ABSTRACT
Objective
A significant proportion of the infants developed recurrent wheezing after an acute bronchiolitis (AB) event. Recent studies have demonstrated protection for recurrent wheeze and lower respiratory morbidity in infants treated with azithromycin during an acute respiratory wheezing. The aim of the present study was to test the hypothesis that administration of azithromycin during an AB event reduces subsequent wheezing and hospital re-admissions.
Methods
This is a secondary analysis of a randomized, double-blinded, placebo-controlled trial, including unpublished data of wheezing and hospitalizations during the initial 6 months following admission for acute viral bronchiolitis. The study was performed in a tertiary University hospital. Infants (<12 months of age) hospitalized with AB were randomized to receive either azithromycin or placebo, administered orally, for 7 days. Families were contacted by telephone at 3 and 6 months after the initial acute event and answered to a standardized questionnaire in order to identify recurrent wheezing and hospital readmissions.
Results
One hundred and four patients were included (Azithromycin group, n= 50; placebo group, n=54). Considering the total of patients contacted 3 months after hospitalization (n=70), the recurrence rate of wheezing in the azithromycin group was significantly lower than in the placebo group (RR = 0.48; CI = 0.24-0.98; p = 0.038).
Conclusion
Azithromycin significantly reduces the risk of subsequent wheezing between 0 and 3 months after hospital admission due to acute bronchiolitis irrespective of the presence of respiratory syncytial virus.
Keywords: Bronchiolitis, Macrolides, Recurrent wheezing, Hospitalization
RESUMO
Objetivo
Uma proporção significativa de lactentes desenvolve sibilância recorrente após um evento de bronquiolite aguda (BA). Estudos recentes demonstraram proteção para sibilância recorrente e menor morbidade respiratória em lactentes tratados com azitromicina durante uma crise de sibilância. O objetivo do presente estudo foi testar a hipótese de que a administração de azitromicina durante um evento BA reduz sibilos e reinternações hospitalares subsequentes.
Métodos
Trata-se de uma análise secundária de um estudo randomizado, duplo-cego, controlado por placebo, incluindo dados não publicados de sibilância e hospitalizações durante os seis meses iniciais após a internação por bronquiolite aguda. O estudo foi realizado em um hospital universitário terciário. Os bebês (<12 meses de idade) hospitalizados com BA foram randomizados para receber azitromicina ou placebo, administrados por via oral, por sete dias. As famílias foram contatadas por telefone aos três e seis meses após o evento agudo inicial, e responderam a um questionário padronizado para identificar sibilos recorrentes e reinternações hospitalares.
Resultados
Cento e quatro pacientes foram incluídos (grupo Azitromicina, n=50; grupo Placebo, n=54). Considerando o total de pacientes contatados com sucesso três meses após a hospitalização (n=70), a taxa de recorrência de sibilância no grupo da azitromicina foi significativamente menor do que no grupo placebo (RR=0,48; CI=0,24-0.98; p=0,038).
Conclusões
A azitromicina reduziu significativamente o risco de sibilância subsequente entre zero e três meses após a admissão hospitalar por bronquiolite aguda.
Descritores: Bronquiolite, Macrolídeos, Sibilância recorrente, Hospitalização
INTRODUCTION
Acute viral bronchiolitis (AB) is the most common lower respiratory tract illness (LRTI) among infants. A previous study has shown that AB is significantly associated with subsequent development of recurrent wheezing and asthma in childhood.(1) Macrolides have a well-established antibacterial effect,(2) covering several agents, including Mycoplasma pneumoniae and Bordetella pertussis. In the past decade, additional immunomodulatory and antiviral properties of macrolides have also been described.(3-5)
Studies showed that some macrolides had a beneficial effect in the treatment of lung diseases associated with recurrent or chronic symptoms, such as cystic fibrosis (CF) and non-CF bronchiectasis.(4-6) Macrolides seem to inhibit interleukin (IL)-8 production, reducing overall neutrophilic inflammation.(7) Recurrent wheezing in young infants is characterized by a neutrophilic airway response.(8,9) Only a few trials have used the immunomodulatory rationale to test the efficacy of macrolides in recurrent wheezing. The most well-designed trials have shown negative results for acute bronchiolitis.(10,11) Instead, recent previous studies have demonstrated a prolonged protection for subsequent recurrent wheeze and lower respiratory morbidity in infants treated with azithromycin during an acute Respiratory Syncytial Virus (RSV) bronchiolitis, through the inhibition of the IL-8 inflammatory pathway.(12,13) In the present study, we have tested the hypothesis that administration of azithromycin during hospitalization for AB reduces the risk of subsequent wheezing episodes and hospital readmission, independent of the viral etiology.
