Abstract
Introduction:
Methamphetamine (MA) triggers neuroinflammation and medications that counteract MA-induced neuroinflammation may reduce MA-induced neurodegeneration and improve neurocognition and treatment outcomes in MA use disorder. We performed a randomized, placebo-controlled trial to determine the safety and efficacy of ibudilast (IBUD), a phosphodiesterase inhibitor that reduces neuroinflammation, for the treatment of MA use disorder.
Methods:
Treatment-seeking volunteers with MA use disorder were randomly assigned to receive 12 weeks of IBUD 50 mg twice daily (N = 64) or placebo (N = 61) with medication management counseling. Participants visited the outpatient research clinic twice weekly to provide urine specimens for drug screens and undergo study assessments. The primary outcome was end of treatment MA-abstinence (EOTA) during weeks 11 and 12 of treatment. Serum IBUID levels were measured for IBUD participants during week 3 of treatment.
Results:
There was no difference in EOTA for IBUD (14%) versus placebo (16%, p > 0.05). There was no correlation between serum IBUD levels and MA use during treatment and mean IBUD levels for participants with (mean = 51.3, SD = 20.3) and without (mean = 54.7, SD = 33.0, p = 0.70) EOTA. IBUD was well tolerated.
Conclusions:
IBUD did not facilitate MA abstinence in this outpatient trial. Whether targeting neuroinflammation, either with IBUD in other subgroups of MA users or clinical trial designs, or with other anti-inflammatory medications, is an effective strategy for treating MA use disorder is not clear.
Keywords: Methamphetamine, neuroinflammation, ibudilast, placebo, randomized clinical trials
Introduction
No effective medication exists for methamphetamine (MA) use disorder. Numerous negative clinical trials have assessed medications with a variety of different mechanisms including antidepressants with serotonergic, noradrenergic, and dopaminergic activity, antiepileptics with effects on gaba and/or glutamate, typical stimulants, atypical stimulants such as modafinil, and other dopamine agonists, cholinergic medications, ACH inhibitors, and opioid antagonists (Brensilver et al., 2013; Morley et al., 2017; Lee et al., 2018). Clinical trials of medications with novel mechanisms of action are needed to identify an effective medication for MA use disorder.
Multiple studies implicate neuroinflammatory processes in the pathophysiology of a variety of neuropsychiatric conditions (Hirsch and Hunot, 2009; Sidoryk-Wegrzynowicz et al., 2011), including MA use disorder (Kohno et al., 2019). Activated glial cells play a central role in neuroinflammation via the secretion of multiple pro-inflammatory mediators (Minghetti et al., 2005). MA activates microglia in preclinical studies and blocking MA-induced glial activation results in attenuated subsequent MA-induced neurodegeneration (Ladenheim, Krasnova et al. 2000; Flora, Lee et al. 2002; Thomas and Kuhn 2005; Fantegrossi, Ciullo et al. 2008; Narita, Suzuki et al. 2008; Thomas, Francescutti-Verbeem et al. 2008). In a human imaging study, a marker for activated microglia was significantly increased in abstinent MA users versus non-using controls and binding levels correlated inversely with the duration of MA abstinence (Sekine, Ouchi et al. 2008). Among human MA users, increased plasma levels of pro-inflammatory cytokines (IFN-α, IL-1β, IL-2, IL-6, TNF-α) and chemokines (MCP-1, MIP-1α, MIP-1β) were significantly associated with greater neurocognitive dysfunction (Loftis et al., 2011a). Neuroinflammation and associated neurocognitive dysfunction are common with HIV infection and MA use increases the risk of neurocognitive impairment among persons with HIV (Soontornniyomkij et al., 2016). Together, these results suggest that medications that counteract MA-induced neuroinflammation and microglial activation may reduce MA-induced neurodegeneration and thereby improve neurocognition and treatment outcomes in MA use disorder. In contrast, other authors have concluded that MA-induced microglial activation is relatively modest and may not be a major contributor to MA-related dopaminergic toxicity (Shaerzadeh et al., 2018). Clinical trials of medications targeting MA-induced microglial activation and neuroinflammation are warranted.
Ibudilast (IBUD) is a non-selective phosphodiesterase inhibitor with preferential inhibition of PDE3A, PDE4, PDE10, and PDE11 (Gibson et al., 2006) that also inhibits glial cell activation (Suzumura et al., 1999) and production of macrophage migration inhibitory factor (Cho et al., 2010). IBUD has been used clinically for over 20 years in Asia for the treatment of bronchial asthma, and more recently for post-stroke dizziness and ocular allergies during which it has proven to be safe and well tolerated (Rolan et al., 2009). IBUD dose-dependently protected against microglial activation and the subsequent cerebrovascular white matter lesions following bilateral ligation of the carotid arteries (an animal model of vascular dementia/cognitive impairment) in rats (Wakita et al., 2003). IBUD also suppressed activated microglia-induced neuronal cell death in vitro via inhibiting production of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α), reactive oxygen species, and nitric oxide and increasing the secretion of anti-inflammatory mediators (IL-10, nerve growth factor, neurotrophin-4, and GDNF) by microglial cells (Mizuno et al., 2004). Clinical trials in patients with multiple sclerosis has demonstrated that IBUD has a neuroprotective effect and slows the progression of brain atrophy (Barkhof et al., 2010; Fox et al., 2018).
Preclinical studies have found that IBUD has effects in multiple rodent models of MA use disorder including reductions in MA reinstatement, locomotor sensitization, and self-administration (Beardsley et al., 2010; Snider et al., 2012; Snider et al., 2013). IBUD was safe and well tolerated in humans when combined with MA and reduced MA subjective effects and improved attention during early MA abstinence in a human laboratory study (DeYoung et al., 2016; Worley et al., 2016; Birath et al., 2017). On the strength of this preclinical and clinical data, we performed a randomized, placebo-controlled trial to determine the safety and efficacy of IBUD for the treatment of MA use disorder.
Methods
Study activities took place at a UCLA outpatient clinic in Los Angeles. All activities were approved by the UCLA Institutional Review Board and an independent Data Safety Monitoring Board. The study was registered with ClinicalTrials.gov, NCT01860807. A CONSORT study flowchart is presented in Figure 1.
