Abstract
Insulin resistance is hypothesized to contribute to increases in blood pressure (BP) through both the renin-angiotensin-aldosterone and sympathetic nervous systems. Prior large-scale studies examining this association are confounded by overt diabetes, obesity, and antihypertensive medication use. In a cross-sectional analysis of 10,810 Hispanic Community Health Study/Study of Latinos (HCHS/SOL) participants without diabetes and not taking anti-hypertensive medications, we examined the associations of insulin resistance, quantified by HOMA-IR, with systolic BP (SBP) and diastolic BP (DBP) overall and stratified by sex and pre-diabetes status in unadjusted and adjusted analyses. The total sample included 52% women, mean (SD) age 37.2 (13.4) years, 39% of participants had pre-diabetes, mean (SD) HOMA-IR 2.8 (2.2). Stage 1 or 2 hypertension was present in 26% of participants; mean (SD) SBP 116.8 (15) mmHg and DPB 71.0 (10.4) mmHg. Overall, there was a significant linear association between HOMA-IR and both SBP (β 2.64±0.44) and DBP (β 1.49±0.35). We found a significant interaction with sex and the association between HOMA-IR and SBP; the association was linear in men and nonlinear in women. There was no statistically significant interaction between pre-diabetes status and the associations between HOMA-IR and BP. In conclusion, in a large community-based sample of Hispanic/Latino adults of diverse national backgrounds not taking anti-hypertensive medications and free from diabetes, insulin resistance was positively associated with both SBP and DBP. Future longitudinal studies, with adequate power to examine sex-specific differences, are needed to examine the independent contribution of insulin resistance to the development of hypertension.
Keywords: insulin resistance, pre-diabetes, blood pressure, hypertension, sex, Hispanic/Latino
INTRODUCTION
There is a significant burden of diabetes and hypertension (HTN) in the United States (US).1 Among US adults, 26 million (9.8%) have diagnosed diabetes, 9.4 million (3.7%) have undiagnosed diabetes and 91.8 million (37.6%) have prediabetes based on NHANES (National Health and Nutrition Examination Survey) 2013-2016 data;1,2 and 45.6% have HTN using the new blood pressure (BP) thresholds from the 2017 American College of Cardiology/ American Heart Association (ACC/AHA) guidelines.3, 4 Diabetes and HTN commonly co-exist especially in Hispanic/Latino individuals who are disproportionally affected by diabetes, have higher proportion of untreated diabetes and HTN, and are at increased risk of HTN related mortality compared to Whites.5-7
Insulin resistance, and the compensatory hyperinsulinemia that accompany it over time, are posited to not only result in the development of diabetes, but also to contribute to the development of elevated BP and HTN through dysregulation of the renal sodium metabolism,8, 9 renin-angiotensin-aldosterone10, 11 and sympathetic nervous systems.12 Although several prior epidemiologic studies have demonstrated associations between insulin resistance and BP and HTN, their findings have been inconsistent.13-15 The extent to which insulin resistance is independently associated with increased BP and HTN in Hispanic/Latino adults, a population disproportionately affected by insulin resistance and diabetes mellitus, is limited to very few studies that report conflicting results.16-18 In the Insulin Resistance Atherosclerosis Study (IRAS), Saad et al. found an association between insulin resistance (lower levels of insulin sensitivity index) and higher systolic BP (SBP) and diastolic BP (DBP) and HTN among nondiabetic Whites and Hispanics;17 whereas other studies report an association in Whites but not Hispanics.16, 18 It is hypothesized that some of this variability may be due to small sample sizes, confounding from concomitant antihypertensive medication use, and nulling of the associations after adjustment for obesity.
In this study, our goal was to examine the associations between insulin resistance and BP early in the spectrum of insulin resistance, prior to the development of overt diabetes. To overcome the limitations of prior studies, we examined the associations between insulin resistance, measured using the homeostasis model assessment of insulin resistance (HOMA-IR), and SBP and DBP in adults without diabetes who were not taking antihypertensive medications from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). In a sensitivity analysis, we repeated all analyses in the subset of participants with a body mass index (BMI) in the normal range (18.5-25 kg/m2).
METHODS
Data from the HCHS/SOL cohort are publicly available to researchers upon application to NHLBI BIOLINCC (National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center).
Study Sample
We studied participants of the HCHS/SOL, a community-based cohort of adults representing diverse Hispanic/Latino backgrounds (Central American, Cuban, Dominican, Mexican, Puerto Rican, and South American).19 The sampling methods and design of HCHS/SOL have been detailed previously.20, 21 In brief, self-identified Hispanic/Latino men and women were recruited between March 2008 to June 2011 from 4 communities in the United States (Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA) using a multi-stage area probability sample design. At each stage of sampling, oversampling occurred, and sampling weights were generated to reflect the probabilities of selection. The institutional review board at each HCHS/SOL center approved the study protocol, and all participants provided written informed consent.
Of the 16,415 HCHS/SOL participants who attended the baseline examination, we excluded individuals with missing data for BP measurement (N=23), BMI (N=41), heart rate (N=6), diabetes status (N=1), glycosylated hemoglobin A1c (HbA1c) (N=131), and (HOMA-IR) (N=92). We also excluded participants who self-reported coronary heart disease or heart failure (N=703) or had missing data (N=406). Individuals taking antihypertensive medications (N=2,172) or missing information on medications (N=359), and those with diabetes (N=1,671) were also excluded.22 The final sample size was 10,810 participants (Figure 1).
Figure 1.
