Abstract
Aims
The aim of this study was to evaluate the association between alcohol consumption status (and its changes) after newly diagnosed atrial fibrillation (AF) and the risk of ischaemic stroke.
Methods and results
Using the Korean nationwide claims and health examination database, we included subjects who were newly diagnosed with AF between 2010 and 2016. Patients were categorized into three groups according to the status of alcohol consumption before and after AF diagnosis: non-drinkers; abstainers from alcohol after AF diagnosis; and current drinkers. The primary outcome was incident ischaemic stroke during follow-up. Non-drinkers, abstainers, and current drinkers were compared using incidence rate differences after the inverse probability of treatment weighting (IPTW). Among a total of 97 869 newly diagnosed AF patients, 51% were non-drinkers, 13% were abstainers, and 36% were current drinkers. During 310 926 person-years of follow-up, 3120 patients were diagnosed with incident ischaemic stroke (10.0 per 1000 person-years). At 5-year follow-up, abstainers and non-drinkers were associated with a lower risk for stroke than current drinkers (incidence rate differences after IPTW, −2.03 [−3.25, −0.82] for abstainers and −2.98 [−3.81, −2.15] for non-drinkers, per 1000 person-years, respectively; and incidence rate ratios after IPTW, 0.75 [0.70, 0.81] for non-drinkers and 0.83 [0.74, 0.93] for abstainers, respectively).
Conclusion
Current alcohol consumption was associated with an increased risk of ischaemic stroke in patients with newly diagnosed AF, and alcohol abstinence after AF diagnosis could reduce the risk of ischaemic stroke. Lifestyle intervention, including attention to alcohol consumption, should be encouraged as part of a comprehensive approach to AF management to improve clinical outcomes.
Keywords: Atrial fibrillation, Alcohol, Alcohol abstinence, Stroke
Graphical Abstract
See page 4769 for the editorial comment for this article ‘Alcohol intake and risk of stroke in atrial fibrillation: the lesser the better, but this is not enough’, by A.M. Russo, https://doi.org/10.1093/eurheartj/ehab657.
Introduction
Alcohol consumption is associated with an increased risk of incident atrial fibrillation (AF).1–3 Not only heavy drinking but also a “low spectrum of alcohol consumption” could be a risk factor for incident AF, as reported in a recent observational study.4 Regarding the risk of AF, alcohol consumption showed a consistent linear dose–response relationship.2–4
While the association and dose–response relationship between alcohol consumption and the risk of AF is well-established, the association between drinking alcohol and stroke is more controversial. In the general population, the overall association of alcohol intake with the risk of stroke was almost null, and the association with ischaemic stroke showed a marginal reduction.5 In several prior studies, the associations between alcohol consumption and cardiovascular risk, including ischaemic stroke, are varied depending on whether the study focused on an immediate effect or long-term effect or alcohol dose in the general population.6–10 The relationship between alcohol consumption and incident stroke in patients with AF per se remains unclear.11
In a recent study, alcohol abstinence reduced AF recurrence in patients with paroxysmal or persistent AF.12 Alcohol abstinence in AF patients with moderate to heavy drinking showed a significant reduction in the recurrence of AF and AF burden compared to those who continue their alcohol consumption. However, this study did not report the reduction of hard clinical endpoints (e.g., stroke). Physicians also need to consider the potentially beneficial effect of low amounts of alcohol on the cardiovascular outcome (at least in the general population), but they should also consider the effect of alcohol on AF management. Considering that a greater burden of AF has associated with a higher risk of ischaemic stroke in patients with AF,13 we still need evidence of whether alcohol abstinence would reduce the risk of ischaemic stroke in patients with AF, especially from a large population-based perspective. These data would help physicians how to make recommendations on drinking alcohol to their patients with newly diagnosed AF.
Implementation of a healthy lifestyle is an important part of guideline-recommended optimal care of patients with AF.14 When a new disease is diagnosed, it can be a good opportunity to intervene with respect to the patient’s lifestyle. Although there was some evidence on the clinical impact of lifestyle modification in patients with AF, we still need more evidence-based recommendations to inform guidelines, e.g. to educate and encourage ‘alcohol abstinence’ among patients who are newly diagnosed with AF.
In this study, we aimed to evaluate the associations between alcohol consumption status (especially abstaining alcohol after newly diagnosed AF) and the risk of ischaemic stroke in a population-based nationwide observational cohort.
Methods
We used data from the Korean National Health Insurance Service (NHIS) and the National Health Screening databases for our analysis.15 The Korean NHIS is a mandatory health insurance coverage provided by the government for the entire Korean population (∼50 million people). The Korean NHIS database includes demographic information as well as information related to the medical records and expenses of enrolees, such as diagnoses, examinations, prescriptions, and procedures. Diagnoses were coded using the International Classification of Diseases, Tenth Revision, Clinical Modification codes. We also used data from the National Health Screening database linked with the Korean NHIS database. All enrolees of the Korean NHIS aged 40 years or older are recommended to undergo a biennial health screening. The health examination includes anthropometric measurement, assessment of blood pressure, laboratory investigations, as well as a self-reported questionnaire on health behaviours such as smoking, alcohol consumption, and physical activity. The present study complied with the Declaration of Helsinki. This study was exempted from review by the institutional review board of Seoul National University Hospital (E-2007-071-1141).
Study design and study population
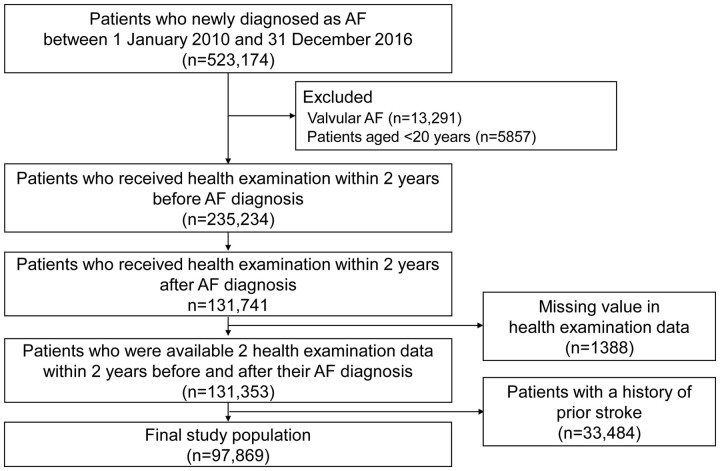
The study design and flowchart of population enrolment are presented in Figures 1 and 2, respectively. We included subjects who were newly diagnosed with AF between 1 January 2010 and 31 December 2016 (n = 523 174). Patients diagnosed with valvular AF and those younger than 20 years of age were excluded. Patients who underwent health examinations within 2 years before and after the diagnosis of AF were included. Patients with missing values in the health examination data were excluded. To enhance accuracy in the diagnosis of stroke as a primary outcome, we excluded patients with a prior history of stroke before the second health examination (index date). Detailed definitions of the diagnoses, namely, AF, valvular AF, and prior stroke, are presented in Supplementary material online, Table S1.15 , 16
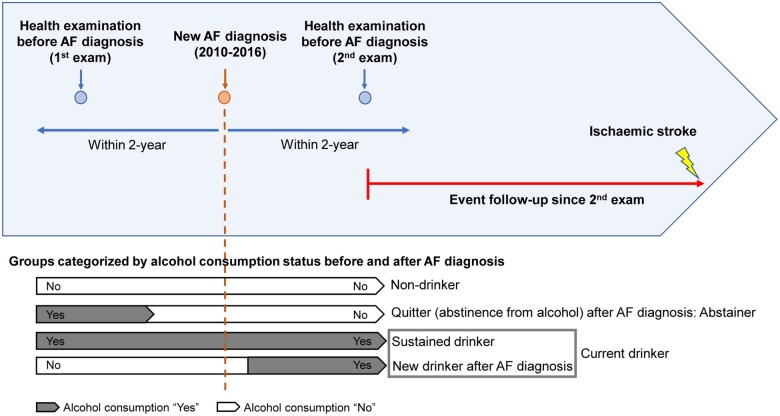
Figure 1.
