). Some of these dynamics and how they may be affected by TDCs are illustrated in Fig. 1.
Figure 1.
Sites of potential chemical interference of the thyroid system of the mother and fetus are denoted 1 through 10 (yellow circles), and range from interaction with central regulation of the HPT axis (1), thyroid gland function (2), hormone distribution (3), metabolism (4–6), tissue concentrations (4 and 7–9), and cellular action (10). The concentrations of THs in peripheral tissues are mediated by TH transporters (not shown) and deiodinating enzymes in all tissues. TH transporters also regulate the transfer of THs from the mother to the fetus via the placenta (7), and transporters in blood-brain and blood-cerebrospinal fluid brain barriers control the uptake of T4 and/or T3 into the developing brain tissue (8). The concentrations of brain THs is further controlled by deiodinase enzymes (9). The primary action of THs in cells is to control gene transcription through activation of nuclear receptors (10); however, it is now known that other mechanisms of cell signaling exist (229). Environmental contaminants have the potential to disrupt several of these sites in the mother, fetus, and the child.
Adapted from Gilbert et al., Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences.
Neurotoxicology. 2012;33(4):842–852, with permission from Elsevier (230).
The spatiotemporal dynamics of TH action differ between rats and humans. In humans, fetal thyroid gland function begins in midpregnancy while the bulk of neural circuits begin to form in the third trimester of pregnancy and extends well beyond adolescence (9, 64, 65). In contrast, rat thyroid function begins a few days before birth and the first few weeks of postnatal life are analogous to brain development in the third trimester of human pregnancy (9). These species differences, both in facets of thyroid biology and in brain development must be considered when interpreting the effects of TDCs in rodents and extrapolating them to humans.
Traditional Rodent Models of Thyroid Disruption During Pregnancy.
Rodent models of developmental hypothyroidism have provided essential information about the hormonal control of brain development. Most of these studies, conducted in rats using manipulations to induce severe TH reduction, model the human condition of cretinism that results from overt maternal hypothyroidism (66). These manipulations have included maternal thyroidectomy, severe iodine deficiency, and exposure to high doses of pharmaceuticals like propylthiouracil (PTU) and methimazole (MMI). Both PTU and MMI inhibit the enzyme thyroperoxidase in the thyroid gland to reduce TH synthesis, and PTU also inhibits DIO1 activity; both are used to treat hyperthyroid patients. Traditionally, a single high dose of either drug (typically 200–500 ppm in drinking water) was delivered to the pregnant dam throughout pregnancy and/or lactation, which severely reduces serum T4/T3 in dams and offspring to a degree near, or below, the lower limit of quantification of many commercial assays (58, 67–80). These high-dose pharmaceutical exposures can also induce overt toxicity in the dam, and developmental (ie, body weight deficits, delayed developmental milestones, growth retardation) and neurotoxicological (ie, smaller brain size, deafness, disturbances of gait, psychomotor deficits, severe learning impairments) defects in the offspring (29, 37, 81). These confounds of overt toxicity make it difficult to dissociate general systemic toxicity from true thyroid-dependent effects. In contrast, most environmental TDCs do not induce either overt hypothyroidism or readily apparent developmental neurotoxicity. In order to determine how TDCs may negatively impact brain development, it is imperative to use animal models that more closely mimic the degree and pattern of hormonal disturbance in human populations. As such, traditional rat models of severe maternal and neonatal hypothyroidism are not the most relevant ways to study the developmental neurotoxicity of a TDC exposure(s).
The Need for Clinically Relevant Animal Models.
In the last decade toxicologists have pursued more clinically relevant approaches for assessing xenobiotics. These include the use of animal models that more accurately reflect human conditions of hypothyroxinemia (reduced serum T4, TSH within normal limits). Instead of a single dose of PTU or MMI administered at 200 ppm or greater, a range of much lower concentrations (0.05– 10 ppm PTU) of these pharmaceuticals are administered to pregnant rats, producing graded levels of serum T4 (29, 82–92). As evidenced by serum T4 and TSH concentrations, these administered doses emulate conditions of both maternal hypothyroxinemia (<3 ppm PTU), as well as moderate and overt hypothyroidism (3 and 10 ppm). It is of note that in these PTU studies, reductions in fetal and neonatal serum T4 were often of greater magnitude than that observed in the dams, and also resulted in dose-dependent reductions T4/T3 concentrations in the brain (83, 86, 93). Importantly, these rat models can exhibit reductions in serum T4 with no clear evidence of overt hypothyroidism, including a normal birth weight, normal weight gain over time, and no delay in developmental milestones such as eye opening.
Despite the lack of obvious somatic changes and complete recovery of thyroid function, offspring aged to adulthood exhibit deficits in brain synaptic physiology. Permanent alterations in hippocampal synaptic transmission resulting from transient developmental TH insufficiencies are indicative of impaired neural circuit function. While the developmental underpinnings of these observations are not completely understood, we discuss 2 neurodevelopmental pathways critical for neural circuit development and how their modulation by TH may contribute to the etiology of these functional impairments.
Synaptic Function in the Hippocampus – A Footprint of Neural Circuit Defects.
A neural circuit is a collection of neurons that are anatomically and functionally linked by synaptic connections. Multiple neural circuits interconnect to form integrated brain networks that relay complex information across brain regions. Excitatory and inhibitory synaptic transmission are the means whereby neurons communicate within and across these neural circuits by way of chemical signaling. A biochemical transmitter released from 1 neuron traverses across a physical gap, the synapse, and is transduced into an electrical signal in the receiving neuron. Glutamate is the primary excitatory neurotransmitter and GABA the primary inhibitory neurotransmitter in the mammalian brain. Electrophysiological recordings in 2 subregions of the hippocampus, the dentate gyrus, and the cornu ammonis (CA)1, in both in vivo and ex vivo slices of hippocampus retrieved from experimental animals, have been widely used to examine dysfunctions in neural circuitry (94–97).
A number of studies have shown that PTU-induced developmental TH insufficiency leads to reductions in both excitatory and inhibitory synaptic transmission within the hippocampus, as well as reductions in synaptic plasticity as quantified by long-term potentiation (LTP) and long-term depression (LTD). These effects, evident in early postweaning offspring and persisting to adulthood (29, 33, 34, 83, 98–103), were associated with reductions in T4/T3 concentrations in the fetal and neonatal brain (85, 86). The absence of similar synaptic dysfunction in severely hypothyroid adult rats exemplifies the vulnerability of the developing brain, both pre and postnatally, to abnormal TH action (31, 104).
Activity-dependent synaptic plasticity in the form of LTP and LTD, are processes shared by the developing and mature brain and both can be readily examined in the hippocampus. LTP is a sustained enhancement in the synaptic response that follows coincident “high-frequency” synaptic activation, while LTD ensues with patterns of “low-frequency” stimulation (105–108). LTP reinforces active synaptic connections, while LTD weakens inactive connections and the irrelevant information they may carry (107). These plasticity processes occur throughout the brain, and during development they are integral to formation and stabilization of neural circuits (106, 108–112).
Developmental PTU exposure impaired synaptic plasticity in the hippocampus of the rat (29, 31–33, 83). Neurotrophins, most notably, brain-derived neurotrophic factor (BDNF) are growth factors that regulate neuronal development, differentiation, survival, and plasticity in the developing nervous system. In the adult nervous system, neurotrophins are essential for the induction and maintenance of LTP (111–113). In adult offspring of PTU-exposed dams, deficits in LTP were accompanied by a dramatic suppression of LTP-induced neurotrophin signaling, and this reduction was restricted to the stimulated dentate gyrus (103). Emerging evidence suggests that the direct T3 target, sonic hedgehog (Shh), plays important roles in the formation and plasticity of neuronal circuits in the hippocampus through its interaction with neurotrophins, specifically BDNF (49, 114). It is possible that these actions induced by TH insufficiency and persisting in the brain of adult offspring, may similarly disrupt the establishment of activity dependent connectivity in developing neural circuits (110, 113, 115–117).
Impairments in synaptic transmission have also been reported in offspring following maternal exposure to perchlorate, a drinking water contaminant, or dietary iodine deficiency. In our hands, serum and/or brain TH reductions with these treatments are largely limited to the fetal period (30, 35). Interestingly, under these conditions, synaptic impairments in hippocampus were also limited to excitatory transmission, while inhibition, LTP, and hippocampal-based learning, all features prominently impaired by PTU exposure, remained intact (29, 30, 35, 83, 103, 118). In a PTU cross-fostering study, the observed learning deficits required a maternal exposure that spanned gestation and lactation and produced T4/T3 reductions in both the fetal and neonatal brain (86, 118). Thus, these differential impairments of neural circuitry suggest that in the rodent, deficiencies in inhibition, plasticity, and learning require extension of TH brain disturbances into the postnatal period life (35, 86–88, 119). Elucidation of the quantitative relationships between serum thyroid hormone profiles in the dam, fetus, and neonate, and how these relate to brain T4/T3, will improve our understanding of how maternal TH dysfunction may affect brain development at different life stages. In the following sections we discuss how abnormal TH action in the developing brain may contribute to deficits in neural circuit formation that manifest as impaired synaptic transmission.
Neuronal Migration and Neural Circuit Formation.
Complex networks of synaptic contacts physically connect neurons located in discrete positions in the brain. Optimal information processing demands that appropriate connectivity occurs among specific neuronal populations within the circuit. A fundamental requirement for proper neurocircuit formation in the developing brain is the precise positioning of migrating neurons in time and space (36, 95, 96, 120, 121); defects in neuronal migration, arising from a number of distinct etiologies, can lead to devastating neurodevelopmental disorders including schizophrenia, autism, ataxia, and epilepsy (122–125).
Cells migrate from sites of origin in specialized progenitor cell niches to their appropriate destination in the brain through radial, tangential, or rostral migration processes. Radial migration is the most prominent mode for excitatory neurons in the cortex and hippocampus (124, 126), and several laboratories have demonstrated that disturbed TH action can affect radial cell migration. Using cell birth dating methods, Lavado-Autric and colleagues (127) reported the presence of heterotopic neurons in the cortex and hippocampus of rat offspring born to iodine-deficient mothers, suggesting that abnormal cell migration occurred. The adult female rats were placed on an iodine-deficient diet for 6 months prior to breeding and exhibited pronounced suppression of serum T4. However, no measures of overt toxicity were evident in the dams or pups, setting these observations apart from previous studies of severe hypothyroidism (eg, (67, 74, 128)). These migration errors were attributed to a disruption in progenitor radial glial cells, a transient population of specialized cells that form a scaffold for migrating neuroblasts (127). In an even milder iodine deficiency regimen, maternal hypothyroxinemia was accompanied by reductions in fetal, but not neonatal, brain T4. Although in this study neuronal migration was not investigated, deficits in excitatory synaptic transmission were observed (35). As transmission deficits in hippocampal circuitry have been linked to disruption in neuronal migration (35, 95, 96, 129), it is plausible that impaired synaptic function with iodine deficiency also stemmed from altered neuronal migration and fetal brain TH deficiencies (35).
A mild state of maternal hypothyroxinemia induced by MMI from gestational day (GD) 13 to GD15 (birth is GD22) also produced heterotopic neurons in the cortex and hippocampus, similar to those seen with iodine deficiency (129). Fetal brain hormones were not assessed in this study, but maternal exposure was brief and presumably offspring serum and brain T4/T3 concentrations had recovered by parturition, thus limiting TH deficits to the prenatal period. Collectively, these findings indicate that a mild, brief deficit in maternal THs during fetal development is sufficient to disrupt neuronal migration, which may permanently alter function in hippocampal circuits. In this respect, it is interesting that the progeny of iodine deficient and MMI-treated dams both show increased susceptibility to audiogenic seizures (129–131).
