Abstract
Objective
Psoriatic nail disease is more common in PsA than in isolated skin psoriasis (PsO). The nail is closely integrated to the DIP joint entheses. US data have shown that those patients with nail disease in PsO are more likely to have systemic enthesitis. We examined whether there was a relationship between nail disease, DIP enthesitis and systemic enthesitis in established PsA.
Methods
Forty-six PsA participants with nail disease underwent US scanning of the nail unit and the DIP entheses along with peripheral entheseal sites according to the Madrid sonographic enthesitis index (MASEI).
Results
At the finger level, there was a mild to moderate correlation between nail US changes and both clinical nail disease and DIP enthesis changes (DIP US) [Spearman correlation (rS) = 0.30, P < 0.001 and rS = 0.16, P < 0.001, respectively]. At the patient level, there was a moderate correlation between the nail US score and nail psoriasis severity index score and DIP US (rS = 0.33, P = 0.024 and rS = 0.43, P = 0.003, respectively). At the patient level, there was also a positive correlation between a higher nail US score and the active peripheral enthesitis score (MASEI-active) (rS = 0.35, P = 0.018). When power Doppler was part of nail US score, similar results were demonstrated at both the finger and patient levels.
Conclusion
This study has demonstrated the utility of nail US imaging and the close relationship, on scanning, between the DIP entheses and the nail unit. In PsA, we have seen a correlation between active US changes at the nail and peripheral enthesitis, which requires further analysis.
Trial registration
ClinicalTrials.gov, https://clinicaltrials.gov, NCT03955861.
Keywords: psoriatic arthritis, onychopathy, psoriasis, ultrasound, enthesitis
Key messages
The nail unit and the DIP joint entheses are integrated on US imaging in PsA.
Active peripheral inflammatory enthesitis appears to be linked to US changes at the nail unit.
Standardization of imaging protocols at the nail and entheses will allow us to characterize PsA more accurately.
Introduction
An enthesis is the insertion of a tendon, ligament, capsule or fascia into bone, and it is implicated in the presentation and prognosis of PsA. Enthesitis is traditionally defined as tenderness, attributable to inflammation, at the site of an enthesis and occurs in ≥30% of patients, with imaging detecting an even higher prevalence of subclinical disease [1]. Those with skin psoriasis (PsO) without arthritis have a higher incidence of subclinical enthesitis on US [1]. It has been suggested that an auto-inflammatory reaction at sites of stress might be a trigger in the development of PsA [2]. This model has been supported by the concept of the entheses organ and its close relationship to the synovium [3].
We know that those with nail disease in PsO are more likely to develop PsA [4]. The nail is functionally integrated with the entheses at the DIP joint, and both US [5] and MRI studies have demonstrated the relationship between the digital extensor tendons and the nail unit in patients with psoriatic disease [6]. The nail is obviously a peripheral site that is predisposed to trauma, and it is therefore an interesting site to examine the importance of the enthesal–synovium complex in PsA. A previous study demonstrated that those with PsO and clinical nail disease had a higher prevalence of US-confirmed systemic enthesitis [7].
In this study, we wished to examine the relationship in those with PsA, to determine whether there were similar findings in established articular disease. We wanted to assess nail disease to clarify whether it was related to enthesitis, both at the local DIP joint and at peripheral sites.
Methods
This study was carried out in a single UK centre in Belfast. It is part of a larger observational trial (clinical trial registration no. NCT03955861) examining the effect of biologic therapy on US-confirmed enthesitis and, as such, all patients recruited were biologic therapy naïve, but were due to commence on biologic treatment. Written informed consent was obtained from all participants according to the Declaration of Helsinki, and the study was approved by the West Midlands–South Birmingham Research Ethics Committee (Research ethics committee Ref18/WM/0369).
Forty-six participants were included. All patients had to be aged ≥18 years and fulfil the classification criteria for PsA (ClASsification criteria for Psoriatic Arthritis (CASPAR)). All patients had clinical fingernail disease, according to the nail psoriasis severity index (NAPSI) score, in at least one fingernail and were able to give informed consent and allow for all 10 fingers to undergo US assessment. Patients were not eligible if they had a history of another current autoimmune rheumatic disease other than PsA, were taking oral CSs or had an i.m. CS injection within 6 weeks of baseline.
