Abstract
Background
Large-scale vaccination against COVID-19 is being implemented in many countries with CoronaVac, an inactivated vaccine. We aimed to assess the immune persistence of a two-dose schedule of CoronaVac, and the immunogenicity and safety of a third dose of CoronaVac, in healthy adults aged 18 years and older.
Methods
In the first of two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials, adults aged 18–59 years in Jiangsu, China, were initially allocated (1:1) into two vaccination schedule cohorts: a day 0 and day 14 vaccination cohort (cohort 1) and a day 0 and day 28 vaccination cohort (cohort 2); each cohort was randomly assigned (2:2:1) to either a 3 μg dose or 6 μg dose of CoronaVac or a placebo group. Following a protocol amendment on Dec 25, 2020, half of the participants in each cohort were allocated to receive an additional dose 28 days (window period 30 days) after the second dose, and the other half were allocated to receive a third dose 6 months (window period 60 days) after the second dose. In the other phase 2 trial, in Hebei, China, participants aged 60 years and older were assigned sequentially to receive three injections of either 1·5 μg, 3 μg, or 6 μg of vaccine or placebo, administered 28 days apart for the first two doses and 6 months (window period 90 days) apart for doses two and three. The main outcomes of the study were geometric mean titres (GMTs), geometric mean increases (GMIs), and seropositivity of neutralising antibody to SARS-CoV-2 (virus strain SARS-CoV-2/human/CHN/CN1/2020, GenBank accession number MT407649.1), as analysed in the per-protocol population (all participants who completed their assigned third dose). Our reporting is focused on the 3 μg groups, since 3 μg is the licensed formulation. The trials are registered with ClinicalTrials.gov, NCT04352608 and NCT04383574.
Findings
540 (90%) of 600 participants aged 18–59 years were eligible to receive a third dose, of whom 269 (50%) received the primary third dose 2 months after the second dose (cohorts 1a-14d-2m and 2a-28d-2m) and 271 (50%) received a booster dose 8 months after the second dose (cohorts 1b-14d-8m and 2b-28d-8m). In the 3 μg group, neutralising antibody titres induced by the first two doses declined after 6 months to near or below the seropositive cutoff (GMT of 8) for cohort 1b-14d-8m (n=53; GMT 3·9 [95% CI 3·1–5·0]) and for cohort 2b-28d-8m (n=49; 6·8 [5·2–8·8]). When a booster dose was given 8 months after a second dose, GMTs assessed 14 days later increased to 137·9 (95% CI 99·9–190·4) for cohort 1b-14d-8m and 143·1 (110·8–184·7) 28 days later for cohort 2b-28d-8m. GMTs moderately increased following a primary third dose, from 21·8 (95% CI 17·3–27·6) on day 28 after the second dose to 45·8 (35·7–58·9) on day 28 after the third dose in cohort 1a-14d-2m (n=54), and from 38·1 (28·4–51·1) to 49·7 (39·9–61·9) in cohort 2a-28d-2m (n=53). GMTs had decayed to near the positive threshold by 6 months after the third dose: GMT 9·2 (95% CI 7·1–12·0) in cohort 1a-14d-2m and 10·0 (7·3–13·7) in cohort 2a-28d-2m. Similarly, in adults aged 60 years and older who received booster doses (303 [87%] of 350 participants were eligible to receive a third dose), neutralising antibody titres had declined to near or below the seropositive threshold by 6 months after the primary two-dose series. A third dose given 8 months after the second dose significantly increased neutralising antibody concentrations: GMTs increased from 42·9 (95% CI 31·0–59·4) on day 28 after the second dose to 158·5 (96·6–259·2) on day 28 following the third dose (n=29). All adverse reactions reported within 28 days after a third dose were of grade 1 or 2 severity in all vaccination cohorts. There were three serious adverse events (2%) reported by the 150 participants in cohort 1a-14d-2m, four (3%) by 150 participants from cohort 1b-14d-8m, one (1%) by 150 participants in each of cohorts 2a-28d-2m and 2b-28d-8m, and 24 (7%) by 349 participants from cohort 3-28d-8m.
Interpretation
A third dose of CoronaVac in adults administered 8 months after a second dose effectively recalled specific immune responses to SARS-CoV-2, which had declined substantially 6 months after two doses of CoronaVac, resulting in a remarkable increase in the concentration of antibodies and indicating that a two-dose schedule generates good immune memory, and a primary third dose given 2 months after the second dose induced slightly higher antibody titres than the primary two doses.
Funding
National Key Research and Development Program, Beijing Science and Technology Program, and Key Program of the National Natural Science Foundation of China.
Translation
For the Mandarin translation of the abstract see Supplementary Materials section.
Introduction
More than 20 vaccines have been approved for use in response to the COVID-19 pandemic,1 with over 6·33 billion doses administered globally as of Oct 3, 2021.2 Following primary vaccination with vaccines including BNT162b23, 4, 5 (Pfizer–BioNTech's mRNA vaccine), mRNA-12734, 6 (Moderna's mRNA vaccine), and ChAdOx1 nCoV-197, 8 (AstraZeneca's non-replicating adenoviral vectored vaccine), neutralising antibody titres and vaccine effectiveness against symptomatic illness have been observed to decrease over time, particularly against the delta (B.1.617.2) variant of SARS-CoV-2, which has become the predominant strain across the globe.9 A booster dose given 6–8 months after the second dose of BNT162b2,10 mRNA-1273,11 and NVX-CoV237312 (Novavax's protein subunit vaccine) greatly increased neutralising antibody concentrations, and thus increased neutralisation capacity against the delta variant. Booster vaccination with BNT162b2 was initiated in Israel in response to a surge of COVID-19 cases caused by the delta variant;13 interim results show that the booster dose significantly reduces rates of confirmed infection and severe illness.14
CoronaVac (Sinovac Life Sciences, Beijing, China), an inactivated vaccine against COVID-19, has been authorised for conditional use in China,15 and is included in WHO's emergency use listing.16 This vaccine has been administered in 26 countries, including China,1 and is increasing the global supply through COVAX.17 In China, 2·21 billion doses of COVID-19 vaccines have been administered as of Oct 3, 2021,18 the vast majority of which are inactivated vaccines. Evidence from real-world studies of CoronaVac in two-dose schedules in Chile,19 Brazil,20 and China21, 22 shows that the vaccine effectively prevents laboratory-confirmed COVID-19, with greater effectiveness against more severe outcomes, including in settings with circulation of variants of concern. However, persistence of CoronaVac vaccine-induced immunity is unknown, and the immunogenicity and safety of a booster dose has not been determined.
Research in context.
Evidence before this study
We used the terms “SARS-CoV-2”, “COVID-19”, “vaccine”, and “clinical trial” to search PubMed and Europe PMC on Sept 29, 2021, without language or date restrictions, to identify seven research articles on the immune persistence of currently approved vaccines or the immunogenicity of additional doses in the general population. Previous research reported that neutralising antibody responses elicited by mRNA vaccines (BNT162b2, developed by Pfizer and BioNTech, and mRNA-1273, developed by Moderna), adenovirus-vectored vaccines (ChAdOx1 nCoV-19, developed by Oxford and AstraZeneca, and Ad26.COV2-S, developed by Janssen), an inactivated vaccine (CoronaVac, developed by SinoVac), and a protein subunit vaccine (NVX-CoV2373, developed by Novavax) persisted for 6–8 months after full-schedule vaccination and declined to varying degrees. Neutralising antibodies against variants of concern started at lower concentrations than they did against the original alpha variant and waned substantially, especially against the beta (B.1.351) variant, whereas neutralising antibody concentrations against other variants of concern were less affected. Neutralisation capacity against the delta (B.1.617.2) variant, mediated by a homologous third dose given 6–8 months after the second dose of mRNA-1273, BNT162b2, or ChAdOx1 nCoV-19, increased multifold and was similar to or higher than the level against the ancestral SARS-CoV-2 after the second dose. Several clinical trials have explored heterologous vaccination schedules with ChAdOx1 nCoV-19 and BNT162b2, BNT162b2 and Ad26.COV2-S, CoronaVac and ChAdOx1 nCoV-19, and CoronaVac and Convidecia (adenovirus type-5-vectored vaccine, developed by CanSino), showing that heterologous vaccination can induce robust immune responses in adults aged 18 years and older. These results indicate flexibility in deploying COVID-19 vaccines in mix-and-match schedules.
