Abstract
Objectives
The main aim of this work was to analyse the potential tumour growth inhibition effects of (−)-epicatechin (EC). Triple-negative breast cancer (TNBC) is an invasive form of cancer characterized by the absence of progesterone receptor, estrogen receptor and human epidermal growth factor receptor 2. Doxorubicin (DOX) is widely used for its anti-tumour activity. EC belongs to the flavanol subfamily and is a candidate molecule for the adjuvant treatment of cancer due to its antiproliferative activities.
Methods
Evaluation of EC effects and pathways involved in a model of TNBC.
Key findings
EC inhibited tumour growth as efficiently as DOX (inhibition rates of 74% and 79% for EC and DOX, respectively). The evaluation of adenosine monophosphate-activated protein kinase (AMPK) and Akt phosphorylation and mTOR expression indicates that EC modulates these pathways, resulting in the inhibition of cell proliferation. Additionally, we found an increase in the survival of EC-treated animals compared with control-treated animals. This effect was similar to the effects induced by DOX (survival rates of 44% and 30% for EC and DOX, respectively).
Conclusion
EC has antiproliferative properties and increases survival in a model of TNBC. These effects may occur through the modulation of deregulated AMPK and Akt/mTOR signalling pathways.
Keywords: epicatechin, mammary gland, tumour, survival
Introduction
Breast cancer is the most common malignant disease in women and is among the leading causes of cancer-related death in women worldwide. According to GLOBOCAN (2018), breast cancer ranked first among all types of cancer in women (11.6%) worldwide (https://gco.iarc.fr/today/data/factsheets/cancers/20-Breast-fact-sheet.pdf), accounting for 2 088 848 new cases and 626 679 deaths in 2018.
The Pan American Health Organization and the World Health Organization (PAHO/WHO) estimate that by 2030, its incidence in America will increase by 46%.[1] Among the different breast cancer subtypes, triple-negative breast cancer (TNBC), which is more prevalent among young women, accounts for 10–20% of newly diagnosed breast cancer cases; it is the most aggressive form of breast cancer[2] and has a poorer prognosis compared with that of other breast cancer subtypes. TNBC is negative for estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) expression. Usually, there is no specific therapy available for patients with this cancer subtype, and they have a poor prognosis.[3]
In the absence of therapeutic targets, chemotherapy plays a vital role in treatment, and many efforts have been made looking for combinations of chemotherapy and new drugs. Doxorubicin (DOX), an anthracycline with anticancer activity, is one of the most effective chemotherapeutic agents against different types of solid tumours, including breast cancer.[4, 5] DOX triggers cell death by apoptosis and necrosis. Its effects are achieved by its interaction with DNA by intercalation and inhibition of topoisomerase II, preventing the DNA double helix from being reformed and stopping the process of replication and transcription. It also increases free radical production, hence contributing to its cytotoxicity. Some of the adverse effects are myelosuppression, nausea/vomiting, mucositis, diarrhoea and cardiotoxicity that progresses to congestive heart failure, which could cause death in patients years after receiving antineoplastic treatment.[6, 7]
The search for new molecules that contribute to breast cancer treatment and reduce side effects is continuous and mandatory. Polyphenols in general and flavonoids, in particular, have attracted attention as anticancer molecule options.[8, 9]
Previously, our group showed that (−)-epicatechin (EC), a flavonoid that belongs to the flavonoid subfamily, possesses diverse pharmacological attributes, including antioxidant and anti-inflammatory effects; also EC supports mitochondrial structure and function in different tissues and cell types, such as skeletal muscle, endothelial cells and cardiomyocytes.[10–15]
EC anti-tumoural effects have been studied in many cancer cell lines.[16–18] Recently, we showed through an isobolographic analysis that EC inhibits the proliferation of A459 lung cancer cells in a concentration-dependent manner and acts synergistically when combined with cisplatin.[19] This effect was also observed in combination with bleomycin in ovarian cancer cells[20] and etoposide as an antileukemic agent.[21] Nevertheless, the exact mechanism by which EC exerts its anti-tumour effects without affecting healthy cells is still under study.
On the other hand, it has been reported that AMPK inhibits cell proliferation and tumour growth by modulating the mTOR pathway and decreasing cyclin D1. Consequently, the present work evaluates the effects of EC, as an anticancer molecule modulating AMPK and Akt/mTOR pathways; this study utilized the triple-negative cell line 4T1 to generate a model of breast tumours, and DOX was used as a positive antineoplastic control.[22]
The main aim of this work was to analyse the potential tumour growth inhibition effects of (−)-EC and possible additive effects with DOX.
We hypothesized that EC would provide a new alternative for the treatment of breast cancer, improving survival and maybe the quality of life
Materials and Methods
4T1 cell line culture
4T1 mammary carcinoma is a transplantable tumour cell line that is highly tumourigenic and invasive, and unlike most tumour models, those generated with 4T1 cells can spontaneously metastasize; this cell type was isolated initially by Fred Miller and colleagues.[23, 24] The 4T1 breast cancer model used here is a basal type, triple-negative ductal carcinoma negative for ER/PR and HER-2.[22] Cells were obtained from ATCC (CRL-2539) and cultured at 37°C in a chamber under an atmosphere of 5% CO2.
Model of mammary tumour
Female BALB/c mice of 20–25 g were used. Mice were maintained with access to food and water ad libitum. To induce breast tumour development, 5 × 103 4T1 cells were subcutaneously inoculated in 10 mice per group in the abdominal mammary gland as previously described by Pulaski and Ostrand-Rosenberg.[25] Animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee and carried out under the N.I.H. Guide for the Care and Use of Laboratory Animals (National Research Council, 2011, https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf) and official Mexican regulations (Diario Oficial de la Federacion (NOM-062-ZOO-199, 1999).[26]
Tumour growth was assessed morphometrically using Vernier callipers, and tumour volumes were calculated according to the formula V = (length) (width) (height). Seven days post-inoculation, tumours were palpable, and mice were randomly assigned to 6 groups: (1) the control group (n = 10) was treated with vehicle (sterilized saline solution); (2) the DOX group (n = 10) was treated with DOX administered by intraperitoneal injection (2 mg/day every other day, cumulative dose of 12 mg/kg; (6 doses in total); (3–5) the EC groups (n = 10 each) were treated with 1, 2 or 3 mg/kg/day of (−)-EC by oral gavage for 15 days; and (6) the DOX plus EC group (n = 10) was treated with DOX (2 mg/day every other day, cumulative dose of 12 mg/kg) and EC 3 mg/kg/day for 15 days.
