Abstract
Background
Emerging data demonstrate that the use of integrase inhibitor (INSTI)-based antiretroviral treatment (ART) is associated with increased weight, but the cardiometabolic health consequences of increased weight remains poorly understood.
Methods
This analysis examined INSTI use (>6 months) at entry among REPRIEVE participants enrolled in High Income and Latin America/Caribbean Global Burden of Disease regions. Primary analyses used linear and logistic regression; secondary analyses used quantile regression to examine differences across the full data distribution. Characteristics of those with and without INSTI use were balanced using inverse probability of treatment weighting.
Results
Among 4500 REPRIEVE participants, 1848 were on an INSTI-based regimen at entry for an average of 2.1 ± 1.8 years. Integrase inhibitor use (vs no INSTI use) was associated with higher odds of obesity (odds ratio [OR], 1.63; 95% confidence interval [CI], 1.4–1.9) and higher mean body mass index ([BMI] +1.5kg/m2; 95% CI, 1.0–1.9) and waist circumference (+3.6cm; 95% CI, 2.6–4.6). Differences in weight related to INSTI use were greater in the upper tails of the distribution (+3.1kg/m2 [95% CI, 1.9–4.4] at the 90th centile vs +0.7kg/m2 [95% CI, 0.2–1.2] at the 50th centile) and among women and nonwhite participants, with sex and race having an additive effect on BMI. Conversely, INSTI use was not associated with differences in glucose, low-density lipoprotein cholesterol, or higher odds of metabolic syndrome or hypertension.
Conclusions
Differences in weight and waist circumference associated with INSTI use are (1) not uniform across people with human immunodeficiency virus, (2) greatest among women and nonwhites, and (3) concentrated at the upper tails of weight distribution. These data identify at-risk subgroups for whom long-term cardiovascular disease outcomes should be carefully assessed.
Keywords: cardiovascular risk, HIV, integrase inhibitors, metabolic syndrome, obesity
INSTI-based regimens are associated with higher weight but not with increased cardiovascular risk for most INSTI users. Differences in weight are not uniform across PWH, and specific subgroups of INSTI users should be monitored for long-term CVD outcomes.
Integrase inhibitor (INSTI)-based antiretroviral regimens (ie, antiretroviral therapy [ART]) are highly effective in suppressing human immunodeficiency virus (HIV) and are now the preferred regimens in most countries [1, 2]. Emerging epidemiologic data from multiple cohort studies and randomized controlled trials (RCTs) have demonstrated significant weight gain associated with INSTIs [3–5]. Although recent studies have started to evaluate cardiovascular disease (CVD) and diabetes risk among small cohorts of individuals taking various combinations of INSTI regimens [6], the cardiometabolic health consequences of weight gain associated with INSTI use remain largely unknown.
In this analysis, we aimed to simultaneously investigate the effects of INSTI use on weight and associated clinically relevant cardiometabolic parameters including central adiposity (waist circumference [WC]), glucose, low-density lipoprotein cholesterol (LDL-C), metabolic syndrome, and hypertension. We leveraged baseline data from the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE), a diverse, global, cohort of people with human immunodeficiency virus (PWH) eligible for primary prevention of CVD. The analysis population was sufficiently large to enable careful balancing of potential confounding characteristics using inverse probability treatment (IPT) weighting methodology, allowing for comparison of groups on similar duration of INSTI and non-INSTI regimens, and for sensitivity analyses assuring similar nucleoside reverse-transcriptase inhibitor (NRTI) use among the comparison groups. The inclusion of natal females and males in the cohort allowed us to assess sex-specific associations between INSTI use and metabolic changes; these factors are important because emerging data suggest that greater weight gain was associated with INSTI use among females [4, 5, 7]. These data, taken together, significantly extend our understanding of increased weight and cardiometabolic effects associated with INSTI use in PWH.
METHODS
Selection of Analysis Population
The inclusion and exclusion criteria for REPRIEVE have been reported previously [8] and trial population characteristics were published (see [9]). In brief, REPRIEVE enrolled a global cohort of PWH on ART between March 2015 and July 2019 with overall low to moderate traditional atherosclerotic cardiovascular disease (ASCVD) risk based on the 2013 American College of Cardiology/American Heart Association pooled cohort equation risk score and LDL-C level (Supplemental Table 1), randomized to pitavastatin calcium or placebo. (ClinicalTrials.gov Identifier NCT02344290.) Institutional review board (IRB) approval was obtained at each site for each participant. To ensure all participants had a nonzero probability of receiving an INSTI, this analysis was restricted to enrollment regions where at least 5% of the enrolled population was using INSTI-based regimens (High Income and Latin America/Caribbean Global Burden of Disease regions) (Supplemental Table 2). Participants with missing body mass index (BMI) at entry were excluded (n = 6) (Supplemental Figure 1). The final analysis set included baseline data from 4500 REPRIEVE participants who had been on a stable ART regimen for at least 6 months. We set the minimum duration of INSTI use to 6 months, in accordance with prior data showing that most weight gain was achieved during the first 6–12 months of initiation of INSTIs [10], and performed sensitivity analyses for longer duration. Information was collected on the composition and duration of ART regimen at entry into REPRIEVE, but information on prior ART history was not collected.
Patient Consent Statement
Each clinical research site obtained IRB/ethics committee approval and any other applicable regulatory entity approvals. Participants were provided with study information, including a discussion of risks and benefits, and were asked to sign the approved declaration of informed consent.
Data Sharing
Research data, with all patient identifiers removed, will be available as per National Institutes of Health policy to other researchers through request to the principal investigator (S.K.G.).
Study Outcomes
The primary outcomes were BMI (kg/m2), calculated based on weight and height at entry using a standard formula, obesity (BMI ≥30kg/m2), and WC (cm), collected according to a standardized operating procedure across all sites [9]. Secondary outcomes included glucose, LDL-C, metabolic syndrome, and hypertension. Glucose and LDL-C were collected in the fasting state and run centrally at Quest Diagnostics. Metabolic syndrome and hypertension were defined using standard criteria (see Supplemental Table 3).
Statistical Analysis
To assess the relationship between INSTI use and outcomes of interest, primary analyses used linear and logistic regressions, secondary analyses used quantile regressions to examine differences across the full data distribution. Analyses were performed within the full sample and separately by natal sex. To control for potential confounding by indication in the choice of ART regimen, we used stabilized IPT weighting; a methodology in which covariates between participants on an INSTI versus not on an INSTI were balanced in a weighted population. A directed acyclic graph was used to assess likely causal relationships between the exposure (INSTI use) and the primary outcomes of interest (BMI and WC) to determine which baseline covariates to include in the propensity model used to estimate the treatment weights. We carefully selected potential confounding variables for inclusion in propensity models and avoided selection of covariates that were part of an outcome measure or covariates that might influence treatment selection [11, 12]. The covariates included in the propensity model were natal sex, age, race, baseline ART duration, CD4 count, estimated glomerular filtration rate, cigarette use, substance use, diet quality, physical activity level, and use of estrogen or testosterone containing medications (Supplemental Figure 2, Supplemental Table 4).
Upon final selection of covariates, propensity scores and stabilized IPT weights were estimated. Weights were constrained via trimming of extreme values at the 1st and 99th percentiles. Standardized differences and graphical distributions were used to assess the balance of covariates between participants on an INSTI versus not on an INSTI in the weighted sample. Analyses by sex used re-estimated weights excluding natal sex from the propensity model.
The effect of specific INSTI-containing regimens was estimated using stabilized IPT weights for each type of INSTI (dolutegravir [DTG], elvitegravir [EVG], or raltegravir [RAL]) using an analogous procedure. The combination model weight was defined as the product of the stabilized weights from each propensity model. Due to the small number of participants on bictegravir (BIC) at entry (n = 7), we were unable to evaluate the potential effects of BIC in this analysis.