Added value of this study
Our phase 2 trial among adults aged 18–59 years provides preliminary evidence of 6-month immune persistence after two two-dose schedules (14-day and 28-day intervals) of CoronaVac and immunogenicity and safety of a third dose of CoronaVac given 2 months or 8 months after the second dose. Neutralising antibody titres induced by two doses of CoronaVac (3 μg formulation) declined to near or below the lower limit of seropositivity after 6 months. A third dose given 8 months after the second dose led to a strong boost in immune response (a three-fold to five-fold increase in neutralising antibody titres 28 days after the second dose). Our phase 2 trial in healthy adults aged 60 years and older found that neutralising antibody titres declined to low concentrations 6 months after the second dose but rapidly rebounded after a third dose given at 8 months after the second dose (an approximate three-fold increase in neutralising antibody titre). Seropositivity after an 8-month third dose was 98–100% regardless of age group. No safety concerns were seen with a third dose; reactogenicity of the vaccine was indistinguishable from reactogenicity of aluminium hydroxide placebo. This study provided data on immune persistence after primary immunisation with CoronaVac, and immunogenicity and safety of a third homologous dose in adults aged 18 years or older.
Implications of all the available evidence
The rapid and robust rebound in immunity induced by a third dose of CoronaVac showed that primary vaccination with two doses induced immune memory in adults aged 18 years and older. A third dose was immunogenic and markedly increased neutralising antibody titres when given 8 months after the second dose. Therefore, a third dose might provide additional benefit, including longer-lasting immunity and higher level of protection, over a two-dose schedule, but such determinations need longer-term study and real-world studies of vaccine effectiveness.
To fill this knowledge gap, we aimed to assess immune persistence after primary immunisation with CoronaVac, and immunogenicity and safety of a third homologous dose, in two population groups: adults aged 18–59 years and adults aged 60 years or older.
Methods
Study design and participants
Our study is built upon two single-centre, double-blind, randomised, placebo-controlled, phase 2 clinical trials of CoronaVac. One trial was initiated in Suining County, Jiangsu province, China, by Jiangsu Provincial Center for Disease Control and Prevention (CDC) on May 3, 2020, among healthy adults aged 18–59 years, and the other was initiated in Renqiu, Hebei province, China, by Hebei Provincial CDC, on June 12, 2020, among healthy adults aged 60 years and older. The designs of the phase 2 trials have been published previously.23, 24 Briefly, key exclusion criteria for trial enrolment included suspected or laboratory-confirmed SARS-CoV-2 infections and known allergy to any vaccine component. A complete list of exclusion criteria is in the protocol (appendix 2 pp 74–76; appendix 3 pp 38–39).
For the trial in adults aged 18–59 years, eligible participants were initially recruited and randomly allocated (1:1) to vaccination cohorts with two-dose schedules, either 14 days apart (cohort 1) or 28 days apart (cohort 2). Within each cohort, participants were randomly allocated (2:2:1) to either a 3 μg group, a 6 μg group, or a placebo group. For the trial in adults aged 60 years and older, eligible participants were assigned (2:2:2:1) sequentially to receive two doses 28 days apart of either 1·5 μg, 3 μg, or 6 μg vaccine or placebo (cohort 3). Randomisation codes for each vaccination schedule cohort were generated individually and randomly assigned using block randomisation developed with SAS version 9.4. Adults aged 18–59 years were assigned with a block size of five and adults aged 60 years and older were assigned with a block size of 14. Concealed random group allocations and blinding codes were kept in signed and sealed envelopes. Investigators, participants, and laboratory staff were masked to group assignment. The randomisation code was assigned to each participant in sequence in the order of enrolment by investigators, who were involved in the rest of the trial.
1·5 μg, 3 μg, or 6 μg doses of CoronaVac (Vero cell, inactivated CN02 strain of SARS-CoV-2 with 1·5, 3, or 6 μg per 0·5 mL of aluminium hydroxide adjuvant) or placebo (0·5 mL of aluminium hydroxide adjuvant) in prefilled syringes were administered by intramuscular injection into the deltoid muscle. To evaluate the immunogenicity of primary vaccination, blood samples were taken before vaccination and at day 28 after the second dose. Interim results of these data have been published.23, 24 For the trial in adults aged 18–59 years, the protocol was amended on Dec 25, 2020, to evaluate the immunogenicity of an additional dose (appendix 2 p 3). The amended protocol was updated on ClinicalTrials.gov. According to the order of the blocks, half of the participants were sequentially allocated to receive an additional dose of the vaccine or placebo at 28 days after the second dose (with a 30-day window period; hereafter cohort 1a-14d-2m and cohort 2a-28d-2m, with 14d and 28d representing the interval in days between the first two doses, and 2m denoting the actual median interval in months between the second and third doses), and the other half were allocated to receive a booster dose 6 months after the second dose (with a 60-day window period; hereafter cohort 1b-14d-8m and cohort 2b-28d-8m, with 8m denoting the actual median interval in months between the second and third doses). For the trial in adults aged 60 years and older, a booster dose was given 6 months after the second dose (with a 90-day window period; cohort 3-28d-8m) per the original protocol (appendix 3 p 41–42). Key exclusion criteria for third doses are shown in appendix 4 (p 3). Written informed consent was obtained from participants both before enrolment and before administration of a third dose of a vaccine in eligible participants. The clinical trial protocol and informed consent forms for the study in adults aged 18–59 years were approved by the Jiangsu Ethics Committee (JSJK2020-A021–02), and those for the study in adults aged 60 years and older were approved by Hebei CDC Ethics Committee (IRB2020–006).
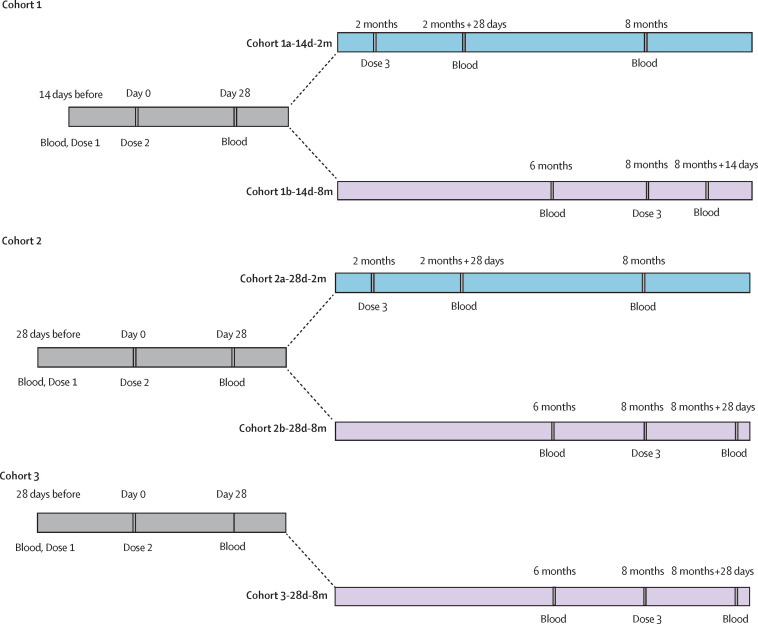
Essential steps and timing for each visit specified in the protocol are shown in appendix 4 (p 4). Participants in each cohort received homologous third doses, vaccine or placebo. Participants were to be withdrawn from the trial if they had an unacceptable adverse event as judged by the investigators and defined by the Guidelines of the National Medical Products Administration for Adverse Event Classification Standards for Clinical Trials of Preventive Vaccines (2019), an unacceptable health status as judged by the investigators, or abnormal clinical manifestations as judged by the investigators, or at the participant's request or for any other reason judged necessary by the investigator. The trial would be suspended under the following conditions as judged by the investigators: occurrence of one or more grade 4 local or systemic adverse reactions related to vaccination or more than 15% of the participants having grade 3 or above adverse reactions, including local reactions, systemic reactions, and vital sign changes. During the trial periods, no active surveillance for natural infection with SARS-CoV-2 was done by this study. SARS-CoV-2 occurring in study participants was required to be reported to the investigator. Under the China Government's COVID-19 prevention and control policy of zero tolerance for local transmission, all infections are identified in a timely manner and reported by local health departments for contact tracing, isolated treatment, and quarantine of close contacts and testing for SARS-CoV-2 RNA.
