Abstract
Background: In the past two decades, many advances have been made to our understanding of interstitial lung disease (ILD) and the way we approach its treatment. Despite this, many questions remain unanswered, particularly those related to how the disease and its therapies impact outcomes that are most important to patients. There is currently a lack of guidance on how to best define and incorporate these patient-centered outcomes in ILD research.
Objectives: To summarize the current state of patient-centered outcomes research in ILD, identify gaps in knowledge and research, and highlight opportunities and methods for future patient-centered research agendas in ILD.
Methods: An international interdisciplinary group of experts was assembled. The group identified top patient-centered outcomes in ILD, reviewed available literature for each outcome, highlighted important discoveries and knowledge gaps, and formulated research recommendations.
Results: The committee identified seven themes around patient-centered outcomes as the focus of the statement. After a review of the literature and expert committee discussion, we developed 28 research recommendations.
Conclusions: Patient-centered outcomes are key to ascertaining whether and how ILD and interventions used to treat it affect the way patients feel and function in their daily lives. Ample opportunities exist to conduct additional work dedicated to elevating and incorporating patient-centered outcomes in ILD research.
Keywords: interstitial lung disease, patient-centered outcomes, health-related quality of life
Contents
- Overview
- Definition
- Specific Patient-centered Outcomes
Introduction
- Methods
- Committee Composition
- Definitions and Outcomes of Interest
- Literature Search and Preparation for In-Person Committee Meeting
- In-Person Meeting and Research Recommendations
- Document Development
Definitions
Considerations and Best Practices for Engaging Patients and Other Key Stakeholders in PCOR in ILD
- Discussion of Specific Patient-centered Outcomes and Research Recommendations
- HRQOL
- Symptoms
- Functional Status
- Psychological and Emotional Well-Being
- Hospitalizations and Survival
- Supplemental Oxygen Needs
- Acquisition of Knowledge
Considerations for Use of Digital Technology to Facilitate PCOR in ILD and Immersion with Clinical Practice
Conclusions
Overview
Interstitial lung disease (ILD) is often a life-altering diagnosis associated with chronic symptoms, disruption of patients’ daily lives, and the prospect of shortened survival. Many ILDs have an unpredictable trajectory, making prognostication challenging and reinforcing the need for care plans that align with patients’ goals, values, and preferences. Traditionally, upper-tier endpoints in ILD therapeutic trials have been measures of lung function, which correlate weakly with outcomes that patients value most, such as symptom burden, day-to-day physical functioning, and quality of life (QOL). In the past two decades, major advances in understanding the pathophysiology of ILD have supported the identification of promising therapies; however, none have demonstrated improvement in patient-centered outcomes. There remains much to learn about how ILD itself and the interventions prescribed to treat it affect the outcomes that are valued most by patients. The need for an improved understanding provides the impetus for developing an agenda around patient-centered outcomes research (PCOR) in ILD. This statement describes the work of a multistakeholder committee whose mission was to assess the current state of PCOR in ILD, to identify important gaps in our understanding of patient-centered outcomes in the field, and to provide recommendations for conducting PCOR in patients with ILD.
Definition
We defined patient-centered outcomes and PCOR in ILD as a collection of reliable and valid endpoints that represent what matters most to individual patients in their day-to-day lives. These outcomes may represent how patients feel, or function, or how they view their QOL. Patient-centered outcomes are determined from the patient perspective and are ideally designed and chosen with patient engagement to ensure they reflect and encompass patients’ priorities, preferences, beliefs, values, hopes, and needs. PCOR is research of any design that seeks to understand or measure patient-centered outcomes.
Specific Patient-centered Outcomes
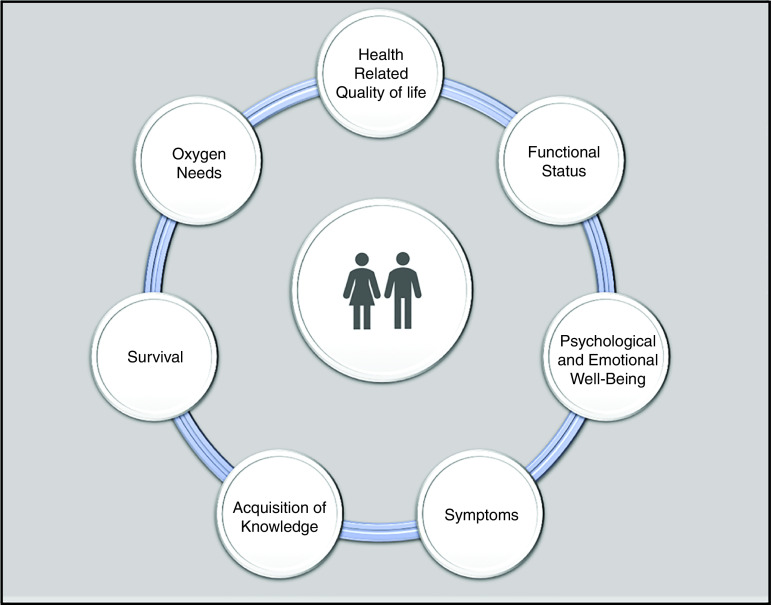
The committee identified several patient-centered outcomes grouped into the following seven themes that form the framework for this statement: 1) health-related QOL (HRQOL), 2) symptoms, 3) psychological and emotional well-being, 4) functional status, 5) oxygen needs, 6) hospitalizations and survival, and 7) acquisition of knowledge (Figure 1). These themes are meant to guide the discussion of PCOR in ILD and provide a starting point to identify gaps and formulate recommendations for research. The committee acknowledges that these themes are not necessarily mutually exclusive (for example, HRQOL may be influenced by a patient’s symptoms or psychological state) but highlighted them distinctly for the purposes of comprehensively evaluating each, highlighting important gaps, and making research recommendations. For each theme, the committee reviewed the current state of research, identified gaps in knowledge, and developed a total of 28 key recommendations as an agenda for future research priorities. These are summarized in Table 1.
Figure 1.
Key patient-centered outcomes of focus in interstitial lung disease.
Table 1.
Key Research Recommendations for PCOR in ILD
| Domain | Key Recommendation |
|---|---|
| HRQOL | • Investigators, sponsors, regulatory agencies, scientific organizations, publishing bodies, and policy makers should come to a consensus to support use of HRQOL PROs in trials. |
| • Studies should use a combination of generic and disease-specific HRQOL questionnaires. These must be carefully chosen on the basis of the context and population included in the study. | |
| • There should be a balance between developing new and better PROs, validating existing newer PROs, and using older, extensively validated ones. | |
| • Development of a new HRQOL measure requires careful consideration and input from a diverse multicultural panel of stakeholders from the outset. | |
| • Studies should include a limited number of VASs to assess major driving components of HRQOL in order to simplify assessments. | |
| • Studies should use composite endpoints including HRQOL, quality-adjusted life-years, or quality-adjusted hospitalization-free survival. | |
| • In trials with disease-modifying drugs aimed at slowing down disease progression, assessing time to deterioration in HRQOL should be considered. | |
| • In RCTs with interventions primarily aimed at the improvement of HRQOL, consider measuring actual values of HRQOL with PROs and comparing these values between intervention groups. | |
| • Studies to determine the clinical utility of the HRQOL PROs for patients with chronic fibrotic ILD are warranted. | |
| Symptoms | • Investigators should define which aspect(s) of which symptom(s) they wish to investigate and how those can be measured appropriately in their study. This will inform the decision of what measurement tool is chosen or whether a new tool needs to be developed. |
| • The choice of which symptom PRO to include in a study should be dependent on the research question and reviewed by multidisciplinary research staff, including patients. One may also consider an HRQOL instrument if the goal is to better understand the impact of the burden of a particular symptom. | |
| • Studies should explore fatigue as an endpoint in ILD. More work is needed to characterize patients’ fatigue in ILD and to develop and validate outcome measures of fatigue in patients with ILD. | |
| • Studies using a mixed-method study design are needed. More work is needed to determine implementation strategies of this study design in the context of a large clinical trial. | |
| • Studies should focus on the development of an individualized approach to symptom measurement based on what an individual patient designates as their most distressing or bothersome symptoms. | |
| Functional status | • When including patient-reported functional status as an outcome in their study, investigators should choose a PRO on the basis of the type of information they want to learn (e.g., someone’s functional capacity from functional performance). |
| • Studies to assess the feasibility, acceptability, and effectiveness of using accelerometers and GPS tracking devices in different patient populations are needed. | |
| • Subjective (questionnaire-based) and objective data are needed if intending to capture a comprehensive understanding of a patient’s functional status. | |
| • More research is needed to identify the most acceptable test of endurance for patients with ILD. | |
| Psychological and emotional well-being | • Studies that measure aspects of psychological and emotional well-being as distinct outcomes instead of predictor variables in models measuring other outcomes (e.g., symptoms, PFTs, HRQOL) are needed. |
| • Studies to assess the validity of currently available measures of depression and anxiety in ILD are needed, and, when appropriate, stakeholder engagement is recommended to modify existing instruments for use in ILD. | |
| • Studies are needed (including summarizing existing and generating new qualitative work) to better understand and determine how to measure other aspects of psychological and emotional well-being (aside from depression and anxiety) that are not currently captured, including coping effects, stress, resilience, satisfaction, interpersonal relationships, and self-esteem. | |
| • Studies of medications targeting improvement in primary psychological endpoints are needed. | |
| Hospitalizations and survival | • Studies are needed that include the following outcomes to capture survival: 1) hospital-free days, 2) disease-related hospitalization, and 3) QUALY. |
| • More work to best define and include a hospitalization endpoint in the context of ILD and its therapies is needed. | |
| Supplemental oxygen needs | • There should be continued efforts to produce standardized and data-driven practice guidelines for supplemental oxygen prescription in ILDs. |
| • Studies should include “oxygen need” as a distinct patient endpoint. More work is needed on how to best define and measure this endpoint. | |
| Acquisition of knowledge | • Studies focused on effects of disease-related education should include an assessment of knowledge as an endpoint. |
| • Further investigation is warranted to determine the best way to deliver disease-related information to patients, with consideration of including stakeholders with expertise in dissemination and implementation science methods to achieve this. |
Definition of abbreviations: GPS = Global Positioning System; HRQOL = health-related quality of life; ILD = interstitial lung disease; PCOR = patient-centered outcomes research; PFTs = pulmonary function tests; PRO = patient-reported outcome; QUALY = quality-adjusted life-years; RCT = randomized controlled trial; VAS = visual analog scale.
Introduction
ILD comprises multiple conditions defined by diffuse inflammation and/or fibrosis of the lung interstitium, typically resulting in physiological restriction and impaired gas exchange. The cause of the ILD may be unknown, be a manifestation of systemic autoimmune disease, or result from an environmental, occupational, avocational, or drug exposure. Regardless of etiology, the majority of patients with ILD experience symptoms including exertional breathlessness, cough, and fatigue. Furthermore, the subset of ILDs that are fibrosing are typically incurable, frequently progressive, and often lethal (1–4). As such, for many patients, ILD is a life-altering condition whose intrusive symptoms rob them of their physical and emotional well-being as they confront the prospect of shortened survival (5–7).
Over the past two decades, investigators have advanced our understanding around the pathogenesis of ILD and have conducted ground-breaking research focused on therapeutic interventions. The discovery of effective therapies for various forms of ILD has enhanced awareness of ILD and generated significant optimism among patients and enthusiasm in the research community (8–13).
PCOR includes all forms of research focused on things that matter most to patients, including symptom frequency and severity, day-to-day physical functioning, emotional well-being, and QOL (14). PCOR might include assessing how a condition or symptom affects patients’ lives or determining whether a therapy has beneficial (or detrimental) impacts on those things most important to patients. Given the recent scientific discoveries in the field, we believe now is the perfect time to take stock of PCOR in ILD (15). This research statement provides an in-depth assessment of several patient-centered outcomes in ILD, reviews the excellent work in PCOR already accomplished, and sets the agenda for future PCOR in ILD.
Methods
Committee Composition
An international committee comprising patients, patient advocates, and experts from various disciplines, including nurses, scientists, and clinician investigators, was assembled by the two co-chairs (K.I.A. and J.J.S.). Potential conflicts of interest were disclosed and managed in accordance with the policies and procedures of the American Thoracic Society. A steering committee of six experts, including the two co-chairs, was identified and charged with overseeing the work of six subcommittees. Each subcommittee was responsible for reviewing relevant literature, presenting findings to the committee at large, and generating a draft manuscript of their findings.
Definitions and Outcomes of Interest
To formulate an initial framework for the statement, every committee member responded to an e-mail survey (developed by the steering committee) that asked for the following: 1) their definition of “patient-centered outcomes research in ILD” and 2) the five patient-centered outcomes they viewed as most important in ILD research. The co-chairs conducted a simple qualitative content analysis to derive a consensus definition of PCOR in ILD (subsequently approved by the committee after review and discussion at the in-person meeting, and upon review of the draft manuscript) and to identify the themes and outcomes the committee would focus on most intensely. Each subcommittee was assigned one or two (of the seven total selected) outcomes.
Literature Search and Preparation for In-Person Committee Meeting
For each of the seven outcome themes, literature searches were conducted with the assistance of a medical librarian. Search results were sent to the subcommittees, who reviewed them, selected relevant publications, and used these to summarize published research on their topic, to identify gaps in understanding, and to make recommendations for advancing PCOR in ILD moving forward. Committee members were encouraged to include additional literature they viewed as relevant to the topic. To promote consistency, each subcommittee used a question guide developed by the co-chairs to review relevant literature (see the online supplement).
In-Person Meeting and Research Recommendations
Each subcommittee presented their findings to the entire committee at an in-person meeting at the American Thoracic Society 2019 International Conference in Dallas. Recommendations for future research were formulated via consensus. Additional topics covered in the discussion, addressed in this statement, and accompanied by research recommendations include general approaches to PCOR and innovative ways of engaging patients with ILD in PCOR research. In accordance with its mission, the committee developed research recommendations, not recommendations for patient care; thus, guideline methodology was not employed to formulate or grade recommendations.
Document Development
Each subcommittee was responsible for writing on their assigned topic and submitting drafts to the co-chairs (K.I.A. and J.J.S.) who reviewed, edited, and collated sections to develop the draft document. The draft was circulated among the committee for comment, review, and approval. Two cycles of review and revision occurred before the final document was submitted for peer review.
Definitions
The committee’s consensus definition for patient-centered outcome research in ILD is “a collection of reliable and valid endpoints for ILD research that represent what matters most to individual patients in their day-to-day lives. These outcomes may represent how patients feel, function, and view their QOL. They are determined from the patient perspective and are ideally designed and chosen with patient engagement to ensure they reflect and encompass patients’ priorities, preferences, beliefs, values, hopes, and needs.”
It is important to recognize the distinction between a patient-centered outcome and a patient-reported outcome (PRO). A patient-centered outcome may or may not be a PRO. For example, patients with ILD value performing various physical activities (e.g., exercising, taking a leisurely walk, or walking to the mailbox to pick up their mail), so a laboratory-based test to measure physical functioning (such as a wearable activity tracker), although not a PRO, is clearly patient centered. The Food and Drug Administration defined a PRO as “any report of the status of a patient’s health that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else” (16).
