Abstract
Aims
At present, there are no guideline recommendations for minimally interrupted use of non-vitamin K antagonist oral anticoagulants (mi-NOAC) during catheter ablation (CA) for atrial fibrillation (AF). Current evidence is predominantly based on observational studies, with continuous use of vitamin K antagonist in the control arm. This quantitative summary reflects the first high-level evidence on contemporary regimens, with continuous NOAC use (c-NOAC) as the current gold standard.
Methods and results
Meta-analysis (Pubmed, Embase, and Web of Science) on prospective, controlled studies comparing contemporary mi-NOAC (without bridging) with c-NOAC. Net adverse clinical events (major bleeding, thrombo-embolic events) were the primary outcome. In addition, we analysed total bleeding, minor bleeding, and silent cerebral embolism.
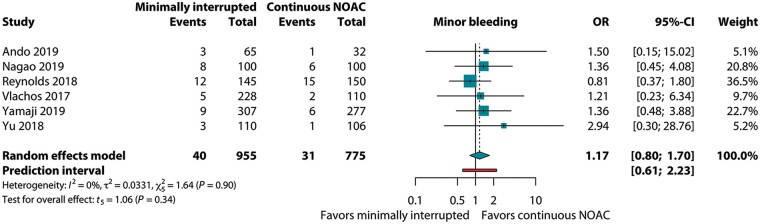
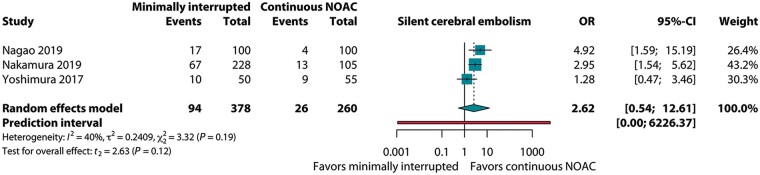
Eight studies (six randomized, two observational) with 2168 patients were summarized. The primary endpoint occurred in 1.0% (18/1835): 1.1% (11/1005) vs. 0.8% (7/830) for the mi-NOAC and c-NOAC groups, respectively; odds ratio (OR) 1.20 [95% confidence interval (CI) 0.49–2.92, P = 0.64]. The OR for total bleeding on mi-NOAC was 1.26 (95% CI 0.97–1.63, P = 0.07). ORs for minor bleeding and silent cerebral embolism were 1.17 (95% CI 0.80–1.70, P = 0.34) and 2.62 (95% CI 0.54–12.61, P = 0.12), respectively.
Conclusion
This synopsis provides a quantitative synthesis of high-level evidence on a contemporary strategy of mi-NOAC in CA for AF, and overall clinical outcomes were not different from continuous NOAC use. Despite preprocedural interruption, there was no sign of lower bleeding rates. Additional higher volume datasets are warranted for more precise treatment effect estimations of this everyday alternative anticoagulation strategy in AF ablation.
Keywords: Atrial fibrillation, Stroke, Bleeding, Catheter ablation, Non-vitamin K antagonist oral anticoagulants, Periprocedural anticoagulation strategy
What’s new?
Minimally interrupted use of non-vitamin K antagonist oral anticoagulants (NOACs) is an everyday used strategy during catheter ablation for atrial fibrillation, but the 2020 ESC guidelines provide no recommendations.
Most of the available evidence is limited to observational data, from the era where comparative studies had continuous vitamin K antagonist in the control arm.
The present meta-analysis provides the first quantitative summary of (predominantly) randomized evidence, with continuous use of NOAC in the control arm, and comparing two peri-procedural strategies meeting the contemporary standards (i.e. no bridging).
Based on currently available evidence, net adverse clinical outcomes were similar, without indication of lower bleeding raters with pre-procedural interruption.
Additional higher volume datasets are warranted for a more precise estimation of treatment effects.
Introduction
Catheter ablation (CA) has become a cornerstone in rhythm control therapy for atrial fibrillation (AF), and optimization of the procedural risk-benefit is a key topic of interest.1–3 Real-world registries report complication rates of up to 6.7%.1–4 As for periprocedural anticoagulation, uninterrupted use of non-vitamin K antagonist oral anticoagulants (NOACs) has replaced the continuous use of vitamin K antagonists (VKAs) as the current standard.1–3,5–8 Alternatively, a temporary interruption of the use of NOACs has been suggested to further improve procedural safety.9,10 As of yet, there are no guideline recommendations on this approach, despite accumulating evidence.2,3
Importantly, the class IIa recommendation in the expert consensus statement to skip one or two NOAC doses prior to CA,1 is primarily based on observational studies, and with the less contemporary strategy of continuous VKA in the control arm.11–13 At present, the largest dataset on major bleeding rates after different NOAC regimens during CA for AF concerns a descriptive meta-analysis, with a design that precludes between-regimen comparisons.14
In terms of level of evidence, randomized controlled studies are warranted to compare outcomes after minimally interrupted NOAC use (mi-NOAC) with the current standard. As thrombo-embolic complications are associated with an eight- to nine-fold increased risk of mortality, it is important to substantiate the expected safety benefit of a minimally interrupted strategy, in relation to the observed thrombo-embolic complications.4
The first randomized comparisons with a continuous NOAC strategy (c-NOAC) as control group reported similar outcomes on mi-NOAC, in modestly sized trials with very low complication rates.9,10 The initial meta-analyses on this issue also included interrupted NOAC regimens with pre- and/or post-procedural heparin bridging, which is not a guideline endorsed approach.1–3,11,15,16 In the abovementioned context, we performed the first systematic review and updated meta-analysis on outcomes after CA for AF focused on controlled studies of contemporary mi-NOAC vs. c-NOAC as the reference.