A different structural phenotype, a periventricular heterotopia, has been described in rats following maternal PTU exposure during the perinatal period and is the result of disruptions in neuronal migration. This defect is characterized by ectopic neurons which form at the lateral ventricular epithelium within the posterior forebrain. The heterotopia is bilateral, comprises mature neurons, increases in size with larger decreases in serum T4, and remains throughout the life of the animals (93, 118, 132). Progeny of PTU-treated dams were also more susceptible to seizures when challenged with a subthreshold dosage of the convulsant pentylenetetrazol (93). Importantly, the heterotopia was present following a developmental exposure to PTU that reduced offspring, but not maternal, serum T4 (85, 93). Distinct from the work of Ausó and colleagues (129), a slightly later temporal period of abnormal brain TH action extending into the early postnatal period was required for periventricular heterotopia formation. Despite different temporal windows of TH insufficiency, iodine deficiency, gestational MMI, and perinatal PTU treatment all result in a disruption of radial glial cells (53, 67, 74, 127–129, 132, 133). Together, these findings indicate that developmental hypothyroxinemia, both in the dams and pups, can induce permanent structural phenotypes caused by abnormal cell migration in the brain. These errors in neuronal migration may contribute to the synaptic dysfunction and seizure sensitivity in adult offspring in response to developmental TH insufficiency (Fig. 2) and are consistent with human findings on heterotopia and clinical manifestations of epilepsy. It is also evident that different brain regions are impacted during distinct developmental windows. Although some alterations in neocortical cytoarchitecture may be more readily apparent than others with routine histopathology, assessment of radial glia progenitors may prove a reliable and sensitive marker of altered cell migration (134).
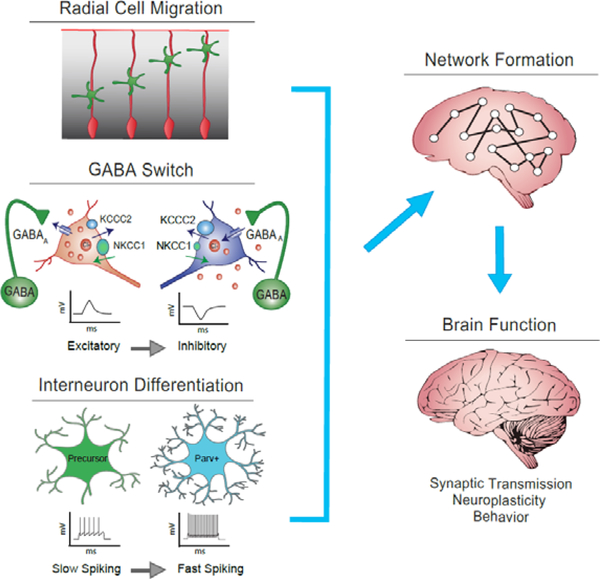
Figure 2.
Thyroid hormone (TH) action may affect three critical neurodevelopmental processes. The normal radial migration of neuroblasts (top panel left, green cells) is mediated by radial glia progenitors (depicted in red): this process is a known target of TH action in rodents. THs may also affect the timing of the transition of GABAergic transmission from excitatory to inhibitory action (GABA switch, middle panel left). This process is mediated by the expression of two potassium-chloride ion transporters (NKCC1 and KCC2), which regulate the intracellular concentrations of chloride ions (orange circles). In the immature state, depicted on the left, intracellular chloride concentrations are high due to a greater expression of NKCCK1 relative to KCC2 chloride transporters. GABA activation under these conditions leads to depolarization of the neuron. A shift in ratio of these chloride transporters increases the chloride concentrations inside the cell and underlies the transitioning to a mature state of GABAergic function depicted on the right. Under these conditions GABA receptor activation now leads to hyperpolarization of the neuron. The final steps in interneuron differentiation that follows the GABA switch that may also be affected by THs is depicted in the bottom left panel. The expression of parvalbumin in interneurons confers a change from slow/irregular spiking to fast-spiking properties that fine tune neural circuitry through control of activity-dependent plasticity. When these three processes are affected alone or in concert by abnormal TH action, it may contribute to abnormal brain circuitry and function observed in rodent models and in children.
Illustration of the GABA switch reprinted from Neuroscience, Vol 279, Ben-Ari, Y. The GABA excitatory/inhibitory developmental sequence: A personal journey, 187–219, 2014, adapted with permission from Elsevier (148).
Role of GABA Assembling Functional Networks.
Interneurons are local circuit neurons whose extensive axonal projections orchestrate the coincident activation of the electrical output of large ensembles of neurons. Interneurons are “pacemaker cells,” synchronizing activity across neural networks that entrain the biological rhythms associated with behavioral and cognitive brain functions (135). This synchronization is largely accomplished by release of the inhibitory neurotransmitter GABA from a relatively small number of interneurons synapsing onto a large number of primarily excitatory glutamatergic, principal neurons (136–140). In the hippocampus of the adult rat, this inhibitory action is reflected in the tests of paired pulse inhibition described above (31). In the developing brain, GABA is also essential to the synchronization of neuronal output as neural circuits begin to form. The maturation of GABAergic neurons develops in parallel with assembly of neural microcircuits as they become integrated into larger neural networks. Selective disruption of GABAergic interneuron maturation impairs the development of neural circuitry and has been implicated in many neurodevelopmental disorders (36, 141–143). Although the precise role THs may play in GABAergic interneuron development and function has yet to be fully elucidated, perturbations in neural circuit development may underlie the alterations in brain function that follow developmental TH insufficiency.
The diverse set of interneurons present in the brain are generated in three main progenitor zones, the ganglionic eminences (see review (144)). Distinct from radial migration of excitatory neurons described above, they migrate tangentially to their sites in neocortex, hippocampus, and striatum. Neurons derived from the medial ganglionic eminence constitute 2 main subtypes of interneurons, those that at maturity express either parvalbumin or somatostatin. Interneuron cell fate determination occurs early in embryonic development and is controlled by a host of transcription factors and other signaling molecules that exhibit strict spatiotemporal dynamics (144). Although largely determined by developmental cues, these cues that drive cell fate determination may also be influenced by developmental TH insufficiency (145).
In addition to the regulation of cell fate, cell activity, and other environmental factors are equally important in the functional maturation of interneurons and their integration into neural networks. In rats, mature neurochemical, morphological, and physiological attributes of GABAergic interneurons are not acquired until late in the postnatal period (105, 135, 143, 146, 147). As such, THs may also modulate the timing and functional maturation of interneurons that occurs postnatally to influence the formation of neural circuits. Here we describe 2 such independent but related GABAergic events, each with significant implications for the formation of neural circuits. The first of these occurs during the early postnatal period as synaptic connections initially form. During this time, brain activity is characterized by large, spontaneous, excitatory depolarizations appearing as synchronous activity across a large number of neurons. These spontaneous events are largely mediated by GABAergic transmission activating rather than inhibiting cell firing—an action opposite to the inhibitory action of GABA observed in the adult brain (148). This transient period of excitatory GABAergic transmission is a robust phenomenon observed throughout a number of developing brain structures.
Excitatory versus inhibitory GABAergic transmission in neurons is determined by the ratio of expression of two potassium-chloride co-transporters, sodium potassium chloride channel 1 (NKCC1) and potassium chloride channel 2 (KCC2). These membrane-bound transporters reside on both glutamatergic cells and interneurons and have opposite roles in chloride ion shuttling. The immature neuron exhibits a preponderance of NKCC1 transporters, which bring chloride ions into the cell to establish a high intracellular chloride concentration (136). A shift in the ratio to a greater number of KCC2 transporters occurs as the cell transitions to a more mature state of functionality and GABA takes on the role of inhibitory transmission seen in the adult. This transition from excitatory to inhibitory action of GABA, known as the GABA switch, represents a very vulnerable time in neural circuit formation, delays in which can negatively impact the formation of neural circuits (see Fig. 2) (136, 137, 140). Many brain regions including the cortex, hippocampus, cerebellum, spinal cord, and sensory organs undergo the GABA switch; each region exhibiting its own distinct temporal transition during development (148, 149).
Do THs play a role in the GABA switch? Although not widely studied, in neuronal cell cultures, GABA neuron development and expression of KCC2 are driven by T3 (150, 151). One means whereby THs may interfere with the GABA switch is through insulin signaling. Insulin-like growth factor (Igf1, IGF-1) is essential for brain development, and there are several similarities in neuroanatomical defects associated with hypothyroidism and IGF-1 deficiency. Importantly, IGF-1 promotes the switch of GABA polarity from excitation to inhibition by altering the ratio of NKCC1 and KCC2 (152) and defines a critical period in developmental plasticity of sensory neocortex (152, 153). The expression of Igf1 is directly regulated by T3 in cortical neurons (49) and preliminary findings of reductions in its expression and the number of Igf1-expressing cells have been reported in the brains of neonatal hypothyroid rats (154, 155). Similarly, maternal exposure to a high dose of MMI reduces both gene and protein expression of KCC2 in the hippocampus on postnatal days 10 and 15 (156). These data suggest that TH insufficiency, possibly through an action on Igf1 signaling, delays the timing of transition of the GABA response from excitatory to inhibitory action with significant ramifications on brain function.
A second interdependent phase of GABAergic maturation is coincident with the polarity switch of GABA function and is essential for the refinement of neural networks. The primitive patterns of synchronous firing driven by the excitatory action of GABA in the initial phase of network formation are “information poor.” These spontaneous patterns serve to forge a substrate for connectivity in nascent neural circuits. Once established, and a shift to inhibitory action of GABA occurs, a transitioning to mature “information-rich” patterns of electrical activity begins. Activity of neural circuits is now not spontaneous but synaptically-mediated, and activity patterns begin transmitting sensory and other biologically meaningful information. This phase is driven by the emergence of phenotypic properties of a prominent subset of GABAergic interneurons that express the calcium binding protein, parvalbumin.
Parvalbumin-expressing neurons represent the largest population of interneurons in the cortex and hippocampus and first appear in these regions of the rat brain the first week after birth (105, 143, 157, 158). The presence of this protein confers a “fast-spiking” signature firing pattern in cells, distinguishing parvalbumin interneurons functionally from all others in the brain (105, 143, 152, 159). It is this high frequency, nonadaptive firing characteristic that lends itself to the “pacemaker” function of these cells by synchronizing neuronal output. The onset of the critical periods of developmental plasticity in sensory intracortical maps is defined by maturation of the activity in these neurons induced by the expression of parvalbumin (160). Selective alterations in the maturation of parvalbumin-expressing interneurons is reflective of a disruption in timing of GABAergic functional development, upsetting the finely tuned balance of excitation and inhibition in brain. It impairs neural circuit formation and may underlie the diverse set of neurological deficits in a number of neurodevelopmental disorders (36, 141–143).
Although only a few studies have directly assessed the role of TH insufficiency in these aspects of GABAergic functional maturation, parvalbumin expression, and thus perhaps normal GABAergic cell differentiation, is altered by abnormal TH action in the brain. In vitro studies in developing neocortical neurons have demonstrated that T3 promotes the development of GABAergic network activity through BDNF-mediated mechanisms (150, 151). The congenitally athyroid Pax8 knockout mouse, which exhibits severe hypothyroidism, displays profound deficiencies in parvalbumin immunostaining in cerebral cortex (161). Mice with a dominant-negative mutation in Thra, which encodes TRα, also display a significant delay in the appearance of parvalbumin-expressing fast-spiking interneurons. The developmental mistiming in GABAergic system is accompanied by seizures and a lowering in the oscillatory frequency of networks recorded ex vivo from cortical slices of mutant animals (162, 163). Consistent with these findings, a recent report of a conditional mouse mutant targeting TRα function only in GABAergic interneurons (ThraAMI/gn) also exhibits reduced parvalbumin expression in the hippocampus, striatum, and cortex; this decrease in parvalbumin-positive cells is not due to a decrease in the total number of GABAergic interneurons (145). These mice also displayed severe seizures around the third postnatal week, observations consistent with an alteration in the late steps of GABAergic interneuron differentiation accompanying abnormal TRα1 activity (145).
