Abstract
HLA class-I antigen processing machinery (APM) plays a crucial role in the synthesis and expression of HLA class-I-tumor antigen-derived peptide complexes; the latter mediate the recognition and elimination of malignant cells by cognate T cells. Defects in HLA class-I APM component expression and/or function are frequently found in cancer cells, providing them with an immune escape mechanism which has relevance in the clinical course of the disease and in the response to T cell-based immunotherapy. The majority of HLA class-I APM defects (>75%) are caused by epigenetic mechanisms or dysregulated signaling and therefore can be corrected by strategies which counteract the underlying mechanisms. Their application in oncology is likely to improve responses to T cell-based immunotherapies, including checkpoint inhibition.
Keywords: Antigen processing machinery, Epigenetics, HLA class-I, Immune escape, Immunosurveillance, T cell recognition
HLA class-I APM component defects in cancer
The resistance to checkpoint inhibitor-based immunotherapy experienced by a high percentage of patients withsolid tumors has rekindled interest in the characterization of the defects in HLA class-I APM component expression and/or function frequently found in cancer cells. Recognition of cancer cells by cognate T cells is mediated by the transmembrane HLA class-I trimolecular complex, which consists of a three-domain heavy (α) chain, a β2-microglobulin (β2M) light chain and a loaded tumor antigen (TA)-derived peptide. These complexes are synthesized by HLA class-I APM, which generates 8–12 amino acid long peptides from TAs and loads them on β2M-associated HLA class-I heavy chain heterodimers. The resulting trimolecular complexes are transported to the cell membrane for presentation to cognate T cells (Figure 1).
Figure 1: Function of HLA Class-I Antigen Processing Machinery.

Ubiquitinated proteins are degraded by proteasome catalytic subunits: β1 (Delta), β2 (Zeta) and β5 (MB1), or cytokine-inducible immunoproteasome catalytic subunits: LMP2, LMP10, and LMP7. The generated peptides enter the ER via the heterodimeric transporter associated with antigen processing complex (TAP1/TAP2). Often following length refinement by ER aminopeptidases (ERAP1, ERAP2), peptides are loaded onto β2M-associated HLA class-I heavy chain with assistance of chaperones tapasin, ERp57, and calreticulin. The resulting β2M-HLA class-I heavy chain-peptide trimers travel to the cell surface; their expression is regulated through clathrin-independent endocytosis. Mutations/deletions of any HLA class-I APM component may impair the synthesis/expression of β2M-HLA class-I heavy chain-peptide trimolecular complexes on the cell surface.
Typically, endogenous proteins are ubiquitinated and subsequently degraded by multi-subunit proteasome complexes in the cytosol. The constitutive proteasome catalytic subunits include β1, β2 and β5. Alternative catalytic subunits comprise the immunoproteasome, namely, low molecular weight protein 2 (LMP2), LMP10 and LMP7 (also known as β1i, β2i/MECL1, and β5i, respectively) to produce peptides with a hydrophobic C terminus (1). The immunoproteasome, which is induced under cell stress and inflammation, primarily through interferon-γ (IFNγ) stimulation, is thought to perform its proteolytic function more efficiently than the proteasome; whether the immunoproteasome plays a more significant role than the proteasome in the cleavage of TAs is unclear at present (1).
Proteasomal degradation products are shuttled by the heterodimeric transporter associated with antigen processing (TAP) complex into the lumen of the endoplasmic reticulum (ER). Peptide lengths are refined by ER aminopeptidase 1 (ERAP1) and ERAP2 (2). Furthermore, newly synthesized HLA class-I heavy chains are stabilized by calnexin in the ER and then associate with β2M. Peptides with the correct length and sequence for HLA class-I allele binding are loaded onto β2M-associated HLA class-I heavy chain dimers with the help of the peptide loading complex (PLC). The latter consists of TAP, oxidoreductase ER resident protein 57 (ERp57) and chaperone molecules calreticulin and tapasin (3). The resulting trimers then travel to the plasma membrane via the Golgi apparatus and are presented to cognate T cells.
HLA class-I trimolecular complexes are removed or recycled from the cell membrane via clathrin-independent endocytosis. These complexes are then internalized by Arf6-GTP-enriched vesicles and reach EEA1+/Rab5+ sorting endosomes (4). Here, they are recycled either quickly to the membrane through the Rab35 regulated early endosomal recycling pathway or moved to the Rab11+ endocytic recycling compartment for slow recycling. Rab22 mediates the formation of tubular recycling endosomes from the endocytic recycling compartment, which undergo Arf6-dependent fusion with the plasma membrane (5). Sub-optimally loaded HLA class-I molecules usually undergo degradation after reaching the sorting endosomes, but may also be recycled via the late endosomal recycling pathway (4).
Defects in HLA class-I APM component expression have been detected in most, if not all of the cancer types analyzed (6,7). HLA class-I heavy chain defects range between a minimum of 36% in urinary bladder cancer and a maximum of 80% in penile cancer, whilst β2M defects range between a minimum of 17% in bone and soft tissue cancer and a maximum of 73% in uveal melanoma (6). Brain cancers often are associated with defects in TAP1 (40%) and TAP2 (88%) (7). Defects in other APM components have been observed in a range of cancer types, however, their extent has been poorly characterized. These defects play a role in disease progression and have a negative impact on the clinical response to T cell-based immunotherapy (6,7).
Analysis of the mechanism(s) underlying these defects has mainly focused on structural mutations in HLA class-I APM genes, although they constitute a small proportion of total HLA class-I APM defects. Indeed, comparison between HLA class-I APM component mutation frequency and the frequency of protein-level downregulation revealed that mutations in HLA class-I APM components constitute at most 25% of defects (8). Nevertheless, HLA class-I APM downregulation by epigenetic mechanisms or dysregulated signaling, which appear to be the most frequent cause of HLA class-I APM defects, have been neglected.
Here, we review HLA class-I APM defects found in malignant cells. Then we describe their underlying mechanisms, emphasizing the major role played by epigenetic and signaling abnormalities. Finally, we discuss the potential impact of these defects on the clinical response to T cell-based immunotherapy as well as the available approaches to restore HLA class-I APM expression and/or function in malignant cells.
Structural mechanisms underlying HLA class-I APM component defects
Defects in HLA class-I alleles found in cancer pertain to loss of the gene products of one or two HLA class-I loci or of the HLA class-I allo-specificities encoded by the genes present in the MHC region of parental chromosome 6. In the absence of one or more HLA class-I allele(s), the diversity of peptides presented on cancer cells generally decreases. Mutations in β2M, as well as TAP1 and tapasin can result in total loss of HLA class-I expression, whilst structural defects in other HLA class-I APM genes generally cause HLA class-I downregulation (8). In view of the co-dominance of the two genes, one of paternal and the other one of maternal origin, encoding APM components as well as β2M, two events are required to cause lack of their expression. Typically, in the case of β2M, the total or partial loss of chromosome 15, which carries the β2M encoding gene, is followed by a structural mutation in the other β2M allele (9–11). In the case of APM, the loss of one component typically results from the combination of a cancer-unrelated germ-line mutation, which targets one encoding allele, with a cancer-related repression of the other allele. HLA class-I APM component mutation frequency is significantly higher in patients with high microsatellite instability (MSI-H) (12). At variance with tumor mutation burden, which is one of the determinants of immunotherapy response (13–16), structural defects in HLA class-I subunits are implicated in primary and acquired resistance to T cell-based immunotherapy. Indeed, homozygous deletions and mutations in B2M are associated with resistance to ICI-based therapy in advanced melanoma patients (17,18). Moreover, loss of heterozygosity of germline HLA class-I heavy chain at one or more loci in advanced cancer patients receiving ICIs is associated with poor overall survival (19). Likewise, knockout of B2m in mouse lung cancer cells confers resistance to anti-PD-1 mAb-based therapy in syngeneic, immunocompetent mouse tumor models (20).
Non-structural mechanisms underlying HLA class-I APM component defects
Non-structural defects are the most frequent cause of HLA class-I APM component downregulation in malignant cells. At least three-fourths of defects in HLA class-I heavy chain, β2M, and APM components are non-structural (8). Most defects occur transcriptionally or post-translationally, and often result in HLA class-I downregulation, which may lead to undetectable HLA class-I expression. These defects can often be corrected by cytokines and/or by demethylating agents, highlighting the role of epigenetic mechanisms in the regulation of HLA class-I APM component expression (Figure 2).