Animal body weight and tumour size (length and width) were measured and recorded every other day. Mice were euthanized 21 days after the injection of tumour cells. Breast tumours were excised, measured (length, width and height), divided into two parts and homogenized for western blot analysis (n = 10) or formalin-fixed, embedded in paraffin and cut into 3.5 µm sections (n = 10) for H&E staining and immunohistochemical techniques using antibodies against cyclin D1 and Ki67; staining was examined by optical microscopy (Nikon Eclipse E600). Immunostaining was performed following the next methodology: Deparaffinized slides were quenched for endogenous peroxidase with 3% H2O2 in methanol. Antigen was retrieved by heating (20 min) in a microwave oven. Blocking of unspecific bindings was performed with 1% bovine serum albumin in phosphate-buffered saline (30 min). The sections were exposed to a 1:50 dilution of anti-cyclin D1 antibody or a 1:150 dilution of anti-Ki67 antibody. Blocking solution was used as a negative control. A peroxidase-labelled secondary antibody was used. Slides were developed with diaminobenzidine reagent. At least 5 photographs were taken randomly per tumour and analysed using ImageJ software.
Survival analysis
Based on tumour volume analysis, a survival test[25] was implemented. In a separate group, 7 days after 4T1 cell inoculation, mice were randomly assigned to treatment groups: (1) vehicle (n = 10), (2) DOX [2 mg/day every other day, cumulative dose of 12 mg/kg] (n = 10), (3) EC [3 mg/kg/day until death (n = 10)] or (4) combination of DOX [2 mg/day every other day, cumulative dose of 12 mg/kg] plus EC [3 mg/kg/day until death (n = 10)]; then, mortality was monitored until all mice died.
Western blot analysis
Based on the results obtained in the volume and survival analyses, where an apparent anti-tumoral EC-induced effect was present, but no additive (acting throughout the same pathway) effects when combined with DOX, in the remaining experiments, we compared only the EC effects vs control.
Based on the reports by Ramirez-Sanchez et al.,[11, 15] we used the following methodology: Mouse breast tumours from different groups were excised and immediately frozen (−80°C until analysis). Tumour tissues were homogenized on ice for 15 s using a polytron and lysis buffer (10% glycerol, 1% NP-40, 20 mM Na-pyrophosphate, 150 mM NaCl, 50 mM HEPES, 20 mM β-glycerol phosphate, 10 mM NaF, 2 mM PMSF, 1 mM EDTA, 1 mM EGTA, 2 mM sodium orthovanadate; pH 7.5 with protease and phosphatase inhibitors). Homogenates were sonicated for 20 min at 4°C and further centrifuged (13 000 g) for 20 min at 4°C. The total protein content in the supernatant was determined via the Bradford method. A total of 30 µg of protein per sample was loaded onto a 4–15% polyacrylamide gel and electrotransferred to a polyvinyl membrane at 18 V for 45 min using a semidry transfer system. Membranes were incubated for 1 h in blocking solution (5% non-fat dry milk in Tris-buffered saline [TBS] plus 0.1% Tween 20 [TBS-T]), followed by overnight incubation at 4°C with primary antibodies against AMPK and Akt and their phosphorylated forms and mTOR1. All antibodies were acquired from Santa Cruz Biotechnology, Santa Cruz, CA, USA. Membranes were washed (3× for 5 min) in TBS-T and incubated for 1 h at 4°C in the presence of horseradish peroxidase-conjugated secondary antibodies diluted 1:1000 in TBS-T. Membranes were again washed in TBS-T, and the immunoblots were developed using an enhanced chemiluminescence detection kit (ImmunoCruz Western Blotting Luminol Reagent). The densitometric intensity of each band was measured using ImageStudio, dividing the value by the corresponding β-tubulin densitometric intensity and these values were divided (phosphorylated/total forms) when activation was analysed (EC vs control).
Materials
Doxorubicin (doxorubicin hydrochloride, 98–102%) was obtained from Sigma-Aldrich, Merck, Co. (Cat. No. D1515).
(−)-EC (>98%) was obtained from Sigma-Aldrich, Merck, Co. (Cat. No. E4018). EC was recrystallized using ethanol.
Statistical analysis
Differences were assessed using ANOVA and Tukey’s post hoc test. Student’s t-test was used as needed. Survival curves (Kaplan–Meier) were evaluated by a log-rank test (Mantel–Cox). Data are expressed as the mean ± SEM, and P ≤ 0.05 was considered significant. Fisher’s exact test was used with categorical data.
Results
(−)-Epicatechin as an inhibitor of tumour growth
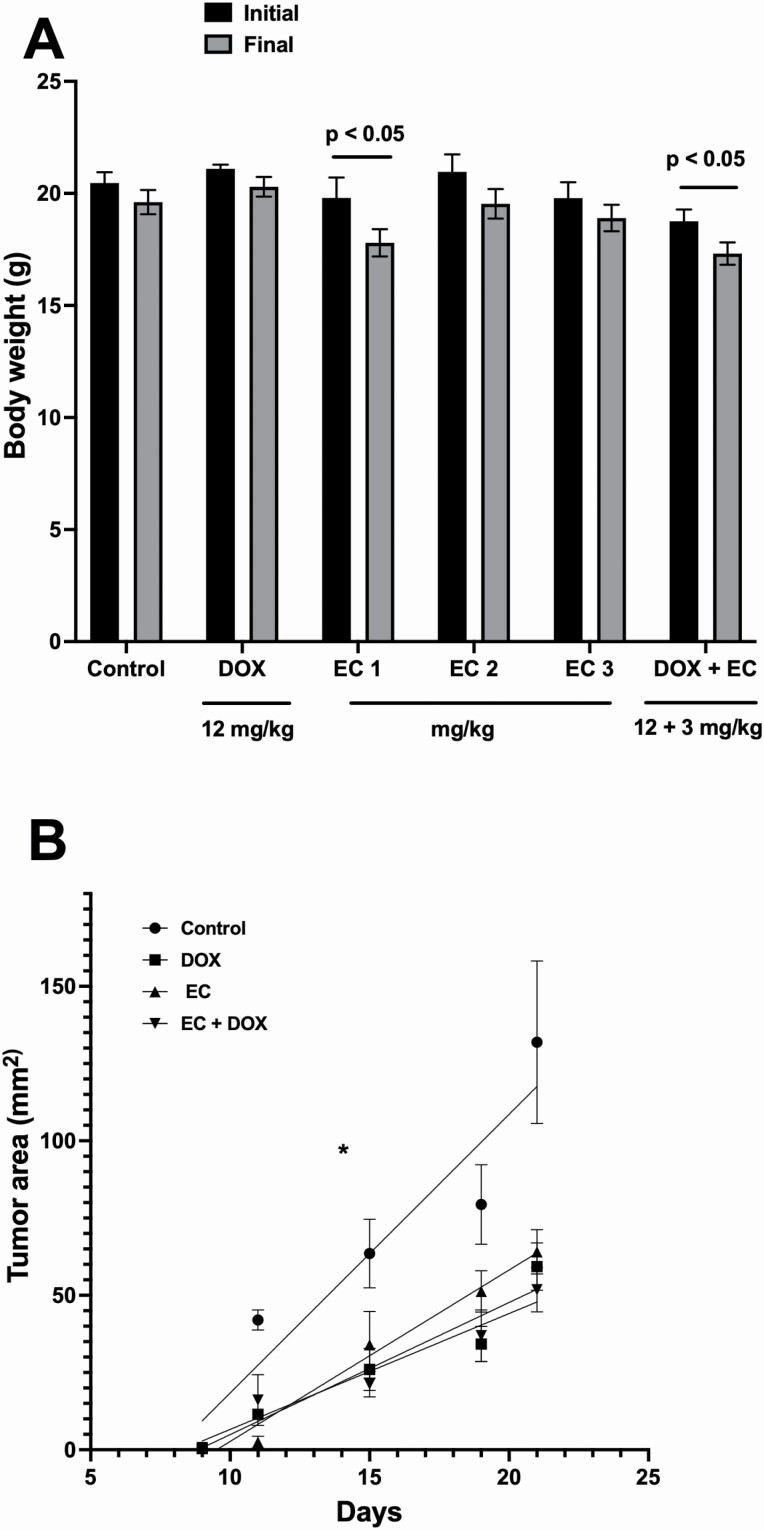
The body weight change in the different groups is shown in Figure 1A. The comparative changes in tumour area (length × width) are shown in Figure 1B.