METHODS
This was a randomized, double-blinded, placebo-controlled trial. Infants with a clinical diagnosis of AB were recruited from the pediatric emergency department or hospital wards of two large, tertiary hospitals during 2 years. Clinical data were recorded and nasopharyngeal samples for viral identification were collected at the time of enrollment. In the total of 184 initial study patients in the two centers,(10) 104 were exclusively from one center were followed by phone for a secondary analysis. After hospitalization, we tried to contact all 104 families by phone calls during the period between discharge and up to 6 months after discharge. A diagnosis of AB was confirmed if children were: (1) <12 months and admitted with prodromal viral symptoms in a first episode of wheezing or crackles with tachypnea; and (2) recruited within 48 hours of hospitalization, with a maximum of 72 hours of a history of lower respiratory tract clinical signs (wheeze and/or respiratory distress).
Main exclusion criteria were: (1) any restrictions to the use of oral macrolides; (2) prescription of macrolide therapy by the attending physician due to clinical and radiologic features consistent with a diagnosis of Chlamydia sp. or Bordetella pertussis respiratory infection; (3) a previous diagnosis of any chronic cardiopulmonary disorder, congenital/acquired immunodeficiency, or neuromuscular disease; and (4) a history of prematurity or other neonatal complications.
Infants were randomized (simple/unrestricted randomization) to receive either a daily oral dose of Azithromycin (10 mg/kg/day) or an equivalent volume of placebo for 7 days. The mechanism used to implement the random allocation sequence was a list generated using numbers 1 and 2 random selected from a sealed opaque envelope, previously to the enrollment of the patients by the first author. The azithromycin group was represented as 1 and the control group as 2. The placebo formula was produced with similar taste and smell of azithromycin. Identical medicine bottle (identified only as 1 or 2) were used. The patients were enrolled by the first author or collaborators and assigned to interventions according to the randomization list. Participants, care providers, and authors who assessed outcomes were blinded to the intervention groups. Medication was administered within 72 hours of the initial clinical symptoms and a blinded study team member supervised the interventions.
All infants were managed according to protocols routinely used by the pediatric staff in the hospital. Infants enrolled in the study could receive additional therapies (other than macrolides) prescribed by the attending pediatricians. Assessment of clinical data included length of stay in the hospital, duration of supplemental oxygen required, and identification of respiratory viruses, described in a previous publication.(10) In addition, secondary data from the initial study (but only one center) were registered in a follow-up protocol during 6 months after the AB episode in order to identify recurrent wheezing and hospital readmissions. For definition of subsequent recurrent wheezing, families were successfully contacted by telephone at 3 and 6 months after the initial acute event and answered to a standardized questionnaire. The question used was: “Did your child have wheezing again after hospital discharge?”
Statistical analysis and ethics
The outcomes were compared between groups by chi-square, Mann-Whitney tests (this latter when variables failed for normality using Kolmogorov Smirnov). In addition, relative risk (RR) with CI95% (Confidence Interval) was calculated. To detect a reduction in subsequent wheezing approximately 50% (.50 vs .22), based on data from a previous study,(12) allowing for a 2-sided 5% significance level and a power of 80%, a sample size of 45 patients per group would be required.(14) The protocol has been approved by the Human Research Ethics Committees. The parents or caregivers of all the infants included in the study signed an informed consent term. The study was registered in the Brazilian Clinical Trials Registry (Nº RBR-257ZBC), which is a joint project of the Brazilian Ministry of Health and the Pan-American Health Organization,(15) and recognized by the World Health Organization Trial Registration Data Set.