Methods
Data sources and search strategy
This meta-analysis was prospectively registered with the PROSPERO international register (CRD42019136982), and performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.17 Pubmed, Embase, and Web of Science were systematically searched for publications up to 1 February 2020. Search terms included but were not limited to AF, catheter ablation, and non-VKA antagonist oral anticoagulants (Supplementary material online, Table S1, detailed search strategy).
Selection criteria
Studies meeting the following criteria were eligible for inclusion:
Randomized or non-randomized comparisons of a minimally interrupted vs. a continuous regimen with any NOAC (i.e. apixaban, dabigatran, rivaroxaban, edoxaban) in the setting of CA for AF.
Outcome data reported both for bleeding and thrombo-embolic events.
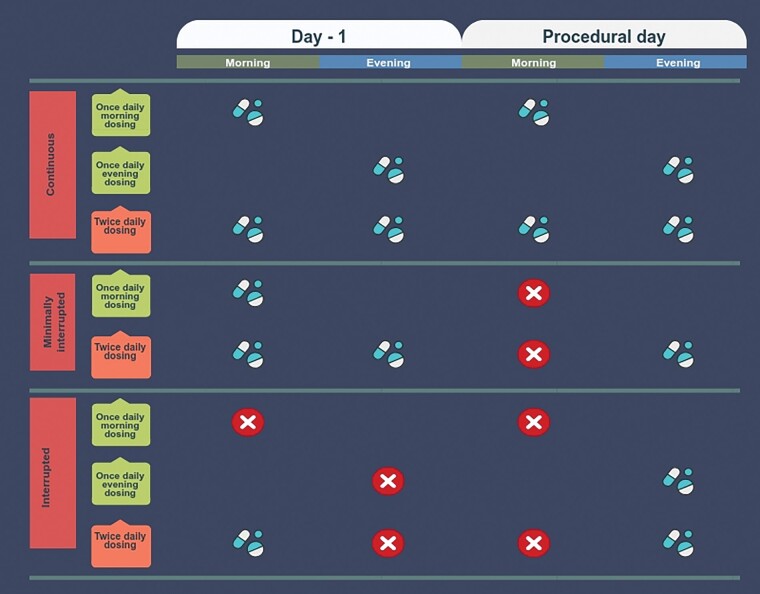
We considered a NOAC strategy minimally interrupted when the morning dose was held on the day of ablation for twice-daily agents.6,7 In the landmark trials on once-daily agents, drug intake in the evening before the day of the procedure was considered a continuous NOAC strategy.5,8 Hence, patients using once-daily agents were considered to be on a minimally interrupted regimen when the last intake was on the morning the day before the procedure (Figure 1). To meet the criteria for contemporary, endorsed, anticoagulation strategies,1–3 we excluded studies that included a bridging protocol with (low-molecular weight) heparin.
Figure 1.
Definition of periprocedural anticoagulation strategies. Overview of periprocedural anticoagulation strategies according to dosing regimen of non-vitamin K antagonist oral anticoagulant.
Endpoint definition
We defined net adverse clinical events (NACE) as the primary endpoint (major bleeding and/or thrombo-embolic events). Bleeding complications were considered as major according to the definition in the respective manuscripts. Thrombo-embolic events consist of a composite of ischaemic stroke, systemic embolism, and transient ischaemic attack. As secondary endpoints, we studied major bleeding and thrombo-embolic events separately. In addition, we analysed total bleeding, minor bleeding, and silent cerebral embolism detected on magnetic resonance (MR) imaging. Bleeding complications not meeting the respective major bleeding criteria were considered as minor bleeding complications.
Data extraction and quality assessment
After removal of duplicates, three investigators (S.P.G.v.V., R.H.J.A.V., and S.W.W.) independently screened all titles and abstracts to identify records that met the inclusion criteria. We extracted study characteristics (i.e. design), patient characteristics, periprocedural characteristics, and outcome data using prespecified data collection forms. Risk of bias was assessed with the Newcastle-Ottawa Scale for observational studies and the Cochrane Collaboration’s tool for randomized trials. Disagreements in selection and risk of bias assessment were resolved by discussion. Events were extracted by the aforementioned investigators independently and discrepancies were cross-checked to ensure they matched with the original manuscripts.
