Abstract
This review will give a brief description of β-mannans, abundance in feedstuffs, utility of supplemental feed β-mannanase, and subsequent animal responses. Soybean products and co-products of processing palm, coconut, and guar seeds are the major sources of β-mannans in poultry and livestock feed. β-Mannans are linear polymers of mannose residues linked by β-1,4 glycosidic bonds and their ingestion elicit undesirable and metabolically costly responses. Web of Science was searched to retrieve published studies for meta-analyses of the impact of supplemental β-mannanase on performance and digestibility in pigs and poultry. The mean difference (MD) between β-mannanase and control on average daily gain (g/d) was +0.23 (P = 0.013; 95% CI of 0.05; 0.41), +10.8 g/d (P = 0.0005; 95% CI of 6.6; 15.0 g/d), and +20.68 (P < 0.000; 95% CI of 17.15; 24.20 g/d) for broiler chickens, nursery pigs, and grow-finish pigs, respectively. The MD on β-mannanase improvement on feed conversion (FCR) was −0.02 (P < 0.0001) with 95% CI (−0.03; −0.02) suggesting a 2-to-3-point FCR improvement in broiler chickens. β-Mannanase improvement on gain to feed (G:F) was +13.8 g/kg (P = 0.027; 2.1; 25.4 g/kg) and +8.77 g/kg (6.32; 11.23 g/kg) in nursery and grow-finish pigs, respectively. β-Mannanase improved apparent metabolizable energy by 47 kcal/kg (P = 0.0004) with 95% CI (28.8; 65.7 kcal/kg) in broiler chickens. The improvement of gross energy digestibility in pigs was 1.08% unit with 95% CI (0.90; 1.26) translating to the release of between 30.6 and 42.8 kcal/kg of digestible energy. Although data were limited, β-mannanase improved egg production in laying hens linked to improved energy metabolism in laying hens linked to improved energy metabolism but had no impact on egg quality. Turkeys may be more adversely affected by β-mannans because of the high protein/amino acids requirements necessitating higher dietary inclusion of soybean meal. However, growth performance and feed efficiency responses of turkeys fed diets supplemented with β-mannanase were variable. In summary, β-mannanase supplementation improved performance linked to energy and nutrient utilization. However, the magnitude of response was variable within and between species indicating further application refinement is warranted to achieve consistent efficacy, and improved understanding of the functional contribution of β-mannans hydrolysis products.
Keywords: β-mannanase, digestibility, feed-induced immune response, growth performance, swine and poultry nutrition
INTRODUCTION
Although pigs and poultry are highly efficient in converting feed to food products, they still excrete significant amounts of undigested nutrients. The peculiarity is that feedstuffs contain anti-nutritional factors (ANF) such as phytic acid or fractions that are not degraded sufficiently or indeed at all by the conditions and the array of digestive enzymes in the gastrointestinal tract (GIT; Kiarie et al., 2013, 2016). The undigested nutrients have negative implications on production efficiency, profitability, and sustainability of farm operations. This inherent digestive inefficiency in monogastric animals is seen as the reason of commercial development and application of exogenous feed enzymes technology. Indeed, the notion for application of exogenous enzymes in animal nutrition was initially suggested almost a century ago (Hervey, 1925); however, the prohibitive cost did not allow their application in animal nutrition until many decades later (Masey O’Neill et al., 2014). The mode of action for most commercial feed enzymes has been characterized as follows: 1) hydrolysis of specific chemical bonds in feedstuffs that are not sufficiently degraded or indeed not at all by the animal’s own enzymes (e.g., mixed salts of phytic acid); 2) elimination of the nutrient encapsulating effect of the cell wall polysaccharides and therefore increased availability of starches, amino acids, and minerals; 3) breakdown of ANF that are present in many feed ingredients (e.g., non-starch polysaccharides [NSP] and phytic acid); 4) solubilization of insoluble NSP for more effective hindgut fermentation and thus improved overall energy utilization; and 5) complementation of the enzymes (e.g., amylase, protease, lipase) produced by young animals where, because of the immaturity of their own digestive system, endogenous enzyme production may be inadequate. The global feed enzymes market size was estimated to account for USD 1.3 billion in 2020 and projected to reach USD 1.9 billion by 2025 (MarketsandMarkets, 2020). In terms of application and commercialization, phytase currently dominates the market, followed by carbohydrases (MarketsandMarkets, 2020). Xylanases and cellulases are the dominant carbohydrases and their utility in monogastric nutrition has been well characterized (Bedford, 1996; Bedford and Schulze, 1998; Adeola and Cowieson, 2011; Slominski, 2011; Masey O’Neill et al., 2014). However, the growing concern regarding animal health and the necessity to enhance nutrient utilization has led to development and utility of other carbohydrases such as β-mannanase, α-galactosidase, and pectinase (Ward, 2021). For example, emerging evidence suggests that some indigestible feed components such as β-mannans are not only anti-nutritive but elicit undesirable and metabolically costly immune responses (Klasing, 2007; Arsenault et al., 2014; Arsenault and Kogut, 2015). As such, supplemental β-mannanase may have nutritional and metabolic benefits. Review on application of single β-mannanase on growth performance and digestibility in broiler chickens and pigs has been reported (Shastak et al., 2015; Saeed et al., 2019; Torres-Pitarch et al., 2019; Kipper et al., 2020). However, these studies were limited in scope in terms of species coverage to document comparative responses within and between species. Moreover, these reviews evaluated some studies in which the tested enzymes were admixture of β-mannanase and other enzymes. Therefore, the objective of the present meta-analysis and systematic review is to evaluate growth performance and digestibility responses of supplementation of single β-mannanase in broilers, laying hens, turkey, nursery pigs, grow-finish pigs and sows feed programs to underscore mechanisms, and suggest opportunities for expanded exploitation.
What are β-Mannans
The NSP are present in high quantities in cereal grain co-products, intermediate to high quantities in both legumes and protein-rich meals, and low to intermediate quantities in cereal grains (Bach Knudsen, 1997). The primary mono-sugars in NSP consist of pentoses (arabinose and xylose); hexoses (glucose, galactose, and mannose); 6-deoxyhexoses (rhamnose and fucose); and uronic acids (glucuronic and galacturonic acids or their 4-O-methyl ethers; Bach Knudsen, 1997). β-mannans and xylans are the two most important hemicelluloses and hence considerable research has been focused on their value-added applications and hydrolysis.
Types and Structure of β-Mannans
β-mannans from plants are polysaccharides of d-mannose units linked together by β-(1-4) glycosidic bonds and are distinct from yeast cell wall backbone of d-mannose units that are linked by α-(1-6) glycosidic bonds substituted by mannan units linked by α-(1-2) and α-(1-3) glycosidic bonds (Chen et al., 2018). Structurally, β-mannans are linear polymers of β-1,4-linked mannose residues backbone without (linear mannan) or with combination of glucose and mannose residues (glucomannan) and occasional side chains of α-1,6-linked galactose residues (galactomannan or galactoglucomannan). Galactomannans are straight-chain polysaccharides with mannose units (main chain) linked by β-(1-4) glycosidic bonds and galactose units (side group) in varying proportions linked by α-(1-6) glycosidic bond (de Vries and Visser, 2001; BeMiller, 2019). Mannose to galactose ratio can vary from 1.0 to 5.3, depending on the sources (de Vries and Visser, 2001). The two rich sources of galactomannans are the endosperm of plant seeds, the vast majority of which originate in the Leguminosae family (de Vries and Visser, 2001). Galactomannans are present in the endosperm of legumes of coconut, coffee, and several palm species (Nishinari et al., 2007). Rich sources of galactomannans for human food and non-food commercial application includes guar or caster bean (Cyamopsis tetragonoloba), tara (Caesalpinia spinosa Kuntze), carob or locust bean (Ceratonia siliqua), and fenugreek (Trigonella foenum-graecum L.; Prajapati et al., 2013; Barak and Mudgil, 2014). Complete hydrolysis of galactomannans requires β-(1-4)-mannanase, β-(1-4)-mannosidase, and α-(1-6)-galactosidase (Bågenholm et al., 2017). Glucomannans are composed of d-mannose and d-glucose units with a ratio of 1.4:1 to 1.6:1 in linear chain arrangement with some branching (Cui et al., 2013). One of the richest sources of glucomannan is the tuber of Amorphophallus konjac which has been cultivated for centuries in China and Japan and used as food (Cui et al., 2013). Pine woods contain glucomannans that have glucose units linked to acetyl groups. Hydrolysis of glucomannan requires β-(1-4)-mannanase, β-(1-4)-mannosidase, and acetyl esterase (de Vries and Visser, 2001). Galactoglucomannans consist of a backbone of mannose and glucose units linked by β-(1-4) and side chains of galactose units linked by α-(1-6). The mannose or glucose units of the backbone are 20% to 30% acetylated at C-2 or C-3. Acetylation and galactose increase solubility of galactoglucomannans. Galactomannans are commonly present in softwood (de Vries and Visser, 2001). Hydrolysis of galactomannans requires microbial β-(1-4)-mannanase, β-(1-4)-mannosidase, α-(1-6)-galactosidase, and acetyl esterase (van Gool, 2012).
Abundance of β-Mannans in Feed Ingredients
The major β-mannans in feedstuffs are in the form of glucomannan and galactomannan. The concentration of total β-mannans in feedstuffs can be as high as 30% dry matter (DM; Table 1). Ranking of feedstuffs in terms of concentration of soluble β-mannans follows the pattern of total β-mannans (Table 2). Soybean products and co-products of processing palm, coconut, and guar seeds are the major sources of β-mannans in poultry and livestock feed. In general, the concentration of β-mannans is low (<0.5% DM, Table 1) in cereals (corn, barley, wheat, sorghum). Cereal co-products from the milling industry such as corn gluten meal, wheat middlings, and wheat bran have slightly higher concentration of β-mannans than parent grains but still less than 1% DM (Table 1; Bach Knudsen, 1997). However, cereal co-products from the ethanol industry have relatively high concentration of β-mannans. This may be due to the potential contamination with residual yeast mannans and the fact that these products have higher concentration of NSP. Indeed, it has been estimated that that distillers dried grains with solubles (DDGS) from corn and wheat contains up to 6% yeast biomass (Alizadeh et al., 2016). Typical analytical approach for determining the concentration of β-mannans in feedstuffs is through estimation of total free mannose sugar (Englyst and Cummings, 1984; Hsiao et al., 2006). Such approach will not obviously distinguish between β-mannans characteristic to plant feedstuffs and yeast cell walls (Chen et al., 2018). This might explain observed variability of β-mannans concentration in corn and wheat DDGS. For example, the concentration of β-mannans in corn DDGS ranges from 0.5% to 2.0% DM and for wheat DDGS from 1.3% to 1.8% DM (Pedersen et al., 2014; Jaworski et al., 2015; Rho et al., 2020).
Table 1.
Concentration of total β-mannans in feedstuffs, % DM
| Feedstuff | Mannose | References | ||
|---|---|---|---|---|
| Minimum | Maximum | Mean | ||
| Copra meal | 30.60 | 34.56 | 32.58 | Saittagaroon et al. (1983); Bach Knudsen (1997) |
| Palm kernel meal | – | – | 30.90 | Bach Knudsen (1997) |
| Guar seed | 20.60 | 22.70 | 21.34 | Hansen et al. (1992) |
| Guar meal | 8.20 | 12.62 | 10.41 | |
| Soybean hulls | – | – | 5.00 | Bach Knudsen (2014) |
| Soybean meal, 44% CP, with hulls | 1.30 | 2.07 | 1.67 | Bach Knudsen (1997) |
| Wheat DDGS | 1.30 | 1.80 | 1.55 | Pedersen et al. (2014) |
| Corn DDGS | 0.47 | 2.00 | 1.28 | Jaworski et al. (2015) |
| Soybean meal, 48% CP, dehulled | 0.65 | 1.31 | 0.98 | Bach Knudsen (1997) |
| Corn gluten feed | 0.40 | 1.20 | 0.80 | Bach Knudsen (1997) |
| Brewer spent grains | – | – | 0.80 | Denstadli et al. (2010) |
| Wheat middlings | 0.30 | 0.80 | 0.55 | Bach Knudsen (1997) |
| Wheat bran | 0.50 | 0.50 | 0.50 | Bach Knudsen (1997) |
| Canola meal | 0.39 | 0.45 | 0.42 | Slominski et al. (1994) |
| Barley | – | – | 0.40 | Bach Knudsen (1997) |
| Wheat | 0.30 | 0.30 | 0.30 | Bach Knudsen (1997) |
| Corn | 0.20 | 0.30 | 0.25 | Bach Knudsen (1997) |
| Sorghum | – | – | 0.10 | Jaworski et al. (2015) |
CP, crude protein.
