Abstract
Aims
Atrial fibrillation (AF) is associated with higher risk of stroke. While the prevalence of AF is low in the general population, risk prediction models might identify individuals for selective screening of AF. We aimed to systematically identify and compare the utility of established models to predict prevalent AF.
Methods and results
Systematic search of PubMed and EMBASE for risk prediction models for AF. We adapted established risk prediction models and assessed their predictive performance using data from 2.5M individuals who attended vascular screening clinics in the USA and the UK and in the subset of 1.2M individuals with CHA2DS2-VASc ≥2. We assessed discrimination using area under the receiver operating characteristic (AUROC) curves and agreement between observed and predicted cases using calibration plots. After screening 6959 studies, 14 risk prediction models were identified. In our cohort, 10 464 (0.41%) participants had AF. For discrimination, six prediction model had AUROC curves of 0.70 or above in all individuals and those with CHA2DS2-VASc ≥2. In these models, calibration plots showed very good concordance between predicted and observed risks of AF. The two models with the highest observed prevalence in the highest decile of predicted risk, CHARGE-AF and MHS, showed an observed prevalence of AF of 1.6% with a number needed to screen of 63. Selective screening of the 10% highest risk identified 39% of cases with AF.
Conclusion
Prediction models can reliably identify individuals at high risk of AF. The best performing models showed an almost fourfold higher prevalence of AF by selective screening of individuals in the highest decile of risk compared with systematic screening of all cases.
Registration
This systematic review was registered (PROSPERO CRD42019123847).
Keywords: Atrial fibrillation, Risk prediction models, Stroke, Selective screening, External validation
Introduction
Atrial fibrillation (AF) is the most frequent sustained cardiac arrhythmia in clinical practice and its prevalence is increasing, due to ageing populations, altered lifestyle habits and increasing levels of adiposity. Over 33.5 million people worldwide are currently diagnosed with AF.1 AF may be categorized in different ways, including by the frequency of the arrhythmia as either paroxysmal, persistent, or permanent. However, all subtypes are associated with an increased risk of stroke and other cardiovascular disease outcomes, which include a five-fold higher risk of cardioembolic stroke.2,3
Risk prediction scores such as CHA2DS2-VASc are recommended to help determine the stroke risk for people who are diagnosed with AF, categorized as low, medium, or high.4 Anticoagulation with either a vitamin K antagonist such as warfarin or a direct oral anticoagulant in high-risk individuals can reduce their stroke risk by around 65%.5 Yet many people with AF currently go undetected, either because they are asymptomatic or have paroxysmal disease not detected at the time of assessment. A recent systematic review of single time-point screening reported a prevalence of undetected AF of 1.4% in adults aged ≥65 years old in the general population.6 However, AF is typically found in up to 20% of cases with ischaemic stroke.7,8 In at least half of such cases, AF is newly diagnosed at the time of the event.9,10 This has prompted interest in implementing national screening programmes to detect people with AF, particularly in individuals who might benefit from anticoagulation.4,11,12
One argument against population-level systematic screening is the low overall prevalence of AF in the general population. Accurate identification of individuals at higher risk of AF could help to target screening, reduce the number needed to screen. Most simply, this involves screening above a certain age threshold given the increased prevalence of AF in older people; over 80% of cases with AF occur in individuals aged over 65 years compared to 2.8% who are aged below 45 years.13 Currently, international guidelines suggest either opportunistic screening in individuals aged 65 years or older, or systematic screening in those aged 75 years or older and individuals at high-risk of stroke since the latter approach has been shown to be particularly cost-effective.14–16
Risk prediction models have been developed to detect either incident or prevalent AF and may be able to more accurately identify populations at high risk of AF to inform selective screening. These have the additional benefit of identifying people who are also at higher risk of stroke and therefore likely to benefit from treatment.17,18 Assessing the predictive performance of such models is necessary before seeking to implement these approaches to determine their comparative accuracy and utility. We conducted a systematic review of established risk prediction models of AF and then evaluated the predictive performance of these models in a large contemporary screened population.
Methods
We conducted a systematic review according to a predefined protocol to identify established prediction model to detect AF. This protocol has been registered prospectively in the international prospective registry for systematic reviews (PROSPERO): CRD42019123847. We report the results of our systematic review consistent with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA).19
Search strategy and eligibility criteria
We searched Medline (via PubMed interface) and EMBASE (via OVID interface) from inception to 1 March 2019 using comprehensive electronic strategies, which incorporated a validated search filter (Supplementary material online, eTable 1). We included articles that: (i) develop risk prediction models for the prevalence or incidence of AF based on multiple risk factors; (ii) used general or screened population as domain, not diseased populations at higher risk of AF; (iii) used a single time-point 12-lead electrocardiogram (ECG) for diagnosing AF; and (iv) published in peer-reviewed journals without any language restrictions.
Screening process and data extraction
Two authors (M.H.F.P. and N.R.J.) independently screened all titles and abstract of the retrieved references and subsequently independently reviewed full-texts for final inclusion in this study. Discrepancies could be resolved in those meetings with the help of a third author (R.B.) where required. We performed backward citation searching using the bibliographies of included studies.
Two authors (M.H.F.P. and N.R.J.) independently extracted the following data from the included studies that report the development of a risk prediction model, based on the CHARMS checklist:20 source of data, setting study, geographic area (country and continent), study years, sample size, modelling method (e.g., logistic model), number of participants with missing data, handling of missing data, investigation of satisfaction of modelling assumptions, selection methods for predictor selection, shrinkage of predictor weights, number of outcome events, number of patients, ascertainment of outcome, number and type of predictors used in the final model, number of outcome events per variable, presentation of model, model performance (calibration and validation).