Statistical analyses
Pooled treatment effects for binary endpoints were compared using odds ratios (ORs) and their 95% confidence intervals (CIs). Summary ORs were estimated using random-effects models and between-study variance was quantified using the τ2 statistic, estimated using the Sidik–Jonkman estimator. Test statistics and CIs were adjusted using the Hartung–Knapp method. In the case of zero cells (i.e. no outcome events), a continuity correction was applied by adding 0.5 to all cells of the contingency table.
We assessed heterogeneity by visual inspection of forest plots, use of the I2 statistic, and its connected χ2 test. Heterogeneity was further investigated using sensitivity analyses. We prospectively defined sensitivity analyses, based on study quality, and study design (randomized, observational).
To present the expected range of true treatment effects for a future similar study, 95% prediction intervals were calculated. A two-sided P-value of <0.05 was considered statistically significant. Statistical analyses were performed with R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria) using the ‘meta’ package.
Results
Study selection
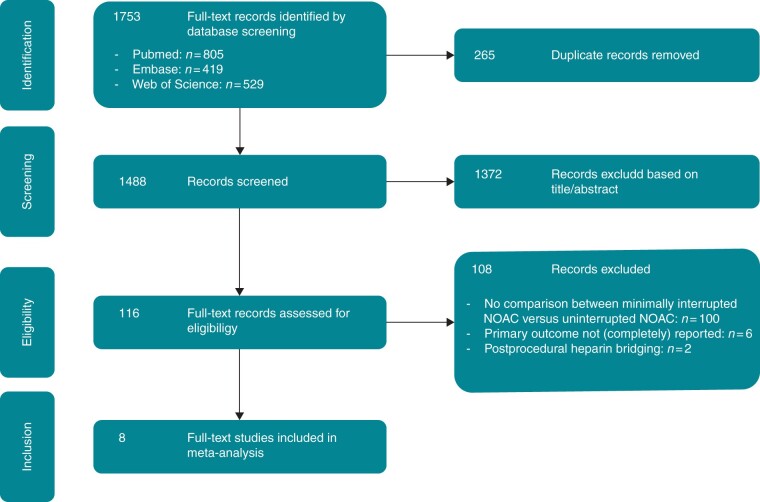
The screening and selection process is shown in Figure 2. We identified 1753 records, of which 1637 were excluded due to duplicate records or unmet inclusion criteria. Among the 116 full-text articles assessed for eligibility, eight were included in the current analysis.9,10,18–23
Figure 2.
PRISMA flow chart. PRISMA diagram of selection process for the current meta-analysis. NOAC, non-vitamin K antagonist oral anticoagulant.
Population and study characteristics
The study, patient and procedural characteristics are displayed in Tables 1 and 2. Table 1 - please align the number of follow-up days in the center of the column. Table 2 - please align the text in the column Preprocedural imaging to the center of the column. Please align the text in the column Radiofrequency ablation to the center of the column. Please align the text of the column Protamine use to the center of the column. Please improve layout of columm Resumption of oral anticoagulation therapy. Average age varied between 58 and 70 years and the average CHA2DS2-VASc score varied between 1.6 and 2.8. Two studies only included patients with paroxysmal AF, in the other studies, the proportion was at least 57%.
Table 1.
Study and patient characteristics of included studies
| Study | Year | Design | Centres | Data collection | No. of patients | Follow-up (days) | Agent(s) (n) | Reduced dose (%) | Age | PAF (%) | Male (%) | CHA2DS2- VASc | CHF (%) | HT (%) | DM (%) | Prior stroke/ TIA (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ando18 | 2019 | RCT | Single | Prospective | 65/32 | 2 | A (65/32) | 0/0 | 66/67 | 100/100 | 75/81 | NR | 8/6 | 56/56 | 14/13 | 9/6 |
| Nagao19 | 2019 | RCT | Single | Prospective | 100/100 | 30 | A (47/51) R/E (53/49) | 41/48 | 70/70 | 59/57 | 62/64 | 2.6/2.8 | 14/15 | 50/55 | 29/33 | 10/7 |
| Nakamura20 | 2019 | Observational | Single | Prospective | 228/105 | 1 |
|
NR | 64/64 | 100/100 | 67/65 | 1.9/1.9 | NR | 46/51 | 10/12 | 6/7 |
| Reynolds9 | 2018 | RCT | Multi | Prospective | 145/150 | 30 | A (145/150) | 2.2/0 | 64/63 | 63/67 | 67/67 | 2.4/2.2 | 9/14 | 70/68 | 24/22 | 3/4 |
| Vlachos21 | 2017 | Observational | Single | Prospective | 228/110 | 90 |
|
NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Yamaji22 | 2019 | RCT | Single | Prospective | 307/277 | 90 |
|
NR | 65/66 | 65/62 | 69/76 | 1.9/1.9 | 7/2 | 47/48 | 11/14 | 4/6 |
| Yoshimura10 | 2017 | RCT | Single | Prospective | 50/55 | 1 | A (50/0) R (0/55) | NR | 59/59 | 62/60 | 82/82 | 1.7/1.7 | 10/7 | 64/78 | 10/13 | 16/13 |
| Yu23 | 2018 | RCT | Multi | Prospective | 110/106 | 30 |
|
NR | 58/59 | 67/63 | 72/76 | 1.7/1.6 | 15/10 | 41/43 | 15/9 | 16/11 |
Numbers displayed as minimally interrupted/uninterrupted strategy with non-vitamin K antagonist oral anticoagulant.