Figure 1.
(A) Treatment-induced body weight changes, initial (black bars) and 21 days after the injection of 4T1 cells (final). Data are expressed as mean ± SEM. n = 10. (B) Analysis of changes in tumour area (length × width in mm2). Data are expressed as mean ± SEM. n = 10, *P < 0.05.
Our results showed that treatment with DOX limited tumour growth up to 79% compared with that in animals that did not receive treatment. Interestingly, compared with the control treatment, EC showed a tumour growth inhibition effect, with 27, 70 and 74% decreases in tumour volume with the administered doses of 1, 2 and 3 mg/kg/day, respectively.
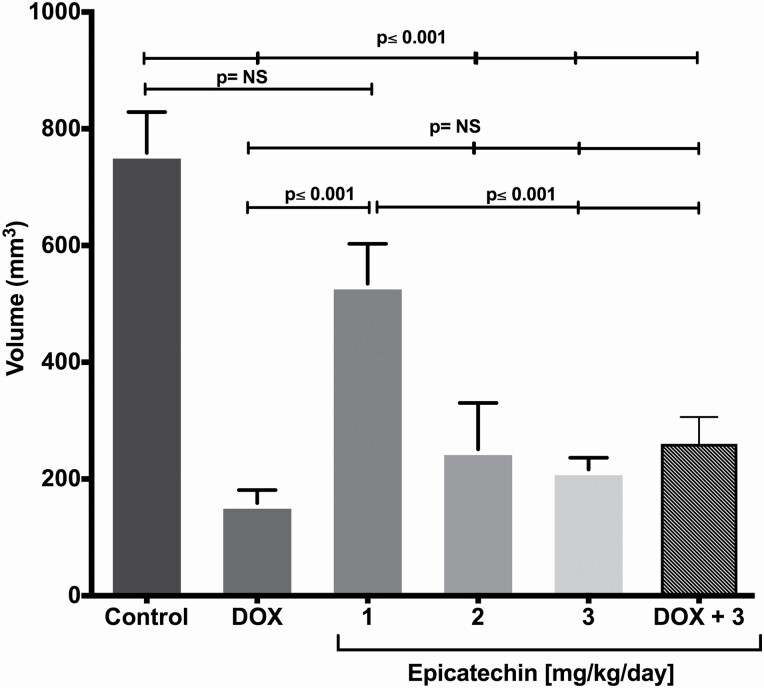
EC doses of 2 and 3 mg/kg/day were as effective as DOX since there were no significant differences among these groups (Figure 2). Since EC at 3 mg/kg/day induced the maximal effect, in the next set of experiments, only this dose was assayed.
Figure 2.
(−)-Epicatechin-induced effects on breast cancer volume. The volume (mm3) obtained 21 days after administration by orthotopic route of 5 × 103 4T1 cells is observed in BALB/c mouse. Doxorubicin (DOX) was used as positive control (12 mg/kg), this presented a smaller tumour volume compared with the control. Increasing doses of EC 1, 2 and 3 mg/kg were administered daily during 15 days. Doses of 2 and 3 mg/kg showed a decrease in tumour volume compared with the control and with the dose of 1 mg/kg. Each bar represents the means ± SEM. n = 10. The data were analysed of variance analysis (ANOVA) followed by the Tukey’s multiple comparisons test. P values <0.05 were statistically significant.
Efficacy of the combination of doxorubicin and (−)-epicatechin
EC was administered simultaneously with DOX (EC 3 mg/kg/day + DOX 12 mg/kg cumulative dose), and the results showed a decrease in tumour size when compared with that in the control group (P = 0.0001); however, the tumour growth in the combination group was not significantly different from that in the groups that were treated with DOX (2 mg/day every other day, cumulative dose of 12 mg/kg) or EC (3 mg/kg/day) alone (Figure 2), suggesting no additive effects of DOX and EC
Effect of (−)-epicatechin on mouse survival
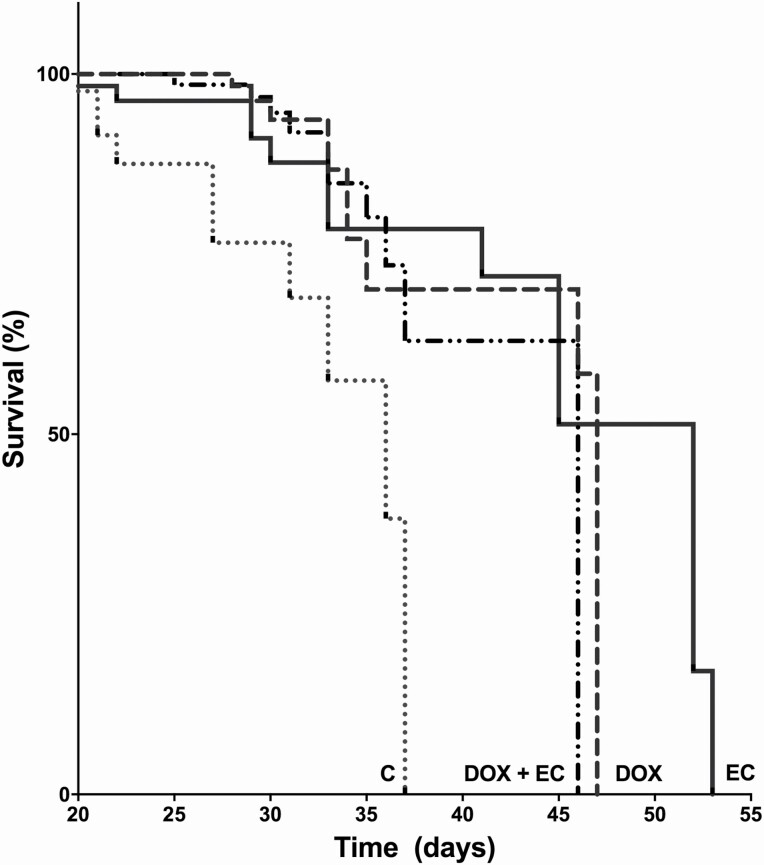
Once we observed the anti-tumour effects of EC, we explored its effects on the survival of the animals and therefore its impact on prognosis. For this, we performed a log-rank test (Mantel–Cox) and found that in the group treated with DOX (2 mg/day every other day, cumulative dose of 12 mg/kg) there was a significant 30% increase in survival (P = 0.0007) with respect to that in the control group, as expected. Interestingly, similar to DOX, EC alone was able to significantly increase survival by 44% compared with that in the control group (P = 0.005). The effect of the DOX + EC combination was not significantly different from that with either compound alone (Figure 3). The area under the curve of the effects (control = 5.6; DOX = 4.3; EC = 11.7 and DOX + EC = 7.4) showed that EC significantly increased survival, indicating its role as an anticancer agent.