Validation cohort
A cohort of self-referred and self-funded individuals who attended commercial vascular screening clinics (Life Line Screening Inc.) between 2008 and 2013 in the USA and UK was used to assess the predictive performance. All individuals completed standardized questionnaires including questions about their age, sex, smoking status, alcohol use, height and weight, history of vascular disease (coronary artery disease, congestive heart failure, stroke, transient ischaemic attack, and peripheral arterial disease), valvular disease, chronic obstructive pulmonary disease, hypertension and use of antihypertensive medication, and diabetes mellitus. Blood pressure was measured as part of the ankle-brachial pressure index assessment. Standard blood pressure cuffs and sphygmomanometers were used, systolic blood pressure (SBP) being measured using a Doppler probe.
Predicted outcome and its ascertainment
The predicted outcome was the prevalence of AF, measured with a single 12-lead ECG. All ECGs were evaluated by physicians who received in-house training.
Statistical analyses (external validation)
Characteristics of the predictor variables in the included models were summarized using standard methods. We excluded participants with an established history of AF prior to screening (N = 285 934), who did not undergo a single 12-lead ECG (N = 356 684), or with inconsistent values for sex (N = 14 287). We used the same population for all analyses to enable comparisons between different models. Some models applied age and body mass index (BMI) restrictions (Supplementary material online, eTable 2). We therefore further excluded participants who were younger than 45 at screening (N = 59 357) or who had a BMI lower than 18 (N = 18 175).
Variables only relevant for predicting incident AF, such as ECG and echocardiographic characteristics, were not included in our assessment of the risk prediction models. Predictors involving biochemical or other blood measurements were not included, since their availability for inclusion in screening programmes or measurement before performing a single ECG might limit the clinical applicability (Supplementary material online, eTable 3). We used proxies whenever possible and appropriate for any predictors that were not available in our dataset. Predictors for which no proxy was found were considered missing (Supplementary material online, eTable 3).
Missing data were imputed if data were missing in <30% (Supplementary material online, eTable 4). We used chained equations and created 20 imputed datasets with 200 iterations.21 BMI was calculated before imputation.22 Post-imputation rounding was applied to limited-range variables (SBP, heart rate, BMI, height, and weight), if needed.23 Analyses were performed in the resulting 20 imputed datasets.
We used the risk equations to calculate the probability of AF for each participant. We used the β-coefficients (predictor weights) of prediction models that were based on logistic regression or time-dependent regression modelling, such as cox regression (Supplementary material online, eTable 5). We also calculated a sum score (total points) for each participant by summing the points assigned to each predictor of the score chart.
We examined the discrimination and calibration indices of the prediction models, assessed using the area under the receiver operating characteristic (AUROC) curve and calibration plots respectively. We calculated the AUROC curve per imputed dataset and results were pooled using Rubin’s rules.24,25 For models that reported the risk equation, we estimated the mean probability per participant across the 20 imputed datasets and subsequently we split the predicted risks in deciles and calculated observed probability with corresponding 95% confidence interval (CI) per decile. We recalibrated the prediction models to the prevalence of AF in our cohort by re-estimating the intercept. This type of recalibration is referred to as ‘update intercept’ or ‘calibration-in-the-large’.26 For this, we fitted a logistic model with a fixed calibration slope and the intercept as the only free parameter.
In addition, for models that reported a score chart, we created bar charts with the observed prevalence of AF by sum score.
We performed additional assessments of discrimination and calibration using participants with CHA2DS2-VASc of two or more, since anticoagulation is recommended for these people if AF is found.14
Test characteristics and reclassification measures
We assessed two possible cut-offs for a selective screening. We assessed test characteristics, such as sensitivity, specificity, positive predictive value, negative predictive value, prevalence, and number needed to screen (NNS), of selective screening of the 10% and 20% individuals at highest predicted risk of AF.
We calculated reclassification measures to assess the ability of the included risk prediction models to correctly identify cases with and without AF compared to the threshold of ≥65 years of age.27 We calculated integrated discrimination improvement (IDI), relative IDI (rIDI), and continuous net reclassification improvement (NRI).27,28 IDI is the absolute difference in discrimination slopes of the risk prediction models and the age threshold. rIDI is the ratio of absolute difference in discrimination slopes of the risk prediction models and the age threshold over the discrimination slope of the age threshold. Continuous NRI is the sum of the net percentages of participants with and without the AF correctly assigned a different predicted risk with the risk prediction models compared to the age threshold. Positive values correspond to improved classification. The reclassification measures were estimated for all 1000 bootstrap replications in each imputed dataset and the median value across the combined 20 datasets is reported (with the 95% CI obtained from the 2.5th and 97.5th percentiles). P-values <0.05 were considered significant. STATA version 15.1 was used for all statistical analyses and R version 3.5.1 was used for constructing the figures.
Sensitivity analyses
We performed additional assessment of the prediction models in complete cases.