Crude and IPT-weighted regression models are presented in the results. Analyses adjusting for propensity score were also conducted showing consistent results. Formal statistical inference was guided by an alpha level of 0.05. Analyses were conducted using SAS software, version 9.4 (TS1M5, SAS/STAT 14.3; SAS Institute Inc., Cary, NC) on a Linux operating environment.
Sensitivity Analyses
To account for differences in duration of entry ART regimen between INSTI users and non-INSTI users, a sensitivity analysis was conducted restricting the analysis population to participants who had been on their entry regimen for 6 months to 5 years. In addition, because INSTI-associated weight gain tends to occur within the first 6–12 months of INSTI initiation, we conducted a sensitivity analysis restricting the population to those who had been on their entry ART regimen for at least 12 months to ensure that INSTI-associated weight differences were not underestimated.
Three sensitivity analyses were conducted to address the potential effects of tenofovir alafenamide (TAF). First, IPT weights were re-estimated to balance NRTI regimens between INSTI users in a reweighted analysis population. Second, we evaluated the effect of INSTIs on outcome measures excluding all TAF users from the analysis population. Third, we evaluated the effects of TAF versus no TAF on outcome measures among INSTI users.
Finally, in post hoc analyses, we examined interaction terms to evaluate for potential differential effects of INSTI use by race (white vs nonwhite), ASCVD risk category (≤/>5%), and obesity (BMI ≥30kg/m2).
RESULTS
Study Population
The final analysis population included 4500 participants, 1848 of whom were on an INSTI-based regimen at entry. Baseline demographics and behavioral characteristics are shown in Table 1. Median age was 51 years. Twenty-three percent were natal female, 93% identified as cisgender, and 40% were black or African American. Table 2 highlights baseline metabolic characteristics, including BMI (27.5kg/m2 [±5.7]), WC (95.5cm [±13.8]), fasting glucose (93.0mg/dL [±14.0]), and LDL-C (108mg/dL [±31]). Metabolic syndrome was present in 28% of the analysis population. Human immunodeficiency virus-related health parameters and information on specific ART regimens are shown in Table 3 and Table 4, respectively. Mean duration of entry ART regimen was 3.8 years overall (±3.4) and 2.1 years (±1.8) among participants on an INSTI-based regimen.
Table 1.
Baseline Demographics and Behavioral Characteristicsa
| Among Analysis Population | Among Participants on INSTIs at Entry | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Total (N = 4500) | Non-INSTI-Containing Entry Regimen (N = 2652) | INSTI-Containing Entry Regimen (N = 1848) | Entry INSTI = DTG (N = 882) | Entry INSTI = EVG (N = 643) | Entry INSTI = Otherb (N = 323) | |
| DemographicsandBehavioral | |||||||
| Age (years) | Median (Q1, Q3) | 51 (46, 55) | 50 (46, 55) | 51 (47, 56) | 52 (47, 56) | 50 (45, 55) | 51 (47, 55) |
| 40–44 | 801 (18%) | 512 (19%) | 289 (16%) | 114 (13%) | 131 (20%) | 44 (14%) | |
| 45–49 | 1,156 (26%) | 699 (26%) | 457 (25%) | 214 (24%) | 163 (25%) | 80 (25%) | |
| 50–54 | 1,235 (27%) | 706 (27%) | 529 (29%) | 239 (27%) | 183 (28%) | 107 (33%) | |
| 55–59 | 886 (20%) | 497 (19%) | 389 (21%) | 212 (24%) | 110 (17%) | 67 (21%) | |
| 60+ | 422 (9%) | 238 (9%) | 184 (10%) | 103 (12%) | 56 (9%) | 25 (8%) | |
| Natal sex | Female | 1040 (23%) | 675 (25%) | 365 (20%) | 174 (20%) | 117 (18%) | 74 (23%) |
| Male | 3460 (77%) | 1977 (75%) | 1483 (80%) | 708 (80%) | 526 (82%) | 249 (77%) | |
| Gender identity | Cisgender | 4200 (93%) | 2492 (94%) | 1708 (92%) | 821 (93%) | 592 (92%) | 295 (91%) |
| Transgender Spectrum | 91 (2%) | 46 (2%) | 45 (2%) | 21 (2%) | 17 (3%) | 7 (2%) | |
| Not reported | 209 (5%) | 114 (4%) | 95 (5%) | 40 (5%) | 34 (5%) | 21 (7%) | |
| Race | Black or African American | 1799 (40%) | 1073 (40%) | 726 (39%) | 357 (40%) | 254 (40%) | 115 (36%) |
| White | 2210 (49%) | 1204 (45%) | 1006 (54%) | 478 (54%) | 348 (54%) | 180 (56%) | |
| Other | 491 (11%) | 375 (14%) | 116 (6%) | 47 (5%) | 41 (6%) | 28 (9%) | |
| Ethnicity | Hispanic or Latino | 569 (13%) | 286 (11%) | 283 (15%) | 123 (14%) | 106 (16%) | 54 (17%) |
| Not Applicable | 1323 (29%) | 1163 (44%) | 160 (9%) | 103 (12%) | 24 (4%) | 33 (10%) | |
| Not Hispanic or Latino | 2608 (58%) | 1203 (45%) | 1405 (76%) | 656 (74%) | 513 (80%) | 236 (73%) | |
| Country of Enrollment | USA/Canada | 3,177 (71%) | 1489 (56%) | 1688 (91%) | 779 (88%) | 619 (96%) | 290 (90%) |
| Brazil | 912 (20%) | 851 (32%) | 61 (3%) | 45 (5%) | 0 (0%) | 16 (5%) | |
| Spain | 179 (4%) | 84 (3%) | 95 (5%) | 58 (7%) | 24 (4%) | 13 (4%) | |
| Haiti | 123 (3%) | 123 (5%) | 0 (0%) | ||||
| Peru | 109 (2%) | 105 (4%) | 4 (<0.5%) | 0 (0%) | 0 (0%) | 4 (1%) | |
| Smoking status | Current | 1271 (28%) | 729 (28%) | 542 (29%) | 273 (31%) | 184 (29%) | 85 (26%) |
| Former | 1300 (29%) | 722 (27%) | 578 (31%) | 262 (30%) | 207 (32%) | 109 (34%) | |
| Never | 1921 (43%) | 1198 (45%) | 723 (39%) | 344 (39%) | 252 (39%) | 127 (40%) | |
| Substance use | Current | 124 (3%) | 78 (3%) | 46 (2%) | 21 (2%) | 20 (3%) | 5 (2%) |
| Former | 1818 (40%) | 900 (34%) | 918 (50%) | 429 (49%) | 320 (50%) | 169 (53%) | |
| Never | 2551 (57%) | 1671 (63%) | 880 (48%) | 430 (49%) | 303 (47%) | 147 (46%) | |
Abbreviations: DTG, dolutegravir; EVG, elvitegravir; INSTI, integrase-strand transfer inhibitor;
All statistics are calculated with data collected from participants.
“Other” race includes participants who self-identify as follows: native or indigenous to the enrollment region; more than 1 race (with no single race noted as predominant); or of unknown race.
Table 2.