Ultrasonography
The US investigator was a rheumatologist with 6 years of experience of musculoskeletal scanning and had European Federation of Societies for Ultrasound in Medicines and Biology (EFSUMB) accreditation. A Toshiba Xario 200 scanner was used for all scans (“Canon, West Sussex, UK”). The investigator was blinded to all clinical findings before scanning the peripheral entheseal sites. All scans were carried out in darkened room, in which it was unlikely to delineate any clinical findings. The patients were asked not to communicate with the scanner during the assessment. After scanning of the peripheral entheseal sites, the clinical fingernail assessment occurred. If a patient had evidence of clinical nail disease (NAPSI ≥ 1) in at least one nail, they then underwent further US imaging of all 10 nail beds. After nail scanning was complete, the remaining clinical assessment occurred.
Entheses scanning protocol
Ultrasonography was carried out with a PLU-1204BT linear (5–18 MHz range) probe. Scanning of the entheses was as per the Madrid sonographic enthesitis index (MASEI) protocol [8], which examines six enthesis locations bilaterally (proximal plantar fascia, distal Achilles tendon, distal and proximal patellar ligaments, distal quadriceps and brachial triceps tendons) in each patient. US examinations of the knee enthesis (quadriceps and patellar sites) were performed with the patient in the supine position, with the knees flexed 30°. For the examination of the Achilles tendon and the plantar fascia, the patient was in the prone position, with the feet hanging over the edge of the examination table at 90° of flexion. Triceps tendon entheses were evaluated with the subjects seated in front of the examiner, shoulders in internal rotation and elbows flexed 90°. The protocol for scanning is included in Supplementary Table S1, available at Rheumatology Advances in Practice online. In brief, each enthesis was evaluated for the following: thickness, structure, calcifications, bursae, erosions and power Doppler signal in both the bursa and at the enthesis insertion. Thickness of the enthesis was measured on the longitudinal plane as the maximum anteroposterior diameter (in millimetres) at the proximal bony insertion.
A structure was defined as pathological if loss of fibrillar pattern, hypoechoic aspect or fusiform thickening of the enthesis occurred; bone erosion was defined as a cortical interruption with a step-down contour defect witnessed on both longitudinal and transverse scan; and an enthesophyte was defined as a step-up bony prominence at the end of normal bone profile. Calcifications were evaluated at the area of the enthesis insertion and classified according to size. Both ossifications and enthesophytes at the enthesis were also included as calcifications according to the protocol.
Blood flow was examined in each enthesis using power Doppler US, the settings of which were standardized with a frequency between 6.6 and 8.8 MHz and a low wall filter. The pulse repetition frequency is automatically set by the Toshiba Xario US machine based on the other parameters to allow for maximum sensitivity with a range between 500 to 1000 Hz, and gain was adjusted to the point where power Doppler signal was not generated under the bone cortex. All power Doppler assessment was carried out with the joint relaxed, including the knee, which was allowed to rest on the examining table, and the Achilles tendon no longer maximally flexed.
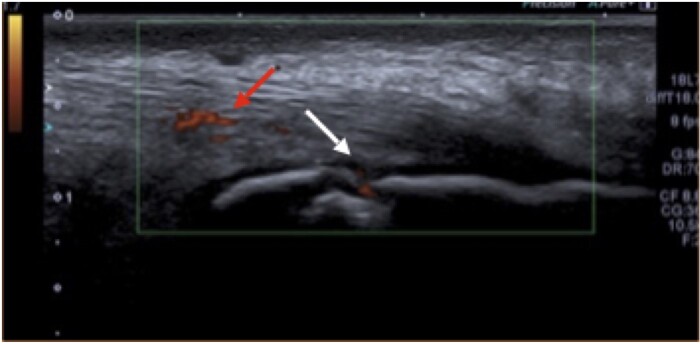
The MASEI score is out of 136. We also broke down the results to assess for the active components that make up the MASEI score (MASEI-active), including a thickened tendon, pathological tendon structure, power Dopplersignal and bursitis. An example of US-confirmed enthesitis at the Achilles tendon is shown in Fig. 1.