Results
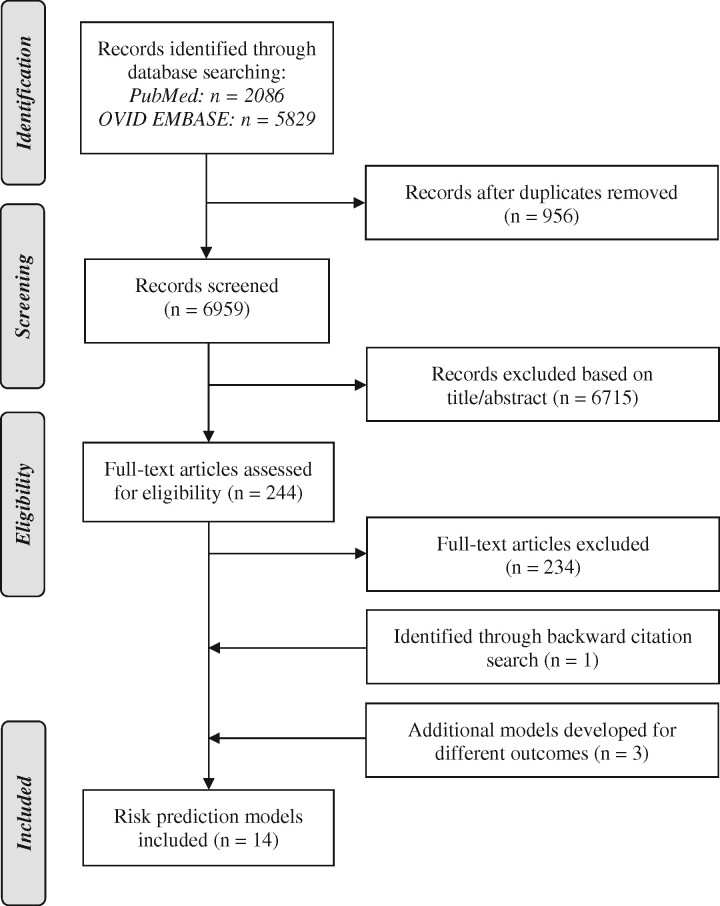
We screened 6961 unique reports identified by our literature search, assessed 249 full-texts, and included 14 studies (Figure 1 and Supplementary material online, eTable 6).4,12,29–40 Six studies used incident AF as predicted outcome,32–37 three used incident AF or atrial flutter,29,30,39 one used prevalent AF,38 and one did not specify the type of AF.31 HATCH was developed to predict progression to sustained AF and CHADS2 and CHA2DS2-VASc were developed to predict the risk of stroke in cases with AF.4,12,40 These three prediction models were included, although not originally designed for detecting AF, because they have been used in a number of subsequent studies for predicting AF and might be used for combined prediction of outcomes.37,38,41,42 Characteristics of model development are provided in Table 1.
Figure 1.
Flowchart.
Table 1.
Selected characteristics of studies assessing different risk prediction models for AF
| Author, year, and study name | Predicted outcome | Country | Cases/participants in derivation cohort (%) | Number of predictorsa |
|---|---|---|---|---|
| Alonso et al., 2013 (CHARGE-AF)29 | Incident AF or atrial flutter | USA | 1186/18 556 (6.39%) | 11 |
| Aronson et al., 2018 (MHS)30 | Incident AF or atrial flutter | Israel | 5660/96 778 (5.8%) | 10 |
| Brunner et al., 2014 (MAYO)31 | AF | — | — | 7 |
| Chamberlain et al., 2011 (ARIC)32 | Incident AF | USA | 515/14 546 (3.54%) | 12 |
| Ding et al., 2017 (JINAN)33 | Incident AF | China | 134/33 186 (0.4%) | 4 |
| Everett et al., 2013 (WHS)34 | Incident AF | USA | 404/13 743 (2.9%) | 6 |
| Hamada et al., 2019 (SEIREI)35 | Incident AF | Japan | 349/65 984 (0.53%) | 7 |
| Kokubo et al., 2017 (SUITA)36 | Incident AF | Japan | 311/6864 (4.5%) | 9 |
| Li et al., 2018 (C2HEST)37 | Incident AF | China | 921/471 446 (0.20%) | 6 |
| Linker et al., 2018 (SAAFE)38 | Prevalent AF | USA | 509/3790 (13.4%) | 13 |
| Schnabel et al., 2009 (FHS)39 | Incident AF or atrial flutter | USA | 457/4764 (9.6%) | 7 |
| de Vos et al., 2010 (HATCH)40 | Progression to sustained AF | — | — | 5 |
| Gage et al., 2001 (CHADS2)12 | Stroke risk | — | — | 5 |
| Lip et al., 2010 (CHA2DS2-VASc)4 | Stroke risk | — | — | 7 |
AF, atrial fibrillation.
Number of predictors of the risk prediction models assessed in the present study are provided.
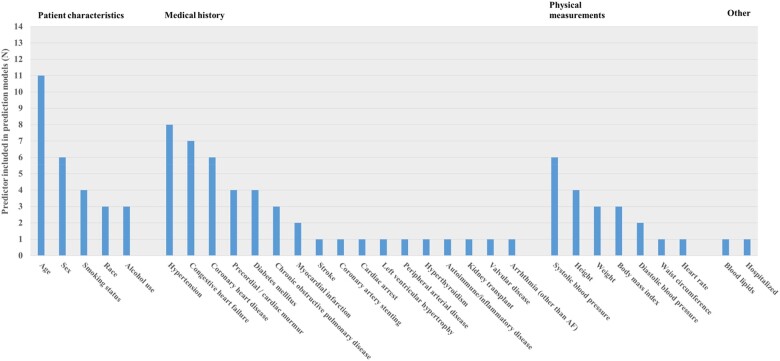
The number of predictors in the models varied from four to thirteen. An overview of predictors of the included prediction models originally developed for detecting AF is provided in Figure 2. Age was used as predictor in all of the models. Other predictors frequently included were hypertension (n = 8), heart failure (n = 7), coronary heart disease (n = 6), sex (n = 6), and SBP (n = 6). Of the fourteen included prediction models, predictor weights of 12 models were reported and score charts of eleven models.
Figure 2.
Included predictors. An overview of predictors used in the eleven risk prediction models that were developed to predict atrial fibrillation.
Validation cohort
The validation cohort consisted of 2 541 702 participants, of whom 10 464 (0.4%) had AF. In total, 1 153 878 (52.4%) participants had a CHA2DS2-VASc score of two or higher of which 5298 (0.5%) of the participants with AF. The mean CHA2DS2-VASc score was two in participants without AF and three in participants with AF. Characteristics of our cohort that were used as predictors in the included prediction models are provided in Table 2.
Table 2.