Baseline Metabolic Characteristicsa
| Among Analysis Population | Among Participants on INSTIs at Entry | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Total (N = 4500) | Non-INSTI-Containing Entry Regimen (N = 2652) | INSTI-Containing Entry Regimen (N = 1848) | Entry INSTI = DTG (N = 882) | Entry INSTI = EVG (N = 643) | Entry INSTI = Otherb (N = 323) | |
| MetabolicCharacteristics | |||||||
| BMI (kg/m²) | Mean (s.d.) | 27.5 (5.7) | 26.9 (5.3) | 28.2 (6.1) | 28.2 (5.9) | 28.5 (6.1) | 27.8 (6.3) |
| Median (Q1, Q3) | 26.5 (23.7, 30.1) | 26.2 (23.4, 29.5) | 27.0 (24.3, 30.8) | 27.0 (24.1, 30.7) | 27.6 (24.5, 31.2) | 26.6 (23.7, 30.3) | |
| 10%, 90% | 21.5, 34.6 | 21.1, 33.5 | 22.0, 35.9 | 22.0, 35.5 | 22.1, 36.7 | 21.5, 35.1 | |
| 30+ | 1,146 (25%) | 589 (22%) | 557 (30%) | 261 (30%) | 208 (32%) | 88 (27%) | |
| <30 | 3,354 (75%) | 2,063 (78%) | 1291 (70%) | 621 (70%) | 435 (68%) | 235 (73%) | |
| Waist circumference (cm) | Mean (s.d.) | 95.5 (13.8) | 94.0 (12.8) | 97.7 (14.8) | 97.8 (14.5) | 98.0 (15.1) | 96.9 (15.0) |
| Median (Q1, Q3) | 94.2 (86.4, 102.6) | 93.0 (85.5, 101.0) | 96.0 (88.0, 105.7) | 96.0 (88.3, 105.5) | 96.0 (88.6, 106.0) | 96.0 (87.0, 105.7) | |
| 10%, 90% | 80.0, 112.5 | 78.9, 109.9 | 81.5, 116.5 | 81.5, 117.0 | 81.7, 116.2 | 80.5, 114.5 | |
| Fasting glucose (mg/dL) | Mean (s.d.) | 93.0 (14.0) | 93.0 (14.0) | 93.1 (14.1) | 93.9 (15.0) | 92.4 (13.2) | 92.5 (13.1) |
| Median (Q1, Q3) | 92 (86, 98) | 92 (86, 98) | 91 (85, 99) | 92 (85, 100) | 91 (85, 98) | 91 (85, 98) | |
| 10%, 90% | 80, 106 | 80, 105 | 79, 108 | 80, 111 | 79, 106 | 79, 105 | |
| Fasting triglycerides (mg/dL) | Mean (s.d.) | 139 (86) | 143 (89) | 134 (83) | 130 (80) | 136 (85) | 139 (86) |
| Median (Q1, Q3) | 116 (83, 168) | 119 (85, 175) | 111 (79, 159) | 110 (78, 154) | 112 (81, 162) | 111 (81, 172) | |
| 10%, 90% | 63, 245 | 63, 250 | 62, 233 | 59, 223 | 63, 233 | 62, 261 | |
| Fasting-derived LDL cholesterol (mg/dL) | Mean (s.d.) | 108 (31) | 108 (31) | 108 (30) | 107 (31) | 111 (30) | 105 (30) |
| Median (Q1, Q3) | 107 (87, 128) | 108 (87, 128) | 107 (87, 129) | 106 (86, 128) | 111 (90, 131) | 105 (84, 126) | |
| 10%, 90% | 70, 147 | 71, 148 | 68, 147 | 68, 145 | 74, 148 | 67, 146 | |
| Fasting HDL cholesterol (mg/dL) | Mean (s.d.) | 50 (16) | 50 (16) | 49 (16) | 49 (16) | 49 (15) | 49 (17) |
| Median (Q1, Q3) | 47 (39, 58) | 47 (39, 58) | 46 (38, 57) | 46 (38, 56) | 46 (39, 57) | 47 (37, 58) | |
| 10%, 90% | 33, 70 | 33, 71 | 32, 68 | 31, 68 | 33, 68 | 31, 69 | |
| HGB (g/dL) | Mean (s.d.) | 14.3 (1.5) | 14.3 (1.5) | 14.4 (1.5) | 14.4 (1.5) | 14.5 (1.4) | 14.5 (1.5) |
| Median (Q1, Q3) | 14.4 (13.4, 15.4) | 14.3 (13.3, 15.3) | 14.6 (13.5, 15.4) | 14.6 (13.5, 15.4) | 14.6 (13.5, 15.5) | 14.5 (13.6, 15.4) | |
| 10%, 90% | 12.4, 16.2 | 12.3, 16.1 | 12.5, 16.2 | 12.4, 16.2 | 12.6, 16.2 | 12.8, 16.2 | |
| eGFR by CKD-EPI (mL/min per 1.73 mm²) | 60 to <90 | 1913 (43%) | 918 (35%) | 995 (54%) | 500 (57%) | 330 (51%) | 165 (51%) |
| <60 | 157 (3%) | 43 (2%) | 114 (6%) | 69 (8%) | 33 (5%) | 12 (4%) | |
| ≥90 | 2428 (54%) | 1691 (64%) | 737 (40%) | 311 (35%) | 280 (44%) | 146 (45%) | |
| Metabolic syndrome | No | 3086 (69%) | 1800 (68%) | 1286 (70%) | 602 (68%) | 461 (72%) | 223 (69%) |
| Yes | 1278 (28%) | 782 (29%) | 496 (27%) | 254 (29%) | 149 (23%) | 93 (29%) | |
| Not evaluable | 136 (3%) | 70 (3%) | 66 (4%) | 26 (3%) | 33 (5%) | 7 (2%) | |
Abbreviations: CKD, chronic kidney disease; DTG, dolutegravir; eGFR, estimated glomerular filtration rate; EPI, epinephrine; EVG, elvitegravir; HDL, high-density lipoprotein; HGB, hemoglobin; INSTI, integrase-strand transfer inhibitor; LDL, low-density lipoprotein cholesterol; s.d., standard deviation.
All statistics are calculated with data collected from participants. Missing data include the following: waist circumference (n = 88); fasting glucose (n = 145); fasting triglycerides (n = 115); fasting-derived LDL cholesterol (n = 142); fasting HDL cholesterol (n = 115); fasting HGB (n = 26).
“Other” INSTI includes participants on raltegravir or bictegravir.
Table 3.
