Abstract
Total-Body (TB) PET/computed tomography (CT) provides a substantial gain in the physical sensitivity of PET, leading to vastly improved image quality or can enable imaging applications that are either not possible with conventional PET or suffer from poor signal-to-noise. The paradigm-shifting promise of TB-PET/CT lies in its capability to perform dynamic, delayed, and low-dose imaging, which have the potential to increase the range of diseases and disorders that can be investigated or managed using PET. Here we discuss the use of TB-PET/CT and describe protocols that take advantages of this unique innovation applied to the needs of the pediatric population.
Introduction
Following developments in the EXPLORER Consortium, in December 2018 the first Total-Body (TB) PET/CT system obtained FDA 510(k) clearance and became commercially available in the US. The main concept underlying this revolutionary idea was to extend the axial field-of-view (FOV) of PET from 16–30 cm, which had been used in conventional whole body (WB) PET/CT scanners, to 194 cm—an axial length long enough to simultaneously image the entire body1. This dramatic increase in the PET system’s axial length corresponds to a significant increase in the total detector volume, which now surrounds the entire patient body. The elongated PET geometry and increase in detector volume lead to a substantial increase in the system’s signal detection efficiency when compared to current conventional PET/CT scanners (approximately 40 times more)2. This gain has the potential to significantly improve PET imaging procedures in pediatric patients in several ways: (1) reduction of acquisition time (whole body scan < 1 minute), or (2) reduction in administrated activity (as low as 1/20th of the standard dose), or (3) delayed imaging (up to 6 radiotracer half-lives), (4) if using conventional uptake time, scan time, and injected dose regimens, providing reconstructed images with much improved signal-to-noise ratio and therefore superior image spatial resolution and quality or (5) an optimized combination of those 3. Furthermore, TB-PET/CT allows imaging the entire body function at the same time, with a temporal resolution less than 1 second per imaging frame 4, which has profound advantages for whole body dynamic imaging applications. In this contribution, we review each of these benefits in turn, but given its current dominant place in the application of pediatric PET we start by separately discussing the potential benefits in oncologic applications.
Pediatric oncology:
Pediatric patients are frequently diagnosed with malignant diseases that are diffuse in the pattern of involvement. For example, hematologic malignancies such as lymphomas and leukemias, which involve the bone marrow and the associated organs such as the lymph nodes and spleen, account for a large number of pediatric cancers.5,6 In addition, pediatric solid tumors can metastasize to many different sites and often children must be assessed for disease in several different parts of the body. Therefore, a modality that allows image acquisition of the entire body will provide significant advantages in the management of such patients. Recent developments of data analysis schemes that provide segmentation of the entire skeleton will likely be well suited for analyzing data generated by TB PET/CT imaging instruments.6 Such approaches have been adopted for image analysis in patients with multiple myeloma and other adult hematologic diseases that primarily involve the bone marrow throughout the skeleton.7
Currently, PET/CT with fluorodeoxyglucose (FDG-PET/CT) is recommended for staging, restaging after neoadjuvant chemotherapy and follow up of pediatric bone and soft tissue tumors, including Ewing Tumor and Osteosarcoma.8 For tumors such as germinal cell tumors, hepatoblastoma and brain tumors, conventional FDG-PET/CT can be used to assess for the presence of recurrence. Furthermore, PET/CT with other imaging agents has great potential in theranostic imaging applications, and could have immediate impact in the near future in patients such as the subset of neuroblastomas that show no MIBG (iodine-123 metaiodobenzylguanidine) uptake but that are candidates for targeted therapy based on diagnostic imaging results, e.g. Lutathera and its imaging diagnostic correlate Ga68-labelled DOTA peptide SSTR receptor.9,10 In all these cases, the improved image quality that can be provided by TB-PET/CT has the potential to increase test sensitivity and (more speculatively), to improve test specificity by improving anatomic localization or by means of parametric imaging/kinetic modelling.
TB PET/CT will also bring significant advantages for imaging research applications in pediatric oncology, by offering the potential for very significant reductions in radiation dose, by enabling studies that could not be done using conventional PET due to low signal or by improving the data quality in current protocols. Lastly, TB-PET/CT imaging protocols using rapid acquisitions may eliminate current logistical barriers in patients who otherwise may require anesthesia to mitigate motion artifacts in long duration scans.
