Abstract
Objective
Reported associations between kidney risk variants (G1 and G2) in APOL1, encoding apolipoprotein L1 (APOL1), and cardiovascular disease have been conflicting. We sought to explore associations of APOL1 risk variants with cause of sudden death using the CVPath Sudden Death Autopsy Registry.
Approach and Results
APOL1 haplotypes and causes of sudden death, as determined through autopsy and histopathology, were obtained for 764 African Americans.
Genotyping revealed APOL1 risk alleles in 452 of 764 (59%) subjects with 347 (77%) subjects carrying one risk allele and 105 (23%) subjects harboring two risk alleles. APOL1 risk allele carrier status was associated with a significantly increased risk of coronary thrombosis due to plaque rupture, versus non-carriers (OR for rupture 1.655, 95% CI 1.079 – 2.539; p = 0.021). Histological examinations showed coronary plaques in carriers of two APOL1 risk alleles had larger necrotic cores compared to non-carriers (necrotic core area/total plaque area: 46.79% ± 6.47% vs. 20.57% ± 5.11%; p = 0.0343 in ruptured plaques, and 41.48% ± 7.49% vs. 18.93% ± 3.97%; p = 0.0342 in non-ruptured plaques), and immunohistochemical and immunofluorescent staining revealed APOL1-positive areas localized primarily to the necrotic core.
Conclusions
APOL1 risk alleles were independently associated with an increased risk of thrombotic coronary death due to plaque rupture. Our results suggest that carriers of both one and two APOL1 risk alleles have greater accumulation of APOL1 protein within culprit plaques and greater necrotic core sizes than non-carriers. These findings suggest that APOL1 plays a role in determining plaque stability.
Keywords: Plaque Rupture, Genetic Variants, Apolipoprotein L1, Coronary Artery Disease, African Americans
Journal Subject Terms: Thrombosis, Sudden Cardiac Death, Genetic, Association Studies
Introduction
African Americans have a three to four times increased lifetime risk for end-stage kidney disease (ESKD) compared to white patients in the United States, in spite of a similar prevalence in earlier stages of chronic kidney disease (CKD) (1–3). Two alleles in the gene encoding apolipoprotein L1 (APOL1), termed G1 and G2, are thought to account for a substantial fraction of the excess ESKD risk in African Americans (4–6). While these variants are absent from European chromosomes, approximately 13% of African Americans have two copies (5, 7), most likely representing positive selection as the two variants provide enhanced lytic potency against Trypanosoma brucei rhodesiense, which is a cause of sleeping sickness (4). The APOL1 G1 allele consists of two missense variants (p.S342G, rs73885319, and p.I384M, rs60910145), which are in nearly complete linkage disequilibrium with each other and therefore nearly always are present together. The G2 allele manifests the deletion of two amino acids in positions 388 and 389 (p.delN388/Y389, rs71785313) of the apolipoprotein L1 protein (APOL1). The exact molecular mechanisms by which APOL1 risk variants cause kidney disease are not fully understood, although a large number of studies investigating APOL1 variant-driven kidney injury have proposed various mechanisms leading to cell injury (6).
CKD and hypertension often develop concomitantly and are strong risk factors for cardiovascular diseases (CVD) (8–10) but whether APOL1 directly affects the risk of CVD independent of its effects on the kidney remains uncertain. APOL1 is found in plasma, vasculature, kidney and liver, as well as other organs (11, 12). Because APOL1 is an apolipoprotein component of dense high-density lipoprotein (HDL3) particles that play a central role in cholesterol transport and attenuate low-density lipoprotein (LDL) oxidation (13), it also has effects on lipid metabolism that might directly influence the development of CVD. However, studies investigating a relationship between the APOL1 risk variants and CVD have generated conflicting results. While some studies found APOL1 risk variants to be associated with a higher risk of developing coronary artery disease (CAD) (14) or with experiencing a major adverse cardiovascular event (15), others did not (16, 17), and some studies did not find associations between APOL1 risk variants and the overall CVD outcome, especially when stroke and heart failure were included, but found an increased cardiovascular mortality associated with increasing numbers of APOL1 risk variants (18, 19). Moreover, carriers of two APOL1 risk variants were shown to have a 80% higher risk of myocardial infarction (MI), compared with carriers of only one risk variant or carriers of the reference allele (19).
The most frequent cause of MI and fatal acute coronary thrombosis is plaque rupture (20–22), occurring by disruption of the fibrous cap overlying the plaque and exposing the highly thrombogenic lipid core to the blood stream. Other, less frequent causes of acute intracoronary thrombosis include plaque erosion and eruptive calcified nodules, also presenting as acute coronary syndrome. Experimental data suggest that plaque rupture is initiated by a lipid-driven inflammation. Oxidized LDL particles stimulate the attraction of monocytes and macrophages that secrete proinflammatory cytokines, enzymes, and reactive oxygen species, weakening the fibrous cap. Macrophages also take up lipids and develop into foam cells that eventually undergo apoptosis (23). This process, along with further lipid accumulation, drives the formation and growth of a necrotic core within the plaque, substantially decreasing plaque stability (24), and plaques with larger cores are especially prone to rupture (21).
Despite a potential impact on reverse cholesterol transport (25), it is unclear if and to what extent APOL1 risk carrier status causes morphologic chances in the vasculature, and associations between APOL1 risk allele carrier status and specific cardiovascular causes of sudden death have never been explored in histopathologic detail. Data from the only autopsy study investigating APOL1 risk allele carrier status and cardiac death suggested that APOL1 risk variants are independently associated with early CVD death (26); however, the study included only 43 CAD cases from a total cohort of 162 African American subjects, limiting analytic power.
Here, we investigated the associations of APOL1 haplotypes (G0, G1, or G2) and cause of sudden death using detailed histopathologic data from a cohort of 764 African American subjects from the CVPath Sudden Death Registry. Due to the role of APOL1in cholesterol physiology, we hypothesized that carriers of APOL1 risk variants might be at increased risk for plaque rupture, as opposed to plaque erosion or eruptive calcified nodules in plaque.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
The CVPath Institute Sudden Cardiac Death registry heart samples are received through an ongoing joint consultation service provided to the Maryland Office of the Chief Medical Examiner. Sudden death was defined as symptoms commencing within 6 hours of death (witnessed arrest) or death occurring within 24 hours after the victim was last seen alive in their normal state of health. Cardiovascular risk factors (smoking, diabetes, hypertension, and hyperlipidemia) were assessed from the subjects’ medical history, obtained from the Medical Examiner’s report. Hypertension was considered present if either postmortem kidney evaluation was positive or if there was a clinical history. In addition, serum samples had previously been analyzed for total cholesterol, HDL cholesterol, and thiocyanate, a marker for cigarette smoking, and blood cells were analyzed for glycosylated hemoglobin, as described elsewhere (27, 28). Comprehensive analysis of each heart sample included coronary artery histology, and percentage cross-sectional luminal narrowing was determined in each artery in all subjects. Cases of unexpected sudden death were stratified into cardiac and non-cardiac causes. Cardiac death was further specified as death associated with CAD or non-CAD-associated death. CAD-associated death was defined as at least one epicardial coronary artery having ≥75% cross-sectional luminal narrowing by an atherosclerotic plaque or coronary thrombosis (i.e., a lesion with a superimposed thrombus) or evidence of MI with no other cause of death noted. Non-CAD-associated death was defined as cardiac causes of death other than those related to CAD, for example, cardiomyopathies. Non-cardiac causes of death included drug overdose, trauma, seizure disorder, and stroke. Information on co-morbidities, such as CKD, was available from autopsy reports. All subjects were genotyped for the APOL1 variants (G0, G1, or G2). For the current study, 764 subjects ≥ 18 years who died suddenly were included. This study was approved by the Institutional Review Board at CVPath Institute (#RP0093).
Genotype Ascertainment
Coding exons of the APOL1 gene were genotyped using Taqman genotyping assays as previously described (29, 30). Positive APOL1 carrier status was defined as heterozygosity or homozygosity for the risk alleles G1, consisting of two coding variants rs73885319 (S342G) and rs60910145 (I384M), and G2, rs71785313 (delN388/Y389). In addition, rs136164 genotypes were assessed by DNA sequencing using the Illumina Infinium Multi-Ethnic Genotyping array. Imputation was performed on the Michigan Server using Trans-Omics for Precision Medicine (TOPMed) data, mapped to human genome build GRCh38.
Assessment of Cholesterol and Creatinine Levels
Serum samples were available in 333 subjects. Total cholesterol was assessed in 179 subjects and HDL cholesterol was assessed in 140 subjects as previously described (27, 28). Serum creatinine was assessed using LabAssay™ Creatinine for Cell biology (FUJIFILM Wako Pure Chemical Corporation).
Histological and Immunohistochemical Analysis
Formalin-fixed and paraffin-embedded coronary arteries were stained using H&E and Movat stains. Area measurements were performed by tracing the contours of EEL (external elastic lamina), IEL (internal elastic lamina), lumen, and the necrotic core in Zen software (Zen 2.0 software, Zeiss, Oberkochen, Germany) using a graphic drawing tablet (Supplemental Figure I). EEL area, IEL area, lumen area, and necrotic core area were calculated by the software. Percentage stenosis was calculated by
Less than 25% cross-sectional stenosis was considered negligible/no atherosclerosis, 25–49% cross-sectional stenosis was considered mild, 50–74% cross-sectional stenosis was considered moderate, and ≥75% cross-sectional stenosis in at least one coronary artery was considered severe atherosclerosis.