HIV-Related Health Status at Baselinea
| Among Analysis Population | Among Participants on INSTIs at Entry | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Total (N = 4500) | Non-INSTI-Containing Entry Regimen (N = 2652) | INSTI-Containing Entry Regimen (N = 1848) | Entry INSTI = DTG (N = 882) | Entry INSTI = EVG (N = 643) | Entry INSTI = Otherb (N = 323) | |
| HIV-RelatedHealthStatus | |||||||
| Nadir CD4 count (cells/mm³) | <50 | 854 (19%) | 461 (17%) | 393 (21%) | 205 (23%) | 103 (16%) | 85 (26%) |
| 50–199 | 1210 (27%) | 701 (26%) | 509 (28%) | 236 (27%) | 178 (28%) | 95 (29%) | |
| 200–349 | 1188 (26%) | 755 (28%) | 433 (23%) | 201 (23%) | 170 (26%) | 62 (19%) | |
| 350+ | 1079 (24%) | 640 (24%) | 439 (24%) | 212 (24%) | 162 (25%) | 65 (20%) | |
| Unknown | 169 (4%) | 95 (4%) | 74 (4%) | 28 (3%) | 30 (5%) | 16 (5%) | |
| Total ART use duration (years) | Mean (s.d.) | 11 (7) | 10 (6) | 12 (8) | 12 (8) | 10 (7) | 14 (7) |
| Median (Q1, Q3) | 10 (5, 16) | 9 (5, 15) | 11 (6, 18) | 11 (6, 18) | 8 (4, 15) | 15 (8, 20) | |
| <5 | 978 (22%) | 576 (22%) | 402 (22%) | 189 (21%) | 181 (28%) | 32 (10%) | |
| 5–10 | 1288 (29%) | 825 (31%) | 463 (25%) | 205 (23%) | 183 (28%) | 75 (23%) | |
| 10+ | 2232 (50%) | 1250 (47%) | 982 (53%) | 487 (55%) | 279 (43%) | 216 (67%) | |
| Unknown | 2 (<0.5%) | 1 (<0.5%) | 1 (<0.5%) | 1 (<0.5%) | 0 (0%) | 0 (0%) | |
| Duration of Entry ART Regimen (years) | Mean (s.d.) | 4 (3) | 5 (4) | 2 (2) | 2 (1) | 2 (1) | 4 (3) |
| Median (Q1, Q3) | 3 (1, 6) | 4 (2, 8) | 1 (1, 3) | 1 (1, 2) | 1 (1, 2) | 4 (2, 6) | |
| 6 months <1 | 815 (18%) | 266 (10%) | 549 (30%) | 287 (33%) | 218 (34%) | 44 (14%) | |
| 1–3 | 1697 (38%) | 766 (29%) | 931 (50%) | 497 (56%) | 359 (56%) | 75 (23%) | |
| 3–5 | 706 (16%) | 487 (18%) | 219 (12%) | 84 (10%) | 60 (9%) | 75 (23%) | |
| 5+ | 1282 (28%) | 1133 (43%) | 149 (8%) | 14 (2%) | 6 (1%) | 129 (40%) | |
| CD4 count (cells/mm³) | 500+ | 3118 (69%) | 1900 (72%) | 1,218 (66%) | 568 (64%) | 440 (68%) | 210 (65%) |
| <500 | 1382 (31%) | 752 (28%) | 630 (34%) | 314 (36%) | 203 (32%) | 113 (35%) | |
| HIV-1 RNA (copies/mL) | 400+ | 74 (2%) | 40 (2%) | 34 (2%) | 14 (2%) | 11 (2%) | 9 (3%) |
| <400 | 4047 (98%) | 2308 (98%) | 1739 (98%) | 838 (98%) | 604 (98%) | 297 (97%) | |
Abbreviations: ART, antiretroviral therapy; DTG, dolutegravir; EVG, elvitegravir; HIV, human immunodeficiency virus; INSTI, integrase-strand transfer inhibitor; RNA, ribonucleic acid; s.d., standard deviation.
All statistics are calculated with data collected from participants.
“Other” INSTI includes participants on raltegravir or bictegravir.
Table 4.
ART Regimen Detailsa
| Among Analysis Population | Among Participants on INSTI at Entry | ||||||
|---|---|---|---|---|---|---|---|
| Regimen Details | Specific Regimen | Total (N = 4500) | Non-INSTI- Containing Entry Regimen (N = 2652) | INSTI-Containing Entry Regimen (N = 1848) | Entry INSTI = DTG (N = 882) | Entry INSTI = EVG (N = 643) | Entry INSTI = Otherb (N = 323) |
| ART regimen (by class) | NRTI + NNRTI | 1638 (36%) | 1638 (62%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| NRTI + INSTI | 1437 (32%) | 0 (0%) | 1437 (78%) | 689 (78%) | 592 (92%) | 156 (48%) | |
| NRTI + PI | 930 (21%) | 930 (35%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| NRTI-sparing | 146 (3%) | 25 (1%) | 121 (7%) | 69 (8%) | 0 (0%) | 52 (16%) | |
| Other NRTI-containing | 349 (8%) | 59 (2%) | 290 (16%) | 124 (14%) | 51 (8%) | 115 (36%) | |
| Entry NRTI | TDF | 2574 (59%) | 1891 (72%) | 683 (40%) | 200 (25%) | 293 (46%) | 190 (70%) |
| ABC | 678 (16%) | 208 (8%) | 470 (27%) | 448 (55%) | 0 (0%) | 22 (8%) | |
| TAF | 668 (15%) | 182 (7%) | 486 (28%) | 107 (13%) | 350 (54%) | 29 (11%) | |
| ZDV | 261 (6%) | 255 (10%) | 6 (0%) | 3 (0%) | 0 (0%) | 3 (1%) | |
| Other NRTI | 173 (4%) | 91 (3%) | 82 (5%) | 55 (7%) | 0 (0%) | 27 (10%) | |
| Entry NNRTI | EFV | 1271 (70%) | 1261 (75%) | 10 (8%) | 0 (0%) | 0 (0%) | 10 (15%) |
| RPV | 337 (19%) | 291 (17%) | 46 (37%) | 43 (72%) | 0 (0%) | 3 (5%) | |
| NVP | 103 (6%) | 103 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| ETR | 97 (5%) | 28 (2%) | 69 (55%) | 17 (28%) | 0 (0%) | 52 (80%) | |
| Entry PI | DRV | 655 (50%) | 377 (38%) | 278 (84%) | 130 (88%) | 44 (86%) | 104 (79%) |
| ATV | 529 (40%) | 493 (50%) | 36 (11%) | 13 (9%) | 7 (14%) | 16 (12%) | |
| LPV | 112 (8%) | 99 (10%) | 13 (4%) | 3 (2%) | 0 (0%) | 10 (8%) | |
| Other PI | 27 (2%) | 23 (2%) | 4 (1%) | 2 (1%) | 0 (0%) | 2 (2%) | |
Abbreviations: ABC, abacavir; ART, antiretroviral therapy; ATV, atazanavir; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; ETR, etravirine; EVG, elvitegravir; INSTI, integrase-strand transfer inhibitor; LPV, lopinavir; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse-transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
All statistics are calculated with data collected from participants. Entry PI does not include boosting agents.
“Other” INSTI includes participants on raltegravir or bictegravir.
Propensity Scores and Inverse Probability Treatment Weighting
Baseline covariates included in the propensity model were successfully balanced between participants on an INSTI versus not on an INSTI via IPT weighting (Supplemental Figures 3 and 4). Stabilized IPT weights ranged from 1.07 to 7.24 after trimming at the 1st and 99th percentiles.
In the sensitivity analysis accounting for differences in NRTI regimens, including TAF and TDF, re-estimated weights successfully balanced NRTI regimens between INSTI users and non-INSTI users in the weighted sample.
Evaluating Integrase Inhibitor Effects on Weight Parameters
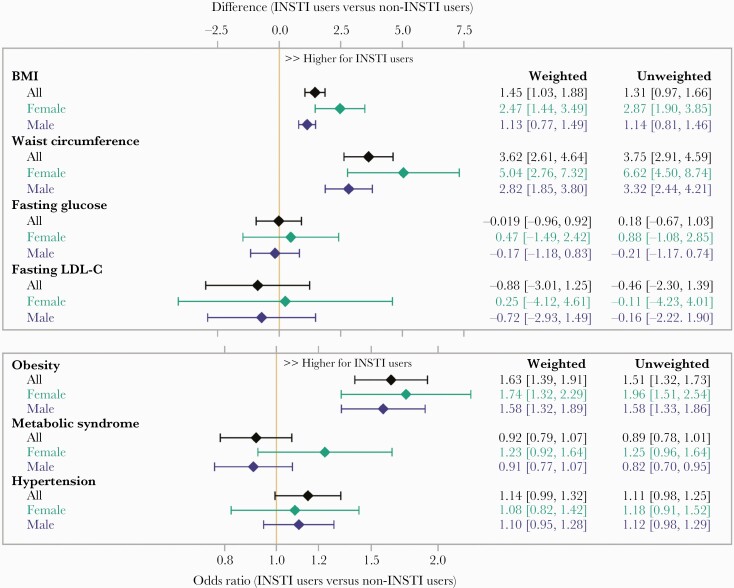
In the overall sample, INSTI use (vs no INSTI use) was associated with a higher mean BMI of +1.5kg/m2 (95% confidence interval [CI], 1.0–1.9), 63% higher odds of obesity (odds ratio [OR], 1.63; 95% CI, 1.4–1.9), and a higher mean WC of +3.6cm (2.6–4.6) (Figure 1). Integrase inhibitor-associated differences in BMI and WC were greatest among females, among whom INSTI use (vs no INSTI use) was associated with a higher mean BMI of + 2.5kg/m2 (95% CI, 1.4–3.5) and 74% higher odds of obesity (OR, 1.74; 95% CI, 1.3–2.3). In comparison, among males, INSTI use was associated with a higher BMI of +1.1kg/m2 (95% CI, 0.8–1.5) and increased odds of obesity of 58% (OR, 1.58; 95% CI, 1.3–1.9). Likewise, INSTI use was associated with a higher mean WC of +5.0cm (95% CI, 2.8–7.3) in females compared to +2.8cm (95% CI, 1.9–3.8) in males.