Imaging Faster
The increase in the axial FOV with TB-PET has two distinct advantages that could allow for significantly shorter imaging times. First, the long cylindrical geometry of TB scanners increases the amount of signal captured by detecting annihilation photons that are emitted with oblique angles. The magnitude of this increase in the scanner’s detection efficiency will also depend upon the patient size and, based on simulation results of an adult-sized phantom, is approximately a 4x fold increase in signal per axial slice. The second advantage of TB-PET over conventional PET instruments is rather straightforward; because of the scanner’s extended axial coverage, only a single bed position is needed. To assume that conventional PET scanning requires 2 minute bed positions to produce adequate image quality, a 30 second image acquisition with TB-PET could be sufficient to provide images with an axial slice with roughly matched signal. Recent imaging reconstruction experiments using data obtained in list-mode from human subjects have demonstrated that whole body imaging can be performed in less than one minute. The quality of images generated is not significantly different from that of a conventional PET/CT scanner.3,11
TB-PET for fast whole-body scanning may eliminate the need for anesthesia in patients able to stay still for sort period but not for the duration of a conventional PET/CT scan. Avoiding anesthesia reduces the risks associated with sedation and intubation, especially for those children that require multiple imaging evaluations during their therapy and disease evaluation. This may enable scanning in outside facilities where sedation is not an option. This procedure can be facilitated using the help of a child life specialist, motivating the children to stay still during acquisition. For such a dedicated short acquisition (in the order of 30–60 seconds), the pediatric protocol needs to include the current dose regimen (for example, 0.1–0.15 mCi per kg up to 8 mCi for FDG). The administered amount of activity may be reduced in proportion to the degree of compliance of the patient to the increased scan time.
Low-Dose Imaging
With the advances made in managing many malignancies, it has been estimated that about 84% of pediatric patients survive their cancer for at least 5 years, compared with only 58% in the mid 1970s.12 Obviously, the survival mostly depends on the type of cancer and the stage of the disease at diagnosis but overall the prognosis has improved for most patients. By now it is well established that exposing pediatric subject to radiation may lead to increased risk of developing secondary malignancy in the future. However, radiation exposure due to frequent performance of diagnostic imaging procedures has accelerated over the past 10 years. Several disciplines in medicine have been advocating for moderation of radiation exposure in the pediatric population. In particular, the Image Gently Campaign began in 2008 and has been successful in increasing radiologist awareness of the importance of decreasing the radiation dose to the pediatric population.
Several pediatric cancers (e.g., Ewing tumor/Osteosarcoma), inflammatory/infectious conditions (e.g., chronic granulomatous disease) and hereditary diseases (e.g., Li-Fraumeni syndrome) may benefit from PET/CT scans for longitudinal follow-up and screening purposes. For example, a diagnostic scheme for many pediatric malignancies may include 3 to 4 PET/CT scans. One PET/CT is usually acquired at staging; a second PET/CT is at mid-treatment (eg. after 2nd chemotherapy cycle for lymphoma) or after neoadjuvant therapy and a third PET/CT after completion of therapy. Often the child may be rescanned a few months or years later when there is high risk of tumor recurrence or when clinical indicators or laboratory test results are suspicious for disease relapse.
Older children and adolescent patients may be able to maintain stillness to the same degree as adults and for this population, particularly where repeated scanning is indicated, it may be appropriate to use TB-PET/CT with a scan time of, say, 20 minutes but with substantially reduced administered activity. However, the lower limit of injected activity remains to be determined and will almost certainly vary by body habitus; for example, for smaller patients, high spatial resolution is desirable, and this will be negatively impacted by the use of less injected activity. Another concern for very low injected activity protocols is the proper administration of the radiotracer, because technologists may be less experienced in the accurate handling of such small amounts of radioactive material.
If appropriate, such protocols may be coupled with a super-low dose CT scan for attenuation correction 13, with the trade-off between low dose and CT image quality being duly noted.
More speculatively, the ability to image with low dose has the potential to broaden the scope with which molecular imaging may be applied. For example, at low doses, the risk to benefit ratio may become much more favorable in musculoskeletal imaging and it is possible to imagine how, say, inflammation imaging could play a role in the management of adolescents and young adults undergoing ligament repair following trauma; there may also be benefits in the management of pediatric patients suffering from autoimmune diseases.
Delayed Imaging
The TB-PET geometric sensitivity gain can be used to scan several half-lives after radiotracer administration. Preliminary data acquired at UC Davis (Figure 1) and at the University of Pennsylvania 11confirmed the feasibility of obtaining diagnostic quality scans many hours after FDG injection. The possibility of delayed imaging over several half-lives is now a reality in the field and it may provide additional clinically useful information and increase tumor detectability in patients with cancer. Different organs and pathologic entities are characterized by different uptake and washout over time. For FDG, at delayed time points the radiotracer concentration in the background tends to decrease, whereas its uptake within tumors is shown to increase substantially.14,15 Therefore, delayed imaging may allow improved detection of small tumor. For example, it is possible to recognize tumor recurrence after radiation therapy in organs that are highly FDG avid such as the brain. Delayed imaging of the brain has shown substantially reduced activity, compared to a standard scan obtained at 60 minutes after radiotracer injection (Figure 2). This may improve detection of recurrent brain tumors. In contrast, inflammatory lesions are often characterized by early wash out.15 Therefore, on delayed images, tumor may appear more prominent while inflammatory changes may be less conspicuous. This could allow differentiation of radiation therapy induced inflammatory changes and tumor recurrence.
