Abstract
Purpose:
Acyclovir is most commonly used for treating ocular Herpes Keratitis, a leading cause of infectious blindness. However, emerging resistance to Acyclovir resulting from mutations in the thymidine kinase gene of Herpes Simplex Virus -1 (HSV-1), has prompted the need for new therapeutics directed against a different viral protein. One novel target is the HSV-1 Processivity Factor which is essential for tethering HSV-1 Polymerase to the viral genome to enable long-chain DNA synthesis.
Methods:
A series of peptides, based on the crystal structure of the C-terminus of HSV-1 Polymerase, were constructed with hydrocarbon staples to retain their alpha-helical conformation. The stapled peptides were tested for blocking both HSV-1 DNA synthesis and infection. The most effective peptide was further optimized by replacing its negative N-terminus with two hydrophobic valine residues. This di-valine stapled peptide was tested for inhibiting HSV-1 infection of human primary corneal epithelial cells.
Results:
The stapled peptides blocked HSV-1 DNA synthesis and HSV-1 infection. The unstapled control peptide had no inhibitory effects. Specificity of the stapled peptides was confirmed by their inabilities to block infection by an unrelated virus. Significantly, the optimized di-valine stapled peptide effectively blocked HSV-1 infection in human primary corneal epithelial cells with selectivity index of 11.6.
Conclusions:
Hydrocarbon stapled peptides that simulate the α-helix from the C-terminus of HSV-1 DNA polymerase can specifically block DNA synthesis and infection of HSV-1 in human primary corneal epithelial cells. These stapled peptides provide a foundation for developing a topical therapeutic for treating human ocular Herpes Keratitis.
Keywords: Herpes keratitis, Herpes simplex virus-1, DNA polymerase, Processivity factor, Hydrocarbon stapled peptide
1. Introduction
Herpes Simplex Virsus-1 (HSV-1) resides in the majority of the human population where it becomes transmitted through saliva and mucosal secretions. Following the primary oral infection, which is often asymptomatic, HSV-1 enters nerve endings and migrates to the cranial trigeminal ganglia (TG). Eventually, within the TG, herpes DNA becomes stably maintained, establishing a non-infectious latent state. The latent herpes DNA will persist indefinitely in the TG, until it is reactivated by one of several physiological stresses, including hormonal or immune fluctuations. Following reactivation, HSV-1 can migrate down the mandibular branch of the TG, as it commonly does, to manifest as typical cold sores on the lip and mouth. Alternatively, Herpes Keratitis (HK) will result if reactivated HSV-1 migrates along the ophthalmic branch of the TG to reach the eye and infect the corneal epithelium (1–4). Notably, HK may also result from persistence of HSV-1 in the corneal epithelium (4).
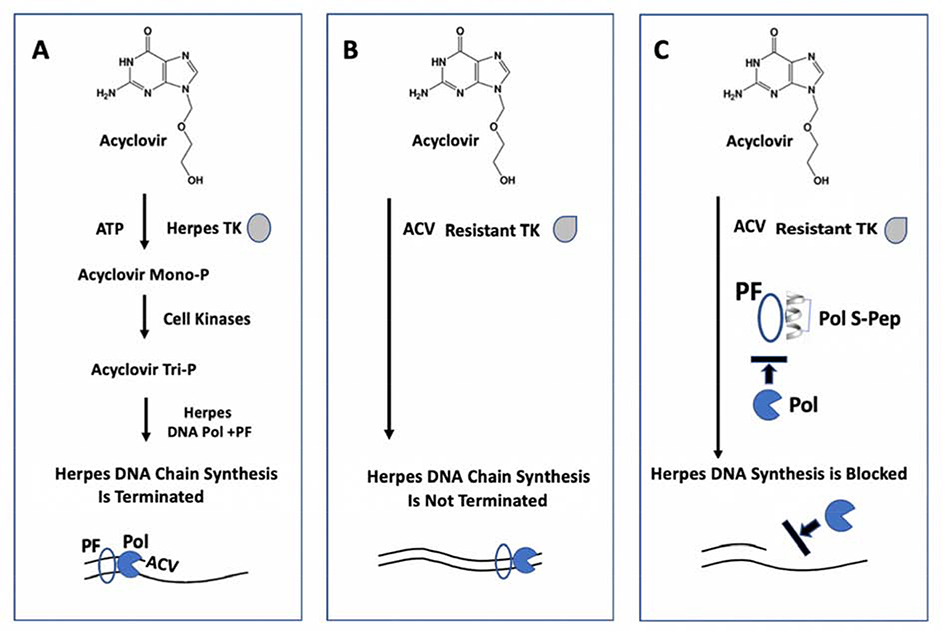
Acyclovir (ACV), a guanosine nucleoside analogue, has served as the gold standard for treating HK. As depicted in Fig. 1A, for ACV to function as an antiviral it initially must be converted to ACV monophosphate by the HSV-1 encoded thymidine kinase (TK). While ACV is extremely effective against oral and genital herpes with negligible drug failures, resistance to ACV has emerged in about 7% in immune-competent HK patients and even greater in immune-compromised HK patients (5–11). Significantly, this resistance to ACV (Fig. 1B) correlates primarily with specific mutations in the TK of HSV-1 isolated from HK patients no longer responsive to the drug (5–9, 12–14). None of the other ACV-related inhibitors (ganciclovir, valacyclovir, famciclovir or vidarabine) can be used when HK becomes unresponsive to ACV, because they likewise are no longer able to be initially phosphorylated by HSV-1 TK. This leaves only trifluridine as an FDA approved drug against ocular HK (reviewed in 15). While trifluridine (Viroptic) has proven effectiveness, its prolonged use is limited by concerns of toxicity related to the fact that, as a fluorinated pyrimidine nucleoside, it can block any DNA polymerase. Trifluridine does not specifically target HSV-1.
Fig. 1. Depiction of how a stapled alpha-helical peptide against HSV-1 Polymerase provides a novel strategy to block viral infection in ocular epithelial cells that are resistant to Acyclovir (ACV).
A. Initial phosphorylation of Acyclovir by HSV-1 Thymidine Kinase (TK) is essential for it to function as a chain terminator of viral DNA synthesis. Pol, polymerase; PF, processivity factor.