41 arterial sections with ≥75% luminal narrowing from 19 carriers of only the reference allele and 23 carriers of at least one APOL1 risk allele (15 carriers of one and 7 carriers of two APOL1 risk alleles), and 26 ruptured plaques with similar percentage of cross-sectional luminal narrowing from 13 carriers of the reference allele and 13 carriers of APOL1 risk alleles (7 carriers of one and 6 carriers of two APOL1 risk alleles) were selected for immunohistochemistry and immunofluorescent staining. For immunohistochemistry, paraffin sections were deparaffinized before antigen retrieval by steam heat in EDTA buffer (pH 8.0). The sections were treated with 3% H2O2 and Dako protein block for 10 min. and incubated with primary antibodies. Areas of foam cell macrophage accumulation were identified with immunohistochemical staining for CD68 (Dako, Carpinteria, CA). APOL1 accumulation was detected by a polyclonal APOL1 rabbit antibody (Sigma). Please see the Major Resources Table (Supplemental Table I). Antibody binding was detected using a streptavidin-alkaline phosphatase 4plus ALP Universal detection system (Biocare Medical, Concord CA) and visualized with a NovaRed chromogen (Vector, Burlingame, California). In addition, apoptotic nuclei were visualized by the terminal deoxynucleotidyl transferase-mediated desoxyuridinetriphosphate nick end-labeling (TUNEL) method, using the CardioTACS™ In Situ Apoptosis Detection Kit (Trevigen) according to the manufacturer’s instructions. Immunohistochemical quantification for CD68 and APOL1, and of apoptotic nuclei (TUNEL) was performed in HALO 3.0 (Indica Labs, Corrales, NM) after digitally scanning the stained slides using Axio.Scan Z.1 slide scanner (Zeiss, Thornwood, NY) and Zen software (Zen 2.6 blue edition software, Zeiss, Oberkochen, Germany).
Immunofluorescent staining and confocal analysis
Coronary arteries were stained against APOL1 (Sigma, St. Louis, MO) and CD68 (Dako, Carpinteria, CA). In addition, a subset of 14 stable plaques (including plaques from 5 carriers of the reference allele, 5 carriers of one, and 4 carriers of two APOL1 risk alleles) and 16 ruptured coronary plaques (from 6 carriers of the reference allele, 5 carriers of one, and 5 carriers of two APOL1 risk alleles) was stained against apolipoprotein A1 (APOA1), detected by a polyclonal APOA1 rabbit antibody (Sigma, St. Louis, MO), and cleaved caspase 3, identified by a monoclonal rabbit antibody (Cell Signaling Technology). Nuclear counterstain was performed with DAPI (Invitrogen). Images were obtained with a confocal laser scanning microscope (LSM 800, Zeiss, Oberkochen, Germany), and staining quantification was performed using Zen software (Zeiss, Oberkochen, Germany), assessing the APOL1-, CD68, APOA1-, and cleaved caspase 3-positive areas within the plaque area, defined as the area between the IEL and the lumen.
Statistical Analysis
Statistical analyses were performed with SPSS version 22.0. Continuous data are presented as means ± SD. Categorical data are presented as numbers and percentages. Statistical differences between continuous variables were determined with one-way ANOVA, followed by Tukey’s Multiple Comparisons testing, and between categorical variables with chi-square tests. Assuming Hardy-Weinberg Equilibrium in both cases and controls, odds ratios were calculated in 2 × 3 contingency tables to determine the probability of rupture occurring in carriers of one or two risk alleles versus non-carriers. Associations between APOL1 risk allele carrier status and outcomes were assessed using logistic regression analysis with adjustment for age, sex, BMI, hypertension, smoking, diabetes mellitus, hyperlipidemia, and kidney disease as covariates. Graphs were created in GraphPad Prism (GraphPad Software, Version 8.4.1).
Results
Baseline characteristics
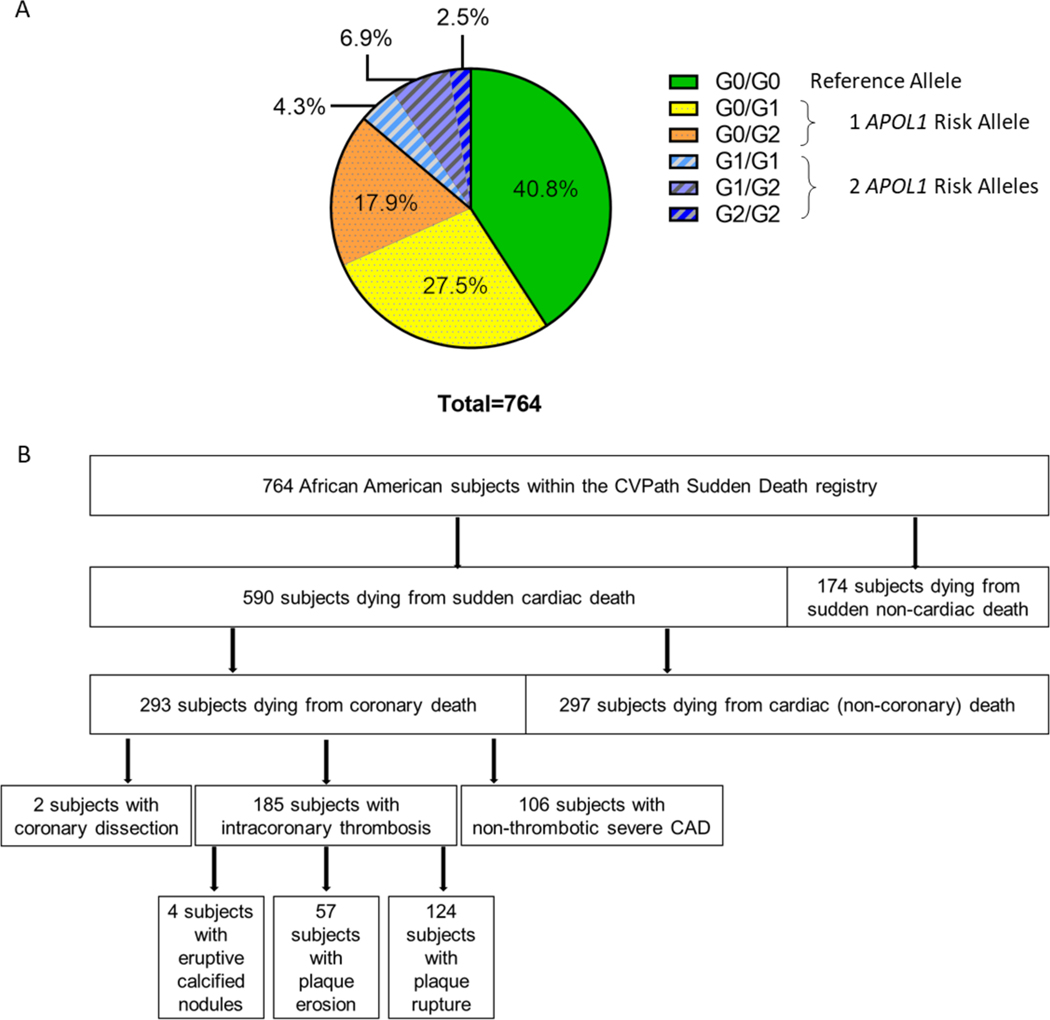
764 African American subjects ≥18 years who died suddenly were genotyped for the APOL1 gene variants. In 105 subjects (13.7%), two APOL1 risk alleles were present, of whom 33 subjects (4.3%) had G1/G1, 53 subjects (6.9%) had G1/G2, and 19 subjects (2.5%) had G2/G2 haplotypes. 347 subjects (45.4%) carried one APOL1 risk allele, with 210 subjects (27.5%) having G0/G1 and 137 subjects (17.9%) having G0/G2 haplotypes. 312 subjects (40.8%) carried G0/G0 wildtype alleles (Figure 1A). These proportions are consistent with those observed in the general African American population (4).
Figure 1: APOL1 Risk Variants and Causes of Death in Subjects within the CVPath Sudden Death Registry.
A. Distribution of APOL1 gene variants in 764 subjects within the CVPath Sudden Death Registry. In 105 subjects (13.7%), two APOL1 risk alleles were present, of whom 33 subjects (4.3%) had G1/G1, 53 subjects (6.9%) had G1/G2, and 19 subjects (2.5%) had G2/G2 haplotypes. 347 subjects (45.4%) carried one APOL1 risk allele, with 210 subjects (27.5%) having G0/G1 and 137 subjects (17.9%) having G0/G2 haplotypes. 312 subjects (40.8%) carried G0/G0 reference alleles. B. Causes of Death of subjects within the study cohort. Overall, 590 subjects died from sudden cardiac death, and 174 subjects died from sudden non-cardiac death. 293 subjects died from sudden coronary deaths, of whom 185 died from intracoronary thrombosis, 106 subjects had severe CAD without acute thrombosis, and 2 subjects had spontaneous coronary artery dissections. Intracoronary thrombosis was caused by plaque rupture in 124 subjects, by plaque erosion in 57 subjects, and by eruptive calcified nodules in 4 subjects.