Considerations and Best Practices for Engaging Patients and Other Key Stakeholders in PCOR in ILD
There are several things to consider when designing a patient-centered research study. The committee unanimously agreed on the utility and importance of engaging patients in all aspects of research, from planning to disseminating results. Patient advisors, integrated at the clinic and organization level, are increasingly viewed as essential members of the research community (17–19). Engaging patients early in the research process helps to determine key patient-centered study outcomes, to identify drivers of and barriers to research participation, and to enable patients to take on central roles in overseeing the conduct of PCOR. Researchers have documented their real-world experiences and recommendations for best practices for engaging patients in research. This includes suggested approaches for patient recruitment, ensuring a diverse patient group of representatives, and providing training materials for patient advisors (20, 21). The PCOR Institute has developed a rubric for investigators who are interested in collaborating with patients in research (22). Other groups, including the Agency for Healthcare Research and Quality, Outcome Measures in Rheumatology, and the European League Against Rheumatism, have provided their experience and recommended best practices for patient engagement in research (18, 23). More research is needed to evaluate the impact of these practices and to identify ways to facilitate uptake of these practices across other disciplines, including ILD (24, 25) (Table 2).
Table 2.
Considerations for Best Practices to Facilitate PCOR and Immersion with Clinical Practice in ILD
| • Patients should be viewed as important stakeholders in ILD research, and patients should be included in the process of research from conception and design through dissemination of results. |
| • We recommend studies to determine the feasibility and optimal use of digital platforms for data collection in ILD research studies and clinical practice. |
| • We recommend studies to determine how the use of digital technology in both research and clinical practice affects patient-centered outcomes |
Definition of abbreviations: ILD = interstitial lung disease; PCOR = patient-centered outcomes research.
The committee stressed the importance of identifying and incorporating all other stakeholders, including individuals or groups who represent, support, or otherwise align with the patient’s voice and their interests. These may be patients’ family members or caregivers, healthcare providers, community members, patient advocacy groups, not-for-profit organizations that support research, and even regulatory agencies such as the Food and Drug Administration and European Medicines Agency (26).
Discussion of Specific Patient-centered Outcomes and Research Recommendations
The seven outcome themes (and associated subthemes) identified by the committee are summarized in Table 3. Below, each theme is addressed in its own subsection of the manuscript, and, for each theme, the following topics are addressed: 1) the current state of research, 2) gaps in knowledge, and 3) recommendations for future research priorities.
Table 3.
Conceptual Framework That Includes the Seven Patient-centered Outcomes and Subcategories of Focus
| Patient-centered Outcome | Subcategories |
|---|---|
| Health-related quality of life | General |
| Disease-specific | |
| Symptoms | Dyspnea |
| Cough | |
| Fatigue | |
| Medication side effects | |
| Psychological and emotional well-being | Depression |
| Anxiety | |
| Grief | |
| Stress | |
| Emotional freedom | |
| Confidence | |
| Functional status | Activities of daily living |
| Capacity to function at home and at work | |
| Ability to leave home to participate in enjoyable activities and/or travel | |
| Oxygen needs | Need for supplemental oxygen prescription and amount of oxygen prescribed |
| Type and availability of oxygen delivery device | |
| Cost and navigating logistics | |
| Hospitalizations and survival | Hospital-free days |
| Acquisition of knowledge | Therapies and clinical trials available |
| Risks and benefits of tests and procedures | |
| Prognosis and level of certainty for what the future holds | |
| Supportive care options |
HRQOL
QOL is defined as an individual’s perception of their position in life, considered in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns (27). Health is one of the important factors affecting QOL, but other factors such as personality traits, beliefs, spiritual and cultural forces/traditions, psychosocial and economic status, social relationships, and living environment also play important roles. HRQOL encompasses all aspects of health or disease (and all associated treatments and evaluations) on overall QOL, but in the literature, HRQOL is often limited to physical and psychological (mental) impacts and social well-being.
Over the last decade, our field has witnessed increasing awareness of the impact of ILD—especially the progressive fibrotic form—on HRQOL (28–32). Patients with ILD have impaired HRQOL in several domains, particularly those that assess physical health. Patients with idiopathic pulmonary fibrosis (IPF; the most common of the progressive fibrosing ILDs) report impaired emotional health and have scores significantly worse than the normative values for the general population (and equal to or greater than those with other chronic illnesses such as heart failure and sleep disorders) on domains that assess depression, anxiety, fatigue, and sleep disturbance (33). Patients with IPF or fibrotic hypersensitivity pneumonitis have shared their perspectives on how their condition impacts other important aspects of their lives, such as family, independence, sexual relations, employment and finances, self-perception and identity, and knowledge and uncertainty (5, 6). Although breathlessness is a strong driver of HRQOL among patients with IPF, variability in HRQOL is not fully explained by indices of breathlessness or tests of pulmonary function, suggesting that measures of HRQOL yield important patient-centered information not captured by routinely used physiological clinical trial endpoints (34). More research is needed to convince stakeholders (regulatory agencies, in particular) of the validity of HRQOL and that it is one of the most important endpoints to assess in therapeutic trials.
Traditionally, therapeutic trials for ILD have employed a combination of generic and respiratory- or disease-specific questionnaires aimed at assessing HRQOL (35, 36). Table 4 provides an overview of some of the measures used in ILD research. Although there is broad consensus that HRQOL is both an important and relevant outcome in ILD research, several factors have shaped the current landscape of pharmaceutical trials, in which HRQOL has yet to be accepted as a top-tier endpoint. One driving factor may be the misguided belief that there is one “ideal” instrument to be used in every study or trial; however, the choice of which instrument(s) to use in a particular instance depends on several things, including the interests of the investigator/research team, the research questions to be answered, and the mechanism (and potential side effects) of the drug/intervention. A general lack (or poor understanding) of the scientific rigor needed to develop, test, and build validity of HRQOL questionnaires has also detracted from enthusiasm. In addition, until recently, there were no ILD-specific questionnaires available, so many questionnaires used for ILD research contained irrelevant questions, and most did not include items capable of capturing cultural differences among subjects. The heterogeneity of phenotypes of patients with ILD and the variable prognoses within the spectrum of ILDs add to the challenge (37). In addition, HRQOL does not necessarily follow a linear path in many patients with progressive ILDs, so statistical analyses require sophistication (38). Impairments in HRQOL may be slight or decline gradually, but periods of steep decline or rapid deterioration (particularly in the final stages of disease) are not uncommon (38).
Table 4.
Patient-reported Outcome Measures Used in ILD
| Patient-reported Outcome Measure | Description | Validated Disease and MCID* | Key Features | References |
|---|---|---|---|---|
| Disease-specific | ||||
| SGRQ | 50-item questionnaire with three domains assessing HRQOL in chronic respiratory disease | IPF (MCID, 4–8) and CTD-ILD (MCID, 4–13) | It is used in many clinical trials and is well validated. It was originally developed for COPD and asthma and is a lengthy, difficult questionnaire. | 72, 164–172 |
| SGRQ-I | IPF-specific version of the original SGRQ; contains 34 items | IPF (MCID, N/A) | Its questions are more relevant for IPF than those of the SGRQ. Its responsiveness and MCID are not known yet. Limited experience. | 173, 174 |
| CAT | Composed of eight symptom items on a 0–5 response scale | IPF (MCID, N/A) and CTD-ILD (MCID, 1–4) | It is a simple and quick instrument that was originally developed for COPD. Limited experience in clinical trials. | 175–178 |
| K-BILD | 15-item health status questionnaire in ILD with three domains | IPF and ILD (MCID, 4–8) | It is brief and was developed in ILD (including patients with IPF). Limited experience in clinical trials, though increasingly used. | 35, 179–182 |
| ATAQ-IPF | 74-item questionnaire with 13 domains assessing HRQOL; designed for IPF | IPF (MCID, N/A) | It covers the patient-identified domains of interest, and is a lengthy, difficult questionnaire. | 183 |
| L-IPF | Modified variant of the ATAQ-IPF, consisting of two modules (symptoms and impacts) | Currently in validation process | It was adapted with feedback from patients and has undergone initial stages of validation testing. | 36 |
| IPF-PROM | Concise questionnaire to assess QOL in IPF | Study is ongoing | It was developed with patients and caregivers. Validation studies are ongoing. | 184, 185 |
| PESaM | Generic and disease-specific module; evaluates patients’ expectations, experiences, and satisfaction with disease-modifying drugs | Currently in validation process | It was developed together with patients with IPF and has undergone initial stages of the validation process. | 186, 187 |
| IPF-PREM | Questionnaire to assess experiences with care delivery | Study is ongoing | It measures experiences of patients. Not available yet. | 188 |
| Domain-specific | ||||
| Dyspnea | ||||
| UCSD-SOBQ | Contains 24 items on a 0–5 response scale assessing dyspnea in the last week | IPF (MCID, 8) and CTD-ILD (MCID, N/A) | It is used in different clinical trials and is valid to assess change in dyspnea in IPF but takes more time to complete compared with other dyspnea measures. It was not originally developed in ILD. | 189–191 |
| mMRC | Consists of one question with five grades for the level of dyspnea | IPF and ILD (MCID, N/A) | It is a quick, easy tool for use in daily practice that relates to disease progression. Its responsiveness in ILD is unclear. It was not originally developed in ILD. | 164, 192, 193 |
| BDI-TDI | BDI (three components of dyspnea on baseline) and TDI (measure changes compared with baseline) | SSc-ILD (TDI MCID, 1.5) | It measures both baseline and change over time. There are few specific instructions included in the instrument. It was not originally developed in ILD. | 194, 195 |
| D-12 | Consists of 12 items on a 0–3 response scale assessing dyspnea | ILD and CTD-ILD (MCID, N/A) | It is a brief, reliable, and valid instrument that was developed using descriptors of dyspnea relevant to patients with a variety of cardiopulmonary diseases, including ILD. Its responsiveness and MCID are not known yet. Limited experience in clinical trials. | 189, 196, 197 |
| Borg scale | Measures level of dyspnea scored on a 0–10 response scale | Not well-validated in ILD | It is useful during 6-min-walk test in daily practice and only measures dyspnea during exertion. It was not originally developed in ILD. | 198 |
| PROMIS Dyspnea Item Banks | Two separate item banks available (functional limitations of dyspnea and severity of dyspnea) | Not well-validated in ILD | It was developed and validated in patients with COPD. Computer adaptive testing is available. Additional pool items are available to assess impact of environment, characteristics of dyspnea, and emotional response to dyspnea (these are not scored but aid in having a more complete understanding of individual respondents). | 199 |
| Cough | ||||
| LCQ | Chronic cough QOL questionnaire with 19 items in three domains | IPF (MCID, 1.3 in chronic cough) | It is reliable, responsive to changes, and easy to complete. Its responsiveness and MCID in ILD are not known yet. It was not originally developed in ILD. | 200–204 |
| CQLQ | Consists of 28 cough-specific questionnaires in six domains | IPF (MCID, 5) | It is a comprehensive, responsive outcome measure with good validity for total score in IPF but not for all domains. It was not originally developed in ILD. | 205 |
| Fatigue | ||||
| FAS | 10-item general fatigue questionnaire | IPF (MCID, N/A) and sarcoidosis (MCID, 4) | It is quick and easy to complete. Limited experience in ILD. It was not originally developed in ILD. | 206–209 |
| MFI | 20-item multidimensional questionnaire with five subscales of fatigue | Sarcoidosis (MCID, N/A) | Limited experience in ILD. | 210 |
| PROMIS fatigue | Asks about experience of fatigue and the impact of fatigue on physical, mental, and social activities | Sarcoidosis (MCID, N/A) | It is available in short form for computer adaptive testing. Limited experience in ILD. | 211 |
| Anxiety/depression | ||||
| HADS | Composed of 14 items assessing anxiety and depression in a general medical population | IPF (MCID, N/A) | It is a reliable screening tool for anxiety and depression and is simple and easy to use. It should not be used as a diagnostic test. It was not originally developed in ILD. | 117, 119, 212, 213 |
| GAD-7 | Seven-item questionnaire assessing generalized anxiety disorder | Not well-validated in ILD yet | It is a valid and efficient tool for screening for generalized anxiety disorder and assessing its severity in clinical practice and research. It is not specific to ILD and was not originally developed in ILD. | 214 |
| Sleep disorders | ||||
| ESS | Consists of eight items on a 0–3 response scale measuring daytime sleepiness | IPF (MCID, N/A) | It is a quick, easy tool for use in daily practice and is well validated and reliable. Limited experience in ILD. It was not originally developed in ILD. | 215–217 |
| SA-SDQ | Consists of 12 items on a 1–5 response scale measuring sleep-related breathing disorders | IPF (MCID, N/A) | It is quick and easy to complete. Limited experience in ILD. It was not originally developed in ILD. | 216, 218 |
| Generic HRQOL questionnaires | ||||
| SF-36 | Generic questionnaire with 36 items measuring functional health and well-being | IPF (MCID, 2–4) and SSc-ILD (MCID, N/A) | It is a widely used and accepted global health assessment measure. It is not specific to ILD and was not originally developed in ILD. | 166, 219–223 |
| EQ-5D-5L | Generic questionnaire assessing health status with five dimensions | ILD (MCID, N/A) | Quality-adjusted life-years can be measured. It was developed to improve the instrument’s sensitivity and to reduce ceiling effects, as compared with the other version. It is not specific to ILD. Limited experience in ILD. It was not originally developed in ILD. | 224–227 |
| PROMIS-29 | Generic measurement of HRQOL across multiple health domains | IPF and other ILD (MCID, N/A) | Still limited use in ILD. | 33, 199, 228–230 |
Definition of abbreviations: ATAQ-IPF = A Tool to Assess Quality of Life in IPF; BDI-TDI = Baseline Dyspnea Index TDI; CAT = COPD Assessment Test; COPD = chronic obstructive pulmonary disease; CQLQ = Cough Quality of Life Questionnaire; CTD-ILD = connective tissue disease–associated ILD; D-12 = Dyspnea-12; EQ-5D-5L = EuroQol-5 Dimension-5 Level; ESS = Epworth Sleepiness Scale; FAS = Fatigue Assessment Scale; GAD-7 = Generalized Anxiety Disorder-7; HADS = Hospital Anxiety and Depression Scale; HRQOL = health-related QOL; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; IPF-PREM = IPF Patient-reported Experience Measure; IPF-PROM = IPF Patient-reported Outcome Measure; K-BILD = King’s Brief Interstitial Lung Disease health status questionnaire; LCQ = Leicester Cough Questionnaire; L-IPF = Living with IPF; MCID = minimal clinically important difference; MFI = Multidimensional Fatigue Inventory; mMRC = modified Medical Research Council dyspnea scale; N/A = not applicable; PESaM = Patient Experiences and Satisfaction with Medication; PROMIS = Patient-reported Outcome Measurement Information System; QOL = quality of life; SA-SDQ = sleep apnea scale of sleep disorders questionnaire; SF-36 = Short Form-36; SGRQ = St. George’s Respiratory Questionnaire; SGRQ-I = IPF-specific version of the SGRQ; SSc-ILD = systemic sclerosis–related ILD; TDI = Transition Dyspnea Index; UCSD-SOBQ = University of California San Diego Shortness of Breath Questionnaire.
The MCID varies depending on the cohort and research.
Ideally, a HRQOL measure should capture 80% of determinants of HRQOL at the group level, and the minimal clinically important difference for its scores in ILD should be rigorously established. The committee suggests that, rather than measuring HRQOL at any one time, time to decline in HRQOL (as defined by any selected change score displayed by a cumulative distribution function plot) may hold more merit. This is particularly relevant in the progressive fibrotic ILDs, in which combination therapies are being studied and effect size margins are small. In other forms of ILD, in which improvement or reversal of disease is achievable, changes in actual values of HRQOL scores could be more easily assessed and evaluated. The same may hold true for interventions directly aimed at improving HRQOL (e.g., ambulatory oxygen) (39).
