Abstract
Objectives
Biologic treatments have revolutionized the management of PsA by significantly improving clinical manifestations and preventing structural damage. Both result in better quality of life and improved physical functioning. Since the introduction of the first TNF inhibitor (TNFi) in the early 2000s, therapeutic options for PsA are increasing steadily, and a new generation of biologics, including anti-IL-17 and anti-IL-23 strategies, allows distinct targeted approaches. The purpose of this study was to investigate whether the demographic, clinical and disease characteristics of PsA patients who are selected for first-line biologic treatment has changed over time since the introduction of biologics.
Methods
Patients with a clinical diagnosis of PsA were included in the KU Leuven BioSPAR registry, a prospective cohort of SpA and PsA patients treated with biologics and targeted synthetic DMARDs (tsDMARDs), such as apremilast and Janus kinase inhibitors. Demographics, prior DMARD use, disease characteristics and disease activity parameters were recorded at the initiation of biologic treatment and subsequently every 3 months for the first 2 years and later every 6 months. The patient data were compared in three treatment periods, corresponding to availability of the first and second generation of TNFi and the third generation of biologics.
Results
Analysis of 185 Caucasian patients with PsA from our prospective cohort showed longer disease duration and higher disease activity, with higher tender joint count, swollen joint count and CRP in the first period compared with the later time periods. The demographic characteristics and prior DMARD use did not change over time. Skin and nail psoriasis were more frequent in earlier compared with the later treatment periods. The bio-DMARD survival rate was similar in the early and later treatment periods.
Conclusion
The population of patients selected for treatment escalation has changed over time since the introduction of biologics. Our results suggest that with years of experience, PsA patients might be considered earlier and for therapy intensification in patients with less active disease in comparison to profiles in the early days of biologic treatment.
Keywords: psoriatic arthritis, epidemiology, observational study, prospective, biologics, drug survival, TNF-blocking agents
Key messages
Changing experience with biologics affects the patient population considered for biologics in daily clinical practice.
Early treatment opens perspectives for a positive effect on the burden of disease, including remission.
Introduction
Biologics have revolutionized the management of rheumatic diseases, thereby significantly improving the outcome and quality of life of our patients. From the early 2000s, inhibition of TNF, a pro-inflammatory cytokine, has proved to be effective for a wide range of inflammatory disorders, including different forms of arthritis, axial SpA, uveitis, IBD and psoriasis. PsA has a high disease burden and can affect patients across a wide age range. Anti-TNF treatment has been a mainstay in the management of the disease for >15 years and has significantly improved the quality of life of our patients.
In the last decades, the therapeutic options for PsA have expanded, and new generations of biologics provide excellent alternatives to anti-TNF. New treatments have given us a better understanding and deeper insight into the pathophysiology of psoriatic disease. Targeting the IL-23/IL-17 pathway has shown great efficacy in PsA [1, 2] and even superiority in resolution of enthesitis [3] and skin psoriasis [4] in comparison to anti-TNF-α therapy. Better control of inflammation thanks to biologics has been beneficial for slowing down radiographic damage in PsA [5, 6]. Moreover, targeting specific pathways has allowed us to aim selectively at different domains beyond the musculoskeletal system, including skin, eye and gastrointestinal tract, depending on the phenotype of an individual patient [7].
The aim of this study was to analyse whether this knowledge and two decades of experience has influenced the use of biologics in patients with PsA over the course of time. We recently demonstrated that, in the context of clinical trials, the target population of PsA patients eligible for treatment with biologics has evolved over time [8]. In this study we addressed the same question in a real-life academic cohort. We aimed to document the demographics, lifestyle factors, disease characteristics and previous treatments in PsA patients eligible for biologics and how these might have changed over time and influenced local management strategies, including therapy escalation.
Methods
Data collection
We identified patients with a clinical diagnosis of PsA from the BioSPAR register, a prospective cohort of patients with SpA and PsA, treated with biologics, apremilast or Janus kinase inhibitors attending the rheumatology department of University Hospitals Leuven. The demographic (race, age, sex, weight, height, BMI and disease duration), clinical [swollen joint count (SJC28 and SJC66), tender joint count (TJC28 and TJC68), presence of enthesitis, dactylitis, skin psoriasis and nail psoriasis], disease activity parameters [psoriasis area severity index (PASI), body surface area, Maastricht ankylosing spondylitis enthesitis score (MASES) and Leeds enthesitis score (LEI), dactylitis score, physician’s global assessment (PGA) and patient’s global assessment on a visual analogue scale (VAS), CRP level, ESR, DAS on 28 joints (DAS28)] data were recorded at the initiation of the first-line biologic treatment. For all patients, DMARD treatment and use of glucocorticoids and NSAIDs were recorded. All data were collected at the time of the initiation of therapy with the first biologic or apremilast. All patients gave informed consent to be included in this prospective database. The protocol was approved by the local ethics committee of UZ Leuven (study S51013, approval of ethical committee B32220084074).
