Abstract
A cluster of a Neisseria meningitidis serogroup C strain causing invasive disease was investigated. Five out of seven cases were associated with a particular discotheque. The strains were indistinguishable, as revealed by pulsed-field gel electrophoresis and sequencing of variable regions of the porA gene, but caused strikingly different clinical presentations during 5 months.
Neisseria meningitidis causes both endemic and epidemic disease (8, 12, 13, 18). Although the vast majority of the estimated 2,600 annual cases of meningococcal disease in the United States are sporadic, the frequency of serogroup C meningococcal clusters has increased in, for example, military camps and universities (6–8). During recent years, however, meningococcal serogroup C spread has also been associated with discotheques, hence the designation “Disco Fever.” A limited outbreak that involved six individuals who attended a dance club in Argentina was reported (4). A similar epidemic involving 10 young adults who visited a nightclub in Australia was described (9).
Characterization of meningococcal strains isolated during outbreaks is crucial in understanding an epidemic. Isolates may change their phenotype by, for example, capsular switching, justifying approaches other than serogroup typing when disease-causing strains are traced (19). In addition to the standard pulsed-field gel electrophoresis (PFGE) (15, 19), multilocus enzyme electrophoresis (13), ribosomal DNA restriction profiles (21), and PCR analysis followed by restriction fragment length polymorphism analysis of the porA gene have, among other methods, been used for characterization of N. meningitidis serogroup C (5, 14).
Blood culturing was performed using aerobic flasks (BacT/Alert; Organon Teknika, Durham, N.C.). Cerebrospinal fluid (CSF) was cultured on Columbia blood agar (Difco, Detroit, Mich.) and chocolate agar, and enrichment culturing was performed with brain heart infusion medium including factors V and X (Difco). Serogrouping was carried out by coagglutination (11), and all isolates were serotyped and serosubtyped with monoclonal antibodies for outer-membrane protein (1). PFGE was done using a contour-clamped homogeneous electric field 2 apparatus (Bio-Rad Laboratories, Richmond, Calif.). For porA gene sequencing, chromosomal DNAs were directly isolated from bacterial suspensions using Dynabeads DNA DIRECT system I (Dynal, Oslo, Norway). The porA gene was amplified by PCR, and variable region 1 (VR1), VR2, and VR3 were labeled with a BigDye Terminator Cycle Sequencing kit followed by sequencing with an ABI PRISM 310 genetic analyzer (Perkin-Elmer, Foster City, Calif.). To assign VR sequences to families (2), deduced amino acid sequences of the VRs were aligned with sequences available in the N. meningitidis PorA VR database (http://mlst.zoo.ox.ac.uk/porA-vr/), where the VR family designation is based on the scheme of Suker et al. (17).
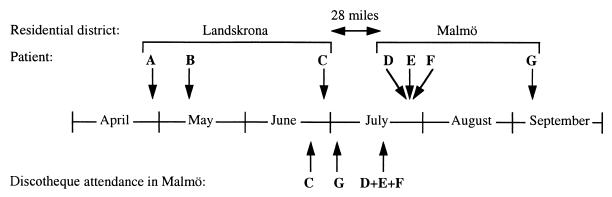
Three patients (Table 1; patients D, E, and F) with N. meningitidis serogroup C disease were admitted to a local hospital on three subsequent days. The first patient (patient D) was a 25-year-old male who fell ill with fever, petechiae, cutaneous bleedings, and hypotension. The patient developed a fulminant septicemia and a fatal disseminated intravascular coagulation within 5 h. The following day, a 21-year-old female attended the hospital due to a swollen knee joint (Table 1; patient E). Septic arthritis was suspected, and N. meningitidis group C was isolated from the joint fluid. The third patient, a 21-year-old male with an artificial eye (enucleation performed due to an uveal tumor at the age of 3 years), suffered from conjunctivitis and displayed symptoms of meningitis (Table 1; patient F). None of these patients knew each other or listed close friends when answering the question of social contacts. However, they had all visited the same discotheque in Malmö on the same night (Fig. 1). The strains from all three patients were phenotypically identical (C2aP1.nst). Genosubtyping showed the same nucleotide sequences in VR1, VR2, and VR3 of the porA gene, namely, those of genosubtypes 5a, 10d, and 36b (Table 1). Healthy individuals who had had contact with patients D, E, and F were either checked with pharyngeal swab cultures or directly prescribed ciprofloxacin. One strain of N. meningitidis with full identity with the invasive isolates was detected among the healthy contacts (Table 1).