Figure 1.
Inverse probability of treatment weighted linear and logistic regression estimates of integrase inhibitors on primary and secondary outcomes of interest. INSTI, integrase-strand transfer inhibitor; LDL-C, low-density lipoprotein cholesterol.
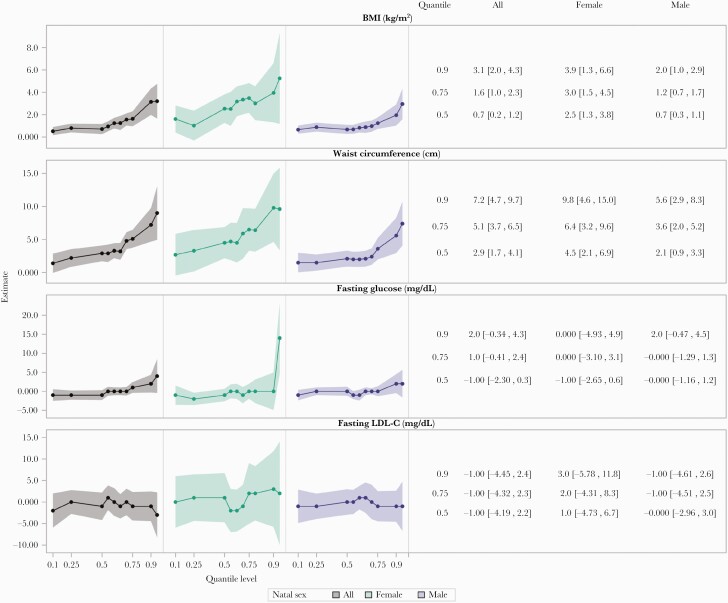
Quantile regressions revealed larger differences between INSTI users and non-INSTI users in the upper tails of the BMI and WC distributions, which were more pronounced among females compared to males. Comparing INSTI to non-INSTI users, differences in BMI of +0.7kg/m2 and +2.5kg/m2 were seen at the 50th centile of BMI among males and females, respectively, whereas differences of +2.0kg/m2 and +3.9kg/m2 were seen for males and females, respectively, at the 90th centile (Figure 2).
Figure 2.
Inverse probability of treatment weighted quantile regression estimates of integrase inhibitors on body mass index (BMI), waist circumference, fasting glucose, and LDL-cholesterol (LDL-C).
A similar effect was seen across the distribution of WC: comparing INSTI to non-INSTI users, differences of +2.1cm and +4.5cm were seen at the 50th centile and 5.6cm and 9.8cm were seen at the 90th centile for males and females, respectively. The IPT-weighted histograms illustrate these findings, highlighting a more skewed and longer tailed distribution of BMI and WC among INSTI users compared to nonusers (Supplemental Figure 5).
Evaluating Integrase Inhibitor Effects on Cardiometabolic Parameters
In the overall sample, INSTI use was not associated with a difference in mean fasting glucose, LDL-C, higher odds of hypertension, or metabolic syndrome (Figure 1). When stratified by natal sex, no association between INSTI use and fasting glucose, LDL-C, or hypertension among males or females was apparent.
Despite marked differences in the upper tails of the BMI and WC distributions in association with INSTI use, differences in the tails of the fasting glucose and LDL-C distributions were largely not apparent except among females, in whom higher fasting glucose associated with INSTI use was apparent at the highest (95th) centile of the glucose distribution (Figure 2 and Supplemental Figure 5). Among participants with the highest measures of BMI (≥30kg/m2), we found no evidence of interaction (P > .05) that would indicate a differential effect of INSTI use on LDL-C, glucose, metabolic syndrome, or hypertension.
Evaluating Effects of Specific Integrase Inhibitor-Containing Regimens
When evaluating the effects of specific INSTI regimens compared with non-INSTI containing regimens, EVG-based regimens were associated with the largest differences in BMI and WC (Table 5). However, higher weight and WC were seen across all INSTI regimens, and differential effects on BMI and WC were not apparent when comparing individual INSTIs to each other. Neither EVG-, DTG-, nor RAL-containing regimens were associated with significant differences in fasting glucose or LDL-C levels at the mean. However, the DTG-containing regimens (vs non-INSTI regimens) were associated with higher odds of hypertension (Table 5).
Table 5.
Estimated Linear and Logistic Regression Parameters of INSTI Use by Type of INSTI on BMI, Waist Circumference, Fasting Glucose, and Fasting LDL-C at REPRIEVE Entry
| Outcome | Overall (n = 4500) | Natal Sex = Female (n = 1040) | Natal Sex = Male (3460) | |||
|---|---|---|---|---|---|---|
| Weighted Estimate (95% CI)a,b | P Valuec | Weighted Estimate (95% CI)a,b | P Valuec | Weighted Estimate (95% CI)a,b | P Valuec | |
| Linear Regressions | ||||||
| BMI (kg/m2) | .19 | .54 | .18 | |||
| EVG vs No INSTI | 1.6 (1.1–2.2) | 3.3 (1.7–4.9) | 1.4 (0.8–1.9) | |||
| DTG vs No INSTI | 1.2 (0.7–1.7) | 2.3 (0.8–3.8) | 1.0 (0.5–1.5) | |||
| RAL vs No INSTI | 0.9 (0.1–1.6) | 2.1 (0.3–3.9) | 0.5 (−0.2 to 1.3) | |||
| Waist Circumference (cm) | .43 | .51 | .51 | |||
| EVG vs No INSTI | 4.1 (2.7 5.5) | 7.3 (3.9–10.7) | 3.5 (1.9–5.0) | |||
| DTG vs No INSTI | 3.5 (2.3–4.8) | 5.0 (1.9–8.2) | 3.3 (2.0–4.7) | |||
| RAL vs No INSTI | 2.6 (0.8–4.5) | 4.9 (1.2–8.5) | 2.1 (−0.02 to 4.2) | |||
| Fasting Glucose (mg/dL) | .13 | .49 | .10 | |||
| EVG vs No INSTI | −0.4 (−1.7 to 0.9) | 0.9 (−2.0 to 3.7) | −0.8 (−2.3 to 0.7) | |||
| DTG vs No INSTI | 0.9 (−0.4 to 2.2) | 0.5 (−3.1 to 4.1) | 0.9 (−0.4 to 2.3) | |||
| RAL vs No INSTI | −0.9 (−2.5 to 0.7) | −1.4 (−4.4 to 1.1) | −0.7 (−2.6 to 1.1) | |||
| Fasting LDL-C (mg/dL) | .04 | .21 | .01 | |||
| EVG vs No INSTI | 2.0 (−0.9 to 4.9) | −1.6 (−8.1 to 5.0) | 3.1 (−0.1 to 6.3) | |||
| DTG vs No INSTI | −1.5 (−4.1 to 1.2) | 2.7 (−3.1 to 8.6) | −2.5 (−5.4 to 0.5) | |||
| RAL vs No INSTI | −3.3 (−7.4 to 0.8) | −6.0 (−14.5 to 2.5) | −2.4 (−7.1 to 2.3) | |||
| Logistic Regressions | ||||||
| Obesity | .13 | .18 | .19 | |||
| EVG vs No INSTI | 1.8 (1.5–2.2) | 2.6 (1.7–4.0) | 1.7 (1.4–2.2) | |||
| DTG vs No INSTI | 1.5 (1.2–1.8) | 1.7 (1.1–2.5) | 1.6 (1.3–2.0) | |||
| RAL vs No INSTI | 1.3 (0.9–1.7) | 1.5 (0.9–2.6) | 1.2 (0.8–1.8) | |||
| Metabolic Syndrome | .04 | 1.0 | .02 | |||
| EVG vs No INSTI | 0.8 (0.6–1.0) | 1.2 (0.8–1.9) | 0.7 (0.5–0.9) | |||
| DTG vs No INSTI | 1.1 (0.9–1.3) | 1.3 (0.9–1.9) | 1.0 (0.8–1.3) | |||
| RAL vs No INSTI | 1.0 (0.8–1.4) | 1.3 (0.7–2.3) | 1.0 (0.7–1.4) | |||
| Hypertension | .0003 | .39 | .0004 | |||
| EVG vs No INSTI | 0.9 (0.7–1.1) | 1.0 (0.7–1.6) | 0.9 (0.7–1.1) | |||
| DTG vs No INSTI | 1.4 (1.2–1.7) | 1.4 (1.0–2.1) | 1.5 (1.2–1.8) | |||
| RAL vs No INSTI | 1.1 (0.8–1.4) | 1.0 (0.6–1.8) | 1.1 (0.8–1.5) | |||
Abbreviations: BMI, body mass index; CI, confidence interval; DTG, dolutegravir; EVG, elvitegravir; INSTI, integrase-strand transfer inhibitor; LDL-C, low-density lipoprotein cholesterol; RAL, raltegravir.
