Abstract
Rationale: People with chronic obstructive pulmonary disease (COPD) have an increased risk of cardiovascular disease and may be more susceptible to air pollution exposure. However, no study has examined the association between long-term fine particulate matter exposure (≤2.5 μm in aerodynamic diameter) and risk of cardiovascular events in this potentially vulnerable population.
Objectives: To estimate the association between long-term fine particulate matter and risk of cardiovascular events among adults with COPD.
Methods: This retrospective cohort study included 169,714 adults with COPD who were members of the Kaiser Permanente Northern California health plan during 2007–2016. Electronic health record data were linked to 1 km modeled particulate matter ≤2.5 μm in aerodynamic diameter exposure estimates. We fit Cox proportional hazard models, adjusting for age, sex, race/ethnicity, calendar year, smoking, body mass index, comorbidities, medications, and socioeconomic status. In low exposure analyses, we examined effects below the current regulation limit (12 μg/m3).
Measurements and Main Results: Among adults with COPD, a 10-μg/m3 increase in 1-year mean fine particulate matter exposure was associated with an elevated risk of cardiovascular mortality (hazard ratio, 1.10; 95% confidence interval [CI], 1.01–1.20). Effects were stronger in low exposure analyses (hazard ratio, 1.88; 95% CI, 1.56–2.27). Fine particulate matter exposure was not associated with acute myocardial infarction or stroke in overall analyses.
Conclusions: Long-term fine particulate matter exposure was associated with an increased risk of cardiovascular mortality among adults with COPD. Current regulations may not sufficiently protect those with COPD.
Keywords: air pollution, cardiovascular diseases, lung diseases
At a Glance Commentary
Scientific Knowledge on the Subject
Long-term fine particulate matter exposure is a known risk factor for cardiovascular mortality in the general population.
What This Study Adds to the Field
Our study adds to the field by providing risk estimates of the association between long-term exposure to particulate matter ≤2.5 μm in aerodynamic diameter and cardiovascular events among adults with chronic obstructive pulmonary disease (COPD). Our findings generally suggest that long-term exposure to particulate matter ≤2.5 μm in aerodynamic diameter is an important modifiable risk factor that should be considered in addition to other traditional cardiovascular risk factors. Furthermore, we found that the current regulation level of 12 μg/m3 for 1-year mean exposure does not appear to be sufficiently protective of adults with COPD.
Chronic obstructive pulmonary disease (COPD) is a group of progressive lung diseases including emphysema and chronic bronchitis. COPD is a leading cause of morbidity and mortality in the United States. Annual medical costs for patients with COPD are nearly twice as high as costs for patients without COPD, after adjusting for comorbidities (1), and the CDC estimates that total U.S. medical costs attributable to having COPD will be $49.0 billion in 2020 (2). Individuals with COPD have higher hospitalization rates (1) and higher risks of cardiovascular disease (CVD) events including cardiovascular mortality (3, 4).
Air pollution, especially particulate matter ≤2.5 μm in aerodynamic diameter (PM2.5), is a modifiable risk factor related to both COPD and CVD. Short-term air pollution exposure has been associated with COPD exacerbations (5) and with CVD events (6–8). Long-term PM2.5 exposure is associated with a larger increase in risk for cardiovascular mortality than short-term PM2.5 exposure (9). Given the importance of long-term PM2.5 exposure as a modifiable risk factor for cardiovascular events and mortality, it is particularly important to study these associations in sensitive populations, such as those with COPD who already have an increased risk of CVD events. However, only a small number of studies (10–16) of long-term PM2.5 exposure and CVD events have included subjects with COPD, and no studies have analyzed the effect of long-term PM2.5 exposure on the risk of CVD events specifically among adults with COPD.
This study examines the relationship of long-term average PM2.5 exposure and risk of cardiovascular events in a large cohort of adults with COPD. This study provides clinicians with relative risk estimates for air pollution exposure so that this modifiable risk factor can be considered in a clinical context in relation to other risk factors that need to be managed. We also examined low concentrations of exposure to determine whether there is evidence that long-term PM2.5 exposure affects CVD risk when exposure concentration is below the current regulation limit of 12.0 μg/m3 for 1-year average exposure (17, 18). Understanding the cardiovascular health effects of PM2.5 exposure among sensitive populations is important for determining regulatory actions for PM2.5 and for shaping individual-level recommendations for behaviors that could reduce personal exposure to PM2.5.
Methods
Study Cohort
This retrospective cohort study includes 169,714 adults with COPD who were members of the Kaiser Permanente Northern California (KPNC) health plan during 2007–2016. KPNC is a large, integrated healthcare system that provides comprehensive medical services to over 4 million members through a nonprofit health plan and nonprofit hospitals and outpatient clinics. To be included in the study cohort, each subject must meet the following criteria: 1) a clinical diagnosis of COPD (discharge codes for COPD: International Classification of Diseases–9, 491 492 496; International Classification of Diseases–10, J41, J43, J44); 2) be an adult (age 18+); 3) at least 1 year of KPNC health plan membership and one outpatient use; 4) lived in the 35-county Northern California region for at least 1 year (see Figure E1 in the online supplement); and 5) a home address that was successfully geocoded and linked to the air pollution exposure data. Study follow-up began on January 1, 2007, with subjects entering the study on the first day that all inclusion criteria were met. Follow-up continued to the first of the following dates: end of membership, relocation out of study region or to an address that could not be successfully geocoded, death, or December 31, 2016. The institutional review board at the Kaiser Foundation Research Institute approved this study.
Air Pollution Exposures
Daily PM2.5 exposures were obtained from a validated state-of-the-art ensemble model with a resolution of 1 km × 1 km across the entire study region from January 1, 2007, to December 31, 2016 (19). The ensemble model integrated three machine learning algorithms and combined discrete PM2.5 daily ground monitoring data with more than 100 predictor variables including satellite-based aerosol optical depth measurements, absorbing aerosol index data, satellite-based surface reflectance data, Goddard Earth Observing System (NASA) chemical transport model outputs, meteorologic data, and land-use data. The ensemble model was validated and performed extremely well, with an R2 of 0.86 for daily PM2.5 predictions and an R2 of 0.89 for 1-year PM2.5 predictions using 10-fold cross-validation (19).