Study design. AF, atrial fibrillation.
Figure 2.
Flowchart of enrolment of the study population. AF, atrial fibrillation.
Alcohol consumption status was evaluated using a self-reported questionnaire at the first and second health examinations. Subjects were categorized into three groups based on the status of alcohol consumption before and after the diagnosis of AF: (i) non-drinkers; (ii) abstainers from alcohol after the diagnosis of AF; and (iii) current drinkers, including sustained (persistent) drinkers and new drinkers after the diagnosis of AF (Figure 1). Data on the frequency of alcohol intake per week (0–7) and amount of alcohol intake per session (0 to ≤32 g, 32 to ≤56 g, 56 to ≤112 g, and >112 g) were obtained from the self-reported questionnaire (Supplementary material online, Methods).3 Based on the frequency per week and amount per session, we classified the amount of alcohol consumption per week as mild (0 to <105 g), moderate (105 to <210 g), and heavy (≥210 g).
Covariates
Covariates included demographic data, comorbidities, and medications of the subjects, and results of the health examination ascertained from the Korean NHIS database. Detailed information is presented in Supplementary material online, Methods and Supplementary material online, Table S1.
Study outcomes and follow-up
The primary outcome was the incidence of ischaemic stroke during follow-up. A detailed definition of the primary outcome is presented in Supplementary material online, Table S1.15 , 16 We defined the index date of follow-up as the second health examination date, which was performed within two years after AF diagnosis (Figure 1). Patients were followed up starting from the index date until the occurrence of ischaemic stroke, death, or end of the study period (31 December 2017), whichever came first (Figure 1).
Statistical analysis
Continuous variables are presented as mean ± standard deviation, and categorical variables are presented as numbers (percentages). One-way analysis of variance and the chi-square test were used to evaluate the significance of the differences among groups categorized by alcohol status. The incidence rate (IR) of ischaemic stroke was calculated by dividing the number of incident cases by the total follow-up period and presented as per 1000 person-years. The hazard ratio (HR) and 95% confidence interval (CI) for ischaemic stroke were analysed using the Cox proportional hazards model.
To compare the three groups, including non-drinker, abstainer, and current drinker, after balancing the differences in baseline characteristics, we performed inverse probability of treatment weighting (IPTW) analysis by using stabilized weights calculated from propensity score (PS).17 , 18 The propensity for being in each group was calculated using a logistic regression model with baseline covariates that included age, sex, comorbidities including hypertension, diabetes mellitus, dyslipidaemia, heart failure, prior myocardial infarction, peripheral artery disease, chronic obstructive pulmonary disease, chronic kidney disease, and cancer; baseline medications including oral anticoagulants (OACs), antiplatelet agents, and statins, baseline body mass index, smoking status, regular exercise, low income, and CHA2DS2-VASc score.19 To evaluate the balance of baseline characteristics among three groups, the maximum absolute standardized difference (ASD) was calculated in each covariate. The maximum ASD of ≤0.1 indicates that there is a negligible difference in a certain covariate among three groups.20 Weighted cumulative incidence curve was presented with weighted IR, IR difference (IRD) with 95% CI, IR ratios (IRR) with 95% CIs with consideration of the IPTW using PS. The risk for ischaemic stroke of the three groups was obtained using weighted Cox proportional hazards regression models with IPTW. HRs are presented with current drinkers as the reference group.
The proportional hazards assumption was tested by graphical inspection of log-minus-log plot and Schoenfeld residuals for Cox models. The results showed parallel log-minus-log survival curves and random patterns in Schoenfeld residuals, indicating no significant departure from the proportionality assumption.
A P-value of <0.05 was considered statistically significant. We used SAS version 9.4 (SAS Institute, Cary, NC) for statistical analyses.
Sensitivity analyses
First, to provide complementary analyses, we performed a multivariable-adjusted Cox proportional hazards model analysis. The multivariable model was adjusted for the same variables that included PS calculation. Before creating a final adjusted model, models that were adjusted sequentially based on a priori considerations for baseline covariates were also evaluated (models 1–5,Supplementary material online, Methods). HRs are presented with non-drinkers as the reference group and also presented with current drinkers as the reference group to more intuitively present the relative risk (RR) of each group compared to current drinkers.
Second, focusing on the beneficial effect of alcohol abstinence after AF diagnosis, PS matching (PSM) analysis was performed between the abstainer group and current drinker group. The propensity for being in each group was calculated using a logistic regression model with baseline covariates included in the final multivariable Cox analysis.19 Patients were matched in a one-to-one manner between the two groups using the greedy, nearest-neighbor method without replacement with a calliper of 0.01 of the PS.21 The balance of baseline characteristics between two groups was evaluated using the ASD. An ASD of ≤0.1 (10%) indicates that study groups were well balanced.20 After PSM between the two groups, the cumulative incidence curve of PS matched cohort was presented with IR, IRD with 95% CI, and IRR with 95% CI.
Third, we performed a competing risk analysis considering death as a competing risk using the Fine and Gray proportional hazards model for the subdistribution of a competing risk.22
Subgroup analyses
The potential effect modification by sex, age (<65 years, 65–74 years, and ≥75 years), underlying stroke risk of patients assessed by the CHA2DS2-VASc score (<3 and ≥3), and baseline smoking status (never smoker, ex-smoker, and current smoker) were evaluated by subgroup analyses and interaction tests. To account for the possible influence of the accumulative alcohol intake, we stratified each group based on alcohol intake per week at the first health examination. In addition, to account for the impact of current alcohol consumption and the risk of ischaemic stroke, we categorized each group based on the frequency of drinking per week, alcohol amount per session, and the total amount of alcohol intake per week at the second health examination. Subgroup analyses performed using multivariable Cox proportional hazard regression methods and P for interaction <0.1 was considered significant interaction.