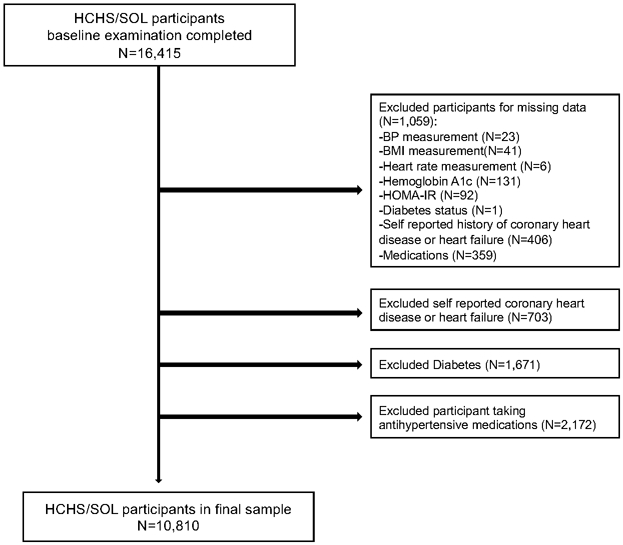
Study Flowchart
HCHS/SOL Study Procedures
At the baseline examination, all study participants completed questionnaires regarding demographic, acculturation and social factors, medical history, and dietary patterns. Physical activity was self-reported using the modified Global Physical Activity Questionnaire (GPAQ) to derive physical activity in minutes/day.23, 24
Participants were asked to fast and refrain from smoking for a minimum of 8-hours before the HCHS/SOL baseline clinic examination. Venous blood and urine specimens were collected, processed, and frozen on site and sent to the HCHS/SOL central laboratory for analysis. A hexokinase enzymatic method (Roche Diagnostics Corp, Indianapolis, IN) was used to assay fasting plasma glucose. Hemoglobin A1C was measured in EDTA whole blood using a Tosoh G7 automated high-performance liquid chromatography analyzer (Tosoh Bioscience Inc, San Francisco, CA). Fasting insulin was measured with 2 commercial immunoassays (ELISA, Mercodia AB, Uppsala, Sweden; and sandwich immunoassay on a Roche Elecsys 2010 Analyzer, Roche Diagnostics, Indianapolis, IN).25 To account for the change in assays, insulin levels collected prior to October 29, 2009 using the Mercodia analyzers were calibrated using the following regression equation: y = 1.00494x – 1.4504, where y = adjusted insulin value, x = original insulin value.
BP Measurement
Height, weight, waist and hip circumferences, heart rate and BP were measured by trained study staff using standardized protocols previously described.26 Prior to BP measurement, the appropriate cuff size was selected based on arm circumference at the midpoint of the upper arm; cuff size was determined as follows: small (17-22ccm), adult (22-32cm), large (32-42cm) and XLarge (42-50cm). BP and heart rate were measured in triplicate while in the seated position following 5 minutes of rest using an automated BP device (Omron model HEM-907 XL; Omron Healthcare Inc, Bannockburn, IL).27 The means of the three measurements (SBP, DBP, heart rate) were used for all calculations. Normal BP was defined as SBP <120 mmHg and DBP <80 mmHg, elevated BP as SBP 120-129 mmHg and DBP <80 mmHg, untreated stage 1 HTN as SBP 130-139 mmHg and/or DBP 80-89 mmHg, and untreated stage 2 HTN as SBP ≥140 mmHg and/or DBP ≥90 mmHg) based on the 2017 ACC/AHA BP guidelines.4
Definition of Pre-diabetes and BMI categories
Pre-diabetes were defined according to the American Diabetes Association guideline as the presence of a fasting plasma glucose ranging from 100-125 mg/dL and/or HbA1c value of 5.7-6.4%.28 Normal blood glucose was a fasting plasma glucose <100 mg/dL and HbA1c <5.7%. Insulin resistance was calculated using the HOMA-IR score [fasting insulin (mU/L) x fasting glucose (mg/dL)/405].29 Normal BMI was defined as BMI 18.5-25 kg/m2, overweight as BMI 25.1-29.9 kg/m2, and obese as BMI≥30 kg/m2. The presence of metabolic syndrome was defined as having any three or more of the following criteria: 1) waist circumference ≥102 cm in men and ≥88 cm in women; 2) triglyceride level ≥150 mg/dL or use of triglyceride lowering medication; 3) HDL-C level <40 mg/dL in men and <50 mg/dL in women; 4) blood pressure ≥130 mmHg systolic or ≥85 mmHg diastolic; and 5) fasting glucose level ≥100 mg/dL.30
Statistical Analyses
The results of all analyses (including model-based means, percentages, and p-values) are weighted using sampling weights to adjust for unequal sampling probability and nonresponse and accounted for the complex survey design in HCHS/SOL.20, 21 Continuous variables are summarized as means [standard deviation (SD)] for and categorical variables as percentage (%). All variables were assessed for normality. HOMA-IR was found to be skewed, and all subsequent analyses were performed using log-base transformed values.
In linear regression analyses, we examined the age- and sex-adjusted univariate associations of SBP and DBP with demographic and clinical characteristics. We examined the associations of insulin resistance, quantified as log(HOMA-IR), with SBP and DBP using multivariable adjusted restricted cubic spline models with 3 knots (placed at the 10th, 50th, and 90th percentile).31 All models were adjusted for HCHS/SOL site, self-identified Hispanic/Latino subgroup, age, sex, and all co-variates that were found to be statistically significant in univariate analyses (P<0.05). Wald tests were used to assess all models for non-linearity and significance, as well as to test for equality and interactions in the pre-defined subgroups of sex (men/women) and pre-diabetes status (normal blood glucose/pre-diabetes).
We tested for interactions between BMI category (normal, overweight, obese) and the association of insulin resistance with SBP or DBP. In sensitivity analyses, we repeated all statistical procedures outlined above including only individuals with a normal BMI to examine the associations of insulin resistance with SBP and DBP in the 2,524 individuals free from the potential confounding effects of excess or inadequate weight.
All analyses were performed using Stata v13.1 (College Station, TX). A two-sided p-value <0.05 was the threshold for statistical significance.