Figure 3.
(−)-Epicatechin (EC) (3 mg/kg/day/15 days) induced effects on mice survival. It shows the comparison of the vehicle against 12 mg/kg DOX, EC and the combination of both EC/DOX, the survival time was similar between the last three groups and different from control. EC induced an increase in survival vs the control group (P ≤ 0.005). Each line represents the mean ± SEM. n = 10. Log-rank test, Kaplan–Meier graph was used for the analysis.
Histology and immunohistochemical analysis of cyclin D1 and Ki67 expression
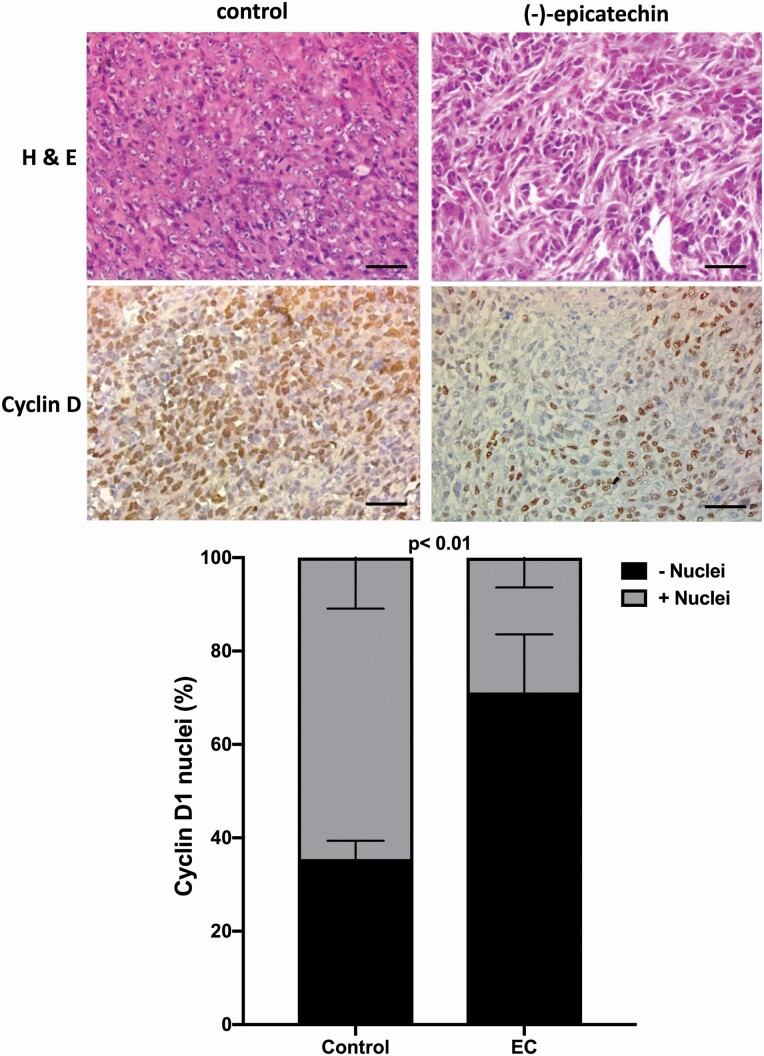
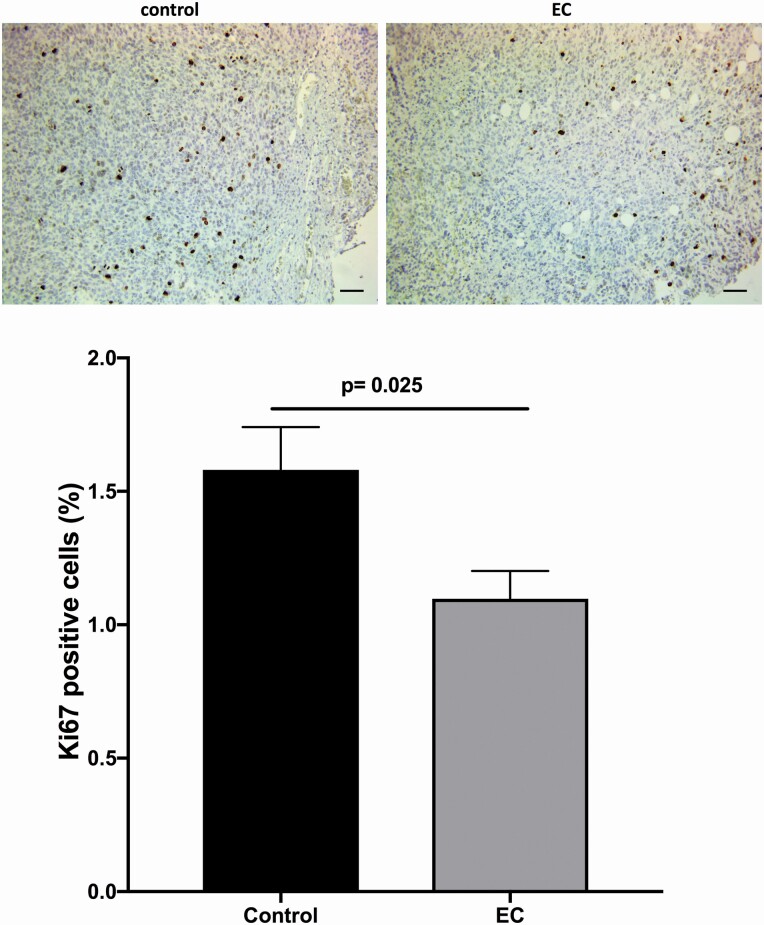
In the histological analysis, a lower cell density and a greater degree of karyorrhexis and karyolysis were observed in the tumours extracted from mice treated with EC; in addition, there was a significant reduction in the proportion of cyclin D1-positive cells in the EC-treated group compared with the control group (28.86% and 64.55%, respectively, P < 0.01) (Figure 4). The percentage of Ki67-positive cells in the control group was relatively low (1.576%), but EC treatment still induced a significant decrease (Figure 5).
Figure 4.
Representative images of (−)-EC (3 mg/kg/day/15 days) induced effects on the number of cells expressing nuclei positive to Cyclin D1. A significant reduction in the number of cyclin D1-positive (graph) cells as compared with the control group (28.9% and 64.6%, respectively, was found, P = 0.01). The data were analysed by t-test. Each bar represents the means ± SEM with n = 10. Bar in images = 50 µm.