We constructed individual-level time-varying 1-year average PM2.5 exposures for every study subject. Exposures were updated monthly from baseline through the end of follow-up and accounted for moving. Complete residential address history of all cohort members from 1 year before the index date to the end of follow-up were obtained from the KPNC historical and current residential address databases. Each address was geocoded to the latitude and longitude coordinates using ArcGIS (Esri) and coordinates were linked with the PM2.5 exposure data.
Cardiovascular Event Outcomes
We examined three primary cardiovascular event endpoints: acute myocardial infarction (AMI), stroke, and cardiovascular mortality. We also considered a combined endpoint defined as having any of those cardiovascular events. Cardiovascular event data were obtained from the electronic health record (EHR) system and from death records, and events were defined using previously validated methods (20, 21). An AMI event was defined as an inpatient hospitalization with a principal discharge diagnosis of AMI based on International Classification of Diseases (ICD) (ICD-9 and ICD-10) codes (ICD-9: 410.x; ICD-10: I21.x-I23.x). A stroke event was defined as an inpatient hospitalization with a principal discharge diagnosis of stroke (ICD-9: 431.x-434.x, 436.x; ICD-10: I60.x, I61.x, I63.x, I64.x). Mortality data were compiled from several sources: official state of California death certificates, health plan in-hospital mortality, Social Security Administration Death Files, and the National Death Index (see online supplemental material for details). Cardiovascular mortality was defined by cause of death codes (ICD-9: 400.x-440.x; ICD-10: I10.x-I70.x). As secondary endpoints, we also examined ischemic heart disease (IHD) mortality (ICD-9: 410.x-414.x; ICD-10: I20.x-I25.x) and cerebrovascular disease mortality (ICD-9: 430.x-438.x; ICD-10: I60.x-I69.x).
Validation of COPD Diagnosis
A total of 100 cohort members with COPD were randomly selected for medical record review. The full Kaiser Permanente medical record for each subject was reviewed to identify 1) COPD medication use, 2) spirometry measurements, and 3) COPD symptoms of chronic cough, exertional dyspnea, wheezing, chest tightness, and/or excessive sputum production noted in the medical record. COPD medication use was determined based on pharmacy records for filled prescriptions. COPD medications included inhaled anticholinergic agents, inhaled β-adrenergic steroids, inhaled corticosteroids, combination inhaled medications, and methylxanthine agents. Spirometry measurements included FEV1 and FVC measurements. Airflow obstruction determined through spirometry was defined as an FEV1/FVC ratio <70% predicted, based on the Global Initiative for Chronic Obstructive Lung Disease criteria (22).
COPD symptoms were noted in the medical record for the majority of subjects: chronic cough (78%), exertional dyspnea (70%), wheezing (59%), chest tightness (54%), and excessive sputum production (41%). Most subjects (89%) had at least one of the symptoms above. We found that 83% of subjects had filled prescriptions for one or more COPD medications. Spirometry was performed in only 35% of the subjects; of those with spirometry, airflow obstruction was found in 74%.
Covariates
Data on age, sex, race/ethnicity, smoking, body mass index (BMI), comorbidities, insurance type, and medication use were obtained from the KPNC EHR. BMI was categorized as defined by the World Health Organization: underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obesity (≥30 kg/m2) (23). Missing categories were included for unknown race/ethnicity (N = 3,133; 1.9%) and unknown BMI (N = 2,785; 1.6%). We excluded four subjects with missing sex. Comorbidities (diabetes, hypertension, hyperlipidemia, and history of stroke or AMI) were determined from the KPNC EHR. Diabetes was determined from the previously validated KPNC Diabetes registry (24, 25), which combines diagnoses, medications, and laboratory results documented in the KPNC EHR (26). Hypertension was determined from diagnosis codes (ICD-9: 401.x-404.x; ICD-10: I10.x-I15.x). Using pharmacy data, hypertensive and statin medication use at baseline were defined as having at least one prescription fill in the last 120 days. Hypertensive medication use (angiotensin-converting enzymeinhibitors, angiotensin antagonists, antiadrenergics, β-adrenergic blockers, calcium channel blockers, chlorthalidone, diuretics, metolazone, and vasodilators) was obtained for those diagnosed with hypertension because some medications could be prescribed for other clinical purposes. Neighborhood high school education was obtained from Census American Community Survey data at the block group level and used as a measure of neighborhood socioeconomic status (SES). Medicaid insurance was used as an indicator of low SES at the individual level.
Statistical Analysis
We fit Cox proportional hazards models to quantify the relationship between long-term PM2.5 exposure and each cardiovascular health outcome. We used the time on study as the time scale and flexibly controlled for age by fitting models stratified by age in 5-year intervals, allowing separate baseline hazards for each age group. All models were adjusted for covariates chosen a priori on the basis of previous epidemiologic studies of air pollution and CVD events (27–29). We fit a set of nested models with increasing levels of adjustment. Model 1 (minimally adjusted model) adjusted for age, sex, race/ethnicity, and calendar year. Model 2 additionally adjusted for smoking, BMI, baseline comorbidities, and medication use. Model 3 (fully adjusted model) additionally adjusted for neighborhood education and Medicaid insurance. We assessed departures from the proportional hazards assumption by including interaction terms between covariates and time (30).
Our primary analysis modeled PM2.5 exposure as a continuous, time-updated variable with a linear effect. To assess potential nonlinearity in the shape of the association between PM2.5 and risk of each cardiovascular event type, we used restricted cubic splines and formally tested for any evidence of a nonlinear relationship using a Wald test for the statistical significance of the spline terms. We assessed effect modification by age (<65 vs. ≥65), sex, race/ethnicity, and neighborhood education. We conducted two analyses to understand the effects of PM2.5 exposure concentrations below 12 μg/m3. First, we fit PM2.5 exposures categorically, using the following categories: low (<9.0 μg/m3), moderate (9.0–11.9 μg/m3), and high (≥12.0 μg/m3). The low exposure cut point was chosen as the lowest level supported by the distribution of our data because only 8.1% of subjects had PM2.5 exposures <8 μg/m3 at baseline. Second, we modeled the linear association between PM2.5 exposure and cardiovascular mortality among subjects with PM2.5 exposures <12 μg/m3 for the entire follow-up. We conducted several sensitivity analyses: excluded subjects with a previous stroke or AMI, baseline 1-year average PM2.5, and imputed missing data. A level of α = 0.05 was used to determine statistical significance. All analyses were conducted using SAS software, version 9.4 (SAS Institute).