B. Mutations in the TK gene of HSV-1 cause resistance to Acyclovir so that it can no longer terminate HSV-1 DNA chain synthesis.
C. A stapled α-helical peptide (Pol S-Pep) that conforms to the C-terminus of HSV-1 DNA Polymerase, blocks viral DNA synthesis by targeting the HSV-1 Processivity Factor (PF), so that it is no longer available to bind and tether the Polymerase to the viral DNA template.
The emergence of ACV resistance in treating HK necessitates the development of a new, safe and effective ocular herpes drug directed against a different HSV-1 target. As depicted in Fig. 1C, one such novel target is the HSV-1 processivity factor protein UL42, which is essential for binding and tethering the HSV-1 Polymerase (UL30) to the template to enable continuous viral DNA synthesis (16, 17). The attractiveness of UL42 is not only its absolute requirement for viral replication, but its specificity for UL30. In this study, we report the construction of a stapled peptide that structurally mimics the C-terminus of UL30 and demonstrate its ability to block UL42-dependent processive DNA synthesis in vitro, as well as HSV-1 infection of human primary corneal epithelial cells.
2. Materials and methods
2.1. Cells
The African green monkey kidney epithelial cells (Vero and BSC-1) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) supplemented with 5% FBS, 2 mM L-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin. Human primary corneal epithelial cells were purchased from ATCC® (PCS-700–010™). These human primary corneal epithelial cells were grown in serum-free corneal epithelial cell medium (ATCC® PCS-700–030™) supplemented with corneal epithelial cell growth kit (ATCC® PCS-700–040™) according to the manufacturer’s instruction.
2.2. Plasmid construction.
Viral genomic DNA was isolated from HSV-1 strain KOS (a kind gift from Dr. Gary H. Cohen) via treatment with Proteinase K at 56°C for 1 h, followed by phenol-chloroform extraction and ethanol precipitation. The viral DNA was used to amplify full-length UL30 and UL42 genes by PCR with the following primer sets: UL30 forward (5’-GACAAGCTTGCGATGTTTTCCGGTGGCGGCGGCCCGCT) and reverse (5’-GTGTCTAGATCATGCTAGAGTATCAAAGGCTCTATG) UL42 forward (5’-GTCAAGCTTGGGATGACGGATTCCCCTGGCGGTGT) and reverse (5’-GACTCTAGATCAGGGGAATCCAAAACCATACGGGGT).
Each of the forward primers contains a HindIII site (underlined) and the original Kozak translation initiation sequences (bold) of the two genes, whereas the reverse primers comprise a XbaI site (underlined). PCR was conducted using Herculase enhanced DNA polymerase according to the manufacturer’s protocol (Agilent Technologies, Inc.). Amplified PCR products were ligated into the HindIII and XbaI sites of pcDNA3.1(+) plasmid (Invitrogen). Both cloned genes were confirmed by DNA sequencing. The vaccinia virus (VV) DNA polymerase E9, and Processivity Factor A20 and D4 genes were cloned as previously described (18). All constructs were used for in vitro translation using the TNT T7 coupled reticulocyte lysate system (Promega).
2.3. Stapled peptides
All peptides were synthesized by Bio-Synthesis, Inc. (Lewisville, TX) with reported purities >95%. Stapled peptides were all-hydrocarbon cross-linked at positions i, i+4 or i, i+7.
2.4. In vitro processive DNA synthesis assay.
Processive DNA synthesis was performed by the enzyme-linked immunosorbent assay (ELISA)-based Rapid Plate Assay (19, 20, 21) using in vitro translated viral DNA polymerase and processivity factor. Briefly, a 5’-biotinylated 100-nucleotide template that contains adenines only at its 5’ distal end was annealed with a 15-nucleotide primer to its 3’ end and attached to streptavidin-coated 96-plate wells (Roche Applied Science). DNA synthesis was carried out in 50 μL reaction mixture containing 100 mM (NH)2S04, 20 mM Tris-HCl (pH 7.5), 3 mM MgCl2, 0.1 mM EDTA, 0.5 mM DTT, 2% glycerol, 5 μM dATP, 5 μM dCTP, 5 μM dGTP, 1 μM digoxigenin-11-dUTP, and in vitro translated UL30 and UL42 proteins. The TNT reticulocyte lysate was used as a negative control. After incubation at 37°C for 30 min, the plate was washed extensively with PBS containing 0.1% Tween-20 (PBST). The wells were then incubated with anti-digoxigenin-peroxidase antibody (Roche) for 1 h at 37°C, followed by washing with PBST. The substrate 2,2′-azino-bis(3-ethylbenzthiazoline)-sulfonate (Roche) was added to allow color development. DNA synthesis was quantified by measuring the absorbance at 405 nm with a microplate reader (Tecan). Experiments were conducted in triplicate and independently repeated at least twice.
2.5. HSV-1 infection of human primary corneal epithelial cells.
Human primary corneal epithelial cells were seeded in a 48-well plate (104 cells/well) in 300 μL growth medium and cultured to 80–90% confluence. The human primary corneal epithelial cells were infected by absorbing HSV-1 (KOS) at ~100 PFU/well in 100 μL growth medium for 1 h. The cells were washed 4 times with PBS to remove unabsorbed virus, followed by treatment with S-pep7B at different dilutions in 300 μL growth medium containing 1% DMSO. At 72 h post-treatment, virus titers in the culture medium were determined by titration via standard plaque reduction assays in 48-well plates using Vero cells as described below.
2.6. Plaque reduction assays.
As previously described (22), cells with ~90% confluence in 48-well plates were infected by absorbing virus (~100 PFU/well) for 1 h in 100 μL growth medium, followed by adding 200 μL culture medium containing DMSO vehicle or serially diluted peptides or Acyclovir (Tocris Bioscience -Fisher Scientific) to each well of the plate.
DMSO was maintained at 1% throughout the treatment. Cells were subsequently fixed and stained with 300 μL PBS containing 4% formaldehyde and 0.2% crystal violet overnight at room temperature. HSV-1 plaques were quantified at ~55 h post-infection of Vero cells. VV (WR strain) infection of BSC-1 cells was analyzed for plaque formation after 24 h. The plaque reduction assays were performed in duplicate and independently repeated twice.