RESULTS
One hundred and four infants fulfilled all eligibility criteria (one center: N=104) and have been included in the trial that evaluated the efficacy of Azithromycin for acute bronchiolitis, as published previously.(10) In the total of patients successfully contacted in the follow-up 3 months after hospitalization (n=70/104), 52.85% (37 of 70) were in the azithromycin group. At 6 months of follow-up we were able to contact 63 subjects (Figure 1). Baseline characteristics of the patients are shown in Table 1.
Figure 1. Flowchart with randomization and follow-up data. N: sample size.
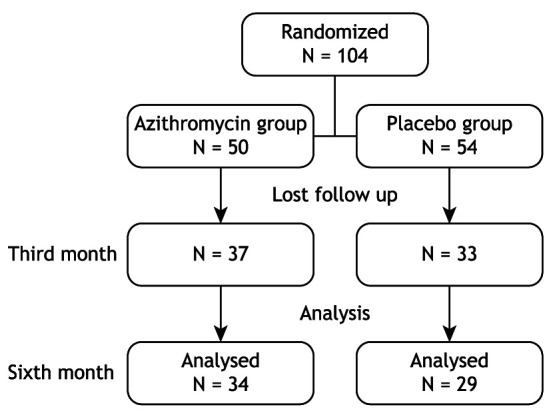
Table 1. Demographic and clinical characteristics of analyzed patients to follow up (n=70).
| Azithromycin Group n=37 | Placebo Group n=33 | P | |
|---|---|---|---|
| Age at enrollment, months, mean (SD) | 3.26 (2.49) | 3.14 (2.29) | 0.843 |
| Weight at enrollment, kg, mean (SD) | 5.72 (1.76) | 5.85 (1.50) | 0.749 |
| Gender, boys, n (%) | 22 (59.5) | 21 (63.6) | 0.720 |
| Use of β2 agonist, n (%) | 9 (24.3) | 11 (33.3) | 0.405 |
| Hypoxemia at admission, n (%) | 37 (100) | 33 (100) | 1.000 |
| Positive for any virus | 20 (54.1) | 22 (66.7) | 0.288 |
| Positive for RSV, n (%) | 17 (45.9) | 21 (63.6) | 0.182 |
| Duration of hospitalization, days, mean (SD) | 5.32 (2.63) | 5.85 (3.30) | 0.464 |
SD: Standard Deviation; RSV: respiratory syncicial virus; n: sample size; p-value: significance level (probability of obtaining test results as extreme as the results observed).
Positive samples for RSV were found in 65 of the 104 (62%) of randomized patients Other viruses identified were Parainfluenza (N=10), Influenza (N=15) and Adenovirus (N=3). In the patients included in the secondary follow-up analysis, RSV was identified in 38/70 (54.3%) of patients. All studied samples were evaluated by direct immunofluorescence, which does not allow the detection of rhinovirus or metapneumovirus.
Recurrent wheezing risk was significantly reduced in infants at 3 months after AB episode (RR=0.48, CI=0.24 – 0.98, p= 0.038). Hospital readmission was not significantly different between the groups.
According to our results in the third month of follow-up, the recurrence rate of wheezing in the azithromycin group was 19.1%, whereas in the placebo group it was 39.5%, showing a significant difference (p =0.038). Analyzing the data at sixth month of follow-up, there was no significant difference between the two groups (p = 0.868). In the group that used azithromycin 25.6% had recurrence of episodes of wheezing while in the placebo group the index was 27.3% (Table 2).
Table 2. Risk of recurrent wheezing and hospital readmission after acute bronchiolitis.
| Azithromycin Group - Placebo Group (%) | RR (CI) | P- value | |
|---|---|---|---|
| 3st month (wheezing) | 19.1-39.5 | 0.48 (0.24-0.98) | 0.038 |
| 3st month (readmission) | 8.5-10.5 | 0.80 (0.21-3.02) | 0.752 |
| 6st month (wheezing) | 25.6-27.3 | 0.93 (0.44-1.99) | 0.868 |
| 6st month (readmission) | 9.3-3.0 | 3.07 (0.36-26.2) | 1.274 |
RR: Relative Risk; CI: Confidence Interval; p-value: significance level (probability of obtaining test results as extreme as the results observed).