A less severe phenotype is produced in mutant mouse models with deficiencies in the TH transporters MCT8 or OATP1C1, alone or in combination with mutations in the deiodinizing enzyme Dio2. The importance of TH transport proteins was first identified in humans with inactivating mutations of SLC16A2 (MCT8). These patients exhibit high serum T3 with low or normal serum T4, intellectual disability with severe neuromotor impairments, and reduced brain T3 concentrations have been reported in postmortem tissue (43, 164–168). A loss of parvalbumin expression in the cerebellum, striatum and cortex was also revealed by immunohistochemistry in postmortem fetal tissue possessing a similar mutation (168). In mice deficient in Slc16a2 (MCT8) similar elevations in serum T3, reduced serum T4, and deficits in brain T4/T3 were observed (55, 169–172), yet no change in parvalbumin expression was evident (169, 172, 173). Mice with mutations of the gene encoding the Slco1c1 (OATP1C1) transporter exhibited no changes in serum T3/T4, yet reductions in brain T4 (171, 173) and lower numbers of parvalbumin positive neurons were present in the somatosensory cortex on postnatal day 12, but not postnatal day 30 (173). The observed relationship between these serum and brain abnormalities also varied with age and reflects the ontogeny of the various brain TH transporters (170, 171, 174).
Targeted deletions of Dio2, which encodes the enzyme responsible for converting T4 to T3 in brain, produce no change in serum T4/T3 but elevate T4 and reduce T3 concentrations in the mouse cortex (175, 176). Reduced parvalbumin immunostaining was reported in neocortex of Dio2–/–mouse at 3 months of age, although in this instance plasma and brain T4 levels were elevated (169). Despite these variations among knockout models, when loss of function mutations of individual transporters are expressed together, or individually combined with deletions of Dio2, reductions in brain T4/T3 and parvalbumin immunostaining are consistently reported across laboratories. Furthermore, these histological features and accompanying motor dysfunction are greatly exacerbated with combined relative to single gene knockouts (169, 172, 173).
Consistent with findings in genetically modified mice, severe TH deficiencies induced by MMI beginning prenatally or at birth also result in deficits in parvalbumin expression in the cortex and hippocampus (177, 178). In the low-dose PTU model, parvalbumin immunostaining was dose dependently reduced in cortex and hippocampus and accompanied by reductions in parvalbumin gene and protein expression (31, 86, 91, 179). Functionally, this transient exposure in early development was accompanied by a reduction in synaptic function and plasticity in the neonatal and adult hippocampus (29, 31–33, 83). Collectively, these data indicate that abnormal maturation of GABAergic neurons, evidenced by a decrease in parvalbumin expression, may impair neural circuit development and appears a common feature of developmental TH insufficiency in humans and rodents (Fig. 2).
Rodent Models and Neurotoxicology of TDCs: One Size May Not Fit All.
Most TDCs have the ability to reduce serum T4 in rats, yet the direct implications to impaired neurodevelopment remain unclear. Studies using PTU and MMI have demonstrated that declines in maternal serum T4 are accompanied by decrements in both fetal and neonatal brain T4/T3 concentrations (86, 132, 180, 181). However, the specificity and potency of pharmaceuticals such as PTU and MMI are distinct from most environmental contaminants; TDCs often being less selective in their mechanism and much less potent in their biological action(s). TDCs comprise chemicals of diverse chemical structures with the potential to compromise TH action in the brain in a variety of ways (Fig. 1). As such, environmental contaminants with weak potency at several distinct biological sites may not produce conditions identical to those achieved with MMI and PTU, even when these drugs are delivered at low doses. It is possible that TDCs with a mode of action similar to PTU/MMI (thyroid peroxidase inhibition/decreased hormone synthesis) may mimic the in vivo effects of these drugs; however, additional information on chemical toxicokinetics is critical to determining how an exposure may affect brain development. As exemplified by perchlorate exposure and iodine deficiency in rats, both with targeted actions within the thyroid gland, the timing and degree of abnormal brain TH action in the fetus and/or pup will dictate the observed neurodevelopment phenotype(s). Defining relationships between measures of hormone in serum vs brain are essential to understanding complex effects of TDCs on brain structure and function. Unfortunately, the in vivo mechanism of action of most environmental contaminants is not known, and, therefore, it is difficult to predict how these xenobiotics may or may not affect serum THs, brain THs, and/or brain TH action.
Distinct from PTU, MMI, and perchlorate, a large number of environmental contaminants including polychlorinated biphenyls and polybrominated diphenyl ethers reduce serum T4 through mechanisms remote from the thyroid gland itself. In exposed rats, the observed reduction in T4 concentrations is largely attributed to the abnormal induction of liver metabolism and enhanced hormone clearance from the body (182–190). Other xenobiotics like perfluoroalkyl substances likely reduce serum T4 by competitive inhibition of thyroid distributing proteins in the blood (191–195). For these chemical groups, as well as for some other TDCs with proposed “extrathyroidal” sites of action (eg, sunscreen UV filter, octyl methoxycinnamate, the antimicrobial triclosan, and the several pesticides) the majority of studies, performed at high doses, do not report hypothyroidism, but rather a milder state of hypothyroxinemia (ie, reductions in serum T4 and absence of the anticipated augmentation in serum TSH) (186–188, 196–204). The understanding of this phenomenon and the implications for the potential developmental neurotoxicity of these contaminants remains inconclusive (205). It may be that the neurological phenotype(s) associated with maternal hypothyroxinemia are distinct from those observed with PTU. Certainly, distinct neurological manifestations exist in mouse models when different components of the thyroid system are selectively targeted. Alternatively, a chemical’s mode of action and/or toxicokinetics may result in a unique fetal and/or postnatal exposure, which would influence the type and severity of the neurodevelopmental outcome (30, 35, 118). As such, developmental neurotoxicity may have been assessed using inappropriate or insensitive metrics. As very little relevant information exists following in vivo developmental exposure to most TDCs, incorporation of brain T4/T3 concentrations and thorough phenotyping may be required to resolve the safety of many chemicals.
Finally, as detailed above in genetically modified mice, complex relationships exist between serum THs, brain THs, and the neurological phenotypes they may produce. These models clearly demonstrate that simple predictions of neurological outcomes from a serum T4 measure is not possible (206). Furthermore, these models reveal redundancies in both the function and expression of transporters and deiodinases in the rodent, which are not observed in humans (56, 169, 207, 208). Therefore, rodents may be less sensitive than humans to xenobiotic interaction with these molecules, with significant implications for species extrapolation in regulatory assessments. It is also not unlikely that TDCs acting at multiple and/or distinct sites of action within the thyroid system may exhibit heterogeneity in serum and brain hormone profiles and complex neurodevelopmental outcomes, further complicating chemical risk assessment and regulation.
Regulatory Testing Strategies.
Regulatory agencies have traditionally identified TDCs in rats by a chemical’s ability to induce histopathological changes in the thyroid gland, and/or reduce serum T4 in developing and/or adult animals (5, 21, 24, 46, 134, 209, 210). Unfortunately, the recommended sampling procedures in these studies do not necessarily include the most appropriate biological window for fetal/neonatal brain development, and TH function has often been assessed with insufficiently sensitive methodologies (209, 211). Both have important implications for current regulatory strategies. In response to these challenges, a number of expert panels have recently been assembled, workshops convened, and research efforts initiated in Europe and elsewhere to address these outstanding regulatory issues (206, 212–214). To date, the consensus from these efforts indicates that a simple and direct translation from serum T4 to adverse neurodevelopmental outcomes appears unlikely. Information on the quantitative relationship(s) between serum and brain TH concentrations is sparse, and therefore, reliance on serum T4 alone as predictive markers of brain malfunction is clearly inadequate (205).
As an alternative approach to in vivo testing, high-throughput in vitro and in silico assays have been developed by various institutions, including the United States Environmental Protection Agency (215–224). These assays were largely designed to characterize a chemical’s interaction with molecules that control TH synthesis, regulation, and metabolism, transporter, deiodinase, and receptor activity (potential biological sites are portrayed in Fig. 1) (215, 225, 226). Despite significant recent advances in high-throughput toxicology, many target sites remain uninterrogated, international quality control measures have yet to be implemented, and the translation to in vivo neurological consequence remains largely unknown. As such, data from in vitro screens for thyroid disruption, although useful for the prioritization of chemical testing, have yet to be incorporated into regulatory decision making. Efforts to translate in vitro/in silico data to in vivo markers of hormone disruption have been advanced through computational modeling, though the complexities of pregnancy, lactation, and development are challenging to replicate mathematically and the accuracy of these models is largely dependent on knowledge of chemical toxicokinetics (85, 227, 228). For more apical outcomes including brain development, a quantitative understanding between serum T4 concentrations, brain T4/T3 concentrations, and neurodevelopmental outcomes is required. Given the sparsity of data in this area, considerable uncertainty remains in the accuracy of in vitro to in vivo translations.
Conclusion.
With advancements in our understanding of how THs control brain development, it has become clear that more clinically relevant models are necessary to properly evaluate the potential health implications of early-life exposure to TDCs. Abnormal TH action in the developing brain results in aberrant neuronal migration and disruption of GABAergic function. Alone or together, these processes may disrupt neural circuitry and underlie cognitive deficits in children born to mothers with mild TH dysfunction. In the regulatory arena, assessments of serum TH profiles in animal studies do provide an informative link to epidemiological studies, yet it is clear that serum measures do not always reflect TH actions in the brain.
Implementation of novel in vitro approaches and augmentation of existing in vivo tests are promising steps forward, but still do not provide any direct information of how an exposure may affect neurodevelopment. To further improve chemical assessment, incorporation of fetal and neonatal brain hormone measures in toxicology guideline studies would greatly clarify if a chemical is of high concern. With further study, it may also be possible to develop better metrics of brain TH action, allowing a direct evaluation of how an exposure is affecting the developing brain. Together, the promise of these approaches is to enhance the evaluation of TDCs in vivo, in order to best protect children’s health.
Acknowledgment:
The authors thank Drs. Terge Svingen and Tom Zoeller for critical reviews and constructive comments on an earlier version of this manuscript. We are grateful to John Havel and Molly Windsor for their contributions to artwork.
Footnotes
This document has been subjected to review by the Center for Public Health and Environmental Assessment and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The interpretations presented here are solely of the authors and do not represent an official position of the US EPA.