Figure 2: Mechanisms Regulating HLA Class-I Antigen Processing Machinery Component Expression in Cancer Cells.

HLA class-I APM components are involved in the synthesis and expression of HLA class-I trimolecular complexes. Their expression is regulated by transcription factors IRF1 and NF-κB, as well as enhanceosome transactivator NLRC5. Overexpression and/or activation of histone deacetylases, DNA methyltransferases, and polycomb repressive complex 2 (PRC2) enzymatic component EZH2 may reduce DNA accessibility of HLA class-I APM component promoter/enhancer regions, preventing transcription factors from binding. Activating mutations or amplifications in the receptor tyrosine kinase/MAPK signaling pathway may inactivate STAT1, downregulating IRF1, NLRC5, and HLA class-I APM component expression. MAPK activation may also inactivate STAT3, leading to HLA class-I APM component downregulation. Type I and type II interferons upregulate HLA class-I APM component expression through JAK/STAT1 activation. cGAS, a cytoplasmic DNA sensor that activates stimulator of interferon genes (STING) and downstream TBK1/IKKε, may also induce HLA class-I APM component transcription. IRF3/IRF7-dependent type I interferon secretion represents one of the underlying mechanisms. The other mechanism is canonical NF-κB activation. The latter is also induced by tumor necrosis factor α (TNFα) and Toll-like receptor (TLR)/interleukin-1 receptor (IL-1R) agonists. Non-canonical NF-κB activation mediated by binding of ligands to TNF receptor superfamily members can also induce HLA class-I APM component transcription. Post-translationally, autophagy receptor NBR1 mediates HLA class-I trimolecular complex degradation via the autophagy-lysosomal pathway, while secreted enzyme PCSK9 mediates degradation of HLA class-I trimolecular complexes via the endosomal-lysosomal pathway.
HLA class-I heavy chain expression is regulated at three major transcription factor binding sites: Enhancer A region, IFN-stimulated response element (ISRE) and SXY module. They are recognized by nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB), interferon regulatory factor 1 (IRF1) and HLA class-I enhanceosome, respectively. The latter consists of regulatory factor X (RFX) family transcription factors (RFX5, RFXAP, RFXANK), activating transcription factor (ATF)/cAMP responsive element binding protein (CREB) family transcription factors (ATF1, CREB1), nuclear factor Y (NFY) transcription factor complex (NFYA, NFYB, NFYC), and enhanceosome transactivator NLR family CARD domain containing 5 (NLRC5) (21,22). The distal promoter displays additional upstream stimulatory factor 1 (USF1), USF2, and specificity protein 1 (Sp1) binding sites in a locus and allele-specific manner (23). Notably, NF-κB enhancer A binding is also locus-specific, with two functional κB sites for HLA-A, one for HLA-B, and none for HLA-C (24). Many of these transcriptional regulators also promote β2M and APM component gene expression (25), along with several others, such as E2F1 in the case of tapasin (26) (Figure 3). In non-malignant cells, these proteins maintain HLA class-I heavy chain, β2M, and APM component expression at a basal level. However, malignant cells may display alterations that i) impair binding of these proteins to the promoter/enhancer region of HLA class-I APM genes, ii) downregulate the expression of these transcriptional regulators, or iii) accelerate post-translational HLA class-I trimolecular complex degradation. The relative frequency of these alterations varies by cancer type.
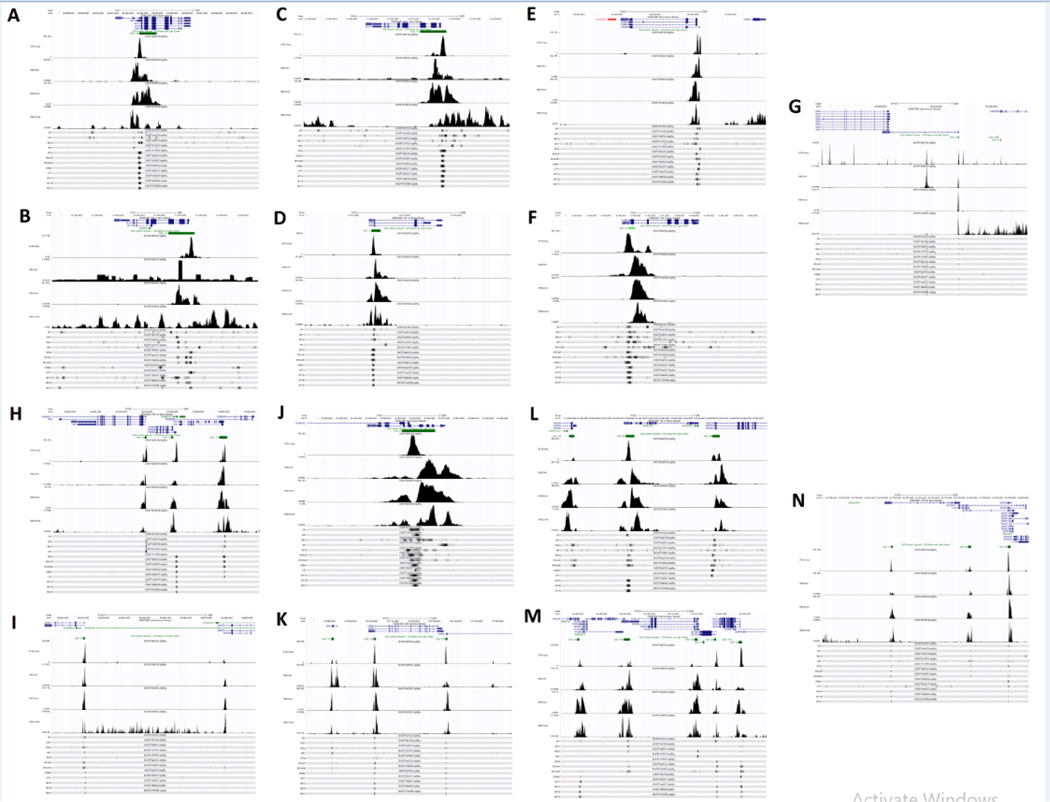
Figure 3: Transcriptional Regulation of HLA Class I APM Components.
Processed ChIP-sequencing and ATAC-sequencing data in the HepG2 hepatocellular carcinoma cell line published as part of the ENCODE project were used to generate tracks on the UCSC Genome Browser (accession ID annotated at the top of each track). GENCODE V36 was used to annotate transcripts and CpG islands were generated using the default method on the UCSC Genome Browser. ATAC-seq, H3K27 acetylation ChIP-seq, H3K4 trimethylation ChIP-seq, H3K27 trimethylation ChIP-seq, SP1 ChIP-seq, E2F1 ChIP-seq, RELA ChIP-seq, IRF1 ChIP-seq, RFX5 ChIP-seq, RFXAP ChIP-seq, RFXANK ChIP-seq, CREB1 ChIP-seq, ATF1 ChIP-seq, NFYA ChIP-seq, NFYB ChIP-seq, and NFYC ChIP-seq tracks are shown. Analysis was performed on HLA-A (A), HLA-B (B), HLA-C (C), B2M (D), PSMB5 (MB1; E), PSMB6 (Delta; F), PSMB7 (Zeta; G), PSMB8 (LMP7; H), PSMB9 (LM P2)/TAP1 bidirectional promoter (H), TAP2 (H), ERAP1 (I), ERAP2 (I), PSMB10 (LMP10; J), CANX (calnexin; K), CALR (calreticulin; L), TAPBP (tapasin; M), and PDIA3 (ERp57; N). HLA-B appears to be dysregulated in this cell line, displaying low levels of H3K27 acetylation and an abnormal distribution/intensity of transcription factor ChIP peaks compared to HLA-B in other cell lines.