Three treatment periods were defined according to the time of the first-line biologic treatment initiation: period 1 from the date the first patient in our records started TNF inhibitor (TNFi) therapy (infliximab, etanercept or adalimumab) until the second generation of anti-TNF (golimumab and certolizumab) was available: 15 September 2000–3 June 2006; period 2 from the date the first patient was started on the second-generation TNFi (golimumab or certolizumab) until the third generation of biologics was available: 4 June 2006– 22 March 2016; and period 3 from the date the first patient was started on the third generation of biologics (ustekinumab or secukinumab) or apremilast until a defined closure date in the BioSPAR registry: 23 March 2016–28 February 2018.
Statistical analysis
Statistical analysis was performed using SPSS statistical software, v.24.0. The basic demographic and clinical data were analysed using descriptive statistics. Continuous data are presented as the mean (S.d.), discrete data as percentages. To compare continuous and normally distributed variables in the three time periods, we used one-way ANOVA. If the test of homogeneity of variances showed significant deviation from normality, robust tests of equality of the means were performed (Welch and Brown–Forsythe), and in the evnt of significant results, non-parametric tests were used. To compare continuous and not-normally distributed variables in the three time periods, we used the Kruskal–Wallis test. For discrete data, the χ2 test was used. Values of P < 0.05 were considered significant. For normally distributed continuous variables, where information was not available for the first time period, Student’s unpaired t-test was used in order to determine the difference between the second and third periods. Significance values have been adjusted by the Bonferroni correction for multiple testing. The log rank test (Mantel–Cox) was used the evaluate drug survival during period 2 compared with period 1, and to compare biologics in monotherapy with biologics in combination with conventional DMARDs.
Results
Out of 407 patients included in the BioSPAR registry, we identified 185 patients with PsA (Table 1). All patients were eligible for reimbursement of biologics in PsA under the Belgian health-care regulations. By definition, all patients had erosive peripheral arthritis. Biologics in PsA are not reimbursed for axial involvement. All patients were Caucasian. Period 1 included 65 patients, slightly more than half of whom were male; aged 45.7 (10.2) years and with a disease duration [mean (S.d.)] of 11.6 (8) years. SJC66 [mean (S.d.)] and TJC68 [mean (S.d.)] were 9.2 (6.3) and 13.5 (11), respectively. The mean CRP level was 26 (28) mg/l. In 30.8% of the patients infliximab was the TNFi of choice, and in 45 patients (69.2%) etanercept was initiated as the first biologic treatment.
Table 1.
Patient characteristics per time period
| Parameter | Time period 1 (15 September 2000–3 June 2006) |
Time period 2 (4 June 2006–22 March 2016) |
Time period 3 (23 March 2016–28 February 2018) |
|---|---|---|---|
| Patient characteristics | |||
| Number per group | 65 | 84 | 36 |
| Male patients, n (%) | 41 (63.1) | 47 (56) | 17 (47.2) |
| Age, mean (S.d.) (median), years | 45.7 (10.2) (47) | 45.4 (13.5) (46.4) | 47.7 (14) (45.3) |
| Weight, mean (S.d.) (median), kg | 81 (13.8) (80.5) | 77.4 (18.7) (74.4) | 80.5 (17.3) (88.3) |
| BMI, mean (S.d.) (median), kg/m2 | 26.9 (3.7) (26.8) | 26 (5.6) (24.4) | 26.8 (5) (26.6) |
| Disease characteristics | |||
| Disease duration, mean (S.d.) (median), years | 11.6 (8) (10.3) | 8.1 (7.3) (6.2) | 8.8 (9) (6.8) |
| Patients with dactylitis, n (%) | 14 (21.5) | 10 (11.9) | 4 (11.1) |