We recommend the following:
To advance the field, investigators, sponsors, regulatory agencies, scientific organizations, publishing bodies, and policymakers should come to a consensus to support use of HRQOL PROs in trials. This includes more established PROs as well as newly developed, simpler PROs when deemed to be appropriate for the target population. Even when used as an exploratory endpoint, these data will likely generate hypotheses and may contribute to the validation and acceptance of these HRQOL PROs in ILD.
Using a combination of generic and disease-specific HRQOL questionnaires is advocated, and these must be carefully chosen on the basis of the context and population included in the study.
To optimize development and use of HRQOL PROs, a balance should be sought between developing new and better PROs, validating existing newer PROs, and using older, extensively validated ones.
Development of a new HRQOL measure requires careful consideration and input from a diverse multicultural panel of stakeholders from the outset.
One possible strategy for simplifying assessments is to include a limited number of visual analog scales to assess major driving components of HRQOL.
Composite endpoints, including HRQOL, quality-adjusted life-years, or quality-adjusted hospitalization-free survival, should be explored.
In trials with disease-modifying drugs aimed at slowing disease progression, assessing time to deterioration in HRQOL should be considered.
In randomized controlled trials with interventions primarily aimed at the improvement of HRQOL, measuring actual values of HRQOL with PROs and comparing these values between intervention groups may be the best method of endpoint assessment.
Further studies to determine the utility in clinical practice of an HRQOL PRO for patients with ILD are warranted.
Symptoms
A patient’s experience of physical symptoms largely shapes their experience of living with ILD. Not uncommonly, symptoms prompt patients to seek evaluation, as symptoms affect their psychological and emotional well-being and QOL. There are a myriad of symptoms associated with ILD, some related to the disease itself and others related to therapy side effects. Many symptoms go unmeasured or unreported in research studies unless they are systematically captured as adverse events. Here, we focus on dyspnea, cough, and fatigue, which are three of the most common symptoms in ILD.
Dyspnea (also commonly referred to as breathlessness or shortness of breath) is the most common symptom of ILD (40–42) and is a strong driver of QOL (7). The emotional and psychological components of dyspnea (i.e., fear, frustration, and anxiety) may be equally or more detrimental to some patients than the physical constraints imposed by dyspnea. Qualitative and mixed-methods studies have shown that patients with ILD describe their dyspnea as gasping, feeling out of breath, or breathing that is shallow or requires effort (43–45). Several things make the measurement of dyspnea challenging, including its multiple dimensions, its variability and dependence on the amount of exertion, and the likelihood that the use of supplemental oxygen confounds its measurement (46). Dyspnea should be included as an endpoint in ILD research. It is important to consider the distinction between the perception of the intensity of breathlessness, the emotional response to the breathlessness, and the impact of breathlessness on activity (47). Several dyspnea-specific scales are available, with each scale measuring different dimensions of the symptom (Table 4).
Cough is a common and often debilitating symptom in patients with ILD. Cough may be a result of the disease itself, a side effect of a therapy, or a comorbid condition (48–50). There are numerous physical, psychological, and social consequences of cough that may influence HRQOL (51, 52). Although identified as an important symptom by patients, cough has generally been excluded as an endpoint in ILD therapy trials (53), either because the mechanism of the intervention is not expected to affect cough or because it is difficult to determine whether cough is due to the ILD or a comorbid condition. Some interventions have targeted cough (54), and there are several questionnaires available that focus on cough should researchers choose to include this as an endpoint in their study (Table 4).
Although prevalent among patients with ILD, fatigue has received little attention as a potential study outcome (55, 56). Fatigue can affect QOL, constrain daily activities, limit productivity, impair physical functioning, and precipitate feelings of depression and guilt (57–60). As with cough, fatigue may be caused by ILD or any of several other contributors (e.g., sleep-disordered breathing, mood disturbance, comorbid physical conditions, and medications used to treat the ILD), and this makes it a more challenging ILD-related endpoint (61). Patients and researchers alike may have difficulty distinguishing breathlessness from fatigue, as do many currently available dyspnea scales. We currently lack an in-depth understanding of fatigue in patients with ILD, which limits our ability to advocate for its inclusion as an endpoint in trials. Investigations are needed to inform understanding of the experiences of fatigue in patients living with ILD, to define the correlates of fatigue in ILD, and to identify optimal management strategies (62).
We recommend the following:
That investigators define which aspect(s) of which symptom(s) they wish to investigate and how those can be measured appropriately in their study. For example, is it a qualitative aspect? Frequency? Severity? Is it the limitations the symptom imposes? This will inform the decision of what measurement instrument is chosen or whether a new measure needs to be developed.
The choice of which symptom PRO to include depends on the research question, and candidate instruments should be reviewed by multidisciplinary research staff, including patients. There are several available PROs for dyspnea and cough used in ILD, and these may be appropriate for use in the design of a research study (Table 4). One may also consider an HRQOL instrument if the goal is to better understand the impact of the burden of a particular symptom.
Fatigue should be explored as an endpoint in appropriate ILD studies. More work is needed to characterize patients’ fatigue in ILD and to develop and validate outcome measures of fatigue in patients with ILD.
Consider a mixed-method study design with a qualitative component that will capture individual patient perspectives of a symptom and help to understand why the change in the symptom score occurred. More work is needed to determine implementation strategies of this study design in the context of a large clinical trial, and this will require the involvement of multidisciplinary stakeholders and those with experience with this methodology and data analysis.
More work is needed to develop an individualized approach to symptom measurement in a research study whereby the symptoms measured are determined at study initiation on the basis of what an individual patient designates as their most distressing or bothersome symptoms.
Functional Status
Functional status is generally defined as the ability to accomplish routine activities of daily living required to maintain health and well-being. Patients greatly value being able to do the things they want or need to do, and in the way they desire (e.g., at a certain pace, without needing to take the time to rest). Even patients with mild or subclinical ILD may experience limitations in their performance of activities of daily living and their physical activity (63), which typically worsen as ILD progresses. Limitations in functional status affect QOL and are strongly associated with mortality.
Functional status has been included as an endpoint in several ILD studies (64–69). There are several questionnaires that assess functional status, with many having been studied in patients with fibrotic ILD. Most functional status questionnaires attempt to distinguish functional capacity (someone’s peak ability) from functional performance (what someone regularly does) (70). Many dyspnea and QOL questionnaires include functional status as a distinct domain (e.g., the physical functioning domain from the Short Form-36, the activity component of the St. George’s Respiratory Questionnaire, or the activity domain of the King’s Brief ILD health status questionnaire) (35, 71, 72).
There are three important challenges in assessing functional status by patient report. First, reduced functional status can result from derangement in any number of different body systems. Second, a patient’s expectations for their functional status may change over time. For example, older, less physically active adults with chronic lung disease are less likely to report impairment in their activities compared with younger adults with chronic disease (67). Expectations are also altered by the presence of comorbid conditions, the use of medications with adverse effects, and patients’ ability or desire to adapt to changes in disease status and how they define or conceptualize functional status. Third, none of the currently available functional status questionnaires were derived specifically for patients with ILD, and, therefore, they may be suboptimal for use in these patients.
Aside from assessing functional status by patient report, there are patient-worn devices and laboratory tests at various stages of use. Portable devices that measure functional status in daily living situations are growing in popularity. They offer a well-rounded assessment of physical activity and performance in “real-world” situations (73) and may have the potential to motivate patients by stimulating physical activity, thus, potentially, improving QOL (74). There are several devices available, and many have been studied in patients with chronic lung diseases, such as chronic obstructive pulmonary disease (COPD) (75). The experience with these devices in patients with ILD is still limited (76).
Accelerometers—portable electronic devices worn on a patient’s wrist, thigh, or waist to detect acceleration along multiple axes (77, 78)—are reported to be well tolerated by patients with ILD and have demonstrated feasibility in small studies that included patients with IPF (73, 79, 80). Accelerometers measure step count, intensity of activity (i.e., metabolic equivalents), energy expenditure, and time spent in sedentary activity (81–84). Pedometers are less expensive devices that measure step count. They frequently underestimate the number of steps in patients with moderate to severe lung disease who have slow walking speed (85–87). Global Positioning System (GPS) trackers, or satellite-based global navigation systems, capture a person’s precise location at any point on earth (88), giving additional information on where and when people are moving. GPS tracking also allows quantification of other psychosocial and geographic factors that inform physical activity and functioning aside from disease severity (i.e., a patient’s “activity space”) (89).
Despite the multiple options for monitoring functional status in daily living situations, operational challenges and considerations remain. For example, these devices are effort and motivation dependent, and there are privacy issues for some of these trackers. The use of these devices may be more challenging for those who have difficulty using technology. Although promoted as patient centered (because they capture information valued by patients), monitoring devices do not provide the patient perspective on physical function. Despite these limitations, these devices have significant potential for providing real-world data on functional status in patients with ILD.
Laboratory-based tests of functional capacity are performed in a healthcare setting because they require specialized equipment and/or personnel. These objective studies offer some understanding of the effects of an intervention on functional status (90). These tests measure aspects of functional status, including endurance, strength, and physical performance. Endurance has been measured using a standardized walk test (usually 6 min in duration) (91), stair/step climbing or repeated sit-to-stand tests (92–94). Cardiopulmonary tests provide a measure of maximal endurance (i.e., functional capacity), with the added advantage of providing detailed information on causes of functional limitation (95, 96). Strength is typically measured using either grip strength (97, 98) or quadriceps force (98, 99). General physical performance can also be measured using four-meter gait speed (100, 101) and up-and-go tests (102).
Drawbacks of laboratory-based tests include the requirement for patient travel, the need for specialized equipment and environments, and/or the need for highly trained personnel. There is also some debate as to whether or not the current standard walk test (6-min-walk test) is a true test of endurance in ILD (103–105). In addition, most laboratory-based tests measure functional capacity, not functional performance, often making it difficult to translate results to day-to-day living.
We recommend the following:
When including patient-reported functional status as an outcome in their study, investigators should choose a PRO on the basis of the type of information they want to learn (e.g., someone’s functional capacity from functional performance).
More research is needed to assess the feasibility and acceptability of using accelerometers and GPS tracking devices in different patient populations and to assess the correlation of the information output from these devices with other patient-centered outcomes.
In any one study, both subjective (questionnaire-based) and objective data are needed if the intent is to capture a comprehensive understanding of a patient’s functional status.
More research is needed to identify the most acceptable test of endurance for patients with ILD.
Psychological and Emotional Well-Being
The committee considered this outcome to represent the collective impact of disease on the emotional well-being and psyche of an individual patient. Psychological and emotional well-being includes, but is not limited to, coping, stress, grief, resilience, satisfaction, depression, anxiety, interpersonal relationships, and self-esteem. Interpersonal relationships may include such aspects as those with family and friends or those related to a person’s sexuality (106–108). Although there has been some attention paid to the assessment of psychological and emotional well-being in ILD, for the most part, it has been narrowly focused around the clinical concepts of depression and anxiety. Depression is a well-documented comorbidity in ILD (109–113). The use of depression and anxiety scales has largely been to assess the prevalence of the problem and the relationship with disease diagnosis or its severity (114). For example, correlations have been reported between depression and anxiety and various factors, including dyspnea, pain, sleep quality, functional status, and FVC (109, 115, 116). Depression and anxiety also have a negative effect on HRQOL in ILD (117–119).
PROs assessing anxiety and depression have been included as endpoints in a small number of ILD studies; most are observational and assess the effects of multidisciplinary palliative interventions for advanced disease or the impact of pulmonary rehabilitation (101, 120–122). Pharmaceutical trials in ILD have largely excluded this endpoint (unless in the context of an HRQOL domain). The most commonly used PROs for psychological and emotional well-being in ILD are documented in Table 4. Validity testing of these measures has not been widely performed in the ILD-specific patient population.
Although anxiety and depression are important, other outcomes also deserve mention; for example, grief is a distinct manifestation that is present in many patients with IPF (123). The impact of social support on psychological well-being has been preliminarily studied (124). Small observational studies in ILD have reported on general well-being indices, patient satisfaction, and perceived stress in the context of a mindfulness-based stress reduction program (125–127). Although this is a terrific start, more research is needed. Targeted qualitative studies integrating more comprehensive assessments of psychological and emotional well-being are warranted. There are limited metrics to measure the global emotional impact of living with ILD. There is also a dearth of literature focused on the positive aspects of adaptation, resilience, and coping (128).
We recommend the following:
Consider measuring aspects of psychological and emotional well-being as distinct outcomes in studies instead of only as predictor variables in models measuring other outcomes (e.g., symptoms, pulmonary function tests, and HRQOL).
More studies are needed to assess the validity of currently available measures of depression and anxiety in ILD and when it is appropriate to use stakeholder engagement to modify existing instruments for use in ILD. If a strong need to generate a new measure of psychological and emotional well-being is identified, this should be designed with stakeholder engagement and developed using baseline qualitative studies that include patients with ILD.
More research is needed (including summarizing existing and generating new qualitative work) to better understand and determine how to measure other aspects of psychological and emotional well-being (aside from depression and anxiety) that are not currently captured, including coping effects, stress, resilience, satisfaction, interpersonal relationships, and self-esteem.
Given the significant psychological impact of ILD on patients, pharmaceutical trials studying medications (aside from ILD disease-modifying medications) targeting improvement in primary psychological endpoints are warranted.
Hospitalizations and Survival
Several observational studies and therapeutic trials have assessed survival as an outcome. These include retrospective studies from the late 1990s that led to significant advances in the field’s understanding of ILD and, in particular, how radiopathological classification affects survival (129, 130); observational studies aimed at identifying individual or combinations of variables that predicted time to death; and late-phase trials of drugs for IPF that included progression-free survival as a primary endpoint (131, 132).
Length of survival is an outcome that holds meaning to almost every patient, but many value quality over duration. So, for many patients with ILD, the relevant question is not “how long will I live with ILD?” but “how long will I be able to live well with ILD?” Of course, “living well” depends entirely on a person’s circumstance, values, and judgments.
The committee recognized the merits of progression-free survival as an important outcome but viewed hospitalization as a serious threat to living well with ILD. Large therapeutic trials for patients with ILD have generally either included an “acute exacerbation” secondary endpoint or no hospitalization-related endpoint at all (8, 9, 12, 13, 133). Few secondary analyses have sought to primarily address the question of respiratory-related and non–respiratory-related hospitalizations (134). Studying this endpoint is challenging, as there are multiple reasons for hospitalization, including an exacerbation of the disease itself, a comorbidity of the disease (such as pulmonary hypertension), a medication-related side effect, or a psychosocial issue. The optimal way to define a hospitalization in ILD and incorporate this outcome into an intervention trial as a high-tier endpoint remains an important area of investigation.
We recommend the following:
The following outcomes are potentially capable of capturing aspects of hospitalizations and/or survival in the most patient-centered way: 1) hospital-free days, 2) disease-related hospitalization, and 3) quality-adjusted life-years.
More work is needed to best define and include a hospitalization endpoint in the context of ILD and its therapies.
