Abstract
A commercially available enzyme-linked immunosorbent assay (ELISA) for the diagnosis of Q fever (PanBio Coxiella burnetii immunoglobulin M [IgM] ELISA, QFM-200) was compared to the indirect fluorescent antibody test (IFAT) for C. burnetii IgM and the complement fixation test (CFT). The ELISA demonstrated 92% agreement with the reference method (IFAT), and gave a sensitivity of 99% (69 of 70 samples) and a specificity of 88% (106 of 121). Specificity can be increased with confirmation by IFAT. CFT was found to have a specificity of 90% (107 of 119), although it was lacking in sensitivity (73%; 51 of 70). No cross-reactivity was observed in the ELISA with serum samples from patients with mycoplasma (n = 6), chlamydia (n = 5), or legionella (n = 4) infections, although 2 of 5 patients with leptospirosis and 1 of 4 samples containing rheumatoid factor (RF) demonstrated positive results in the ELISA. Results indicate that the performance of the PanBio C. burnetii (Q fever) IgM ELISA (F = 187) is superior to that of CFT (F = 163), and consequently the ELISA should be a useful aid in the diagnosis of acute Q fever.
Coxiella burnetii is the causative agent of Q fever, a worldwide zoonosis. Infection in humans results from the inhalation of contaminated aerosols. Although the clinical signs of acute infection vary, typical symptoms include fever, headache, myalgia, muscle cramps, hepatitis, and respiratory complications (8). Chronic manifestations may include endocarditis and granulomatous hepatitis (8).
Diagnosis of acute Q fever relies mainly on the detection of specific antibodies to C. burnetii, as culture of the organism is extremely hazardous (3). Commonly used serological methods include the complement fixation test (CFT), the indirect immunofluorescent antibody test (IFAT), and the enzyme-linked immunosorbent assay (ELISA).
IFAT and CFT, although frequently used, have a number of disadvantages. Both tests are subjective, are not standardized between laboratories, are inconvenient for large-scale screening, and cannot be automated. Furthermore, paired sera are usually required when CFT is to be used for the diagnosis of acute infection, as complement fixation (CF) antibodies are often not present early in infection but can persist for long periods after illness (4, 16).
These limitations led to the development of ELISAs that detect antibodies to C. burnetii (1, 7, 17), including a commercial ELISA (PanBio, Brisbane, Australia) for the detection of immunoglobulin M (IgM) antibodies (2). A study of this IgM ELISA was criticized, as the ELISA was not compared to IFAT but to CFT only (D. Raoult, Letter, J. Clin. Microbiol. 36:3446, 1998). In this study, we have compared the performance of the PanBio C. burnetii (Q fever) IgM ELISA to the reference method (IFAT) and CFT with sera from patients with acute Q-fever or other infections.
A total of 191 serum samples were included in the study. Of these, 167 were from patients with clinically suspected Q fever that were submitted to the Centre for Infectious Diseases and Microbiology Laboratory Services at the Institute of Clinical Pathology and Medical Research, Westmead, Australia. These specimens consisted of 22 paired and 43 single serum samples from patients diagnosed with Q fever as determined by the IgM IFAT result. Of the paired sera, five acute-phase (S1) sera and all second (S2) sera were positive by IgM IFAT. The remaining 80 single serum samples were from patients determined not to have Q fever, based on negative IgM IFAT results. A further 24 sera with rheumatoid factor (RF) or from patients with infections other than Q fever were also tested. These comprised 4 single sera falsely positive for IgM IFAT prior to IgG-RF depletion and 20 single IgM IFAT-negative sera from patients with serologically confirmed Mycoplasma pneumoniae (n = 6), Chlamydia psittaci (n = 5), Leptospira sp. (n = 5), and Legionella pneumophila (n = 4) infections. These sera were tested with the PanBio C. burnetii (Q fever) IgM ELISA and the CFT.