Figure 1:
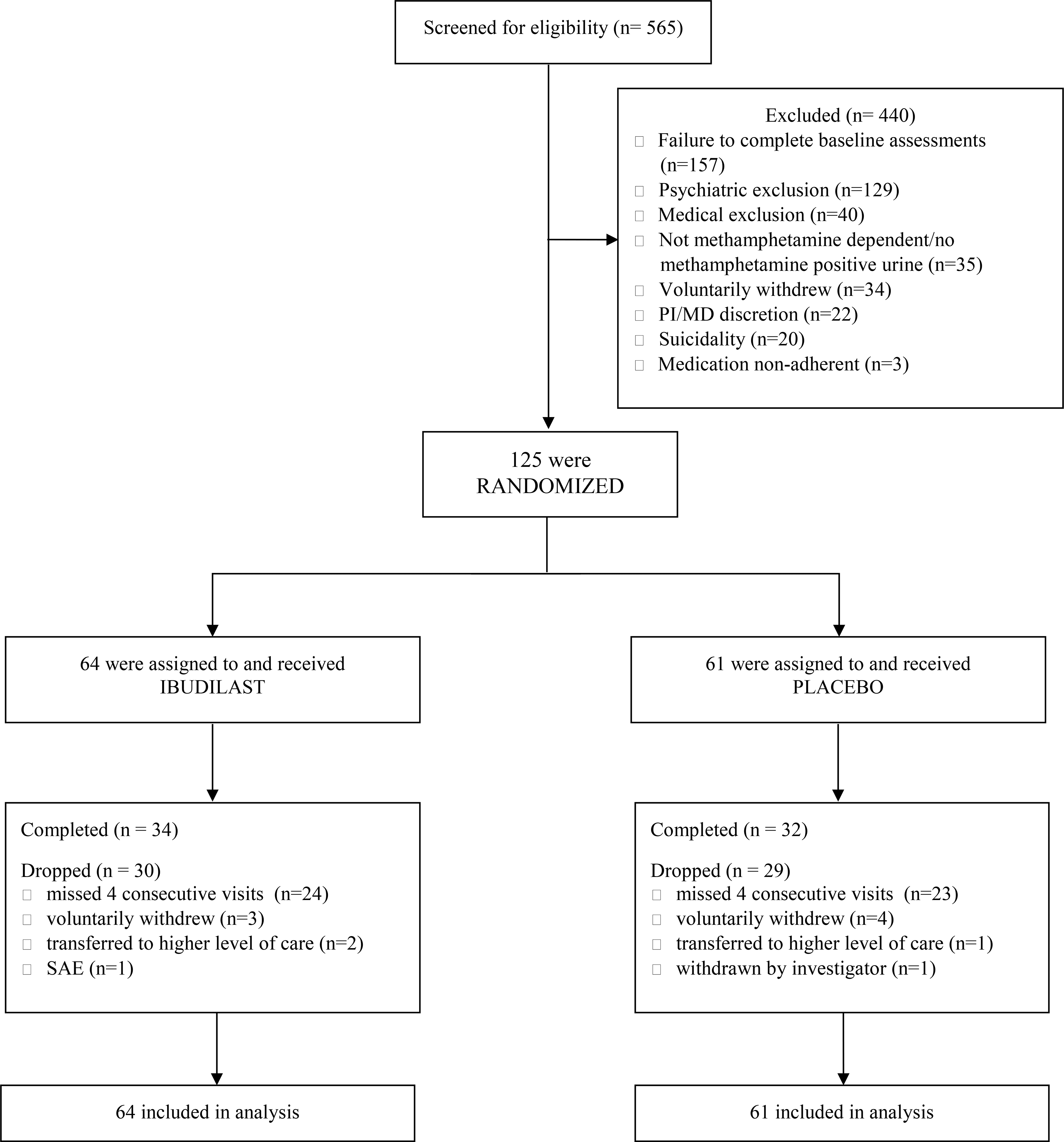
CONSORT Flow Diagram
Design
This randomized, double-blind, placebo-controlled Phase II clinical trial recruited participants from October 2013 through August 2017 and compared outcomes for IBUD and placebo conditions. Treatment-seeking volunteers with MA use disorder underwent screening and eligibility assessments during a two-week placebo lead-in and were prescribed 12.5 mg riboflavin BID as part of a high value contingency management (CM) behavioral intervention aimed at assessing participants’ ability to adhere to medication taking and initiate MA abstinence prior to the start of medication (Bisaga et al., 2005; Anderson et al., 2009; Bisaga et al., 2010). Participants deemed eligible by the study clinician, were randomized to IBUD or placebo, stratified by baseline HIV serostatus. Within each strata an urn randomization procedure (Stout et al., 1994) was used to provide multivariate balance across conditions by MA abstinent versus not abstinent as confirmed via urine drug screen during the second week of the lead-in period, (MA abstinence was confirmed with at least two urine drug screens negative for presence of MA and no urine drug screen positive for MA, while all other urine drug screen results were considered not abstinent), gender (biologic male versus female), ethnicity (Hispanic versus not Hispanic, marijuana dependence (dependent versus not as assessed by the SCID), and baseline cognitive function (score of ≥26 versus <26) as assessed by the Montreal Cognitive Assessment (MOCA) tool to determine cognitive impairment (Nasreddine et al., 2005). A staff member not directly involved in the research maintained the randomization key and program off-site. Participants and study staff who had any participant contact were blind to treatment assignment.
Following randomization, participants underwent dose escalation to IBUD 50 mg/placebo BID over three days and completed twice-weekly clinic visits for medication management counseling, urine drug tests, and safety/medication adherence monitoring during the 12-week medication phase. Participants then completed four additional weeks of medical and safety assessments; the full duration of the trial was 16 weeks. Participants were reimbursed in gift cards, up to $595, for time spent completing study assessments and transportation to/from the clinic.
Participants
In total, 565 participants completed the informed consent process; 440 screen-failed and 125 were randomized and received IBUD or placebo. Of the 125 randomized participants, 66 completed and 59 dropped (Figure 1). Participants were recruited via websites, newspapers, radio, and referrals. Interested individuals called a toll-free number, completed telephone pre-screening, were provided study information, and if eligible, were scheduled to meet with a study clinician to complete the informed consent process. The 125 randomized participants (21% HIV positive) were methamphetamine-dependent, treatment-seeking volunteers who met the following eligibility criteria:
Inclusion criteria were: 1) 18 years of age or older, 2) meet DSM-IV criteria for MA dependence verified by the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID),(American Psychiatric Association, 2000; First, 2002), 3) a MA-positive urine drug screen at one or more visit during the two week lead-in period, 4) seeking treatment for MA problems, 5) willing and able to comply with study procedures, 6) provide written informed consent, 7) English speaking, 8) live within 35 miles of the clinical research site, and 9) if female of childbearing potential, not pregnant or lactating and willing to use a medically reliable method of birth control during the trial (e.g., birth control pills, Depo-Provera, and/or condoms with spermicide).