Figure 5.
Representative images of (−)-EC (3 mg/kg/day/15 days) induced effects on the number of cells expressing nuclei positive to Ki67. A significant reduction in the number of Ki67-positive (graph) cells as compared with the control group (P = 0.025). Bar = 50 µm. The data were analysed by t-test. Each bar represents the means ± SEM with n = 10.
Western blot analysis
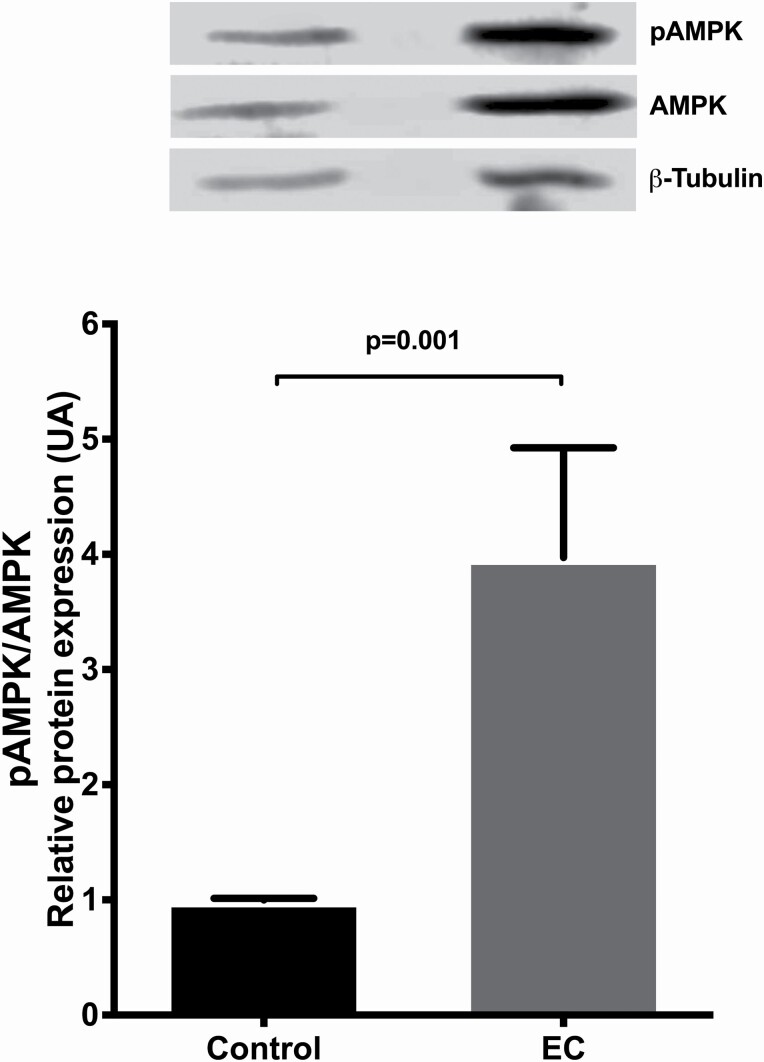
To identify the pathways through which EC exerts its effect on tumour cells, the activation of proteins of the AMPK and Akt/mTOR signalling pathways were analysed by western blotting. We found no differences in the AMPK total protein when compare control vs. EC-treated groups (data not shown). The results showed that the pAMPK/AMPK ratio (indicating activation level) was significantly higher in the EC-treated group than in the control group (P = 0.001) (Figure 6).
Figure 6.
Representative western blot of (−)-EC (3 mg/kg/day/15 days) induced effects on activation (phosphorylation) of AMPK. Data are presented as means ± SEM. The densitometric intensity of each band was measured using ImageStudio, dividing the value by the corresponding β-tubulin densitometric intensity and these values were divided (phosphorylated/total forms). The results were expressed as the optical density of the reading of the pAMPK/β-tubulin/AMPK/β-tubulin ratios. The data were analysed by t-test. Each bar represents the means ± SEM (n = 10).
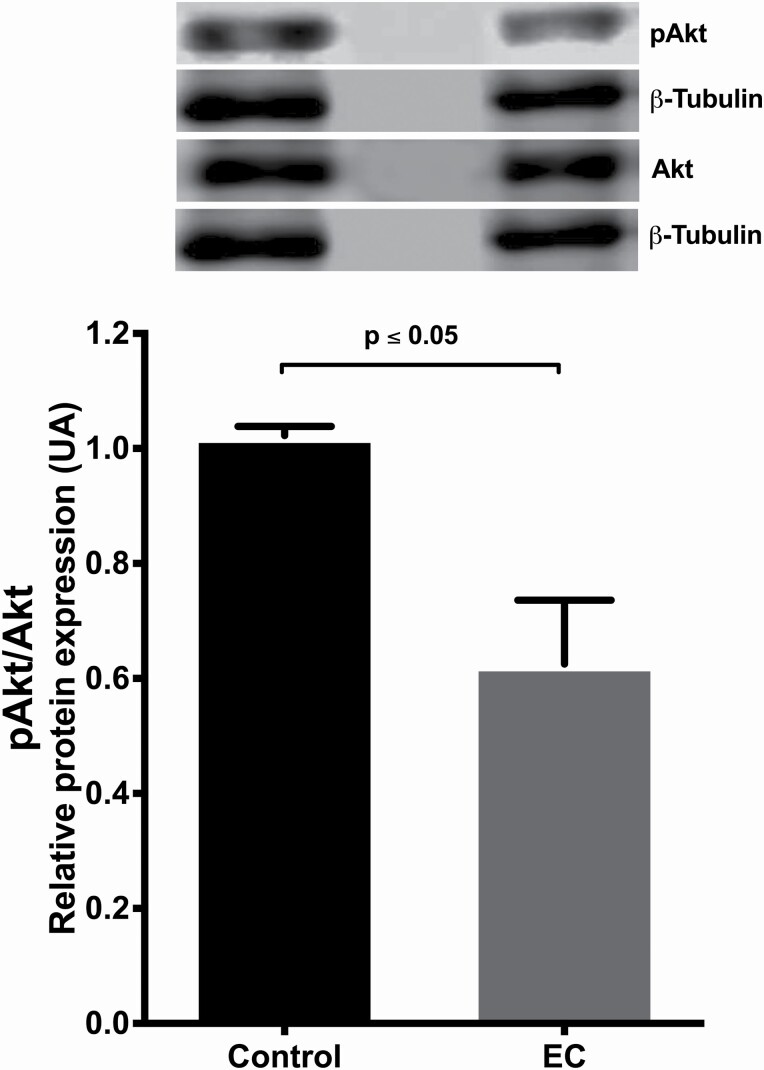
On the other hand, when analysing the activation (phosphorylation) of the Akt protein, it was found that in the EC-treated group, the pAkt/Akt ratio decreased significantly compared with that in the control group (P = 0.05) (Figure 7).