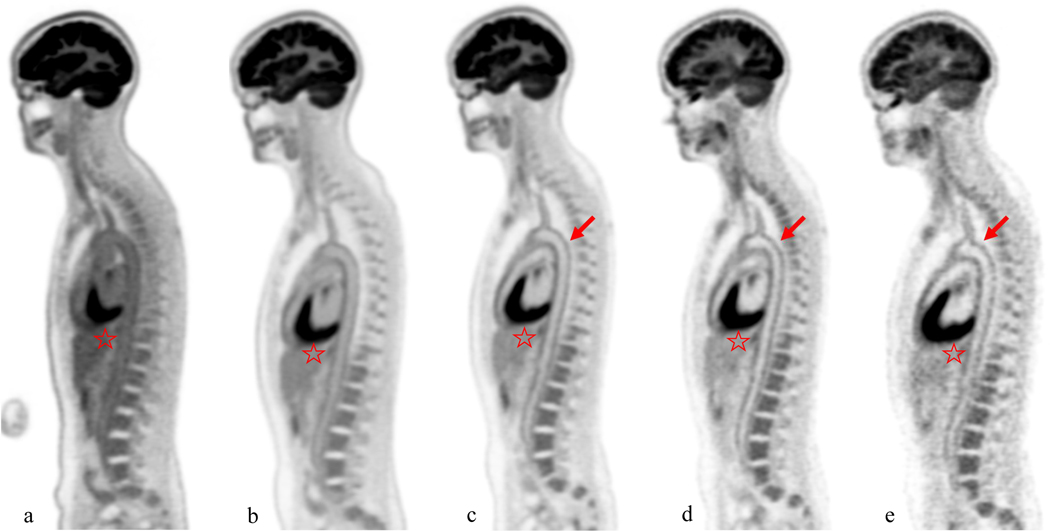
Figure 1:
Female, 41 year-old, 53 kg healthy volunteer subject. Sagittal slice images at the level of the aortic arch were obtained at 40 minutes (a), 90min (b), 3 h (c), 6 h (d), and 9 h (e) after injection of 10mCi of 18F-FDG. The aorta demonstrates the decreasing blood pool activity over time, leading to progressively higher signal to noise at the level of the aortic wall (arrows) on delayed images. Also brain and liver uptake (star) decreases on delayed images. However, the noise increases on delayed images leading to image quality at 9 hours comparable to image quality of a conventional PET/CT scan obtained at 60 minute.
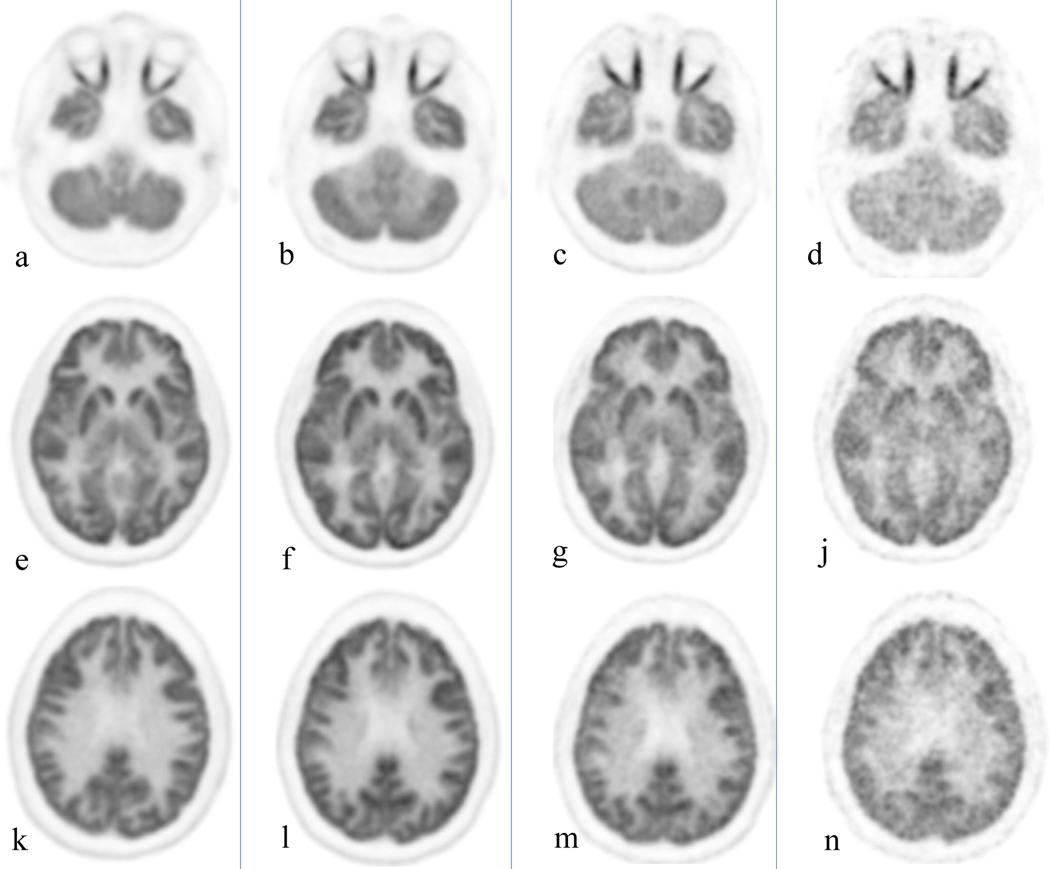
Figure 2:
Healthy volunteer, total body PET/CT 20 minute acquisition at 40 minutes, 3, 6 and 9 hours after administration of 10 mCi of 18F-FGD. Cuts were selected at the orbit level (a-d), basal ganglia (e-j) and upper aspect of the ventricles (k-n). Brain uptake slowly decreases over time; the increase noise level starts to become clinically evident at 9 hours.