References
- 1.IPCS. Global assessment of the state-of-the-science of endocrine disruptors. (ed. Damstra T, Barlow S, Bergman A, Kavlock R, Van Der Kraak G. International Program on Chemical Saftety, WHO/PCS/EDC/02.2. 2002. [Google Scholar]
- 2.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015;36(6):E1–e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nature Reviews Endocrinology. 2016;13:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.La Merrill MA, Vandenberg LN, Smith MT, Goodson W. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. 2020;16(1):45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browne P, Van Der Wal L, Gourmelon A. OECD approaches and considerations for regulatory evaluation of endocrine disruptors. Mol Cell Endocrinol 2020;504:110675. [DOI] [PubMed] [Google Scholar]
- 6.Delange F. Endemic Cretinism. In: Braverman LE, Utiger RD, eds. The Thyroid: A Fundamental and Clinical Text. Seventh ed. Philadelphia: Lippincott-Raven; 1996:756–767. [Google Scholar]
- 7.Taylor PN, Zouras S, Min T, Nagarahaj K, Lazarus JH, Okosieme O. Thyroid Screening in Early Pregnancy: Pros and Cons. Front Endocrinol (Lausanne). 2018;9:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glinoer D, Delange F. The potential repercussions of maternal, fetal, and neonatal hypothyroxinemia on the progeny. Thyroid. 2000;10(10):871–887. [DOI] [PubMed] [Google Scholar]
- 9.Zoeller R, Rovet J. Timing of thyroid hormone action in the developing brain - clinical observations and experimental findings. J Neuroendocrinol. 2004;16(10):809–818. [DOI] [PubMed] [Google Scholar]
- 10.Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18(3):404–433. [DOI] [PubMed] [Google Scholar]
- 11.Rovet JF, Willoughby KA. Maternal thyroid function during pregnancy: Effects on the developing fetal brain. Maternal Influences on Fetal Neurodevelopment: Springer; 2010:55–77. [Google Scholar]
- 12.Willoughby KA, McAndrews MP, Rovet JF. Accuracy of episodic autobiographical memory in children with early thyroid hormone deficiency using a staged event. Dev Cogn Neurosci. 2014;9c:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willoughby KA, McAndrews MP, Rovet JF. Effects of maternal hypothyroidism on offspring hippocampus and memory. Thyroid. 2014;24(3):576–584. [DOI] [PubMed] [Google Scholar]
- 14.Korevaar TI, Muetzel R, Medici M, Chaker L, Jaddoe VW, de Rijke YB, Steegers EA, Visser TJ, White T, Tiemeier H, Peeters RP. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol 2016;4(1):35–43. [DOI] [PubMed] [Google Scholar]
- 15.Wheeler SM, McLelland VC, Sheard E, McAndrews MP, Rovet JF. Hippocampal Functioning and Verbal Associative Memory in Adolescents with Congenital Hypothyroidism. Front Endocrinol (Lausanne). 2015;6:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willoughby KA, McAndrews MP, Rovet J. Effects of early thyroid hormone deficiency on children’s autobiographical memory performance. J Int Neuropsychol Soc 2013;19(4):419–429. [DOI] [PubMed] [Google Scholar]
- 17.Man EB, Brown JF, Serunian SA. Maternal hypothyroxinemia: psychoneurological deficits of progeny. Ann Clin Lab Sci 1991;21(4):227–239. [PubMed] [Google Scholar]
- 18.Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf). 2003;59(3):282–288. [DOI] [PubMed] [Google Scholar]
- 19.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 1999;341(8):549–555. [DOI] [PubMed] [Google Scholar]
- 20.Wheeler SM, Willoughby KA, McAndrews MP, Rovet JF. Hippocampal size and memory functioning in children and adolescents with congenital hypothyroidism. J Clin Endocrinol Metab 2011;96(9):E1427–1434. [DOI] [PubMed] [Google Scholar]
- 21.Brucker-Davis F. Effects of environmental synthetic chemicals on thyroid function. Thyroid. 1998;8(9):827–856. [DOI] [PubMed] [Google Scholar]
- 22.Boas M, Feldt-Rasmussen U, Skakkebaek NE, Main KM. Environmental chemicals and thyroid function. Eur J Endocrinol 2006;154(5):599–611. [DOI] [PubMed] [Google Scholar]
- 23.Mughal BB, Fini JB, Demeneix BA. Thyroid-disrupting chemicals and brain development: an update. Endocr Connect. 2018;7(4):R160–r186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leemans M, Couderq S, Demeneix B, Fini JB. Pesticides With Potential Thyroid Hormone-Disrupting Effects: A Review of Recent Data. Front Endocrinol (Lausanne). 2019;10:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect 2011;119(6):878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Street ME, Angelini S, Bernasconi S, Burgio E, Cassio A, Catellani C, Cirillo F, Deodati A, Fabbrizi E, Fanos V, Gargano G, Grossi E, Iughetti L, Lazzeroni P, Mantovani A, Migliore L, Palanza P, Panzica G, Papini AM, Parmigiani S, Predieri B, Sartori C, Tridenti G, Amarri S. Current Knowledge on Endocrine Disrupting Chemicals (EDCs) from Animal Biology to Humans, from Pregnancy to Adulthood: Highlights from a National Italian Meeting. Int J Mol Sci 2018;19(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghassabian A, Trasande L. Disruption in Thyroid Signaling Pathway: A Mechanism for the Effect of Endocrine-Disrupting Chemicals on Child Neurodevelopment. Front Endocrinol (Lausanne). 2018;9:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padula AM, Monk C, Brennan PA, Borders A, Barrett ES, McEvoy CT, Foss S, Desai P, Alshawabkeh A, Wurth R, Salafia C, Fichorova R, Varshavsky J, Kress A, Woodruff TJ, Morello-Frosch R, on behalf of program collaborators for Environmental influences on Child Health O. A review of maternal prenatal exposures to environmental chemicals and psychosocial stressors—implications for research on perinatal outcomes in the ECHO program. J Perinatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilbert M, Sui L. Dose-dependent reductions in spatial learning and synaptic function in the dentate gyrus of adult rats following developmental thyroid hormone insufficiency. Brain Res 2006;1069(1):10–22. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert M, Sui L. Developmental exposure to perchlorate alters synaptic transmission in hippocampus of the adult rat. Environ Health Perspect 2008;116(6):752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert ME, Sui L, Walker MJ, Anderson W, Thomas S, Smoller SN, Schon JP, Phani S, Goodman JH. Thyroid hormone insufficiency during brain development reduces parvalbumin immunoreactivity and inhibitory function in the hippocampus. Endocrinology. 2007;148(1):92–102. [DOI] [PubMed] [Google Scholar]
- 32.Sui L, Anderson WL, Gilbert ME. Impairment in short-term but enhanced long-term synaptic potentiation and ERK activation in adult hippocampal area CA1 following developmental thyroid hormone insufficiency. Toxicol Sci. 2005;85(1):647–656. [DOI] [PubMed] [Google Scholar]
- 33.Sui L, Gilbert ME. Pre- and postnatal propylthiouracil-induced hypothyroidism impairs synaptic transmission and plasticity in area CA1 of the neonatal rat hippocampus. Endocrinology. 2003;144(9):4195–4203. [DOI] [PubMed] [Google Scholar]
- 34.Taylor MA, Swant J, Wagner JJ, Fisher JW, Ferguson DC. Lower thyroid compensatory reserve of rat pups after maternal hypothyroidism: correlation of thyroid, hepatic, and cerebrocortical biomarkers with hippocampal neurophysiology. Endocrinology. 2008;149(7):3521–3530. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert M, Hedge J, Valentin-Blasini L, Blount B, Kannan K, Tietge J, Zoeller RT, Crofton KM, Jarrett JM, Fisher JW. An animal model of marginal iodine deficiency during development: the thyroid axis and neurodevelopmental outcome. Toxicol Sci 2013;132(1):177–195. [DOI] [PubMed] [Google Scholar]
- 36.Levitt P, Eagleson KL, Powell EM. Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends Neurosci 2004;27(7):400–406. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz HL. Effect of thyroid hormone on growth and development. In: Molecular Basis of Thyroid Hormone Action In: Oppenheimer JH, and Samuels HH, ed. New York: Academic Press; 1983:413–444. [Google Scholar]
- 38.Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol 2004;151 Suppl 3:U25–37. [DOI] [PubMed] [Google Scholar]
- 39.Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab 2007;3(3):249–259. [DOI] [PubMed] [Google Scholar]
- 40.Bernal J. Thyroid Hormones in Brain Development and Function. . In: De Groot LJ, Beck-Peccoz GC P, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan MN R, Rebar R, Singer F, Vinik A, and Weickert MO,, eds. Endotext. South Dartmouth (MA). MDText.com, Inc.; 2015. [Google Scholar]
- 41.Zoeller R, Tan SW, Tyl RW. General background on the hypothalamic-pituitary-thyroid (HPT) axis. Crit Rev Toxicol 2007;37(1–2):11–53. [DOI] [PubMed] [Google Scholar]
- 42.Gilbert M, Zoeller R. Thyroid hormone - impact on the developing brain: possible mechanisms of neurotoxicity. In: Harry G, Tilson H, eds. Neurotoxicology, 3rd edition Vol 3. New York: Informa Healthcare USA, Inc; 2010:79–111. [Google Scholar]
- 43.Bernal J, Guadano-Ferraz A, Morte B. Thyroid hormone transporters--functions and clinical implications. Nat Rev Endocrinol 2015;11(7):406–417. [DOI] [PubMed] [Google Scholar]
- 44.Groeneweg S, van Geest FS, Peeters RP, Heuer H, Visser WE. Thyroid Hormone Transporters. Endocr Rev 2020;41(2). [DOI] [PubMed] [Google Scholar]
- 45.OECD. Test No. 421: Reproduction/Developmental Toxicity Screening Test. [Google Scholar]
- 46.US EPA. Guidance for Thyroid Assays in Pregnant Animals, Fetuses and Postnatal Animals, and Adult Animals. Vol https://www.epa.gov/sites/production/files/2015-06/documents/thyroid_guidance_assay.pdf: Office of Pesticide Programs, Health Effects Division, Washington DC; 2005. [Google Scholar]
- 47.Oppenheimer JH, Schwartz HL. Molecular basis of thyroid hormone-dependent brain development. Endocr Rev 1997;18(4):462–475. [DOI] [PubMed] [Google Scholar]
- 48.Gil-Ibanez P, Bernal J, Morte B. Thyroid hormone regulation of gene expression in primary cerebrocortical cells: role of thyroid hormone receptor subtypes and interactions with retinoic acid and glucocorticoids. PLoS One. 2014;9(3):e91692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gil-Ibanez P, Garcia-Garcia F, Dopazo J, Bernal J, Morte B. Global Transcriptome Analysis of Primary Cerebrocortical Cells: Identification of Genes Regulated by Triiodothyronine in Specific Cell Types. Cereb Cortex. 2017;27(1):706–717. [DOI] [PubMed] [Google Scholar]
- 50.Chatonnet F, Flamant F, Morte B. A temporary compendium of thyroid hormone target genes in brain. Biochim Biophys Acta 2015;1849(2):122–129. [DOI] [PubMed] [Google Scholar]
- 51.Mourouzis I, Lavecchia AM, Xinaris C. Thyroid Hormone Signalling: From the Dawn of Life to the Bedside. J Mol Evol 2020;88(1):88–103. [DOI] [PubMed] [Google Scholar]
- 52.Bernal J. Thyroid hormone regulated genes in cerebral cortex development. J Endocrinol 2017;232(2):R83–r97. [DOI] [PubMed] [Google Scholar]