TABLE 1.
Summary of cases, contacts with healthy individuals, and unrelated cases
| Patient, healthy contact, or unrelated patient | Age (yr) | Gendera | Date of presentation (day/mo/yr) | Time (days) between discotheque visit and disease | Relationship to patient(s) | Clinical presentation | Site(s) yielding positive culture(s) | Serogroup | Serotype:serosubtype | PFGE pattern |
porA gene subtypeb
|
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VR1 | VR2 | VR3 | |||||||||||
| Patients | |||||||||||||
| A | 9 | M | 30/04/1992 | Sore throat, septis | Blood | C | 2a:nst | I | 5a | 10d | 36b | ||
| B | 2 | F | 11/05/1992 | Septis | Blood | C | 2a:nst | I | 5a | 10d | 36b | ||
| C | 24 | M | 28/06/1992 | ≈5 | Septis, meningitis | Blood | C | 2a:nst | I | 5a | 10d | 36b | |
| D | 25 | M | 23/07/1992 | 5 | Septisc | Blood, CSF | C | 2a:nst | I | 5a | 10d | 36b | |
| E | 21 | F | 24/07/1992 | 6 | Arthritis | Knee joint | C | 2a:nst | I | 5a | 10d | 36b | |
| F | 21 | M | 25/07/1992 | 7 | Conjunctivitis, meningitis | Blood, CSF, pharynx, socket | C | 2a:nst | I | 5a | 10d | 36b | |
| G | 21 | M | 09/09/1992 | ≈65 | Arthritis, fever | Knee joint, pharynx | C | 2a:nst | I | 5a | 10d | 36b | |
| Healthy contacts | |||||||||||||
| 1 | 25 | M | 26/07/1992 | Boyfriend of patient E | Pharynx | C | 2a:nst | I | 5a | 10d | 36b | ||
| 2 | 16 | M | 26/07/1992 | Brother of patient D | Pharynx | C | 15:P1.6 | II | 18 | 25b | 38a | ||
| 3 | 24 | M | 26/07/1992 | Friend of patient D | Pharynx | C | 4:nst | III | 5a | 10a | 36b | ||
| 4–10 | 1–52 | 26–28/07/1992 | Contact with patients D, E, and F | —d | |||||||||
| Unrelated patients | |||||||||||||
| 1 | 1 | F | 30/07/1992 | CSF | C | 2b:nst | IV | 5 | 2b | 36b | |||
| 2 | 17 | F | 18/12/1992 | CSF | C | 15:P1.15 | V | 19 | 15a | 36 | |||
| 3 | 24 | M | 30/12/1992 | Blood | C | nt:P1.7,9 | VI | 7 | 9 | 35a | |||
| 4 | 37 | F | 30/12/1992 | CSF | C | 2a:nst | VII | 5 | 2 | 36b | |||
Abbreviations: F, female; M, male.
The amino acid sequences of VR1 to VR3 (with subtypes in parentheses) were as follows: VR1, PLQNIQPQVTKR (5), PLQNIQQPQVTKR (5a), AQAANGGASGQVKVTKVTKA (7), PPSKGQTGNKVTKG (18), and PPSKSQPQVKVTKA (19); VR2, HFVQQTPKSQPTLVP (2), HFVQQPPKSQPTLVP (2b), YVDEQSKYHA (9), HFVQNKQNQPPTLVP (10a), HFVQNKQNKQNQPPTLVP (10d), HYTRQNNTDVFVP (15a), and TYTEGSSGVFTPVP (25b); and VR3, LLGSGSDQ (35a), LLGSTSDE (36), LLGSGSDE (36b), and LLGRIGEDDE (38a).
Fatal case.
No positive pharyngeal specimens.
FIG. 1.
Chronology of occurrences of meningococcal disease and discotheque attendance. Upper arrows indicate dates when patients fell ill, and lower arrows indicate dates when patients C to F attended the discotheque. The distance between the two residential districts is shown.