Fig. 1.
US of an Achilles tendon with enthesitis
Finger scanning protocol
Patients had all 10 fingers scanned at a grey-scale frequency of 18 MHz in the longitudinal plane. An assessment of the nails, extensor tendons and DIP joints was made by placing the hands on the dorsal side. Inflammation was assessed with power Doppler, with a wall filter of three, Doppler frequency of 8.8 MHz and a pulse repetition frequency range again between 500 to 1000 Hz to allow for maximum sensitivity, and a gain adjusted to the point where power Doppler signal was not generated under the bone cortex.
Nail score
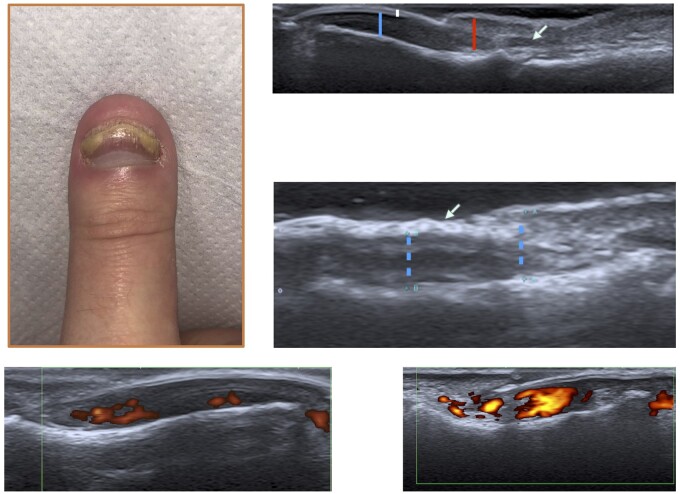
Scanning of the nail unit was as per the measurements previously proposed by members of the Group for Research and Assessment of Psoriasis and PsA (GRAPPA) [9] (Supplementary Fig. S1, available at Rheumatology Advances in Practice online). Accordingly, the nail bed thickness was measured as the distance between the ventral plate and the bone margin of the distal phalanx. The nail matrix thickness was measured at the proximal end of the nail bed up to the ventral portion of the nail skin fold. The matrix envelops the proximal nail plate, with pathological changes extending up to the nail fold; therefore, we felt that measurement of the matrix should include up to the nail fold to capture any changes at this site. The nail plate itself was graded from zero to two based on the Wortsman criteria [10]. A normal nail plate morphology was graded zero. A focal hyperechoic involvement of the ventral plate (type I) or loosening of the borders of the ventral plate (type II) was graded one. Wavy plates (type III) and loss of definition of both plates (type IV) were graded two. We also assessed the power Doppler signal at the nail unit. This was graded based on previous work by Gutierrez et al. [11]. The scoring was as follows: if no significant signal was detected, it was graded as zero; if a confluent signal was detected in <50% of the nail bed area, this was graded as one, and if a confluent signal was seen in >50% of the nail bed area, a grade of two was given. A semi-quantitative nail score was developed by adding the measurement of the nail matrix and nail bed (in millimetres) to the grade of the nail plate (Nail US). We then added the Doppler score to this total to obtain a Nail USD score. This was to allow comparison, because the standardization of nail power Doppler scanning has not been clarified, and we wanted to assess the impact on the overall results. An example of US nail scanning and pathology, including power Doppler grading, is shown in Fig. 2.
Fig. 2.
Nail disease US imaging
DIP joint score (DIPUS)
The DIP was graded from zero to three where the extensor tendon is attached to the distal phalanx of the joint looking at the bone profile, tendon insertion and power Doppler. In accordance with Outcome Measures in Rheumatology (OMERACT) for entheses [12], a score of one was given if there was evidence at the enthesis insertion of a loss of normal fibrillary architecture or a thickened tendon. A further score of one was given if there was evidence of enthesophytes at the bony insertion or other bony changes, including erosions or calcifications. Finally a score of zero or one was given for the absence or presence of power Doppler signal at the extensor tendon enthesis insertion. This resulted in a maximum score of 30.