Results
This retrospective cohort study included 169,714 KPNC adults with COPD. The characteristics of the study cohort at baseline are given in Table 1. Adults in this cohort were generally older (mean age = 60.8 yr; SD = 15.6) and mostly female (56.2%), and the cohort was diverse (65.5% white and 34.5% other race/ethnicity groups). Many subjects were overweight (32.9%) or obese (38.1%), and hypertension (53.1%) and hyperlipidemia (47.4%) were common comorbidities. Some subjects had diabetes (17.9%), and a smaller number had a history of stroke (8.9%) or AMI (6.5%). Most subjects had 1-year average PM2.5 exposures that were below the current regulation limit of 12 μg/m3 at baseline (81.3%).
Table 1.
Characteristics of the Study Cohort at Baseline
| Characteristic | Cohort [n (%)] (N = 169,714) |
|---|---|
| Sex | |
| F | 95,350 (56.2) |
| M | 74,364 (43.8) |
| Age, mean (SD), yr | 60.8 (15.6) |
| Race/ethnicity* | |
| White, non-Hispanic | 109,157 (65.5) |
| Hispanic white | 15,541 (9.3) |
| Black | 12,838 (7.7) |
| American Indian/Alaska native | 1,076 (0.7) |
| Asian/Pacific Islander | 15,369 (9.2) |
| Multiple races | 12,600 (7.6) |
| Hispanic ethnicity | 19,501 (11.5) |
| Neighborhood education, mean (SD) | |
| Percentage with less than high school education | 14.0 (12.1) |
| Smoking* | |
| Never | 62,553 (37.60) |
| Former | 62,259 (37.42) |
| Current | 41,553 (24.98) |
| BMI*, kg/m2 | |
| Underweight (<18.5) | 3,901 (2.3) |
| Normal (18.5–24.9) | 44,448 (26.6) |
| Overweight (25.0–29.9) | 54,964 (32.9) |
| Obese (≥30.0) | 63,616 (38.1) |
| Comorbidities | |
| Hypertension | 90,094 (53.1) |
| Hyperlipidemia | 80,386 (47.4) |
| Diabetes | 30,292 (17.9) |
| Medications | |
| Hypertensive medication† | 86,695 (96.3) |
| Statin medication | 68,591 (40.4) |
| History of CVD | |
| Acute myocardial infarction | 11,055 (6.5) |
| Stroke | 15,158 (8.9) |
| 1-yr average PM2.5 at baseline, μg/m3 | |
| <9.0 | 31,734 (18.70) |
| 9.0–11.9 | 107,846 (63.55) |
| ≥12 | 30,134 (17.76) |
Definition of abbreviations: BMI = body mass index; CVD = cardiovascular disease; PM2.5 = particulate matter ≤2.5 μm in aerodynamic diameter.
Missing data at baseline: race/ethnicity = 3,133; smoking = 3,349; BMI = 2,785.
Hypertensive medication use among those with hypertension.
Subjects were followed for a maximum of 10 years, with more than 1 million total person-years of follow-up in the analyses and an average follow-up time of 6.5 years per person. A total of 17,770 subjects (10.5%) had at least one CVD event during follow-up. The most common CVD event was cardiovascular mortality (10,169 events), followed by AMI (5,970 events), IHD mortality (5,309 events), stroke (4,269 events), and cerebrovascular mortality (917 events). Most AMI hospitalization events were nonfatal (94%) and most stroke hospitalization events were also nonfatal (89%).
Table 2 shows the hazard ratios (HRs) representing the relative risk for each cardiovascular event per 10-μg/m3 increase in 1-year average PM2.5 exposure. The strongest association was with increased risk of cardiovascular mortality, with an HR of 1.27 (95% confidence interval [CI], 1.17–1.38) in the minimally adjusted model and an HR of 1.10 (95% CI, 1.01–1.20) in the fully adjusted model. The outcomes of AMI, IHD mortality, and the combined endpoint of any cardiovascular event were associated with long-term PM2.5 exposure in minimally adjusted models but not in fully adjusted models. Stroke and cerebrovascular mortality were not associated with PM2.5 exposure in any of the models. We found no violations of the proportional hazards assumption and no evidence of nonlinearity in the association of 1-year average PM2.5 exposure and cardiovascular mortality when we assessed the relationship using restricted cubic splines (P value for nonlinearity 0.344) (Figure E2). In sensitivity analyses, we found that results were similar when we restricted analyses to subjects with no known history of AMI or stroke, and when we imputed missing covariate data instead of using missing categories; results were slightly stronger when we used baseline PM2.5 exposures instead of time-updated PM2.5 exposures (Tables E1 and E2).
Table 2.
Relative Risk of Cardiovascular Events per 10-μg/m3 Increase in 1-Year Average PM2.5 Exposure among 169,714 Subjects with COPD
| Outcomes | Number of Events | Model 1* | Model 2† | Model 3‡ |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Any cardiovascular event§ | 17,770 | 1.18 (1.11–1.26) | 1.07 (1.00–1.14) | 1.01 (0.95–1.08) |
| Cardiovascular mortality | 10,169 | 1.27 (1.17–1.38) | 1.16 (1.07–1.26) | 1.10 (1.01–1.20) |
| AMI | 5,970 | 1.16 (1.04–1.30) | 1.01 (0.90–1.13) | 0.94 (0.84–1.06) |
| IHD mortality | 5,309 | 1.32 (1.18–1.49) | 1.18 (1.05–1.33) | 1.09 (0.97–1.23) |
| Stroke | 4,269 | 0.98 (0.86–1.12) | 0.90 (0.79–1.03) | 0.88 (0.77–1.02) |
| Cerebrovascular mortality | 917 | 1.04 (0.78–1.39) | 1.01 (0.76–1.35) | 1.00 (0.74–1.34) |
Definition of abbreviations: AMI = acute myocardial infarction; CI = confidence interval; COPD = chronic obstructive pulmonary disease; HR = hazard ratio; IHD = ischemic heart disease; PM2.5 = particulate matter ≤2.5 μm in aerodynamic diameter.
Model 1: Adjusted for age, sex, race/ethnicity, and calendar year.
Model 2: Adjusted for age, sex, race/ethnicity, calendar year, smoking, body mass index, baseline comorbidities, and medications.