DISCUSSION
A relevant proportion (30-40%) of infants hospitalized for acute bronchiolitis in the first year of life present recurrent wheezing episodes after the first hospital admission.(16) In the present study, the treatment with Azithromycin at the time of an admission for acute bronchiolitis showed relevant protection for recurrence of wheezing 3 months after hospitalization for AB (RR = 0.48 (CI = 0.24-0.98)). The same effect was not observed 6 months after hospital discharge.
Data from previous studies(12,13,17,18) support the hypothesis that recurrent wheezing, or even childhood asthma, could be prevented by interventions to prevent acute severe viral bronchiolitis. This concept is supported by studies demonstrating reductions in recurrent wheezing among preterm infants who received Palivizumab.(19,20) Although Palivizumab can be an effective intervention for the prevention of severe RSV bronchiolitis and subsequent wheezing its use has some limitations. Palivizumab is recommended for a high-risk group of preterm infants, especially because it is quite expensive and requires monthly intra-muscular injections during the virus season.(19) Therefore, there is a need to identify other interventions that could be used in children with bronchiolitis to prevent the common and costly event of post-bronchiolitis wheezing. Conventional asthma controller medications have shown limited efficacy for the prevention of post-RSV recurrent wheezing.(20-23) Seeing that neutrophils are predominant inflammatory cells in the airways of AB,(8,9) a medication with anti-neutrophilic properties would have, theoretically, the mechanistic rationale to serve as a potential intervention for the prevention of post-RSV recurrent wheezing.
To the best of our knowledge, there are few previous trials using macrolides as a treatment for prevention of post-bronchiolitis wheezing.(10,13,24) The treatment with clarithromycin among children hospitalized for RSV bronchiolitis was initially reported to be associated with shorter length of stay and fewer readmissions for wheezing during the period of follow-up. Subsequent communications refuted the efficacy of macrolides at the time of acute bronchiolitis, but it did not investigate its impact on recurrent wheezing.(10) A recent clinical trial testing preschool children showed that early use of Azithromycin for 5 days, when children had signs and symptoms of lower respiratory tract illness, reduced the risk of a LRTI to progress into severe disease.(13)
A recent meta-analysis has suggested that macrolide therapy may be safe and effective in achieving better outcomes in childhood reactive airway diseases. Treatment with Azithromycin may decrease the need for short-acting β2-agonists among preschool children with recurrent wheezing.(25)
The unique pharmacokinetic properties of Azithromycin might explain the differential effect observed in the risk of post-bronchiolitis wheezing. In general, Azithromycin accumulates in lung tissue, resulting in alveolar macrophages and bronchoalveolar lavage fluid concentrations higher than in serum concentrations.(3,26,27) Moreover, the intracellular accumulation property of Azithromycin results in a long half-life in the airway because it persists in measurable quantities in human airway macrophages for three weeks after the last dose of an 8-day course.(26) However, the correct doses and duration of macrolides needed to provide long-term anti-inflammatory effects are not yet clear.
Co-detection of upper airway virus and bacteria in children was associated with an increased risk of experiencing asthma exacerbations.(28) Therefore, the beneficial effects of Azithromycin detected in our study could, instead of being purely anti-neutrophilic, it is also mediated by antimicrobial properties. Interestingly, results from experimental studies using human respiratory cells showed that Azithromycin treatment inhibited rhinovirus replication,(12,29) and that Clarithromycin reduced RSV and influenza titers.(24)
In the present study, we included otherwise healthy infants admitted by AB, which is the first cause of hospitalization in infants. However, the findings at 6 months follow-up limits our conclusions to more long-term efficacy. Future trials with longer treatment and follow-up should be designed to determine the long-term effects of macrolides on wheezing and asthma. Our research question was investigated among a group of patients experiencing the most severe disease since all infants required hospitalization and these children generally experience the greatest morbidity in terms of subsequent wheezing and asthma. Relative high levels of response of participants during follow-up further strengthen our findings. Finally, we evaluated the potential effects of Azithromycin for an important and highly prevalent clinical endpoint (recurrent wheezing).