Clinical assessment
The clinical assessment included the NAPSI, which was carried out after scanning the peripheral entheseal sites but before the nail scanning. The psoriasis area and severity index (PASI), tender/swollen 68/66 joint score, patient global activity visual analogue score (VAS), disease activity in PsA (DAPSA), Leeds enthesitis index (LEI) and the Spondyloarthritis Research Research Consortium of Canada enthesitis index (SPARCC) were also calculated. Assessment was carried out by the same trained rheumatologist who had carried out the US assessment.
Assessment of inter- and intra-observer variation
All US images were stored and reassessed. For each patient, the MASEI scores, DIP joint grading and nail unit US was analysed separately to allow for comparison. This also served as an intra-observer variation analysis for all 460 images. Three patients had their stored nail and DIP (n = 30) images reassessed by a second investigator to allow for an analysis of inter-observer variation.
Statistical analysis
Continuous variables are presented as a mean value with S.d. The Spearman correlation (rS) was used to analyse the relationship between the NAPSI, Nail US, Nail USD, DIPUS and MASEI score. This was performed at both the patient level, with an overall score for all 10 nails when comparing finger findings with peripheral enthesitis, and also for each individual nail for all 46 patients (460 nails) when assessing only finger characteristics. We also assessed differences in those nails with or without nail disease after differences in subjects were taken into consideration. To determine whether there was a significant difference in Nail US scores, we used a general linear model, with the patient as a random categorical variable and NAPSI as a covariate. For the ordinal DIP score outcome, we used an ordinal regression analysis to compare results depending on presence or absence of nail disease. A value of P < 0.05 was considered statistically significant. For inter- and intra-observer analysis, continuous variables were assessed using a coefficient repeatability score to demonstrate the smallest possible change that would be deemed to represent a true difference in the observations. For the ordinal DIP US values, a linear weighted κ was used to assess both inter- and intra-observer agreement. Statistical analyses were performed using the IBM SPSS Statistics v.26.0.
Results
Clinical characteristics
Of the 46 patients, 26 (56.5%) were female; the mean (S.d.) age was 44.7 (12.02) years, BMI was 28.88 (6.20) kg/m2, and average disease duration was 7.23 (6.05) years. Of the 46 patients, 26 were on DMARDs (Table 1).
Table 1.
Baseline characteristics
| Patient characteristics (n = 46) | Results |
|---|---|
| Female, n (%) | 26 (56.5) |
| BMI, kg/m2 | 28.88 (6.20) |
| Age, years | 44.7 (12.02) |
| Duration of PsA, years | 7.23 (6.05) |
| Number on DMARD, n (%) | 26 (56.5) |
| MTX | 19 |
| SSZ | 4 |
| LEF | 2 |
| MTX and SSZ | 1 |
| Tender joint score/68 | 11.37 (11.59) |
| Swollen joint score/66 | 4.10 (4.71) |
| Patient global activity VAS/100 | 54.00 (23.92) |
| PASI | 3.01 (4.12) |
| LEI/6 | 0.91 (1.15) |
| SPARCC/16 | 2.28 (2.08) |
| DAPSA | 27.21 (16.80) |
| NAPSI/80 | 11.09 (10.57) |
Results are given as the mean (S.d.) unless stated otherwise.
DAPSA: disease activity in psoriatic arthritis; LEI: Leeds enthesitis index; NAPSI: nail psoriasis severity index; PASI: psoriasis area and severity index; SPARCC: Spondyloarthritis Research Consortium of Canada enthesitis index; VAS: visual analogue score.