2.7. Cytotoxicity and cell viability assays.
Cells were grown in 96-well plates to ~80% confluence and treated with peptides at different concentrations in 150 μL of growth medium containing 1% DMSO. At 24 h post-treatment, 100 μL of the culture medium was used for the lactate dehydrogenase (LDH) cytotoxicity assay as previously reported (22). In addition, the cells from the same treatment were assessed with an ATP-based cell viability assay. Briefly, after the growth medium was completely removed, the cells were lysed in 100 μL of 1% Triton X-100 in PBS per well at room temperature. After 10 min, 5 μl of lysate was used for ATP-based luciferase/luminescence assay according to the manufacture’s protocol (Invitrogen, USA). The assays were performed in triplicate and independently repeated twice.
2.8. Analysis of HSV-1 DNA replication in infected cells treated with S-pep7B stapled peptide.
Confluent Vero cells in 48-well plates were infected by absorbing 105 PFU of HSV-1 (MOI ~1) for 1 h in 100 μL of DMEM medium containing 5% FBS. Unabsorbed virus was removed by washing the wells 4 times with PBS. Cells were then treated with S-pep7B peptide in triplicate at different dilutions in 300 μL growth medium containing 1% DMSO. At 4 h post-treatment, cells of each triplicate treatment were combined and lysed with 20 mM Tris buffer (pH 7.5) containing 20 mM EDTA, 0.5% SDS, and 0.5 mg/ml proteinase K. Total DNA was then prepared from the lysed cells with phenol/chloroform extraction and ethanol precipitation, and used for UL42 gene amplification by PCR using the primers described above for plasmid construction. After agarose gel electrophoresis, UL42 DNA levels were quantitated using an image capture and analysis system (G:Box Chemi HR, Syngene) and normalized to that of the cellular house keeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase), which was amplified using primers (forward: 5′-ACATCATCCCTGCCTCTAC-3′, and reverse: 5′-TCAAAGGTGGAGGAGTGG-3′, a kind gift from Dr. Yan Yuan). The assays were independently repeated twice.
2.9. Data analysis
Half-maximal (EC50, IC50, and CC50) values were obtained by nonlinear regression fitting to a variable slope, four parameter dose-response model using the Prizm 6 software (GraphPad Software, LaJolla, CA).
3. Results
3.1. Constructing stapled peptides that mimic the α-helical domain of the extreme C-terminus of UL30 DNA Polymerases.
Stapled α-helical peptides have emerged as a new class of therapeutics applicable for targeting protein-protein interactions (24, 25, 26, 27). Hydrocarbon stapling is a method of constraining an α-helical peptide by crosslinking two amino acid sidechains. Positioning of the staple on the α-helical peptide relies upon knowing the co-crystal structure of the protein from which it is derived in contact with its target protein. Moreover, unlike the typically disordered structure of unconstrained natural peptides, introduction of the staple as a brace to stabilize the helical structure decreases susceptibility by acting as a protease shield that can extend residence-time (23). Hydrocarbon stapled peptides share key characteristics of both small-molecule (e.g., cell permeability) and large biological drugs (e.g., highly specific target binding), and are increasingly being developed as therapeutics in many medical areas including cancer, metabolism, neuroscience, and infectious disease (26, 27).
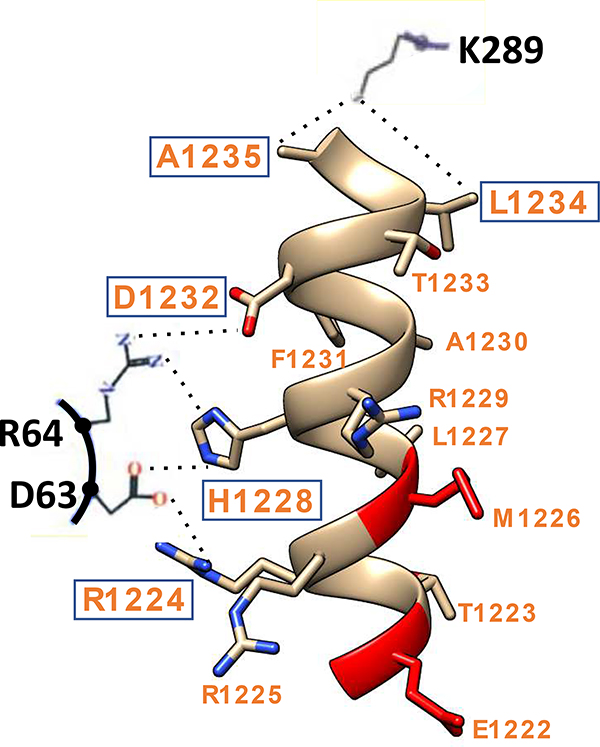
Our object was to design stapled peptides that can prevent the HSV-1 processivity factor UL42 from interacting with its cognate Polymerase UL30 as a means of blocking viral DNA synthesis and infection. The co-crystal structure of UL42 and UL30 (28) revealed that the C-terminal 36 amino acids of UL30 forms an αβα structure that binds to UL42. The extreme 15 amino acid C-terminal α-helix of UL30 is largely buried in a deep groove of UL42, with the interface contributed by electrostatic, hydrophobic, and hydrogen bonding interactions (Fig. 2). Based on this structure, we designed a series of stapled peptides that mimic the extreme C-terminal α-helix of UL30.
Fig. 2.
Interaction between HSV-1 DNA Polymerase (UL30) and Processivity Factor (UL42). The extreme C-terminus of UL30 forms an α-helix (shown as orange ribbon with residues indicated) that makes multiple interactions with Ul42. Hydrogen bonds between three residues (K289, R64, D63, in black) of UL42 with residues R1224, H1228, D1232, L1234, and A1235 (boxed) of UL30 C-terminus are indicated as dotted lines. This interactive depiction is based on the UL30/UL42 co-crystal structure of HSV-1 (28).