RESULTS
The demographic and clinical characteristics of the HCHS/SOL participants included in the current analysis are shown in Table 1. The mean (SD) age was 37.2 (13.4) years and 52% were women. Overall, 39% had pre-diabetes, 35% were obese, and 22% had metabolic syndrome. In this study population that did not include any individuals taking anti-hypertensive medications; 59% of individuals had normal BP, 15% had elevated BP, 17% had untreated stage 1 HTN, and 9% had untreated stage 2 HTN based on the 2017 ACC/AHA BP guidelines.4 BMI, SBP, DBP, mean arterial pressure, pulse pressure, cholesterol levels (total, LDL, triglycerides), HbA1c, fasting glucose, HOMA-IR were significantly higher in individuals with pre-diabetes compared to those with normal blood glucose (Table 1). Demographic and clinic characteristics stratified by the 2017 ACC/AHA BP categories are shown in Table S1.
Table 1:
Demographics and Clinical Characteristics Overall and by Pre-Diabetes Status in the Hispanic Community Health Study/Study of Latinos at Visit 1 (2008-2011)
| Characteristics at Visit 1 | Overall (n = 10,810) |
Normal Blood Glucose (n = 5,972) |
Pre-diabetes (n = 4,838) |
P value |
|---|---|---|---|---|
| Demographics | ||||
| Female, % | 52.0 | 54.7 | 47.8 | <0.001 |
| Age, yrs, mean (SD) | 37.2 (13.4) | 33.2 (11.9) | 43.4 (13.2) | <0.001 |
| Annual Family Income ≥$40,000, % | 22.1 | 23.3 | 20.4 | 0.083 |
| Years living in U.S., yrs, mean (SD) | 18.6 (13.4) | 17.4 (12.3) | 20.6 (14.7) | <0.001 |
| Health Insurance, % | 46.3 | 46.8 | 45.6 | 0.436 |
| Hispanic/Latin Background, % | 0.181 | |||
| Dominican | 9.9 | 11.1 | 8.0 | |
| Central/South American | 13.2 | 13.3 | 13.1 | |
| Cuban | 19.1 | 18.2 | 20.6 | |
| Mexican | 39.0 | 38.3 | 40.0 | |
| Puerto-Rican | 14.3 | 14.2 | 14.4 | |
| Other or more than 1 heritage | 4.5 | 4.9 | 4.0 | |
| Clinical Characteristics | ||||
| 2017 ACC/AHA BP Category *, % | <0.001 | |||
| Normal | 59.3 | 67.4 | 46.5 | |
| Elevated | 14.9 | 13.8 | 16.6 | |
| Stage 1 Hypertension | 16.9 | 13.6 | 22.1 | |
| Stage 2 Hypertension | 9.0 | 5.2 | 14.9 | |
| CKD, % | 2.3 | 1.4 | 3.6 | <0.001 |
| Metabolic Syndrome, % | 21.6 | 10.2 | 39.5 | <0.001 |
| Current smoker, % | 21.6 | 21.3 | 22.1 | 0.522 |
| Alcohol use, drinks/week, mean (SD) | 5.4 (8.0) | 5.5 (8.1) | 5.2 (7.8) | 0.359 |
| TPA, metabolic min/day, mean (SD) | 145.1 (196.2) | 146.8 (193.6) | 142.4 (200.4) | 0.528 |
| Clinical Measures | ||||
| Body mass index, kg/m2, mean. (SD) | 28.7 (5.8) | 27.7 (5.6) | 30.1 (5.8) | <0.001 |
| Waist/hip ratio, mean (SD) | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | <0.001 |
| Heart rate, bpm, mean (SD) | 64.9 (9.4) | 64.5 (9.2) | 65.7 (9.8) | <0.001 |
| Systolic BP, mmHg, mean (SD) | 116.8 (15.0) | 113.7 (13.5) | 121.6 (15.9) | <0.001 |
| Diastolic BP, mmHg, mean (SD) | 71.0 (10.4) | 69.1 (9.8) | 74.1 (10.5) | <0.001 |
| MAP, mmHg, mean (SD) | 86.3 (11.1) | 84.0 (10.3) | 89.9 (11.4) | <0.001 |
| Pulse pressure, mmHg, mean (SD) | 45.8 (10.2) | 44.7 (9.2) | 47.5 (11.3) | <0.001 |
| Laboratory Measures | ||||
| Total cholesterol, mg/dL, mean (SD) | 191.6 (41.7) | 183.6 (39.1) | 204.1 (42.6) | <0.001 |
| HDL cholesterol, mg/dL, mean (SD) | 48.9 (12.8) | 50.0 (12.8) | 47.3 (12.7) | <0.001 |
| LDL cholesterol, mg/dL, mean (SD) | 118.6 (35.4) | 112.4 (33.3) | 128.4 (36.4) | <0.001 |
| Triglycerides, mg/dL, mean (SD) | 122.8 (91.0) | 107.2 (75.8) | 147.0 (106.3) | <0.001 |
| Proteinuria, % | 5.5 | 5.0 | 6.3 | 0.027 |
| HbA1c, %, mean (SD) | 5.4 (0.4) | 5.2 (0.3) | 5.6 (0.3) | <0.001 |
| Fasting glucose, mg/dL, mean (SD) | 93.1 (8.1) | 89.6 (5.7) | 98.4 (8.4) | <0.001 |
| HOMA-IR, mean (SD) | 2.8 (2.2) | 2.3 (1.6) | 3.5 (2.6) | <0.001 |
BP, blood pressure; CKD, chronic kidney disease (eGFR <60 mL/min/1.73m2); Global Physical Activity Questionnaire was used to derive total physical activity (TPA) in min/day; MAP, mean arterial pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; proteinuria (urine albumin/creatinine >30mg/g); HbA1c, hemoglobin A1c; HOMA-IR, Homeostasis model assessment-Insulin Resistance
2017 ACC/AHA Blood Pressure Category: normal BP (<120 mmHg and DBP <80 mmHg), elevated BP (SBP 120-129 mmHg and DBP <80 mmHg), stage 1 hypertension (SBP 130-139 mmHg or DBP 80-89 mmHg), stage 2 hypertension (SBP ≥140 mmHg or DBP ≥90 mmHg)
The associations of clinical and metabolic characteristics with SBP and DBP are shown in Table 2. Both SBP and DBP were significantly associated with male sex, age, BMI, waist/hip ratio, protein-urine albumin/creatine, heart rate, alcohol use, all lipid measures, HbA1c, and fasting glucose.