Supplemental Oxygen Needs
The committee chose to highlight supplemental oxygen needs as a separate outcome given the multiple emotional, physical, and economic complexities of oxygen use and the potential significance that the prescription of oxygen has on patients’ emotional well-being and QOL. The committee considers oxygen as a prescription medication. Because of the lack of controlled long-term trials of supplemental oxygen in ILD, it has historically been difficult to provide guidance around appropriate timing and criteria for oxygen prescription, and most recommendations have been extrapolated from the COPD literature (1, 135–138). Recently, groups of experts have formulated recommendations for oxygen to ILD and put forth initial guidelines on the use of home oxygen therapy, while simultaneously highlighting the need for additional research to guide clinical practice (139, 140).
Some healthcare professionals may be more inclined to prescribe oxygen to their patients with ILD for symptomatic relief alone (141); however, it is also important to understand that the initiation of oxygen can have potential detrimental effects on both patients and their families (142). Oxygen is frequently viewed as a marker of disease progression, and, for some, it brings with it the stigma of being sick. As oxygen needs increase, the patient’s ability to live “carefree” declines, benchmarking a significant transition in the patient’s disease journey (143).
Patients have described the multiple potential benefits of oxygen, including improved physical symptoms, increased functional capacity, relief of psychological symptoms, and improved QOL (144, 145). Despite the nearly ubiquitous use of supplemental oxygen among patients with fibrotic ILD, there are only limited data to suggest that these benefits offset the burdens that oxygen users experience related to the psychosocial ramifications, potential mechanical problems, access to the correct type of delivery device, economic considerations, and lack of education about the equipment at the patient, informal caregiver, and provider levels (39, 146–148). The number of patients using oxygen in any study is characterized in patient demographics. To our knowledge, the need for supplemental oxygen (as in a new prescription for oxygen during the time course of the study) as an endpoint in a therapeutic or longitudinal study has yet to be used. This may be in part because guidelines for oxygen prescription are not yet widely established.
We recommend the following:
Continued efforts to produce standardized and data-driven practice guidelines for supplemental oxygen prescription in ILDs.
When designing a longitudinal study or therapeutic trial, consider inclusion of “oxygen need” as a patient-centered endpoint in the trial. More work is needed on how to best define and measure this endpoint.
Acquisition of Knowledge
Although knowledge acquisition of ILDs in the lay community has increased substantially over the last two decades, ILD remains a relatively enigmatic condition compared with asthma and COPD. Like people with any medical condition, patients with ILD value an improved understanding of why they developed ILD, where in the lung ILD occurs, which tests are required to make the diagnosis and to follow the condition over time, what treatments are available and why one therapeutic approach might be chosen over another, and many more aspects about their potentially life-altering condition (149–151).
Investigators have conducted studies to assess the specific informational/educational needs of patients with ILD (specifically those with IPF) and in what formats and settings the knowledge could/should be delivered (152, 153). The subcommittee recognized the importance of making sure patients with ILD (and their caregivers) are knowledgeable about all aspects of ILD and found a paucity of research on the effects of the acquisition of knowledge on outcomes of importance to patients with ILD. Three studies have tested the effect of education and support programming, designed for dyads of patients with IPF and their caregivers, on various behavioral health outcomes, including stress, anxiety, and HRQOL (154–156). Although disease-related education was provided in these studies, only one focused on the acquisition of knowledge, with an assessment of knowledge gained from before to after program completion. The substantial challenges with diagnostic ambiguity and paucity of robust evidence to guide management or prognosticate are barriers to providing sufficient education to patients living with ILD. There is also limited experience determining the most effective and patient-centered way of delivering this information.
We recommend the following:
Moving forward, for investigations focusing on the effects of disease-related education, the committee strongly believed that some assessment of knowledge should be performed.
Further investigation is warranted to determine the best way to deliver disease-related information to patients, with consideration of including stakeholders with expertise in dissemination and implementation science methods to achieve this.
Considerations for Use of Digital Technology to Facilitate PCOR In ILD and Immersion with Clinical Practice
As we outlined in detail in this statement, use of PROs is one method of measuring and reporting patient-centered outcomes. New technologies may facilitate different ways of implementing PROs in both research and clinical practice. It is important to recognize the potential role of digital technology in the collection of patient-reported data now more than ever. The recent coronavirus disease (COVID-19) pandemic and need for social distancing (157) has increased the need to establish remote avenues of data collection and study visits. This holds true for both research studies and clinical office visits. The committee advocates that the value of PROs extends beyond research studies to clinical practice. There is value in following outcomes in real-world practice simultaneously with our research efforts to formulate data-driven patient-centered recommendations for our patients.
Given the amount of information needed to be collected during any visit and being mindful of the burden that this places on the patient, we should strongly consider how to achieve this using simpler methods of detecting change in outcomes. This information may be gathered using a shorter questionnaire or through a visual analog scale. An example of an innovative way to assess health status with the shortest possible PRO is computer adaptive testing (CAT). With CAT, questions are tailored to the individual patient. The questions are drawn from an item response theory–based item bank (a large set of questions measuring the same construct, e.g., fatigue). The questions are ranked in order of difficulty. With each response, the computer refines a person’s score and determines what the next relevant (most informative) question would be. Irrelevant questions are skipped, so the number of questions is kept to a minimum (4–10 items), without losing precision (158). Some preliminary studies outside of ILD have evaluated the use of PRO collection using CAT questionnaires before clinic visits with positive results (159, 160); however, more work is needed, and, to our knowledge, this has not yet been tested in the ILD patient population specifically.
Another way of implementing digital technologies is to administer electronic PROs. Instead of spending valuable time in the clinic on completing questionnaires, patients can do this at home online or in the clinic on a computer or tablet before the consultation. A potential advantage in ILD is that this minimizes the number of in-person visits that are needed, something that may benefit those with advanced and functionally limiting forms of the disease and that is also of value in the current age of physical distancing (161). This will also allow patients to self-evaluate the effect of changes in management. In addition to the option for completing PROs electronically from home, there is also opportunity to collect clinical data such as home spirometry and pulse oximetry/oxygen saturation values. Home monitoring of patients with IPF using a combination of spirometry and PROs has been piloted, with promising results of feasibility (162). More work is needed in the broader ILD patient population, including larger samples with varying age, education levels, and socioeconomic backgrounds to determine widespread feasibility. In addition, with the rise of telehealth visits and patient portals (in which patients have access to their own electronic health records), there is opportunity to evaluate this approach in real-world settings.
In addition to addressing the feasibility of using these technologies and platforms for the collection of patient-centered outcome data in ILD, there is little research on how using digital methods of data collection in ILD affect patient-centered outcomes (163). Over the past few years, there are many initiatives and applications catering to digital home assessment for research and clinical care but very little research about their impact on patient well-being, medical outcomes, or implication for healthcare consumption, and economic burden. We have little understanding about which of these platforms is best suited for patients with ILD and what the optimal design should be. More research is needed to address these gaps (Table 2).
Conclusions
ILDs are often chronic and debilitating conditions that affect many aspects of a person’s life. There has been great progress made in our understanding of disease pathophysiology and the identification of therapeutic targets; however, additional research is needed to advance our understanding of how ILD affects patients on a day-to-day basis and to determine how best to measure those effects. This document provides an overview of several common patient-centered outcomes identified by our interdisciplinary expert committee (including patients). This overview should not be seen as an all-inclusive list. It is up to us as researchers and clinicians to continue looking at the patients as individuals who have their own set of goals, values, and beliefs. Listening to patients, hearing what they say, and working together has set a strong foundation for patient-centered research in ILD. Moving forward in partnership, our future interventions must continue to address the outcomes that are most important to those who will potentially benefit from them—patients.
Acknowledgments
This official research statement was prepared by an ad hoc subcommittee of the ATS Assembly on Clinical Problems.
Members of the subcommittee are as follows:
Kerri I. Aronson, M.D., M.S. (Co-Chair)1
Jeffrey J. Swigris, D.O., M.S. (Co-Chair)2
Sabrina Bajwah, Ph.D.3
Pauline Bianchi, R.N., B.S.N.4
Tamera J. Corte, M.B. B.S., Ph.D.5,6
Sonye K. Danoff, M.D., Ph.D.7
Joyce S. Lee, M.D.8
Kathleen O. Lindell, R.N, Ph.D.9
Toby M. Maher, M.D., Ph.D.10,11
Fernando J. Martinez, M.D., M.S.1
Paula M. Meek, R.N, Ph.D.12
Ganesh Raghu, M.D.13
Glenda Rouland14
Rick Rudell14
Anne-Marie Russell, Ph.D., M.Sc.15,16
Christopher J. Ryerson, M.D., M.A.S.17
Monika M. Safford, M.D.18
Jamie S. Sheth, M.D.19
Atsushi Suzuki, M.D.20
Marlies S. Wijsenbeek, M.D., Ph.D.21
1Division of Pulmonary and Critical Care Medicine and 18Division of General Internal Medicine, Department of Medicine, Weill Cornell Medicine Cornell University, New York, New York; 2Interstitial Lung Disease Program, National Jewish Health, Denver, Colorado; 3King’s College London, London, United Kingdom; 4Research and Development and 14Patient Ambassador Program, Pulmonary Fibrosis Foundation, Chicago, Illinois; 5Department of Respiratory Medicine, Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; 6Medical School, University of Sydney, Sydney, New South Wales, Australia; 7Division of Pulmonary and Critical Care Medicine, Department of Medicine, School of Medicine, John Hopkins University, Baltimore, Maryland; 8Department of Medicine and Division of Pulmonary Sciences and Critical Care Medicine, University of Colorado Denver, Aurora, Colorado; 9College of Nursing, Medical University of South Carolina, Charleston, South Carolina; 10Inflammation, Repair, and Development Section, National Heart and Lung Institute, Imperial College London, London, United Kingdom; 11National Institute for Health Research Respiratory Clinical Research Facility, Royal Brompton Hospital, London, United Kingdom; 12College of Nursing, University of Utah, Salt Lake City, Utah; 13Center for Interstitial Lung Diseases, Department of Medicine and Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington; 15College of Medicine and Health, University of Exeter, Exeter, United Kingdom; 16Imperial College Healthcare National Health Service Trust, London, United Kingdom; 17Department of Medicine, University of British Columbia, Vancouver, British Columbia, Canada; 19Division of Pulmonary and Critical Care Medicine, University of Michigan, Ann Arbor, Michigan; 20Department of Respiratory Medicine, Graduate School of Medicine, Nagoya University, Nagoya, Japan; and 21Department of Respiratory Medicine, Erasmus Medical Center, Rotterdam, the Netherlands
Acknowledgment
The authors thank the patients, Rick Rudell and Glenda Rouland, for their participation and invaluable contributions in the development of this research statement. The authors thank Drew N. Wright, Assistant Librarian at the Samuel J. Wood Library, Weill Cornell Medical College, for his assistance with the literature reviews for this research statement.
Footnotes
An Executive Summary of this document is available at https://www.atsjournals.org/doi/suppl/10.1164/rccm.202105-1193ST.
This official research statement of the American Thoracic Society was approved September 2021
This is a corrected version of the document; it was updated on September 13, 2021. See erratum: Am J Respir Care Med 2021;204:616; https://www.atsjournals.org/doi/full/10.1164/rccm.v204erratum1.
This statement is dedicated to the memory of Mrs. Glenda Rouland.
This document has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author Disclosures: P.B. was an employee of Veracyte. T.J.C. served on an advisory committee for Ad Alta, Bristol-Myers Squibb, Boehringer Ingelheim, and Roche; served as a consultant for Bristol-Myers Squibb, Boehringer Ingelheim, Promedior, and Roche; and received research support from Actelion, Avalyn Pharma, Biogen, Bristol-Myers Squibb, Boehringer Ingelheim, Galapagos, and Roche. S.K.D. served on an advisory committee for Boehringer Ingelheim; served as a consultant for Boehringer Ingelheim; served on a data safety and monitoring board for Galapagos and Galecto; received research support from Boehringer Ingelheim and Genentech/Roche; and was an employee of Pulmonary Fibrosis Foundation. J.S.L. served on an advisory committee for Boehringer Ingelheim, Celgene, Galapagos, and the Pulmonary Fibrosis Foundation; served as a consultant for Bonac, Eleven P15, and United Therapeutics; and received research support from Boehringer Ingelheim and the National Institutes of Health. T.M.M. served on an advisory committee for Boehringer Ingelheim, GlaxoSmithKline, Roche, and Veracyte; served as a consultant for Blade Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Galapagos, Galecto, IQVIA, Pilant, Respivant, Roche, and Theravance; served on a data safety and monitoring board for AstraZeneca, Fibrogen, and United Therapeutics; served as a speaker for Boehringer Ingelheim, Galapagos, and Roche; and received research support from AstraZeneca and GlaxoSmithKline. F.J.M. served on an advisory committee for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Bridge Biotherapeutics, CSL Behring, DevPro, Gala, GlaxoSmithKline, IQVIA, Novartis, Polarean, Sanofi, Shionogi, Teva, Veracyte, and Zambon; served as a consultant for AbbVie, Afferent/Merck, AstraZeneca, Biogen, Boehringer Ingelheim, Bridge Biotherapeutics, Bristol-Myers Squibb, Chiesi, Genentech, Gilead, GlaxoSmithKline, Nitto, Patara/Respivent, Promedior, ProTerrixBio, Raziel, Sanofi, Sunovion, Teva, twoXR, United Therapeutics, and Verona; served on a data safety and monitoring board for Abbvie, Biogen, Boehringer Ingelheim, GlaxoSmithKline, Medtronic, and NACE/Haymarket; served as a speaker for Academy for Continuing Healthcare Learning, AstraZeneca, Boehringer Ingelheim, Brooklyn Methodist Hospital, Canadian Respiratory Network, Chiesi, CME Outfitters, Dartmouth University, France Foundation, Integritas, Integrity Communication, MD Magazine, Miller Communications, National Association for Continuing Education, New York University, PeerView, Physician Education Resource, Projects in Knowledge, Rare Diseases Healthcare Communications, Rockpointe, Vindico, and WebMD/MedScape; received research support from Afferent/Merck, AstraZeneca, Bayer, Biogen, Boehringer Ingelheim, Chiesi, Gilead, GlaxoSmithKline, National Institutes of Health, Nitto, Patara/Respivent, Promedior, ProMetic, Sanofi/Regeneron, Veracyte, Verona, and Zambon; and received author royalties from UpToDate. G. Raghu served as a consultant for Belleorphan, Biogen, Bristol-Myers Squibb, Boehringer Ingelheim, Fibrogen, Genentech, Gilead, Nitto, Novartis, Promedior, Pure Tech Health, Respivant, Roche, Sanofi, United Therapeutics, Veracyte, and Zambon; and served on a data and safety monitoring board for Avalyn. A.-M.R. served on an advisory committee for Boehringer Ingelheim; served as a consultant for Roche, Interstitial Lung Disease Interdisciplinary Network, and the Irish Lung Fibrosis Association; served as a speaker for Boehringer Ingelheim, Roche, and the Irish Lung Fibrosis Association; and received research support from Boehringer Ingelheim, National Institute of Health Research, and Pulmonary Fibrosis Trust UK. C.J.R. served as a consultant for Boehringer Ingelheim, Roche, and Veracyte; served as a speaker for Boehringer Ingelheim and Roche; and received research support from Boehringer Ingelheim and Galapagos. M.M.S. received research support from Amgen. J.S.S. served on an advisory committee for Boehringer Ingelheim and Genentech; served as a consultant for Boehringer Ingelheim; served as a speaker for Boehringer Ingelheim and Genentech; received research support from Boehringer Ingelheim and Genentech; and has an intellectual property/patent unsold with Live Fully, Inc. M.S.W. served on an advisory committee for Boehringer Ingelheim, Bristol-Myers Squibb, Galapagos, Galecto, Novartis, and Roche; served as a consultant for Respivant; served on a data safety and monitoring board for Galapagos and Savara; served as a speaker for Boehringer Ingelheim, Galapagos, and Roche; and received research support from Boehringer Ingelheim, Galapagos, and Roche. K.I.A., J.J.S., S.B., K.O.L., P.M.M., G. Rouland, R.R., and A.S. reported no commercial or relevant noncommercial interests.