HLA class-I APM component downregulation by epigenetic mechanisms
Repressive hypermethylation of DNA at the 5-position of cytosine in HLA class-I heavy chain, β2m and APM component encoding gene promoter regions has been described in several cancer cell lines and surgically removed patient tumors (27–29). Treatment with DNA methyltransferase (DNMT) inhibitors reverses DNA methylation, and enhances cancer cell susceptibility to T cell-based immunotherapy through restoration of HLA class-I APM component expression (30,31). Similar results have been obtained with inhibitors of enhancer of zeste homolog 2 (EZH2), a histone methyltransferase enzyme (32), which is overexpressed in many cancer types (33). EZH2 inhibition reverses repressive trimethylation of histone H3 at lysine 27 (H3K27me3) and upregulates HLA class-I expression on head and neck squamous cell carcinoma (HNSCC) cells. The resulting improvement in TA presentation to cognate cytotoxic T cells restores the efficacy of anti-programmed cell death protein-1 (anti-PD-1)-based immunotherapy in HNSCC models (34). Furthermore, histone deacetylase (HDAC) inhibitors upregulate HLA class-I APM component expression in breast and Merkel cell carcinoma cell lines in vitro (35,36). Enhanced histone acetylation at lysine(s) represents the underlying mechanism, as this process increases accessibility of HLA class-I APM chromatin to transcription factors and possibly reverses the repressive chromatin state induced by lack of histone acetylation (37). The induced HLA class-I upregulation enhanced the clinical efficacy of immune checkpoint inhibition (ICI) in a Merkel cell carcinoma patient (38). Identifying other cancer types where DNMTs, EZH2, and HDACs regulate HLA class-I APM expression is essential in the rational design of clinical trials combining epigenetic therapy with T cell-based immunotherapy, a strategy which has become increasingly popular. Epigenetic agents display a wide range of immunostimulatory activities beyond HLA class-I APM component upregulation. They may increase TA expression including cancer/testis antigens (CTAs), induce type I IFN signaling by activating endogenous retroviruses (ERVs), and inhibit MYC-mediated immunosuppression (39). Moreover, when combined with ICIs they may enhance exhausted T cell rejuvenation (40). Early-stage clinical trials suggest that the combination of ICIs and epigenetic agents is well-tolerated; however, further testing is required to prove greater efficacy of the combinatorial therapy as compared to ICIs alone (39,41).
HLA class-I APM component downregulation by interferon signaling defects
IFN signaling plays an important role in HLA class-I trimolecular complex synthesis by upregulating genes such as TAP and ERAP (42). IFN stimulation activates Janus kinases (JAKs) and signal transducer and activator of transcription 1 (STAT1), inducing the expression of IRF1 and NLRC5, which promote transcription of HLA class-I APM genes (43). Defective IFN signaling, which affects HLA class-I APM component expression and consequently HLA class-I trimolecular complex expression, has been described in many cancer types (44,45). Embryonic transcription factor double homeobox 4 (DUX4) upregulation decreases JAK1/JAK2 and STAT1 signaling, reducing constitutive and IFNγ-inducible HLA class-I APM gene expression in DUX4+ cancer cells (46). Additionally, Src homology region 2 domain-containing phosphatase 2 (SHP2) depletion in HNSCC cells activates JAK1/JAK2 and STAT1, and upregulates HLA class-I expression (47). Lastly, hypoxia inhibits STAT1 and IFN-inducible gene expression in renal cell carcinoma cells. Indeed, von Hippel-Lindau (VHL) mutations, which are known to stabilize hypoxia-inducible factor 1 (HIF-1), upregulate the transcriptional repressor stimulated by retinoic acid 13 (STRA13), which inhibits STAT1 transcription. Thus, VHL mutations are associated with HLA class-I APM component downregulation (48). Defects in HLA class-I APM expression caused by this mechanism can be potentially corrected with chemotherapy or radiotherapy, which exert their antitumor activity not only through cytotoxicity, but also through induction or enhancement of a TA-specific immune response (49,50). Both chemotherapy and radiotherapy increase IFN signaling in several cancer types. The resulting HLA class-I upregulation can improve the recognition and elimination of cancer cells by cognate T cells in pre-clinical models (51,52). Several ongoing clinical trials are combining chemo- and/or radiotherapy with ICI or adoptive T cell therapy (49,53). More recently, stimulator of interferon gene (STING) agonists, promoting type I IFN secretion, have emerged as another potential strategy to correct defects in IFN-inducible genes (54).
HLA class-I APM component downregulation by NF-κB signaling defects
NF-κB signaling can be activated by the binding of ligands to the tumor-necrosis factor receptor superfamily (TNFRSF) and to Toll-like receptors (TLRs) via canonical and non-canonical pathways (44,55). Members of the NF-κB family of transcription factors (Canonical: NF-κB1 p50, RELA, and c-REL; Non-canonical: NF-κB2 p52, and RELB), can migrate from the cytosol to the nucleus following NF-κB activation and bind to the Enhancer A region of HLA class-I APM component encoding genes. Abnormalities in downstream signaling of the NF-κB pathway cause HLA class-I downregulation in neuroblastoma (56,57). NEDD4 Binding Protein 1 (N4BP1) and TNFα-induced protein 3 interacting protein 1 (TNIP1) inhibit NF-κB-mediated HLA class-I upregulation (57). Their depletion upregulates HLA class-I expression and increases neuroblastoma cell susceptibility to recognition and elimination by cognate TA-specific CD8+ T cells. Furthermore, transforming growth factor β (TGFβ)-mediated HLA class-I downregulation has been described in prostate cancer (58). This phenotype is likely to be mediated by the upregulation of the transcription factor Snail (SNAI1), which downregulates HLA class-I by targeting NF-κB/p65.
HLA class-I APM component downregulation by oncogenes
Various oncogenes may contribute to MHC class-I downregulation including receptor tyrosine kinase (RTK)/mitogen-activated protein kinase (MAPK) pathway components, cyclin-dependent kinase 4/6 (CDK4/6), and c-Myc. The effect of the former is most prominent in cases of activating mutations or amplifications in RTK/MAPK pathway genes (epidermal growth factor receptor [EGFR], KRAS, BRAF, SHP2, and MEK) (59). KRAS mutations are associated with HLA class-I downregulation in NSCLC tumors (60). MAPK inhibition enhances HLA class-I expression on several types of cancer cell lines including breast, esophagus, lung, stomach, liver and pancreas (61,62). This effect is mediated by STAT1 and STAT3 activation by the MAPK inhibitor (59,63). Anaplastic lymphoma kinase (ALK) and RET, two RTKs that are associated with MAPK and phosphoinositide 3-kinase (PI3K) pathway activation, are mutated in various cancer types (64).Inhibition of these molecules can suppress MAPK signaling and upregulate HLA class-I expression (64). Human epidermal growth factor receptor 2 (HER2/neu) overexpression is also associated with HLA class-I downregulation due to impaired LMP2, TAP1, TAP2, and tapasin transcription (26). These defects and the resulting decrease in cancer cell susceptibility to cytotoxic T cell-mediated lysis can be corrected in HER2/neu+ cells by IFNγ treatment (65). Similarly, HER2 downregulation induced by a small interfering RNA, or MAPK pathway inhibition can upregulate HLA class-I expression (66,67). Targeting RTKs with mAbs can also inhibit MAPK signaling. The anti-EGFR mAb cetuximab upregulates HLA class-I expression by head and neck cancer cells in patients’ tumors displaying a post-treatment volume reduction (68). Moreover, CDK4/6 are involved in MHC class-I downregulation in breast cancer cells. Their inhibition stimulates the production of type III interferons and induces STAT1 phosphorylation by downregulating DNMT1. Consequently, MHC class I APM components are upregulated, and a synergy is observed with anti-PD-L1 mAbs in transgenic mouse tumor models (69). Lastly, c-Myc transfection downregulates HLA class-I subunits at the mRNA level in melanoma cells (70) likely by binding to the initiator (Inr) element of their promoters (71).
HLA class-I downregulation by lysosomal degradation
HLA class-I expression is downregulated in a large percentage of pancreatic ductal adenocarcinoma (PDAC) tumors (72). This abnormality is caused by the autophagy-lysosomal degradation pathway (73), a result which corroborates the previously described MHC class-I antigen upregulation by autophagy inhibition in a murine melanoma cell line (74). The autophagy cargo receptor NBR1 associates with HLA class-I trimolecular complexes mediating their transport to lysosomes where they are degraded (73). Inhibition of lysosomal degradation by chloroquine restored MHC class-I antigen expression on murine PDAC models and enhanced the efficacy of T cell-based immunity triggered by ICIs. The same restoration is likely to occur in humans; hydroxychloroquine, administered with the rationale of inhibiting autophagy-mediated cancer cell survival, in combination with chemotherapy, resulted in increased immune cell infiltration compared to chemotherapy alone in a Phase II trial in PDAC patients (75). Whether this effect was mediated through HLA class-I upregulation remains to be proven.