Characteristics of variables used as predictors in the prediction cohort
| All participants (N = 2 541 702) | Participants with AF (N = 10 464) | Participants without AF (N = 2 531 238) | |
|---|---|---|---|
| Age (years) | 64.8 ± 9.6 | 72.9 ± 9.4 | 64.8 ± 9.6 |
| Female sex | 1 648 242 (64.8) | 4315 (41.2) | 1 643 927 (64.9) |
| Current smoker | 219 444 (9.7) | 751 (8.3) | 218 693 (9.7) |
| Former smoker | 693 974 (30.6) | 3340 (36.7) | 690 634 (30.5) |
| Never smoked | 1 357 094 (59.8) | 5012 (55.1) | 1 352 082 (59.8) |
| Medical history | |||
| Hypertension | 1 015 663 (41.8) | 5014 (51.9) | 1 010 649 (41.8) |
| Antihypertensive medication | 1 023 749 (43.4) | 5317 (56.5) | 1 018 432 (43.3) |
| DM | 276 051 (11.9) | 1622 (17.7) | 274 429 (11.8) |
| CHDa | 137 508 (6.2) | 1156 (12.9) | 136 352 (6.1) |
| Valvular disease | 76 985 (4.0) | 494 (6.9) | 76 491 (4.0) |
| CHF | 20 847 (0.9) | 426 (4.8) | 20 421 (0.9) |
| COPD | 64 592 (3.4) | 486 (6.8) | 64 106 (3.4) |
| PAD | 91 823 (3.7) | 938 (9.5) | 90 885 (3.6) |
| Stroke or TIA | 78 048 (3.5) | 819 (9.4) | 77 229 (3.5) |
| Physical measurements | |||
| Height (m) | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 |
| Weight (kg) | 79.1 ± 18.2 | 86.5 ± 21.1 | 79.1 ± 18.2 |
| BMI (kg/m2) | 27.9 ± 5.3 | 28.9 ± 5.7 | 27.9 ± 5.3 |
| SBP (mmHg) | 133 ± 19.7 | 139 ± 21.2 | 133 ± 19.7 |
| Heart rate (beats/min) | 66 ± 10.3 | 77 ± 16.7 | 66 ± 10.3 |
| CHA2DS2-VASc of ≥2 | 1 153 878 (52.4) | 5298 (60.9) | 1 148 580 (52.4) |
| Mean CHA2DS2-VASc | 2 ± 1.3 | 3 ± 1.6 | 2 ± 1.3 |
Values are mean ± SD for continuous variables and n (%) for categorical variables.
AF, atrial fibrillation; BMI, body mass index; CHD, coronary heart disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; PAD, peripheral arterial disease; SBP, systolic blood pressure; TIA, transient ischaemic attack.
CHD is defined as previous myocardial infarction or a coronary intervention (bypass, angioplasty, or stenting).
Predictive performance in validation cohort
Discrimination
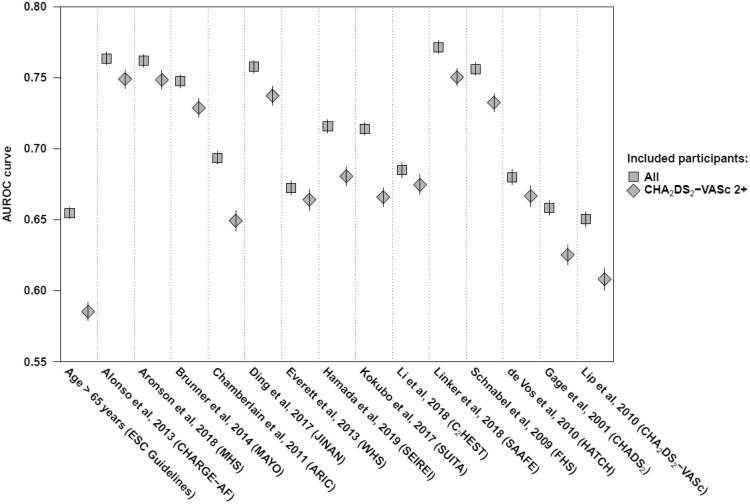
For discrimination in all participants, AUROC curves were between 0.71 and 0.77 in eight models,29–31,33,35,36,38,39 and between 0.65 and 0.69 in six models.4,12,32,34,37,40 (Figure 3 and Supplementary material online, eTable 7). All models showed a statistically significant better discrimination compared with the age threshold of 65 years or older suggested for opportunistic screening in the current European Society of Cardiology (ESC) guidelines.14 All the models also had a statistically significant better discrimination than both CHADS2 and CHA2DS2-VASc.4,12
Figure 3.
Discriminative performance. Squares represent the AUROC curves in the analysis of all 2.5M participants and diamonds in 1.2M participants with CHA2DS2-VASc of two or more.4 The vertical bars represent the 95% CIs. The AUROC curves are based on the regression equation in 12 prediction models,29–40 and on the point chart for two prediction models.4,12 Values are provided in Supplementary material online, eTable 9.
In participants with CHA2DS2-VASc scores of two or higher, AUROC curves were between 0.73 and 0.75 in six studies,29–31,33,38,39 and between 0.65 and 0.68 in six studies.32,34–37,40 The AUROC curve for the age threshold was 0.59 (95% CI 0.58-0.59).14 (Figure 3 and Supplementary material online, eTable 7). The difference in discrimination between age alone and all other models was also statistically significant.