References
- 1. Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. American Thoracic Society; European Respiratory Society; Japanese Respiratory Society; Latin American Thoracic Society. Diagnosis of idiopathic pulmonary fibrosis. an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med . 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 2. Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med . 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raghu G, Remy-Jardin M, Ryerson CJ, Myers JL, Kreuter M, Vasakova M, et al. Diagnosis of hypersensitivity pneumonitis in adults: an official ATS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med . 2020;202:e36–e69. doi: 10.1164/rccm.202005-2032ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cottin V, Hirani NA, Hotchkin DL, Nambiar AM, Ogura T, Otaola M, et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev . 2018;27:180076. doi: 10.1183/16000617.0076-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swigris JJ, Stewart AL, Gould MK, Wilson SR. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes . 2005;3:61. doi: 10.1186/1477-7525-3-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aronson KI, Hayward BJ, Robbins L, Kaner RJ, Martinez FJ, Safford MM. ‘It’s difficult, it’s life changing what happens to you’ patient perspective on life with chronic hypersensitivity pneumonitis: a qualitative study. BMJ Open Respir Res . 2019;6:e000522. [Google Scholar]
- 7. Duck A, Spencer LG, Bailey S, Leonard C, Ormes J, Caress A-L. Perceptions, experiences and needs of patients with idiopathic pulmonary fibrosis. J Adv Nurs . 2015;71:1055–1065. doi: 10.1111/jan.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, et al. SENSCIS Trial Investigators. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med . 2019;380:2518–2528. doi: 10.1056/NEJMoa1903076. [DOI] [PubMed] [Google Scholar]
- 9. King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 10. Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al. Scleroderma Lung Study II Investigators. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med . 2016;4:708–719. doi: 10.1016/S2213-2600(16)30152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Scleroderma Lung Study Research Group. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med . 2006;354:2655–2666. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 12. Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. INBUILD Trial Investigators. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med . 2019;381:1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 13. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 14. Feemster LC, Saft HL, Bartlett SJ, Parthasarathy S, Barnes T, Calverley P, et al. American Thoracic Society Behavioral Sciences and Health Services Research Assembly and Nursing Assembly. Patient-centered outcomes research in pulmonary, critical care, and sleep medicine: an official American Thoracic Society workshop report. Ann Am Thorac Soc . 2018;15:1005–1015. doi: 10.1513/AnnalsATS.201806-406WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Russell A-M, Sprangers MAG, Wibberley S, Snell N, Rose DM, Swigris JJ. The need for patient-centred clinical research in idiopathic pulmonary fibrosis. BMC Med . 2015;13:240. doi: 10.1186/s12916-015-0475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Food and Drug Administration. Silver Spring, MD: Food and Drug Administration; 2009. https://www.fda.gov/media/77832/download [Google Scholar]
- 17. Natafgi N, Tafari AT, Chauhan C, Bekelman JE, Mullins CD. Patients’ early engagement in research proposal development (PEER-PD): patients guiding the proposal writing. J Comp Eff Res . 2019;8:441–453. doi: 10.2217/cer-2018-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strategy 1: working with patient and families as advisors. Rockville, MD: Agency for Healthcare Research and Quality; 2013 Jun [reviewed 2017 Dec; accessed 2019 Oct 17]. Available from: https://www.ahrq.gov/patient-safety/patients-families/engagingfamilies/strategy1/index.html.
- 19. Sharma AE, Willard-Grace R, Willis A, Zieve O, Dubé K, Parker C. et al. “How can we talk about patient-centered care without patients at the table?” Lessons learned from patient advisory councils. J Am Board Fam Med . 2016;29:775–784. doi: 10.3122/jabfm.2016.06.150380. [DOI] [PubMed] [Google Scholar]
- 20. Vat LE, Ryan D, Etchegary H. Recruiting patients as partners in health research: a qualitative descriptive study. Res Involv Engagem . 2017;3:15. doi: 10.1186/s40900-017-0067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Portalupi LB, Lewis CL, Miller CD, Whiteman-Jones KL, Sather KA, Nease DE, Jr, et al. Developing a patient and family research advisory panel to include people with significant disease, multimorbidity and advanced age. Fam Pract . 2017;34:364–369. doi: 10.1093/fampra/cmw138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sheridan S, Schrandt S, Forsythe L, Hilliard TS, Paez KA. Advisory Panel on Patient Engagement (2013 Inaugural Panel) The PCORI engagement rubric: promising practices for partnering in research. Ann Fam Med . 2017;15:165–170. doi: 10.1370/afm.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamilton CB, Leese JC, Hoens AM, Li LC. Framework for advancing the reporting of patient engagement in rheumatology research projects. Curry Rheumatol Rep . 2017;19:38. doi: 10.1007/s11926-017-0666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kirwan JR, de Wit M, Frank L, Haywood KL, Salek S, Brace-McDonnell S, et al. Emerging guidelines for patient engagement in research. Value Health . 2017;20:481–486. doi: 10.1016/j.jval.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 25. Kendell C, Urquhart R, Petrella J, MacDonald S, McCallum M. Evaluation of an advisory committee as a model for patient engagement. Patient Exp J . 2014;1:62–70. [Google Scholar]
- 26.Macaulay A. Commanda FW. Freeman WL. Mccabe L. Robbins M. Twohig PL. In: Gibson N, editor. Leawood, KS: North American Primary Care Research Group; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. The WHOQOL Group. The World Health Organization Quality of Life Assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med . 1995;41:1403–1409. doi: 10.1016/0277-9536(95)00112-k. [DOI] [PubMed] [Google Scholar]
- 28. Lubin M, Chen H, Elicker B, Jones KD, Collard HR, Lee JS. A comparison of health-related quality of life in idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. Chest . 2014;145:1333–1338. doi: 10.1378/chest.13-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Natalini JG, Swigris JJ, Morisset J, Elicker BM, Jones KD, Fischer A, et al. Understanding the determinants of health-related quality of life in rheumatoid arthritis-associated interstitial lung disease. Respir Med . 2017;127:1–6. doi: 10.1016/j.rmed.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Russell A-M, Ripamonti E, Vancheri C. Qualitative European survey of patients with idiopathic pulmonary fibrosis: patients’ perspectives of the disease and treatment. BMC Pulm Med . 2016;16:10. doi: 10.1186/s12890-016-0171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Belkin A, Swigris JJ. Patient expectations and experiences in idiopathic pulmonary fibrosis: implications of patient surveys for improved care. Expert Rev Respir Med . 2014;8:173–178. doi: 10.1586/17476348.2014.880056. [DOI] [PubMed] [Google Scholar]
- 32. Bajwah S, Higginson IJ, Ross JR, Wells AU, Birring SS, Riley J, et al. The palliative care needs for fibrotic interstitial lung disease: a qualitative study of patients, informal caregivers and health professionals. Palliat Med . 2013;27:869–876. doi: 10.1177/0269216313497226. [DOI] [PubMed] [Google Scholar]
- 33. Yount SE, Beaumont JL, Chen S-Y, Kaiser K, Wortman K, Van Brunt DL, et al. Health-related quality of life in patients with idiopathic pulmonary fibrosis. Lung . 2016;194:227–234. doi: 10.1007/s00408-016-9850-y. [DOI] [PubMed] [Google Scholar]
- 34. Swigris JJ, Kuschner WG, Jacobs SS, Wilson SR, Gould MK. Health-related quality of life in patients with idiopathic pulmonary fibrosis: a systematic review. Thorax . 2005;60:588–594. doi: 10.1136/thx.2004.035220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patel AS, Siegert RJ, Brignall K, Gordon P, Steer S, Desai SR, et al. The development and validation of the King’s Brief Interstitial Lung Disease (K-BILD) health status questionnaire. Thorax . 2012;67:804–810. doi: 10.1136/thoraxjnl-2012-201581. [DOI] [PubMed] [Google Scholar]
- 36. Swigris JJ, Andrae DA, Churney T, Johnson N, Scholand MB, White ES, et al. Development and initial validation analyses of the Living with Idiopathic Pulmonary Fibrosis Questionnaire. Am J Respir Crit Care Med . 2020;202:1689–1697. doi: 10.1164/rccm.202002-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kreuter M, Bendstrup E, Russell A-M, Bajwah S, Lindell K, Adir Y, et al. Palliative care in interstitial lung disease: living well. Lancet Respir Med . 2017;5:968–980. doi: 10.1016/S2213-2600(17)30383-1. [DOI] [PubMed] [Google Scholar]
- 38. Rajala K, Lehto JT, Sutinen E, Kautiainen H, Myllärniemi M, Saarto T. Marked deterioration in the quality of life of patients with idiopathic pulmonary fibrosis during the last two years of life. BMC Pulm Med . 2018;18:172. doi: 10.1186/s12890-018-0738-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Visca D, Mori L, Tsipouri V, Fleming S, Firouzi A, Bonini M, et al. Effect of ambulatory oxygen on quality of life for patients with fibrotic lung disease (AmbOx): a prospective, open-label, mixed-method, crossover randomised controlled trial. Lancet Respir Med . 2018;6:759–770. doi: 10.1016/S2213-2600(18)30289-3. [DOI] [PubMed] [Google Scholar]
- 40. Behr J, Kreuter M, Hoeper MM, Wirtz H, Klotsche J, Koschel D, et al. Management of patients with idiopathic pulmonary fibrosis in clinical practice: the INSIGHTS-IPF registry. Eur Respir J . 2015;46:186–196. doi: 10.1183/09031936.00217614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bajwah S, Ross JR, Peacock JL, Higginson IJ, Wells AU, Patel AS, et al. Interventions to improve symptoms and quality of life of patients with fibrotic interstitial lung disease: a systematic review of the literature. Thorax . 2013;68:867–879. doi: 10.1136/thoraxjnl-2012-202040. [DOI] [PubMed] [Google Scholar]
- 42. Carvajalino S, Reigada C, Johnson MJ, Dzingina M, Bajwah S. Symptom prevalence of patients with fibrotic interstitial lung disease: a systematic literature review. BMC Pulm Med . 2018;18:78. doi: 10.1186/s12890-018-0651-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Simon PM, Schwartzstein RM, Weiss JW, Fencl V, Teghtsoonian M, Weinberger SE. Distinguishable types of dyspnea in patients with shortness of breath. Am Rev Respir Dis . 1990;142:1009–1014. doi: 10.1164/ajrccm/142.5.1009. [DOI] [PubMed] [Google Scholar]
- 44. Smith J, Albert P, Bertella E, Lester J, Jack S, Calverley P. Qualitative aspects of breathlessness in health and disease. Thorax . 2009;64:713–718. doi: 10.1136/thx.2008.104869. [DOI] [PubMed] [Google Scholar]
- 45. Vázquez-García JC, Balcázar-Cruz CA, Cervantes-Méndez G, Mejía-Alfaro R, Cossío-Alcántara J, Ramírez-Venegas A. Descriptors of breathlessness in Mexican Spanish [in Spanish and English] Arch Bronconeumol . 2006;42:211–217. doi: 10.1016/s1579-2129(06)60448-5. [DOI] [PubMed] [Google Scholar]
- 46. Mura M, Ferretti A, Ferro O, Zompatori M, Cavalli A, Schiavina M, et al. Functional predictors of exertional dyspnea, 6-min walking distance and HRCT fibrosis score in idiopathic pulmonary fibrosis. Respiration . 2006;73:495–502. doi: 10.1159/000089656. [DOI] [PubMed] [Google Scholar]
- 47. Speakman L, Walthall H. Assessment and management of refractory breathlessness in interstitial lung disease. Br J Community Nurs . 2017;22:434–439. doi: 10.12968/bjcn.2017.22.9.434. [DOI] [PubMed] [Google Scholar]
- 48. Ryerson CJ, Abbritti M, Ley B, Elicker BM, Jones KD, Collard HR. Cough predicts prognosis in idiopathic pulmonary fibrosis. Respirology . 2011;16:969–975. doi: 10.1111/j.1440-1843.2011.01996.x. [DOI] [PubMed] [Google Scholar]
- 49. Tobin RW, Pope CE, II, Pellegrini CA, Emond MJ, Sillery J, Raghu G. Increased prevalence of gastroesophageal reflux in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 1998;158:1804–1808. doi: 10.1164/ajrccm.158.6.9804105. [DOI] [PubMed] [Google Scholar]
- 50. Theodore AC, Tseng C-H, Li N, Elashoff RM, Tashkin DP. Correlation of cough with disease activity and treatment with cyclophosphamide in scleroderma interstitial lung disease: findings from the Scleroderma Lung Study. Chest . 2012;142:614–621. doi: 10.1378/chest.11-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brignall K, Jayaraman B, Birring SS. Quality of life and psychosocial aspects of cough. Lung . 2008;186:S55–S58. doi: 10.1007/s00408-007-9034-x. [DOI] [PubMed] [Google Scholar]
- 52. van Manen MJG, Birring SS, Vancheri C, Cottin V, Renzoni EA, Russell A-M, et al. Cough in idiopathic pulmonary fibrosis. Eur Respir Rev . 2016;25:278–286. doi: 10.1183/16000617.0090-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Saketkoo LA, Mittoo S, Frankel S, LeSage D, Sarver C, Phillips K, et al. OMERACT Connective Tissue Disease–Interstitial Lung Diseases Working Group; OMERACT Connective Tissue Disease-Interstitial Lung Diseases Working Group. Reconciling healthcare professional and patient perspectives in the development of disease activity and response criteria in connective tissue disease-related interstitial lung diseases. J Rheumatol . 2014;41:792–798. doi: 10.3899/jrheum.131251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Myall KJ, Kavanagh JE, Birring SS. Idiopathic pulmonary fibrosis-associated cough: mechanisms and management. Pulm Pharmacol Ther . 2019;56:100–103. doi: 10.1016/j.pupt.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 55. Schoenheit G, Becattelli I, Cohen AH. Living with idiopathic pulmonary fibrosis: an in-depth qualitative survey of European patients. Chron Respir Dis . 2011;8:225–231. doi: 10.1177/1479972311416382. [DOI] [PubMed] [Google Scholar]
- 56. Mittoo S, Frankel S, LeSage D, Strand V, Shah AA, Christopher-Stine L, et al. Patient perspectives in OMERACT provide an anchor for future metric development and improved approaches to healthcare delivery in connective tissue disease related interstitial lung disease (CTD-ILD) Curr Respir Med Rev . 2015;11:175–183. doi: 10.2174/1573398X11666150619182624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Michielsen HJ, Drent M, Peros-Golubicic T, De Vries J. Fatigue is associated with quality of life in sarcoidosis patients. Chest . 2006;130:989–994. doi: 10.1378/chest.130.4.989. [DOI] [PubMed] [Google Scholar]
- 58. Bahmer T, Watz H, Develaska M, Waschki B, Rabe KF, Magnussen H, et al. Physical activity and fatigue in patients with sarcoidosis. Respiration . 2018;95:18–26. doi: 10.1159/000481827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kotecha J, Atkins C, Wilson A. Patient confidence and quality of life in idiopathic pulmonary fibrosis and sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2016;33:341–348. [PubMed] [Google Scholar]