Weighted estimates are estimated using the product of inverse probability of treatment weights (IPTW) of each type of INSTI.
Covariates used to create IPTW included the following: natal sex, age, race, CD4 count, estimated glomerular filtration rate (</>90), smoking status, substance use, estrogen-containing preparations, testosterone-containing preparations, diet quality, and physical activity level. Sex-stratified regressions used re-estimated IPTW excluding natal sex.
P values represent contrast results evaluating whether INSTI effects differed by type of INSTI.
Sensitivity Analyses
The overall results did not differ when the analysis population was restricted to participants on their entry regimen for 6 months to 5 years. When restricted to participants on their entry regimen for at least 12 months, INSTI-associated differences in BMI and WC were only slightly higher compared to the differences seen in the unrestricted population (+1.7kg/m2 vs +1.5kg/m2 and +4.2cm vs +3.6cm).
When type of NRTI regimen—including TDF and TAF—was balanced between INSTI users and nonusers in a reweighted sample, the associations between INSTI use and higher BMI and WC remained consistent, as did the lack of associations between INSTI use and fasting glucose, metabolic syndrome, and hypertension. In this reweighted sample, INSTI use was associated with lower LDL-C levels at the mean (−3.3mg/dL [95% CI, −5.5 to −1.0]). When TAF users were excluded from the analysis population, the overall results were not different (Table 6). Among INSTI users only, TAF use was associated with higher BMI, WC, and LDL-C but not with increased fasting glucose or odds of metabolic syndrome or hypertension (Supplemental Table 5).
Table 6.
Effects of INSTI-Based Regimens on BMI, Waist Circumference, Fasting Glucose, LDL-C, Metabolic Syndrome, and Hypertension, With and Without Participants on TAF-Based Regimensa
| Outcome | Effects of INSTI-Based Regimens With TAF Users | Effects of INSTI-Based Regimens Without TAF Users | ||
|---|---|---|---|---|
| Crude Estimate (95% CI) | Weighted Estimate (95% CI)b | Crude Estimate (95% CI) | Weighted Estimate (95% CI)b | |
| Linear Regressionsc | ||||
| BMI (kg/m2) | 1.3 (1.0–1.7) | 1.5 (1.0–1.9) | 1.1 (0.8–1.5) | 1.4 (0.9–1.9) |
| Waist circumference (cm) | 3.8 (2.9–4.6) | 3.6 (2.6–4.6) | 3.3 (2.3–4.2) | 3.4 (2.2–4.5) |
| Fasting glucose (mg/dL) | 0.9 (−0.7 to 1.0) | −0.02 (−1.0 to 0.9) | 0.2 (−0.7 to 1.2) | −0.01 (−1.0 to 1.0) |
| Fasting LDL (mg/dL) | −0.5 (−2.3 to 1.4) | −0.9 (−3.0 to 1.3) | −2.5 (0.02) | −1.6 (−4.0 to 0.7) |
| Logistic Regressionsd | ||||
| Crude OR (95% CI) | Weighted OR (95% CI) | Crude OR (95% CI) | Weighted OR (95% CI) | |
| Obesity | 1.5 (1.3–1.4) | 1.6 (1.4–1.9) | 1.4 (1.2–1.7) | 1.6 (1.3–1.9) |
| Metabolic syndrome | 0.9 (0.8–1.0) | 0.9 (0.8–1.1) | 0.9 (0.8–1.0) | 0.9 (0.8–1.1) |
| Hypertension | 1.1 (1.0–1.3) | 1.1 (1.0–1.3) | 1.1 (1.0–1.3) | 1.2 (1.0–1.4) |
Abbreviations: BMI, body mass index; CI, confidence interval; INSTI, integrase-strand transfer inhibitor; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio; TAF, tenofovir alafenamide.
Weighted estimates are estimated using inverse probability of treatment weights (IPTW).
Covariates used to create IPTW included the following: natal sex, age, race, CD4 count, estimated glomerular filtration rate (</>90), smoking status, substance use, estrogen-containing preparations, testosterone-containing preparations, diet quality, and physical activity level. Sex-stratified regressions used re-estimated IPTW excluding natal sex.
Estimates represent absolute difference (INSTI use vs no INSTI use).
Estimates represent odds ratios (INSTI use vs no INSTI use).
In a post hoc analysis evaluating for differential effects of INSTI use and BMI by race (white vs nonwhite), in addition to the significant interaction between natal sex and INSTI use, we found significant interaction between race and INSTI use (P = .0007). Taken together, the smallest effect of INSTI use on BMI was seen among white men (+0.8kg/m2 [95% CI, 0.4–1.2]) then nonwhite men (+1.6kg/m2 [95% CI, 1.1–2.1]), white women (+1.9kg/m2 [95% CI, 0.1–3.6]), and finally nonwhite women (+3.3kg/m2 [95% CI, 2.2–4.5]) (Supplemental Figure 6). Finally, no differential effects of INSTIs by ASCVD risk score, on BMI, WC, glucose, or LDL, were seen (P for interaction >.05).
DISCUSSION
In this baseline analysis of 4500 REPRIEVE participants with approximately 2000 participants on an INSTI-based entry regimen for an average of 2.1 years, we observed that INSTI use was associated with higher BMI and WC and greater odds of obesity. These associations were most pronounced among women. Despite these findings, INSTI use was not associated with a difference in fasting glucose, LDL-C, metabolic syndrome, or hypertension overall.