Table 2.
Concentration of soluble β-mannans in common feedstuffs, % as is1
| Item | n | Minimum | Maximum | Mean |
|---|---|---|---|---|
| Palm kernel meal | 4 | 3.56 | 7.27 | 4.83 |
| Guar meal, ~40% CP | 8 | 3.33 | 5.83 | 4.62 |
| Soyhulls | 2 | 4.29 | 4.61 | 4.45 |
| Copra meal (coconut meal) | 1 | – | – | 3.36 |
| Guar meal, >47% CP | 3 | 1.33 | 2.38 | 1.79 |
| Sunflower, expeller | 1 | – | – | 0.56 |
| Soybean cake | 3 | 0.43 | 0.72 | 0.53 |
| Soybean meal, 44% CP, with hulls | 7 | 0.25 | 0.87 | 0.53 |
| Soybean meal, full fat, with hulls | 15 | 0.28 | 0.70 | 0.47 |
| Sunflower meal, ≤32% CP, with hulls | 7 | 0.35 | 0.46 | 0.41 |
| Soybean meal, 48% CP, dehulled | 58 | 0.19 | 0.67 | 0.39 |
| Soybean meal, fermented | 2 | 0.39 | 0.39 | 0.39 |
| Corn DDGS | 12 | 0.15 | 0.73 | 0.38 |
| Sunflower, >32% CP, dehulled | 15 | 0.28 | 0.50 | 0.38 |
| Barley | 5 | 0.25 | 0.31 | 0.28 |
| Oats | 1 | – | – | 0.21 |
| Wheat middlings | 2 | 0.17 | 0.20 | 0.19 |
| Rice | 1 | – | – | 0.18 |
| Wheat | 15 | 0.07 | 0.28 | 0.18 |
| Wheat bran | 4 | 0.14 | 0.23 | 0.17 |
| Cassava/Tapioca | 2 | 0.12 | 0.18 | 0.15 |
| Beet flour | 1 | – | – | 0.15 |
| Rice bran | 1 | – | – | 0.13 |
| Rapeseed meal | 11 | 0.09 | 0.25 | 0.12 |
| Corn gluten meal | 2 | 0.07 | 0.16 | 0.11 |
| Lentils | 2 | 0.10 | 0.11 | 0.11 |
| Oats, dehulled | 2 | 0.06 | 0.15 | 0.11 |
| Sorghum | 3 | 0.09 | 0.12 | 0.11 |
| Corn meal (maize screenings) | 3 | 0.05 | 0.14 | 0.10 |
| Corn germ meal | 1 | – | – | 0.10 |
| Hominy | 2 | 0.08 | 0.12 | 0.10 |
| Corn | 33 | 0.06 | 0.15 | 0.09 |
| Rapeseed, expeller | 1 | – | – | 0.09 |
| Peas | 5 | 0.06 | 0.08 | 0.07 |
| Faba beans | 3 | 0.05 | 0.05 | 0.05 |
| Rapeseed, whole | 2 | 0.05 | 0.06 | 0.05 |
1Data from Elanco internal survey database.CP, crude protein.
Soybean meal (SBM) and its derivatives are a major protein (amino acids) source for poultry and livestock across the globe (Kiarie et al., 2020; Kiarie et al., 2021). The SBM contains appreciable amounts of carbohydrates (approximately 40%) of which >50% is in the form of NSP. The concentration of β-mannans in SBM ranges from 0.7% DM in dehulled SBM (~48 crude protein) to 2.1% DM in 44% crude protein SBM (hulled; Table 1). β-mannans in SBM are mainly associated with the hull (~5% β-mannans) and are heat-resistant compounds that survive the drying-toasting phase of processing soybeans (Hsiao et al., 2006). However, it is noteworthy that majority of β-mannans in soy hulls are soluble (Table 2). Canola/rapeseed meal is another important protein feedstuff but has low β-mannans concentration (less than 0.5% DM, Table 1; Slominski et al., 1994). Large quantities of guar beans (C. tetragonoloba) are produced and processed mainly in India and Pakistan for galactomannan-rich gum extraction from endosperm and residue left over from processing is converted into meal (Prajapati et al., 2013). Guar meal has a good amino acid profile with crude protein content of 33% to >45% and is used to replace conventional feedstuffs in monogastric and ruminant feeds (Conner, 2002; Lee et al., 2005; Hussain et al., 2012). However, a major limitation of guar meal application in monogastric diets is high and variable concentration of β-mannans that can range from 8% to 13% DM (Table 1) depending on residual gum in the meal (Hansen et al., 1992). The concentration of soluble β-mannans in guar meal ranges from 1.8% to 4.6% (Table 2); suggesting a significant portion of total β-mannans in guar meal are soluble. Copra and palm kernel meals are defatted co-products from coconut and palm processing, respectively, and are an attractive choice for feed cost reduction in tropical countries (Saittagaroon et al., 1983; Khanongnuch et al., 2006; Ibuki et al., 2014; Stein et al., 2015; Jang et al., 2020b). The concentration of β-mannans in copra and palm kernel exceeds 30% DM (Table 1; Saittagaroon et al., 1983; Bach Knudsen, 1997). However, as indicated in Table 2, comparatively, the proportion of soluble β-mannans in copra and kernel meal are much lower than for guar meal and soyhulls.
Implications of Presence of β-Mannans in Feed
Like other dietary fiber fractions, β-mannans are not degraded sufficiently or indeed at all by the conditions and repertoire of endogenous digestive enzymes in monogastric upper GIT (Bach Knudsen, 2011; Knudsen, 2014). Feed β-mannans have been associated with negative influences on voluntary feed intake, nutrient utilization, health, and metabolic processes through a variety of variably characterized mechanisms related to GIT and systemic responses (van Nevel et al., 2005). The following subsection will review some of these mechanisms and associated impact on animal performance and health.
β-Mannans and Digestive Physiology
Fibrous indigestible complexes can impede normal digestion and absorption processes of nutrients (Slominski, 2011). Soluble β-mannans have been demonstrated to increase viscosity of intestinal contents accompanied with decreased nutrient (glucose, lipids) and water absorption in pig and chicken models (Rainbird et al., 1984; Lee et al., 2003; Blackburn and Johnson, 2007; Rainbird et al., 2007). For example, perfusion of guar gum in isolated porcine jejunal loops reduced net absorption of glucose and maltose from 74.2% to 41.4% and 71.1% to 35.0%, respectively (Rainbird et al., 1984). Correlation analysis showed an inverse linear relationship between glucose absorption and dietary guar gum concentration (Ellis et al., 2007). The peculiarity is that viscous digesta impairs diffusion and convective transport of digestive enzymes, effectively reducing contact with substrates (Angkanaporn et al., 1994). Highly viscous diets cause pasty excreta as a result of increased water consumption leading to poor litter quality (Bedford and Schulze, 1998; Daskiran et al., 2004). The consequences of low and variable nutrient absorption are increased feed cost, proliferation of gut pathogens, poor feed efficiency and nutrient loading and emissions into the environment (Kiarie et al., 2013, 2016). Moreover, fibrous fractions increase visceral organ weight and consequently increase utilization of dietary energy and amino acids for maintenance at the expense of tissue deposition (growth; Cant et al., 1996; Agyekum et al., 2012). Pigs-fed diets with high levels of dietary fiber have greater intestinal mass and a lower dressing percentage than pigs-fed diets with relatively lower concentration of dietary fiber (Agyekum et al., 2012). Feeding carob tree seeds, a rich source of β-mannans, resulted in shorter villi and a lower jejunum villus height/crypt depth ratio in piglets, indicating faster renewal rate (van Nevel et al., 2005). Increased mass of the GIT and portal vein drained viscera implies greater utilization of digestible energy to meet maintenance energy requirements subsequently resulting in less energy being available for protein and fat deposition (Just et al., 1983).
β-Mannans and Feed-Induced Immune Response
The primary functions of the GIT are to digest and absorb nutrients and to excrete waste products. However, the GIT is also involved in numerous immune and endocrine functions. Indeed, the GIT has dual and opposing roles as the site of nutrient absorption and host defense (MacDonald and Monteleone, 2005; Okumura and Takeda, 2017). Optimal nutrient digestion and absorption require a large surface area and a thin epithelium that has the potential to compromise host defense. Many infectious diseases involve the GIT, and the investment by the GIT in protecting itself is evident by the abundance of lymphoid tissue and immune cells it harbors (MacDonald and Monteleone, 2005; Okumura and Takeda, 2017). The primary intestinal cellular barrier in preventing antigens encountering the immune system is the single layer of epithelium with an expanded surface area due to millions of fingerlike villi. Each epithelial cell maintains intimate association with its neighbors and seals the surface of the gut with tight junctions (Okumura and Takeda, 2017). The GIT epithelial barrier, therefore, represents a highly dynamic structure that limits but does not exclude, antigens from entering the tissues, whereas the immune system constantly samples gut antigens (MacDonald and Monteleone, 2005). In the upper GIT, the bulk of the antigen exposure comes from the diet, whereas in the lower GIT (terminal ileum to distal colon) antigenic load will emanate from abundant and highly complex commensal microflora (MacDonald and Monteleone, 2005).
In general, biologists view immunity and metabolism as distinct processes. However, it is increasingly been recognized that the goal of efficient animal protein production is often blunted by any level of immune system stimulation (Klasing, 2007; Arsenault et al., 2014; Arsenault and Kogut, 2015). Unlike other dietary fiber fractions, β-mannans are akin to the mannose residues that cover the surface of most cells and play important role in various biological mechanisms such as immune response, adhesion, infection, and signal transduction (Arsenault et al., 2017). As such, β-mannans can be recognized by the host immune system as Pathogen Associated Molecular Patterns through several cell surface receptors in the GIT. It is plausible that fragments of β-mannans may either bind to gut epithelia and exert localized and/or systemic immune system effects, or be absorbed into the bloodstream, with the potential to exert systemic effects (Zhang and Tizard, 1996; Duncan et al., 2002; Arsenault and Kogut, 2015; Arsenault et al., 2017; Tiwari et al., 2020). Consequently, feed β-mannans have been associated with provocation of an intestinal immune response leading to wasteful energy utilization and depression in animal performance (Daskiran et al., 2004; Gabler and Spurlock, 2008; Arsenault et al., 2017). This phenomenon has been named feed-induced immune response (FIIR) and is linked to unproductive energy expenditure due to inappropriate activation of the immune system. For example, β-mannans from Aloe Vera exhibited similar chemical properties to SBM β-mannans and were shown to stimulate nitric oxide synthesis through macrophage mannose receptor activation (Karaca et al., 1995; Ramamoorthy et al., 1996). In principle, the animal immune system mistakes feed β-mannans for harmful microorganisms and use up vital energy mounting an immune response through increased proliferation of monocytes and macrophages resulting in higher production of cytokines and acute-phase proteins (Wu et al., 2005; Arsenault et al., 2017).