Model 3: Adjusted for age, sex, race/ethnicity, calendar year, smoking, body mass index, baseline comorbidities, medications, neighborhood education, and Medicaid insurance.
Any cardiovascular event includes AMI, stroke, or cardiovascular mortality.
Figure 1 illustrates the results of our analysis of effect modification. We found statistically significant differences between men and women (P = 0.007), in which risks were increased among men (HR, 1.21; 95% CI, 1.08–1.36) but not women (HR, 0.98; 95% CI, 0.87–1.11). We also found stronger effects in low-SES neighborhoods than in high-SES neighborhoods (P = 0.034). We found no differences by age (P = 0.393) or by race/ethnicity (P = 0.975).
Figure 1.
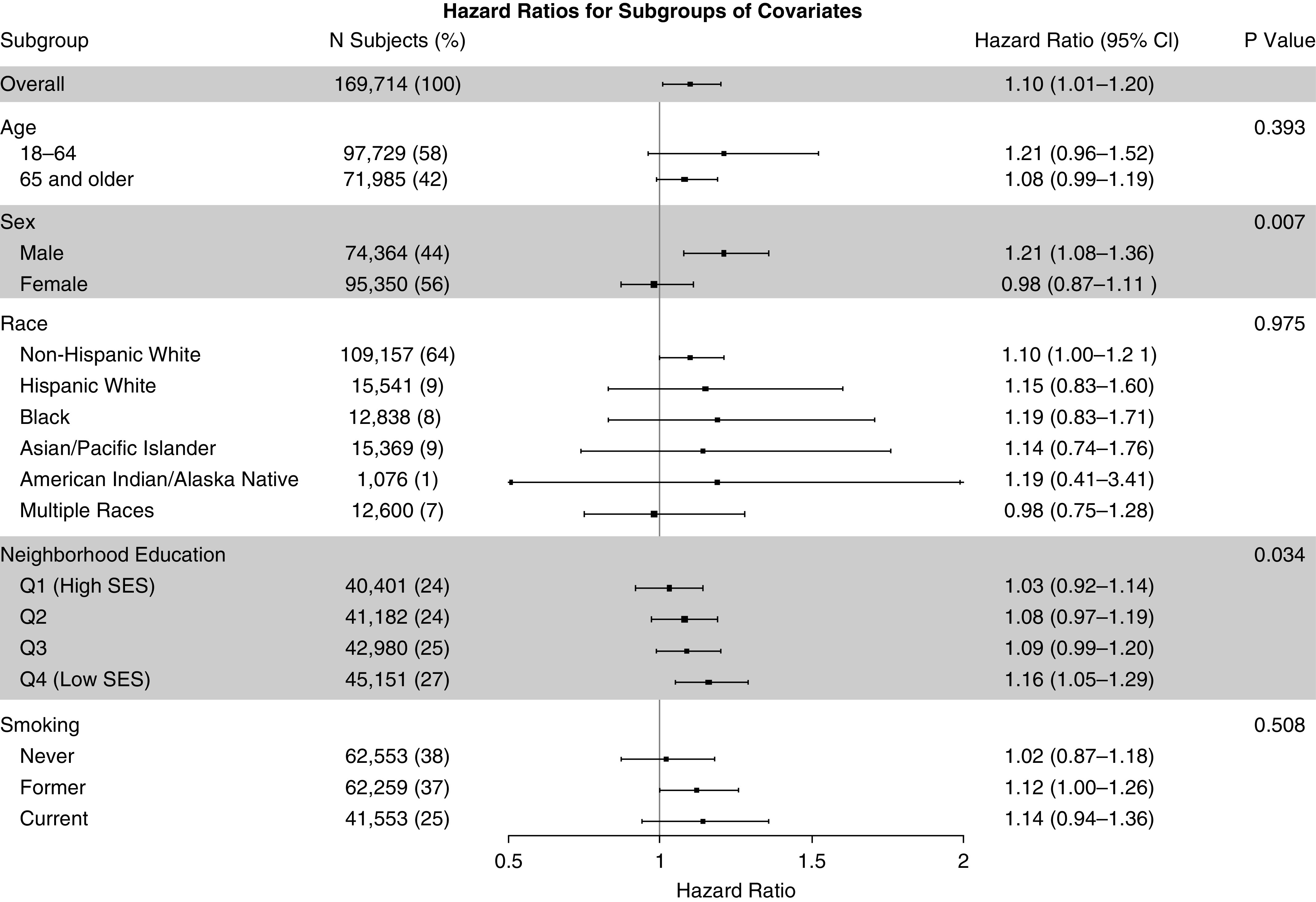
Relative risk of cardiovascular mortality per 10-μg/m3 increase in 1-year average exposure to particulate matter ≤2.5 μm in aerodynamic diameter, overall and by subgroup. P values were computed using interaction terms, and the P value for neighborhood education was computed as the interaction trend. CI = confidence interval; Q = quartile; SES = socioeconomic status.
Analyses of Exposure Concentrations below 12 μg/m3
Figure 2 shows the relative risk of cardiovascular mortality when PM2.5 exposures were modeled in categories above and below 12 μg/m3, overall and within the high-risk subgroups identified in our effect modification analyses. Compared with those with low exposures (<9 μg/m3), we found an increased risk of cardiovascular mortality at PM2.5 exposures of 9.0–11.9 μg/m3 among all subjects (HR, 1.05; 95% CI, 1.00–1.10) and among men (HR, 1.07; 95% CI, 1.01–1.14). Results in the low-SES group were suggestive but not statistically significant (see Table E3 for numeric results).
Figure 2.