The PanBio C. burnetii (Q fever) IgM ELISA was performed according to the manufacturer's instructions. Sera were diluted 1/100 in the diluent provided, which contained goat anti-human IgG to remove competing IgG and RF. The diluted sera were then transferred to the purified phase II C. burnetii whole-cell antigen-coated microwells and incubated for 20 min at 37°C (100 μl/well). After washing with phosphate-buffered saline (PBS) containing 0.05% Tween 20, bound IgM (if present) reacted during a 20-min, 37°C incubation with anti-human IgM peroxidase conjugate (100 μl/well). The plate was then washed and a 10-min incubation with tetramethylbenzidine substrate (100 μl/well) was performed. The reaction was stopped by the addition of 100 μl of 1 M phosphoric acid to each well, and the strips were read with a microtiter plate reader at a wavelength of 450 nm. Results were determined by comparison with an IgM reference serum provided (cut-off calibrator). The initial calibrator value in the IgM ELISA was increased by 50% to obtain optimal specificity and sensitivity. A positive sample was defined as having a sample absorbance/calibrator absorbance ratio (ELISA ratio) of ≥1.0; a negative sample had a ratio of <1.0.
IFAT was modified from the methods previously described (7, 12) by the addition of an RF-IgG depletion step using sheep anti-human IgG. Briefly, phase II antigen (Nine Mile strain; Commonwealth Serum Laboratories, Melbourne, Australia) was diluted, dropped onto the wells of a glass microscope slide, allowed to dry, and fixed with acetone. After depletion of IgG and RF by treatment with sheep anti-human IgG (RF absorbent; Dade Behring, Marburg, Germany), five fourfold dilutions of serum from 1/12 to 1/3,072 in PBS were reacted with antigen on the slides for 3 h at 37°C and then washed with PBS. Bound antibody was then detected via a 30-min incubation with fluorescein-labelled sheep anti-human IgM F(ab′)2 fragment conjugate (Silenus; AMRAD, Melbourne, Australia). After being washed and dried, slides were mounted with a coverslip and examined using an incident light fluorescence microscope (Carl Zeiss, Oberkochen, Germany). Antibody titers were defined as the inverse of the highest dilution with definite staining of C. burnetii membranes. A positive IgM result was defined as having an end point titer of ≥48.
CFT was performed as previously described (14). After determining the optimal dilutions of C. burnetii phase II antigen, complement, and hemolysin via checkerboard titrations, serial dilutions of serum were prepared in veronal-buffered saline and 2 U each of antigen and guinea pig complement were added. After an overnight incubation at 4°C, sensitized sheep cells (2%) were added and incubated for 1 h at 37°C with intermittent shaking. The highest dilution with ≥75% fixation was defined as the end point. A positive result was defined as having an end point titer of ≥32.
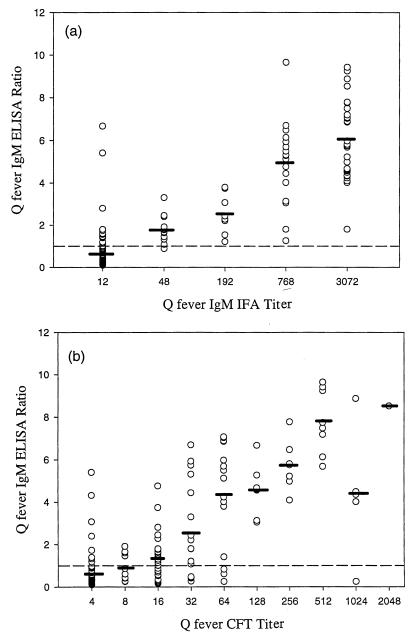
The individual assay values obtained in the PanBio C. burnetii (Q fever) IgM ELISA showed good correlation with IFAT and CFT (Fig. 1). There was a significant correlation between the IFAT or CFT titer and the mean IgM ELISA ratio (analysis of variance was as follows: for ELISA versus CFT, F = 43.2 and P < 0.0001; for ELISA versus IFAT, F = 157.5 and P < 0.0001). Excellent agreement between ELISA and IFAT was obtained using the modified calibrator, with 92% (175 of 191) serum samples giving the same result by both methods. The agreement between CFT and IFAT was 83% (158 of 189). As measured by the sum of sensitivity and specificity (F value) (9, 22) compared to IFAT, the overall performance of the ELISA (F = 187) was significantly better than CFT (F = 163) (Fisher's exact test, P = 0.0004).