Systematic and Meta-analyses of Animal Responses to Supplemental β-Mannanase
Web of Science (clarivate.com) was used to search for publications that evaluated supplementation of feed β-mannanase in pigs and poultry. Once all publications were collected, only those fulfilling the following selection criteria were retained: 1) tested single β-mannanase as standalone treatment; 2) in-vivo swine and poultry studies including a control treatment group with the same dietary composition as β-mannanase group; 3) published in English; 4) reported growth performance results (feed intake, growth, and feed efficiency); 5) reported other parameters such as digestibility, carcass data, etc.; 6) reported sample variance (standard deviation or standard error of the mean [SEM]), sample size (n), age or body weight (BW) weight range, sex, and duration of the study. Each study was assigned a publication number from which the information was extracted and stored in an Excel workbook. Where more than one β-mannanase dosage was used in a study, each dosage and associated data was entered in a separate row. Similarly, if a study reported more than one experiment, each tested β-mannanase dose and associated control was entered in a separate row. Additional information such as main ingredients, use of additional β-mannans such as DDGS, soy hulls, copra meal, and guar meal were entered. Separate workbooks were created for broiler chickens, nursery pigs, grow-finishing pigs, sows, and turkeys. Meta-analyses were conducted on growth performance and energy digestibility in broiler chickens, nursery, and grow-finish pigs based on higher number of studies. Other responses and data on laying hens, turkeys, and sows were systematically reviewed. For meta-analyses, the metafor package in R was used to conduct meta-analysis and to construct forest plots (Viechtbauer, 2010). The independent variables (y) included in the random models of the meta-analysis were: average daily feed intake (ADFI), average daily gain (ADG), feed efficiency (feed conversion [FCR] for broilers and gain to feed [G:F] for pigs), apparent metabolizable energy (AMEn) in broilers, and apparent total tract digestibility (ATTD) of gross energy in pigs. Mean difference (MD) was the effect size and was calculated by subtracting the mean of the control group from β-mannanase supplemented group (Bougouin et al., 2014). The MD allows easy effect size interpretation because it is in the original units of the response variable in question. Publication number was included as a random effect in all models (µ) and the error term (e) was also included in the model. The validity and robustness of meta-analysis conclusions can be affected by publication bias and the presence of outlier observations. In this context, outliers were identified and removed using the test of I2 heterogeneity as described by Viechtbauer and Cheung (2010). The pooled SEM of each study was considered for standardization and weighting of the different comparisons. Forest plots were constructed to show the MD effect size estimate, its confidence intervals (CIs) and weighting (Torres-Pitarch et al., 2019).
Broiler Chickens
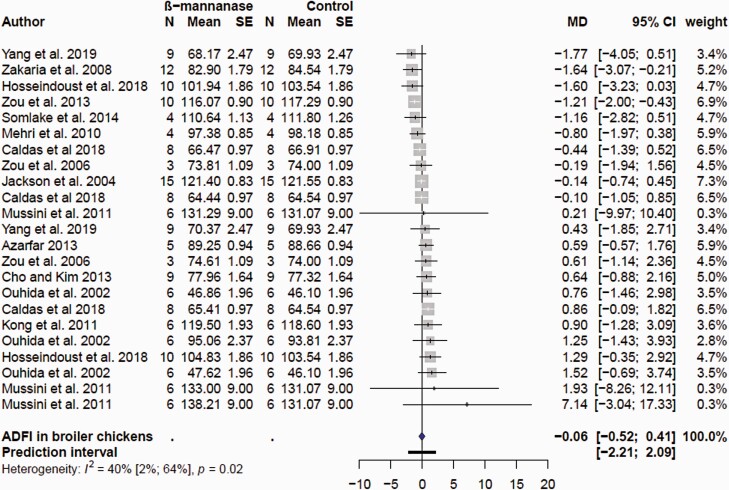
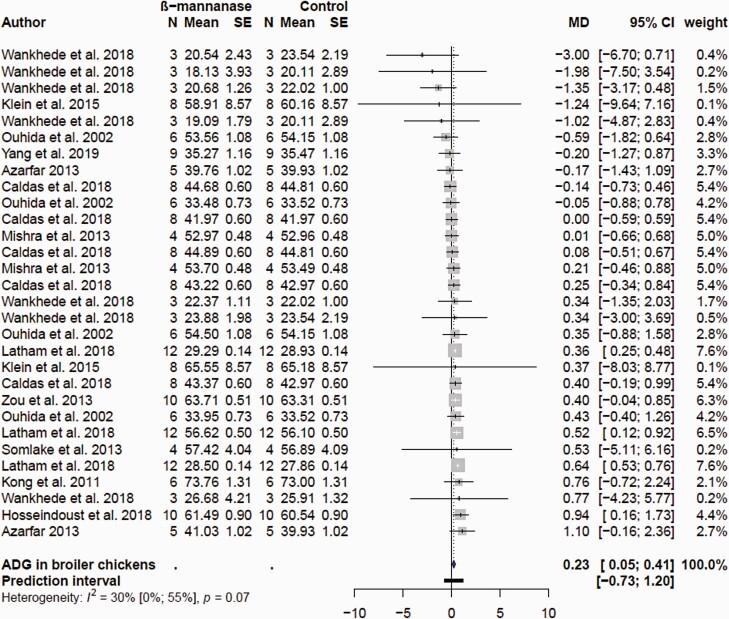
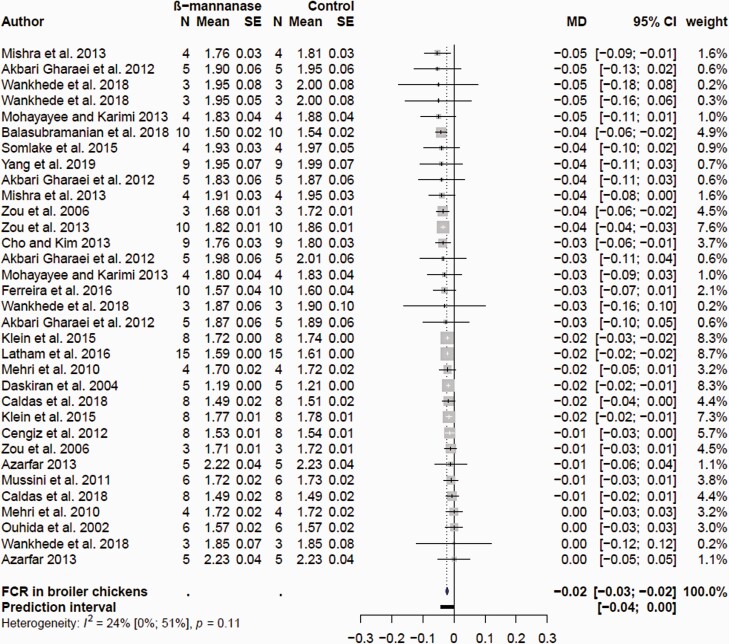
The searches collectively resulted in 28 research articles for growth performance in broiler chickens. Data entry based on previously described criteria resulted in 48, 73, and 87 comparisons for ADFI, ADG, and FCR, respectively. The final list of comparisons included in the meta-analyses after removal of influential observations is shown in Supplementary Table S1. A total of 23, 30, and 33 comparisons for ADFI, ADG, and FCR were used in the final model highlighting tremendous variability in response of supplemental feed β-mannanase. The mean ADFI, ADG, and FCR in the control and β-mannanase supplementation treatment groups and corresponding MD estimates are presented as forest plots in Figures 1, 2, and 3, respectively. Supplemental β-mannanase had no effect on ADFI (P = 0.795); the mean MD was −0.059 with 95% CI (−0.52; 0.41 g/d; Figure 1). β-Mannanase supplementation had a consistently positive effect on ADG and FCR (Figure 2), but the effect sizes were variable. The MD effect of β-mannanase on ADG ranged from −3.0 to 1.0 g/d (Figure 1) reflecting variation of responses among the individual studies. The weighted pooled MD improvement of β-mannanase over the control on ADG was 0.23 g/d (P = 0.013) with 95% CI (0.05; 0.41 g/d). This suggested supplementation with β-mannanase from hatch to 42 days of age would translate to between 2.1 and 17.2 g extra weight per bird or between 210 and 1,720 kg in 100,000 flocks. The mean MD β-mannanase improvement on FCR was −0.02 (P < 0.0001) with 95% CI (−0.03; −0.02) suggesting a 2-to-3-point FCR improvement (Figure 3). Variable growth performance responses to supplemental fiber degrading enzymes in poultry are well documented and are linked to animal, diet, and enzyme factors (Ravindran, 2013). With exception of few studies where additional β-mannans in form of corn DDGS and guar meal were added, most studies used corn and SBM.
Figure 1.
Forest plots showing mean difference (MD) effect and confidence interval of β-mannanase supplementation on average daily feed intake (ADFI, g/d) in broiler chickens.
Figure 2.
Forest plots showing mean difference (MD) effect and confidence interval of β-mannanase supplementation on average daily gain (ADG, g/d) in broiler chickens.
Figure 3.
Forest plots showing mean difference (MD) effect and confidence interval of β-mannanase supplementation on feed conversion (FCR, g/g) in broiler chickens.
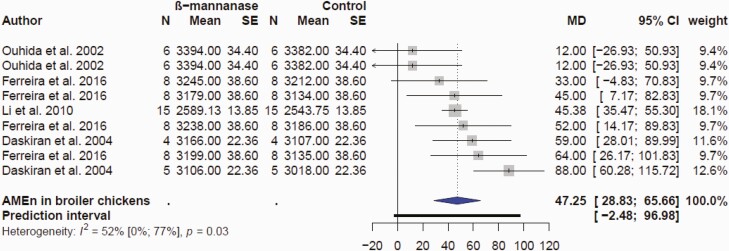
Because β-mannanase hydrolyzes all types of β-mannans, it could be effective in improving nutrient utilization. A meta-analysis was conducted to characterize the impact of β-mannanase supplementation on AMEn responses; the mean MD between β-mannanase and control was 47 kcal/kg (P = 0.0004) with 95% CI (28.8; 65.7 kcal/kg; Figure 4). This corroborated data showing broilers fed low-energy diets (3,003, 3,080, and 3,157 kcal/kg for starter, grower, and finisher, respectively) supplemented with β-mannanase performed slightly better than broilers fed high energy diets (3,146, 3,223, and 3,300 kcal for starter, grower, and finisher, respectively) without β-mannanase (McNaughton et al., 1998). Specifically, β-mannanase improved AME by 143 kcal/kg concomitant with 3% improvement on growth and FCR. β-Mannanase supplementation has been demonstrated to have positive effects on performance and nutrient digestibility in poultry fed corn-SBM-based diets as well as diets containing guar meal, copra meal, and other β-mannan-containing components (Daskiran et al., 2004; Jackson et al., 2004; Ferreira et al., 2016; Balasubramanian et al., 2018; Caldas et al., 2018; Mohammadigheisar et al., 2021). Some of these positive effects have been ascribed to mechanisms that are different compared to the modes of action of other NSP carbohydrases such as reducing FIIR. β-Mannanase supplementation significantly improved protein, lipid, and metabolizable energy utilization in broilers fed corn-SBM-copra meal-based diets (Sundu et al., 2006). The authors suggested that β-mannanase might have decreased digesta flow rate due to the greater hydrolysis of copra meal leading to improved nutrient utilization. It has also been demonstrated that β-mannanase improved digestibility of valine, methionine, and leucine in broiler chickens fed corn-SBM diet (Mohammadigheisar et al., 2021).
Figure 4.
Forest plots showing mean difference effect (MD) and confidence interval of β-mannanase supplementation on apparent metabolizable energy corrected for nitrogen (AMEn, kcal/kg) in broiler chickens.
Turkeys
Turkeys may be more adversely affected by β-mannans because of the high protein/amino acids requirements especially during early growth phases necessitating higher dietary inclusion of SBM. Therefore, β-mannanase may be particularly effective in turkey diets. To test this hypothesis, experiments have been designed to determine the effect of β-mannanase supplementation in corn-SBM diets on the growth performance of market turkeys and to compare the efficacy of β-mannanase supplementation in diets containing SBM-44 and SBM-48 (Odetallah et al., 2002). The data indicated that β-mannanase supplementation improved growth performance but to a great extent in birds fed SBM-44%. Specifically, β-mannanase improved BW and FCR by 1% and 3% in hens and 2.5% and 4% in toms, respectively (Odetallah et al., 2002). β-mannanase has also been demonstrated to improve growth, feed efficiency, and BW uniformity in 20 weeks old toms (Jackson et al., 2006). Corn DDGS is often incorporated in larger quantities in turkey feeding programs. Because of higher concentration of β-mannans (Table 1), the effects of supplementing β-mannanase in turkey diets containing corn DDGS have also been investigated. β-mannanase improved live weight and feed efficiency in turkeys fed corn-SBM diet without or with 15% corn DDGS over 12 weeks (Jackson et al., 2008). Supplemental β-mannanase had no effects on growth performance in turkey hens fed wheat/corn/pork meal/SBM without or with 30% wheat DDGS from hatch to 21 days of age (Opoku et al., 2015b). However, β-mannanase improved nitrogen retention and AME in diets without wheat DDGS but had negative effects on these parameters in diets with wheat DDGS (Opoku et al., 2015a). In a follow-up study, turkey hens were fed wheat/corn/pork meal/SBM plus 30% wheat DDGS without or with β-mannanase for 72 days (Opoku et al., 2015b). There were no diet effects on growth performance and mortality throughout the entire study. However, birds fed β-mannanase had higher nitrogen retention compared with the control birds (Opoku et al., 2015b). As earlier discussed, a greater portion of β-mannans in wheat and corn DDGS is likely due to residual yeast and therefore unresponsive to commercially available feed β-mannanase.