Exclusion criteria were: 1) a medical condition that, in the study physician’s judgment, may interfere with safe study participation (e.g., active TB; unstable cardiac, renal, or liver disease; uncontrolled hypertension; unstable diabetes), 2) CD4 count < 50 cells/mm3 (suggestive of advanced HIV infection), 3) AST, ALT, or GGT > 3 times upper normal limit, 4) A corrected QT of > 450 msecs in men or > 460 msec in women on at least two ECGs during the baseline period, or clinical risk factors for Torsades de Pointes (e.g. (e.g., heart failure, hypokalemia, family history of Long QT Syndrome), or requiring ongoing treatment with concomitant medication(s) with established risk of Torsades de Pointes (e.g. Amiodarone, Arsenic trioxide, Astemizole, Bepridil, Chloroquine, Chlorpromazine, Cisapride, Citalopram, Clarithromycin, Disopyramide, Dofetilide, Domperidone, Droperidol, Erythromycin, Flecainide, Halofantrine, Haloperidol, Ibutilide, Levomethadyl, Mesoridazine, Methadone, Moxifloxacin, Pentamidine, Pimozide, Probucol, Procainamide, Quinidine, Sotalol, Sparfloxacin, Terfenadine, Thioridazine, Vandetanib), 5) current ongoing treatment with psychotropic medications (e.g., antidepressants, antipsychotics, antiepileptics, sedative/hypnotics, narcotic analgesics), 6) a neurological disorder (e.g., organic brain disease, dementia) or a medical condition which would make study agent compliance difficult or which would compromise informed consent, 7) a major psychiatric disorder not due to substance abuse (e.g., schizophrenia, bipolar disorder) as assessed by the SCID, 8) attempted suicide in the past 3 years and/or serious suicidal intention or plan in the past year as assessed by the C-SSRS, 9) currently on prescription medication that is contraindicated for use with IBUD including alpha or beta agonists, theophylline, or other sympathomimetics, 10) current dependence on cocaine, opiates, alcohol, or benzodiazepines as defined by DSM-IV-TR, 11) alcohol dependence within the past year, 12) greater than one urine specimens during the lead-in with a riboflavin concentration of < 900 ng/ml as assessed via UV fluorescence, 13) a history of sensitivity to IBUD, 14) any other circumstances that, in the opinion of the investigators, would compromise participant safety, or 15) current participation in another clinical trial.
Study Medication
Study medication, IBUD 10 mg capsules, and matching placebo capsules were obtained from MediciNova. To minimize nausea, participants took IBUD or placebo 20 mg twice daily for 3 days and then increased to 50 mg twice daily on day 4 and through the remainder of the 12-week medication period. Medication was blister packaged along with riboflavin tablets for adherence monitoring purposes and participants took riboflavin 12.5 mg twice daily along with the study capsules. Participants met weekly with a study clinician for medication adherence counseling and received a $5 gift card for bringing their medication blister package each week for a pill count.
Instead of an intensive cognitive behavioral therapy counseling platform that has previously been used in stimulant pharmacotherapy trials, we used a less intensive medically-based counseling platform -- Medical Management (MM) counseling -- as used in the COMBINE study of alcohol pharmacotherapy (Pettinati and National Institute on Alcohol Abuse and Alcoholism (U.S.), 2004; Pettinati et al., 2005) which we have adapted for stimulant trials. The MM counseling sessions were delivered weekly by a study physician/nurse or counselor following a manual developed for the trial. A primary focus of MM counseling was to help clinicians provide education, support, and strategies to ensure that participants are medication compliant and core components included: (1) providing participants strategies for taking their medications and staying in treatment, (2) educating participants about MA dependence and pharmacotherapy, (3) supporting their efforts to reduce or stop MA use, and (4) making direct recommendations that participants change their drug use behaviors.
Study Assessments
Participants provided urine specimens at each clinic visit which were assessed for MA-metabolites using standard point of care immunoassay drug screen urine cups with a threshold of 300ng/mL. A randomly timed serum specimen was collected from each participant at their clinic visit during week 3 of the medication period for serum IBUD level determination. Substance use and mental health disorders were assessed at baseline by a study clinician using the Structured Clinical Interview for the DSM-IV-TR (Spitzer et al., 1995). Self-reported drug, alcohol, and tobacco use were assessed using the time-line follow-back method (Sobell et al., 1986).
Data Analysis
The primary outcome was end of treatment MA abstinence during the final two weeks of the 12-week medication treatment period (weeks 11 and 12) defined as at least one of the two weekly urine specimens each week available for analysis and no urine specimens positive for MA. Participants with at least one MA positive urine specimen during week 11 or 12 and participants missing both urine specimens during either of the two final weeks were considered not MA abstinent. Pre-study power calculations for the primary study outcome, end of treatment abstinence during the final two weeks of treatment (weeks 11 and 12), indicated 55 participants in each group (total N=110) were required to achieve 80% power to detect a between-group difference with an effect size of 0.5 and an alpha = 0.05. We planned to enroll 140 participants but due to budgetary constraints, accrual was closed after surpassing 110 participants with the final sample size being 125 participants. Secondary outcomes included treatment retention, frequency of adverse events, and evaluation of MA use outcomes by levels of baseline MA use and medication adherence.
Baseline demographic and clinical characteristics among participants assigned to IBUD versus placebo were described and compared using t tests and Chi square analysis. Generalized estimating equations were used to test for an association between treatment group assignment and the probability of providing a MA-negative urine drug screen during the 12-week treatment period. Aggregate measures of MA-use were calculated including the treatment effectiveness score, defined as the mean number of MA-negative urine drug screens for participants in the IBUD versus placebo groups, and the joint probability index at weeks 6 and 12, defined as the probability of a MA-negative urine specimen for the treatment group at the time point, adjusted for retention rates (Ling et al., 1997). IBUD serum levels were described (mean, standard deviation, range) for IBUD participants. Mean days retained in treatment was calculated for participants in the IBUD versus placebo groups and retention in the two treatment groups was compared, adjusting for co-variates, using a Cox regression model. The frequency of adverse events in the IBUD versus placebo groups was described and compared using Chi square analysis.
Results
Participant demographics and clinical characteristics
There were no significant differences in baseline clinical or demographic characteristics of participants randomly assigned to the IBUD versus placebo groups (Table 1). Approximately one-quarter of participants were women, about half were white and half were Hispanic, 21% were HIV positive, and the baseline frequency of MA use days was relatively high at 22 of the past 30 days.
Table 1:
Demographics and clinical characteristics of participants randomized to ibudilast (IBUD) versus placebo (PLA) conditions
| IBUD (N=64) | PLA (N=61) | P value | |
|---|---|---|---|
| Age, mean years (SD) | 38.8 (8.89) | 40.1 (11) | 0.50 |
| Gender, % (N) | |||
| Men | 76.6% (49) | 70.5% (43) | 0.60 |
| Women | 23.4% (15) | 29.5% (18) | |
| Race, % (N) | |||
| African American | 10.9% (7) | 11.5% (7) | 0.80 |
| American Indian/Alaska Native | 3.12% (2) | 3.28% (2) | |
| Asian | 6.25% (4) | 3.28% (2) | |
| Unknown/Not Reported | 32.8% (21) | 31.1% (19) | |
| More than One Race | 1.56% (1) | 0% (0) | |
| Native Hawaiian/Other Pacific Islander | 0% (0) | 1.64% (1) | |
| White | 45.3% (29) | 49.2% (30) | |
| Ethnicity, % (N) | |||
| Hispanic or Latino | 46.9% (30) | 49.2% (30) | 0.90 |
| Not Hispanic or Latino | 53.1% (34) | 50.8% (31) | |
| Substance use in past 30 days, mean days (SD) | |||
| Methamphetamine | 22.3 (8.97) | 22.1 (9.37) | 0.90 |
| Cannabis | 10.17 (13.1) | 6.11 (10.7) | 0.06 |
| Alcohol | 3.11 (6.38) | 5.59 (9.43) | 0.09 |
| Cigarette smoker, % (N) | |||
| Smoker | 68.8% (44) | 55.7% (34) | 0.20 |
| Non-smoker | 31.2% (20) | 44.3% (27) | |
| HIV serostatus | |||
| HIV positive | 21.9% (14) | 21.3% (13) | 1.00 |
| HIV negative | 78.1% (50) | 78.7% (48) |
Lead-in period (visits −6 thru −1)
There was no significant difference in the proportion of participants achieving MA abstinence, the mean number of MA negative urine drug screens, or the probability of providing an MA-negative urine drug screen during the two-week pre-medication lead-in period between participants in the IBUD versus placebo groups (Table 2, p > 0.05 for all comparison, Figure 2, p = 0.11 for GEE model). Methamphetamine use was more frequent during the lead-in among participants with higher baseline MA use frequency (>18/30 days) than lower frequency (<=18/30 days). Among participants with higher baseline MA use (>18/30 days), MA use was more frequent among participants assigned to IBUD than those assigned to placebo although the difference was not statistically significant (p = 0.61, Table 2, Figure 2).