The enzyme proprotein convertase subtilisin/kexin type 9 (PCSK9), which is involved in regulation of cholesterol levels by low-density lipoprotein (LDL) receptor lysosomal trafficking, has also been reported to regulate HLA class-I levels (76,77). Specifically, PCSK9 is secreted by malignant and non-malignant cells, binds to the extracellular alpha-1 region of HLA class-I heavy chain and mediates trimolecular complex degradation via the endosomal-lysosomal pathway. PCSK9 knockdown or inhibition of its function with blocking mAbs restored MHC class-I expression and improved responses to ICI-based therapy in multiple syngeneic, immunocompetent mouse tumor models (76,77).
HLA class-I APM component regulation by microRNAs
Several microRNAs (miRNAs) binding to the 3’-untranslated regions (3’UTRs) of HLA class-I APM components regulate their expression. Despite limited investigation of miRNA-mediated HLA class-I heavy chain regulation due to low DNA sequence homology of these genes with their orthologs, HLA-C is negatively regulated by miR-148a. However, the binding affinity is highly dependent on the germline HLA-C 3’UTR variant (78). Additionally, TAP1 is negatively regulated by miR-26b-5p and miR-21–3p in melanoma cells. Overexpression of these miRNAs downregulates both TAP1 and HLA class-I trimolecular complex expression (79). TAP2, instead, is negatively regulated by miRNA-125a in esophageal adenocarcinoma cells. These cells display concomitant TAP2 and HLA class-I trimolecular complex downregulation upon transfection of a miRNA-125a mimic (80). Other APM components are also negatively regulated by miRNAs, including PSMB8 (LMP7) targeted by miR-451a (81), PDIA3 (ERp57) targeted by miR-148a and miR-330–5p (82,83), (CANX) (calnexin) targeted by miR-711 (84), and (CALR) calreticulin targeted by miR-455 and miR-27a (85,86). Furthermore, NLRC5 is negatively regulated by miR-34a in HPV16-positive cervical cancer cells (87). This activity is likely to influence HLA class-I expression as NLRC5 exclusively transactivates HLA class-I APM component genes (88) and miR-34a inhibition enhances MHC class-I antigen expression in mouse neuroblastoma cells (89).
HLA class-I APM component modulation by cytokines
In addition to IFNs and TGFβ, other cytokines modulate HLA class-I expression. For instance, cytokines inducing NF-κB activation such as tumor necrosis factor α (TNFα) enhance HLA class-I expression on cancer cells in an allele specific manner due to the differential ability of NF-κB to bind to κB sites among HLA class-I heavy chain loci promoter regions (90,91). Furthermore, IL-2, IL-12, and IL-27 enhance HLA class-I expression by papillary thyroid cancer, melanoma, and small cell lung cancer cells, respectively (92–94). While the underlying mechanism is not characterized for IL-2 or IL-12, STAT1 or STAT3 phosphorylation may regulate IL-27-mediated HLA class-I upregulation, which is accompanied by enhanced TAP1 and TAP2 expression. The role of IL-10 in HLA class-I modulation remains controversial; downregulation in monocytes and upregulation in papillary thyroid cancer cells have been reported (95,96).
HLA class-I APM component modulation by the tumor microenvironment
Abnormal conditions within the tumor microenvironment (TME) are also believed to influence HLA class-I APM component expression by cancer cells (97). Inadequate angiogenesis results in low blood supply to rapidly proliferating malignant cells in the TME, decreasing oxygen levels and causing hypoxia (98). In this environment, hypoxia-inducible factors (HIFs) are not degraded and can relocate to the nucleus to drive transcriptional modulation of HLA class-I APM components (99). Similarly, adenosine triphosphate (ATP) levels are significantly increased at tumor sites due to the glycolytic metabolism of cancer cells (100,101). As the TAP complex is regulated by ATP hydrolysis in the cytoplasm (102), its function is likely to be influenced.
Concluding Remarks
Experimental evidence convincingly indicates that non-structural mechanisms are the major cause of HLA class-I APM downregulation, which is frequently present in most cancer types. These abnormalities have clinical relevance, as shown by their association with the clinical course of the disease and with response to T cell-based immunotherapies, including ICI therapy, which are at the forefront of cancer treatment (103–106). Nevertheless, the role of HLA class-I APM defects in resistance to T cell-based immunotherapies (107) and in disease recurrence following a clinical response (108,109) has often been overlooked, likely because these defects are assumed to be rare, given the low frequency of structural mutations in HLA class-I APM component encoding genes (8). As a result, the value of HLA class-I APM defects as biomarkers to identify patients who may benefit from T cell-based immunotherapy has been explored to a limited extent, despite the reported association of HLA class-I APM component expression level with clinical response to ICI therapy in some cancer types (110,111) and its increased efficacy when combined with strategies that correct HLA class-I APM abnormalities in targeted cancer cells (30,31,34,38,51,52,73,76,77,98). Similarly, HLA class-I alleles expressed by targeted cancer cells, with few exceptions (112), have not been taken into account when assessing the immunogenicity and clinical relevance of neoantigens present in malignant cells, although they are crucial for their presentation to cognate T cells. Therefore, systematic studies to explore the value of HLA class-I APM component expression level as a biomarker on malignant cells, to identify potential responders to ICI-based therapy, and those who may require HLA class-I APM restoration to achieve a response, are warranted (see Outstanding Questions’ box).
Outstanding Questions.
What is the frequency and impact of defects in HLA class-I APM components in various cancer types on HLA class-I trimolecular complex expression?
How do defects in HLA class-I alleles impact response to immune checkpoint inhibitors?
Does the evaluation of mutation load in malignant tumors in the context of HLA class-I alleles expressed by cancer cells and APM component expression by cancer cells improve its correlation with clinical response to ICI therapy?
Can more quantitative diagnostic assays be developed to assess the extent of HLA class-I APM component downregulation in patients?
What is the level of downregulation or loss of function of HLA class-I APM that has clinical relevance?
What is the role of HLA class-I recycling components in the expression and functionality of HLA class-I molecules in malignant cells?
Several examples have been described in which tumors displaying HLA class-I APM component defects have recurred following a clinical response to T cell-based immunotherapy (108,109). Furthermore, the available evidence, although limited, suggests that the frequency of HLA class-I APM defects in metastases is increased in patients treated with T cell-based immunotherapy (27). It is likely that a cancer cell subpopulation can develop novel escape mechanism(s) under the selective pressure imposed by an immunotherapy. When the targeted restricting HLA class-I allele is lost, the host’s immune system can change the TA and the restricting HLA class-I allele (113). These clinical findings emphasize the need to develop strategies which maintain the expression and function of HLA class-I APM components to enhance recognition and elimination of cancer cells by cognate T cells. However, strategies which may have beneficial effects by upregulating HLA class-I expression, could have broad effects and therefore also upregulate molecules which have a negative impact upon immune effector cell-cancer cell interactions, such as radiotherapy-induced upregulation of programmed death ligand-1 (114,115).
From a practical viewpoint, analysis of HLA class-I APM expression and function will greatly benefit from standardization of the methodology used to quantitate expression levels in malignant and normal cells in formalin-fixed, paraffin-embedded tissues. The latter represent the standard to score the HLA class-I APM component expression level in malignant cells. It is also important to consider that HLA class-I APM component expression and source of defects will differ among patients even with the same cancer type, as well as between primary and metastatic tumors (116). Therefore, mechanisms and potential interventions may have to be individualized. Furthermore, mAbs that detect shared HLA class-I epitopes are not practical for determining selective allele loss (117); therefore, selection of a panel of mAbs to identify specific HLA class-I alleles is critical. Finally, HLA class-I APM component expression levels may not necessarily correspond to functionality of HLA class-I trimolecular complexes. Variance in the repertoire of TA-derived peptides presented by HLA class-I alleles, due to differential expression of immunoproteasome subunits, is associated with patient outcomes in various cancer types (118,119). Assessment of the efficiency of peptide presentation by malignant cells before and/or after HLA class-I APM expression restoration will help determine a patient’s likelihood of clinical response to T cell-based immunotherapy.
Highlights.
HLA class-I APM component defects are present in most, if not all, cancer types at high frequency.
The mechanisms underlying these defects are predominantly non-structural and therefore, are potentially correctable with rationally designed therapeutic strategies.
Restoration or upregulation of HLA class-I APM component expression in malignant cells enhances the efficacy of T cell-mediated immunity, including that triggered by immune checkpoint inhibitors.
HLA class-I APM component expression is a potential biomarker for the selection of patients who may benefit from immune checkpoint inhibitor-based therapy.