Laying Hens
Two Hy-Line strains (W36 and W77) were fed high and low AME corn-SBM diets from 18 through 66 weeks of age (Jackson et al., 1999). There was no interaction between β-mannanase, diet energy level, and strain on egg production. Independent of diet energy level and strain, birds fed β-mannanase had higher hen-day production from 30 weeks of age to the end of the experiment (Jackson et al., 1999). Although FCR was not reported, β-mannanase birds showed higher feed intake between 43 and 54 weeks but there was no diet effect on BW (Jackson et al., 1999). The authors opined that insulin-driven metabolism explained increased feed intake and corresponding increase in egg production. β-mannanase improved FCR in laying hens fed a low energy corn-SBM diet (−120 kcal/kg AME) to the level of control hens fed diet with adequate AME over a 12-week study (Wu et al., 2005). Moreover, β-mannanase increased average egg production and egg mass of hens in weeks 5 to 8 but had no effects on mortality, BW, and BW uniformity. β-mannanase improved FCR due to reduced feed intake and larger eggs in 65-week old Lohmann LSL hens fed barley-SBM-based diets (Torki et al., 2016). However, there was no diet effect on egg production rate, mortality, and BW over the 8-week trial (Torki et al., 2016). Supplementation of β-mannanase in corn-SBM diets without or with 4% or 8% guar meal improved FCR in Hy-Line W-36 hens in the second cycle (98 weeks old), which was linked to reduced feed intake but not egg production rate or egg weight (Hasani et al., 2019). Hy-line brown (84 weeks old) laying hens were fed high or low (−100 kcal/kg) AME corn/wheat/SBM/DDGS diets without or with β-mannanase added in low AME diet at two levels (0.04% and 0.08%; Kim et al., 2017c). The 4-week trial showed no dietary effects on egg production, egg weight, feed intake, FCR, BW, and nitrogen retention. However, hens fed low energy diet with 0.08% β-mannanase had similar AMEn to hens fed the high energy diet. There was no interaction between supplemental β-mannanase (0% or 0.04%) and dietary AME concentration (2,650, 2,750, or 2,850 kcal/kg) on egg production, gross energy, and nutrient retention in 68-wk-old Hy-line brown layers (Shim et al., 2018). However, the hens fed β-mannanase had higher egg production, egg mass, and retained more gross energy and crude protein. Supplemental β-mannanase (800,000 U/kg) in lower AME and crude protein diets resulted in comparable egg production and egg mass to high AME and crude protein diets during early (30 weeks) and late (>62 weeks) laying cycle in Hy-Line brown laying hens (Zheng et al., 2020). The effect of dietary β-mannans (1.05% vs. 2.33%) and supplemental β-mannanase (0%, 0.04%, and 0.08%) was investigated for 8 weeks in 52-wk-old Hy-line brown hens (Ryu et al., 2017). There was no interaction between β-mannans and β-mannanase on egg production, egg weight, egg mass, feed intake, and FCR. However, β-mannans increased feed intake and the two β-mannanase doses equally improved egg production, egg weight, egg mass, and FCR (Ryu et al., 2017). The effects of β-mannanase were linked to improved retention of DM, crude protein and β-mannans and concentration of blood glucose. Thus, overall, the increased egg production in hens fed diets supplemented with β-mannanase could be linked to improved energy availability and metabolism.
Dietary β-mannanase supplementation increased egg weight at early stages of production (18–30 weeks of age) in Hy-line brown hens but had no impact on egg specific gravity throughout the 66-wk trial (Jackson et al., 1999). In contrast, β-mannanase did not have an effect on egg quality measurements (egg specific gravity, egg weight) in 98-wk-old Hy-Line W-36 hens in the second cycle of laying (Wu et al., 2005). Similarly, there was no effect of diet on indices of egg quality (Haugh unit, yolk color, shell weight, and thickness) in 65-wk-old LSL lite hens fed barley-based diets without or with β-mannanase (Torki et al., 2016). Interestingly, β-mannanase increased egg yolk cholesterol and blood triglyceride in laying hens in the second cycle of laying (Hasani et al., 2019). Egg yolk color, Haugh unit, eggshell color, and strength was similar in 84-wk-old Hy-line brown hens fed high AME diet or low AME diet supplement with 0.04% and 0.08% β-mannanase (Kim et al., 2017c). However, hens receiving a low AME diet with 0.04% β-mannanase had thicker eggshells than hens fed other diets. Supplemental β-mannanase (800,000 U/kg) had no effects on egg quality measures (shell color, thickness, strength, yolk color, and Haugh unit) during early and late lay in Hy-line brown hens (Zheng et al., 2020).
Sows
Lactating sows have the challenge of adequate feed intake to support nutrient requirements for maintenance, growth, and milk production (NRC, 2012). Overcoming factors that may limit feed intake during lactation will reduce BW loss, improve litter weight gain, and future sow reproductive performance. While increasing nutrient density may overcome poor feed intake in lactating sows, this approach increases feed cost. An alternative is to use fibrous feed ingredients from ethanol, milling, and vegetable oil industries. However, use of these feedstuffs requires use of fiber degrading enzymes for effective utilization. Lactating multi-parous sows were fed corn-SBM diet containing 2% palm kernel meal and 8% corn DDGS without and with two levels of β-mannanase (400 and 800 U/kg; Kim et al., 2018). β-mannanase prevented BW loss during lactation, however, the diets had no effect on lactation backfat loss and feed intake, wean to estrus interval and litter performance (Kim et al., 2018). In a second experiment within the same study, lactating multi-parous sows were fed corn-SBM diet containing 2% palm kernel meal and 8% corn DDGS, formulated for 3,300 or 3,350 kcal/kg metabolizable energy (ME) and fed without or with or 400 U/kg of β-mannanase. There was no interaction between ME level and β-mannanase on sow and litter performance or main effects on litter performance. However, sows fed high ME and β-mannanase diets had lower lactation BW loss and higher milk fat content (Kim et al., 2018). Sows fed β-mannanase had higher total tract digestibility of dry matter and gross energy, suggesting improvements in nutrient utilization.
Nursery and Finishing Pigs
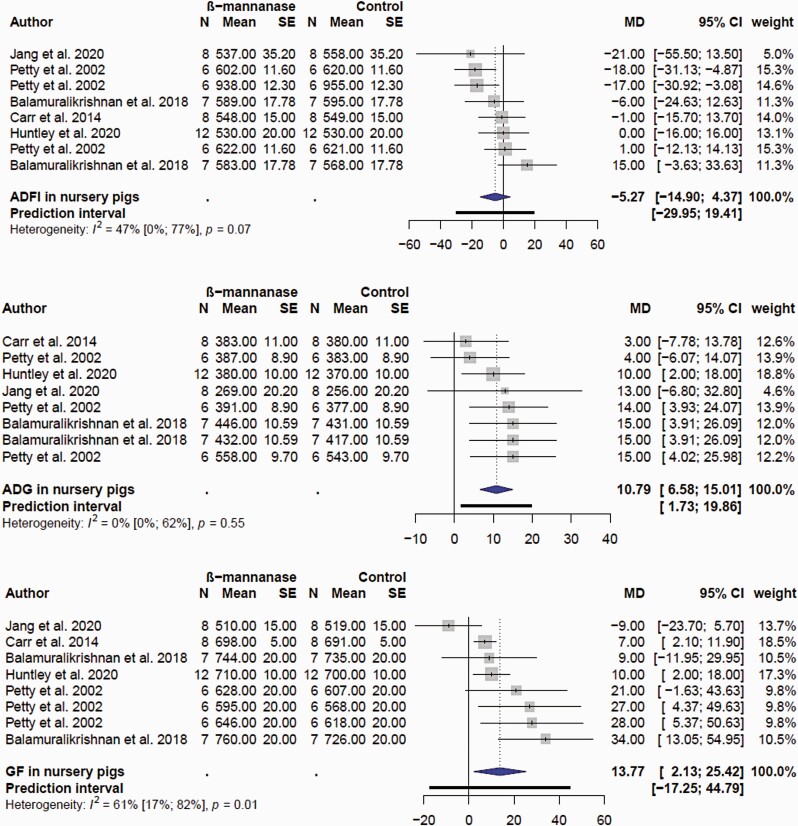
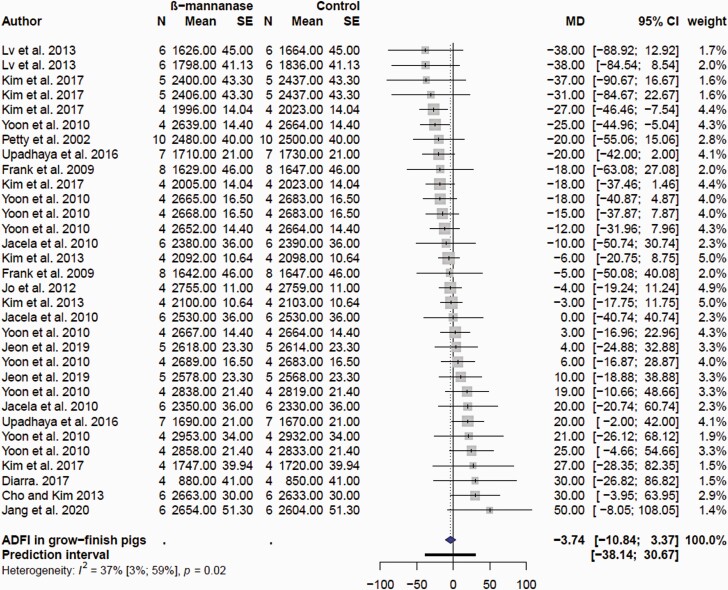
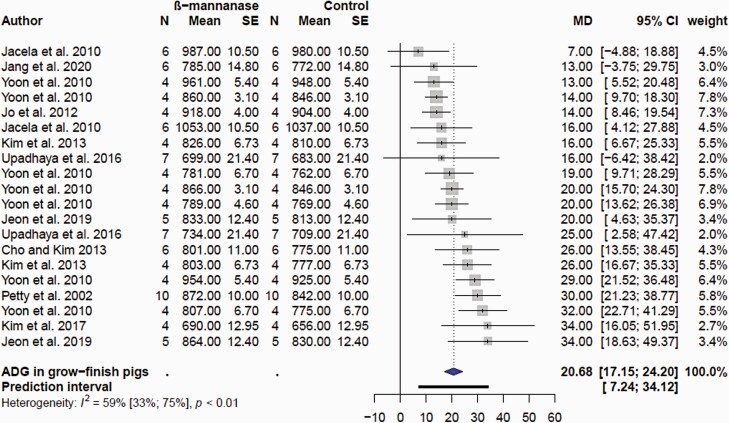
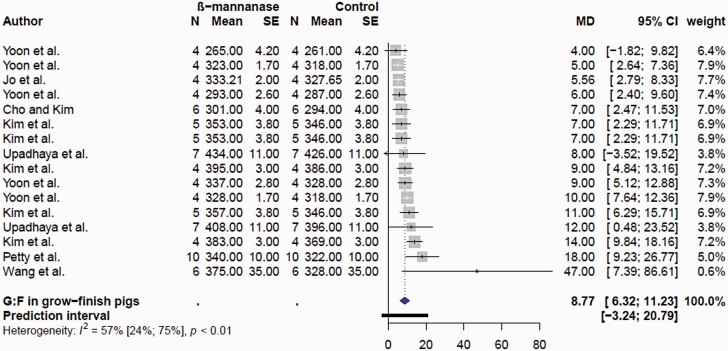
For the purpose of the meta-analyses, the effects of β-mannanase on growth performance were categorized in nursery (wean to ≥30 kg BW) and growing finishing (≥ 30 kg BW to slaughter) pigs. There were only eight comparisons for the nursery pig performance, reflecting the low number of published articles (Supplementary Table S2). There was no (P = 0.237) effect of β-mannanase on ADFI in nursery pigs (Figure 5A). The mean MD of ADFI was −5.7 g/d with 95% CI of (−14.9; 4.4). However, supplemental β-mannanase improved ADG (Figure 5B) and G:F (Figure 5C) in nursery pigs. The mean MD of ADG and G:F was 10.8 g/d (P = 0.0005; 95% CI of 6.6; 15.0 g/d) and 13.8 g/kg (P = 0.027; 2.1; 25.4 g/kg). In grow-finish pigs, β-mannanase had no effect (P = 0.292) on ADFI; the mean MD was −3.73 g/d; 95% CI of −10.8; 3.37 g/d; Figure 6). β-Mannanase improved (P < 0.0001) ADG and G:F in grow-finish pigs (Figures 7 and 8). The mean MD for ADG and G:F was 20.68 g/d (17.15; 24.20 g/d) and 8.77 g/kg (6.32; 11.23 g/kg) respectively (Figures 7 and 8). Based on the NRC estimate that a 25 kg growing pig gaining an average of 861 g/d will reach 135 kg BW, it follows that a pig fed β-mannanase will be between 2.1 and 3 kg heavier at lower feed consumption. Comparatively, the available data seems to suggest the magnitude of β-mannanase on growth was larger in grow-finish pigs than nursery pigs. Overall, this indicated that pigs fed β-mannanase will attain market/slaughter weight faster and with less feed consumption. Younger pigs have a limitation on feed consumption due to gut capacity (Nyachoti et al., 2004) and there is an expectation they would benefit more from feed enzyme supplementation. However, with respect to β-mannanase responses, it appears the improvement seen in feed efficiency is due to increased growth rate and not feed intake. This might suggest enhanced nutrient utilization and metabolism linked to levels of β-mannans.