Table 2:
Methamphetamine (MA) use outcomes during two-week pre-medication lead-in period and 12-week medication treatment period for ibudilast (IBUD) versus placebo (PLA) groups in the total sample and by baseline MA use frequency
| By Baseline Methamphetamine Use | ||||||
|---|---|---|---|---|---|---|
| Total Sample | <= 18/30 days | > 18/30 days | ||||
| IBUD N=64 | PLA N=61 | IBUD N=18 | PLA N=19 | IBUD N=46 | PLA N=42 | |
| Lead-In Period | ||||||
| End of Lead-in MA Abstinent, % (N) | 13% (8) | 16% (10) | 33% (6) | 26% (5) | 4% (2) | 12% (5) |
| MA negative urines, mean (SD) | 1.1 (1.5) | 1.4 (1.8) | 2.7 (1.4) | 3.2 (1.8) | 0.4 (0.9) | 0.7 (1.2) |
| Treatment Period | ||||||
| End of Treatment MA abstinent, % (N) | 14% (9) | 16% (10) | 22% (4) | 32% (6) | 11% (5) | 10% (4) |
| Treatment Effectiveness Score, mean (SD) | 4.5 (6.2) | 6.1 (7.6) | 7.8 (5.8) | 12.3 (6.9) | 3.2 (5.9) | 3.2 (6.0) |
| Joint Probability Index, week 6 | 0.22 | 0.33 | 0.28 | 0.63 | 0.20 | 0.19 |
| Joint Probability Index, week 12 | 0.17 | 0.21 | 0.28 | 0.37 | 0.13 | 0.14 |
Bold is p < 0.05
Figure 2:
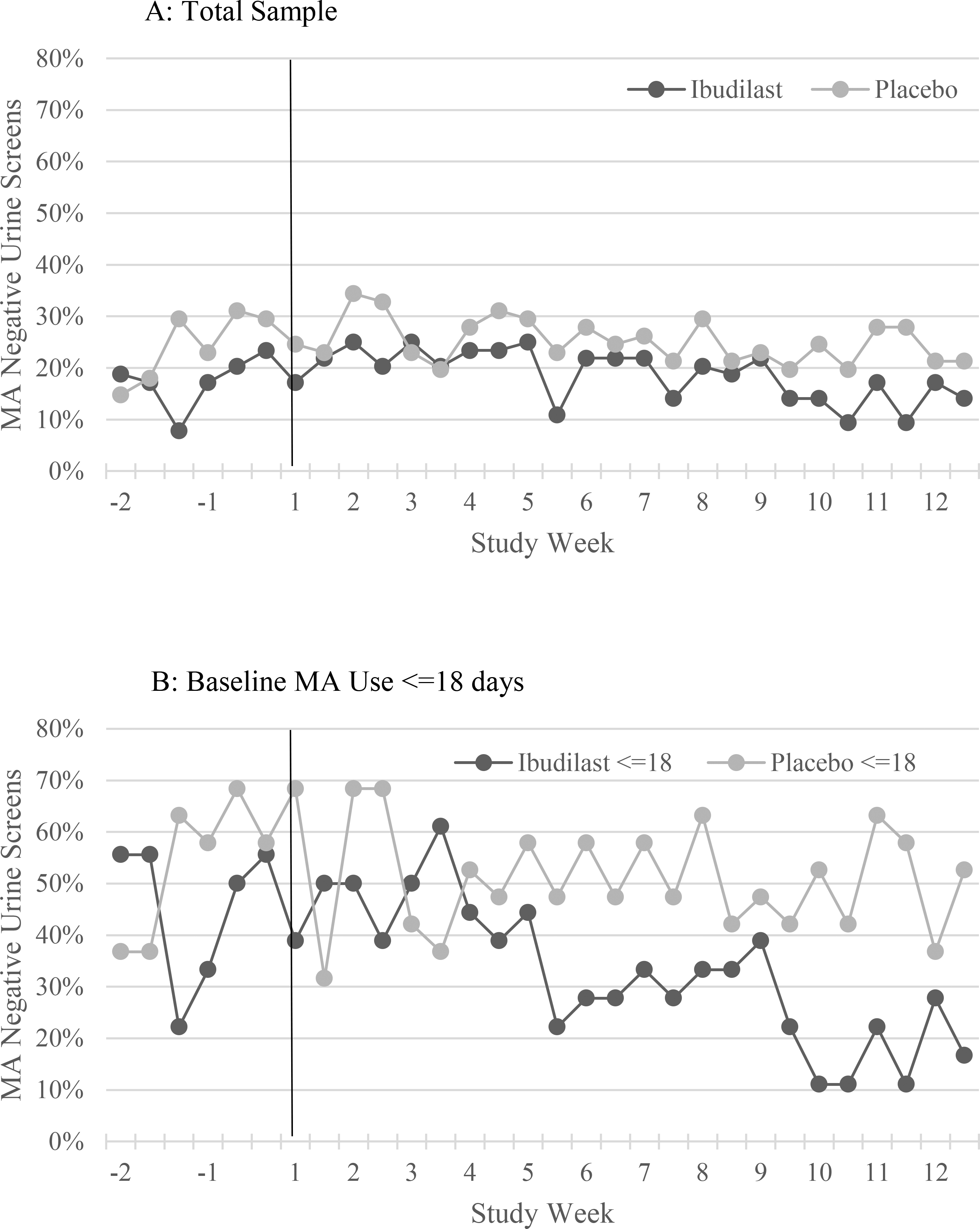
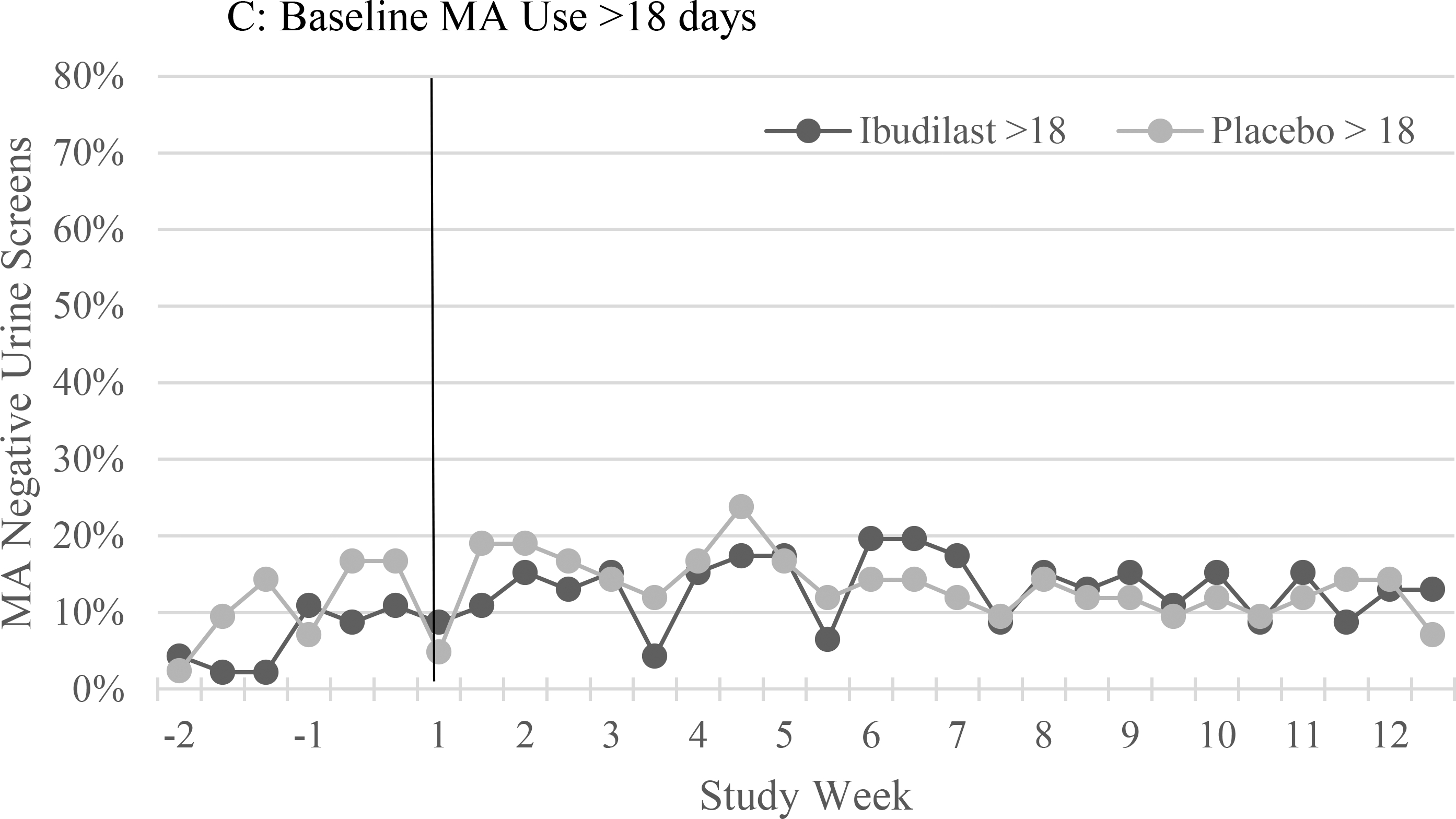
The proportion of urine drug screens negative for methamphetamine (MA) during the two week lead-in period (weeks −2 and −1) and the 12 week medication treatment period (weeks 1–12) for ibudilast versus placebo in: (A) the total sample, and participants with methamphetamine use at baseline on (B) ≤18 of the past 30 days, and (C) >18 days of the past 30 days.
Medication adherence
Serum IBUD levels at week 3 of the 12-week treatment period were available for 59 of the 64 (92%) participants in the IBUD group. The mean IBUD concentration was 54.1 ng/mL (S.D. = 31.3 ng/mL, range 6.76 ng/mL – 124.00 ng/mL). Serum IBUD concentration in one of the participants was Below the Limit of Quantitation. Sixty-four percent (N = 38) of IBUD participants with serum specimens available had a level greater than 40 ng/ml. There were no significant differences in IBUD levels by baseline clinical or demographic characteristics (Table 3).
Table 3:
Serum ibudilast levels at week 3 of the 12-week medication treatment period among participants assigned to the ibudilast group by baseline demographic and clinical characteristics
| N | Ibudilast, mean (SD) ng/mL | P value | |
|---|---|---|---|
| Age | |||
| 18 – 34 years | 24 | 47.0 (30.0) | 0.22 |
| 35 – 44 years | 18 | 63.9 (35.1) | |