Information on age, sex, and body mass index (BMI) was available for all subjects and did not significantly differ between APOL1 risk allele carriers and non-carriers (Table 1) or between carriers of zero, one, or two APOL1 alleles (Supplemental Table II). There were also no differences in the frequency of other cardiovascular risk factors, such as hypertension, hyperlipidemia, diabetes, and smoking, as well as in history of kidney disease. However, the proportion of dialysis patients was significantly higher in carriers versus non-carriers of APOL1 risk alleles (5.8% vs. 2.6%; p=0.0357).
Table 1.
Descriptive characteristics of subjects byAPOL1 carrier status.
| Total Study Cohort, n=764 | Reference Allele, n=312 | ≥1 APOL1 Risk Allele, n=452 | p-value* (unadjusted) | |
|---|---|---|---|---|
| Demographics | ||||
| Age (mean ± SD) | 47.18 ± 12.75 | 47.57 ± 13.44 | 46.91 ± 12.25 | 0.4821 |
| Male Sex | 524 (68.6%) | 206 (66.0%) | 318 (70.4%) | 0.2052 |
| BMI, kg/m2 (mean ± SD) | 30.03 ± 8.36 | 30.51 ± 8.68 | 29.73 ± 8.13 | 0.2390 |
|
| ||||
| Risk factors | ||||
| Hypertension | 488 (63.9%) | 193 (61.9%) | 295 (65.3%) | 0.3353 |
| Hyperlipidemia | 120 (15.7%) | 44 (14.1%) | 76 (16.8%) | 0.3113 |
| Diabetes Mellitus | 117 (15.3%) | 47 (15.1%) | 70 (15.5%) | 0.8733 |
| Smoking | 65 (8.5%) | 24 (7.7%) | 41 (9.1%) | 0.5020 |
| Kidney disease | 60 (7.9%) | 19 (6.1%) | 41 (9.1%) | 0.1322 |
| Dialysis | 34 (4.5%) | 8 (2.6%) | 26 (5.8%) | 0.0357 |
| History of drug abuse | 120 (15.7%) | 53 (17.0%) | 67 (14.8%) | 0.4190 |
|
| ||||
| Cause of Death | ||||
| Sudden Cardiac Death | 590 (77.2%) | 231 (74.0%) | 359 (79.4%) | 0.0810 |
| Coronary Death | 293 (38.4%) | 111 (35.6%) | 182 (40.3%) | 0.1908 |
| Intracoronary Thrombosis | 185 (24.2%) | 62 (19.9%) | 123 (27.2%) | 0.0199 |
| Rupture | 124 (16.2%) | 39 (12.5%) | 85 (18.8%) | 0.0202 |
| Erosion | 57 (7.5%) | 22 (7.1%) | 35 (7.7%) | 0.7205 |
| Calcified Nodules | 4 (0.5%) | 1 (0.3%) | 3 (0.7%) | 0.5182 |
| Non-thrombotic CAD | 106 (13.9%) | 47 (15.1%) | 59 (13.1%) | 0.4293 |
| Coronary Dissection | 2 (0.3%) | 2 (0.6%) | 0 (0.0%) | NA |
| Cardiac but Non-Coronary Death | 297 (38.9%) | 120 (38.5%) | 177 (39.2%) | 0.8458 |
| Sudden Non-Cardiac Death | 174 (22.8%) | 81 (26.0%) | 93 (20.6%) | 0.0810 |
|
| ||||
| Degree of Atherosclerosis | ||||
| None (0–24% cross-sectional stenosis) | 271 (35.5%) | 110 (35.3%) | 161 (35.6%) | 0.9179 |
| Mild (25–50% cross-sectional stenosis) | 74 (9.7%) | 33 (10.5%) | 41 (9.1%) | 0.4890 |
| Moderate (50–74% cross-sectional stenosis) | 122 (16.0%) | 51 (16.3%) | 71 (15.7%) | 0.8129 |
| Severe (≥75% cross-sectional stenosis) | 279 (38.9%) | 118 (37.8%) | 179 (39.6%) | 0.6196 |
p-values were calculated by comparison between carriers of the reference allele and carriers of ≥1 APOL1 risk allele. NA: not available.
Kidney function, histopathology, and cholesterol levels
To evaluate markers of kidney function, we assessed serum creatinine values in a subset of 333 subjects within the cohort with available serum samples and found no differences among the three haplotypes (Supplemental Table II). Histopathologic analysis of kidney specimens from 45 subjects carrying two APOL1 risk alleles and 46 carriers with only the reference allele, matched for age and sex, revealed a significantly higher percentage of sclerotic glomeruli, and a trend towards a higher number of sclerotic glomeruli as well as a higher amount of microcystic dilation and glomerular density (Supplemental Table III). Total cholesterol levels were determined in 179 randomly selected subjects of 333 subjects with serum samples available, and were not different between carriers of only the reference allele and carriers of one or two APOL1 risk alleles (Supplemental Table II), in line with previous studies (14, 18, 19, 31). In addition, HDL cholesterol levels were available for 140 subjects, and serum concentrations of apolipoprotein A1 (APOA1), the primary component of the HDL particle, were available for 48 subjects. However, we found no association between APOL1 genotype and HDL cholesterol or APOA1 serum levels.
Carriers of APOL1 risk alleles showed an increased risk of sudden coronary death due to plaque rupture
We evaluated associations between cause of death and APOL1 risk allele carrier status in our cohort (Table 1). Figure 1B shows the causes of death from 764 African American subjects within the CVPath Sudden Death Registry. Overall, 590 subjects (77.2%) experienced sudden cardiac death, and 174 subjects (22.8%) experienced sudden non-cardiac death. Sudden coronary death occurred in 293 sudden cardiac death subjects (38.4% of the total cohort), of whom 185 (63.1%) died from intracoronary thrombosis, 106 (36.2%) from severe CAD without acute thrombosis, and 2 (0.7%%) from spontaneous coronary artery dissections. More specifically, intracoronary thrombosis was caused by plaque rupture in 124 subjects (67.0 % of those with coronary thrombosis), by plaque erosion in 57 subjects (30.8% of those with coronary thrombosis), and by eruptive calcified nodules in 4 subjects (2.1%).
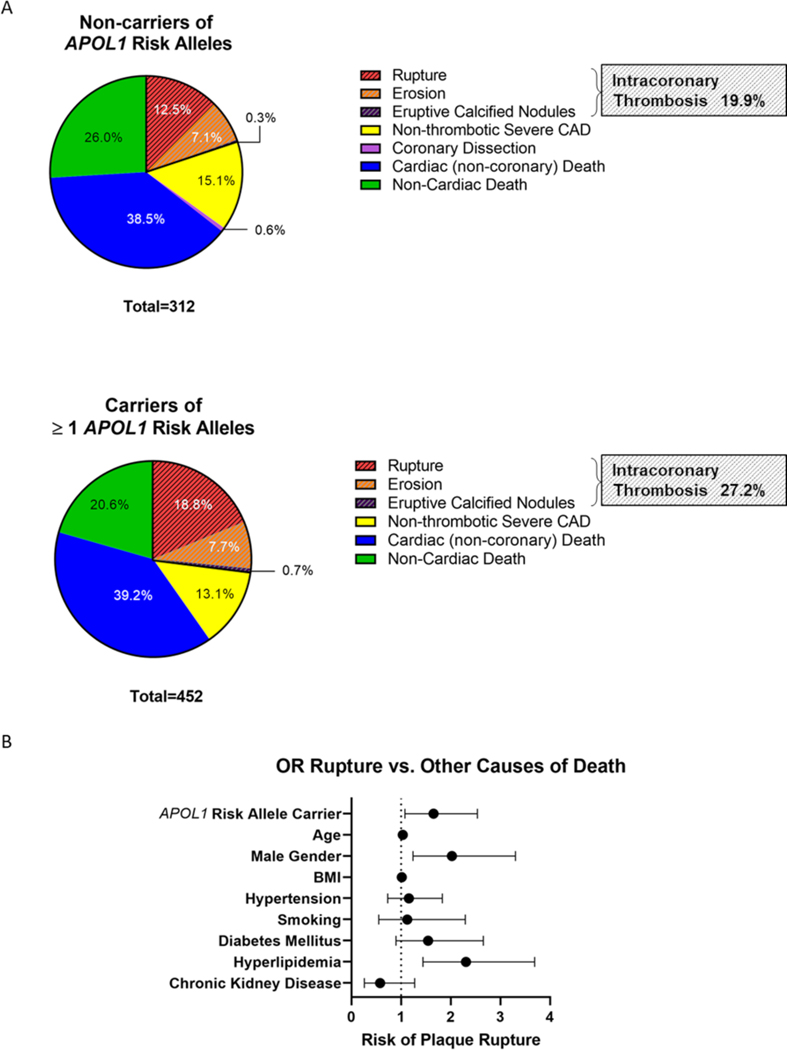
Among 590 subjects who died from sudden cardiac death, there was a higher proportion of carriers of at least one APOL1 risk allele versus the 174 subjects with non-cardiac death, although this association was not statistically significant (359 carriers out of 590 subjects (60.9%) with sudden cardiac death versus 93 carriers out of 174 subjects (53.5%) with non-cardiac death; p=0.081; Table 1). This was mostly driven by an increased likelihood of intracoronary thrombosis among carriers compared with non-carriers of APOL1 risk alleles (123 out of 452 carriers [27.2%] vs. 62 out of 312 non-carriers [19.9%]; p=0.0199, Figure 2A; Table 1). When further specifying the causes of intracoronary thrombosis on a histopathology level, we found a significantly higher proportion of subjects who died from plaque rupture among carriers than among non-carriers (85 out of 452 carriers [18.8%] vs. 39 out of 312 non-carriers [12.5%]; p=0.0202, Figure 2A, Table 1), whereas there was no difference in the proportions of subjects who died from plaque erosion (35 out of 452 carriers [7.7%] vs. 22 out of 312 non-carriers [7.1%]; p=0.7205) or eruptive calcified nodules (3 out of 452 carriers [0.7%] vs. 1 out of 312 non-carriers [0.3%]; p=0.5182) (Table 1). Of note, we found a stepwise increase in the proportion of subjects with rupture among carriers of zero, one, and two APOL1 risk alleles (12.5% vs. 17.6% vs. 22.9%; p=0.0295; Supplemental Table II). Considering that patients with ESKD on dialysis have an increased risk of cardiovascular events and sudden cardiac death (32), we compared causes of death in dialysis patients versus non-dialysis patients. In line with previous reports (33, 34), dialysis patients were more likely to die from eruptive calcified nodules, while there were no differences in plaque rupture or plaque erosion (Supplemental Table IV). Overall, sudden coronary death was not different between dialysis and non-dialysis subjects.