Our data extend findings from prior studies demonstrating greater weight gain associated with INSTI-based regimens as observed in several cohort studies and RCTs including ADVANCE [4, 6], NAMSAL [13], and other studies [3, 5, 7, 14–16]. Our analysis has one of the largest sample sizes to date and includes a multinational population. Leveraging the trial size, we performed novel analyses and demonstrated that the greater weight associated with INSTI use was most striking in the upper tail of the BMI distribution. These data on weight distribution highlight the important observation that for both men and women, those with higher BMI are at greater risk to be differentially affected by INSTI use. Further work is needed to identify the mechanistic factors contributing to increased weight associated with INSTI use among this smaller group of individuals, who will need to be carefully monitored for CVD and metabolic complications.
We further demonstrate a significant association between INSTI use and higher WC in sex-stratified analyses. Moreover, the sex-stratified analyses demonstrate a more skewed distribution of WC among women on an INSTI, relative to men. Waist circumference, as an indicator of body fat distribution, is an important overall determinant of increased risk of cardiometabolic disease, above and beyond BMI [17]. In prior studies, AIDS Clinical Trials Group (ACTG) A5257 demonstrated sex differences on WC with RAL [18], and the Women’s Interagency HIV Study (WIHS) showed INSTI effects on WC among women without a male comparator [19]. We extend these data by examining the distribution tails, the association of WC with multiple CVD indices, and the effects of multiple INSTI regimens on WC simultaneously in both males and females. It is notable that WC does not permit delineation of visceral and subcutaneous fat, which differ in terms of CVD risk promotion. Further studies on the relative distribution, composition, and functionality of added weight are needed to better understand (1) the mechanism of weight and WC gain associated with INSTI use in women and (2) associated future metabolic and CVD risks [20].
Prior studies have demonstrated differential effects of INSTI use by race [4, 5, 18, 21]. In this study, we examined the impact of race in conjunction with sex, demonstrating a differential effect of INSTIs on BMI between white and nonwhite participants. These differential effects of INSTI use by race and sex were additive, with the greatest effect among nonwhite women. Thus, heightened awareness and focus on the potential side effects of weight gain are needed in women and nonwhite individuals with HIV who are taking INSTIs.
This analysis helps to fill an important knowledge gap in the field as to whether increased weight with INSTI use is associated with increased CVD risk. Our data did not identify any differences in key cardiometabolic parameters including fasting glucose, LDL-C, metabolic syndrome, and hypertension, comparing our overall populations of INSTI versus non-INSTI users, nor any major overall differences in sex-stratified models, among patients with an average 2-year duration of INSTI use. In comparison, in a recent analysis among a subset of ADVANCE trial participants, INSTI-associated weight gain resulted in increased CVD and type 2 diabetes risk [6]. However, this prior analysis did not have a non-INSTI comparator group and was restricted to sub-Saharan Africa where obesity and diabetes rates are on the rise among the general population [6, 22]; therefore, one cannot attribute the increased risk seen in this population solely to INSTI use. An analysis of the WIHS cohort showed slight increases in HbA1c (+0.05 vs −0.06) and blood pressure compared with non-INSTI users [23]. More importantly, however, the WIHS study enrolled only women in the United States and included a significant percentage of PWH with diabetes, all of whom were studied before 2017. In contrast, our study includes a large multinational cohort of PWH on more contemporary regimens, including TAF and newer INSTIs.
We performed sensitivity analyses to understand whether the INSTI effect on BMI and WC was related to TAF use. First, we balanced entry NRTI regimens and showed a similar association of INSTI use and higher BMI and WC. Second, we excluded TAF users from the overall analysis population and again found that the association of INSTI use with higher BMI and WC remained significant. In contrast to the primary analysis, INSTI use was associated with lower LDL-C when TAF was excluded. Moreover, when evaluating TAF among INSTI users only in this study, TAF use was associated with higher BMI, WC, and LDL-C but not with higher fasting glucose or odds of metabolic syndrome or hypertension. These sensitivity analyses demonstrate that both INSTI use and TAF use are associated with higher BMI and WC, and that the INSTI effects on weight occur independent of TAF use. However, the differential effects of TAF in these analyses may be due to the known cholesterol-lowering and potential weight-suppressing effects of TDF used more frequently among participants not on TAF [6, 24, 25].
These results suggest that the modest overall weight gain associated with INSTI use may be generally well tolerated from a metabolic and cardiovascular perspective in healthier PWH without known CVD. At the same time, these data point out the much larger differences associated with INSTI use at the highest quantiles of weight and WC. These data are a cautionary note that such patients may be a unique group, at greatest risk for increased weight and WC, and related CVD complications, associated with INSTI use, and thus should be monitored carefully over time. Longitudinal analyses of REPRIEVE will permit for assessment of the relationship of longer duration INSTI use to major adverse cardiac event among a primary CVD prevention cohort of PWH.
The majority of prior studies investigating INSTI effects on weight do not include multiple INSTIs in the same study to allow for comparison between INSTI agents. In our current study, among different INSTIs, IPT-weighted regressions showed that compared with non-INSTI containing regimens, EVG-containing regimens exhibited the greatest association with higher BMI, higher odds of obesity, and higher WC, although weight increases were associated consistently with use of all individual INSTIs. The DTG-containing regimens were associated with greater odds of hypertension and elevated fasting glucose levels in the upper tail of the distribution. These findings are consistent with prior reports raising concern for hyperglycemia with DTG [26]. It is still uncertain whether this is a class effect or DTG-specific effect. These findings on individual INSTIs require confirmation in further studies.
This analysis has strengths and some limitations. We analyzed baseline data from REPRIEVE, which recruited a diverse population of PWH eligible for primary CVD prevention, for whom it is critical to understand the CVD risk of increased weight and WC associated with contemporary INSTI use. We were able to consider the effects of important variables, including diet quality and physical activity in our analyses. However, we did not have access to pre-ART weight but examined associations of INSTI use with metabolic parameters over a broad range of current weight. This study was cross-sectional and was not a randomized trial of INSTI versus non-INSTI-based regimens. To account for this, we performed a rigorous analysis using IPT weighting methodology that successfully balanced measured confounders between INSTI users and nonusers in a weighted analysis population, reducing potential bias of the treatment effect estimates [27]. We performed sensitivity analyses to account for differences in ART duration and NRTI regimens—specifically, TAF—between groups. Although larger effects of INSTI use on metabolic dysregulation might be seen given a longer duration of follow-up and in a population with pre-existing CVD and related comorbidities, we did not see any differential effects in sensitivity analyses with longer duration of entry NRTI or by ASCVD risk stratification.
CONCLUSIONS
In conclusion, in this large multinational cohort of PWH, including a large number of female participants, INSTI-based regimens were associated with higher BMI, higher odds of obesity, and higher WC but not with differences in key cardiometabolic risk factors. Sex-stratified differences show that the effects are more consistent and concerning in women, in whom they may be more likely to be related to metabolic risk, with a similar worrisome signal for nonwhites. These data provide a degree of reassurance that, in general, for most PWH at low to moderate traditional CVD risk, higher weights associated with INSTI use are not associated with significant metabolic and cardiovascular risk. However, our data highlight several at-risk subgroups for whom such changes may be very concerning and for whom long-term CVD outcomes should be carefully assessed.
Supplementary Material
Acknowledgments
We thank the participants of the study for their willingness to volunteer their time and effort and their dedication to research.
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Financial support. This study is funded through National Institutes of Health (NIH) Grants U01HL123336, to the Clinical Coordinating Center, and U01HL123339, to the Data Coordinating Center, and funding from ViiV Healthcare, KOWA Pharmaceuticals, and Gilead Sciences. The National Institute of Allergy and Infectious Diseases (NIAID) is funding this study through the following grants: UM1 AI068636, which supports the ACTG Leadership and Operations Center; UM1 AI068634, which supports the ACTG Statistical and Data Management Center; UM1 AI106701, which supports the ACTG Laboratory Center; NIH P30DK 050561, KOWA Pharmaceuticals, and Gilead Sciences (to S.K.G.).