| 45 - older | 17 | 53.9 (27.6) | |
| Gender | |||
| Men | 45 | 53.6 (30.6) | 0.80 |
| Women | 14 | 56.1 (34.7) | |
| Race | |||
| African American | 7 | 34.7 (30.9) | 0.19 |
| American Indian/Alaska Native | 2 | 65.5 (45.8) | |
| Asian | 4 | 33.1 (18.6) | |
| Unknown/Not Reported | 18 | 65.1 (35.1) | |
| More than One Race | 1 | 74.3 (NA) | |
| Native Hawaiian/Other Pacific Islander | 0 | NA (NA) | |
| White | 27 | 53.5 (27.6) | |
| Ethnicity | |||
| Hispanic or Latino | 26 | 55.7 (34.3) | 0.70 |
| Not Hispanic or Latino | 33 | 53.0 (29.2) | |
| Baseline Methamphetamine Use | |||
| > 18/30 days | 41 | 50.8 (30.9) | 0.20 |
| <= 18/30 days | 18 | 61.8 (31.8) | |
| Cigarette smoker, % (N) | |||
| Smoker | 40 | 52.3 (29.4) | 0.60 |
| Non-smoker | 19 | 58.0 (35.6) | |
| HIV serostatus | |||
| HIV positive | 14 | 51.1 (25.9) | 0.60 |
| HIV negative | 45 | 55.1 (33.0) |
Methamphetamine use outcomes
There were no statistically significant differences between IBUD and placebo for the primary study outcome, MA abstinence at end of treatment (week 11 and 12, the final two weeks of the medication treatment period, Table 2). There was no significant association between treatment group and the probability of providing MA negative urine drug screens during the 12 week treatment period in a GEE model controlling for gender, baseline past 30 day MA use, and number of MA negative urine drug screens during the two-week lead-in period (p = 0.34, Figure 2). Baseline MA use frequency (p = 1.66 × 10−19) and MA negative drug screens during the lead-in (p = 0.04), but not gender, were significantly associated with MA negative drug screens during the treatment period in the GEE model. Secondary outcomes, including treatment effectiveness score and joint probability index at weeks 6 and 12, favored placebo over IBUD in the total sample but did not achieve statistical significance (Table 2). Among participants with methamphetamine use on <=18 of the past 30 days at baseline, the proportion of participants achieving end of treatment MA abstinence (p = 0.04), treatment effectiveness score (p = 0.04) and joint probability index at week 6 (p = 0.03) were significantly higher for placebo compared to IBUD (Table 2). In separate GEE models predicting MA negative urine drug screens during treatment there was no significant effect for IBUD compared to placebo among HIV positive (p = 0.07) or HIV negative (p = 0.16) participants.