Figure 5.
Forest plots showing mean difference effect and confidence interval of β-mannanase supplementation on average daily feed intake (ADFI, g/d), average daily gain (ADG, g/d), and gain efficiency (G:F, g/kg) in nursery pigs.
Figure 6.
Forest plots showing mean difference effect and confidence interval of β-mannanase supplementation on average daily feed intake (ADFI, g/d) in grow-finish pigs.
Figure 7.
Forest plots showing mean difference effect and confidence interval of β-mannanase supplementation on average daily gain (ADG, g/d) in grow-finish pigs.
Figure 8.
Forest plots showing mean difference effect and confidence interval of β-mannanase supplementation on gain efficiency (G:F, g/kg) in grow-finish pigs.
Studies involving corn-SBM diets indicated minimal impact of supplemental β-mannanase on growth performance in nursery and grow/finishing pigs. For example, β-mannanase in corn-SBM diets improved growth performance in some studies (Pettey et al., 2002; Bass et al., 2010; Lv et al., 2013) but not in others (Jacela et al., 2010; Jo et al., 2012; Carr et al., 2014; Upadhaya et al., 2016; Balamuralikrishnan et al., 2018; Huntley et al., 2020; Jang et al., 2020a). The responses of supplemental β-mannanase have been much more consistent in corn-SBM diets containing additional β-mannans. In a series of four experiments, supplemental β-mannanase (200–600 U/kg) improved ADG and blood glucose in growing-finishing pigs fed corn-SBM-based diets containing 10% to 15% corn DDGS (Yoon et al., 2010). Improved ADG and G:F were also observed in growing-finishing pigs fed corn-SBM with 5% to 6% corn DDGS with 0.05% β-mannanase (Wang et al., 2009; Bass et al., 2010). Supplementing β-mannanase in corn-SBM diets formulated with corn DDGS or palm kernel meal or combination of corn DDGS and palm kernel meal improved ADG and G:F in growing-finishing pigs (Kim et al., 2013; Kim et al., 2017b; Jeon et al., 2019). A study in which DDGS and soy hulls were formulated in a corn-SBM diet failed to show positive effects of β-mannanase on growth performance of finishing pigs (Cho and Kim, 2013). Incorporation of 10% to 25% copra meal in corn-SBM diets with 0.10% β-mannanase had no impact on growth performance of grow-finishing pigs (Kim et al., 2017a). Supplemental β-mannanase (0.10%) improved ADG in grow-finishing pigs fed corn-SBM diets with 6% or 12% palm kernel meal compared to pigs fed control diet without palm kernel (Jang et al., 2020b). However, pigs fed corn-SBM diet with 18% palm kernel meal with β-mannanase had poor ADG and ADFI compared with control without copra meal. This perhaps indicates an excess amount of β-mannans and bulkiness exceeding the gut capacity of the pig to eat more to maintain energy and nutrient intake. Feeding nursery pigs corn-SBM diet with 10% copra meal reduced ADFI and ADG but supplemental β-mannanase was not beneficial (Huntley et al., 2020). In the contrary, supplemental β-mannanase improved growth performance in nursery pigs fed corn-SBM diet with 30% palm kernel meal (Diarra, 2017). As previously discussed, research in pigs has demonstrated that β-mannans interfere with glucose metabolism and insulin secretion rates. It has been suggested that the reduction in glucose absorption and circulating insulin growth factor I (IGF-I) concentrations due to presence of dietary β-mannans may be ameliorated by β-mannanase. Thus, elevated blood glucose and circulating concentrations of IGF-I have been linked to the growth responses seen in pigs fed with β-mannanase (Pettey et al., 2002; Yoon et al., 2010; Kim et al., 2013; Kim et al., 2017b).
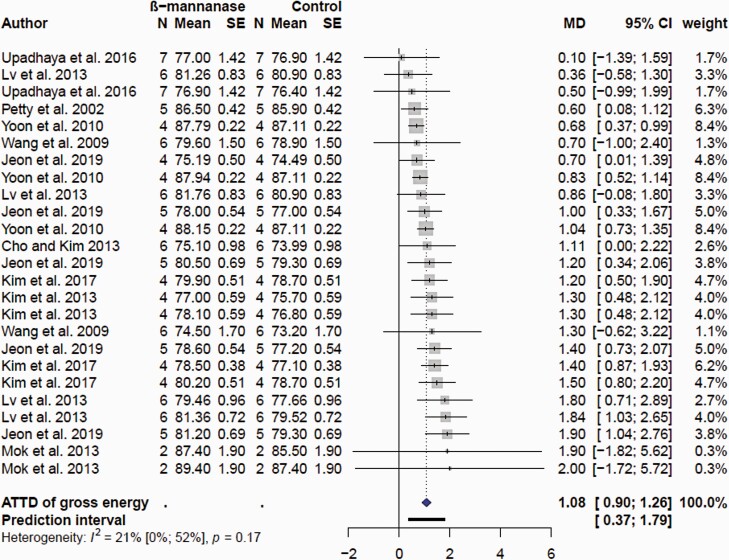
Meta-analyses of available literature indicated that supplemental β-mannanase improved (P < 0.001) ATTD of gross energy in pigs (Figure 9). The mean MD improvement of ATTD of gross energy was 1.08% unit with 95% CI (0.90; 1.26). According to the NRC, a digestible energy (DE) of 3,400 kcal/kg is recommended for growing pigs; it follows that supplemental β-mannanase will release between 30.6 and 42.8 kcal/kg of digestible energy. Dietary supplementation with β-mannanase can directly target β-1,4-mannans in feed ingredients and improve the nutrient digestibility (Pettey et al., 2002; Yoon et al., 2010). Interaction was observed between SBM type (hulled and dehulled) and β-mannanase on ATTD of energy and crude protein in nursery pigs, with supplementation improving digestibility to a greater extent in nursery pigs fed a corn-based diet formulated with hulled SBM (Balamuralikrishnan et al., 2018). In contrast, although adding β-mannanase in a corn-SBM diet with 15% corn DDGS increased β-mannan digestibility there was no improvement on ileal digestibility of gross energy and crude protein (Tiwari et al., 2018). Similarly, β-mannanase had no effect on energy and nitrogen balance in nursery pigs fed corn-SBM plus 10% soyhulls diet (Huntley et al., 2018). Supplementation of β-mannanase in corn diets formulated with hulled or dehulled SBM had no effect on ATTD of gross energy and crude digestibility in growing pigs (Jo et al., 2012; Upadhaya et al., 2016). Similarly, supplementing β-mannanase in corn-SBM with 6% DDGS did not affect ATTD of nutrients in growing-finishing pigs (Wang et al., 2009). However, growing-finishing pigs may benefit from supplemental fiber degrading enzymes when fed higher levels of fibrous feedstuffs rich in β-mannans. Jeon et al (2019) used a semi-purified diet containing corn, wheat, SBM, corn DDGS, and palm kernel meal to investigate the impact of supplemental β-mannanase on digestible energy content. A significant response was only noted for palm kernel meal; however, the DE between control and treated was 33, 35, 33, 43, and 72 kcal/kg for corn, wheat, SBM, corn DDGS, and palm kernel meal, respectively (Jeon et al., 2019). This suggested a stronger response of β-mannanase as dietary β-mannans increased. Growing-finishing pigs fed β-mannanase in corn-SBM diets containing 10% to 15% corn DDGS had improved ATTD of dry matter, crude protein, gross energy (Yoon et al., 2010). Supplementing β-mannanase in corn-SBM diets with corn DDGS or palm kernel meal or corn DDGS and palm kernel meal improved ATTD of gross energy in growing-finishing pigs (Kim et al., 2013, 2017b; Jeon et al., 2019). It has been shown that β-mannanase improved nutrient digestibility in pigs fed corn-SBM diets containing up to 12% palm kernel meal (Mok et al., 2013, 2015; Jang et al., 2020b). However, supplemental β-mannanase did not improve energy and nutrient digestibility in pigs fed corn-SBM diets containing more than 15% palm kernel meal (Kwon and Kim, 2015; Jang et al., 2020b). Formulating 10% to 25% copra meal in corn-SBM diets with 0.10% β-mannanase linearly reduced ATTD of crude protein in grow-finishing pigs (Kim et al., 2017a). Whereas, supplemental 0.05% β-mannanase did not improve ATTD of energy in corn-SBM diet formulated with combination of DDGS (6%) and soy hulls (2%; Cho and Kim, 2013). These studies suggested there is a limitation for overcoming the negative effects of β-mannans on nutrient digestibility.
Figure 9.
Forest plots showing mean difference effect and confidence interval of β-mannanase supplementation on apparent total tract digestibility (ATTD) of gross energy (%) in grow-finish pigs.
Conclusions and Future Perspectives
Feed represents more than 60% of the variable cost of producing pork and poultry products with provision of dietary energy and protein (amino acids) representing more than 90% of this cost. In the context of burgeoning human population and attendant demand for food, feed supply is challenged by limited availability of natural resources, climate change pressure, and food-feed-biofuel competition. Monogastric animals are increasingly being fed significant quantities of cost-effective alternative high fiber feedstuffs unacceptable for human consumption. Moreover, the sector is under pressure to produce animal food products in ways that are ethical, environmentally sustainable, and wholesome. For example, animal agriculture uses a significant amount of antibiotics for therapy, prevention of bacterial infection, and growth promotion. There are growing concerns around the world on indiscriminate use of antibiotics and linkage to the emergency of antibiotic-resistant human and animal pathogens. The actions taken by regulatory bodies, producers, and consumers will eliminate or reduce the total use of antibiotics in poultry and pig production placing greater emphasis on dietary strategies for promoting and maintaining healthy and functional GIT. The dietary fiber fractions vary widely among feedstuffs; however, they can be considered alike from a nutritional viewpoint as they are not degraded sufficiently or indeed at all by the conditions and the repertoire of endogenous digestive enzymes in the monogastric upper gut. The consequences of variable and low nutrient digestibility range from economic through increased feed costs, proliferation of pathogens in the gut, poor feed efficiency to ecological through nutrient loading and emissions into the environment. Moreover, β-mannans induce FIIR leading to increased use of nutrients and energy for non-productive purposes. The current review revealed that supplemental β-mannanase improved animal performance (egg production, growth, FCR, reduction of BW loss in lactating sows) linked to increased nutrient and energy digestibility. Moreover, investigations have indicated that supplemental β-mannanase can reduce FIIR with implications for improved energy utilization efficiency. However, there is still variability in the data that require further investigations. Evolving the utility of β-mannanase in feed programs will be driven by understanding of its implications to animal nutrition and gastrointestinal physiology. For example, the GIT is populated with diverse assemblages of microbiota that play a critical role not only for the overall well-being of the animal, but also for its nutrition, performance, and quality of its products. In this context, there is a clear need to understand the role of β-mannanase in influencing gut health through modulation of the gastrointestinal microbiota. This is particularly important as the industry gears toward reducing antimicrobial usage. Moreover, much progress is warranted to achieve consistent enzyme efficacy, including an improved understanding of the utilization and energetic contribution of fiber hydrolysis products. Unraveling the relation between specific β-mannanase hydrolysis products and gastrointestinal development, immunity, and microbial colonization will help to develop nutritional strategies to steer gastrointestinal health and function for enhanced performance, especially under suboptimal environmental conditions and antibiotic-free feeding programs.