The relatively small sample size during the follow-up may be considered a limitation to our findings. Although the overall trend toward improved clinical outcomes is encouraging that we cannot firmly conclude that Azithromycin intervention during AB definitely reduces the occurrence of recurrent wheezing. Another recent publication showed similar results to our study. In this clinical trial, 40 infants with the first episode of RSV wheezing received either Azithromycin or placebo for 14 days, with Azithromycin reducing IL-8 levels in nasal lavage, and significantly reducing the time for the third episode of wheezing.(12)
In summary, the results of our trial showed that treatment with Azithromycin during AB hospitalization resulted in reduction of recurrent wheezing episodes, but this effect is not sustained at six months after AB hospitalization. Considering the important clinical impact of our findings and the risk of increased widely use of macrolides in this group of patients, further studies should try to better define which infants could be better responders to macrolides and whether severity is also a factor associated with efficacy of treatment.
Footnotes
Study carried out in the Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre (RS) Brasil.
TRIAL REGISTRATION: Brazilian Clinical Trial Registry: RBR-257ZBC.
Financial support: Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul.
REFERENCES
- 1.Zorc JJ, Hall CB. Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125(2):342–349. doi: 10.1542/peds.2009-2092. [DOI] [PubMed] [Google Scholar]
- 2.Lakoš AK, Pangercic A, Gasparic M, Kukuruzovic MM, Kovacic D, Barsic B. Safety and effectiveness of azithromycin in the treatment of respiratory infections in children. Curr Med Res Opin. 2012;28(1):155–162. doi: 10.1185/03007995.2011.639355. [DOI] [PubMed] [Google Scholar]
- 3.Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23(3):590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedlander AL, Albert RK. Chronic macrolide therapy in inflammatory airways diseases. Chest. 2010;138(5):1202–1212. doi: 10.1378/chest.10-0196. [DOI] [PubMed] [Google Scholar]
- 5.Pinto LA, Camozzato C, Avozani M, Machado DC, Jones MH, Stein RT, et al. Effect of clarithromycin on the cell profile of bronchoalveolar lavage fluid in mice with neutrophil-predominant lung disease. Rev Hosp Clin Fac Med Sao Paulo. 2004;59(3):99–103. doi: 10.1590/S0041-87812004000300002. [DOI] [PubMed] [Google Scholar]
- 6.Johnston SL. Macrolide antibiotics and asthma treatment. J Allergy Clin Immunol. 2006;117(6):1233–1236. doi: 10.1016/j.jaci.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 7.Desaki M, Takizawa H, Ohtoshi T, Kasama T, Kobayashi K, Sunazuka T, et al. Erythromycin suppresses nuclear factor-kappaB and activator protein-1 activation in human bronchial epithelial cells. Biochem Biophys Res Commun. 2000;267(1):124–128. doi: 10.1006/bbrc.1999.1917. [DOI] [PubMed] [Google Scholar]
- 8.Everard ML, Swarbrick A, Wrightham M, McIntyre J, Dunkley C, James PD, et al. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child. 1994;71(5):428–432. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitrez PM, Pinto LA, Machado DC, Tsukazan MT, Jones MH, Stein RT. Upper airway cellular pattern in infants with acute bronchiolitis: neutrophils or eosinophils? J Pediatr. 2003;79(5):443–448. doi: 10.2223/JPED.1078. [DOI] [PubMed] [Google Scholar]
- 10.Pinto LA, Pitrez PM, Luisi F, de Mello PP, Gerhardt M, Ferlini R, et al. Azithromycin therapy in hospitalized infants with acute bronchiolitis is not associated with better clinical outcomes: a randomized, double-blinded, and placebo-controlled clinical trial. J Pediatr. 2012;161(6):1104–1108. doi: 10.1016/j.jpeds.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 11.McCallum GB, Morris PS, Grimwood K, Maclennan C, White AV, Chatfield MD, et al. Three-weekly doses of azithromycin for indigenous infants hospitalized with bronchiolitis: a multicentre, randomized, placebo-controlled trial. Front Pediatr. 2015;3:32. doi: 10.3389/fped.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beigelman A, Isaacson-Schmid M, Sajol G, Baty J, Rodriguez OM, Leege E, et al. Randomized trial to evaluate azithromycin’s effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2015;135(5):1171-8.e1. doi: 10.1016/j.jaci.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacharier LB, Guilbert TW, Mauger DT, Boehmer S, Beigelman A, Fitzpatrick AM, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses a randomized clinical trial. JAMA. 2015;314(19):2034–2044. doi: 10.1001/jama.2015.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UBC: University of British Columbia . UBC: University of British Columbia. Vancouver: University of British Columbia; 2019. [cited 2019 Jan 1]. https://www.stat.ubc.ca/~rollin/stats/ssize/ homepage on the Internet. Available from: [Google Scholar]