Figure 7.
Representative western blot of (−)-EC (3 mg/kg/day/15 days) induced effects on activation (phosphorylation) of AKT. The phosphorylated Akt/β-tubulin quotient on Akt/β-tubulin is presented, showing a decrease in activation in relation to control P = 0.003. The densitometric intensity of each band was measured using ImageStudio, dividing the value by the corresponding β-tubulin densitometric intensity and these values were divided (phosphorylated/total forms). The results were expressed as the optical density of the reading of the pAkt/β-tubulin/Akt/β-tubulin ratios. The data were analysed by t-test. Each bar represents the means ± SEM. P values ≤ 0.05 were considered statistically significant (n = 10).
We found no differences in the AktK total protein when compare control vs EC-treated groups (data not shown).
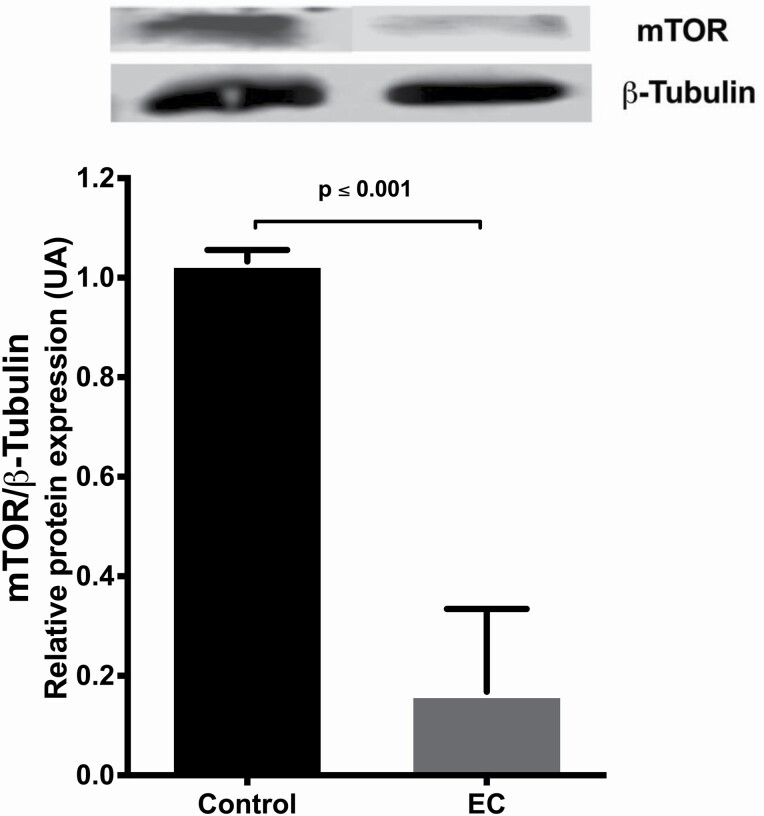
The expression of mTOR decreased in the EC-treated group as compared with the control group (P = 0.001) (Figure 8).
Figure 8.
Representative western blot of (−)-EC (3 mg/kg/day/15 days) induced effects on mTOR relative expression. Data are presented as means ± SEM of the optical density of the reading of mTOR bands ratio on β-tubulin. The data were analysed by t-test, n = 10. P values ≤ 0.05 were considered statistically significant.
Discussion
In this work, we studied the effect of (−)-EC on the development of TNBC induced by 4T1 cells in BALB/c mice. Our results show that EC inhibits tumour growth. Using a dose of 3 mg/kg/day, it was possible to reduce the tumour volume by up to 74% compared with that in the group that did not receive treatment. This effect was similar to that generated by one of the most used anticancer agents in treating solid tumours, DOX. These results are relevant when considering the multiple adverse effects that conventional DOX chemotherapy induces, including cardiotoxicity which is a limiting factor in its use. This adverse effect has been associated with the accumulation of the drug in the mitochondria, disrupting mitochondrial functions such as bioenergetics, enzymatic inhibition and membrane peroxidation.[27–29] In particular, TNBC is a therapeutic challenge due to its difficult diagnosis and treatment. Although it has a high response rate to anthracycline treatment, it is also associated with low survival.[30] Therefore, the search for new therapeutic alternatives to reduce tumour growth and simultaneously reduce the adverse effects of currently available chemotherapy is necessary.
Polyphenols are molecules that have been shown to have beneficial effects; in particular, (−)-EC supports mitochondrial function in different models of healthy cells, tissues and pathological states.[11, 13, 31]
In the present work, the effects of EC in a solid murine tumour were explored, and the anticancer capacity of EC was confirmed to be as efficient as that of DOX. These results have great potential for application since EC is considered a GRAS (generally recognized as safe) substance by the FDA, and there are no adverse effects reported in the literature.[32]
In the search for the molecular mechanisms through which EC acts, we analysed the signalling pathways AMPK and Akt/mTOR as possibly responsible for the regulation of cell proliferation.
Our results showed that in the presence of EC, the activation (phosphorylation) of AMPK was increased compared with that in vehicle control mice, while Akt (phosphorylation) was diminished. These findings coincide with what was proposed by Chaube et al.[33]: homeostasis and adaptation to metabolic stress in cancer cells are mainly due to the integral response exerted by the activation of the energy sensor AMPK. Moreover, the constitutive phosphorylation of Akt in cancer cells activates different targets for tumour development as a pathway to chemoresistance in cancer cells.[34] PI3K/Akt/mTOR pathway inhibition has been associated with high survival rates in different types of cancer.[35, 36]
Additionally, existing evidence suggests that the AMPK-p38-PGC1α axis increases mitochondrial biogenesis and, therefore the oxidative metabolism of substrates other than glucose. Additionally, an increase in phosphorylated AMPK is related to a good prognosis in cancer patients.[33] It was also reported that AMPK activation downregulates the mTOR pathway in breast cancer following its activation by metformin.[35] Our results agree with this proposal since EC downregulated the expression of mTOR, reducing cell proliferation. The reported results all together are consistent with the so-called Warburg effect, which explains that cancer cells depend mainly on the energy obtained by glycolysis, probably as a consequence of mitochondrial dysfunction.[37] Consequently, in this study, EC might have exerted its function by improving mitochondrial function and changing the energy dependence of cells. Previously, Elbaz et al. reported that EC stimulated mitochondrial respiration and oxygen consumption in PANC-1 cells.[38]
In this work, we wondered whether the combination of DOX with EC would have additive effects on tumour volume; we found that the simultaneous administration of DOX (3 mg/day every other day, cumulative dose of 12 mg/kg) with EC did not induce a more significant effect than that when both compounds were administered individually.
It is possible that the doses used were high or reached their maximum effect, limiting the possibility of increased effects with the combination. DOX is a widely studied drug that regulates energy, oxidative and genotoxic stress, which involves inhibiting AMPK partially via the intercommunication between the Akt and MAPK pathways as a consequence of DNA damage.[39]
The convergence in signalling pathways between EC and DOX could explain the lack of additive effects between them when evaluating tumour volume. However, it is possible that the stepwise or intercalated administration of both compounds would lead to the different impacts. In this regard, even when there was no enhancement of the effect of DOX by EC, the combination could be interesting because it can reduce the cardiotoxic effect of DOX.