For participants who received their third dose 28 days after the second dose (cohort 1a-14d-2m and cohort 2a-28d-2m), blood samples were collected on day 28 and month 6 after the third dose to evaluate immunogenicity and immune persistence of the third dose. For participants who received their third dose 6 months after the second dose (cohort 1b-14d-8m, cohort 2b-28d-8m, and cohort 3-28d-8m), blood samples were collected at month 6 after the second dose to evaluate the immune persistence of the second dose, and on day 28 after the third dose to assess immunogenicity of the third dose (with the exception of cohort 1b-14d-8m, in which samples were collected on day 14 after the third dose; figure 1 ; appendix 4 p 4).
Figure 1.
Trial process timeline
Blood=blood sample taken.
Safety information after the third dose was obtained by the same methods as for the first two doses, as described previously.23 Participants were required to record injection-site adverse events (eg, pain, redness, and swelling), or systemic adverse events (eg, allergic reactions, cough, and fever) on diary cards for 7 days after their third dose. For days 8–28, unsolicited adverse reactions were collected by spontaneous reporting from participants in all cohorts. We planned to collect serious adverse events until 6 months after the third dose for participants in cohorts 1 and 2, and until 1 year after third dose for participants in cohort 3. The cut-off day of this report was 6 months after the second dose for participants in cohort 1b-14d-8m, cohort 2b-28d-8m, and cohort 3-28d-8m, and 6 months after the third dose for participants in cohort 1a-14d-2m and cohort 2a-28d-2m. Reported adverse events were graded according to China National Medical Products Administration guidelines.23 Serious adverse events were coded by the Medical Dictionary for Regulatory Activities (MedDRA) System Organ Class. The existence of causal associations between adverse events and vaccination was determined by the investigators.
Immunological assessment methods and related procedures are described in appendix 4 (p 5). Neutralising antibodies against infectious SARS-CoV-2 (virus strain SARS-CoV-2/human/CHN/CN1/2020, GenBank accession number MT407649.1) were quantified using a microcytopathogenic effect assay.23 Several measures were taken to control the quality of the microcytopathogenic effect assay, including virus back-titration for each batch of tests to determine whether the amount of virus was within the range of 32–320 tissue culture infectious dose (TCID50) per 50 μL.25 Two types of positive antibody control, a negative antibody control, a serum toxicity control, and a cell control were included for each test. Blood samples taken at baseline and 28 days after the second dose had been tested previously, and the neutralising antibody titres were comparable between the group aged 18–59 years and those aged 60 years or older.23, 24
Blood samples taken 6 months after the second dose or 14 days, 28 days, or 6 months after the third dose were tested in our analyses. However, neutralising antibody titres of sera obtained on day 28 after the third dose from participants in the older age group were approximately two-fold higher (352·8 [95% CI 266·4–441·1] in cohort 3-28d-8m) than titres from participants in the younger age group (143·1 [95% CI 110·8–184·7] in cohort 2b-28d-8m) who had been immunised with the same vaccination schedule. To verify the stability and reliability of the neutralising antibody test results, we retested a convenient random sample of specimens from 100 adults in the younger age group and 100 adults in the older age group. In the group of younger adults, neutralising antibody titres were consistent between the first test and the retest. Accordingly, the results of the first test were used in our analysis for this population. In the group of older adults, neutralising antibody titres were significantly lower in the retests than they were in the first tests. Considering the acceptable results of serum samples in younger adults and older adults in the retests, and the consistence of our procedures with the protocol after evaluation, we used the retest results of the 100 adults in the older age group, which we believe to be more reliable, in our analyses. Due to repeated freezing and thawing, and insufficient quantity of sera, we were unable to retest specimens from the other adults in the older age group. A detailed description of retest procedures and results for the older adults is provided in appendix 4 (pp 9–11).
Outcomes
The primary immunological outcomes of the two phase 2 trials have been reported previously;23, 24 here, we report the results of prespecified secondary and exploratory immunological outcomes. Secondary immunological outcomes included geometric mean titres (GMTs), geometric mean increases (GMIs), and seropositivity of neutralising antibodies to infectious SARS-CoV-2 28 days after the third dose (for cohort 1a-14d-2m and cohort 2a-28d-2m). Exploratory immunological outcomes included GMTs and seropositivity at 6 months after the second dose (for cohort 1b-14d-8m, cohort 2b-28d-8m, and cohort 3-28d-8m) and at 14 days (for cohort 1b-14d-8m) or 28 days (for cohort 2b-28d-8m and cohort 3-28d-8m) after the third dose. The additional outcome of GMTs and seropositivity at 6 months after the third dose for cohort 1a-14d-2m and cohort 2a-28d-2m was a post-hoc analysis. To assess the immunogenicity of a third dose, we included the participants who received their assigned third doses and had available antibody results on day 28 after the third dose (day 14 after the third dose for cohort 1b-14d-8m); defined as the per-protocol analysis set of third doses. To assess the immune persistence of primary two-dose series we included participants who completed 6-month follow-up after two doses for cohort 1b-14d-8m, cohort 2b-28d-8m, and cohort 3-28d-8m; to assess the immune persistence of primary three-dose series we included participants who completed 6-month follow-up after three doses for cohort 1a-14d-2m and cohort 2a-28d-2m; defined as the immune persistence analysis set. We defined seropositivity as a titre of 8 or greater for neutralising antibodies to infectious SARS-CoV-2. Primary safety endpoints included any adverse reactions within 28 days after dose three in all cohorts. Secondary safety endpoints were serious adverse events occurring from the first dose to 6 months after the third dose in all vaccination cohorts. A complete list of outcomes is provided in appendix 4 (pp 6–7). Given that the 3 μg dose is the licensed formulation, and owing to space constraints, we mainly present results for the 3 μg group in the main text and provide detailed results for other intervention groups in tables and appendix 4.
Statistical analysis
The sample size was determined following requirements of the National Medical Products Administration, China's regulatory authority for vaccines. We assessed immunological endpoints in the per-protocol population, which included all participants who completed their assigned third doses and had antibody results available according to the protocol. In addition, we assessed the immune persistence of primary immunisation in the immune-persistence analysis set, which included participants who completed 6-month follow-up after two doses for cohort 1b-14d-8m, cohort 2b-28d-8m, and cohort 3-28d-8m and who completed 6-month follow-up after three doses for cohort 1a-14d-2m and cohort 2a-28d-2m. Serious adverse events were evaluated in the safety population, which included all participants who received at least one dose of study vaccine from the beginning of the vaccination schedule. Safety assessments for the third dose were done in a safety population data set of all participants who received a third dose.
The demographics of participants who received the third dose were summarised for vaccination cohorts, and Pearson χ2 test or Fisher's exact test were used to analyse categorical outcomes. We calculated 95% CIs for all categorical outcomes using the Clopper-Pearson method. We calculated GMTs and corresponding 95% CIs on the basis of the standard normal distribution of log-transformed antibody titres. For the third dose given at 28 days after the second dose (cohort 1a-14d-2m and cohort 2a-28d-2m), GMIs were calculated using antibody titres before vaccination and at 28 days after the third dose (taking prevaccination as baseline). For the booster dose given 6 months after the second dose (cohort 1b-14d-8m, cohort 2b-28d-8m, and cohort 3-28d-8m), GMIs were calculated using antibody titres before (ie, 6 months after the second dose) and at 28 days or 14 days after the third (booster) dose (taking pre-booster as baseline). ANOVA models with log-transformation (per GMT and GMI as above) were used to detect differences among groups. Post-hoc generalised liner mixed models (GLMM) were done to compare antibody concentrations induced by the third dose among participants in the four groups in cohorts 1 and 2, accounting for age, sex, dose group, vaccine schedule, interactions of dose and schedule, sampling time, and a random intercept for each participant.