| Patients with enthesitis, n (%) | 8 (12.3) | 18 (21.4) | 6 (16.7) |
| Patients with skin psoriasis, n (%)* | 51 (78.5) | 49 (58.3) | 17 (47.2) |
| Patients with nail psoriasis, n (%)* | 41 (63.1) | 28 (33.3) | 11 (30.6) |
| Disease activity | |||
| SJC28, mean (S.d.) (median) | 5 (3.5) (4) | 3.71 (3.6) (3.0) | 2.1 (1.9) (2.0) |
| SJC66, mean (S.d.) (median) | 9.2 (6.2) (8) | 6 (4.8) (5) | 4.8 (3.7) (5.0) |
| TJC28, mean (S.d.) (median) | 5.8 (5) (4) | 5.4 (5.56) (3.5) | 3.0 (4.4) (2) |
| TJC68, mean (S.d.) (median)* | 13.5 (11) (12) | 10 (9.6) (7.5) | 7.5 (8.3) (5.5) |
| CRP level, mean (S.d.) (median), mg/l* | 26 (28) (18.3) | 14.7 (18) (8) | 13.7 (16) (10.6) |
| ESR, mean (S.d.) (median), mm | 28.9 (26) (21) | 24.2 (21.6) (17) | 19.5 (20.6) (13.5) |
| DAS28-CRP | NA | 4.1 (1.2) (4.0) | 4.7 (3.4) (4) |
| PGA, mean (S.d.) (median), VAS 0–10 cm | NA | 4.2 (2.0) (4.0) | 3.4 (2.2) (3) |
| VAS patient, mean (S.d.) (median), VAS 0–10 cm | NA | 6.5 (2.7) (6.0) | 7 (1.5) (7) |
| PASI, mean (S.d.) (median), 0–72 scale | NA | 1.66 (4.2) 0 | 1.9 (3.8) 0 |
| Body surface area, mean (S.d.) (median), % | NA | 1.1 (4.6) 0 | 0.06 (0.25) 0 |
| Dactylitis score (0–60), mean (S.d.) (median) | NA | 0.58 (2.9) 0 | 0.87 (2.0) 0 |
| LEI, mean (S.d.) (median), 0–6 scale | NA | 0.73 (1.7) (0) | 0.81 (2) (0) |
| MASES, mean (S.d.) (median), 0–13 scale | NA | 0.53 (1.4) (0) | 0.81 (2) (0) |
| Treatment, prior to biologic initiation | |||
| Patients receiving any DMARD, n (%) | 40 (61.5) | 63 (75) | 25 (69.4) |
| Patients receiving MTX, n (%) | 36 (55.4) | 53 (63.1) | 21 (58.3) |
| Patients receiving SSZ, n (%) | 8 (12.3) | 8 (9.5) | 6 (16.7) |
| Patients receiving LEF, n (%)* | 3 (4.6) | 21 (25) | 9 (25) |
| Patients receiving glucocorticoids, n (%) | 19 (29.2) | 20 (23.8) | 9 (25) |
| Patients receiving NSAIDs, n (%) | 42 (64.6) | 51 (60.7) | 23 (63.9) |
| Patients receiving a combination of DMARDs, n (%) | 11 (16.7) | 19 (22.6) | 12 (33.3) |
| Biologic treatment, n | 65 | 84 | 36 |
| Infliximab, n (%) | 20 (30.8) | 10 (11.9) | 0 |
| Etanercept, n (%) | 45 (69.2) | 66 (78.6) | 8 (22.2) |
| Adalimumab, n (%) | 0 | 3 (3.6) | 1 (2.8) |
| Certolizumab, n (%) | NA | 0 | 0 |
| Golimumab, n (%) | NA | 5 (6.0) | 0 |
| Apremilast, n (%) | NA | NA | 24 (66.7) |
| Ustekinumab, n (%) | NA | NA | 1 (2.8) |
| Secukinumab, n (%) | NA | NA | 2 (5.6) |
| Treatment discontinuation, n (%) | 36 (55.4) | 19 (22.6) | 8 (22.2%) |
Statistically significant difference between the groups p< 0.05.
DAS28-CRP: disease activity Score on 28 joints; MASES: Maastricht ankylosing spondylitis enthesitis score; NA: not assessed; PASI: psoriasis area severity index; PGA: physician’s global assessment; SJC28: swollen joint count on 28 joints; SJC66: swollen joint count on 66 joints; TJC28: tender joint count on 28 joints; TJC68: tender joint count on 68 joints; VAS patient: patient’s global assessment on visual analogue scale.
Period 2 included 84 patients, with equal sex distribution and a disease duration of 8.1 (7.3) years. SJC66 and TJC68 were 6 (4.8) and 10 (9.6), respectively. Mean CRP levels were 14.7 (18) mg/l. In the majority of the patients the first TNF inhibitors were still the treatment of choice, with 78.6% being treated with etanercept and 11.9% with infliximab; whereas in 5 patients (6%) golimumab was initiated as first-line biologic therapy, and in 3 (3.6%) patients adalimumab.
Period 3 included 36 patients, half of whom were male, with disease duration of 8.8 (9) years. SJC66 and TJC68 were 4.8 (3.7) and 7.5 (8.3), respectively. The average CRP levels were 13.7 (16) mg/l. In the majority of these patients (66.7%) apremilast was initiated; 8 (22.2%) patients were started on etanercept, 2 (5.6%) on secukinumab, 1 (2.8%) on ustekinumab and 1 (2.8%) on adalimumab.