A, apixaban; CHF, congestive heart failure; D, dabigatran; DM, diabetes mellitus; E, edoxaban; HT, hypertension; NR, not reported; PAF, paroxysmal atrial fibrillation; RCT, randomized controlled trial; R, rivaroxaban; TIA, transient ischaemic attack.
Table 2.
Periprocedural characteristics of included studies
| Study | Year | Preprocedural imaging | Target ACT (s) | Radiofrequency ablation (%) | Protamine use (%) | Resumption of oral anticoagulation therapy |
|---|---|---|---|---|---|---|
| Ando18 | 2019 | TOE | 300–350 | 0 | 100 | Evening after procedure |
| Nagao19 | 2019 | TOE/CT | 300 | 100 | 100 | Evening after procedure (A) or morning after procedure (R + E) |
| Nakamura20 | 2019 | NR | 300–350 | 0 | NR | Evening after procedure (uninterrupted group) or morning after procedure (minimally interrupted group) |
| Reynolds9 | 2018 | TOE (46%) | >300 | 52 | 90 | Evening after procedure |
| Vlachos21 | 2017 | TOE | 300–400 | 100 | NR | Evening after procedure |
| Yamaji22 | 2019 | NR | 300–400 | 100 | 100 | Evening after procedure (A + D) or morning after procedure (R + E) |
| Yoshimura10 | 2017 | TOE | >300 | 100 | NR | NR |
| Yu23 | 2018 | TOE/ICE | 350–400 | 100 | NR | Evening after procedure |
A, apixaban; ACT, activated clotting time; CT, computed tomography; D, dabigatran; E, edoxaban; ICE, intracardiac echography; NR, not reported; R, rivaroxaban; TOE, transesophageal echocardiography.
The total study population consists of 2168 patients, with 1233 (56.9%) on mi-NOAC and 935 (43.1%) on c-NOAC. For the mi-NOAC group, apixaban was most commonly used (n = 556, 45.1%). Rivaroxaban, dabigatran, and edoxaban were used in 23.2%, 20.2%, and 11.5% of patients, respectively. For the c-NOAC group, proportions were 35.3%, 33.3%, 23.9%, and 7.6% for apixaban, rivaroxaban dabigatran, and edoxaban, respectively.
All included controlled studies were prospective in design, six were randomized trials and two were observational studies. In six studies the risk of bias was low, in two studies (one randomized, one observational) risk of bias was considered moderate (Supplementary material online, Table S2).10,21
Primary endpoint
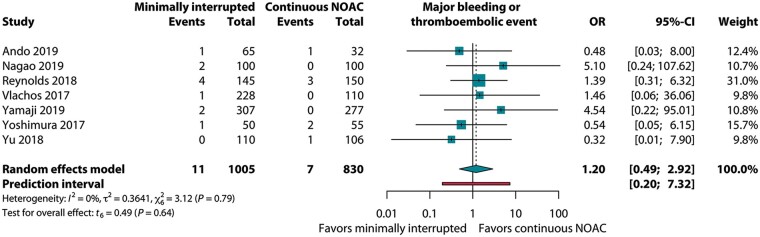
A total of seven studies reported data on both major bleeding and thrombo-embolic complications. Overall, the primary endpoint was observed in 1.0% (18/1835) of the patients. Rates were 1.1% (11/1005) and 0.8% (7/830) for the mi-NOAC and c-NOAC groups, respectively; OR 1.20 (95% CI 0.49–2.92, P = 0.64, I2 = 0%), as displayed in Figure 3.
Figure 3.
Pooled estimate of primary endpoint in patients on minimally interrupted NOAC vs. continuous NOAC. Primary endpoint: major bleeding or thrombo-embolic events. CI, confidence interval; NOAC, non-vitamin K antagonist oral anticoagulant; OR, odds ratio.
Secondary endpoints
In the seven studies reporting on major bleeding, the overall rate was 0.8% (14/1835), without significant difference between mi-NOAC and c-NOAC: OR 1.04 (95% CI 0.43–2.51, P = 0.91, I2 = 0%, Supplementary material online, Figure S1). Cardiac tamponade was observed in 0.55% (10/1835).