Comparisons were done between groups by group t-tests with log-transformation and Bonferroni correction done as a post-hoc test if variance was significant. Hypothesis testing was two-sided, and we considered p values of less than 0·05 to be significant. We used R software version 3.6.0 for all analyses. The clinical trial is supervised by an independent data monitoring committee that consists of an independent statistician, a clinician, and an epidemiologist. Detailed information on the members is provided in appendix 4 (p 8). The trials are registered with ClinicalTrials.gov, NCT04352608 and NCT04383574.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
On May 3, 2020, 600 participants aged 18–59 years were enrolled into the phase 2 trial, of whom 540 (90%) were eligible and allocated to receive third doses (appendix 4 pp 13–14). Of these 540 participants, 139 (26%) participants were allocated to cohort 1a-14d-2m and 130 (24%) participants were allocated to cohort 2a-28d-2m; cohort 1a-14d-2m received a third dose at a median of 2 months (IQR 56–56 days) and cohort 2a-28d-2m received a third dose at a median of 2 months (IQR 51–51 days) after the second dose. 135 (97%) of 139 participants from cohort 1a-14d-2m and 124 (95%) of 130 participants from cohort 2a-28d-2m completed blood sampling to assess immune persistence for 6 months after dose three. Separately, 147 (25%) of the 600 participants assigned to cohort 1b-14d-8m and 138 (23%) assigned to cohort 2b-28d-8m were followed up for 6 months after the second dose, and 141 participants in cohort 1b-14d-8m (26% of the 540 participants eligible for a third dose) and 130 participants in cohort 2b-28d-8m (24% of the 540 participants eligible for a third dose) received a third dose at month 8 after the second dose for immunogenic evaluation (figure 1).
On June 12, 2020, 350 participants aged 60 years and older were enrolled in the phase 2 trial and 303 (87%) were allocated to receive third doses at month 8 after the second dose (appendix 4 p 15). 98 (32%) of the 303 participants were included in the immunogenicity analysis as described in the Methods (two participants were excluded due to protocol violation). The demographic characteristics of these 98 participants were similar to the other participants in the same age group (appendix 4 pp 9–11). All participants in the older age group were included in the safety analyses.
No natural infections were reported in any cohort. There were 141 minor protocol deviations in cohort 1b-14d-8m, including 141 participants given third doses 9–11 days outside of the prespecified time window, which did not result in exclusion of participants from the analysis (appendix 3 p 12). Mean ages of participants were between 40·4 years (SD 10·3) and 45·7 years (9·7) in cohorts 1 and 2 (adults aged 18–59 years old), and between 66·3 years (SD 4·4) and 67·1 years (4·7) in cohort 3 (adults aged 60 years and older; table 1 ). At baseline, none of the participants in any cohort had detectable neutralising antibodies (Figure 2, Figure 3 ).
Table 1.
Baseline demographic characteristics in the safety population of participants who received the third dose
| 1·5 μg group | 3 μg group | 6 μg group | Placebo group | |
|---|---|---|---|---|
| Cohort 1a-14d-2m (a third dose at month 2 after the second dose) | ||||
| Number of participants | NA | 55 | 58 | 26 |
| Age, years | NA | 45·2 (9·1) | 44·7 (8·6) | 44·3 (8·6) |
| Male | NA | 29 (53%) | 20 (34%) | 10 (38%) |
| Female | NA | 26 (47%) | 38 (66%) | 16 (62%) |
| Cohort 1b-14d-8m (a third dose at month 8 after the second dose) | ||||
| Number of participants | NA | 55 | 56 | 30 |
| Age, years | NA | 40·4 (10·3) | 42·4 (8·8) | 44·8 (6·9) |
| Male | NA | 24 (44%) | 27 (48%) | 12 (40%) |
| Female | NA | 31 (56%) | 29 (52%) | 18 (60%) |
| Cohort 2a-28d-2m (a third dose at month 2 after the second dose) | ||||
| Number of participants | NA | 54 | 50 | 26 |
| Age, years | NA | 42·5 (8·6) | 40·7 (9·4) | 44·0 (7·7) |
| Male | NA | 34 (63%) | 26 (52%) | 14 (54%) |
| Female | NA | 20 (37%) | 24 (48%) | 12 (46%) |
| Cohort 2b-28d-8m (a third dose at month 8 after the second dose) | ||||
| Number of participants | NA | 52 | 50 | 28 |
| Age, years | NA | 44·3 (9·5) | 43·1 (9·9) | 45·7 (9·7) |
| Male | NA | 23 (44%) | 26 (52%) | 11 (39%) |
| Female | NA | 29 (56%) | 24 (48%) | 17 (61%) |
| Cohort 3-28d-8m (a third dose at month 8 after the second dose) | ||||
| Number of participants | 85 | 90 | 81 | 47 |
| Age, years | 66·3 (4·4) | 66·4 (4·4) | 66·3 (4·4) | 67·1 (4·7) |
| Male | 41 (48%) | 44 (49%) | 37 (46%) | 27 (57%) |
| Female | 44 (52%) | 46 (51%) | 44 (54%) | 20 (43%) |
Data are n (%) or mean (SD). NA=not applicable.
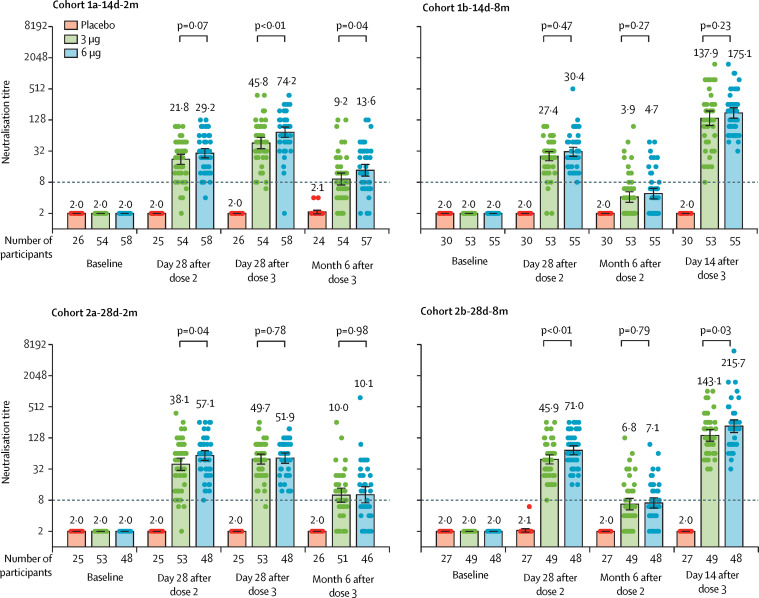
Figure 2.
Level of neutralising antibodies to infectious SARS-CoV-2 in adults aged 18–59 years
Dots are reciprocal neutralising antibody titres for individuals in the per-protocol population. Numbers above the bars are GMTs, and the error bars indicate the 95% CI. The dotted horizontal line represents the seropositivity threshold. Titres lower than the limit of detection (1/4) are presented as half the limit of detection. Numbers above the short horizontal lines are p values of comparisons between 3 μg group and 6 μg group. GMT=geometric mean titre.
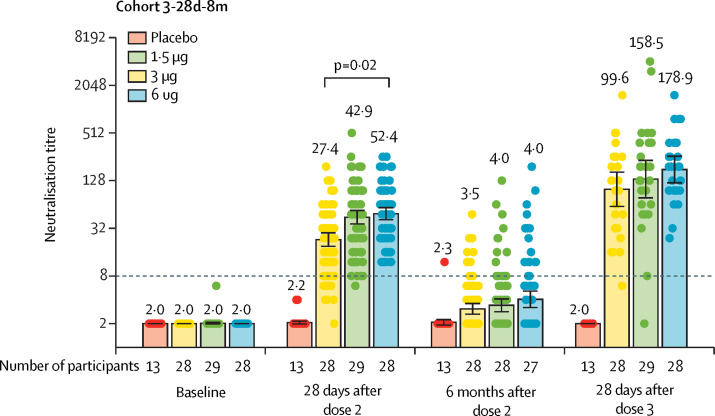
Figure 3.