- 53.Berbel P, Navarro D, Roman GC. An evo-devo approach to thyroid hormones in cerebral and cerebellar cortical development: etiological implications for autism. Front Endocrinol (Lausanne). 2014;5:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flamant F, Koibuchi N, Bernal J. Editorial: “Thyroid Hormone in Brain and Brain Cells”. Front Endocrinol (Lausanne). 2015;6:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharlin DS, Ng L, Verrey F, Visser TJ, Liu Y, Olszewski RT, Hoa M, Heuer H, Forrest D. Deafness and loss of cochlear hair cells in the absence of thyroid hormone transporters Slc16a2 (Mct8) and Slc16a10 (Mct10). Sci Rep 2018;8(1):4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vancamp P, Darras VM. From zebrafish to human: A comparative approach to elucidate the role of the thyroid hormone transporter MCT8 during brain development. Gen Comp Endocrinol 2018;265:219–229. [DOI] [PubMed] [Google Scholar]
- 57.Galton VA, de Waard E, Parlow AF, St Germain DL, Hernandez A. Life without the iodothyronine deiodinases. Endocrinology. 2014;155(10):4081–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barez-Lopez S, Obregon MJ, Bernal J, Guadano-Ferraz A. Thyroid Hormone Economy in the Perinatal Mouse Brain: Implications for Cerebral Cortex Development. Cereb Cortex. 2017:1–11. [DOI] [PubMed] [Google Scholar]
- 59.St Germain DL, Galton VA, Hernandez A. Minireview: Defining the roles of the iodothyronine deiodinases: current concepts and challenges. Endocrinology. 2009;150(3):1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan S, Franklyn JA, Kilby MD Maternal thyroid hormones and fetal brain development. Curr Opinion Endocrinology and Diabetes. 2005;12:23–30. [Google Scholar]
- 61.Chan SY, Vasilopoulou E, Kilby MD. The role of the placenta in thyroid hormone delivery to the fetus. Nature Clinical Practice Endocrinology & Metabolism. 2009;5(1):45–54. [DOI] [PubMed] [Google Scholar]
- 62.Landers K, Richard K. Traversing barriers – How thyroid hormones pass placental, blood-brain and blood-cerebrospinal fluid barriers. Mol Cell Endocrinol 2017;458:22–28. [DOI] [PubMed] [Google Scholar]
- 63.Calvo R, Obregón MJ, Ruiz de Oña C, Escobar del Rey F, Morreale de Escobar G. Congenital hypothyroidism, as studied in rats. Crucial role of maternal thyroxine but not of 3,5,3’-triiodothyronine in the protection of the fetal brain. J Clin Invest 1990;86(3):889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Somerville LH. Searching for Signatures of Brain Maturity: What Are We Searching For? Neuron 2016;92(6):1164–1167. [DOI] [PubMed] [Google Scholar]
- 65.Chan S, Kilby MD. Thyroid hormone and central nervous system development. J Endocrinol 2000;165(1):1–8. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz HL, Ross ME, Oppenheimer JH. Lack of effect of thyroid hormone on late fetal rat brain development. Endocrinology. 1997;138(8):3119–3124. [DOI] [PubMed] [Google Scholar]
- 67.Navarro D, Alvarado M, Navarrete F, Giner M, Obregon MJ, Manzanares J, Berbel P. Gestational and early postnatal hypothyroidism alters VGluT1 and VGAT bouton distribution in the neocortex and hippocampus, and behavior in rats. Front Neuroanat 2015;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Navarro D, Alvarado M, Morte B, Berbel D, Sesma J, Pacheco P, Morreale de Escobar G, Bernal J, Berbel P. Late Maternal Hypothyroidism Alters the Expression of Camk4 in Neocortical Subplate Neurons: A Comparison with Nurr1 Labeling. Cereb Cortex. 2013. [DOI] [PubMed] [Google Scholar]
- 69.Morte B, Diez D, Auso E, Belinchon MM, Gil-Ibanez P, Grijota-Martinez C, Navarro D, de Escobar GM, Berbel P, Bernal J. Thyroid hormone regulation of gene expression in the developing rat fetal cerebral cortex: prominent role of the Ca2+/calmodulin-dependent protein kinase IV pathway. Endocrinology. 2010;151(2):810–820. [DOI] [PubMed] [Google Scholar]
- 70.Rami A, Rabie A. Delayed synaptogenesis in the dentate gyrus of the thyroid-deficient developing rat. Dev Neurosci 1990;12(6):398–405. [DOI] [PubMed] [Google Scholar]
- 71.Rami A, Patel AJ, Rabie A. Thyroid hormone and development of the rat hippocampus: morphological alterations in granule and pyramidal cells. Neuroscience. 1986;19(4):1217–1226. [DOI] [PubMed] [Google Scholar]
- 72.Li GH, Post J, Koibuchi N, Sajdel-Sulkowska EM. Impact of thyroid hormone deficiency on the developing CNS: cerebellar glial and neuronal protein expression in rat neonates exposed to antithyroid drug propylthiouracil. Cerebellum. 2004;3(2):100–106. [DOI] [PubMed] [Google Scholar]
- 73.Koibuchi N, Chin WW. Thyroid hormone action and brain development. Trends Endocrinol Metab 2000;11(4):123–128. [DOI] [PubMed] [Google Scholar]
- 74.Martinez-Galan JR, Pedraza P, Santacana M, Escobar del Ray F, Morreale de Escobar G, Ruiz-Marcos A. Early effects of iodine deficiency on radial glial cells of the hippocampus of the rat fetus. A model of neurological cretinism. J Clin Invest 1997;99(11):2701–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martinez-Galan JR, Pedraza P, Santacana M, Escobar del Rey F, Morreale de Escobar G, Ruiz-Marcos A. Myelin basic protein immunoreactivity in the internal capsule of neonates from rats on a low iodine intake or on methylmercaptoimidazole (MMI). Brain Res Dev Brain Res 1997;101(1–2):249–256. [DOI] [PubMed] [Google Scholar]
- 76.Alvarez-Dolado M, Ruiz M, Del Rio JA, Alcantara S, Burgaya F, Sheldon M, Nakajima K, Bernal J, Howell BW, Curran T, Soriano E, Munoz A. Thyroid hormone regulates reelin and dab1 expression during brain development. J Neurosci 1999;19(16):6979–6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iniguez MA, Rodriguez-Pena A, Ibarrola N, Aguilera M, Munoz A, Bernal J. Thyroid hormone regulation of RC3, a brain-specific gene encoding a protein kinase-C substrate. Endocrinology. 1993;133(2):467–473. [DOI] [PubMed] [Google Scholar]
- 78.Madeira MD, Cadete-Leite A, Andrade JP, Paula-Barbosa MM. Effects of hypothyroidism upon the granular layer of the dentate gyrus in male and female adult rats: a morphometric study. J Comp Neurol 1991;314(1):171–186. [DOI] [PubMed] [Google Scholar]
- 79.Madeira MD, Sousa N, Lima-Andrade MT, Calheiros F, Cadete-Leite A, Paula-Barbosa MM. Selective vulnerability of the hippocampal pyramidal neurons to hypothyroidism in male and female rats. J Comp Neurol 1992;322(4):501–518. [DOI] [PubMed] [Google Scholar]
- 80.Mohan V, Sinha RA, Pathak A, Rastogi L, Kumar P, Pal A, Godbole MM. Maternal thyroid hormone deficiency affects the fetal neocorticogenesis by reducing the proliferating pool, rate of neurogenesis and indirect neurogenesis. Exp Neurol 2012;237(2):477–488. [DOI] [PubMed] [Google Scholar]
- 81.Goldey ES, Kehn LS, Rehnberg GL, Crofton KM. Effects of developmental hypothyroidism on auditory and motor function in the rat. Toxicol Appl Pharmacol 1995;135(1):67–76. [DOI] [PubMed] [Google Scholar]
- 82.Royland JE, Parker JS, Gilbert ME. A genomic analysis of subclinical hypothyroidism in hippocampus and neocortex of the developing rat brain. J Neuroendocrinol 2008;20(12):1319–1338. [DOI] [PubMed] [Google Scholar]
- 83.Gilbert ME. Impact of low-level thyroid hormone disruption induced by propylthiouracil on brain development and function. Toxicol Sci 2011;124(2):432–445. [DOI] [PubMed] [Google Scholar]
- 84.Boyes WK, Degn L, George BJ, Gilbert ME. Moderate perinatal thyroid hormone insufficiency alters visual system function in adult rats. Neurotoxicology. 2018;67:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hassan I, El-Masri H, Kosian PA, Ford J, Degitz SJ, Gilbert ME. Neurodevelopment and Thyroid Hormone Synthesis Inhibition in the Rat: Quantitative Understanding Within the Adverse Outcome Pathway Framework. Toxicol Sci 2017;160(1):57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O’Shaughnessy KL, Wood CR, Ford RL, Kosian PA, Hotchkiss MG, Degitz SJ, Gilbert ME. Thyroid Hormone Disruption in the Fetal and Neonatal Rat: Predictive Hormone Measures and Bioindicators of Hormone Action in the Developing Cortex. Toxicol Sci 2018;166(1):163–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bastian TW, Prohaska JR, Georgieff MK, Anderson GW. Fetal and neonatal iron deficiency exacerbates mild thyroid hormone insufficiency effects on male thyroid hormone levels and brain thyroid hormone-responsive gene expression. Endocrinology. 2014;155(3):1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bastian TW, Anderson JA, Fretham SJ, Prohaska JR, Georgieff MK, Anderson GW. Fetal and neonatal iron deficiency reduces thyroid hormone-responsive gene mRNA levels in the neonatal rat hippocampus and cerebral cortex. Endocrinology. 2012;153(11):5668–5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bastian TW, Prohaska JR, Georgieff MK, Anderson GW. Perinatal iron and copper deficiencies alter neonatal rat circulating and brain thyroid hormone concentrations. Endocrinology. 2010;151(8):4055–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Axelstad M, Hansen PR, Boberg J, Bonnichsen M, Nellemann C, Lund SP, Hougaard KS, U H. Developmental neurotoxicity of Propylthiouracil (PTU) in rats: relationship between transient hypothyroxinemia during development and long-lasting behavioural and functional changes. Toxicol Appl Pharmacol 2008;232(1):1–13. [DOI] [PubMed] [Google Scholar]
- 91.Shiraki A, Akane H, Ohishi T, Wang L, Morita R, Suzuki K, Mitsumori K, Shibutani M. Similar distribution changes of GABAergic interneuron subpopulations in contrast to the different impact on neurogenesis between developmental and adult-stage hypothyroidism in the hippocampal dentate gyrus in rats. Arch Toxicol 2012;86(10):1559–1569. [DOI] [PubMed] [Google Scholar]
- 92.Shiraki A, Saito F, Akane H, Takeyoshi M, Imatanaka N, Itahashi M, Yoshida T, Shibutani M. Expression alterations of genes on both neuronal and glial development in rats after developmental exposure to 6-propyl-2-thiouracil. Toxicol Lett 2014;228(3):225–234. [DOI] [PubMed] [Google Scholar]
- 93.Gilbert ME, Ramos RL, McCloskey DP, Goodman JH. Subcortical band heterotopia in rat offspring following maternal hypothyroxinaemia: structural and functional characteristics. J Neuroendocrinol 2014;26(8):528–541. [DOI] [PubMed] [Google Scholar]
- 94.Rebesco J, Stevenson I, Koerding K, Solla S, Miller L. Rewiring Neural Interactions by Micro-Stimulation. Front Syst Neurosci 2010;4(39). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pan Y-H, Wu N, Yuan X-B. Toward a Better Understanding of Neuronal Migration Deficits in Autism Spectrum Disorders. Frontiers in Cell and Developmental Biology. 2019;7(205). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fleck M, Hirotsune S, Gambello MJ, Phillips-Tansey E, Suares G, Mervis RF, Wynshaw-Boris A, McBain CJ. Hippocampal abnormalities and enhanced excitability in a murine model of human lissencephaly. J Neurosci 2000;20(7):2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.James Chrobak, György B. Operational Dynamics in the Hippocampal-entorhinal Axis. Neurosci Biobehav Rev 1998;22(2):303–310. [DOI] [PubMed] [Google Scholar]