All invasive N. meningitidis group C strains (n = 11) collected in the surveillance area (Skåne, Sweden; population, 1.1 million people) during 1992 were analyzed. Four strains isolated from patients A, B, C, and G displayed the same PFGE patterns and VRs in their porA genes as the strains associated with the discotheque (Table 1). Patient C was a 24-year-old male who also had visited the same discotheque 3 weeks before patients D, E, and F (Fig. 1). He was admitted with septicemia and menigitis complicated by convulsions and required respirator therapy for 6 days. Patient G, a 21-year-old male who had visited the discotheque in July, fell ill in early September. A few days before his admission to the hospital, he had gotten a splinter in his right ankle, accompanied by local tenderness and arthritis in his right knee joint. Surprisingly, meningococci with full identity with the cluster strain were isolated from his knee joint. Two additional patients carrying the cluster strain were detected in our study; patient A was a 9-year-old boy (Table 1) who initially presented with a sore throat, followed by a fulminant septicemia. Due to necrosis in a finger, amputation of the two distal phalanges had to be performed. Suspected cerebral damage resulting in a considerably delayed puberty was observed after 4 years. Patient B was a 2-year-old girl suffering from septicemia without any manifest sequelae. No epidemiological connection was found either between patients A, B, and C (except for living in the same city [Landskrona, Sweden; population, 37,000]) or between the different disco attendees. The genetic diversity within the group C meningococci in the surveillance area is illustrated by the characterization of the four unrelated cases during 1992 (Table 1).
A relationship between alcohol consumption, tobacco smoke exposure, male gender, and meningococcal disease is statistically proven (4, 7). These hallmarks are, however, less prominent during sporadic serogroup C meningococcal disease (13, 16). Except for environmental factors, it is presently obscure why patients are differently affected by the same strain. Various disease manifestations from fulminant septicemia to less severe disease, including primary arthritis (20) and conjunctivitis (3), were observed in our study (Table 1). The meningeal tropism and interactions with most human cell types by N. meningitidis (10) suggests that a patient's phenotype as well as acquired specific and nonspecific immunity plays a crucial role in the clinical outcome. In conclusion, we found that porA gene sequencing (genosubtyping) together with PFGE provided meaningful information during a meningococcal outbreak with the cluster strain lasting 5 months.
REFERENCES
- 1.Abdillahi H, Poolman J T. Whole-cell ELISA for typing Neisseria meningitidis with monoclonal antibodies. FEMS Microbiol Lett. 1987;48:367–371. [PubMed] [Google Scholar]
- 2.Arhin F F, Moreau F, Coulton J W, Mills E L. Sequencing of porA from clinical isolates of Neisseria meningitidis defines a subtyping scheme and its genetic regulation. Can J Microbiol. 1998;1:56–63. [PubMed] [Google Scholar]
- 3.Barquet N, Gasser I, Domingo P, Moraga F A, Macaya A, Elcuaz R. Primary meningococcal conjunctivitis: report of 21 patients and review. Rev Infect Dis. 1990;12:838–847. doi: 10.1093/clinids/12.5.838. [DOI] [PubMed] [Google Scholar]
- 4.Cookson S T, Corrales J L, Lotero J O, Regueira M, Binsztein N, Reeves M W, Ajello G, Jarvis W R. Disco fever: epidemic meningococcal disease in northeastern Argentina associated with disco patronage. J Infect Dis. 1998;178:266–269. doi: 10.1086/517450. [DOI] [PubMed] [Google Scholar]