Level of neutralising antibodies to infectious SARS-CoV-2 in adults aged 60 years and older
Dots are reciprocal neutralising antibody titres for individuals in the per-protocol population. Numbers above the bars are GMTs, and the error bars indicate the 95% CI. The dotted horizontal line represents the seropositivity threshold. Titres lower than the limit of detection (1/4) are presented as half the limit of detection. Numbers above the short horizontal lines are p values of comparisons between 1.5 μg group, 3 μg group, and 6 μg group. Only the p values indicating significant difference are marked. GMT=geometric mean titre.
A third dose of CoronaVac given at month 2 after the second dose moderately increased neutralising antibody levels induced by the first two doses. In the 3 μg group, the GMT in cohort 1a-14d-2m on day 28 after dose 2 was 21·8 (95% CI 17·3–27·6) and on day 28 after dose 3 was 45·8 (35·7–58·9), and in cohort 2a-28d-2m GMT on day 28 after dose 2 was 38·1 (95% CI 28·4–51·1) and on day 28 after dose 3 was 49·7 (39·9–61·9; figure 2; table 2 ). GMIs of neutralising antibodies from baseline to 28 days after the third dose were 22·9 (95% CI 17·8–29·4) for cohort 1a-14d-2m and 24·8 (19·9–31·0) for cohort 2a-28d-2m (table 2). Seropositivity rates in all vaccination groups in cohorts 1a-14d-2m and 2a-28d-2m were above 95% at 28 days after three doses (table 2).
Table 2.
Immunogenicity assessment on day 28 after the third dose
| 1·5 μg group | 3 μg group | 6 μg group | Placebo | p value† | p value† | |
|---|---|---|---|---|---|---|
| Cohort 1a-14d-2m | ||||||
| Seropositivity | NA | 53/54 (98%; 90·11–99·95) | 57/58 (98%; 90·76–99·96) | 0/26 (0·00–13·23) | <0·0001 | 1·00 |
| GMT (95% CI) | NA | 45·8 (35·7–58·9) | 74·2 (59·0–93·3) | 2·0 (2·0–2·0) | <0·0001 | 0·0053 |
| GMI (95% CI) | NA | 22·9 (17·8–29·4) | 37·1 (29·5–46·6) | 1·0 (1·0–1·0) | <0·0001 | 0·0052 |
| Cohort 1b-14d-8m‡ | ||||||
| Seropositivity | NA | 53/53 (100%; 93·28–100·00) | 55/55 (100%; 93·51–100·00) | 0/30 (0·00–11·57) | <0·0001 | 1·00 |
| GMT (95% CI) | NA | 137·9 (99·9–190·4) | 175·1 (138·8–221·0) | 2·0 (2·0–2·0) | <0·0001 | 0·23 |
| GMI (95% CI) | NA | 35·1 (24·3–50·7) | 36·9 (28·5–47·8) | 1·0 (1·0–1·0) | <0·0001 | 0·82 |
| Cohort 2a-28d-2m | ||||||
| Seropositivity | NA | 52/53 (98%; 89·93–99·95) | 48/48 (100%; 92·60–100·00) | 0/25 (0·00–13·72) | <0·0001 | 1·00 |
| GMT (95% CI) | NA | 49·7 (39·9–61·9) | 51·9 (41·3–65·3) | 2·0 (2·0–2·0) | <0·0001 | 0·78 |
| GMI (95% CI) | NA | 24·8 (19·9–31·0) | 26·0 (20·7–32·7) | 1·0 (1·0–1·0) | <0·0001 | 0·78 |
| Cohort 2b-28d-8m | ||||||
| Seropositivity | NA | 49/49 (100%; 92·75–100·00) | 48/48 (100%; 92·60–100·00) | 0/27 (0·00–12·77) | <0·0001 | 1·00 |
| GMT (95% CI) | NA | 143·1 (110·8–184·7) | 215·7 (162·6–286·2) | 2·0 (2·0–2·0) | <0·0001 | 0·03 |
| GMI (95% CI) | NA | 21·2 (15·3–29·2) | 30·4 (21·5–43·0) | 1·0 (1·0–1·0) | <0·0001 | 0·24 |
| Cohort 3-28d-8m§ | ||||||
| Seropositivity | 27/28 (96%; 81·65–99·91) | 29/29 (100%; 88·06–100·00) | 27/28 (96%; 81·65–99·91) | 0/13 (0·00–24·71) | <0·0001 | 0·49 |
| GMT (95% CI) | 99·6 (62·0–159·9) | 158·5 (99·0–253·7) | 178·9 (125·2–255·6) | 2·0 (2·0–2·0) | <0·0001 | 0·37 |
| GMI (95% CI) | 28·2 (16·8–47·4) | 39·7 (23·6–66·6) | 44·2 (27·2–71·9) | 0·9 (0·7–1·1) | <0·0001 | 0·77 |
Data are n/N (%; 95% CI) unless otherwise stated. ANOVA model with log-transformation (per GMT and GMI as above) was used to detect the difference among groups. Comparison between groups was conducted by group t-test with log-transformation. GMT=geometric mean titre. GMI=geometric mean increase. NA=not applicable.
p values are for comparisons among all groups.
p values are for comparisons between the 3 μg group and the 6 μg group.
Immunogenicity was assessed on day 14 after the third dose.
p values for comparisons between the 1·5 μg group and the 3 μg group were 0·49 for seropositivity, 0·18 for GMTs, and 0·37 for GMIs; p values for comparisons between the 1·5 μg group and the 6 μg group were 1·00 for seropositivity, 0·06 for GMTs, and 0·22 for GMIs.
Results of immune persistence analysis from cohort 1a-14d-2m and cohort 2a-28d-2m show that, by 6 months after the third dose, the GMT was approximately 10 and seropositivity remained above 50% (appendix 4 pp 16–17). GMTs in cohort 1a-14d-2m on day 28 (p=0·0053) and at month 6 (p=0·039) after the third dose were significantly higher in the 6 μg group than in the 3 μg group, whereas there was no significant difference between the two doses at either timepoint in cohort 2a-28d-2m (appendix 4 pp 16–17).
Regardless of the interval between the first two doses, neutralising antibody titres declined to below the seropositive cutoff by 6 months after the second dose (GMT 3·9 [95% CI 3·1–5·0] in cohort 1b-14d-8m and 6·8 [5·2–8·8] in cohort 2b-28d-8m; figure 2). In the immune persistence analysis set, at month 6 after the second dose, ten (17%) of 59 participants in cohort 1b-14d-8m and 19 (35%) of 54 participants in cohort 2b-28d-8m were seropositive (appendix 4 pp 18–19).
In post-hoc analyses, after administering a booster dose at 8 months after the second dose, GMTs increased to 137·9 (95% CI 99·9–190·4) in cohort 1b-14d-8m 14 days later, and to 143·1 (110·8–184·7) in cohort 2b-28d-8m 28 days later (figure 2). Neutralising antibody concentrations 14 days after dose 3 were approximately five-fold higher than neutralising antibody concentrations on day 28 after the second dose in cohort 1b-14d-8m (from a GMT of 27·4 to 137·9 in the 3 μg group and from a GMT of 30·4 to 175·1 in the 6 μg group), and in cohort 2b-28d-8m, neutralising antibody titres 28 days after the third dose were approximately three-fold higher than neutralising antibody titres 28 days after the second dose (from a GMT of 45·9 to 143·1 in the 3 μg group; table 2, figure 2). Seropositivity on day 14 after the third dose in cohort 1b-14d-8m and on day 28 after the third dose in cohort 2b-28d-8m was 100% for both doses (table 2). GMIs from before to after the booster dose were 35·1 (95% CI 24·3–50·7) in cohort 1b-14d-8m and 21·2 (15·3–29·2) in cohort 2b-28d-8m (table 2).
In GLMM models, neutralisation titres decreased with increasing age (appendix 4 p 21). Immune responses induced by 6 μg doses were better than those induced by 3 μg doses, and a third dose significantly raised antibody levels compare with 28 days after dose 2. The vaccination schedule used in cohort 2b-28d-8m produced the best immunogenicity (appendix 4 p 21).