- 60. Curt GA. The impact of fatigue on patients with cancer: overview of FATIGUE 1 and 2. Oncologist . 2000;5:9–12. doi: 10.1634/theoncologist.5-suppl_2-9. [DOI] [PubMed] [Google Scholar]
- 61. Raghu G, Rochwerg B, Zhang Y, Garcia CAC, Azuma A, Behr J, et al. American Thoracic Society; European Respiratory society; Japanese Respiratory Society; Latin American Thoracic Association. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. an update of the 2011 clinical practice guideline. Am J Respir Crit Care Med . 2015;192:e3–e19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 62. Kahlmann V, Moor CC, Wijsenbeek MS. Managing fatigue in patients with interstitial lung disease. Chest . 2020;158:2026–2033. doi: 10.1016/j.chest.2020.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Axelsson GT, Putman RK, Araki T, Sigurdsson S, Gudmundsson EF, Eiriksdottir G, et al. Interstitial lung abnormalities and self-reported health and functional status. Thorax . 2018;73:884–886. doi: 10.1136/thoraxjnl-2017-210956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gomes-Neto M, Silva CM, Ezequiel D, Conceição CS, Saquetto M, Machado AS. Impact of pulmonary rehabilitation on exercise tolerance and quality of life in patients with idiopathic pulmonary fibrosis: a systematic review and meta-analysis. J Cardiopulm Rehabil Prev . 2018;38:273–278. doi: 10.1097/HCR.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 65. Dowman L, Hill CJ, Holland AE. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev . 2014;(10):CD006322. doi: 10.1002/14651858.CD006322.pub3. [DOI] [PubMed] [Google Scholar]
- 66. Cox NS, McDonald CF, Alison JA, Mahal A, Wootton R, Hill CJ, et al. Telerehabilitation versus traditional centre-based pulmonary rehabilitation for people with chronic respiratory disease: protocol for a randomised controlled trial. BMC Pulm Med . 2018;18:71. doi: 10.1186/s12890-018-0646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Berry CE, Han MK, Thompson B, Limper AH, Martinez FJ, Schwarz MI, et al. Older adults with chronic lung disease report less limitation compared with younger adults with similar lung function impairment. Ann Am Thorac Soc . 2015;12:21–26. doi: 10.1513/AnnalsATS.201407-312OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Visca D, Tsipouri V, Mori L, Firouzi A, Fleming S, Farquhar M, et al. Ambulatory oxygen in fibrotic lung disease (AmbOx): study protocol for a randomised controlled trial. Trials . 2017;18:201. doi: 10.1186/s13063-017-1912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Raghu G, van den Blink B, Hamblin MJ, Brown AW, Golden JA, Ho LA, et al. Effect of recombinant human pentraxin 2 vs placebo on change in forced vital capacity in patients with idiopathic pulmonary fibrosis: a randomized clinical trial. JAMA . 2018;319:2299–2307. doi: 10.1001/jama.2018.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Leidy NK. Functional status and the forward progress of merry-go-rounds: toward a coherent analytical framework. Nurs Res . 1994;43:196–202. [PubMed] [Google Scholar]
- 71. Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ . 1992;305:160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George’s Respiratory Questionnaire. Am Rev Respir Dis . 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 73. Hur SA, Guler SA, Khalil N, Camp PG, Guenette JA, Swigris JJ, et al. Minimal important difference for physical activity and validity of the international physical activity questionnaire in interstitial lung disease. Ann Am Thorac Soc . 2019;16:107–115. doi: 10.1513/AnnalsATS.201804-265OC. [DOI] [PubMed] [Google Scholar]
- 74. Hendriks CMR, Deenstra DD, Elfferich MDP, Strookappe B, Wijnen P, De Vries J, et al. Experience with activity monitors of patients with COPD, sarcoidosis and pulmonary fibrosis in the Netherlands. Psychol Behav Sci Int J . 2019;12:1–6. [Google Scholar]
- 75. Watz H, Pitta F, Rochester CL, Garcia-Aymerich J, ZuWallack R, Troosters T, et al. An official European Respiratory Society statement on physical activity in COPD. Eur Respir J . 2014;44:1521–1537. doi: 10.1183/09031936.00046814. [DOI] [PubMed] [Google Scholar]
- 76. Bahmer T, Kirsten A-M, Waschki B, Rabe KF, Magnussen H, Kirsten D, et al. Prognosis and longitudinal changes of physical activity in idiopathic pulmonary fibrosis. BMC Pulm Med . 2017;17:104. doi: 10.1186/s12890-017-0444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen KY, Bassett DR., Jr The technology of accelerometry-based activity monitors: current and future. Med Sci Sports Exerc . 2005;37(11)(Suppl):S490–S500. doi: 10.1249/01.mss.0000185571.49104.82. [DOI] [PubMed] [Google Scholar]
- 78. Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Quantifying physical activity in daily life with questionnaires and motion sensors in COPD. Eur Respir J . 2006;27:1040–1055. doi: 10.1183/09031936.06.00064105. [DOI] [PubMed] [Google Scholar]
- 79. Wallaert B, Monge E, Le Rouzic O, Wémeau-Stervinou L, Salleron J, Grosbois J-M. Physical activity in daily life of patients with fibrotic idiopathic interstitial pneumonia. Chest . 2013;144:1652–1658. doi: 10.1378/chest.13-0806. [DOI] [PubMed] [Google Scholar]
- 80. Marcoux V, Wang M, Burgoyne SJ, Fell CD, Ryerson CJ, Sajobi TT, et al. Mobile health monitoring in patients with idiopathic pulmonary fibrosis. Ann Am Thorac Soc . 2019;16:1327–1329. doi: 10.1513/AnnalsATS.201904-335RL. [DOI] [PubMed] [Google Scholar]
- 81. Nakayama M, Bando M, Araki K, Sekine T, Kurosaki F, Sawata T, et al. Physical activity in patients with idiopathic pulmonary fibrosis. Respirology . 2015;20:640–646. doi: 10.1111/resp.12500. [DOI] [PubMed] [Google Scholar]
- 82. Atkins C, Baxter M, Jones A, Wilson A. Measuring sedentary behaviors in patients with idiopathic pulmonary fibrosis using wrist-worn accelerometers. Clin Respir J . 2018;12:746–753. doi: 10.1111/crj.12589. [DOI] [PubMed] [Google Scholar]
- 83. Fitting JW, Frascarolo P, Jéquier E, Leuenberger P. Resting energy expenditure in interstitial lung disease. Am Rev Respir Dis . 1990;142:631–635. doi: 10.1164/ajrccm/142.3.631. [DOI] [PubMed] [Google Scholar]
- 84. Sehgal S, Small B, Highland KB. Activity monitors in pulmonary disease. Respir Med . 2019;151:81–95. doi: 10.1016/j.rmed.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 85. Turner LJ, Houchen L, Williams J, Singh SJ. Reliability of pedometers to measure step counts in patients with chronic respiratory disease. J Cardiopulm Rehabil Prev . 2012;32:284–291. doi: 10.1097/HCR.0b013e31825c49f2. [DOI] [PubMed] [Google Scholar]
- 86. Moy ML, Janney AW, Nguyen HQ, Matthess KR, Cohen M, Garshick E, et al. Use of pedometer and Internet-mediated walking program in patients with chronic obstructive pulmonary disease. J Rehabil Res Dev . 2010;47:485–496. doi: 10.1682/jrrd.2009.07.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Moy ML, Weston NA, Wilson EJ, Hess ML, Richardson CR. A pilot study of an Internet walking program and pedometer in COPD. Respir Med . 2012;106:1342–1350. doi: 10.1016/j.rmed.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 88. Krenn PJ, Titze S, Oja P, Jones A, Ogilvie D. Use of global positioning systems to study physical activity and the environment: a systematic review. Am J Prev Med . 2011;41:508–515. doi: 10.1016/j.amepre.2011.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Root ED, Graney B, Baird S, Churney T, Fier K, Korn M, et al. Physical activity and activity space in patients with pulmonary fibrosis not prescribed supplemental oxygen. BMC Pulm Med . 2017;17:154. doi: 10.1186/s12890-017-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schneeberger T, Gloeckl R, Welte T, Kenn K. Pulmonary rehabilitation outcomes after single or double lung transplantation in patients with chronic obstructive pulmonary disease or interstitial lung disease. Respiration . 2017;94:178–185. doi: 10.1159/000477351. [DOI] [PubMed] [Google Scholar]
- 91. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med . 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 92. Rammaert B, Leroy S, Cavestri B, Wallaert B, Grosbois JM. Home-based pulmonary rehabilitation in idiopathic pulmonary fibrosis. Rev Mal Respir . 2011;28:e52–e57. doi: 10.1016/j.rmr.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 93. Rusanov V, Shitrit D, Fox B, Amital A, Peled N, Kramer MR. Use of the 15-steps climbing exercise oximetry test in patients with idiopathic pulmonary fibrosis. Respir Med . 2008;102:1080–1088. doi: 10.1016/j.rmed.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 94. Briand J, Behal H, Chenivesse C, Wémeau-Stervinou L, Wallaert B. The 1-minute sit-to-stand test to detect exercise-induced oxygen desaturation in patients with interstitial lung disease. Ther Adv Respir Dis . 2018;12:1753466618793028. doi: 10.1177/1753466618793028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ochmann U, Kotschy-Lang N, Raab W, Kellberger J, Nowak D, Jörres RA. Long-term efficacy of pulmonary rehabilitation in patients with occupational respiratory diseases. Respiration . 2012;84:396–405. doi: 10.1159/000337271. [DOI] [PubMed] [Google Scholar]
- 96. American Thoracic Society; American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med . 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 97. Dalichau S, Demedts A, im Sande A, Möller T. Improvement of lasting effects in outpatient pulmonary rehabilitation with special regard to exercise therapy and sports [in German] Rehabilitation (Stuttg) 2010;49:30–37. doi: 10.1055/s-0029-1246154. [DOI] [PubMed] [Google Scholar]
- 98. Hanada M, Sakamoto N, Ishimatsu Y, Kakugawa T, Obase Y, Kozu R, et al. Effect of long-term treatment with corticosteroids on skeletal muscle strength, functional exercise capacity and health status in patients with interstitial lung disease. Respirology . 2016;21:1088–1093. doi: 10.1111/resp.12807. [DOI] [PubMed] [Google Scholar]
- 99. Perez-Bogerd S, Wuyts W, Barbier V, Demeyer H, Van Muylem A, Janssens W, et al. Short and long-term effects of pulmonary rehabilitation in interstitial lung diseases: a randomised controlled trial. Respir Res . 2018;19:182. doi: 10.1186/s12931-018-0884-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. DePew ZS, Karpman C, Novotny PJ, Benzo RP. Correlations between gait speed, 6-minute walk distance, physical activity, and self-efficacy in patients with severe chronic lung disease. Respir Care . 2013;58:2113–2119. doi: 10.4187/respcare.02471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ryerson CJ, Cayou C, Topp F, Hilling L, Camp PG, Wilcox PG, et al. Pulmonary rehabilitation improves long-term outcomes in interstitial lung disease: a prospective cohort study. Respir Med . 2014;108:203–210. doi: 10.1016/j.rmed.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 102. Vainshelboim B, Kramer MR, Myers J, Unterman A, Izhakian S, Oliveira J. 8-Foot-Up-and-Go Test is associated with hospitalizations and mortality in idiopathic pulmonary fibrosis: a prospective pilot study. Lung . 2019;197:81–88. doi: 10.1007/s00408-018-0189-4. [DOI] [PubMed] [Google Scholar]
- 103. Sanges S, Giovannelli J, Sobanski V, Morell-Dubois S, Maillard H, Lambert M, et al. Factors associated with the 6-minute walk distance in patients with systemic sclerosis. Arthritis Res Ther . 2017;19:279. doi: 10.1186/s13075-017-1489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Brown AW, Nathan SD. The value and application of the 6-minute-walk test in idiopathic pulmonary fibrosis. Ann Am Thorac Soc . 2018;15:3–10. doi: 10.1513/AnnalsATS.201703-244FR. [DOI] [PubMed] [Google Scholar]
- 105. Sanchez-Morillo D, Lara-Doña A, Priego-Torres B, Morales-Gonzalez M, Montoro-Ballesteros F, Leon-Jimenez A. Portable oxygen therapy: is the 6-minute walking test overestimating the actual oxygen needs? J Clin Med . 2020;9:E4007. doi: 10.3390/jcm9124007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zapf D. Emotion work and psychological well-being. Human Resource Management Review . 2002;12:237–268. [Google Scholar]
- 107. Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol . 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- 108. Fredrickson BL, Joiner T. Positive emotions trigger upward spirals toward emotional well-being. Psychol Sci . 2002;13:172–175. doi: 10.1111/1467-9280.00431. [DOI] [PubMed] [Google Scholar]
- 109. Ryerson CJ, Arean PA, Berkeley J, Carrieri-Kohlman VL, Pantilat SZ, Landefeld CS, et al. Depression is a common and chronic comorbidity in patients with interstitial lung disease. Respirology . 2012;17:525–532. doi: 10.1111/j.1440-1843.2011.02122.x. [DOI] [PubMed] [Google Scholar]
- 110. Collard HR, Ward AJ, Lanes S, Cortney Hayflinger D, Rosenberg DM, Hunsche E. Burden of illness in idiopathic pulmonary fibrosis. J Med Econ . 2012;15:829–835. doi: 10.3111/13696998.2012.680553. [DOI] [PubMed] [Google Scholar]
- 111. Akhtar AA, Ali MA, Smith RP. Depression in patients with idiopathic pulmonary fibrosis. Chron Respir Dis . 2013;10:127–133. doi: 10.1177/1479972313493098. [DOI] [PubMed] [Google Scholar]
- 112. Tzouvelekis A, Karampitsakos T, Kourtidou S, Bouros E, Tzilas V, Katsaras M, et al. Impact of depression on patients with idiopathic pulmonary fibrosis. Front Med (Lausanne) . 2020;7:29. doi: 10.3389/fmed.2020.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yuan X-Y, Zhang H, Huang L-R, Zhang F, Sheng X-W, Cui A. Evaluation of health-related quality of life and the related factors in a group of Chinese patients with interstitial lung diseases. PLoS One . 2020;15:e0236346. doi: 10.1371/journal.pone.0236346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Holland AE, Fiore JF, Jr, Bell EC, Goh N, Westall G, Symons K, et al. Dyspnoea and comorbidity contribute to anxiety and depression in interstitial lung disease. Respirology . 2014;19:1215–1221. doi: 10.1111/resp.12360. [DOI] [PubMed] [Google Scholar]
- 115. Ryerson CJ, Berkeley J, Carrieri-Kohlman VL, Pantilat SZ, Landefeld CS, Collard HR. Depression and functional status are strongly associated with dyspnea in interstitial lung disease. Chest . 2011;139:609–616. doi: 10.1378/chest.10-0608. [DOI] [PubMed] [Google Scholar]
- 116. Hur SA, Guler SA, Khalil N, Camp PG, Guenette JA, Ryerson CJ. Impact of psychological deficits and pain on physical activity of patients with interstitial lung disease. Lung . 2019;197:415–425. doi: 10.1007/s00408-019-00242-3. [DOI] [PubMed] [Google Scholar]
- 117. Lee YJ, Choi SM, Lee YJ, Cho Y-J, Yoon HI, Lee J-H, et al. Clinical impact of depression and anxiety in patients with idiopathic pulmonary fibrosis. PLoS One . 2017;12:e0184300. doi: 10.1371/journal.pone.0184300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. De Vries J, Kessels BL, Drent M. Quality of life of idiopathic pulmonary fibrosis patients. Eur Respir J . 2001;17:954–961. doi: 10.1183/09031936.01.17509540. [DOI] [PubMed] [Google Scholar]