FIG. 1.
Comparison of Q-fever IgM ELISA ratios with IgM IFAT titers (a) and Q-fever IgM ELISA ratios with CFT titers (b). Mean ELISA ratios are shown by horizontal bars. The cut-off value (ratio = 1.0) is shown by a broken line.
As observed in studies with research ELISAs (6, 15), the ELISA demonstrated a higher sensitivity than CFT (99% versus 73%) (Table 1), and this difference was statistically significant (Fisher's exact test, P < 0.0001). The ELISA detected IgM in 5 acute-phase sera before CFT (Table 2). Similarly, these studies showed that ELISA may be a more sensitive method for Q-fever diagnosis than IFAT (6, 15), and this was observed in this study, in which ELISA detected IgM in one more acute-phase serum than IFAT (Table 2).
TABLE 1.
Specificity of Q fever IgM ELISA and CFT with IgM IFAT as the reference methoda
| Disease state (IgM IFAT negative) | No. of negative results/no. of IgM IFAT-negative sera (%)
|
|
|---|---|---|
| ELISA | CFT | |
| Positive RF | 3/4 (75) | 2/4 (50) |
| M. pneumoniae infection | 6/6 (100) | 6/6 (100) |
| C. psittaci infection | 5/5 (100) | 3/3 (100)b |
| Leptospirosis | 3/5 (60) | 5/5 (100) |
| Legionellosis | 4/4 (100) | 4/4 (100) |
| IgM IFAT negativec | 85/97 (88) | 87/97 (90) |
| Total IgM IFAT negative | 106/121 (88) | 107/119 (90) |
The IgM IFAT positive sera included 43 single sera positive by IgM IFAT and 5 S1 and 22 S2 sera from patients with Q fever. The sensitivity (positive results/IgM IFAT-positive sera) was as follows: ELISA, 69/70 (99%); CFT, 51/70 (73%).
Two sera from patients with C. psittaci infections were not included in CFT analysis due to insufficient quantity of serum.
The 97 negative IgM IFAT sera include the 80 single sera from patients found not to have Q fever on the basis of a negative IgM IFAT result and the 17 IgM IFAT negative acute-phase samples of serum pairs from patients diagnosed with Q fever.
TABLE 2.
Detection of Q fever infection in paired sera
| Test | No. positive (%)
|
|
|---|---|---|
| Acute-phase sera (S1) | Second sera (S2) | |
| IgM IFATa | 5/22 (23) | 22/22 (100) |
| IgM ELISA | 6/22b (27) | 22/22 (100) |
| CFT | 1/22 (5) | 22/22 (100) |
All paired sera showed at least a fourfold rise in titer by IgM IFAT.
One acute serum was IgM ELISA positive (ratio, 1.78) and IgM IFAT negative (ratio, <12).
The ELISA used in this study showed a slightly lower specificity than CFT (88 versus 90%), although this difference was not statistically significant (Fisher's exact test, P = 0.6838 (Table 1). As previously reported (8, 16), the CFT demonstrated good specificity (90%) when IFAT was used as the reference method (Table 1). It is interesting to note that when a cut-off titer of 64 was used, the CFT specificity increased to 95% (113 of 119), while the sensitivity decreased to 56% (39 of 70). CFT specificity and sensitivity are related to the level of CF titer selected, and this work demonstrated the inherent difficulty in selecting a single CF titer suggestive of recent Q-fever infection (7). Q fever is difficult to diagnose by CFT with a single convalescent-phase serum because antibodies can persist, with titers falling at variable rates (13). Furthermore, CFT is not specific for Q-fever IgM, as are IgM IFAT and IgM ELISA.