Calibration
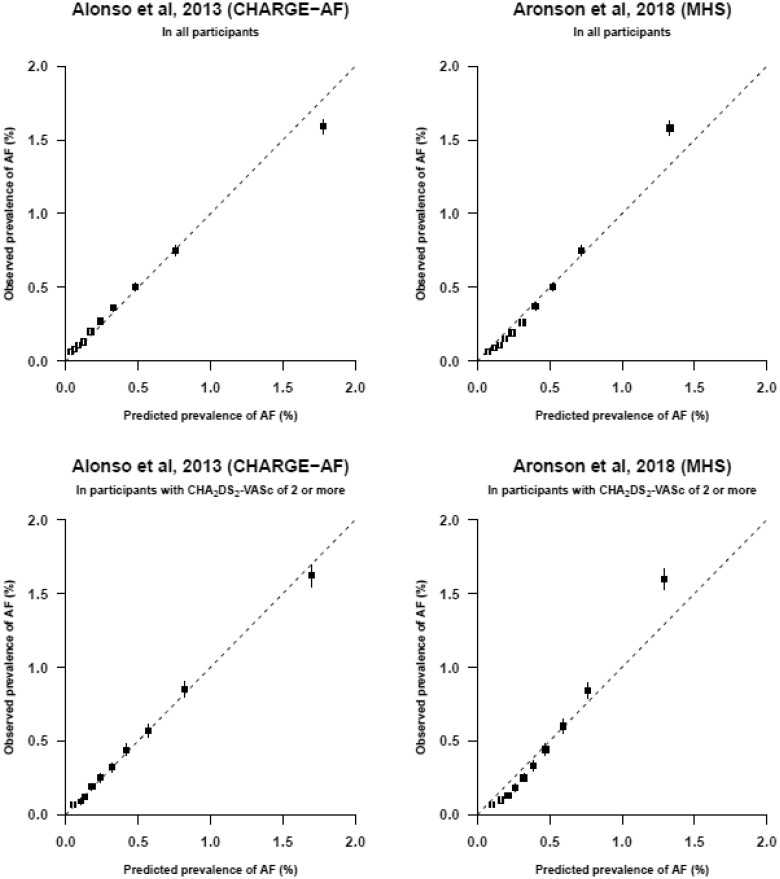
Calibration showed good correspondence between predicted and observed risks of AF in six of the eight models with AUROC curves >0.70.29–31,33–36,39 (Figure 4 and Supplementary material online, eFigure 1). The two models with the highest observed prevalence in the highest decile of predicted risk were CHARGE-AF and MHS. An observed prevalence of AF of 1.6% was found in this decile (Figure 4).29,30 Prevalences were predicted accurately across all deciles of predicted risk except for the highest decile, where CHARGE-AF overestimated the observed prevalence (1.8% vs. 1.6%) and MHS underestimated the observed prevalence of AF (1.3% vs. 1.6%). In participants with CHA2DS2-VASc scores of two or higher, calibration plots showed similar results (Figure 4).
Figure 4.
Calibration plots. Calibration plots of the two risk prediction models with the highest observed prevalence of AF in the highest decile of predicted risk: CHARGE-AF and MHS.29,30 To construct the calibration plots, data of all 2.5M participants (top row) and 1.2M participants with CHA2DS2-VASc of two or more (bottom row) were used. Mean predicted risk against the observed risk of AF across deciles of predicted risk (after recalibration with adjusting the intercept) is shown. The boxes represent the mean predicted risk for each decile and the vertical lines represent the 95% confidence intervals. The dotted diagonal line indicates perfect calibration. Boxes above the diagonal line indicate underestimation of risk and below the diagonal line overestimation of risk. The prevalences and number of cases of each decile are provided in Supplementary material online, eTable 9.
The predictors included in CHARGE-AF are age, ethnicity, height, weight, SBP, diastolic blood pressure, smoking, antihypertensive medication use, diabetes, heart failure and myocardial infarction, of which ethnicity and diastolic blood pressure were not included in the present analysis. The predictors included in MHS are age, sex, BMI, myocardial infarction, peripheral arterial disease, treated hypertension, SBP, chronic obstructive lung disease, female with autoimmune or inflammatory disease and heart failure by age group, of which female with autoimmune or inflammatory disease was not included in the present analysis. Other calibration plots are provided in Supplementary material online, eFigure 1. The bar charts showed increasing observed prevalence with increasing sum scores (Supplementary material online, eFigure 2).
Test characteristics
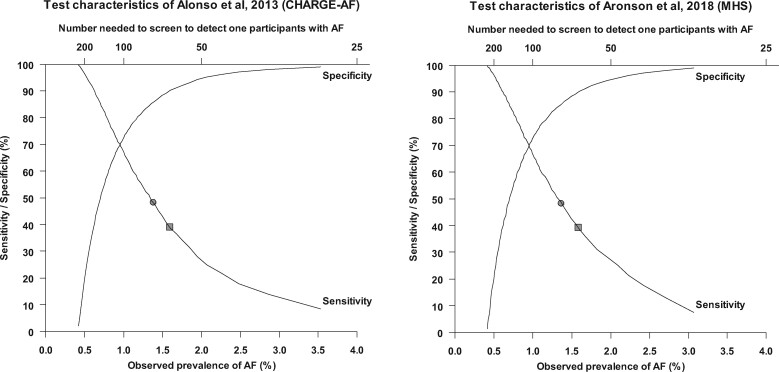
We assessed selective screening of participants in the highest decile and highest two deciles of predicted risk. The prevalence of AF in the highest decile of predicted risk varied from 1.0% to 1.6% with corresponding NNS of 96 to 63 across the 12 prediction models (Supplementary material online, eTable 10). CHARGE-AF and MHS showed the highest observed prevalence of 1.6% by selective screening of these 10% highest risk cases. This identified 39% of cases with prevalent AF with a specificity of 90%.
The prevalence of AF in the highest two deciles of predicted risk varied from 0.9% to 1.3% with corresponding NNS of 107 to 76 across the 12 prediction models. CHARGE-AF and MHS showed the highest observed prevalence of 1.3% by selective screening of these 20% highest risk cases. This identified 48% of cases with prevalent AF with a specificity of 85% (Supplementary material online, eTable 10). Observed prevalence, NNS, sensitivity and specificity for other cut-offs of predicted risk using CHARGE-AF and MHS are shown in Figure 5.
Figure 5.
Test characteristics. Graph showing the sensitivity and specificity and corresponding observed prevalence and number needed to screen to detect 1 participant with AF using the prediction model developed by Alonso et al. 2013 (left) and Aronson et al. 2018 (right). The squares and circles correspond to selective screening of participants in the highest decile and highest two decile of predicted risk, respectively.