Acknowledgements
This work was supported by the National Institutes of Health grant RO1DE028172, by the National Cancer Institute grants R01CA230275, R03CA219603 and R03CA253319 and by the Department of Defense grant W81XWH-20–1-0315.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferrington DA, Gregerson DS. Immunoproteasomes: Structure, Function, and Antigen Presentation. Prog Mol Biol Transl Sci [Internet]. 2012;109. Available from: https://pubmed.ncbi.nlm.nih.gov/22727420/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saveanu L, Carroll O, Lindo V, Del Val M, Lopez D, Lepelletier Y, et al. Concerted Peptide Trimming by Human ERAP1 and ERAP2 Aminopeptidase Complexes in the Endoplasmic Reticulum. Nat Immunol [Internet]. 2005;6(7). Available from: https://pubmed.ncbi.nlm.nih.gov/15908954/?from_single_result=PMID%3A+15908954 [DOI] [PubMed] [Google Scholar]
- 3.Blees A, Januliene D, Hofmann T, Koller N, Schmidt C, Trowitzsch S, et al. Structure of the human MHC-I peptide-loading complex. Nature. 2017. November;551(7681):525–8. [DOI] [PubMed] [Google Scholar]
- 4.Montealegre S, van Endert PM. Endocytic Recycling of MHC Class I Molecules in Non-professional Antigen Presenting and Dendritic Cells. Front Immunol [Internet]. 2018;9:3098. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30666258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weigert R, Yeung AC, Li J, Donaldson JG. Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol Biol Cell [Internet]. 2004. August [cited 2021 Jun 2];15(8):3758–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15181155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai L, Michelakos T, Yamada T, Fan S, Wang X, Schwab JH, et al. Defective HLA class I antigen processing machinery in cancer. Cancer Immunol Immunother [Internet]. 2018. June;67(6):999–1009. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29487978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seliger B, Ferrone S. HLA Class I Antigen Processing Machinery Defects in Cancer Cells-Frequency, Functional Significance, and Clinical Relevance With Special Emphasis on Their Role in T Cell-Based Immunotherapy of Malignant Disease. Methods Mol Biol [Internet]. 2020;2055. Available from: https://pubmed.ncbi.nlm.nih.gov/31502159/ [DOI] [PubMed] [Google Scholar]
- 8.Cai L, Michelakos T, Yamada T, Fan S, Schwab JH, Ferrone CR, et al. HLA Class I Antigen-Processing Machinery in Cancer. In: Cancer Immunotherapy Principles and Practice [Internet]. New York, NY: Springer Publishing Company; [cited 2021 Feb 5]. Available from: http://connect.springerpub.com/lookup/doi/10.1891/9781617052736.0004 [Google Scholar]
- 9.Maleno I, Aptsiauri N, Cabrera T, Gallego A, Paschen A, López-Nevot MA, et al. Frequent loss of heterozygosity in the β2-microglobulin region of chromosome 15 in primary human tumors. Immunogenetics [Internet]. 2011. February [cited 2020 Oct 26];63(2):65–71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21086121 [DOI] [PubMed] [Google Scholar]
- 10.Sucker A, Zhao F, Real B, Heeke C, Bielefeld N, Maβen S, et al. Genetic evolution of T-cell resistance in the course of melanoma progression. Clin Cancer Res [Internet]. 2014. December 15 [cited 2020 Oct 26];20(24):6593–604. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25294904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao F, Sucker A, Horn S, Heeke C, Bielefeld N, Schrörs B, et al. Melanoma Lesions Independently Acquire T-cell Resistance during Metastatic Latency. Cancer Res [Internet]. 2016. [cited 2020 Oct 26];76(15):4347–58. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27261508 [DOI] [PubMed] [Google Scholar]
- 12.Ozcan M, Janikovits J, von Knebel Doeberitz M, Kloor M. Complex pattern of immune evasion in MSI colorectal cancer. Oncoimmunology [Internet]. 2018. [cited 2021 Feb 15];7(7):e1445453. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29900056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science [Internet]. 2015. October 9 [cited 2021 May 27];350(6257):207–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26359337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science [Internet]. 2015. April 3 [cited 2021 May 27];348(6230):124–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25765070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samstein RM, Lee C-H, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet [Internet]. 2019. [cited 2021 May 27];51(2):202–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30643254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med [Internet]. 2014. December 4 [cited 2021 May 27];371(23):2189–99. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25409260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med [Internet]. 2016. September 1 [cited 2021 May 27];375(9):819–29. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27433843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sade-Feldman M, Jiao YJ, Chen JH, Rooney MS, Barzily-Rokni M, Eliane J-P, et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun [Internet]. 2017. [cited 2021 May 27];8(1):1136. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29070816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science [Internet]. 2018. [cited 2021 May 27];359(6375):582–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29217585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gettinger S, Choi J, Hastings K, Truini A, Datar I, Sowell R, et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov [Internet]. 2017. [cited 2021 May 27];7(12):1420–35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29025772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Florea ID, Karaoulani C. Epigenetic Changes of the Immune System with Role in Tumor Development. In 2018. p. 203–18. Available from: http://link.springer.com/10.1007/978-1-4939-8751-1_11 [DOI] [PubMed] [Google Scholar]
- 22.Jongsma MLM, Guarda G, Spaapen RM. The regulatory network behind MHC class I expression. Mol Immunol [Internet]. 2019;113:16–21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29224918 [DOI] [PubMed] [Google Scholar]
- 23.van den Elsen PJ. Expression regulation of major histocompatibility complex class I and class II encoding genes. Front Immunol [Internet]. 2011. [cited 2021 Jun 1];2:48. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22566838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gobin SJ, Keijsers V, van Zutphen M van den EP. The role of enhancer A in the locus-specific transactivation of classical and nonclassical HLA class I genes by nuclear factor kappa B. J Immunol. 161(5):2276–83. [PubMed] [Google Scholar]
- 25.Meissner TB, Li A, Biswas A, Lee KH, Liu YJ, Bayir E, et al. NLR Family Member NLRC5 Is a Transcriptional Regulator of MHC Class I Genes. Proc Natl Acad Sci U S A [Internet]. 2010;107(31). Available from: https://pubmed.ncbi.nlm.nih.gov/20639463/?from_single_result=20639463&show_create_notification_links=False [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bukur J, Herrmann F, Handke D, Recktenwald C, Seliger B. Identification of E2F1 as an Important Transcription Factor for the Regulation of Tapasin Expression. J Biol Chem [Internet]. 2010;285(40). Available from: https://pubmed.ncbi.nlm.nih.gov/20663889/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang C-C, Pirozzi G, Wen S-H, Chung I-H, Chiu B-L, Errico S, et al. Multiple structural and epigenetic defects in the human leukocyte antigen class I antigen presentation pathway in a recurrent metastatic melanoma following immunotherapy. J Biol Chem. 2015. October;290(44):26562–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nie Y, Yang G, Song Y, Zhao X, So C, et al. DNA Hypermethylation Is a Mechanism for Loss of Expression of the HLA Class I Genes in Human Esophageal Squamous Cell Carcinomas. Carcinogenesis [Internet]. 2001;22(10). Available from: https://pubmed.ncbi.nlm.nih.gov/11577000/ [DOI] [PubMed] [Google Scholar]
- 29.Fonsatti E, Sigalotti L, Coral S, Colizzi F, Altomonte M, Maio M. Methylation-regulated expression of HLA class I antigens in melanoma. Int J cancer [Internet]. 2003. June;105(3):430–3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12704681 [DOI] [PubMed] [Google Scholar]
- 30.Serrano A, Tanzarella S, Lionello I, Mendez R, Traversari C, Ruiz-Cabello F, et al. Rexpression of HLA class I antigens and restoration of antigen-specific CTL response in melanoma cells following 5-aza-2’-deoxycytidine treatment. Int J cancer [Internet]. 2001. October;94(2):243–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11668505 [DOI] [PubMed] [Google Scholar]
- 31.Fonsatti E, Nicolay HJM, Sigalotti L, Calabrò L, Pezzani L, Colizzi F, et al. Functional up-regulation of human leukocyte antigen class I antigens expression by 5-aza-2’-deoxycytidine in cutaneous melanoma: immunotherapeutic implications. Clin Cancer Res [Internet]. 2007. June;13(11):3333–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17545540 [DOI] [PubMed] [Google Scholar]