We found no statistically significant difference in the age of patients, mean weight or BMI at the time of initiation of biologic treatment in a comparison of the three time periods (Table 2). The proportion of male/female patients was also similar. The disease duration was significantly longer in the first compared with the third (P = 0.017) time period. There was no significant difference in disease duration between periods 2 and 3 (P = 0.19). Furthermore, patients in period 1 had more tender joints than did patients in period 3 (P = 0.012). The number of swollen joints in the patient population in period 1 was significantly higher than in patients in time periods 2 and 3 (P = 0.001 and P = 0.0001, respectively). Skin and nail psoriasis were also significantly more frequent in the first compared with second and third treatment periods (P < 0.0001 for both). However, the extent of skin involvement was relatively low (body surface area < 3% and PASI < 10) and comparable in the second and third time periods (data for period 1 were not available). In our study population, the proportion of patients with dactylitis or enthesitis was relatively low and similar in all three time periods. CRP levels, but not ESR, differed significantly between the time periods, being higher in period 1 than in periods 2 (P = 0.006) and 3 (P = 0.023).
Table 2.
Statistical analysis: significant differences between the periods
| Parameter | Δ P1P2P3e | ΔP1P2f | ΔP1P3g | ΔP2P3h | |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Male patientsc | |||||
| Theoretical χ2 value | 2.41 | ||||
| Two-sided P-value | 0.3 | ||||
| Age, yearsa | |||||
| F-value | 0.45 | Group difference | 0.29 | −2.01 | −2.3 |
| Two-sided P-value | 0.8 | 95% CI | [−4.6, 5.2] | [−8.2, 4.1] | [−8.2, 3.6] |
| P-value | 1.0 | 0.72 | 0.62 | ||
| Weight, kga | Group difference | 3.9 | 0.8 | 3.08 | |
| F-value | 1.05 | 95% CI | [−2.7, 10.5] | [−7.5, 9.1] | [−11.1, 4.5] |
| Two-sided P-value | 0.35 | P-value | 0.35 | 0.97 | 0.64 |
| BMI, kg/m2a | Group difference | 0.79 | 0.09 | 0.7 | |
| F-value | 0.52 | 95% CI | [−1.2, 2.7] | [−2.5, 2.7] | [−3.2, 1.8] |
| Two-sided P-value | 0.59 | P-value | 0.6 | 1 | 0.8 |
| Disease characteristics | |||||
| Disease duration, yearsb | |||||
| Test statistic | 9.3 | Test statistic | 24.6 | 26.6 | 2.03 |
| Two-sided P-value | 0.009 | Adjusted P-value* | 0.017 | 0.05 | 1.0 |
| Patients with dactylitisc | |||||
| Theoretical χ2 value | 2.3 | ||||
| Two-sided P-value | 0.31 | ||||
| Patients with enthesitisc | |||||
| Theoretical χ2 value | 0.81 | ||||
| Two-sided P-value | 0.66 | ||||
| Patients with skin psoriasisc | |||||
| Theoretical χ2 value | 16.4 | Theoretical χ2 value | 13.2 | 13.5 | 0.41 |
| Two-sided P-value | 0.0001 | Two-sided P-value | 0.0001 | 0.0001 | 0.53 |
| Patients with nail psoriasisc | |||||
| 19.6 | Theoretical χ2 value | 17.6 | 10.2 | 91 | |
| 0.0001 | Two-sided P-value | 0.0001 | 0.001 | 0.14 | |
| Disease activity | |||||
| SJC66b | |||||
| Test statistic | 16.4 | Test statistic | 28.1 | 40.3 | 12.2 |
| Two-sided P-value | 0.0001 | Adjusted P-value | 0.004 | 0.001 | 0.71 |
| TJC68a | Group difference | 3.4 | 6.0 | 2.6 | |
| F-value | 4.4 | 95% CI | [−0.56, 7.33] | [1.08, 10.9] | [−2.02, 7.22] |
| Two-sided P-value | 0.013 | P-value | 0.11 | 0.012** | 0.56 |
| CRP level, mg/lb | |||||
| Test statistic | 11.7 | Test statistic | 27 | 28.9 | 1.7 |
| Two-sided P-value | 0.003 | Adjusted P-value | 0.005 | 0.02 | 1.0 |
| ESR, mma | Group difference | 4.7 | 9.4 | 4.7 | |
| F-value | 1.7 | 95% CI | [−5.2, 14.6] | [−2.7, 21.5] | [−6.4, 15.8] |
| Two-sided P-value | 0.19 | P-value | 0.5 | 0.16 | 0.6 |
| PGA, score 0–10d | |||||
| F-value | NA | NA | NA | NA | 0.22 |
| Two-sided P-value | NA | NA | NA | NA | 0.64 |
| VAS patient, score 0–10d | |||||
| Test statistic | NA | NA | NA | NA | 2.7 |