Among the 59 participants in the IBUD group with week 3 serum IBUD levels available, there was no significant correlation between serum IBUD levels and treatment effectiveness score (Correlation coefficient = 0.008, p = 1.00) and there was no significant difference between IBUD levels for participants with (mean = 51.3, SD = 20.3, N = 9) and without (mean = 54.7, SD = 33.0, N = 50, p = 0.70) end of treatment MA abstinence.
Treatment Retention
There was no difference in mean number of days retained in treatment for participants in the IBUD (mean = 58.8, SD = 28.6) versus placebo (mean = 57.0, SD = 29.7) groups (t = 0.03, df = 100, p = 0.70). There was no significant difference in treatment retention for participants in the IBUD versus placebo groups (p = 0.68) in a multivariate Cox regression model controlling for gender and baseline methamphetamine use frequency (Figure 3).
Figure 3:
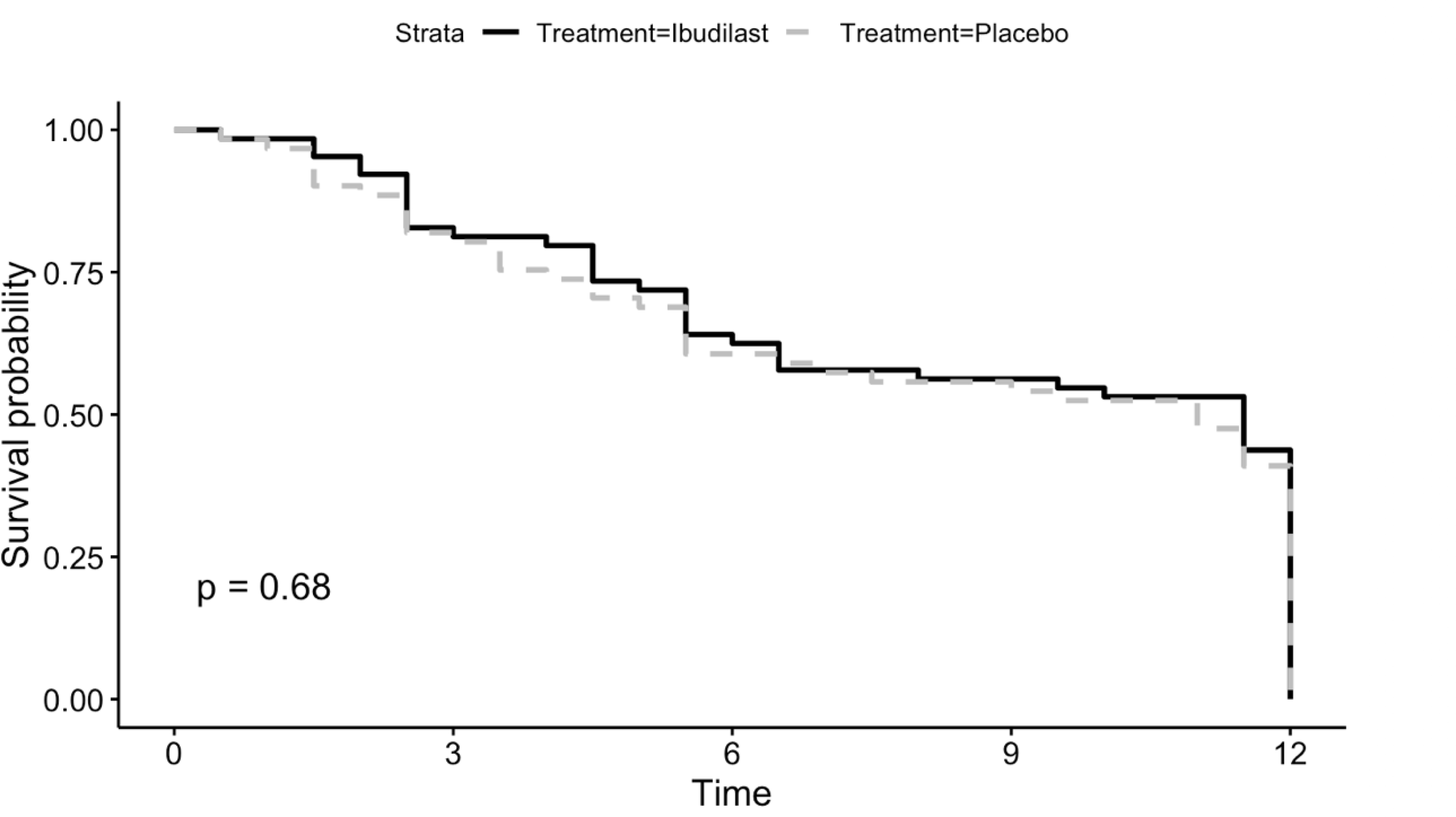
Cox regression survival curve for retention during the 12-week treatment period in the ibudilast versus placebo groups.
Adverse Events
There was no significant difference in the proportion of participants reporting at least one adverse event in the IBUD (57/64, 89%) versus placebo (51/61, 84%) groups (Chi sq = 0.4, df = 1, p = 0.50). There were 3 serious adverse events among participants, two in the IBUD group (a seizure and a suicide attempt) and one in the placebo group (chest pain). The majority of adverse events were mild to moderate and typical of IBUD and treatment for methamphetamine use (Table 4).
Table 4:
Frequency of most commonly reported adverse events among participants in the ibudilast versus placebo groups
| Adverse event | Ibudilast | Placebo | Total |
|---|---|---|---|
| Headache | 32 | 11 | 43 |
| Nausea | 11 | 9 | 20 |
| Insomnia | 10 | 5 | 15 |
| Diarrhea | 8 | 7 | 15 |
| Upper respiratory tract infection | 7 | 6 | 13 |
| Dyspepsia | 8 | 3 | 11 |
| Back pain | 3 | 8 | 11 |
| Depressed mood | 4 | 5 | 9 |
| Abdominal pain | 7 | 1 | 8 |
| Fatigue | 6 | 2 | 8 |
| Dizziness | 2 | 5 | 7 |
| Dry mouth | 5 | 1 | 6 |
| Vomiting | 5 | 1 | 6 |
Discussion
IBUD was well tolerated among participants with MA use disorder during 12 weeks of outpatient treatment but the trial failed to demonstrate any significant difference between IBUD and placebo in reducing MA use or increasing retention. The average days with MA use in the past 30 days at baseline among participants was 22 days and 70% of participants reported MA use on more than 18 of the past 30 days at baseline, which is higher than in previous studies (Heinzerling et al., 2010; Anderson et al., 2015). The high frequency of pre-treatment MA use suggests that we enrolled a sample with high severity of MA use and this may account for the low numbers of participants achieving MA abstinence at the end of treatment in both the IBUD and placebo groups (approximately 15%). Still we found no evidence that IBUD might be effective in participants with less frequent baseline MA use and in fact MA use outcomes were significantly better for placebo compared to IBUD among participants with MA use on 18 or fewer days per month at baseline. This finding may be due to chance given this was a post hoc analysis of the small number of participants in the <=18/30 days group.