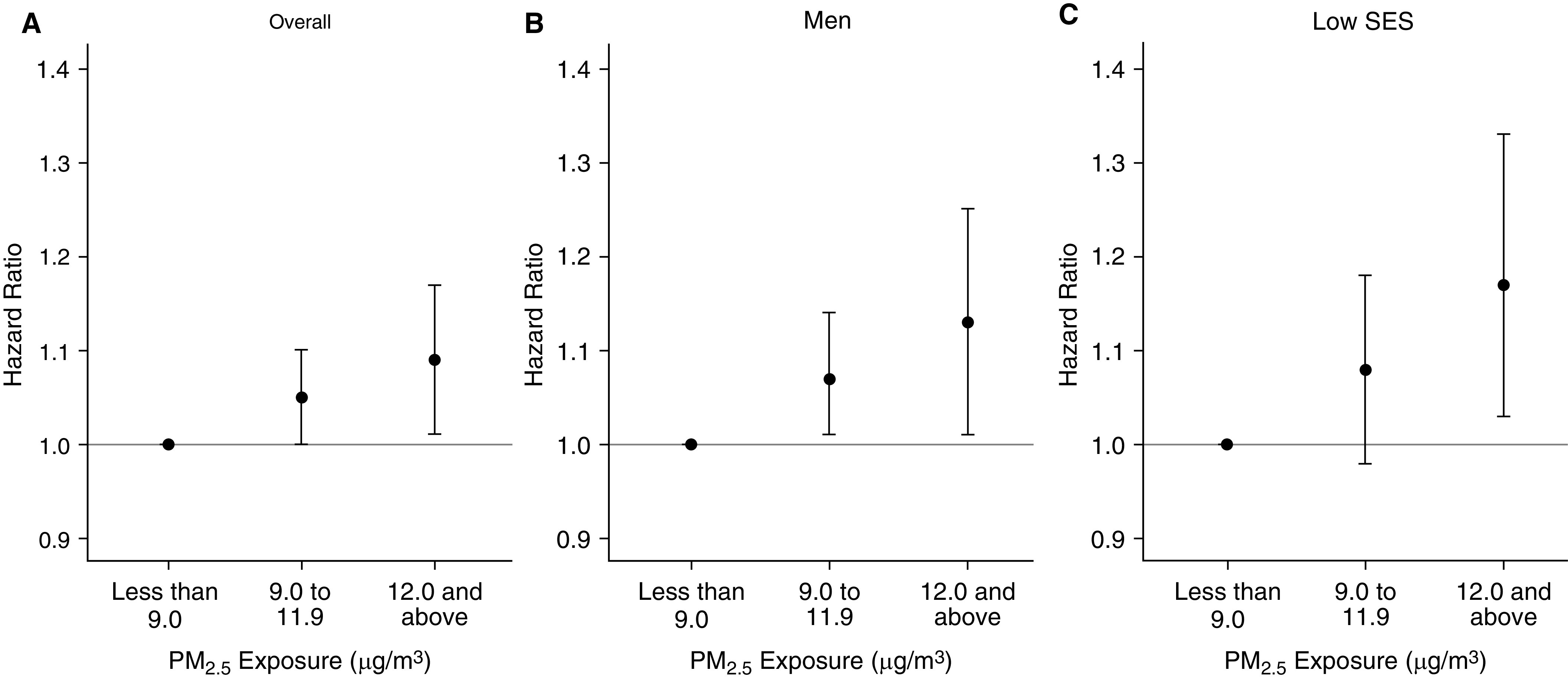
Relative risk of cardiovascular mortality for categories of particulate matter ≤2.5 μm in diameter above and below the current regulation limit of 12 μg/m3, overall and among high-risk subgroups. PM2.5 = particulate matter ≤2.5 μm in aerodynamic diameter; SES = socioeconomic status.
Table 3 shows the low exposure analyses for subjects with time-varying PM2.5 exposure below 12 μg/m3 for the entire follow-up. We found much stronger linear associations for all models and all outcomes. In fully adjusted models, we found that 1-year average PM2.5 exposure was associated with increased risks of cardiovascular mortality (HR, 1.88; 95% CI, 1.56–2.27), AMI (HR, 1.52; 95% CI, 1.17–1.96), IHD mortality (HR, 1.84; 95% CI, 1.42–2.39), and the combined endpoint of any cardiovascular event (HR, 1.62; 95% CI, 1.40–1.88). Stroke and cerebrovascular mortality associations were suggestive of higher risk. We further explored the differences between the main analyses and the low exposure analyses in the online supplemental material (Figures E3 and E4 and Table E4).
Table 3.
Low Exposure Analysis of Relative Risk of Cardiovascular Events per 10-μg/m3 Increase in 1-Year Average PM2.5 Exposure among 91,699 Subjects with COPD Who Have Time-Varying PM2.5 Exposure below the Current Regulation Limit of 12 μg/m3 for Entire Follow-Up
| Outcomes | Number of Events | Model 1* | Model 2† | Model 3‡ |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Any cardiovascular event§ | 9,594 | 1.99 (1.72–2.30) | 1.75 (1.51–2.01) | 1.62 (1.40–1.88) |
| Cardiovascular mortality | 5,733 | 2.33 (1.93–2.80) | 2.02 (1.68–2.43) | 1.88 (1.56–2.27) |
| AMI | 3,097 | 1.91 (1.48–2.46) | 1.64 (1.27–2.12) | 1.52 (1.17–1.96) |
| IHD mortality | 2,968 | 2.42 (1.87–3.13) | 2.02 (1.56–2.61) | 1.84 (1.42–2.39) |
| Stroke | 2,230 | 1.56 (1.16–2.10) | 1.39 (1.03–1.88) | 1.30 (0.97–1.76) |
| Cerebrovascular mortality | 536 | 1.82 (0.99–3.34) | 1.60 (0.87–2.94) | 1.52 (0.82–2.81) |
For definition of abbreviations, see Table 2.
Model 1: Adjusted for age, sex, race/ethnicity, and calendar year.
Model 2: Adjusted for age, sex, race/ethnicity, calendar year, smoking, body mass index, baseline comorbidities, and medications.
Model 3: Adjusted for age, sex, race/ethnicity, calendar year, smoking, body mass index, baseline comorbidities, medications, neighborhood education, and Medicaid.
Any cardiovascular event includes AMI, stroke, or cardiovascular mortality.
Discussion
In this large cohort study of adults with COPD, we found evidence that long-term PM2.5 exposure was associated with an elevated risk of cardiovascular mortality. We also found stronger associations among men and among those living in low-SES neighborhoods. In our low exposure analyses, we found evidence of increased risk of cardiovascular events at PM2.5 exposure concentrations lower than the current regulation limit of 12 μg/m3 for 1-year mean exposure. Thus, current regulations do not appear to be sufficiently protective of adults with COPD.