- 32.Burr ML, Sparbier CE, Chan KL, Chan Y-C, Kersbergen A, Lam EYN, et al. An Evolutionarily Conserved Function of Polycomb Silences the MHC Class I Antigen Presentation Pathway and Enables Immune Evasion in Cancer. Cancer Cell [Internet]. 2019. [cited 2020 Dec 7];36(4):385–401.e8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31564637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim KH, Roberts CWM. Targeting EZH2 in cancer. Nat Med [Internet]. 2016. February;22(2):128–34. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26845405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou L, Mudianto T, Ma X, Riley R, Uppaluri R. Targeting EZH2 Enhances Antigen Presentation, Antitumor Immunity, and Circumvents Anti-PD-1 Resistance in Head and Neck Cancer. Clin Cancer Res [Internet]. 2020;26(1):290–300. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31562203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gameiro SR, Malamas AS, Tsang KY, Ferrone S, Hodge JW. Inhibitors of histone deacetylase 1 reverse the immune evasion phenotype to enhance T-cell mediated lysis of prostate and breast carcinoma cells. Oncotarget [Internet]. 2016. February;7(7):7390–402. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26862729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritter C, Fan K, Paschen A, Reker Hardrup S, Ferrone S, Nghiem P, et al. Epigenetic Priming Restores the HLA class-I Antigen Processing Machinery Expression in Merkel Cell Carcinoma. Sci Rep [Internet]. 2017;7(1). Available from: https://pubmed.ncbi.nlm.nih.gov/28536458/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Görisch SM, Wachsmuth M, Tóth KF, Lichter P, Rippe K. Histone acetylation increases chromatin accessibility. J Cell Sci [Internet]. 2005. December 15 [cited 2020 Nov 13];118(Pt 24):5825–34. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16317046 [DOI] [PubMed] [Google Scholar]
- 38.Ugurel S, Spassova I, Wohlfarth J, Drusio C, Cherouny A, Melior A, et al. MHC class-I downregulation in PD-1/PD-L1 inhibitor refractory Merkel cell carcinoma and its potential reversal by histone deacetylase inhibition: a case series. Cancer Immunol Immunother [Internet]. 2019. June [cited 2020 Oct 27];68(6):983–90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30993371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Topper MJ, Vaz M, Marrone KA, Brahmer JR, Baylin SB. The emerging role of epigenetic therapeutics in immuno-oncology. Nat Rev Clin Oncol [Internet]. 2020. [cited 2021 Jun 1];17(2):75–90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31548600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghoneim HE, Fan Y, Moustaki A, Abdelsamed HA, Dash P, Dogra P, et al. De Novo Epigenetic Programs Inhibit PD-1 Blockade-Mediated T Cell Rejuvenation. Cell [Internet]. 2017. June 29 [cited 2021 Jun 1];170(1):142–157.e19. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28648661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morera L, Lübbert M, Jung M. Targeting histone methyltransferases and demethylases in clinical trials for cancer therapy. Clin Epigenetics [Internet]. 2016. [cited 2021 Jun 1];8:57. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27222667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Girdlestone J. Regulation of HLA Class I Loci by Interferons. Immunobiology [Internet]. 1995;193(2–4). Available from: https://pubmed.ncbi.nlm.nih.gov/8530148/?from_single_result=8530148&show_create_notification_links=False [DOI] [PubMed] [Google Scholar]
- 43.Meissner TB, Li A, Biswas A, Lee K-H, Liu Y-J, Bayir E, et al. NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proc Natl Acad Sci U S A [Internet]. 2010. August;107(31):13794–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20639463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cornel AM, Mimpen IL, Nierkens S. MHC Class I Downregulation in Cancer: Underlying Mechanisms and Potential Targets for Cancer Immunotherapy. Cancers (Basel) [Internet]. 2020. July;12(7). Available from: http://www.ncbi.nlm.nih.gov/pubmed/32630675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castro F, Cardoso AP, Gonçalves RM, Serre K, Oliveira MJ. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front Immunol [Internet]. 2018. [cited 2020 Oct 27];9:847. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29780381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chew GL, Campbell AE, De Neef E, Sutliff NA, Shadle SC, Tapscott SJ, et al. DUX4 Suppresses MHC Class I to Promote Cancer Immune Evasion and Resistance to Checkpoint Blockade. Dev Cell [Internet]. 2019;50(5). Available from: https://pubmed.ncbi.nlm.nih.gov/31327741/?from_single_result=31327741&show_create_notification_links=False [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xi S, Dyer KF, Kimak M, Zhang Q, Gooding WE, Chaillet JR, et al. Decreased STAT1 Expression by Promoter Methylation in Squamous Cell Carcinogenesis. J Natl Cancer Inst [Internet]. 2006;98(3). Available from: https://pubmed.ncbi.nlm.nih.gov/16449678/?from_single_result=16449678&show_create_notification_links=False [DOI] [PubMed] [Google Scholar]
- 48.Ivanov SV, Salnikow K, Ivanova AV, Bai L, Lerman MI. Hypoxic repression of STAT1 and its downstream genes by a pVHL/HIF-1 target DEC1/STRA13. Oncogene [Internet]. 2007. February 8 [cited 2021 Feb 15];26(6):802–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16878149 [DOI] [PubMed] [Google Scholar]
- 49.Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol Off J Eur Soc Med Oncol [Internet]. 2019. [cited 2021 Feb 15];30(2):219–35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30608567 [DOI] [PubMed] [Google Scholar]
- 50.Spiotto M, Fu YX, Weichselbaum RR. The intersection of radiotherapy and immunotherapy: Mechanisms and clinical implications. Sci Immunol. 2016;1(3):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wan S, Pestka S, Jubin RG, Lyu YL, Tsai Y-C, Liu LF. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PLoS One [Internet]. 2012;7(3):e32542. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22396773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med [Internet]. 2006. May;203(5):1259–71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16636135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Formenti SC, Rudqvist N-P, Golden E, Cooper B, Wennerberg E, Lhuillier C, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med [Internet]. 2018. December;24(12):1845–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30397353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang M, Chen P, Wang L, Li W, Chen B, Liu Y, et al. cGAS-STING, an important pathway in cancer immunotherapy. J Hematol Oncol [Internet]. 2020. [cited 2021 Feb 15];13(1):81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32571374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun S-C. The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol. 2017. September;17(9):545–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lorenzi S, Forloni M, Cifaldi L, Antonucci C, Citti A, Boldrini R, et al. IRF1 and NF-kB Restore MHC Class I-restricted Tumor Antigen Processing and Presentation to Cytotoxic T Cells in Aggressive Neuroblastoma. PLoS One [Internet]. 2012;7(10). Available from: https://pubmed.ncbi.nlm.nih.gov/23071666/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spel L, Nieuwenhuis J, Haarsma R, Stickel E, Bleijerveld OB, Altelaar M, et al. Nedd4-Binding Protein 1 and TNFAIP3-Interacting Protein 1 Control MHC-1 Display in Neuroblastoma. Cancer Res [Internet]. 2018;78(23). Available from: https://pubmed.ncbi.nlm.nih.gov/30213788/ [DOI] [PubMed] [Google Scholar]
- 58.Chen X-H, Liu Z-C, Zhang G, Wei W, Wang X-X, Wang H, et al. TGF-β and EGF induced HLA-I downregulation is associated with epithelialmesenchymal transition (EMT) through upregulation of snail in prostate cancer cells. Mol Immunol. 2015. May;65(1):34–42. [DOI] [PubMed] [Google Scholar]
- 59.Brea EJ, Oh CY, Manchado E, Budhu S, Gejman RS, Mo G, et al. Kinase Regulation of Human MHC Class I Molecule Expression on Cancer Cells. Cancer Immunol Res [Internet]. 2016. [cited 2020 Sep 23];4(11):936–47. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27680026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He XP, Song FJ, Liu XY, Wang Z, Li XX, Liu FY, et al. The Relationship Between KRAS Gene Mutations and HLA Class I Antigen Downregulation in the Metastasis of Non-Small Cell Lung Cancer. J Int Med Res [Internet]. 2013;41(5). Available from: https://pubmed.ncbi.nlm.nih.gov/23975858/ [DOI] [PubMed] [Google Scholar]