While the CFT did not demonstrate reactivity with sera from patients with infections other than Q fever, two of the four samples containing RF gave a positive result. This is probably due to the presence of either anticomplementary factors or persistent CF antibody from past infection in the sera. No cross-reactivity was observed in the ELISA with samples from patients with legionellosis (Table 1), although the C. burnetii antigen has been reported to express on its surface epitopes of the common bacterial antigen and eukaryotic chaperoning protein (5, 11, 19, 20), which has extensive homology with proteins from other prokaryotes, such as L. pneumophila (10). No cross-reactivity in the ELISA with samples from patients with mycoplasma or chlamydia infection was observed (Table 1). The ELISA did however show elevated levels in sera from two of five leptospirosis patients and in one of four RF-positive sera (Table 1), although these results may not be a true representation of the cross-reactivity in these disease groups due to the low sample number. It would be interesting to investigate this cross-reactivity further with a larger number of samples from these groups. In a recent study of the PanBio Leptospira IgM ELISA (21), sera from 3 of 34 patients with Q-fever infections were reactive, though it could not be determined whether this was due to cross-reactivity or persistent antibody from a previous leptospiral infection. A previously published study of the PanBio C. burnetii (Q fever) IgM ELISA reported a specificity (20 of 23 samples; 87%) similar to that found in this study, with cross-reactivity observed with sera from patients with M. pneumoniae and Bordetella pertussis infections but not Epstein-Barr virus or cytomegalovirus infections (2). Cross-reactivity with M. pneumoniae has been suggested by Stallman and Allan (18).
The ELISA described in this study provides a standardized method for Q-fever diagnosis, with a total incubation time of 50 min, suitability for large-scale screening, and the potential for automation. The results presented suggest that the PanBio C. burnetii (Q fever) IgM ELISA is a sensitive and specific alternative for the diagnosis of Q fever. Samples can be screened in the ELISA, with positive results confirmed by IFAT if necessary, thus reducing the number of IFAT tests performed.
Acknowledgments
We thank David J. Judd and Eric Kapsalis for technical assistance.
REFERENCES
- 1.Cowley R, Fernandez F, Freemantle W, Rutter D. Enzyme immunoassay for Q fever: comparison with complement fixation and immunofluorescence tests and dot immunoblotting. J Clin Microbiol. 1992;30:2451–2455. doi: 10.1128/jcm.30.9.2451-2455.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devine P, Doyle C, Lambkin G. Combined determination of Coxiella burnetii-specific immunoglobulin M (IgM) and IgA improves specificity in the diagnosis of acute Q fever. Clin Diagn Lab Immunol. 1997;4:384–386. doi: 10.1128/cdli.4.3.384-386.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dupuis G, Péter O, Luthy R, Nicolet J, Peacock M, Burgdorfer W. Serological diagnosis of Q fever endocarditis. Eur Heart J. 1986;7:1062–1066. doi: 10.1093/oxfordjournals.eurheartj.a062016. [DOI] [PubMed] [Google Scholar]
- 4.Dupuis G, Péter O, Peacock M, Burgdorfer W, Haller E. Immunoglobulin responses in acute Q fever. J Clin Microbiol. 1985;22:484–487. doi: 10.1128/jcm.22.4.484-487.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dwyer D E, Gibbons V L, Brady L M, Cunningham A L. Serological reaction to Legionella pneumophila group 4 in a patient with Q fever. J Infect Dis. 1988;158:499–500. [PubMed] [Google Scholar]
- 6.Embil J, Williams J C, Marrie T J. The immune response in cat-related outbreak of Q fever as measured by the indirect immunofluorescence test and the enzyme-linked immunosorbent assay. Can J Microbiol. 1990;36:292–296. doi: 10.1139/m90-050. [DOI] [PubMed] [Google Scholar]