The mean PASI score was 3.01 (4.12), tender joint 11.37 (11.59), swollen joint 4.10 (4.71), patient global VAS score 54.00 (23.92), LEI 0.91 (1.15), SPARCC 2.28 (2.08), and a NAPSI score of 11.09 (10.57). The DAPSA composite disease score mean was 27.21 (16.80).
US
There was excellent intra- and inter-observer reliability for both finger and peripheral enthesitis scanning. In terms of the MASEI score and MASEI-active score, the intra-observer coefficient repeatability (CR) measurement was r = 1.87 and r = 0.65, respectively. In summary, for the MASEI score, which has a maximum value of 136, the true difference in intra-reader measurements is 1.87 (95% CI). For the nail scanning, the intra-observer CR for Nail US and Nail USD was r = 0.34 and r = 0.50, respectively. The inter-observer CR for Nail US and Nail USD was r = 0.458 and r = 0.687, respectively. For the DIP readings, the linear weighted κ for the intra-reader and inter-reader analysis was 0.92 (0.89–0.95) and 0.79 (0.63–0.95), respectively.
The mean score for each patient for the MASEI and MASEI-active was 21.30 (10.16) and 9.43 (5.52), respectively. The Nail US, Nail USD and DIPUS was 54.35 (8.75), 62.61 (10.02) and 6.17 (6.02), respectively (Table 2).
Table 2.
US characteristics
| US (n = 46) | Result, mean (S.d.) |
|---|---|
| MASEI | 21.30 (10.16) |
| MASEI-active | 9.43 (5.52) |
| DIPUS | 6.17 (6.02) |
| Nail US | 54.35 (8.75) |
| Nail USD | 62.61 (10.02) |
DIPUS: DIP US score; MASEI: Madrid sonographic enthesitis index; MASEI-active: active elementary lesions of the Madrid sonographic enthesitis index; Nail US: nail US score; Nail USD: nail US score with power Doppler assessment.
Supplementary Table S2, available at Rheumatology Advances in Practice online, demonstrates a breakdown of all entheseal elementary lesions noted at each enthesis site as per the MASEI score. Supplementary Tables S3 and S4, available at Rheumatology Advances in Practice online, describe the prevalence of the various lesions noted on US at the extensor tendon entheses and nail unit.
The relationship between the nail, DIP entheses and systemic enthesitis at the patient level is shown in Table 3, and the relationship between the nail and DIP entheses at the finger level is shown in Table 4.
Table 3.
The relationship between the nail, DIP entheses and systemic enthesitis at the patient level
| Relationship | n = 46 | r S (95% CI) | P-value |
|---|---|---|---|
| Witdout Doppler signal as part of tde nail US score | |||
| Nail disease | NAPSI–Nail US | 0.33 (0.04, 0.57) | 0.024 |
| Nail and systemic enthesitis | NAPSI–MASEI | 0.12 (−0.18, 0.40) | 0.418 |
| NAPSI–MASEI-active | 0.22 (−0.08, 0.48) | 0.148 | |
| Nail US–MASEI | 0.25 (−0.04, 0.50) | 0.099 | |
| Nail US–MASEI-active | 0.35 (0.07, 0.58) | 0.018 | |
| Nail and DIP | NAPSI–DIPUS | 0.09 (−0.21, 0.37) | 0.548 |
| Nail US–DIPUS | 0.43 (0.16, 0.64) | 0.003 | |
| DIP and systemic enthesitis | DIPUS–MASEI | 0.19 (−0.11, 0.46) | 0.196 |
| DIPUS–MASEI-active | 0.09 (−0.21, 0.37) | 0.554 | |
| With Doppler signal as part of the nail US score at the patient level | |||
| Nail disease | NAPSI and Nail USD | 0.34 (0.06, 0.57) | 0.020 |
| Nail and enthesitis | Nail USD–MASEI | 0.27 (−0.02, 0.52) | 0.070 |
| Nail USD–MASEI-active | 0.36 (0.08, 0.59) | 0.013 | |
| Nail and DIP | Nail USD—DIPUS | 0.44 (0.17, 0.65) | 0.002 |
All significant correlations are shown in bold.