The possibility of scanning at delayed time points also open the door to adopting novel radiotracers that may play a major role in assessing children with benign and malignant disorders. It will facilitate the use of radiotracers with short half-lives, such as those labeled with O-15, C-11 or N-13. TB-PET/CT may also allow detection of activity several weeks after injection of radiotracers with longer half-lives such as Zr-89 16.
Improved Image Quality
The increase in signal-to-noise ratio provided by TB-PET improves both the contrast of small structures and the uniformity of large structures (Figure 3). As such, the detection of small and/or low-contrast normal structures as well as disease sites is improved. In our recent experience, we were able to better identify several anatomical structures such as the spinal cord, the pituitary and adrenal glands, as well as small soft tissue tumor deposits (Figure 4). The improved resolution gained by this approach opens the door to a more precise quantification of small structures. For example, the uptake in the adrenal glands at 60 minutes in normal subjects is often higher than the liver background. On conventional PET/CT scanners (particularly the older models), the adrenals are difficult to resolve and often their level of uptake is underestimated due to the partial volume effect.
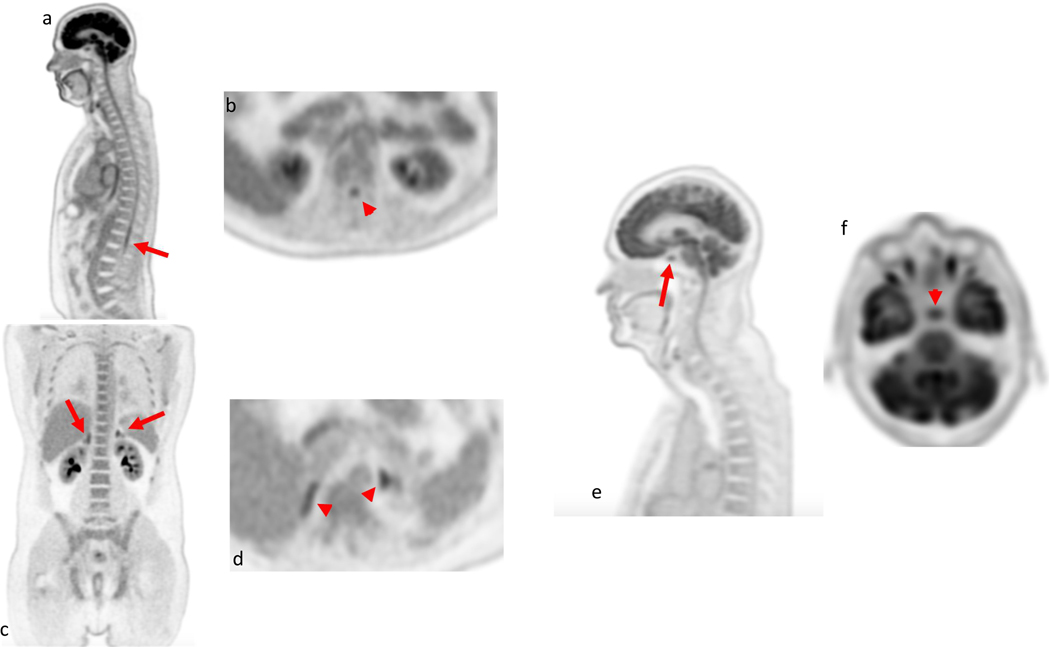
Figure 3:
Several small anatomic structures are nicely demonstrated in these healthy volunteers scanned at 90 minutes after administration of 10 mCi of 18F-FDG. The spinal cord (arrow and arrow ahead) is well seen including the conus midollaris at the level of the upper lumbar spine on both the sagittal images (a) and axial images (b); The adrenals glands (arrows and arrow heads) are visualized with uptake higher than liver background in presented coronal (c) and axial images (d). The pituitary gland (arrow and arrow ahead) has FDG uptake higher than liver background and is visualized in the sagittal (e) and in the axial (f) images.