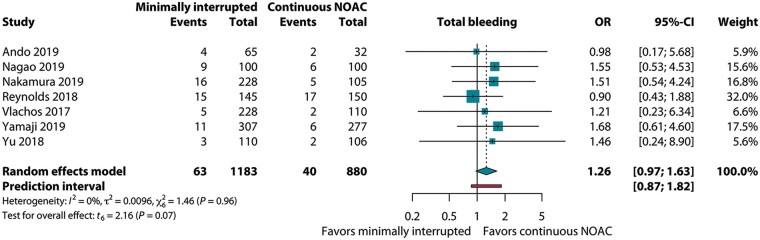
For total bleeding, reported in seven studies, the OR of mi-NOAC vs. c-NOAC was 1.26 (95% CI 0.97–1.63, P = 0.07, I2 = 0%, Figure 4). This was paralleled by the six studies reporting on groin bleedings: 4.0% (38/955) vs. 3.0% (23/775) for the mi-NOAC and c-NOAC strategies, respectively (OR 1.45; 95% CI 0.93–2.28, P = 0.09, I2 = 0%). With regard to minor bleeding, the OR was 1.17 (95% CI 0.80–1.70, P = 0.34, I2 = 0%, Figure 5).
Figure 4.
Pooled estimate of total bleeding in patients on minimally interrupted NOAC vs. continuous NOAC. CI, confidence interval; NOAC, non-vitamin K antagonist oral anticoagulant; OR, odds ratio.
Figure 5.
Pooled estimate of minor bleeding in patients on minimally interrupted NOAC vs. continuous NOAC. CI, confidence interval; NOAC, non-vitamin K antagonist oral anticoagulant; OR, odds ratio.
Thrombo-embolic complications, reported in eight studies, were observed in 0.3% of the patients (6/2168), OR 1.00 (95% CI 0.59–1.69, P = 1.00, I2 = 0%, Supplementary material online, Figure S2) and in the three studies with data on silent cerebral embolism, the OR was 2.62 (95% CI 0.54–12.61, P = 0.12, I2 = 40%, Figure 6).
Figure 6.
Pooled estimate of silent cerebral embolism in patients on minimally interrupted NOAC vs. continuous NOAC. CI, confidence interval; NOAC, non-vitamin K antagonist oral anticoagulant; OR, odds ratio.
Sensitivity analyses and bias
Analyses restricted to randomized trials showed similar results: the OR for the primary endpoint was 1.17 (95% CI 0.39–3.48), without signs for interaction compared to observational data (P for interaction = 0.90). Also for the secondary endpoints, these analyses showed similar results.
A sensitivity analysis restricted to the studies with a low risk of bias showed an OR of 1.38 (95% CI 0.35–5.35, P = 0.12, I2 = 0%) for the primary endpoint. Visual inspection of the funnel plot did not suggest publication bias for the primary endpoint (Supplementary material online, Figure S3).
Discussion
The present meta-analysis was undertaken to provide contemporary, higher level evidence on outcomes after ablation for AF with use of a minimally interrupted NOAC regimen in comparison to the current gold standard.1–3 Where previous evidence has been limited to observational studies, and with continuous use of VKA in the control arm,12,13 the present analysis is based on predominantly randomized evidence, with continuous NOAC use as the reference. Overall complication rates were low, without a difference in NACE (major bleeding, thrombo-embolic events) between minimally interrupted and uninterrupted NOAC use. Despite preprocedural interruption, there were no signs of lower bleeding rates with this alternative regimen.
This synopsis reflects the first summary of outcomes related to these two everyday anticoagulation strategies, and provides an initial impression for our daily decision-making. However, additional, larger datasets are warranted to address whether a minimally interrupted regimen confers a potential safety benefit without compromising thrombo-embolic risk.
Interrupted non-vitamin K antagonist oral anticoagulant regimens vs. continuous vitamin K antagonist
Hypothetically, a temporal interruption of NOAC intake may reduce anticoagulant activity at the time of the procedure, and reduce bleeding complications. In the era when continuous VKA was the comparator of interest, meta-analyses on outcomes after interrupted NOAC regimens were inconclusive and showed trends towards about 20% lower major bleeding rates, but with point estimates of ORs for thrombo-embolic complications in the opposite direction.11,24 Importantly, these synopses on interrupted NOAC regimens also included non-contemporary regimens with preprocedural discontinuations of up to 48 h.11–13
The most comprehensive available synopsis on major bleeding rates with different NOAC regimens is a descriptive meta-analysis, which reported weighted mean incidences for major bleeding of 1.02% for uninterrupted NOAC use, 1.49% for mildly interrupted NOAC regimes, and 1.17% for interrupted regimes.14 As the vast majority of the studies in this descriptive meta-analysis had a control group with VKA use, this precluded comparisons between the different NOAC regimens. This also holds true for thrombo-embolic events, which were 0.46% and 0.16% for mildly interrupted and uninterrupted NOAC regimens, respectively.14 Although in absolute terms this is a small difference, thrombo-embolic complications have a profound clinical impact, with an eight- to nine-fold higher risk of mortality, according to a recent, large registry including over 60 000 ablations.4
In this context, controlled comparisons are warranted to substantiate the risk-benefit of a minimally interrupted approach.