Results
A total of 97 869 patients newly diagnosed with AF (mean age 61.3 ± 12.3 years, 62.4% men, mean CHA2DS2-VASc score 2.3 ± 1.5) were included. The mean time interval between the first health examination and diagnosis of AF, between diagnosis of AF and the second health examination and between the first and second health examinations was 1.1 ± 0.5, 0.9 ± 0.5 and 2.0 ± 0.5 years, respectively.
Among the total study population, 51% (n = 49 781) were non-drinkers, 13% (n = 12 789) were abstainers after AF diagnosis, and 36% (n = 35 299) were current drinkers. The baseline characteristics of the study population are shown in Table 1 and Supplementary material online, Table S2. Current drinkers were younger and more likely to be men than abstainers and non-drinkers. Current drinkers had the lowest mean CHA2DS2-VASc score among the three groups, with a lower prevalence of most comorbidities. The prevalence of heart failure, peripheral artery disease, chronic obstructive pulmonary disease, chronic kidney disease, and cancer was higher in non-drinker and abstainers than current drinkers. The maximum ASD of these comorbidities before IPTW was ≥0.1 (Table 1). In association with a relatively low CHA2DS2-VASc score, current drinkers demonstrated a lower prevalence of OAC use than non-drinkers and abstainers. Current drinkers included a larger proportion of current smokers and a lower proportion of subjects in the low-income category compared to non-drinkers and abstainers. Furthermore, current drinkers included a larger proportion of subjects who performed regular exercise than the other groups. Among current drinkers, 58.8% were mild drinkers, 23.5% were moderate drinkers, and 17.8% were heavy drinkers at the second health examination.
Table 1.
Baseline characteristics before and after inverse probability of treatment weighting
| Pre-IPTW |
Post-IPTW |
|||||||
|---|---|---|---|---|---|---|---|---|
| Non-drinkers (n = 49 781) | Abstainers (n = 12 789) | Current drinkers (n = 35 299) | Maximum ASD | Non-drinkers (n = 50 833) | Abstainers (n = 12 636) | Current drinkers (n = 34 277) | Maximum ASD | |
| Age, years | 64.6 ± 11.2 | 60.1 ± 12.3 | 56.9 ± 12.2 | 0.654 | 60.6 ± 12.9 | 61.1 ± 12.3 | 60.7 ± 12.3 | 0.044 |
| <65 years | 22 110 (44.4) | 7693 (60.2) | 25 086 (71.1) | 29 070 (57.2) | 7245 (57.3) | 20 012 (58.4) | ||
| 65-74 years | 18 022 (36.2) | 3618 (28.3) | 7880 (22.3) | 14 948 (29.4) | 3621 (28.7) | 9565 (27.9) | ||
| ≥75 years | 9649 (19.4) | 1478 (11.6) | 2333 (6.6) | 6814 (13.4) | 1769 (14.0) | 4698 (13.7) | ||
| Male sex | 19 565 (39.3) | 10 192 (79.7) | 31 274 (88.6) | 1.196 | 32 221 (63.4) | 7923 (62.7) | 22 269 (65.0) | 0.047 |
| CHA2DS2-VASc score | 2.8 ± 1.5 | 2.1 ± 1.4 | 1.8 ± 1.2 | 0.745 | 2.3 ± 1.5 | 2.3 ± 1.4 | 2.3 ± 1.4 | 0.027 |
| 0 | 2002 (4.0) | 1283 (10.0) | 4655 (13.2) | 4818 (9.5) | 1022 (8.1) | 2698 (7.9) | ||
| 1 | 8673 (17.4) | 3306 (25.9) | 12 158 (34.4) | 12 867 (25.3) | 3061 (24.2) | 9089 (26.5) | ||
| 2 | 12 065 (24.2) | 3575 (28.0) | 9760 (27.7) | 12 617 (24.8) | 3405 (27.0) | 9081 (26.5) | ||
| ≥3 | 27 041 (54.3) | 4625 (36.2) | 8726 (24.7) | 20 531 (40.4) | 5146 (40.7) | 13 407 (39.1) | ||
| Comorbidities | ||||||||
| Hypertension | 32 014 (64.3) | 8226 (64.3) | 23 203 (65.7) | 0.029 | 32 693 (64.3) | 8138 (64.4) | 21 841 (63.7) | 0.014 |
| Diabetes mellitus | 10 886 (21.9) | 2779 (21.7) | 6896 (19.5) | 0.057 | 10 397 (20.5) | 2604 (20.6) | 6722 (19.6) | 0.024 |
| Dyslipidaemia | 5160 (10.4) | 1279 (10.0) | 4099 (11.6) | 0.051 | 5457 (10.7) | 1369 (10.8) | 3657 (10.7) | 0.005 |
| Heart failure | 12 944 (26.0) | 3543 (27.7) | 7179 (20.3) | 0.173 | 12 045 (23.7) | 3009 (23.8) | 8476 (24.7) | 0.024 |
| Prior MI | 2476 (5.0) | 718 (5.6) | 1339 (3.8) | 0.086 | 2352 (4.6) | 600 (4.8) | 1721 (5.0) | 0.018 |
| PAD | 11 023 (22.1) | 2309 (18.1) | 5862 (16.6) | 0.140 | 9735 (19.2) | 2459 (19.5) | 6552 (19.1) | 0.008 |
| COPD | 10 699 (21.5) | 2546 (19.9) | 4883 (13.8) | 0.201 | 9274 (18.2) | 2320 (18.4) | 6495 (19.0) | 0.018 |
| CKD | 8490 (17.1) | 1541 (12.1) | 2776 (7.9) | 0.281 | 6537 (12.9) | 1631 (12.9) | 4523 (13.2) | 0.009 |
| Cancer | 3415 (6.9) | 1239 (9.7) | 1039 (2.9) | 0.279 | 2895 (5.7) | 705 (5.6) | 2143 (6.3) | 0.028 |
| Medication | ||||||||
| OAC | 12 283 (24.7) | 3767 (29.5) | 7579 (21.5) | 0.184 | 11 992 (23.6) | 3049 (24.1) | 8286 (24.2) | 0.012 |
| Warfarin | 7712 (15.5) | 2630 (20.6) | 5370 (15.3) | 0.151 | 7984 (15.7) | 2029 (16.0) | 5545 (16.2) | 0.015 |
| DOAC | 4571 (9.2) | 1137 (8.9) | 2202 (6.2) | 0.110 | 4008 (7.9) | 1020 (8.1) | 2743 (8.0) | 0.006 |
| Aspirin | 10 348 (20.8) | 2537 (19.8) | 7650 (21.7) | 0.045 | 10 461 (20.6) | 2693 (21.3) | 7041 (20.5) | 0.018 |
| P2Y12 inhibitor | 2722 (5.5) | 649 (7.1) | 1605 (4.6) | 0.042 | 2549 (5.0) | 626 (5.0) | 1827 (5.3) | 0.016 |
| Statin | 8924 (17.9) | 1856 (14.5) | 4915 (13.9) | 0.109 | 7979 (15.7) | 2039 (16.1) | 5386 (15.7) | 0.011 |
| Health exam parameter | ||||||||
| BMI (kg/m2) | 24.5 ± 3.3 | 24.4 ± 3.3 | 24.8 ± 3.2 | 0.141 | 24.6 ± 3.4 | 24.6 ± 3.3 | 24.5 ± 3.2 | 0.013 |
| ≥25 kg/m2 | 20 166 (40.5) | 5222 (40.8) | 16 396 (46.5) | 0.120 | 21 665 (42.6) | 5350 (42.3) | 14 283 (42.0) | 0.013 |
| Smoking status | ||||||||
| Never smoker | 38 900 (78.1) | 6652 (52.0) | 11 526 (32.7) | 1.029 | 29 261 (57.6) | 7329 (58.0) | 19 403 (56.6) | 0.008 |
| Smoker | 10 881 (21.9) | 6137 (48.0) | 23 773 (67.3) | 21 572 (42.4) | 5307 (42.0) | 14 783 (43.4) | ||
| Regular exercise | 9574 (19.2) | 3069 (24.0) | 9311 (26.4) | 0.170 | 11 449 (22.5) | 2801 (22.2) | 7851 (22.9) | 0.017 |
| Low income | 8936 (18.0) | 2107 (16.5) | 5267 (14.9) | 0.081 | 8640 (17.0) | 2173 (17.2) | 6028 (17.6) | 0.015 |
Continuous variables are presented as mean ± standard deviation, and categorical variables are presented as number (percentage).