As shown in Fig. 3, peptide staples were introduced at positions i, i+4 or i, i+7 in order to stabilize the α-helix (3.6 amino acids/turn). Based on the UL30-UL42 co-crystal structure (28), the staples were positioned on the solvation-side of the α-helix of UL30 in order to prevent interfering with residues on the contact side required for making hydrogen bonds with UL42. Specifically, as shown in Fig. 2, these UL42 residues are D63, R64 and K289 (depicted in black) that make hydrogen bonds (dotted lines) with UL30 residues R1224, H1228, D1232, L1234, and A1235 (depicted in orange and boxed).
Fig. 3.
Stapled peptide constructs based on the sequence of the extreme C-terminus of HSV-1 UL30 DNA polymerase. The native peptide (N-pep) comprises the C-terminal 14 amino acids residues 1222 to 1235 of UL30. The amino acids of the UL30 peptides that are known to make contact with HSV-1 Processivity Factor (UL42) are boxed. Stapled peptides (S-pep1 to 8) were generated by all-hydrocarbon crosslinking at positions i, i+4 or i, i+7. X = (S)-2-(4-pentenyl) alanine; R8=(R)-2-(7-octenyl) alanine.
3.2. Stapled peptides of UL30 Polymerase block processive DNA synthesis in a mechanistic assay.
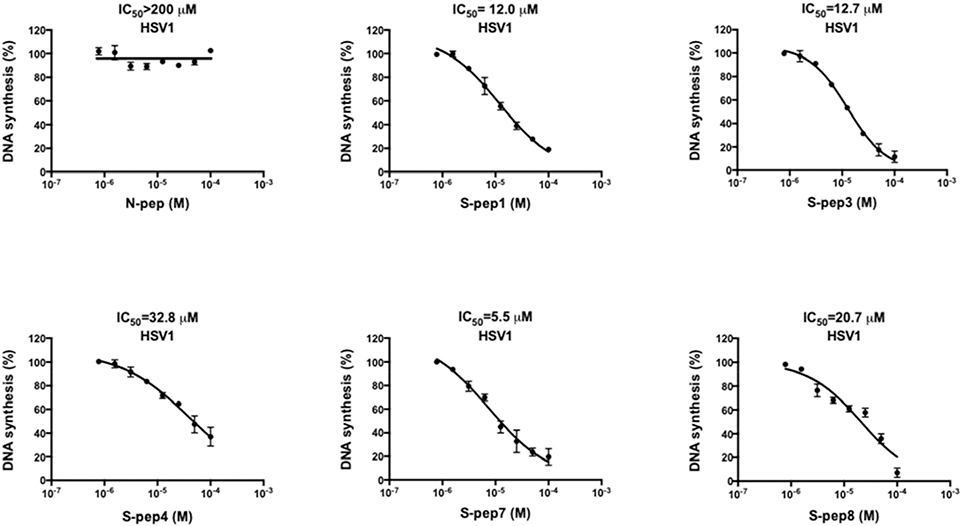
We first tested if the UL30 stapled peptides could block in vitro processive DNA synthesis directed by recombinant UL30 and UL42 proteins in our mechanistic Rapid Plate Assay (19, 20, 21). As shown in Fig. 4, the native control peptide N-pep (ETRRMLHRAFDTLA) was totally incapable of inhibiting processive DNA synthesis in vitro (IC50>200 μM). Interestingly, Digard et al., (29), demonstrated that a similar native peptide with an extra N-terminal glutamic acid residue (EETRRMLHRAFDTLA) also exhibited very poor activity in blocking DNA synthesis in vitro, whereas a slightly longer peptide (ATAEETRRMLHRAFDTLA) could inhibit DNA synthesis with an IC50=30 μM. Notably this same study (29) did not examine inhibition of HSV-1 cellular infection by these two native peptides.
Fig. 4.
Inhibition of HSV-1 processive DNA synthesis by stapled peptides in a mechanistic in vitro DNA processivity assay. HSV-1 UL30 DNA Polymerase and UL42 Processivity Factor were translated in vitro and then used to conduct processive DNA synthesis in the presence of increasing amounts of Native (N) or Stapled (S) peptides. DNA synthesis was quantified via product-dependent colorimetry. The data represents mean ±SD from at least two independent experiments in triplicate
To explore stapled peptides capable of inhibiting processive DNA synthesis, we initially produced three comparable constructs (S-pep1, S-pep2, and S-pep3) linking positions 1222 and 1226 (Fig. 3). S-pep1 effectively inhibited processive DNA synthesis at IC50=12.0 μM (Fig. 4), suggesting the adoption of a stable helical structure capable of binding to UL42. A similar result was obtained for S-pep3 (IC50=12.7 μM, Fig. 4), which extends two additional amino acids (Ala1220 and Glu1221) N-terminal of the staple (Fig. 3). This result with S-pep3 indicated that the very N-terminus of the stapled peptide can be modified and still maintain its ability to contact the UL42 processivity factor and block DNA synthesis. Elimination of the last four C-terminal amino acids (Asp1232-Ala1235) produced S-pep2, (Fig. 3) which lost activity (>200 μM), substantiating the importance of residues Leu1234 and Ala1235 that are known to make contact with Lys289 of UL42 (28). In support of the requirement of Leu1234 for peptide activity, the i, i+7 staple introduced at the corresponding Leu1227 and Leu1234 positions resulted in an inactive S-pep6 (>200 μM).
Accordingly, each of the remaining peptides in this series (S-pep4, S-pep5, S-pep7, S-pep8), purposely avoided introducing staples at residues known to contact the target protein UL42 (Fig. 3). As shown in Fig. 4, S-pep4, S-pep7, and S-pep8 were able to inhibit processive DNA synthesis in vitro, with IC50 = 32.8 μM, 5.5 μM, and 20.7 μM, respectively. Interestingly, S-pep5 was the only stapled peptide that failed to inhibit processive DNA synthesis, even though all residues known to contact the UL42 target remained intact. Comparison of S-pep5 with S-pep1 and S-pep3 suggests that in the context of this particular linkage where position 1222 connects with 1226, Thr1223 needs to be juxtaposed to residue Arg1224, perhaps to maintain an essential bond angle for contacting Asp63 of the UL42 target protein.