Minimally interrupted vs. continuous non-vitamin K antagonist oral anticoagulant
The first report on this issue described a subgroup comparison on 511 patients, as part of a larger network meta-analysis.25 A subsequent meta-analysis on the impact of a minimally interrupted strategy reported on all studies with any form of continuous anticoagulation therapy in the control arm (including VKA) and presented a subgroup analysis restricted to a comparison with continuous NOAC use.16 However, studies with pre- and/or post-procedural bridging were included, which is not in line with current recommendations.1–3 This limitation also applies to the only available meta-analysis that strictly focused on studies with continuous NOAC use in the control arm.15
Our present analysis not only includes new controlled studies, with an up-to-date synthesis of all the available (predominantly) randomized head-to-head comparisons, but is also restricted to endorsed regimens of anticoagulation. We found no difference in NACE between minimally interrupted and continuous use of NOACs, and sensitivity analyses confirmed the consistency of the results. This was our primary outcome parameter in view of the low anticipated number of events, and the rationale that, ideally, the optimal anticoagulation strategy further reduces major bleeding complications without additional thrombo-embolic complications.
Bleeding rates
Overall, major bleeding rate was 0.8%, without difference between strategies, and cardiac tamponade accounted for 0.55%.
Considering that major bleeding rates are about 2–3%, with 0.7–0.8% cardiac tamponade, in the large multicentre randomized landmark trials comparing different continuous anticoagulation regimens, the observed rates in the present synopsis are rather low.8,26 This marked contrast may be related to patient selection, factors related to study organization/conduct, and centre experience.4 As for the minimally interrupted group, major bleeding rates were lower than in other reports,11,15,16,24 which may also relate to the exclusion of studies with bridging. All in all, the abovementioned aspects may have precluded the detection of significant differences with the currently available evidence.
Interestingly, despite the anticipation that a minimal interruption of NOAC intake may reduce bleeding complications, current findings show no indication of (a trend of) lower bleeding rates. In fact, for total bleeding, we observed an OR of 1.26 (95% CI 0.97–1.63, P = 0.07) for a minimally interrupted strategy, paralleled by an OR of 1.45 (95% CI 0.93–2.28, P = 0.09) for groin bleeding.
A potential explanation may be the more unstable anticoagulant activity with a minimally interrupted NOAC regimen. Pre-procedural anticoagulant activity will be lower, followed by a steeper rise in activity after heparin administration, on average requiring a higher dose to reach the target activated clotting time than with an uninterrupted regimen.9,20,23 Similarly, after the heparin effect has faded, post-procedural NOAC resumption will induce a steeper increase in anticoagulant activity after a minimally interrupted regimen.
Thrombo-embolic complications
In this synopsis, there was a 0.3% complication rate, which is comparable to that observed in the key randomized trials on continuous anticoagulation therapy in ablation for AF.8,26 In eight controlled studies with 2168 patients we observed no difference between the NOAC regimens studied. This is in contrast with the rates observed in the descriptive meta-analysis on 8362 NOAC users, with 0.46% thrombo-embolic events for mildly interrupted and 0.16% for uninterrupted NOAC regimens.14 As mentioned before, the design of summarized studies precluded between-regimen comparisons. As for silent cerebral embolism, we found an OR of 2.62 (95% CI 0.54–12.61, P = 0.12). Current evidence with focus on MR imaging suggests that complication rates seem higher in the minimally interrupted group.15,16 This may be an indication of suboptimal anticoagulant activity. However, these analyses often included regimens with heparin bridging, while switching from anticoagulants is known to increase complications. Further research is warranted, also because little is known about the long-term clinical impact of these subclinical events.
Implications
This synopsis of (predominantly) randomized data provides the most comprehensive available evidence to assess the impact and support the use of a contemporary minimally interrupted NOAC regimen, in an era where a continuous NOAC regimen has become the standard.1–3
On the one hand, bleeding rates and patient numbers in our current quantitative summary may have been too low to demonstrate a safety benefit. On the other hand, our findings may be a first indication that preprocedural interruption does not reduce bleeding.
In terms of level of evidence, this analysis on controlled studies summarizes the highest quality of data available, but it reflects 2168 patients. In that context, the aforementioned descriptive meta-analysis is also very informative, with mean weighted incidences assessed for different NOAC regimens in 8362 patients.14 Although limited by lack of a control group, the reported complication rates form an additional source to guide clinical decision-making.
From a scientific perspective, additional evidence on a minimally interrupted strategy is warranted for more precise estimations of the potential risk and benefit for thrombo-embolic and bleeding complications, respectively. From a practical point of view, however, the organization and conduct of a randomized controlled trial on this topic seems somewhat unrealistic, considering the required sample size with the currently rather low complication rates. In daily practice, both strategies are likely to yield similar net results in the majority of patients, when performed in experienced centres, with low complication rates. Although there was no sign of lower bleeding with pre-procedural interruption in the group as a whole, it may improve safety in patients at high risk of bleeding.