AF, atrial fibrillation; ASD, absolute standardized difference; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DOAC, direct oral anticoagulant;IPTW, inverse probability of treatment weighting; MI, myocardial infarction; OAC, oral anticoagulant; PAD, peripheral artery disease.
Alcohol status and ischaemic stroke
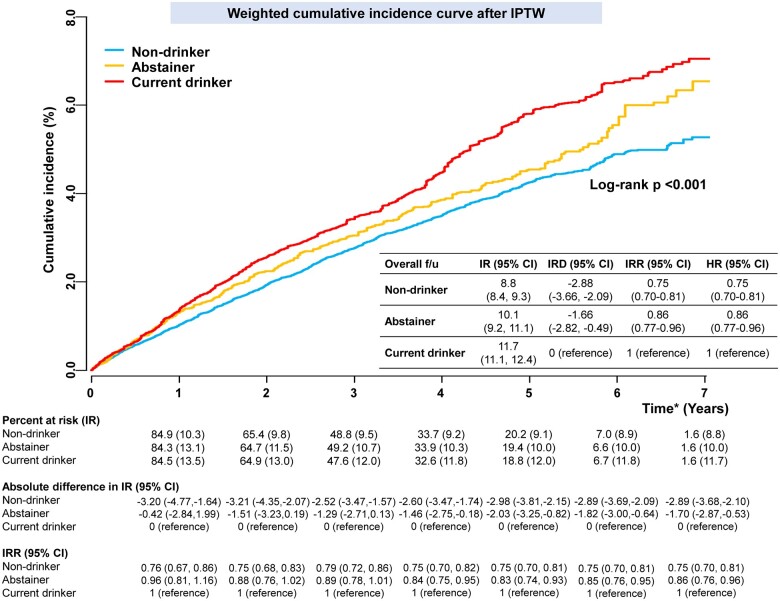
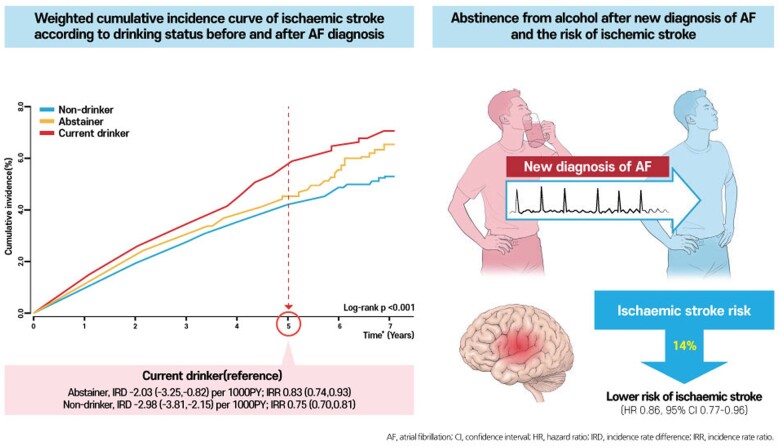
During 310 926 person-years of follow-up, 3120 patients were diagnosed with incident ischaemic stroke (10.0 per 1000 person-years). After IPTW, the baseline characteristics of the three groups were well-balanced (Table 1). Weighted cumulative incidence curves are presented in Figure 3. At 5 years, the weighted IR of stroke was lower in non-drinkers and abstainers than in current drinkers (IRDs, −2.98 [−3.81, −2.15] for non-drinkers vs. current drinkers and −2.03 [−3.25 to −0.82] for abstainers vs. current drinkers). Consistent with the results of the main analysis, IRRs at 5 years after IPTW suggested that abstainers and non-drinkers were associated with a lower risk for stroke than current drinkers (IRRs, 0.75 [0.70, 0.81] for non-drinkers vs. current drinkers, and 0.83 [0.74, 0.93] for abstainers vs. current drinkers). The results of weighted Cox proportional hazards regression models with IPTW were also consistent with the main results.
Figure 3.
Weighted cumulative incidence curves of non-drinkers, abstainers, and current drinkers after inverse probability of treatment weighting. Incidence rate and incidence rate difference, per 1000 person-years. *Time after the second health examination (within 2-year after the date of AF diagnosis). CI, confidence interval; HR, hazard ratio; IPTW, inverse probability of treatment weighting; IR, incidence rate; IRD, incidence rate difference; IRR, incidence rate ratio.