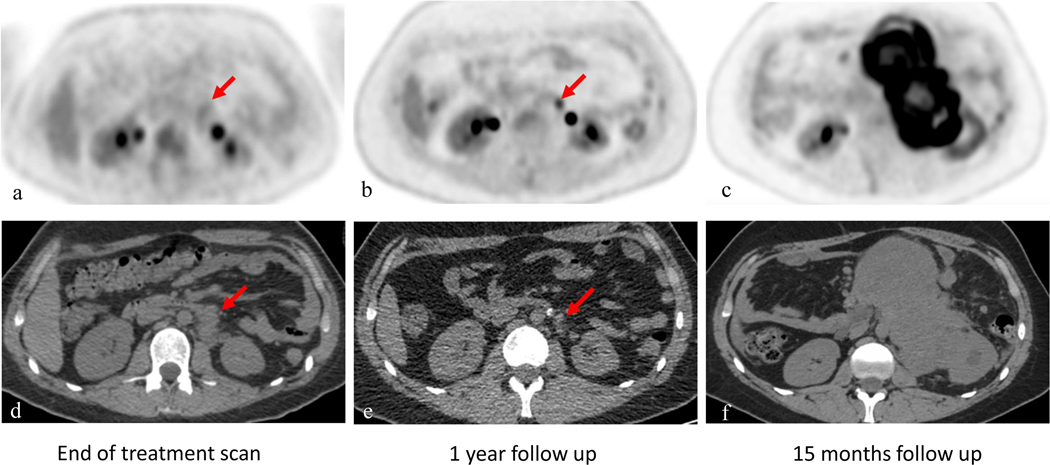
Figure 4:
18 year-old male with right testicular rhabdomyosarcoma. PET images of the left retroperitoneal mass (red arrow) are shown on the top (a-c), CT correlated are seen on the bottom (d-f); Figure a and d from conventional PET/CT scanner obtained after treatment. Follow up scan obtained one year later using TB-PET/CT (b and e) shows decrease in size of retroperitoneal disease but increase focal uptake in this area (arrow) without any clinical suspicion of disease recurrence. In this clinical scenario, PET/CT finding non consistent with clinical picture, a close follow up scan in 3 months was obtained (c and f) demonstrating extensive retroperitoneal disease and confirming the prior TB scan of recurrent disease.
The improved uniformity leads to improved assessment of the larger organs. For example, the increase signal to noise ratio decreases the apparent heterogeneity of uptake within the liver and therefore improves the overall performance of FDG-PET in this organ. In our experience with TB PET/CT, the prevalent random foci of apparent uptake in the liver due to background noise has been low, both decreasing the false positive/uncertain results and facilitating the detection of small metastases.
An improvement in the known role of FDG-PET/CT in assessing therapy response is also seen using TB PET/CT. For example, as shown on Fig. 5, we were able to detect small photopenic areas within the homogenously activated bone marrow.
Figure 5:
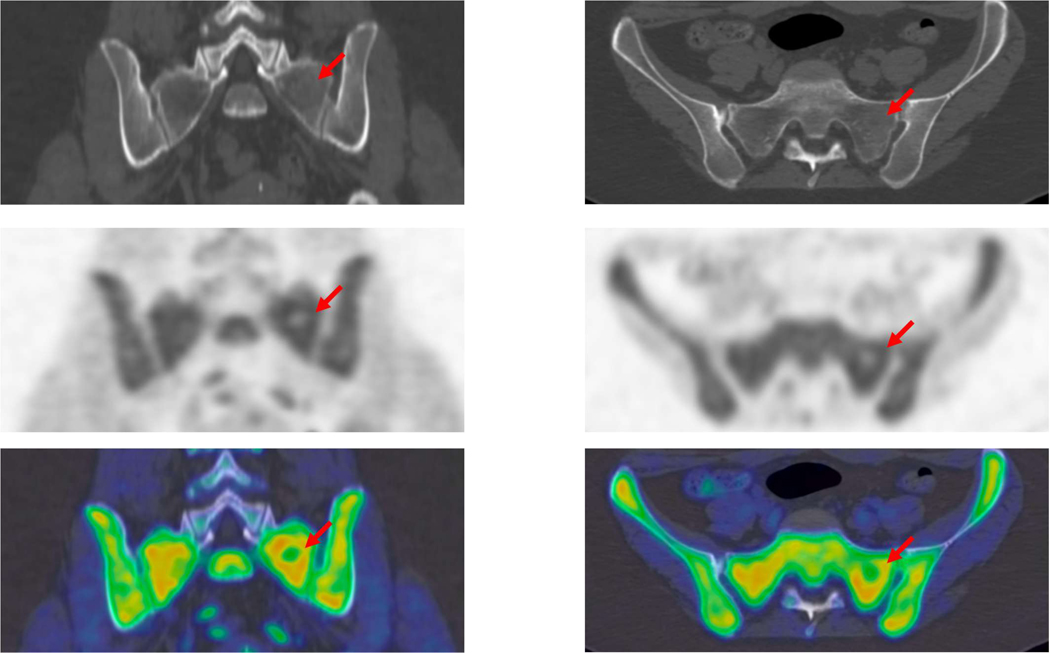
19 year-old female with history of right adrenal gland adrenocorticocarcinoma with several bone metastases. First PET/CT was obtained after chemotherapy. A very subtle area of lucency surrounded by minimal sclerosis (arrow) correlates with area of photopenia on the correlating PET images. These findings probably represent response to treatment. The high spatial resolution and improved signal-to-noise ratio allow for detection of small photopenic region which can be often useful for therapy response.