Medication non-adherence is common among MA users and may contribute to the negative findings in many MA pharmacotherapy trials (Lee et al., 2018). Defining a cut-off serum level to define adherence in our sample is difficult as validated therapeutic reference ranges for IBUD are not available and we collected a randomly timed serum specimen at each participant’s outpatient clinic visit as opposed to a trough specimen. Previously, a single randomly timed plasma drug level providing a “snapshot” of medication adherence was strongly associated with end of treatment MA abstinence in a clinical trial of bupropion with an outpatient design similar to that employed in the current IBUD trial. Using a cut-off of 40 ng/ml, which is two standard deviations below the mean trough serum concentration for IBUD 50 mg twice a day (Medicinova, personal communication), 64% of IBUD participants were medication adherent, higher than adherence rates (32%−47%) in previous MA clinical trials (Heinzerling et al., 2014; Anderson et al., 2015). The lack of any association between IBUD levels and MA use outcomes suggests that although medication adherence was still low, non-adherence is unlikely to explain the lack of effect of IBUD on MA use observed in the current trial.
The study has several limitations. The study was designed to determine whether IBUD facilitates MA abstinence at the end of a relatively short 12-week course of treatment. MA-induced inflammation has been implicated in the pathogenesis of a variety of health complications of chronic MA use, including cognitive dysfunction, cardiomyopathy, and increased risk of Parkinson’s disease (Loftis et al., 2011b; Huckans et al., 2015; De Virgilio et al., 2016; Schurer et al., 2017; Papageorgiou et al., 2019), and whether IBUD’s anti-inflammatory effects may have positive effects on MA-associated conditions even in the absence of reductions in MA use is not known. As described above, the baseline MA use frequency in the sample was high, reflecting severe MA use disorder and potentially higher levels of neuroinflammation among participants, which may have overwhelmed any potential effects of IBUD on MA use. Furthermore, the importance of microglial inflammation, which is targeted by IBUD, in MA-induced neurotoxicty, versus other potential mediators of MA’s neurotoxic effects such as oxidative stress, is not clear (Yang et al., 2018). The study used a low intensity medication management counseling platform as opposed to more intensive cognitive behavioral therapies used in previous MA clinical trials with the intent of avoiding masking of medication effects with too potent behavioral therapy. Whether IBUD may reduce MA use when combined with more intensive behavioral therapies is also unknown. Lastly, the study was powered to detect a moderate effect size and may have failed to detect smaller effects for IBUD, which could be important in light of the paucity of effective treatments available for MA use disorder.
In conclusion, IBUD was not effective in facilitating MA abstinence during this outpatient clinical trial. Whether targeting neuroinflammation, either with IBUD in other subgroups of MA users or clinical trial designs, such as to sustain abstinence in MA users with lower baseline MA use frequency or in the context of highly structured behavioral support, or with other anti-inflammatory medications, is an effective strategy for developing medications for MA use disorder is not clear. The extent to which MA-induced microglial activation is responsible for clinically relevant dopaminergic dysfunction in MA users also is not clear (Shaerzadeh et al., 2018). Despite numerous clinical trials, an effective medication for MA use disorder remains elusive suggesting that a reframing of our approach to medication development for MA use problems, including novel medication targets and clinical trial designs, is needed.
Acknowledgements:
The study was funded by the National Institute on Drug Abuse (DA035054). Study medication and matching placebo was provided by MediciNova, Inc.
Footnotes
Compliance with Ethical Standards:
Conflict of Interest: Dr. Heinzerling has received study medication from MediciNova and DeNovo, has been a scientific advisor for Alkermes and Indivior, and is a founder of BDH Pharma, LLC. Dr. Briones is a founder of BDH Pharma, LLC. Dr. Shoptaw has received study medication from Gilead. No other authors declare potential conflicts.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References:
- American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR, Fourth Edition, Text Revision Edition. Washington D.C.: American Psychiatric Association. [Google Scholar]
- Anderson AL, Li SH, Markova D, Holmes TH, Chiang N, Kahn R, Campbell J, Dickerson DL, Galloway GP, Haning W, Roache JD, Stock C, Elkashef AM (2015) Bupropion for the treatment of methamphetamine dependence in non-daily users: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend 150:170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC 3rd, Elkashef AM (2009) Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend 104:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhof F, Hulst HE, Drulovic J, Uitdehaag BM, Matsuda K, Landin R (2010) Ibudilast in relapsing-remitting multiple sclerosis: a neuroprotectant? Neurology 74:1033–1040. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Shelton KL, Hendrick E, Johnson KW (2010) The glial cell modulator and phosphodiesterase inhibitor, AV411 (ibudilast), attenuates prime- and stress-induced methamphetamine relapse. Eur J Pharmacol 637:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birath JB, Briones M, Amaya S, Shoptaw S, Swanson AN, Tsuang J, Furst B, Heinzerling K, Obermeit L, Maes L, McKay C, Wright MJ (2017) Ibudilast may improve attention during early abstinence from methamphetamine. Drug Alcohol Depend 178:386–390. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Cheng WY, Levin FR, Mariani JJ, Raby WN, Nunes EV (2010) A placebo-controlled trial of memantine for cocaine dependence with high-value voucher incentives during a pre-randomization lead-in period. Drug Alcohol Depend 111:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, Vosburg SK, Nunes EV (2005) Utility of lead-in period in cocaine dependence pharmacotherapy trials. Drug Alcohol Depend 77:7–11. [DOI] [PubMed] [Google Scholar]
- Brensilver M, Heinzerling KG, Shoptaw S (2013) Pharmacotherapy of amphetamine-type stimulant dependence: an update. Drug Alcohol Rev 32:449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Crichlow GV, Vermeire JJ, Leng L, Du X, Hodsdon ME, Bucala R, Cappello M, Gross M, Gaeta F, Johnson K, Lolis EJ (2010) Allosteric inhibition of macrophage migration inhibitory factor revealed by ibudilast. Proceedings of the National Academy of Sciences 107:11313–11318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Virgilio A, Greco A, Fabbrini G, Inghilleri M, Rizzo MI, Gallo A, Conte M, Rosato C, Ciniglio Appiani M, de Vincentiis M (2016) Parkinson’s disease: Autoimmunity and neuroinflammation. Autoimmunity reviews 15:1005–1011. [DOI] [PubMed] [Google Scholar]