DIPUS: DIP US score; MASEI: Madrid sonographic enthesitis index; MASEI-active: active elementary lesions of the Madrid sonographic enthesitis index; Nail US: nail US score; Nail USD: nail US score with power Doppler assessment; NAPSI: nail psoriasis severity index; rS: Spearman correlation.
Table 4.
The relationship between the nail and DIP entheses at the finger level
| Relationship | n = 460 | r S (95% CI) | P-value |
|---|---|---|---|
| Nail disease | NAPSI and Nail US | 0.30 (0.22, 0.38) | <0.001 |
| NAPSI and Nail USD | 0.27 (0.18, 0.35) | <0.001 | |
| Nail and the DIP entheses | DIPUS and Nail US | 0.16 (0.07, 0.25) | 0.001 |
| DIPUS and Nail USD | 0.18 (0.09, 0.27) | <0.001 | |
| DIPUS and NAPSI | 0.05 (−0.04, 0.14) | 0.285 |
All significant correlations are shown in bold.
DIPUS: DUP US score; Nail US: nail US score; Nail USD: nail US score with power Doppler assessment; NAPSI: nail psoriasis severity index; rS: Spearman correlation.
The nail and the DIP entheses
There was a weakly positive correlation between the NAPSI and nail US changes (Nail US) at the finger level (rS = 0.30, P < 0.001), and this was also correlated at the patient level overall (rS = 0.33, P = 0.024). There was a moderately significant relationship between the DIP enthesitis changes (DIPUS) and the Nail US at the patient level, with a weaker correlation at the finger level (rS = 0.43, P = 0.003 and rS = 0.16, P = 0.001, respectively). There was no significant relationship with nail disease alone (NAPSI) and the DIPUS score at patient or the finger level (rS = 0.09, P = 0.548 and rS = 0.05, P = 0.285, respectively).
The nail and peripheral systemic enthesitis
There was a mild to moderately significant correlation between the Nail US and the active component of the MASEI score (MASEI-active; rS = 0.35, P = 0.018). The Nail US score had a positive correlation with an overall MASEI score, but this was not statistically significant (rS = 0.25, P = 0.099). In terms of NAPSI and both MASEI and MASEI-active (rS = 0.12, P = 0.418 and rS = 0.22, P = 0.148 respectively), a higher nail score appeared to be more closely linked, albeit weakly, to a higher active peripheral enthesitis score, but again this was not statistically significant.
Enthesitis at the DIP joint and systemic enthesitis
There was a very weak positive correlation between the degree of DIP enthesitis and peripheral enthesitis, but this did not reach significance for either the overall MASEI or the MASEI-active score (rS = 0.19, P = 0.196 and rS = 0.09, P = 0.554, respectively).
Power Doppler
When power Doppler was added to the Nail US assessment (Nail USD), the strength of relationship with active peripheral enthesitis, NAPSI and the DIPUS score was similar at the patient level (rS = 0.36, P = 0.014, rS = 0.34, P 0.022 and rS = 0.44, P = 0.002, respectively) to the scoring without power Doppler. Similar weak correlation patterns were also seen at the finger level for Nail USD and its relationship with the DIPUS score and NAPSI assessment (rS = 0.18, P < 0.001 and rS = 0.27, P < 0.001, respectively) in comparison to without power Doppler assessment. The Nail USD and its relationship with the overall MASEI score was weakly positive but not statistically significant (rS = 0.27, P = 0.074).
Comparing outcomes based on presence or absence of nail disease
When comparing outcomes at the finger level based on those nails with a NAPSI ≥ 1 vs no psoriatic nail disease, the Nail US and Nail USD were both 0.27 points higher in those nails with psoriatic changes vs those without [0.27 (0.08–0.47), P = 0.007 and 0.27 (0.03–0.52), P = 0.030, respectively]. The DIP score was 0.12 points higher in the nail disease group at the finger level, but this was not statistically significant [0.12 (−0.39 to 0.63, P = 0.65)].