Supplementary Material
ACKNOWLEDGMENTS
Contributions of the current and former students and associates of EGK Monogastric Nutrition Research Laboratory at University of Guelph appreciated. EGK developed the concepts and had the overall editorial responsibility whereas MM, SS, and KL provided critical review of the manuscript.
Conflict of interest statement. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
LITERATURE CITED
- Adeola, O., and Cowieson A. J.. . 2011. BOARD-INVITED REVIEW: opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 89:3189–3218. doi: 10.2527/jas.2010-3715. [DOI] [PubMed] [Google Scholar]
- Agyekum, A. K., Slominski B. A., and Nyachoti C. M.. . 2012. Organ weight, intestinal morphology, and fasting whole-body oxygen consumption in growing pigs fed diets containing distillers dried grains with solubles alone or in combination with a multienzyme supplement. J. Anim. Sci. 90:3032–3040. doi: 10.2527/jas.2011-4380. [DOI] [PubMed] [Google Scholar]
- Alizadeh, M., Rodriguez-Lecompte J. C., Rogiewicz A., Patterson R., and Slominski B. A.. . 2016. Effect of yeast-derived products and distillers dried grains with solubles (DDGS) on growth performance, gut morphology, and gene expression of pattern recognition receptors and cytokines in broiler chickens. Poult. Sci. 95:507–517. doi: 10.3382/ps/pev362. [DOI] [PubMed] [Google Scholar]
- Angkanaporn, K., Choct M., Bryden W. L., and Annison E. F.. . 1994. Effects of wheat pentosan on endogenous aminoacids losses in chickens. J. Sci. Food Agr. 66:399–404. [Google Scholar]
- Arsenault, R. J., and Kogut M. H.. . 2015. Immunometabolism and the kinome peptide array: A new perspective and tool for the study of gut health. Front. Vet. Sci. 2:44. doi: 10.3389/fvets.2015.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault, R. J., Lee J. T., Latham R., Carter B., and Kogut M. H.. . 2017. Changes in immune and metabolic gut response in broilers fed β-mannanase in β-mannan-containing diets. Poult. Sci. 96:4307–4316. doi: 10.3382/ps/pex246. [DOI] [PubMed] [Google Scholar]
- Arsenault, R. J., Trost B., and Kogut M. H.. . 2014. A Comparison of the chicken and turkey proteomes and phosphoproteomes in the development of poultry-specific immuno-metabolism kinome peptide arrays. Front. Vet. Sci. 1:22. doi: 10.3389/fvets.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach Knudsen, K. E. 1997. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim. Feed Sci. Technol. 67(4):319–338. doi: 10.1016/S0377-8401(97)00009-6 [DOI] [Google Scholar]
- Bach Knudsen, K. E. 2011. Triennial Growth Symposium: Effects of polymeric carbohydrates on growth and development in pigs. J. Anim. Sci. 89:1965–1980. doi: 10.2527/jas.2010-3602. [DOI] [PubMed] [Google Scholar]
- Bågenholm, V., Reddy S. K., Bouraoui H., Morrill J., Kulcinskaja E., Bahr C. M., Aurelius O., Rogers T., Xiao Y., Logan D. T., . et al. 2017. Galactomannan catabolism conferred by a polysaccharide utilization locus of Bacteroides ovatus: Enzyme synergy and crystal structure of a β-mannanase. J. Biol. Chem. 292:229–243. doi: 10.1074/jbc.M116.746438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balamuralikrishnan, B., Lee J. H., and Kim I. H.. . 2018. Effects of dietary β-mannanase supplementation of soybean meal on the performance of weanling pigs. Anim. Nutr. Feed Technol. 18(1):13–23. doi: 10.5958/0974-181X.2018.00002.1 [DOI] [Google Scholar]
- Balasubramanian, B., Ingale S. L., Park J. H., Rathi P. C., Shanmugam S., and Kim I. H.. . 2018. Inclusion of dietary β-mannanase improves performance and ileal digestibility and reduces ileal digesta viscosity of broilers fed corn-soybean meal based diet. Poult. Sci. 97:3097–3101. doi: 10.3382/ps/pey157. [DOI] [PubMed] [Google Scholar]
- Barak, S., and Mudgil D.. . 2014. Locust bean gum: processing, properties and food applications – a review. Int. J. Biol. Macromol. 66:74–80. doi: 10.1016/j.ijbiomac.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Bass, B. E., Frank J. W., Johnson Z. B., Maxwell C. V., and Lee J. H.. . 2010. Mannanase addition to nursery pig diets improves growth performance. Fayetteville (AR): University of Arkansas. [Google Scholar]
- Bedford, M. R. 1996. The effect of enzymes on digestion. J. Appl. Poult. Res. 5(4):370–378. [Google Scholar]
- Bedford, M. R., and Schulze H.. . 1998. Exogenous enzymes for pigs and poultry. Nutr. Res. Rev. 11:91–114. doi: 10.1079/NRR19980007. [DOI] [PubMed] [Google Scholar]
- BeMiller, J. N. 2019. 4 – Polysaccharides: occurrence, structures, and chemistry. In: BeMiller J. N., editor. Carbohydrate chemistry for food scientists. 3rd ed. St. Paul (MN): AACC International Press; p. 75–101. [Google Scholar]
- Blackburn, N. A., and Johnson I. T.. . 2007. The effect of guar gum on the viscosity of the gastrointestinal contents and on glucose uptake from the perfused jejunum in the rat. Br. J. Nutr. 46:239–246. doi: 10.1079/bjn19810029. [DOI] [PubMed] [Google Scholar]
- Bougouin, A., Appuhamy J. A., Kebreab E., Dijkstra J., Kwakkel R. P., and France J.. . 2014. Effects of phytase supplementation on phosphorus retention in broilers and layers: a meta-analysis. Poult. Sci. 93:1981–1992. doi: 10.3382/ps.2013-03820. [DOI] [PubMed] [Google Scholar]
- Caldas, J. V., Vignale K., Boonsinchai N., Wang J., Putsakum M., England J. A., and Coon C. N.. . 2018. The effect of β-mannanase on nutrient utilization and blood parameters in chicks fed diets containing soybean meal and guar gum. Poult. Sci. 97:2807–2817. doi: 10.3382/ps/pey099. [DOI] [PubMed] [Google Scholar]
- Cant, J. P., McBride B. W., and W. J.Croom, Jr. 1996. The regulation of intestinal metabolism and its impact on whole animal energetics. J. Anim. Sci. 74:2541–2553. doi: 10.2527/1996.74102541x. [DOI] [PubMed] [Google Scholar]
- Carr, S. N., Allee G. L., Rincker P. J., Fry R. S., and Boler D. D.. . 2014. Effects of endo-1,4-β-d-mannanase enzyme (Hemicell HT 1.5 ×) on the growth performance of nursery pigs. Prof. Anim. Sci. 30(4):393–399. doi: 10.15232/pas.2014-01326 [DOI] [Google Scholar]
- Chen, J., Robb C. S., Unfried F., Kappelmann L., Markert S., Song T., Harder J., Avcı B., Becher D., Xie P., . et al. 2018. Alpha- and beta-mannan utilization by marine Bacteroidetes. Environ. Microbiol. 20:4127–4140. doi: 10.1111/1462-2920.14414. [DOI] [PubMed] [Google Scholar]
- Cho, J. H., and Kim I. H.. . 2013. Effects of beta mannanase and xylanase supplementation in low energy density diets on performances, nutrient digestibility, blood profiles and meat quality in finishing pigs. Asian J. Anim. Vet. Adv. 8(4):622–630. [Google Scholar]
- Conner, S. R. 2002. Characterization of guar meal for use in poultry rations. College Station (TX): Texas A&M University. [Google Scholar]
- Cui, S. W., Wu Y., and Ding H.. . 2013. 5 – The range of dietary fibre ingredients and a comparison of their technical functionality. In: Delcour J. A. and Poutanen K., editors, Fibre-rich and wholegrain foods. Cambridge (UK): Woodhead Publishing; p. 96–119. [Google Scholar]
- Daskiran, M., Teeter R. G., Fodge D., and Hsiao H. Y.. . 2004. An evaluation of endo-β-d-mannanase (Hemicell) effects on broiler performance and energy use in diets varying in β-mannan content1. Poult. Sci. 83(4):662–668. doi: 10.1093/ps/83.4.662 [DOI] [PubMed] [Google Scholar]
- Denstadli, V., Ballance S., Knutsen S. H., Westereng B., andSvihus. B.. 2010. Influence of graded levels of brewers dried grains on pellet quality and performance in broiler chickens. Poult. Sci. 89(12):2640–2645. doi: 10.1093/ps/83.4.662 [DOI] [PubMed] [Google Scholar]
- de Vries, R. P., and Visser J.. . 2001. Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol. Mol. Biol. Rev. 65:497–522, table of contents. doi: 10.1128/MMBR.65.4.497-522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra, S. S. 2017. Effects of enzyme products in the diet on growth, dressing-out percent and organ weights of light pigs fed copra-meal-based diets. Anim. Prod. Sci. 57(4):683–689. doi: 10.1071/AN15545 [DOI] [Google Scholar]
- Duncan, C. J., Pugh N., Pasco D. S., and Ross S. A.. . 2002. Isolation of a galactomannan that enhances macrophage activation from the edible fungus Morchella esculenta. J. Agric. Food Chem. 50:5683–5685. doi: 10.1021/jf020267c. [DOI] [PubMed] [Google Scholar]
- Ellis, P. R., Roberts F. G., Low A. G., and Morgan L. M.. . 2007. The effect of high-molecular-weight guar gum on net apparent glucose absorption and net apparent insulin and gastric inhibitory polypeptide production in the growing pig: Relationship to rheological changes in jejunal digesta. Br. J. Nutr. 74(4):539–556. doi: 10.1079/bjn19950157. [DOI] [PubMed] [Google Scholar]
- Englyst, H. N., and Cummings J. H.. . 1984. Simplified method for the measurement of total non-starch polysaccharides by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 109(7):937–942. doi: 10.1039/AN9840900937 [DOI] [PubMed] [Google Scholar]
- Ferreira, H. C., Jr, Hannas M. I., Albino L. F., Rostagno H. S., Neme R., Faria B. D., M. L.Xavier, Jr, and Rennó L. N.. . 2016. Effect of the addition of β-mannanase on the performance, metabolizable energy, amino acid digestibility coefficients, and immune functions of broilers fed different nutritional levels. Poult. Sci. 95:1848–1857. doi: 10.3382/ps/pew076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabler, N. K., and Spurlock M. E.. . 2008. Integrating the immune system with the regulation of growth and efficiency. J. Anim. Sci. 86(14 Suppl):E64–E74. doi: 10.2527/jas.2007-0466. [DOI] [PubMed] [Google Scholar]
- Hansen, R. W., Byrnes S. M., and Johnson A. D.. . 1992. Determination of galactomannan (gum) in guar (Cyamopsis tetragonolobus) by high performance liquid chromatography. J. Sci. Food Agr. 59(3):419–421. doi: 10.1002/jsfa.2740590322 [DOI] [Google Scholar]
- Hasani, M., Rezaei M., Ansari Pirsaraei Z., and Yussefi Kelarikolaei K.. . 2019. Effects of different levels of guar meal and β-mannanase on performance, yolk cholesterol concentration and blood lipid parameters of laying hens in second-cycle of production. Iran. J. Appl. Anim. Sci. 9(2):309–313. [Google Scholar]
- Hervey, G. W. 1925. A nutritional study upon a fungus enzyme. Science 62:247. doi: 10.1126/science.62.1602.247. [DOI] [PubMed] [Google Scholar]
- Hsiao, H. Y., Anderson D. M., and Dale N. M.. . 2006. Levels of β-mannan in soybean meal. Poult. Sci. 85(8):1430–1432. doi: 10.1093/ps/85.8.1430 [DOI] [PubMed] [Google Scholar]
- Huntley, N. F., Gould S. A., Patience J. F., and Plaizier J.. . 2020. Evaluation of the effect of β-mannanase supplementation and mannans on nursery pig growth performance and serum acute-phase protein concentrations. Can. J. Anim. Sci. 100(1):111–118. doi: 10.1139/cjas-2018-0248 [DOI] [Google Scholar]