The improved image quality may also redefine the role of PET/CT in the characterization of skeletal lesions. For example, the gold standard for staging of osteosarcoma with suspected bone metastasis is MRI. However, TB-PET/CT may play a major role in this domain in the near future.
A concern with is that the ability to detect smaller and less intense foci of uptake may result in reduced specificity, since optimal methods of interpreting such findings have yet to be established (Fig. 6). Further experience is needed to clarify this concern.
Figure 6:
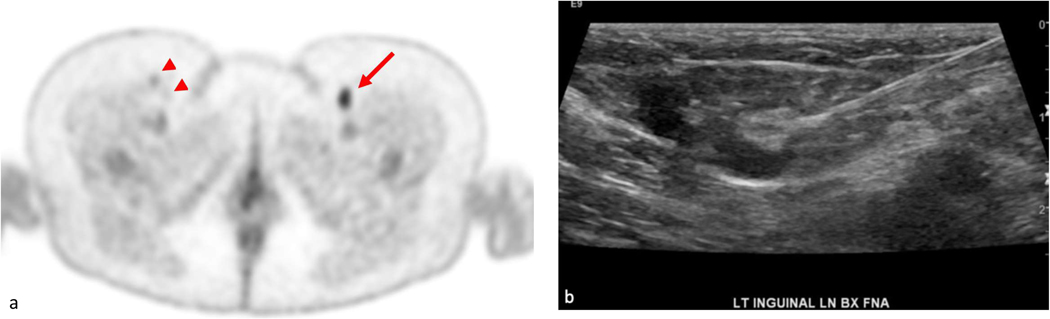
47 year old female affected by diffuse large B-cell lymphoma; axial image through the lower pelvic region was acquired 90 minutes after injection of 5 mCi of 18F-FDG; this image demonstrates focally increased uptake in the left inguinal region (SUV max of 5.4). In addition, bilateral mildly FDG avid lymph nodes were noted (arrowheads). The highly FDG avid left inguinal lymph node was demonstrated to be reactive in nature by ultrasound and fine needle aspiration (image b).
High-quality, high-resolution TB-PET/CT may be of great value in assessing cardiovascular disorders that involve diffuse segments of the cardiovascular system throughout the body.17,18 This could be particularly relevant in assessing inflammatory vasculitis and in detecting systemic clot formation in the venous system throughout the body.
Total-body Dynamic Imaging
TB PET/CT allows for simultaneous full body coverage for most of the pediatric population and therefore allows total body dynamic imaging. This is the first time that time activity curves of each part of the body can be obtained at the same time. Necessarily, the application space for this new technique is currently unexplored. However, a number of potential new uses can be considered. For example, kinetic modeling with perfusion agents could be used to determine quantitative measures of perfusion throughout the body and this may have value in diseases where multiple organs are involved, e.g. Duchenne’s 19,20 or sickle cell disease 21.
Time-activity curves could be assessed to determine dosimetry and to predict on and off-target effects for theranostics, and possibly also for therapeutics. This is possible with standard PET/CT but would substantially benefit from TB PET/CT. Time-activity curves could also be used to perform network analyses for both normal physiologic and pathologic processes, allowing elucidation of physiological connections between different organs. Such information could be used as a diagnostic tool, or to monitor toxicity during chemotherapy or immunotherapy. In metastatic cancer, time activity curves and parametric image derived using kinetic models could be correlated with therapy response and may prove useful to for the separation of responders and non-responders.
The ability to simultaneously study the kinetic interplay between organs, may lead to better understanding of coordination between the brain function and other physiological activities. This could substantially enhance our knowledge about the critical role of the central nervous system in effective function of many organs including the cardiopulmonary, gastrointestinal and hematopoietic systems.