Reclassification measures
Reclassification measures demonstrated a significant improvement of the CHARGE-AF and MHS prediction models compared to the age threshold of 65 years.14 For the CHARGE-AF risk prediction model, the IDI was 0.0048 (95% CI 0.0046–0.0051; P < 0.00001), rIDI was 1.84 corresponding to an 184% improved classification, and the NRI was 0.6201 (95% CI 0.6011–0.6387; P < 0.00001). For the MHS risk prediction model, the IDI was 0.0021 (95% CI 0.0020–0.0022; P < 0.00001), rIDI was 0.80 corresponding to an 80% improved classification, and the NRI was 0.4447 (0.4258–0.4643; P < 0.00001).
Sensitivity analysis
Discrimination values were only marginally decreased in subsets with complete cases (Supplementary material online, eTable 8).
Discussion
Our study is the first to compare the performance of all established risk prediction models for prevalent AF. We conducted an external validation in a large contemporary screened population who underwent a single time point 12-lead ECG to detect AF. Eight models showed AUROC curves of >0.70 and in seven of these, there was good concordance of predicted and observed risks. Several common predictors were included in most models, such as age, hypertension and heart failure. The two models with the highest observed prevalence of AF in the highest decile of predicted risk were developed in the CHARGE-AF and MHS cohorts.29,30 The observed prevalence of AF in the highest deciles across the two models was 1.6%, with a number needed to screen to detect one case with AF of 63. This was almost four-fold higher than the overall prevalence and 25-fold higher than the lowest decile of predicted risk. These prediction models showed better discriminative performance compared to an age threshold of 65 years, CHADS2 and CHA2DS2-VASc. Application of these risk models therefore may be able to inform more selective opportunistic or systematic screening.
Unselected population screening is likely to detect only small numbers of people with AF. For example, the recent Apple Heart Study screened nearly 420,000 people using smartwatch technology with an irregular pulse notification system.43 Possible cases wore an ECG patch for seven days to confirm a diagnosis of AF. Irregular pulse notifications were received by 0.16% of people aged under 40 but 3.1% of those aged ≥65 years. Of those who received a notification, 18% of people under 40 years were diagnosed with AF but 35% of those aged ≥65 years. If screening is to be both cost effective and clinically relevant, it must be targeted at high-risk groups.
Different types of screening for AF in the population have been suggested, including systematic screening where participants are invited to have an ECG and opportunistic screening where pulse palpation is performed followed by an ECG if an irregular pulse is found.44–47 These strategies were informed by randomized trials which used an age threshold for case selection rather than a prediction model with multiple predictors. Our results show that age alone is not the best discriminator of AF risk. Two previous studies also compared risk prediction models to the age criterion of 65 years of age and over and found better discrimination when prediction models were used.34,38
A previous external validation compared nine prediction models to age for predicting the 3-year risk of incident AF using data from the ARIC study. Five models were significantly better than age alone but the CHADS2 and CHA2DS2-VASc scores were not.38 We found comparable results of discriminative indices for predicting prevalent AF, indicating that predictors for prevalent and incident AF overlap and the same models might be used for selection of high-risk cases in both situations.
Strengths and limitations
We conducted a comprehensive literature search to identify all established prediction models, according to a prespecified protocol. We are the first external validation using the outcome prevalent AF, an outcome relevant for a selective screening protocol with a single ECG. A large contemporary screened population of 2.5M participants was used for validation of included models. Included models were validated in the same participants enabling direct comparison of predictive performance. Missing data were handled with multiple imputation and did not affect our findings. Both risk equations and point charts were used for validation if reported. Point charts are easier to apply but contemporary presentation formats, such as webtools and smartphone apps, might use more complicated equations to estimate risks more precisely. We recalibrated risks to update the risk prediction models to the setting of our cohort, with its prevalence of AF.
Most included models were not developed to predict prevalent AF, and this might have influenced predictive performance. Some predictors were not available and for some we used proxies if a direct match was not available which might also have influenced predictive performance. Participants in our cohort were self-referred and self-funded, which might influence generalizability of our findings and might indicate the need to update (the intercept of) the models to new settings before implementation.26 Participants were also relatively young and healthy compared to most people who develop AF, which may impact on the external validity of these results to the wider public. Nonetheless, we include data on over 10 000 cases of AF within the population. It is also important to note that studies such as AppleWatch demonstrate a trend to increased screening in younger participants.43 Auscultatory or oscillometric sphygmomanometers are recommended in international guidelines to measure SBP and results might have been influenced by using Doppler probes.48 Recall bias cannot be excluded for predictors that were self-reported. Symptoms of AF were not recorded. ECG was performed only once in the screened participants, therefore cases of paroxysmal AF are likely to have been missed.45 However, given stroke risk increases with frequency of AF, people detected on single-timepoint ECG are more likely to benefit from anticoagulation compared to people with brief episodes of paroxysmal AF, who are most likely to be missed by this approach to screening. Data on use of anticoagulant drugs were not available, but participants with a reported history of AF were excluded from the analyses. The prevalence of AF in our population was lower compared with other populations, possibly making targeted screening more worthwhile in different settings.6
Implications for practice and future research
Recent cohort studies have re-affirmed the importance of using stroke risk assessment tool, such as CHA2DS2-VASc, to guide anticoagulation decisions and not to withhold this treatment based on high baseline bleeding risk alone.49,50 However, the relatively poor performance of CHA2DS2-VASc for predicting either AF prevalence or incidence hampers the possibility of using a single score for prediction of AF diagnosis and risk stratification of outcomes, such as stroke or systemic thromboembolism. Using CHA2DS2-VASc for selection of cases was recently applied by the REHEARSE-AF trial, a randomized controlled trial of AF screening using the AliveCor Kardia smartphone device in people with a CHA2DS2-VASc score ≥2. Among 1001 participants, 19 were diagnosed with AF in the AliveCor Kardia arm compared to 5 in the control arm at a cost per AF diagnosis of $10 780 in the intervention arm.51 Our findings suggest that future research should consider using alternative prediction models, such as CHARGE-AF or MHS to limit screening to high-risk populations and reduce the number needed to screen. Future research will determine how many strokes could be prevented by improved cardiovascular risk management in cases in whom AF is detected by a selective screening programme and whether that leads to a cost-effective screening programme for AF. This might also help determining a threshold probability for selective screening.