- 98.Opazo M, Gianini A PF, Azkcona G, Alarcón L, Lizana R, Noches V,, Gonzalez PAPM, Mora S, Rosenthal D, Eugenin E, Naranjo D, Bueno SM,, Kalergis AMRC, . Maternal hypothyroxinemia impairs spatial learning and synaptic nature and function in the offspring. Endocrinology. 2008;149(10):5097–5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van Wijk N, Rijntjes E, van de Heijning BJ; . Perinatal and chronic hypothyroidism impair behavioural developmental in male and female rats. . Exp Physiol 2008;93:1199–1209. [DOI] [PubMed] [Google Scholar]
- 100.Wang Y, Wei W, Song B, Wang Y, Dong J, Min H, Chen J. Developmental hypothyroxinemia caused by mild iodine deficiency leads to HFS-induced LTD in rat hippocampal CA1 region: involvement of AMPA receptor. Mol Neurobiol 2014;50(2):348–357. [DOI] [PubMed] [Google Scholar]
- 101.Wang Y, Wei W, Wang Y, Dong J, Song B, Min H, Teng W, Chen J. Neurotoxicity of developmental hypothyroxinemia and hypothyroidism in rats: Impairments of long-term potentiation are mediated by phosphatidylinositol 3-kinase signaling pathway. Toxicol Appl Pharmacol 2013;271(2):257–265. [DOI] [PubMed] [Google Scholar]
- 102.Gilbert M. Alterations in synaptic transmission and plasticity in area CA1 of adult hippocampus following developmental hypothyroidism. Brain Res Dev Brain Res 2004;148(1):11–18. [DOI] [PubMed] [Google Scholar]
- 103.Gilbert M, Sanchez-Huerta K, Wood C. Mild Thyroid Hormone Insufficiency During Development Compromises Activity-Dependent Neuroplasticity in the Hippocampus of Adult Male Rats. Endocrinology. 2016;157(2):774–787. [DOI] [PubMed] [Google Scholar]
- 104.Sanchez-Huerta K, Pacheco-Rosado J, Gilbert ME. Adult onset-hypothyroidism: alterations in hippocampal field potentials in the dentate gyrus are largely associated with anaesthesia-induced hypothermia. J Neuroendocrinol 2015;27(1):8–19. [DOI] [PubMed] [Google Scholar]
- 105.Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature. 2013;501(7468):543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron 2004;44(1):5–21. [DOI] [PubMed] [Google Scholar]
- 107.Bruel-Jungerman E, Davis S, Laroche S. Brain plasticity mechanisms and memory: a party of four. Neuroscientist 2007;13(5):492–505. [DOI] [PubMed] [Google Scholar]
- 108.Cohen-Cory S. The developing synapse: construction and modulation of synaptic structures and circuits. Science. 2002;298(5594):770–776. [DOI] [PubMed] [Google Scholar]
- 109.Wong ROL, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nature Reviews Neuroscience. 2002;3(10):803–812. [DOI] [PubMed] [Google Scholar]
- 110.Park H, Poo M-m. Neurotrophin regulation of neural circuit development and function. Nature Reviews Neuroscience. 2013;14(1):7–23. [DOI] [PubMed] [Google Scholar]
- 111.Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci 1996;19:289–317. [DOI] [PubMed] [Google Scholar]
- 112.Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol 2010;70(5):304–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol 2005;76(2):99–125. [DOI] [PubMed] [Google Scholar]
- 114.Yao PJ, Petralia RS, Mattson MP. Sonic Hedgehog Signaling and Hippocampal Neuroplasticity. Trends Neurosci 2016;39(12):840–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Watson DJ, Ostroff L, Cao G, Parker PH, Smith H, Harris KM. LTP enhances synaptogenesis in the developing hippocampus. Hippocampus. 2016;26(5):560–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chakraborty G, Magagna-Poveda A, Parratt C, Umans JG, MacLusky NJ, Scharfman HE. Reduced hippocampal brain-derived neurotrophic factor (BDNF) in neonatal rats after prenatal exposure to propylthiouracil (PTU). Endocrinology. 2012;153(3):1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu D, Teng W, Shan Z, Yu X, Gao Y, Wang S, Fan C, Wang H, Zhang H. The effect of maternal subclinical hypothyroidism during pregnancy on brain development in rat offspring. Thyroid. 2010;20(8):909–915. [DOI] [PubMed] [Google Scholar]
- 118.O’Shaughnessy KL, Kosian PA, Ford JL, Oshiro WM, Degitz SJ, Gilbert ME. Developmental Thyroid Hormone Insufficiency Induces a Cortical Brain Malformation and Learning Impairments: A Cross-Fostering Study. Toxicol Sci 2018;163(1):101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spring SR, Bastian TW, Wang Y, Kosian P, Anderson GW, Gilbert ME. Thyroid hormone-dependent formation of a subcortical band heterotopia (SBH) in the neonatal brain is not exacerbated under conditions of low dietary iron (FeD). Neurotoxicol Teratol 2016;56:41–46. [DOI] [PubMed] [Google Scholar]
- 120.Thompson BL, Levitt P. Now you see it, now you don’t--closing in on allostasis and developmental basis of psychiatric disorders. Neuron 2010;65(4):437–439. [DOI] [PubMed] [Google Scholar]
- 121.Ghashghaei HT, Lai C, Anton ES. Neuronal migration in the adult brain: are we there yet? Nature Reviews Neuroscience. 2007;8(2):141–151. [DOI] [PubMed] [Google Scholar]
- 122.Del Pino I, Rico B, Marin O. Neural circuit dysfunction in mouse models of neurodevelopmental disorders. Curr Opin Neurobiol 2018;48:174–182. [DOI] [PubMed] [Google Scholar]
- 123.Watrin F, Manent JB, Cardoso C, Represa A. Causes and consequences of gray matter heterotopia. CNS Neurosci Ther 2015;21(2):112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Metin C, Vallee RB, Rakic P, Bhide PG. Modes and mishaps of neuronal migration in the mammalian brain. J Neurosci 2008;28(46):11746–11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gleeson JG, Walsh CA. Neuronal migration disorders: from genetic diseases to developmental mechanisms. Trends Neurosci 2000;23(8):352–359. [DOI] [PubMed] [Google Scholar]
- 126.Kanatani S, Tabata H, Nakajima K. Neuronal migration in cortical development. J Child Neurol 2005;20(4):274–279. [DOI] [PubMed] [Google Scholar]
- 127.Lavado-Autric R, Auso E, Garcia-Velasco JV, Arufe Mdel C, Escobar del Rey F, Berbel P, G MdE. Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J Clin Invest 2003;111(7):1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rami A, Rabie A. Effect of thyroid deficiency on the development of glia in the hippocampal formation of the rat: an immunocytochemical study. Glia. 1988;1(5):337–345. [DOI] [PubMed] [Google Scholar]
- 129.Auso E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, B. P A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology. 2004;145(9):4037–4047. [DOI] [PubMed] [Google Scholar]
- 130.van Middlesworth L. Audiogenic seizures in rats after severe prenatal and perinatal iodine depletion. Endocrinology. 1977; 100:242–245. [DOI] [PubMed] [Google Scholar]
- 131.Van Middlesworth L, Norris CH. Audiogenic seizures and cochlear damage in rats after perinatal antithyroid treatment. Endocrinology. 1980;106(6):1686–1690. [DOI] [PubMed] [Google Scholar]
- 132.O’Shaughnessy K, Thomas SE, Spring SR, Ford JL, Ford RL, Gilbert ME. A transient window of hypothyroidism alters neural progenitor cells and results in abnormal brain development. Sci Rep 2019;9(1):4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Berbel P, Navarro D, Auso E, Varea E, Rodriguez AE, Ballesta JJ, Salinas M, Flores E, Faura CC, de Escobar GM. Role of late maternal thyroid hormones in cerebral cortex development: an experimental model for human prematurity. Cereb Cortex. 2010;20(6):1462–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.O’Shaughnessy KL, Gilbert ME. Thyroid disrupting chemicals and developmental neurotoxicity - New tools and approaches to evaluate hormone action. Mol Cell Endocrinol 2019:110663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cossart R. The maturation of cortical interneuron diversity: how multiple developmental journeys shape the emergence of proper network function. Curr Opin Neurobiol 2011;21(1):160–168. [DOI] [PubMed] [Google Scholar]
- 136.Medina I, Friedel P, Rivera C, Kahle KT, Kourdougli N, Uvarov P, Pellegrino C. Current view on the functional regulation of the neuronal K(+)-Cl(−) cotransporter KCC2. Front Cell Neurosci 2014;8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Khalilov I, Chazal G, Chudotvorova I, Pellegrino C, Corby S, Ferrand N, Gubkina O, Nardou R, Tyzio R, Yamamoto S, Jentsch TJ, Hubner CA, Gaiarsa JL, Ben-Ari Y, Medina I. Enhanced Synaptic Activity and Epileptiform Events in the Embryonic KCC2 Deficient Hippocampus. Front Cell Neurosci 2011;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105(4):521–532. [DOI] [PubMed] [Google Scholar]
- 139.Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist. 2012;18(5):467–486. [DOI] [PubMed] [Google Scholar]
- 140.Ben-Ari Y, Khalilov I, Represa A, Gozlan H. Interneurons set the tune of developing networks. Trends Neurosci 2004;27(7):422–427. [DOI] [PubMed] [Google Scholar]
- 141.Thompson BL, Levitt P. The clinical-basic interface in defining pathogenesis in disorders of neurodevelopmental origin. Neuron 2010;67(5):702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kast RJ, Levitt P. Precision in the development of neocortical architecture: From progenitors to cortical networks. Prog Neurobiol 2019;175:77–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Butt SJ, Stacey JA, Teramoto Y, Vagnoni C. A role for GABAergic interneuron diversity in circuit development and plasticity of the neonatal cerebral cortex. Curr Opin Neurobiol 2017;43:149–155. [DOI] [PubMed] [Google Scholar]
- 144.Hu JS, Vogt D, Sandberg M, Rubenstein JL. Cortical interneuron development: a tale of time and space. Development. 2017;144(21):3867–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Richard S, Guyot R, Rey-Millet M, Prieux M, Markossian S, Aubert D, Flamant F. A Pivotal Genetic Program Controlled by Thyroid Hormone during the Maturation of GABAergic Neurons. iScience. 2020;23(3):100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nomura T, Musial TF, Marshall JJ, Zhu Y, Remmers CL, Xu J, Nicholson DA, Contractor A. Delayed Maturation of Fast-Spiking Interneurons Is Rectified by Activation of the TrkB Receptor in the Mouse Model of Fragile X Syndrome. J Neurosci 2017;37(47):11298–11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Koh DX, Sng JC. HDAC1 negatively regulates Bdnf and Pvalb required for parvalbumin interneuron maturation in an experience-dependent manner. J Neurochem 2016;139(3):369–380. [DOI] [PubMed] [Google Scholar]
- 148.Ben-Ari Y. The GABA excitatory/inhibitory developmental sequence: a personal journey. Neuroscience. 2014;279:187–219. [DOI] [PubMed] [Google Scholar]