Global Disease Assessment
PET imaging is the only modality that allows for assessment of the global disease activity in the entire body system. As such, total body imaging will make it possible to generate a single value that represents the overall ongoing disease process throughout its course at baseline and following therapeutic interventions.7,22 This potential applies to both benign and malignant disorders and will substantially enhance the role of PET imaging in the day-to-day practice of medicine and research domains in the future. Global disease assessment has been employed to assess patients with focal and diffuse disease processes that involve multiple organs.23–30 In particular, this approach will be of great importance in pediatric patients with hematologic malignancies and diseases that affect the musculoskeletal system, such as metabolic bone disorders and inflammatory bone abnormalities. Therefore, we predict that TB PET/CT will have a major impact on the management of both benign and malignant disorders that are systemic in nature.6
Conclusion
In the current diagnostic paradigm, the substantial physical sensitivity gain is the main advantage of TB PET/CT. Therefore, it is up to the imaging community to tailor protocols that take advantage of this unique innovation as it relates to the needs of the pediatric population. However, the paradigm-shifting promise of TB PET/CT really lies in its capability to perform dynamic, delayed and low-dose imaging, which have the potential to vastly increase the range of diseases and pathologies that can be investigated or managed using molecular imaging.
During the coming years, we expect that pediatric imaging specialists will develop and adopt innovative molecular imaging protocols that will lead to substantial enhancement of the understanding and management of serious and/or chronic diseases in the pediatric population.
Acknowledgements:
We would like to acknowledge Dr. Yasser Gaber Abdelhafez for the help in imaging post-processing. We would like to thank all the EXPLORER team at UC Davis and UPenn.
The authors acknowledge Cancer Support Grant P30: In Vivo Translational Imaging Shared Ressource 5P30CA093373–17. Support for this work was provided in part by the National Cancer Institute under grants R01CA206187 and R01CA249422.
References
- 1.Cherry SR, Jones T, Karp JS, Qi J, Moses WW, Badawi RD. Total-Body PET: Maximizing Sensitivity to Create New Opportunities for Clinical Research and Patient Care. J Nucl Med. 2018;59(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poon JK, Dahlbom ML, Moses WW, et al. Optimal whole-body PET scanner configurations for different volumes of LSO scintillator: a simulation study. Phys Med Biol. 2012;57(13):4077–4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badawi RD, Shi H, Hu P, et al. First Human Imaging Studies with the EXPLORER Total-Body PET Scanner. J Nucl Med. 2019;60(3):299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Cherry SR, Xie Z, Shi H, Badawi RD, Qi J. Subsecond total-body imaging using ultrasensitive positron emission tomography. Proc Natl Acad Sci U S A. 2020;117(5):2265–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaatsch P Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36(4):277–285. [DOI] [PubMed] [Google Scholar]
- 6.Hoilund-Carlsen PF, Edenbrandt L, Alavi A. Global disease score (GDS) is the name of the game! Eur J Nucl Med Mol Imaging. 2019;46(9):1768–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raynor WY, Zadeh MZ, Kothekar E, Yellanki DP, Alavi A. Evolving Role of PET-Based Novel Quantitative Techniques in the Management of Hematological Malignancies. PET Clin. 2019;14(3):331–340. [DOI] [PubMed] [Google Scholar]
- 8.Costelloe CM, Chuang HH, Daw NC. PET/CT of Osteosarcoma and Ewing Sarcoma. Semin Roentgenol. 2017;52(4):255–268. [DOI] [PubMed] [Google Scholar]
- 9.Kong G, Hofman MS, Murray WK, et al. Initial Experience With Gallium-68 DOTA-Octreotate PET/CT and Peptide Receptor Radionuclide Therapy for Pediatric Patients With Refractory Metastatic Neuroblastoma. J Pediatr Hematol Oncol. 2016;38(2):87–96. [DOI] [PubMed] [Google Scholar]
- 10.Hennrich U, Kopka K. Lutathera((R)): The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals (Basel). 2019;12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pantel AR, Viswanath V, Daube-Witherspoon ME, et al. PennPET Explorer: Human Imaging on a Whole-Body Imager. J Nucl Med. 2020;61(1):144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Society AC. Cancer Facts & Figures 2020. https://www.cancer.org/cancer/cancer-in-children/key-statistics.html#references. Published 2020. Accessed 03/04, 2020.