In summary, these data demonstrate that UL30 stapled peptides have a very high probability of functioning as inhibitors of processive DNA synthesis when residues that are required to contact the UL42 target protein are preserved. Conversely, there is a perfect correlation between the inability of UL30 stapled peptides to block processive DNA synthesis when linkers replace residues required to contact the UL42 target protein.
3.3. UL30 Polymerase stapled peptides block HSV-1 plaque formation in Vero cells.
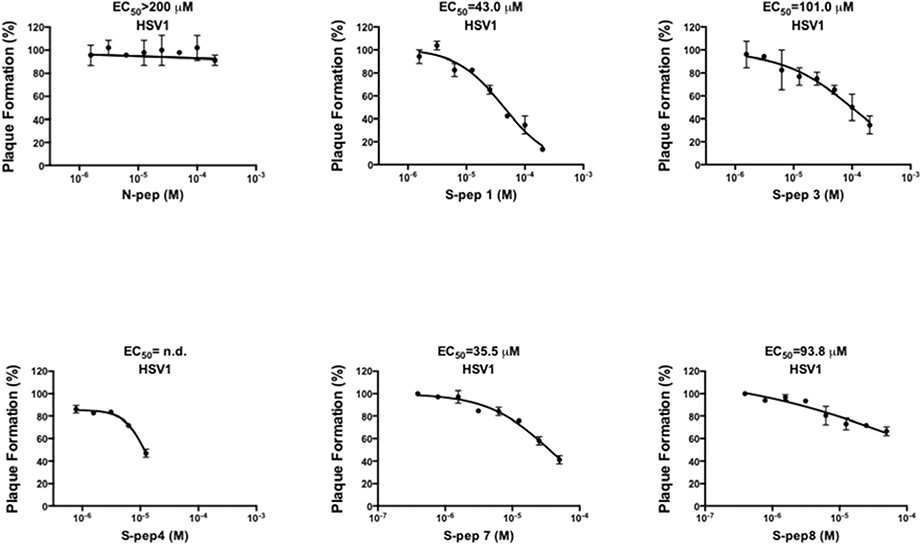
Next, standard viral plaque reduction assays were conducted in Vero cells to determine if any of the five stapled peptides (S-pep1/3/4/7/8) that were able to block processive DNA synthesis, could also block HSV-1 infection. To minimize possible interference of HSV-1 cell attachment and entry by the peptides, cells were first infected by absorbing the virus for 1 h, followed by treatment with peptides at serial dilutions. Consistent with the DNA synthesis observation, the native peptide N-pep showed no inhibition of HSV-1 infection (EC50>200 μM, Fig. 5). By contrast, inhibition was observed for S-pep1 (EC50=43.0 μM) and S-pep7 (EC50=35.5 μM)) (Fig. 5) revealing their potential as candidates for further optimization. The EC50 values of both S-pep3 and S-pep8 were approximately 100 μM (Fig. 5), which was considered too high to justify any further optimization. S-pep4 was toxic at 12.5 μM, which precluded its continuation for improvement.
Fig. 5.
Inhibition of HSV-1 plaque formation by stapled peptides in Vero cells. HSV-1 (~100 PFU) was first absorbed onto near confluent Vero cells for 1 h to allow cell attachment and entry of the virus. After the absorption, increasing concentrations of peptides were added. Following 55 h treatment, viral plaques were counted and used to calculate the EC50 values.
3.4. A di-valine analog of S-pep7 blocks processive DNA synthesis more effectively and exhibits increased anti-viral potency against HSV-1.
We investigated if we could optimize our most favorable stapled peptide (S-pep7) from the primary pool of constructs presented in Fig. 3. Results of the in vitro processive DNA synthesis assay (Fig. 4) indicated that the extreme C-terminus of the stapled peptides comprising Leucine-Alanine cannot be altered or perturbed. This was made evident by comparing S-pep1 with S-pep2 and S-pep4 with S-pep6. By contrast, the extreme N-terminus was able to be modified without affecting DNA synthesis, as revealed by comparing S-pep1 with S-pep3. Hence, being able to change the N-terminus of the stapled peptides without diminishing their abilities to block DNA synthesis, provided the rational for testing amino acid substitutions that could potentially increase antiviral potency in HSV-1 infected cells.
Since S-pep7 was the most effective stapled peptide capable of blocking both HSV-1 processive DNA synthesis (IC50=5.5 μM) and cellular infection (EC50=35.5 μM), we chose to incorporate alterations at its N-terminus that could enhance cellular uptake. Our predictions were based on biophysical features known to affect cellular uptake of stapled peptides that include hydrophobicity, charge, amphipathicity, and structure (30; 31). Hence, as shown in Fig. 6A, we replaced the negatively charged N-terminal Glu1222 with one and two hydrophobic Val residues to form S-pep7A and S-pep7B, respectively, or with one and two positively charged Arg residues to form S-pep7C and S-pep7D, respectively. When compared to the parental S-pep7 (IC50=5.5 μM, Fig. 4), each of the N-terminal modified peptides had a comparable increase in ability to block HSV-1processive DNA synthesis in vitro, with IC50 values ranging between 1.0 μM (S-pep7B) to 2.0 μM (S-pep7D) (Fig. 6B). We next inquired whether the stapled peptides could also block HSV-1 from infecting Vero cells. Viral plaque reduction assays revealed that both the single and double Val substitutions had increased antiviral potency against HSV-1, with EC50 = 9.6 μM and 9.8 μM for S-pep7A and S-pep7B, respectively (Fig. 6C, left and middle) compared to parental S-pep7 with EC50 = 35.5 μM (Fig. 5). As a direct comparison, we also tested Acyclovir in blocking HSV-1 plaque formation, which displayed an EC50=0.81 μM (Fig. 6C, right). It is noted that while S-pep7A and S-pep7B were less potent in blocking HSV-1 plaque formation in Vero cells, both peptides had IC50 values around 1 μM in the in vitro processive DNA synthesis mechanistic assay (Fig. 6B), suggesting that these two peptides can be further optimized to improve their cellular antiviral potency close to that of Acyclovir. Formation of viral plaques could not be determined for S-pep7C and S-pep7D due to cellular toxicity (below).