- DeYoung DZ, Heinzerling KG, Swanson AN, Tsuang J, Furst BA, Yi Y, Wu YN, Moody DE, Andrenyak DM, Shoptaw SJ (2016) Safety of Intravenous Methamphetamine Administration During Ibudilast Treatment. Journal of clinical psychopharmacology 36:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MBS RL; Gibbon M; and Williams JBW (2002) Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research. [Google Scholar]
- Fox RJ et al. (2018) Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis. The New England journal of medicine 379:846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson LC, Hastings SF, McPhee I, Clayton RA, Darroch CE, Mackenzie A, Mackenzie FL, Nagasawa M, Stevens PA, Mackenzie SJ (2006) The inhibitory profile of Ibudilast against the human phosphodiesterase enzyme family. Eur J Pharmacol 538:39–42. [DOI] [PubMed] [Google Scholar]
- Heinzerling KG, Swanson AN, Hall TM, Yi Y, Wu Y, Shoptaw SJ (2014) Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction 109:1878–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzerling KG, Swanson AN, Kim S, Cederblom L, Moe A, Ling W, Shoptaw S (2010) Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend 109:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8:382–397. [DOI] [PubMed] [Google Scholar]
- Huckans M, Fuller BE, Chalker AL, Adams M, Loftis JM (2015) Plasma Inflammatory Factors Are Associated with Anxiety, Depression, and Cognitive Problems in Adults with and without Methamphetamine Dependence: An Exploratory Protein Array Study. Frontiers in psychiatry 6:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Link J, Dennis LE, McCready H, Huckans M, Hoffman WF, Loftis JM (2019) Neuroinflammation in addiction: A review of neuroimaging studies and potential immunotherapies. Pharmacology, biochemistry, and behavior 179:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Jenner L, Harney A, Cameron J (2018) Pharmacotherapy for amphetamine dependence: A systematic review. Drug Alcohol Depend 191:309–337. [DOI] [PubMed] [Google Scholar]
- Ling W, Shoptaw S, Wesson D, Rawson RA, Compton M, Klett CJ (1997) Treatment effectiveness score as an outcome measure in clinical trials. NIDA research monograph 175:208–220. [PubMed] [Google Scholar]
- Loftis JM, Choi D, Hoffman W, Huckans MS (2011a) Methamphetamine causes persistent immune dysregulation: a cross-species, translational report. Neurotox Res 20:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Choi D, Hoffman W, Huckans MS (2011b) Methamphetamine causes persistent immune dysregulation: a cross-species, translational report. Neurotoxicity research 20:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghetti L, Ajmone-Cat MA, De Berardinis MA, De Simone R (2005) Microglial activation in chronic neurodegenerative diseases: roles of apoptotic neurons and chronic stimulation. Brain Res Brain Res Rev 48:251–256. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Kurotani T, Komatsu Y, Kawanokuchi J, Kato H, Mitsuma N, Suzumura A (2004) Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology 46:404–411. [DOI] [PubMed] [Google Scholar]
- Morley KC, Cornish JL, Faingold A, Wood K, Haber PS (2017) Pharmacotherapeutic agents in the treatment of methamphetamine dependence. Expert opinion on investigational drugs 26:563–578. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society 53:695–699. [DOI] [PubMed] [Google Scholar]
- Papageorgiou M, Raza A, Fraser S, Nurgali K, Apostolopoulos V (2019) Methamphetamine and its immune-modulating effects. Maturitas 121:13–21. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, National Institute on Alcohol Abuse and Alcoholism (U.S.) (2004) Medical management treatment manual : a clinical research guide for medically trained clinicians providing pharmacotherapy as part of the treatmemt for alcohol dependence. Bethesda, MD: U.S. Dept. of Health and Human Services, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism. [Google Scholar]
- Pettinati HM, Weiss RD, Dundon W, Miller WR, Donovan D, Ernst DB, Rounsaville BJ (2005) A structured approach to medical management: a psychosocial intervention to support pharmacotherapy in the treatment of alcohol dependence. Journal of studies on alcohol:170–178; discussion 168–179. [DOI] [PubMed] [Google Scholar]
- Rolan P, Hutchinson M, Johnson K (2009) Ibudilast: a review of its pharmacology, efficacy and safety in respiratory and neurological disease. Expert Opinion on Pharmacotherapy 10:2897–2904. [DOI] [PubMed] [Google Scholar]
- Schurer S, Klingel K, Sandri M, Majunke N, Besler C, Kandolf R, Lurz P, Luck M, Hertel P, Schuler G, Linke A, Mangner N (2017) Clinical Characteristics, Histopathological Features, and Clinical Outcome of Methamphetamine-Associated Cardiomyopathy. JACC Heart failure 5:435–445. [DOI] [PubMed] [Google Scholar]
- Shaerzadeh F, Streit WJ, Heysieattalab S, Khoshbouei H (2018) Methamphetamine neurotoxicity, microglia, and neuroinflammation. Journal of neuroinflammation 15:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M, Wegrzynowicz M, Lee E, Bowman AB, Aschner M (2011) Role of astrocytes in brain function and disease. Toxicol Pathol 39:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, Hendrick ES, Beardsley PM (2013) Glial cell modulators attenuate methamphetamine self-administration in the rat. Eur J Pharmacol 701:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, Vunck SA, van den Oord EJ, Adkins DE, McClay JL, Beardsley PM (2012) The glial cell modulators, ibudilast and its amino analog, AV1013, attenuate methamphetamine locomotor activity and its sensitization in mice. Eur J Pharmacol 679:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E (1986) The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addictive behaviors 11:149–161. [DOI] [PubMed] [Google Scholar]
- Soontornniyomkij V, Kesby JP, Morgan EE, Bischoff-Grethe A, Minassian A, Brown GG, Grant I (2016) Effects of HIV and Methamphetamine on Brain and Behavior: Evidence from Human Studies and Animal Models. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 11:495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R, Williams J, Gibbbon M, First M (1995) The Structured Clinical Interview for DSM-IV. Washington, D.C.: American Psychiatric Press. [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK (1994) Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol, Supplement s12:70–75. [DOI] [PubMed] [Google Scholar]
- Suzumura A, Ito A, Yoshikawa M, Sawada M (1999) Ibudilast suppresses TNFalpha production by glial cells functioning mainly as type III phosphodiesterase inhibitor in the CNS. Brain research 837:203–212. [DOI] [PubMed] [Google Scholar]
- Wakita H, Tomimoto H, Akiguchi I, Lin JX, Ihara M, Ohtani R, Shibata M (2003) Ibudilast, a phosphodiesterase inhibitor, protects against white matter damage under chronic cerebral hypoperfusion in the rat. Brain research 992:53–59. [DOI] [PubMed] [Google Scholar]
- Worley MJ, Swanson AN, Heinzerling KG, Roche DJ, Shoptaw S (2016) Ibudilast attenuates subjective effects of methamphetamine in a placebo-controlled inpatient study. Drug Alcohol Depend 162:245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wang Y, Li Q, Zhong Y, Chen L, Du Y, He J, Liao L, Xiong K, Yi CX, Yan J (2018) The Main Molecular Mechanisms Underlying Methamphetamine- Induced Neurotoxicity and Implications for Pharmacological Treatment. Frontiers in molecular neuroscience 11:186. [DOI] [PMC free article] [PubMed] [Google Scholar]