Primary care computer software systems currently use electronic alerts based on CHA2DS2-VASc to help healthcare professionals identify people to consider for opportunistic screening. Such software providers may wish to consider updating their diagnostic algorithms to use a more accurate risk score, such as CHARGE-AF or MHS.
Conclusions
We identified 14 potential models for predicting prevalent AF, all of which outperformed an age threshold of 65 years, CHADS2 and CHA2DS2-VASc. The CHARGE-AF and MHS risk scores had the highest observed prevalence of AF in the highest decile of predicted risk (1.6%). Using these prediction models could reduce the number needed to screen to detect one case with AF using single time point ECG. Our study showed that established prediction models are able to identify reliably individuals at higher risk of AF. Application of these risk models therefore may be able to inform more selective opportunistic or systematic screening.
Supplementary material
Supplementary material is available at European Journal of Preventive Cardiology online.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Life Line Screening for providing data for these analyses. The authors would like to acknowledge Doron Aronson, MD for providing additional data.
Funding
A.H. is funded by the UK Health Research (NIHR) Oxford Biomedical Research Centre (BRC). S.L. reports grants from UK Medical Research Council and from the CDC Foundation (with support from Amgen) outside the submitted work. N.R.J. is supported by a Doctoral Research Fellowship grant from the Wellcome Trust (grant number 203921/Z/16/Z).
Conflict of interest: The authors declare no conflicts of interest. This study was designed, conducted and reported independently of Life Line Screening and all funding sources. Life Line Screening provided data at no cost.
Data availability
Data from large population-based studies conducted by the Nuffield Department of Population Health can be shared with bona fide researchers on application to the principal investigators of this study. Details of the departmental data access policy can be found at https://www.ndph.ox.ac.uk/data-access.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim Y-H, McAnulty JH, Zheng Z-J, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJL. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gómez-Outes A, Lagunar-Ruíz J, Terleira-Fernández AI, Calvo-Rojas G, Suárez-Gea ML, Vargas-Castrillón E. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 2016;68:2508–2521. [DOI] [PubMed] [Google Scholar]
- 3. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 4. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 5. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 6. Lowres N, Neubeck L, Redfern J, Freedman SB. Screening to identify unknown atrial fibrillation. A systematic review. Thromb Haemost 2013;110:213–222. [DOI] [PubMed] [Google Scholar]
- 7. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham study. Arch Intern Med 1987;147:1561–1564. [PubMed] [Google Scholar]
- 8. Jung YH, Kim YD, Kim J, Han SW, Lee KY. Atrial fibrillation in patients with first-ever stroke: incidence trends and antithrombotic therapy before the event. PLoS One 2018;13:e0209198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friberg L, Rosenqvist M, Lindgren A, Terent A, Norrving B, Asplund K. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke 2014;45:2599–2605. [DOI] [PubMed] [Google Scholar]
- 10. Wilson D, Ambler G, Shakeshaft C, Banerjee G, Charidimou A, Seiffge D, White M, Cohen H, Yousry T, Salman R, Lip GYH, Muir K, Brown MM, Jäger HR, Werring DJ. Potential missed opportunities to prevent ischaemic stroke: prospective multicentre cohort study of atrial fibrillation-associated ischaemic stroke and TIA. BMJ Open 2019;9:e028387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 12. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 13. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham heart study: a cohort study. Lancet 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H-C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GYH, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 15. Hobbs F, Fitzmaurice D, Mant J, Murray E, Jowett S, Bryan S, Raftery J, Davies M, Lip G. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess 2005;9:1–71. [DOI] [PubMed] [Google Scholar]
- 16. Moran PS, Teljeur C, Ryan M, Smith SM. Systematic screening for the detection of atrial fibrillation. Cochrane Database Syst Rev 2016;6:CD009586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Renda G, Ricci F, Patti G, Aung N, Petersen SE, Gallina S, Hamrefors V, Melander O, Sutton R, Engstrom G, Caterina RD, Fedorowski A. CHA2DS2VASc score and adverse outcomes in middle-aged individuals without atrial fibrillation. Eur J Prev Cardiol 2019;26:1987–1997. [DOI] [PubMed] [Google Scholar]
- 18. Chousou PA, Pugh PJ, Vassiliou VS. CHA2DS2-VASc score use in sinus rhythm: can it predict cardiovascular events? Eur J Prev Cardiol 2019;26:1985–1986. [DOI] [PubMed] [Google Scholar]
- 19. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moons KGM, de Groot JAH, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, Reitsma JB, Collins GS. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med 2014;11:e1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 22. Morris TP, White IR, Royston P, Seaman SR, Wood AM. Multiple imputation for an incomplete covariate that is a ratio. Stat Med 2014;33:88–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodwell L, Lee KJ, Romaniuk H, Carlin JB. Comparison of methods for imputing limited-range variables: a simulation study. BMC Med Res Methodol 2014;14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol 2009;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rubin DB. Inference and missing data. Biometrika 1976;63:581–592. [Google Scholar]
- 26. Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. New York: Springer-Verlag; 2009. [Google Scholar]
- 27. Pencina MJ, D' Agostino RB, D' Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the roc curve to reclassification and beyond. Stat Med 2008;27:157–172. [DOI] [PubMed] [Google Scholar]