On the other hand, we evaluated cyclin D1 and Ki67 as proliferation markers and found a significant reduction in the number of cyclin D1-positive cells in tumours of animals treated with EC compared with those treated with the control (64% and 29% cell positives, respectively). Additionally, the number of Ki67-positive cells was lower in the EC-treated group than in the control group. These results indicate that the proliferation of tumour cells is reduced when the mice are treated with EC, and therefore, the tumour size is smaller.
To evaluate the impact of EC treatment on the survival of animals, we explored the effects of DOX (2 mg/day every other day, cumulative dose of 12 mg/kg) and EC. Interestingly, the survival rate was significantly higher in animals treated with EC than in animals treated with vehicle (P = 0.005), and it was even slightly higher than that in the group treated with DOX, even when there were no significant differences (P = 0.900).
In summary, our results showed that (−)-EC has the anti-tumour capacity and that these effects might be related to the regulation of cell proliferation, the promotion of cell cycle arrest and the inhibition of cyclin D1 and Ki67 expression.
We showed that phosphorylation of Akt is inhibited, and AMPK activation is increased with EC treatment. These results suggest that EC can be used as an alternative in the treatment of cancer. The decrease in tumour size and the increase in survival induced by (−)-EC opens several possibilities, including the use of EC after/before DOX therapy and/or the decrease in DOX dose to reduce adverse effects and toxicity.
The results reported here warrant the implementation of more work, including a clinical trial to demonstrate the relevance of (−)-EC as a coadjuvant in chemotherapy.
Conclusions
(−)-EC increases the survival of animals with triple-negative breast tumours by inhibiting tumour growth; this effect is as potent as that of DOX, an antineoplastic widely used in the treatment of solid tumours. (−)-EC may exert its effects inhibiting the tumour growth through the regulation of AMPK and the Akt/mTOR pathway.
Funding
This work was supported by the National Institute of Health (NIH DK98717, AG47326) to Dr F.V. and Consejo Nacional de Ciencia y Tecnologia (253769) to Dr G.C.
Author Contributions
G.A., P.O.-V., P.C., R.M.-V. and J.P.-D. developed model, performed assays and wrote preliminary versions of the manuscript. E.M., F.V., G.C. and N.N. analysed data, wrote the manuscript and edited manuscript versions.
Conflict of Interest
Dr F.V. is a co-founder and stockholder (Dr G.C.) of Cardero Therapeutics, Inc. The co-authors have no disclosures.
Contributor Information
Georgina Almaguer, Escuela Superior de Medicina, Instituto Politécnico Nacional, Plan de San Luis y Díaz Mirón, México City, México.
Pilar Ortiz-Vilchis, Escuela Superior de Medicina, Instituto Politécnico Nacional, Plan de San Luis y Díaz Mirón, México City, México.
Paola Cordero, Escuela Superior de Medicina, Instituto Politécnico Nacional, Plan de San Luis y Díaz Mirón, México City, México.
Rocío Martinez-Vega, Escuela Superior de Medicina, Instituto Politécnico Nacional, Plan de San Luis y Díaz Mirón, México City, México.
Javier Perez-Durán, Escuela Superior de Medicina, Instituto Politécnico Nacional, Plan de San Luis y Díaz Mirón, México City, México; Department of Human Genetics and Genomics, Instituto Nacional de Perinatología, Mexico City, Mexico.
Eduardo Meaney, Escuela Superior de Medicina, Instituto Politécnico Nacional, Plan de San Luis y Díaz Mirón, México City, México.
Francisco Villarreal, Department of Medicine, School of Medicine, University of California, San Diego, La Jolla, CA, USA.
Guillermo Ceballos, Escuela Superior de Medicina, Instituto Politécnico Nacional, Plan de San Luis y Díaz Mirón, México City, México.
Nayelli Nájera, Escuela Superior de Medicina, Instituto Politécnico Nacional, Plan de San Luis y Díaz Mirón, México City, México.
References
- 1. PAHO Observes Breast Cancer Awareness Month. www.paho.org/cancer
- 2. Thakur KK, Bordoloi D, Kunnumakkara AB. Alarming burden of triple-negative breast cancer in India. Clin Breast Cancer 2018; 18: e393–9. 10.1016/j.clbc.2017.07.013 [DOI] [PubMed] [Google Scholar]
- 3. Yamamoto Y, Iwase H. Clinicopathological features and treatment strategy for triple-negative breast cancer. Int J Clin Oncol 2010; 15: 341–51. 10.1007/s10147-010-0106-1 [DOI] [PubMed] [Google Scholar]
- 4. Muggia FM, Green MD. New anthracycline antitumor antibiotics. Crit Rev Oncol Hematol 1991; 11: 43–64. 10.1016/1040-8428(91)90017-7 [DOI] [PubMed] [Google Scholar]
- 5. Giordano SH, Lin YL, Kuo YFet al. Decline in the use of anthracyclines for breast cancer. J Clin Oncol 2012; 30: 2232–9. 10.1200/JCO.2011.40.1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Niu X, Zhang Z, Zhong Y. Hydrogel loaded with self-assembled dextran sulfate-doxorubicin complexes as a delivery system for chemotherapy. Mater Sci Eng C Mater Biol Appl 2017; 77: 888–94. 10.1016/j.msec.2017.04.013 [DOI] [PubMed] [Google Scholar]
- 7. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 2003; 97: 2869–79. 10.1002/cncr.11407 [DOI] [PubMed] [Google Scholar]
- 8. Braakhuis AJ, Campion P, Bishop KS. Reducing breast cancer recurrence: the role of dietary polyphenolics. Nutrients 2016; 8: 547. 10.3390/nu8090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qureshi WA, Zhaoa R, Wang Het al. Co-delivery of doxorubicin and quercetin via mPEG–PLGA copolymer assembly for synergistic anti-tumor efficacy and reducing cardio-toxicity. Sci Bull 2016; 61: 1689–98. 10.1007/s11434-016-1182-z. [DOI] [Google Scholar]