Sensitivity analyses
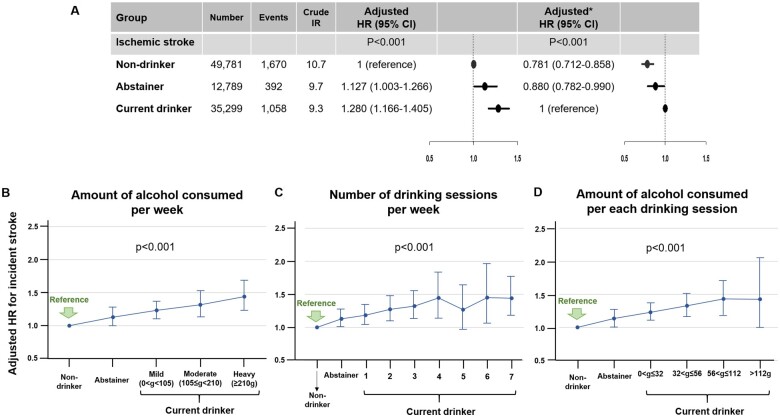
In a multivariable-adjusted Cox proportional hazards model analysis, results were consistent with the main analysis (Figure 4A). Results of a final fully adjusted model were consistently observed in all adjusted models (Supplementary material online, Figure S1).
Figure 4.
The risk of incident ischaemic stroke according to alcohol consumption status. (A) The risk of incident ischaemic stroke in abstainers and current drinkers compared to non-drinkers. *With current drinkers as the reference group. Incidence rate, per 1000 person-years. (B) Stratifying current drinkers according to the amount of alcohol consumed per week. (C) Stratifying current drinkers according to the number of drinking sessions per week. (D) Stratifying current drinkers according to the alcohol consumed per each drinking session adjusted for age, sex, baseline body mass index, heavy drinker, regular exercise, low income, comorbidities including hypertension, diabetes mellitus, dyslipidaemia, heart failure, prior myocardial infarction, peripheral artery disease, chronic kidney disease, chronic obstructive pulmonary disease, cancer, CHA2DS2-VASc score, baseline medications including oral anticoagulants (warfarin or DOAC), anti-platelet agents (aspirin or P2Y12 inhibitor) and statin. CI, confidence interval; DOAC, direct oral anticoagulant; HR, hazard ratio; IR, incidence rate.
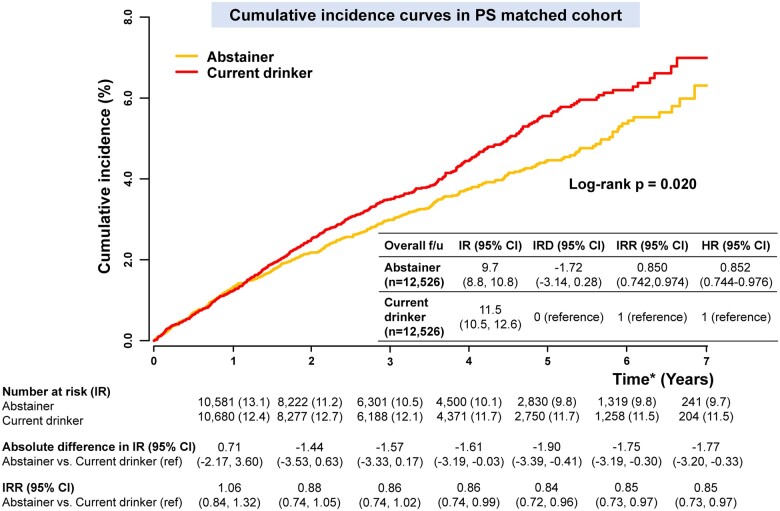
After PSM, the baseline characteristics of abstainers and current drinkers were well-balanced (Supplementary material online, Table S3). Cumulative incidence curves of the PS-matched cohort are presented in Figure 5. At 5 years, the IR of stroke was lower in abstainers than in current drinkers (IRD, −1.90 [−3.39, −0.41]). IRR estimated after PSM suggested that abstainers were associated with a lower risk for stroke compared to current drinkers (IRR, 0.85 [0.74, 0.97]), consistent with the main results.
Figure 5.
Cumulative incidence curves in the propensity score matched cohort. Incidence rate and incidence rate difference, per 1000 person-years. *Time after the second health examination (within 2 years after the date of atrial fibrillation diagnosis). CI, confidence interval; HR, hazard ratio; IR, incidence rate; IRD, incidence rate difference; IRR, incidence rate ratio.
The crude event numbers and IRs of all-cause death and cardiovascular and non-cardiovascular death were different among the three groups (Supplementary material online, Table S4). The results were also consistent when adjusting for the competing risks of death (Supplementary material online, Table S5).
Subgroup analyses
In each subgroup stratified, according to sex, age, CHA2DS2-VASc score, and smoking status, the overall results were consistent with the main results. Furthermore, there were no significant interactions with respect to ischaemic stroke between different groups categorized by alcohol status and all subgroups (Supplementary material online, Figure S2).
Alcohol consumption amount at the first health examination
To account for the influence of previous cumulative alcohol intake, abstainers and current drinkers were stratified according to the amount of alcohol intake per week as described in the Methods section (mild [0 to <105 g], moderate [105 to <210 g], and heavy [≥210 g]). Event numbers and crude IRs are presented in Supplementary material online, Table S6. As shown in Supplementary material online, Figure S3, abstainers who consumed mild or moderate amounts of alcohol per week and stopped drinking after the diagnosis of AF had a similar risk of ischaemic stroke as non-drinkers. Ex-heavy drinkers showed a significantly higher risk of ischaemic stroke than non-drinkers, despite the cessation of drinking after the diagnosis of AF (adjusted HR 1.433, 95% CI 1.122–1.831). All current drinkers showed a higher risk of ischaemic stroke compared to non-drinkers, regardless of the amount of alcohol consumed previously.
Alcohol consumption amount at the second health examination
Current drinkers were stratified according to the frequency of drinking per week, the amount of alcohol consumed per session, and the total amount of alcohol intake per week at the second health examination. Event numbers and crude IRs are presented in Supplementary material online, Table S7. Among current drinkers, a greater amount of alcohol consumption per week was associated with a higher risk of ischaemic stroke (Figure 4B). Both frequent drinking and binge drinking per each drinking session were significant risk factors for incident ischaemic stroke (Figure 4C and D).
Discussion
In this study on a large nationwide cohort of patients with incident AF, our principal findings were as follows: (i) a substantial proportion (36%) of subjects with newly diagnosed AF remained current drinkers, and 13% stopped drinking after the diagnosis; (ii) current drinkers with any amount of alcohol intake (even mild-to-moderate intake) had a significantly higher risk of ischaemic stroke compared to non-drinkers; and (iii) abstinence from alcohol after the diagnosis of AF was associated with a lower risk of ischaemic stroke compared to current drinkers (Graphical abstract). These observations have implications for promoting lifestyle modifications as part of the holistic and integrated approach in the care of patients with AF.14 , 23
Alcohol abstinence after atrial fibrillation diagnosis could reduce the risk of ischaemic stroke. Lifestyle intervention, including attention to alcohol consumption, should be encouraged as part of a comprehensive approach to atrial fibrillation management to improve clinical outcomes.