Yet, appreciating the aforementioned profound prognostic impact of a neurological complication after ablation,4 and the fact that an ablation is primarily performed to improve quality of life, it remains important to collect additional supportive evidence that skipping one or two doses does not cause harm, for example in specific groups at higher thrombo-embolic risk. In that context, well-documented datasets from national registries, including a broad spectrum of centres with varying volume load and experience, can provide valuable information.4 This underscores the need to participate in these registries and share procedural data. Information from these large-scale datasets may help further optimize patient-tailored periprocedural anticoagulation.
In terms of other initiatives to reduce periprocedural complications, our focus may need to shift towards other strategies to prevent bleeding such as a more individualized heparin regimen, protocol-driven strategies of protamine use, and more attention for the timing of re-initiation of NOAC intake. Notwithstanding these pharmacological aspects, echo-guided vascular access, methodology of groin compression, and other non-pharmacological issues are also all of importance.27
Limitations
Although current data reflects the highest level of currently available evidence, there are some limitations in terms of generalizability. First, the majority of studies was single-centre. Second, six of the included studies were performed in Asian populations, known for their favourable response to NOACs, and their higher thrombo-embolic and bleeding risk than non-Asians.28 From a scientific perspective, however, a higher risk population is where differences between two different anticoagulation regimens may emerge.
In terms of design, two of the incorporated studies were primarily undertaken as studies with a laboratory outcome measure, scoring clinical complications as secondary outcome.18,22 As a general limitation for most meta-analyses on CA for AF, there unfortunately was no uniform bleeding definition among the studies. Finally, we identified one study that reported on bleeding and thrombo-embolic events, according to our prespecified search criteria, but without reporting major bleeding.20 As such, it could not be used for the primary endpoint.
Conclusion
This quantitative synopsis summarizes the comprehensive, comparative evidence on a contemporary strategy of minimally interrupted NOAC use in CA for AF and shows that overall clinical outcomes were not different from continuous NOAC use. Despite preprocedural interruption, there was no sign of lower bleeding rates. Although this analysis provides higher level evidence than previously available, additional dedicated, higher volume multicentre studies are warranted for more precise treatment effect estimations of this everyday alternative anticoagulation strategy in AF ablation.
Supplementary material
Supplementary material is available at Europace online.
Conflict of interest: S.P.G.v.V. received consulting fees/honorariums from Bayer and Daiichi Sankyo, outside the submitted work. G.B.C. received professional fees for speaking, teaching, and consultancy for Medtronic, Abbott, Biosense Webster, Biotronik. E.P.N. received honorariums from Eli Lilly, outside the submitted work. M.A.B. received consulting fees/honorariums from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, and Daiichi Sankyo, outside the submitted work. All remaining authors have declared no conflicts of interest.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L. et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018;20:e1–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C. et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 3. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr. et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125–51. [DOI] [PubMed] [Google Scholar]
- 4. Cheng EP, Liu CF, Yeo I, Markowitz SM, Thomas G, Ip JE. et al. Risk of mortality following catheter ablation of atrial fibrillation. J Am Coll Cardiol 2019;74:2254–64. [DOI] [PubMed] [Google Scholar]
- 5. Cappato R, Marchlinski FE, Hohnloser SH, Naccarelli GV, Xiang J, Wilber DJ. et al. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J 2015;36:1805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kirchhof P, Haeusler KG, Blank B, De Bono J, Callans D, Elvan A. et al. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur Heart J 2018;39:2942–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Calkins H, Willems S, Gerstenfeld EP, Verma A, Schilling R, Hohnloser SH. et al. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med 2017;376:1627–36. [DOI] [PubMed] [Google Scholar]
- 8. Hohnloser SH, Camm J, Cappato R, Diener HC, Heidbuchel H, Mont L. et al. Uninterrupted edoxaban vs. vitamin K antagonists for ablation of atrial fibrillation: the ELIMINATE-AF trial. Eur Heart J 2019;40:3013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reynolds MR, Allison JS, Natale A, Weisberg IL, Ellenbogen KA, Richards M. et al. A prospective randomized trial of apixaban dosing during atrial fibrillation ablation: the AEIOU trial. JACC Clinical Electrophysiology 2018;4:580–8. [DOI] [PubMed] [Google Scholar]