Table 2:
Association of clinical and metabolic characteristics with systolic and diastolic blood pressure
| Systolic Blood Pressure (mmHg) |
Diastolic Blood Pressure (mmHg) |
|||
|---|---|---|---|---|
| Characteristics at Visit 1 | B-coefficient (95% CI) | P value | B-coefficient (95% CI) |
P value |
| Male Sex | 9.06 (8.37 - 9.76) | <0.001 | 3.17 (2.61 - 3.73) | <0.001 |
| Age (per 10 years) | 4.92 (4.66 - 5.19) | <0.001 | 2.45 (2.24 - 2.66) | <0.001 |
| Annual Family Income ≥$40,000 | −0.66 (−1.55 - 0.24) | 0.149 | −0.49 (−1.21 - 0.23) | 0.183 |
| Years living in U.S. (per 5-year increase) | −0.07 (−0.20 - 0.06) | 0.302 | 0.13 (0.02 - 0.24) | 0.017 |
| Currently Has Health Insurance, % | −0.05 (−0.74 - 0.64) | 0.894 | −0.52 (−1.07 - 0.03) | 0.062 |
| Body mass index (per 1 kg/m2 increase) | 0.25 (0.18 - 0.31) | <0.001 | 0.55 (0.49 - 0.60) | <0.001 |
| Waist/hip ratio (per 0.2 increase) | 1.90 (0.88 - 2.92) | <0.001 | 4.78 (4.02 - 5.54) | <0.001 |
| Current smoker | −0.73 (−1.54 - 0.09) | 0.082 | −0.20 (−0.88 - 0.49) | 0.572 |
| Proteinuria | 5.45 (3.70 - 7.20) | <0.001 | 3.82 (2.65 - 5.00) | <0.001 |
| Chronic kidney disease (eGFR <60) | 2.82 (0.04 - 5.61) | 0.047 | 0.48 (−1.22 - 2.17) | 0.582 |
| Heart rate (per 5 bpm increase) | 0.57 (0.39 - 0.75) | <0.001 | 1.50 (1.37 - 1.64) | <0.001 |
| Alcohol use (per 1 drink per week increase) | 0.14 (0.09 - 0.19) | <0.001 | 0.12 (0.07 - 0.17) | <0.001 |
| TPA (per 100 metabolic min/day increase) | 0.001 (−0.193 - 0.195) | 0.993 | −0.24 (−0.39 - −0.09) | 0.002 |
| Total cholesterol (per 10 mg/dL increase) | 0.33 (0.24 - 0.43) | <0.0001 | 0.39 (0.32 - 0.46) | <0.001 |
| HDL cholesterol (per 10 mg/dL increase) | 0.22 (−0.03 - 0.47) | 0.080 | −0.71 (−0.92 - −0.50) | <0.001 |
| LDL cholesterol (per 10 mg/dL increase) | 0.29 (0.18 - 0.40) | <0.001 | 0.40 (0.32 - 0.48) | <0.001 |
| Triglycerides (per 10 mg/dL increase) | 0.11 (0.07 - 0.16) | <0.001 | 0.18 (0.15 - 0.22) | <0.001 |
| HbA1c (per 1 % increase) | 1.64 (0.48 - 2.80) | 0.006 | 2.90 (1.99 - 3.80) | <0.001 |
| Fasting glucose (per 10 mg/dL increase) | 1.94 (1.46 - 2.41) | <0.001 | 1.77 (1.38 - 2.15) | <0.001 |
| HOMA IR (per 1unit increase) | 0.80 (0.63 - 0.97) | <0.001 | 1.18 (0.99 - 1.37) | <0.001 |
All are adjusted for age and sex, age is adjusted for only sex and sex is adjusted for only age.
HDL, high density lipoprotein; LDL, low density lipoprotein; proteinuria (urine albumin/creatinine >30mg/g); HbA1c, hemoglobin A1c; Global Physical Activity Questionnaire was used to derive total physical activity (TPA) in minutes/day (min/day); HOMA-IR, Homeostasis model assessment-Insulin Resistance; eGFR, estimated glomerular filtration rate;
Associations between Insulin Resistance and Systolic and Diastolic Blood Pressure
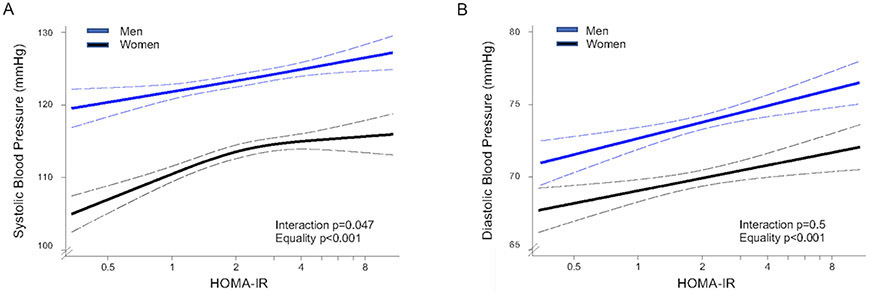
In multivariable-adjusted analyses, insulin resistance was positively associated with both SBP (β 2.64±0.44) and DBP (β 1.49±0.35) (p<0.001 for both). There was a significant interaction between sex and the association of HOMA-IR with SBP (p=0.047). In men, the association of HOMA-IR with SBP was positive and linear (linear trend p <0.001, multi-variable adjusted β-coefficient 2.56±0.53), but in women it was nonlinear (overall trend p<0.001, multi-variable adjusted β 3.62±0.69 before the inflection point) (Figure 2A). For DBP there was no significant interaction with sex, and the association with HOMA-IR was positive and linear (multi-variable adjusted β 1.49±0.35) for both men and women (Figure 2B).