In the immune persistence analysis of cohort 3-28d-8m, in the 3 μg group, neutralising antibody titres had declined to below the seropositive cutoff at 6 months after the second dose (from 40·8 [95% CI 33·8–49·3] at day 28 after dose 2 to 3·4 [2·9–4·1]), and 17 (18%) of 98 participants were seropositive (appendix 4 p 20). A booster dose given 8 months after the second dose increased the GMT to 158·5 (95% CI 96·9–259·2) 28 days after the booster dose (figure 3, table 2). The GMI from before to after the booster dose was 39·7 (95% CI 23·6–66·6; table 2). GMTs on day 28 after the third dose were highest in the 6 μg group (p<0·0001) and similar between the 3 μg group and the 1·5 μg group (p=0·18; table 2).
Severities of solicited local and systemic adverse reactions reported within 28 days after the third dose were grade 1–2 in all vaccination cohorts in both trials. The most common reported reaction was injection-site pain (table 3 ; appendix 4 pp 22–28). Taking the 3 μg group as an example, the incidences of adverse reactions within 28 days after the third dose in primary three-dose regimens were five (9%) of 55 participants in cohort 1a-14d-2m and three (6%) of 54 participants in cohort 2a-28d-2m; not higher than the incidence of adverse reactions within 28 days after each previous dose (table 3; appendix 4 pp 22–23, 25–26). The overall incidence of any adverse reaction within 28 days after the booster dose (3 μg) was ten (18%) of 55 participants in cohort 1b-14d-8m, eight (15%) of 52 in cohort 2b-28d-8m, and five (6%) of 90 in cohort 3-28d-8m (table 3; appendix 4 p 24, 27–28).
Table 3.
Adverse reactions within 28 days after the third dose
|
Cohort 1a-14d-2m |
Cohort 1b-14d-8m |
Cohort 2a-28d-2m |
Cohort 2b-28d-8m |
Cohort 3–28d-8m |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 μg (N=55) | 6 μg (n=58) | Placebo (N=26) | 3 μg (N=55) | 6 μg (N=56) | Placebo (N=30) | 3 μg (N=54) | 6 μg (N=50) | Placebo (N=26) | 3 μg (N=52) | 6 μg (N=50) | Placebo (N=28) | 1·5 μg (N=85) | 3 μg (N=90) | 6 μg (N=81) | Placebo (N=47) | |
| Any adverse reaction | ||||||||||||||||
| Grade 1 | 5 (9%) | 5 (9%) | 0 | 10 (18%) | 13 (23%) | 3 (10%) | 3 (6%) | 1 (2%) | 0 | 7 (13%) | 10 (20%) | 1 (4%) | 3 (4%) | 3 (3%) | 3 (4%) | 2 (4%) |
| Grade 2 | 1 (2%) | 1 (2%) | 0 | 1 (2%) | 0 | 1 (3%) | 0 | 0 | 0 | 1 (2%) | 1 (2%) | 1 (4%) | 1 (1%) | 2 (2%) | 2 (2%) | 1 (2%) |
| Systemic diseases and injection site adverse reactions | ||||||||||||||||
| Injection site pain | 3 (5%) | 5 (9%) | 0 | 8 (1%) | 9 (16%) | 0 | 1 (2%) | 1 (2%) | 0 | 6 (12%) | 7 (14%) | 0 | 1 (1%) | 2 (2%) | 2 (2%) | 1 (2%) |
| Injection site swelling | 0 | 0 | 0 | 0 | 0 | 1 (3%) | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Injection site itch | 0 | 0 | 0 | 0 | 1 (2%) | 2 (7%) | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Injection site erythema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
| Fever | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 0 | 0 | 1 (2%) | 1 (2%) | 1 (4%) | 0 | 0 | 0 | 0 |
| Fatigue | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 1 (2%) | 0 | 0 | 1 (2%) | 2 (4%) | 0 | 0 | 1 (1%) | 0 | 1 (2%) |
| Respiratory, thoracic, and mediastinal disorders | ||||||||||||||||
| Cough | 0 | 1 (2%) | 0 | 0 | 2 (4%) | 0 | 2 (4%) | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 1 (1%) | 1 (2%) |
| Runny nose | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 0 | 1 (1%) | 0 |
| Oropharyngeal pain | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Laryngeal stimulation | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nervous system disorders | ||||||||||||||||
| Dizziness | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 1 (1%) | 0 | 0 |
| Headache | 0 | 1 (2%) | 0 | 1 (2%) | 2 (4%) | 1 (3%) | 0 | 0 | 0 | 1 (2%) | 1 (2%) | 1 (4%) | 0 | 0 | 1 (1%) | 0 (0%) |
| Gastrointestinal disorders | ||||||||||||||||
| Diarrhoea | 1 (2%) | 0 | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 1 (2%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Nausea | 1 (2%) | 1 (2%) | 0 | 1 (2%) | 0 | 0 | 1 (2%) | 0 | 0 | 0 | 2 (4%) | 0 | 1 (1%) | 1 (1%) | 0 | 0 |
| Musculoskeletal and connective tissue disorders | ||||||||||||||||
| Muscle pain | 1 (2%) | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Myalgia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 1 (1%) | 0 | 0 | 0 |
| Skin and subcutaneous tissue disorders | ||||||||||||||||
| Rash | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Eye disorders | ||||||||||||||||
| Periorbital oedema | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Data are n (%), representing the total number of participants who had adverse reactions (ie, adverse events related to vaccination).
Serious adverse events were reported in one (2%) of 60 participants in the 3 μg group and two (3%) of 60 participants in the 6 μg group in cohort 1a-14d-2m, in two (3%) of 60 participants in the 3 μg group and two (3%) of 60 in the 6 μg group in cohort 1b-14d-8m, and in no participant in the 30 μg group and one (2%) of 60 in the 6 μg group in each of cohorts 2a-28d-2m and 2b-28d-8m (appendix 4 pp 29–30). No participant in the placebo group reported a serious adverse event. From the beginning of immunisation to 28 days after dose 3 in cohort 3-28d-8m, ten (10%) of 100 participants in the 1·5 μg group, five (5%) of 101 in the 3 μg group, seven (7%) of 99 in the 6 μg group, and two (4%) of 49 in the placebo group had non-fatal serious adverse events (appendix 4 pp 30–31). No serious adverse event in either trial was considered by the investigators to be related to vaccination, and no prespecified trial-halting rules were met.
Discussion
Our study showed that the initial neutralising antibody response from two doses of CoronaVac declined to near or below the lower limit of seropositivity after 6 months. A third dose of CoronaVac (3 μg) given 8 months after the second dose led to a strong boost in immunity, with neutralising GMTs increasing to approximately 140 among adults aged 18–59 years and 159 among adults aged 60 years and older 14–28 days after the booster dose. These increases correspond to roughly three-fold to five-fold increases in neutralising antibody titres compared with titres 28 days after a second dose. Seropositivity 28 days after a third dose at 8 months was 98–100% regardless of age group. By contrast, a third dose given 2 months after the second dose induced much lower neutralising antibody titres. Reactogenicity of the third dose was indistinguishable from reactogenicity of the previous two doses, regardless of age group.
Decreases over time of vaccine-induced neutralising antibodies against ancestral SARS-CoV-2 have been observed with other COVID-19 vaccines, but at a much lower magnitude. For example, following vaccination with Moderna's mRNA-1273 vaccine, neutralising antibodies declined but remained detectable among all participants on days 90 and 180 after a second dose.6, 26 SARS-CoV-2 spike protein-specific memory B cells are detectable in most patients with COVID-19 and in people who are naive to SARS-CoV-2 after receiving two doses of COVID-19 vaccines.27, 28 This study is the first to show that the antibody response mediated by a third dose of CoronaVac given 2 months after the second dose rebounded only moderately and degraded to near the seropositive threshold after 6 months. This observation is probably because the interval between the two doses was short and the memory B cells were immature. However, a third dose of CoronaVac given 8 months after the second dose appears to effectively augment the potency, breadth, and likely duration of anamnestic responses against SARS-CoV-2.29 Compared with the 3 μg formulation of CoronaVac, which is approved for use, the 1·5 μg formulation produced similar neutralising antibody titres by day 28 after the third dose for adults aged 60 years and older. Whether the 1·5 μg formulation could serve as a booster dose needs further study due to the small sample size in the analysis of this dose (28 participants).