- 13.Fahey FH, Palmer MR, Strauss KJ, Zimmerman RE, Badawi RD, Treves ST. Dosimetry and adequacy of CT-based attenuation correction for pediatric PET: phantom study. Radiology. 2007;243(1):96–104. [DOI] [PubMed] [Google Scholar]
- 14.Basu S, Kung J, Houseni M, Zhuang H, Tidmarsh GF, Alavi A. Temporal profile of fluorodeoxyglucose uptake in malignant lesions and normal organs over extended time periods in patients with lung carcinoma: implications for its utilization in assessing malignant lesions. Q J Nucl Med Mol Imaging. 2009;53(1):9–19. [PubMed] [Google Scholar]
- 15.Houshmand S, Salavati A, Hess S, Werner TJ, Alavi A, Zaidi H. An update on novel quantitative techniques in the context of evolving whole-body PET imaging. PET Clin. 2015;10(1):45–58. [DOI] [PubMed] [Google Scholar]
- 16.Berg E, Gill H, Marik J, et al. Total-Body PET and Highly Stable Chelators Together Enable Meaningful (89)Zr-Antibody PET Studies up to 30 Days After Injection. J Nucl Med. 2020;61(3):453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beheshti M, Saboury B, Mehta NN, et al. Detection and global quantification of cardiovascular molecular calcification by fluoro18-fluoride positron emission tomography/computed tomography--a novel concept. Hell J Nucl Med. 2011;14(2):114–120. [PubMed] [Google Scholar]
- 18.Schmall JP, Karp JS, Alavi A. The Potential Role of Total Body PET Imaging in Assessment of Atherosclerosis. PET Clin. 2019;14(2):245–250. [DOI] [PubMed] [Google Scholar]
- 19.Doorenweerd N, Dumas EM, Ghariq E, et al. Decreased cerebral perfusion in Duchenne muscular dystrophy patients. Neuromuscul Disord. 2017;27(1):29–37. [DOI] [PubMed] [Google Scholar]
- 20.Quinlivan RM, Lewis P, Marsden P, et al. Cardiac function, metabolism and perfusion in Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 1996;6(4):237–246. [DOI] [PubMed] [Google Scholar]
- 21.Nath KA, Katusic ZS, Gladwin MT. The perfusion paradox and vascular instability in sickle cell disease. Microcirculation. 2004;11(2):179–193. [DOI] [PubMed] [Google Scholar]
- 22.Raynor WY, Al-Zaghal A, Zadeh MZ, Seraj SM, Alavi A. Metastatic Seeding Attacks Bone Marrow, Not Bone: Rectifying Ongoing Misconceptions. PET Clin. 2019;14(1):135–144. [DOI] [PubMed] [Google Scholar]
- 23.Basu S, Zaidi H, Salavati A, Hess S, Carlsen PF, Alavi A. FDG PET/CT methodology for evaluation of treatment response in lymphoma: from “graded visual analysis” and “semiquantitative SUVmax” to global disease burden assessment. Eur J Nucl Med Mol Imaging. 2014;41(11):2158–2160. [DOI] [PubMed] [Google Scholar]
- 24.Marin-Oyaga VA, Salavati A, Houshmand S, et al. Feasibility and performance of an adaptive contrast-oriented FDG PET/CT quantification technique for global disease assessment of malignant pleural mesothelioma and a brief review of the literature. Hell J Nucl Med. 2015;18(1):11–18. [DOI] [PubMed] [Google Scholar]
- 25.Fardin S, Gholami S, Samimi S, Rook AH, Alavi A. Global Quantitative Techniques for Positron Emission Tomographic Assessment of Disease Activity in Cutaneous T-Cell Lymphoma and Response to Treatment. JAMA Dermatol. 2016;152(1):103–105. [DOI] [PubMed] [Google Scholar]
- 26.Peter J, Houshmand S, Werner TJ, Rubello D, Alavi A. Applications of global quantitative 18F-FDG-PET analysis in temporal lobe epilepsy. Nucl Med Commun. 2016;37(3):223–230. [DOI] [PubMed] [Google Scholar]
- 27.Saboury B, Parsons MA, Moghbel M, et al. Quantification of aging effects upon global knee inflammation by 18F-FDG-PET. Nucl Med Commun. 2016;37(3):254–258. [DOI] [PubMed] [Google Scholar]
- 28.Khosravi M, Peter J, Wintering NA, et al. 18F-FDG Is a Superior Indicator of Cognitive Performance Compared to 18F-Florbetapir in Alzheimer’s Disease and Mild Cognitive Impairment Evaluation: A Global Quantitative Analysis. J Alzheimers Dis. 2019;70(4):1197–1207. [DOI] [PubMed] [Google Scholar]
- 29.Raynor WY, Jonnakuti VS, Zirakchian Zadeh M, et al. Comparison of methods of quantifying global synovial metabolic activity with FDG-PET/CT in rheumatoid arthritis. Int J Rheum Dis. 2019;22(12):2191–2198. [DOI] [PubMed] [Google Scholar]
- 30.Seraj SM, Ayubcha C, Zadeh MZ, Alavi A, Hunt SJ. The Evolving Role of PET-Based Novel Quantitative Techniques in the Interventional Radiology Procedures of the Liver. PET Clin. 2019;14(4):419–425. [DOI] [PubMed] [Google Scholar]