Figure 2. Associations of insulin resistance with systolic and diastolic blood pressure by sex.
Multivariable-adjusted model showing association between HOMA-IR and systolic blood pressure [A], diastolic blood pressure [B] by sex. Models were adjusted for any variable with p<0.05 in the univariate analysis: sex, age, years living in the US, body mass index, waist/hip ratio, urine albumin/creatine, heart rate, alcohol use, total physical activity, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, HbA1c, fasting glucose. The dashed black curves represent the upper and lower 95% confidence limits.
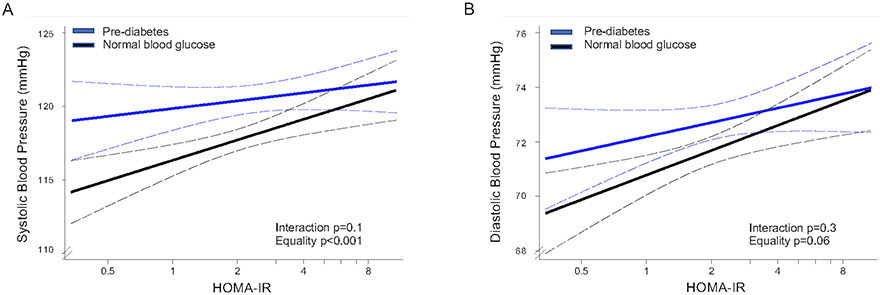
When examined by pre-diabetes status (normal blood glucose vs. presence of pre-diabetes), there was no significant interaction between pre-diabetes status and the association of HOMA-IR with either SBP or DBP. In participants with and without prediabetes there was a significant, positive linear association between SBP and HOMA-IR (multi-variable adjusted β 2.64±0.44) (Figure 3A). For DBP, there was a significant linear association with HOMA-IR only in participants with normal blood glucose (multi-variable adjusted β 1.47±0.44) (Figure 3B).
Figure 3. Association of insulin resistance with systolic and diastolic blood pressure by pre-diabetes status.
Multivariable-adjusted linear model showing association between HOMA-IR and systolic blood pressure [A], diastolic blood pressure [B] by pre-diabetes status (normal blood glucose vs. pre-diabetes). Model was adjusted for any variable with p<0.05 in the univariate analysis: sex, age, years living in the US, body mass index, waist/hip ratio, urine albumin/creatine, heart rate, alcohol use, total physical activity, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, HbA1c, fasting glucose. The dashed black curves represent the upper and lower 95% confidence limits.
Sensitivity Analyses Examining Individuals with a Normal BMI
There was no statistically significant interaction between BMI category (normal, overweight, obese) and the association of HOMA-IR with SBP or DBP (p>0.5). In sensitivity analyses including only normal BMI individuals (N=2,524), the association between HOMA-IR and both SBP (β 1.94±0.76) and DBP (β 1.04±0.46) was positive, linear and statistically significant. There was no statistically significant interaction between sex or pre-diabetes status and the association of HOMA-IR with either SBP or DBP (p>0.05).
DISCUSSION
In the largest US community-based sample of Hispanics/Latino adults of diverse national backgrounds across the age spectrum with a relatively high (~40%) burden of untreated elevated BP and HTN we demonstrate that insulin resistance was positively associated with both SBP and DBP in individuals without diabetes. This association was not confounded by antihypertensive medications and was independent of abnormal BMI. We also demonstrate a sex-specific interaction exists in the association between insulin resistance and SBP; in women the association was non-linear and stronger than the non-linear association seen in men. Further, we found a linear association between insulin resistance and SBP in individuals with both normal blood glucose and prediabetes, whereas the association with DBP was only significant in individuals with normal blood glucose.
The mechanisms through which insulin resistance alters BP are not fully understood. The hypersecretion of high levels of insulin in response to insulin resistance is known to affect renal sodium excretion,8, 9 the sympathetic nervous system,12 and the renin-angiotensin-aldosterone system.10, 11 Insulin resistance has also been associated with impaired endothelium-dependent vasodilatation, atherosclerosis, and inflammation.32 Over the long-term, these toxic effects of insulin resistance on the vasculature may contribute to BP elevation, and as shown in the current study this appears to be independent of diabetes status. The association between insulin resistance and DBP in individuals with normal blood glucose also suggests that insulin resistance may affect DBP even prior to the development of the hyperinsulinemia associated with pre-diabetes. These findings may help us understand why DBP is the strongest predictor of coronary heart disease in young and middle-age adults.33, 34
Consistent with previous studies, we found a positive association between insulin resistance and BP.13-15 Unlike prior work, we were able to examine this association in a large number of individuals not taking antihypertensive medications, thus eliminating potential confounding effects of therapies such as thiazide diuretics and beta-blockers, which are known to increase insulin resistance.17, 35-37 It is also known that visceral adipose tissue secretes cytokines that may result in inflammation and contribute to insulin resistance.38-40 Unlike prior studies which reported attenuation or nulling of the association between insulin resistance and BP after adjustment for BMI,41-43 the associations remained significant after multivariable adjustment that included BMI, as well as in sensitivity analyses examining only individuals with a normal BMI. This consistency lends strength to the hypothesis that the relationship between insulin resistance and BP is independent of the contribution of obesity to insulin resistance. It is possible that the underlying changes in adipose tissue resulting in insulin resistance may also contribute to BP elevation, and future studies are needed to examine the potential direct effects of metabolically active adipose on BP.