- 149.Cherubini E, Griguoli M, Safiulina V, Lagostena L. The depolarizing action of GABA controls early network activity in the developing hippocampus. Mol Neurobiol 2011;43(2):97–106. [DOI] [PubMed] [Google Scholar]
- 150.Westerholz S, de Lima AD, Voigt T. Regulation of early spontaneous network activity and GABAergic neurons development by thyroid hormone. Neuroscience. 2010;168(2):573–589. [DOI] [PubMed] [Google Scholar]
- 151.Westerholz S, de Lima AD, Voigt T. Thyroid hormone-dependent development of early cortical networks: temporal specificity and the contribution of trkB and mTOR pathways. Front Cell Neurosci 2013;7:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Baroncelli L, Cenni MC, Melani R, Deidda G, Landi S, Narducci R, Cancedda L, Maffei L, Berardi N. Early IGF-1 primes visual cortex maturation and accelerates developmental switch between NKCC1 and KCC2 chloride transporters in enriched animals. Neuropharmacology. 2017;113(Pt A):167–177. [DOI] [PubMed] [Google Scholar]
- 153.Dyer AH, Vahdatpour C, Sanfeliu A, Tropea D. The role of Insulin-Like Growth Factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience. 2016;325:89–99. [DOI] [PubMed] [Google Scholar]
- 154.Kline S. T emporal Localization of Insulin-Like Growth Factor 1 (Igf1) Positive Cells in Developing Hypothyroid Brain. Vol MS. All Theses, Dissertations, and Other Capstone Projects. Paper 516.: Minnisota State University; 2015. [Google Scholar]
- 155.Graber-Feesi C, Kline S, Sharlin D. Developmental hypothyroidism results in a brain region-specific reduction in insulin-like growth factor 1 positive cells. Paper presented at: Endocrine Society Abstract2017; Orlando, FL [Google Scholar]
- 156.Sawano E, Takahashi M, Negishi T, Tashiro T. Thyroid hormone-dependent development of the GABAergic pre- and post-synaptic components in the rat hippocampus. Int J Dev Neurosci 2013;31(8):751–761. [DOI] [PubMed] [Google Scholar]
- 157.Ueno H, Takao K, Suemitsu S, Murakami S, Kitamura N, Wani K, Okamoto M, Aoki S, Ishihara T. Age-dependent and region-specific alteration of parvalbumin neurons and perineuronal nets in the mouse cerebral cortex. Neurochem Int 2018;112:59–70. [DOI] [PubMed] [Google Scholar]
- 158.Song J, Sun J, Moss J, Wen Z, Sun GJ, Hsu D, Zhong C, Davoudi H, Christian KM, Toni N, Ming GL, Song H. Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus. Nat Neurosci 2013;16(12):1728–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Duan ZRS, Che A, Chu P, Modol L, Bollmann Y, Babij R, Fetcho RN, Otsuka T, Fuccillo MV, Liston C, Pisapia DJ, Cossart R, De Marco García NV. GABAergic Restriction of Network Dynamics Regulates Interneuron Survival in the Developing Cortex. Neuron 2020;105(1):75–92.e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci 2005;6(11):877–888. [DOI] [PubMed] [Google Scholar]
- 161.Horn S, Kersseboom S, Mayerl S, Muller J, Groba C, Trajkovic-Arsic M, Ackermann T, Visser TJ, Heuer H. Tetrac can replace thyroid hormone during brain development in mouse mutants deficient in the thyroid hormone transporter mct8. Endocrinology. 2013;154(2):968–979. [DOI] [PubMed] [Google Scholar]
- 162.Hadjab-Lallemend S, Wallis K, van Hogerlinden M, Dudazy S, Nordstrom K, Vennstrom B, Fisahn A. A mutant thyroid hormone receptor alpha1 alters hippocampal circuitry and reduces seizure susceptibility in mice. Neuropharmacology. 2010;58(7):1130–1139. [DOI] [PubMed] [Google Scholar]
- 163.Wallis K, Sjogren M, van Hogerlinden M, Silberberg G, Fisahn A, Nordstrom K, Larsson L, Westerblad H, Morreale de Escobar G, Shupliakov O, Vennstrom B. Locomotor deficiencies and aberrant development of subtype-specific GABAergic interneurons caused by an unliganded thyroid hormone receptor alpha1. J Neurosci 2008;28(8):1904–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Friesema EC, Jansen J, Milici C, Visser TJ. Thyroid hormone transporters. Vitam Horm 2005;70:137–167. [DOI] [PubMed] [Google Scholar]
- 165.Masnada S, Groenweg S, Saletti V, Chiapparini L, Castellotti B, Salsano E, Visser WE, Tonduti D. Novel mutations in SLC16A2 associated with a less severe phenotype of MCT8 deficiency. 2019;34(6):1565–1575. [DOI] [PubMed] [Google Scholar]
- 166.Matheus MG, Lehman RK, Bonilha L, Holden KR. Redefining the Pediatric Phenotype of X-Linked Monocarboxylate Transporter 8 (MCT8) Deficiency: Implications for Diagnosis and Therapies. J Child Neurol 2015;30(12):1664–1668. [DOI] [PubMed] [Google Scholar]
- 167.Visser TJ, Liu Y, Olszewski RT, Hoa M, Heuer H, Forrest D, Groeneweg S, Visser WE, Visser TJ. Disorder of thyroid hormone transport into the tissues. Sci Rep 2017;31(2):241–253. [DOI] [PubMed] [Google Scholar]
- 168.Lopez-Espindola D, Morales-Bastos C, Grijota-Martinez C, Liao XH, Lev D, Sugo E, Verge CF, Refetoff S, Bernal J, Guadano-Ferraz A. Mutations of the thyroid hormone transporter MCT8 cause prenatal brain damage and persistent hypomyelination. J Clin Endocrinol Metab 2014;99(12):E2799–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Barez-Lopez S, Grijota-Martinez C, Auso E, Fernandez-de Frutos M, Montero-Pedrazuela A, Guadano-Ferraz A. Adult Mice Lacking Mct8 and Dio2 Proteins Present Alterations in Peripheral Thyroid Hormone Levels and Severe Brain and Motor Skill Impairments. PLoS One. 2019;29(11):1669–1682. [DOI] [PubMed] [Google Scholar]
- 170.Ferrara AM, Liao XH, Gil-Ibanez P, Marcinkowski T, Bernal J, Weiss RE, Dumitrescu AM, Refetoff S. Changes in thyroid status during perinatal development of MCT8-deficient male mice. Endocrinology. 2013;154(7):2533–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mayerl S, Visser TJ, Darras VM, Horn S, Heuer H. Impact of Oatp1c1 deficiency on thyroid hormone metabolism and action in the mouse brain. Endocrinology. 2012;153(3):1528–1537. [DOI] [PubMed] [Google Scholar]
- 172.Harder L, Dudazy-Gralla S, Muller-Fielitz H, Hjerling Leffler J, Vennstrom B, Heuer H, Mittag J. Maternal thyroid hormone is required for parvalbumin neurone development in the anterior hypothalamic area. J Neuroendocrinol 2018;30(3):e12573. [DOI] [PubMed] [Google Scholar]
- 173.Mayerl S, Muller J, Bauer R, Richert S, Kassmann CM, Darras VM, Buder K, Boelen A, Visser TJ, Heuer H. Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J Clin Invest 2014;124(5):1987–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Harder L, Dudazy-Gralla S, Muller-Fielitz H, Hjerling Leffler J, Vennstrom B, Heuer H, Mittag J. Maternal thyroid hormone is required for parvalbumin neurone development in the anterior hypothalamic area. 2018;30(3):e12573. [DOI] [PubMed] [Google Scholar]
- 175.Muller J, Heuer H. Expression pattern of thyroid hormone transporters in the postnatal mouse brain. Front Endocrinol (Lausanne). 2014;5:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Galton VA, Schneider MJ, Clark AS, St Germain DL. Life without thyroxine to 3,5,3’-triiodothyronine conversion: studies in mice devoid of the 5’-deiodinases. Endocrinology. 2009;150(6):2957–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Barez-Lopez S, Bosch-Garcia D, Gomez-Andres D, Pulido-Valdeolivas I, Montero-Pedrazuela A, Obregon MJ, Guadano-Ferraz A. Abnormal motor phenotype at adult stages in mice lacking type 2 deiodinase. PLoS One. 2014;9(8):e103857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Berbel P, Marco P, Cerezo JR, DeFelipe J. Distribution of parvalbumin immunoreactivity in the neocortex of hypothyroid adult rats. Neurosci Lett 1996;204(1–2):65–68. [DOI] [PubMed] [Google Scholar]
- 179.Sawano E, Negishi T, Aoki T, Murakami M, Tashiro T. Alterations in local thyroid hormone signaling in the hippocampus of the SAMP8 mouse at younger ages: association with delayed myelination and behavioral abnormalities. J Neurosci Res 2013;91(3):382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gilbert ME, Goodman JH, Gomez J, Johnstone AFM, Ramos RL. Adult hippocampal neurogenesis is impaired by transient and moderate developmental thyroid hormone disruption. Neurotoxicology. 2017;59:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab 2000;85(11):3975–3987. [DOI] [PubMed] [Google Scholar]
- 182.Morreale de Escobar GM, Obregon MJ, del Rey FE. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab 2004;18(2):225–248. [DOI] [PubMed] [Google Scholar]
- 183.Hood A, Klaassen CD. Differential effects of microsomal enzyme inducers on in vitro thyroxine (T(4)) and triiodothyronine (T(3)) glucuronidation. Toxicol Sci 2000;55(1):78–84. [DOI] [PubMed] [Google Scholar]
- 184.Klaassen CD, Hood AM. Effects of microsomal enzyme inducers on thyroid follicular cell proliferation and thyroid hormone metabolism. Toxicol Pathol 2001;29(1):34–40. [DOI] [PubMed] [Google Scholar]
- 185.Hood A, Allen ML, Liu Y, Liu J, Klaassen CD. Induction of T(4) UDP-GT activity, serum thyroid stimulating hormone, and thyroid follicular cell proliferation in mice treated with microsomal enzyme inducers. Toxicol Appl Pharmacol 2003;188(1):6–13. [DOI] [PubMed] [Google Scholar]
- 186.Kolaja KL, Hood AM, Klaassen CD. The UDP-glucuronyltransferase inducers, phenobarbital and pregnenolone-16alpha-carbonitrile, enhance thyroid-follicular cell apoptosis: association with TGF-beta1 expression. Toxicol Lett 1999;106(2–3):143–150. [DOI] [PubMed] [Google Scholar]
- 187.Zhou T, Taylor MM, DeVito MJ, Crofton KM. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol Sci 2002;66(1):105–116. [DOI] [PubMed] [Google Scholar]
- 188.Goldey ES, Kehn LS, Lau C, Rehnberg GL, Crofton KM. Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol Appl Pharmacol 1995;135(1):77–88. [DOI] [PubMed] [Google Scholar]
- 189.Paul KB, Hedge JM, Bansal R, Zoeller RT, Peter R, DeVito MJ, Crofton KM. Developmental triclosan exposure decreases maternal, fetal, and early neonatal thyroxine: a dynamic and kinetic evaluation of a putative mode-of-action. Toxicology. 2012;300(1–2):31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhou T, Ross DG, DeVito MJ, Crofton KM. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol Sci 2001;61(1):76–82. [DOI] [PubMed] [Google Scholar]
- 191.Crofton KM, Zoeller RT. Mode of action: neurotoxicity induced by thyroid hormone disruption during development--hearing loss resulting from exposure to PHAHs. Crit Rev Toxicol 2005;35(8–9):757–769. [DOI] [PubMed] [Google Scholar]
- 192.Mariussen E. Neurotoxic effects of perfluoroalkylated compounds: mechanisms of action and environmental relevance. Arch Toxicol 2012;86(9):1349–1367. [DOI] [PubMed] [Google Scholar]
- 193.Weiss JM, Andersson PL, Lamoree MH, Leonards PE, van Leeuwen SP, Hamers T. Competitive binding of poly- and perfluorinated compounds to the thyroid hormone transport protein transthyretin. Toxicol Sci 2009;109(2):206–216. [DOI] [PubMed] [Google Scholar]