- 61.Mimura K, Kua L-F, Shiraishi K, Kee Siang L, Shabbir A, Komachi M, et al. Inhibition of mitogen-activated protein kinase pathway can induce upregulation of human leukocyte antigen class I without PD-L1-upregulation in contrast to interferon-$γ$ treatment. Cancer Sci [Internet]. 2014. October;105(10):1236–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25154680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mimura K, Shiraishi K, Mueller A, Izawa S, Kua L-F, So J, et al. The MAPK pathway is a predominant regulator of HLA-A expression in esophageal and gastric cancer. J Immunol [Internet]. 2013. December;191(12):6261–72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24244023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang S-H, Keam B, Ahn Y-O, Park H-R, Kim M, Kim TM, et al. Inhibition of MEK with trametinib enhances the efficacy of anti-PD-L1 inhibitor by regulating anti-tumor immunity in head and neck squamous cell carcinoma. Oncoimmunology [Internet]. 2019. [cited 2020 Dec 7];8(1):e1515057. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30546955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oh CY, Klatt MG, Bourne C, Dao T, Dacek MM, Brea EJ, et al. ALK and RET Inhibitors Promote HLA Class I Antigen Presentation and Unmask New Antigens within the Tumor Immunopeptidome. Cancer Immunol Res [Internet]. 2019. [cited 2020 Sep 23];7(12):1984–97. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31540894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herrmann F, Lehr HA, Drexler I, Sutter G, Hengstler J, Wollscheid U, et al. HER-2/neu-mediated Regulation of Components of the MHC Class I Antigen-Processing Pathway. Cancer Res [Internet]. 2004;64(1). Available from: https://pubmed.ncbi.nlm.nih.gov/14729627/ [DOI] [PubMed] [Google Scholar]
- 66.Maruyama T, Mimura K, Sato E, Watanabe M, Mizukami Y, Kawaguchi Y, et al. Inverse correlation of HER2 with MHC class I expression on oesophageal squamous cell carcinoma. Br J Cancer [Internet]. 2010. August;103(4):552–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20628381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Inoue M, Mimura K, Izawa S, Shiraishi K, Inoue A, Shiba S, et al. Expression of MHC Class I on breast cancer cells correlates inversely with HER2 expression. Oncoimmunology [Internet]. 2012. October;1(7):1104–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23170258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srivastava RM, Trivedi S, Concha-Benavente F, Hyun-Bae J, Wang L, Seethala RR, et al. Stat1-induced HLA class i upregulation enhances immunogenicity and clinical response to anti-EGFR mab cetuximab therapy in HNC patients. Cancer Immunol Res. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature [Internet]. 2017. [cited 2021 Jun 1];548(7668):471–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28813415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Versteeg R, Noordermeer IA, Krüse-Wolters M, Ruiter DJ, Schrier PI. c-myc down-regulates class I HLA expression in human melanomas. EMBO J [Internet]. 1988. April [cited 2021 Jun 1];7(4):1023–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3402430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Griffioen M, Peltenburg LT, van Oorschot DA, Schrier PI. C-myc represses transiently transfected HLA class I promoter sequences not locus-specifically. Immunobiology [Internet]. 1995. July [cited 2021 Jun 1];193(2–4):238–47. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8530149 [DOI] [PubMed] [Google Scholar]
- 72.Michelakos T, Cai L, Villani V, Sabbatino F, Kontos F, Fernández-Del Castillo C, et al. Tumor microenvironment immune response in pancreatic ductal adenocarcinoma patients treated with neoadjuvant therapy. J Natl Cancer Inst [Internet]. 2020. June 4 [cited 2020 Oct 27]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/32497200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature [Internet]. 2020;581(7806):100–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32376951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li B, Lei Z, Lichty BD, Li D, Zhang GM, Feng ZH, et al. Autophagy facilitates major histocompatibility complex class I expression induced by IFN-γ in B16 melanoma cells. Cancer Immunol Immunother. 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeh HJ, Bahary N, Boone BA, Singhi AD, Miller-Ocuin JL, Normolle DP, et al. A Randomized Phase II Preoperative Study of Autophagy Inhibition with High-Dose Hydroxychloroquine and Gemcitabine/Nab-Paclitaxel in Pancreatic Cancer Patients. Clin Cancer Res [Internet]. 2020;26(13). Available from: https://pubmed.ncbi.nlm.nih.gov/32156749/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu X, Bao X, Hu M, Chang H, Jiao M, Cheng J, et al. Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature [Internet]. 2020. November 11 [cited 2020 Nov 16]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/33177715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell [Internet]. 2004. August [cited 2020 Nov 16];15(8):3542–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15146059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kulkarni S, Savan R, Qi Y, Gao X, Yuki Y, Bass SE, et al. Differential microRNA regulation of HLA-C expression and its association with HIV control. Nature [Internet]. 2011. April 28 [cited 2021 Jun 1];472(7344):495–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21499264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lazaridou M-F, Massa C, Handke D, Mueller A, Friedrich M, Subbarayan K, et al. Identification of microRNAs Targeting the Transporter Associated with Antigen Processing TAP1 in Melanoma. J Clin Med [Internet]. 2020. August 20 [cited 2021 Jun 1];9(9). Available from: http://www.ncbi.nlm.nih.gov/pubmed/32825219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mari L, Hoefnagel SJM, Zito D, van de Meent M, van Endert P, Calpe S, et al. microRNA 125a Regulates MHC-I Expression on Esophageal Adenocarcinoma Cells, Associated With Suppression of Antitumor Immune Response and Poor Outcomes of Patients. Gastroenterology [Internet]. 2018. [cited 2021 Jun 1];155(3):784–98. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29885883 [DOI] [PubMed] [Google Scholar]
- 81.Liu Y, Yang H-Z, Jiang Y-J, Xu L-Q. miR-451a is downregulated and targets PSMB8 in prostate cancer. Kaohsiung J Med Sci [Internet]. 2020. July [cited 2021 Jun 1];36(7):494–500. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32128987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao S, Wen Z, Liu S, Liu Y, Li X, Ge Y, et al. MicroRNA-148a inhibits the proliferation and promotes the paclitaxel-induced apoptosis of ovarian cancer cells by targeting PDIA3. Mol Med Rep [Internet]. 2015. September [cited 2021 Jun 1];12(3):3923–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26004124 [DOI] [PubMed] [Google Scholar]
- 83.Kim B-K, Yoo H-I, Choi K, Yoon SK. miR-330–5p inhibits proliferation and migration of keratinocytes by targeting Pdia3 expression. FEBS J [Internet]. 2015. December [cited 2021 Jun 1];282(24):4692–702. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26402295 [DOI] [PubMed] [Google Scholar]
- 84.Zhao N, Mi L, Zhang X, Xu M, Yu H, Liu Z, et al. Enhanced MiR-711 transcription by PPARγ induces endoplasmic reticulum stress-mediated apoptosis targeting calnexin in rat cardiomyocytes after myocardial infarction. J Mol Cell Cardiol [Internet]. 2018. [cited 2021 Jun 1];118:36–45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29522763 [DOI] [PubMed] [Google Scholar]
- 85.Colangelo T, Polcaro G, Ziccardi P, Pucci B, Muccillo L, Galgani M, et al. Proteomic screening identifies calreticulin as a miR-27a direct target repressing MHC class I cell surface exposure in colorectal cancer. Cell Death Dis [Internet]. 2016. February 25 [cited 2021 Jun 1];7(2):e2120. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26913609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Belmont PJ, Chen WJ, Thuerauf DJ, Glembotski CC. Regulation of microRNA expression in the heart by the ATF6 branch of the ER stress response. J Mol Cell Cardiol [Internet]. 2012. May [cited 2021 Jun 1];52(5):1176–82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22326432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li J, Yu L, Shen Z, Li Y, Chen B, Wei W, et al. miR-34a and its novel target, NLRC5, are associated with HPV16 persistence. Infect Genet Evol [Internet]. 2016. [cited 2021 Jun 1];44:293–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27423514 [DOI] [PubMed] [Google Scholar]
- 88.Ludigs K, Seguín-Estévez Q, Lemeille S, Ferrero I, Rota G, Chelbi S, et al. NLRC5 exclusively transactivates MHC class I and related genes through a distinctive SXY module. PLoS Genet [Internet]. 2015. March [cited 2021 Jun 1];11(3):e1005088. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25811463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu Y, Pei W, Hu Y, Li P, Sun C, Du J, et al. MiR34a Regulates Neuronal MHC Class I Molecules and Promotes Primary Hippocampal Neuron Dendritic Growth and Branching. Front Cell Neurosci [Internet]. 2020. [cited 2021 Jun 1];14:573208. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33192317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pfizenmaier K, Scheurich P, Schlüter C, Krönke M. Tumor necrosis factor enhances HLA-A,B,C and HLA-DR gene expression in human tumor cells. J Immunol [Internet]. 1987. February 1 [cited 2021 Jun 1];138(3):975–80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2433337 [PubMed] [Google Scholar]