Potential conflicts of interest. J. L. reports consulting fees from ViiV Healthcare and Gilead Sciences, Inc. as well as investigator-initiated research grant support from ViiV Healthcare related to this work. C. M. reports personal fees from ViiV Healthcare and Gilead Sciences for participation in advisory board meetings outside the submitted work. K. V. F. reports personal fees from Gilead Sciences outside the submitted work. M. V. Z. reports grants from NIH and Gilead Sciences during the conduct of the study. C. J. F. reported receiving grants from ViiV Healthcare, Janssen Pharmaceuticals, Merck, CytoDyn, Amgen, and Abbvie outside the submitted work. E. T. O. reports grants from the NIH during the conduct of the study as well as person fees from Merck, ViiV Healthcare, and Theratechnologies outside the submitted work. N. L. O. reports grants from the NIH/National Heart, Lung, and Blood Institute (NHLBI) outside the submitted work. P. K. reports grant funding from Eli Lilly, Merck, Gilead, and GSK; stock in Pfizer, J&J, Gilead, Merck, and GSK; and serves on the advisory board for Gilead, GSK, Merck, TheraTechnologies, and J&J. J. A. A. reports institutional research support for clinical trials from Atea, Emergent Biosolutions, Frontier Technologies, Gilead Sciences, GlaxoSmithKline, Janssen, Merck, Pfizer, Regeneron, and Viiv Healthcare and personal fees for advisory boards from GlaxoSmithKline and Merck, all outside the submitted work. E. M. reports institutional research support from Merck and ViiV Healthcare and personal fees for advisory boards from Janssen, Gilead Sciences, Merck, and ViiV Healthcare, all outside the submitted work. J. S. C. reports personal advisory fees from Merck, outside the submitted work. H. J. R. reports grants from NIH/NHLBI and KOWA Pharmaceuticals during the conduct of the study as well as grants from NIH/NIAID and NIH/NHLBI outside the submitted work. S. K. G. reports grants from NIH, KOWA Pharmaceuticals, Gilead Sciences, and ViiV Healthcare during the conduct of the study as well as personal consulting fees from TheraTechnologies and VIiV Healthcare outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented at Virtual CROI, March 6–10, 2021.
References
- 1. Unites States Department of Health and Human Services Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Available at: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/what-start-initial-combination-regimens-antiretroviral-naive. Accessed February 15, 2021.
- 2. World Health Organization. Update of Recommendations on First- and Second-line Antiretroviral Regimens. Geneva, Switzerland: World Health Organization, 2019. [Google Scholar]
- 3. Norwood J, Turner M, Bofill C, et al. Brief report: weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr 2017; 76:527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–15. [DOI] [PubMed] [Google Scholar]
- 5. Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020; 71:1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCann K, Shah S, Hindley L, et al. Implications of weight gain with newer anti-retrovirals: 10-year predictions of cardiovascular disease and diabetes. AIDS 2021; 35:1657–65. [DOI] [PubMed] [Google Scholar]
- 7. Gomez M, Seybold U, Roider J, et al. A retrospective analysis of weight changes in HIV-positive patients switching from a tenofovir disoproxil fumarate (TDF)- to a tenofovir alafenamide fumarate (TAF)-containing treatment regimen in one German university hospital in 2015-2017. Infection 2019; 47:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grinspoon SK, Fitch KV, Overton ET, et al. ; REPRIEVE Investigators. Rationale and design of the randomized trial to prevent vascular events in HIV (REPRIEVE). Am Heart J 2019; 212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grinspoon SK, Douglas PS, Hoffmann U, Ribaudo HJ.. Leveraging a landmark trial of primary cardiovascular disease prevention in human immunodeficiency virus: introduction from the reprieve coprincipal investigators. J Infect Dis 2020; 222:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bourgi K, Rebeiro PF, Turner M, et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis 2020; 70:1267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Austin PC, Stuart EA.. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015; 34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brookhart MA, Schneeweiss S, Rothman KJ, et al. Variable selection for propensity score models. Am J Epidemiol 2006; 163:1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calmy A, Tovar Sanchez T, Kouanfack C, et al. ; New Antiretroviral and Monitoring Strategies in HIV-infected Adults in Low-Income Countries (NAMSAL) ANRS 12313 Study Group. Dolutegravir-based and low-dose efavirenz-based regimen for the initial treatment of HIV-1 infection (NAMSAL): week 96 results from a two-group, multicentre, randomised, open label, phase 3 non-inferiority trial in Cameroon. Lancet HIV 2020; 7:e677–87. [DOI] [PubMed] [Google Scholar]
- 14. Ruderman SA, Crane HM, Nance RM, et al. Brief report: weight gain following ART initiation in ART-naïve people living with HIV in the current treatment era. J Acquir Immune Defic Syndr 2021; 86:339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taramasso L, Ricci E, Menzaghi B, et al. ; CISAI Study Group. Weight gain: a possible side effect of all antiretrovirals. Open Forum Infect Dis 2017; 4:ofx239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lahiri CD, Xu Y, Wang K, et al. Weight and body mass index change after switching to integrase inhibitors or tenofovir alafenamide among women living with HIV. AIDS Res Hum Retroviruses 2021; 37:461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klein S, Allison DB, Heymsfield SB, et al. ; Association for Weight Management and Obesity Prevention; NAASO; Obesity Society; American Society for Nutrition; American Diabetes Association. Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care 2007; 30:1647–52. [DOI] [PubMed] [Google Scholar]
- 18. Bhagwat P, Ofotokun I, McComsey GA, et al. Changes in waist circumference in HIV-infected individuals initiating a raltegravir or protease inhibitor regimen: effects of sex and race. Open Forum Infect Dis 2018; 5:ofy201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kerchberger AM, Sheth AN, Angert CD, et al. Weight gain associated with integrase stand transfer inhibitor use in women. Clin Infect Dis 2020; 71:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gorwood J, Bourgeois C, Pourcher V, et al. The integrase inhibitors dolutegravir and raltegravir exert proadipogenic and profibrotic effects and induce insulin resistance in human/simian adipose tissue and human adipocytes. Clin Infect Dis 2020; 71:e549–60. [DOI] [PubMed] [Google Scholar]
- 21. Surial B, Mugglin C, Calmy A, et al. ; Swiss HIV Cohort Study. Weight and metabolic changes after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in people living with HIV: a cohort study. Ann Intern Med 2021; 174:758–67. [DOI] [PubMed] [Google Scholar]
- 22. Group NCDRFCAW. Trends in obesity and diabetes across Africa from 1980 to 2014: an analysis of pooled population-based studies. Int J Epidemiol 2017; 46:1421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Summers NA, Lahiri CD, Angert CD, et al. Metabolic changes associated with the use of integrase strand transfer inhibitors among virally controlled women. J Acquir Immune Defic Syndr 2020; 85:355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Milinkovic A, Berger F, Arenas-Pinto A, Mauss S.. Reversible effect on lipids by switching from tenofovir disoproxil fumarate to tenofovir alafenamide and back. AIDS 2019; 33:2387–91. [DOI] [PubMed] [Google Scholar]
- 25. Erlandson KM, Carter CC, Melbourne K, et al. Weight change following antiretroviral therapy switch in people with viral suppression: pooled data from randomized clinical trials. Clin Infect Dis 2021; 73:1440–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lamorde M, Atwiine M, Owarwo NC, et al. Dolutegravir-associated hyperglycaemia in patients with HIV. Lancet HIV 2020; 7:e461–2. [DOI] [PubMed] [Google Scholar]
- 27. Webster-Clark M, Stürmer T, Wang T, et al. Using propensity scores to estimate effects of treatment initiation decisions: state of the science. Stat Med 2021; 40:1718–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.