| Two-sided P-value | NA | NA | NA | NA | 0.1 |
| PASI, 0–72 scaled | |||||
| Test statistic | NA | NA | NA | NA | 0.13 |
| Two-sided P-value | NA | NA | NA | NA | 0.7 |
| Body surface area, %d | NA | NA | 0.25 | ||
| F-value | NA | NA | NA | NA | 0.12 |
| Two-sided P-value | NA | NA | |||
| Dactylitis score, 0–60d | |||||
| Test statistic | NA | NA | NA | NA | 1.1 |
| Two-sided P-value | NA | NA | NA | NA | 0.3 |
| Leuven enthesitis score, 0–99 scaled | |||||
| F-value | NA | NA | NA | NA | 0.94 |
| Two-sided P-value | NA | NA | NA | NA | 0.33 |
| MASES, 0–60 scaled | |||||
| F-value | NA | NA | NA | NA | 2.7 |
| Two-sided P-value | NA | NA | NA | NA | 0.1 |
| DAS28-CRPd | |||||
| Test statistic | NA | NA | NA | NA | 5.4 |
| Two-sided P-value | NA | NA | NA | NA | 0.2 |
| Treatment, prior to biologic initiation | |||||
| Patients receiving DMARD treatmentc | |||||
| Theoretical χ2 value | 1.56 | ||||
| Two-sided P-value | 0.5 | ||||
| Patients receiving MTXc | |||||
| Theoretical χ2 value | 0.4 | ||||
| Two-sided P-value | 0.82 | ||||
| Patients receiving SSZ c | |||||
| Theoretical χ2 value | 1.24 | ||||
| Two-sided P-value | 0.54 | ||||
| Patients receiving LEFc | |||||
| Theoretical χ2 value | 10.8 | Theoretical χ2 value | 10.2 | 8.2 | 0.001 |
| Two-sided P-value | 0.004 | Two-sided P-value | 0.01 | 0.04 | 0.97 |
| Patients receiving glucocorticoidsc | |||||
| Theoretical χ2 value | 0.83 | ||||
| Test statistic | 0.662 | ||||
| Two-sided P-value | |||||
| Patients receiving NSAIDsc | |||||
| Theoretical χ2 value | 1.1 | ||||
| Two-sided P-value | 0.57 | ||||
| Patients receiving combination DMARD treatmentc | |||||
| Theoretical χ2 value | |||||
| Two-sided P-value | 2.8 | ||||
| 0.24 | |||||
One-way ANOVA was used to compare continuous and normally distributed data in the three time periods.
The Kruskal–Wallis test was used to compare continuous and not-normally distributed data in the three time periods.
Chi-square test for independence was used to compare discrete data in the tree time periods.
If ≥40% of the values were missing for the first time period, Student’s unpaired t-test was used to compare the difference between the second and third periods in all the statistical tests; the P-value is two-sided.
e Global differences between the 3 periods
f Difference between period 1 and 2
g Difference between period 1 and 3
h Difference between period 2 and 3
After performing the Kruskal–Wallis test, the significance level was adjusted for P-value in the pairwise comparisons.
The significance level according to Bonferroni correction was set at 0.017. The P-value of 0.012 is therefore significant in this subgroup analysis.
DAS28-CRP: disease activity Score on 28 joints; MASES: Maastricht ankylosing spondylitis enthesitis score; NA: not assessed; PASI: psoriasis area severity index; PGA: physician’s global assessment; SJC28: swollen joint count on 28 joints; SJC66: swollen joint count on 66 joints; TJC28: tender joint count on 28 joints; TJC68: tender joint count on 68 joints; VAS patient: patient’s global assessment on visual analogue scale.
We compared the clinical scores for psoriasis (PASI and body surface area), enthesitis (LEI and MASES) and disease activity (PGA, patient VAS and DAS28-CRP) between periods 2 and 3, because data for the first time period were not available. No meaningful differences were found.
In all time periods, almost two-thirds of all the patients were receiving DMARDs and more than half were receiving MTX at initiation of the biologic therapy, with a mean (S.d.) dose of 16.3 (4) mg/week. The proportions of patients treated with MTZ, SSZ, a combination of DMARDs in addition to glucocorticoids and NSAIDs were comparable in three time groups. A significantly lower proportion of patients in the first time period was treated with LEF (P = 0.004).