- 7.Field P R, Hunt J G, Murphy A M. Detection and persistence of specific IgM antibody to Coxiella burnetii by enzyme-linked immunosorbent assay: a comparison with immunofluorescence and complement fixation tests. J Infect Dis. 1983;148:477–487. doi: 10.1093/infdis/148.3.477. [DOI] [PubMed] [Google Scholar]
- 8.Fournier P E, Marrie T J, Raoult D. Diagnosis of Q fever. J Clin Microbiol. 1998;36:1823–1834. doi: 10.1128/jcm.36.7.1823-1834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greiner M, Sohr D, Gobel P. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J Immunol Methods. 1995;185:123–132. doi: 10.1016/0022-1759(95)00121-p. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman P S, Houston L, Butler C A. Legionella pneumophila htpAB heat shock operon: nucleotide sequence and expression of the 60-kilodalton antigen in L. pneumophila-infected HeLa cells. Infect Immun. 1990;58:3380–3387. doi: 10.1128/iai.58.10.3380-3387.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoover T A, Vodkin M H. Cloning and expression of Coxiella burnetii DNA. In: Williams J C, Thompson H A, editors. Q fever: the biology of Coxiella burnetii. Boca Raton, Fla: CRC Press; 1991. pp. 285–310. [Google Scholar]
- 12.Hunt J G, Field P R, Murphy A M. Immunoglobulin responses to Coxiella burnetii (Q fever): single-serum diagnosis of acute infection, using an immunofluorescence technique. Infect Immun. 1983;39:977–981. doi: 10.1128/iai.39.2.977-981.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy A M, Field P R. The persistence of complement-fixing antibodies to Q fever (Coxiella burnetii) after infection. Med J Aust. 1970;1:1148–1150. doi: 10.5694/j.1326-5377.1970.tb84481.x. [DOI] [PubMed] [Google Scholar]
- 14.Murphy A M, Magro L. IgM globulin response in Q fever (Coxiella burnetii) infections. Pathology. 1980;12:391–396. doi: 10.3109/00313028009077101. [DOI] [PubMed] [Google Scholar]
- 15.Péter O, Dupuis G, Bee D, Luthy R, Nicolet J, Burgdorfer W. Enzyme-linked immunosorbent assay for diagnosis of chronic Q fever. J Clin Microbiol. 1988;26:1978–1982. doi: 10.1128/jcm.26.10.1978-1982.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Péter O, Dupuis G, Burgdorfer W, Peacock M. Evaluation of the complement fixation and indirect immunofluorescence tests in the early diagnosis of primary Q fever. Eur J Clin Microbiol Infect Dis. 1985;4:394–396. doi: 10.1007/BF02148690. [DOI] [PubMed] [Google Scholar]
- 17.Péter O, Dupuis G, Peacock M G, Burgdorfer W. Comparison of enzyme-linked immunosorbent assay and complement fixation and indirect fluorescent-antibody tests for detection of Coxiella burnetii antibody. J Clin Microbiol. 1987;25:1063–1067. doi: 10.1128/jcm.25.6.1063-1067.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stallman N D, Allan B C. A survey of antibodies to Mycoplasma pneumoniae in Queensland. Med J Aust. 1970;1:800–802. doi: 10.5694/j.1326-5377.1970.tb116816.x. [DOI] [PubMed] [Google Scholar]
- 19.Vodkin M H, Williams J C. A heat shock operon in Coxiella burnetii produces a major antigen homologous to a protein in both mycobacteria and Escherichia coli. J Bacteriol. 1988;170:1227–1234. doi: 10.1128/jb.170.3.1227-1234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams J C, Hoove T A, Waag D M, Bhatnagar N, Bolt C R, Scott G H. Antigenic structure of Coxiella burnetii: a comparison of lipopolysaccharide and protein antigens as vaccines against Q fever. Ann N Y Acad Sci. 1990;590:370–380. doi: 10.1111/j.1749-6632.1990.tb42243.x. [DOI] [PubMed] [Google Scholar]
- 21.Winslow W E, Merry D J, Pirc M L, Devine P L. Evaluation of a commercial enzyme-linked immunosorbent assay for detection of immunoglobulin M antibody in the diagnosis of leptospiral infection. J Clin Microbiol. 1997;35:1938–1942. doi: 10.1128/jcm.35.8.1938-1942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu H, Lohr J, Greiner M. The selection of ELISA cut-off points for testing antibody to Newcastle disease by two-graph receiver operating characteristic (TG-ROC) analysis. J Immunol Methods. 1997;208:61–64. doi: 10.1016/s0022-1759(97)00128-2. [DOI] [PubMed] [Google Scholar]