Figure 2. APOL1 Risk Allele Carrier Status and Cause of Death.
A) Intracoronary thrombosis was evident in 19.9% of carriers of the reference alleles, compared to 27.2% of carriers of at least one APOL1 risk allele (p=0.0199). This difference was mostly driven by an increased likelihood of plaque rupture in carriers versus non-carriers of APOL1 risk variants (18.8% vs. 12.5%, p=0.0202). B) APOL1 risk allele carrier status significantly increased the risk for plaque rupture compared with any other causes of death (OR 1.655, 95% CI 1.079–2.539; adjusted p=0.021) in a logistic regression analysis with adjustment for age, sex, BMI, hypertension, smoking, diabetes mellitus, hyperlipidemia, and CKD.
Interestingly, there were no differences in the degree of atherosclerosis, percentage of cross-sectional narrowing in left anterior descending (LAD), left circumflex artery (LCA), and right coronary artery (RCA), and no differences in the number of diseased coronary arteries among the three haplotypes (Supplemental Table II).
We performed logistic regression analyses to further investigate the association between carrier status of the APOL1 risk alleles and plaque rupture (Figure 2B). Each model contained nine independent variables (age, sex, BMI, hypertension, smoking, diabetes mellitus, hyperlipidemia, CKD, and APOL1 risk allele carrier status). Carriers of at least one APOL1 risk allele were more likely to die from plaque rupture compared to carriers of the reference allele (OR 1.655, 95% CI 1.079–2.539; adjusted p=0.021; Figure 2B, Table 2). To explore the risk per copy variant number, the analyses were repeated using an additive model. With every additional copy of risk alleles, the risk for plaque rupture increased by a factor of 1.4 (OR 1.436, 95% CI 1.078–1.913; adjusted p=0.014) in a logistic regression analysis with adjustment for the above-mentioned covariates.
Table 2.
Characteristics of subjects with plaque rupture compared to subjects who died from other causes
| Variables | Rupture n=124 | Control (Death from other causes) n=640 | p-value (unadjusted) | p-value (adjusted) | Odds Ratio Plaque Rupture | 95% C.I. | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower | Upper | ||||||
| Age (mean ± SD) | 52.02 ± 11.61 | 46.23 ± 12.75 | <0.0001 | <0.0001 | 1.035 | 1.019 | 1.052 |
| Male Sex | 99 (79.8%) | 425 (66.4%) | 0.003 | 0.005 | 2.025 | 1.240 | 3.306 |
| BMI, kg/m2 (mean ± SD) | 30.33 ± 7.06 | 29.97 ± 8.59 | 0.662 | 0.239 | 1.015 | 0.990 | 1.040 |
| Hypertension | 88 (71.0%) | 400 (62.5%) | 0.072 | 0.532 | 1.158 | 0.731 | 1.833 |
| Smoking | 11 (8.9%) | 54 (8.4%) | 0.874 | 0.744 | 1.126 | 0.552 | 2.296 |
| Diabetes Mellitus | 26 (21.0%) | 91 (14.2%) | 0.056 | 0.116 | 1.545 | 0.898 | 2.658 |
| Hyperlipidemia | 36 (29.0%) | 84 (13.1%) | <0.0001 | <0.0001 | 2.308 | 1.443 | 3.690 |
| CKD | 9 (7.3%) | 51 (8.0%) | 0.788 | 0.174 | 0.579 | 0.263 | 1.274 |
| APOL1 Risk Allele Carrier | 85 (68.5%) | 367 (57.3%) | 0.020 | 0.021 | 1.655 | 1.079 | 2.539 |
Necrotic core size was larger in carriers of APOL1 risk variants
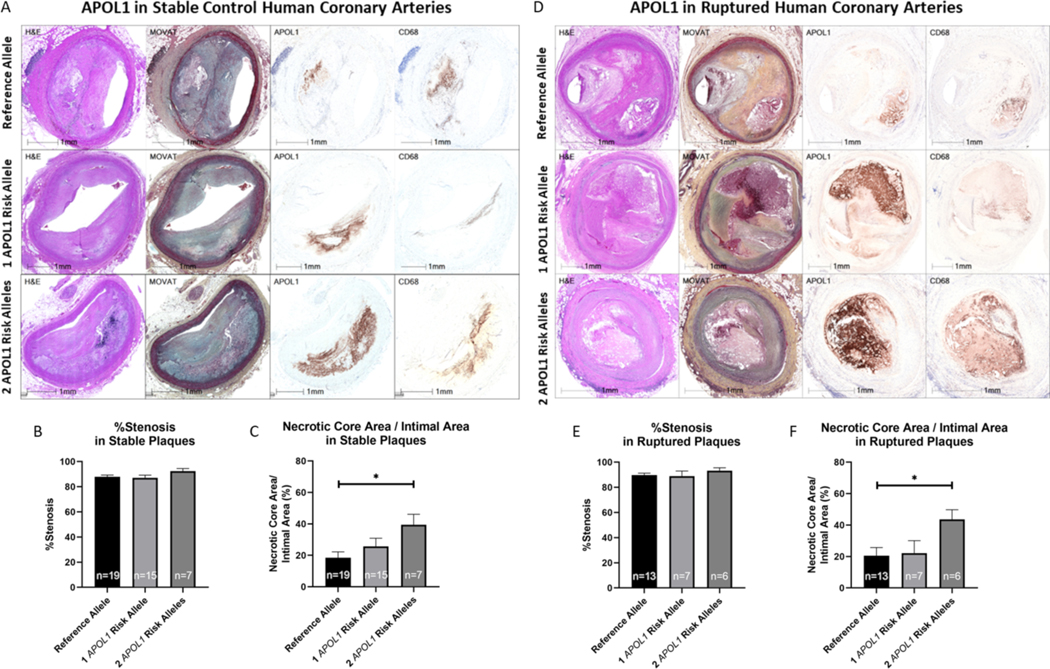
Next, we sought to identify key factors that may have impacted the increased risk of plaque rupture in carriers of APOL1 risk alleles. Several plaque characteristics have been associated with plaque vulnerability, i.e., with a higher risk for plaque rupture. Besides plaque burden, necrotic core size is an important determinant of plaque stability (35). Thus, we assessed within stable plaque types the percentage of cross-sectional luminal narrowing and necrotic core size in 41 arterial sections with ≥75% luminal narrowing from 19 carriers of only the reference allele and 23 carriers of at least one APOL1 risk allele (15 carriers of one and 7 carriers of two APOL1 risk alleles), matched for age and sex, who were randomly selected from the total study cohort (Figure 3A). While the percentage of cross-sectional luminal narrowing was not different in carriers of the reference allele and carriers of one or two APOL1 risk alleles (Figure 3B), the size of the necrotic core was significantly greater in stable plaques of carriers of two APOL1 risk alleles compared with carriers of only the reference allele (39.46% ± 6.64% vs. 18.44% ± 3.79% of the plaque; p=0.0335; Figure 3C). Likewise, in 26 ruptured plaques with similar percentage of cross-sectional luminal narrowing from 13 carriers of the reference allele and 13 carriers of APOL1 risk alleles (7 carriers of one and 6 carriers of two APOL1 risk alleles) (Figure 3D, E), the relative necrotic core size was significantly greater in carriers of two APOL1 risk alleles than in carriers of only the reference allele (43.56% ± 6.19% vs. 20.57% ± 5.11%; p=0.0484; Figure 3F).
Figure 3: Histopathology and Immunostaining of Stable and Ruptured Human Coronary Arteries from Carriers and Non-Carriers of APOL1 Risk Alleles.