- 119. Matsuda T, Taniguchi H, Ando M, Kondoh Y, Kimura T, Kataoka K, et al. Depression is significantly associated with the health status in patients with idiopathic pulmonary fibrosis. Intern Med . 2017;56:1637–1644. doi: 10.2169/internalmedicine.56.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wallaert B, Duthoit L, Drumez E, Behal H, Wemeau L, Chenivesse C, et al. Long-term evaluation of home-based pulmonary rehabilitation in patients with fibrotic idiopathic interstitial pneumonias. ERJ Open Res . 2019;5:00045-2019. doi: 10.1183/23120541.00045-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bajwah S, Ross JR, Wells AU, Mohammed K, Oyebode C, Birring SS, et al. Palliative care for patients with advanced fibrotic lung disease: a randomised controlled phase II and feasibility trial of a community case conference intervention. Thorax . 2015;70:830–839. doi: 10.1136/thoraxjnl-2014-206583. [DOI] [PubMed] [Google Scholar]
- 122. Swigris JJ, Fairclough DL, Morrison M, Make B, Kozora E, Brown KK, et al. Benefits of pulmonary rehabilitation in idiopathic pulmonary fibrosis. Respir Care . 2011;56:783–789. doi: 10.4187/respcare.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Reynolds -Sandford D. Reynolds P. Jersmann H. Schubert O.. Differentiating between depression, anxiety and a grief reaction in idiopathic pulmonary fibrosis (IPF) patients and their caregivers. Is it time to rethink how and what we measure? Eur Respir J . 2019;54:PA1309. [Google Scholar]
- 124. Han B, Yan B, Zhang J, Zhao N, Sun J, Li C, et al. The influence of the social support on symptoms of anxiety and depression among patients with silicosis. ScientificWorldJournal . 2014;2014:724804. doi: 10.1155/2014/724804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sgalla G, Cerri S, Ferrari R, Ricchieri MP, Poletti S, Ori M, et al. Mindfulness-based stress reduction in patients with interstitial lung diseases: a pilot, single-centre observational study on safety and efficacy. BMJ Open Respir Res . 2015;2:e000065. doi: 10.1136/bmjresp-2014-000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Magnani D, Lenoci G, Balduzzi S, Artioli G, Ferri P. Effectiveness of support groups to improve the quality of life of people with idiopathic pulmonary fibrosis a pre-post test pilot study. Acta Biomed . 2017;88:5–12. doi: 10.23750/abm.v88i5-S.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Jeong SO, Uh S-T, Park S, Kim H-S. Effects of patient satisfaction and confidence on the success of treatment of combined rheumatic disease and interstitial lung disease in a multidisciplinary outpatient clinic. Int J Rheum Dis . 2018;21:1600–1608. doi: 10.1111/1756-185X.13331. [DOI] [PubMed] [Google Scholar]
- 128. Shah RJ, Collard HR, Morisset J. Burden, resilience and coping in caregivers of patients with interstitial lung disease. Heart Lung . 2018;47:264–268. doi: 10.1016/j.hrtlng.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Bjoraker JA, Ryu JH, Edwin MK, Myers JL, Tazelaar HD, Schroeder DR, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 1998;157:199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 130. Daniil ZD, Gilchrist FC, Nicholson AG, Hansell DM, Harris J, Colby TV, et al. A histologic pattern of nonspecific interstitial pneumonia is associated with a better prognosis than usual interstitial pneumonia in patients with cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med . 1999;160:899–905. doi: 10.1164/ajrccm.160.3.9903021. [DOI] [PubMed] [Google Scholar]
- 131. King TE, Jr, Brown KK, Raghu G, du Bois RM, Lynch DA, Martinez F, et al. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2011;184:92–99. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]
- 132. Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, et al. Idiopathic Pulmonary Fibrosis Study Group. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2004;350:125–133. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]
- 133. Flaherty KR, Brown KK, Wells AU, Clerisme-Beaty E, Collard HR, Cottin V, et al. Design of the PF-ILD trial: a double-blind, randomised, placebo-controlled phase III trial of nintedanib in patients with progressive fibrosing interstitial lung disease. BMJ Open Respir Res . 2017;4:e000212. doi: 10.1136/bmjresp-2017-000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ley B, Swigris J, Day B-M, Stauffer JL, Raimundo K, Chou W, et al. Pirfenidone reduces respiratory-related hospitalizations in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2017;196:756–761. doi: 10.1164/rccm.201701-0091OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hardinge M, Annandale J, Bourne S, Cooper B, Evans A, Freeman D, et al. British Thoracic Society Home Oxygen Guideline Development Group; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for home oxygen use in adults. Thorax . 2015;70:i1–i43. doi: 10.1136/thoraxjnl-2015-206865. [DOI] [PubMed] [Google Scholar]
- 136. Hayes D, Jr, Wilson KC, Krivchenia K, Hawkins SMM, Balfour-Lynn IM, Gozal D, et al. Home oxygen therapy for children: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med . 2019;199:e5–e23. doi: 10.1164/rccm.201812-2276ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. McDonald CF, Whyte K, Jenkins S, Serginson J, Frith P. Clinical practice guideline on adult domiciliary oxygen therapy: executive summary from the Thoracic Society of Australia and New Zealand. Respirology . 2016;21:76–78. doi: 10.1111/resp.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lacasse Y, Bernard S, Maltais F. Eligibility for home oxygen programs and funding across Canada. Can Respir J . 2015;22:324–330. doi: 10.1155/2015/280604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Jacobs SS, Krishnan JA, Lederer DJ, Ghazipura M, Hossain T, Tan AM, et al. Home oxygen therapy for adults with chronic lung disease: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med . 2020;202:e121–e141. doi: 10.1164/rccm.202009-3608ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lim RK, Humphreys C, Morisset J, Holland AE, Johannson KA. O2 Delphi Collaborators. Oxygen in patients with fibrotic interstitial lung disease: an international Delphi survey. Eur Respir J . 2019;54:1900421. doi: 10.1183/13993003.00421-2019. [DOI] [PubMed] [Google Scholar]
- 141. Khor YH, Goh NSL, McDonald CF, Holland AE. Oxygen therapy for interstitial lung disease: physicians’ perceptions and experiences. Ann Am Thorac Soc . 2017;14:1772–1778. doi: 10.1513/AnnalsATS.201705-372OC. [DOI] [PubMed] [Google Scholar]
- 142. Graney BA, Wamboldt FS, Baird S, Churney T, Fier K, Korn M, et al. Informal caregivers experience of supplemental oxygen in pulmonary fibrosis. Health Qual Life Outcomes . 2017;15:133. doi: 10.1186/s12955-017-0710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Swigris JJ. Transitions and touchpoints in idiopathic pulmonary fibrosis. BMJ Open Respir Res . 2018;5:e000317. doi: 10.1136/bmjresp-2018-000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Khor YH, Goh NSL, McDonald CF, Holland AE. Oxygen therapy for interstitial lung disease: a mismatch between patient expectations and experiences. Ann Am Thorac Soc . 2017;14:888–895. doi: 10.1513/AnnalsATS.201611-934OC. [DOI] [PubMed] [Google Scholar]
- 145. Graney BA, Wamboldt FS, Baird S, Churney T, Fier K, Korn M, et al. Looking ahead and behind at supplemental oxygen: a qualitative study of patients with pulmonary fibrosis. Heart Lung . 2017;46:387–393. doi: 10.1016/j.hrtlng.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 146. Jacobs SS, Lederer DJ, Garvey CM, Hernandez C, Lindell KO, McLaughlin S, et al. Optimizing home oxygen therapy. an official American Thoracic Society workshop report. Ann Am Thorac Soc . 2018;15:1369–1381. doi: 10.1513/AnnalsATS.201809-627WS. [DOI] [PubMed] [Google Scholar]
- 147. Lindell KO, Collins EG, Catanzarite L, Garvey CM, Hernandez C, Mclaughlin S, et al. Equipment, access and worry about running short of oxygen: key concerns in the ATS patient supplemental oxygen survey. Heart Lung . 2019;48:245–249. doi: 10.1016/j.hrtlng.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 148. Cullen DL, Stiffler D. Long-term oxygen therapy: review from the patients’ perspective. Chron Respir Dis . 2009;6:141–147. doi: 10.1177/1479972309103046. [DOI] [PubMed] [Google Scholar]
- 149. Ramadurai D, Corder S, Churney T, Graney B, Harshman A, Meadows S, et al. Idiopathic pulmonary fibrosis: Educational needs of health-care providers, patients, and caregivers. Chron Respir Dis . 2019;16:1479973119858961. doi: 10.1177/1479973119858961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ramadurai D, Corder S, Churney T, Graney B, Harshman A, Meadows S, et al. Understanding the informational needs of patients with IPF and their caregivers: ‘You get diagnosed, and you ask this question right away, what does this mean?’. BMJ Open Qual . 2018;7:e000207. doi: 10.1136/bmjoq-2017-000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Albright K, Walker T, Baird S, Eres L, Farnsworth T, Fier K, et al. Seeking and sharing: why the pulmonary fibrosis community engages the Web 2.0 environment. BMC Pulm Med . 2016;16:4. doi: 10.1186/s12890-016-0167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Morisset J, Dubé B-P, Garvey C, Bourbeau J, Collard HR, Swigris JJ, et al. The unmet educational needs of patients with interstitial lung disease: setting the stage for tailored pulmonary rehabilitation. Ann Am Thorac Soc . 2016;13:1026–1033. doi: 10.1513/AnnalsATS.201512-836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Holland AE, Fiore JF, Jr, Goh N, Symons K, Dowman L, Westall G, et al. Be honest and help me prepare for the future: what people with interstitial lung disease want from education in pulmonary rehabilitation. Chron Respir Dis . 2015;12:93–101. doi: 10.1177/1479972315571925. [DOI] [PubMed] [Google Scholar]
- 154. Lindell KO, Olshansky E, Song M-K, Zullo TG, Gibson KF, Kaminski N, et al. Impact of a disease-management program on symptom burden and health-related quality of life in patients with idiopathic pulmonary fibrosis and their care partners. Heart Lung . 2010;39:304–313. doi: 10.1016/j.hrtlng.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lindell KO, Nouraie M, Klesen MJ, Klein S, Gibson KF, Kass DJ, et al. Randomised clinical trial of an early palliative care intervention (SUPPORT) for patients with idiopathic pulmonary fibrosis (IPF) and their caregivers: protocol and key design considerations. BMJ Open Respir Res . 2018;5:e000272. doi: 10.1136/bmjresp-2017-000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. van Manen MJG, van ’t Spijker A, Tak NC, Baars CT, Jongenotter SM, van Roon LR, et al. Patient and partner empowerment programme for idiopathic pulmonary fibrosis. Eur Respir J . 2017;49:1601596. doi: 10.1183/13993003.01596-2016. [DOI] [PubMed] [Google Scholar]
- 157. Hollander JE, Carr BG. Virtually perfect? Telemedicine for COVID-19. N Engl J Med . 2020;382:1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 158. Smith AB, Hanbury A, Retzler J. Item banking and computer-adaptive testing in clinical trials: Standing in sight of the PROMISed land. Contemp Clin Trials Commun . 2019;13:005. doi: 10.1016/j.conctc.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Borowsky PA, Kadri OM, Meldau JE, Blanchett J, Makhni EC. The remote completion rate of electronic patient-reported outcome forms before scheduled clinic visits: a proof-of-concept study using patient-reported outcome measurement information system computer adaptive test questionnaires. J Am Acad Orthop Surg Glob Res Rev . 2019;3:e19.00038. doi: 10.5435/JAAOSGlobal-D-19-00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Shufelt C, Dzubur E, Joung S, Fuller G, Mouapi KN, Van Den Broek I, et al. A protocol integrating remote patient monitoring patient reported outcomes and cardiovascular biomarkers. NPJ Digit Med . 2019;2:84. doi: 10.1038/s41746-019-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Johannson KA. Remote monitoring in idiopathic pulmonary fibrosis: home is where the Bluetooth-enabled spirometer is. Am J Respir Crit Care Med . 2020;202:316–317. doi: 10.1164/rccm.202005-1532ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Moor CC, Wapenaar M, Miedema JR, Geelhoed JJM, Chandoesing PP, Wijsenbeek MS. A home monitoring program including real-time wireless home spirometry in idiopathic pulmonary fibrosis: a pilot study on experiences and barriers. Respir Res . 2018;19:105. doi: 10.1186/s12931-018-0810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Moor CC, Mostard RLM, Grutters JC, Bresser P, Aerts JGJV, Chavannes NH, et al. Home monitoring in patients with idiopathic pulmonary fibrosis: a randomized controlled trial. Am J Respir Crit Care Med . 2020;202:393–401. doi: 10.1164/rccm.202002-0328OC. [DOI] [PubMed] [Google Scholar]
- 164. Tzanakis N, Samiou M, Lambiri I, Antoniou K, Siafakas N, Bouros D. Evaluation of health-related quality-of-life and dyspnea scales in patients with idiopathic pulmonary fibrosis: correlation with pulmonary function tests. Eur J Intern Med . 2005;16:105–112. doi: 10.1016/j.ejim.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 165. Beretta L, Santaniello A, Lemos A, Masciocchi M, Scorza R. Validity of the Saint George’s Respiratory Questionnaire in the evaluation of the health-related quality of life in patients with interstitial lung disease secondary to systemic sclerosis. Rheumatology (Oxford) . 2007;46:296–301. doi: 10.1093/rheumatology/kel221. [DOI] [PubMed] [Google Scholar]
- 166. Swigris JJ, Brown KK, Behr J, du Bois RM, King TE, Raghu G, et al. The SF-36 and SGRQ: validity and first look at minimum important differences in IPF. Respir Med . 2010;104:296–304. doi: 10.1016/j.rmed.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Wallace B, Kafaja S, Furst DE, Berrocal VJ, Merkel PA, Seibold JR, et al. Reliability, validity and responsiveness to change of the Saint George’s Respiratory Questionnaire in early diffuse cutaneous systemic sclerosis. Rheumatology (Oxford) . 2015;54:1369–1379. doi: 10.1093/rheumatology/keu456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Swigris JJ, Esser D, Wilson H, Conoscenti CS, Schmidt H, Stansen W, et al. Psychometric properties of the St George’s Respiratory Questionnaire in patients with idiopathic pulmonary fibrosis. Eur Respir J . 2017;49:1601788. doi: 10.1183/13993003.01788-2016. [DOI] [PubMed] [Google Scholar]
- 169. Glaspole IN, Chapman SA, Cooper WA, Ellis SJ, Goh NS, Hopkins PM, et al. Health-related quality of life in idiopathic pulmonary fibrosis: data from the Australian IPF Registry. Respirology . 2017;22:950–956. doi: 10.1111/resp.12989. [DOI] [PubMed] [Google Scholar]
- 170. Suzuki A, Kondoh Y, Swigris JJ, Ando M, Kimura T, Kataoka K, et al. Performance of the St George’s Respiratory Questionnaire in patients with connective tissue disease-associated interstitial lung disease. Respirology . 2018;23:851–859. doi: 10.1111/resp.13293. [DOI] [PubMed] [Google Scholar]