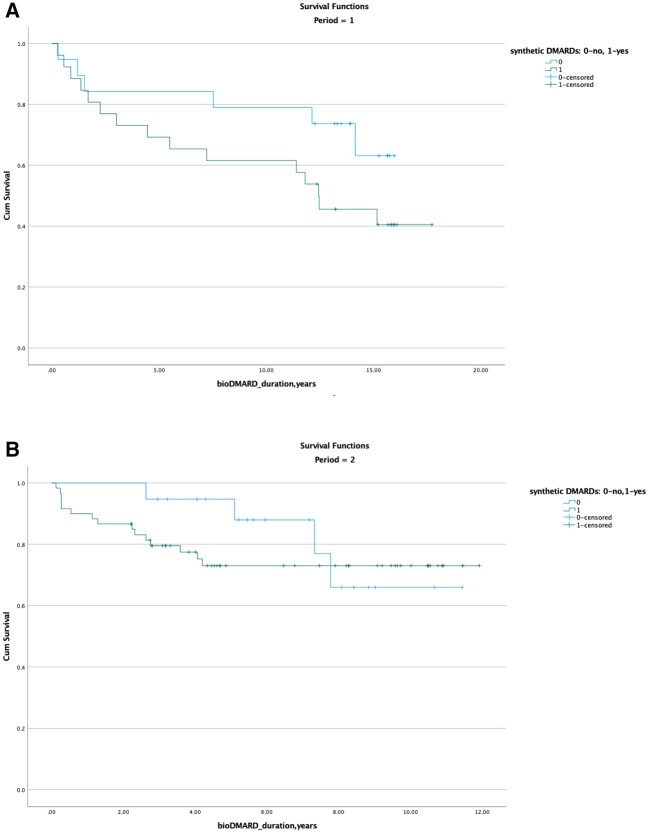
We compared the survival rate of the first initiated biologic treatment in the first and second time periods. Period 3 was excluded owing to a short duration of follow-up (2 years). The mean survival time of a biologic in monotherapy during the first period was 12.7 years (95% CI: 10.3, 15.2) and slightly lower during the second period (9.69 years; 95% CI: 8.2, 11.1). The mean survival time of a biologic in combination with a conventional DMARD during the first period was 10.9 years (95% CI: 8.3, 13.6) and during the second period 9.2 years (95% CI: 8.0, 10.4). The drug survival rates were not significantly different between the groups (Fig. 1). The drug persistence was 68.7% and 71.6% after 10 years of treatment in the periods 1 and 2, respectively, and 54.8% after 15 years in the first time period. The biologic persistence was similar in patients treated with and without synthetic DMARDs during periods 1 and 2 (log rank P = 0.544; Fig. 2).
Fig. 1.
Drug survival curves of bio-DMARD use in patients with PsA for time periods 1 and 2
The x-axis shows the duration (in years) of the first biologic treatment, and the y-axis shows the cumulative survival rate. The P-value determined by log rank test is 0.636 (non-significant). Owing to the short duration (2 years) and small number of patients, the third time period was excluded from the analysis. In the first time period, 12 patients who interrupted the biologic treatment owing to the end of a study protocol were also excluded from analysis. Censored observations define patients who were still on their first biologic treatment at the time of the completion of the study, on 1 June 2018 (indicated by vertical stripes).
Fig. 2.
Drug survival of bio-DMARD use for time periods 1 (A) and 2 (B) based on concomitant use of synthetic DMARDs
The x-axis shows the duration (in years) of the first biologic treatment, and the y-axis shows the cumulative survival rate. The P-value determined by log rank test is 0.112 (non-significant). In the first time period, 15 patients who interrupted biologic treatment owing to the end of a study protocol were excluded from the analysis. Censored observations define patients who were still on their first biologic treatment at time of completion of the study, on 1 June 2018 (indicated by vertical stripes). 0: not concomitant synthetic DMARD; 1: concomitant synthetic DMARD.
Discussion
We performed a comparative analysis of baseline characteristics of patients with PsA registered in our prospective academic BioSPAR cohort according to the time of initiation of the first biologic DMARD. Some notable differences were recorded in the disease activity components. Interestingly, patients initiated on anti-TNF therapy in the beginning of the 2000s (period 1) had higher disease activity, with more tender and swollen joints and higher CRP levels, than in the later time periods. Furthermore, patients in the first period had a longer disease duration, although the difference was significant only between periods 1 and 3. These data suggest that initially, patients with more severe/active established disease were selected for therapy escalation with novel biologic DMARDs. The difference for TJC was significant only between periods 1 and 3. This might be attributable to a shorter duration of the third period (<2 years of follow-up), a smaller number of patients or, possibly, a shift from TNFi toward anti-IL-12/-23, anti-IL-17 inhibitors and apremilast.