Representative images of H&E and Movat staining, as well as immunohistochemistry for APOL1 and CD68 (A, D) are shown in stable (A) and ruptured (D) coronary plaques. No differences were observed in percentage cross-sectional stenosis in 41 stable plaques from 19 carriers of the reference allele, 15 carriers of one APOL1 risk allele and 7 carriers of two APOL1 risk alleles (B) and in 26 ruptured coronary plaques from 13 carriers of the reference allele, 7 carriers of one APOL1 risk allele and 6 carriers of two APOL1 risk alleles (E). Carriers of two APOL1 risk alleles had significantly larger necrotic cores compared with carriers of the reference allele, both in stable (C) and in ruptured coronary artery plaques (F).
APOL1 accumulated in coronary plaques of risk allele carriers
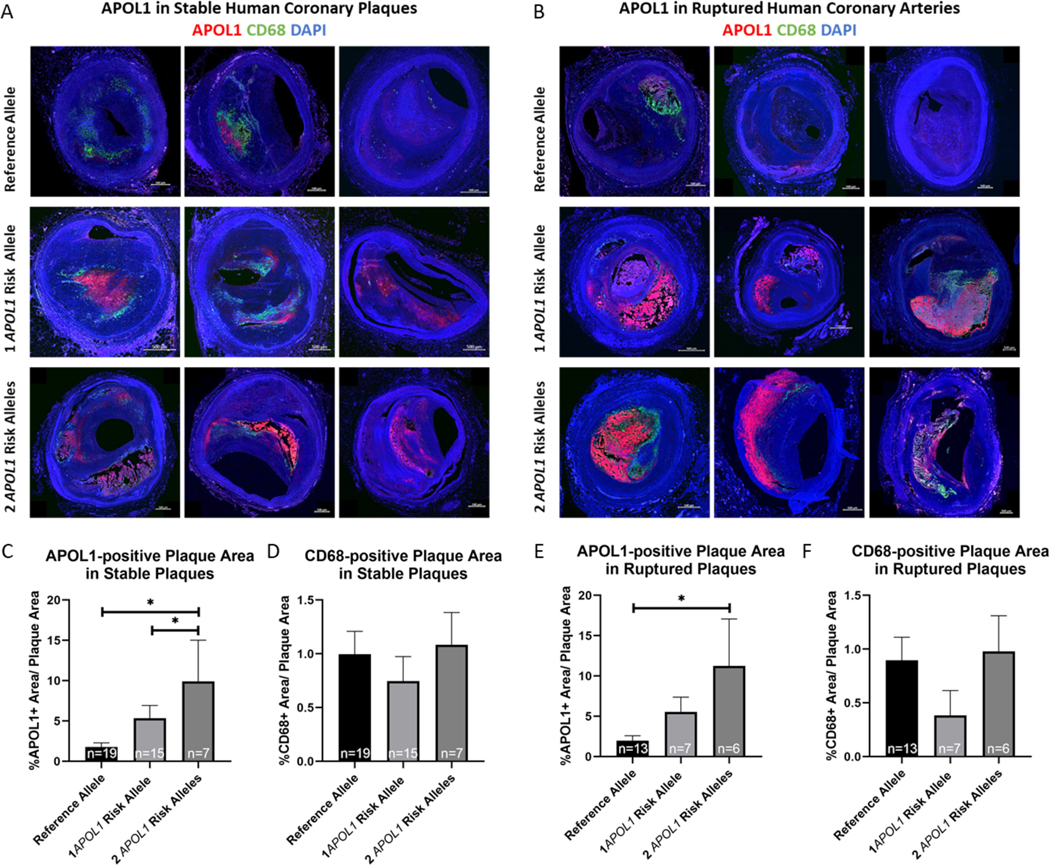
APOL1 is expressed in various cell types within many organs including the vasculature, kidney, lung, pancreas, and others (12, 36, 37). APOL1 protein is associated with the HDL particle, so it may play a role in reverse cholesterol transport as suggested by a study in APOL1-G1 and APOL1-G2 transgenic mice (25). Thus, we performed immunohistochemical and immunofluorescent staining for APOL1 protein to determine associations between APOL1 content of the plaque and carrier status in ruptured and stable plaques (Figure 3A, D, Figure 4A, B) as mentioned above. APOL1-positive staining was primarily located in the necrotic core of plaques. Carriers of two APOL1 risk alleles had a greater APOL1-positive plaque area than non-carriers in both non-thrombotic CAD plaques (9.92% ± 5.10% vs. 1.79% ± 0.51%; p=0.013; Figure 4C) and in ruptured plaques (11.25% ± 5.83% vs. 1.98% ± 0.61%; p=0.027; Figure 4D). In addition, APOL1-positive plaque area was larger in carriers of one APOL1 risk allele than in carriers of the reference allele in stable CAD plaques (5.36% ± 1.57% vs. 1.79% ± 0.51%; p=0.033; Figure 4C). We performed immunofluorescent staining against apolipoprotein A1 (APOA1), which is the primary protein associated with the HDL particle, in a subset of 14 stable plaques and 16 ruptured coronary plaques (Supplemental Figure II). However, we observed no difference in APOA1-positive staining among the three haplotypes.
Figure 4: Confocal Microscopy Images of Stable and Ruptured Human Coronary Arteries from Carriers and Non-Carriers of APOL1 Risk Alleles.
Representative confocal microscopy images with staining against APOL1 (red) CD68 (green), and DAPI (blue) in stable (A) and in ruptured (B) coronary plaques. APOL1-positive areas were larger in carriers of two APOL1 risk alleles than in non-carriers of APOL1 risk alleles in stable coronary artery plaques (including 19 carriers of the reference allele, 15 carriers of one APOL1 risk allele and 7 carriers of two APOL1 risk allele) (C) and in ruptured plaques (including 13 carriers of the reference allele, 7 carriers of one APOL1 risk allele and 6 carriers of two APOL1 risk alleles) (E). There was no difference in macrophage inflammation, as assessed by staining against CD68 in stable (D) and in ruptured coronary plaques (F).
Another important determinant of plaque vulnerability is macrophage infiltration and apoptosis (38). We assessed the presence of macrophages by immunohistochemical and immunofluorescent staining against the macrophage marker CD68 (Figure 3A, D, Figure 4A, B). We found no difference in CD68-positive plaque area between carriers of the reference allele and carriers of one or two APOL1 risk alleles, either in non-thrombotic CAD plaques (1.00% ± 0.21% vs. 0.75% ± 0.88% vs. 1.08% ± 0.79%; p=0.405; Figure 4E), or in ruptured plaques (0.90% ± 0.21% vs. 0.38% ± 0.23% vs. 0.98% ± 0.33%; p=0.189; Figure 4F). To investigate whether APOL1 risk alleles might drive apoptosis in coronary plaques, we performed immunofluorescent staining against cleaved caspase 3, an executioner caspase in apoptosis, as well as TUNEL staining in a subset of stable (5 reference allele, 5 one risk allele, and 4 two risk allele carriers) and ruptured coronary plaques (6 reference allele, 5 one risk allele, and 5 two risk allele carriers) (Supplemental Figure III). Cleaved caspase 3-positive staining co-localized with CD68 and was found primarily around the necrotic core, supporting the concept of apoptotic macrophages driving enlargement of the necrotic core. There were, however, no differences in the amount of cleaved caspase 3-positivity between carriers of the reference allele and carriers of one or two APOL1 risk alleles. Likewise, there was no difference in TUNEL staining among the three haplotypes.
No association between eQTL rs136164 genotypes and cause of death
Rs136164 is a known expression quantitative trait locus (eQTL) for APOL1 in arteries, explaining a fraction of the genetic variance of the gene expression phenotype. We assessed the frequencies of the reference allele versus the alternative allele of rs136164 in a subset of 375 subjects within our cohort (Supplemental Table V). The distribution of APOL1 risk alleles was similar in homozygous carriers of the rs136164 reference allele, heterozygous subjects, and homozygous carriers of the rs136164 alternative allele. However, the causes of death were not different in carriers of the reference allele and in carriers of the alternative allele, suggesting that rs136164 and indirectly the abundance of intracellular APOL1 expression appears to not have a strong impact on atherosclerotic outcomes.
Discussion
This is the first autopsy study investigating in detail associations between APOL1 risk variants and cardiovascular outcome in African American subjects. Carriers of at least one APOL1 risk allele had an increased risk of intracoronary thrombosis, driven by plaque rupture, compared with carriers of only the reference allele. This was true in both the unadjusted analysis and in the adjusted logistic regression model, taking age, sex, BMI, hypertension, smoking, diabetes mellitus, and hyperlipidemia into account. Importantly, we observed a gene-dose dependent effect of APOL1 risk variants for the increased risk of plaque rupture. Further, both ruptured and non-ruptured plaques of carriers of two APOL1 risk variants had larger necrotic cores compared with carriers of the reference allele, along with larger APOL1-positive plaque areas in carriers of at least one APOL1 risk allele. APOL1 risk variants might directly trigger plaque vulnerability by accumulating APOL1 protein in the plaque, suggesting that APOL1 risk variants might have an effect on lipid metabolism in addition to their effect on the kidneys, mediating an increased risk of plaque rupture.