- Huntley, N. F., Nyachoti C. M., and Patience J. F.. . 2018. Lipopolysaccharide immune stimulation but not β-mannanase supplementation affects maintenance energy requirements in young weaned pigs. J. Anim. Sci. Biotechnol. 9:47. doi: 10.1186/s40104-018-0264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain, M., Rehman A. U., and Khalid M. F.. . 2012. Feeding value of guar meal and the application of enzymes in improving nutritive value for broilers. World’s Poult. Sci. J. 68(2):253–268. doi: 10.1017/S0043933912000311 [DOI] [Google Scholar]
- Ibuki, M., Fukui K., and Yamauchi K.. . 2014. Effect of dietary mannanase-hydrolysed copra meal on growth performance and intestinal histology in broiler chickens. J. Anim. Physiol. Anim. Nutr. (Berl). 98:636–642. doi: 10.1111/jpn.12105. [DOI] [PubMed] [Google Scholar]
- Jacela, J. Y., Dritz S. S., DeRouchey J. M., Tokach M. D., Goodband R. D., and Nelssen J. L.. . 2010. Effects of supplemental enzymes in diets containing distillers dried grains with solubles on finishing pig growth performance. Prof. Anim. Sci. 26(4):412–424. doi: 10.15232/S1080-7446(15)30623-9 [DOI] [Google Scholar]
- Jackson, M. E., Fodge D. W., and Hsiao H. Y.. . 1999. Effects of beta-mannanase in corn-soybean meal diets on laying hen performance. Poult. Sci. 78:1737–1741. doi: 10.1093/ps/78.12.1737. [DOI] [PubMed] [Google Scholar]
- Jackson, M. E., Geronian K., Knox A., McNab J., and McCartney E.. . 2004. A dose-response study with the feed enzyme beta-mannanase in broilers provided with corn-soybean meal based diets in the absence of antibiotic growth promoters. Poult. Sci. 83:1992–1996. doi: 10.1093/ps/83.12.1992. [DOI] [PubMed] [Google Scholar]
- Jackson, M. E., Mitchell J., and Mathis G.. . 2006. Effect of formulating of β-mannanase (Hemicell Feed Enzyme) into turkey tom diets varying in amino acid density Poultry Science Association No. 85. Edmonton (Canada): Poultry Science Association University of Alberta; p. 129–130. [Google Scholar]
- Jackson, M. E., Stephens K. R., and Mathis G. F.. . 2008. The effect of β-mannanase (Hemicell Feed Enzyme) and high levels of distillers dried grains on turkey hen performance Poultry Science Annual Meeting No. 87. Niagara Falls (Ontario, Canada): Poultry Science; p. 65–66. [Google Scholar]
- Jang, J.-C., Kim K. H., Jang Y. D., and Kim Y. Y.. . 2020a. Effects of dietary β-mannanase supplementation on growth performance, apparent total tract digestibility, intestinal integrity, and immune responses in weaning pigs. Animals. 10(4):703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, J.-C., Kim K. H., Kim D. H., Jang S. K., Hong J. S., Heo P. S., and Kim Y. Y.. . 2020b. Effects of increasing levels of palm kernel meal containing β-mannanase to growing-finishing pig diets on growth performance, nutrient digestibility, and pork quality. Livest. Sci. 238:104041. doi: 10.1016/j.livsci.2020.104041 [DOI] [Google Scholar]
- Jaworski, N. W., Lærke H. N., Bach Knudsen K. E., and Stein H. H.. . 2015. Carbohydrate composition and in vitro digestibility of dry matter and nonstarch polysaccharides in corn, sorghum, and wheat and coproducts from these grains. J. Anim. Sci. 93:1103–1113. doi: 10.2527/jas.2014-8147. [DOI] [PubMed] [Google Scholar]
- Jeon, S. M., Hosseindoust A., Choi Y. H., Kim M. J., Kim K. Y., Lee J. H., Kil D. Y., Kim B. G., and Chae B. J.. . 2019. Comparative standardized ileal amino acid digestibility and metabolizable energy contents of main feed ingredients for growing pigs when adding dietary β-mannanase. Anim. Nutr. 5:359–365. doi: 10.1016/j.aninu.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo, J. K., Ingale S. L., Kim J. S., Kim Y. W., Kim K. H., Lohakare J. D., Lee J. H., and Chae B. J.. . 2012. Effects of exogenous enzyme supplementation to corn- and soybean meal-based or complex diets on growth performance, nutrient digestibility, and blood metabolites in growing pigs. J. Anim. Sci. 90:3041–3048. doi: 10.2527/jas.2010-3430. [DOI] [PubMed] [Google Scholar]
- Just, A., Fernandez J. A., Jorgensen H., Fernández J., and Jørgensen H.. . 1983. The net energy value of diets for growth in pigs in relation to the fermentative processes in the digestive-tract and the site of absorption of the nutrients. Livest. Prod. Sci. 10(2):171–186. doi: 10.1016/0301-6226(83)90033-7 [DOI] [Google Scholar]
- Karaca, K., Sharma J. M., and Nordgren R.. . 1995. Nitric-oxide production by chicken macrophages activated by Acemannan, a complex carbohydrate extracted from Aloe vera. Int. J. Immunopharmacol. 17(3):183–188. doi: 10.1016/0192-0561(94)00102-t [DOI] [PubMed] [Google Scholar]
- Khanongnuch, C., Sa-nguansook C., and Lumyong S.. . 2006. Nutritive quality of β-mannanase treated copra meal in broiler diets and effectiveness on some fecal bacteria. Int. J. Poult. Sci. 5:1087–1091. doi: 10.3923/ijps.2006.1087.1091 [DOI] [Google Scholar]
- Kiarie, E. G., Mohammadigheisar M., Kakhki R. A. M., and Madsen M. H.. . 2021. Impact of feeding modified soy protein concentrate in the starter phase on growth performance and gastrointestinal responses in broiler chickens through to day 42 of age. Poult. Sci. 100:101147. doi: 10.1016/j.psj.2021.101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiarie, E. G., Parenteau I. A., Zhu C., Ward N. E., and Cowieson A. J.. . 2020. Digestibility of amino acids, energy, and minerals in roasted full-fat soybean and expelled-extruded soybean meal fed to growing pigs without or with multienzyme supplement containing fiber-degrading enzymes, protease, and phytase. J. Anim. Sci. 98(6). doi: 10.1093/jas/skaa174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiarie, E., Romero L. F., and Nyachoti C. M.. . 2013. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr. Res. Rev. 26:71–88. doi: 10.1017/S0954422413000048. [DOI] [PubMed] [Google Scholar]
- Kiarie, E., Walsh M. C., and Nyachoti C. M.. . 2016. Performance, digestive function, and mucosal responses to selected feed additives for pigs. J. Anim. Sci. 94(Suppl 3):169–180. doi: 10.2527/jas.2015-9835 [DOI] [Google Scholar]
- Kim, J. S., Hosseindoust A., Ju I. K., Yang X., Lee S. H., Noh H. S., Lee J. H., and Chae B. J.. . 2018. Effects of dietary energy levels and β-mannanase supplementation in a high mannan-based diet during lactation on reproductive performance, apparent total tract digestibility and milk composition in multiparous sows. Ital. J. Anim. Sci. 17(1):128–134. doi: 10.1080/1828051X.2017.1345663 [DOI] [Google Scholar]
- Kim, J. S., Ingale S. L., Hosseindoust A. R., Lee S. H., Lee J. H., and Chae B. J.. . 2017b. Effects of mannan level and β-mannanase supplementation on growth performance, apparent total tract digestibility and blood metabolites of growing pigs. Animal. 11:202–208. doi: 10.1017/S1751731116001385. [DOI] [PubMed] [Google Scholar]
- Kim, J. S., Ingale S. L., Lee S. H., Kim K. H., Kim J. S., Lee J. H., and Chae B. J.. . 2013. Effects of energy levels of diet and β-mannanase supplementation on growth performance, apparent total tract digestibility and blood metabolites in growing pigs. Anim. Feed Sci. Technol. 186(1):64–70. doi: 10.1016/j.anifeedsci.2013.08.008 [DOI] [Google Scholar]
- Kim, M. C., Kim J. H., Pitargue F. M., Koo D. Y., Choi H. S., and Kil D. Y.. . 2017c. Effect of dietary β-mannanase on productive performance, egg quality, and utilization of dietary energy and nutrients in aged laying hens raised under hot climatic conditions. Asian-Australas. J. Anim. Sci. 30:1450–1455. doi: 10.5713/ajas.17.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. J., Nam S. O., Jeong J. H., Fang L. H., Yoo H. B., Yoo S. H., Hong J. S., Son S. W., Ha S. H., and Kim Y. Y.. . 2017a. Various levels of copra meal supplementation with β-mannanase on growth performance, blood profile, nutrient digestibility, pork quality and economical analysis in growing-finishing pigs. J. Anim. Sci. Technol. 59:19. doi: 10.1186/s40781-017-0144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipper, M., Andretta I., Quadros V. R., Schroeder B., Pires P. G. S., Franceschina C. S., Hickmann F. M. W., and França I.. . 2020. Performance responses of broilers and pigs fed diets with β-mannanase. Braz. J. Anim. Sci. Zootec. 49:e20180177. doi: 10.37496/rbz4920180177 [DOI] [Google Scholar]
- Klasing, K. C. 2007. Nutrition and the immune system. Br. Poult. Sci. 48:525–537. doi: 10.1080/00071660701671336. [DOI] [PubMed] [Google Scholar]
- Knudsen, K. E. B. 2014. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets1. Poult. Sci. 93(9):2380–2393. doi: 10.3382/ps.2014-03902. [DOI] [PubMed] [Google Scholar]
- Kwon, W. B., and Kim B. G.. . 2015. Effects of supplemental beta-mannanase on digestible energy and metabolizable energy contents of copra expellers and palm kernel expellers fed to pigs. Asian-Australas. J. Anim. Sci. 28:1014–1019. doi: 10.5713/ajas.15.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. T., Bailey C. A., and Cartwright A. L.. . 2003. β-Mannanase ameliorates viscosity-associated depression of growth in broiler chickens fed guar germ and hull fractions. Poult. Sci 82(12):1925–1931. doi: 10.1093/ps/82.12.1925 [DOI] [PubMed] [Google Scholar]
- Lee, J. T., Connor-Appleton S., Bailey C. A., and Cartwright A. L.. . 2005. Effects of guar meal by-product with and without beta-mannanase Hemicell on broiler performance. Poult. Sci. 84:1261–1267. doi: 10.1093/ps/84.8.1261. [DOI] [PubMed] [Google Scholar]
- Lv, J. N., Chen Y. Q., Guo X. J., Piao X. S., Cao Y. H., and Dong B.. . 2013. Effects of Supplementation of β-mannanase in corn-soybean meal diets on performance and nutrient digestibility in growing pigs. Asian-Australas. J. Anim. Sci. 26:579–587. doi: 10.5713/ajas.2012.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald, T. T., and Monteleone G.. . 2005. Immunity, inflammation, and allergy in the gut. Science 307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- MarketsandMarkets. 2020. Feed enzymes market by type (phytase, carbohydrase, and protease), livestock (poultry, swine, ruminants, and aquatic animals), source (microorganism, plant, and animal), form (dry and liquid), and region – global forecast to 2025. Hadapsar (India): MarketsandMarkets™ Research Private Ltd. [Google Scholar]
- Masey O’Neill, H. V., Smith J. A., and Bedford M. R.. . 2014. Multicarbohydrase enzymes for non-ruminants. Asian-Australas. J. Anim. Sci. 27:290–301. doi: 10.5713/ajas.2013.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton, J. L., Hsiao H., Anderson D., and Fodge D. W.. . 1998. Corn/soy/fat diets for broilers, Beta-mannanase and improved feed conversion Poultry Science Association No. 77. University Park (PA): Poultr. Sci., Pennsylvania State College; p. 153. [Google Scholar]