- 15.ReBEC: Registro Brasileiro de Ensaios Clínicos ReBEC: Registro Brasileiro de Ensaios Clínicos. 2019. [cited 2019 Jan 1]. homepage on the Internet. Available from: http://www.ensaiosclinicos.gov.br/
- 16.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stokholm J, Chawes BL, Vissing NH, Bjarnadóttir E, Pedersen TM, Vinding RK, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4(1):19–26. doi: 10.1016/S2213-2600(15)00500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandhane PJ, Paredes Zambrano de Silbernagel P, Aung YN, Williamson J, Lee BE, Spier S, et al. Treatment of preschool children presenting to the emergency department with wheeze with azithromycin: a placebo-controlled randomized trial. PLoS One. 2017;12(8):e0182411. doi: 10.1371/journal.pone.0182411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshihara S, Kusuda S, Mochizuki H, Okada K, Nishima S, Simões EA. Effect of palivizumab prophylaxis on subsequent recurrent wheezing in preterm infants. Pediatrics. 2013;132(5):811–818. doi: 10.1542/peds.2013-0982. [DOI] [PubMed] [Google Scholar]
- 20.Simões EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151(1):34–42. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 21.Fox GF, Everard ML, Marsh MJ, Milner AD. Randomised controlled trial of budesonide for the prevention of post-bronchiolitis wheezing. Arch Dis Child. 1999;80(4):343–347. doi: 10.1136/adc.80.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clavenna A, Sequi M, Cartabia M, Fortinguerra F, Borghi M, Bonati M. Effectiveness of nebulized beclomethasone in preventing viral wheezing: an RCT. Pediatrics. 2014;133(3):e505–12. doi: 10.1542/peds.2013-2404. [DOI] [PubMed] [Google Scholar]
- 23.Brodlie M, Gupta A, Rodriguez-Martinez CE, Castro-Rodriguez JA, Ducharme FM, McKean MC. Leukotriene receptor antagonists as maintenance and intermittent therapy for episodic viral wheeze in children. Cochrane Database Syst Rev. 2015;(10):CD008202. doi: 10.1002/14651858.CD008202.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tahan F, Ozcan A, Koc N. Clarithromycin in the treatment of RSV bronchiolitis: a double-blind, randomised, placebo-controlled trial. Eur Respir J. 2007;29(1):91–97. doi: 10.1183/09031936.00029206. [DOI] [PubMed] [Google Scholar]
- 25.Lei W-T, Lin HH, Tsai M-C, Hung H-H, Cheng Y-J, Liu S-J, et al. The effects of macrolides in children with reactive airway disease: a systematic review and metaanalysis of randomized controlled trials. Drug Des Devel Ther. 2018;12:3825–3845. doi: 10.2147/DDDT.S183527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matzneller P, Krasniqi S, Kinzig M, Sörgel F, Hüttner S, Lackner E, et al. Blood, Tissue, and Intracellular Concentrations of Azithromycin during and after End of Therapy. Antimicrob Agents Chemother. 2013;57(4):1736–1742. doi: 10.1128/AAC.02011-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beigelman A, Mikols CL, Gunsten SP, Cannon CL, Brody SL, Walter MJ. Azithromycin attenuates airway inflammation in a mouse model of viral bronchiolitis. Respir Res. 2010;11(1):90. doi: 10.1186/1465-9921-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kloepfer KM, Lee WM, Pappas TE, Kang TG, Vrtis RF, Evans MD, et al. Detection of Pathogenic Bacteria During Rhinovirus Infection is Associated with Increased Respiratory Symptoms and asthma Exacerbations. J Allergy Clin Immunol. 2014;133(5):1301–1307. doi: 10.1016/j.jaci.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gielen V, Johnston LS, Edwards MR. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur Respir J. 2010;36(3):646–654. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]