Fig. 6.
Effect of substituting and adding amino acids at the N-terminus of S-pep7. (A) Amino acid substitutions or additions introduced at the N-terminus of S-pep7. The N-terminal Glu1222 in S-pep7 was replaced by a single Val (S-pep7A), two Val (S-pep7B), a single Arg (S-pep7C), or two Arg (S-pep7D). All altered amino acids are italicized and underlined. (B) In vitro processive DNA synthesis conducted by recombinant proteins of HSV-1 UL42 and UL30 in the presence of increasing concentrations of each modified S-peptide. (C) HSV-1 plaque reduction assay. Following 1 h absorption of HSV-1 onto Vero cells, S-pep7A and S-pep7B were added at increasing concentrations and plaques counted after 55 h (left and middle). For direct comparison, inhibition of HSV-1 plaques by Acyclovir (ACV) was also performed (right). (D) Cytotoxicity of S-pep7B. Vero cells were treated with S-pep7B at two-fold serial dilutions for 24h and measured for intracellular ATP content and LDH leakage. Data represents mean ±SD obtained from at least two independent experiments performed in triplicate. (E) Specificity of S-pep7B as shown by its inability to block both in vitro processive DNA synthesis conducted by vaccinia virus proteins (Left) and vaccinia virus infection (Right). The data represents mean ±SD from at least two independent experiments performed in duplicate.
Cellular cytotoxicity of the N-terminal substituted stapled peptides was assessed. S-pep7A caused observable cell death at 50 μM in the plaque assays. Both the single and double Arg substitutions (S-pep7C and S-pep7D) exhibited dramatic cytotoxicity at 5 μM (data not shown), which is consistent with the concept that positively charged stapled peptide can trigger cell lysis (30). By contrast, S-pep7B showed no visible cytotoxicity in the plaque reduction assays. Specifically, the measurements of intracellular ATP content and LDH leakage produced similar cytotoxicity information: CC50 values of 114 μM for ATP and 126 μM for LDH (Fig. 6D).
We next evaluated the specificity of S-pep7B by testing its antiviral activity directed against vaccinia virus (VV), which is completely unrelated to HSV-1. As shown in Fig. 6E (left), S-pep7B completely failed to block in vitro DNA synthesis conducted by recombinantly expressed polymerase and processivity factor of VV (IC50>50 μM). Correspondingly, S-pep7B did not inhibit VV from infecting cells (Fig. 6E, right), confirming its antiviral specificity as a herpes virus inhibitor.
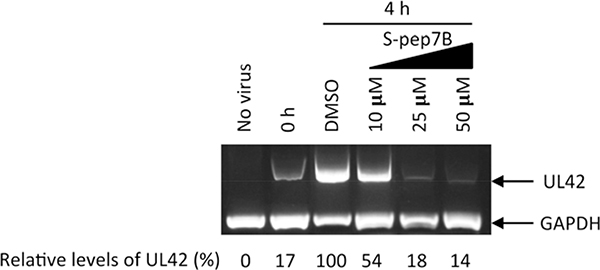
We next needed to ascertain if the HSV-1 antiviral activity by S-pep7B is specifically due to the inhibition of viral DNA replication. Following 1 h absorption of HSV-1, cells were treated with vehicle DMSO or increasing concentrations of S-pep7B. At 4 h post-treatment, viral genomic DNA was extracted from the cells and then used for amplification of the UL42 gene. As shown in Fig. 7, the UL42 DNA levels in infected cells were greatly reduced by S-pep7B in a dose-dependent manner. At 50 μM, S-pep7B completely suppressed the viral DNA level to that of time point 0 h, which was the start of S-pep7B treatment (Fig. 7, leftmost lane). This result is consistent with the plaque assay showing that no HSV-1 plaques were formed in the presence of 50 μM S-pep7B (Fig. 6C, center). These data clearly indicate that S-pep7B blocks HSV-1 infection by preventing the viral DNA replication.
Fig. 7.
Inhibition of HSV-1 DNA replication by S-pep7B in infected cells. Confluent Vero cells were infected by absorbing HSV-1 (MOI ~1) for 1 h (marked as time point 0 h), followed by treatment with vehicle DMSO or S-pep7B at indicated concentrations. At 4 h post-treatment, viral genomic DNA was extracted from the cells and then used for amplification of the UL42 gene. Following agarose gel electrophoresis, relative % levels of UL42 DNA were determined after being normalized to that of the cellular house-keeping gene GAPDH. The level of UL42 DNA from vehicle treatment was arbitrarily set at 100.
3.5. S-pep7B blocks HSV-1 infection in human primary corneal epithelial cells.
Corneal infection by HSV-1 is the most frequent cause of vision loss by herpes keratitis. We tested if S-pep7B could block HSV-1 infection in human primary corneal epithelial cells. Following 1 h absorption of HSV-1, the cells were treated with S-pep7B at increasing concentrations for 72 h. Unlike the clearly defined viral plaques formed on Vero cells following HSV-1 infection (presented above), distinctly quantifiable plaques were not apparent on human primary corneal epithelial cells due to morphological differences. To circumvent this issue, the amount of virus produced in the culture media of HSV-1 infected human primary corneal epithelial cells was collected and then quantitated by titration on Vero cells to evaluate plaque reduction by S-pep7B.
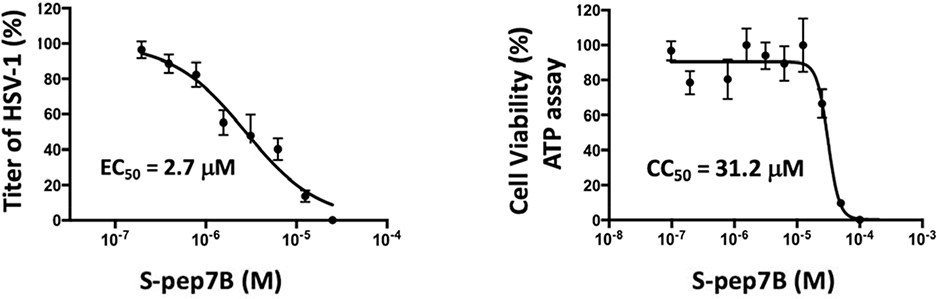
As shown in Fig. 8 Left-Panel, HSV-1 infection of human primary corneal epithelial cells was blocked by S-pep7B at an EC50=2.7 μM. As shown in Fig. 8 Right-Panel, when the human primary corneal epithelial cells were tested for cytotoxicity in the presence of increasing concentrations of S-pep7B, a CC50=31.2 μM was obtained by ATP measurement. The determined Selectivity Index (SI)=11.6 for S-pep7B in the human primary corneal epithelial cells proved to be identical for that obtained in Vero cells. Significantly, these results clearly demonstrate that S-pep7B is able to inhibit HSV-1 replication in human primary corneal epithelial cells.