Recent analysis of baseline patient characteristics from randomized controlled trials (RCTs) by our group showed similar results [8]. PsA patients started on biologics from earlier trials had more active disease, with more tender and swollen joints, higher CRP levels and longer disease duration, in comparison to later RCTs. It is clear that the changing demographics at inclusion in RCTs over time is reflected in the real-world setting, initiating biologic DMARDs in patients with less extended disease over time.
However, it is difficult to compare populations from real time cohorts and clinical trials. PsA patients included in trials have predominantly polyarticular disease, CRP levels above normal, and more than two- thirds have extended skin psoriasis (body surface area > 3%) [9–13]. These patients are carefully selected and represent only a part of the whole PsA population. In contrast, patients from prospective cohorts better reflect the daily practice situation and often present oligoarticular disease, with lower SJC, TJC and CRP levels [14–16]. This setting completes the whole clinical spectrum of PsA. Therefore real-world evidence provides additional valuable information about the PsA population and complements data from RCTs [17].
Of note, significantly more patients in our cohort had skin and nail psoriasis in period 1 compared with the later time periods. The degree of skin involvement was mild; however, PASI and body surface area were missing for the majority of patients in period 1.
Enthesitis and dactylitis are distinctive features of PsA, and >50% of patients will develop dactylitis during the course of the disease [18]. However, the proportion of patients with documented enthesitis and dactylitis in our cohort was relatively low and similar in the three time periods, as opposed to the data from RCTs, where more than half of patients have entheseal involvement, especially in the later trials [12, 13, 19]. Assessment of enthesitis in the setting of a clinical trial is likely to differ from the more pragmatic evaluation in routine clinical practice.
The demographics of our patient population did not change significantly over time, with a similar male/female ratio, mean age and a mean BMI being slightly above normal, as previously reported in the literature [16, 20]. Possibly, these characteristics had less impact on therapeutic decisions compared with disease activity parameters. Most of our patients were receiving synthetic DMARDs. This might be explained, in part, by the need to fulfil the reimbursement criteria to be treated with biologics in Belgium. This treatment was comparable in the three time groups, with the exception of fewer patients being treated with LEF in the last time period.
We also report drug survival rates for the first-line biologic therapy in our cohort with ≤18 years of follow-up. Patients in our cohort had a survival rate of 70%, similar in earlier and later time periods. After 15 years of follow-up, more than half of our patients, despite having established disease, were on their first biologic treatment. Long-term persistence of first-line biologics in PsA has been reported in the Danish DANBIO cohort, with 8-year TNFi survival slightly <40% [16], and the British Biologics registry (BSRBR), being at 50% at 8 years of follow-up [21]. French retrospective analysis of first-line TNFi showed a 10-year survival rate of 50% [22]. Despite similar patient characteristics and prior DMARD treatment, the biologic persistence rate in our cohort was higher than reported in the literature.
Although higher TJC, SJC and a higher baseline level of inflammation are predictive of response to anti-TNF therapy [14], drug persistence in our patients with more active established disease was similar to that for the patients with shorter disease duration and lower SJC. High drug persistence in our single-centre university cohort might have been attributable to uniformity of follow-up and choice of treatment modalities, in addition to rigorous patient selection for biologic treatment by the same experienced academic clinicians (K.d.V. and R.L.) over a period of 20 years.
Conclusion
The population of patients selected for treatment escalation has changed significantly over time since the introduction of biologics. Although patient demographics (age, sex and BMI) were similar in all time periods, disease duration and objective disease activity characteristics (swollen and tender joints, psoriatic nail and skin involvement and CRP level) were lower in the later time periods. Our results suggest that in a clinical setting with years of experience, PsA patients might be considered earlier and with less active disease for therapy intensification, to obtain tight disease control and to prevent long-term damage.
Acknowledgements
A.I. is the recipient of a fellowship from PARTNER, an international fellowship program to study disease mechanisms in PsA (www.partner-fellows.de).
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: A.I. is the recipient of a fellowship from PARTNER, an international fellowship program to study disease mechanisms in psoriatic arthritis (www.partner-fellows.de). Leuven Research and Development, the technology transfer office of KU Leuven, has received consultancy, speaker fees and research grants on behalf of R.L. from Abbvie, Amgen (formerly Celgene), Boehringer-Ingelheim, Eli-Lilly, Galapagos, Janssen, Kabi-Fresenius, MSD, Novartis, Pfizer, Biosplice Therapeutic (formerly Samumed), Sandoz and UCB. K.d.V received consultancy, speakers fees and research grants from Amgen (formerly Celgene), MSD, Eli Lilly, Galapagos, Janssen, Kabi-Fresenius, Novartis, Pfizer, Sandoz and UCB. The remaining authors have declared no conflicts of interest.