This study provides clinicians with relative risk estimates for PM2.5 exposure so that this modifiable risk factor can be considered in a clinical context in relation to other risk factors that need to be managed. For the traditional cardiovascular risk factors in the general population, the risk of cardiovascular disease is estimated to increase by 2.00 for hypertension, 1.74 for diabetes, 1.64 for current smoking, and 1.31 for high cholesterol (31). In our low exposure analyses, we found increased risks for major cardiovascular events (any cardiovascular event, cardiovascular mortality, AMI, and IHD mortality) ranging from 1.52 to 1.88 for a 10-μg/m3 increase in 1-year mean PM2.5 exposure when exposures were below 12 μg/m3. Though these risk estimates cannot be directly compared, our findings generally suggest that long-term PM2.5 exposure is an important modifiable risk factor that should be considered in addition to other traditional cardiovascular risk factors.
There currently are no directly comparable studies of the association between long-term PM2.5 and CVD events in a population of adults with COPD. However, our findings among people with COPD are generally consistent results reported in the general population. A 2013 meta-analysis found that a 10-μg/m3 increase in long-term PM2.5 was associated with an 11% increase in the risk of cardiovascular mortality (32). Notably, relative risks of cardiovascular mortality have varied widely in individual studies, with some studies reporting strong effects with HRs of 1.76 (95% CI, 1.25–2.47) (27), 1.48 (95% CI, 1.46–1.49) (33), and 1.54 (95% CI, 1.12–2.10) (34) and other studies reporting much weaker or null effects (12, 35–37). Another related study of Medicare beneficiaries with COPD examined a slightly different exposure–outcome relationship (long-term PM10 and all-cause mortality) and reported an HR of 1.22 (95% CI, 1.17–1.27) for an increase of 10 μg/m3 PM10 over the previous 4 years (38).
We can also compare our results for effect modification to those reported in studies of the general population. We found evidence of effect modification by sex, with stronger effects among men than among women. In the general population, a few studies have reported no effect modification by sex (39, 40) and a few have reported slightly stronger associations among men than among women (36, 41). A study of elderly adults with COPD found stronger effects of long-term PM10 exposure on all-cause mortality among men and among non-white subjects (38). We also found stronger effects in low-SES neighborhoods compared with high-SES neighborhoods, consistent with several studies in the general population (42–44). We found no effect modification by age, which was consistent with several other studies (27, 36, 39). We also found no effect modification by race/ethnicity. Most air pollution cohorts have little diversity and are unable to assess whether susceptibility differs by race, but a few previous studies have reported stronger effects for PM-induced cardiovascular mortality among Hispanic, Black, and other non-white subjects (40, 42). Our finding of no differences by race may be because all study cohort participants were members of the same health plan and had equal access to health care.
Inflammation and oxidative stress are key mechanisms by which PM2.5 acts to increase the risk of cardiovascular disease. Reactive oxygen species are generated in pulmonary and vascular tissue when exposed to particulate air pollution (45, 46). Activation of reactive oxygen species–dependent pathways can affect vascular inflammation, atherosclerosis, basal vasomotor balance, coagulation and thrombosis, and platelet activation (47). Inflammatory cytokines are also activated in response to air pollution exposures, including increased levels of circulating proinflammatory mediators (48, 49). Furthermore, these PM2.5-induced increases in neutrophils, eosinophils, and inflammatory cytokines and decreases in antioxidant levels have also been demonstrated in rats with preexisting COPD (50). Given these mechanisms of oxidative stress and inflammation, populations of subjects with a history of COPD may have increased susceptibility to the effects of PM2.5 on cardiovascular events.
Strengths and Limitations
This study has a number of strengths and limitations. A major strength of our study is the use of high-resolution, time-varying PM2.5 exposures based on participants’ home addresses, using detailed address histories and accounting for moving. Many previous studies are limited by using address data aggregated to the ZIP code or census tract level, central site monitoring, or low-resolution air pollution estimates. The use of high-resolution modeled PM2.5 estimates at the home address reduces exposure misclassification compared with central site monitoring (51). However, we do not have data on time–activity patterns, time spent outdoors, or indoor air pollution exposure measurements. Californians over 18 years of age have been shown to spend on average >65% of daily time at their home residence (52). In addition, outdoor particles can readily penetrate indoors in California, where many homes lack air conditioning, causing higher air exchange rates between indoors and outdoors and often similar ambient contributions of PM2.5 to indoor and personal concentrations (53, 54). Furthermore, indoor and outdoor particulate matter differ in sources, chemical composition, and temporal patterns, which could lead to different health effects (55). Our study focused on 1-year mean exposures because that is the time window regulated in both California and the United States (17, 18). We did not examine time windows longer than 1 year, which would be of interest in future work. We could not adjust for previous years of PM2.5 exposure because of the high correlation of long-term averages at the same location, which would cause multicollinearity. We did not have data available on physical activity, diet (e.g., fruit and antioxidant intake), alcohol use, marital status, or detailed smoking history (e.g., lifetime pack-years, years quit), which could be confounders. However, our study controls for nearly all key confounders, including BMI, clinical comorbidities, and medication use, which are not available in many previously studied air pollution cohorts. We adjusted for these key covariates as measured at baseline only because they could be on the causal pathway between exposure and outcome, to avoid overadjustment. Another strength is the use of a large, diverse, representative cohort with well-characterized data available from electronic health records. However, because subjects were not brought into a research laboratory, we do not have detailed spirometry measurements or COPD severity data, which may also be important factors in the susceptibility to air pollution exposures among adults with COPD.
Conclusions
In this large cohort study of adults with COPD, long-term PM2.5 exposure was associated with an increased risk of cardiovascular mortality. These risks were higher among men and among those living in low-SES neighborhoods. We also found evidence of increased risk of cardiovascular events at PM2.5 exposure concentrations lower than the current regulation limit of 12.0 μg/m3 for 1-year mean exposure, suggesting that current regulations are not sufficiently protective of adults with COPD.
Footnotes
Supported by the Kaiser Permanente Northern California Community Benefit grant and the National Institute of Environmental Health Sciences grant R01 ES029557 Particulate Air Pollution, Cardiovascular Events, and Susceptibility Factors.