Large studies investigating associations between APOL1 risk variants and CVD have yielded conflicting results. Data from 1959 participants in the Jackson Heart Study Cohort and from 749 participants in the Women’s Health Initiative suggested an increased risk for cardiovascular events among carriers of two APOL1 risk alleles compared with carriers of the reference allele after controlling for traditional CVD risk factors and CKD (OR 2.17, p=0.0009 in the Jackson Heart Study and OR 1.98, p=0.008 in Women’s Health Initiative) (15), and findings from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study suggested an association between APOL1 high risk status and incident composite CVD, defined as stroke or CAD, in non-diabetic subjects (39). Data from the Million Veteran Program, including 30,903 African Americans, showed that subjects with two APOL1 risk variants had a slightly higher risk of developing CAD compared with carriers of the reference allele (HR, 1.11 [95% CI, 1.01–1.21], p=0.039) when only individuals were considered who did not have kidney disease at baseline (14). However, this association was lost when all individuals regardless of CKD at baseline were included and both CVD and renal outcomes were modeled (14). On the other hand, according to data from 2,571 African Americans enrolled in the Systolic Blood Pressure Intervention Trial (SPRINT), APOL1 risk variants were not significantly associated with prevalent CVD (16). Similarly, the American Study of Kidney Disease and Hypertension (AASK), which enrolled 693 subjects with prevalent kidney disease, did not detect a significant relationship between APOL1 and participant survival (5). Lastly, a recent meta-analysis with combined data from eight cohorts including 21,305 subjects failed to show associations between APOL1 risk variants and incident CVD or death independent of CKD (17). Importantly, some of the ongoing controversy regarding the role of APOL1 risk variants can be attributed to different definitions of cardiovascular outcome. This is especially true when heart failure is included in the composite endpoint, as this may be difficult to adjudicate in the setting of concomitant CKD, and so heart failure was not included in the present analysis.
We did not observe associations between APOL1 risk variants and the presence or severity of CAD in our cohort. Neither the degree of atherosclerosis, the mean cross-sectional luminal narrowing, nor the number of diseased coronary arteries were different in risk allele carriers and non-carriers, under both recessive and dominant models. However, carriers of the APOL1 risk variants had a higher likelihood of plaque rupture compared with non-carriers in our study in a gene-dose dependent manner. Of note, the unequivocal diagnosis of plaque rupture can only be made by histology, whereas clinical MI cases also comprise plaque erosions and eruptive calcified nodules; the prevalence of both pathologies were similar among APOL1 risk allele carriers and non-carriers in the present study, under various genetic models. Given the CVPath registry population consists of cases of suspected sudden cardiac death referred from the Maryland Office of the Chief Medical Examiner, the proportion of subjects with abundant risk factors is high and therefore it may not be surprising that plaque types were overall not different between carriers and non-carriers.
In the present study, 13% of subjects had two copies of G1 or G2 risk variants, which is in line with the 12–13% frequencies observed in unselected African American populations (4). In contrast to previous findings, for example from SPRINT (16) and AASK (5), we did not observe an association between APOL1 risk allele carrier status and history of kidney disease but the proportion of subjects chronic on dialysis was significantly higher in carriers of two APOL1 risk alleles, supporting the association between APOL1 risk alleles and ESRD (4–6). However, with only 7.9% of subjects manifesting kidney disease, the overall frequency of CKD was low in our cohort, as opposed to 35.2% of SPRINT subjects having CKD (16) and CKD being an inclusion criterion in AASK (5). The low frequency of CKD in our cohort might be related to a significantly younger age (mean age of 47.18 ± 12.75 years) as opposed to a mean age of 63.9 years in SPRINT(16). Moreover, both SPRINT and AASK were also highly enriched for subjects with hypertension, which has been highly associated with an increased risk for CKD, whereas only 63.9% of subjects in our study cohort had history of hypertension. Of note, only homozygous or compound heterozygous haplotypes have been associated with an increased risk of nephropathy (with the rare exception of HIV nephropathy in South Africa (40), where a single copy of the G1 allele increases risk). Thus, an additional stress factor (“second hit”) seems to be a necessary trigger (7, 41). Nevertheless, our results suggest APOL1 risk alleles have an effect on CAD that is independent of the presence of CKD.
The inverse correlation between plasma HDL cholesterol levels and the likelihood of developing coronary artery disease is well known. Some studies have shown associations between APOL1 risk alleles and circulating total cholesterol, HDL, and LDL levels (14, 42), while other studies did not find significant differences in total cholesterol, HDL, VLDL or LDL concentrations by APOL1 haplotypes (18, 19, 31). APOL1 risk variants have been associated with lower plasma concentrations of medium-sized HDL (43), which is thought to be the form most protective against CVD (44, 45), although the causality of the association between HDL and CVD is still uncertain (46). Apolipoprotein A1 (APOA1) is the primary component of HDL particles and plays a central role in reverse cholesterol transport, involving efflux of free cholesterol from macrophages or other cells to APOA1 and HDL (47). APOA1 serum levels were similar regardless of APOL1 genotype in a study by Kozlitina et al. (11). Unfortunately, we were not able to perform an extensive analysis of lipoprotein subclasses due to the limited amount of serum that was available from these autopsy cases. Nevertheless, we found no differences in HDL and APOA1 serum levels between carriers of APOL1 risk alleles and the reference allele in a selected subset of our study cohort. In addition, the amount of APOA1 within stable and ruptured coronary plaques did not differ among the three APOL1 haplotypes.
We found no differences in the causes of death in a subset of 375 subjects within our cohort by genotypes for rs136164, an eQTL for APOL1 in arteries. This data suggests that a trait that alters the abundance of APOL1 expression does not alone explain why plaques become more unstable and likely to rupture in APOL1 risk allele carriers. While rs136164 has been associated with focal segmental glomerulosclerosis and HIV-associated nephropathy (48), to the best of our knowledge it has not been associated with cardiovascular diseases.
It has been shown that plasma levels of circulating APOL1 do not differ significantly according to the number of APOL1 risk alleles (49). Likewise, APOL1 genotypes did not alter APOL1 expression levels in a global gene expression analysis in human primary renal tubule cell lines (50). Interestingly, endogenous APOL1 expression was even higher in HeLa cells carrying the reference allele compared with cells carrying the G1 and G2 risk variants, but G0 cells still showed lower lipid droplet content when compared to G1 and G2 cells (25). These data suggest APOL1 risk variants impact the function rather than the amount of apolipoprotein L1. Thus, the increased amount of APOL1 protein in carriers of two APOL1 risk variants in our study likely represents extracellular APOL1 accumulated within the plaque, perhaps because of an impaired cholesterol efflux from macrophages.
As a component of HDL, APOL1 has been suggested to play a role in reverse cholesterol transport. Ryu et al. showed in a transgenic mice study that APOL1 risk variants are associated with increased intracellular cholesterol content, with repression of the cholesterol transporters ABCA1 and ABCG1, and with decreased reverse cholesterol transport via suppressed ATP-binding cassette transporter gene expression (25). Therefore, APOL1 risk variants might promote foam cell formation, driving the formation and growth of a necrotic core and decreasing plaque stability. Indeed, subjects with two APOL1 risk alleles had significantly larger necrotic cores, along with greater plaque areas enriched for APOL1 in our study. An important driver for enlargement of the necrotic core is macrophage apoptosis. However, we found no differences in apoptosis by APOL1 haplotype. In line with our results, in vitro experiments by Opeyemi et al. showed that expression of G1 or G2 APOL1 does not increase apoptosis in T-Rex-293 cells (51). Likewise, there was no increased expression of cleaved caspase 3 in mice with podocyte specific APOL1 risk allele expression (52). While there is mounting evidence that APOL1 risk variants drive cytotoxic effects (53–55), these data suggest apoptosis is not the primary mechanism induced by the risk variants.
APOL1 is part of the trypanolytic machinery. Its cytotoxicity is thought to result from cationic pore formation at the plasma membrane which is initiated at acidic pH and increases at more neutral conditions, eventually leading to trypanolysis (56). A similar mechanism is thought to occur in mammalian cells (57). APOL1 expression is strongly induced by proinflammatory agents, including lipopolysaccharide, Toll-like receptor agonists, type I interferon, tumor necrosis factor, and other cytokines (58, 59). While overexpression of APOL1 in cells is generally cytotoxic, increased cytotoxicity has been reported with APOL1 risk variants (51, 52, 54, 60–62). However, the fundamental differences between APOL1 genotypes at the molecular level remain incompletely understood. One hypothesis is that there are endogenous proteins that bind and restrict APOL1 toxicity, supported by the fact that the risk alleles confer differential binding to the APOL1-inhibiting serum resistance antigen in trypanosomes (57). On the other hand, APOL1 risk alleles have been shown to induce cell death through mitochondrial translocation and opening of the inner mitochondrial membrane permeability transition pore (63) as well as by mitochondrial fission (50). Apolipoprotein L3 (APOL3) is another member of the apolipoprotein L family that is able to kill trypanosomes. A recent study by Uzureau et al. showed that truncation of the C-terminal helix of APOL1 (APOL1Δ) or deletion of APOL3 induces reorganization of actomyosin activity in podocytes, resulting in a reduced cell size and adherence, and an increase of cellular mobility through reduced Golgi phosphatidylinositol-4-phosphate [PI(4)P] synthesis, suggesting inactivation of the Golgi phosphatidylinositol 4-kinase PI4KB (64). Concomitantly, increased membrane fission of mitochondria, Golgi apparatus, and ER tubules was observed. Importantly, reduction of PI(4)P synthesis was also observed in podocytes and kidney glomeruli from carriers of APOL1 risk variants. Thus, the authors of the study concluded from these findings that APOL1 risk variants might cause an APOL1Δ-like phenotype. Considering that PI(4)P is not only expressed in podocytes, this mechanism might affect other cell types with intense intracellular membrane trafficking. Further research is warranted to study the effects in cells of cardiovascular origin.