It is increasingly recognized that there are sex-specific differences in the association between BP and HTN, though prior studies have reported conflicting results.44-47 We found a sex-specific interaction in the association between insulin resistance and SBP, with a stronger, non-linear association in females. This is consistent with the findings of Goodfriend et al. who reported an association between insulin resistance in normotensive women but not in men.45 These findings suggest that the underlying physiologic mechanism of blood pressure and insulin regulation may differ in men and women and may partially explain the heightened cardiovascular risk seen in women with diabetes relative to men with a similar risk factor profile.48, 49
Our study has several strengths and limitations that should be considered. First, our study population included individuals aged 18-76 years, with a mean age of 37 years. This represents an age group most likely to benefit from interventions aimed at preventing the development of HTN, a major cause of morbidity and mortality. Next, the use of HOMA-IR as a measure of insulin resistance has been extensively validated for the evaluation of insulin resistance in individuals with and without diabetes.29, 50-54 Although multiple statistical tests were performed, all analyses were prespecified, and the consistency of our findings across the different models argue against a type I error as an explanation for our findings. Our sample was composed of an ethnically diverse population of Hispanic/Latino women and men, a group that has a heightened risk of diabetes and hypertension, which has historically been understudied. Despite the large sample size, we did not have the power to study these associations by Hispanic/Latino subgroup. Our findings also require confirmation in other populations of different ethnic backgrounds. Another limitation was the cross-sectional nature of our data, which precludes us from investigating potential causal associations between insulin resistance and the development of elevated BP and overt HTN. Lastly, although BP was measured using a rigorous protocol, the measurements were all made during one visit, rather than over at least 2 separate visits as recommended by the ACC/AHA guidelines.4
In summary, this study provides a unique opportunity to examine the association between insulin resistance and BP in a large, ethnically diverse, community-based cohort of Hispanics/Latino adults free from the potentially confounding effects of overt diabetes, abnormal BMI, and anti-hypertensive medications. We found strong, positive associations between insulin resistance and both SBP and DBP as well as sex-based differences in some associations. Our findings support the hypothesis that insulin resistance adversely affects the vasculature early in the disease process, before the development of diabetes, and thus may contribute to the development of HTN, particularly in women. Future, longitudinal sex-specific studies are needed to examine whether there is a casual relationship between insulin resistance and the development of elevated BP and HTN.
PERSPECTIVES
In light of the rising burden of both HTN and diabetes it is imperative that we better understand the associations between insulin resistance and BP. Prior large-scale studies examining these associations have reported conflicting results and their interpretation is limited by potential confounding from overt diabetes, abnormal BMI, and antihypertensive medication use. In this large, ethnically diverse, community-based cohort of Hispanics/Latino adults, insulin resistance was associated with both SBP and DBP suggesting that insulin resistance may adversely affect the vasculature prior to the development of diabetes independent of confounders. Our findings also suggest the association of insulin resistance and SBP is stronger in women.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new:
There are sex-differences in the association between insulin resistance and systolic blood pressure.
What is relevant:
Insulin resistance is associated with systolic and diastolic blood pressure in individuals without diabetes independent of potential confounders such as antihypertensive medications and abnormal body mass index.
Summary:
The positive association between insulin resistance and both SBP and DBP in individuals without diabetes suggests that insulin resistance may adversely affect the vasculature prior to the development of dysglycemia, and this association may be stronger in women than men.
ACKNOWLEDGEMENTS
The authors thank the participants and staff of HCHS/SOL for their important contributions to this research. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services
FUNDING
The Hispanic Community Health Study/Study of Latinos is a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (HHSN268201300001I / N01-HC-65233), University of Miami (HHSN268201300004I / N01-HC-65234), Albert Einstein College of Medicine (HHSN268201300002I / N01-HC-65235), University of Illinois at Chicago (HHSN268201300003I / N01-HC-65236 Northwestern Univ), and San Diego State University (HHSN268201300005I / N01-HC-65237). The following Institutes/Centers/Offices have contributed to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, NIH Institution-Office of Dietary Supplements. The authors report the following sources of funding: NIH/NHLBI K23-HL136853 and R01-HL153382 (NAB) and R00-HL-107642 (SC), NIH T32HL116273 (OQ), NIH K23HL151867 (OQ), NIH/NHLBI K01 HL144607 (FR), NIH/NHLBI K01-HL-137557 (DBH), and a grant from the Ellison Foundation (SC), the Barbra Streisand Women's Cardiovascular Research and Education Program, the Linda Joy Pollin Women's Heart Health Program, the Erika J. Glazer Women's Heart Research Initiative (OQ, NBM, SC).