- 91.Johnson DR. Locus-specific constitutive and cytokine-induced HLA class I gene expression. J Immunol [Internet]. 2003. February 15 [cited 2021 Jun 1];170(4):1894–902. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12574356 [DOI] [PubMed] [Google Scholar]
- 92.Hu J-Q, Lei B-W, Wen D, Ma B, Zhang T-T, Lu Z-W, et al. IL-2 enhanced MHC class I expression in papillary thyroid cancer with Hashimoto’s thyroiditis overcomes immune escape in vitro. J Cancer [Internet]. 2020. [cited 2021 Jun 1];11(14):4250–60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32368308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yue FY, Geertsen R, Hemmi S, Burg G, Pavlovic J, Laine E, et al. IL-12 directly up-regulates the expression of HLA class I, HLA class II and ICAM-1 on human melanoma cells: a mechanism for its antitumor activity? Eur J Immunol [Internet]. 1999. [cited 2021 Jun 1];29(6):1762–73. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10382738 [DOI] [PubMed] [Google Scholar]
- 94.Carbotti G, Nikpoor AR, Vacca P, Gangemi R, Giordano C, Campelli F, et al. IL-27 mediates HLA class I up-regulation, which can be inhibited by the IL-6 pathway, in HLA-deficient Small Cell Lung Cancer cells. J Exp Clin Cancer Res [Internet]. 2017. [cited 2021 Jun 1];36(1):140. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29020964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moreau P, Adrian-Cabestre F, Menier C, Guiard V, Gourand L, Dausset J, et al. IL-10 selectively induces HLA-G expression in human trophoblasts and monocytes. Int Immunol [Internet]. 1999. May [cited 2021 Jun 1];11(5):803–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10330285 [DOI] [PubMed] [Google Scholar]
- 96.Lu Z-W, Hu J-Q, Liu W-L, Wen D, Wei W-J, Wang Y-L, et al. IL-10 Restores MHC Class I Expression and Interferes With Immunity in Papillary Thyroid Cancer With Hashimoto Thyroiditis. Endocrinology [Internet]. 2020. [cited 2021 Jun 1];161(10). Available from: http://www.ncbi.nlm.nih.gov/pubmed/32348468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maggs L, Ferrone S. Improving the clinical significance of preclinical immunotherapy studies through incorporating tumor microenvironment-like conditions. Clin Cancer Res [Internet]. 2020. June; Available from: http://www.ncbi.nlm.nih.gov/pubmed/32571789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature [Internet]. 2006. May;441(7092):437–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16724055 [DOI] [PubMed] [Google Scholar]
- 99.Sethumadhavan S, Silva M, Philbrook P, Nguyen T, Hatfield SM, Ohta A, et al. Hypoxia and hypoxia-inducible factor (HIF) downregulate antigen-presenting MHC class I molecules limiting tumor cell recognition by T cells. PLoS One [Internet]. 2017. [cited 2020 Oct 27];12(11):e0187314. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29155844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased Level of Extracellular ATP at Tumor Sites: In Vivo Imaging with Plasma Membrane Luciferase. El Khoury J, editor. PLoS One [Internet]. 2008. July;3(7):e2599. Available from: 10.1371/journal.pone.0002599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim S-Y. Cancer Energy Metabolism: Shutting Power off Cancer Factory. Biomol Ther (Seoul) [Internet]. 2018. January 1 [cited 2020 Oct 27];26(1):39–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29212305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schmitt L, Tampé R. Structure and Mechanism of ABC Transporters. Curr Opin Struct Biol [Internet]. 2002;12(6). Available from: https://pubmed.ncbi.nlm.nih.gov/12504680/ [DOI] [PubMed] [Google Scholar]
- 103.Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol Off J Eur Soc Med Oncol [Internet]. 2019;30(1):44–56. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30395155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Plesca I, Tunger A, Müller L, Wehner R, Lai X, Grimm M-O, et al. Characteristics of Tumor-Infiltrating Lymphocytes Prior to and During Immune Checkpoint Inhibitor Therapy. Front Immunol [Internet]. 2020;11:364. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32194568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature [Internet]. 2014. November;515(7528):577–81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25428507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miller JFAP, Sadelain M. The journey from discoveries in fundamental immunology to cancer immunotherapy. Cancer Cell [Internet]. 2015. April;27(4):439–49. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25858803 [DOI] [PubMed] [Google Scholar]
- 107.Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol [Internet]. 2020. [cited 2021 Feb 16];20(1):25–39. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31570880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Donia M, Harbst K, van Buuren M, Kvistborg P, Lindberg MF, Andersen R, et al. Acquired Immune Resistance Follows Complete Tumor Regression without Loss of Target Antigens or IFN$γ$ Signaling. Cancer Res [Internet]. 2017. September;77(17):4562–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28655789 [DOI] [PubMed] [Google Scholar]
- 109.Aptsiauri N, Ruiz-Cabello F, Garrido F. The transition from HLA-I positive to HLA-I negative primary tumors: the road to escape from T-cell responses. Curr Opin Immunol. 2018;51:123–32. [DOI] [PubMed] [Google Scholar]
- 110.Rodig SJ, Gusenleitner D, Jackson DG, Gjini E, Giobbie-Hurder A, Jin C, et al. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci Transl Med [Internet]. 2018. [cited 2021 Mar 1];10(450). Available from: http://www.ncbi.nlm.nih.gov/pubmed/30021886 [DOI] [PubMed] [Google Scholar]
- 111.Ladányi A, Papp E, Mohos A, Balatoni T, Liszkay G, Oláh J, et al. Role of the anatomic site in the association of HLA class I antigen expression level in metastases with clinical response to ipilimumab therapy in patients with melanoma. J Immunother cancer [Internet]. 2020. [cited 2021 Mar 1];8(1). Available from: http://www.ncbi.nlm.nih.gov/pubmed/32554608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cummings AL, Gukasyan J, Lu HY, Grogan T, Sunga G, Fares CM, et al. Mutational landscape influences immunotherapy outcomes among patients with non-small-cell lung cancer with human leukocyte antigen supertype B44. Nat Cancer [Internet]. 2020. December 16 [cited 2021 Feb 20];1(12):1167–75. Available from: http://www.nature.com/articles/s43018-020-00140-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Raffaghello L, Prigione I, Bocca P, Morandi F, Camoriano M, Gambini C, et al. Multiple defects of the antigen-processing machinery components in human neuroblastoma: immunotherapeutic implications. Oncogene [Internet]. 2005. July 7 [cited 2020 Nov 13];24(29):4634–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15897905 [DOI] [PubMed] [Google Scholar]
- 114.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest [Internet]. 2014. February [cited 2021 Mar 3];124(2):687–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24382348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gong J, Le TQ, Massarelli E, Hendifar AE, Tuli R. Radiation therapy and PD-1/PD-L1 blockade: the clinical development of an evolving anticancer combination. J Immunother cancer [Internet]. 2018. [cited 2021 Mar 3];6(1):46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29866197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mizukami Y, Kono K, Maruyama T, Watanabe M, Kawaguchi Y, Kamimura K, et al. Downregulation of HLA Class I molecules in the tumour is associated with a poor prognosis in patients with oesophageal squamous cell carcinoma. Br J Cancer [Internet]. 2008. November;99(9):1462–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18841157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Z, Marincola FM, Rivoltini L, Parmiani G, Ferrone S. Selective histocompatibility leukocyte antigen (HLA)-A2 loss caused by aberrant pre-mRNA splicing in 624MEL28 melanoma cells. J Exp Med [Internet]. 1999. July 19 [cited 2021 Jun 1];190(2):205–15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10432284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tripathi SC, Peters HL, Taguchi A, Katayama H, Wang H, Momin A, et al. Immunoproteasome Deficiency Is a Feature of Non-Small Cell Lung Cancer With a Mesenchymal Phenotype and Is Associated With a Poor Outcome. Proc Natl Acad Sci U S A [Internet]. 2016;113(11). Available from: https://pubmed.ncbi.nlm.nih.gov/26929325/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kalaora S, Lee JS, Barnea E, Levy R, Greenberg P, Alon M, et al. Immunoproteasome expression is associated with better prognosis and response to checkpoint therapies in melanoma. Nat Commun [Internet]. 2020;11. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7021791/#CR17 [DOI] [PMC free article] [PubMed] [Google Scholar]