- 10. Yoshimura A, Iriki Y, Ichiki H, Oketani N, Okui H, Maenosono R. et al. Evaluation of safety and efficacy of periprocedural use of rivaroxaban and apixaban in catheter ablation for atrial fibrillation. J Cardiol 2017;69:228–35. [DOI] [PubMed] [Google Scholar]
- 11. Zhao Y, Yang Y, Tang X, Yu X, Zhang L, Xiao H.. New oral anticoagulants compared to warfarin for perioperative anticoagulation in patients undergoing atrial fibrillation catheter ablation: a meta-analysis of continuous or interrupted new oral anticoagulants during ablation compared to interrupted or continuous warfarin. J Interv Card Electrophysiol 2017;48:267–82. [DOI] [PubMed] [Google Scholar]
- 12. Providencia R, Marijon E, Albenque JP, Combes S, Combes N, Jourda F. et al. Rivaroxaban and dabigatran in patients undergoing catheter ablation of atrial fibrillation. Europace 2014;16:1137–44. [DOI] [PubMed] [Google Scholar]
- 13. Hohnloser SH, Camm AJ.. Safety and efficacy of dabigatran etexilate during catheter ablation of atrial fibrillation: a meta-analysis of the literature. Europace 2013;15:1407–11. [DOI] [PubMed] [Google Scholar]
- 14. Gorla R, Dentali F, Crippa M, Marazzato J, Di Minno MND, Grandi AM. et al. Perioperative safety and efficacy of different anticoagulation strategies with direct oral anticoagulants in pulmonary vein isolation: a meta-analysis. JACC Clinical Electrophysiology 2018;4:794–806. [DOI] [PubMed] [Google Scholar]
- 15. Mao YJ, Wang H, Huang PF.. Peri-procedural novel oral anticoagulants dosing strategy during atrial fibrillation ablation: a meta-analysis. Pacing Clin Electrophysiol 2020;43:1104–14. [DOI] [PubMed] [Google Scholar]
- 16. Mao YJ, Wang H, Huang PF.. Meta-analysis of the safety and efficacy of using minimally interrupted novel oral anticoagulants in patients undergoing catheter ablation for atrial fibrillation. J Interv Card Electrophysiol 2021;60:407–17. [DOI] [PubMed] [Google Scholar]
- 17. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- 18. Ando M, Inden Y, Yoshida Y, Sairaku A, Yanagisawa S, Suzuki H. et al. Differences in prothrombotic response between the uninterrupted and interrupted apixaban therapies in patients undergoing cryoballoon ablation for paroxysmal atrial fibrillation: a randomized controlled study. Heart Vessels 2019;34:1533–41. [DOI] [PubMed] [Google Scholar]
- 19. Nagao T, Suzuki H, Matsunaga S, Nishikawa Y, Harada K, Mamiya K. et al. Impact of periprocedural anticoagulation therapy on the incidence of silent stroke after atrial fibrillation ablation in patients receiving direct oral anticoagulants: uninterrupted vs. interrupted by one dose strategy. Europace 2019;21:590–7. [DOI] [PubMed] [Google Scholar]
- 20. Nakamura R, Okishige K, Shigeta T, Nishimura T, Kurabayashi M, Yamauchi Y. et al. Clinical comparative study regarding interrupted and uninterrupted dabigatran therapy during perioperative periods of cryoballoon ablation for paroxysmal atrial fibrillation. J Cardiol 2019;74:150–5. [DOI] [PubMed] [Google Scholar]
- 21. Vlachos K, Efremidis M, Bazoukis G, Letsas KP, Saplaouras A, Georgopoulos S. et al. Safety and efficacy of DOACs vs acenocoumarol in patients undergoing catheter ablation of atrial fibrillation. Clin Cardiol 2017;40:847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamaji H, Murakami T, Hina K, Higashiya S, Kawamura H, Murakami M. et al. Activated clotting time on the day of atrial fibrillation ablation for minimally interrupted and uninterrupted direct oral anticoagulation therapy: equential changes, differences among direct oral anticoagulants, and ablation safety outcomes. J Cardiovasc Electrophysiol 2019;30:2823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu HT, Shim J, Park J, Kim TH, Uhm JS, Kim JY. et al. When is it appropriate to stop non-vitamin K antagonist oral anticoagulants before catheter ablation of atrial fibrillation? A multicentre prospective randomized study. Eur Heart J 2019;40:1531–7. [DOI] [PubMed] [Google Scholar]
- 24. Wu S, Yang YM, Zhu J, Wan HB, Wang J, Zhang H. et al. Meta-analysis of efficacy and safety of new oral anticoagulants compared with uninterrupted vitamin K antagonists in patients undergoing catheter ablation for atrial fibrillation. Am J Cardiol 2016;117:926–34. [DOI] [PubMed] [Google Scholar]
- 25. Yang P, Wang C, Ye Y, Huang T, Yang S, Shen W. et al. Interrupted or uninterrupted oral anticoagulants in patients undergoing atrial fibrillation ablation. Cardiovasc Drugs Ther 2020;34:371–81. [DOI] [PubMed] [Google Scholar]
- 26. Cardoso R, Willems S, Gerstenfeld EP, Verma A, Schilling R, Hohnloser SH. et al. Uninterrupted anticoagulation with non-vitamin K antagonist oral anticoagulants in atrial fibrillation catheter ablation: Lessons learned from randomized trials. Clin Cardiol 2019;42:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Winkle RA. Periprocedural DOAC anticoagulation interruption strategies for atrial fibrillation ablation: can a physician actually choose the one they like? JACC Clin Electrophysiol 2018;4:807–9. [DOI] [PubMed] [Google Scholar]
- 28. Chao TF, Chen SA, Ruff CT, Hamershock RA, Mercuri MF, Antman EM. et al. Clinical outcomes, edoxaban concentration, and anti-factor Xa activity of Asian patients with atrial fibrillation compared with non-Asians in the ENGAGE AF-TIMI 48 trial. Eur Heart J 2019;40:1518–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.