The strengths of the present study lie in the inclusion of a large number of patients who were available for serial health examination before and after the diagnosis of AF. Based on the availability of the serial self-reported questionnaires before and after the diagnosis of AF, we identified non-drinkers, abstainers, and current drinkers and demonstrated the association between each alcohol intake status and the risk of ischaemic stroke in the patients after the diagnosis of AF.
Alcohol consumption has been reported to be associated with an increased risk of incident AF, and a linear dose–response relationship is well established between the two parameters.1–4 Binge drinking, as well as habitual alcohol consumption, has been implicated in the increased risk of developing AF.3 Excessive alcohol consumption causes acute electrophysiological effects, including decreased atrial effective refractory period and slow intra-atrial conduction.24 Binge drinking also increases the susceptibility to atrial arrhythmias by mishandling of calcium in the sarcoplasmic reticulum.25
Alcohol exposure may lead to atrial electrical and structural remodelling. The atrial tissue has been reported to be more susceptible to toxicity even at lower doses of alcohol than doses associated with alcohol-induced cardiomyopathy in the ventricles.26 Thus, regular mild-to-moderate consumption of alcohol has been associated with lower atrial voltages or the presence of low-voltage zones, conduction slowing, and enlargement of the left atrium.27–29 In a recent randomized clinical trial that included regular drinkers with AF, abstainers showed significantly lower recurrence and burden of AF compared to sustained drinkers.12 Proactive abstinence from alcohol intake is therefore recommended in patients with AF to control AF itself. Moreover, the reduction of recurrence and burden of AF might lower AF-related complications.13
Although the amount of alcohol intake shows a linear relationship with incident AF, and alcohol abstinence is an important modifiable factor for effective rhythm control in patients with AF, there has been limited evidence on recommendations regarding optimal care of such patients, especially in relation to stroke prevention. In the general population, the association between alcohol intake and the risk of stroke showed a U-shaped relationship, wherein light to moderate alcohol consumption was associated with a reduced risk of stroke compared to abstinence.5 , 7 , 10 , 30 However, there has been no definitive answer to the question of whether a U-shaped relationship exists between alcohol intake and the risk of stroke in the population with AF.
A previous study reported that heavy drinking was associated with an increased risk of the composite of thromboembolism and death in the population with AF.11 However, this result was mainly driven by death, and the numbers were insufficient to address the beneficial effect of abstinence from alcohol and establish a dose–response relationship for the risk of stroke.11 Al-Khalili et al.31 reported that prior alcohol-related hospitalization was associated with a two-fold increased risk of ischaemic stroke in young patients with AF (18–64 years, median 55 years) at low risk for ischaemic stroke stratified by CHA2DS2-VASc score (0 in men and 1 in women). However, a direct relationship between alcohol intake and ischaemic stroke was not demonstrated in this prior study.31
The present study showed that any amount of current alcohol intake in patients with AF was associated with a higher risk of ischaemic stroke compared to non-drinkers. Moreover, there was a linear dose–response relationship between the amount of current alcohol intake and the risk of ischaemic stroke. Furthermore, abstinence from alcohol might reduce the risk of ischaemic stroke in patients with newly diagnosed AF.
The differences in the population with AF compared to the general population could be explained by several mechanisms. In previous studies, light to moderate regular alcohol intake was also reported to cause electrical and structural atrial remodelling, which eventually led to atrial myopathy.27–29 The latter refers to atrial fibrotic remodelling with electrical and autonomic remodelling, associated with an increased risk of stroke.32 In patients with AF, the presence of any AF events lasting over 6 min was found to be associated with an increased risk of stroke, which was even greater in cases with the AF burden lasting over 24 h.33 Thus, reduction in the recurrence and the burden of arrhythmia by alcohol abstinence might be beneficial in preventing stroke in patients with AF.12 Although the cumulative incidence between abstainer and current drinker groups was not significantly different in the early period (<1 year), the absolute difference of IR and IRR after 2-year follow-up remained consistent. We assumed that the beneficial effect of alcohol abstinence, such as reduction of AF burden and atrial reverse remodelling, might need time to present as a risk reduction of hard endpoint (e.g. stroke).
To evaluate the risk of stroke in patients with AF, the impact of age is crucial.34–36 Before IPTW, current drinkers were younger than abstainers and non-drinkers, and the crude IRs of ischaemic stroke were higher in non-drinkers and abstainers than current drinkers (10.7, 9.7, and 9.3 per 1000 person-years, respectively, in Figure 4A). After IPTW, age differences were well-balanced, and weighted IRs of ischaemic stroke were highest in current drinkers (11.7 per 1000 person-years) than in abstainers and non-drinkers (10.1 per 1000 person-years and 8.8 per 1000 person-years, respectively). Hence, although age is a strong factor associated with the risk of stroke, non-drinkers and abstainers showed significantly lower risks of stroke than current drinkers after balancing the baseline characteristics. In the subgroup analysis by age, the P-for-interaction was not significant; however, the beneficial effect of abstinence from alcohol was attenuated in patients aged 75 years or older. This finding could be explained by the strong association between age and the risk of stroke.34–36 Age is the strongest risk factor for AF-related stroke and is also associated with irreversible remodelling of a substrate in the left atrium.37–40
Given the increased focus on integrated care of patients with AF, lifestyle changes are part of the ‘C’ criterion of the ABC pathway, Focusing on the management of comorbidities.14 , 23 , 41 The results of the present study provide support for lifestyle changes in the management of patients with AF, wherein proactive reduction and abstinence from alcohol consumption were associated with better outcomes.
Study limitations
This study had several limitations. First, alcohol intake could affect comorbidities, such as hypertension and obesity, and these conditions could also be modifiable risk factors associated with stroke.14 To minimize this, we adjusted for the comorbidities evaluated at the second examination; however, a possibility of residual confounding persists. Second, alcohol status was evaluated using a self-reported questionnaire, which could be associated with recall bias. Nonetheless, many published retrospective and prospective observational studies, or even randomized controlled trials, have collected information about alcohol intake using similar self-reported questionnaires.1–3 , 12 Third, the beneficial effect of alcohol abstinence and the dose–response relationship between the amount of alcohol intake and the risk of stroke might be mediated by atrial electrical/structural remodelling and the burden of arrhythmia. However, this aspect could not be evaluated, considering the inherent limitation of the database. Lastly, the impact of heavy drinking in the general population on the risk of stroke differs according to the subtype of stroke (ischaemic vs. haemorrhagic).5 , 30 For example, the RR of haemorrhagic stroke was higher (RR, 2.18) than that of ischaemic stroke (RR 1.69) due to heavy drinking (>60 g/day).30 Furthermore, the association between alcohol consumption and the RR of stroke was J-shaped for ischaemic and linear-shaped for haemorrhagic stroke.30 In the present study, we focused on ischaemic stroke, especially since data on haemorrhagic stroke could not be differentiated from claims databases of intracranial haemorrhage due to other causes.