Fig. 8.
(Left Panel) Inhibition of HSV-1 infection of human primary corneal epithelial cells by S-pep7B. Following 1 h absorption of HSV-1 onto human primary corneal epithelial cells, the cells were washed thoroughly to remove any unabsorbed virus and then treated with S-pep7B at different dilutions. At 72 h post-treatment, virus titers in the culture media were determined by plaque reduction assays using Vero cells. (Right Panel) S-pep7B cytotoxicity in the primary cells was determined after 24 h by measuring ATP content. All data represents mean±SD obtained from two independent experiments in duplicate.
4. Discussion
Herpes Keratitis (HK) is caused by a recurrent HSV-1 infection of the ocular surface that results in progressive corneal scarring and loss of vision. While Acyclovir is the most commonly used drug for treating HK, it falls short of guaranteeing full protection due to an increase in viral resistance as a consequence of mutations arising in the TK gene of HSV-1. This has produced a considerable effort to discover new antivirals, including both small molecules and peptides to treat ocular HSV infections (Reviewed in 32, 33).
One novel target is the HSV-1 Processivity Factor (PF) UL42 which is essential for tethering HSV-1 Polymerase UL30 to the viral genome to enable long-chain DNA synthesis. The attractive feature about PFs is that they function only with their cognate polymerases, making them specific drug targets (34). In this study, we have engineered and tested stapled peptides from the C-terminus of UL30 to target the UL42 PF of HSV-1. Most of the stapled peptides were able to effectively block processive DNA synthesis in a mechanistic assay. By comparison, the unstapled peptide had no inhibitory effect, thereby supporting the premise that critically positioned staples on the UL30 peptide reinforce the α-helical conformation required to promote specific interaction with the UL42 PF target. Furthermore, peptides with the hydrocarbon staple linked to UL30 residues known to hydrogen-bond with the UL42 PF failed to block processive DNA synthesis, reinforcing the importance of key contact residues as depicted in the UL30-UL42 co-crystal structure (28).
Optimization of the most promising stapled peptide (S-pep7) was explored by altering the N-terminus. Whereas the addition of positively charged arginine residues proved toxic in Vero cells, the introduction of hydrophobic di-valine to form S-pep7B, increased the potency several-fold. Notably, S-pep7B exhibited a potency (EC50 =2.7 μM) and SI=11.6 in blocking HSV-1 infection in human primary corneal epithelial cells. Future strategies can be explored to generate analogs of S-pep7B with greater antiviral potency in blocking HSV-1 infection.
In summary, we have shown that a stapled peptide, designed in conformity to the α-helix of the C-terminus of HSV-1 DNA Polymerase can block infection in human primary corneal epithelial cells by specifically targeting the viral processivity factor. This unique and specific antiviral mechanism demonstrated by these stapled peptides provides the potential for developing a new drug to treat human ocular Herpes Keratitis, especially in cases where the medical need is unmet due to Acyclovir resistance.
Acknowledgements:
The National Eye Institute Grant 1R41EY026849 to R.P.R., and funding from the University of Pennsylvania Institute of Translational Medicine and Therapeutics (An Institutional Clinical and Translational Science Award from National Center for Advancing Translational Sciences/NIH/DHHS) to R.P.R. and V.L. supported this work.
Footnotes
Disclosure: We claim that there is no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Rajasagi NK, Rouse BT. Application of our understanding of pathogenesis of herpetic stromal keratitis for novel therapy. Mic Inf, 2018. 017.12.014 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Dujaili LJ, Clerkin PP, Clement C, McFerrin HE, Bhattacharjee PS, Varnell ED, Kaufman HE, Hill JM. 2011. Ocular herpes simplex virus: how are latency, reactivation, recurrent disease and therapy interrelated? Future Microbiol. 6:877–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dix RD. Pathogenesis of herpes simplex ocular disease. 2002. Vol. 2. Capter 89. Lippincott, Williams and Wilkins, Philadelphia, PA. [Google Scholar]
- 4.Polcicova K, Biswas PS, Banerjee K, Wisner TW, Rouse BT, Johnson DC. 2005. Herpes keratitis in the absence of anterograde transport of virus from sensory ganglia to the cornea. Proc Natl Acad Sci USA. 102:11462–11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piret J, Boivin G. 2011. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 55:459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burrel S, Boutolleau D, Azar G, Doan S, Deback C, Cochereau I, Agut H, Gabison EE. 2013. Phenotypic and genotypic characterization of acyclovir-resistant corneal HSV-1 isolates from immunocompetent patients with recurrent herpetic keratitis. J Clin Virol. 58:321–324. [DOI] [PubMed] [Google Scholar]
- 7.Duan R, de Vries RD, Osterhaus AD, Remeijer L, Verjans GM. 2008. Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J Infect Dis. 198:659–663. [DOI] [PubMed] [Google Scholar]
- 8.Duan R, de Vries RD, van Dun JM, van Loenen FB, Osterhaus AD, Remeijer L, Verjans GM. 2009. Acyclovir susceptibility and genetic characteristics of sequential herpes simplex virus type 1 corneal isolates from patients with recurrent herpetic keratitis. J Infect Dis. 200:1402–1414. [DOI] [PubMed] [Google Scholar]