The present study has several limitations. First, as this was an autopsy study, we were unable to include or adjust for clinical markers, such as systolic and diastolic blood pressure, estimate glomerular filtration rate, albuminuria, and the subjects’ medication, as we did not have such information for all subjects. Second, the frequency of some cardiovascular risk factors, especially of smoking status and hyperlipidemia, might be underestimated, due to a lack of reporting in the autopsy reports. Third, the sample size in our study was modest. Nevertheless, this is, to our knowledge, the first autopsy study investigating associations between APOL1 risk allele carrier status and coronary outcome on a histopathology level, which gains its strength by the ability to report coronary pathologies in a precise manner.
Conclusion
APOL1 risk variants were associated with an increased risk of dying from coronary thrombosis, driven by an increased risk of plaque rupture. We found greater accumulation of APOL1 protein within the plaque. Our results suggest APOL1 risk allele carrier status is an independent risk factor for the development of unstable CAD perhaps through effects on necrotic core development which increases risk of dying from plaque rupture, but this will require experimental confirmation.
Supplementary Material
Highlights.
APOL1 risk allele carrier status was associated with a significantly increased risk of coronary thrombosis due to plaque rupture in a gene-dose dependent manner.
Carriers of APOL1 risk alleles had larger necrotic cores compared with non-carriers, along with pronounced accumulation of apolipoprotein 1 within culprit plaques, suggesting APOL1 risk allele carrier status is associated with plaque vulnerability.
In addition to their effect on the kidneys, APOL1 risk variants might have an effect on lipid metabolism, mediating an increased risk of plaque rupture.
a). Acknowledgements
We would like to thank Ms. Wei Han and Mrs. Lila Adams for their assistance in immunohistochemistry and immunofluorescence staining.
b) Sources of Funding
This study is supported by Merck Inc. and CVPath Institute Research Fund.
c) Disclosure
R.V. and A.V.F. have received institutional research support from NIH (HL141425), Leducq Foundation Grant; AWS COVID-19 Diagnostic Development Initiative Grant; 480 Biomedical; 4C Medical; 4Tech; Abbott; Accumedical; Amgen; Biosensors; Boston Scientific; Cardiac Implants; Celonova; Claret Medical; Concept Medical; Cook; CSI; DuNing, Inc; Edwards LifeSciences; Emboline; Endotronix; Envision Scientific; Lutonix/Bard; Gateway; Lifetech; Limflo; MedAlliance; Medtronic; Mercator; Merill; Microport Medical; Microvention; Mitraalign; Mitra assist; NAMSA; Nanova; Neovasc; NIPRO; Novogate; Occulotech; OrbusNeich Medical; Phenox; Profusa; Protembis; Qool; Recor; Senseonics; Shockwave; Sinomed; Spectranetics; Surmodics; Symic; Vesper; W.L. Gore; Xeltis. A.V.F. has received honoraria from Abbott Vascular; Biosensors; Boston Scientific; Celonova; Cook Medical; CSI; Lutonix Bard; Sinomed; Terumo Corporation; and is a consultant to Amgen; Abbott Vascular; Boston Scientific; Celonova; Cook Medical; Lutonix Bard; Sinomed. R.V. has received honoraria from Abbott Vascular; Biosensors; Boston Scientific; Cook Medical; Cordis; CSI; Lutonix Bard; Medtronic; OrbusNeich Medical; CeloNova; SINO Medical Technology; ReCore; Terumo Corporation; W. L. Gore; Spectranetics; and is a consultant Abbott Vascular; Boston Scientific; Celonova; Cook Medical; Cordis; CSI; Edwards Lifescience; Lutonix Bard; Medtronic; OrbusNeich Medical; ReCore; Sinomededical Technology; Spectranetics; Surmodics; Terumo Corporation; W. L. Gore; Xeltis. L.G. is supported by NIH (HL141425) and Leducq Foundation Grant. P. S. D.V. was supported by American Heart Association grant number 18CDA34110116. TH, KZL, and JBK were supported by the NIDDK Intramural Research Program, under project Z01 DK43308. However, none of these entities provided financial support for this study. The other authors declare no competing interests.
Abbreviations
- APOL1
apolipoprotein L1
- ESKD
end-stage kidney disease
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- CAD
coronary artery disease
- MI
myocardial infarction
- EEL
external elastic lamina
- IEL
internal elastic lamina
- LAD
left anterior descending
- LCA
left circumflex artery
- RCA
right coronary artery
- APOA1
apolipoprotein A1
References
- 1.Kiberd BA, Clase CM. Cumulative risk for developing end-stage renal disease in the US population. J Am Soc Nephrol. 2002;13:1635–1644. [DOI] [PubMed] [Google Scholar]
- 2.Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14:2902–2907. [DOI] [PubMed] [Google Scholar]
- 3.Lipworth L, Mumma MT, Cavanaugh KL, Edwards TL, Ikizler TA, Tarone RE, McLaughlin JK, Blot WJ. Incidence and predictors of end stage renal disease among low-income blacks and whites. PLoS One. 2012;7:e48407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopp JB, Rosenberg AZ. One Actor, Many Roles: Histopathologies Associated With APOL1 Genetic Variants. Adv Anat Pathol. 2019;26:215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kronenberg F. Apolipoprotein L1 and apolipoprotein A-IV and their association with kidney function. Curr Opin Lipidol. 2017;28:39–45. [DOI] [PubMed] [Google Scholar]
- 8.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352. [DOI] [PubMed] [Google Scholar]
- 10.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. [DOI] [PubMed] [Google Scholar]
- 11.Kozlitina J, Zhou H, Brown PN, Rohm RJ, Pan Y, Ayanoglu G, Du X, Rimmer E, Reilly DF, Roddy TP, Cully DF, Vogt TF, Blom D, Hoek M. Plasma Levels of Risk-Variant APOL1 Do Not Associate with Renal Disease in a Population-Based Cohort. J Am Soc Nephrol. 2016;27:3204–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madhavan SM, O’Toole JF, Konieczkowski M, Ganesan S, Bruggeman LA, Sedor JR. APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol. 2011;22:2119–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson WS, Silva RA, Chantepie S, Lagor WR, Chapman MJ, Kontush A. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler Thromb Vasc Biol. 2009;29:870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bick AG, Akwo E, Robinson-Cohen C, et al. Association of APOL1 Risk Alleles With Cardiovascular Disease in Blacks in the Million Veteran Program. Circulation. 2019;140:1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito K, Bick AG, Flannick J, et al. Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circ Res. 2014;114:845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langefeld CD, Divers J, Pajewski NM, Hawfield AT, Reboussin DM, Bild DE, Kaysen GA, Kimmel PL, Raj DS, Ricardo AC, Wright JT Jr., , Sedor JR, Rocco MV, Freedman BI. Apolipoprotein L1 gene variants associate with prevalent kidney but not prevalent cardiovascular disease in the Systolic Blood Pressure Intervention Trial. Kidney Int. 2015;87:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grams ME, Surapaneni A, Ballew SH, et al. APOL1 Kidney Risk Variants and Cardiovascular Disease: An Individual Participant Data Meta-Analysis. J Am Soc Nephrol. 2019;30:2027–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen TK, Appel LJ, Grams ME, Tin A, Choi MJ, Lipkowitz MS, Winkler CA, Estrella MM. APOL1 Risk Variants and Cardiovascular Disease: Results From the AASK (African American Study of Kidney Disease and Hypertension). Arterioscler Thromb Vasc Biol. 2017;37:1765–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukamal KJ, Tremaglio J, Friedman DJ, Ix JH, Kuller LH, Tracy RP, Pollak MR. APOL1 Genotype, Kidney and Cardiovascular Disease, and Death in Older Adults. Arterioscler Thromb Vasc Biol. 2016;36:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies MJ. The pathophysiology of acute coronary syndromes. Heart. 2000;83:361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. [DOI] [PubMed] [Google Scholar]
- 22.Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists’ view. European heart journal. 2013;34:719–728. [DOI] [PubMed] [Google Scholar]
- 23.Clarke MC, Bennett MR. Cause or consequence: what does macrophage apoptosis do in atherosclerosis? : Am Heart Assoc; 2009. [DOI] [PubMed] [Google Scholar]
- 24.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryu JH, Ge M, Merscher S, Rosenberg AZ, Desante M, Roshanravan H, Okamoto K, Shin MK, Hoek M, Fornoni A, Kopp JB. APOL1 renal risk variants promote cholesterol accumulation in tissues and cultured macrophages from APOL1 transgenic mice. PLoS One. 2019;14:e0211559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughson MD, Hoy WE, Mott SA, Bertram JF, Winkler CA, Kopp JB. APOL1 Risk Variants Independently Associated With Early Cardiovascular Disease Death. Kidney Int Rep. 2018;3:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276–1282. [DOI] [PubMed] [Google Scholar]