Discussion
This is the first study, to our knowledge, that has assessed the relationship between nail disease and systemic peripheral enthesitis in established PsA. A previous study in psoriasis and pre-articular disease did not find a significant correlation between DIP enthesitis and Achilles tendon enthesitis [13]. In this study, we attempted to assess enthesitis both peripherally, at multiple sites, and at all fingers. We wanted to investigate the argument that in those with more significant nail disease, both clinically and on imaging, there was a relationship with enthesitis, both locally and systemically.
We were initially able to demonstrate the feasibility and repeatability of using US to assess the nail bed and that it was correlated with clinical nail disease. Once again, we witnessed the relationship on imaging between DIP joint entheses and the nail unit. This supports the idea that imaging gives us a more detailed picture of what is occurring subclinically, and US has been shown to be a valuable tool to assess multiple domains in PsA and, potentially, help to stratify patients. Previous work has demonstrated a relationship between clinical nail disease and DIP enthesitis at the finger level but not the patient level in PsA [14]; however, we did not see a relationship at either level between clinical nail disease and DIP enthesitis.
We observed a relationship between changes at the nail unit on US and the active component of the MASEI score, which was a surrogate for active systemic enthesitis. We also demonstrated that those with a higher NAPSI score had a positive but not significant correlation with active systemic enthesitis. Changes at the nail matrix, nail bed and nail plate morphology on US reflect active disease and respond to treatment, as do the active lesions of enthesitis on US assessment. These subclinical changes that we detected are interesting, but we need to understand what they mean, both in terms of active disease and with chronic damage over time. A more detailed analysis of active peripheral enthesitis, including a rating of Doppler signal, might yield a clearer relationship. Classifying elemental lesions that make up the NAPSI score and how they relate to enthesitis might also yield interesting results and help to stratify patients.
Nail power Doppler signal and its relevance to disease is still under debate. The power Doppler rating that was also assessed and added to the Nail US score was based on a modified version of previous work by Gutierrez et al. [11], in which they had noted that power Doppler was not rated at the nail bed unless the nail bed measures >3 mm. Although this value will yield high sensitivity for an abnormal nail bed, it is still at this stage arbitrary because nail US is a new research tool. Angiogenesis dysregulation is closely linked to PsA and its pathogenesis; therefore, we chose to investigate power Doppler. We felt that this cohort, who had some degree of nail disease, were more likely to have subclinical findings even in those neighbouring clinically normal nails. We wanted to study how this correlated with US scoring and found that it served to reinforce but not necessarily add to the relationship with the DIP entheses and widespread active enthesitis. Studies to date have noted a wide variation in Doppler signal detected at the nail bed [15] and a lack of definite correlation with clinical improvement [16]. The cut-off point for nail matrix measurements is also an area of debate, with our work using the nail fold as detailed in the Methods section. All this highlights a need, as imaging technology evolves, to standardize and validate nail US scanning.
This study was limited by the fact that it was performed in a single centre, and we did not have a comparator arm including conditions such as OA. We also used scores that were developed for this study and are adapted from previous work by experts in the field but have not been validated elsewhere, such as the DIPUS and Nail US tools. Further work is needed to clarify whether the relationship between clinical nail disease and active systemic enthesitis, which has been demonstrated in psoriasis, is a pre-articular phenomenon. Nail disease might also be a manifestation of a particular cohort of PsA patients in whom an auto-inflammatory trigger, owing to local tissue factors, is a driver of disease development.
Funding: This study has been supported by funding from the Psoriasis and Psoriatic Alliance and the British Medical Association.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
The data underlying this article are available in this article, and any extra information will be shared upon reasonable request to the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology Advances in Practice online.