- 194.Butenhoff JL, Chang SC, Ehresman DJ, York RG. Evaluation of potential reproductive and developmental toxicity of potassium perfluorohexanesulfonate in Sprague Dawley rats. Reprod Toxicol 2009;27(3–4):331–341. [DOI] [PubMed] [Google Scholar]
- 195.Chang S, Butenhoff JL, Parker GA, Coder PS, Zitzow JD, Krisko RM, Bjork JA, Wallace KB, Seed JG. Reproductive and developmental toxicity of potassium perfluorohexanesulfonate in CD-1 mice. Reprod Toxicol 2018;78:150–168. [DOI] [PubMed] [Google Scholar]
- 196.Chang S, Thibodeaux JR, Eastvold ML, Ehresman DJ, Bjork JA, Froehlich JW, Lau C, Singh RJ, Wallace KB, Butenhoff JL. Thyroid hormone status and pituitary function in adult rats given oral doses of perfluorooctanesulfonate (PFOS). Toxicology. 2008;243(3):330–339. [DOI] [PubMed] [Google Scholar]
- 197.Axelstad M, Boberg J, Hougaard KS, Christiansen S, Jacobsen PR, Mandrup KR, Nellemann C, Lund SP, Hass U. Effects of pre- and postnatal exposure to the UV-filter octyl methoxycinnamate (OMC) on the reproductive, auditory and neurological development of rat offspring. Toxicol Appl Pharmacol 2011;250(3):278–290. [DOI] [PubMed] [Google Scholar]
- 198.Kortenkamp A, Martin O, Baynes A, Silva E, Axelstad M, Hass U. Supporting the organization of a workshop on thyroid disruption – Final report. Framework contract ENV.A.3/FRA/2014/0029 on implementation of the community strategy on endocrine disruptors. . : Brunel University London and DTU National Food Institute Denmark. European Commission; 2017. [Google Scholar]
- 199.Louis GW, Hallinger DR, Braxton MJ, Kamel A, Stoker TE. Effects of chronic exposure to triclosan on reproductive and thyroid endpoints in the adult Wistar female rat. J Toxicol Environ Health A. 2017;80(4):236–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gilbert ME, Crofton KM. Developmental exposure to a commercial PCB mixture (Aroclor 1254) produces a persistent impairment in long-term potentiation in the rat dentate gyrus in vivo. Brain Res 1999;850(1–2):87–95. [DOI] [PubMed] [Google Scholar]
- 201.Gilbert ME, Mundy WR, Crofton KM. Spatial learning and long-term potentiation in the dentate gyrus of the hippocampus in animals developmentally exposed to Aroclor 1254. Toxicol Sci 2000;57(1):102–111. [DOI] [PubMed] [Google Scholar]
- 202.Goldey ES, Crofton KM. Thyroxine replacement attenuates hypothyroxinemia, hearing loss, and motor deficits following developmental exposure to Aroclor 1254 in rats. Toxicol Sci 1998;45(1):94–105. [DOI] [PubMed] [Google Scholar]
- 203.Herr DW, Goldey ES, Crofton KM. Developmental exposure to Aroclor 1254 produces low-frequency alterations in adult rat brainstem auditory evoked responses. Fundam Appl Toxicol 1996;33(1):120–128. [DOI] [PubMed] [Google Scholar]
- 204.Axelstad M, Boberg J, Nellemann C, Kiersgaard M, Jacobsen PR, Christiansen S, Hougaard KS, Hass U. Exposure to the widely used fungicide mancozeb causes thyroid hormone disruption in rat dams but no behavioral effects in the offspring. Toxicol Sci 2011;120(2):439–446. [DOI] [PubMed] [Google Scholar]
- 205.Ramhoj L, Hass U, Gilbert ME, Wood C, Svingen T, Usai D, Vinggaard AM, Mandrup K, Axelstad M. Evaluating thyroid hormone disruption: investigations of long-term neurodevelopmental effects in rats after perinatal exposure to perfluorohexane sulfonate (PFHxS). Sci Rep 2020;10(1):2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Axelstad M, Ramhøj L, Scholze M, Martin O, Köhrle J, Kortenkamp A, Directorate-General ECE, Health BUIoE, Societies, Denmark NFITUo. Development of a Study Protocol for Thyroid Disruptor Testing in the Mammalian System: Deliverable 17 : Revised Study Protocol with Recommendations for Follow-up Activities. Publications Office of the European Union. [Google Scholar]
- 207.Kortenkamp A, Axelstad M, Baig A, Bergman A, Bornehag C, Cenijn P, Christiansen S, Demeneix D, Derakhshan A, Fini J, Frädrich C, Hamers T, Hellwig L, Köhrle J, Korevaa rT, Lindberg J, Martin O, Meima M, Mergenthaler P, Nikolov N, Du Pasquier D, Peeters R, Platzack B, Ramhøj L, Remaud S, Renko K, Scholze M, Stachelscheid H, Svingen T, Wagenaars F, Wedebye E, Zoeller R. Removing critical gaps in chemical test methods by developing new assays for the identification of thyroid hormone system-disrupting chemicals – the ATHENA project. Int J Mol Sci 2020;submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.St Germain DL, Hernandez A, Schneider MJ, Galton VA. Insights into the role of deiodinases from studies of genetically modified animals. Thyroid. 2005;15(8):905–916. [DOI] [PubMed] [Google Scholar]
- 209.Galton VA, Wood ET, St Germain EA, Withrow CA, Aldrich G, St Germain GM, Clark AS, St Germain DL. Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient rodent brain during development. Endocrinology. 2007;148(7):3080–3088. [DOI] [PubMed] [Google Scholar]
- 210.Li A, Makris SL, Marty MS, Strauss V, Gilbert ME, Blacker A, Zorrilla LM, Coder PS, Hannas B, Lordi S, Schneider S. Practical considerations for developmental thyroid toxicity assessments: What’s working, what’s not, and how can we do better? Regul Toxicol Pharmacol 2019;106:111–136. [DOI] [PubMed] [Google Scholar]
- 211.US EPA. NonMonotonic Dose Response: State of the Science. Appendix C: Mammalian Studies Describing the Effects of Chemicals That Disrupt the Thyroid Signaling Pathways.: https://www.epa.gov/sites/production/files/2016-01/documents/appendix_c_mammalian_thyroid_v_7.1.pdf; https://www.epa.gov/sites/production/files/2016-01/documents/nmdr.pdf; 2014.
- 212.Beekhuijzen M, Schneider S, Barraclough N, Hallmark N, Hoberman A, Lordi S, Moxon M, Perks D, Piersma AH, Makris SL. The urgency for optimization and harmonization of thyroid hormone analyses and their interpretation in developmental and reproductive toxicology studies. Reprod Toxicol 2018;80:126–130. [DOI] [PubMed] [Google Scholar]
- 213.HESI-DART-ETS. Thyroid Hormone Assessment: Implications for Developmental and Reproductive Toxicology. https://hesiglobalorg/event/hesi-dart-committee-thyroid-hormone-assessment-workshop/). 2019.
- 214.CEFIC. LRI-EMSG59: Species Comparison in liver-mediated thyroid adn thyroid-related toxicities. Part 1: Characterization of liver-mediated thyroid toxicity in the rat. http://cefic-lriorg/wp-content/uploads/2018/06/EMSG59-Thyroid-LRI-RfP-2018-for-publicationpdf),. 2019. [Google Scholar]
- 215.ATHENA. Assays for the identification of thyroid hormone axis-disrupting chemicals: Elaborating novel assessment strategies. . https://athenaedctestmethods.net/ 2020.
- 216.Noyes PD, Friedman KP, Browne P, Haselman JT, Gilbert ME, Hornung MW, Barone S Jr., Crofton KM, Laws SC, Stoker TE, Simmons SO, Tietge JE, Degitz SJ. Evaluating Chemicals for Thyroid Disruption: Opportunities and Challenges with in Vitro Testing and Adverse Outcome Pathway Approaches. Environ Health Perspect 2019;127(9):95001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Olker JH, Korte JJ, Denny JS, Hartig PC, Cardon MC, Knutsen CN, Kent PM, Christensen JP, Degitz SJ, Hornung MW. Screening the ToxCast Phase 1, Phase 2, and e1k Chemical Libraries for Inhibitors of Iodothyronine Deiodinases. Toxicol Sci 2019;168(2):430–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hallinger DR, Murr AS, Buckalew AR, Simmons SO, Stoker TE, Laws SC. Development of a screening approach to detect thyroid disrupting chemicals that inhibit the human sodium iodide symporter (NIS). Toxicol In Vitro. 2017;40:66–78. [DOI] [PubMed] [Google Scholar]
- 219.Stoker TE, Dong H, Wade MG. Application of a nonradioactive assay for high throughput screening for inhibition of thyroid hormone uptake via the transmembrane transporter MCT8. Environ Sci Technol 2017;40:234–242. [DOI] [PubMed] [Google Scholar]
- 220.Wang J, Hallinger DR, Murr AS, Buckalew AR, Lougee RR, Richard AM, Laws SC, Stoker TE. High-throughput screening and chemotype-enrichment analysis of ToxCast phase II chemicals evaluated for human sodium-iodide symporter (NIS) inhibition. Environ Int 2019;126:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Dong H, Atlas E, Wade MG. Development of a non-radioactive screening assay to detect chemicals disrupting the human sodium iodide symporter activity. Toxicol In Vitro. 2019;57:39–47. [DOI] [PubMed] [Google Scholar]
- 222.Dong H, Godlewska M, Wade MG. A rapid assay of human thyroid peroxidase activity. Toxicol In Vitro. 2020;62:104662. [DOI] [PubMed] [Google Scholar]
- 223.Paul Friedman K, Watt ED, Hornung MW, Hedge JM, Judson RS, Crofton KM, Houck KA, Simmons SO. Tiered High-Throughput Screening Approach to Identify Thyroperoxidase Inhibitors Within the ToxCast Phase I and II Chemical Libraries. Toxicol Sci 2016;151(1):160–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Paul KB, Hedge JM, Rotroff DM, Hornung MW, Crofton KM, Simmons SO. Development of a thyroperoxidase inhibition assay for high-throughput screening. Chem Res Toxicol 2014;27(3):387–399. [DOI] [PubMed] [Google Scholar]
- 225.Paul-Friedman K, Martin M, Crofton KM, Hsu CW, Sakamuru S, Zhao J, Xia M, Huang R, Stavreva DA, Soni V, Varticovski L, Raziuddin R, Hager GL, Houck KA. Limited Chemical Structural Diversity Found to Modulate Thyroid Hormone Receptor in the Tox21 Chemical Library. Environ Health Perspect 2019;127(9):97009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.OECD. New scoping document on in vitro and ex vivo assays for the identification of modulators of thyroid hormone signaling. In: OECD Environment, Health and Safety Publications Series on Testing and Assessment. . 2014. [Google Scholar]
- 227.Murk AJ, Rijntjes E, Blaauboer BJ, Clewell R, Crofton KM, Dingemans MM, Furlow JD, Kavlock R, Kohrle J, Opitz R, Traas T, Visser TJ, Xia M, Gutleb AC. Mechanism-based testing strategy using in vitro approaches for identification of thyroid hormone disrupting chemicals. Toxicol In Vitro. 2013;27(4):1320–1346. [DOI] [PubMed] [Google Scholar]
- 228.Hassan I, El-Masri H, Ford J, Brennan A, Handa S, Paul Friedman K, Gilbert ME. Extrapolating In Vitro Screening Assay Data for Thyroperoxidase Inhibition to Predict Serum Thyroid Hormones in the Rat. Toxicol Sci 2020;173(2):280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Leonard JA, Tan YM, Gilbert M, Isaacs K, El-Masri H. Estimating Margin of Exposure to Thyroid Peroxidase Inhibitors Using High-Throughput in vitro Data, High-Throughput Exposure Modeling, and Physiologically Based Pharmacokinetic/Pharmacodynamic Modeling. Toxicol Sci 2016;151(1):57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Davis PJ, Goglia F, Leonard JL. Nongenomic actions of thyroid hormone. Nat Rev Endocrinol 2016;12(2):111–121. [DOI] [PubMed] [Google Scholar]
- 231.Gilbert ME, Rovet J, Chen Z, Koibuchi N Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology. 2012;33(4):842–852. [DOI] [PubMed] [Google Scholar]