- Mohammadigheisar, M., Shouldice V. L., Balasubramanian B., and Kim I. H.. . 2021. Effect of dietary supplementation of β-mannanase on growth performance, carcass characteristics, excreta microflora, blood constituents, and nutrient ileal digestibility in broiler chickens. Anim. Biosci. 34:1342–1349. doi: 10.5713/ab.20.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok, C. H., Kong C., and Kim B. G.. . 2015. Combination of phytase and β-mannanase supplementation on energy and nutrient digestibility in pig diets containing palm kernel expellers. Anim. Feed Sci. Technol. 205:116–121. doi: 10.1016/j.anifeedsci.2015.04.012 [DOI] [Google Scholar]
- Mok, C. H., Lee J. H., and Kim B. G.. . 2013. Effects of exogenous phytase and β-mannanase on ileal and total tract digestibility of energy and nutrient in palm kernel expeller-containing diets fed to growing pigs. Anim. Feed Sci. Technol. 186(3):209–213. doi: 10.1016/j.anifeedsci.2013.10.008 [DOI] [Google Scholar]
- Nishinari, K., Takemasa M., Zhang H., and Takahashi R.. . 2007. 2.19 – Storage plant polysaccharides: Xyloglucans, galactomannans, glucomannans. In: Kamerling H., editor, Comprehensive glycoscience. Oxford (UK): Elsevier; p. 613–652. [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th revision ed. Washington (DC): National Academy of Sciences Press. [Google Scholar]
- Nyachoti, C. M., Zijlstra R. T., de Lange C. F. M., and Patience J. F.. . 2004. Voluntary feed intake in growing-finishing pigs: A review of the main determining factors and potential approaches for accurate predictions. Can. J. Anim. Sci. 84(4):549–566. doi: 10.4141/A04-001 [DOI] [Google Scholar]
- Odetallah, N. H., Ferket P. R., Grimes J. L., and McNaughton J. L.. . 2002. Effect of mannan-endo-1,4-beta-mannosidase on the growth performance of turkeys fed diets containing 44 and 48% crude protein soybean meal. Poult. Sci. 81:1322–1331. doi: 10.1093/ps/81.9.1322. [DOI] [PubMed] [Google Scholar]
- Okumura, R., and Takeda K.. . 2017. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 49:e338. doi: 10.1038/emm.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opoku, E. Y., Classen H. L., and Scott T. A.. . 2015a. Effects of wheat distillers dried grains with solubles with or without protease and β-mannanase on the performance of turkey hen poults. Poult. Sci. 94:207–214. doi: 10.3382/ps/peu049. [DOI] [PubMed] [Google Scholar]
- Opoku, E. Y., Classen H. L., and Scott T. A.. . 2015b. Evaluation of inclusion level of wheat distillers dried grains with solubles with and without protease or β-mannanase on performance and water intake of turkey hens. Poult. Sci. 94:1600–1610. doi: 10.3382/ps/pev088. [DOI] [PubMed] [Google Scholar]
- Pedersen, M. B., Dalsgaard S., Bach Knudsen K. E., Yu S., and Lærke H. N.. . 2014. Compositional profile and variation of distillers dried grains with solubles from various origins with focus on non-starch polysaccharides. Anim. Feed Sci. Technol. 197:130–141. doi: 10.1016/j.anifeedsci.2014.07.011 [DOI] [Google Scholar]
- Pettey, L. A., Carter S. D., Senne B. W., and Shriver J. A.. . 2002. Effects of beta-mannanase addition to corn-soybean meal diets on growth performance, carcass traits, and nutrient digestibility of weanling and growing-finishing pigs. J. Anim. Sci. 80:1012–1019. doi: 10.2527/2002.8041012x. [DOI] [PubMed] [Google Scholar]
- Prajapati, V. D., Jani G. K., Moradiya N. G., Randeria N. P., Nagar B. J., Naikwadi N. N., and Variya B. C.. . 2013. Galactomannan: A versatile biodegradable seed polysaccharide. Int. J. Biol. Macromol. 60:83–92. doi: 10.1016/j.ijbiomac.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Rainbird, A. L., Low A. G., and Zebrowska T.. . 1984. Effect of guar gum on glucose and water absorption from isolated loops of jejunum in conscious growing pigs. Br. J. Nutr. 52:489–498. doi: 10.1079/bjn19840116. [DOI] [PubMed] [Google Scholar]
- Rainbird, A. L., Low A. G., and Zebrowska T.. . 2007. Effect of guar gum on glucose and water absorption from isolated loops of jejunum in conscious growing pigs. Brit. J. Nutr. 52(3):489–498. doi: 10.1079/BJN19840116 [DOI] [PubMed] [Google Scholar]
- Ramamoorthy, L., Kemp M. C., and Tizard I. R.. . 1996. Acemannan, a beta-(1,4)-acetylated mannan, induces nitric oxide production in macrophage cell line RAW 264.7. Mol. Pharmacol. 50:878–884. [PubMed] [Google Scholar]
- Ravindran, V. 2013. Feed enzymes: The science, practice, and metabolic realities. J. Appl. Poult. Res. 22(3):628–636. doi: 10.3382/japr.2013-00739 [DOI] [Google Scholar]
- Rho, Y., Patterson R., Joye I., Martinez M., Squires E. J., and Kiarie E. G.. . 2020. Fiber degrading enzymes increased monosaccharides release and fermentation in corn distillers dried grains with solubles and wheat middlings steeped without or with protease. Transl. Anim. Sci. 4:txaa153. doi: 10.1093/tas/txaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu, M. H., Hosseindoust A., Kim J. S., Choi Y. H., Lee S. H., Kim M. J., Lee J. H., and Chae B. J.. . 2017. β-Mannanase derived from Bacillus subtilis WL-7 improves the performance of commercial laying hens fed low or high mannan-based diets. J. Poult. Sci. 54:212–217. doi: 10.2141/jpsa.0160021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed, M., Aya_an T., Alagawany M., El-Hack M., Abdel-Latif M., and Patra A.. . 2019. The role of β-mannanase (Hemicell) in improving poultry productivity, health and environment. Braz. J. Poult. Sci. 21. doi: 10.1590/1806-9061-2019-1001. [DOI] [Google Scholar]
- Saittagaroon, S., Kawakishi S., and Namiki M.. . 1983. Characterisation of polysaccharides of copra meal. J. Sci. Food Agr. 34(8):855–860. doi: 10.1002/jsfa.2740340813 [DOI] [Google Scholar]
- Shastak, Y., Ader P., Feuerstein D., Ruehle R., and Matuschek M.. . 2015. β-Mannan and mannanase in poultry nutrition. World’s Poult. Sci. J. 71(1):161–174. doi: 10.1017/S0043933915000136 [DOI] [Google Scholar]
- Shim, Y. H., Ryu M. H., Hosseindoust A., Kim J. S., Choi Y. H., Kim M. J., Lee J. H., and Chae B. J.. . 2018. Evaluation of β-mannanase and different dietary energy levels on egg production, apparent digestibility and blood metabolites of laying hen. Anim. Nutr. Feed Technol. 18(2):131. doi: 10.5958/0974-181X.2018.00013.6 [DOI] [Google Scholar]
- Slominski, B. A. 2011. Recent advances in research on enzymes for poultry diets. Poult. Sci. 90:2013–2023. doi: 10.3382/ps.2011-01372. [DOI] [PubMed] [Google Scholar]
- Slominski, B. A., Campbell L. D., and Guenter W.. . 1994. Carbohydrates and dietary fiber components of yellow- and brown-seeded canola. J. Agric. Food Chem. 42(3):704–707. doi: 10.1021/jf00039a020. [DOI] [Google Scholar]
- Stein, H. H., Casas G. A., Abelilla J. J., Liu Y., and Sulabo R. C.. . 2015. Nutritional value of high fiber co-products from the copra, palm kernel, and rice industries in diets fed to pigs. J. Anim. Sci. Biotechnol. 6:56. doi: 10.1186/s40104-015-0056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundu, B., Kumar A., and Dingle J.. . 2006. Response of broiler chicks fed increasing levels of copra meal and enzymes. Int. J. Poult. Sci. 5:13–18. doi: 10.3923/ijps.2006.13.18. [DOI] [Google Scholar]
- Tiwari, U. P., Chen H., Kim S. W., and Jha R.. . 2018. Supplemental effect of xylanase and mannanase on nutrient digestibility and gut health of nursery pigs studied using both in vivo and in vitro models. Anim. Feed Sci. Technol. 245:77–90. doi: 10.1016/j.anifeedsci.2018.07.002 [DOI] [Google Scholar]
- Tiwari, U. P., Fleming S. A., Abdul Rasheed M. S., Jha R., and Dilger R. N.. . 2020. The role of oligosaccharides and polysaccharides of xylan and mannan in gut health of monogastric animals. J. Nutr. Sci. 9:e21. doi: 10.1017/jns.2020.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torki, M., Mirzaee M., and Habibian M.. . 2016. Effects of barley cultivar and dietary supplemental enzyme on performance, egg quality traits, and selected blood parameters of laying hens. Poult. Sci. J. 4(1):1–12. [Google Scholar]
- Torres-Pitarch, A., Manzanilla E. G., Gardiner G. E., O’Doherty J. V., and Lawlor P. G.. . 2019. Systematic review and meta-analysis of the effect of feed enzymes on growth and nutrient digestibility in grow-finisher pigs: Effect of enzyme type and cereal source. Anim. Feed Sci. Technol. 251:153–165. doi: 10.1016/j.anifeedsci.2018.12.007. [DOI] [Google Scholar]
- Upadhaya, S. D., Park J. W., Lee J. H., and Kim I. H.. . 2016. Efficacy of β-mannanase supplementation to corn-soya bean meal-based diets on growth performance, nutrient digestibility, blood urea nitrogen, faecal coliform and lactic acid bacteria and faecal noxious gas emission in growing pigs. Arch. Anim. Nutr. 70:33–43. doi: 10.1080/1745039X.2015.1117697. [DOI] [PubMed] [Google Scholar]
- Van Nevel, C. J., Decuypere J. A., Dierick N. A., and Molly K.. . 2005. Incorporation of galactomannans in the diet of newly weaned piglets: effect on bacteriological and some morphological characteristics of the small intestine. Arch. Anim. Nutr. 59:123–138. doi: 10.1080/17450390512331387936. [DOI] [PubMed] [Google Scholar]
- Viechtbauer, W. 2010. Conducting meta-analyses in R with the metafor package. 36(3):48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- Viechtbauer, W., and Cheung M. W.. . 2010. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods. 1:112–125. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- Wang, J. P., Hong S. M., Yan L., Yoo J. S., Lee J. H., Jang H. D., Kim H. J., and Kim I. H.. . 2009. Effects of single or carbohydrases cocktail in low-nutrient-density diets on growth performance, nutrient digestibility, blood characteristics, and carcass traits in growing–finishing pigs. Livest. Sci. 126(1):215–220. doi: 10.1016/j.livsci.2009.07.003 [DOI] [Google Scholar]
- Ward, N. E. 2021. Debranching enzymes in corn/soybean meal-based poultry feeds: a review. Poult. Sci. 100(2):765–775. doi: 10.1016/j.psj.2020.10.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, G., Bryant M. M., Voitle R. A., and Roland D. A.. . 2005. Effects of β-mannanase in corn-soy diets on commercial leghorns in second-cycle hens. Poult. Sci. 84(6):894–897. doi: 10.1093/ps/84.6.894 [DOI] [PubMed] [Google Scholar]
- Yoon, S. Y., Yang Y. X., Shinde P. L., Choi J. Y., Kim J. S., Kim Y. W., Yun K., Jo J. K., Lee J. H., Ohh S. J., . et al. 2010. Effects of mannanase and distillers dried grain with solubles on growth performance, nutrient digestibility, and carcass characteristics of grower-finisher pigs. J. Anim. Sci. 88:181–191. doi: 10.2527/jas.2008-1741. [DOI] [PubMed] [Google Scholar]
- Zhang, L., and Tizard I. R.. . 1996. Activation of a mouse macrophage cell line by Acemannan: The major carbohydrate fraction from Aloe vera gel. Immunopharmacology 35:119–128. doi: 10.1016/s0162-3109(96)00135-x. [DOI] [PubMed] [Google Scholar]
- Zheng, L., Cho S., Kang C., Lee K., Kim K., and An B.. . 2020. Effects of β-mannanase on egg production performance, egg quality, intestinal microbiota, viscosity, and ammonia concentration in laying hens. Braz. J. Poult. Sci. 22. doi: 10.1590/1806-9061-2019-1180. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.