Conclusion
Current alcohol consumption was associated with an increased risk of ischaemic stroke in patients with newly diagnosed AF. Compared to non-drinkers, current drinkers with mild amount of alcohol consumption still showed a significantly higher risk of ischaemic stroke and a linear dose–response relationship between the amount of current alcohol intake and the risk of ischaemic stroke. Furthermore, alcohol abstinence after the diagnosis of AF could reduce the risk of ischaemic stroke. Lifestyle interventions, including attention to alcohol consumption, should be encouraged as part of a comprehensive approach in the management of patients with a new diagnosis of AF to improve clinical outcomes.
Supplementary material
Supplementary material is available at European Heart Journal online.
Declaration of Helsinki
The present study complied with the Declaration of Helsinki. This study was exempted from review by the institutional review board of Seoul National University Hospital (E-2007-071-1141).
Funding
This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, Republic of Korea, the Ministry of Food and Drug Safety) (Project Number: 202013B14) and by the Korea National Research Foundation funded by the Ministry of Education, Science and Technology (grant 2020R1F1A106740).
Conflict of interest: S.-R.L., J.-H.J., K.-D.H., and S.O. report no conflict of interest. E.-K.C. reports research grant from Bayer, BMS/Pfizer, Biosense Webster, Chong Kun Dang, Daiichi-Sankyo, Dreamtech Co., Ltd., Medtronic, Samjinpharm, Sanofi-Aventis, Seers Technology, Skylabs, and Yuhan. G.Y.H.L. reports consultant for BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo; but no fees are received personally; no other relationships or activities that could appear to have influenced the submitted work.
Data availability
All data generated and analyzed during the current study are available at the National Health Insurance Data Sharing Service (accessed at https://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do).
Supplementary Material
Contributor Information
So-Ryoung Lee, Department of Internal Medicine, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul 03080, Republic of Korea.
Eue-Keun Choi, Department of Internal Medicine, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul 03080, Republic of Korea; Department of Internal Medicine, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 03080, Republic of Korea.
Jin-Hyung Jung, Department of Medical Statistics, College of Medicine, Catholic University of Korea, 222 Banpo-daero, Seoucho-gu, Seoul 06591, Republic of Korea.
Kyung-Do Han, Statistics and Actuarial Science, Soongsil University, 369 Sangdo-ro, Dongjak-gu, Seoul 06978, Republic of Korea.
Seil Oh, Department of Internal Medicine, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul 03080, Republic of Korea; Department of Internal Medicine, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 03080, Republic of Korea.
Gregory Y H Lip, Department of Internal Medicine, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 03080, Republic of Korea; Liverpool Centre for Cardiovascular Science, University of Liverpool and Liverpool Chest and Heart Hospital, Thomas Dr, Liverpool L14 3PE, UK; Department of Clinical Medicine, Aalborg University, Aalborg 9000, Denmark.
References
- 1. Mukamal KJ, Tolstrup JS, Friberg J, Jensen G, Grønbaek M. Alcohol consumption and risk of atrial fibrillation in men and women: the Copenhagen City Heart Study. Circulation 2005;112:1736–1742. [DOI] [PubMed] [Google Scholar]
- 2. Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol 2014;64:281–289. [DOI] [PubMed] [Google Scholar]
- 3. Kim YG, Han KD, Choi JI, Boo KY, Kim DY, Lee KN, Shim J, Kim JS, Kim YH. Frequent drinking is a more important risk factor for new-onset atrial fibrillation than binge drinking: a nationwide population-based study. Europace 2020;22:216–224. [DOI] [PubMed] [Google Scholar]
- 4. Csengeri D, Sprünker NA, Di Castelnuovo A, Niiranen T, Vishram-Nielsen JK, Costanzo S, Söderberg S, Jensen SM, Vartiainen E, Donati MB, Magnussen C, Camen S, Gianfagna F, Løchen ML, Kee F, Kontto J, Mathiesen EB, Koenig W, Stefan B, de Gaetano G, Jørgensen T, Kuulasmaa K, Zeller T, Salomaa V, Iacoviello L, Schnabel RB. Alcohol consumption, cardiac biomarkers, and risk of atrial fibrillation and adverse outcomes. Eur Heart J 2021;42:1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systemic review and meta-analysis. BMJ 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pai JK, Mukamal KJ, Rimm EB. Long-term alcohol consumption in relation to all-cause and cardiovascular mortality among survivors of myocardial infarction: the Health Professionals Follow-up Study. Eur Heart J 2012;33:1598–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee SJ, Cho YJ, Kim JG, Ko Y, Hong KS, Park JM, Kang K, Park TH, Park SS, Lee KB, Cha JK, Kim DH, Lee J, Kim JT, Lee J, Lee JS, Jang MS, Han MK, Gorelick PB, Bae HJ; CRCS-5 Investigators. Moderate alcohol intake reduces risk of ischemic stroke in Korea. Neurology 2015;85:1950–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mostofsky E, Chahal HS, Mukamal KJ, Rimm EB, Mittleman MA. Alcohol and immediate risk of cardiovascular events: a systematic review and dose-response meta-analysis. Circulation 2016;133:979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, Paige E, Paul DS, Sweeting M, Burgess S, Bell S, Astle W, Stevens D, Koulman A, Selmer RM, Verschuren WMM, Sato S, Njølstad I, Woodward M, Salomaa V, Nordestgaard BG, Yeap BB, Fletcher A, Melander O, Kuller LH, Balkau B, Marmot M, Koenig W, Casiglia E, Cooper C, Arndt V, Franco OH, Wennberg P, Gallacher J, de la Cámara AG, Völzke H, Dahm CC, Dale CE, Bergmann MM, Crespo CJ, van der Schouw YT, Kaaks R, Simons LA, Lagiou P, Schoufour JD, Boer JMA, Key TJ, Rodriguez B, Moreno-Iribas C, Davidson KW, Taylor JO, Sacerdote C, Wallace RB, Quiros JR, Tumino R, Blazer DG, Linneberg A, Daimon M, Panico S, Howard B, Skeie G, Strandberg T, Weiderpass E, Nietert PJ, Psaty BM, Kromhout D, Salamanca-Fernandez E, Kiechl S, Krumholz HM, Grioni S, Palli D, Huerta JM, Price J, Sundström J, Arriola L, Arima H, Travis RC, Panagiotakos DB, Karakatsani A, Trichopoulou A, Kühn T, Grobbee DE, Barrett-Connor E, van Schoor N, Boeing H, Overvad K, Kauhanen J, Wareham N, Langenberg C, Forouhi N, Wennberg M, Després J-P, Cushman M, Cooper JA, Rodriguez CJ, Sakurai M, Shaw JE, Knuiman M, Voortman T, Meisinger C, Tjønneland A, Brenner H, Palmieri L, Dallongeville J, Brunner EJ, Assmann G, Trevisan M, Gillum RF, Ford I, Sattar N, Lazo M, Thompson SG, Ferrari P, Leon DA, Smith GD, Peto R, Jackson R, Banks E, Di Angelantonio E, Danesh J; Emerging Risk Factors Collaboration/EPIC-CVD/UK Biobank Alcohol Study Group. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 2018;391:1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang W, Kang DW, Ha SY, Lee SH. Drinking patterns and risk of ischemic stroke in middle-aged adults: do beneficial drinking habits indeed exist? Stroke 2021;52:164–171. [DOI] [PubMed] [Google Scholar]