- 171. Kreuter M, Swigris J, Pittrow D, Geier S, Klotsche J, Prasse A, et al. The clinical course of idiopathic pulmonary fibrosis and its association to quality of life over time: longitudinal data from the INSIGHTS-IPF registry. Respir Res . 2019;20:59. doi: 10.1186/s12931-019-1020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Swigris JJ, Wilson H, Esser D, Conoscenti CS, Stansen W, Kline Leidy N, et al. Psychometric properties of the St George’s Respiratory Questionnaire in patients with idiopathic pulmonary fibrosis: insights from the INPULSIS trials. BMJ Open Respir Res . 2018;5:e000278. doi: 10.1136/bmjresp-2018-000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Yorke J, Jones PW, Swigris JJ. Development and validity testing of an IPF-specific version of the St George’s Respiratory Questionnaire. Thorax . 2010;65:921–926. doi: 10.1136/thx.2010.139121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Prior TS, Hoyer N, Shaker SB, Davidsen JR, Yorke J, Hilberg O, et al. Validation of the IPF-specific version of St. George’s Respiratory Questionnaire. Respir Res . 2019;20:199. doi: 10.1186/s12931-019-1169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Nagata K, Tomii K, Otsuka K, Tachikawa R, Otsuka K, Takeshita J, et al. Evaluation of the chronic obstructive pulmonary disease assessment test for measurement of health-related quality of life in patients with interstitial lung disease. Respirology . 2012;17:506–512. doi: 10.1111/j.1440-1843.2012.02131.x. [DOI] [PubMed] [Google Scholar]
- 176. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J . 2009;34:648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 177. Suzuki A, Kondoh Y, Swigris JJ, Matsuda T, Kimura T, Kataoka K, et al. Performance of the COPD Assessment Test in patients with connective tissue disease-associated interstitial lung disease. Respir Med . 2019;150:15–20. doi: 10.1016/j.rmed.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 178. Matsuda T, Taniguchi H, Ando M, Kondoh Y, Kimura T, Kataoka K, et al. COPD Assessment Test for measurement of health status in patients with idiopathic pulmonary fibrosis: a cross-sectional study. Respirology . 2017;22:721–727. doi: 10.1111/resp.12936. [DOI] [PubMed] [Google Scholar]
- 179. Patel AS, Siegert RJ, Keir GJ, Bajwah S, Barker RD, Maher TM, et al. The minimal important difference of the King’s Brief Interstitial Lung Disease Questionnaire (K-BILD) and forced vital capacity in interstitial lung disease. Respir Med . 2013;107:1438–1443. doi: 10.1016/j.rmed.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 180. Nolan CM, Birring SS, Maddocks M, Maher TM, Patel S, Barker RE, et al. King’s Brief Interstitial Lung Disease questionnaire: responsiveness and minimum clinically important difference. Eur Respir J . 2019;54:1900281. doi: 10.1183/13993003.00281-2019. [DOI] [PubMed] [Google Scholar]
- 181. Wapenaar M, Patel AS, Birring SS, Domburg RTV, Bakker EW, Vindigni V, et al. Translation and validation of the King’s Brief Interstitial Lung Disease (K-BILD) questionnaire in French, Italian, Swedish, and Dutch. Chron Respir Dis . 2017;14:140–150. doi: 10.1177/1479972316674425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Sinha A, Patel AS, Siegert RJ, Bajwah S, Maher TM, Renzoni EA, et al. The King’s Brief Interstitial Lung Disease (KBILD) questionnaire: an updated minimal clinically important difference. BMJ Open Respir Res . 2019;6:e000363. doi: 10.1136/bmjresp-2018-000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Swigris JJ, Wilson SR, Green KE, Sprunger DB, Brown KK, Wamboldt FS. Development of the ATAQ-IPF: a tool to assess quality of life in IPF. Health Qual Life Outcomes . 2010;8:77. doi: 10.1186/1477-7525-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Russell A-M, Wickremasinghe M, Saketkoo LA, Borril Z, Fletcher S, Adamali HI, et al. preliminary testing of the idiopathic pulmonary fibrosis patient reported outcome measure (IPF PRoM) [abstract] Am J Respir Crit Care Med. 2018;197:A7707. [Google Scholar]
- 185. Russell A-M, Doyle AM, Fleming S, Burdett C, Ross D, Aden Z, et al. Development of a patient reported outcome measure (PRoM) in idiopathic pulmonary fibrosis (IPF): incorporating a research partnership with patients [abstract] Am J Respir Crit Care Med . 2015;191:A5190. [Google Scholar]
- 186. Kimman ML, Rotteveel AH, Wijsenbeek M, Mostard R, Tak NC, van Jaarsveld X, et al. Development and pretesting of a questionnaire to assess patient experiences and satisfaction with medications (PESaM Questionnaire) Patient . 2017;10:629–642. doi: 10.1007/s40271-017-0234-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Kimman ML, Wijsenbeek MS, van Kuijk SMJ, Wijnsma KL, van de Kar NCAJ, Storm M, et al. PESaM Collaborating Group. Validity of the Patient Experiences and Satisfaction with Medications (PESaM) Questionnaire. Patient . 2019;12:149–162. doi: 10.1007/s40271-018-0340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Russell AM, Sonecha S, Datta A, Hewitt R, Howell I, Elliott A, et al. P276 Development of patient reported experience measure (PREM) for idiopathic pulmonary fibrosis (IPF) Thorax . 2016;71:A238.1–A238. [Google Scholar]
- 189. Swigris JJ, Yorke J, Sprunger DB, Swearingen C, Pincus T, du Bois RM, et al. Assessing dyspnea and its impact on patients with connective tissue disease-related interstitial lung disease. Respir Med . 2010;104:1350–1355. doi: 10.1016/j.rmed.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Swigris JJ, Han M, Vij R, Noth I, Eisenstein EL, Anstrom KJ, et al. The UCSD shortness of breath questionnaire has longitudinal construct validity in idiopathic pulmonary fibrosis. Respir Med . 2012;106:1447–1455. doi: 10.1016/j.rmed.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: the UCSD shortness of breath questionnaire. Chest . 1998;113:619–624. doi: 10.1378/chest.113.3.619. [DOI] [PubMed] [Google Scholar]
- 192. Rajala K, Lehto JT, Sutinen E, Kautiainen H, Myllärniemi M, Saarto T. mMRC dyspnoea scale indicates impaired quality of life and increased pain in patients with idiopathic pulmonary fibrosis. ERJ Open Res . 2017;3:00084-2017. doi: 10.1183/23120541.00084-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Kato K, Kataoka K, et al. A simple assessment of dyspnoea as a prognostic indicator in idiopathic pulmonary fibrosis. Eur Respir J . 2010;36:1067–1072. doi: 10.1183/09031936.00152609. [DOI] [PubMed] [Google Scholar]
- 194. Khanna D, Tseng C-H, Furst DE, Clements PJ, Elashoff R, Roth M, et al. Scleroderma Lung Study Investigators. Minimally important differences in the Mahler’s Transition Dyspnoea Index in a large randomized controlled trial: results from the Scleroderma Lung Study. Rheumatology (Oxford) . 2009;48:1537–1540. doi: 10.1093/rheumatology/kep284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Mahler DA, Witek TJ., Jr The MCID of the transition dyspnea index is a total score of one unit. COPD . 2005;2:99–103. doi: 10.1081/copd-200050666. [DOI] [PubMed] [Google Scholar]
- 196. Yorke J, Moosavi SH, Shuldham C, Jones PW. Quantification of dyspnoea using descriptors: development and initial testing of the Dyspnoea-12. Thorax . 2010;65:21–26. doi: 10.1136/thx.2009.118521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Yorke J, Swigris J, Russell A-M, Moosavi SH, Ng Man Kwong G, Longshaw M, et al. Dyspnea-12 is a valid and reliable measure of breathlessness in patients with interstitial lung disease. Chest . 2011;139:159–164. doi: 10.1378/chest.10-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc . 1982;14:377–381. [PubMed] [Google Scholar]
- 199. Yount SE, Choi SW, Victorson D, Ruo B, Cella D, Anton S, et al. Brief, valid measures of dyspnea and related functional limitations in chronic obstructive pulmonary disease (COPD) Value Health . 2011;14:307–315. doi: 10.1016/j.jval.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 200. Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MDL, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ) Thorax . 2003;58:339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chung KF, Widdicombe J, editors. Berlin, Germany: Springer; 2009. Pharmacology and therapeutics of cough. [PubMed] [Google Scholar]
- 202. Key AL, Holt K, Hamilton A, Smith JA, Earis JE. Objective cough frequency in idiopathic pulmonary fibrosis. Cough . 2010;6:4. doi: 10.1186/1745-9974-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Scholand MB, Wolff R, Crossno PF, Sundar K, Winegar M, Whipple S, et al. Severity of cough in idiopathic pulmonary fibrosis is associated with MUC5 B genotype. Cough . 2014;10:3. doi: 10.1186/1745-9974-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Saunders P, Saini G, Marshall RP, Fahy WA, Duggan A-M, Wells AU, et al. Cough related quality of life in patients with idiopathic pulmonary fibrosis: initial findings from the PROFILE cohort. Am J Respir Crit Care Med . 2017;195:A1543. [Google Scholar]
- 205. Lechtzin N, Hilliard ME, Horton MR. Validation of the Cough Quality-of-Life Questionnaire in patients with idiopathic pulmonary fibrosis. Chest . 2013;143:1745–1749. doi: 10.1378/chest.12-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. De Vries J, Michielsen H, Van Heck GL, Drent M. Measuring fatigue in sarcoidosis: the Fatigue Assessment Scale (FAS) Br J Health Psychol . 2004;9:279–291. doi: 10.1348/1359107041557048. [DOI] [PubMed] [Google Scholar]
- 207. de Kleijn WPE, De Vries J, Wijnen PAHM, Drent M. Minimal (clinically) important differences for the Fatigue Assessment Scale in sarcoidosis. Respir Med . 2011;105:1388–1395. doi: 10.1016/j.rmed.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 208. Drent M, Lower EE, De Vries J. Sarcoidosis-associated fatigue. Eur Respir J . 2012;40:255–263. doi: 10.1183/09031936.00002512. [DOI] [PubMed] [Google Scholar]
- 209. Atkins CP, Gilbert D, Brockwell C, Robinson S, Wilson AM. Fatigue in sarcoidosis and idiopathic pulmonary fibrosis: differences in character and severity between diseases. Sarcoidosis Vasc Diffuse Lung Dis . 2016;33:130–138. [PubMed] [Google Scholar]
- 210. Thunold RF, Løkke A, Cohen AL, Ole H, Bendstrup E. Patient reported outcome measures (PROMs) in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2017;34:2–17. doi: 10.36141/svdld.v34i1.5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Egerton T, Riphagen II, Nygård AJ, Thingstad P, Helbostad JL. Systematic content evaluation and review of measurement properties of questionnaires for measuring self-reported fatigue among older people. Qual Life Res . 2015;24:2239–2255. doi: 10.1007/s11136-015-0963-1. [DOI] [PubMed] [Google Scholar]
- 212. Stern AF. The Hospital Anxiety and Depression Scale. Occup Med (Lond) . 2014;64:393–394. doi: 10.1093/occmed/kqu024. [DOI] [PubMed] [Google Scholar]
- 213. Glaspole IN, Watson AL, Allan H, Chapman S, Cooper WA, Corte TJ, et al. Determinants and outcomes of prolonged anxiety and depression in idiopathic pulmonary fibrosis. Eur Respir J . 2017;50:1700168. doi: 10.1183/13993003.00168-2017. [DOI] [PubMed] [Google Scholar]
- 214. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med . 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 215. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep . 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 216. Lancaster LH, Mason WR, Parnell JA, Rice TW, Loyd JE, Milstone AP, et al. Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest . 2009;136:772–778. doi: 10.1378/chest.08-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Mermigkis C, Stagaki E, Tryfon S, Schiza S, Amfilochiou A, Polychronopoulos V, et al. How common is sleep-disordered breathing in patients with idiopathic pulmonary fibrosis? Sleep Breath . 2010;14:387–390. doi: 10.1007/s11325-010-0336-5. [DOI] [PubMed] [Google Scholar]
- 218. Weatherwax KJ, Lin X, Marzec ML, Malow BA. Obstructive sleep apnea in epilepsy patients: the Sleep Apnea Scale of the Sleep Disorders Questionnaire (SA-SDQ) is a useful screening instrument for obstructive sleep apnea in a disease-specific population. Sleep Med . 2003;4:517–521. doi: 10.1016/j.sleep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 219. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care . 1992;30:473–483. [PubMed] [Google Scholar]
- 220. Chang JA, Curtis JR, Patrick DL, Raghu G. Assessment of health-related quality of life in patients with interstitial lung disease. Chest . 1999;116:1175–1182. doi: 10.1378/chest.116.5.1175. [DOI] [PubMed] [Google Scholar]
- 221. Martinez TY, Pereira CA, dos Santos ML, Ciconelli RM, Guimarães SM, Martinez JA. Evaluation of the short-form 36-item questionnaire to measure health-related quality of life in patients with idiopathic pulmonary fibrosis. Chest . 2000;117:1627–1632. doi: 10.1378/chest.117.6.1627. [DOI] [PubMed] [Google Scholar]
- 222. Tomioka H, Imanaka K, Hashimoto K, Iwasaki H. Health-related quality of life in patients with idiopathic pulmonary fibrosis--cross-sectional and longitudinal study. Intern Med . 2007;46:1533–1542. doi: 10.2169/internalmedicine.46.6218. [DOI] [PubMed] [Google Scholar]
- 223. Khanna D, Clements PJ, Furst DE, Chon Y, Elashoff R, Roth MD, et al. Scleroderma Lung Study Group. Correlation of the degree of dyspnea with health-related quality of life, functional abilities, and diffusing capacity for carbon monoxide in patients with systemic sclerosis and active alveolitis: results from the Scleroderma Lung Study. Arthritis Rheum . 2005;52:592–600. doi: 10.1002/art.20787. [DOI] [PubMed] [Google Scholar]
- 224. Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res . 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Mulhern B, Feng Y, Shah K, Janssen MF, Herdman M, van Hout B, et al. Comparing the UK EQ-5D-3L and English EQ-5D-5L value sets. Pharmacoeconomics . 2018;36:699–713. doi: 10.1007/s40273-018-0628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: An EQ-5D-5L value set for England. Health Econ . 2018;27:7–22. doi: 10.1002/hec.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Szentes BL, Kreuter M, Bahmer T, Birring SS, Claussen M, Waelscher J, et al. Quality of life assessment in interstitial lung diseases: a comparison of the disease-specific K-BILD with the generic EQ-5D-5L. Respir Res . 2018;19:101. doi: 10.1186/s12931-018-0808-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. PROMIS Cooperative Group. The Patient-reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap Cooperative Group during its first two years. Med Care . 2007;45:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Schifferdecker KE, Yount SE, Kaiser K, Adachi-Mejia A, Cella D, Carluzzo KL, et al. A method to create a standardized generic and condition-specific patient-reported outcome measure for patient care and healthcare improvement. Qual Life Res . 2018;27:367–378. doi: 10.1007/s11136-017-1675-5. [DOI] [PubMed] [Google Scholar]
- 230.Dimmock AEF, Lehmann H, Dimmock TH, Danoff SK, Chuang CH, Cordova F, et al. Assessment of quality of life in a cohort of patients with idiopathic pulmonary fibrosis using PROMIS-29 [abstract] Am J Respir Crit Care Med 2017195A7702. [Google Scholar]