Supplementary Material
References
- 1. Macchioni P, Salvarani C, Possemato N. et al. Ultrasonographic and clinical assessment of peripheral enthesitis in patients with psoriatic arthritis, psoriasis, and fibromyalgia syndrome: the ULISSE study. J Rheumatol 2019;46:904–11. [DOI] [PubMed] [Google Scholar]
- 2. McGonagle D. Enthesitis: an autoinflammatory lesion linking nail and joint involvement in psoriatic disease. J Eur Acad Dermatol Venereol 2009;23:9–13. [DOI] [PubMed] [Google Scholar]
- 3. Kaeley GS, Eder L, Aydin SZ, Gutierrez M, Bakewell C.. Enthesitis: a hallmark of psoriatic arthritis. Semin Arthritis Rheum 2018;48:35–43. [DOI] [PubMed] [Google Scholar]
- 4. Langenbruch A, Radtke MA, Krensel M. et al. Nail involvement as a predictor of concomitant psoriatic arthritis in patients with psoriasis. Br J Dermatol 2014;171:1123–8. [DOI] [PubMed] [Google Scholar]
- 5. Aydin SZ, Castillo-Gallego C, Ash ZR. et al. Ultrasonographic assessment of nail in psoriatic disease shows a link between onychopathy and distal interphalangeal joint extensor tendon enthesopathy. Dermatology 2012;225:231–5. [DOI] [PubMed] [Google Scholar]
- 6. Scarpa R, Soscia E, Peluso R. et al. Nail and distal interphalangeal joint in psoriatic arthritis. J Rheumatol 2006;33:1315–9. [PubMed] [Google Scholar]
- 7. Ash ZR, Tinazzi I, Gallego CC. et al. Psoriasis patients with nail disease have a greater magnitude of underlying systemic subclinical enthesopathy than those with normal nails. Ann Rheum Dis 2012;71:553–6. [DOI] [PubMed] [Google Scholar]
- 8. De Miguel E, Cobo T, Muñoz-Femández S. et al. Validity of enthesis ultrasound assessment in spondyloarthropathy. Ann Rheum Dis 2009;68:169–74. [DOI] [PubMed] [Google Scholar]
- 9. Cunha JS, Qureshi AA, Reginato AM.. Nail enthesis ultrasound in psoriasis and psoriatic arthritis: a report from the 2016 GRAPPA annual meeting. J Rheumatol 2017;44:688–90. [DOI] [PubMed] [Google Scholar]
- 10. Gutierrez M, Wortsman X, Filippucci E. et al. High-frequency sonography in the evaluation of psoriasis: nail and skin involvement. J Ultrasound Med 2009;28:1569–74. [DOI] [PubMed] [Google Scholar]
- 11. Gutierrez M, Di geso L, Salaffi F. et al. Development of a preliminary US power Doppler composite score for monitoring treatment in PsA. Rheumatology (Oxford) 2012;51:1261–8. [DOI] [PubMed] [Google Scholar]
- 12. Terslev L, Naredo E, Iagnocco A. et al. Defining enthesitis in spondyloarthritis by ultrasound: results of a delphi process and of a reliability reading exercise. Arthritis Care Res 2014;66:741–8. [DOI] [PubMed] [Google Scholar]
- 13. Moya Alvarado P, R, Crespo E, Muñoz-Garza FZ. et al. Subclinical enthesopathy of extensor digitorum tendon is highly prevalent and associated with clinical and ultrasound alterations of the adjacent fingernails in patients with psoriatic disease. J Eur Acad Dermatology Venereol 2018;32:1728–36. [DOI] [PubMed] [Google Scholar]
- 14. Acosta-Felquer ML, Ruta S, Rosa J. et al. Ultrasound entheseal abnormalities at the distal interphalangeal joints and clinical nail involvement in patients with psoriasis and psoriatic arthritis, supporting the nail-enthesitis theory. Semin Arthritis Rheum 2017;47:338–42. [DOI] [PubMed] [Google Scholar]
- 15. Fassio A, Giovannini I, Idolazzi L. et al. Nail ultrasonography for psoriatic arthritis and psoriasis patients: a systematic literature review. Clin Rheumatol 2020;39:1391–404. [DOI] [PubMed] [Google Scholar]
- 16. Gutierrez M, Di geso L, Salaffi F. et al. Development of a preliminary US power Doppler composite score for monitoring treatment in PsA. Rheumatology (Oxford) 2012;51:1261–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in this article, and any extra information will be shared upon reasonable request to the corresponding author.