Significant rebound in antibody concentration induced by homologous booster doses has been reported for other vaccines. Neutralisation titres against ancestral SARS-CoV-2 increased approximately four-fold after a homologous booster dose compared with titres following primary series with BNT162b2,10 mRNA-1273,11 and NVX-CoV2373,12 with similarly long intervals (6–8 months) between the booster dose and primary vaccination. A nine-fold increase in spike protein-binding antibody was observed after a 6-month homologous booster dose of Ad26.COV2-S.30
Heterologous prime–boost regimens appear to induce higher levels of immune response than homologous booster doses. Vaccination with mRNA vaccines and adenovirus-vectored vaccines31, 32 or inactivated vaccines and adenovirus-vectored vaccines33 have shown strong short-term immune responses and tolerable reactogenicity. Wanlapakorn and colleagues34 found that CoronaVac and AZD1222 vaccine recipients had higher neutralising antibody activity against the original wild-type virus and the beta (B.1.351) variant of concern than did recipients of two doses of CoronaVac or AZD1222, suggesting that heterologous immunisation might be considered an alternative to homologous boosting for immunisation programmes. Long-term effectiveness of boosting remains unevaluated because of the newness of COVID-19 vaccine booster dosing.
SARS-CoV-2 continues to evolve and produce variants, among which the delta variant has become predominant.9 Although we did not perform neutralisation testing in vitro against emerging variants of concern, high neutralising antibody titres against the ancestral strain are believed to be important for protection against novel circulating SARS-CoV-2 variants that potentially can lead to immune escape.35 Several studies have reported in-vitro neutralisation titres against variants for CoronaVac, but results varied greatly. Vacharathit and colleagues, using a live-virus microneutralisation assay, identified 22-fold and 32-fold reductions in neutralising antibodies against the beta and delta variants, respectively, compared with ancestral SARS-CoV-2.36 Wang and colleagues reported a three-fold reduction in neutralising antibody titres against the beta variant, using a pseudovirus neutralisation assay.37 Another study reported 5·7-fold, 4·3-fold, and 3·7-fold reductions of neutralising antibody titres against beta, gamma (P.1), and delta variants, respectively.29 Of note, it is difficult to directly compare these estimates because of the differences in study design and laboratory methods.38 Determining the neutralisation ability of CoronaVac to emerging variants and evaluating the protection level in risk groups such as immunosuppressed individuals or elderly people are important research endeavours.
Decreased effectiveness of mRNA vaccines against SARS-CoV-2 infection with circulating variants has been seen in real-world studies in the USA, but effectiveness against hospitalisation was sustained.39, 40 Two doses of CoronaVac showed good effectiveness in a setting with co-circulating alpha and gamma variants in Chile: the vaccine was 66% effective against COVID-19 and nearly 90% effective against severe outcomes.19 A test-negative case-control study done in Brazil showed that the adjusted vaccine effectiveness against hospital admission was above 55% in older adults during a time of extensive transmission of the gamma variant.20 During local outbreaks caused by the delta variant in China, two studies with small sample sizes showed that inactivated vaccines were 70·2% effective against illness of moderate or worse severity41 and could lower the risk of progressing to severe disease by 88%.22 Protection against variants and persistence in protection with CoronaVac need to be continually evaluated in real-world studies.
Interim protection results from booster programmes in Israel showed that booster doses effectively reduced breakthrough infections, including breakthroughs of the delta variant.14 Considering sustained protection of primary immunisation with COVID-19 vaccines against severe outcomes42 and equity in vaccine deployment, WHO currently prioritises completion of primary immunisation over booster dose strategies to protect more people from COVID-19 due to global shortage of supply of COVID-19 vaccines,43 although the US Centers for Disease Control and Prevention has issued booster recommendations for specific populations.44
During the trials, participants were masked to study group assignment and participants in placebo groups could be vaccinated immediately after completion of the phase 2 trial for adults aged 18–59 years and completion of follow-up for 28 days after the booster dose for adults aged 60 years and older. Since strict non-pharmaceutical interventions have been maintained to date across mainland China, the risk of infection was very low for participants in the placebo group. Maintenance of the placebo groups until the end of the trial was approved by Jiangsu Ethics Committee (JSJK2020-A021–02) and Hebei CDC Ethics Committee (IRB2020–006).
Our study has several limitations. First, establishment of SARS-CoV-2 spike protein-specific immune memory, in addition to inducing durable antibodies, might be important for a successful COVID-19 vaccine. For example, T-cell immunity elicited by inactivated vaccines might contribute to protection.45, 46 However, T-cell responses and neutralisation tests in vitro against emerging variants were not assessed in our study, and these need to be further explored. Second, we report the results of interim analyses, and long-term follow-up is ongoing to identify a satisfactory duration of immunity induced by the booster dose and to assess longer-term safety. Third, a population at greatest risk of immunosenescence (ie, adults aged 80 years and older) was not evaluated in this study. Larger, multicentre studies will be needed to assess primary outcomes among subpopulations for whom our study had relatively small proportions. Fourth, although neutralising antibodies are related to protection, actual protection from infection with current and emerging variants will need to be monitored with real-world observational studies. Further research to identify correlates of protection and to determine whether different vaccines have different correlates is important.
In conclusion, our study found that a two-dose schedule of CoronaVac generated good immune memory. Although neutralising antibody titres decreased to near or below the lower limit of seropositivity 6 months after the second dose, a third dose given 8 months after the second dose was highly effective at recalling a SARS-CoV-2-specific immune response, leading to a significant rebound in antibody levels. Our study indicates that a homologous booster dose might provide longer-lasting immunity and higher levels of protection than a two-dose schedule, but additional study is needed to monitor neutralisation ability and effectiveness against variants.
Data sharing
The individual participant-level data that underlie the results reported in this Article (text, tables, figures, and appendices) will be shared after de-identification. This clinical trial is ongoing, and all the individual participant data cannot be available until the immune persistence evaluation is done. The data will be available immediately after publication and finalisation of the completed clinical study report for at least 1 year. Supporting clinical documents, including the study protocol and statistical analysis plan, and the informed consent form will be available immediately following the publication of this Article for at least 1 year. Information on how to access supporting clinical documents is available online for adults aged 18–59 years at http://www.jshealth.com/ and for adults aged 60 years and older at http://www.hebeicdc.cn/kygz/22506.jhtml. Researchers who provide a scientifically sound proposal will be allowed access to the de-identified individual participant data. Proposals should be sent to the corresponding authors. These proposals will be reviewed and approved by the sponsor, investigators, and collaborators on the basis of scientific merit. To gain access, data requestors will need to sign a data access agreement.
Declaration of interests
HY received research funding from Sanofi Pasteur, GlaxoSmithKline, Yichang HEC Changjiang Pharmaceutical Company, and Shanghai Roche Pharmaceutical Company; none of this research funding is related to development of COVID-19 vaccines. GZ, LeW and WY are employees of Sinovac Biotech and LiW and DJ are employees of Sinovac Life Sciences. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
The study was supported by grants from National Key Research and Development Program (2020YFC0849600), Beijing Science and Technology Program (Z201100005420023), and Key Program of the National Natural Science Foundation of China (82130093). We thank Dr Lance Rodewald from Chinese Center for Disease Control and Prevention for his comments and English language editing.
Contributors
GZ, QW, HP, ML, JY, YZ, FZ, HY, and WY designed the study and contributed to data collection, data analysis, data interpretation, and writing of the manuscript. GZ, QW, HP, and ML verified the data. ZW, KC, LeW, and BH collected data and revised the manuscript. DJ and LiW did the laboratory assays and revised the manuscript. XD, WZ, and WL analysed the data and revised the manuscript. All authors had full access to all of the data (including statistical reports and tables) in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors had final responsibility for the decision to submit the manuscript for publication.