- 28.Burke AP, Farb A, Pestaner J, Malcom GT, Zieske A, Kutys R, Smialek J, Virmani R. Traditional risk factors and the incidence of sudden coronary death with and without coronary thrombosis in blacks. Circulation. 2002;105:419–424. [DOI] [PubMed] [Google Scholar]
- 29.Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22:2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.David VA, Binns-Roemer EA, Winkler CA. Taqman Assay for Genotyping CKD-Associated APOL1 SNP rs60910145: A Cautionary Note. Kidney Int Rep. 2019;4:184–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freedman BI, Rocco MV, Bates JT, Chonchol M, Hawfield AT, Lash JP, Papademetriou V, Sedor JR, Servilla K, Kimmel PL, Wall BM, Pajewski NM. APOL1 renal-risk variants do not associate with incident cardiovascular disease or mortality in the Systolic Blood Pressure Intervention Trial. Kidney Int Rep. 2017;2:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 33.Chin CY, Matsumura M, Maehara A, et al. Coronary Plaque Characteristics in Hemodialysis-Dependent Patients as Assessed by Optical Coherence Tomography. Am J Cardiol. 2017;119:1313–1319. [DOI] [PubMed] [Google Scholar]
- 34.Torii S, Sato Y, Otsuka F, et al. Eruptive Calcified Nodules as a Potential Mechanism of Acute Coronary Thrombosis and Sudden Death. J Am Coll Cardiol. 2021;77:1599–1611. [DOI] [PubMed] [Google Scholar]
- 35.Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–1292. [DOI] [PubMed] [Google Scholar]
- 36.Duchateau PN, Pullinger CR, Orellana RE, Kunitake ST, Naya-Vigne J, O’Connor PM, Malloy MJ, Kane JP. Apolipoprotein L, a new human high density lipoprotein apolipoprotein expressed by the pancreas. Identification, cloning, characterization, and plasma distribution of apolipoprotein L. J Biol Chem. 1997;272:25576–25582. [DOI] [PubMed] [Google Scholar]
- 37.Duchateau PN, Pullinger CR, Cho MH, Eng C, Kane JP. Apolipoprotein L gene family: tissue-specific expression, splicing, promoter regions; discovery of a new gene. J Lipid Res. 2001;42:620–630. [PubMed] [Google Scholar]
- 38.Carr SC, Farb A, Pearce WH, Virmani R, Yao JS. Activated inflammatory cells are associated with plaque rupture in carotid artery stenosis. Surgery. 1997;122:757–763; discussion 763–754. [DOI] [PubMed] [Google Scholar]
- 39.Gutiérrez OM, Irvin MR, Chaudhary NS, et al. APOL1 Nephropathy Risk Variants and Incident Cardiovascular Disease Events in Community-Dwelling Black Adults. Circ Genom Precis Med. 2018;11:e002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasembeli AN, Duarte R, Ramsay M, Mosiane P, Dickens C, Dix-Peek T, Limou S, Sezgin E, Nelson GW, Fogo AB, Goetsch S, Kopp JB, Winkler CA, Naicker S. APOL1 Risk Variants Are Strongly Associated with HIV-Associated Nephropathy in Black South Africans. J Am Soc Nephrol. 2015;26:2882–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siemens TA, Riella MC, Moraes TP, Riella CV. APOL1 risk variants and kidney disease: what we know so far. J Bras Nefrol. 2018;40:388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duchateau PN, Movsesyan I, Yamashita S, Sakai N, Hirano K, Schoenhaus SA, O’Connor-Kearns PM, Spencer SJ, Jaffe RB, Redberg RF, Ishida BY, Matsuzawa Y, Kane JP, Malloy MJ. Plasma apolipoprotein L concentrations correlate with plasma triglycerides and cholesterol levels in normolipidemic, hyperlipidemic, and diabetic subjects. J Lipid Res. 2000;41:1231–1236. [PubMed] [Google Scholar]
- 43.Freedman BI, Langefeld CD, Murea M, Ma L, Otvos JD, Turner J, Antinozzi PA, Divers J, Hicks PJ, Bowden DW, Rocco MV, Parks JS. Apolipoprotein L1 nephropathy risk variants associate with HDL subfraction concentration in African Americans. Nephrol Dial Transplant. 2011;26:3805–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parish S, Offer A, Clarke R, Hopewell JC, Hill MR, Otvos JD, Armitage J, Collins R. Lipids and lipoproteins and risk of different vascular events in the MRC/BHF Heart Protection Study. Circulation. 2012;125:2469–2478. [DOI] [PubMed] [Google Scholar]
- 45.Akinkuolie AO, Paynter NP, Padmanabhan L, Mora S. High-density lipoprotein particle subclass heterogeneity and incident coronary heart disease. Circ Cardiovasc Qual Outcomes. 2014;7:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet. 2014;384:618–625. [DOI] [PubMed] [Google Scholar]
- 47.Sorci-Thomas MG, Thomas MJ. Why targeting HDL should work as a therapeutic tool, but has not. J Cardiovasc Pharmacol. 2013;62:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Limou S, Nelson GW, Lecordier L, An P, O’HUigin CS, David VA, Binns-Roemer EA, Guiblet WM, Oleksyk TK, Pays E, Kopp JB, Winkler CA. Sequencing rare and common APOL1 coding variants to determine kidney disease risk. Kidney Int. 2015;88:754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruggeman LA, O’Toole JF, Ross MD, Madhavan SM, Smurzynski M, Wu K, Bosch RJ, Gupta S, Pollak MR, Sedor JR, Kalayjian RC. Plasma apolipoprotein L1 levels do not correlate with CKD. J Am Soc Nephrol. 2014;25:634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma L, Ainsworth HC, Snipes JA, et al. APOL1 Kidney-Risk Variants Induce Mitochondrial Fission. Kidney Int Rep. 2020;5:891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olabisi OA, Zhang JY, VerPlank L, Zahler N, DiBartolo S, 3rd, Heneghan JF, Schlöndorff JS, Suh JH, Yan P, Alper SL, Friedman DJ, Pollak MR. APOL1 kidney disease risk variants cause cytotoxicity by depleting cellular potassium and inducing stress-activated protein kinases. Proc Natl Acad Sci U S A. 2016;113:830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beckerman P, Bi-Karchin J, Park AS, et al. Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nat Med. 2017;23:429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chun J, Zhang JY, Wilkins MS, Subramanian B, Riella C, Magraner JM, Alper SL, Friedman DJ, Pollak MR. Recruitment of APOL1 kidney disease risk variants to lipid droplets attenuates cell toxicity. Proc Natl Acad Sci U S A. 2019;116:3712–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lan X, Jhaveri A, Cheng K, Wen H, Saleem MA, Mathieson PW, Mikulak J, Aviram S, Malhotra A, Skorecki K, Singhal PC. APOL1 risk variants enhance podocyte necrosis through compromising lysosomal membrane permeability. Am J Physiol Renal Physiol. 2014;307:F326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Datta S, Kataria R, Zhang JY, Moore S, Petitpas K, Mohamed A, Zahler N, Pollak MR, Olabisi OA. Kidney Disease-Associated APOL1 Variants Have Dose-Dependent, Dominant Toxic Gain-of-Function. J Am Soc Nephrol. 2020;31:2083–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomson R, Finkelstein A. Human trypanolytic factor APOL1 forms pH-gated cation-selective channels in planar lipid bilayers: relevance to trypanosome lysis. Proc Natl Acad Sci U S A. 2015;112:2894–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friedman DJ, Pollak MR. APOL1 and Kidney Disease: From Genetics to Biology. Annu Rev Physiol. 2020;82:323–342. [DOI] [PubMed] [Google Scholar]
- 58.Zhaorigetu S, Wan G, Kaini R, Jiang Z, Hu CA. ApoL1, a BH3-only lipid-binding protein, induces autophagic cell death. Autophagy. 2008;4:1079–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nichols B, Jog P, Lee JH, Blackler D, Wilmot M, D’Agati V, Markowitz G, Kopp JB, Alper SL, Pollak MR, Friedman DJ. Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int. 2015;87:332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lan X, Wen H, Saleem MA, Mikulak J, Malhotra A, Skorecki K, Singhal PC. Vascular smooth muscle cells contribute to APOL1-induced podocyte injury in HIV milieu. Exp Mol Pathol. 2015;98:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng D, Weckerle A, Yu Y, Ma L, Zhu X, Murea M, Freedman BI, Parks JS, Shelness GS. Biogenesis and cytotoxicity of APOL1 renal risk variant proteins in hepatocytes and hepatoma cells. J Lipid Res. 2015;56:1583–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wen H, Kumar V, Lan X, Shoshtari SSM, Eng JM, Zhou X, Wang F, Wang H, Skorecki K, Xing G, Wu G, Luo H, Malhotra A, Singhal PC. APOL1 risk variants cause podocytes injury through enhancing endoplasmic reticulum stress. Biosci Rep. 2018;38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shah SS, Lannon H, Dias L, Zhang JY, Alper SL, Pollak MR, Friedman DJ. APOL1 Kidney Risk Variants Induce Cell Death via Mitochondrial Translocation and Opening of the Mitochondrial Permeability Transition Pore. J Am Soc Nephrol. 2019;30:2355–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uzureau S, Lecordier L, Uzureau P, et al. APOL1 C-Terminal Variants May Trigger Kidney Disease through Interference with APOL3 Control of Actomyosin. Cell Rep. 2020;30:3821–3836.e3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.