Author Contributions: Conception and design: S.E.A. and S.S. Acquisition of data: K.D. and J.S. Analysis and interpretation: S.E.A., K.D., N.S.L., S.K.V.D.E., J.S., and S.S. Drafting manuscript: S.E.A. and N.S.L. Revising manuscript: K.D., S.K.V.D.E., J.S. and S.S.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202007-2901OC on March 4, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Patel JG, Coutinho AD, Lunacsek OE, Dalal AA. COPD affects worker productivity and health care costs. Int J Chron Obstruct Pulmon Dis . 2018;13:2301–2311. doi: 10.2147/COPD.S163795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease ControlCOPD Costs. U.S Department of Health and Human Services; 2018. [updated 2018 Feb 21. accessed 2020 Jun 24]. Available from: https://www.cdc.gov/copd/infographics/copd-costs.html.
- 3. Sidney S, Sorel M, Quesenberry CP, Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: kaiser permanente medical care program. Chest . 2005;128:2068–2075. doi: 10.1378/chest.128.4.2068. [DOI] [PubMed] [Google Scholar]
- 4. Morgan AD, Zakeri R, Quint JK. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Ther Adv Respir Dis . 2018;12:1753465817750524. doi: 10.1177/1753465817750524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arbex MA, de Souza Conceição GM, Cendon SP, Arbex FF, Lopes AC, Moysés EP, et al. Urban air pollution and chronic obstructive pulmonary disease-related emergency department visits. J Epidemiol Community Health . 2009;63:777–783. doi: 10.1136/jech.2008.078360. [DOI] [PubMed] [Google Scholar]
- 6. Zhang C, Ding R, Xiao C, Xu Y, Cheng H, Zhu F, et al. Association between air pollution and cardiovascular mortality in Hefei, China: a time-series analysis. Environ Pollut . 2017;229:790–797. doi: 10.1016/j.envpol.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 7. Chen C, Zhu P, Lan L, Zhou L, Liu R, Sun Q, et al. Short-term exposures to PM2.5 and cause-specific mortality of cardiovascular health in China. Environ Res . 2018;161:188–194. doi: 10.1016/j.envres.2017.10.046. [DOI] [PubMed] [Google Scholar]
- 8. Ballester F, Rodríguez P, Iñíguez C, Saez M, Daponte A, Galán I, et al. Air pollution and cardiovascular admissions association in Spain: results within the EMECAS project. J Epidemiol Community Health . 2006;60:328–336. doi: 10.1136/jech.2005.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux AV, et al. American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation . 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 10. Bai L, Shin S, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, et al. Exposure to ambient air pollution and the incidence of congestive heart failure and acute myocardial infarction: a population-based study of 5.1 million Canadian adults living in Ontario. Environ Int . 2019;132:105004. doi: 10.1016/j.envint.2019.105004. [DOI] [PubMed] [Google Scholar]
- 11. Carey IM, Anderson HR, Atkinson RW, Beevers S, Cook DG, Dajnak D, et al. Traffic pollution and the incidence of cardiorespiratory outcomes in an adult cohort in London. Occup Environ Med . 2016;73:849–856. doi: 10.1136/oemed-2015-103531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cesaroni G, Badaloni C, Gariazzo C, Stafoggia M, Sozzi R, Davoli M, et al. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ Health Perspect . 2013;121:324–331. doi: 10.1289/ehp.1205862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen H, Burnett RT, Copes R, Kwong JC, Villeneuve PJ, Goldberg MS, et al. Ambient fine particulate matter and mortality among survivors of myocardial infarction: population-based cohort study. Environ Health Perspect . 2016;124:1421–1428. doi: 10.1289/EHP185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gan WQ, Koehoorn M, Davies HW, Demers PA, Tamburic L, Brauer M. Long-term exposure to traffic-related air pollution and the risk of coronary heart disease hospitalization and mortality. Environ Health Perspect . 2011;119:501–507. doi: 10.1289/ehp.1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim H, Kim J, Kim S, Kang SH, Kim HJ, Kim H, et al. Cardiovascular effects of long-term exposure to air pollution: a population-based study with 900 845 person-years of follow-up. J Am Heart Assoc . 2017;6:e007170. doi: 10.1161/JAHA.117.007170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qiu H, Sun S, Tsang H, Wong CM, Lee RS, Schooling CM, et al. Fine particulate matter exposure and incidence of stroke: a cohort study in Hong Kong. Neurology . 2017;88:1709–1717. doi: 10.1212/WNL.0000000000003903. [DOI] [PubMed] [Google Scholar]
- 17.California Air Resources BoardInhalable particulate matter and health (PM2.5 and PM10). Sacramento, California: California Air Resources Board; 2020. [updated 2020; accessed 2020 Jun 17]. Available from: https://ww2.arb.ca.gov/resources/inhalable-particulate-matter-and-health [Google Scholar]
- 18.United States Environmental Protection AgencyIntegrated Science Assessment (ISA) for particulate matter (final report, 2019). Washington, DC: US EPA; 2019 [Google Scholar]
- 19. Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J. et al. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ Int . 2019;130:104909. doi: 10.1016/j.envint.2019.104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med . 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 21. Iribarren C, Round AD, Lu M, Okin PM, McNulty EJ. Cohort study of ECG left ventricular hypertrophy trajectories: ethnic disparities, associations with cardiovascular outcomes, and clinical utility. J Am Heart Assoc . 2017;6:e004954. doi: 10.1161/JAHA.116.004954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Global Initiative for Chronic Obstructive Lung Disease – GOLD. Fontana, WI: Global Initiative for Chronic Obstructive Lung Disease; 2007. https://goldcopd.org/ [Google Scholar]
- 23.World Health Organization. Obesity: preventing and managing the global epidemic. Geneva, Switzerland: World Health Organization; 2000. [PubMed] [Google Scholar]
- 24. Moffet HH, Adler N, Schillinger D, Ahmed AT, Laraia B, Selby JV. et al. Cohort Profile: the Diabetes Study of Northern California (DISTANCE): objectives and design of a survey follow-up study of social health disparities in a managed care population. Int J Epidemiol . 2009;38:38–47. doi: 10.1093/ije/dyn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA . 2002;287:2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 26. Karter AJ, Ackerson LM, Darbinian JA, D’Agostino RB, Jr, Ferrara A, Liu J, et al. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes registry. Am J Med . 2001;111:1–9. doi: 10.1016/s0002-9343(01)00742-2. [DOI] [PubMed] [Google Scholar]