- 5.Derrick J P, Urwin R, Suker J, Feavers I M, Maiden M C. Structural and evolutionary inference from molecular variation in Neisseria porins. Infect Immun. 1999;67:2406–2413. doi: 10.1128/iai.67.5.2406-2413.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edmond M B, Hollis R J, Houston A K, Wenzel R P. Molecular epidemiology of an outbreak of meningococcal disease in a university community. J Clin Microbiol. 1995;33:2209–2211. doi: 10.1128/jcm.33.8.2209-2211.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imrey P B, Jackson L A, Ludwinski P H, England III A C, Fella G A, Fox B C, Isdale L B, Reeves M W, Wenger J D. Meningococcal carriage, alcohol consumption, and campus bar patronage in a serogroup C meningococcal disease outbreak. J Clin Microbiol. 1995;33:3133–3137. doi: 10.1128/jcm.33.12.3133-3137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson L A, Schuchat A, Reeves M W, Wenger J D. Serogroup C meningococcal outbreaks in the United States. An emerging threat. JAMA. 1995;273:383–389. [PubMed] [Google Scholar]
- 9.Jelfs J, Jalaludin B, Munro R, Patel M, Kerr M, Daley D, Neville S, Capon A. A cluster of meningococcal disease in western Sydney, Australia initially associated with a nightclub. Epidemiol Infect. 1998;120:263–270. doi: 10.1017/s0950268898008681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nassif X. Interaction mechanisms of encapsulated meningococci with eucaryotic cells: what does this tell us about the crossing of the blood-brain barrier by Neisseria meningitidis? Curr Opin Microbiol. 1999;2:71–77. doi: 10.1016/s1369-5274(99)80012-5. [DOI] [PubMed] [Google Scholar]
- 11.Olcén P, Danielsson D, Kjellander J. The use of protein A-containing staphylococci sensitized with anti-meningococcal antibodies for grouping Neisseria meningitidis and demonstration of meningococcal antigen in cerebrospinal fluid. Acta Pathol Microbiol Scand B. 1975;83:387–396. doi: 10.1111/j.1699-0463.1975.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 12.Peltola H. Meningococcal disease: still with us. Rev Infect Dis. 1983;5:71–91. doi: 10.1093/clinids/5.1.71. [DOI] [PubMed] [Google Scholar]
- 13.Raymond N J, Reeves M, Ajello G, Baughman W, Gheesling L L, Carlone G M, Wenger J D, Stephens D S. Molecular epidemiology of sporadic (endemic) serogroup C meningococcal disease. J Infect Dis. 1997;176:1277–1284. doi: 10.1086/514123. [DOI] [PubMed] [Google Scholar]
- 14.Speers D J, Jelfs J. Typing of Neisseria meningitidis by restriction analysis of the amplified porA gene. Pathology. 1997;29:201–205. doi: 10.1080/00313029700169864. [DOI] [PubMed] [Google Scholar]
- 15.Strathdee C A, Tyler S D, Ryan J A, Johnson W M, Ashton F E. Genomic fingerprinting of Neisseria meningitidis associated with group C meningococcal disease in Canada. J Clin Microbiol. 1993;31:2506–2508. doi: 10.1128/jcm.31.9.2506-2508.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stuart J M, Cartwright K A, Dawson J A, Rickard J, Noah N D. Risk factors for meningococcal disease: a case control study in southwest England. Community Med. 1988;10:139–146. [PubMed] [Google Scholar]
- 17.Suker J, Feavers I M, Achtman M, Morelli G, Wang J F, Maiden M C. The porA gene in serogroup A meningococci: evolutionary stability and mechanism of genetic variation. Mol Microbiol. 1994;12:253–265. doi: 10.1111/j.1365-2958.1994.tb01014.x. [DOI] [PubMed] [Google Scholar]
- 18.van Deuren M, Brandtzaeg P, van der Meer J W. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin Microbiol Rev. 2000;13:144–166. doi: 10.1128/cmr.13.1.144-166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogel U, Morelli G, Zurth K, Claus H, Kriener E, Achtman M, Frosch M. Necessity of molecular techniques to distinguish between Neisseria meningitidis strains isolated from patients with meningococcal disease and from their healthy contacts. J Clin Microbiol. 1998;36:2465–2470. doi: 10.1128/jcm.36.9.2465-2470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells M, Gibbons R B. Primary meningococcal arthritis: case report and review of the literature. Mil Med. 1997;162:769–772. [PubMed] [Google Scholar]
- 21.Woods T C, Helsel L O, Swaminathan B, Bibb W F, Pinner R W, Gellin B G, Collin S F, Waterman S H, Reeves M W, Brenner D J, Broome C V. Characterization of Neisseria meningitidis serogroup C by multilocus enzyme electrophoresis and ribosomal DNA restriction profiles (ribotyping) J Clin Microbiol. 1992;30:132–137. doi: 10.1128/jcm.30.1.132-137.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]