- 10. Moreno-Ulloa A, Cid A, Rubio-Gayosso Iet al. Effects of (−)-epicatechin and derivatives on nitric oxide mediated induction of mitochondrial proteins. Bioorg Med Chem Lett 2013; 23: 4441–6. 10.1016/j.bmcl.2013.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramirez-Sanchez I, De los Santos S, Gonzalez-Basurto Set al. (−)-Epicatechin improves mitochondrial-related protein levels and ameliorates oxidative stress in dystrophic δ-sarcoglycan null mouse striated muscle. FEBS J 2014; 281: 5567–80. 10.1111/febs.13098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamazaki KG, Andreyev AY, Ortiz-Vilchis Pet al. Intravenous (−)-epicatechin reduces myocardial ischemic injury by protecting mitochondrial function. Int J Cardiol 2014; 175: 297–306. 10.1016/j.ijcard.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ortiz-Vilchis P, Ortiz-Flores M, Pacheco Met al. The cardioprotective effects of (−)-epicatechin are mediated through arginase activity inhibition in a murine model of ischemia/reperfusion. Eur J Pharmacol 2018; 818: 335–42. 10.1016/j.ejphar.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 14. Ortiz-Vilchis P, Yamazaki KG, Rubio-Gayosso Iet al. Co-administration of the flavanol (−)-epicatechin with doxycycline synergistically reduces infarct size in a model of ischemia reperfusion injury by inhibition of mitochondrial swelling. Eur J Pharmacol 2014; 744: 76–82. 10.1016/j.ejphar.2014.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramírez-Sánchez I, Rodríguez A, Moreno-Ulloa Aet al. (−)-Epicatechin-induced recovery of mitochondria from simulated diabetes: potential role of endothelial nitric oxide synthase. Diabetes Vasc Dis Res 2016; 13: 201–10. 10.1177/1479164115620982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tan X, Hu D, Li Set al. Differences of four catechins in cell cycle arrest and induction of apoptosis in LoVo cells. Cancer Lett 2000; 158: 1–6. 10.1016/s0304-3835(00)00445-6 [DOI] [PubMed] [Google Scholar]
- 17. Kim D, Mollah ML, Kim K. Induction of apoptosis of SW480 human colon cancer cells by (−)-epicatechin isolated from Bulnesia sarmienti. Anticancer Res 2012; 32: 5353–62. [PubMed] [Google Scholar]
- 18. Papiez MA, Baran J, Bukowska-Straková Ket al. Antileukemic action of (−)-epicatechin in the spleen of rats with acute myeloid leukemia. Food Chem Toxicol 2010; 48: 3391–7. 10.1016/j.fct.2010.09.010 [DOI] [PubMed] [Google Scholar]
- 19. Varela-Castillo O, Cordero P, Gutiérrez-Iglesias Get al. Characterization of the cytotoxic effects of the combination of cisplatin and flavanol (−)-epicatechin on human lung cancer cell line A549. An isobolographic approach. Exp Oncol 2018; 40: 19–23. [PubMed] [Google Scholar]
- 20. Hosseinimehr SJ, Rostamnejad M, Ghaffarirad V. Epicatechin enhances antiproliferative effect of bleomycin in ovarian cancer cell. Res Mol Med 2013; 1: 25–8. 10.18869/acadpub.rmm.1.3.25. [DOI] [Google Scholar]
- 21. Papiez M, Bukowska-Straková K, Krzysciak W. (−)-Epicatechin enhances etoposide-induced antileukaemic effect in rats with acute myeloid leukaemia. Anticancer Res 2012; 32: 2905–13. [PubMed] [Google Scholar]
- 22. Bao L, Haque A, Jackson Ket al. Increased expression of P-glycoprotein is associated with doxorubicin chemoresistance in the metastatic 4T1 breast cancer model. Am J Pathol 2011; 178: 838–52. 10.1016/j.ajpath.2010.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heppner GH, Dexter DL, DeNucci Tet al. Heterogeneity in drug sensitivity among tumor cell subpopulations of a single mammary tumor. Cancer Res 1978; 38: 3758–63. [PubMed] [Google Scholar]
- 24. Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res 1992; 52: 1399–405. 10.1002/0471142735.im2002s39. [DOI] [PubMed] [Google Scholar]
- 25. Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol 2000; 39: 1–16. 10.1002/0471142735.im2002s39. [DOI] [PubMed] [Google Scholar]
- 26. Nom-062-Zoo-199. Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. Nom-062-Zoo 1999: 1–58. https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf. (31 July 2019, date last accessed).
- 27. Jung K, Reszka R. Mitochondria as subcellular targets for clinically useful anthracyclines. Adv Drug Deliv Rev 2001; 49: 87–105. 10.1016/s0169-409x(01)00128-4 [DOI] [PubMed] [Google Scholar]
- 28. Gorini S, De Angelis A, Berrino Let al. Chemotherapeutic drugs and mitochondrial dysfunction: focus on doxorubicin, trastuzumab, and sunitinib. Oxid Med Cell Longev 2018; 2018: 7582730. 10.1155/2018/7582730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hasinoff BB, Schnabl KL, Marusak RAet al. Dexrazoxane (ICRF-187) protects cardiac myocytes against doxorubicin by preventing damage to mitochondria. Cardiovasc Toxicol 2003; 3: 89–99. 10.1385/ct:3:2:89 [DOI] [PubMed] [Google Scholar]
- 30. Wahba HA, El-Hadaad HA. Current approaches in treatment of triple-negative breast cancer. Cancer Biol Med 2015; 12: 106–16. 10.7497/j.issn.2095-3941.2015.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tanabe K, Tamura Y, Lanaspa MAet al. Epicatechin limits renal injury by mitochondrial protection in cisplatin nephropathy. Am J Physiol Renal Physiol 2012; 303: F1264–74. 10.1152/ajprenal.00227.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. CFSAN/Office of Food Additive Safety. Agency Response Letter GRAS Notice No. GRN 000497. U.S. Food and Drug Administration 2014: 1–6. https://wayback.archive-it.org/7993/20170606194720/https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm404928.htm. (30 June 2019, date last accessed).
- 33. Chaube B, Malvi P, Singh SVet al. AMPK maintains energy homeostasis and survival in cancer cells via regulating p38/PGC-1α-mediated mitochondrial biogenesis. Cell Death Discov 2015; 1: 15063. 10.1038/cddiscovery.2015.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu HG, Ai YW, Yu LLet al. Phosphoinositide 3-kinase/Akt pathway plays an important role in chemoresistance of gastric cancer cells against etoposide and doxorubicin induced cell death. Int J Cancer 2008; 122: 433–43. 10.1002/ijc.23049 [DOI] [PubMed] [Google Scholar]
- 35. Gomez-Pinillos A, Ferrari AC. mTOR signaling pathway and mTOR inhibitors in cancer therapy. Hematol Oncol Clin North Am 2012; 26: 483–505, vii. 10.1016/j.hoc.2012.02.014 [DOI] [PubMed] [Google Scholar]
- 36. Dimitrova V, Arcaro A. Targeting the PI3K/AKT/mTOR signaling pathway in medulloblastoma. Curr Mol Med 2015; 15: 82–93. 10.2174/1566524015666150114115427 [DOI] [PubMed] [Google Scholar]
- 37. Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer 2011; 11: 325–37. 10.1038/nrc3038 [DOI] [PubMed] [Google Scholar]
- 38. Elbaz HA, Lee I, Antwih DAet al. Epicatechin stimulates mitochondrial activity and selectively sensitizes cancer cells to radiation. PLoS One 2014; 9: e88322. 10.1371/journal.pone.0088322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheng J, Shuai X, Gao Jet al. Prognostic significance of AMPK in human malignancies: a meta-analysis. Oncotarget 2016; 7: 75739–48. 10.18632/oncotarget.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]