- 28. Leening MJ, Vedder MM, Witteman JC, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician's guide. Ann Intern Med 2014;160:122–131. [DOI] [PubMed] [Google Scholar]
- 29. Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aronson D, Shalev V, Katz R, Chodick G, Mutlak D. Risk score for prediction of 10-year atrial fibrillation: a community-based study. Thromb Haemost 2018;118:1556–1563. [DOI] [PubMed] [Google Scholar]
- 31. Brunner KJ, Bunch TJ, Mullin CM, May HT, Bair TL, Elliot DW, Anderson JL, Mahapatra S. Clinical predictors of risk for atrial fibrillation: implications for diagnosis and monitoring. Mayo Clin Proc 2014;89:1498–1505. [DOI] [PubMed] [Google Scholar]
- 32. Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, Ambrose M, Alonso A. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the atherosclerosis risk in communities [ARIC] study). Am J Cardiol 2011;107:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ding L, Li J, Wang C, Li X, Su Q, Zhang G, Xue F. Incidence of atrial fibrillation and its risk prediction model based on a prospective urban Han Chinese cohort. J Hum Hypertens 2017;31:574–579. [DOI] [PubMed] [Google Scholar]
- 34. Everett BM, Cook NR, Conen D, Chasman DI, Ridker PM, Albert CM. Novel genetic markers improve measures of atrial fibrillation risk prediction. Eur Heart J 2013;34:2243–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hamada R, Muto S. Simple risk model and score for predicting of incident atrial fibrillation in japanese. J Cardiol 2019;73:65–72. [DOI] [PubMed] [Google Scholar]
- 36. Kokubo Y, Watanabe M, Higashiyama A, Nakao YM, Kusano K, Miyamoto Y. Development of a basic risk score for incident atrial fibrillation in a Japanese general population—the Suita study. Circ J 2017;81:1580–1588. [DOI] [PubMed] [Google Scholar]
- 37. Li YG, Pastori D, Farcomeni A, Yang PS, Jang E, Joung B, Wang YT, Guo YT, Lip GYH. A simple clinical risk score (C2HEST) for predicting incident atrial fibrillation in Asian subjects: derivation in 471,446 Chinese subjects, with internal validation and external application in 451,199 Korean subjects. Chest 2018;261:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Linker DT, Murphy TB, Mokdad AH. Selective screening for atrial fibrillation using multivariable risk models. Heart 2018;104:1492–1499. [DOI] [PubMed] [Google Scholar]
- 39. Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB, Newton-Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ Sr. Development of a risk score for atrial fibrillation (Framingham heart study): a community-based cohort study. Lancet 2009;373:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen R-JS, van den Heijkant AC, Allessie MA, Crijns HJGM. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol 2010;55:725–731. [DOI] [PubMed] [Google Scholar]
- 41. Suenari K, Chao TF, Liu CJ, Kihara Y, Chen TJ, Chen SA. Usefulness of hatch score in the prediction of new-onset atrial fibrillation for Asians. Medicine 2017;96:e5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Christophersen IE, Yin X, Larson MG, Lubitz SA, Magnani JW, McManus DD, Ellinor PT, Benjamin EJ. A comparison of the CHARGE-AF and the CHA2DS2-VASc risk scores for prediction of atrial fibrillation in the Framingham heart study. Am Heart J 2016;178:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L, Hung G, Lee J, Kowey P, Talati N, Nag D, Gummidipundi SE, Beatty A, Hills MT, Desai S, Granger CB, Desai M, Turakhia MP. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hald J, Poulsen PB, Qvist I, Holm L, Wedell-Wedellsborg D, Dybro L, Frost L. Opportunistic screening for atrial fibrillation in a real-life setting in general practice in Denmark—the atrial fibrillation found on routine detection (AFFORD) non-interventional study. PLoS One 2017;12:e0188086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation 2015;131:2176–2184. [DOI] [PubMed] [Google Scholar]
- 46. González Blanco V, Pérula de Torres LÁ, Martín Rioboó E, Martínez Adell MÁ, Parras Rejano JM, González Lama J, Ruiz Moruno J, Martín Alvarez R, Fernández García JÁ, Ruiz de Castroviejo J, Roldán Villalobos A, Ruiz Moral R. Opportunistic screening for atrial fibrillation versus detecting symptomatic patients aged 65 years and older: a cluster-controlled clinical trial. Med Clin 2017;148:8–15. [DOI] [PubMed] [Google Scholar]
- 47. Fitzmaurice DA, Hobbs FDR, Jowett S, Mant J, Murray ET, Holder R, Raftery JP, Bryan S, Davies M, Lip GYH, Allan TF. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ 2007;335:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stergiou GS, Alpert B, Mieke S, Asmar R, Atkins N, Eckert S, Frick G, Friedman B, Graßl T, Ichikawa T, Ioannidis JP, Lacy P, McManus R, Murray A, Myers M, Palatini P, Parati G, Quinn D, Sarkis J, Shennan A, Usuda T, Wang J, Wu CO, O’Brien E. A universal standard for the validation of blood pressure measuring devices: association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) collaboration statement. Hypertension 2018;71:368–374. [DOI] [PubMed] [Google Scholar]
- 49. Gamble DT, Buono R, Mamas MA, Leslie S, Bettencourt-Silva JH, Clark AB, Bowles KM, Metcalf AK, Potter JF, Myint PK. Does prior antithrombotic therapy influence recurrence and bleeding risk in stroke patients with atrial fibrillation or atrial flutter? Eur J Prev Cardiol 2020;27:729–737. [DOI] [PubMed] [Google Scholar]
- 50. Proietti M, Vitolo M, Boriani G. Tailored oral anticoagulant prescription in patients with atrial fibrillation: use and misuse of clinical risk prediction scores. Eur J Prev Cardiol 2020;27:726–728. [DOI] [PubMed] [Google Scholar]
- 51. Halcox JPJ, Wareham K, Cardew A, Gilmore M, Barry JP, Phillips C, Gravenor MB. Assessment of remote heart rhythm sampling using the AliveCor Heart Monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017;136:1784–1794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from large population-based studies conducted by the Nuffield Department of Population Health can be shared with bona fide researchers on application to the principal investigators of this study. Details of the departmental data access policy can be found at https://www.ndph.ox.ac.uk/data-access.