Data availability statement
This study is based on data collected in a prospective longitudinal observational cohort (BioSPAR) approved by the Ethical Review Board of UZLEUVEN. The authors own the data. Extracts of the data can be shared after written request and approval of the ethical review board and as long as the criteria for GDPR are fulfilled.
References
- 1. Kavanaugh A, Puig L, Gottlieb AB. et al. Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician-reported spondylitis: post-hoc analyses from two phase III, multicentre, double-blind, placebo-controlled studies (PSUMMIT-1/PSUMMIT-2). Ann Rheum Dis 2016;75:1984–8. [DOI] [PubMed] [Google Scholar]
- 2. Mease P, van der Heijde D, Landewé R. et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis 2018;77:890–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Araujo EG, Englbrecht M, Hoepken S. et al. Effects of ustekinumab versus tumor necrosis factor inhibition on enthesitis: Results from the enthesial clearance in psoriatic arthritis (ECLIPSA) study. Semin Arthritis Rheum 2019;48:632–7. [DOI] [PubMed] [Google Scholar]
- 4. Reich K, Gooderham M, Thaçi D. et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet 2019;394:576–86. [DOI] [PubMed] [Google Scholar]
- 5. Schett G, Coates LC, Ash ZR, Finzel S, Conaghan PG.. Structural damage in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: traditional views, novel insights gained from TNF blockade, and concepts for the future. Arthritis Res Ther 2011;13(Suppl 1):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu D, Li C, Zhang S. et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford) 2020;59:3172–80. [DOI] [PubMed] [Google Scholar]
- 7. FitzGerald O, Ritchlin C.. Opportunities and challenges in the treatment of psoriatic arthritis. Best Pract Res Clin Rheumatol 2018;32:440–52. [DOI] [PubMed] [Google Scholar]
- 8. Vandendorpe AS, de Vlam K, Lories R.. Evolution of psoriatic arthritis study patient population characteristics in the era of biological treatments. RMD Open 2019;5:e000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Antoni CE, Kavanaugh A, Kirkham B. et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum 2005;52:1227–36. [DOI] [PubMed] [Google Scholar]
- 10. Mease PJ, Goffe BS, Metz J. et al. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 2000;356:385–90. [DOI] [PubMed] [Google Scholar]
- 11. Kavanaugh A, McInnes I, Mease P. et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum 2009;60:976–86. [DOI] [PubMed] [Google Scholar]
- 12. Mease PJ, Fleischmann R, Deodhar AA. et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 2014;73:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ritchlin C, Rahman P, Kavanaugh A. et al. on behalf of the PSUMMIT 2 Study Group. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 2014;73:990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eder L, Chandran V, Schentag CT. et al. Time and predictors of response to tumour necrosis factor-α blockers in psoriatic arthritis: an analysis of a longitudinal observational cohort. Rheumatology (Oxford) 2010;49:1361–6. [DOI] [PubMed] [Google Scholar]
- 15. Aaltonen K, Heinonen A, Joensuu J. et al. Effectiveness and drug survival of TNF-inhibitors in the treatment of psoriatic arthritis: A prospective cohort study. Semin Arthritis Rheum 2017;46:732–9. [DOI] [PubMed] [Google Scholar]
- 16. Glintborg B, Østergaard M, Dreyer L. et al. Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor α therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheum 2011;63:382–90. [DOI] [PubMed] [Google Scholar]
- 17. Kim HS, Lee S, Kim JH.. Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. J Korean Med Sci 2018;33:e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK.. Psoriatic arthritis (PSA) – an analysis of 220 patients. Q J Med 1987;62:127–41. [PubMed] [Google Scholar]
- 19. Kavanaugh A, Mease PJ, Gomez-Reino JJ. et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis 2014;73:1020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gladman DD, Chandran V.. Observational cohort studies: lessons learnt from the University of Toronto Psoriatic Arthritis Program. Rheumatology (Oxford) 2011;50:25–31. [DOI] [PubMed] [Google Scholar]
- 21. Fagerli KM, Kearsley-Fleet L, Watson KD. et al. Long-term persistence of TNF-inhibitor treatment in patients with psoriatic arthritis. Data from the British Society for Rheumatology Biologics Register. RMD open 2018;4:e000596.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Soubrier AS, Bele-Philippe P, Cortet B. et al. Treatment response, drug survival and safety of anti-tumour necrosis factor a therapy in 193 patients with psoriatic arthritis: a twelve-year “real life” experience. Joint Bone Spine 2015;82:31–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study is based on data collected in a prospective longitudinal observational cohort (BioSPAR) approved by the Ethical Review Board of UZLEUVEN. The authors own the data. Extracts of the data can be shared after written request and approval of the ethical review board and as long as the criteria for GDPR are fulfilled.