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang NY, Tsao CW, American Heart Association Council on E, Prevention Statistics C, Stroke Statistics S. Heart disease and stroke statistics-2021 update: A report from the american heart association. Circulation. 2021;143:e254–e743 [DOI] [PubMed] [Google Scholar]
- 2.Mendola ND CT-C, Gu Q, Eberhardt MS, Saydah S Prevalence of total, diagnosed, and undiagnosed diabetes among adults: United states, 2013–2016. NCHS Data Brief, no 319. Hyattsville, MD: National Center for Health Statistics. 2018 [PubMed] [Google Scholar]
- 3.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr., Whelton PK Potential u.S. Population impact of the 2017 acc/aha high blood pressure guideline. J Am Coll Cardiol. 2018;71:109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT Jr. 2017 acc/aha/aapa/abc/acpm/ags/apha/ash/aspc/nma/pcna guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation. 2018;138:e426–e483 [DOI] [PubMed] [Google Scholar]
- 5.Gu A, Yue Y, Desai RP, Argulian E Racial and ethnic differences in antihypertensive medication use and blood pressure control among us adults with hypertension: The national health and nutrition examination survey, 2003 to 2012. Circ Cardiovasc Qual Outcomes. 2017;10 [DOI] [PubMed] [Google Scholar]
- 6.Al Kibria GM Racial/ethnic disparities in prevalence, treatment, and control of hypertension among us adults following application of the 2017 american college of cardiology/american heart association guideline. Prev Med Rep. 2019;14:100850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hypertension-related mortality among hispanic subpopulations--united states, 1995-2002. MMWR Morb Mortal Wkly Rep. 2006;55:177–180 [PubMed] [Google Scholar]
- 8.DeFronzo RA The effect of insulin on renal sodium metabolism. A review with clinical implications. Diabetologia. 1981;21:165–171 [DOI] [PubMed] [Google Scholar]
- 9.Skott P, Hother-Nielsen O, Bruun NE, Giese J, Nielsen MD, Beck-Nielsen H, Parving HH Effects of insulin on kidney function and sodium excretion in healthy subjects. Diabetologia. 1989;32:694–699 [DOI] [PubMed] [Google Scholar]
- 10.Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, Stas S, Sowers JR Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. American journal of physiology. Heart and circulatory physiology. 2007;293:H2009–2023 [DOI] [PubMed] [Google Scholar]
- 11.Zhou MS, Schulman IH, Zeng Q Link between the renin-angiotensin system and insulin resistance: Implications for cardiovascular disease. Vascular medicine (London, England). 2012;17:330–341 [DOI] [PubMed] [Google Scholar]
- 12.Fossum E, Hoieggen A, Reims HM, Moan A, Rostrup M, Eide I, Kjeldsen SE High screening blood pressure is related to sympathetic nervous system activity and insulin resistance in healthy young men. Blood pressure. 2004;13:89–94 [DOI] [PubMed] [Google Scholar]
- 13.Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, Pedrinelli R, Brandi L, Bevilacqua S Insulin resistance in essential hypertension. N Engl J Med. 1987;317:350–357 [DOI] [PubMed] [Google Scholar]
- 14.Ferrannini E, Natali A, Capaldo B, Lehtovirta M, Jacob S, Yki-Jarvinen H Insulin resistance, hyperinsulinemia, and blood pressure: Role of age and obesity. European group for the study of insulin resistance (egir). Hypertension. 1997;30:1144–1149 [DOI] [PubMed] [Google Scholar]
- 15.Saad MF, Lillioja S, Nyomba BL, Castillo C, Ferraro R, De Gregorio M, Ravussin E, Knowler WC, Bennett PH, Howard BV, et al. Racial differences in the relation between blood pressure and insulin resistance. N Engl J Med. 1991;324:733–739 [DOI] [PubMed] [Google Scholar]
- 16.Mitchell BD, Haffner SM, Hazuda HP, Valdez R, Stern MP The relation between serum insulin levels and 8-year changes in lipid, lipoprotein, and blood pressure levels. American journal of epidemiology. 1992;136:12–22 [DOI] [PubMed] [Google Scholar]
- 17.Saad MF, Rewers M, Selby J, Howard G, Jinagouda S, Fahmi S, Zaccaro D, Bergman RN, Savage PJ, Haffner SM Insulin resistance and hypertension: The insulin resistance atherosclerosis study. Hypertension. 2004;43:1324–1331 [DOI] [PubMed] [Google Scholar]
- 18.Shetterly SM, Rewers M, Hamman RF, Marshall JA Patterns and predictors of hypertension incidence among hispanics and non-hispanic whites: The san luis valley diabetes study. Journal of hypertension. 1994;12:1095–1102 [PubMed] [Google Scholar]
- 19.Conomos MP, Laurie CA, Stilp AM, Gogarten SM, McHugh CP, Nelson SC, Sofer T, Fernandez-Rhodes L, Justice AE, Graff M, Young KL, Seyerle AA, Avery CL, Taylor KD, Rotter JI, Talavera GA, Daviglus ML, Wassertheil-Smoller S, Schneiderman N, Heiss G, Kaplan RC, Franceschini N, Reiner AP, Shaffer JR, Barr RG, Kerr KF, Browning SR, Browning BL, Weir BS, Aviles-Santa ML, Papanicolaou GJ, Lumley T, Szpiro AA, North KE, Rice K, Thornton TA, Laurie CC Genetic diversity and association studies in us hispanic/latino populations: Applications in the hispanic community health study/study of latinos. Am J Hum Genet. 2016;98:165–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavange LM, Kalsbeek WD, Sorlie PD, Aviles-Santa LM, Kaplan RC, Barnhart J, Liu K, Giachello A, Lee DJ, Ryan J, Criqui MH, Elder JP Sample design and cohort selection in the hispanic community health study/study of latinos. Ann Epidemiol. 2010;20:642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Schneiderman N, Raij L, Talavera G, Allison M, Lavange L, Chambless LE, Heiss G Design and implementation of the hispanic community health study/study of latinos. Ann Epidemiol. 2010;20:629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page MM, Watkins PJ Provocation of postural hypotension by insulin in diabetic autonomic neuropathy. Diabetes. 1976;25:90–95 [DOI] [PubMed] [Google Scholar]
- 23.Bull FC, Maslin TS, Armstrong T Global physical activity questionnaire (gpaq): Nine country reliability and validity study. J Phys Act Health. 2009;6:790–804 [DOI] [PubMed] [Google Scholar]