- 11. Overvad TF, Rasmussen LH, Skjøth F, Overvad K, Albertsen IE, Lane DA, Lip GYH, Larsen TB. Alcohol intake and prognosis of atrial fibrillation. Heart 2013;99:1093–1099. [DOI] [PubMed] [Google Scholar]
- 12. Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara S, Prabhu S, Stub D, Azzopardi S, Vizi D, Wong G, Nalliah C, Sugumar H, Wong M, Kotschet E, Kaye D, Taylor AJ, Kistler PM. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med 2020;382:20–28. [DOI] [PubMed] [Google Scholar]
- 13. Go AS, Reynolds K, Yang J, Gupta N, Lenane J, Sung SH, Harrison TN, Liu TI, Solomon MD. Association of burden of atrial fibrillation with risk of ischemic stroke in adults with paroxysmal atrial fibrillation: the KP-RHYTHM study. JAMA Cardiol 2018;3:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 15. Choi EK. Cardiovascular research using the Korean National Health Information Database. Korean Circ J 2020;50:754–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee SR, Choi EK, Han KD, Jung JH, Oh S, Lip GYH. Edoxaban in Asian patients with atrial fibrillation: effectiveness and safety. J Am Coll Cardiol 2018;72:838–853. [DOI] [PubMed] [Google Scholar]
- 17. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee BK, Lessler J, Stuart EA. Weight trimming and propensity score weighting. PLoS One 2011;6:e18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014;33:1242–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parsons LS. Reducing bias in a propensity score matched-pair sample using Greedy matching techniques. In Proceedings of the Twenty-Sixth Annual SAS Users Group International Conference. Cary, NC: SAS Institute Inc 2001.
- 22. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 23. Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol 2017;14:627–628. [DOI] [PubMed] [Google Scholar]
- 24. Gould L, Reddy CV, Becker W, Oh KC, Kim SG. Electrophysiologic properties of alcohol in man. J Electrocardiol 1978;11:219–226. [DOI] [PubMed] [Google Scholar]
- 25. Yan J, Thomson JK, Zhao W, Gao X, Huang F, Chen B, Liang Q, Song LS, Fill M, Ai X. Role of stress kinase JNK in binge alcohol-evoked atrial arrhythmia. J Am Coll Cardiol 2018;71:1459–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fernández-Solà J. Cardiovascular risks and benefits of moderate and heavy alcohol consumption. Nat Rev Cardiol 2015;12:576–587. [DOI] [PubMed] [Google Scholar]
- 27. Voskoboinik A, Wong G, Lee G, Nalliah C, Hawson J, Prabhu S, Sugumar H, Ling LH, McLellan A, Morton J, Kalman JM, Kistler PM. Moderate alcohol consumption is associated with atrial electrical and structural changes: insights from high-density left atrial electroanatomic mapping. Heart Rhythm 2019;16:251–259. [DOI] [PubMed] [Google Scholar]
- 28. Qiao Y, Shi R, Hou B, Wu L, Zheng L, Ding L, Chen G, Zhang S, Yao Y. Impact of alcohol consumption on substrate remodeling and ablation outcome of paroxysmal atrial fibrillation. J Am Heart Assoc 2015;4:e002349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. MaManus DD, Yin X, Gladstone R, Vittinghoff E, Vasan RS, Larson MG, Benjamin EJ, Marcus GM. Alcohol consumption, left atrial diameter, and atrial fibrillation. J Am Heart Assoc 2016;5:e004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reynolds K, Lewis B, Nolen JDL, Kinney GL, Sathya B, He J, Lewis BL. Alcohol consumption and risk of stroke: a meta-analysis. JAMA 2003;289:579–588. [DOI] [PubMed] [Google Scholar]
- 31. Al-Khalili F, Benson L, Friberg L. Alcohol-related hospitalization is associated with increased risk of ischaemic stroke among low-risk patients with atrial fibrillation. Europace 2018;20:19–24. [DOI] [PubMed] [Google Scholar]
- 32. Shen MJ, Arora R, Jalife J. Atrial myopathy. JACC Basic Transl Sci 2019;4:640–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Uittenbogaart SB, Lucassen WAM, van Etten-Jamaludin FS, de Groot JR, van Weert HCPM. Burden of atrial high-rate episodes and risk of stroke: a systematic review. Europace 2018;20:1420–1427. [DOI] [PubMed] [Google Scholar]
- 34. Chao TF, Liu CJ, Wang KL, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chen TJ, Lip GY, Chen SA. Should atrial fibrillation patients with 1 additional risk factor of the CHA2DS2-VASc score (beyond sex) receive oral anticoagulation? J Am Coll Cardiol 2015;65:635–642. [DOI] [PubMed] [Google Scholar]
- 35. Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen AM, Gislason GH, Torp-Pedersen C. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ 2011;342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim TH, Yang PS, Yu HT, Jang E, Uhm JS, Kim JY, Pak HN, Lee MH, Joung B, Lip GYH. Age threshold for ischemic stroke risk in atrial fibrillation. Stroke 2018;49:1872–1879. [DOI] [PubMed] [Google Scholar]
- 37. Dun W, Boyden PA. Aged atria: electrical remodeling conducive to atrial fibrillation. J Interv Card Electrophysiol 2009;25:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol 2008;1:62–73. [DOI] [PubMed] [Google Scholar]
- 39. Xu GJ, Gan TY, Tang BP, Chen ZH, Jiang T, Song JG, Guo X, Li JX. Age-related changes in cellular electrophysiology and calcium handling for atrial fibrillation. J Cell Mol Med 2013;17:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jansen HJ, Moghtadaei M, Mackasey M, Rafferty SA, Bogachev O, Sapp JL, Howlett SE, Rose RA. Atrial structure, function and arrhythmogenesis in aged and frail mice. Sci Rep 2017;7:44336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Proietti M, Romiti GF, Olshansky B, Lane DA, Lip GYH. Improved outcomes by integrated care of anticoagulated patients with atrial fibrillation using the simple ABC (Atrial Fibrillation Better Care) pathway. Am J Med 2018;131:1359–1366. e6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed during the current study are available at the National Health Insurance Data Sharing Service (accessed at https://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do).