- 9.van Velzen M, Missotten T, van Loenen FB, Meesters RJ, Luider TM, Baarsma GS, Osterhaus AD, Verjans GM. 2013. Acyclovir-resistant herpes simplex virus type 1 in intra-ocular fluid samples of herpetic uveitis patients.J Clin Virol 57:215–221. [DOI] [PubMed] [Google Scholar]
- 10.van Velzen M, van de Vijver DA, van Loenen FB, Osterhaus AD, Remeijer L, Verjans GM. 2013. Acyclovir prophylaxis predisposes to antiviral-resistant recurrent herpetic keratitis. J Infect Dis. 208:1359–1365. [DOI] [PubMed] [Google Scholar]
- 11.James SH, Prichard MN. 2013. A possible pitfall in acyclovir prophylaxis for recurrent herpetic keratitis? J Infect Dis. 208:1353–1355. [DOI] [PubMed] [Google Scholar]
- 12.Roozbahani M, Hammersmith KM. Management of herpes simplex virus epithelial keratitis. Curr Opin Ophthalmol. 2018. 29:360–364. Review. [DOI] [PubMed] [Google Scholar]
- 13.Choong K, Walker NJ, Apel AJ, Whitby M. 2010. Aciclovir-resistant herpes keratitis. Clin Experiment7Ophthalmol. 38:309–313. [DOI] [PubMed] [Google Scholar]
- 14.Andrei G1, Snoeck R. 2013. Herpes simplex virus drug-resistance: new mutations and insights. Curr Opin Infect Dis. 26:551–260. [DOI] [PubMed] [Google Scholar]
- 15.White ML MD, Chodosh J, 2014. Herpes Simplex Virus Keratitis: A Treatment Guideline – Harvard Medical School. One Network. [Google Scholar]
- 16.Digard P, Coen DM. 1990. A novel functional domain of an alpha-like DNA polymerase. The binding site on the herpes simplex virus polymerase for the viral UL42 protein. J Biol Chem. 265:17393–17396. [PubMed] [Google Scholar]
- 17.Digard P, Bebrin WR, Weisshart K, Coen DM. 1993. The extreme C terminus of herpes simplex virus DNA polymerase is crucial for functional interaction with processivity factor UL42 and for viral replication. J Virol. 67:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Druck Shudofsky AM, Silverman JE, Chattopadhyay D, Ricciardi RP. 2010. Vaccinia virus D4 mutants defective in processive DNA synthesis retain binding to A20 and DNA. J Virol. 84:12325–12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin K, Ricciardi RP. 2000. A rapid plate assay for the screening of inhibitors against herpesvirus DNA polymerases and processivity factors. J Virol Methods. 88:219–225. [DOI] [PubMed] [Google Scholar]
- 20.Ricciardi RP, Lin K, Chen X, Dorjsuren D, Shoemaker R, Sei S. 2005. Rapid screening of chemical inhibitors that block processive DNA synthesis of herpesviruses: potential application to high-throughput screening. Methods Mol Biol. 292:481–492. [DOI] [PubMed] [Google Scholar]
- 21.Ciustea M, Silverman JE, Druck Shudofsky AM, Ricciardi RP. 2008. Identification of non-nucleoside DNA synthesis inhibitors of vaccinia virus by high-throughput screening. J Med Chem. 51:6563–6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuth M, Huang L, Saw YL, Schormann N, Chattopadhyay D, Ricciardi RP. 2011. Identification of inhibitors that block vaccinia virus infection by targeting the DNA synthesis processivity factor D4. J Med Chem. 54:3260–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schafmeister Christian E., Po Julia, and Verdine Gregory L.. An All-Hydrocarbon Cross-Linking System for Enhancing the Helicity and Metabolic Stability of Peptides. J. Am. Chem. Soc, 2000, 122 (24), pp 5891–5892 [Google Scholar]
- 24.Walensky LD and Bird GH. Hydrocarbon-stapled peptides: principles, practice, and progress. J. Med. Chem. 2014, 57: 6275–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cromm PM, Spiegel J, Grossmann TN, 2015. Hydrocarbon stapled peptides as modulators of biological function. ACS Chem. Biol. 10:1362–1375. [DOI] [PubMed] [Google Scholar]
- 26.Hillman RA, Nadraws JW, Bertucci MA, 2018. The hydrocarbon staple & beyond: recent advances towards stapled peptide therapeutics that target protein-protein interactions. Curr. Top. Med. Chem.18:611–624. [DOI] [PubMed] [Google Scholar]
- 27.Ali AM, Atmaj J, Van Oosterwijk N, Groves MR, Dömling A, 2019. Stapled peptides inhibitors: a new window for target drug discovery. Comput. Struct. Biotechnol. J. 17:263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuccola HJ, Filman DJ, Coen DM, Hogle JM. 2000. The crystal structure of an unusual processivity factor, herpes simplex virus UL42, bound to the C terminus of its cognate polymerase. Mol Cell. 5:267–278. [DOI] [PubMed] [Google Scholar]
- 29.Digard P, Williams KP, Hensley P, Brooks IS, Dahl CE, Coen DM. 1995. Specific inhibition of herpes simplex virus DNA polymerase by helical peptides corresponding to the subunit interface. Proc Natl Acad Sci USA. 92:1456–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bird GH, Mazzola E, Opoku-Nsiah K, Lammert MA, Godes M, Neuberg DS, Walensky LD. 2016. Biophysical determinants for cellular uptake of hydrocarbon-stapled peptide helices. Nat Chem Biol. 12: 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakagami K, Masuda T, Kawano K, Futaki S, 2018. Importance of net hydrophobicity in the cellular uptake of all-hydrocarbon stapled peptides. Mol. Pharm. 15:1332–1340. [DOI] [PubMed] [Google Scholar]
- 32.Koganti R, Yadavalli T, Shukla D. 2019. Current and Emerging Therapies for Ocular Herpes Simplex Virus Type-1 Infections. Microorganisms. 7:429–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brandt CR. 2014. Peptide therapeutics for treating ocular surface infections. J Ocul Pharmacol Ther. 30:691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin K, Dai CY, Ricciardi RP. 1998. Cloning and functional analysis of Kaposi’s sarcoma-associated herpesvirus DNA polymerase and its processivity factor. J Virol. 72:6228–6232. [DOI] [PMC free article] [PubMed] [Google Scholar]