- 24.Evenson KR, Sotres-Alvarez D, Deng YU, Marshall SJ, Isasi CR, Esliger DW, Davis S Accelerometer adherence and performance in a cohort study of us hispanic adults. Med Sci Sports Exerc. 2015;47:725–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diaz KM, Goldsmith J, Greenlee H, Strizich G, Qi Q, Mossavar-Rahmani Y, Vidot DC, Buelna C, Brintz CE, Elfassy T, Gallo LC, Daviglus ML, Sotres-Alvarez D, Kaplan RC Prolonged, uninterrupted sedentary behavior and glycemic biomarkers among us hispanic/latino adults: The hchs/sol (hispanic community health study/study of latinos). Circulation. 2017;136:1362–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daviglus ML, Talavera GA, Aviles-Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RC, LaVange L, Penedo F, Perreira K, Pirzada A, Schneiderman N, Wassertheil-Smoller S, Sorlie PD, Stamler J Prevalence of major cardiovascular risk factors and cardiovascular diseases among hispanic/latino individuals of diverse backgrounds in the united states. JAMA. 2012;308:1775–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Assaad MA, Topouchian JA, Darne BM, Asmar RG Validation of the omron hem-907 device for blood pressure measurement. Blood pressure monitoring. 2002;7:237–241 [DOI] [PubMed] [Google Scholar]
- 28.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020. January;43(Suppl 1):S14–S31. doi: 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 30.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, et al. ; American Heart. Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. [DOI] [PubMed] [Google Scholar]
- 31.H FE Regression modeling strategies: With applications to linear models, logistic regression, and survival analysis. New York: Springer. 2001 [Google Scholar]
- 32.Bansilal S, Farkouh ME, Fuster V Role of insulin resistance and hyperglycemia in the development of atherosclerosis. Am J Cardiol. 2007;99:6b–14b [DOI] [PubMed] [Google Scholar]
- 33.Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D Does the relation of blood pressure to coronary heart disease risk change with aging? The framingham heart study. Circulation. 2001;103:1245–1249 [DOI] [PubMed] [Google Scholar]
- 34.Khattar RS, Swales JD, Dore C, Senior R, Lahiri A Effect of aging on the prognostic significance of ambulatory systolic, diastolic, and pulse pressure in essential hypertension. Circulation. 2001;104:783–789 [DOI] [PubMed] [Google Scholar]
- 35.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007; 369: 201–207. [DOI] [PubMed] [Google Scholar]
- 36.Eriksson JW, Jansson PA, Carlberg B, Hägg A, Kurland L, Svensson MK, Ahlström H, Ström C, Lönn L, Ojbrandt K, Johansson L, Lind L. Hydrochlorothiazide, but not Candesartan, aggravates insulin resistance and causes visceral and hepatic fat accumulation: the mechanisms for the diabetes preventing effect of Candesartan (MEDICA) Study. Hypertension. 2008. December;52(6):1030–7. [DOI] [PubMed] [Google Scholar]
- 37.Zillich AJ, Garg J, Basu S, Bakris GL, Carter BL. Thiazide diuretics, potassium, and the development of diabetes: a quantitative review. Hypertension. 2006; 48: 219–22. [DOI] [PubMed] [Google Scholar]
- 38.Makki K, Froguel P, Wolowczuk I Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013 [DOI] [PubMed] [Google Scholar]
- 40.Gregor MF, Hotamisligil GS Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445 [DOI] [PubMed] [Google Scholar]
- 41.Liese AD, Mayer-Davis EJ, Chambless LE, Folsom AR, Sharrett AR, Brancati FL, Heiss G Elevated fasting insulin predicts incident hypertension: The aric study. Atherosclerosis risk in communities study investigators. Journal of hypertension. 1999;17:1169–1177 [DOI] [PubMed] [Google Scholar]
- 42.Arnlov J, Pencina MJ, Nam BH, Meigs JB, Fox CS, Levy D, D'Agostino RB, Vasan RS. Relations of insulin sensitivity to longitudinal blood pressure tracking: Variations with baseline age, body mass index, and blood pressure. Circulation. 2005;112:1719–1727. [DOI] [PubMed] [Google Scholar]
- 43.Fagot-Campagna A, Balkau B, Simon D, Ducimetiere P and Eschwege E. Is insulin an independent risk factor for hypertension? The Paris Prospective Study. International journal of epidemiology. 1997;26:542–50. [DOI] [PubMed] [Google Scholar]
- 44.Rana BK, Insel PA, Payne SH, Abel K, Beutler E, Ziegler MG, Schork NJ, O'Connor DT Population-based sample reveals gene-gender interactions in blood pressure in white americans. Hypertension. 2007;49:96–106 [DOI] [PubMed] [Google Scholar]
- 45.Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res. 1999;7:355–362 [DOI] [PubMed] [Google Scholar]
- 46.Player MS, Mainous AG 3rd, Diaz VA, Everett CJ Prehypertension and insulin resistance in a nationally representative adult population. J Clin Hypertens (Greenwich). 2007;9:424–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kidambi S, Kotchen JM, Krishnaswami S, Grim CE, Kotchen TA Hypertension, insulin resistance, and aldosterone: Sex-specific relationships. J Clin Hypertens (Greenwich). 2009;11:130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A, Hemingway H Type 2 diabetes and incidence of cardiovascular diseases: A cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters SA, Huxley RR, Woodward M Diabetes as risk factor for incident coronary heart disease in women compared with men: A systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551 [DOI] [PubMed] [Google Scholar]
- 50.Bonora E, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, Saggiani F, Poli M, Perbellini S, Raffaelli A, Cacciatori V, Santi L, Targher G, Bonadonna R, Muggeo M Homa-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: Prospective data from the verona diabetes complications study. Diabetes Care. 2002;25:1135–1141 [DOI] [PubMed] [Google Scholar]
- 51.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, Bonadonna RC, Muggeo M Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: The bruneck study. Diabetes Care. 2007;30:318–324 [DOI] [PubMed] [Google Scholar]
- 52.Wallace TM, Levy JC, Matthews DR Use and abuse of homa modeling. Diabetes Care. 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 53.Wallace TM, Matthews DR The assessment of insulin resistance in man. Diabetic medicine : a journal of the British Diabetic Association. 2002;19:527–534 [DOI] [PubMed] [Google Scholar]
- 54.Tabak AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimaki M, Witte DR Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: An analysis from the whitehall ii study. Lancet. 2009;373:2215–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.