- 27. Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med . 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 28. Atkinson RW, Carey IM, Kent AJ, van Staa TP, Anderson HR, Cook DG. Long-term exposure to outdoor air pollution and incidence of cardiovascular diseases. Epidemiology . 2013;24:44–53. doi: 10.1097/EDE.0b013e318276ccb8. [DOI] [PubMed] [Google Scholar]
- 29. Hoffmann B, Weinmayr G, Hennig F, Fuks K, Moebus S, Weimar C, et al. Air quality, stroke, and coronary events: results of the heinz nixdorf recall study from the ruhr region. Dtsch Arztebl Int . 2015;112:195–201. doi: 10.3238/arztebl.2015.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosmer DW, Lemeshow S, May S. Applied survival analysis. Hoboken, NJ: Wiley Blackwell; 2011. [Google Scholar]
- 31. Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet . 2020;395:795–808. doi: 10.1016/S0140-6736(19)32008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, et al. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ Health . 2013;12:43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pun VC, Kazemiparkouhi F, Manjourides J, Suh HH. Long-term PM2.5 exposure and respiratory, cancer, and cardiovascular mortality in older US adults. Am J Epidemiol . 2017;186:961–969. doi: 10.1093/aje/kwx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hvidtfeldt UA, Geels C, Sørensen M, Ketzel M, Khan J, Tjønneland A, et al. Long-term residential exposure to PM2.5 constituents and mortality in a Danish cohort. Environ Int . 2019;133:105268. doi: 10.1016/j.envint.2019.105268. [DOI] [PubMed] [Google Scholar]
- 35. Hart JE, Garshick E, Dockery DW, Smith TJ, Ryan L, Laden F. Long-term ambient multipollutant exposures and mortality. Am J Respir Crit Care Med . 2011;183:73–78. doi: 10.1164/rccm.200912-1903OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beelen R, Stafoggia M, Raaschou-Nielsen O, Andersen ZJ, Xun WW, Katsouyanni K, et al. Long-term exposure to air pollution and cardiovascular mortality: an analysis of 22 European cohorts. Epidemiology . 2014;25:368–378. doi: 10.1097/EDE.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 37. Lipsett MJ, Ostro BD, Reynolds P, Goldberg D, Hertz A, Jerrett M, et al. Long-term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am J Respir Crit Care Med . 2011;184:828–835. doi: 10.1164/rccm.201012-2082OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zanobetti A, Bind MA, Schwartz J. Particulate air pollution and survival in a COPD cohort. Environ Health . 2008;7:48. doi: 10.1186/1476-069X-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pope CA, III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA . 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ostro BD, Feng WY, Broadwin R, Malig BJ, Green RS, Lipsett MJ. The impact of components of fine particulate matter on cardiovascular mortality in susceptible subpopulations. Occup Environ Med . 2008;65:750–756. doi: 10.1136/oem.2007.036673. [DOI] [PubMed] [Google Scholar]
- 41. Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. Air pollution and mortality in the Medicare population. N Engl J Med . 2017;376:2513–2522. doi: 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Y, Shi L, Lee M, Liu P, Di Q, Zanobetti A, et al. Long-term exposure to PM2.5 and mortality among older adults in the southeastern US. Epidemiology . 2017;28:207–214. doi: 10.1097/EDE.0000000000000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chi GC, Hajat A, Bird CE, Cullen MR, Griffin BA, Miller KA, et al. Individual and neighborhood socioeconomic status and the association between air pollution and cardiovascular disease. Environ Health Perspect . 2016;124:1840–1847. doi: 10.1289/EHP199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kioumourtzoglou MA, Schwartz J, James P, Dominici F, Zanobetti A. PM2.5 and mortality in 207 US cities: modification by temperature and city characteristics. Epidemiology . 2016;27:221–227. doi: 10.1097/EDE.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dellinger B, Pryor WA, Cueto R, Squadrito GL, Hegde V, Deutsch WA. Role of free radicals in the toxicity of airborne fine particulate matter. Chem Res Toxicol . 2001;14:1371–1377. doi: 10.1021/tx010050x. [DOI] [PubMed] [Google Scholar]
- 46. González-Flecha B. Oxidant mechanisms in response to ambient air particles. Mol Aspects Med . 2004;25:169–182. doi: 10.1016/j.mam.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 47. Møller P, Jacobsen NR, Folkmann JK, Danielsen PH, Mikkelsen L, Hemmingsen JG, et al. Role of oxidative damage in toxicity of particulates. Free Radic Res . 2010;44:1–46. doi: 10.3109/10715760903300691. [DOI] [PubMed] [Google Scholar]
- 48. van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, et al. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)) Am J Respir Crit Care Med . 2001;164:826–830. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- 49. Törnqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, et al. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med . 2007;176:395–400. doi: 10.1164/rccm.200606-872OC. [DOI] [PubMed] [Google Scholar]
- 50. Wang J, Li Y, Zhao P, Tian Y, Liu X, He H, et al. Exposure to air pollution exacerbates inflammation in rats with preexisting COPD. Mediators Inflamm . 2020;2020:4260204. doi: 10.1155/2020/4260204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miller KA, Spalt EW, Gassett AJ, Curl CL, Larson TV, Avol E, et al. Estimating ambient-origin PM2.5 exposure for epidemiology: observations, prediction, and validation using personal sampling in the Multi-Ethnic Study of Atherosclerosis. J Expo Sci Environ Epidemiol . 2019;29:227–237. doi: 10.1038/s41370-018-0053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol . 2001;11:231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 53. Koenig JQ, Mar TF, Allen RW, Jansen K, Lumley T, Sullivan JH, et al. Pulmonary effects of indoor- and outdoor-generated particles in children with asthma. Environ Health Perspect . 2005;113:499–503. doi: 10.1289/ehp.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Meng QY, Turpin BJ, Korn L, Weisel CP, Morandi M, Colome S, et al. Influence of ambient (outdoor) sources on residential indoor and personal PM2.5 concentrations: analyses of RIOPA data. J Expo Anal Environ Epidemiol . 2005;15:17–28. doi: 10.1038/sj.jea.7500378. [DOI] [PubMed] [Google Scholar]
- 55. Ebelt ST, Wilson WE, Brauer M. Exposure to ambient and nonambient components of particulate matter: a comparison of health effects. Epidemiology . 2005;16:396–405. doi: 10.1097/01.ede.0000158918.57071.3e. [DOI] [PubMed] [Google Scholar]