Supplementary Materials
References
- 1.UNICEF COVID-19 vaccine market dashboard. https://www.unicef.org/supply/covid-19-vaccine-market-dashboard
- 2.Our World in Data Statistics and research, coronavirus (COVID-19) vaccinations. https://ourworldindata.org/covid-vaccinations
- 3.Favresse J, Bayart JL, Mullier F, et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerg Microbes Infect. 2021;10:1495–1498. doi: 10.1080/22221751.2021.1953403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nanduri S, Pilishvili T, Derado G, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (delta) variant—national healthcare safety network, March 1–August 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1163–1166. doi: 10.15585/mmwr.mm7034e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doria-Rose N, Suthar MS, Makowski M, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for COVID-19. N Engl J Med. 2021;384:2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flaxman A, Marchevsky NG, Jenkin D, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002) Lancet. 2021;398:981–990. doi: 10.1016/S0140-6736(21)01699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z, Azman AS, Chen X, et al. Landscape of SARS-CoV-2 genomic surveillance, public availability extent of genomic data, and epidemic shaped by variants: a global descriptive study. medRxiv. 2021 https://www.medrxiv.org/content/10.1101/2021.09.06.21263152v1 published online Sept 8. (preprint). [Google Scholar]
- 10.Falsey AR, Frenck RW, Jr, Walsh EE, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021;385:1627–1629. doi: 10.1056/NEJMc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moderna Business updates, second quarter 2021 financial results. Aug 5, 2021. https://investors.modernatx.com/static-files/c43de312-8273-4394-9a58-a7fc7d5ed098
- 12.NOVAVAX Second quarter 2021 financial results and operational highlights. Aug 5, 2021. https://filecache.investorroom.com/mr5ir_novavax/115/Q2-2021-Earnings-Slides.pdf
- 13.Israel National News Booster program expands: Israel expands third vaccine dose to all adults over 30. Aug 24, 2021. https://www.israelnationalnews.com/News/News.aspx/312324
- 14.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.China Food and Drug Administration Conditional use approval for CoronaVac. https://www.nmpa.gov.cn/yaopin/ypjgdt/20210206154636109.html
- 16.WHO Regulation and prequalification. https://www.who.int/teams/regulation-prequalification/eul/covid-19
- 17.Gavi. the Vaccine Alliance COVAX global supply forecast. Sept 8, 2021. https://www.gavi.org/sites/default/files/covid/covax/COVAX-Supply-Forecast.pdf
- 18.China Food and Drug Administration COVID-19 vaccination status (as of October 3) http://www.gov.cn/xinwen/2021-10/04/content_5640964.htm
- 19.Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranzani OT, Hitchings MDT, Dorion M, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of COVID-19 in Brazil: test negative case-control study. BMJ. 2021;374 doi: 10.1136/bmj.n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang M, Yi Y, Li Y, et al. Effectiveness of inactivated COVID-19 vaccines against COVID-19 pneumonia and severe illness caused by the B.1.617.2 (delta) variant: evidence from an outbreak in Guangdong, China. SSRN. 2021 doi: 10.7326/M21-3509. https://ssrn.com/abstract=3895639 published online Aug 5. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Z, Tao B, Li Z, et al. Effectiveness of inactive COVID-19 vaccines against severe illness in B.1.617.2 (delta) variant-infected patients in Jiangsu, China. medRxiv. 2021 doi: 10.1016/j.ijid.2022.01.030. https://www.medrxiv.org/content/10.1101/2021.09.02.21263010v1.full published online Sept 5. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:803–812. doi: 10.1016/S1473-3099(20)30987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO Polio laboratory manual, 4th ed. 2004. https://apps.who.int/iris/handle/10665/68762
- 26.Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384:80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goel RR, Apostolidis SA, Painter MM, et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang K, Cao Y, Zhou Y, et al. A third dose of inactivated vaccine augments the potency, breadth, and duration of anamnestic responses against SARS-CoV-2. medRxiv. 2021 doi: 10.1093/procel/pwae033. https://www.medrxiv.org/content/10.1101/2021.09.02.21261735v1 published online Sept 5. (preprint). [DOI] [PubMed] [Google Scholar]
- 30.Sadoff J, Le Gars M, Cardenas V, et al. Durability of antibody responses elicited by a single dose of Ad26.COV2.S and substantial increase following late boosting. medRxiv. 2021 https://www.medrxiv.org/content/10.1101/2021.08.25.21262569v1 published online Aug 26. (preprint). [Google Scholar]
- 31.Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021;27:1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borobia AM, Carcas AJ, Pérez-Olmeda M, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Hou L, Guo X, et al. Heterologous prime–boost immunization with CoronaVac and Convidecia. medRxiv. 2021 doi: 10.1101/2021.09.03.21263062. published online Sept 6. (preprint). [DOI] [Google Scholar]
- 34.Wanlapakorn N, Suntronwong N, Phowatthanasathian H, et al. Immunogenicity of heterologous prime/booster-inactivated and adenoviral-vectored COVID-19 vaccine: real-world data. Research Square. 2021 doi: 10.1016/j.vaccine.2022.04.043. https://www.researchsquare.com/article/rs-785693/v1 published online Sept 10. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi A, Koch M, Wu K, et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med. 2021 doi: 10.1038/s41591-021-01527-y. published online Sept 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vacharathit V, Aiewsakun P, Manopwisedjaroen S, et al. CoronaVac induces lower neutralising activity against variants of concern than natural infection. Lancet Infect Dis. 2021;21:1352–1354. doi: 10.1016/S1473-3099(21)00568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang G-L, Wang Z-Y, Duan L-J, et al. Susceptibility of circulating SARS-CoV-2 variants to neutralization. N Engl J Med. 2021;384:2354–2356. doi: 10.1056/NEJMc2103022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, Chen Z, Azman AS, et al. Neutralizing antibodies against SARS-CoV-2 variants induced by natural infection or vaccination: a systematic review and pooled meta-analysis. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab646. published online July 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tenforde MW, Self WH, Naioti EA, et al. Sustained effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 associated hospitalizations among adults—United States, March–July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1156–1162. doi: 10.15585/mmwr.mm7034e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eli S, David H, Vajeera D, et al. New COVID-19 cases and hospitalizations among adults, by vaccination status—New York, May 3–July 25, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1150–1155. doi: 10.15585/mmwr.mm7034e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li XN, Huang Y, Wang W, et al. Efficacy of inactivated SARS-CoV-2 vaccines against the delta variant infection in Guangzhou: a test-negative case-control real-world study. Emerg Microbes Infect. 2021;10:1751–1759. doi: 10.1080/22221751.2021.1969291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krause PR, Fleming TR, Peto R, et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet. 2021;398:1377–1380. doi: 10.1016/S0140-6736(21)02046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WHO Interim statement on COVID-19 vaccine booster doses. Aug 10, 2021. https://www.who.int/news/item/10-08-2021-interim-statement-on-covid-19-vaccine-booster-doses [PMC free article] [PubMed]
- 44.Centres for Disease Control and Prevention CDC statement on ACIP booster recommendations. Sept 24, 2021. https://www.cdc.gov/media/releases/2021/p0924-booster-recommendations-.html
- 45.Quast I, Tarlinton D. B cell memory: understanding COVID-19. Immunity. 2021;54:205–210. doi: 10.1016/j.immuni.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng Y, Li Y, Yang R, Tan W. SARS-CoV-2-specific T cell immunity to structural proteins in inactivated COVID-19 vaccine recipients. Cell Mol Immunol. 2021;18:2040–2041. doi: 10.1038/s41423-021-00730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The individual participant-level data that underlie the results reported in this Article (text, tables, figures, and appendices) will be shared after de-identification. This clinical trial is ongoing, and all the individual participant data cannot be available until the immune persistence evaluation is done. The data will be available immediately after publication and finalisation of the completed clinical study report for at least 1 year. Supporting clinical documents, including the study protocol and statistical analysis plan, and the informed consent form will be available immediately following the publication of this Article for at least 1 year. Information on how to access supporting clinical documents is available online for adults aged 18–59 years at http://www.jshealth.com/ and for adults aged 60 years and older at http://www.hebeicdc.cn/kygz/22506.jhtml. Researchers who provide a scientifically sound proposal will be allowed access to the de-identified individual participant data. Proposals should be sent to the corresponding authors. These proposals will be reviewed and approved by the sponsor, investigators, and collaborators on the basis of scientific merit. To gain access, data requestors will need to sign a data access agreement.